Background.
Ex vivo perfusion technology has been actively developed to solve the problem of severe donor shortage. In this study, the ex vivo metabolic characteristics of porcine donation after circulatory death (DCD) liver in short-term perfusion using whole or diluted blood were compared with those of the in vivo transplanted state to evaluate their initial response to resuscitation.
Methods.
The porcine DCD model was constructed by clamping the thoracic aorta. After 60 min of blood flow cessation, retrieved livers were flushed with 500 mL of heparin saline (20 000 IU/L) followed by perfusion with 500 mL of cold histidine-tryptophan-ketoglutarate solution. The liver grafts were immersed in cold histidine-tryptophan-ketoglutarate solution for 60 min. Subsequently, normothermic ex vivo perfusion was performed with 20 000 IU/L of heparin added to the collected blood (whole blood group) or medium mixed with 10% whole blood (dilution group) for 3 h. Blood from the portal vein, the hepatic artery, and infra hepatica inferior vena cava was collected hourly and metabolomic analyses were performed. The other liver graft was heterotopically transplanted as a control (in vivo group). Each experiment was conducted once.
Results.
The guanosine levels demonstrated similar fluctuating trends in the whole blood and in vivo groups. In contrast, the levels increased during the perfusion in the diluted blood group. Fluctuations in choline metabolism demonstrated similar trends in the whole blood and in vivo groups.
Conclusions.
Ex vivo machine perfusion with whole blood over a short time resulted in a metabolic trend similar to that in the in vivo model. Further studies in this regard are warranted to progress in the utilization of DCD organs.
INTRODUCTION
Donor shortage is a severe problem globally. One solution is the transplantation of liver grafts via a donation after circulatory death (DCD). Recently, it has been reported that reperfusion injury of livers from a controlled DCD (c-DCD) or uncontrolled DCD (u-DCD) following transplantation could be reduced with normothermic ex vivo perfusion using diluted blood.1,2 This ex vivo perfusion method can be further improved by maintaining the metabolism of the extracted liver and culturing it for a week.3 Additionally, it was highlighted that it is crucial to maintain the metabolism of the extracted liver ex vivo similar to that of an in vivo system.
While it has been reported that the ex vivo system with culture medium-based diluted blood works in small animal models,4,5 most clinical cases are currently perfused normothermally with whole blood-based perfusate with anticoagulant.2,6 However, ex vivo perfusion is difficult to perform in livers with prolonged circulatory arrest and large amounts of microthrombi. Furthermore, the metabolism of these livers has not been fully characterized, although investigations into the viscosity of the perfusate and analysis of the metabolic state during perfusion have been studied.7-9 Previously, we reported a method for continuous resuscitation of the ischemic liver between the initiation of machine perfusion and vascular anastomosis in the recipient in a porcine u-DCD model.10 The aim of this study was to characterize the metabolism of resuscitated livers in a porcine u-DCD model by comparing diluted blood perfusion with whole blood-based perfusion over a short duration. Furthermore, we compared the metabolism of resuscitation in vivo by transplanting heterotopically with those in the 2 types of ex vivo perfusions.
MATERIALS AND METHODS
Experimental Design
A DCD liver model with 60 min of circulatory arrest was constructed in pigs as reported previously.10 The extracted liver grafts were flushed with 500 mL of heparin saline (20 000 IU/L) and replaced with 500 mL of cold histidine-tryptophan-ketoglutarate solution. Subsequently, normothermic ex vivo perfusion was performed using our custom-made system with 2 types of perfusion solutions. For the dilution group, the perfusion solution consisted of 10% diluted donor blood, Leibovitz’s Medium (L-15 medium, Thermo fisher scientific, Waltham, MA), and pig serum (26250, Thermo fisher scientific, Waltham, MA) (total volume, 2000 mL). For the whole blood group, the perfusion solution consisted of whole blood from a donor and heparin (5000 IU/L, NIPRO, Osaka, Japan) (total volume, 1300 mL). Each experiment was conducted once.
The fluids flowing in and out of the liver were sampled every hour immediately after perfusion was initiated for metabolomic analysis. As a control group, heterotopic transplantations were also performed (in vivo group, N = 1). Under general anesthesia, blood from the portal vein (PV) and infra hepatica inferior vena cava (IH-IVC) was collected for 3 h after resuming blood flow and analyzed similar to that in the other groups (Figure 1). Metabolomic analysis was performed with 1 case in each group.
FIGURE 1.
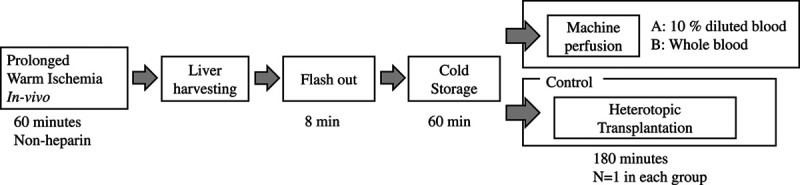
Experimental design.
Animals and Study Design
The experiments with pig livers were approved by the Ethics Committee for Animal Experimentation of the Keio University School of Medicine (Approval number. 16072[0]), and the procedures were performed according to the Guidelines of Keio University on Animal Use. The pigs were female juvenile 3-way hybrid pigs (Landrace, Large White; 25–30 kg). The pigs were anesthetized, and cannulation tubes were inserted into the right carotid artery for reagent administration and blood collection. The animals underwent liver resection while being infused with Ringer’s solution (Lactec D, Otsuka Pharmaceutical Factory, Inc., Japan).
Porcine DCD Model
The preparation of the porcine DCD model and subsequent liver removal was performed as described previously.10 In brief, following anesthesia, the thoracic aorta was clamped after performing thoracotomy. By clamping the thoracic aorta, blood flow from the diaphragm to the lower body can be blocked and blood can be collected through the middle cardiac vein to the upper body while the liver is in cardiac arrest. A tube was inserted into the carotid artery beforehand, and blood was collected using blood pressure and hydrocephalic differences. Approximately 500 mL of saline solution was infused to maintain the blood pressure, while the blood was being collected. Blood collection tubes were filled with heparin saline to prevent clotting.
The blood collection bag contained sodium citrate as an anticoagulant, and the bag was stirred slowly during blood collection to prevent clotting. Finally, the liver was placed in the abdominal cavity to establish an ischemic liver model; 60 min after the aortic clamp, the liver was removed.
The in vivo group was treated with ectopic liver transplantation as previously reported.10 Briefly, the recipient underwent laparotomy, and the PV and IH-IVC of the donor liver grafts were subjected to end-to-lateral anastomosis with the PV and IH-IVC of the recipient. Subsequently, the donor HA in the form of a Carrel patch was sutured to the recipient’s aorta. After all treatments were completed, blood flow was switched to the transplanted liver by ligating the PV and HA in the recipient while measuring blood pressure using a Mediate catheter (Nippon Covidien, Shizuoka, Japan) (Figure 2).
FIGURE 2.
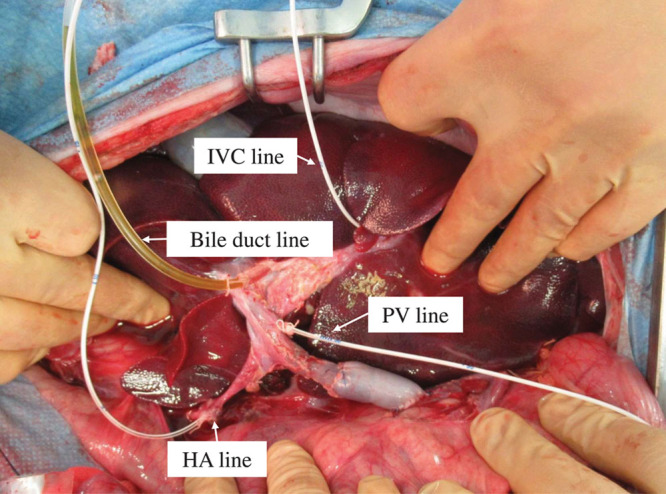
In vivo settings. HA, hepatic artery; IVC, inferior vena cava; PV, portal vein.
Ex vivo Perfusion System
We developed an experimental machine to perfuse the large animal liver ex vivo (Figure 3). The perfusion circuit included a reservoir bag (Corning, NY), silicone tube (Asone, Osaka, Japan), tubing pump (custom made, SCREEN Holdings, Japan), artificial lung (HMO11000, Getinge, Sweden), thermostat (Asone, Osaka, Japan), and chiller (Ohm Electric, Tokyo, Japan). A hydraulic head differential was used for inflow to the liver, and a reservoir bag was placed 80 cm from the liver. The perfusate collected in the reactor was delivered to the artificial lung by a tubing pump; subsequently, it was oxygenated, warmed to 37°C, and returned to the reservoir bag.
FIGURE 3.
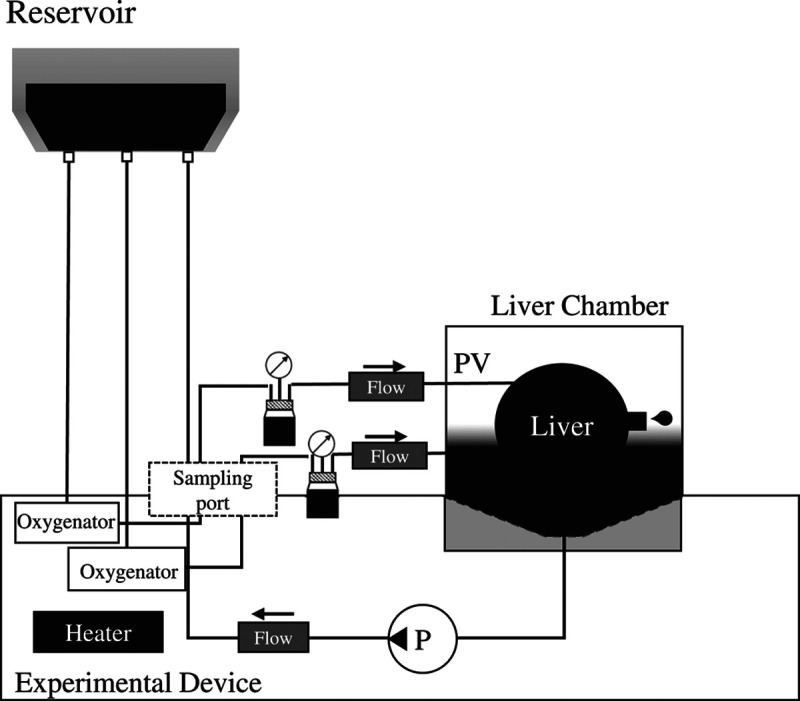
Perfusion settings of the ex vivo machine. HA, hepatic artery; PV, portal vein.
Biochemical Assays
To evaluate the oxygen delivery of each group, the partial pressures of oxygen of the hepatic artery and the IH-IVC were measured by using a blood analyzer (i-stat, Abbott Laboratories, Chicago, IL) and the oxygen partial pressure of the blood inflowing into the artery was compared with that of the output blood from the IVC. The dissolved oxygen partial pressure of the fluid entering the artery was compared with the dissolved oxygen partial pressure of the fluid coming out of the IVC. Lactate and glucose were measured in situ using a blood analyzer (i-stat, Abbott Laboratories, Chicago, IL).
Metabolomic Analysis
Perfusate samples were collected every hour from the PV inflow and IVC outflow after reperfusion of blood or perfusate and compared with the in vivo conditions. Metabolomic analyses were performed using liquid Chromatography–mass spectrometry and Capillary electrophoresis–mass spectrometry to quantify 110 water and fat-soluble compounds.
RESULTS
In the whole blood group during 3 h of mechanical perfusion at the setting of reservoir with a water head difference of 80 cm, the mean PV flow rate was 383 (SD, 18.5) mL/min and the mean HA flow rate was 44 (SD, 3.6) mL/min. Meanwhile, in the 10% dilution group, the mean PV flow rate 354 (SD, 16.9) mL/min and the mean HA flow rate was 47 (SD, 1.8) mL/min. Metabolic analysis of the perfusate was performed under these conditions.
Blood Analyses
Figure 4 shows the results of the blood analyses. The pH of 10% diluted blood was more acidic than the in vivo perfusion solution. The pH of whole blood was kept stable at the same level of the blood in vivo, even though the Pco2 of the whole blood was lower than that of whole blood in vivo and the same as in the 10% diluted perfusion solution.
FIGURE 4.
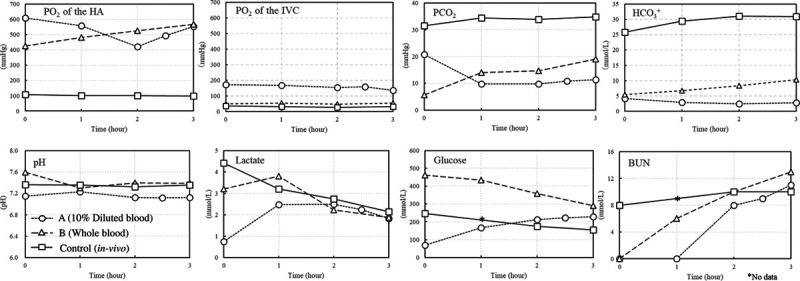
Comparison of blood analyses. BUN, blood urea nitrogen; HA, hepatic artery; IVC, inferior vena cava.
To evaluate the oxygenation delivery of each group, we compared the partial pressure of dissolved oxygen in the inlet into the arterial line with the outlet of the IH-IVC. In the control, the Po2 in the arterial blood remained stable at around 100 mm Hg, while in the IH-IVC, the partial pressure decreased to nearly 30 mm Hg whereas, in mechanical perfusion group, oxygen gas was supplied to the oxygenator, and the Po2 was >400 mm Hg, but the Po2 in IH-IVC was >130 mm Hg in the 10% diluted blood group and near 50 mm Hg in whole blood.
In the 2 mechanical perfusion groups, using whole blood tended to be alkalotic and using 10% diluted blood tended to be acidotic in the first few minutes, but during mechanical perfusion, the pH of the whole blood was closer to that in vivo, while the pH of 10% diluted blood remained acidotic.
The pH of the whole blood group was kept at the same level as that in vivo 1 h after starting perfusion, although CO2 partial pressure and bicarbonate concentration were lower during mechanical perfusion compared with that in vivo. The values of lactate and glucose were measured as central carbon metabolism. Lactate tended to have an initial increase and then a gradual decrease in the whole blood, while it was eliminated in the first hour in 10% diluted blood and began to decrease after 2 h. Blood urea nitrogen, the final product of urea metabolism, was stable in the controls but continued to be gradually stored in the ex vivo perfusate.
Results of the Metabolomic Analyses
The quantified values from the metabolomic analyses were compared with determining which perfusate was most similar to the blood in vivo (Figure 5). Regarding guanosine, an indicator of reperfusion injury, the controls had the highest initial level, while it increased during normothermic perfusion in 10% diluted blood and reached the same level as that in in vivo conditions at 3 h. Ex vivo perfusion in whole blood resulted in the lowest average level of guanosine; it increased from 0.5 µmol/L to 1.0 µmol/L over 1 h and dropped subsequently.
FIGURE 5.
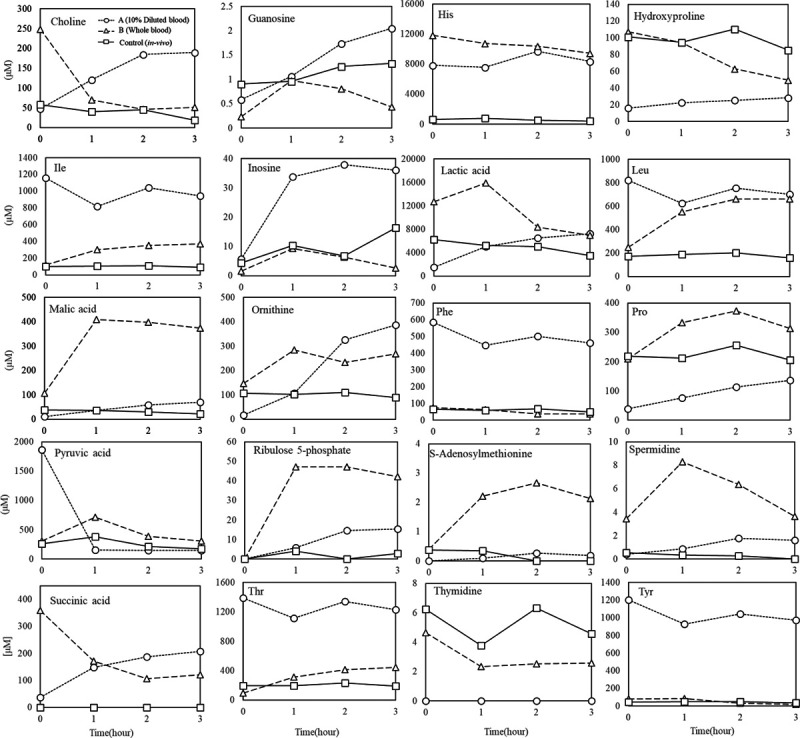
Comparison of metabolomic analyses. His, histidine; Ile, isoleucine; Leu, leucine; Phe, phenylalanine; Pro, proline; Thr, threonine; Tyr, tyrosine.
Lactate variability in whole blood ex vivo perfusion was high initially but gradually decreased, and lactate production in 10% diluted blood revealed that it was expelled from the cells into the fluid. Some of the amino acids, such as isoleucine, inosine, phenylalanine, and pyruvic acid, contained in the medium used in 10% diluted blood, and tended to remain in the perfusate, while others approached the values observed in vivo through metabolism. Regarding choline, its initial level was high in the whole blood perfusion and decreased over time as it was taken up by the hepatocytes in the first hour, remaining at a level of ~50 µmol/L, which was the same level as that observed in vivo, while its level in the 10% diluted blood perfusion increased over time. The concentration of bile acids and related substances was constant during the in vivo perfusion; however, during the whole blood ex vivo perfusion, it decreased over time even though the initial levels were comparable to those in vivo, and in 10% diluted blood, the levels were initially very low. Detailed results of the metabolomic analysis can be found in Figure S1 (SDC, http://links.lww.com/TXD/A332).
DISCUSSION
In this study, we performed mechanical perfusion using 2 types of perfusion fluid and in vivo heterotopic transplantation as a control to analyze the metabolic profiles of DCD livers and evaluate their initial response to resuscitation (N = 1 in each group). For normothermic perfusion, blood products were used as the perfusate and with a temperature range equivalent to the living body temperature.6 However, it has been reported that metabolism can be suppressed by reducing the perfusion temperature, and studies have been conducted to investigate the metabolic status of the perfusate in ex vivo perfusion.11,12 Based on these studies, metabolic studies in pigs are of great value because the metabolism of pigs is similar to that of humans. The required components of ex vivo perfusion were examined by comparing perfusion ex vivo with 10% diluted blood and whole blood collected from donors.
The Composition Related to Reperfusion Injury
In in vivo perfusion, the guanosine level remains around 0.9 µmol/L. In ex vivo perfusion, the guanosine level after 3 h was lower in whole blood and increased to 2 µmol/L in 10% diluted blood. Because guanosine is oxidized by reactive oxygen species, it is used as a marker of oxidative stress derived from ischemia-reperfusion injury. Although the Po2 and dissolved oxygen was comparable between whole blood and diluted blood, the concentration of erythrocytes may have affected the generation of guanosine. Some studies have examined the minimum concentration of oxygen delivery required for normothermic perfusion.12,13
Many studies have also correlated liver function and lactate gradient after liver transplantation,14-17 and high lactate clearance after reperfusing blood flow is expected to predict good liver function. In this study, we observed that the lactate concentration tended to increase during perfusion with 10% diluted blood but decrease during perfusion with whole blood. Further studies are warranted in this regard.
Metabolism Involved in ATP Synthesis
L-15 medium used in 10% diluted blood contains galactose instead of glucose and high levels of pyruvate. The metabolomic analysis revealed no significant changes in 6-carbohydrate, which is believed to be galactose; however, the pyruvate level (initially 1800 µmol/L) decreased to approximately 200 µmol/L, the biologic equivalent, in 1 h Its conversion target was lactic acid, which suggests that the conversion of pyruvate, a substrate of the citric acid circuit, to lactate is adjusted in a biomimetic manner even when the substrate of the citric acid circuit, pyruvate, is administered in excess to promote ATP synthesis.
Methods such as the use of insulin and glucagon to control long-term glucose concentrations have also been studied,3 and it is expected that more progress will be achieved in future studies on how to control the central carbon metabolism. Some components in the L-15 medium, such as phenylalanine and isoleucine, were not adjusted by metabolism, unlike pyruvate; thus, suggesting that the amino acids were selectively consumed immediately after reperfusion of blood flow.
Choline Metabolism
In vivo studies have gradually elucidated the correlation between blood choline levels and liver function.18,19 In vivo studies have discussed the activity of liver function using blood choline concentration as an indicator of its vital activity. However, in ex vivo perfusion, the evaluation based on the incoming and outgoing balance of the extracted liver is expected to be a very useful indicator in the assessment of liver function since ex vivo perfusion can be used to directly evaluate liver function. In ex vivo perfusion, there was a linear increase of choline in 10% diluted blood and a plateau in whole blood perfusion at the same level as that in in vivo perfusion; thus, suggesting that ex vivo perfusion using whole blood may be a promising way to assess the choline metabolism to mimic in vivo conditions.
Bile Acid Metabolism
Bile production was observed in all groups. When comparing ex vivo whole blood and in vivo perfusion, the initial blood bile acid concentration was comparable; however, the concentration decreased over time in ex vivo perfusion. Since this level was maintained during the in vivo perfusion, it is likely that components or systems that enhance bile acid metabolism instead of the enterohepatic circulation during ex vivo perfusion are necessary for liver metabolism. Detailed results of the metabolomic analysis can be found in Figure S1 (SDC, http://links.lww.com/TXD/A332).
In conclusion, there was a tendency toward oxidative damage in perfusion with 10% diluted blood prepared on a L-15 medium basis despite higher dissolved oxygen content compared with in vivo blood. In contrast, in ex vivo perfusion with whole blood, markers of oxidative damage were also low; thus, suggesting that sufficient metabolic maintenance is possible.
These analyses are expected to reconstitute an in vitro environment closely similar to the in vivo environment for a longer period of time. We hope that this metabolic analysis will contribute to further progress in the utilization of DCD organs.
Footnotes
Published online 10 June, 2021.
This work was supported by a grant from SCREEN Holdings Co., Ltd.
E.K. is a technical adviser for SCREEN Holdings. The other authors declare no conflicts of interest.
S.Y., M.O., S.T., H.K., J.I., and E.K. designed the study and contributed to the data collection, analysis, and interpretation of data. T.K. and S.N. contributed to the interpretation of data and critically reviewed the article. All authors approved the final version of the article and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
REFERENCES
- 1.St Peter SD, Imber CJ, Lopez I, et al. Extended preservation of non-heart-beating donor livers with normothermic machine perfusion. Br J Surg. 2002; 89:609–616. [DOI] [PubMed] [Google Scholar]
- 2.Nasralla D, Coussios CC, Mergental H, et al. ; Consortium for Organ Preservation in Europe. A randomized trial of normothermic preservation in liver transplantation. Nature. 2018; 557:50–56. [DOI] [PubMed] [Google Scholar]
- 3.Eshmuminov D, Becker D, Bautista Borrego L, et al. An integrated perfusion machine preserves injured human livers for 1 week. Nat Biotechnol. 2020; 38:189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perk S, Izamis ML, Tolboom H, et al. A metabolic index of ischemic injury for perfusion-recovery of cadaveric rat livers. PLoS One. 2011; 6:e28518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishikawa J, Oshima M, Iwasaki F, et al. Hypothermic temperature effects on organ survival and restoration. Sci Rep. 2015; 5:9563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ravikumar R, Jassem W, Mergental H, et al. Liver transplantation after ex vivo normothermic machine preservation: a phase 1 (First-in-Man) clinical trial. Am J Transplant. 2016; 16:1779–1787. [DOI] [PubMed] [Google Scholar]
- 7.Izamis ML, Tolboom H, Uygun B, et al. Resuscitation of ischemic donor livers with normothermic machine perfusion: a metabolic flux analysis of treatment in rats. PLoS One. 2013; 8:e69758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor CT. Mitochondria and cellular oxygen sensing in the HIF pathway. Biochem J. 2008; 409:19–26. [DOI] [PubMed] [Google Scholar]
- 9.Jia Y, Cui R, Wang C, et al. Metformin protects against intestinal ischemia-reperfusion injury and cell pyroptosis via TXNIP-NLRP3-GSDMD pathway. Redox Biol. 2020; 32:101534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshimoto S, Torai S, Yoshioka M, et al. Continuous resuscitation for porcine liver transplantation from donor after cardiac death. Transplant Proc. 2019; 51:1463–1467. [DOI] [PubMed] [Google Scholar]
- 11.Nösser M, Gassner JMGV, Moosburner S, et al. Development of a rat liver machine perfusion system for normothermic and subnormothermic conditions. Tissue Eng Part A. 2020; 26:57–65. [DOI] [PubMed] [Google Scholar]
- 12.Dondossola D, Santini A, Lonati C, et al. Human red blood cells as oxygen carriers to improve ex-situ liver perfusion in a rat model. J Clin Med. 2019; 8:E1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bodewes SB, van Leeuwen OB, Thorne AM, et al. Oxygen transport during ex situ machine perfusion of donor livers using red blood cells or artificial oxygen carriers. Int J Mol Sci. 2020; 22:E235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Theodoraki K, Arkadopoulos N, Fragulidis G, et al. Transhepatic lactate gradient in relation to liver ischemia/reperfusion injury during major hepatectomies. Liver Transpl. 2006; 12:1825–1831. [DOI] [PubMed] [Google Scholar]
- 15.Perilli V, Aceto P, Sacco T, et al. Usefulness of postreperfusion lactate clearance for predicting early graft recovery in liver transplant patients: a single center study. Minerva Anestesiol. 2018; 84:1142–1149. [DOI] [PubMed] [Google Scholar]
- 16.Bral M, Gala-Lopez B, Thiesen A, et al. Determination of minimal hemoglobin level necessary for normothermic porcine ex situ liver perfusion. Transplantation. 2018; 102:1284–1292. [DOI] [PubMed] [Google Scholar]
- 17.Linares-Cervantes I, Kollmann D, Goto T, et al. Impact of different clinical perfusates during normothermic ex situ liver perfusion on pig liver transplant outcomes in a DCD model. Transplant Direct. 2019; 5:e437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corbin KD, Zeisel SH. Choline metabolism provides novel insights into nonalcoholic fatty liver disease and its progression. Curr Opin Gastroenterol. 2012; 28:159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu ZY, Yishake D, Fang AP, et al. Serum choline is associated with hepatocellular carcinoma survival: a prospective cohort study. Nutr Metab (Lond). 2020; 17:25. [DOI] [PMC free article] [PubMed] [Google Scholar]


