Abstract
The screening of novel probiotic strains from various food sources including fruits, vegetables, herbs, and traditional fermented foods, have been of growing concern recently. Most of these potential probiotic lactic acid bacteria isolates were distinguished from the commercial probiotics based on multiple therapeutic effects and functionalities. Recent in vitro and in vivo investigates have also verified the usage of probiotics to lower the risk of diseases. Application of these novel strains in fermented dairy products is also an emerging trend to improve the physical and quality characteristics, functional properties, and safety of dairy products. Moreover, since dairy products are one of the highest consumed products in the globe, the dispatch channels for fermented dairy products are already established. Therefore, incorporating novel probiotic strains into fermented dairy products might be the most feasible approach for their delivery. In this context, our aim is to discuss the feasibility of dairy products as delivery vehicles for novel probiotic strains. Thus, we summarize the scientific evidence that points to a dynamic future for the production of fermented dairy-based probiotics.
Keywords: Probiotics, Traditional fermented food, Probiotic carrier, Fermented dairy product, Functional effect
Introduction
The paradigm of food consumption behavior has drastically changed, and consumers tend to purchase foods that promote health and lower the risk of diseases. This has inevitably led to an increase in functional food development (Turkmen et al. 2019). Functional foods are described as foods that are able to modulate targeted body functions through enhancing some physiological responses, reducing the risk of disease or both, that extend beyond nutritional and energy supply (ILSI 2006). Furthermore, functional foods can be classified as fresh, fortified, and enriched foods, encompassing a very broad range of products including probiotics, prebiotics, and synbiotic (Turkmen et al. 2019). Probiotics have a wide range of possible health benefits, which has led to an upsurge of probiotic consumption globally (Champagne et al. 2018). The probiotic industry is expected to raise $57.4 billion by 2022 while sustaining a compound annual growth rate (CAGR) of 7.7% during the 2016–2022 projection period (Allied Market Research 2019). Probiotics are live microorganisms that provide human health benefits when given in sufficient quantities (FAO/WHO 2006). Many probiotics belong to the lactic acid bacteria (LAB) genera of Lactobacillus. Other probiotic genera include Leuconostoc, Lactococcus, Pediococcus, Enterococcus, and Streptococcus (Le and Yang 2018).
Traditionally, fermented dairy products have commonly been used as a primary tool for supplying probiotics to consumers all over the globe. Moreover, numerous probiotic strains have been isolated from fermented milk (James and Wang 2019). Recently, consumers have become more conscious of health and nutrition, and consequently, different strains with various therapeutic properties are constantly being identified from traditional fermented foods from many countries. These traditional fermented foods are produced from a range of raw materials, including fish, beef, pork, seafood, soybeans, fruits, and vegetables. Korean kimchi, the Chinese serofluid dish, boza (a Turkish fermented cereal-based beverage), and hantak (Indian fermented fish) are some of the traditional fermented foods evaluated for their probiotic activities (Sornplang and Piyadeatsoontorn 2016). Based on in vivo studies, LAB isolated from fermented foods are involved in the modulation of obesity and type 2 diabetes (Lee et al. 2018), hypocholesterolemic activity by lowering plasma cholesterol (Jo et al. 2015) and immunomodulation (Lee et al. 2014a). Some LAB contain beneficial compounds and are associated with antioxidant activity, antimicrobial activity, angiotensin-converting enzyme (ACE) inhibitory activity, exopolysaccharide (EPS)-producing activity, bacteriocin production, and anti-inflammatory activity (James and Wang 2019). Therefore, incorporation of these LAB in fermented dairy products might deliver benefits to the consumer. In this regard, we discuss the feasibility of using LAB from ethnic food sources in dairy products.
Lactic acid bacteria in traditional fermented Asian foods
The LAB in Asian traditional fermented foods include Lactobacillus casei, Lb. plantarum, Lb. brevis, Lb. paracasei, Leuconostoc mesenteroides, Leu. kimchi, Weissella koreenis, W. confusa, W. cibaria, and Pediococcus pentosaceus many of which are considered to be potential probiotics (Nuraida 2015; Kim et al. 2020; Kwun et al. 2020). Table 1 shows the lactic acid bacteria isolated from various fermented foods and their functional activities. These isolated LAB, having beneficial effects, can be adopted in commercial food manufacture and industrial applications. In particular, several probiotics have been identified as possible sources of enzymes such as alkaline protease, esterase, and amylase (Zhao et al. 2019). Some studies have proposed that probiotics to be used as feed additives to boost livestock health, such as swine, cattle, and poultry. Additionally, certain probiotics are applied to various types of functional foods and introduced as probiotic carrier foods that enable consumers to ingest significant quantities of probiotic bacteria for therapeutic benefits. In this context, fermented dairy products are considered the key vehicle for probiotics in different region of the world.
Table 1.
The functional properties of lactic acid bacteria isolated from different fermented foods
| Microorganism | Origin | Functional property | Country | Reference |
|---|---|---|---|---|
| Lb. plantarum KLAB21 | Kimchi | Anti-mutagenic activity | Korea | Park and Rhee (2001) |
| Lb. plantarum SY11 and Lb. plantarum SY12 | Kimchi | Anti-allergic activity | Korea | Lee et al. (2014b) |
| Lb. plantarum sp. | Kimchi | Atopic dermatitis inhibition activity | Korea | Won et al. (2011) |
| Lb. plantarum 200661 | Kimchi | Anticavity activity | Korea | Lim et al. (2020) |
| Leu. kimchii GJ2 | Kimchi | Cholesterol lowering activity | Korea | Jo et al. (2015) |
| Lc. lactis NK34 | Kimchi | Antioxidant and β-galactosidase activity | Korea | Son et al. (2017) |
| Lb. paraplantarum SC61 | Jangajii (fermented vegetable) | Antioxidant and immunostimulatory activity | Korea | Son et al. (2018) |
| Lb. buchneri KU200793 | Kimchi | Neuroprotective activity | Korea | Cheon et al. (2020) |
| Lb. plantarum Ln4 | Kimchi | Modulate obesity and type 2 diabetes | Korea | Lee et al. (2018) |
| Leu. citreum EFEL2061 | Kimchi | Immunomodulatory activity | Korea | Kang et al. (2016) |
| Lb. plantarum AAS3 | Hentak (fermented fish) | Hemolytic, DNase, and gelatinase activities | India | Aarti and Khusro (2019) |
| Lb. brevis LAP2 | Hentak (fermented fish) | Proteolytic activity and antioxidant activity | India | Aarti et al. (2017) |
| Lb. paraplantarum MTCC 9483 | Gundruck (fermented leafy green vegetable) | Anti-inflammatory activity | Nepal | Devi et al. (2018) |
| Lb. plantarum JLK0142 | Fermented tofu (fermented soy milk) | Immunomodulatory activity | China | Wang et al. (2018) |
| Lb. s193 and s292 | Funazushi (fermented soybean) | Anti-inflammatory activity | Japan | Okada et al. (2018) |
| Lb. plantarum S-SU5 and P. pentosaceus S-SU6 | Fermented fish | Antioxidant activity and anti-inflammatory activity | Japan | Kuda et al. (2014) |
| Lb. plantarum (B0040, B0110) and W. cibaria (B0145) | Fermented mustard | Immunopotentiating activity and anti-mutagenic activity | Taiwan | Chang et al. (2015) |
| Lb. plantarum K68 | Fu-tsai (fermented mustard) | Anti-inflammatory and immunomodulatory activity | Taiwan | Liu et al. (2011) |
| Lb. plantarum MB 427 | ||||
| Lactobacillus sp. | Mandai | Shortened duration of diarrhea and induced secretion of IgA and IgG | Indonesia | Nuraida (2015) |
| Lb. plantarum sa28k, Lb. acidophilus FNCC116, and Lb. casei FNCC262 | Sauerkraut | Assimilating cholesterol and lowering serum cholesterol | Indonesia | Nuraida (2015) |
| W. hellenica BCC 7293 | Nham (fermented pork sausage) | Bacteriocin production | Thailand | Woraprayote et al. (2015) |
Names of genera are abbreviated as Lb., Lactobacillus; Lc., Lactococcus; Leu., Leuconostoc; W., Weissella; P., Pediococcus
Trends and probiotic cultures in the current dairy market
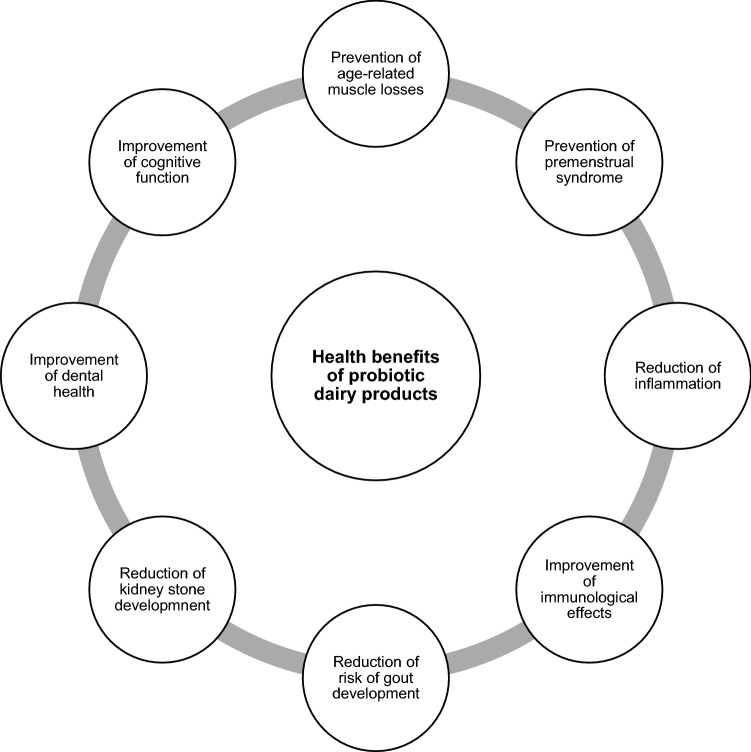
Milk is a rich source of macro-and micronutrients, and thus provides the nutrients and energy needed for growth. Milk products play a major role in regulating public health and are the main component of the food pyramid. In South Asia, India is the main supplier of raw milk required for dairy products, with a record production of $26.9 billion in 2014 (Nozaki 2017). Milk products are the major contributors to the functional food market (60%) in Europe, with yoghurt accounting for the largest segment of the probiotic market (Vella et al. 2013). In fact, yoghurt has been widely recognized for its nutritional properties and health benefits and has been consumed for thousand years (Min et al. 2019). Lb. delbrueckii subsp. bulgaricus and S. thermophilus are used as starter cultures in conventional yoghurt productions. Nevertheless, these bacteria are not acid and bile salt tolerance and do not withstand the passage across the gastrointestinal tract (GIT). However, in response to the increasing consumer interest in health, the food industry has developed fermented dairy probiotic products supplemented with probiotics. Figure 1 indicates the health benefits associated with probiotic dairy products. As a result of these benefits, milk consumption has also increased in countries in East Asia, where milk consumption was lower in the period from 1981 to 2000 (FAO 2009). In the global dairy market, China is becoming more competitive and the entire industry has gradually shifted from scale-up development to efficiency upgrading. In China, gross dairy sector revenue was 359.04 billion yuan (0.53 billion USD) in 2017, with an increase of 7.85% (Daxue Consulting 2020). Table 2 shows the dairy-based probiotics currently available in the global market and the constituent probiotic species.
Fig. 1.
Health benefits of probiotic dairy products
Table 2.
Dairy probiotic products available in the global market
| Product category | Brand name | Manufacturer | Probiotic strains |
|---|---|---|---|
| Fermented milk |
Yakult Yakult |
Yakult Honsha Korea Yakult Co., Ltd |
Lb. casei Shirota Lb. paracasei |
| Rela | Ingman foods Oy | Lb. reuteri | |
| Chamyto | Nestle Co., Ltd | Lb. johnsonii and Lb. helveticus | |
| Activia | Danone Co., Ltd | B. animalis DN173010 | |
| Vigor‐Club | Vigor Co | Lb. acidophilus and Lb. casei | |
| Batavito | Batavo | Lb. casei | |
| Bob Sponja | Batavo | Lb. casei | |
| Yoghurt (set type, stirred, and drinking) | Activia (stirred, drinkable) | Danone Co., Ltd | B. animalis DN173010 |
| Actimel (drinkable) | Danone Co., Ltd | Lb. casei Immunitas | |
| Activia (creamy yoghurt) | Danone Co., Ltd | B. lactis | |
| Hellus |
Tallinna Piimatööstuse AS |
Lb. fermentum ME-3 | |
| Jovita Probiotisch | H&J Bruggen | Lactobacillus strain | |
| ProViva | Skanemejerier | Lb. plantarum | |
| Rela | Ingman Foods | Lb. reuteri | |
|
Revital Active |
Olma | Lb. reuteri | |
| Olma | Lb. reuteri | ||
| Vifit | Campina | Lactobacillus strain | |
|
Vitamel Doctors choice |
Campina Bio foods Ltd |
Lb. casei, B. bifidum, Lb. acidophilus Lb. acidophilus, and S. thermophilus |
|
| Cheese | Kraft | Kraft Heinz Co | B. lactis and Lb. rhamnosus |
| Saputo | Saputo Inc | ||
| Miyoko’s | Nestle Co. Ltd |
Names of genera are abbreviated as Lb., Lactobacillus; Lc., Lactococcus; W., Weissella; B., Bifidobacterium
Application of novel probiotic strains in fermented dairy products
As consumers are aware of the health-promoting effect of the diet, the food industry is trying to develop or modify dairy foods to give the consumer maximum health benefits while preserving the product's traditional mouth feeling. In this scenario, milk companies may find multiple opportunities for the use of novel probiotics. Table 3 shows the application of novel probiotics in fermented dairy products. In latter discussed the various application of novel probiotics for the preparation of fermented dairy products.
Table 3.
Dairy fermented products incorporated with potential probiotics isolated from traditional fermented Asian foods
| Microorganism | Origin | Functional property | Dairy product | Reference | Concentration and shelf life of the product |
|---|---|---|---|---|---|
| Lb. plantarum DKL119 | Korean kimchi | Cold-shock induced cryotolerance, and β-galactosidase and proteolytic activity | Yoghurt | Cho et al. (2013) | 15 d, 1.0 × 109 cfu/mL |
| Lc. lactis strains | Herbs, fruits, and vegetables | Antilisterial activity, adjunct cultures in cheese to enhance the flavor | Cheese | Ho et al. (2018) | 108–109 cfu/mL, no shelf life study |
| Lb. plantarum strains | Herbs, fruits, and vegetables | Antifungal activity | Cottage cheese | Cheong et al. (2014) | 108–109 cfu/mL, 29 days |
| Lb. plantarum SY11 and L. plantarum SY12 | Kimchi | Anti-allergic effects | Yoghurt | Lee et al. (2014a, b) | No shelf-life study |
| Lb. plantarum Lb41 | Kimchi | Anti-inflammatory activity and anticancer activity | Cottage cheese | Jeon et al. (2016) | 109 cfu/mL, 28 days |
| Lb. plantarum RS20D | Pao cai (Chinese homemade fermented vegetable) | Immunoregulatory activity and exopolysaccharide producing activity | Yoghurt | Zhu et al. (2019) | No shelf life study |
| Lc. lactis SL6 | Kimchi | Antioxidant activity | Fermented skim milk | Kim et al. (2017) | No shelf life study |
| Lb. plantarum JLK0142 | Tofu | Antioxidant, ACE inhibition, α-amylase and β-glucosidase inhibitory activity, and antitumor activity | Cheddar cheese | Wang et al. (2019) | No shelf life study |
| Lb. plantarum L67 | Kimchi | Anti-allergic activity | Yoghurt | Song et al. (2016) | No shelf life study |
| Gluconobacter oxydans and Dekkera anomala | Kombucha | Antihypertensive activity | Fermented milk | Elkhtab et al. (2017) | No shelf life study |
| W. cibaria D30 | Kimchi | Antioxidant and antilisterial activity | Cottage cheese | Kariyawasam et al. (2019) | 106 cfu/g, 28 days |
| Lb. brevis KU200019 | Jeotgal | Antioxidant activity | Fermented milk | Kariyawasam et al. (2020) | 108 cfu/mL, 28 days |
Names of genera are abbreviated as Lb., Lactobacillus; Lc., Lactococcus; W., Weissella
Bio-preservative and antagonistic properties
In dairy fermentation, lactic acid production by probiotic bacteria plays a crucial role in the preservation of the food, preventing food spoilage and the proliferation of pathogens (Othman et al. 2017). Probiotic isolates have shown antagonistic activity towards food-borne pathogens and organisms that cause food spoilage. LAB produces a broad spectrum of antimicrobial metabolites, including primary and secondary metabolites (Choi et al. 2018). The important metabolites produced are ethanol, hydrogen peroxide, free fatty acids, bacteriocins, and antifungal compounds, all of which may play an antagonistic role against bacteria, viruses, and fungi (Shokryazdan et al. 2017). Recently, there is a global trend of increasing resistance to use of chemical preservatives, while natural compounds are gaining popularity. The antimicrobial compounds produced by LAB are appealing in this context as they can promote food bio-preservation, while eliminating some of the side effects of the chemical preservatives. In certain dairy products Lc. lactis strains with antimicrobial properties have appeared as promising bio-preservatives particularly for the prevention of listeriosis which is a serious infection caused by Listeria monocytogenes. L. monocytogenes is a post-processing contaminant in refrigerated ready-to-eat foods like soft cheeses as it can grow at low temperatures and is highly acid and salt tolerant (Ho et al. 2018). However, many scientific investigations confirmed the feasibility of enhancing the safety of dairy probiotic foods through the incorporation of novel probiotic strains derived from traditional fermented foods. For example, Ho et al. (2018) isolated Lc. lactis strains from herbs, fruits, and vegetables and successfully incorporated the bacterium in cheese to inhibit the proliferation of well-known foodborne pathogen L. monocytogenes. Similarly, W. cibaria D30, derived from Korean kimchi, was effectively used as a bio-preservative in ready-to-eat cottage cheese (Kariyawasam et al. 2019).
In addition, despite the use of yeast and molds in the processing of many fermented food products, including dairy products, they are a major cause of spoilage for many processed dairy foods. Ryu et al. (2014) was reported the antifungal activity of Lb. plantarum HD1 isolated from Korean kimchi and potential use as a powerful bio-preservative system which is capable of preventing fungal spoilage and mycotoxin formation in the food. Furthermore, Cheong et al. (2014) have reported the antifungal activity of LAB isolated from various herbs, fruits, and vegetables and potential for use as bio-preservatives in cheese.
Therapeutic properties
Typically, milk products fermented with Lb. delbrueckii subsp. bulgaricus and S. thermophilus starter strains may not play a vital role in the human GIT as they are unable to colonize in a human epithelial cell layer (Mcfarland 2015). Nonetheless, probiotics have been documented for restoring GIT microbiota and offering numerous health benefits (James and Wang 2019).
The role of probiotics in mitigating and reducing the duration of diarrhea, particularly antibiotic associated diarrhea is well documented. Another, indication is the administration of Lactobacillus reuteri DSM 17938, which decreases the duration of diarrhea and improves the chance of recovery in healthy children (Urbanska and Szajewska 2014). In addition, the consumption of yoghurt comprising the probiotic Lb. rhamnosus GR-1 and Lb. reuteri RC-14 address mild diarrhea in HIV/AIDS patients (Anukam et al. 2008). Furthermore, fermented milk can minimize the risk of common cold as well as its duration (Makino et al. 2010). One research reported that children consuming Lb. rahmnosus GG (LGG) supplemented milk had less days of respiratory issues than the participants in the control group (Kumpu et al. 2013).
In addition to primary health benefits, probiotics have also shown several health benefits, including improved immunomodulatory activity, anti-proliferative activity against tumor cells, reduced risk of colon cancers, and antigenotoxicity, anti-mutagenicity, and hypocholesterolemic effects (Vasiljevic et al. 2008; Jang et al. 2019). As various probiotics isolated from traditional fermented foods with various therapeutic effects current trend is to incorporate these probiotic bacteria along with starter culture during fermentation to accentuate the therapeutic properties.
Lee et al. (2014b) and Song et al. (2016) reported the utilization of Lb. plantarum species isolated from Korean kimchi as yoghurt starters that could help in the prevention of Th2-related immune disorders. Moreover, some authors suggested the use of Lb. plantarum, Lb. brevis, and W. cibaria as adjunct cultures in fermented dairy products to enhance the therapeutic properties including immunomodulatory activity, antitumor activity, antioxidant activity, and anti-inflammatory activity (Table 3). Cai et al. (2019) demonstrated the antidiabetic scores of probiotics isolated from pickles, P. acidilactici 004 and Lb. plantarum 152 were higher than those of the LGG, which suggested that these two strains are promising probiotics with antidiabetic properties. Moreover, in vitro and in vivo studies of Lee et al. (2018) suggested that Lb. plantarum Ln4 isolated from kimchi has attenuates diet-induced obesity and insulin resistance, highlighting the potential of Ln4 as a therapeutic probiotic agent for metabolic disorders.
Moreover, Lim et al. (2020) demonstrated the anti-cavity activity of Lb. plantarum 200661 derived from Korean Kimchi. Furthermore, author has suggested the use of this novel strain as adjunct in cheese-like products to mediate the dental health issues among young children. Lee et al. (2019) was reported the prevention of oxidative damage of DNA by probiotic S. cerevisiae strains; KU200270, KU200280, and KU200284 isolated from cucumber jangajji. Furthermore, S. cerevisiae KU200284 was demonstrated for potential use in starter culture for kefir milk production (Hong et al. 2019). Thus, these novel strains can be incorporated into fermented dairy products along with starter cultures in order to obtain various health benefits.
Physical properties
Textural properties of fermented dairy products are important attributes in determining consumer acceptance. Attributes such as viscosity, springiness, smoothness, thickness, and structural resistance are considered important. Therefore, many methods have been used in the cheese and yoghurt manufacturing industries, including increasing the total solid content by adding fat, sugars, or proteins, and addition of stabilizers such as starch, pectin, and gelatin. However, consumers are reluctant to accept these methods due to side effects associated with them. Moreover, in some countries the amount of stabilizer used is strictly regulated for yogurt manufacture (Zhu et al. 2019). Consequently, the use of EPS-producing probiotic bacteria to enhance the textural properties of food has become a popular approach as it is possible to combine textural improvement with certain health benefits. Some investigators have reported probiotics with a high EPS-production ability and capability of improving the texture of yoghurt (Han et al. 2016). Zhu et al. (2019) stated that the EPS-producing Lb. plantarum derived from conventional fermented vegetable products is capable of optimizing the texture of yoghurt and is associated with elevated immunomodulatory activity. Chen et al. (2019) was also reported the textural improving and immunoregulatory activity of EPS producing probiotics isolated from fermented vegetables in Taiwan. Therefore, these studies confirmed the feasibility of incorporating probiotics isolated from traditional fermented products into fermented milk products to improve the textural properties of these products.
Detoxification properties
Food security has become a major public health concern. Contaminated food intake is considered to be the primary route of human exposure to harmful compounds. Milk and milk derivatives have been reported as a significant source of aflatoxin M1 (AFM1) to humans. AFM1 is a known potent carcinogenic substance that regularly poses a public health concern (Panwar et al. 2018). However, there is no effective method available to mitigate the problem of AFM1 from milk and milk products. Panwar et al. (2018) described the feasibility of probiotic lactobacilli mitigating the toxic effects of AFM1. The propensity for detoxification of milk products may be attributed to the LAB possessing affinity to certain heavy metals and organic contaminants (Reid 2015). Furthermore, Monachese et al. (2012) showed the ability of probiotic yoghurt to sequester toxic heavy metals. This is a new application of dairy products. Studies conducted by Zhang et al. (2014) confirmed the potential of fermented skimmed milk to accelerate the degradation of the organophosphate pesticides malathion, diazinon, fenitrothion, and chlorpyrifos. Thus, dairy-based probiotics have the potential to detoxify some carcinogenic compounds and can be used to enrich food products.
Merits of probiotic dairy foods as probiotic carriers and recent advancement of probiotic dairy foods
Dairy products are by far the most probiotic food category available on the market. The success relates to the multiple benefits of dairy products as probiotic carriers and their technological advancements. These benefits and recent advances are discussed in the following sections.
High viability of probiotics
The most important criteria for using any probiotic food is that the viability and activity of the probiotics is maintained until the end of the shelf-life of the product since an adequate number of viable cells needs to be administered and survive in the gut long enough to colonize the gut properly and deliver the putative health benefits associated with probiotics (Mcfarland 2015). Effective promotion of probiotic products often demands the guarantee that there will be a sufficient amount of viable probiotic cells during the shelf life of the product. The major crucial key factors affecting probiotic viability are probiotic strain, processing temperatures, processing procedures, and storage conditions (Fig. 2). Therefore, selection of a suitable food carrier can ensure the maximum viability of probiotics throughout storage, which is essential to deliver the optimal therapeutic benefits to the consumer.
Fig. 2.
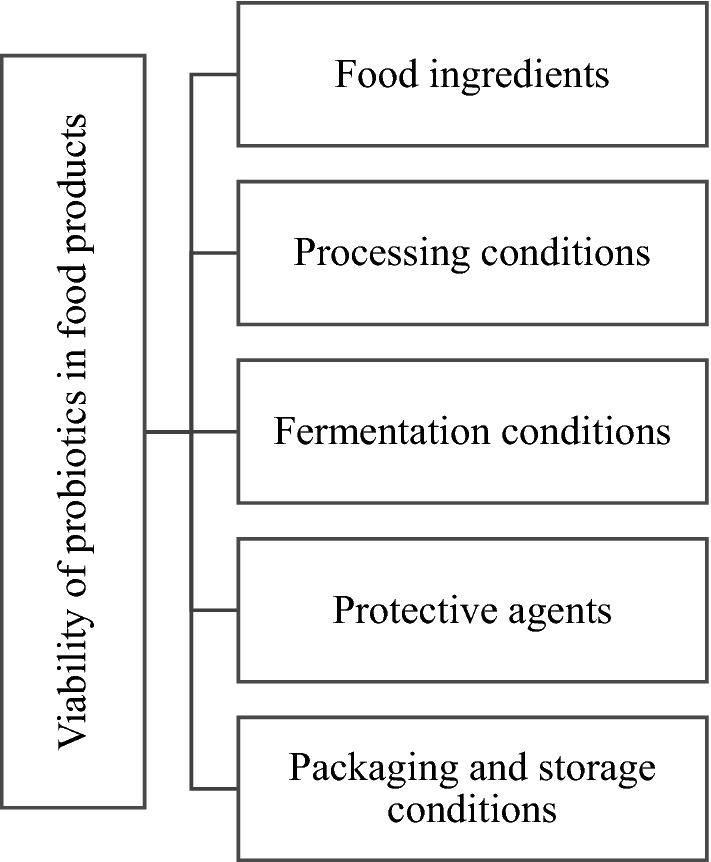
Factors affecting viability of probiotics in foods
(Adapted from Tripathi and Giri 2014)
Dairy fermented foods have been reported to be suitable for probiotics addition, as the fermentative process facilitates optimization of microbial viability; and consumers are familiar with the fact that these products contain living microorganisms. The refrigerated storage also helps to stabilize the probiotic cultures throughout the storage period. In addition, extensive changes in technology or manufacturing process do not require to include the probiotic cultures in the dairy food matrix, as the matrix has the ability to protect probiotics through the gastrointestinal tract (Granato et al. 2019). In particular, cheese has beneficial characteristics, such as high fat, water activity, and pH, which can ensure the maximum viability of probiotic strains during shelf-life and even through the gastric transit (Kariyawasam et al. 2019; Makelainen et al. 2009). Previous authors have reported the successful incorporation of various probiotic cultures isolated from traditional fermented foods as adjuncts in fermented milk products to enhance the therapeutic value, while maintaining the live probiotic count during storage (Ho et al. 2018; Jeon et al. 2016). Jeon et al. (2016) reported cottage cheese containing Lb. plantarum Lb4, isolated from Korean kimchi with 9 log cfu/mL survival rate at the 28 days of the refrigerated storage. Papadopoulou and Chorianopoulo (2016) reported the fresh cheese added with the probiotic strain Lb. plantarum T57, isolated from traditional Greek products. The probiotic viability was 7.5 log cfu/g at the end of the shelf-life. Ice cream also has good potential for use as a probiotic carrier because of its neutral pH and high total solid level providing protection for probiotic cells (Ergin et al. 2016). However, the different technical steps taken during the production of ice cream could have a negative impact on probiotic viability. Extra attention should therefore be paid to technical steps, such as overrun where air is incorporated, storage at freezing temperatures, and how the bacterial inoculum is added to the product. Several strategies, including strain selection, prebiotic incorporation, microencapsulation, the addition of the probiotic at an optimum inoculation level, pH adjustment, adjustment of the level of cream fermentation, and freezing control, have been proposed to improve the survival of the probiotics in ice cream (Granato et al. 2019).
The major limitation of yogurts and fermented milk in probiotic viability is the low pH because most of the probiotic cultures have an optimal growth pH between 5 and 9. Therefore, it is advised to select probiotic strains that are more resistant to the acidity of the medium (Granato et al. 2019). Probiotic cultures isolated from traditional fermented foods generally have high resistance to acidic environments. Kariyawsam et al. (2020) reported fermented milk incorporated with Lb. brevis KU200019 (Korean jeotgal isolate) and observed a high survival rate of over 8 log cfu/mL and high antioxidant activity. Another approach is the use of whey protein concentrates (WPC), probiotic whey beverages presented a significant contribution to the survival of the probiotic cultures. The main reasons are the buffering capacity of the WPC, delaying the post acidification of the products; and the sulfur amino acid release during the heat treatment, lowering the redox potential of the media (Shori 2016). Even a significant increase in probiotic whey beverages is due to the health effects of the whey proteins. Rosa et al. (2020) reported antiproliferative and apoptotic effects of probiotic whey dairy beverages in human prostate cell lines.
In addition, the microencapsulation of probiotic culture appears to offer a good technological alternative for use in the dairy sector and to enhance probiotic viability. The most foodstuff containing microencapsulated probiotics are milk based foods and account for 49%. The common techniques used for probiotic microencapsulation are freeze drying, spray drying, emulsion, and extrusion (Iravani et al. 2015). Different types of cheeses were recently evaluated as a carrier of probiotic cultures, including cheddar, pecorino, fresh cream, pasta filata, soft cheese, coalho, minas frescal, and prato (Granato et al. 2013).
High sensory quality
Sensory quality is one of the most important element that determines the marketability of the food product. Dairy products are consumed by all age groups, and their nutritional and sensory properties mainly influence this consumption. The market for dairy desserts has increased in recent years and a wide range of ready to eat dairy desserts have been introduced the probiotic market (Buriti et al. 2016).
In addition, there are many studies discussing the organoleptic properties of probiotic foods, all of which indicate improvement in acceptability following the introduction of probiotics to milk products (Granato et al. 2010). Yerlikaya and Ozer (2014) reported probiotic fresh white cheese using co-culture with S. thermophiles and was observed incorporation of co-culture did not have any negative impact on the sensory properties including cheese components. Moreover, Lee et al (2014b) reported potential use of Lb. plantarum SY11 and Lb. plantarum SY12 as yoghurt starters with no defects in sensory properties.
The dairy industry has also taken the lead by producing hybrid dairy products that combine dairy and fruit to offer healthier and more appealing products to the consumer. Various authors reported that fortification of plain yoghurt with fruit pieces and pulp, including that of carrot, strawberry, acai, and pomegranate, enhanced the microbiological, sensory, and physicochemical properties of the yoghurt (Kiros et al. 2016). Ranadheera et al. (2012) reported fruit based material could lead to enhance the sugar content and consequently, contribute for appealing aroma and flavor. Besides the addition of fruit pulps, some other authors reported addition of plant extracts such as Inula britannica to cheddar cheese to enhance the functional properties and red ginseng extract to milk to enhance the antioxidant activity of milk and observed enhanced flavor and aroma in those products (Jung et al. 2015; Lee et al. 2016). Jang et al (2018) reported quality improvement of probiotic yoghurt using Lb. plantarum NK181 (Korean jeotgal isolate) and ginseng extract powder. Thus, dairy products can be modified to enhance the functional properties while maintaining the palatable sensorial properties.
Low cost of production
The development of novel non-dairy probiotic products has its own unique challenges. Essentially, many factors should be strictly regulated in order to maintain a viable dose of microorganisms capable of providing health benefits. The factors to be taken into account are the appropriate selection of cultures and their concentrations, adequate processing step at which inoculums are applied, regulation of processing methods, transport and storage temperatures. In addition, food producers need to optimize the specific formulas for each product, taking in to consideration the required sensory, physical and chemical properties, prolonged shelf-life, and chemical consistency, as each food matrix is distinctive (Dey 2018). Moreover, qualitative and quantitative investigations of both the product and the customers are needed before launching these products into the market (Jousse 2008), industrializing novel non-dairy fermented products is a time-consuming and expensive process.
Synbiotic application
Synbiotic foods are foods that contain both probiotics and prebiotics and improve host health by enhancing the viability and implantation of beneficial bacterial microflora in the GIT (Mohanty et al. 2018). The global demand for synbiotic food has grown tremendously, and consequently, the addition of prebiotics including oligosaccharides, inulin, and pyrodextrins to various food sources has also increased. Moreover, some authors reported that the regular consumption of synbiotic yoghurt (which is enriched with bifidobacteria and Lb. rhamnosus GG) boosted the gut health of healthy adults and aging population suffering from constipation (Granata et al. 2013). In addition, Kulka et al. (2015) reported that synbiotic yoghurt containing the probiotic bacteria S. thermophilus, Lb. delbrueckii subsp. bulgaricus, and B. animalis subsp. lactis BB-12 and inulin greatly decreased the duration of fever and increased children’s physical and mental activity.
New formulation of milk-based dairy desserts has made using the inulin due to various health and technological properties. In particular, inulin has incorporated into dairy desserts as a fat replacer and subsequently, to reduce the calorific value of the products. The success in the market implies the maintenance of the mouthfeel and texture of traditional full-fat dairy products (Buriti et al. 2016). Balthazar et al. (2017) reported the prebiotics to replace milk fat and developed a sheep milk-based ice cream with improved nutritional and physiological values. Kariyawasam et al. (2020) reported the utilization of prebiotics to enhance anti-adhesion of pathogenic organism to HT-29 cell line in vitro and improved Lb. brevis KU200019 viability in fermented skim milk.
In addition, academia and industry attention is focused to feasibility use of various starches along with dairy based desserts to enhance the nutritional quality. Parussolo et al. (2017) evaluated feasibility use of yacon flour as a prebiotic ingredient. The addition of yacon enhanced the probiotic viability (above 107 cfu/g) and high sensory scores, demonstrating that this ingredient has potential for use as a prebiotic in the dairy food matrix.
Perspectives and conclusion
Dairy probiotic products have been widely explored by industry and scientific researchers due to their health appeal and the continuous increase in consumer demand. This success is due primarily to the well-known dairy product history as being healthy products and well-established market channels around the world. However, in order to keep a high demand in the functional food market, there must be consistent, convincing, and truthful health claims with appropriate sensory and nutritional appeal and beneficial properties when consumed on a regular basis. In this context, therapeutic effects, textural qualities, sensory properties, and other technological advances of probiotic dairy products can be enhanced by applying beneficial probiotics isolated from traditional fermented foods, as discussed in this review. Although the scientific community has continuously studied the beneficial effects of probiotic microorganisms isolated from various traditional fermented foods and even the possible use of these organisms in dairy probiotic products, these studies are limited to laboratory level. Food companies must therefore use this approach at industrial level to re-engineer products and processes in order to ensure the quality, safety and therapeutic advancement of highly consumed dairy foods.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aarti C, Khusro A. Functional and technological properties of exopolysaccharide producing autochthonous Lactobacillus plantarum strain AAS3 from dry fish based fermented food. LWT-Food Sci Technol. 2019;114:108387. [Google Scholar]
- Aarti C, Khusro A, Varghese R, Arasu MV, Agastian P, Dhabi NAA, Ilavenil S, Choi KC. In vitro studies on probiotic and antioxidant properties of Lactobacillus brevis strain LAP2 isolated from Hentak, a fermented fish product of North-East India. LWT-Food Sci Technol. 2017;6:438–446. [Google Scholar]
- Allied Market Research (2019) Probiotics market by ingredient, function, application, and end use. https://www.alliedmarketresearch.com/probiotics-market. Accessed 11 Jun 2019
- Anukam KC, Osazuwa EO, Osadolor HB, Bruce AW, Reid G. Yogurt containing probiotic Lactobacillus rhamnosus GR-1 and L. reuteri RC-14 helps resolve moderate diarrhea and increases CD4 count in HIV/AIDS patients. J Clin Gastroenterol. 2008;42:239–243. doi: 10.1097/MCG.0b013e31802c7465. [DOI] [PubMed] [Google Scholar]
- Balthazar CF, Silva HA, Vieira AH, Neto RPC, Cappato LP, Coimbra PT, Moraes J, Andrade MM, Calado VMA, Granato D, Freitas MQ, Tavares MIB, Raices RSL, Silva MC, Cruz AG. Assessing the effects of different prebiotic dietary oligosaccharides in sheep milk ice cream. Food Res Int. 2017;91:38–46. doi: 10.1016/j.foodres.2016.11.008. [DOI] [PubMed] [Google Scholar]
- Buriti FCA, Bedani R, Saad SMI. Probiotic and prebiotic dairy desserts. In: Watson RR, Preedy VR, editors. Probiotics, prebiotics and synbiotics. London: Academic Press; 2016. pp. 345–360. [Google Scholar]
- Cai T, Wu H, Qin J, Qiao J, Yang Y, Yong Wu, Qiao D, Xu H, Cao Y. In vitro evaluation by PCA and AHP of potential antidiabetic properties of lactic acid bacteria isolated from traditional fermented food. LWT-Food Sci Technol. 2019;115:108455. [Google Scholar]
- Champagne CP, da Cruz AG, Daga M. Strategies to improve the functionality of probiotics in supplements and foods. Curr Opin Food Sci. 2018;22:160–166. [Google Scholar]
- Chang CK, Wang SC, Chiu CK, Chen SY, Chen ZT, Duh PD. Effect of lactic acid bacteria isolated from fermented mustard on immunopotentiating activity. Asian Pac J Trop Biomed. 2015;5:281–286. [Google Scholar]
- Chen YC, Wu YJ, Hu CY. Monosaccharide composition influence and immunomodulatory effects of probiotic exopolysaccharides. Int J Biol Macromol. 2019;133:575–582. doi: 10.1016/j.ijbiomac.2019.04.109. [DOI] [PubMed] [Google Scholar]
- Cheon MJ, Lim SM, Lee NK, Paik HD. Probiotic properties and neuroprotective effects of Lactobacillus buchneri KU200793 isolated from Korean fermented foods. Int J Mol Sci. 2020;21:1227. doi: 10.3390/ijms21041227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong EY, Sandhu A, Jayabalan J, Le TTK, Nhiep NT, Ho HTM, Zwielehner J, Bansal N, Turner MS. Isolation of lactic acid bacteria with antifungal activity against the common cheese spoilage mould Penicillium commune and their potential as biopreservatives in cheese. Food Control. 2014;46:91–97. [Google Scholar]
- Cho Y, Hong S, Kim C. Isolation and characterization of lactic acid bacteria from kimchi, Korean traditional fermented food to apply into fermented dairy products. Food Sci Anim Resour. 2013;33:75–82. [Google Scholar]
- Choi AR, Patra JK, Kim WJ, Kang SS. Antagonistic activities and probiotic potential of lactic acid bacteria derived from a plant-based fermented food. Front Microbiol. 2018;9:1963. doi: 10.3389/fmicb.2018.01963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daxue Consulting (2020) Dairy products China: the world’s largest dairy market by 2022. https://daxueconsulting.com/china-dairy-market. Accessed 12 Feb 2020
- Devi SM, Kurrey NK, Halami PM. In vitro anti-inflammatory activity among probiotic Lactobacillus species isolated from fermented foods. J Funct Foods. 2018;47:19–27. [Google Scholar]
- Dey G. Non-dairy probiotic foods: innovations and market trends. Food and beverage industries. In: Panda SK, Shetty PH, editors. Innovations in technologies for fermented food and beverage industries. Cham: Springer; 2018. pp. 159–173. [Google Scholar]
- Elkhtab E, El-Alfy M, Shenana M, Mohamed A, Yousef AE. New potentially antihypertensive peptides liberated in milk during fermentation with selected lactic acid bacteria and kombucha cultures. J Dairy Sci. 2017;100:9508–9520. doi: 10.3168/jds.2017-13150. [DOI] [PubMed] [Google Scholar]
- Ergin F, Atamer Z, Arslan AA, Gocer EMC, Demir M, Samtlebe M, Hinrichs J, Kücükcetin A. Application of cold- and heat-adapted Lactobacillus acidophilus in the manufacture of ice cream. Int Dairy J. 2016;59:72–79. [Google Scholar]
- FAO, WHO . Report on joint FAO/WHO expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Argentina: Cordoba; 2006. [Google Scholar]
- Food and Agriculture Organization of the United nations (FAO) (2009) Smallholder dairy development: lessons learned in Asia. https://www.fao.org. Accessed 12 Feb 2020
- Granata M, Brandi G, Borsari A, Gasbarri R, Gioia DDI. Synbiotic yogurt consumption by healthy adults and the elderly: the fate of bifidobacteria and LGG probiotic strain. Int J Food Sci Nutr. 2013;64:162–168. doi: 10.3109/09637486.2012.718742. [DOI] [PubMed] [Google Scholar]
- Granato D, Branco GF, Cruz AG, de Faria AFJ, Shah NP. Probiotic dairy products as functional foods. Compr Rev Food Sci Food Saf. 2010;9:455–470. doi: 10.1111/j.1541-4337.2010.00120.x. [DOI] [PubMed] [Google Scholar]
- Granato D, Nazzaro F, Pimentel TC, Esmerino EA, da Cruz AG. Probiotic food development an updated review based on technological advancement. In: Anderson JR, Ferranti P, Berry E, editors. Encyclopedia of food security and sustainability. Amsterdam: Elsevier; 2019. pp. 422–428. [Google Scholar]
- Han X, Yang Z, Jing X, Yu P, Zhang Y, Yi H, Zhang L. Improvement of the texture of yogurt by use of exopolysaccharide producing lactic acid bacteria. Biomed Res Int. 2016;2016:7945675. doi: 10.1155/2016/7945675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho VTT, Lo R, Bansal N, Turner MS. Characterisation of Lactococcus lactis isolates from herbs, fruits and vegetables for use as biopreservatives against Listeria monocytogenes in cheese. Food Control. 2018;85:472–483. [Google Scholar]
- Hong JY, Lee NK, Yi SH, Hong SP, Paik HD. Short communication: Physicochemical features and microbial community of milk kefir using a potential probiotic Saccharomyces cerevisiae KU200284. J Dairy Sci. 2019;102:10845–10849. doi: 10.3168/jds.2019-16384. [DOI] [PubMed] [Google Scholar]
- International Life Science Institute of North America (2006) https://www.ilsi.org. Accessed 12 Feb 2020
- Iravani S, Korbekandi H, Mirmohammadi SV. Technology and potential applications of probiotic encapsulation in fermented milk products. J Food Sci Technol. 2015;52:4679–4696. doi: 10.1007/s13197-014-1516-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James A, Wang Y. Characterization, health benefits and applications of fruits and vegetable probiotics. CYTA J Food. 2019;17:770–780. [Google Scholar]
- Jang HJ, Jung J, Yu HS, Lee NK, Paik HD. Evaluation of the quality of yogurt using ginseng extract powder and probiotic Lactobacillus plantarum NK181. Korean J Food Sci Anim Resour. 2018;38:1160–1167. doi: 10.5851/kosfa.2018.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang HJ, Lee NK, Paik HD. Probiotic characterization of Lactobacillus brevis KU15153 showing antimicrobial and antioxidant effect isolated from kimchi. Food Sci Biotechnol. 2019;28:1521–1528. doi: 10.1007/s10068-019-00576-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon EB, Son SH, Jeewanthi RKC, Lee NK, Paik HD. Characterization of Lactobacillus plantarum Lb41, an isolate from kimchi and its application as a probiotic in cottage cheese. Food Sci Biotechnol. 2016;25:1129–1133. doi: 10.1007/s10068-016-0181-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo SY, Choi EA, Lee JJ, Chang HC. Characterization of starter kimchi fermented with Leuconostoc kimchii GJ2 and its cholesterol-lowering effects in rats fed a high-fat and high-cholesterol diet. J Sci Food Agric. 2015;95:2750–2756. doi: 10.1002/jsfa.7018. [DOI] [PubMed] [Google Scholar]
- Jousse F. Modeling to improve the efficiency of product and process development. Compr Rev Food Sci Food Saf. 2008;7:175–181. [Google Scholar]
- Jung JE, Yoon HJ, Yu HS, Lee NK, Jee HS, Paik HD. Short communication: physicochemical and antioxidant properties of milk supplemented with red ginseng extract. J Dairy Sci. 2015;98:95–99. doi: 10.3168/jds.2014-8476. [DOI] [PubMed] [Google Scholar]
- Kang H, Moon JS, Lee MG, Han NS. Immunomodulatory effects of Leuconostoc citreum EFEL2061 isolated from kimchi, a traditional Korean food, on the Th2 type-dominant immune response in vitro and in vivo. J Funct Foods. 2016;20:79–87. [Google Scholar]
- Kariyawasam KMGMM, Jeewanthi RKC, Lee NK, Paik HD. Characterization of cottage cheese using Weissella cibaria D30: physicochemical, antioxidant, and antilisterial properties. J Dairy Sci. 2019;102:3887–3893. doi: 10.3168/jds.2018-15360. [DOI] [PubMed] [Google Scholar]
- Kariyawasam KMGMM, Yang SJ, Lee NK, Paik HD. Probiotic properties of Lactobacillus brevis KU200019 and synergistic activity with fructooligosaccharides in antagonistic activity against foodborne pathogens. Food Sci Anim Resour. 2020;40:297–310. doi: 10.5851/kosfa.2020.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Lee JY, Balolong MP, Kim JE, Paik HD, Kang DK. Identification and characterization of a novel antioxidant peptide from bovine skim milk fermented by Lactococcus lactis SL6. Korean J Food Sci Anim Resour. 2017;37:402–409. doi: 10.5851/kosfa.2017.37.3.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HY, Bae WY, Yu HS, Chang KH, Hong YH, Lee NK, Paik HD. Inula britannica fermented with probiotic Weissella cibaria D30 exhibited anti-inflammatory effect and increased viability in RAW 264.7 cells. Food Sci Biotechnol. 2020;29:569–578. doi: 10.1007/s10068-019-00690-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiros E, Seifu E, Bultosa G, Solomon WK. Effect of carrot juice and stabilizer on the physicochemical and microbiological properties of yoghurt. LWT-Food Sci Technol. 2016;69:191–196. [Google Scholar]
- Kuda T, Kawahara M, Nemoto M, Takahashi H, Kimura B. In vitro antioxidant and anti-inflammation properties of lactic acid bacteria isolated from fish intestines and fermented fish from the Sanriku Satoumi region in Japan. Food Res Int. 2014;64:248–255. doi: 10.1016/j.foodres.2014.06.028. [DOI] [PubMed] [Google Scholar]
- Kulka RT, Kotch JB, Jensen ET, Savage E, Weber DJ. Randomized, double-blind, placebo-controlled study of synbiotic yogurt effect on the health of children. J Pediatr. 2015;166:1475–1481. doi: 10.1016/j.jpeds.2015.02.038. [DOI] [PubMed] [Google Scholar]
- Kumpu M, Lehtoranta L, Roivainen M, Rönkkö E, Ziegler T, Venermo MS, Kautiainen H, Järvenpää S, Kekkonen R, Hatakka K, Korpela R, Pitkäranta A. The use of the probiotic Lactobacillus rhamnosus GG and viral findings in the nasopharynx of children attending day care. J Med Virol. 2013;85:1632–1638. doi: 10.1002/jmv.23623. [DOI] [PubMed] [Google Scholar]
- Kwun SY, Bae YW, Yoon JA, Park EH, Kim MD. Isolation of acid tolerant lactic acid bacteria and evaluation of α-glucosidase inhibitory activity. Food Sci Biotechnol. 2020;29:1125–1130. doi: 10.1007/s10068-020-00760-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le B, Yang SH. Isolation of Weissella strains as potent probiotics to improve antioxidant activity of salted squid by fermentation. J Appl Biol Chem. 2018;61:93–100. [Google Scholar]
- Lee H, Kim DY, Lee M, Jang JY, Choue R. Immunomodulatory effects of kimchi in chinese healthy college students: a randomized controlled trial. Clinic Nutr Resear. 2014;3:98–105. doi: 10.7762/cnr.2014.3.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NK, Kim SY, Han KJ, Eom SJ, Paik HD. Probiotic potential of Lactobacillus strains with anti-allergic effects from kimchi for yogurt starters. LWT-Food Sci Technol. 2014;58:130–134. [Google Scholar]
- Lee NK, Jeewanthi RKC, Park EH, Paik HD. Short communication: physicochemical and antioxidant properties of cheddar-type cheese fortified with Inula britannica extract. J Dairy Sci. 2016;99:83–88. doi: 10.3168/jds.2015-9935. [DOI] [PubMed] [Google Scholar]
- Lee E, Jung SR, Lee SY, Lee NK, Paik HD, Lim SL. Lactobacillus plantarum strain Ln4 attenuates diet-induced obesity, insulin resistance, and changes in hepatic mRNA levels associated with glucose and lipid metabolism. Nutrients. 2018;10:643. doi: 10.3390/nu10050643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NK, Hong JY, Yi SH, Hong SP, Lee JE, Paik HD. Bioactive compounds of probiotic Saccharomyces cerevisiae strains isolated from cucumber jangajji. J Funct Foods. 2019;58:324–329. [Google Scholar]
- Lim SM, Lee NK, Paik HD. Antibacterial and anticavity activity of probiotic Lactobacillus plantarum 200661 isolated from fermented foods against Streptococcus mutans. LWT-Food Sci Technol. 2020;118:108840. [Google Scholar]
- Liu CF, Tseng KC, Chiang SS, Lee BH, Hsu WH, Pan TM. Immunomodulatory and antioxidant potential of Lactobacillus exopolysaccharides. J Sci Food Agric. 2011;91:2284–2291. doi: 10.1002/jsfa.4456. [DOI] [PubMed] [Google Scholar]
- Makelainen H, Forssten S, Olli K, Granlund L, Rautonen N, Ouwehand AC. Probiotic Lactobacilli in a semi-soft cheese survive in the simulated human gastrointestinal tract. Int Dairy J. 2009;19:675–683. [Google Scholar]
- Makino S, Ikegami S, Kume A, Horiuchi H, Sasaki H, Orii N. Reducing the risk of infection in the elderly by dietary intake of yoghurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1. Br J Nutr. 2010;104:998–1006. doi: 10.1017/S000711451000173X. [DOI] [PubMed] [Google Scholar]
- Mcfarland LV. From yaks to yogurt: the history, development, and current use of probiotics. Clin Infect Dis. 2015;15:85–90. doi: 10.1093/cid/civ054. [DOI] [PubMed] [Google Scholar]
- Min M, Bunt CR, Mason SL, Hussain MA. Non-dairy probiotic food products: an emerging group of functional foods. Crit Rev Food Sci Nutr. 2019;59:2626–2641. doi: 10.1080/10408398.2018.1462760. [DOI] [PubMed] [Google Scholar]
- Mishra SS, Behera PK, Kar B, Ray RC. Advances in probiotics, prebiotics and nutraceuticals. In: Panda S, Shetty P, editors. Innovations in technologies for fermented food and beverage industries food microbiology and food safety. Cham: Springer; 2018. [Google Scholar]
- Mohanty D, Misra S, Mohapatra S, Sahu PS. Prebiotics and synbiotics: recent concepts in nutrition. Food Biosci. 2018;26:152–160. [Google Scholar]
- Monachese M, Burton JP, Reid G. Bioremediation and tolerance of humans to heavy metals through microbial processes: a potential role for probiotics? Appl Environ Microbiol. 2012;78:6397–6404. doi: 10.1128/AEM.01665-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki Y (2017) Future trends of growing demand for milk and dairy products and milk supply in India. Mitsui and Co Global Strategic Studies Institute monthly report. Mitsui & Co Global Strategic Studies Institute
- Nuraida L. A review: health promoting lactic acid bacteria in traditional Indonesian fermented foods. Food Sci Hum Wellness. 2015;4:47–55. [Google Scholar]
- Okada Y, Tsuzuki Y, Takeshi T, Furuhashi H, Higashiyama M, Watanabe C, Shirakabe K, Kurihara C, Komoto S, Tomita K, Nagao S, Miura S, Hokari R. Novel probiotics isolated from a Japanese traditional fermented food, funazushi, attenuates DSS-induced colitis by increasing the induction of high integrin αv/β8-expressing dendritic cells. J Gastroenterol. 2018;53:407–418. doi: 10.1007/s00535-017-1362-x. [DOI] [PubMed] [Google Scholar]
- Othman M, Ariff AB, Wasoh H, Kapri MR, Halim M. Strategies for improving production performance of probiotic Pediococcus acidilactici viable cell by overcoming lactic acid inhibition. AMB Express. 2017;7:215. doi: 10.1186/s13568-017-0519-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panwar R, Kumar N, Kashyap V, Ram C, Kapila R. Aflatoxin M1 detoxification ability of probiotic Lactobacilli of Indian origin in in vitro digestion model. Probiotics Antimicrob Proteins. 2018;11:460–469. doi: 10.1007/s12602-018-9414-y. [DOI] [PubMed] [Google Scholar]
- Papadopoulou OS, Chorianopoulos NG. Production of a functional fresh cheese enriched with the probiotic strain Lb. plantarum T571 isolated from traditional Greek product. Curr Res Nutr Food Sci. 2016;J4:169–181. [Google Scholar]
- Park HD, Rhee CH. Antimutagenic activity of Lactobacillus plantarum KLAB21 isolated from kimchi Korean fermented vegetables. Biotechnol Lett. 2001;23:1583–1589. [Google Scholar]
- Parussolo G, Busatto RT, Schmitt J, Pauletto R, Schons PF, Ries EF. Synbiotic ice cream containing yacon flour and Lactobacillus acidophilus NCFM. LWT-Food Sci Technol. 2017;82:192–198. [Google Scholar]
- Ranadheera CS, Evans CA, Adams MC, Baines SK. In vitro analysis of gastrointestinal tolerance and intestinal cell adhesion of probiotics in goat’s milk ice cream and yogurt. Food Res Int. 2012;49:619–625. [Google Scholar]
- Reid G. The growth potential for dairy probiotics. Int Dairy J. 2015;49:16–22. [Google Scholar]
- Rosa LS, Santos ML, Abreu JP, Balthazar CF, Rocha RS, Silva HLA, et al. Antiproliferative and apoptotic effects of probiotic whey dairy beverages in human prostate cell lines. Food Res Int. 2020;137:109450. doi: 10.1016/j.foodres.2020.109450. [DOI] [PubMed] [Google Scholar]
- Ryu EH, Yang EJ, Woo ER, Chang HC. Purification and characterization of antifungal compounds from Lactobacillus plantarum HD1 isolated from kimchi. Food Microbiol. 2014;41:19–26. doi: 10.1016/j.fm.2014.01.011. [DOI] [PubMed] [Google Scholar]
- Shokryazdan P, Jahromi MF, Liang JB, Ho YW. Probiotics: from isolation to application. J Am Coll Nutr. 2017;36:666–676. doi: 10.1080/07315724.2017.1337529. [DOI] [PubMed] [Google Scholar]
- Shori AB. Influence of food matrix on the viability of probiotic bacteria: a review based on dairy and non-dairy beverages. Food Biosci. 2016;13:1–8. [Google Scholar]
- Son SH, Jeon HL, Jeon EB, Lee NK, Park YS, Kang DK, Paik HD. Potential probiotic Lactobacillus plantarum Ln4 from kimchi: evaluation of β-galactosidase and antioxidant activities. LWT-Food Sci Technol. 2017;85:181–186. [Google Scholar]
- Son SH, Yang SJ, Jeon HL, Yu HS, Lee NK, Park YS, Paik HD. Antioxidant and immunostimulatory effect of potential probiotic Lactobacillus paraplantarum SC61 isolated from Korean traditional fermented food, jangajji. Microb Pathog. 2018;125:486–492. doi: 10.1016/j.micpath.2018.10.018. [DOI] [PubMed] [Google Scholar]
- Song S, Lee SJ, Park DJ, Sejong OH, Lim KY. The anti-allergic activity of Lactobacillus plantarum L67 and its application to yogurt. J Dairy Sci. 2016;99:9372–9382. doi: 10.3168/jds.2016-11809. [DOI] [PubMed] [Google Scholar]
- Sornplang P, Piyadeatsoontorn S. Probiotic isolates from unconventional sources: a review. J Anim Sci Technol. 2016;58:1–11. doi: 10.1186/s40781-016-0108-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi MK, Giri SK. Probiotic functional foods: survival of probiotics during processing and storage. J Funct Foods. 2014;9:225–241. [Google Scholar]
- Turkmen N, Akal C, Özer B. Probiotic dairy-based beverages: a review. J Funct Foods. 2019;53:62–75. [Google Scholar]
- Urbanska M, Szajewska H. The efficacy of Lactobacillus reuteri DSM 17938 in infants and children: a review of the current evidence. Eur J Pediatr. 2014;173:1327–1337. doi: 10.1007/s00431-014-2328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasiljevic T, Shah NP. Probiotics—from Metchnikoff to bioactives. Int Dairy J. 2008;18:714–728. [Google Scholar]
- Vella MN, Stratton LM, Sheeshka J, Alison M. Exploration of functional food consumption in older adults in relation to food matrices, bioactive ingredients, and health. J Nutr Gerontol Geriatr. 2013;32:122–144. doi: 10.1080/21551197.2013.781419. [DOI] [PubMed] [Google Scholar]
- Wang J, Wu T, Fang X, Min W, Yang Z. Characterization and immunomodulatory activity of an exopolysaccharide produced by Lactobacillus plantarum JLK0142 isolated from fermented dairy tofu. Int J Biol Macromol. 2018;115:985–993. doi: 10.1016/j.ijbiomac.2018.04.099. [DOI] [PubMed] [Google Scholar]
- Wang J, Wu T, Fang X, Yang Z. Manufacture of low-fat cheddar cheese by exopolysaccharide producing Lactobacillus plantarum JLK0142 and its functional properties. J Dairy Sci. 2019;102:1–4. doi: 10.3168/jds.2018-15154. [DOI] [PubMed] [Google Scholar]
- Won TJ, Kim B, Lim YT, Song DS, Park SY, Park ES, Lee DI, Hwang KW. Oral administration of Lactobacillus strains from kimchi inhibits atopic dermatitis in NC/Nga mice. J Appl Microbiol. 2011;110:1195–1202. doi: 10.1111/j.1365-2672.2011.04981.x. [DOI] [PubMed] [Google Scholar]
- Woraprayote W, Pumpuang L, Tosukhowong A, Roytrakul S, Perez RH, Zendo T, Sonomoto K, Benjakul S, Visessanguan W. Two putatively novel bacteriocins active against gram-negative foodborne pathogens produced by Weissella hellenica BCC 7293. Food Control. 2015;55:176–184. [Google Scholar]
- Yerlikaya O, Ozer E. Production of probiotic fresh white cheese using co-culture with Streptococcus thermophilus. Food Sci Technol. 2014;34:471–477. [Google Scholar]
- Zhang YH, Xu D, Liu JQ, Zhao XH. Enhanced degradation of five organophosphorus pesticides in skimmed milk by lactic acid bacteria and its potential relationship with phosphatase production. Food Chem. 2014;164:173–178. doi: 10.1016/j.foodchem.2014.05.059. [DOI] [PubMed] [Google Scholar]
- Zhao W, Liu Y, Latta M, Ma W, Wu Z, Chen P. Probiotics database: a potential source of fermented foods. Int J Food Prop. 2019;22:198–217. [Google Scholar]
- Zhu Y, Wang X, Pan W, Shen X, He Y, Yin H, Zhou K, Zou L, Chen S, Liu S. Exopolysaccharides produced by yogurt-texture improving Lactobacillus plantarum RS20D and the immunoregulatory activity. Int J Biol Macromol. 2019;121:342–349. doi: 10.1016/j.ijbiomac.2018.09.201. [DOI] [PubMed] [Google Scholar]