Abstract
This research was carried out to evaluate the effect of viscozyme pre-treatment followed by hydrodistillation (E-HD) on extraction yield, extraction time and quality of methyl cinnamate basil (O. canum) oil. The viscozyme, as a multienzyme acting on cell wall, was used at different concentrations (0.5% and 1%, v/w) for 30, 60 and 90 min at 50 °C and pH 5 before hydrodistillation (HD). Oxygenated monoterpenes/monoterpene hydrocarbons ratio was used as a quality index for the obtained essential oil. Enzymatic pretreatment at 1% enzyme concentration for 90 min followed by HD increased yield of essential oil by 44.5% with high quality index and decreased HD time from 180 to 30 min. Thirty-nine volatile compounds, comprising > 99% of the essential oil were identified and quantified by Gas Chromatography Mass Spectrum (GC-MS). The major components of essential oil were (E)-methyl cinnamate, camphor, trans-β-caryophyllene and 1,8-cineole. Results confirm the effectiveness of the enzyme pretreatment, which enhance extraction of O. canum essential oil in a short time, with a high quality.
Keywords: Ocimum canum, Viscozyme assisted extraction, Hydrodistillation, Volatile oil
Introduction
The Lamiaceae family is a rich source of spices with functional ingredients that are used as food flavorings and antioxidants (Sakkas and Papadopoulou 2017).
Basil is an aromatic plant with delicate aroma (Shah et al. 2018). It belongs to this family. It is often referred as the ‘King of Herbs’ (Capurso et al. 2018). Varga et al. (2017) recognized four chemotypes of basil: methyl chavicol-rich, linalool-rich, methyl eugenol-rich, and methyl cinnamate-rich. O. canum is widespread in Asia and Africa. Its oil is rich in methyl cinnamate (Vieira and Simon 2000). The characteristic aroma of O. canum oil is attributed to the presence of 1,8-cineole, methyl cinnamate, camphor and linalool. Methyl cinnamate is in demand as a flavor and fragrance compound (Wesolowska and Jadczak 2016).
Basil essential oil is generally obtained by steam or hydrodistillation of flowering tops and leaves of the plants (Charles and Simon 1990). Traditional techniques are insufficient to extract volatile oil from spices completely. These techniques have some deficiencies such as extensive time of extraction besides higher operational costs (da Silva Moura et al. 2020). Therefore, it is important to have new techniques that increase the extractability of these volatiles (Baby and Ranganathan 2016a).
Recent innovative extraction techniques are developed to meet the demands on the economic perspective and production of essential oils with good quality (Mohamad et al. 2019). Enzymes have been used as a pretreatment for the extraction of volatile oil from plant material followed by hydrodistillation or steam distillation (Gai et al. 2013). Treatment with enzymes (cellulase, pectinase or hemicellulase) or an enzyme complex containing a wide range of carbohydrases is required to hydrolyze the cell wall, increase cell wall permeability, enhance the release of oil and improve the extraction efficiency of the target compounds from the plant matrix (Boulila et al. 2015; Zhang et al. 2018). Although, enzyme pretreatment technique results in a decrease in extraction time, increase the yield of essential oil and avoids unfavorable changes in heat labile compounds (Polmann et al. 2019; Reis et al. 2019, 2020), there are no reports on the application of this technique to extract the essential oil from O. canum aerial parts have been published.
The purpose of the present research was to select the proper extraction conditions using combination of enzyme pretreatment and hydrodistillation technique to obtain higher yield of methyl cinnamate basil oil in a short time with high quality.
Materials and methods
Materials and chemicals
Aerial parts (leaves and flowering tops) of O. canum were collected during the flowering stage from the Experimental Station of Medicinal Plants, Horticulture Department, Faculty of Agriculture, Cairo University, Egypt in June 2019. A voucher specimen was deposited at the Herbarium of Orman Botanic Garden, Giza, Egypt. The voucher number is CO.06-05-02-06. The herbs were separated manually and dried under ambient shade conditions (moisture content 10.5%, on dry weight basis), then crushed, grounded into powder and passed through 18 mesh sieve. Anhydrous sodium sulphate, n-alkanes (C8–C20), 2,2-diphenyl-1-picrylhydrazyl (DPPH), butylated hydroxytoluene (BHT) and Viscozyme L. (cellulolytic enzyme mixture) were obtained from Sigma-Aldrich, USA. The activity of the enzyme was 100 Fungal Beta-Glucanase Units (FBG)/g.
Essential oil extraction
Hydrodistillation (HD)
Each extraction process was performed by HD using 50 grams of air dried and crushed aerial parts of basil herb that were immersed in 950 mL of distilled water in a 2 L flask. Process was conducted with a Clevenger type apparatus and lasted 3 h (until no more essential oil was recovered) (Council of Europe 2005).
Viscozyme pre-treatment combined with hydrodistillation (E-HD)
Fifty grams of basil powder were mixed with 500 mL of citrate buffer (0.1 M), pH 5. Viscozyme solution was added to the mixture at concentrations of 0.5% and 1%, v/w of basil powder. The blend was then incubated for 30, 60 and 90 min at 50 °C using shaker (G-25, New Brunswick Scientific Company, New Jersy) at 120 rpm. The enzyme was deactivated after enzymatic treatment by heating in water bath (HumanLab Instrument Co., model DWB-22, Korea) at 90 °C for 5 min (Haider et al. 2018). HD was performed using a Clevenger trap apparatus after addition of distilled water (500 mL). HD process was continued till no more essential oil was recovered.
The volume of volatile oil collected in the trap by the preceding extraction methods was recorded. The obtained essential oil was dehydrated with anhydrous sodium sulphate, before storing in dark airtight bottles at 4 °C until further analysis. The yield of oil was expressed as volume/dry material percentage (v/w %). Extraction was carried out in triplicate. The volatile oil obtained by the HD without enzyme pre-treatment was designated as the control sample.
Chemical composition of essential oil
Essential oil components were determined by GC-MS (TRACE GC Ultra Gas Chromatograph (THERMO Scientific Corp., USA). Thermo mass detector (ISQ Single Quadrupole Mass Spectrometer) was used. The temperatures of injection port and detector were set at 240 °C. One μL of the diluted samples (1:10 hexane, v/v) was injected into the GC at a split ratio of 1:10. Helium flow rate was 1.3 mL/min. The separation was performed on TR-5MS column (30 m × 0.25 mm i.d. 0.25 μm film thickness) using temperature programming as follows: initial temperature was held at 60 °C for 1 min and increased by 3.0 °C/min to 240 °C and held for 1 min. The analysis was performed using electron ionization at 70 eV, using a spectral range of m/z 40-450. Identification was carried out by Kovats indices in reference to n-alkanes (C8–C20), mass spectra of authentic standards, Wiley spectral library collection and NIST library.
Antioxidant activity
DPPH radical inhibition efficiency of the investigated essential oils was determined according to Malterud et al. (1993). The absorbance of the samples was measured using the UV-visible spectrophotometer (Unico UV-2000, USA) at 517 nm. Butylated hydroxytoluene was used as a standard. The concentration of the sample required to inhibit 50% of the free radicals (IC50) was calculated.
Statistical analysis
The % yield of essential oils was expressed as mean ± standard deviation. The data were statistically analyzed using COSTAT. Differences between means were determined by analysis of variance (ANOVA) and Duncan’s test at P < 0.05.
Results and discussion
Effect of enzyme pretreatment followed by hydrodistillation on the yield and HD time of O. canum essential oil
Enzyme types, enzyme concentration and extraction time are the most important independent parameters that can influence the yield of oil (Gai et al. 2013).
The yield of basil essential oil is considerably important in the international market (Wogiatzi et al. 2011). The % yield of oil obtained by hydrodistillation only (without enzyme pretreatment) was 0.83±0.01% (Table 1). This result is in accordance with that reported by Fun and Baerheim Svendsen (1990).
Table 1.
Chemical constituents (as relative area %) of Ocimum canum extracted by different techniques
| Compound | RTa | RIb | HDc | Viscozyme Enzyme 0.5% | Viscozyme Enzyme 1% | ||||
|---|---|---|---|---|---|---|---|---|---|
| E-HD1d | E-HD2 | E-HD3 | E-HD4 | E-HD5 | E-HD6 | ||||
| Monoterpenes hydrocarbons | |||||||||
| α-Thujene | 4.16 | 918 | – | – | 0.20 | – | – | – | – |
| α-Pinene | 4.34 | 935 | 0.31 | 0.51 | 0.93 | 0.62 | 0.57 | 0.29 | 0.33 |
| Camphene | 4.76 | 957 | 0.67 | 0.72 | 1.31 | 0.86 | 0.77 | 0.52 | 0.56 |
| α-Phellandrene | 5.31 | 1006 | 0.27 | 0.31 | 0.45 | 0.34 | 0.31 | 0.16 | 0.46 |
| β-Pinene | 5.46 | 963 | 0.54 | 0.68 | 1.15 | 0.79 | 0.69 | 0.43 | 0.16 |
| α-Terpinene | 6.60 | 1017 | – | 0.22 | 0.27 | 0.24 | 0.20 | 0.17 | – |
| D-Limonene | 6.96 | 1020 | 0.90 | 0.86 | 0.99 | 0.91 | 0.77 | 0.31 | 0.54 |
| γ-Terpinene | 7.94 | 1060 | 0.33 | 0.51 | 0.65 | 0.53 | 0.44 | 0.31 | 0.35 |
| (+) 2-Carene | 8.87 | 1091 | 0.18 | – | – | – | – | – | – |
| Oxygenated monoterpenes | |||||||||
| 1.8-Cineole | 7.08 | 1045 | 5.58 | 6.73 | 10.51 | 7.44 | 6.42 | 5.37 | 5.08 |
| cis-β-Terpineol | 8.44 | 1072 | 0.22 | 0.21 | 0.23 | 0.21 | – | – | – |
| Linalool | 9.50 | 1104 | 1.50 | 1.74 | 1.64 | 1.65 | 1.37 | 1.31 | 1.37 |
| Camphor | 11.49 | 1144 | 9.29 | 10.35 | 14.26 | 10.45 | 9.44 | 10.27 | 9.28 |
| endo-Borneol | 12.43 | 1164 | – | 0.23 | 0.29 | 0.24 | 0.22 | 0.25 | 0.23 |
| Terpinen-4-ol | 12.74 | 1172 | 1.30 | 1.78 | 2.11 | 1.89 | 1.58 | 1.72 | 1.71 |
| α-Terpineol | 13.46 | 1190 | 0.48 | 0.50 | 0.39 | 0.45 | 0.47 | 0.45 | 0.47 |
| Bornyl acetate | 16.92 | 1282 | 0.22 | – | – | – | – | – | – |
| Thymol | 17.65 | 1298 | 3.56 | – | – | – | – | – | 1.08 |
| Phenylpropanoids | |||||||||
| (Z)-Methyl cinnamate | 18.25 | 1303 | 2.56 | 2.66 | 2.37 | 3.57 | 1.90 | 1.41 | 3.65 |
| Eugenol | 20.29 | 1356 | 0.27 | 0.37 | – | 0.44 | 0.19 | – | – |
| (E)-Methyl cinnamate | 21.82 | 1395 | 51.28 | 46.16 | 38.23 | 44.46 | 47.94 | 48.04 | 46.78 |
| Sesquiterpenes hydrocarbons | |||||||||
| α-Copaene | 20.48 | 1364 | 0.30 | 0.17 | 0.31 | 0.18 | 0.38 | 0.38 | 0.45 |
| β-Bourbonene | 20.79 | 1372 | – | – | – | – | 0.17 | 0.17 | 0.17 |
| β-Elemene | 21.12 | 1384 | 0.55 | 0.71 | 0.68 | 0.72 | 0.73 | 0.92 | 0.94 |
| trans-β-Caryophyllene | 22.32 | 1400 | 4.11 | 6.30 | 6.48 | 6.29 | 6.55 | 7.04 | 6.71 |
| β-Cubebene | 22.78 | 1416 | 0.23 | 0.32 | 0.33 | 0.32 | 0.34 | 0.36 | 0.37 |
| α-Guaiene | 22.95 | 1439 | 0.49 | 0.61 | 0.58 | 0.58 | 0.69 | 0.90 | 0.86 |
| α-Humulene | 23.82 | 1454 | 0.60 | 0.82 | 0.82 | 0.81 | 0.90 | 1.05 | 0.99 |
| cis-Muurola-4-(15),5-diene | 24.11 | 1465 | – | 0.39 | 0.38 | 0.42 | 0.41 | 0.35 | 0.33 |
| (+) epiBicyclosesquiphellandrene | 24.88 | 1463 | 2.92 | 3.62 | 3.35 | 3.53 | 3.92 | 4.34 | 4.29 |
| γ-Elemene | 25.68 | 1477 | 1.31 | 1.83 | 1.71 | 1.80 | 1.95 | 1.85 | 1.95 |
| D-Guaiene | 25.68 | 1483 | 0.54 | 0.64 | 0.55 | 0.61 | 0.74 | 1.11 | 1.04 |
| Germacrene D | 25.95 | 1489 | 0.99 | 1.09 | 1.03 | 1.03 | 1.24 | 1.44 | 1.37 |
| γ-Muurolene | 26.22 | 1496 | 1.03 | 2.09 | 2.14 | 2.13 | 2.15 | 1.87 | 1.84 |
| σ-Cadinene | 26.40 | 1526 | – | 0.21 | 0.20 | 0.21 | 0.26 | 0.31 | 0.27 |
| Oxygenated sesquiterpenes | |||||||||
| (−)-Spathulenol | 28.81 | 1578 | 0.98 | 0.45 | 0.31 | 0.41 | 0.44 | 0.55 | 0.67 |
| Caryophyllene oxide | 28.94 | 1581 | 0.81 | 0.72 | 0.79 | 0.66 | 0.61 | 0.74 | 0.82 |
| Cubenol (4-epi-cubedol) | 30.25 | 1627 | 0.49 | 0.59 | 0.50 | 0.56 | 0.57 | 0.52 | 0.50 |
| 10-Epi-α-Cadinol | 31.36 | 1630 | 4.12 | 4.63 | 3.64 | 4.37 | 4.37 | 3.96 | 3.78 |
| α-Cadinol | 31.87 | 1659 | 0.37 | 0.27 | 0.22 | 0.25 | 0.27 | 0.27 | 0.27 |
| Compound | HDc | Viscozyme enzyme 0.5% | Viscozyme enzyme 1% | ||||
|---|---|---|---|---|---|---|---|
| E-HD1d | E-HD2 | E-HD3 | E-HD4 | E-HD5 | E-HD6 | ||
| Total oxygenated compounds (%) | 83.03 | 77.39 | 75.49 | 77.05 | 75.79 | 74.86 | 75.69 |
| Total non-oxygenated compounds (%) | 16.27 | 22.61 | 24.51 | 22.92 | 24.18 | 24.28 | 23.98 |
| Monoterpenes Hydrocarbons | 3.2 | 3.81 | 5.95 | 4.29 | 3.75 | 2.19 | 2.4 |
| Oxygenated Monoterpenes | 22.15 | 21.54 | 29.43 | 22.33 | 19.50 | 19.37 | 19.22 |
| Sesquiterpenes Hydrocarbons | 13.07 | 18.8 | 18.56 | 18.63 | 20.43 | 22.09 | 21.58 |
| Oxygenated Sesquiterpenes | 6.77 | 6.66 | 5.46 | 6.25 | 6.26 | 6.04 | 6.04 |
| O/H ratioe | 6.92 | 5.65 | 4.95 | 5.20 | 5.20 | 8.84 | 8.01 |
| Total identified compounds (%) | 99.30 | 100.00 | 100.00 | 99.97 | 99.97 | 99.14 | 99.67 |
| % yield | 0.83±0.01 cd | 0.8±0.015d | 0.9±0.03c | 1.0±0.01b | 0.8±0.01d | 0.9±0.025c | 1.2±0.1a |
| Enzyme reaction time (min) | - | 30 | 60 | 90 | 30 | 60 | 90 |
| Hydrodistillation Time (min) | 180 | 80 | 40 | 40 | 60 | 30 | 30 |
| Total Extraction Time (min) | 180 | 110 | 100 | 130 | 90 | 90 | 120 |
aRetention time (min)
bRetention indices relative to C8–C20 n-alkanes on TR-5MS column
cHydrodistillation
dEnzyme pretreatment followed by hydrodistillation
eO/H oxygenated monoterpene/monoterpene hydrocarbon ratio
The yield of essential oil obtained from the aerial parts of O. canum plant by E-HD combined technique varied from 0.8% to 1.2% according to enzyme concentration and enzyme incubation time as shown in Table 1.
Increasing enzyme concentration from 0.5% to 1% did not significantly (P>0.05) affect the oil yield after either 30 min or 60 min of enzyme incubation time. Extending enzyme incubation time to 90 min significantly increased the oil yield at each enzyme concentration used. This increase could be attributed to the ability of viscozyme to attack cell wall β-glucan and liberate cell contents including essential oil (Gil-Chávez et al. 2013; Baby and Ranganathan 2016a). The maximal yield (1.2 ± 0.1%) was recorded for the E-HD6 treatment with 1% viscozyme, 90 min incubation time followed by 30 min HD. This increment of oil yield represented about 45% increase relative to oil yield that obtained by the traditional technique (HD only). The effect of the interactions of the investigated parameters (enzyme concentration and incubation time) was found to be significant at the highest levels of both. Sowbhagya et al. (2010) and Baby and Ranganathan (2016a) found that increasing enzyme concentration of each of cellulase, pectinase, protease and viscozyme >1% did not significantly (P>0.05) increase the recovery of essential oil from celery and cardamom seeds. They recommended enzyme concentration 1% and enzyme incubation time of 90 min for better yield.
Increasing enzyme concentration (v/w of basil) at each incubation time decreased HD time required to achieve highest yield of oil. Pretreating basil aerial parts with viscozyme at a concentration of 1% (v/w) for 60 min (E-HD5) or 90 min (E-HD6) decreased the HD time to 30 min only. These enzyme treatments decreased HD time (thermal treatment) to 16.6% of its original level.
Effect of enzyme pretreatment followed by hydrodistillation on the chemical constituents of the essential oil
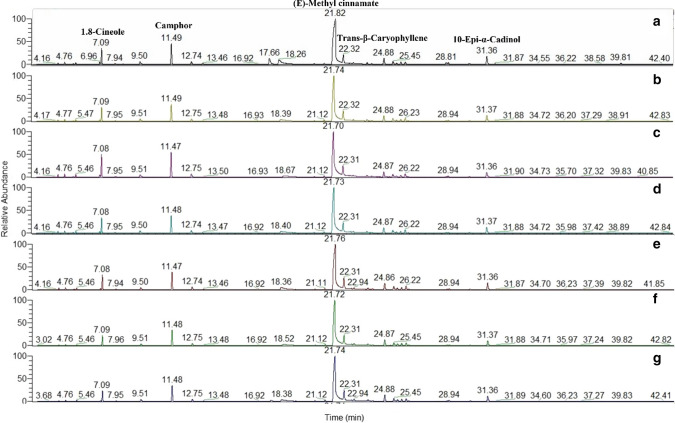
The volatile constituents that distinctive the O. canum essential oil are shown in Fig. 1. and Table 1.
Fig. 1.
GC Chromatograms of Ocimum canum Sims volatile oil extracted by a hydrodistillation (HD) for 180 min; b Enzyme pretreatment (0.5%, 30 min) followed by HD 80 min; c Enzyme pretreatment (0.5%, 60 min) followed by HD 40 min; d Enzyme pretreatment (0.5%, 90 min) followed by HD 40 min; e Enzyme pretreatment (1%, 30 min) followed by HD 60 min; f Enzyme pretreatment (1%, 60 min) followed by HD 30 min; g Enzyme pretreatment (1%, 90 min) followed by HD 30 min
Thirty-nine aroma compounds were identified in the essential oil of O. canum comprising >99% of the total oil. The components of the essential oil extracted by the investigated techniques are generally similar with significant difference in their percentage.
O. canum essential oil composition was dominated with oxygenated monoterpenes (19.22–29.43%), followed by sesquiterpene hydrocarbons (13.07–22.09%), oxygenated sesquiterpenes (5.46–6.77%) and monoterpene hydrocarbons (2.19–5.95%) (Table 1). This result is in agreement with Fun and Baerheim Svendsen (1990).
(E)-methyl cinnamate (38.23–51.28%) was the most predominant compound present in this basil essential oil type, followed by camphor (9.28–14.26%), 1,8-cineole (5.08–10.51%), (Z)-methyl cinnamate (1.41–3.65%), terpinen-4-ol (1.30–2.11%). Sesquiterpene hydrocarbons, contained trans-β-caryophyllene (4.11–7.04%), (+)-epibicyclosesquiphellandrene (2.92–4.34%), germacrene D (0.99–1.44%) and γ-muurolene (1.03–2.15%). On the other hand, 10-Epi-α-cadinol (3.64–4.63%) represented the oxygenated sesquiterpenes. These results are in agreement with Adam et al. (2009). They reported that the main compounds in basil essential oil were (E)-methyl cinnamate (43.4–62.3%) and (Z)-methyl cinnamate (8.1–8.6%), 1,8-cineole (2.8–10.3%) and linalool (4.6–21.9%).
Hydrodistilled oil contained 19.8% of sesquiterpene hydrocarbons and oxygenated sesquiterpenes, while this value ranged from 24% to 28% in the oil samples extracted with enzyme pretreatment.
Results in Table 1 indicated that endo-borneol, cis-muurola-4-(15),5-diene and σ-cadinene, were not detected in the hydrodistilled basil essential oil. (+)-2- Carene (0.18%) and bornyl acetate (0.22%) were detected only in the hydrodistilled oil. Meanwhile, thymol was found only in the hydrodistilled oil and the oil obtained by enzyme pretreatment at 1% concentration for 90 min followed by hydrodistillation for 30 min (E-HD6). On the other hand, β-bourbonene was found only in essential oil samples extracted with the assistance of 1% viscozyme regardless incubation time used. The oil samples extracted by E-HD combined technique exhibit increased trans β-caryophyllene content (> 50%) compared to control (obtained without enzyme pretreatment).
The total oxygenated compounds (%) represented >74% (oxygenated monoterpenes >19%, oxygenated sesquiterpenes <7% and phenylpropanoids >40%), whereas, the total non-oxygenated compounds (%) did not exceed 25%. The distinctive fragrance of the volatile oil depends mainly on the level of oxygenated compounds (Parthasarathy and Prasath 2012). Pretreatment with enzymes helps increase oxygenated components level in the extracted volatile oils of cardamom (Chandran et al. 2012; Nadar et al. 2018), Fructus forsythiae (Jiao et al. 2012), thyme and rosemary (Hosni et al. 2013).
Results in Table 1 indicated that oxygenated monoterpene/monoterpene hydrocarbons ratio (O/H) in the essential oils obtained from enzyme pretreated samples (1% enzyme for 60 and 90 min) exceeded 8 instead of 6.92 in the case of the oil extracted by HD only. From results of Fun and Baerheim Svendsen (1990) it could be concluded that this ratio was 5.37 in the O. canum hydrodistilled oil. Oxygenated compounds are more useful to enhance the scent of essential oil than monoterpene hydrocarbons (Ferhat et al. 2006). Enzyme pre-treatment at proper conditions resulted in higher recovery of the target oil with higher quality (Charoensiddhi and Anprung 2010). Therefore, this pretreatment improved the yield of essential oil and saved processing time and energy as reported by Rashmi Bhardwaj and Gupta (2017).
Antioxidant activity of O. canum essential oil
The IC50 values of the oil samples against DPPH radicals ranged from 139.33 to 154.81 μg/mL regardless the extraction conditions used. IC50 value of the BHT standard was 80 μg/mL. The antioxidant power of the investigated oils was almost the same. This could be attributed to the similarity of chemical composition of the oils. The antioxidant activity of oil is conferred by its major constituents (de Araújo Couto et al. 2019). Koroch et al. (2017) reported that methyl-E-cinnamate showed a very low antioxidant activity. Selvi et al. (2015) found that radical scavenging activity (IC50) of O. canum oil against DPPH was 523.55 μg/mL.
Conclusion
From the obtained results it can be inferred that enzyme assisted extraction of O. canum essential oil led to a higher yield with an improved quality. Pretreatment with viscozyme resulted in an increase (44.5%) in the yield of essential oil and reduction of HD time by 83% compared to the control that obtained by HD only. Enzyme pretreatment enhanced the recovery of oxygenated monoterpenes over the monoterpene hydrocarbons. The results of the study demonstrate that the enzymatic approach combined with HD is appropriate for essential oil extraction with high yield and quality in a short time.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adam F, Vahirua-Lechat I, Deslandes E, Bessiere JM, Menut C. Aromatic plants of French Polynesia. III. Constituents of the essential oil of leaves of Ocimum basilicum L. J Essent Oil Res. 2009;21:237–240. doi: 10.1080/10412905.2009.9700158. [DOI] [Google Scholar]
- Baby KC, Ranganathan TV. Effect of enzyme pre-treatment on extraction yield and quality of cardamom (Elettaria cardamomum maton.) volatile oil. Ind Crop Prod. 2016;89:200–206. doi: 10.1016/j.indcrop.2016.05.017. [DOI] [Google Scholar]
- Baby KC, Ranganathan TV. Effect of enzyme pre-treatment on extraction yield and quality of fennel (Foeniculum vulgare) volatile oil. Biocatal Agric Biotechnol. 2016;8:248–256. doi: 10.1016/j.bcab.2016.10.001. [DOI] [Google Scholar]
- Boulila A, Hassen I, Haouari L, Mejri F, Amor IB, Casabianca H, Hosni K. Enzyme-assisted extraction of bioactive compounds from bay leaves (Laurus nobilis L.) Ind Crop Prod. 2015;74:485–493. doi: 10.1016/j.indcrop.2015.05.050. [DOI] [Google Scholar]
- Capurso A, Crepaldi G, Capurso C (2018) Benefits of the Mediterranean Diet in the Elderly Patient, Practical Issues in Geriatrics. Springer International Publishing AG, part of Springer Nature. 10.1007/978-3-319-78084-9_14
- Chandran J, Padmakumari Amma KP, Menon N, Purushothaman J, Nisha P. Effect of enzyme assisted extraction on quality and yield of volatile oil from black pepper and cardamom. Food Sci Biotechnol. 2012;21:1611–1617. doi: 10.1007/s10068-012-0214-y. [DOI] [Google Scholar]
- Charles DJ, Simon JE. Comparison of extraction methods for the rapid determination of essential oil content and composition of basil (Ocimum spp.) J Am Soc Hortic Sci. 1990;115:458–462. doi: 10.21273/jashs.115.3.458. [DOI] [Google Scholar]
- Charoensiddhi S, Anprung P. Charcaterization of bael fruit (Aegle marmelos (L.) Correa) hydrolysate as affected by enzyme treatment. J Food Biochem. 2010;34:1249–1267. doi: 10.1111/j.1745-4514.2009.00333.x. [DOI] [Google Scholar]
- Council of Europe . European Pharmacopeia. 5. Strasbourg: European Council, European Directorate for the Quality of The Medicines (EDQM); 2005. p. 1. [Google Scholar]
- da Silva Moura E, D’Antonino Faroni LR, Fernandes Heleno F, Aparecida Zinato Rodrigues A, Figueiredo Prates LH, Ribeiro Lopes, de Queiroz ME. Optimal extraction of Ocimum basilicum essential oil by association of ultrasound and hydrodistillation and its potential as a biopesticide against a major stored grains pest. Molecules. 2020;25:2781. doi: 10.3390/molecules25122781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araújo Couto HGS, Blank AF, de Oliveira-e-Silva AM, de Lima Nogueira PC, de Fátima Arrigoni-Blank M, de Castro Nizio DA, Andreza de Oliveira Pinto J. Essential oils of basil chemotypes: major compounds, binary mixtures, and antioxidant activity. Food Chem. 2019;293:446–454. doi: 10.1016/j.foodchem.2019.04.078. [DOI] [PubMed] [Google Scholar]
- Ferhat MA, Meklati BY, Smadja J, Chemat F. An improved microwave Clevenger apparatus for distillation of essential oils from orange peel. J Chromatogr A. 2006;1112:121–126. doi: 10.1016/j.chroma.2005.12.030. [DOI] [PubMed] [Google Scholar]
- Fun CE, Baerheim Svendsen A. Composition of the essential oils of Ocimum basilicum var. canum Sims and O. gratissimum L. grown on Aruba. Flavour Fragr J. 1990;5:173–177. doi: 10.1002/ffj.2730050308. [DOI] [Google Scholar]
- Gai QY, Jiao J, Wei FY, Luo M, Wang W, Zu YG, Fu YJ. Enzyme-assisted aqueous extraction of oil from Forsythia suspense seed and its physicochemical property and antioxidant activity. Ind Crop Prod. 2013;51:274–278. doi: 10.1016/j.indcrop.2013.09.014. [DOI] [Google Scholar]
- Gil-Chávez GJ, Villa JA, Ayala-Zavala JF, Heredia JB, Sepulveda D, Yahia EM, González-Aguilar GA. Technologies for extraction and production of bioactive compounds to be used as nutraceuticals and food ingredients: an overview. Compr Rev Food Sci F. 2013;12:5–23. doi: 10.1111/1541-4337.12005. [DOI] [Google Scholar]
- Haider W, Sultana B, Mushtaq M, Bhatti IA. Multi-response optimization of enzyme-assisted maceration to enhance the yield and antioxidant activity of Cassia fistula pods extracts. J Food Meas Charact. 2018;12:2685–2694. doi: 10.1007/s11694-018-9886-1. [DOI] [Google Scholar]
- Hosni K, Hassen I, Chaâbane H, Jemli M, Dallali S, Sebei H, Casabianca H. Enzyme-assisted extraction of essential oils from thyme (Thymus capitatus L.) and rosemary (Rosmarinus officinalis L.): impact on yield chemical composition and antimicrobial activity. Ind Crop Prod. 2013;47:291–299. doi: 10.1016/j.indcrop.2013.03.023. [DOI] [Google Scholar]
- Jiao J, Fu YJ, Zu YG, Luo M, Wang W, Zhang L, Li J. Enzyme-assisted microwave hydro-distillation essential oil from Fructus forsythia, chemical constituents, and its antimicrobial and antioxidant activities. Food Chem. 2012;134:235–243. doi: 10.1016/j.foodchem.2012.02.114. [DOI] [Google Scholar]
- Koroch AR, Simon JM, Juliani HR. Essential oil composition of purple basils, their reverted green varieties (Ocimum basilicum) and their associated biological activity. Ind Crop Prod. 2017;107:526–530. doi: 10.1016/j.indcrop.2017.04.066. [DOI] [Google Scholar]
- Malterud KE, Farbrot TL, Huse AE, Sund RB. Antioxidant and radical scavenging effects of anthraquinones and anthrones. Pharmacology. 1993;47:77–85. doi: 10.1159/000139846. [DOI] [PubMed] [Google Scholar]
- Mohamad N, Ramli N, Abd-Aziz S, Ibrahim MF (2019) Comparison of hydro-distillation, hydro-distillation with enzyme-assisted and supercritical fluid for the extraction of essential oil from pineapple peels. 3 Biotech 9:234. 10.1007/s13205-019-1767-8 [DOI] [PMC free article] [PubMed]
- Nadar SS, Rao P, Rathod VK. Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: a review. Food Res Int. 2018;108:309–330. doi: 10.1016/j.foodres.2018.03.006. [DOI] [PubMed] [Google Scholar]
- Parthasarathy VA, Prasath D. Cardamom. In: Peter KV, editor. Handbook of Herbs and Spices. UK: Second edition, Woodhead Publishing Limited; 2012. [Google Scholar]
- Polmann G, Badia V, Frena M, Teixeira GL, Rigo E, Block JM, Feltes MMC. Enzyme-assisted aqueous extraction combined with experimental designs allow the obtaining of a high-quality and yield pecan nut oil. LWT. 2019;113:108283. doi: 10.1016/j.lwt.2019.108283. [DOI] [Google Scholar]
- Rashmi Bhardwaj S, Gupta PK. A comparative study of Cymbopogon citratus volatile oil by conventional method versus enzyme pre-treatment. J Essent Oil Bear Pl. 2017;20:744–751. doi: 10.1080/0972060X.2017.1328289. [DOI] [Google Scholar]
- Reis NS, Brito AR, Pacheco CSV, Costa LCB, Gross E, Santos TP, Costa AR, Silva EGP, Oliveira RA, Aguiar-Oliveira E, Oliveira JR, Franco M. Improvement in menthol extraction of fresh leaves of Mentha arvensis by the application of multi-enzymatic extract of Aspergillus niger. Chem Eng Comm. 2019;206:387–397. doi: 10.1080/00986445.2018.1494580. [DOI] [Google Scholar]
- Reis NS, Santana NB, Tavares IMC, Lessa OA, Santos LR, Pereira NE, Soares GA, Oliveira RA, Oliveira JR, Franco M. Enzyme extraction by lab-scale hydrodistillation of ginger essential oil (Zingiber officinale Roscoe): chromatographic and micromorphological analyses. Ind Crop Prod. 2020;146:112210. doi: 10.1016/j.indcrop.2020.112210. [DOI] [Google Scholar]
- Sakkas H, Papadopoulou C. Antimicrobial activity of basil, oregano, and thyme essential oils. J Microbiol Biotechnol. 2017;27:429–438. doi: 10.4014/jmb.1608.08024. [DOI] [PubMed] [Google Scholar]
- Selvi MT, Thirugnanasampandan R, Sundarammal S. Antioxidant and cytotoxic activities of essential oil of Ocimum canum Sims. from India. J Saudi Chem Soc. 2015;19:97–100. doi: 10.1016/j.jscs.2011.12.026. [DOI] [Google Scholar]
- Shah S, Rastogi S, Shasany AK (2018) Genomic resources of ocimum. In: Shasany AK, Kole C (Eds) The ocimum genome, Compendium of plant genomes, 1st edn. Springer Nature Switzerland AG, pp. 99–110. 10.1007/978-3-319-97430-9_8
- Sowbhagya HB, Srinivas P, Krishnamurthy N. Effect of enzymes on extraction of volatiles from celery seeds. Food Chem. 2010;120:230–234. doi: 10.1016/j.foodchem.2009.10.013. [DOI] [Google Scholar]
- Varga F, Carović-Stanko K, Ristić M, Grdiša M, Liber Z, Šatović Z. Morphological and biochemical intraspecific characterization of Ocimum basilicum L. Ind Crops Prod. 2017;109:611–618. doi: 10.1016/j.indcrop.2017.09.018. [DOI] [Google Scholar]
- Vieira RF, Simon JE. Chemical Characterization of basil (Ocimum Spp.) found in the markets and used in traditional medicine in Brazil. Econ Bot. 2000;54:207–216. doi: 10.1007/BF02907824. [DOI] [Google Scholar]
- Wesolowska A, Jadczak D. Composition of the essential oils from inflorescences, leaves and stems of Ocimum basilicum ‘Cinnamon’ cultivated in North-western Poland. J Essent Oil Bear Pl. 2016;19:1037–1042. doi: 10.1080/0972060X.2016.1197801. [DOI] [Google Scholar]
- Wogiatzi E, Papachatzis A, Kalorizou H, Chouliara A, Chouliaras N. Evaluation of essential oil yield and chemical components of selected basil cultivars. Biotechnol Biotec Eq. 2011;25:2525–2527. doi: 10.5504/BBEQ.2011.0067. [DOI] [Google Scholar]
- Zhang W, Leong SM, Zhao F, Zhao F, Yang T, Liu S. Viscozyme L pretreatment on palm kernels improved the aroma of palm kernel oil after kernel roasting. Food Res Int. 2018;107:172–181. doi: 10.1016/j.foodres.2018.02.023. [DOI] [PubMed] [Google Scholar]