Abstract
This study aimed to develop a gluten-free cracker by substituting wheat flour (WF) with riceberry flour (RB) combined with cheese’s milk proteins to replace gluten. The effect of substitution of WF with RB at 50 and 100% (RB50 and RB100, respectively) on cracker properties were evaluated. The results showed that water activity, hunter colour value (L*), hardness, and thickness decreased with an increase in the RB content. This related to a compact structure and a weaker crystallinity of RB cracker when compared with WF-based cracker. The substitution of WF with RB50 and RB100 in cracker significantly (P < 0.05)increased the phytochemical content resulting in increased antioxidant properties.The synergistic effect of the bioactive compound of RB and the bioactive peptides originating from cheese's milk proteins on antioxidant capacity was observed under simulated GI digestion. The greatest increase in the ABTS•+ radical scavenging activity and reducing power was observed in the RB100 cracker at the intestinal digestive phase with the values of 15.74 ± 0.27 and 3.70 ± 0.06 mg Trolox eq./g sample, respectively. The results suggest that the substituted 100% RB combined with cheese’s milk proteins have a potentiality to be developed into a novel gluten-free product with enhanced antioxidant properties.
Keywords: Antioxidant activity, Riceberry flour, Cheese, Cracker, Gastrointestinal transit
Introduction
Crackers are low-moisture baked products made with soft wheat flour which are highly consumed worldwide. However, they are high in calories but low in nutritional value because they usually contain high levels of rapidly digesting carbohydrates, low levels of fibre, and high fat content (Giarnetti et al. 2015). Moreover, coeliac disease patients who are suffering from gluten intolerance are unable to consume them. Thus, food manufacturers try to develop gluten-free (GF) products and also fortify them with bioactive compounds that have high antioxidant properties in order to improve the nutritional value and health benefits of the products (Mir et al. 2017). Dark-coloured rice is one of the most suitable cereal crops for gluten-free and functional food products because it lacks the prolamine and contains high amounts of phytochemical compounds, especially anthocyanin as a major active compound for antioxidation resulting in improved health benefits (Sompong et al. 2011; Settapramote et al. 2018).
Riceberry rice (Oryza sativa L.) is a Thai dark purple rice which has been reported to be a good antioxidants source because it contains high levels of phenolic compounds, with anthocyanins and proanthocyanins as the major compounds (Yodmanee et al. 2011). Nowadays, an anthocyanin-enriched riceberry rice is one of the most popular rice types among consumers because it has shown health-promoting properties including anti-inflammation (Min et al. 2010), cholesterol and LDL reduction (Zawistowski et al. 2009), anti-AGEs activity (Daiponmak et al. 2014), antioxidant effects and inhibiting the growth of cancer cells (Chen et al. 2006). Riceberry flour (RB) has been used as a raw material to increase nutrient density in many food products such as bread (Thiranusornkij et al. 2019), noodles (Thao and Niwat 2017), and biscuits (Klunklin and Savage 2018a) which showed the increase of bioactive compounds such as total phenolic compounds, total anthocyanins and antioxidant activities. Thus RB, being naturally gluten-free, has often been proposed to improve the nutritional value and quality of food products for celiac patients and consumers who are health conscious. However, the elasticity, consistency and viscosity of the dough formation and the quality of the product obtained from gluten in wheat cannot be produced by the rice protein, which has a profound impact on the physicochemical properties (Klunklin and Savage 2018a).
Many studies have investigated the addition of protein in gluten-free products in order to improve the physicochemical properties and nutritional value (Nammakuna et al. 2015). Cheese is a dairy product derived from milk; it contains caseins and whey proteins as the major milk proteins. These proteins have highly functional properties for food processing such as emulsification, water-holding capacity, foaming, thickening and gelling characteristics (Meza et al. 2009). Moreover, they have high nutritional values and the bioactive peptides encrypted in their sequences have been a particular focus as an antioxidants source (Corrochano et al. 2019).
Despite much research into gluten-free baked products, an understanding of the effects of the combination of rice flour and milk protein to overcome the limitations of the absence of gluten protein in rice flour on cracker properties is still needed. In addition, most studies present antioxidant activities of rice flour-based products, but there are no studies concerning the effects of digestive conditions on those activities. Therefore, this study aimed to study the effects of riceberry flour combined with cheese’s milk protein on the physicochemical, microstructure, sensory evaluation, and antioxidant properties under in vitro gastrointestinal digestion of the produced crackers.
Materials and methods
Materials
Ricebery rice flour (RB) was purchased from a local supermarket and cottage cheese with a protein content of 12.5% was made from raw cow milk (the protein content was measured according to AOAC (2000)). Porcine pepsin, porcine pancreatin, 2,4,6-Trinitrobenzenesulfonic acid (TNBS), 2, 2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), 2, 4, 6-tripyridyl-s-triazine (TPTZ), cyanidin-3-glucoside (C3G) and 6-hydroxy-2, 5, 7, 8- tetramethylchroman-2-carboxylic acid (Trolox) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Preparation of cracker
Different formulations of crackers were obtained by making composite flour with riceberry flour (RB) and/or wheat flour (WF). The control cracker contained wheat flour (40 g), sugar (14.4 g), sodium bicarbonate (0.5 g), salt (0.6 g), oat (12 g), rice bran oil (2 g) and water (10.5 g). Riceberry flour was used to substitute 50 and 100% (w/w) of wheat flour, assigned as RB50 and RB100, respectively. All formulations were supplemented with cottage cheese (20 g). All the ingredients were mixed by a mixer at speed 2 for 4 min. Subsequently, the dough was allowed to rest under ambient conditions for 30 min and then the cracker dough was flattened to a thickness of 3 mm and was then cut out to rectangular shape of 3 × 6 cm by a sharp knife. Then, they were baked at 150 °C for 15 min. After cooling for 1 h, crackers were packaged in sealed air-tight plastic bags under ambient conditions until analysis.
Proximate composition
Cracker were analysed for nutritional composition including moisture, ash, fat, and crude protein according to AOAC (2000). The content of total carbohydrates was determined by subtracting the moisture, ash, fat, and crude protein content from the total matter.
Phytochemical composition
Total phenolic compound content
Total phenolic compound content (TPC) was measured by the method of Matthäus (2002) with some adaptations. One hundred microliter of the sample was mixed with 2 mL of 2% Na2CO3, followed by standing for 2 min. Then, 100 µL of Folin–Ciocalteau reagent that was diluted with methanol (1:1) was added. The absorbance was measured at 750 nm after allowing to stand at room temperature for 30 min. TPC content was calculated based on the gallic acid standard curve and was expressed as micrograms of gallic acid equivalent per gram of sample (µg GAE/ g sample).
Total anthocyanin content
The total anthocyanin content (TAC) in the cracker was measured by using a pH differential method. The sample was added to two buffer reagents that were 0.025 M potassium chloride; pH 1.0 and 0.4 M sodium acetate; pH 4.5. The absorbance of the sample was measured at 510 nm and 700 nm and was then calculated using Eq. (1). The content of total anthocyanin was expressed as microgram cyaniding-3-glucoside per gram sample (μg C3G/g).
| 1 |
Gamma-aminobutyric acid (GABA) content
The GABA content of the crackers was evaluated by a method described by Karladee and Suriyong (2012) with some modifications. One gram of sample was dissolved with 80% ethanol (1:4), followed by mixing. Then, 1 mL of the filtered sample was boiled at 80 °C in a water bath to evaporate the ethanol. To the sample, 0.5 mL distilled water was added, and then centrifuged at 10,000 rpm for 10 min. The top portion of supernatant was separated and evaporated. Then, 0.2 mL of 0.2 M borate buffer and 1.0 mL of 6% phenol were added, followed by mixing and cooling on ice for 5 min. Afterwards, 0.4 mL of 10% NaOCl was added and mixed for 1 min, followed by standing on ice for 5 min. The solution was then boiled for 10 min. The absorbance was measured at a wavelength of 630 nm. GABA content was calculated and expressed as micrograms of GABA per gram of sample (µg GABA/ g sample).
Physical properties
The physical properties of the crackers were evaluated including the thickness, colour, water activity (aw) and texture characteristics. Thickness of crackers was measured by a vernier calliper in six replicates for each batch. Water activity of the crackers was determined using an AQUALAB instrument (Series 3, Decagon, USA), calibrated in the range 0.1–0.95 with distilled water. The L*, a* and b* colour values of the different crackers were measured using a colourimeter (Labscan, XE, Hunter Lab. Inc., USA) with L*a*b* scale coordinates. The texture analysis of the samples was performed by the three-point bending test using a TA-XT plus texture analyser (Stable Microsystems, Godalming, UK). The test speed was fixed at 1 mms−1. The force exerted on the sample was recorded. The hardness’ (maximum height of the force peak on the first compression cycle) measurements were taken after 1 day of storage. An average of six values was taken for each set of samples.
Scanning electron microscope (SEM)
The cracker’s microstructure was analysed using a scanning electron microscope (FEI Quanta 250). Prior to the SEM measurement, the crackers were dried at 50 °C and kept in a desiccator until further use. Crackers were mounted on a slide and separately placed on a sample holder using double-sided scotch tape and afterwards were placed on to a microscope. The microstructure of samples was observed under high vacuum at an accelerating voltage of 10 kV.
X-ray diffraction (XRD)
The X-ray diffraction patterns of the crackers were carried out by using an X-ray diffractometer (D8 Advance, Bruker, Germany) at the operating voltage and current of 40 kV and 30 mA, respectively. The diffraction intensities were swept on the cracker between 10 and 30° (2θ) at a scanning rate of 0.1 min−1 with a step size of 0.05°. The crystallinity degree was calculated as the ratio of the area under each crystalline peak to the total area under the XRD pattern.
In vitro pepsin–pancreatin simulated gastrointestinal (GI) digestion
Cracker samples were subjected to in vitro simulated gastrointestinal digestion conditions as described by Helal and Tagliazucchi (2018) with some adaptations. In the gastric phase simulation, the pH of the samples was adjusted to 2.5 with 0.5 M HCl. Pepsin was added to the samples (2000 U/mL), followed by incubation at 37 °C for 2 h. Afterwards, the sample was subjected to the simulated intestinal phase. The pH of the sample was adjusted to 7.5 with 20% Na2CO3 and then pancreatin and bile salts were added (final concentration: 0.8 g/L and 10 mM, respectively), followed by incubation at 37 °C for 3 h. Aliquots of the samples were collected before and after each simulated digestive phase for determination of α-amino acid and antioxidant activity. To terminate the digestion, the aliquots of the samples were boiled for 10 min, followed by cooling at room temperature. The sample was then centrifuged at 11,000 g for 15 min. The supernatants were collected and stored at 4 °C until use.
Determination of α-amino acid content
The α-amino acid content of crackers under simulated GI tract was determined by TNBS (2,4,6-Trinitrobenzenesulfonic acid) method of Adler-Nissen (1979) with some modification. Initially, fifty microliters of each sample were mixed with 0.5 mL of 0.2125 M phosphate buffer; pH 8.2 and 0.5 mL of 0.005% TNBS reagent. The reaction was then incubated at 50 °C for 1 h. One mililiter of 0.1 M HCl was added to terminate the reaction, followed by cooling at room temperature for 30 min. The absorbance was monitored at 420 nm. The results were expressed as milligram leucine equivalent per gram of sample.
Determination of antioxidant activities
ABTS•+ radical cation scavenging activity assay.
The ABTS•+ radical cation scavenging activities of the samples were determined as described by Wiriyaphan et al. (2012). Briefly, 20 μL of samples were added to 1.980 mL of diluted ABTS•+ radical cation solution. The mixture was shaken for 30 s and left in the dark for 5 min. The absorbance of the solution was measured at 734 nm. The degree of ABTS•+ radical cation scavenging activity of samples was expressed as milligram trolox equivalent per gram of sample.
Ferric reducing antioxidant power (FRAP) assay
The FRAP assay of samples was determined by the method of Wiriyaphan et al. (2012) with a slight modification. One millilitre of FRAP reagent and 0.1 mL of samples were added, immediately followed by mixing. The absorbance was measured at 593 nm after standing at 37 °C for 20 min. The ferric reducing antioxidant power of the samples was expressed as microgram trolox equivalent per gram of sample.
Sensory evaluation
A total of 40 volunteering, untrained panellists participated in the test. All were recruited among the staff and student population of the Rajamangala University of Technology Isan. All panellists declared no food allergies. The panellists were instructed to rinse their mouths with water between samples. For each sample, the panellists were asked to rate their preference for its colour, odour, taste, texture, and overall acceptability on three separate 9-box structured hedonic scales, where 1 = extremely dislike and 9 = extremely like.
Statistical analysis
All analyses and enumerations were replicated at least three times. The data were subjected to statistical analysis by one-way ANOVA, using a complete randomized design and sample difference was analysed by Duncan’s Multiple Range Test (DMRT). The level of significance was assigned at P < 0.05.
Results and discussion
The nutritional compositions and calories of cracker are presented in Table 1. The incorporation of RB at 50 and 100% significantly increased ash, fat, and total carbohydrate contents while crude protein content decreased (P < 0.05) when compared with WF-based cracker. This high carbohydrate content may be attributed to low moisture content and higher calories in RB50 and RB100 cracker.
Table 1.
Proximate compositions, calories and phytochemical content of the cracker containing different levels of riceberry flour
| Formulation | |||
|---|---|---|---|
| WF | RB50 | RB100 | |
| Proximate composition (% dry basis) | |||
| Moisture | 11.79 ± 0.93b | 5.87 ± 0.06a | 5.71 ± 0.02a |
| Ash | 2.13 ± 0.08a | 2.24 ± 0.03b | 2.28 ± 0.01b |
| Fat NS | 10.71 ± 0.18 | 11.55 ± 0.36 | 10.83 ± 1.07 |
| Crude protein | 13.35 ± 0.71b | 11.33 ± 0.04a | 11.61 ± 0.23a |
| Total carbohydrate | 62.03 ± 0.98a | 69.01 ± 0.39b | 69.57 ± 1.07b |
| Calories (kcal/100 g) | 397.87 ± 2.70a | 425.35 ± 1.78b | 422.24 ± 5.22b |
| Phytochemical content | |||
| TPC content (µg GAE/g) | 292.7 ± 14.8c | 747.5 ± 6.7b | 812.7 ± 7.4a |
| TAC (µg C3G/g) | N.D | 70.9 ± 1.4b | 387.7 ± 7.5a |
| GABA content (µg GABA/g) | 76.4 ± 17.0c | 150.0 ± 11.1b | 224.1 ± 35.4a |
WF: Wheat flour; RB50: 50% Riceberry flour; RB100: 100% Riceberry flour; N.D.: not detected
Values are expressed as mean ± standard deviation (n = 3)
Means in each row with different letters were significantly different at P < 0.05
NS were none significantly different (P ≥ 0.05)
Phytochemical content
The results showed that the TPC, TAC and GABA contents significantly (P < 0.05) increased when the incorporation of RB increased (Table 1). The substitution of WF with 100% RB exhibited the highest values of TPC (812.7 ± 7.4 μg GAE/g), TAC (387.7 ± 7.5 µg C3G/g), and GABA content (224.1 ± 35.4 µg GABA/g). The results indicate that RB exhibited a higher potential source of bioactive compounds than wheat which resulted in enhanced antioxidant activity. These results are similar to previous studies supporting that RB contains significant amounts of total phenolic compounds unlike white rice or wheat, in which the main compound has been reported as anthocyanins (Sompong et al. 2011). Kittibunchakul et al. (2017) analyzed the GABA content of RB by HPLC which exhibited the GABA content at 8.59 ± 1.51 mg GABA/100 g dry weight. The application of RB in food products caused increased amounts of TPC, TAC, and GABA resulting in enhanced antioxidant activity which was observed in foods such as noodles (Thao and Niwat 2017), and biscuits (Klunklin and Savage 2018a).
Physical properties of cracker
The physical properties of cracker are an important characteristic in determining consumer acceptance. The physical characteristics (colour, aw, thickness and texture) of crackers incorporated with RB and WF are shown in Table 2. Significant differences among crackers (P < 0.05) were observed in all physical parameters. The colour testing of crackers revealed that the lightness (L*), redness (a*) and yellowness (b*) values significantly decreased with an increase in RB levels (P < 0.05). This was affected by the natural colour of riceberry rice that has been shown to have high anthocyanin pigment resulting in darkening of the crackers. Sirichokworrakit et al. (2015) and Klunklin and Savage (2018b) reported similar effects on riceberry noodles and biscuits, respectively. Water activity (aw) affects the shelf life of the products. The values of aw found in this work ranged from 0.4 to 0.6, which reduced the possibility of microbial proliferation, thus indicating a long shelf life of the products. The highest value was found in WF-based cracker (0.61) while the lowest value of aw was detected in the cracker mixed with RB (0.40). The thickness of crackers decreased with an increase in RB content when compared to WF-based control cracker (Table 1). Similarly, the study of Klunklin and Savage (2018b) on the physicochemical properties of purple rice biscuits indicated that the incorporation of purple rice flour affected the thickness due to the protein contents where the thickness of biscuits decreased with a decrease in protein content. For the texture characteristics, the hardness confirmed that as the incorporation of RB increased, the hardness decreased substantially. All crackers that incorporated RB required significantly less force to snap than did the control. According to the texture of the cracker incorporated with RB, it was crumbly, brittle, and had low expansion. This was due to rice flour lacking gluten which is the most important protein contributing to water retention, formation of elastic structures that entrap air and expansion of dough and the end products (Sivaramakrishnan et al. 2004; Nammakuna et al. 2009; Yeboah-Awudzi et al. 2018). Even the addition of milk protein from cheese to replace the gluten did not have an effect on improving texture in RB-based cracker. This may be due to the higher percentage of protein in the dough lowering its hydration, leading to a crumbly texture (Maache-Rezzoug et al. 1998).
Table 2.
Physical and textural characteristics of the cracker containing different levels of riceberry flour
| Formulation | Thickness(mm) | Hardness (N) | aw | Colour | ||
|---|---|---|---|---|---|---|
| L* | a* | b* | ||||
| WF | 6.3 ± 0.42a | 23.7 ± 0.2a | 0.61 ± 0.00a | 59.86 ± 0.26a | 8.42 ± 0.02a | 14.62 ± 0.14a |
| RB50 | 5.2 ± 0.30b | 6.7 ± 0.4b | 0.41 ± 0.00b | 48.68 ± 0.64b | 6.82 ± 0.07c | 6.66 ± 0.30b |
| RB100 | 3.5 ± 0.18c | 2.3 ± 0.5c | 0.40 ± 0.01b | 42.23 ± 0.05c | 7.53 ± 0.03b | 3.22 ± 0.14c |
Values are expressed as mean ± standard deviation (n = 6)
Means in each column with different letters were significantly different at P < 0.05
Microstructure of cracker
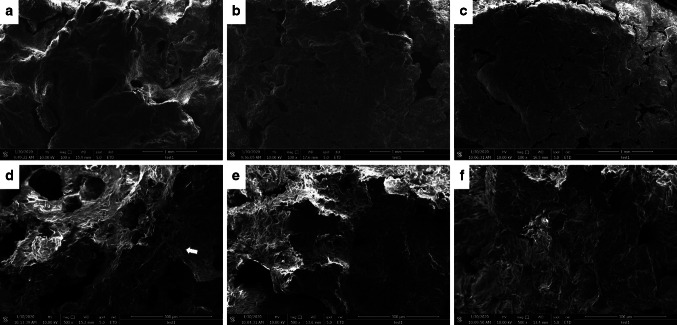
In order to investigate the microstructural changes of crackers corresponding to that of the physical properties, a surface and cross-sectional analysis of the cracker was observed using SEM. Compared with RB-based cracker, WF-based cracker showed a certain roughness on dry surface as shown in Fig. 1a, b and c. For the cross-section morphology, the WF-based cracker revealed many large pores and much larger starch granules, when the substitution percentage of WF for RB increased, samples were more compact and showed a finer structure (Fig. 1d, e and f). These findings were consistent with the results of physical properties that riceberry incorporating cracker exhibited the least expansion and decreased hardness. This result is due to an essential structure-building protein of gluten in wheat flour which can help entrap gas in the dough resulting in larger gas bubbles, but lacked in rice flour (Nammakuna et al. 2015; Yeboah-Awudzi et al. 2018). This observation is similar with Jiamjariyatam (2019) who observed that the morphology of puffed crackers was more compact and with more homogenous air cell walls when the RB percentage was increased.
Fig. 1.
Microstructure of crackers from those of riceberry flour (RB) and wheat flour (WF) control. a–c represented the surface of crackers incorporated with WF, RB50, and RB100, respectively. d–f represented the cross-section of crackers with the same formulations, respectively
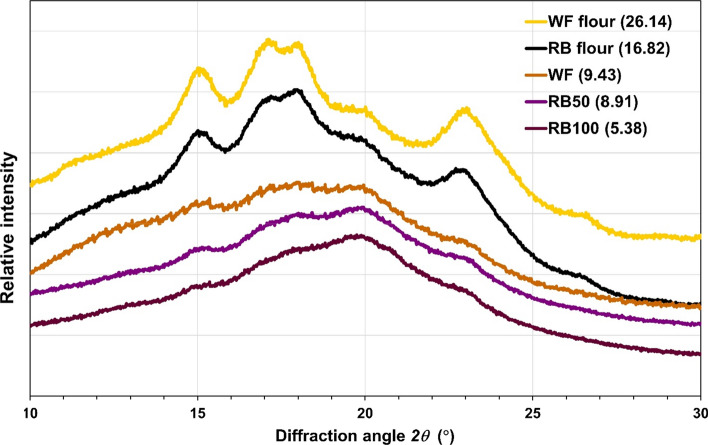
XRD analysis
Crystallinity is one of the factors that affect the physical properties of foods. To examine the influence of substitution of WF with RB on the crystallinity of the cracker, X-ray diffraction analysis was investigated. The X-ray diffraction patterns of unbaked WF and RB flour exhibited a typical A-type XRD pattern with the diffraction peaks at 2θ = 15°, 17°, 18° and 23° (Fig. 2), which corresponds with that of cereal starches (Thuengtung et al. 2019). The crystallinity of WF was significantly higher than RB. After the baking process, A-type crystallinity of all crackers was almost completely destroyed, indicating that the baking process destroyed the crystalline structure of the starch granules. The crystallinity of all crackers considerably decreased with an increase in RB content with the RB100 cracker showing the lowest of crystallinity degree (5.38%). These results suggest that the changes in the crystalline structure of WF and RB flour due to the baking process contributed to the physical properties such as thickness and hardness. These results are consistent with the microstructure results that RB incorporated cracker showed a finer structure which was more homogenous leading to the different physical properties of the cracker.
Fig. 2.
XRD pattern with the crystallinity degrees of riceberry flour (RB flour), wheat flour (WF flour), WF, RB50, and RB100 cracker. Data in brackets indicate the degree of crystallinity (%)
Antioxidant activity of cracker under simulated GI digestion
Inclusion of in vitro findings would help to better understand the properties of RB and cheese’s milk protein as a potential antioxidant source for products. The α-amino acid content of crackers significantly increased with increasing order of the simulated GI digestive phases (P < 0.05), as shown in Fig. 3a. This finding suggested that peptide bonds of protein-fortified crackers were broken with pepsin into smaller fractions and the pancreatin may future hydrolyse some of the peptides being more completely broken down and possibly amino acids (Corrochano et al. 2019). As shown in Figs. 3b and c, in vitro simulated GI digestion modified the antioxidant capacity. All methods revealed that the substitution of WF with RB considerably enhanced antioxidant properties in crackers with increased levels of RB (P < 0.05) which strongly related to the phytochemical content. The greatest increase in the ABTS•+ radical cation scavenging activity and reducing power was observed after the incorporation of RB100 at the intestinal digestive phase with the value of 15.74 ± 0.27 and 3.70 ± 0.06 mg Trolox eq./g sample, respectively. These results might have been influenced by the high amount of phenolic compounds, especially anthocyanins (cyanidin-3-glucoside, pelargonidin-3-glucoside), vitamin E and γ-Oryzanol in RB, which have strong antioxidant effects (Yodmanee et al. 2011; Settapramote et al. 2018). These compounds are able to capture free radicals by the donation of phenolic hydrogen atoms, leading to its antioxidant activity (Chen et al. 1996). The trend of antioxidant activity of enriched RB-based products was similar to other studies, such as in the noodles (Sirichokworrakit et al. 2015), bread (Thiranusornkij et al. 2019), and biscuits (Klunklin and Savage 2018b) which showed the increase of bioactive compounds such as total phenolic compounds, total anthocyanins and antioxidant activities. In addition to RB levels, the trend changes during digestion also showed that the antioxidant capacity continues to increase with the orderly digestion stage. The changing trend of scavenging activity and reducing power correlated with the increase in α-amino acid content under GI digestive phase, indicating that the bioactive peptides formed during digestion could strongly contribute to higher scavenging of ABTS•+ radical cation and reducing power. These results are similar with those of Iskandar et al. (2015) and Corrochano et al. (2019), who showed ABTS•+ radical cation inhibition of whey proteins after the simulated GI digestion was significantly higher than that of the intact protein. This was confirmed by evaluation of the non-digested of crackers that were used as a reference, which exhibited antioxidant activity, but at a level that was far less than that of the hydrolysed samples (data not shown). This finding indicates that the combination of enriched RB and cheese’s milk protein can enhance the antioxidant capacity of the products.
Fig. 3.
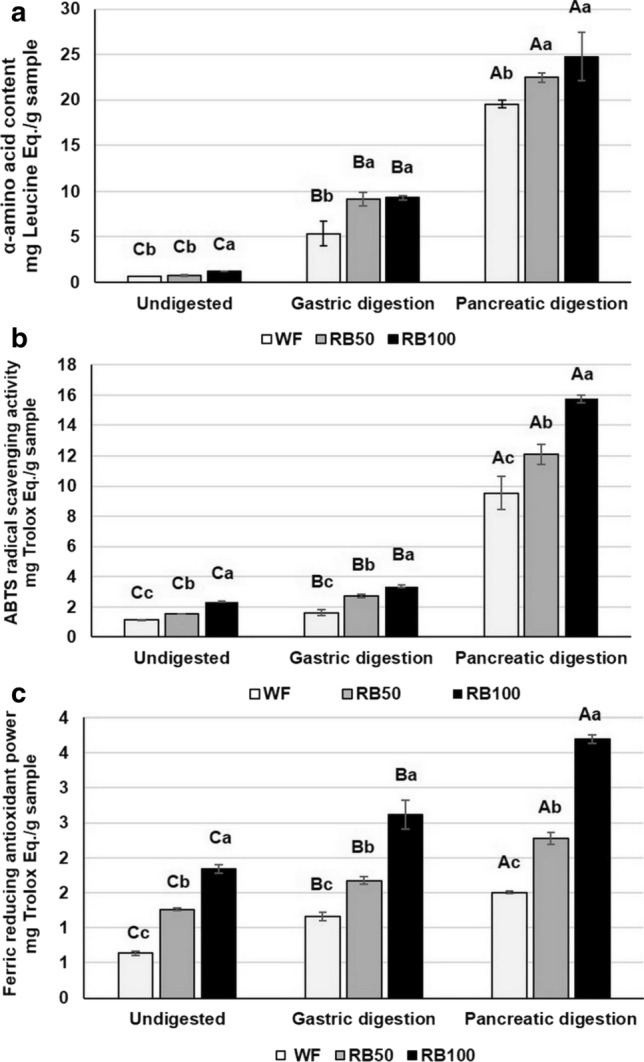
α-amino acid and antioxidant activity of crackers, changes in α-amino acid (a), ABTS radical scavenging activity (b), and ferric reducing antioxidant power (c) of crackers during order in vitro gastric and intestinal conditions Data are expressed as mean ± standard deviation (n = 3). A,B different superscript capital letters denote significant differences between the formulation of crackers for the same sampling period of the in vitro assay (P < 0.05). a,b different lowercase superscript letters denote significant differences between different sampling periods of the in vitro assay for the same formulation of crackers (P < 0.05)
Sensory evaluation
Sensory evaluation results of crackers are shown in Table 3. Crackers incorporating different levels of RB were evaluated for their sensory qualities in all attributes including colour, odour, taste, texture, and overall acceptability. The results showed no statistically variation (P ≥ 0.05) in all parameters between the control WF-based crackers and RB cracker. However, the score of colour, odour, and taste of RB-based cracker was lower than WF cracker. The scores relating to the physical properties such as colour revealed a darkening because RB contains a high anthocyanin pigment. Surprisingly, the term “texture” was used to describe the consumer’s preference for the RB100 crackers, which refers to the hardness characteristic of the cracker. The score of texture of the RB100 substitutions was slightly higher than the control cracker even though the texture of the cracker incorporated with RB was crumbly, brittle, and had low expansion. Additionally, the addition of RB in the crackers significantly increased overall acceptability score from 6.82 ± 0.98 (control) to 7.12 ± 1.11 (RB100) indicating that RB had developed a satisfying flavour in the product. The results conclude that the replacement of wheat flour with riceberry flour will not affect consumer’s acceptance of the gluten-free crackers.
Table 3.
Sensorial analysis of cracker using different proportions of RB
| Formulation | ColourNS | OdourNS | TasteNS | TextureNS | Overall AcceptabilityNS |
|---|---|---|---|---|---|
| WF | 7.40 ± 1.15 | 7.70 ± 0.82 | 6.9 0 ± 1.12 | 7.17 ± 1.08 | 6.82 ± 0.98 |
| RB50 | 7.05 ± 0.93 | 7.45 ± 0.87 | 7.05 ± 1.06 | 7.17 ± 0.95 | 6.87 ± 1.11 |
| RB100 | 7.25 ± 1.03 | 7.47 ± 0.96 | 6.50 ± 1.35 | 7.37 ± 0.95 | 7.12 ± 1.11 |
Values are expressed as mean ± standard deviation (n = 40)
NS were none significantly different (P ≥ 0.05)
Conclusion
The finding of this study suggested that the synergistic effect of the bioactive compounds of the substituted 100% RB and bioactive peptides originating from cheese’s milk protein significantly enhanced the antioxidant capacity under simulated GI digestion whereas RB50 showed all outcome lesser than RB100. The consideration of the cracker properties based on physical, microstructural, and crystallinity degree of RB together with cheese’s milk protein cannot replace gluten to improve the physical properties. However, the overall acceptability of RB100 by consumer was considerably better than that of RB50 and WF. This study indicated that the substution of WF with 100% RB in cracker supplemented with cheese’s milk protein could be developed as a functional food in gluten-free products by promoting antioxidant activity and acceptability by the consumers.
Acknowledgments
We would like to thank RMUTI Scientific research equipment centre for facilities support.
Author contributions
The authors confirm contribution to the paper as follows: Araya Ranok designed and performed. the experiments, supervised the research, analysis and interpretation of results, and prepared. the manuscript. Pornchanok Dissamal performed the experiments. Chanida Kupradit, Chompoonuch Khongla, and Sumalee Musika contributed to sample preparation, Seksan. Mangkalanan prepared the draft manuscript. All authors reviewed the results and approved the final version of the manuscript.
Funding
Not applicable.
Availability of data and material
Author elects to not share data.
Compliance with ethical standards
Conflicts of interest
There are no conflicts of interest to declare.
Consent for publication
The manuscript does not contain data from any individual person.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Araya Ranok, Email: araya7799.bc@gmail.com.
Pornchanok Dissamal, Email: Pummachat.c@gmail.com.
Chanida Kupradit, Email: lego7823@hotmail.com.
Chompoonuch Khongla, Email: chompoonuch.2840@gmail.com.
Sumalee Musika, Email: musika_noi@hotmail.com.
Seksan Mangkalanan, Email: sek.mangkalanan@gmail.com.
References
- Adler-Nissen J. Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid. J Agric Food Chem. 1979;27(6):1256–1262. doi: 10.1021/jf60226a042. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Chan PT, Ho KY, Fung KP, Wang J. Antioxidant activity of natural flavonoids is governed by number and location of their aromatic hydroxyl groups. Chem Phys Lipids. 1996;79(2):157–163. doi: 10.1016/0009-3084(96)02523-6. [DOI] [PubMed] [Google Scholar]
- Chen PN, Kuo WH, Chiang CL, Chiou HL, Hsieh YS, Chu SC. Black rice anthocyanins inhibit cancer cells invasion via repressions of MMPs and u-PA expression. Chem Biol Interact. 2006;163(3):218–229. doi: 10.1016/j.cbi.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Corrochano AR, Sariçay Y, Arranz E, Kelly PM, Buckin V, Giblin L. Comparison of antioxidant activities of bovine whey proteins before and after simulated gastrointestinal digestion. J Dairy Sci. 2019;102(1):54–67. doi: 10.3168/jds.2018-14581. [DOI] [PubMed] [Google Scholar]
- Daiponmak W, Senakun C, Siriamornpun S. Antiglycation capacity and antioxidant activities of different pigmented Thai rice. Int J Food Sci. 2014;49(8):1805–1810. doi: 10.1111/ijfs.12487. [DOI] [Google Scholar]
- Giarnetti M, Paradiso V, Caponio F, Summo C, Pasqualone A. Fat replacement in shortbread cookies using an emulsion filled gel based on inulin and extra virgin olive oil. LWT - Food Sci Technol. 2015;63(1):339–345. doi: 10.1016/j.lwt.2015.03.063. [DOI] [Google Scholar]
- Helal A, Tagliazucchi D. Impact of in-vitro gastro-pancreatic digestion on polyphenols and cinnamaldehyde bioaccessibility and antioxidant activity in stirred cinnamon-fortified yogurt. LWT - Food Sci Technol. 2018;89:164–170. doi: 10.1016/j.lwt.2017.10.047. [DOI] [Google Scholar]
- Iskandar MM, Lands LC, Sabally K, Azadi B, Meehan B, Mawji N, Kubow S. High hydrostatic pressure pretreatment of whey protein isolates improves their digestibility and antioxidant capacity. Foods (Basel, Switz) 2015;4(2):184–207. doi: 10.3390/foods4020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiamjariyatam R. Use of riceberry bran to reduce oil absorption in puffed cracker. Int Food Res J. 2019;26(2):441–450. [Google Scholar]
- Karladee D, Suriyong S. γ-Aminobutyric acid (GABA) content in different varieties of brown rice during germination. SciAsia. 2012;38:13–17. doi: 10.2306/scienceasia1513-1874. [DOI] [Google Scholar]
- Kittibunchakul S, Thiyajai P, Suttisansanee U, Santivarangkna C. Determination of GABA content in Thai brown rice by an optimized enzyme-based method. Chiang Mai J Sci. 2017;44(1):132–143. [Google Scholar]
- Klunklin W, Savage G. Biscuits: a substitution of wheat flour with purple rice flour. Adv J Food Sci Technol. 2018;2(3):81–97. doi: 10.22606/afse.2018.23001. [DOI] [Google Scholar]
- Klunklin W, Savage G. Effect of substituting purple rice flour for wheat flour on physicochemical characteristics, digestibility, and sensory evaluation of biscuits. J Food Qual. 2018;2018:1–8. doi: 10.1155/2018/8052847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maache-Rezzoug Z, Bouvier JM, Allaf K, Patras C. Effect of principal ingredients on rheological behaviour of biscuit dough and on quality of biscuits. J Food Eng. 1998;35(1):23–42. doi: 10.1016/S0260-8774(98)00017-X. [DOI] [Google Scholar]
- Matthäus B. Antioxidant activity of extracts obtained from residues of different oilseeds. J Agric Food Chem. 2002;50(12):3444–3452. doi: 10.1021/jf011440s. [DOI] [PubMed] [Google Scholar]
- Meza BE, Verdini RA, Rubiolo AC. Viscoelastic behaviour of heat-treated whey protein concentrate suspensions. Food Hydrocoll. 2009;23(3):661–666. doi: 10.1016/j.foodhyd.2008.03.015. [DOI] [Google Scholar]
- Min SW, Ryu SN, Kim DH. Anti-inflammatory effects of black rice, cyanidin-3-O-β-d-glycoside, and its metabolites, cyanidin and protocatechuic acid. Int Immunopharmacol. 2010;10(8):959–966. doi: 10.1016/j.intimp.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Mir SA, Bosco SJD, Shah MA, Santhalakshmy S, Mir MM. Effect of apple pomace on quality characteristics of brown rice based cracker. J Saudi Soc. 2017;16(1):25–32. doi: 10.1016/j.jssas.2015.01.001. [DOI] [Google Scholar]
- Nammakuna N, Suwansri S, Thanasukarn P, Ratanatriwong P. Effect of hydrocolloids on qualities of rice crackers made of mixed-flour-blend. As J Food Agro-Ind. 2009;2(04):780–787. [Google Scholar]
- Nammakuna N, Barringer S, Ratanatriwong P. The effects of protein isolates and hydrocolloids complexes on dough rheology, physicochemical properties and qualities of gluten-free crackers. Food Sci Nutr. 2015;4(2):143–155. doi: 10.1002/fsn3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settapramote N, Laokuldilok T, Boonyawan D, Utama-ang N. Physiochemical, antioxidant activities and anthocyanin of riceberry rice from different locations in Thailand. FAB J. 2018;6:84–94. [Google Scholar]
- Sirichokworrakit S, Phetkhut J, Khommoon A. Effect of partial substitution of wheat flour with riceberry flour on quality of noodles. Procedia Soc Behav Sci. 2015;197:1006–1012. doi: 10.1016/j.sbspro.2015.07.294. [DOI] [Google Scholar]
- Sivaramakrishnan H, Senge B, Chattopadhyay P. Rheological properties of rice dough for making rice bread. J Food Eng. 2004;62:37–45. doi: 10.1016/S0260-8774(03)00169-9. [DOI] [Google Scholar]
- Sompong R, Siebenhandl-Ehn S, Linsberger-Martin G, Berghofer E. Physicochemical and antioxidative properties of red and black rice varieties from Thailand. China Sri Lanka Food Chem. 2011;124(1):132–140. doi: 10.1016/j.foodchem.2010.05.115. [DOI] [Google Scholar]
- Thao N, Niwat C. Effect of germinated colored rice on bioactive compounds and quality of fresh germinated colored rice noodle. KMUTNB Int J Appl Sci Technol. 2017;11(1):27–37. doi: 10.14416/j.ijast.2017.12.008. [DOI] [Google Scholar]
- Thiranusornkij L, Thamnarathip P, Chandrachai A, Kuakpetoon D, Adisakwattana S. Comparative studies on physicochemical properties, starch hydrolysis, predicted glycemic index of Hom Mali rice and Riceberry rice flour and their applications in bread. Food Chem. 2019;283:224–231. doi: 10.1016/j.foodchem.2019.01.048. [DOI] [PubMed] [Google Scholar]
- Thuengtung S, Matsushita Y, Ogawa Y. Comparison between microwave-cooking and steam-cooking on starch properties and in vitro starch digestibility of cooked pigmented rice. J Food Process Eng. 2019;42(6):1–9. doi: 10.1111/jfpe.13150. [DOI] [Google Scholar]
- Wiriyaphan C, Chitsomboon B, Yongsawadigul J. Antioxidant activity of protein hydrolysates derived from threadfin bream surimi by products. Food Chem. 2012;132(1):104–111. doi: 10.1016/j.foodchem.2011.10.040. [DOI] [PubMed] [Google Scholar]
- Yeboah-Awudzi M, Lutterodt HE, Kyereh E, Reyes V, Sathivel S, Manful J, King JM. Effect of bambara groundnut supplementation on the physicochemical properties of rice flour and crackers. J Food Sci Technol. 2018;55(9):3556–3563. doi: 10.1007/s13197-018-3281-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yodmanee S, Karrila T, Pakdeechanuan P. Physical, chemical and antioxidant properties of pigmented rice grown in Southern Thailand. Int Food Res J. 2011;18(3):901–906. [Google Scholar]
- Zawistowski J, Kopeć A, Kitts D. Effects of a black rice extract (Oryza sativa L indica) on cholesterol levels and plasma lipid parameters in Wistar Kyoto rats. J Funct Foods. 2009;1(1):50–56. doi: 10.1016/j.jff.2008.09.008. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Author elects to not share data.