Abstract
Inadequate sensitivity has been the primary limitation for implementing high-throughput sequencing for studies of tick-borne agents. Here we describe the development of TBDCapSeq, a sequencing assay that uses hybridization capture probes that cover the complete genomes of the eleven most common tick-borne agents found in the United States. The probes are used for solution-based capture and enrichment of pathogen nucleic acid followed by high-throughput sequencing. We evaluated the performance of TBDCapSeq to surveil samples that included human whole blood, mouse tissues, and field-collected ticks. For Borrelia burgdorferi and Babesia microti, the sensitivity of TBDCapSeq was comparable and occasionally exceeded the performance of agent-specific quantitative PCR and resulted in 25 to > 10,000-fold increase in pathogen reads when compared to standard unbiased sequencing. TBDCapSeq also enabled genome analyses directly within vertebrate and tick hosts. The implementation of TBDCapSeq could have major impact in studies of tick-borne pathogens by improving detection and facilitating genomic research that was previously unachievable with standard sequencing approaches.
Subject terms: Infectious-disease diagnostics, Next-generation sequencing
Introduction
Early detection is critical for prompt and effective treatment of acute infectious diseases. Molecular assays are the optimal method for early and rapid detection of pathogenic agents. For tick-borne diseases (TBD), the lack of accurate early diagnosis can result in delayed treatment, augment morbidity, and increase the likelihood of developing persistent symptoms1,2. For some agents of TBD, such as Anaplasma phagocytophilum and Babesia microti, molecular diagnostic assays are highly useful3. For diagnosis of Lyme disease, however, the low sensitivity of molecular assays in the majority of clinical presentations has prevented their extensive implementation as an effective diagnostic tool4–7. The exception is synovial fluid in patients with Lyme arthritis, where PCR has 70 to 85% sensitivity8,9. The primary challenges in molecular diagnoses of Borrelia burgdorferi sensu stricto (s.s.) infection include low pathogen burden coupled with transient spirochetaemia10,11. As a result, serology remains the primary means of diagnosis for Lyme disease and the majority of TBD11–13. There is nonetheless a need for molecular assays for differential diagnosis of TBD that can complement serology.
PCR and unbiased high-throughput sequencing (UHTS) are the primary molecular assays employed for detection of infectious agents14,15. The advantages of UHTS over PCR include the lack of dependence for analogous primer and template sequences, the capacity to detect all agents, and the ability to accurately identify novel species or strains15–20. However, PCR generally retains an advantage in sensitivity, cost and simplicity. The sensitivity of UHTS can be enhanced with deeper sequencing, but this approach is currently financially unfeasible. The recent implementation of capture-based sequencing assays has had a major impact on the utility of high-throughput sequencing (HTS) for pathogen detection21–23. This approach uses agent-specific probes to selectively capture the template of interest prior to sequencing and results in a remarkable increase in sequencing reads for the captured template when compared to UHTS. Our Center has developed probe-based capture assays for viruses (VirCapSeq) and bacteria (BacCapSeq) that result in assay sensitivity equal or greater to real-time PCR24,25. Dual barcoding enables simultaneous testing of 50 samples in a single run without compromising sensitivity and providing cost efficiency. Thus, employment of capture assays would provide substantial improvement in detection of tick-borne agents over UHTS and PCR. In addition, the far greater capacity of capture-sequencing assays to generate complete genome sequences relative to UHTS can lead to increased understanding of how strain diversity impacts disease. For B. burgdorferi s.s., work in animal models has implicated several strains with increased likelihood of systemic dissemination26. In particular, plasmid content and specific OspC types have been associated with enhanced pathogenesis27–30. Different strains within B. burgdorferi s.s. can be distinguished that impact pathogenicity and virulence in humans26,31,32. B. burgdorferi s.s. RST1 strains, which appear to account for approximately 40% of Lyme borreliosis cases in the northeastern US, are more likely to disseminate hematogenously and are associated with a higher risk of antibiotic-refractory Lyme arthritis33,34. Similarly, certain variations in OspC have been shown to be associated with disseminated infection in humans31,35. Unfortunately, assay limitations have prevented comprehensive genomic analyses of B. burgdorferi s.s. in human specimens and, at present, there is a great paucity of B. burgdorferi s.s. genomic data obtained directly from patient samples. This limits our understanding of how strain diversity could influence the development of Lyme disease-associated syndromes such as neuroborreliosis, Lyme arthritis and post-treatment Lyme disease syndrome. In this work, we address the need for diagnostic improvement combined with genetic typing with the development of Tick-Borne Disease Capture Sequencing assay (TBDCapSeq). We demonstrate that TBDCapSeq is capable of enhanced targeted detection of all major agents of TBD found in the United States that also provides invaluable genomic data that can be used to augment our understanding of strain variation and its importance to tick-borne disease.
Results
To assess the performance of TBDCapSeq, we selected pathogen-infected ticks (larvae, nymphs and adults), mouse tissues, human whole blood, as well as controls (Table 1). These samples were available from other published or ongoing studies, and provided an opportunity to evaluate TBDCapSeq on different sample matrices36–38. For this study, we primarily focused on samples that were infected with B. burgdorferi s.s. or B. microti, as these agents are among the most frequent causes of tick-borne illness in the US.
Table 1.
List of samples analyzed by TBDCapSeq.
| Experiment | Sample type (N) | Number of samples |
|---|---|---|
| 1 | Mouse tissues; heart (3) ear (3) bladder (3); larval ticks (2) | 11a |
| 2 | Mouse tissues; heart (3) ear (3) bladder (3) ankle (3); larval ticks (2) | 14a |
| 3 | Ticks; Ixodes scapularis (16), Amblyomma americanum (2 pools), Dermacentor variabilis (3) | 20 |
| 4 | Whole blood; Babesia microti contrived samples (16); healthy controls (2) | 18 |
| 5 | Whole blood; Babesiosis (19); healthy control (1); salmon sperm (1) | 21 |
| 6 | Whole blood; acute Lyme disease (15); acute babesiosis (3); healthy control (1) | 19 |
| 7 | Whole blood; Borrelia burgdorferi contrived samples (10); healthy controls (2) | 12 |
aEach sample was analyzed by unbiased high-throughput sequencing and TBDCapSeq.
Mouse tissues
To evaluate the utility of TBDCapSeq with respect to UHTS for testing tissue samples, we examined heart, ear, and bladder tissues from three C3H mice infected with 105 spirochetes of the N40 D10E9 strain of B. burgdorferi s.s. (Table 2). All mice were culture positive. We also tested two replete larval ticks that fed on these mice. Prior to sequencing, we used an ospA qPCR assay to estimate the B. burgdorferi s.s. burden for all samples. In murine tissues, the Ct ranged from 29.48 to 35.49 (corresponding to approximately 2500 to 25 copies of ospA). The quantity of B. burgdorferi s.s. in the ticks was > 100 fold higher, with Cts of 21.17 and 22.25 (both > 5 × 105). All 11 samples were sequenced together in two pools on a single Illumina flow cell. One pool was subjected to capture with the TBDCapSeq probes. The second pool was sequenced using a standard unbiased approach. We observed a substantial increase in the number of B. burgdorferi s.s. reads using TBDCapSeq when compared to UHTS. After normalization, the increase in B. burgdorferi s.s. reads in murine tissues with TBDCapSeq ranged from 1133-fold to 9332-fold. We also observed a sizable increase in B. burgdorferi s.s. reads from the larval ticks with TBDCapSeq (125-fold and 140-fold).
Table 2.
Read enrichment using TBDCapSeq compared to unbiased high-throughput sequencing (UHTS) in experiment 1.
| Sample type | ospA Ct | Normalized reads (Borrelia reads per million total reads) | Fold enrichment of reads with TBDCapSeq over UHTS | |
|---|---|---|---|---|
| TBDCap seq | UHTS | |||
| Replete larval tick | 21.17 | 544,328 | 5707 | 140 |
| Replete larval tick | 22.25 | 323,316 | 2578 | 125 |
| Mouse 1, Heart | 33.62 | 1272 | 0.067 | 1890 |
| Mouse 1, Ear | 31.40 | 20,819 | 7.27 | 2863 |
| Mouse 1, Bladder | 32.62 | 2930 | 0.35 | 8338 |
| Mouse 2, Heart | 34.23 | 4790 | 1.83 | 2615 |
| Mouse 2, Ear | 29.64 | 34,353 | 13 | 2648 |
| Mouse 2, Bladder | 35.49 | 392 | 0.34 | 1133 |
| Mouse 3, Heart | 33.47 | 2009 | 0.062 | 3213 |
| Mouse 3, Ear | 29.48 | 3546 | 1.01 | 3494 |
| Mouse 3, Bladder | NDa | 829 | 0.09 | 9332 |
aND = not done.
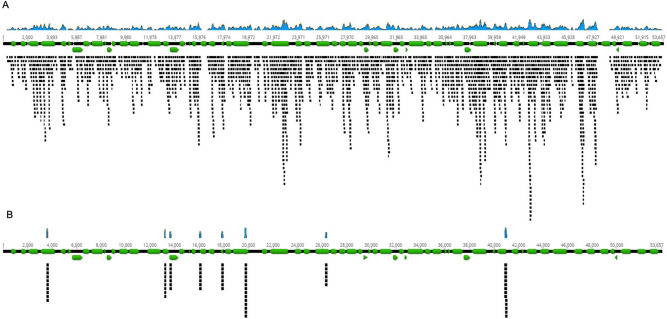
Next, we examined tissues from three C57BL/6 mice (one mouse infected with B. burgdorferi s.s. N40 D10E9, one mouse infected with B. burgdorferi s.s. B31, and one mouse infected with both N40 D10E9 and B31) and two replete larval ticks (one tick infected with B. burgdorferi s.s. B31, and a negative control). Tick and mouse samples were processed blinded as to their infection status in this experiment, since our primary aim was to demonstrate the improvements in detection and strain identification achieved by TBDCapSeq. Samples were sequenced on two separate flow cells, one using UHTS and another using TBDCapSeq. We also determined B. burgdorferi s.s. burden by qPCR. Again, TBDCapSeq generated a remarkable increase in reads with TBDCapSeq relative to UHTS (Table 3). In murine samples with detectable B. burgdorferi s.s. reads using both sequencing approaches, we obtained a 6550-fold to 52,000-fold enrichment using TBDCapSeq over UHTS. In three murine samples with a low bacterial load (Cts 35 to 37, corresponding to approximately 25 to 6 ospA copies), two samples did not generate any Borrelia reads with UHTS, and the third produced only a single Borrelia read. With TBDCapSeq, we generated between 9817 and 26,671 Borrelia reads for these samples. One sample (bladder tissue-mouse 1) was negative by both qPCR and UHTS. With TBDCapSeq, we obtained 781 non-ribosomal reads (out of 1329), originating from the chromosome and multiple linear and circular plasmids. For example, we obtained 74 reads from 8 regions within the lp54 plasmid, accounting for 1005 nt, or 2.1% of the total plasmid sequence (Fig. 1). We also obtained a notable improvement in genome recovery (Table 4). In samples with a qPCR Ct of approximately 35, we were able to recover nearly 20% of the B. burgdorferi s.s. genome. In two samples with a higher B. burgdorferi s.s. burden (qPCR Cts between 30 and 31, corresponding to approximately 2000 to 1000 ospA copies), B. burgdorferi s.s. reads accounted for > 9.5% of the total reads generated by TBDCapSeq, and we were able to assemble > 95% of each genomic segment. For the two larval ticks, we assembled the complete B. burgdorferi s.s. genome for tick 1. The other tick did not yield any Borrelia reads, in agreement with the corresponding ospA-negative qPCR data.
Table 3.
Read enrichment using TBDCapSeq compared to unbiased sequencing in experiment 2.
| ospA Ct | Sample type | Sequencing type | Total reads | # of reads mapped to B31 | % of reads mapped to B31 | Normalized reads (Borrelia reads per million total reads) | Fold enrichment |
|---|---|---|---|---|---|---|---|
| 35.15 | Heart Tissue- Mouse 1 | Unbiased | 10,473,737 | 0 | 0 | 0 | 1603.10 |
| TBDCAPSEQ | 13,922,800 | 22,319 | 0.18 | 1603.10 | |||
| 34.06 | Ear Tissue- Mouse 1 | Unbiased | 11,451,201 | 22 | 0 | 1.921 | 6550.90 |
| TBDCAPSEQ | 11,160,954 | 140,453 | 1.4 | 12,584.30 | |||
| 33.99 | Ankle Tissue- Mouse 1 | Unbiased | 9,663,953 | 3 | 0 | 0.31 | 52,212.80 |
| TBDCAPSEQ | 2,454,532 | 39,729 | 1.938 | 16,185.98 | |||
| ND | Bladder Tissue- Mouse 1 | Unbiased | 8,675,559 | 0 | 0 | 0 | 101.3 |
| TBDCAPSEQ | 13,120,042 | 1329 | 0.01 | 101.3 | |||
| 31 | Heart Tissue- Mouse 2 | Unbiased | 8,956,637 | 32 | 0 | 0 | 46,207.90 |
| TBDCAPSEQ | 12,485,504 | 576,929 | 5.06 | 46,207.90 | |||
| 33.38 | Ear Tissue- Mouse 2 | Unbiased | 10,377,529 | 35 | 0 | 3.37 | 8804.90 |
| TBDCAPSEQ | 11,411,883 | 338,619 | 3.27 | 29,672.50 | |||
| 30.49 | Ankle Tissue- Mouse 2 | Unbiased | 7,332,169 | 46 | 0.001 | 6.27 | 13,893 |
| TBDCAPSEQ | 10,915,727 | 950,856 | 9.5 | 87,108.80 | |||
| 36.67 | Bladder Tissue- Mouse 2 | Unbiased | 10,477,826 | 1 | 0 | 0.1 | 6691 |
| TBDCAPSEQ | 14,041,858 | 9817 | 0.08 | 699.1 | |||
| 30.29 | Heart Tissue- Mouse 3 | Unbiased | 8,959,037 | 131 | 0.002 | 14.62 | 1137.03 |
| TBDCAPSEQ | 13,331,701 | 2,141,384 | 17.46 | 160,623.50 | |||
| 32.2 | Ear Tissue- Mouse 3 | Unbiased | 8,148,936 | 37 | 0.001 | 4.54 | 10,938.30 |
| TBDCAPSEQ | 13,089,033 | 650,000 | 5.52 | 49,659.90 | |||
| 32.83 | Ankle Tissue- Mouse 3 | Unbiased | 7,253,259 | 18 | 0 | 2.48 | 10,517.70 |
| TBDCAPSEQ | 11,386,644 | 297,007 | 2.88 | 26,083.80 | |||
| 36.32 | Bladder Tissue- Mouse 3 | Unbiased | 8,663,429 | 0 | 0 | 0 | 2466.70 |
| TBDCAPSEQ | 10,812,361 | 26,671 | 0.27 | 2466.70 | |||
| 24.03 | Tick (fed larva 1) | Unbiased | 7,014,321 | 7657 | 0.122 | 1091.62 | 68.89 |
| TBDCAPSEQ | 64,314,285 | 4,836,678 | 8.129 | 75,203.79 | |||
| NDc | Tick (fed larva 2) | Unbiased | 8,210,936 | 0 | 0 | 0 | 0 |
| TBDCAPSEQ | 63,077,105 | 1335a | 0 | 0 | |||
| ND | Salmon sperm DNA | Unbiased | 8,214,254 | 0 | 0 | 0 | 0 |
| TBDCAPSEQ | 694,238 | 31b | 0 | 0 |
aAll mapped to 16S and 23S rRNA of Rickettsia buchneri.
bAll represented non-Borrelia 16S rRNA reads.
cND = not done.
Figure 1.
Mapping of B. burgdorferi s.s. reads obtained by TBDCapSeq to the Lp54 plasmid. All quality filtered reads were mapped directly to the B31 Lp54 sequence (accession number NC_001857, www.ncbi.nlm.nih.gov). (A) reads obtained from the bladder tissue of mouse 3. (B) reads from the bladder tissue of mouse 1. The sample was negative by qPCR and UHTS. The black horizontal line represents the contiguous linear length of the plasmid with the numbers representing the nucleotide positions within the plasmid. Green arrows represent open reading frames. Assemblies were performed in Geneious v 10.0.9 (www.geneious.com).
Table 4.
Genome recovery of B. burgdorferi in murine tissues with TBDCapSeq.
| Accession number | Chromosome/plasmid | % coverage Mouse 1, Heart (qPCR Ct 35.15) | % coverage Mouse 2, Heart (qPCR Ct 31) | % coverage Mouse 3, Heart (qPCR Ct 30.29) |
|---|---|---|---|---|
| NC_000948 | cp32-1 | 51.73 | 98.92 | 99.63 |
| NC_000949 | cp32-3 | 47.17 | 99.24 | 99.27 |
| NC_000950 | cp-32-4 | 53.49 | 99.47 | 100.00 |
| NC_000951 | cp32-6 | 56.23 | 99.34 | 99.35 |
| NC_000952 | cp32-7 | 48.81 | 97.98 | 99.96 |
| NC_000953 | cp32-8 | 51.28 | 98.99 | 99.56 |
| NC_000954 | cp32-9 | 56.18 | 99.14 | 99.60 |
| NC_000955 | lp21 | 1.22 | 99.87 | 100.00 |
| NC_000956 | lp56 | 34.23 | 98.69 | 99.49 |
| NC_000957 | lp5 | 2.70 | 100.00 | 100.00 |
| NC_001318 | CHR | 20.42 | 97.93 | 98.55 |
| NC_001849 | lp17 | 35.41 | 99.67 | 99.72 |
| NC_001850 | lp25 | 21.83 | 94.77 | 98.54 |
| NC_001851 | lp28-1 | 0.89 | 100.00 | 99.63 |
| NC_001852 | lp28-2 | 18.49 | 98.34 | 98.90 |
| NC_001853 | lp28-3 | 15.71 | 95.97 | 99.18 |
| NC_001854 | lp28-4 | 16.78 | 97.97 | 98.22 |
| NC_001855 | Lp36 | 5.21 | 99.36 | 99.24 |
| NC_001856 | lp38 | 2.18 | 97.56 | 99.03 |
| NC_001857 | lp54 | 38.08 | 99.54 | 99.58 |
| NC_001903 | cp26 | 19.39 | 98.80 | 98.18 |
| NC_001904 | cp9 | 5.51 | 17.47 | 22.28 |
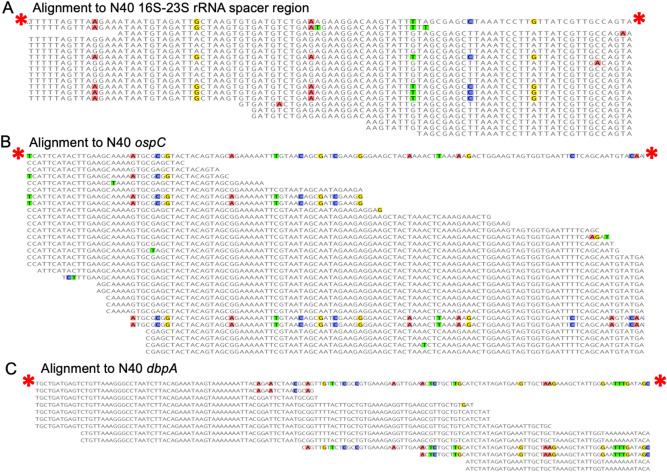
Next, we examined the assembled sequences of 16S rRNA-23S rRNA spacer region, and ospC and dbpA to determine the genotype of the infecting strains. Quality filtered reads were mapped to reference sequences from multiple B. burgdorferi s.s. strains (B31, N40, JD1, ZS7, WI39, and 297). Infecting strains were correctly identified as N40 in mouse 1, B31 in mouse 2 and tick 1, and a mix of both N40 and B31 strains present in mouse 3 (Fig. 2).
Figure 2.
Identification of an infection with multiple strains of B. burgdorferi s.s. by TBDCapSeq. Shown are alignments of TBDCapSeq reads to fragments of (A) 16S-23S rRNA spacer region, (B) dbpA, and (C) ospC from the N40 strain (accession numbers NC_017416, NC_013130, NC_017401, www.ncbi.nlm.nih.gov). The reference N40 sequence for each gene is indicated with red asterisks. Variant nucleotides are indicated with colors. All three alignments contain a mix of two types of sequences, one identical to strain N40 reference sequence and a variant sequence identical to strain B31 reference sequence. Assemblies were performed in Geneious v 10.0.9 (www.geneious.com).
Genotyping in ticks
To demonstrate the utility of TBDCapSeq for studies of ticks and detection of agents other than B. burgdorferi s.s., we selected nucleic acids from 18 individual ticks and two tick pools for TBDCapSeq analysis. These samples were all previously examined for the presence of tick-borne pathogens by UHTS or multiplex qPCR37,38. We selected 10 adult and 5 I. scapularis nymphs with infections with either one, two, or three agents. We also examined three individual D. variabilis, and two pools of A. americanum (pool 1, adults, N = 12; pool 2, nymphs, N = 11). The resulting Illumina reads were put through our regular bioinformatics pipeline, consisting of read filtration, contig assembly and homology searches through Blast. The TBDCapSeq results were 100% congruent with results obtained previously with UHTS or qPCR (Supplementary Table S1). We anticipated that the enrichment for pathogen-specific reads may not be as substantial compared to our experiments with mouse tissues, as the tick TNA had been subjected to > 3 freeze/thaw cycles prior to TBDCapSeq analysis. Nonetheless, in comparison to the previously obtained UHTS data, the fold enrichment of the sequencing reads with TBDCapSeq ranged from 20-fold to 74-fold for the five I. scapularis pathogens. We were also able to generate extended contigs that facilitated genomic analyses, including complete genome sequences from two tick samples positive for Powassan virus lineage II, and a 22 Kb B. miyamotoi chromosome contig from a B. miyamotoi-infected tick.
The presence of probes targeting rRNA genes of known pathogens also enabled detection of other closely related species. The rRNA probes for rickettsial pathogens resulted in the detection of the endosymbiont Rickettsia buchneri in 12 out of 15 I. scapularis samples, Rickettsia amblyommatis in both A. americanum pools, and R. monacensis in one of the two D. variabilis ticks. Ribosomal RNA probes also facilitated the detection of a novel Babesia species in one of the A. americanum pools. Analysis of assembled 18S rRNA and 28S rRNA contigs revealed that these sequences were most closely related to an unclassified Babesia species detected in white tailed deer from Texas (accession number HQ264120)39.
In some instances, highly homologous rRNA sequences also complicated species analysis. Initial BlastN analyses of sequences obtained from Borrelia-positive ticks identified 16S and 23S rRNA sequences as both B. burgdorferi s.s. and B. miyamotoi. Similar confounds occurred with Babesia-positive ticks, resulting with concurrent BlastN results to both of B. microti and B. odocoilei ribosomal genes. This applied only to sequences originating from portions of rRNA genes with high homology across species. To definitively delineate the species, examination of non-rRNA genes always resulted in the correct species identification.
Pathogen detection in blood
We sought to determine the performance of TBDCapSeq on pathogens present in human blood samples. We first established the limits of detection of TBDCapSeq for B. burgdorferi s.s. and B. microti relative to qPCR, by quantifying and then serially diluting each pathogen in sterile human blood. For both agents, > 90% of every complete genomic segment (chromosome or plasmid) was recovered at parasitic/bacterial loads of approximately 50,000 genomic copies. In samples with the pathogen concentration reduced to approximately 1000 genomic copies, we still recovered > 25% of every segment from either agent.
B. microti samples 809-7 (Ct 38.1) and 813-8 (Ct 38.86) were the lowest dilutions tested on TBDCapSeq that were also positive by qPCR for B. microti (Supplementary Table S2). Both samples were estimated to contain 1–10 genomes of B. microti. We recovered > 1 Kb of sequence from each of the four B. microti chromosomes with TBDCapSeq from these samples. We also examined one subsequent dilution, 809-8, that was negative by qPCR, but were unable to identify unique B. microti reads in this sample.
B. burgdorferi s.s. sample LYM-846 (ospA Ct 36.65, flaB Ct 39.84) was the lowest qPCR positive sample tested and TBDCapSeq generated reads within in all major segments of the genome. The next dilution, LYM-847, was negative with both qPCR assays, but we were able to map 21 reads to cp32, lp17 and lp36 plasmids (Table 5).
Table 5.
Comparison of the TBDCapSeq performance to qPCR on serially diluted B. burgdorferi N40 strain.
| LYM-841 | LYM-842 | LYM-843 | LYM-844 | LYM-845 | LYM-846 | LYM-847 | LYM-849 | LYM-850 | Extraction control | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| flaB qPCR Ct | ospA qPCR Ct | flaB qPCR Ct | ospA qPCR Ct | flaB qPCR Ct | ospA qPCR Ct | flaB qPCR Ct | ospA qPCR Ct | flaB qPCR Ct | ospA qPCR Ct | flaB qPCR Ct | ospA qPCR Ct | flaB qPCR Ct | ospA qPCR Ct | flaB qPCR Ct | ospA qPCR Ct | flaB qPCR Ct | ospA qPCR Ct | flaB qPCR Ct | ospA qPCR Ct | |
| 23.64 | 20.4 | 28.58 | 24.66 | 32.65 | 29.48 | 33.95 | 31.51 | 36.54 | 33.4 | 39.84 | 36.65 | NA | NA | NA | NA | NA | NA | NA | NA | |
| Raw Reads | 35,063,602 | 22,486,207 | 16,710,629 | 23,554,898 | 19,813,832 | 18,287,462 | 17,456,214 | 19,060,206 | 18,835,547 | 18,978,900 | ||||||||||
| Filtered reads | 27,287,508 | 16,310,431 | 11,124,968 | 16,189,953 | 13,617,696 | 12,295,407 | 10,615,240 | 13,187,179 | 12,985,727 | 13,215,357 | ||||||||||
| Reads mapped to B31 | 11,268,361 | 1,001,704 | 57,908 | 10,826 | 4954 | 491 | 73 | 35* | 45* | 32* | ||||||||||
| % of reads mapped to B31 | 41.382 | 6.153 | 0.522 | 0.067 | 0.036 | 0.004 | 0.001 | 0 | 0 | 0 | ||||||||||
| Genomic Segment | Mapped reads (% coverage) | |||||||||||||||||||
| cp32-1 | 44,225 (94.41) | 4449 (91.58) | 318 (71.82) | 51 (26.73) | 29 (18.75) | 1 (0.46) | 2 (0.46) | 0 (0) | 0 (0) | 0 (0) | ||||||||||
| cp32-3 | 63,853 (97.82) | 6482 (96.34) | 399 (74.48) | 75 (26.37) | 42 (19.53) | 7 (1.86) | 1 (.047) | 0 (0) | 0 (0) | 0 (0) | ||||||||||
| cp-32-4 | 75,778 (95.07) | 7811 (93.78) | 546 (73.9) | 99 (30.35) | 81 (19.50) | 3 (1.41) | 1 (0.47) | 0 (0) | 0 (0) | 0 (0) | ||||||||||
| cp32-6 | 106,855 (90.73) | 10,875 (88.53) | 771 (75.0) | 119 (32.55) | 63 (20.16) | 4 (1.42) | 0 | 0 (0) | 0 (0) | 0 (0) | ||||||||||
| cp32-7 | 43,573 (94.3) | 4750 (92.13) | 311 (68.99) | 71 (26.35) | 34 (14.34) | 1 (1.38) | 0 | 0 (0) | 0 (0) | 0 (0) | ||||||||||
| cp32-8 | 43,055 (96.57) | 4351 (94.15) | 371 (72.19) | 48 (27.67) | 34 (17.30) | 2 (0.92) | 3 (0.46) | 0 (0) | 0 (0) | 0 (0) | ||||||||||
| cp32-9 | 76,973 (904.82) | 7672 (93.71) | 632 (73.78) | 82 (28.73) | 56 (19.38) | 5 (0.93) | 0 | 0 (0) | 0 (0) | 0 (0) | ||||||||||
| lp21 | 3,980 (15.03) | 356 (13.76) | 12 (9.94) | 0 | 0 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||||||||||
| lp56 | 153,761 (69.57) | 14,724 (67.47) | 1030 (52.87) | 191 (21.47) | 97 (13.83) | 5 (1.20) | 0 | 0 (0) | 0 (0) | 0 (0) | ||||||||||
| lp5 | 10,735 (45.83) | 729 (44.17) | 79 (32.48) | 8 (13.01) | 9 (10.35) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||||||||||
| CHR | 7,521,512 (99.23) | 677,488 (99.12) | 38,572 (60.80) | 7251 (15.61) | 3171 (8.13) | 330 (0.90) | 52 (0.16) | 35 (0.04) | 45 (0.04) | 31 (0.04) | ||||||||||
| lp17 | 720,149 (99.4) | 56,660 (99.31) | 3196 (95.81) | 662 (60.04) | 383 (39.14) | 38 (4.49) | 3 (0.84) | 0 (0) | 0 (0) | 0 (0) | ||||||||||
| lp25 | 340,478 (100) | 26,586 (99.8) | 1584 (74.39) | 244 (21.15) | 185 (16.74) | 12 (1.15) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||||||||||
| lp28-1 | 8018 (10.46) | 534 (8.04) | 45 (3.04) | 8 (0.83) | 0 (0) | 1 (6.79) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||||||||||
| lp28-2 | 505,520 (99.72) | 42,815 (99.64) | 2115 (72.49) | 246 (16.71) | 192 (17.80) | 33 (3.30) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||||||||||
| lp28-3 | 176,090 (86.8) | 14,477 (85.84) | 615 (45.17) | 280 (24.52) | 27 (5.73) | 34 (2.85) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||||||||||
| lp28-4 | 220,059 (91.96) | 17,158 (91.6) | 1034 (64.07) | 328 (23.01) | 27 (5.88) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||||||||||
| lp36 | 82,139 (34.98) | 6399 (32.37) | 380 (20.54) | 51 (5.78) | 31 (2.45) | 0 (0) | 11 (0.71) | 0 (0) | 0 (0) | 0 (0) | ||||||||||
| lp38 | 5485 (9.98) | 404 (9.47) | 13 (6.48) | 7 (2.53) | 0 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||||||||||
| lp54 | 724,635 (97.87) | 62,690 (97.45) | 3731 (76.25) | 739 (26.62) | 380 (17.78%) | 15 (1.06) | 0 (0) | 0 (0) | 0 (0) | 1 (0.27) | ||||||||||
| cp26 | 339,463 (98.74) | 34,089 (97.41) | 2128 (78.52) | 266 (18.39) | 113 (10.13) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||||||||||
| cp9 | 2025 (27.17) | 205 (21.32) | 26 (14.26) | 0 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||||||||||
*All reads mapped to non-Borrelia 16S rRNA.
NA = no amplification.
Clinical specimens
We examined a panel of 14 whole blood samples from patients diagnosed with acute babesiosis (Supplementary Table S3). For five patients, we also analyzed samples collected at a post-treatment follow up visit. The parasitic load was determined by qPCR. Ten samples had high parasitemia as determined by qPCR, with a Ct range of 17.84 to 23.41 (Corresponding to ~ 107 to ~ 2.5 × 105 copies of coxA). TBDCapSeq analysis of these samples resulted in a remarkable enrichment for Babesia reads. Between 81.2 and > 98.9% of all reads from these samples mapped to B. microti. Consequently, we were able to assemble the complete sequence (> 99.8% coverage) of the four B. microti chromosomes from each of these samples. Comparison of these assembled sequences to the B. microti reference strain RI revealed only a limited number of mostly synonymous nucleotide substitutions. Notable exceptions were several ORFs predominately on chromosome 4 that displayed a greater number of nucleotide variations when compared to the reference sequences. Among them were the Bmn 1-11 and Bmn 1-15 genes that encode putative immunogenic outer surface proteins (Supplementary Fig. S1).
Three samples with lower Babesia read counts by TBDCapSeq had low parasitemia as determined by qPCR (Cts 33.69 to 38.47). Five samples, all from follow up visits, were negative for B. microti by qPCR, but we obtained > 100 Babesia non rRNA reads from each sample with TBDCapSeq.
Next, we examined 18 blood samples from patients with an erythema migrans (EM) (N = 15) or acute babesiosis (N = 3) (Supplementary Table S4). All Lyme disease samples were negative by an ospA qPCR. The TBDCapSeq data from this pool were heavily biased towards babesiosis samples with high pathogen burden. The two babesiosis samples with high parasitemia (Ct < 18) accounted for > 87% of all reads on the flow cell, with > 97% of reads from each sample mapping to B. microti. In two of the EM samples, we were able to identify B. burgdorferi s.s. reads, although at low quantities. Sample LYM-904 had 60 non-ribosomal reads, all mapping to cp32 plasmids. LYM-912 had 57 reads, with the majority (N = 24) mapping to Cp32. Neither sample contained chromosomal reads outside of rRNA genes.
Discussion
In this study, we demonstrated the utility of TBDCapSeq for simultaneous detection and genotyping of tick-borne disease agents in a wide range of sample matrices. Although our primary focus was on detection of B. burgdorferi s.s. and B. microti, in our experiments with field-collected ticks we demonstrated the utility of TBDCapSeq for simultanous detection and differentiation of other tick-borne agents. We found TBDCapSeq to be markedly superior in performance to UHTS, and in some instances, it exceeded the sensitivity of qPCR. This was a crucial finding, as assay sensitivity is one of the primary concerns with molecular assays targeting tick-borne agents. For pathogens such as B. microti or A. phagocytophilum, molecular detection in the acute stage can be straight forward3. However, the paucity of spirochetes in blood has proven to be a considerable challenge for molecular detection of B. burgdorferi sensu lato. Serology has been shown to be more useful in Lyme disease diagnosis, but currently employed serologic assays can also suffer from intrinsic limitations, including inadequate sensitivity and specificity for samples collected early in disease as well as subjectivity in data interpretation11,40–42. As a result, alternative platforms for diagnosis of acute Lyme disease have been pursued, including metabolomics, transcriptomics and modifications of PCR such as digital droplet PCR43–45. In specimens with active bacteremia, TBDCapSeq can address the sensitivity limitations of other molecular assays and provide a new approach for genomic and pathogenesis studies of B. burgdorferi s.s. and other tick-borne agents. The limit of detection of TBDCapSeq was at, or below, the detection limits of qPCR. This promising result can potentially be further magnified with modifications to sample preparations and sequencing protocols. For example, increasing the sample volume may partially offset the paucity of spirochetes in liquid specimens and enhance detection. In our experiments, we used nucleic acids extracted from only 200 μl of whole blood, in contrast to other molecular studies of B. burgdorferi s.s. that typically employ much greater sample volumes for spirochete detection (20 ml of whole blood or 1 ml of platelet-rich plasma)44,46. Increasing sequencing depth may also result in greater yield of pathogen-specific reads. In future tests, we will seek to further enhance assay performance in order to determine its utility on complex specimens that typically yield limited data with molecular assays.
Our strategy for identification and quantification of pathogen sequences consisted primarily of mapping sequencing reads directly to a reference genome. For B. burgdorferi s.s., this approach could potentially underestimate the actual number of reads due to mismatches in polymorphic sequences. To account for strain differences, for several samples where the infecting strain was determined to be other than B31, we mapped the reads to other B. burgdorferi s.s. strains. We did not detect a significant difference in the number of Borrelia reads when mapping to these other strains, and occasionally, the output was lower due to the fact that for some strains the complete genome sequence has not yet been deposited in GenBank. We also observed that in all samples, because of high sequence homology, a small subset of ribosomal reads originating from the host (mouse, human or tick) or environmental bacteria mapped to conserved regions in rRNA genes of the reference pathogen genome. As a result, ribosomal reads were omitted from our analyses, particularly in samples with a low pathogen burden.
The combination of genome-level analysis with unparalleled detection capability offered by TBDCapSeq can have immense implications on studies of TBD. This assay could offer valuable new insights into our understanding of TBD by facilitating analysis of previously challenging specimens.
Methods
Probe design
TBDCapseq capture probes were designed to target the most common agents of TBD found in the US (Supplementary Table S5). A reference sequence for every genetic segment of each agent was used as template for probe design. To account for the high degree of heterogeneity in B. burgdorferi s.s. plasmid sequences we included three strains representing disparate OspC types (A, K and E). Probes were designed along the entire nucleotide sequence of every genomic segment, with a total of 106 plasmid and chromosomal sequences used in the design. The final set consisted of > 400,000 probes. Probes were manufactured by Roche Sequencing Solutions as previously described24,25.
Samples
We analyzed 7 TBDCapSeq runs, designated as experiments 1–7. Tissue samples analyzed in experiments 1 and 2 were obtained from C3H mice infected by needle inoculation with 1 × 105 of cultured infectious strains of B. burgdorferi s.s. (N40, B31, or both). Larval ticks were infected by feeding on the B. burgdorferi s.s.-infected mice and were then frozen. Mice were bred and maintained in the Tufts University Animal Facility. All experiments were performed following the guidelines of the American Veterinary Medical Association (AVMA) as well as the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All procedures were performed with approval of the Tufts University Institutional Animal Care and Use Committee. Euthanasia was performed in accordance with guidelines provided by the AVMA and was approved by the Tufts University IACUC. All methods were in accordance with ARRIVE guidelines. For analyses of unfed nymphs and adult ticks in experiment 3, we used cDNA generated in previous tick studies37,38.
Whole blood samples were obtained from patients presenting with a tick-borne illness. Acute babesiosis cases with parasitemia by blood smear (Babesia microti confirmed by PCR), Lyme disease cases (serological CDC criteria or erythema migrans diagnosed by a physician) and controls (serology negative for Lyme disease) were enrolled at Stony Brook University Hospital (IRB#1210472). Additional whole blood specimens from patients diagnosed with early localized Lyme disease (all with erythema migrans) were obtained from Columbia University’s Lyme and Tick-Borne Diseases Research Center at Columbia University.
For sensitivity tests, we generated contrived whole blood samples by spiking-in quantified pathogens followed by serial dilutions. For tests with B. burgdorferi s.s., we serially diluted a culture of an infectious N40 strain. For experiments with B. microti, we serially diluted B. microti from a pair of clinical whole blood samples. For both agents, samples were initially diluted 1:10 four times, followed by multiple subsequent 1:5 dilutions.
To estimate pathogen burden, we used 5 μl of template in quantitative PCR (qPCR) assays for B. burgdorferi s.s. (ospA and flaB) and B. microti (coxA)37,47. All qPCR assays were performed using the TaqMan universal PCR master mix (Applied Biosystems).
Sample extractions
Nucleic acid extractions were performed using multiple methods. For extraction of mouse tissues, 1 ml phosphate buffered saline and 1 μl of Dx solution (Qiagen) was added to tissue fragments followed by addition of 1 mm glass beads and homogenization. Ten μl of proteinase K was added, incubated 65 °C for 30 min, followed by centrifugation of cellular debris. Total nucleic acid (TNA) from 250 μl of the supernatant from mouse tissue samples in experiments 1 and 2 (Table 1) were extracted using the Easy Mag extraction platform (Biomerieux) and eluted in 40 μl. TNA from all tick samples examined in experiment 3 were also extracted using the EasyMag. DNA from whole blood (experiments 4–7) was extracted using 200 μl of each sample with the QIAamp DNA Blood Mini Kit (Qiagen) and eluted in 50 μl of water.
To establish detection thresholds and determine the vigor of the workflow, we used pre-characterized tick-borne pathogen-free samples, including salmon sperm DNA, and whole blood specimens obtained from the Columbia University Pathology department.
HTS sequencing and liquid capture methods
DNA concentrations were measured with the Qubit High Sensitivity Double-stranded DNA kit and Qubit 2.0 Fluorometer (Invitrogen). Dual indexed libraries were prepared with the Kapa Hyperplus kit (Roche) using 25–50 ng of input material and the recommended adaptor concentrations and cycling parameters. Amplified libraries were quantified on a TapeStation 4200 using the D1000 kit (Agilent Technologies). Measured concentrations were used to pool libraries at 150 ng per library. After quantification on the TapeStation 4200, 1 μg of the pool was mixed with 5 μg of COT Human DNA (Thermo Fisher Scientific) and 2000 pmol of Blocking Oligo pool (Roche). The mixture was fully dehydrated at 60 °C in a vacuum centrifuge. The dried pool was resuspended in 7.5 μl Hybridization Buffer and 3 μl Hybridization Component A (Roche) to a volume of 10.5 μl and heated at 95 °C for 5 min before the addition of 4.5 μl of custom biotinylated TBD SeqCap EZ Probe pool (Roche). The mixture was again heated at 95 °C for 5 min before being incubated at 47 °C for 16–20 h. After incubation, the probes were pulled down using magnetic streptavidin SeqCap Capture beads (Roche) and washed with buffers of decreasing stringency (Wash Buffers I, II, III, and IV, Roche). The probe-bound DNA was eluted in water and amplified by 16 cycles of PCR using Illumina universal primers (Kapa HiFi HotStart Ready Mix, Roche) using Illumina Universal Primers. Finally, the amplified pool was quantified (Agilent Tapestation) and sequenced on an Illumina NextSeq2000 platform that generated 150 bp long single end reads.
Bioinformatic analyses
The fastq files were adapter trimmed using Cutadapt program (v 3.0)48. Adaptor trimming was followed by generation of quality reports using FastQC software, (v 0.11.5)49 which were used to determine trimming and filtering criteria based on the average quality scores, read length, homopolymeric reads, nucleotide bias and quality scores at the ends of the reads. The reads were quality filtered and end-trimmed with PRINSEQ software (v 0.20.3)50. To determine the abundance of B. burgdorferi s.s. reads, a database was created by downloading the reference sequences of the 21 plasmids and the linear chromosome of the B31 strain from the NCBI RefSeq database. The reference sequences were used to create a Bowtie2 index and the quality filtered reads were mapped to the database using Bowtie2 mapper (v 2.2.9)51. The bam files containing mapped reads were parsed using a set of custom scripts that utilize the SAMtools and BEDTools for extracting depth and breadth of coverage for each genomic and plasmid sequence. A similar process was followed for B. microti strain RI by downloading the 4 chromosomes, mitochondrion and apicoplast sequences from NCBI RefSeq database. Pathogen DNA free controls were used to discern the quantity of mis-assigned reads for each run.
For experiment 3 only, we followed our standard pipeline for agent identification24,38. Host reads were removed by mapping quality filtered reads against tick reference database using Bowtie2 mapper. The host-subtracted reads were de-novo assembled using MIRA (4.0) assembler52. Contigs and unique singletons were subjected to homology search using Megablast against the complete GenBank nucleotide database. Sequences that showed poor or no homology at the nucleotide level were screened with BLASTX against the viral GenBank protein database. The blast reports were annotated with the taxonomic information from NCBI taxonomy database and the reports were used to identify accession numbers for candidate genomes for mapping the reads and determining the genomic coverage and depth.
Supplementary Information
Acknowledgements
We thank Joel Garcia and Sydney Silverman for their assistance with clinical samples and sequencing. This study was supported in part by the Intramural Research Program of the NIH, National Institute of Allergy and Infectious Diseases and by a grant from the Steven & Alexandra Cohen Foundation.
Author contributions
R.T. designed the study and wrote the manuscript. K.J., T.T., A.M., S.S.V., T.B., V.K., and R.T. analyzed the data. K.J., T.T., S.S.V., A.G., V.K., and C.G. provided technical assistance. A.M., B.F., N.M., T.B., V.K., L.M., L.H., W.I.L., and R.T. contributed to study design. All authors reviewed and approved the manuscript.
Disclaimer The content of this publication does not necessarily reflect the views of or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Data availability
All high-throughput sequencing data has been deposited in the Sequence Read Archive under BioProject ID PRJNA723600. Babesia sequences were deposited in GenBank under accession numbers MW665112-MW665119.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-91956-z.
References
- 1.Bratton RL, Corey R. Tick-borne disease. Am. Fam. Physician. 2005;71:2323–2330. [PubMed] [Google Scholar]
- 2.Hirsch AG, et al. Risk factors and outcomes of treatment delays in lyme disease: A population-based retrospective cohort study. Front. Med. (Lausanne) 2020;7:560018. doi: 10.3389/fmed.2020.560018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanchez E, Vannier E, Wormser GP, Hu LT. Diagnosis, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: A review. JAMA. 2016;315:1767–1777. doi: 10.1001/jama.2016.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forselv KJN, et al. Does more favourable handling of the cerebrospinal fluid increase the diagnostic sensitivity of Borrelia burgdorferi sensu lato-specific PCR in Lyme neuroborreliosis? Infect. Dis. (Lond.) 2018;50:297–302. doi: 10.1080/23744235.2017.1399315. [DOI] [PubMed] [Google Scholar]
- 5.Barstad B, et al. Direct molecular detection and genotyping of Borrelia burgdorferi sensu lato in cerebrospinal fluid of children with lyme neuroborreliosis. J. Clin. Microbiol. 2018 doi: 10.1128/JCM.01868-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liveris D, et al. Comparison of five diagnostic modalities for direct detection of Borrelia burgdorferi in patients with early Lyme disease. Diagn. Microbiol. Infect. Dis. 2012;73:243–245. doi: 10.1016/j.diagmicrobio.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li X, et al. Burden and viability of Borrelia burgdorferi in skin and joints of patients with erythema migrans or lyme arthritis. Arthritis Rheum. 2011;63:2238–2247. doi: 10.1002/art.30384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X, et al. Burden and viability of Borrelia burgdorferi in skin or joints, of patients with erythema migrans or lyme arthritis. Arthritis Rheum. 2011 doi: 10.1002/art.30384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marques AR. Laboratory diagnosis of Lyme disease: Advances and challenges. Infect. Dis. Clin. North Am. 2015;29:295–307. doi: 10.1016/j.idc.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benach JL, et al. Spirochetes isolated from the blood of two patients with Lyme disease. N Engl J Med. 1983;308:740–742. doi: 10.1056/NEJM198303313081302. [DOI] [PubMed] [Google Scholar]
- 11.Aguero-Rosenfeld ME, Wang G, Schwartz I, Wormser GP. Diagnosis of lyme borreliosis. Clin. Microbiol. Rev. 2005;18:484–509. doi: 10.1128/CMR.18.3.484-509.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biggs HM, et al. Diagnosis and management of tickborne rickettsial diseases: Rocky mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis - United States. MMWR Recomm. Rep. 2016;65:1–44. doi: 10.15585/mmwr.rr6502a1. [DOI] [PubMed] [Google Scholar]
- 13.Connally NP, et al. Testing practices and volume of non-Lyme tickborne diseases in the United States. Ticks Tick Borne Dis. 2016;7:193–198. doi: 10.1016/j.ttbdis.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pritt BS, et al. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. Lancet Infect. Dis. 2016;16:556–564. doi: 10.1016/S1473-3099(15)00464-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gu W, Miller S, Chiu CY. Clinical metagenomic next-generation sequencing for pathogen detection. Annu. Rev. Pathol. 2019;14:319–338. doi: 10.1146/annurev-pathmechdis-012418-012751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller S, et al. Laboratory validation of a clinical metagenomic sequencing assay for pathogen detection in cerebrospinal fluid. Genome Res. 2019;29:831–842. doi: 10.1101/gr.238170.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blauwkamp TA, et al. Analytical and clinical validation of a microbial cell-free DNA sequencing test for infectious disease. Nat. Microbiol. 2019;4:663–674. doi: 10.1038/s41564-018-0349-6. [DOI] [PubMed] [Google Scholar]
- 18.Samarkos M, Mastrogianni E, Kampouropoulou O. The role of gut microbiota in Clostridium difficile infection. Eur. J. Intern. Med. 2018;50:28–32. doi: 10.1016/j.ejim.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Hong DK, et al. Liquid biopsy for infectious diseases: Sequencing of cell-free plasma to detect pathogen DNA in patients with invasive fungal disease. Diagn. Microbiol. Infect. Dis. 2018;92:210–213. doi: 10.1016/j.diagmicrobio.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 20.Schlaberg R, et al. Validation of metagenomic next-generation sequencing tests for universal pathogen detection. Arch. Pathol. Lab. Med. 2017;141:776–786. doi: 10.5858/arpa.2016-0539-RA. [DOI] [PubMed] [Google Scholar]
- 21.Wylie TN, Wylie KM, Herter BN, Storch GA. Enhanced virome sequencing using targeted sequence capture. Genome Res. 2015;25:1910–1920. doi: 10.1101/gr.191049.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naccache SN, et al. Distinct Zika virus lineage in Salvador, Bahia, Brazil. Emerg. Infect. Dis. 2016;22:1788–1792. doi: 10.3201/eid2210.160663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Metsky HC, et al. Capturing sequence diversity in metagenomes with comprehensive and scalable probe design. Nat. Biotechnol. 2019;37:160–168. doi: 10.1038/s41587-018-0006-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Briese T, et al. Virome capture sequencing enables sensitive viral diagnosis and comprehensive virome analysis. MBio. 2015;6:e01491–e11415. doi: 10.1128/mBio.01491-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allicock OM, et al. BacCapSeq: A platform for diagnosis and characterization of bacterial infections. MBio. 2018 doi: 10.1128/mBio.02007-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wormser GP, et al. Borrelia burgdorferi genotype predicts the capacity for hematogenous dissemination during early Lyme disease. J. Infect. Dis. 2008;198:1358–1364. doi: 10.1086/592279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cerar T, et al. Differences in genotype, clinical features, and inflammatory potential of Borrelia burgdorferi sensu stricto strains from Europe and the United States. Emerg. Infect. Dis. 2016;22:818–827. doi: 10.3201/eid2205.151806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khatchikian CE, et al. Public health impact of strain specific immunity to Borrelia burgdorferi. BMC Infect. Dis. 2015;15:472. doi: 10.1186/s12879-015-1190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Purser JE, Norris SJ. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc. Natl. Acad. Sci. USA. 2000;97:13865–13870. doi: 10.1073/pnas.97.25.13865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brisson D, Baxamusa N, Schwartz I, Wormser GP. Biodiversity of Borrelia burgdorferi strains in tissues of Lyme disease patients. PLoS ONE. 2011;6:e22926. doi: 10.1371/journal.pone.0022926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanincova K, et al. Multilocus sequence typing of Borrelia burgdorferi suggests existence of lineages with differential pathogenic properties in humans. PLoS ONE. 2013;8:e73066. doi: 10.1371/journal.pone.0073066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pepin KM, et al. Geographic variation in the relationship between human Lyme disease incidence and density of infected host-seeking Ixodes scapularis nymphs in the Eastern United States. Am. J. Trop. Med. Hyg. 2012;86:1062–1071. doi: 10.4269/ajtmh.2012.11-0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strle K, Jones KL, Drouin EE, Li X, Steere AC. Borrelia burgdorferi RST1 (OspC type A) genotype is associated with greater inflammation and more severe Lyme disease. Am. J. Pathol. 2011;178:2726–2739. doi: 10.1016/j.ajpath.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones KL, McHugh GA, Glickstein LJ, Steere AC. Analysis of Borrelia burgdorferi genotypes in patients with Lyme arthritis: High frequency of ribosomal RNA intergenic spacer type 1 strains in antibiotic-refractory arthritis. Arthritis Rheum. 2009;60:2174–2182. doi: 10.1002/art.24812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seinost G, et al. Four clones of Borrelia burgdorferi sensu stricto cause invasive infection in humans. Infect. Immun. 1999;67:3518–3524. doi: 10.1128/IAI.67.7.3518-3524.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma B, McCarthy JE, Freliech CA, Clark MM, Hu LT. Genetic background amplifies the effect of immunodeficiency in antibiotic efficacy against Borrelia burgdorferi. J. Infect. Dis. 2020 doi: 10.1093/infdis/jiaa719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tokarz R, et al. Detection of Anaplasma phagocytophilum, Babesia microti, Borrelia burgdorferi, Borrelia miyamotoi, and Powassan virus in ticks by a multiplex real-time reverse transcription-PCR assay. mSphere. 2017 doi: 10.1128/mSphere.00151-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tokarz R, et al. Microbiome analysis of Ixodes scapularis ticks from New York and Connecticut. Ticks Tick Borne Dis. 2019;10:894–900. doi: 10.1016/j.ttbdis.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 39.Holman PJ, Carroll JE, Pugh R, Davis DS. Molecular detection of Babesia bovis and Babesia bigemina in white-tailed deer (Odocoileus virginianus) from Tom Green County in central Texas. Vet. Parasitol. 2011;177:298–304. doi: 10.1016/j.vetpar.2010.11.052. [DOI] [PubMed] [Google Scholar]
- 40.Seriburi V, Ndukwe N, Chang Z, Cox ME, Wormser GP. High frequency of false positive IgM immunoblots for Borrelia burgdorferi in clinical practice. Clin. Microbiol. Infect. 2012;18:1236–1240. doi: 10.1111/j.1469-0691.2011.03749.x. [DOI] [PubMed] [Google Scholar]
- 41.Fallon BA, Pavlicova M, Coffino SW, Brenner C. A comparison of lyme disease serologic test results from 4 laboratories in patients with persistent symptoms after antibiotic treatment. Clin. Infect. Dis. 2014;59:1705–1710. doi: 10.1093/cid/ciu703. [DOI] [PubMed] [Google Scholar]
- 42.Ang CW, Notermans DW, Hommes M, Simoons-Smit AM, Herremans T. Large differences between test strategies for the detection of anti-Borrelia antibodies are revealed by comparing eight ELISAs and five immunoblots. Eur. J. Clin. Microbiol. Infect. Dis. 2011;30:1027–1032. doi: 10.1007/s10096-011-1157-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bouquet J, et al. Longitudinal transcriptome analysis reveals a sustained differential gene expression signature in patients treated for acute lyme disease. MBio. 2016;7:e00100-00116. doi: 10.1128/mBio.00100-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Das S, Hammond-McKibben D, Guralski D, Lobo S, Fiedler PN. Development of a sensitive molecular diagnostic assay for detecting Borrelia burgdorferi DNA from the blood of Lyme disease patients by digital PCR. PLoS ONE. 2020;15:e0235372. doi: 10.1371/journal.pone.0235372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fitzgerald BL, et al. Host metabolic response in early Lyme disease. J. Proteome Res. 2020;19:610–623. doi: 10.1021/acs.jproteome.9b00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mosel MR, et al. Molecular microbiological and immune characterization of a cohort of patients diagnosed with early Lyme disease. J. Clin. Microbiol. 2020 doi: 10.1128/JCM.00615-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tokarz R, Tagliafierro T, Ian Lipkin W, Marques AR. Characterization of a Monanema nematode in Ixodes scapularis. Parasit. Vectors. 2020;13:371. doi: 10.1186/s13071-020-04228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. ENBnet J. 2011;17:10–12. doi: 10.14806/ej.17.1.200. [DOI] [Google Scholar]
- 49.Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (2010).
- 50.Schmieder R, Edwards R. Quality control and preprocessing of metagenomic datasets. Bioinformatics. 2011;27:863–864. doi: 10.1093/bioinformatics/btr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheverux B, Wetter T, Suhai S. Genome sequence assembly using trace signals and additional sequence information. Comput. Sci. Biol. 1999;99:45–56. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All high-throughput sequencing data has been deposited in the Sequence Read Archive under BioProject ID PRJNA723600. Babesia sequences were deposited in GenBank under accession numbers MW665112-MW665119.