Abstract
Dengue virus (DENV) causes dengue fever (DF) and dengue hemorrhagic fever in humans. Some DF patients suddenly develop severe symptoms around the defervescent period. Although the pathogenic mechanism of the severe symptoms has not been fully elucidated, the viremia level in the early phase has been shown to correlate with the disease severity. One of the hypotheses is that a phenomenon called antibody-dependent enhancement (ADE) of infection leads to high level of viremia. To examine the plausibility of this hypothesis, we examined the relationship between in vitro ADE activity and in vivo viral load quantity in six patients with dengue diseases. Blood samples were collected at multiple time points between the acute and defervescent phases, and the balance between neutralizing and enhancing activities against the autologous and prototype viruses was examined. As the antibody levels against DENV were rapidly increased, ADE activity was decreased over time or partially maintained against some viruses at low serum dilution. In addition, positive correlations were observed between ADE activity representing in vitro progeny virus production and viremia levels in patient plasma samples. The measurement of ADE activity in dengue-seropositive samples may help to predict the level of viral load in the subsequent DENV infection.
Subject terms: Dengue virus, Viral host response
Introduction
Dengue virus (DENV), belonging to the family Flaviviridae, genus Flavivirus, is distributed throughout tropical and subtropical areas of the world1. DENV is transmitted by Aedes mosquito species and causes dengue fever (DF) and severe dengue in humans. Approximately 3.9 billion people are under the risk of infection2. An estimated 390 million people are infected with DENV annually, and 100 million of these individuals show clinical symptoms3. Therefore, dengue is one of the most important mosquito-borne viral diseases worldwide, and it should be controlled to the greatest extent possible.
The four serotypes of DENV (DENV-1, DENV-2, DENV-3 and DENV-4) are genetically distinct, and there is complicated immunological cross-reactivity among them4. Secondary heterotypic infection has been epidemiologically demonstrated to increase the risk of severe forms—namely, dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS)5. Patients showing dengue with warning signs have a risk of developing disease severity, with the emergence of severity usually occurring around the defervescence phase, beginning at days 3–7 of illness6,7. The mortality rate of cases with DSS is much higher than that of cases without DSS8. Although a mechanism associated with the severity and a surrogate marker predicting the deterioration have not been fully identified yet, high levels of viremia have been shown to be related to disease severity6,9–13. Moreover, a recently published meta-analysis revealed that there was an association between disease severity and viremia duration14. On the other hand, some studies have reported finding no association between disease severity and high viremia levels15,16. Antibody-dependent enhancement of infection (ADE) that increases the viremia level has been proposed as one of the pathogenic mechanisms in DHF/DSS17; in the case of ADE the increase occurs by viral internalization via Fc gamma receptors18. Recently, a potential relationship between ADE and human disease severity in DENV infection has been reported19. However, it is still unclear whether in vitro ADE can be used for the prediction in subsequent clinical outcomes.
Enhancing antibodies (EAbs), which exclusively play a role of the ADE phenomenon20, may be associated with an increase in the viremia level in DENV infection21,22. In contrast, neutralizing antibodies (NAbs) have a biological function to decrease the viremia level to protect the host from DENV infection23, while most NAbs show ADE activity at subneutralizing doses24. These functional antibodies are supposed to be introduced by one of three routes: (i) DENV infection, (ii) maternal antibody from a DENV-seropositive mother and (iii) other flavivirus infection. We have previously demonstrated that a DENV-immune serum (polyclonal form) could be represented with a cocktail of functional monoclonal EAbs and NAbs25. Therefore, the balance activity between EAbs and NAbs might be critical to control the outcome (protection or pathogenesis). We previously developed a simple method to detect the balance between the enhancing and neutralizing activities26, and demonstrated that mouse monoclonal EAbs and NAbs competed over the neutralizing activities in vitro25,27. Specifically, the neutralizing activity of an NAb was reduced in the presence of a sufficient level of an EAb, suggesting that the relative capacity for neutralization might be easily affected by the balance between NAbs and EAbs.
In the present study, we evaluated the balance between neutralizing and enhancing activities in sera collected from dengue patients at multiple time points between the acute and defervescent phases. The six autologous viruses isolated from the respective patients were used as assay antigens, allowing us to examine the balance antibody assay with autologous combinations between patient sera and virus antigens. We also measured the number of viral RNA copies in plasma samples collected at multiple time points, and revealed a correlation between the in vitro ADE activity and in vivo viral load quantity.
Results
Balance between the neutralizing and enhancing activities against autologous viruses
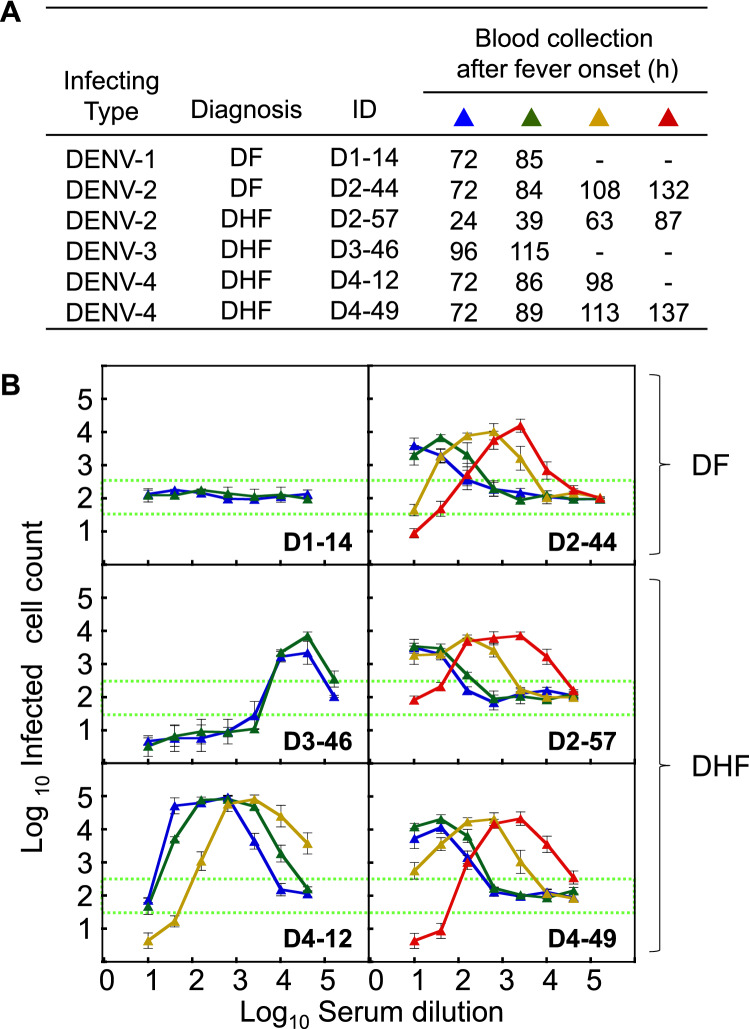
Six hospitalized adult patients, who had been enrolled in the previous study13, were recruited into the present study, and an autologous DENV clinical strain was successfully isolated from each of them. The demographic information (infecting serotype, diagnosis [DF or DHF], ID number and sample collection period [h] after the fever onset) is shown in Fig. 1A. Serum samples, which were collected at several time points between the acute and defervescent phases, were subjected to an antibody assay to determine the balance between neutralizing and enhancing activities (NAb/EAb-balance assay) using each autologous virus. As shown in Fig. 1B, five patients—i.e., all of the enrolled patients except for patient D1-14—showed dose (dilution)-dependent antibody activity patterns displaying neutralizing and enhancing activities. Since the abscissa indicates serum dilutions, increases in antibody levels over time would cause the dose-dependent antibody activity curve to shift from the left to the right. Therefore, the comparison of the antibody activity curves indicated an increase in antibody levels. Antibody titers determined by a conventional neutralization test support an evidence of the shifts with time gaining (Table 1), indicating that their neutralizing antibody titers rapidly increased by 6 ~ 78-fold during the early disease stage. The increase of their antibody levels (up to 64-fold) was confirmed by the enzyme-linked immunosorbent assay (ELISA), too (Supplementary Table S1). Similar dose-dependent patterns were observed in patients D2-44, D2-57 and D4-49, even though these patients had different infecting serotypes and diagnoses. On the other hand, patients D1-14 and D3-46 did not show a remarkable shift during the observation period, as neutralizing antibody titers showed < twofold increase (Table 1). As a patient D1-14 was classified as primary infection by IgG/IgM immunochromatography tests in the previous study13, no functional antibody activity was observed in 72 and 85 h samples. Although patient D3-46 showed strong neutralization with a wide range of low-middle serum dilutions (1:10 ~ 1:2560) (Fig. 1B), this patient was diagnosed with DHF with severe clinical symptoms.
Figure 1.
Balance between neutralizing and enhancing activities against autologous virus. (A) Demographic data for the six dengue patients enrolled in this study. Blood samples were collected on the day of hospitalization and the following time points at 12–24 h intervals until a maximum 137 h after fever onset. (B) NAb/EAb balance activity against the autologous virus. The NAb/EAb-balance assay was performed with a combination of autologous virus and serum. The blue, green, yellow and red triangles, corresponding to (A), indicate the hours after fever onset. The abscissa indicates the serum dilutions and the ordinate shows the numbers of infected cells (both expressed as log10). Each data point represents the average of two separate assays; error bars indicate the SDs. Dotted lines indicate the mean numbers of infected cells plus or minus three times the SD (mean ± 3SD), to define neutralizing or enhancing activity, respectively.
Table 1.
Neutralizing antibody titers against the autologous virus.
| Hours | Patient ID | ||||||
|---|---|---|---|---|---|---|---|
| D1-14 | D2-44 | D2-57 | D3-46 | D4-12 | D4-49 | ||
| Period after fever onset | 24 | – | – | 1:73 | – | – | – |
| 39 | – | – | 1:150 | – | – | – | |
| 63 | – | – | 1:331 | – | – | – | |
| 72 | 1:70a | 1:234 | – | – | 1:188 | 1:38 | |
| 84 | – | 1:1912 | – | – | – | – | |
| 85 | 1:62 | – | – | – | – | – | |
| 86 | – | – | – | – | 1:260 | – | |
| 87 | – | – | 1:2867 | – | – | – | |
| 89 | – | – | – | – | – | 1:104 | |
| 96 | – | – | – | 1:25,553 | – | – | |
| 98 | – | – | – | – | 1:1173 | – | |
| 108 | – | 1:1213 | – | – | – | – | |
| 113 | – | – | – | – | – | 1:417 | |
| 115 | – | – | – | 1:46,414 | – | – | |
| 132 | – | 1:3500 | – | – | – | – | |
| 137 | – | – | – | – | – | 1:2952 | |
| Infection history | Primary | Secondary | Secondary | Secondary | Secondary | Secondary | |
aNeutralizing antibody titer was expressed as the maximum serum dilution showing > 75% focus reduction (FRNT75).
Balance between neutralizing and enhancing activities against four prototype viruses
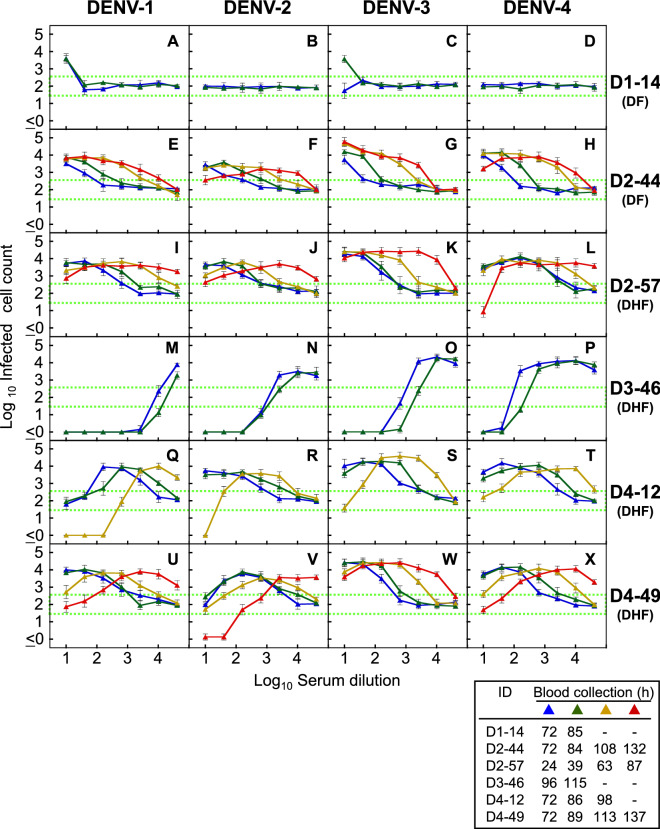
Dose-dependent neutralizing/enhancing activity curves were obtained against four prototype laboratory strains (DENV-1: Mochizuki; DENV-2: NGC; DENV-3: H87; and DENV-4: H241). Enhancing activity against at least one prototype strain was observe in all patients, of whom four patients (D2-44, D2-57, D4-12 and D4-49) showed remarkable shifts in the antibody levels against all four prototype DENVs (Fig. 2). Although patients D4-12 and D4-49 were currently infected with DENV-4, they displayed higher levels of cross-neutralization against heterologous DENV-2 and/or DENV-1 (Fig. 2Q,R,V). Similarly, patient D2-57, who was currently infected with DENV-2, showed cross-neutralization against DENV-4 (Fig. 2L). These results suggest that our assay system may be able to reveal the previous infection history in dengue patients by comparing their cross-neutralizing activities. On the other hand, shift of antibody level was limited in patients D1-14 and D3-46 (Fig. 2A–D,M–P). Patient D3-46 displayed strong neutralization against all serotypes (Fig. 2M–P), while patient D1-14 showed cross-reactive enhancing activities against prototypes DENV-1 and DENV-3 at 1:10 serum dilution (Fig. 2A,C). Based on these findings, it seems likely that a history of previous exposure to and infection by DENV affected the antibody response patterns in our patients.
Figure 2.
Balance between neutralizing and enhancing activities against four prototype viruses. The NAb/EAb-balance assay was performed with a combination of serum (D1-14: A–D, D2-44: E–H, D2-57: I–L, D3-46: M–P, D4-12: Q–T and D4-49: U–X) and four prototype viruses (DENV-1: Mochizuki strain; DENV-2: NGC strain; DENV-3: H87 strain; and DENV-4: H241 strain). The blue, green, yellow and red triangles, corresponding to Fig. 1A, indicate the hours after fever onset. The abscissa indicates the serum dilutions and the ordinate shows the numbers of infected cells (both expressed as log10). Each data point represents the average of two separate assays; error bars indicate the SDs. Dotted lines indicate the mean numbers of infected cells plus or minus three times the SD (mean ± 3SD), to define neutralizing or enhancing activity, respectively.
Evaluation of progeny virus titers by NAb/EAb balance assay
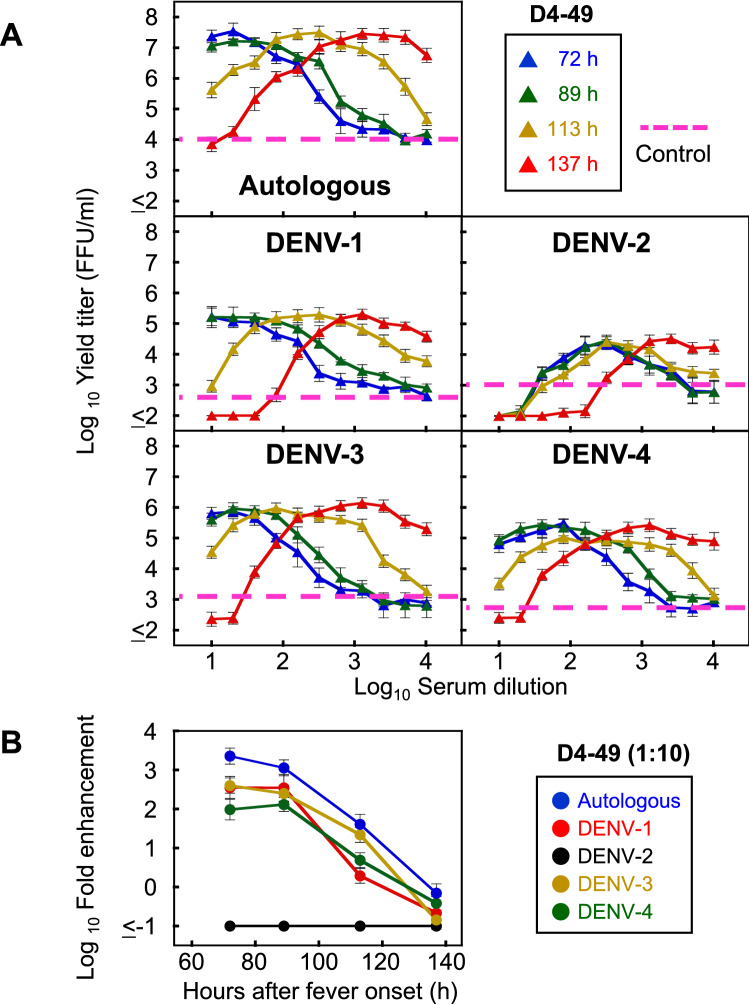
To evaluate the levels of progeny virus secreted from the infected cells using the NAb/EAb balance assay system, patient D4-49 was selected as a representative patient with a range of neutralizing and enhancing activities (Figs. 1 and 2). Culture supernatants were harvested from the infected cells 24 h after the set-up of the NAb/EAb-balance assay, and were titrated on Vero cells. The dose-dependent patterns of progeny virus titers (Fig. 3A) were roughly similar to those from the infected-cell counts (Figs. 1B and 2U–X), with minor differences at lower serum dilutions. Specifically, the defervescent sample (137 h) displayed lower yield titer than the control in all five strains at 1:10 serum dilution (Fig. 3A). Furthermore, fold enhancements were calculated from progeny virus titers at 1:10 serum dilutions (closer to the situation in vivo) (Fig. 3A), and were plotted as the ordinate against time (h) as the abscissae (Fig. 3B). The time-course patterns tended to decline in parallel, except for DENV-2. The autologous virus showed the higher fold enhancement (> 1000 fold on 72 h) than the other four prototype assay antigens. In contrast, patient D4-49 was currently infected with DENV-4, but this patient’s antibody reaction against DENV-2 was much higher than those against other serotypes, showing strong neutralizing activity against prototype DENV-2 at 137 h after fever onset (Fig. 2V). This finding suggests that the patient had been previously infected with DENV-2. Similar declining trends were observed in graphic data based on infected-cell counts as the ordinate (Supplementary Fig. S1).
Figure 3.
Progeny virus infectivity in the NAb/EAb balance assay. (A) Progeny virus titers obtained from the NAb/EAb-balance assay. The NAb/EAb-balance assay was conducted with combinations of patient D4-49 serum (twofold serial serum dilutions, starting from 1:10) and five DENV strains (the autologous, DENV-1: Mochizuki, DENV-2: NGC, DENV-3: H87 and DENV-4: H241 strain). Culture supernatants were harvested at 24 h after the mixture of serum, virus and K562 cells. The infective titers (FFU/ml) were determined on Vero cells. The blue, green, yellow and red triangles, corresponding to Fig. 1A, indicate the hours after fever onset. The abscissa indicates the serum dilutions and the ordinate shows the progeny virus titers (both expressed as log10). Dotted lines indicate the mean progeny virus titers calculated from four negative (no serum) controls. (B) Decreasing trend of ADE at low serum dilution with time progression. Fold enhancement was calculated from the infective progeny virus titers obtained in (A) (specifying data at 1:10 serum dilution), and was expressed in log10 as the increase in the progeny virus titer relative to the negative control. The fold enhancement was plotted as the ordinate against time (h) as the abscissa.
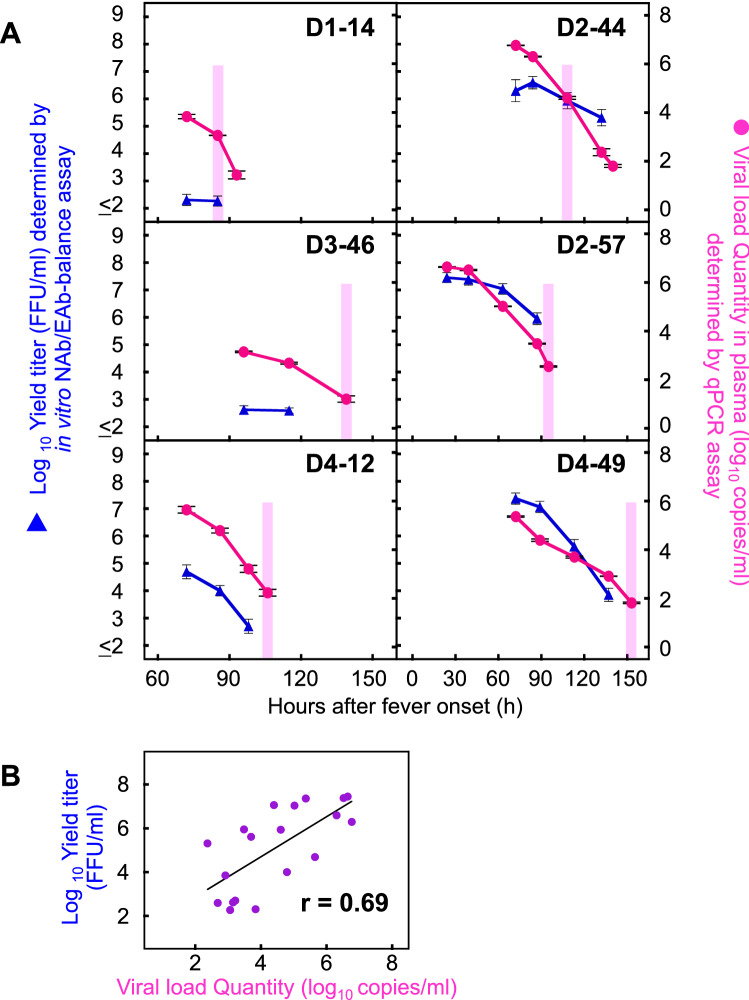
Relationship between the in vitro infective progeny virus and in vivo viral load
Using the same method described above, the infection-enhanced progeny virus titers were determined with the autologous combination (using 1:10 serum dilution) in the other five patients (D1-14, D2-44, D2-57, D3-46 and D4-12) (blue triangles in Fig. 4A). In addition, the quantity of viral RNA was directly determined from the corresponding clinical plasma samples (red circles in Fig. 4A). Both the ADE progeny virus titer (blue triangle) and viral RNA copy number (red circle) basically declined in parallel over time. Significant correlation was observed between the ADE progeny virus titers and the viral RNA copy numbers (r = 0.69; P < 0.05) (Fig. 4B). These results indicate that there might be positive correlations between (i) the in vitro progeny virus titers obtained from the NAb/EAb-balance assay and (ii) the in vivo viral quantity in the plasma. In contrast, no correlations were observed between the laboratory results (the intensity of ADE activity) and the clinical diagnosis (DF or DHF). Patients D2-57, D3-46, D4-12 and D4-49 deteriorated around the defervescent phase (pink shading in Fig. 4A), but the levels of progeny virus titers and viral load in D3-46 (DHF patient) were not as high as those in other DHF patients. Likewise, D2-44 showed DF manifestations, but the levels of progeny virus titers and viral load were as high as those of DHF patients.
Figure 4.
Relationship between in vitro ADE activity and in vivo viral load quantity. (A) The NAb/EAb-balance assay was conducted with a combination of serum diluted at 1:10 and the corresponding autologous virus. Culture supernatants were harvested at 24 h after the mixture of serum, virus and K562 cells. The infective titers were determined on Vero cells and plotted as the left ordinates (blue triangles: expressed as log10 FFU/ml). The number of viral RNA copies in plasma samples collected at same time points as well as one extra time point (D1-14: 93 h, D2-44: 140 h, D3-46: 139 h, D2-57: 95 h, D4-12: 106 h and D4-49: 153 h) were determined by real time RT-PCR, and plotted as the right ordinates (red circles: expressed as log10 copies/ml). The abscissae indicate time (h) after fever onset. Pink shading indicates the beginning of defervescence on the clinical observation. (B) Correlation between the ADE progeny virus titers and the viral RNA copy numbers. The correlation coefficient (r) was estimated for the individual progeny virus titers and qPCR values obtained in Fig. 4A (however, following samples were excluded; D1-14: 93 h, D2-44: 140 h, D3-46: 139 h, D2-57: 95 h, D4-12: 106 h and D4-49: 153 h). The abscissa and ordinate indicate the qPCR values and the progeny virus titers, respectively. Linear regression lines and r values are presented in the panel.
Discussion
In the present study, we analyzed the balance between neutralizing and enhancing activities in serum samples which were collected from dengue patients at multiple time points between the acute and defervescent phases (Figs. 1 and 2). Patient D3-46 showed DHF manifestations in spite of the strong neutralization against both autologous and prototype viruses, and the dose-dependent curve was not meaningfully shifted over 96–115 h in this patient (Figs. 1B and 2M–P, and Table 1). These findings suggest that the strong neutralization revealed by the in vitro antibody assays did not completely neutralize the autologous virus in vivo (Fig. 4A), which led to the development of severe disease. In contrast, DF patient D2-44 did not show severe manifestations, even though the ADE activity patterns of this patient were as high as those of the DHF patients (D2-57 and D4-49). Therefore, when samples from post-hospitalized patients were used, it was still difficult to predict disease outcomes by measuring the balance between neutralizing and enhancing activities. Although other factors might be involved in determining the immune correlation (such as the treatment conditions in the hospital, current infecting serotype and previous infection history, etc.), the most valuable factor (and also the most difficult) is the collection of samples in the pre-infection or pre-hospitalization period through prospective cohort studies28–31. In regard to the relationship between pre-infection sera and disease severity, Kliks et al. have reported that the level of virus yield induced by ADE was significantly higher in a severely symptomatic group than an asymptomatic one22.
The ADE activities shown by the low serum dilution (1:10) were gradually decreased over time (Fig. 3B and Supplementary Fig. S1). These results suggest that neutralizing antibodies were immediately produced after the infection (Table 1), and then the original enhancing activity might have been competitively suppressed by the appearance of neutralizing antibodies25,27. However, some cases did not show a decreasing trend in the heterologous combinations (Supplementary Fig. S1). For instance, patient D2-44 displayed the decreasing trend against DENV-2 (both autologous and NGC strains) and DENV-4, but exhibited an increasing or flat trend against DENV-1 and DENV-3 (Supplementary Fig. S1). This suggests that patient D2-44 may have had different antibody reactions to different serotypes. Therefore, monitoring the balance between NAb and EAb activities against all serotypes is important for the readiness upon secondary heterotypic infection in seropositive individuals. On the other hand, the opposite trend was observed in the high-serum dilution (1:2560)—that is, the ADE activities were basically increased over time (Supplementary Fig. S2). This suggests that the induced neutralizing antibodies still possess the potential ADE activity at sub-neutralizing doses.
The first approved dengue vaccine, Sanofi’s Dengvaxia, has been introduced into approximately 20 endemic countries since 201532. However, WHO SAGE revised their recommendation to say that only seropositive individuals, who have previously been infected with DENV, can be inoculated with Dengvaxia33. Seronegative populations should not be vaccinated34,35, because a risk of vaccine-induced infection enhancement cannot be excluded36–38. Although there are several assay kits to detect dengue antibodies in a quantitative or qualitative manner (e.g., ELISA, immunochromatography assays, etc.), few rapid test kits are available to detect the functional antibody activity. Only an assay with the capacity to detect ADE activity would be able to provide a risk assessment for vaccine recipients. In the present study, five (D2-44, D3-46, D2-57, D4-12 and D4-49) of six patients were confirmed to be seropositive (classified as secondary infection). In contrast, patient D1-14 (classified as primary infection) showed no enhancing antibody activity against the autologous DENV-1, prototype DENV-2 and prototype DENV-4 strains (Figs. 1 and 2). This patient might show cross-reactive enhancing activities against the prototype DENV-1 and prototype DENV-3 strains (Fig. 2A,C). Thus, such individuals might consider to suspend the vaccination until their antibody levels are elevated sufficiently, if they have a plan to get Dengvaxia in the near future.
A previous study showed that ADE contributed to an increase in viremia in an animal model39. High viremia levels in dengue patients have also been reported to correlate with disease severity6,9–13, although the significance of the correlation is dependent on the disease day14. Since the viremia in humans is caused by secretion of the progeny viruses from the infected-host cells, the viral titers in the supernatant obtained from the in vitro NAb/EAb-balance assay might correlate to the clinical viral load. Interestingly, in the present study, significant positive correlations were observed between progeny virus titers (focus forming units [FFU]/ml) in the NAb/EAb-balance assay and in vivo viremia levels (copies/ml) in plasma samples (Fig. 4B). This result suggests that measurement of the infective progeny virus titers (or the infected cell counts) in the NAb/EAb-balance assay may enable us to predict the viral load in the subsequent DENV infection. Since patient D3-46 showed neutralizing activity rather than enhancing activity against autologous virus in the lower serum dilutions (Fig. 1B), the viral load quantity in this patient also might have been lower than those in other patients (Fig. 4A).
In conclusion, we revealed that the in vitro progeny virus titers obtained from an NAb/EAb-balance assay were significantly correlated with the viral load in patient plasma samples determined by qPCR assay. This suggests that the present NAb/EAb-balance assay might be used to predict the viral load that would result from a subsequent infection with DENV. However, we could not find a relationship between the ADE activity (viremia level) and disease severity during the early and defervescent phases. The major limitation of this study was that only six patients were enrolled, since the sample was limited to patients in whom the virus isolation was successful. Nonetheless, we believe that the analysis of the combination of autologous virus and serum is meaningful to understand the relationship between neutralization/ADE and disease outcome. Another limitation was that the present ADE experiments were performed using only K562 cells, which express Fc gamma receptor IIa but not Fc gamma receptor I. A multidirectional analysis using another cell line expressing Fc gamma receptor I may need to be performed to measure antibody-dependent neutralization and enhancement.
Materials and methods
Blood samples
The present study was conducted using the serum and plasma collected from confirmed-dengue patients at the Hospital for Tropical Diseases, Bangkok, Thailand, in a previous study13. The patients were diagnosed based on the World Health Organization (WHO) criteria of 199740. The infection history (primary or secondary infection) of patients was determined based on the results of IgG and IgM antibody presence in acute sera using Panbio Dengue Duo Cassette (Abbott Inc., Chicago, IL) in accordance with the manufacturer’s protocol. Heat inactivation of sera was performed at 56 °C for 30 min. Six dengue patients were enrolled from whom clinical virus isolates were obtained. All subjects gave their informed consent for inclusion into the study before they participated. This study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of the Faulty of Tropical Medicine, Mahidol University, Thailand (FTM ECF-019-04).
Cells
African green monkey kidney Vero cells (CCL-81; American Type Culture Collection, Manassas, VA) were cultivated in Eagle’s minimum essential medium supplemented with 10% fetal bovine serum (FBS) and 60 µg/mL kanamycin41. Human erythroleukemia K562 cells were cultivated in RPMI 1640 medium supplemented with 10% FBS, 100 units/mL penicillin, and 100 µg/mL streptomycin42. All cell lines were cultivated in a humidified atmosphere of 5% CO2: 95% air at 37 °C.
Virus isolation and typing
Vero cells were inoculated with the diluted patient sera (1:10) collected in the acute phase, and then incubated at 37 °C for 7 days. Within five blind passages, viral RNA was extracted from the supernatant of the infected cells, and the serotype was determined by PCR using type-specific primers following a previous report43.
Viruses
Six clinical isolates (1 strain of DENV-1, 2 of DENV-2, 1 of DENV-3, and 2 of DENV-4) and four prototype lab strains (DENV-1: Mochizuki strain; DENV-2: New Guinea C [NGC] strain; DENV-3: H87 strain; and DENV-4: H241 strain)44 were used in this study. The culture supernatants harvested from the infected Vero cells were used as live virus sources for the neutralization test and the antibody assay measuring the balance between neutralizing and enhancing activities, and as antigens for ELISA (see below).
Neutralization test against autologous DENV isolate
The Vero cell focus reduction neutralization test of the serum samples was performed with autologous DENV isolates, essentially as described previously45. Briefly, mixtures of the virus and two-fold serial dilutions of serum samples (starting from 1:10) were incubated at 4 °C overnight. Vero cell monolayers were inoculated with the virus–antibody mixture and incubated at 37 °C for 3 days. After fixation and immunostaining using 4G2 antibody, the foci were counted. The neutralizing activities are expressed as percentages of focus reduction calculated relative to the results for virus controls without test samples.
ELISA for measuring antibodies to autologous DENV isolate
Antibody levels in serum samples were measured by a conventional ELISA as described previously42. Briefly, 96-well microplates sensitized with each autologous DENV were incubated serially with the corresponding two-fold serial dilutions of serum samples (starting from 1:100), anti-human IgG (H + L) alkaline phosphatase conjugate (Promega, Madison, WI), and then p-nitrophenyl phosphate. The end-point titers were expressed as the maximum serum dilution that displayed an optic density (OD) value of ≥ 0.3.
Antibody assay for the balance between neutralizing and enhancing activities (NAb/EAb-balance assay)
The NAb/EAb-balance assay was conducted using semi-adherent K562 cells as described previously26. Briefly, serial dilutions of sera (starting from 1:10 dilution) were mixed with each DENV strain in a poly-L lysine-coated 96-well microplate and incubated at 37 °C for 2 h. K562 cells (1 × 105 cells per well) were then added to the mixtures and incubated at 37 °C for 2 days. After immunostaining (see below), the infected cells were counted. The cut-off values for neutralizing and enhancing activities were calculated from the means ± three standard deviations (SD) of infected cell counts obtained with eight negative controls adjusted for approximately 100 infected cells. When the number of infected cells was higher than the mean + 3SD, it was defined as enhancing activity. In contrast, when the infected cell number was lower than the mean − 3SD, it was defined as neutralizing activity.
In addition, enhancing activity was also evaluated by titrating the progeny virus infectivity in the culture supernatant of the infected cells. The supernatants were harvested at 24 h after the inoculation, and their titers were determined on Vero cells by counting infectious foci after immunostaining (see below) and expressed as FFU/ml.
Immunostaining
Immunochemical staining was performed essentially as described previously41. Briefly, cells were fixed with acetone/methanol (1:1) and incubated serially with a mouse monoclonal antibody D1-4G2 (flavivirus group cross-reactive) purchased from American Type Culture Collection (Manassas, VA), biotinylated anti-mouse IgG, ABC (avidin-biotinylated peroxidase complex) reagent, and VIP substrate (Vector Laboratories, Burlingame, CA).
Quantification of the viral RNA copy number in plasma samples
The viral RNA copy number in plasma samples was determined by following a previous study13. Briefly, viral RNA was extracted from 70 μl plasma using a QIAamp Viral RNA Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol, then subjected to quantitative real-time RT-PCR using a One-Step SYBR PrimeScript RT-PCR kit II (Takara Bio, Japan). The PCR mixture was mixed with 2 μl of extracted RNA and DENV-specific primer46 before running on a CFX96TM real-time PCR cycler (BioRad, Hercules, CA, USA) under cycle conditions of 42 °C for 5 min, 95 °C for 10 s followed by 45 cycles of 95 °C for 5 s, 55 °C for 30 s and 72 °C for 30 s. The viral load quantity was determined by linear regression of the cycle threshold value against the known viral titers quantified by focus forming unit assay.
Statistical analysis
Correlation coefficients were estimated on the basis of the Pearson product-moment correlation coefficient (r). A probability (p) less than 0.05 was considered statistically significant.
Supplementary Information
Acknowledgements
This research was supported by the Japan Initiative for Global Research Network on Infectious Diseases (J-GRID) from the Ministry of Education, Culture, Sport, Science & Technology in Japan, and the Japan Agency for Medical Research and Development (AMED) (JP18fm0108003, 19fm0108003 and 20wm0125010h0001).
Author contributions
A.Y., E.K. and T.S. designed and performed research; A.Y., J.P., E.K. and T.S. analyzed data; H.A.I, W.P., E.K. and T.S. contributed reagents and materials; and A.Y. and T.S. wrote the paper with critical input from all authors.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-91793-0.
References
- 1.World Health Organization. Dengue and severe dengue. https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (2020).
- 2.Brady OJ, et al. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl. Trop. Dis. 2012;6:e1760. doi: 10.1371/journal.pntd.0001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatt S, et al. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guzman MG, Harris E. Dengue. Lancet. 2015;385:453–465. doi: 10.1016/s0140-6736(14)60572-9. [DOI] [PubMed] [Google Scholar]
- 5.Guzman MG, Gubler DJ, Izquierdo A, Martinez E, Halstead SB. Dengue infection. Nat. Rev. Dis. Primers. 2016;2:16055. doi: 10.1038/nrdp.2016.55. [DOI] [PubMed] [Google Scholar]
- 6.Wang WK, et al. High levels of plasma dengue viral load during defervescence in patients with dengue hemorrhagic fever: implications for pathogenesis. Virology. 2003;305:330–338. doi: 10.1006/viro.2002.1704. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Dengue guidelines for diagnosis, treatment, prevention and control: new edition. https://apps.who.int/iris/bitstream/handle/10665/44188/9789241547871_eng.pdf (2009). [PubMed]
- 8.Anders KL, et al. Epidemiological factors associated with dengue shock syndrome and mortality in hospitalized dengue patients in Ho Chi Minh City, Vietnam. Am. J. Trop. Med. Hyg. 2011;84:127–134. doi: 10.4269/ajtmh.2011.10-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaughn DW, et al. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J. Infect. Dis. 2000;181:2–9. doi: 10.1086/315215. [DOI] [PubMed] [Google Scholar]
- 10.Libraty DH, et al. Differing influences of virus burden and immune activation on disease severity in secondary dengue-3 virus infections. J. Infect. Dis. 2002;185:1213–1221. doi: 10.1086/340365. [DOI] [PubMed] [Google Scholar]
- 11.Clapham HE, Tricou V, Van Vinh Chau N, Simmons CP, Ferguson NM. Within-host viral dynamics of dengue serotype 1 infection. J. R. Soc. Interface. 2014;11:20140094. doi: 10.1098/rsif.2014.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waggoner JJ, et al. Viremia and clinical presentation in Nicaraguan patients infected with Zika virus, chikungunya virus, and dengue virus. Clin. Infect. Dis. 2016;63:1584–1590. doi: 10.1093/cid/ciw589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imad HA, et al. Cytokine expression in dengue fever and dengue hemorrhagic fever patients with bleeding and severe hepatitis. Am. J. Trop. Med. Hyg. 2020;102:943–950. doi: 10.4269/ajtmh.19-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morsy S, et al. The association between dengue viremia kinetics and dengue severity: A systemic review and meta-analysis. Rev. Med. Virol. 2020;30:1–10. doi: 10.1002/rmv.2121. [DOI] [PubMed] [Google Scholar]
- 15.de la Cruz-Hernandez SI, et al. Determination of viremia and concentration of circulating nonstructural protein 1 in patients infected with dengue virus in Mexico. Am. J. Trop. Med. Hyg. 2013;88:446–454. doi: 10.4269/ajtmh.12-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perdomo-Celis F, Salgado DM, Narvaez CF. Magnitude of viremia, antigenemia and infection of circulating monocytes in children with mild and severe dengue. Acta Trop. 2017;167:1–8. doi: 10.1016/j.actatropica.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 17.Guzman MG, Vazquez S. The complexity of antibody-dependent enhancement of dengue virus infection. Viruses. 2010;2:2649–2662. doi: 10.3390/v2122649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurtado-Monzón AM, et al. The role of anti-flavivirus humoral immune response in protection and pathogenesis. Rev. Med. Virol. 2020;30:e2100. doi: 10.1002/rmv.2100. [DOI] [PubMed] [Google Scholar]
- 19.Katzelnick LC, et al. Antibody-dependent enhancement of severe dengue disease in humans. Science. 2017;358:929–932. doi: 10.1126/science.aan6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamanaka A, Kotaki T, Konishi E. A mouse monoclonal antibody against dengue virus type 1 Mochizuki strain targeting envelope protein domain II and displaying strongly neutralizing but not enhancing activity. J. Virol. 2013;87:12828–12837. doi: 10.1128/jvi.01874-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halstead SB, O’Rourke EJ. Antibody-enhanced dengue virus infection in primate leukocytes. Nature. 1977;265:739–741. doi: 10.1038/265739a0. [DOI] [PubMed] [Google Scholar]
- 22.Kliks SC, Nisalak A, Brandt WE, Wahl L, Burke DS. Antibody-dependent enhancement of dengue virus growth in human monocytes as a risk factor for dengue hemorrhagic fever. Am. J. Trop. Med. Hyg. 1989;40:444–451. doi: 10.4269/ajtmh.1989.40.444. [DOI] [PubMed] [Google Scholar]
- 23.Halstead SB. Neutralization and antibody-dependent enhancement of dengue viruses. Adv. Virus Res. 2003;60:421–467. doi: 10.1016/s0065-3527(03)60011-4. [DOI] [PubMed] [Google Scholar]
- 24.Morens DM, Halstead SB, Marchette NJ. Profiles of antibody-dependent enhancement of dengue virus type 2 infection. Microb. Pathog. 1987;3:231–237. doi: 10.1016/0882-4010(87)90056-8. [DOI] [PubMed] [Google Scholar]
- 25.Yamanaka A, Konishi E. Dengue-immune humans have higher levels of complement-independent enhancing antibody than complement-dependent neutralizing antibody. Jpn. J. Infect. Dis. 2017;70:579–581. doi: 10.7883/yoken.jjid.2016.379. [DOI] [PubMed] [Google Scholar]
- 26.Konishi E, Tabuchi Y, Yamanaka A. A simple assay system for infection-enhancing and -neutralizing antibodies to dengue type 2 virus using layers of semi-adherent K562 cells. J. Virol. Methods. 2010;163:360–367. doi: 10.1016/j.jviromet.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 27.Yamanaka A, Konishi E. Complement-independent dengue virus type 1 infection-enhancing antibody reduces complement-dependent and -independent neutralizing antibody activity. Vaccine. 2016;34:6449–6457. doi: 10.1016/j.vaccine.2016.11.021. [DOI] [PubMed] [Google Scholar]
- 28.Kliks SC, Nimmanitya S, Nisalak A, Burke DS. Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am. J. Trop. Med. Hyg. 1988;38:411–419. doi: 10.4269/ajtmh.1988.38.411. [DOI] [PubMed] [Google Scholar]
- 29.Halstead SB, et al. Dengue hemorrhagic fever in infants: Research opportunities ignored. Emerg. Infect. Dis. 2002;8:1474–1479. doi: 10.3201/eid0812.020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chau TN, et al. Dengue in Vietnamese infants–results of infection-enhancement assays correlate with age-related disease epidemiology, and cellular immune responses correlate with disease severity. J. Infect. Dis. 2008;198:516–524. doi: 10.1086/590117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Libraty DH, et al. A prospective nested case-control study of Dengue in infants: Rethinking and refining the antibody-dependent enhancement dengue hemorrhagic fever model. PLoS Med. 2009;6:e1000171. doi: 10.1371/journal.pmed.1000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization. Background paper on dengue vaccines. http://www.who.int/immunization/sage/meetings/2018/april/2_DengueBackgrPaper_SAGE_Apr2018.pdf (2018).
- 33.World Health Organization. Revised SAGE recommendation on use of dengue vaccine. https://www.who.int/immunization/diseases/dengue/revised_SAGE_recommendations_dengue_vaccines_apr2018/en/ (2018).
- 34.Halstead SB. Dengvaxia sensitizes seronegatives to vaccine enhanced disease regardless of age. Vaccine. 2017;35:6355–6358. doi: 10.1016/j.vaccine.2017.09.089. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization. Dengue vaccine: WHO position paper—September 2018. Weekly epidemiological record 93, 457–476; https://apps.who.int/iris/bitstream/handle/10665/274315/WER9336.pdf?ua=1 (2018).
- 36.Huisman W, Martina BE, Rimmelzwaan GF, Gruters RA, Osterhaus AD. Vaccine-induced enhancement of viral infections. Vaccine. 2009;27:505–512. doi: 10.1016/j.vaccine.2008.10.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hadinegoro SR, et al. Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N. Engl. J. Med. 2015;373:1195–1206. doi: 10.1056/nejmoa1506223. [DOI] [PubMed] [Google Scholar]
- 38.Dans AL, Dans LF, Lansang MAD, Silvestre MAA, Guyatt GH. Controversy and debate on dengue vaccine series-paper 1: Review of a licensed dengue vaccine: Inappropriate subgroup analyses and selective reporting may cause harm in mass vaccination programs. J. Clin. Epidemiol. 2018;95:137–139. doi: 10.1016/j.jclinepi.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 39.Goncalvez AP, Engle RE, St. Claire M, Purcell RH, Lai CJ. Monoclonal antibody-mediated enhancement of dengue virus infection in vitro and in vivo and strategies for prevention. Proc. Natl. Acad. Sci. USA. 2007;104:9422–9427. doi: 10.1073/pnas.0703498104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.World Health Organization. Dengue Haemorrhagic Fever: Diagnosis, Treatment, Prevention and Control. 2nd ed. WHO. https://www.who.int/csr/resources/publications/dengue/itoviii.pdf?ua=1 (1997).
- 41.Konishi E, Fujii A. Dengue type 2 virus subviral extracellular particles produced by a stably transfected mammalian cell line and their evaluation for a subunit vaccine. Vaccine. 2002;20:1058–1067. doi: 10.1016/s0264-410x(01)00446-7. [DOI] [PubMed] [Google Scholar]
- 42.Yamanaka A, Kosugi S, Konishi E. Infection-enhancing and -neutralizing activities of mouse monoclonal antibodies against dengue type 2 and 4 viruses are controlled by complement levels. J. Virol. 2008;82:927–937. doi: 10.1128/jvi.00992-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lanciotti RS, Calisher CH, Gubler DJ, Chang GJ, Vorndam AV. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J. Clin. Microbiol. 1992;30:545–551. doi: 10.1128/jcm.30.3.545-551.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Konishi E, Kosugi S, Imoto J. Dengue tetravalent DNA vaccine inducing neutralizing antibody and anamnestic responses to four serotypes in mice. Vaccine. 2006;24:2200–2207. doi: 10.1016/j.vaccine.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 45.Konishi E, Terazawa A, Fujii A. Evidence for antigen production in muscles by dengue and Japanese encephalitis DNA vaccines and a relation to their immunogenicity in mice. Vaccine. 2003;21:3713–3720. doi: 10.1016/s0264-410x(03)00376-1. [DOI] [PubMed] [Google Scholar]
- 46.Shu PY, et al. Development of group- and serotype-specific one-step SYBR green I-based real-time reverse transcription-PCR assay for dengue virus. J. Clin. Microbiol. 2003;41:2408–2416. doi: 10.1128/jcm.41.6.2408-2416.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.