Abstract
Background
The Coronavirus disease 2019 (COVID-19) pandemic has led to widespread implementation of public health measures, such as stay-at-home orders, social distancing, and masking mandates. In addition to decreasing spread of severe acute respiratory syndrome coronavirus 2, these measures also impact the transmission of seasonal viral pathogens, which are common triggers of chronic obstructive pulmonary disease (COPD) exacerbations. Whether reduced viral prevalence mediates reduction in COPD exacerbation rates is unknown.
Methods
We performed retrospective analysis of data from a large, multicenter health care system to assess admission trends associated with community viral prevalence and with initiation of COVID-19 pandemic control measures. We applied difference-in-differences analysis to compare season-matched weekly frequency of hospital admissions for COPD prior to and after implementation of public health measures for COVID-19. Community viral prevalence was estimated using regional Centers for Disease Control and Prevention test positivity data and correlated to COPD admissions.
Results
Data involving 4422 COPD admissions demonstrated a season-matched 53% decline in COPD admissions during the COVID-19 pandemic, which correlated to community viral burden (r = 0.73; 95% confidence interval, 0.67-0.78) and represented a 36% greater decline over admission frequencies observed in other medical conditions less affected by respiratory viral infections (incidence rate ratio 0.64; 95% confidence interval, 0.57-0.71, P < .001). The post-COVID-19 decline in COPD admissions was most pronounced in patients with fewer comorbidities and without recurrent admissions.
Conclusion
The implementation of public health measures during the COVID-19 pandemic was associated with decreased COPD admissions. These changes are plausibly explained by reduced prevalence of seasonal respiratory viruses.
Keywords: Community viral infections, COPD, COVID, Exacerbations
Clinical Significance.
-
•
Chronic obstructive pulmonary disease (COPD) admissions declined by 53% during the COVID-19 pandemic.
-
•
COPD admissions decrease was 36% greater than admissions for other common diagnoses.
-
•
Patients with fewer comorbidities and single admissions had greater admission decrease.
-
•
Public health measures during the pandemic led to decline in respiratory virus prevalence.
-
•
Decline in respiratory viruses is associated with decrease in COPD admissions.
Alt-text: Unlabelled box
Introduction
Prior to the Coronavirus disease 2019 (COVID-19) pandemic, chronic obstructive pulmonary disease (COPD) was the fourth leading cause of death worldwide and a common cause of hospital admissions in the United States.1, 2, 3, 4 COPD exacerbations cause a staggering burden of morbidity, mortality, and cost to the health care system.5, 6, 7
The recent COVID-19 pandemic has led to a significant interruption in health care delivery, with reduced admissions for COPD and other non-COVID illnesses.8, 9, 10, 11, 12, 13 This is due to a variety of factors, including avoidance of crowded hospitals due to fear of contracting severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; the virus responsible for COVID-19), reallocation of hospital resources to treat rising numbers of COVID-19 patients, and utilization of telemedicine to manage chronic diseases at home. There is compelling evidence indicating that public health measures (such as stay-at-home orders, social distancing, and masking mandates with strict limitations on large gatherings to curb the spread of COVID-19) are also effective at decreasing the transmission of seasonal viral pathogens.14 , 15
It is plausible that measures taken to reduce the spread of SARS-CoV-2 have also resulted in reductions in COPD exacerbations, due in large part to reduced burden of non-SARS-CoV-2 community virus transmission. Respiratory viral infections are the most commonly identified trigger for COPD exacerbations. They are implicated in approximately 50% of cases, and result in a disease course that tends to be more severe and longer in duration.16, 17, 18
We aimed to determine whether public health measures related to the COVID-19 pandemic were associated with reduced seasonal viral infections and COPD-related hospital admissions. Using data from a large, multicenter health care system, we hypothesized that the public health measures related to the COVID-19 pandemic decreased hospital admissions for COPD exacerbations.
Materials and Methods
Study Design and Data Sources
We conducted a difference-in-differences analysis of retrospective data to compare weekly hospital admissions for COPD pre- vs post-COVID-19 public health measures. Stay-at-home mandate and masking mandate were instituted in the state of Maryland on March 30, 2020 and April 18, 2020, respectively. April 1, 2020 was selected as the start of the post-COVID-19 period. A difference-in-differences model estimates the average effect among an exposure group while controlling for factors unrelated to the exposure of interest that would affect both exposed and unexposed groups. In this case, a key difference between the groups is the association to respiratory viral infections as triggers for destabilization and subsequent hospitalization. We obtained hospital data from the University of Maryland Medical System, which consists of 13 hospitals throughout the state of Maryland. Patients with a primary diagnosis of COPD for their hospital admission were identified using International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes (Supplementary Table 1a, available online). We selected a control group of primary diagnoses that would account for a similar reservation among patients to seek hospital care due to a fear of COVID-19 transmission. The control group included all admissions with a primary diagnosis of myocardial infarction, diabetes mellitus, or congestive heart failure, and were identified using ICD-10-CM codes (Supplementary Table 1b, available online). The control group diagnoses were also selected for informative epidemiologic aspects. Both congestive heart failure and myocardial infarction admission rates experience seasonal variability and are associated with trends in viral infections, although less robustly than observed in COPD.19, 20, 21, 22 As such, selection of these diagnoses was an intentionally conservative approach. Diabetes, on the other hand, demonstrates little seasonality, although it warrants emphasis that all analyses were seasonally controlled. Only the patients with the above ICD-10-CM codes were included in the study. The analyses were restricted to include only data from April 1 through September 30 in 2018 through 2020 to ensure a seasonal match. The period prior to implementation of public health measures included data from 2018 and 2019, and the period after implementation included data from 2020. The study was approved by the University of Maryland institutional review board (HP-00093452).
Table 1s-a.
. ICD-10 Codes Used for Primary COPD Admission Diagnosis
| COPD Category | ICD-10 Code | ICD-10-Code Description |
|---|---|---|
| Bronchitis | J40 | Bronchitis, not specified as acute or chronic |
| J410 | Simple chronic bronchitis | |
| J411 | Mucopurulent chronic bronchitis | |
| J418 | Mixed simple and mucopurulent chronic bronchitis | |
| J42 | Unspecified chronic bronchitis | |
| Emphysema | J430 | Unilateral pulmonary emphysema [MacLeods syndrome] |
| J431 | Panlobular emphysema | |
| J432 | Centrilobular emphysema | |
| J438 | Other emphysema | |
| J439 | Emphysema, unspecified | |
| COPD | J440 | Chronic obstructive pulmonary disease with (acute) lower respiratory infection |
| J441 | Chronic obstructive pulmonary disease w (acute) exacerbation | |
| J449 | Chronic obstructive pulmonary disease, unspecified |
Table 1s-b.
ICD-10 Codes Used for Non-COPD Primary Admission Diagnosis
| Comorbidity | ICD-10 Code |
|---|---|
| Myocardial infarction | I21, I22, I252 |
| Congestive heart failure | I110, I130, I132, I255, I420, I425, I426, I427, I428, I429, I43, I50, P290 |
| Diabetes Mellitus | E080, E081, E086, E088, E089, E090, E091, E096, E098, E099, E100, E101, E106, E108, E109, E110, E111, E116, E118, E119, E130, E131, E136, E138, E139, E082, E083, E084, E085, E092, E093, E094, E095, E102, E103, E104, E105, E112, E113, E114, E115, E132, E133, E134, E135 |
Outcomes
The primary outcome was weekly hospital admissions. Secondary outcomes included weekly counts of in-hospital mortality, severe exacerbations (identified as requiring intensive care unit admission), and hospital length of stay. Additionally, we calculated community viral percent positivity to assess the correlation between viral trends and COPD admissions. Data on respiratory virus incidence were provided by the Centers for Disease Control and Prevention's (CDC) National Respiratory and Enteric Virus Surveillance System and the CDC FluView report. Respiratory virus results are reported weekly from participating laboratories, and the number of tests and positive tests for the included respiratory viruses were provided from the middle Atlantic and South Atlantic census regions encompassing January 1, 2018 through October 1, 2020. Viruses reported included respiratory syncytial virus, human metapneumovirus, seasonal coronaviruses (including 229E, HKU1, NL63, and OC43), human parainfluenza virus (types 1-3), rhinovirus, and influenza.23
Ascertainment of Patient Characteristics
Patient characteristics were extracted from the electronic health records to assess for any imbalance between time periods or exposure groups. We also used ICD-10-CM codes to generate Charlson Comorbidity Index scores, and diagnoses of morbid obesity, tobacco use, alcohol-related disorders, substance-related disorders, and social challenges (Supplementary Tables 1c, d, available online).
Statistical Analysis
The primary analysis of the change in weekly hospital admissions applied a negative binomial model, adjusting for an observed imbalance between periods in patient age and a weekly time trend. We included a dummy variable for the admitting hospital as a random effect in the model to account for variation in public health measure adherence and socioeconomic compositions among the catchment areas. We used Pearson's correlation coefficients to evaluate the association between community viral percent positivity data with admissions for COPD, congestive heart failure, diabetes mellitus, and myocardial infarction.
The in-hospital mortality and severe exacerbation models were fit using negative binomial regression. We used a quantile regression model to evaluate the effect of the COVID-19-related public health measures on the median hospital length-of-stay days among COPD patients. In all models, we adjusted for patient age and accounted for the hospital as a random effect.
We performed 2 subgroup analyses to assess heterogeneity of effect based on comorbidities and recurrent admissions. The comorbidities were identified at the time of admission, and reported using the Charlson Comorbidity Index (CCI), which was stratified between patients with a CCI ≤3 and patients with a CCI >3 (Supplementary Tables 1c).24 Patients were classified as having recurrent admissions if they had more than one admission for the same primary diagnosis during the study period. The subgroup term was interacted with the comparison groups and exposure for a difference-in-difference-in-differences model. With this approach, we accounted for the subgroup covariate as an interaction term to the difference-in-differences equation. This model ascertained if the difference-in-differences estimate changed between subgroups.
We performed 2 sensitivity analyses. In the first analysis, we added patients who had a primary admission diagnosis of COVID-19 and a secondary diagnosis of COPD (Supplementary Table 1e, available online) to the COPD group. The intention of this analysis was to determine if the decline in COPD admissions was due to related COVID-19 diagnosis taking precedence in the diagnosis. In the second analysis, we rebuilt 3 versions of our primary difference-in-differences model, one model for each control group's primary admission diagnosis—congestive heart failure, diabetes mellitus, or myocardial infarction. The purpose of this sensitivity analysis was to evaluate if our estimates were robust across the various control groups.
Difference-in-differences estimates for the negative binomial models are reported as an incidence rate ratio (IRR) with 95% confidence intervals (CIs). The difference-in-differences estimates for the quantile regression model are reported as the change in median days of hospital stay with 95% CIs. CIs were calculated using bootstrap methods.
The analyses were performed with R Version 4.0.0 (Vienna, Austria). For the primary analysis, we considered a 2-tailed P < .05 as statistically significant. We did not adjust for multiple testing, and all secondary outcomes should be considered exploratory. We did not impute for missing data.
Results
There were 18,587 admissions during the specified periods: 13,695 occurred prior to the implementation of COVID-19-related public health measures (pre-COVID-19), and 4892 observations occurred afterward (post-COVID-19). There were 4422 hospital admissions for COPD, 6414 for congestive heart failure, 4217 for diabetes, and 3534 for myocardial infarction.
The characteristics for the COPD patients and the control group during the periods under consideration are provided in Table 1 . Notably, the average age of COPD patients admitted during the post-COVID-19 period was older (62.7 ± 16.5 years) compared with the pre-COVID-19 period (55.3 ± 24.4 years). There were no significant differences in sex, race, or distribution of CCI scores. The mean age of all patients with COPD admission was 57 ± 23 years, and more than half were female (n = 2499, 57%). The majority of these patients had 3 or fewer comorbidities (n = 3683, 83%).
Table 1.
Characteristics of the Study Sample
| COPD Admissions |
CHF, DM, and MI Admissions |
|||
|---|---|---|---|---|
| Variable | Pre-COVID-19 | Post-COVID-19 | Pre- COVID-19 | Post- COVID-19 |
| No. of admissions | 3572 | 850 | 10,123 | 4042 |
| Age (years) | 55.3 ± 24.4 | 62.7 ± 16.5 | 64.4 ± 17.3 | 63.5 ± 17.8 |
| Male sex, n (%) | 1546 (43) | 381 (45) | 5792 (57) | 2369 (59) |
| Race/ethnicity, n (%) | ||||
| White | 1848 (52) | 457 (54) | 5330 (53) | 2148 (53) |
| Black | 1554 (44) | 369 (43) | 4285 (42) | 1674 (41) |
| Other race | 176 (5) | 24 (3) | 508 (5) | 220 (5) |
| Hispanic | 101 (3) | 19 (2) | 246 (2) | 112 (3) |
| Charlson Comorbidity Index, n (%) | ||||
| 0-3 | 2999 (84) | 684 (81) | 5663 (56) | 2299 (57) |
| 4 or more | 573 (16) | 166 (19.5) | 4460 (44) | 1743 (43) |
| Recurrent admissions, n (%) | 804 (23) | 209 (25) | 1996 (20) | 719 (18) |
| Morbidly obese, n (%) | 368 (10) | 101 (12) | 1319 (13) | 567 (14) |
| Tobacco use, n (%) | 1096 (31) | 311 (37) | 1951 (19) | 827 (21) |
| Alcohol-related disorder, n (%) | 199 (6) | 59 (7) | 423 (4) | 172 (4) |
| Social challenges, n (%) | 98 (3) | 41 (5) | 228 (2) | 152 (4) |
| Substance-related disorder, n (%) | 277 (8) | 85 (10) | 595 (6) | 232 (6) |
CHF = congestive heart failure; COPD = chronic obstructive pulmonary disease; DM = diabetes mellitus; MI = myocardial infarction.
Primary Outcome
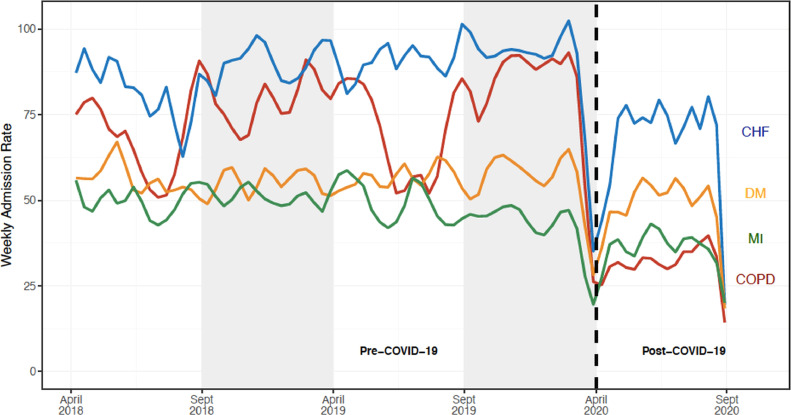
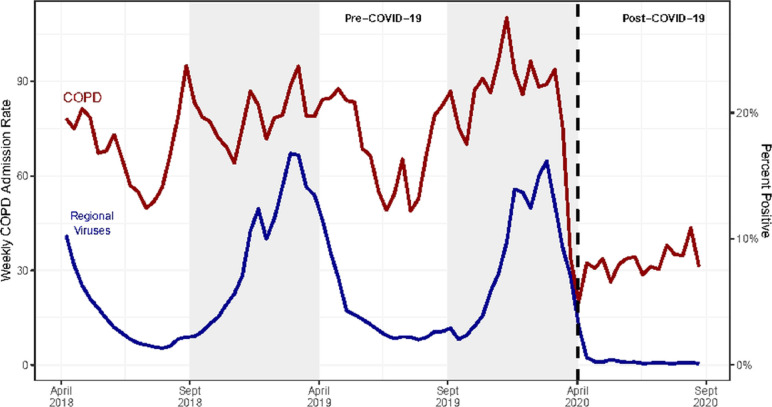
The post-COVID crude weekly COPD admissions decreased by 53% compared with the pre-COVID-19 seasonally comparable period (31.5 vs 67.4 admissions/week, respectively). Compared with declines observed in other medical conditions, weekly COPD admissions demonstrated a 36% greater decline (IRR 0.64; 95% CI, 0.57-0.71, P < .001; Table 2 , Figure 1 ). To ensure that the results were not attributable to concomitant SARS-CoV-2 infection, a sensitivity analysis was performed including admissions for primary diagnosis of COVID-19 with a secondary diagnosis of COPD included in the COPD group. The findings remained significant even with this conservative approach (Supplementary Table 3, available online). The results were also consistent within subsets of the control group admitted for congestive heart failure (IRR 0.66; 95% CI, 0.59-0.75), diabetes mellitus (IRR 0.57; 95% CI, 0.51-0.65), and myocardial infarction (IRR 0.69; 95% CI, 0.60-0.77) (Supplementary Table 4, available online). In addition, there was an apparent temporal correlation between weekly COPD admissions and community viral percent positivity (r 0.73; 95% CI, 0.67-0.78) (Figure 2 ).
Table 2.
Primary and Secondary Outcome Measures
| Pre-COVID-19 | Post-COVID-19 | Difference-in-Differences | P Value | |
|---|---|---|---|---|
| Primary outcome: weekly admissions | Incidence rate ratio (95% CI) | |||
| COPD admissions | 67.4 | 31.5 | 0.64 (0.57-0.71) | < .001 |
| CHF, DM, MI admissions | 191.0 | 149.7 | ||
| Secondary outcomes | ||||
| Weekly in-hospital mortality | Incidence rate ratio (95% CI) | |||
| COPD admissions | 0.3 | 0.1 | 0.66 (0.20-2.13) | – |
| CHF, DM, MI admissions | 3.8 | 3.1 | ||
| Weekly intensive care unit admissions | Incidence rate ratio (95% CI) | |||
| COPD admissions | 3.2 | 2.7 | 1.01 (0.72-1.35) | – |
| CHF, DM, MI admissions | 38.9 | 31.3 | ||
| Median hospital length of stay (IQR) | Difference in median days (95% CI) | |||
| COPD admissions | 2 (1-3) | 2 (1-4) | 0.00 (−0.62-0.62) | – |
| CHF, DM, MI admissions | 3 (2-5) | 3 (2-5) |
CHF = congestive heart failure; CI = confidence interval; COPD = chronic obstructive pulmonary disease; COVID-19 = chronic obstructive pulmonary disease 2019; DM = diabetes mellitus; IQR = interquartile range; MI = myocardial infarction.
Figure 1.
Weekly hospital admission rates for chronic obstructive pulmonary disease, congestive heart failure, diabetes mellitus, and myocardial infarction. Our analyses compared the post-intervention period (April 1, 2020 through September 30, 2020) with a season-matched pre-intervention period comprising of April 1, 2018 through September 30, 2018 and April 1, 2019 through September 30, 2019. The gray shaded areas denote data that were not included in the season-matched analysis. The pandemic time period was associated with a 53% decline in COPD admissions (from 67.4 to 31.5 admissions/week), which represented a 36% greater decline than that observed in other medical indications evaluated (incidence rate ratio 0.64; 95% confidence interval, 0.57-0.71, P < .001). CHF = congestive heart failure; COPD = chronic obstructive pulmonary disease; DM = diabetes mellitus; MI = myocardial infarction.
Figure 2.
Weekly chronic obstructive pulmonary disease (COPD) admissions rates and the percent positive for regional non-SARS-COV-2 human coronavirus and statewide human metapneumovirus. COPD admission and viral data are reported from April 1, 2018 through September 30, 2020. Community viral testing positivity rates were obtained from the Centers for Disease Control and Prevention (CDC) for the Southern and Mid-Atlantic regions. Viruses include influenza, parainfluenza, respiratory syncytial virus, human non-SARS coronaviruses, and human metapneumovirus. Admission rates for COPD correlated to community viral percent positivity (r = 0.64, 95% confidence interval, 0.57-0.69).
Secondary Outcomes
There were no significant differences in in-hospital mortality (0.3 vs 0.1 deaths/week pre- vs post-COVID-19; IRR 0.66; 95% CI, 0.20-2.13) or in severe COPD exacerbations requiring intensive care unit stay (3.2 vs 2.7 exacerbations/week pre- vs post-COVID-19; IRR 1.01; 95% CI, 0.72-1.35) between the pre- and post-COVID-19 periods (Table 2). Similarly, there were no significant differences in median hospital length of stay for COPD admissions.
Subgroup Analyses
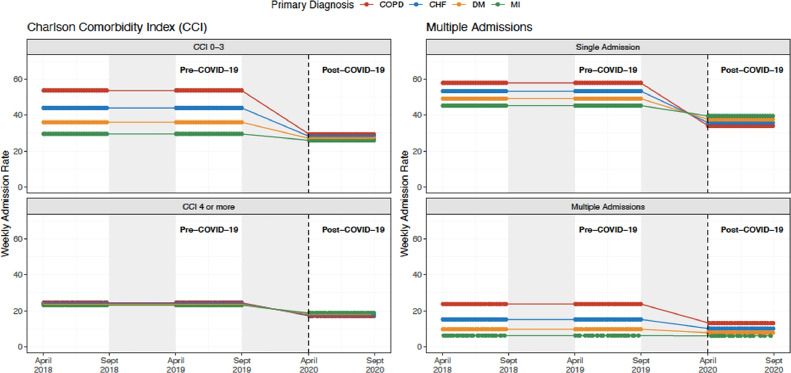
The difference in COPD admissions pre- vs post-COVID-19 period was most pronounced for patients with 3 or fewer comorbidities, assessed using CCI (IRR 0.60; 95% CI, 0.49-0.75) (Figure 3 , Supplementary Table 5, available online)). The reduction in COPD admissions associated with the post COVID-19 period was greater for patients without recurrent admissions than for patients with recurrent admissions (IRR 0.66; 95% CI, 0.53-0.81) (Supplementary Table 5).
Figure 3.
Weekly hospital admission rates for chronic obstructive pulmonary disease, congestive heart failure, diabetes mellitus, and myocardial infarction stratified by Charlson Comorbidity Index and recurrent admissions. Our analyses compared the post-intervention period (April 1, 2020 through September 30, 2020) with a season-matched pre-intervention period comprising April 1, 2018 through September 30, 2018 and April 1, 2019 through September 30, 2019. The gray shaded areas denote data that were not included in the season-matched analysis. Declines in COPD admission rates were most pronounced in the subgroups of patients with fewer comorbidities and without repeat admissions. CHF = congestive heart failure; COPD = chronic obstructive pulmonary disease; DM = diabetes mellitus; MI = myocardial infarction.
Discussion
This study documents a substantial decline in weekly COPD admissions occurring during the COVID-19 pandemic. The reduction is greater in COPD admissions than that observed in other medical conditions less strongly associated with respiratory viral infections. This observation can be plausibly explained by reductions in community respiratory viral infections consequent to measures taken to control SARS-CoV-2 transmission.
Our study includes data from a large, multihospital health system, which allows us to minimize potential bias due to hospital resource allocation, such as admission limitation of non-COVID-19 patients or transfer of such patients to accommodate COVID-19 patients. All hospitals were located in a single state and therefore, we assume more homogeneity in the observed public health measures. We included each of the 13 hospitals in the system as a random effect in our models to account for possible differences in the sociodemographics of the hospital catchments that may influence the study findings. In addition, our data included weekly admission trends dating back to 2018, allowing for a granular evaluation of changes over time. We also assessed other possible causes of the decrease in COPD admissions, including a subgroup analysis comparing patients with varying degrees of comorbidities and frequency of exacerbations, and by correlating our findings with respiratory viral trends. The difference-in-differences models was seasonally matched and included other diagnostic conditions to account for hospital avoidance due to fear of COVID-19 transmission.
There have been prior publications that described declines in hospital admissions during the pandemic. One large study described reductions in emergency department presentations ranging from 41.5% to 63.5%, with greater reductions observed in areas more heavily affected by the pandemic.13 Birkmeyer et al12 examined admission frequencies across a large nationwide medical group and found substantial declines to a low of 42.8% below normal. In that study, COPD admissions decreased more than any other diagnostic group to a nadir reduction of 68.6%, and despite admissions for some other conditions returning to pre-pandemic levels, COPD admissions remained 40.1% lower than baseline.12 A variety of mechanisms have been proposed to explain declines in medical admissions for various major non-COVID-19 conditions, but the apparently greater decline in COPD admissions has been inadequately explained.
The decrease in admissions for all causes relates to a global reduction in hospital and emergency department visits, likely due to patients’ fear of contracting SARS-CoV-2 virus in these settings.13 , 25 , 26 There has also been a shift toward telemedicine, and more patients are treating symptoms at home than in the hospital, both of which may contribute to lowering overall hospitalization.27 , 28 However, there are additional factors that may play a role specific to the COPD population. These include improvements in air quality during the pandemic, potential increased adherence to COPD controller medications, and changes in rates of smoking during the pandemic.29, 30, 31 Overall, the degree of reduction from each of these measures is likely small, and fails to explain such a large, immediate reduction in COPD admissions.
As viral infections account for 40%-50% of COPD exacerbations, the most likely explanation for the large decrease in COPD admissions is the reduction of respiratory virus prevalence in the population.4 , 17 Exacerbations caused by a virus also tend to be more severe and longer-lasting than non-viral exacerbations (median 13 days, compared with 6 days, respectively).17 Recent CDC data demonstrate notable reductions of respiratory viral infections in 2020 compared with 2018 and 2019 combined. 23 Statewide curfews, social distancing measures (including closure of indoor restaurants and limitations on large gatherings), and campaigns for frequent handwashing and mask-wearing likely contributed to this decrease in respiratory viral infection prevalence.14 , 15 , 32 Our analysis shows strong temporal correlation between COPD admission frequencies and respiratory virus burden in Maryland (Figure 2). The decrease in respiratory virus positivity was beyond expected seasonal decrease during the post-COVID period compared with the pre-COVID period (years 2018 and 2019). The correlation between respiratory virus burden and our control group conditions was substantially weaker, suggesting the decrease in respiratory viral prevalence appears likely to be the greatest contributor to the decreased frequency of COPD admissions seen during the COVID-19 pandemic.
Our data also show that the reduction in COPD admissions was greatest in those with fewer comorbidities. COPD exacerbations occurring during the COVID-19 pandemic may have been associated with a patient's underlying poor health condition at baseline. In addition, a change in engagement with the health care system during the pandemic, such as avoidance of crowded emergency department visits, may have led to a shift toward outpatient management of COPD exacerbations, especially in those with fewer comorbidities and less severe disease. Those patients with fewer comorbidities may have been able to manage exacerbations at home better than those with multiple comorbidities and baseline poor physiologic reserve. Often, these patients tend to be younger, and this likely explains the shift in mean age of COPD patients in the post-COVID-19 group. Older patients with more comorbidities and more severe COPD experience more frequent exacerbations, regardless of respiratory viral infections, and may not experience the decrease seen in those with fewer comorbidities.
A few limitations of our study deserve mention. Our analysis is based on hospital admission data, creating a possibility for coding variability among providers and possible misdiagnosis. However, the use of ICD codes in health services research is widely accepted, and we have employed ICD codes consistent with prior studies.33, 34, 35 Our data did not permit granular evaluation of whether COPD exacerbations complicated by COVID could influence ICD codes chosen, but we performed a sensitivity analysis in which the case definition of COPD exacerbation was modified to include admissions in which COVID was listed as a primary diagnosis with COPD listed as a comorbidity. Results remained significant, suggesting there was not a major issue related to misclassification bias. Another limitation involves the inclusion of control diagnoses that manifest a degree of seasonality related to community respiratory viral infections. This was an intentionally conservative approach that may underestimate the true magnitude of the reduction in COPD exacerbations attributable to respiratory viral infections. Finally, the lack of statistical significance observed in the secondary endpoints could reflect power limitations rather than a true lack of association.
Conclusions
There is a significant decrease in weekly COPD admissions during the COVID-19 pandemic, likely due to a decrease in respiratory virus prevalence from public health measures being taken during the pandemic. There may be opportunity to judiciously apply control measures outside of pandemic conditions to reduce the burden of disease imparted by community respiratory viral infections.
Footnotes
Funding: None.
Conflicts of Interest: None.
Authorship: Conception and design of the study: JYS, NNO, MS, PZ, RMR; Analysis and interpretation of the data: JYS, NNO, BK, JGW, JFS, ZZ, MS, PZ, CLB, MD, RMR; Drafting the initial draft: JYS, NNO, RMR; Reviewing and editing of the manuscript: JYS, NNO, BK, JGW, JFS, ZZ, MS, PZ, CLB, MD, RMR; Guarantors of the manuscript: RMR, JYS, NNO.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amjmed.2021.05.008.
Additional Methods
Rationale for Control Group Criteria
A difference-in-differences model estimates the average effect among an exposure group while controlling for factors unrelated to the exposure of interest that would affect both exposed and unexposed groups. For this study, that means the average association between COVID-19 public health measures on weekly COPD hospital admissions. The model requires a control group of patients that exhibit a similar pre-exposure (COVID-19 public health measures) trend. For our study, this control group comprised hospital admissions for a primary diagnosis of myocardial infarction (MI), diabetes mellitus (DM), or congestive heart failure (CHF), and were identified using ICD-10-CM codes. In addition to being common admission diagnoses in our system, these were selected for informative epidemiologic aspects. Both CHF and MI admission rates experience seasonal variability and are associated with trends in viral infections, although less robustly than observed in COPD.(1-4) As such, selection of these diagnoses was an intentionally conservative approach. Diabetes, on the other hand, demonstrates little seasonality.
Ascertainment of Patient Characteristics
Patient characteristics were extracted from the electronic health records to assess for any imbalance between time periods or exposure groups. We also used ICD-10-CM codes to generate Charlson Comorbidity Index scores, and diagnoses of morbid obesity, tobacco use, alcohol-related disorders, substance-related disorders, and social challenges (Table 1s,c -d ).
Table 1s-c.
ICD-10 Codes Used to Determine Charlson Comorbidity Index
| Comorbidity | CCI Point | ICD-10 Code |
|---|---|---|
| Myocardial infarction | 1 | I21, I22, I252 |
| Congestive heart failure | 1 | I110, I130, I132, I255, I420, I425, I426, I427, I428, I429, I43, I50, P290 |
| Peripheral vascular disease | 1 | I70, I71, I731, I738, I739, I771, I790, I791, I798, K551, K558, K559, Z958, Z959 |
| Cerebrovascular disease | 1 | G45, G46, H340, H341, H342, I60, I61, I62, I63, I64, I65, I66, I67, I68 |
| Dementia | 1 | F01, F02, F03, F04, F05, F061, F068, G132, G138, G30, G310, G311, G312, G914, G94, R4181, R54 |
| Chronic pulmonary disease | 1 | J40, J41, J42, J43, J44, J45, J46, J47, J60, J61, J62, J63, J64, J65, J66, J67, J684, J701, J703 |
| Rheumatic disease | 1 | M05, M06, M315, M32, M33, M34, M351, M353, M360 |
| Peptic ulcer disease | 1 | K25, K26, K27, K28 |
| Liver disease, mild | 1 | B18, K700, K701, K702, K703, K709, K713, K714, K715, K717, K73, K74, K760, K762, K763, K764, K768, K769, Z944 |
| Diabetes without chronic complications | 1 | E080, E081, E086, E088, E089, E090, E091, E096, E098, E099, E100, E101, E106, E108, E109, E110, E111, E116, E118, E119, E130, E131, E136, E138, E139 |
| Renal disease, mild to moderate | 1 | I129, I130, I1310, N03, N05, N181, N182, N183, N184, N189, Z940 |
| Diabetes with chronic complications | 2 | E082, E083, E084, E085, E092, E093, E094, E095, E102, E103, E104, E105, E112, E113, E114, E115, E132, E133, E134, E135 |
| Hemiplegia or paraplegia | 2 | G041, G114, G800, G801, G802, G81, G82, G83 |
| Any malignancy | 2 | C00, C01, C02, C03, C04, C05, C06, C07, C08, C09, C10, C11, C12, C13, C14, C15, C16, C17, C18, C19, C20, C21, C22, C23, C24, C25, C26, C27, C28, C29, C30, C31, C32, C33, C34, C37, C38, C39, C40, C41, C43, C45, C46, C47, C48, C49, C50, C51, C52, C53, C54, C55, C56, C57, C58, C60, C61, C62, C63, C76, C801, C81, C82, C83, C84, C85, C88, C90, C91, C92, C93, C94, C95, C96, C97, C98, C99 |
| Liver disease, moderate to severe | 3 | I850, I864, K704, K711, K721, K729, K765, K766, K767 |
| Renal disease, severe | 3 | I120, I1311, I132, N185, N186, N19, N250, Z49, Z992 |
| HIV infection, no AIDS | 3 | B20 |
| Metastatic solid tumor | 6 | C77, C78, C79, C800, C802 |
| AIDS (in addition to HIV code) | 6 | B37, C53, B38, B45, A072, B25, G934, B00, B39, A073, C46, C81, C82, C83, C84, C85, C86, C87, C88, C89, C90, C91, C92, C93, C94, C95, C96, A31, A15, A16, A17, A18, A19, B59, Z8701, A812, A021, B58, R64 |
Table 1s-d.
ICD-10 Codes Used to Classify Patient Characteristics
| Comorbidity | ICD-10 Code |
|---|---|
| Alcohol-related disorder | F10, K70 |
| Substance-related disorder | F11, F13, F14, F15, F16, F18, F19 |
| Tobacco use | F17 |
| Social challenges | Z55, Z56, Z57, Z59, Z60, Z62, Z63, Z64, Z65 |
| Morbidly obese | E6601, Z6841, Z6842, Z6843, Z6844, Z6845 |
Additional Statistical Analysis Details
The difference-in-differences model compared the trends in hospital admissions before (pre-COVID-19) and after public health measures related to the COVID-19 pandemic (post-COVID-19 period). The parallel trends assumption was confirmed using an equivalence test with bounds of ±2% and a negative binomial model that included an interaction between the study group and months from study onset using the pre-COVID-19 data (Table 2s).
We performed two subgroup analyses to assess heterogeneity of effect based on co-morbidities and recurrent admissions. The comorbidities were identified at the time of admission, and reported using the Charlson Comorbidity Index (CCI) which was stratified between patients with a CCI <3 and patients with a CCI >3 (Table 1s-c).(5) Patients were classified as having recurrent admissions if they had more than one admission for the same primary diagnosis during the study period. The subgroup term was interacted with the comparison groups and exposure for a difference-in-difference-in-differences model. With this approach, we accounted for the subgroup covariate as an interaction term to the difference-in-differences equation. This model ascertained if the difference-in-differences estimate changed between subgroups. Sensitivity analyses included patients with a primary admission diagnosis of COVID-19 (Table 1s-e ) to the COPD group, and our primary comparison with subsets of patients with the primary admission diagnosis of congestive heart failure, diabetes, or myocardial infarction.
Table 1s-e.
ICD-10 Code Used to Determine Primary COVID-19 Admission Diagnosis
| Diagnosis Category | ICD-10 Code | ICD-10-Code Description |
|---|---|---|
| COVID-19 | U071 | COVID-19 |
Table 2s.
Test for Parallel Trends in Pre-COVID Period
| COPD vs. | Incidence rate ratio (95% CI) | Equivalence Test P Value |
|---|---|---|
| CHF + DM + MI | 0.996 (0.995 to 0.998) | <0.001 |
| CHF | 0.996 (0.995 to 0.997) | <0.001 |
| DM | 0.995 (0.994 to 0.996) | <0.001 |
| MI | 0.997 (0.995 to 0.998) | <0.001 |
Table 3s.
Sensitivity Analysis of COPD Admissions, Including Admissions with Primary COVID-19 Diagnosis with Secondary COPD Diagnosis
| Pre-COVID-19 | Post-COVID-19 | Difference-in-Differences | |
|---|---|---|---|
| Primary outcome: weekly admissions rates | Incidence rate ratio (95% CI) | ||
| COPD admissions | 67.4 | 43.9 | 0.86 (0.79 to 0.96) |
| CHF, DM, MI admissions | 191.0 | 149.7 | |
Table 4s.
Sensitivity Analysis of Weekly Hospital Admissions Using a Diagnosis-Specific Subgroups as Controls
| CHF control | Pre-COVID-19 | Post-COVID-19 | Difference-in-Differences Incidence rate ratio (95% CI) |
|---|---|---|---|
| COPD admissions | 67.4 | 31.5 | 0.66 (0.59 to 0.75) |
| CHF admissions | 87.0 | 66.8 | Ref (1.00) |
| DM control | |||
| COPD admissions | 67.4 | 31.5 | 0.57 (0.51 to 0.65) |
| DM admissions | 55.3 | 47.6 | Ref (1.00) |
| MI control | |||
| COPD admissions | 67.4 | 31.5 | 0.69 (0.60 to 0.78) |
| MI admissions | 48.7 | 35.4 | Ref (1.00) |
Table 5s.
Subgroup Analysis of Weekly Hospital Admissions using a Differences-in-Difference-in-Differences Model
| Subgroup | COPD Admissions |
CHF, DM, and MI Admissions |
Difference-in-Differences-in-Differences | P Value | ||
|---|---|---|---|---|---|---|
| Pre-COVID-19 | Post-COVID-19 | Pre-COVID-19 | Post-COVID-19 | |||
| Charlson Comorbidity Index | Incidence rate ratio (95% CI) | |||||
| 0-3 | 56.6 | 25.3 | 107.0 | 85.1 | 0.60 (0.49 to 0.75) | <0.001 |
| 4 or more | 10.8 | 6.2 | 84.2 | 64.6 | Ref (1.00) | |
| Recurrent admissions | Incidence rate ratio (95% CI) | |||||
| Only 1 admission during study period | 52.2 | 23.7 | 153.1 | 123.4 | 0.66 (0.53 to 0.81) | <0.001 |
| More than 1 admission during study period | 15.2 | 8.04 | 37.7 | 26.6 | Ref (1.00) | |
CHF = congestive heart failure; COPD = chronic obstructive pulmonary disease; DM = diabetes mellitus; MI = myocardial infarction.
Difference-in-differences estimates for the negative binomial models are reported as an incidence rate ratio with 95% confidence intervals. The difference-in-differences estimates for the quantile regression model is reported as the change in median days of hospital stay with 95% confidence intervals. Confidence intervals were calculated using bootstrap methods.
References
-
1.
Spencer FA, Goldberg RJ, Becker RC, Gore JM. Seasonal distribution of acute myocardial infarction in the second National Registry of Myocardial Infarction. J Am Coll Cardiol 1998; 31: 1226-1233.
-
2.
Bhaskaran K, Hajat S, Haines A, Herrett E, Wilkinson P, Smeeth L. Effects of ambient temperature on the incidence of myocardial infarction. Heart 2009; 95: 1760-1769.
-
3.
Stewart S, McIntyre K, Capewell S, McMurray JJV. Heart failure in a cold climate Seasonal variation in heart failure-related morbidity and mortality. Journal of the American College of Cardiology 2002; 39: 760–766.
-
4.
Martinez-Selles M, Garcia Robles JA, Prieto L, Serrano JA, Munoz R, Frades E, Almendral J. Annual rates of admission and seasonal variations in hospitalizations for heart failure. Eur J Heart Fail 2002; 4: 779-786.
-
5.
Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. Journal of Clinical Epidemiology 1994; 47: 1245–1251.
References
- 1.Centers for Disease Control and Prevention (CDC). Selected respiratory diseases among adults aged 18 and over, by selected characteristics: United States, 2018. National Health Interview Survey: Tables of Summary Health Statistics. Available at: https://ftp.cdc.gov/pub/Health_Statistics/NCHS/NHIS/SHS/2018_SHS_Table_A-2.pdf. Accessed September 30, 2020.
- 2.Centers for Disease Control and Prevention (CDC). National Center for Health Statistics. Chronic obstructive pulmonary disease (COPD) includes: chronic bronchitis and emphysema. Available at: https://www.cdc.gov/nchs/fastats/copd.htm. Accessed October 25, 2020.
- 3.Agency for Healthcare Research and Quality (AHRQ). HCUP Fast Stats–Most Common Diagnoses for Inpatient Stays. Available at: https://www.hcup-us.ahrq.gov/faststats/NationalDiagnosesServlet. Accessed October 20, 2020.
- 4.McManus TE, Marley AM, Baxter N, et al. Respiratory viral infection in exacerbations of COPD. Respir Med. 2008;102(11):1575–1580. doi: 10.1016/j.rmed.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1418–1422. doi: 10.1164/ajrccm.157.5.9709032. [DOI] [PubMed] [Google Scholar]
- 6.Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57(10):847–852. doi: 10.1136/thorax.57.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soler-Cataluna JJ, Martinez-Garcia MA, Roman Sanchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–931. doi: 10.1136/thx.2005.040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berghaus TM, Karschnia P, Haberl S, Schwaiblmair M. Disproportionate decline in admissions for exacerbated COPD during the COVID-19 pandemic. Respir Med. 2020 Aug 14 doi: 10.1016/j.rmed.2020.106120. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baeza-Martínez C, Zamora-Molina L, Olea-Soto J, Soler-Sempere MJ, García-Pachón E. Reduction in hospital admissions for COPD exacerbation during the Covid-19 pandemic. Open Respir Arch. 2020;2(3):201–202. doi: 10.1016/j.opresp.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kansagra AP, Goyal MS, Hamilton S, Albers GW. Collateral effect of Covid-19 on stroke evaluation in the United States. N Engl J Med. 2020;383(4):400–401. doi: 10.1056/NEJMc2014816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Filippo OD, D'Ascenzo F, Angelini F, et al. Reduced rate of hospital admissions for ACS during Covid-19 outbreak in northern Italy. N Engl J Med. 2020;383(1):88–89. doi: 10.1056/NEJMc2009166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Birkmeyer JD, Barnato A, Birkmeyer N, Bessler R, Skinner J. The impact of the COVID-19 pandemic on hospital admissions in the United States. Health Aff (Millwood) 2020;39(11):2010–2017. doi: 10.1377/hlthaff.2020.00980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeffery MM, D'Onofrio G, Paek H, et al. Trends in emergency department visits and hospital admissions in health care systems in 5 states in the first months of the COVID-19 pandemic in the US. JAMA Intern Med. 2020;180(10):1328–1333. doi: 10.1001/jamainternmed.2020.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olsen SJ, Azziz-Baumgartner E, Budd AP, et al. Decreased influenza activity during the COVID-19 pandemic — United States, Australia, Chile, and South Africa, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(37):1305–1309. doi: 10.15585/mmwr.mm6937a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan JY, Conceicao EP, Sim XYJ, Wee LEI, Aung MK, Venkatachalam I. Public health measures during COVID-19 pandemic reduced hospital admissions for community respiratory viral infections. J Hosp Infect. 2020;106(2):387–389. doi: 10.1016/j.jhin.2020.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilkinson TM, Hurst JR, Perera WR, Wilks M, Donaldson GC, Wedzicha JA. Effect of interactions between lower airway bacterial and rhinoviral infection in exacerbations of COPD. Chest. 2006;129(2):317–324. doi: 10.1378/chest.129.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seemungal T, Harper-Owen R, Bhowmik A, et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(9):1618–1623. doi: 10.1164/ajrccm.164.9.2105011. [DOI] [PubMed] [Google Scholar]
- 18.Papi A, Bellettato CM, Braccioni F, et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006;173(10):1114–1121. doi: 10.1164/rccm.200506-859OC. [DOI] [PubMed] [Google Scholar]
- 19.Spencer FA, Goldberg RJ, Becker RC, Gore JM. Seasonal distribution of acute myocardial infarction in the second National Registry of Myocardial Infarction. J Am Coll Cardiol. 1998;31(6):1226–1233. doi: 10.1016/s0735-1097(98)00098-9. [DOI] [PubMed] [Google Scholar]
- 20.Bhaskaran K, Hajat S, Haines A, Herrett E, Wilkinson P, Smeeth L. Effects of ambient temperature on the incidence of myocardial infarction. Heart. 2009;95(21):1760–1769. doi: 10.1136/hrt.2009.175000. [DOI] [PubMed] [Google Scholar]
- 21.Stewart S, McIntyre K, Capewell S, McMurray JJV. Heart failure in a cold climate seasonal variation in heart failure-related morbidity and mortality. J Am Coll Cardiol. 2002;39(5):760–766. doi: 10.1016/s0735-1097(02)01685-6. [DOI] [PubMed] [Google Scholar]
- 22.Martinez-Selles M, Garcia Robles JA, Prieto L, et al. Annual rates of admission and seasonal variations in hospitalizations for heart failure. Eur J Heart Fail. 2002;4(6):779–786. doi: 10.1016/s1388-9842(02)00116-2. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention (CDC). The National Respiratory and Enteric Virus Surveillance System (NREVSS). Available at: https://www.cdc.gov/surveillance/nrevss/index.html. Accessed November 7, 2020.
- 24.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 25.Lazzerini M, Barbi E, Apicella A, Marchetti F, Cardinale F, Trobia G. Delayed access or provision of care in Italy resulting from fear of COVID-19. Lancet Child Adolesc Health. 2020;4(5):e10–e11. doi: 10.1016/S2352-4642(20)30108-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenbaum L. The untold toll - the pandemic's effects on patients without Covid-19. N Engl J Med. 2020;382(24):2368–2371. doi: 10.1056/NEJMms2009984. [DOI] [PubMed] [Google Scholar]
- 27.Chudasama YV, Gillies CL, Zaccardi F, et al. Impact of COVID-19 on routine care for chronic diseases: a global survey of views from healthcare professionals. Diabetes Metab Syndr. 2020;14(5):965–967. doi: 10.1016/j.dsx.2020.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679–1681. doi: 10.1056/NEJMp2003539. [DOI] [PubMed] [Google Scholar]
- 29.Maryland Department of the Environment . 2020. Air monitoring: quality of air monthly reports. Available at https://mde.state.md.us/programs/Air/AirQualityMonitoring/Pages/AQSummary.aspx Accessed October 26, 2020. [Google Scholar]
- 30.Kaye L, Theye B, Smeenk I, Gondalia R, Barrett MA, Stempel DA. Changes in medication adherence among patients with asthma and COPD during the COVID-19 pandemic. J Allergy Clin Immunol Pract. 2020;8(7):2384–2385. doi: 10.1016/j.jaip.2020.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee JJ, Wang MP, Yang SC. Will the tobacco industry ultimately triumph in the midst of COVID-19 pandemic?: a call for nurses' action in tobacco control. Int J Nurs Stud. 2021;115 doi: 10.1016/j.ijnurstu.2020.103726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chow A, Hein AA, Kyaw WM. Unintended consequence: influenza plunges with public health response to COVID-19 in Singapore. J Infect. 2020;81(2):e68–e69. doi: 10.1016/j.jinf.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hasegawa K, Gibo K, Tsugawa Y, Shimada YJ, Camargo CA. Age-related differences in the rate, timing, and diagnosis of 30-day readmissions in hospitalized adults with asthma exacerbation. Chest. 2016;149(4):1021–1029. doi: 10.1016/j.chest.2015.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goto T, Faridi MK, Gibo K, et al. Trends in 30-day readmission rates after COPD hospitalization, 2006-2012. Respir Med. 2017;130:92–97. doi: 10.1016/j.rmed.2017.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holguin F, Folch E, Redd SC, Mannino DM. Comorbidity and mortality in COPD-related hospitalizations in the United States, 1979 to 2001. Chest. 2005;128(4):2005–2011. doi: 10.1378/chest.128.4.2005. [DOI] [PubMed] [Google Scholar]