Abstract
Younger age at first exposure (AFE) to repetitive head impacts while playing American football increases the risk for later-life neuropsychological symptoms and brain alterations. However, it is not known whether AFE is associated with cortical thickness in American football players. Sixty-three former professional National Football League players (55.5 ± 7.7 years) with cognitive, behavioral, and mood symptoms underwent neuroimaging and neuropsychological testing. First, the association between cortical thickness and AFE was tested. Second, the relationship between clusters of decreased cortical thickness and verbal and visual memory, and composite measures of mood/behavior and attention/psychomotor speed was assessed. AFE was positively correlated with cortical thickness in the right superior frontal cortex (cluster-wise P value [CWP] = 0.0006), the left parietal cortex (CWP = 0.0003), and the occipital cortices (right: CWP = 0.0023; left: CWP = 0.0008). A positive correlation was found between cortical thickness of the right superior frontal cortex and verbal memory (R = 0.333, P = 0.019), and the right occipital cortex and visual memory (R = 0.360, P = 0.012). In conclusion, our results suggest an association between younger AFE and decreased cortical thickness, which in turn is associated with worse neuropsychological performance. Furthermore, an association between younger AFE and signs of neurodegeneration later in life in symptomatic former American football players seems likely.
Keywords: cortical thinning, repetitive head impacts, traumatic brain injury, verbal memory, visual memory
Introduction
Repetitive head impacts (RHIs) and resulting concussions and/or subconcussions are frequently observed in contact sports, such as American football (Pellman et al. 2003; Nathanson et al. 2016). Evidence further suggests that exposure to RHI while playing professional football may increase the risk for developing neuropsychological symptoms later in life (Stamm, Bourlas, et al. 2015a; Alosco, Kasimis, et al. 2017b; Alosco et al. 2018; Alosco and Stern 2019; Roberts et al. 2019; Plessow et al. 2020). In this context, the age at first exposure (AFE) to tackle football has been suggested as a potential effect modifier for the development of neuropsychological symptoms (Stamm, Bourlas, et al. 2015a; Alosco, Kasimis, et al. 2017b; Alosco et al. 2018; Alosco and Stern 2019). More specifically, AFE to tackle football prior to the age of 12 years is associated with greater later-life neuropsychiatric and cognitive impairment in former American football players (Stamm, Bourlas, et al. 2015a; Alosco, Kasimis, et al. 2017b). However, a study in active athletes participating in collegiate football or in other contact sports such as lacrosse, wrestling, ice hockey, and soccer, found no association between AFE and current neurocognitive performance (Caccese et al. 2019). Nonetheless, a recent imaging study revealed an association between younger AFE and smaller bilateral hippocampal and posterior corpus callosum (CC) volumes for both former and active fighters (Bryant et al. 2020). A study on symptomatic former professional football players revealed altered white matter (WM) microstructure of the CC in those who had started to play tackle football before the age of 12 years (Stamm, Koerte, et al. 2015b). Further, the earlier they had started to play, the smaller their bilateral thalamus, and decreased right thalamic volume was correlated with worse visual memory (Schultz et al. 2018). Taken together, these findings suggest that earlier exposure to RHI while playing football may increase the risk of later-life neuropsychological symptoms as well as alterations in WM microstructure and subcortical gray matter (GM) volume.
Some of the symptoms observed in former athletes exposed to RHI, such as impaired executive function, may also suggest an involvement of cortical GM. Although cortical thinning physiologically occurs with increasing age (Fjell et al. 2009; Thambisetty et al. 2010), accelerated cortical thinning in locally characteristic patterns has been associated with neurodegenerative diseases, such as Alzheimer’s disease (Lerch et al. 2005, 2008; Kang et al. 2019). Cortical thinning is associated with accelerated cognitive decline in patients with dementia (Dickerson et al. 2009; Querbes et al. 2009; Eskildsen et al. 2013). Importantly, there is increasing evidence of cortical thinning in former athletes who participated in contact sports including soccer (Koerte et al. 2016), rugby (Wojtowicz et al. 2018), and American football (Adler et al. 2018). More specifically, a small study in former professional soccer players reported an association between exposure to RHI and cortical thinning with increasing age (Koerte et al. 2016). Former collegiate American football players showed decreased cortical thickness in the frontal and temporal cortex (Adler et al. 2018). Another study investigated a small cohort of former Canadian Football League players and reported decreased cortical thickness in the anterior temporal lobe (Goswami et al. 2016). Further, in the same study, decreased orbitofrontal cortical thickness was associated with increased aggression and task errors indicative of impulsivity (Goswami et al. 2016). However, whether younger AFE is an effect modifier of the association between RHI and cortical thinning later in life remains unknown.
The aim of this study is to investigate the association between AFE to tackle football and cortical thickness in symptomatic former professional football players. We further assess whether regional decrease in cortical thickness is associated with later-life neuropsychological performance and neuropsychiatric symptoms.
Methods
Study Design and Participants
This study is part of the Diagnosing and Evaluating Traumatic Encephalopathy using Clinical Tests (DETECT) project, a cohort study aimed at developing biomarkers for the in-vivo diagnosis of chronic traumatic encephalopathy. The DETECT protocol included neurological and psychiatric examinations, assessment of exposure to RHI, neuropsychological testing, self-report measure of neuropsychiatric symptoms, and neuroimaging by multi-sequence magnetic resonance imaging (MRI) in former National Football League (NFL) players and healthy controls (the current study includes data only from the former NFL group). Participant evaluations were conducted from 2011 to 2015. The study and its procedures were approved by the Boston University Medical Campus and Partners Healthcare Institutional Review Boards. All study participants provided written informed consent prior to enrollment.
The following inclusion criteria were defined for enrollment of NFL players in the DETECT project: 1) male sex, 2) age between 40 and 69 years, 3) minimum of 12 years of participation in organized tackle football, 4) minimum of 2 years of play in the NFL at positions known to have extensive head impacts based on helmet accelerometer data (i.e., offensive and defensive linemen, defensive backs, linebackers, running backs), and 5) a self-report of cognitive, behavioral, and mood symptoms for a minimum of 6 months prior to study participation. Additionally, the following exclusion criteria were defined: 1) a history of concussion within 1 year of study enrollment, 2) contraindications for MRI, 3) presence of another disease affecting the central nervous system, and 4) a primary language other than English.
Overall, 96 former NFL players were enrolled in the DETECT project. Thirty-three players were excluded from analyses of MRI data. More specifically, in 4 subjects T1-weighted data was not available, 15 scans were excluded due to severe motion artifacts, 13 cases were excluded due to failed post-processing, and 1 case was excluded due to a lesion likely stemming from small infarction. The final sample consisted of 63 former NFL players. Importantly, the included cases did not differ from the excluded cases on demographics (age, body mass index [BMI], school years) or clinical outcome measures (factor scores).
Exposure to RHI
To retrospectively quantify RHI exposure, we used the following self-reported measures: AFE to tackle football (age in years) and total number of years played.
Neuropsychological Testing
Study participants underwent a comprehensive neuropsychological test battery to evaluate neurocognitive function across several domains, including attention, executive function, episodic memory, language, and visuospatial abilities. Raw test scores were converted into standardized scores using normative data that account for age, sex, and/or educational attainment. Participants also completed self-report questionnaires to assess neurobehavioral dysregulation, as well as depression and anxiety symptoms. To reduce the risk of Type I error, four factor scores were derived from the outcome measures using principal component analysis. These factor scores are based on rational and empirical grounds, and their derivation has been previously reported (Alosco, Jarnagin, et al. 2017a).
The four factors reflected a composition of the following tests and scores: 1) “mood and behavior” (Apathy Evaluation Scale (Marin 1996), Beck Depression Inventory-II (Beck et al. 1996), Beck Hopelessness Scale (Beck et al. 1974), Barratt Impulsivity Scale (Patton et al. 1995), Behavior Rating Inventory of Executive Function—Adult Version—Behavioral Regulation Index (Roth et al. 2005), Center for Epidemiologic Studies Depression Scale (Radloff 1977), Hamilton Depression Rating Scale (Hamilton 1960), and Brown-Goodwin Lifetime History of Aggression (Alosco, Jarnagin, et al. 2017a)), 2) “attention and psychomotor speed” (Controlled Oral Word Association Test-FAS (Benton and Hamsher 1989), Wechsler Adult Intelligence Scale–Revised—Digit Symbol Coding test (Wechsler 1997), Delis-Kaplan Executive Function System—Color Word Interference Test, and Trail Making Test—Parts A and B (Delis et al. 2001)), 3) “verbal memory” (Neuropsychological Assessment Battery—Story Learning Phrase Unit—Immediate and Delayed Recall (Petermann and Jäncke 2016), and List Learning Immediate and Delayed Recall), and 4) “visual memory” (Rey-Osterrieth Complex Figure—Boston Qualitative Scoring System—Immediate Recall Presence and accuracy, and Delayed Recall Presence and Accuracy (Stern et al. 1999)).
Magnetic Resonance Imaging
Image Acquisition
Participants underwent cranial MRI on a 3-Tesla scanner (Magnetom Verio, Siemens Healthcare, Erlangen, Germany) in supine position using a 32-channel head array. As part of a multi-sequence neuroimaging protocol, a three-dimensional T1-weighted Magnetization-Prepared Rapid Gradient Echo (MP-RAGE) sequence was acquired with the following parameters: repetition time/echo time = 1800 ms/3.36 ms, inversion time = 1100 ms, voxel size = 1 × 1 × 1 mm3, acquisition matrix = 256 × 256, flip angle = 7 degrees. No contrast agent wasused.
Image Processing and Calculation of Cortical Thickness
The raw MP-RAGE data from the scanner were first converted from dicom to nrrd file format, followed by axis alignment and centering. Thorough visual inspection for completeness, distortion, and motion artifacts were performed using 3D Slicer (http://www.slicer.org; version 4.5, Surgical Planning Laboratory, Brigham and Women’s Hospital, Boston, MA,USA).
Brain segmentations and extractions of cortical thickness were performed in FreeSurfer (http://surfer.nmr.mgh.harvard.edu; version 5.3, Laboratory for Computational Neuroimaging, Charlestown, MA, USA) (Fischl 2012). Our semi-automated, step-wise approach using in-house scripts included removal of non-brain tissue, automated Talairach transformation, grayscale intensity normalization, and correction for any inhomogeneities in the magnetic field, automated topology correction, and surface deformation correction according to intensity gradients in order to optimally delineate the GM/WM and GM/cerebrospinal fluid boundary (Dale et al. 1999; Fischl and Dale 2000). Subsequent steps included surface inflation, registration to a common spherical atlas, and parcellation of the cortex with regards to sulcal and gyral patterns according to the Desikan-Killiany atlas (Fischl et al. 1999). Visual quality inspections were conducted after each step and on the final maps before performing computational analyses for cortical thickness.
Cortical thickness was determined as the closest distance between the GM/WM boundary and the pial surface at each vertex of the cortical mantle (Fischl and Dale 2000). Surface-based smoothing was applied to the maps in order to improve the signal-to-noise ratio (with a default Gaussian kernel approximation of 10 mm).
Statistical Analysis
Descriptive statistics were calculated for participant characteristics including demographics, AFE to tackle football, total number of years played, and neuropsychological testing as represented by the four factor scores (mood and behavior, attention and psychomotor speed, verbal memory, and visual memory).
First, whole-brain analyses of cortical thickness were performed using the QDEC package as implemented in FreeSurfer (http://surfer.nmr.mgh.harvard.edu/fswiki/FsTutorial/QdecGroupAnalysis_freeview). A correlation of cortical thickness with AFE was tested to identify clusters of cortical thickness that are associated with AFE using QDEC. This analysis was corrected for age, BMI, education (represented by school years), and the total number of years played. In addition, a cluster-wise Monte Carlo simulation was applied (threshold of 0.05) to adjust for multiple comparisons. A cluster-wise P value (CWP) and the mean cortical thickness ± standard deviation (SD) were extracted from each detected statistically significant cluster on a single-subject level that survived correction for multiple comparisons.
Second, to assess the relationship between cortical thickness in the identified clusters with neuropsychological function as represented by the four factor scores, post-hoc analyses were performed using SPSS (version 26.0; IBM SPSS Statistics for Windows, Armonk, NY, USA). For these post-hoc analyses, we conducted partial correlation analyses while correcting for age, BMI, education, race, and total number of years played. The level of statistical significance was set at P < 0.05, and results of these correlation analyses were plotted using GraphPad Prism (version 7.0; GraphPad Software Inc., La Jolla, CA,USA).
Results
Cohort Characteristics
The final sample consisted of 63 former NFL players. Table 1 shows cohort characteristics including demographics. The study participants began to participate in tackle football at a mean age of 11.4 ± 2.6 years.
Table 1.
Cohort characteristics
| Number of NFL players included | 63 males | |
|---|---|---|
| Age (in years, mean ± SD [range]) |
55.5 ± 7.7 [40.0–68.0] |
|
| BMI (in kg/m2, mean ± SD [range]) |
32.5 ± 4.4 [25.2–44.7] |
|
| Education (in school years, mean ± SD [range]) |
16.4 ± 1.0 [15.0–20.0] |
|
| Race (% of subjects) |
White | 65.1 |
| Black/African American | 31.7 | |
| Unknown/Other | 3.2 | |
| Start age to play football (in years, mean ± SD [range]) |
11.4 ± 2.6 [6.0–17.0] |
|
| Total number of years played (mean ± SD [range]) |
18.7 ± 3.3 [12.0–25.0] |
|
| Position played (% of subjects) |
Offensive | 36.5 |
| Defensive | 63.5 | |
| Exact role (% of subjects) | Offensive line | 27.0 |
| Linebacker | 25.4 | |
| Defensive line | 19.0 | |
| Defensive back | 19.0 | |
| Running back | 4.8 | |
| Tight end | 3.2 | |
| Offensive skill | 1.6 | |
This table gives an overview of cohort characteristics including demographics of the 63 former NFL players included in this study.
Associations between Cortical Thickness and AFE to RHI
A positive statistically significant correlation between AFE and cortical thickness was found in the left parietal cortex (supramarginal gyrus and superior parietal lobule; CWP = 0.0003), in the right superior frontal cortex (posterior superior frontal cortex and dorsal precentral gyrus, CWP = 0.0006), as well as in the bilateral occipital cortex (cuneal cortex and pericalcarine cortex and lingual gyrus; left: CWP = 0.0008; right: CWP = 0.0023) (Figs 1 and 2). These findings suggest that the younger a former NFL player began to play football, the thinner the cortex in these brain regions later inlife.
Figure 1.
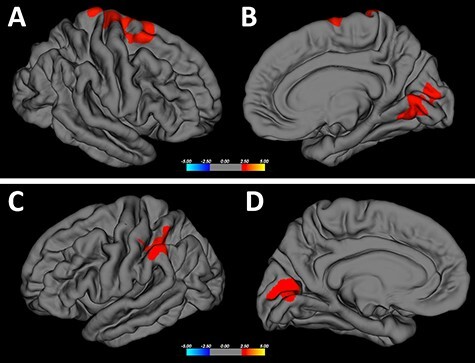
Correlation between cortical thickness and AFE to tackle football—I. Clusters with statistically significant correlations between cortical thickness and AFE are highlighted in “red” (P < 0.05). Analyses were corrected for age, BMI, education (represented by school years), and total number of years played. Furthermore, a cluster-wise Monte Carlo simulation was applied (threshold of 0.05) to adjust for multiple comparisons. The legend represents the color code for P values, i.e., the strength of correlations within the clusters. Statistically significant clusters are located in the right superior frontal cortex (CWP = 0.0006), left parietal cortex (CWP = 0.0003), and bilateral occipital cortex (left hemisphere: CWP = 0.0008; right hemisphere: CWP = 0.0023). (A) right hemisphere, lateral view; (B) right hemisphere, mesial view; (C) left hemisphere, lateral view; and (D) left hemisphere, mesialview.
Figure 2.
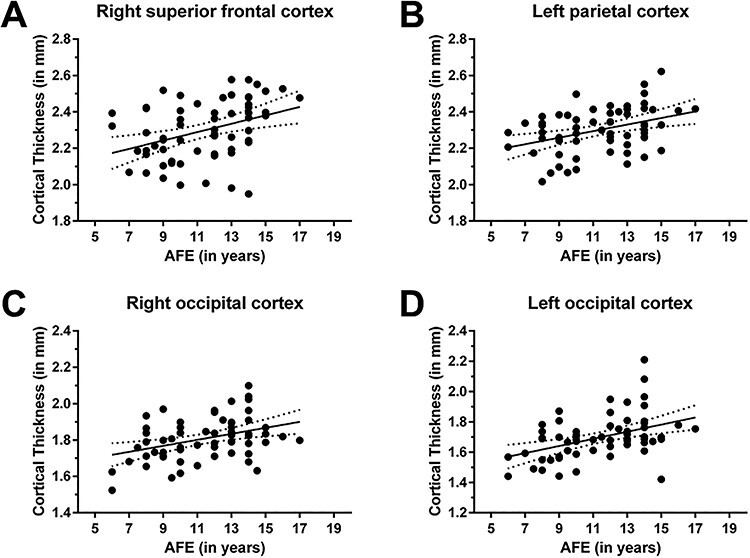
Correlation between cortical thickness and AFE to tackle football—II. Clusters with statistically significant associations between cortical thickness and the AFE are located in the right superior frontal cortex (CWP = 0.0006; A), left parietal cortex (CWP = 0.0003; B), and bilateral occipital cortex (right occipital cortex: CWP = 0.0023; C; left occipital cortex: CWP = 0.0008; D).
Associations between Cortical Thickness and Neuropsychological Function
“Verbal memory” was statistically significantly positively correlated with cortical thickness in the right superior frontal cortex cluster (R = 0.333, P = 0.019; Fig. 3), and “visual memory” was statistically significantly positively correlated with cortical thickness in the cluster of the right occipital cortex (R = 0.360, P = 0.012; Fig. 3). These findings suggest that thinner cortex in these areas is associated with lower neuropsychological performance regarding “verbal memory” and “visual memory”.
Figure 3.
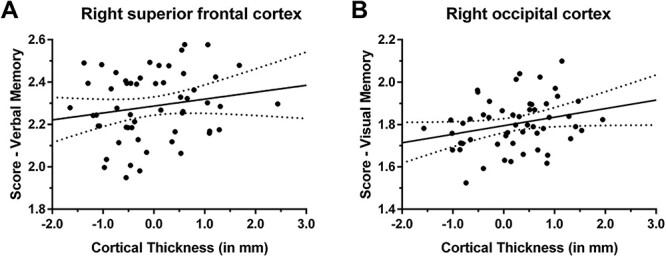
Correlation between cortical thickness and neuropsychological function. The cortical thickness of clusters with statistically significant correlations between cortical thickness and AFE to tackle football was associated with the factor scores of neuropsychological testing. A statistically significant correlation was revealed between “verbal memory” and cortical thickness of the right superior frontal cortex (R = 0.333, P = 0.019; A), and between “visual memory” and cortical thickness of the right occipital cortex (R = 0.360, P = 0.012; B). The line of best fit is shown together with 95% confidence intervals.
Discussion
This study investigated associations between cortical thickness, AFE to tackle football, and later-life neuropsychological function in symptomatic former professional football players. There are two main findings: First, the younger a former football player was when exposure to tackle football began, the thinner the cortex in four brain regions (right superior frontal cortex, left parietal cortex, and bihemispheric occipital cortex) in later life. Second, the thinner the cortex in two of these regions, the worse the individual’s neuropsychological performance (thinner cortex in the right superior frontal cortex was associated with lower “verbal memory” and decreased cortical thickness in the right occipital cortex was associated with lower “visual memory”).
Reduced Cortical Thickness and Earlier Exposure to American Football
A positive association between cortical thickness and AFE to tackle football was revealed in four cortical clusters (right superior frontal cortex, left parietal cortex, and bihemispheric occipital cortex), such that the younger a former NFL player began to play tackle football, the thinner the cortex in these regions, after controlling for age, BMI, education, and the total years of football played.
Earlier exposure to RHI was thus associated with decreased cortical thickness in widespread brain areas later in life. Possible underlying pathomechanisms include: 1) an increased risk of impaired neurodevelopment due to exposure to RHI during a critical period of brain development, and 2) an increased risk for later-life neurodegeneration. Of note, study participants began to participate in tackle football at an average age of 11.4 ± 2.6 years. Thus, exposure to RHI started either before or during early puberty, which is considered a critical period of neurodevelopment (Lebel et al. 2008; Tamnes et al. 2010; Chang et al. 2015). Specifically, important physiological processes for proper brain development in adolescence and particularly during puberty on the way to adulthood include dendritic arborization and synaptic pruning (Huttenlocher and Dabholkar 1997; Giedd 2008; Petanjek et al. 2011), as well as increases in intracortical myelination (Giedd 2008; Tamnes et al. 2017). Thus, not surprisingly, previous studies have repeatedly demonstrated that age is likely to have a major impact on recovery from brain injury, such as concussion, with adolescents experiencing more severe and prolonged postconcussive symptoms compared with adults (Field et al. 2003; Covassin et al. 2012; Zuckerman et al. 2012; Moore et al. 2016). Of note, physiological developmental processes during adolescence do not occur uniformly across the human brain (Gogtay et al. 2004; Giedd 2008). Maturation processes of the cerebral cortex seem to follow a hetero-chronic pattern with considerable regional variations (Gogtay et al. 2004; Whitford et al. 2007; Giedd 2008; Lebel et al. 2008; Brown et al. 2012). Thus, there is an interval of several years during adolescence in which various brain regions may be especially vulnerable to injury.
Disruptions to essential processes of brain development due to sports-related RHI may lead to alterations in brain structure and function, which may also put the brain at increased vulnerability to neurodegeneration later in life (Solomon 2018; Alosco and Stern 2019). Cortical thickness is also known to decrease with increasing age as part of the normal aging process (Thambisetty et al. 2010). Interestingly, a study investigating longitudinal changes of cortical volume, surface area, and thickness from late childhood to early adulthood (7 to 29 years), reported that the parietal lobe showed the greatest decrease in both cortical surface area and thickness (Thambisetty et al. 2010). Furthermore, in a longitudinal study among healthy elderly individuals (60 to 84 years), the inferior parietal lobe was among the regions with the most prominent decrease in cortical thickness over time (Thambisetty et al. 2010). Hence, the cluster of decreased cortical thickness in the left parietal cortex observed in this study may be an expression of accelerated decrease in cortical thickness. Such accelerated decrease in cortical thickness may be interpreted as accelerated aging, possibly triggered by exposure to RHI during the critical neurodevelopmental phase in adolescence.
There are, however, studies that do not reveal an association of AFE to American football with neurocognitive performance or with measures of brain structure and function (Solomon et al. 2016; Caccese et al. 2019). Specifically, among 45 former NFL players, none of the neurological, neuroradiological, or neuropsychological outcome measures yielded a significant relationship with years of exposure to pre-high-school football (Solomon et al. 2016). Furthermore, among 4376 active male athletes with 3462 participating in American football, neurocognitive performance as measured by the Immediate Post-Concussion Assessment and Cognitive Testing test was not associated with the estimated age of first exposure (Caccese et al. 2019). Thus, although evidence accumulated indicates that AFE may be an important factor for later-life alterations in brain health (Alosco and Stern 2019), results from this study need to be confirmed in future studies.
Association of Cortical Thickness and Neuropsychological Function
In two of the four clusters where cortical thickness was associated with AFE, a post-hoc analysis revealed an association between cortical thickness and cognitive function. More specifically, the thinner the cortex, the worse an individual’s verbal memory (for the cluster in the right superior frontal cortex) and visual memory (for the cluster in the right occipital cortex). These findings suggest a relationship between altered brain structure and later-life cognitive decline.
The cluster in the right occipital cortex included parts of the cuneal cortex, pericalcarine cortex, and lingual gyrus. These structures are key areas of visual processing (Bogousslavsky et al. 1987; Beason-Held et al. 1998; Machielsen et al. 2000). Thus, associations between worse performance in visual memory and decreased cortical thickness in this area seem reasonable. Our results are in line with a previous study that reported a correlation between visual memory decline and decreased GM volume of the right-hemispheric middle occipital gyrus in individuals with history of brain injury (Lauer et al. 2017).
Further, decreased performance in verbal memory correlated with decreased cortical thickness in a cluster in the right superior frontal cortex, comprised of parts of the dorsal precentral gyrus and posterior superior frontal gyrus. Although the left-sided superior frontal gyrus has been attributed to higher cognitive functions and particularly to working memory (Boisgueheneuc et al. 2006), the role of the right-sided homolog is less clear. It has been associated with non-verbal functions such as control of impulsive response (Sadeh et al. 2015; Hu et al. 2016). Specifically, in patients with traumatic brain injury, changes in the superior frontal gyrus were associated with impairment in task switching (Leunissen et al. 2014). Taken together, verbal memory decline related to decreased cortical thickness in the right superior frontal cortex remains not fully understood and needs to be elucidated in future work. However, not all individuals exposed to RHI from American football develop later-life neurological disorders or symptoms. Among those that do, there is significant heterogeneity in the presence and severity of symptoms. Therefore, other RHI and non-RHI (e.g., age, vascular risk factors, genetics, racial identity, and related social determinants of health) are likely at play that increase an individual’s susceptibility to the late effects ofRHI.
Limitations
There are limitations to this study that need to be considered when interpreting the results. First, the cross-sectional design of the DETECT study precludes a more definitive link between RHI, particularly AFE, and cortical thinning as well as later-life neuropsychological symptoms. Thus, future studies using a longitudinal design, ideally obtaining imaging at several time points over a time span of several years, are needed to facilitate our understanding of RHI-related changes in brain structure and function. Second, total years played did not have an effect on the association of AFE and cortical thickness. Future studies need to assess the effect of more specific exposure variables (e.g., position played, number of games played, number of practices, etc.). Third, although we accounted for many of the known effects on cortical thickness (age, BMI, education), we acknowledge that there may be additional factors that play a role. Future studies should also consider investigating additional factors such as pre-existing psychiatric and medical comorbidities that may play a role in the decline of cortical thickness in former professional football players. In addition, early life experiences are complex and include many potential factors, beyond—or interacting with—AFE. Future studies on the effects of AFE in athletes would benefit from examining a variety of social and environmental determinants of brain health. Fourth, results from this study cannot be generalized beyond the specific sample investigated, that is, former football players who played professionally in the 1970s to 1990s. Future studies should examine the impact of variables such as era and level of play (i.e., professional, college, high school). Moreover, the lack of a comparison group with asymptomatic former professional football players reflects a limitation. In addition, due to the cross-sectional design of the study, we cannot rule out differences in cortical thickness before athletes began to play tackle football. A future longitudinal study could investigate further healthy controls compared with former football players to distinguish normal from accelerated aging and the role of AFE in modifying later-life cortical thinning.
Conclusions
This study revealed an association between younger AFE to tackle football and decreased cortical thickness in various brain areas including the right superior frontal cortex, left parietal cortex, and bihemispheric occipital cortex. Additionally, in two of the four regions worse verbal and visual memory were associated with decreased cortical thickness. Taken together, findings from this study suggest an association between younger AFE to tackle football and signs of neurodegeneration later in life. Longitudinal studies are needed to confirm these findings.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Notes
Conflict of Interest: Robert A. Stern, PhD, reports the following disclosures: He is a paid consultant to Biogen. He receives royalties for published neuropsychological tests from Psychological Assessment Resources, Inc. He has stock options as a member of the Board of Directors for King-Devick Technologies,Inc.
Alexander P. Lin, PhD, reports the following disclosures: He is a paid consultant to Agios Pharmaceuticals, Biomarin, and Moncton MRI. He is a co-founder of BrainSpec,Inc.
All other authors do not have conflicts of interests to report.
Funding Statement
National Institute of Neurologic Disorders and Stroke (R01 NS078337 to PI: R.A.S.); National Institutes of Health (R01 NS100952 to I.K.K., R01 HD090641 to S.B.); the German Academic Exchange Service (Deutscher Akademischer Austeuschdienst to P.W.W.); the Ludwig-Maximilians-University Munich (P.W.W.).
Ethics Approval Statement
The study and its procedures were approved by the Boston University Medical Campus and Partners Healthcare Institutional Review Boards.
Patient Consent Statement
All study participants provided written informed consent prior to enrollment.
Permission to Reproduce Material from Other Sources
Not applicable.
Clinical Trial Registration
Not applicable.
Contributor Information
David Kaufmann, cBRAIN, Department of Child and Adolescent Psychiatry, Psychosomatics and Psychotherapy, Ludwig-Maximilians-Universität, 80337 Munich, Germany; Psychiatry Neuroimaging Laboratory, Brigham and Women's Hospital, Harvard Medical School, Boston, MA 02215, USA; Department of Radiology, Charité Universitätsmedizin, 10117 Berlin, Germany.
Nico Sollmann, cBRAIN, Department of Child and Adolescent Psychiatry, Psychosomatics and Psychotherapy, Ludwig-Maximilians-Universität, 80337 Munich, Germany; Psychiatry Neuroimaging Laboratory, Brigham and Women's Hospital, Harvard Medical School, Boston, MA 02215, USA; Department of Diagnostic and Interventional Neuroradiology, Klinikum rechts der Isar, Technische Universität München, 81675 Munich, Germany; TUM-Neuroimaging Center, Klinikum rechts der Isar, Technische Universität München, 81675 Munich, Germany.
Elisabeth Kaufmann, cBRAIN, Department of Child and Adolescent Psychiatry, Psychosomatics and Psychotherapy, Ludwig-Maximilians-Universität, 80337 Munich, Germany; Psychiatry Neuroimaging Laboratory, Brigham and Women's Hospital, Harvard Medical School, Boston, MA 02215, USA; Department of Neurology, University Hospital, LMU Munich, 81377 Munich, Germany.
Rosanna Veggeberg, Psychiatry Neuroimaging Laboratory, Brigham and Women's Hospital, Harvard Medical School, Boston, MA 02215, USA.
Yorghos Tripodis, Department of Biostatistics, Boston University School of Public Health, Boston, MA 02118, USA; Boston University Alzheimer’s Disease Center and Boston University CTE Center, Boston University School of Medicine, Boston, MA 02118, USA.
Pawel P Wrobel, cBRAIN, Department of Child and Adolescent Psychiatry, Psychosomatics and Psychotherapy, Ludwig-Maximilians-Universität, 80337 Munich, Germany; Psychiatry Neuroimaging Laboratory, Brigham and Women's Hospital, Harvard Medical School, Boston, MA 02215, USA.
Janna Kochsiek, cBRAIN, Department of Child and Adolescent Psychiatry, Psychosomatics and Psychotherapy, Ludwig-Maximilians-Universität, 80337 Munich, Germany; Psychiatry Neuroimaging Laboratory, Brigham and Women's Hospital, Harvard Medical School, Boston, MA 02215, USA.
Brett M Martin, Data Coordinating Center, Boston University School of Public Health, Boston, MA 02118, USA.
Alexander P Lin, Psychiatry Neuroimaging Laboratory, Brigham and Women's Hospital, Harvard Medical School, Boston, MA 02215, USA; Department of Radiology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA; Center for Clinical Spectroscopy, Department of Radiology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA.
Michael J Coleman, Psychiatry Neuroimaging Laboratory, Brigham and Women's Hospital, Harvard Medical School, Boston, MA 02215, USA.
Michael L Alosco, Boston University Alzheimer’s Disease Center and Boston University CTE Center, Boston University School of Medicine, Boston, MA 02118, USA; Department of Neurology, Boston University School of Medicine, Boston, MA 02118, USA.
Ofer Pasternak, Psychiatry Neuroimaging Laboratory, Brigham and Women's Hospital, Harvard Medical School, Boston, MA 02215, USA; Department of Radiology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA.
Sylvain Bouix, Psychiatry Neuroimaging Laboratory, Brigham and Women's Hospital, Harvard Medical School, Boston, MA 02215, USA.
Robert A Stern, Boston University Alzheimer’s Disease Center and Boston University CTE Center, Boston University School of Medicine, Boston, MA 02118, USA; Department of Neurology, Boston University School of Medicine, Boston, MA 02118, USA; Department of Neurosurgery, Boston University School of Medicine, Boston, MA 02118, USA; Department of Anatomy and Neurobiology, Boston University School of Medicine, Boston, MA 02118, USA.
Martha E Shenton, Psychiatry Neuroimaging Laboratory, Brigham and Women's Hospital, Harvard Medical School, Boston, MA 02215, USA; Department of Radiology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA; VA Boston Healthcare System, Brockton Division, Brockton, MA 02301, USA.
Inga K Koerte, cBRAIN, Department of Child and Adolescent Psychiatry, Psychosomatics and Psychotherapy, Ludwig-Maximilians-Universität, 80337 Munich, Germany; Psychiatry Neuroimaging Laboratory, Brigham and Women's Hospital, Harvard Medical School, Boston, MA 02215, USA; Department of Psychiatry, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114, USA; Graduate School of Systemic Neurosciences, Ludwig-Maximilians-Universität, 82152 Munich, Germany.
References
- Adler CM, Delbello MP, Weber W, Williams M, Duran LRP, Fleck D, Boespflug E, Eliassen J, Strakowski SM, Divine J. 2018. MRI evidence of neuropathic changes in former college football players. Clin J Sport Med. 28:100–105. [DOI] [PubMed] [Google Scholar]
- Alosco ML, Jarnagin J, Tripodis Y, Platt M, Martin B, Chaisson CE, Baugh CM, Fritts NG, Cantu RC, Stern RA. 2017a. Olfactory function and associated clinical correlates in former National Football League players. J Neurotrauma. 34:772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alosco ML, Kasimis AB, Stamm JM, Chua AS, Baugh CM, Daneshvar DH, Robbins CA, Mariani M, Hayden J, Conneely Set al. . 2017b. Age of first exposure to American football and long-term neuropsychiatric and cognitive outcomes. Transl Psychiatry. 7:e1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alosco ML, Mez J, Tripodis Y, Kiernan PT, Abdolmohammadi B, Murphy L, Kowall NW, Stein TD, Huber BR, Goldstein LEet al. . 2018. Age of first exposure to tackle football and chronic traumatic encephalopathy. Ann Neurol. 83:886–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alosco ML, Stern RA. 2019. Youth exposure to repetitive head impacts from tackle football and long-term neurologic outcomes: a review of the literature, knowledge gaps and future directions, and societal and clinical implications. Semin Pediatr Neurol. 30:107–116. [DOI] [PubMed] [Google Scholar]
- Beason-Held LL, Purpura KP, Krasuski JS, Maisog JM, Daly EM, Mangot DJ, Desmond RE, Optican LM, Schapiro MB, Vanmeter JW. 1998. Cortical regions involved in visual texture perception: a fMRI study. Cogn Brain Res. 7:111–118. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri WF. 1996. Comparison of Beck depression inventories -IA and -II in psychiatric outpatients. J Pers Assess. 67:588–597. [DOI] [PubMed] [Google Scholar]
- Beck AT, Weissman A, Lester D, Trexler L. 1974. The measurement of pessimism: the hopelessness scale. J Consult Clin Psychol. 42:861–865. [DOI] [PubMed] [Google Scholar]
- Benton A, Hamsher K. 1989. Multilingual Aphasia Examination. AJA Associates, Iowa City, IA. [Google Scholar]
- Bogousslavsky J, Miklossy J, Deruaz JP, Assal G, Regli F. 1987. Lingual and fusiform gyri in visual processing: a clinico-pathologic study of superior altitudinal hemianopia. J Neurol Neurosurg Psychiatry. 50:607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Boisgueheneuc F, Levy R, Volle E, Seassau M, Duffau H, Kinkingnehun S, Samson Y, Zhang S, Dubois B. 2006. Functions of the left superior frontal gyrus in humans: a lesion study. Brain. 129:3315–3328. [DOI] [PubMed] [Google Scholar]
- Brown TT, Kuperman JM, Chung Y, Erhart M, McCabe C, Hagler DJ, Venkatraman VK, Akshoomoff N, Amaral DG, Bloss CSet al. . 2012. Neuroanatomical assessment of biological maturity. Curr Biol. 22:1693–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant BR, Narapareddy BR, Bray MJC, Richey LN, Krieg A, Shan G, Peters ME, Bernick CB. 2020. The effect of age of first exposure to competitive fighting on cognitive and other neuropsychiatric symptoms and brain volume. Int Rev Psychiatry. 32:89–95. [DOI] [PubMed] [Google Scholar]
- Caccese JB, DeWolf RM, Kaminski TW, Broglio SP, McAllister TW, McCrea M, Buckley TA, Hoy AM, Hazzard JB, Kelly LAet al. . 2019. Estimated age of first exposure to American football and neurocognitive performance amongst NCAA male student-athletes: a cohort study. Sports Med. 49:477–487. [DOI] [PubMed] [Google Scholar]
- Chang WP, Lu HC, Shyu BC. 2015. Treatment with direct-current stimulation against cingulate seizure-like activity induced by 4-aminopyridine and bicuculline in an in vitro mouse model. Exp Neurol. 265:180–192. [DOI] [PubMed] [Google Scholar]
- Covassin T, Elbin RJ, Harris W, Parker T, Kontos A. 2012. The role of age and sex in symptoms, neurocognitive performance, and postural stability in athletes after concussion. Am J Sports Med. 40:1303–1312. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. 1999. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage. 9:179–194. [DOI] [PubMed] [Google Scholar]
- Delis D, Kaplan E, Kramer J. 2001. Delis-Kaplan Executive Function System (D-KEFS). San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, Grodstein F, Wright CI, Blacker D, Rosas HDet al. . 2009. The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex. 19:497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskildsen SF, Coupé P, García-Lorenzo D, Fonov V, Pruessner JC, Collins DL. 2013. Prediction of Alzheimer’s disease in subjects with mild cognitive impairment from the ADNI cohort using patterns of cortical thinning. Neuroimage. 65:511–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Collins MW, Lovell MR, Maroon J. 2003. Does age play a role in recovery from sports-related concussion? A comparison of high school and collegiate athletes. J Pediatr. 142:546–553. [DOI] [PubMed] [Google Scholar]
- Fischl B. 2012. FreeSurfer. Neuroimage. 62:774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM. 2000. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 97:11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. 1999. Cortical surface-based analysis: II. Inflation, flattening, and a surface-based coordinate system. Neuroimage. 9:195–207. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat DH, Greve DN, Fischl Bet al. . 2009. High consistency of regional cortical thinning in aging across multiple samples. Cereb Cortex. 19:2001–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN. 2008. The teen brain: insights from neuroimaging. J Adolesc Health. 42:335–343. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, Herman DH, Clasen LS, Toga AWet al. . 2004. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 101:8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami R, Dufort P, Tartaglia MC, Green RE, Crawley A, Tator CH, Wennberg R, Mikulis DJ, Keightley M, Davis KD. 2016. Frontotemporal correlates of impulsivity and machine learning in retired professional athletes with a history of multiple concussions. Brain Struct Funct. 221:1911–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. 1960. A rating scale for depression. J Neurol Neurosurg Psychiatry. 23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Ide JS, Zhang S, Li CSR. 2016. The right superior frontal gyrus and individual variation in proactive control of impulsive response. J Neurosci. 36:12688–12696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. 1997. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 387:167–178. [DOI] [PubMed] [Google Scholar]
- Kang SH, Park YH, Lee D, Kim JP, Chin J, Ahn Y, Park SB, Kim HJ, Jang H, Jung YHet al. . 2019. The cortical neuroanatomy related to specific neuropsychological deficits in Alzheimer’s continuum. Dement Neurocognitive Disord. 18:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerte IK, Mayinger M, Muehlmann M, Kaufmann D, Lin AP, Steffinger D, Fisch B, Rauchmann BS, Immler S, Karch Set al. . 2016. Cortical thinning in former professional soccer players. Brain Imaging Behav. 10:792–798. [DOI] [PubMed] [Google Scholar]
- Lauer J, Moreno-López L, Manktelow A, Carroll EL, Outtrim JG, Coles JP, Newcombe VF, Sahakian BJ, Menon DK, Stamatakis EA. 2017. Neural correlates of visual memory in patients with diffuse axonal injury. Brain Inj. 31:1513–1520. [DOI] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. 2008. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 40:1044–1055. [DOI] [PubMed] [Google Scholar]
- Lerch JP, Pruessner J, Zijdenbos AP, Collins DL, Teipel SJ, Hampel H, Evans AC. 2008. Automated cortical thickness measurements from MRI can accurately separate Alzheimer’s patients from normal elderly controls. Neurobiol Aging. 29:23–30. [DOI] [PubMed] [Google Scholar]
- Lerch JP, Pruessner JC, Zijdenbos A, Hampel H, Teipel SJ, Evans AC. 2005. Focal decline of cortical thickness in Alzheimer’s disease identified by computational neuroanatomy. Cereb Cortex. 15:995–1001. [DOI] [PubMed] [Google Scholar]
- Leunissen I, Coxon JP, Caeyenberghs K, Michiels K, Sunaert S, Swinnen SP. 2014. Task switching in traumatic brain injury relates to cortico-subcortical integrity. Hum Brain Mapp. 35:2459–2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machielsen WCM, Rombouts SARB, Barkhof F, Scheltens P, Witter MP. 2000. fMRI of visual encoding: reproducibility of activation. Hum Brain Mapp. 9:156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin RS. 1996. Apathy: concept, syndrome, neural mechanisms, and treatment. Semin Clin Neuropsychiatry. 1:304–314. [DOI] [PubMed] [Google Scholar]
- Moore DR, Pindus DM, Raine LB, Drollette ES, Scudder MR, Ellemberg D, Hillman CH. 2016. The persistent influence of concussion on attention, executive control and neuroelectric function in preadolescent children. Int J Psychophysiol. 99:85–95. [DOI] [PubMed] [Google Scholar]
- Nathanson JT, Connolly JG, Yuk F, Gometz A, Rasouli J, Lovell M, Choudhri T. 2016. Concussion incidence in professional football. Orthop J Sports Med. 4:232596711562262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. 1995. Factor structure of the barratt impulsiveness scale. J Clin Psychol. 51:768–774. [DOI] [PubMed] [Google Scholar]
- Pellman EJ, Viano DC, Tucker AM, Casson IR, Waeckerle JF, Maroon JC, Lovell MR, Collins MW, Kelly DF, Valadka ABet al. . 2003. Concussion in professional football: reconstruction of game impacts and injuries. Neurosurgery. 53:799–814. [DOI] [PubMed] [Google Scholar]
- Petanjek Z, Judaš M, Šimić G, Rašin MR, Uylings HBM, Rakic P, Kostović I. 2011. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci U S A. 108:13281–13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petermann F, Jäncke L. 2016. Neuropsychological assessment battery (NAB) - Aussagekraft und anwendungen der deutschsprachigen adaptation. Zeitschrift fur Neuropsychol. 27:129–131. [Google Scholar]
- Plessow F, Pascual-Leone A, McCracken CM, Baker J, Krishnan S, Baggish A, Connor A, Courtney TK, Nadler LM, Speizer FEet al. . 2020. Self-reported cognitive function and mental health diagnoses among former professional American-style football players. J Neurotrauma. 37:1021–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querbes O, Aubry F, Pariente J, Lotterie JA, Dmonet JF, Duret V, Puel M, Berry I, Fort JC, Celsis P. 2009. Early diagnosis of Alzheimers disease using cortical thickness: impact of cognitive reserve. Brain. 132:2036–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. 1977. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Measur. 1:385–401. [Google Scholar]
- Roberts AL, Pascual-Leone A, Speizer FE, Zafonte RD, Baggish AL, Taylor H, Nadler LM, Courtney TK, Connor A, Grashow Ret al. . 2019. Exposure to American football and neuropsychiatric health in former National Football League players: findings from the football players health study. Am J Sports Med. 47:2871–2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth R, Isquith P, Gioia G. 2005. Behavior Rating Inventory of Executive Function - Adult Version (BRIEF-A). Lutz, FL: Psychological Assessment Resources. [Google Scholar]
- Sadeh N, Spielberg JM, Miller MW, Milberg WP, Salat DH, Amick MM, Fortier CB, McGlinchey RE. 2015. Neurobiological indicators of disinhibition in posttraumatic stress disorder. Hum Brain Mapp. 36:3076–3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz V, Stern RA, Tripodis Y, Stamm J, Wrobel P, Lepage C, Weir I, Guenette JP, Chua A, Alosco MLet al. . 2018. Age at first exposure to repetitive head impacts is associated with smaller thalamic volumes in former professional American football players. J Neurotrauma. 35:278–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon G. 2018. Chronic traumatic encephalopathy in sports: a historical and narrative review. Dev Neuropsychol. 43:279–311. [DOI] [PubMed] [Google Scholar]
- Solomon GS, Kuhn AW, Zuckerman SL, Casson IR, Viano DC, Lovell MR, Sills AK. 2016. Participation in pre-high school football and neurological, neuroradiological, and neuropsychological findings in later life. Am J Sports Med. 44:1106–1115. [DOI] [PubMed] [Google Scholar]
- Stamm JM, Bourlas AP, Baugh CM, Fritts NG, Daneshvar DH, Martin BM, McClean MD, Tripodis Y, Stern RA. 2015a. Age of first exposure to football and later-life cognitive impairment in former NFL players. Neurology. 84:1114–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamm JM, Koerte IK, Muehlmann M, Pasternak O, Bourlas AP, Baugh CM, Giwerc MY, Zhu A, Coleman MJ, Bouix Set al. . 2015b. Age at first exposure to football is associated with altered Corpus callosum white matter microstructure in former professional football players. J Neurotrauma. 32:1768–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern R, Javorsky D, Singer E, Singer N, Duke L, Somerville J, Thompson J, Kaplan E. 1999. Boston Qualitative Scoring System (BQSS) for the Rey-Osterrieth Complex Figure. Odessa, FL: Psychological Assessment Resources (PAR). [Google Scholar]
- Tamnes CK, Herting MM, Goddings AL, Meuwese R, Blakemore SJ, Dahl RE, Güroğlu B, Raznahan A, Sowell ER, Crone EAet al. . 2017. Development of the cerebral cortex across adolescence: a multisample study of inter-related longitudinal changes in cortical volume, surface area, and thickness. J Neurosci. 37:3402–3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes CK, Østby Y, Fjell AM, Westlye LT, Due-Tønnessen P, Walhovd KB. 2010. Brain maturation in adolescence and young adulthood: regional age-related changes in cortical thickness and white matter volume and microstructure. Cereb Cortex. 20:534–548. [DOI] [PubMed] [Google Scholar]
- Thambisetty M, Wan J, Carass A, An Y, Prince JL, Resnick SM. 2010. Longitudinal changes in cortical thickness associated with normal aging. Neuroimage. 52:1215–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. 1997. WAIS-III Administration and Scoring Manual. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Whitford TJ, Rennie CJ, Grieve SM, Clark CR, Gordon E, Williams LM. 2007. Brain maturation in adolescence: concurrent changes in neuroanatomy and neurophysiology. Hum Brain Mapp. 28:228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtowicz M, Gardner AJ, Stanwell P, Zafonte R, Dickerson BC, Iverson GL. 2018. Cortical thickness and subcortical brain volumes in professional rugby league players. NeuroImage Clin. 18:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman S, Lee Y, Odom M, Solomon G, Sills A, Forbes J. 2012. Recovery from sports-related concussion: days to return to neurocognitive baseline in adolescents versus young adults. Surg Neurol Int. 3:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


