Abstract
Loneliness and wisdom have opposing impacts on health and well-being, yet their neuro-cognitive bases have never been simultaneously investigated. In this study of 147 healthy human subjects sampled across the adult lifespan, we simultaneously studied the cognitive and neural correlates of loneliness and wisdom in the context of an emotion bias task. Aligned with the social threat framework of loneliness, we found that loneliness was associated with reduced speed of processing when angry emotional stimuli were presented to bias cognition. In contrast, we found that wisdom was associated with greater speed of processing when happy emotions biased cognition. Source models of electroencephalographic data showed that loneliness was specifically associated with enhanced angry stimulus-driven theta activity in the left transverse temporal region of interest, which is located in the area of the temporoparietal junction (TPJ), while wisdom was specifically related to increased TPJ theta activity during happy stimulus processing. Additionally, enhanced attentiveness to threatening stimuli for lonelier individuals was observed as greater beta activity in left superior parietal cortex, while wisdom significantly related to enhanced happy stimulus-evoked alpha activity in the left insula. Our results demonstrate emotion-context driven modulations in cognitive neural circuits by loneliness versus wisdom.
Keywords: emotion bias, temporo-parietal junction, insula, EEG, happiness, social threat
Introduction
The concept of loneliness has been part of human history since ancient times, described by Ovid in 80 AD as the experience of painful, intense longing for social connection (Ovid 2005). Loneliness is distinct from an individual’s objective social network in that it reflects the individual’s subjective distress about their extent of social isolation, hence, the phrase “alone in a crowd” (Cacioppo et al. 2015; Bhatti and ul Haq 2017). Psychological research also suggests that loneliness results from the discrepancy between the interpersonal interactions that are desired versus those that are achieved (Peplaum et al. 1979). Research over the last decade has shown that loneliness is an important psychosocial determinant of health; it is associated with considerable physical and mental health risks, acute stress reactivity and immune system dysfunction, increased risk for suicide, accelerated cognitive decline and dementia, and consequently, heightened morbidity and mortality (Cacioppo et al. 2011; Perissinotto et al. 2012; Boss et al. 2015; Holt-Lunstad et al. 2015; Kuiper et al. 2015; Beutel et al. 2017; Brown et al. 2018; Lara et al. 2019; Jeste, Lee, and Cacioppo 2020). Hence, it is vital to understand the neurobiological bases of loneliness that are the focus of recent research (Weiss 1974; Cacioppo, Capitanio, et al. 2014; Spreng et al. 2020).
Neurobiologically, loneliness is considered within a social evolutionary framework (Cacioppo, Hawkley, et al. 2006; Cacioppo, Capitanio, et al. 2014). According to the social neuroscience model, the brain evolved to put individuals into a short-term self-preservation mode when alone without any mutual protection or assistance. Important among the range of behavioral effects are heightened implicit vigilance for social threats along with increased anxiety, hostility, and social withdrawal to avoid predation (Meng et al. 2020). Notably, increased depressive symptomatology is observed with heightened loneliness as a nonverbal means of signaling the need for support and connection (Cacioppo, Hughes, et al. 2006; Qualter et al. 2010; Cacioppo et al. 2015; Fried et al. 2015). The priming for social threats, in turn, may lead to attentional biases such that the individual acts negatively toward others and may fuel further feelings of social isolation (Rotenberg et al. 2002; Cacioppo, Cacioppo, et al. 2014). Evidence from functional magnetic resonance imaging and electroencephalography (EEG) studies suggest that loneliness enhances attention to negative social stimuli (Somerville et al. 2006; Cacioppo et al. 2009, 2013, 2016; Cacioppo, Balogh, et al. 2015). This research implicates activity in brain regions that are involved in both attentive and social processing with loneliness, with the temporoparietal junction (TPJ) as a key node for perceiving social-affective states (Cacioppo et al. 2009, 2016; Zaki et al. 2009; Kanai et al. 2012; Cacioppo, Cacioppo, et al. 2014; Cacioppo, Capitanio, et al. 2014).
While loneliness maybe a negative trait, at the opposite end of the spectrum is the positive personality trait of wisdom. Wisdom can be defined as the ability to think and act using knowledge, experience, insight, and empathy toward others (Sternberg 1990; Ardelt 2000; Baltes and Staudinger 2000; Jeste, Lee, Palmer, et al. 2020). Based on systematic literature reviews, wisdom is a complex trait that includes dimensions of pro-social behaviors, such as empathy and compassion, emotional regulation, self-reflection, a balance between acceptance of uncertainty and decisiveness, and social advising (Meeks and Jeste 2009; Bangen et al. 2013; Jeste and Lee 2019; Lee et al. 2019, 2020; Jeste, Di Somma, et al. 2020). Notably, wisdom is consistently associated with better health and subjective well-being (Webster et al. 2014; Ardelt and Edwards 2016; Ardelt and Jeste 2018; Ardelt and Ferrari 2019; Lee et al. 2019; Van Patten et al. 2019). This research also shows that both loneliness and wisdom are partially malleable traits (Lee et al. 2019, 2020), and that there is an inverse relationship between wisdom and loneliness across the adult lifespan, now replicated across several studies (Lee et al. 2019; Jeste, Di Somma, et al. 2020; Nguyen et al. 2020). Complementary qualitative research suggests that loneliness is linked to reduced compassion and other components of wisdom, due to negative biases about oneself and others, leading to avoidance of social interactions (Morlett Paredes et al. 2020). Thus, while loneliness and wisdom may appear to be disparate constructs at the outset, an underlying sense of wisdom in social contexts, whether one is physically alone or with others, may impart immunity to the perception of loneliness (Ardelt and Jeste 2018). That wisdom may serve as a protective factor against loneliness that highlights the importance of studying the neural and cognitive correlates of these two constructs within a unified framework. However, no study to-date has simultaneously probed the neuro-cognitive correlates of loneliness and wisdom.
Here, we undertook an adult lifespan study investigating the association of loneliness and wisdom with cognitive/neural processing during an emotion bias task. This task probed attention to target in the presence of different emotional stimuli, including positive, negative, threatening, and neutral emotion stimuli that served as distractors on the task (López-Martín et al. 2013, 2015; Thai et al. 2016). The different types of emotions embedded in the task, thereby, allowed investigation of differential emotional responsivity in the context of loneliness versus wisdom.
Aligned with the social threat model for loneliness (Cacioppo et al. 2016), we hypothesized that loneliness would affect cognitive and neural processing when threatening emotional distractors were presented. Specifically, in the context of the emotion bias task, we hypothesized that threatening faces would be more distracting for lonelier individuals, and hence, greater loneliness would be associated with reduced response speed to the attended target. In contrast, given that pro-social empathic behaviors underlie wisdom, we hypothesized an association of wisdom with the cognitive and neural processing of positive emotion stimuli. Specifically, we hypothesized that in the presence of happy emotion stimuli, individuals reporting greater wisdom would be more alert and thereby have accelerated attentive response speeds.
Neurally, brain networks underlying social-affective processing, attentive processing, and most recently the default mode network have been associated with loneliness (Cacioppo et al. 2009, 2016; Kanai et al. 2012; Cacioppo, Cacioppo, et al. 2014; Cacioppo, Capitanio, et al. 2014; Layden et al. 2017; Spreng et al. 2020). Across these studies, both task-related and resting state neural activities have been investigated. Here, we inform this literature by exploring neural processing on an emotion bias task that included both threatening and positive stimuli. In this context, we hypothesized that loneliness would be associated with neural activity in brain regions associated with social-emotional affective processing, such as the TPJ, and with attentional allocation to the threatening emotions.
With regards to wisdom, while there has not been a prior study investigating the neural correlates of this construct, the components of wisdom have been related to activity in brain regions important for cognitive and socio-emotional control, with a prominent role for the insula that is implicated in empathy, as well as emotional and cognitive regulation (Adolphs 2002; Carr et al. 2003; Hennenlotter et al. 2005; Craig 2009; Meeks and Jeste 2009; Menon and Uddin 2010; Salomon et al. 2016; Gogolla 2017). Hence, for wisdom, we hypothesized an association with cognitive and neural processing of positive emotion stimuli with particular involvement of the insula. Prior studies specifically show that individuals with greater self-reported empathy, which is the prosocial component of wisdom, have higher activity within insular cortex when processing positive stimuli. Thus, within the unifying framework of the same emotion bias task, yet in the context of different emotions, threatening versus positive, we suggest distinct cognitive and neural correlates for the constructs of loneliness and wisdom.
Materials and Methods
Participants
A total of 147 healthy adult human subjects (mean and standard deviation [SD] of age 40.7 ± 22.6 years, age range 18–84 years, 87 [59%] females) participated in this cross-sectional study and completed behavioral self-report assessments and an emotion bias cognitive task with simultaneous EEG. Participants were recruited using the Research Match registry for research participation and using on-campus flyers at the University of California at San Diego. Inclusion/exclusion criteria for the study were participant age of 18 years or older, and healthy adult status with no current diagnosis of a major neuropsychiatric diagnosis and/or current use of psychotropic medications. For older adults >60 years of age, participants were confirmed to have a Mini-Mental State Examination score >26 to verify the absence of apparent cognitive impairment (Arevalo-Rodriguez et al. 2015). All participants had normal/corrected-to-normal vision and hearing and no participant reported color blindness. Majority of participants (139 of 147) were right-handed. All participants had at least a high-school education. All participants provided written informed consent for the study protocol approved by the University of California at San Diego institutional review board (UCSD IRB #180140). Five additional participants were excluded from the study who had a clinical psychiatric diagnosis and were also on a current neuropsychiatric medication regimen. The study did not use any specific cut-off thresholds for psychiatric disorders, other than the participants self-reporting that they had a clinical diagnosis and were taking psychotropic medications. All data were collected in the year prior to COVID-19 research restrictions.
Behavioral Assessments
Participants reported basic demographics, including age, gender, race, and socio-economic status (SES). Race was reported as 1 of 7 categories (Caucasian; Black/African American; Native Hawaiian/Other Pacific Islander; Asian; American Indian/Alaska Native; More than one race; Unknown or not reported). SES composite scores were assessed using the family affluence scale; this scale measures individual wealth based on ownership of objects of value (e.g., car/computer) and produces a composite score ranging from 0 (low affluence) to 9 (high affluence) (Boudreau and Poulin 2009). In addition, all participants completed self-report ratings of anxiety on the Generalized Anxiety Disorder 7-item scale: GAD-7 (Spitzer et al. 2006), and ratings of depression on the 9-item Patient Health Questionnaire: PHQ-9 (Kroenke et al. 2001). Additionally, all participants reported loneliness on the 20-item UCLA-3 Loneliness Scale (Russell 1996), and rated wisdom on the 24-item San Diego Wisdom Scale (SD-WISE; Thomas et al. 2019). The SD-WISE scale was developed based on the psychological and neurobiological models of wisdom and is a measurement model composed of individual wisdom subdomains of decisiveness, emotional regulation, insight, pro-social behaviors, self-reflection, social advising, and tolerance for divergent values. It has been shown to be a reliable psychometric measure with convergent and discriminant validity, and social desirability bias is not found to be a significant factor in scale completion (Thomas et al. 2019). Participants also completed the 6-item Lubben Social Network Scale (Lubben 1988, 2006).
Emotional Bias Assessment
Participants played a game-like assessment named “Face Off,” adapted from prior studies of attention bias in emotional contexts (López-Martín et al. 2013, 2015; Thai et al. 2016). The task integrated a standardized set of culturally diverse faces from the NimStim database (Tottenham et al. 2009). We used an equivalent number of male and female faces, each face with four sets of emotions, either neutral, positive (happy), negative (sad), or threatening (angry), presented on equivalent number of trials. An arrow was superimposed on the face on each trial, occurring either in the upper or lower central visual field on equal number of trials, and participants responded to the direction of the arrow (left/right). Participants completed 144 trials presented over three equipartitioned blocks with shuffled, but equivalent number of emotion trials in each block; a practice set of 4-trials preceded the main task. Each trial initiated with a central fixation “+” for 500 ms followed by a face stimulus with a superimposed arrow of 300-ms duration. Participants responded within an ensuing 1-s response window, followed by positive or negative feedback provided for accuracy. Feedback was provided in the form of a happy/sad emoticon for 200-ms duration followed by a 500-ms intertrial interval (ITI). All participants received summary block accuracy feedback. This assessment was deployed on the “BrainE” Unity-based platform developed by the Neural Engineering & Translation Labs (NEATLabs) (Misra et al. 2018; Balasubramani et al. 2020). The Lab Streaming Layer (LSL; Kothe et al. 2019) protocol was used to time-stamp each stimulus/response event during the task. Study participants engaged with the assessment on a Windows-10 laptop sitting at a comfortable viewing distance. Figure 1A summarizes the task design and trial timings.
Figure 1.
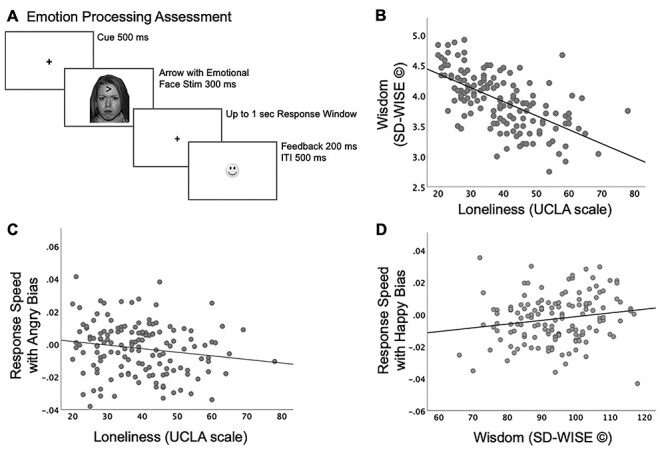
(A) Emotion bias task design. (B) Relationship between wisdom versus loneliness (n = 147, rs = −0.632, P < 0.0001). (C) Relationship between loneliness and response speed in the presence of angry emotion distractors (rs = −0.179, P = 0.03). (D) Relationship between wisdom and response speed in the presence of happy emotion distractors (rs = 0.196, P = 0.02). Response speeds in the presence of angry/happy distractors are calculated relative to speed when neutral emotion stimuli were presented to each subject.
Electroencephalography
EEG data were collected simultaneous to the emotional bias assessment using a 24-channel SMARTING device with a semi-dry and wireless electrode layout. Data were acquired at 500-Hz sampling frequency at 24-bit resolution. Cognitive event markers were integrated using LSL and data files were stored in xdf format.
Cognitive Task Performance Metrics
Cognitive task data were analyzed for each type of emotional stimulus, that is, neutral, happy, sad, and angry. For each stimulus, signal detection sensitivity was computed as d’ = z(Hits) − z(False Alarms) (Heeger and Landy 2009); all d’ values were divided by max theoretical d’ of 4.65 to obtain scaled d’ in the 0–1 range. Stimulus response speeds were calculated as log10(1/RT), where RT is response time in milliseconds. As there can be general speed of processing differences across the lifespan, we analyzed all behavioral metrics for the emotional stimuli (happy/sad/angry) relative to performance on the neutral emotion stimuli in each participant. Outliers >3 median absolute deviations away from the median of each performance metric across subjects were removed prior to statistical analyses.
Neural Analyses
We applied a uniform processing pipeline to all EEG data acquired simultaneous to the cognitive task (Balasubramani et al. 2020). This included: 1) data preprocessing, 2) computing event-related spectral perturbations (ERSP) for all channels, and 3) cortical source localization of the EEG data filtered within relevant theta (3–7 Hz), alpha (8–12 Hz), and beta (13–30 Hz) frequency bands.
1) Data preprocessing was conducted using the EEGLAB toolbox in MATLAB (Delorme and Makeig 2004). EEG data were resampled at 250 Hz and filtered in the 1–45 Hz range to exclude ultraslow DC drifts at <1 Hz and high-frequency noise produced by muscle movements and external electrical sources at >45 Hz. EEG data were average referenced and epoched to the emotional task stimuli as informed by the LSL time-stamps in the −1.5- to +1.5-s stimulus time window. Channel data with activity >5 SD compared with mean activity (up to 3 channels in 3 participants) were spherically interpolated to nearest neighbors to correct for the outlier activity. Epoched data were cleaned using the autorej function in EEGLAB to remove noisy trials (>5 SD outliers rejected over max 8 iterations; 6.3 ± 5.6% of trials rejected per participant). Additionally, EEG data were cleaned by excluding signals estimated to be originating from nonbrain sources, such as electrooculographic, electromyographic, or unknown sources, using the Sparse Bayesian learning (SBL) algorithm (https://github.com/aojeda/PEB) explained below (Ojeda et al. 2018, 2019).
2) For ERSP calculations, we performed time–frequency decomposition of the epoched data using the continuous wavelet transform (cwt) function in MATLAB’s signal processing toolbox. Baseline time–frequency data in the −750- to −550-ms time window prior to stimulus presentation were subtracted from the epoched trials (at each frequency) to observe the event-related synchronization (ERS) and event-related desynchronization modulations (Pfurtscheller 1999).
3) Cortical source localization was performed to map the underlying neural source activations for the ERSPs using the block-SBL algorithm (Ojeda et al. 2019) implemented in a recursive fashion (Ojeda et al. 2018, 2019). This is a two-step algorithm in which the first-step is equivalent to the conventionally applied low-resolution electromagnetic tomography method (LORETA; Pascual-Marqui et al. 1994). LORETA estimates sources subject to smoothness constraints, that is, nearby sources tend to be co-activated, which may produce source estimates with a high number of false positives that are not biologically plausible. To guard against this, SBL applies sparsity constraints in the second step wherein blocks of irrelevant sources are pruned. Source space activations were estimated and the root mean square signals were partitioned into cortical regions of interest (ROIs) and artifact sources. ROIs were based on the standard 68 brain region Desikan–Killiany atlas (Desikan et al. 2006) using the Colin-27 head model (Holmes et al. 1998). Activations from artifact sources contributing to EEG noise from nonbrain sources, such as electrooculographic, electromyographic, or unknown sources, were removed to clean the EEG data. Cleaned subject-wise trial-averaged EEG data were then specifically filtered in theta, alpha, and beta bands and separately source localized in each of the three frequency bands to estimate their band-specific cortical ROI source signals. The source signal envelopes were computed in MATLAB (envelop function) by a spline interpolation over the local maxima separated by at least one-time sample; we used this spectral amplitude signal for all neural analyses presented here. We focused on poststimulus encoding in the 100–500 ms range for all frequency bands; this epoch window was chosen as it encompassed the peak global activity of emotional stimulus processing across all stimuli yet did not overlap with motor responses that may generate artifacts; we confirmed that there were no significant differences in global peak neural processing latencies for the different emotion stimuli across subjects. Additionally, we performed outlier removal on the cortical source level data; outlier values determined to be greater than 5 SD away from the mean cortical activity within that ROI were removed from analyses.
Statistical Analyses
We used linear multiple regression to parse the demographic and behavioral self-report factor predictors of loneliness and wisdom. Modeled factors included demographic factors of age, gender, race, SES, mental health factors of anxiety and depression, as well as social network score. Additionally, wisdom was included as a predictor in the loneliness model, and vice versa. Loneliness and wisdom were log-transformed to obtain normal target distributions.
The performance metrics on the emotion bias task for happy/angry/sad stimuli were all calculated relative to performance on neutral stimuli to minimize the effect of age-differences across participants. These performance metrics on the happy/angry/sad stimuli were then compared using repeated measures analyses of variance (rm-ANOVA) with covariates of age and gender. Emotion-related performance metrics were correlated with loneliness and wisdom, and also with anxiety/depression symptom scores using Spearman’s correlations; we confirmed that Spearman correlations were appropriate based on the Anderson–Darling test for normality (Anderson and Darling 1952).
Neural source activations in the theta, alpha, and beta frequency bands were related to loneliness and wisdom using linear robust regression models. Loneliness and wisdom were log-transformed to obtain normal target distributions. The neural models included covariates as informed by the significant covariates that emerged in the behavioral regression models above across the entire age range. Specifically, the neural model for loneliness accounted for covariates of social network score and wisdom, and similarly, the neural model for wisdom accounted for the covariates of social network score and loneliness. Significant neural activations from independent robust regression models were then fit in a final multiple linear robust regression, to account for comparisons across multiple source activations. Furthermore, to confirm the robustness of the neural models, we checked for consistency of results with and without outliers removed. For all regression models, effect sizes for significant predictors are reported in the text as f2 (0.02 small, 0.15 medium, and 0.35 large) (Selya et al. 2012).
Results
Demographic and Behavioral Predictors of Loneliness and Wisdom
Distributions for the demographic and behavioral self-reports for the 147 study participants are shown in Table 1. Metrics are shown separated by younger, middle-aged, and older adults, and across all participants. The younger adult age bin cut-off was based on the literature on neurodevelopmental trajectories (Lebel et al. 2008; Insel 2014; BrainSpan: Atlas of the Developing Human Brain 2020). Metrics were compared for differences across age bins using the χ2 (chi-square) statistic for gender and the nonparametric Kruskal–Wallis test for behavioral self-reports. Loneliness, wisdom, anxiety, and depression were all significantly different (P < 0.02) across age bins, while gender and social network scores did not statistically differ across age bins. In post-hoc Mann–Whitney tests, younger adults reported significantly greater loneliness than both middle-aged and older adults (P < 0.048); there were no differences in loneliness reported by middle-aged and older adults (P = 0.28). For wisdom, older adults reported significantly higher wisdom than younger adults (P = 0.005), and there were no other significant differences for wisdom across age bins. Significantly different anxiety scores were found for each age bin, with younger adults reporting highest symptom severity compared with both middle-aged and older adults (P < 0.006); additionally, middle-aged adults showed greater anxiety than older adults (P = 0.014). Similarly, depression scores were highest for younger adults relative to both middle-aged and older adults (P < 0.047); there were no significant differences in depression scores between middle-aged and older adults (P = 0.14).
Table 1.
Demographic and behavioral self-reports for younger, middle-aged, and older adults, as well as across all study participants (n = 147). Apart from count and gender, all metrics are reported as mean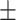 SD. Metrics were compared for differences across age bins using the χ2 (chi-square) statistic for gender and the nonparametric Kruskal–Wallis test for behavioral self-reports
SD. Metrics were compared for differences across age bins using the χ2 (chi-square) statistic for gender and the nonparametric Kruskal–Wallis test for behavioral self-reports
| Demographics and behaviors | 18–25 | 26–64 | 65–84 | All | P-value |
|---|---|---|---|---|---|
| Count (%) Gender (Male %) Loneliness Wisdom Anxiety Depression Social network |
62 (42.2) 25 (40.3) 42.19 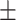 10.56 10.563.82 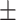 0.40 0.404.43 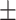 3.61 3.614.46 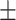 4.22 4.2217.55 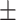 5.33 5.33 |
42 (28.6) 16 (38.1) 38.48 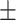 12.34 12.343.90 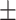 0.44 0.443.02 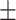 4.17 4.172.86 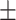 3.87 3.8716.86 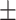 6.38 6.38 |
43 (29.3) 19 (44.2) 36.12 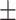 12.77 12.774.06 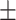 0.50 0.501.28 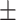 2.13 2.131.91 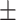 2.91 2.9117.48 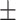 5.71 5.71 |
147 60 (40.8) 39.35 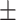 11.96 11.963.92 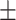 0.45 0.453.10 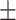 3.65 3.653.25 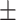 3.91 3.9117.33 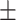 5.73 5.73 |
0.85 0.01 0.02 <0.0001 0.003 0.88 |
We investigated demographic and behavioral factors determining loneliness and wisdom using multiple linear regression models. The model for loneliness included demographic factors of age, gender, race, SES, and mental health factors of anxiety and depression, as well as social network score, and wisdom. Similarly, the model for wisdom included the four demographic factors, two mental health factors, social network, and loneliness scores.
The overall model for loneliness was significant (R2=0.56, F8,133=21.23, P < 0.0001, Supplementary Table 1). Only two factors, social network (f2 = 0.20, β = −0.018, 95% CI [−0.025, −0.012], P < 0.0001) and wisdom (f2 = 0.27, β = −0.276, 95% CI [−0.367, −0.185], P < 0.0001) significantly predicted loneliness across the entire adult lifespan; f2 denotes effect size. Neither demographic nor mental health factors significantly predicted loneliness in this healthy adult population, although a positive trend toward significance was observed for depression severity (f2 = 0.03, β = 0.012, 95% CI [−0.001, 0.025], P = 0.067). Wisdom was a consistently significant negative predictor of loneliness for all age groups; social network score was significant for younger and middle-aged adults but not older adults (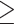 65 years old). In younger adults, depression and race emerged as additionally significant predictors (Supplementary Table 1).
65 years old). In younger adults, depression and race emerged as additionally significant predictors (Supplementary Table 1).
The overall model for wisdom across the adult lifespan was significant (R2=0.39, F8,133=10.82, P < 0.0001, Supplementary Table 1). Only loneliness consistently emerged as a significant factor predictor for wisdom (f2 = 0.21, β = −0.005, 95% CI [−0.006, −0.003], P < 0.0001). Neither demographic nor mental health factors significantly predicted wisdom when all ages were included in the model. Gender was an additional significant predictor only in middle-aged adults, and anxiety was a significant negative predictor only in older adults (Supplementary Table 1).
Figure 1B shows the inverse relationship for wisdom versus loneliness in our sample. Additionally, we correlated loneliness to the wisdom subscales; in order of negative correlation strength with loneliness, these wisdom subscales were pro-social behaviors (Spearman’s rs = −0.645, P < 0.0001), decisiveness (rs = −0.527, P < 0.0001), emotional regulation (rs = −0.511, P < 0.0001), social advising (rs = −0.360, P < 0.0001), insight (rs = −0.242, P < 0.003), and tolerance for divergent values (rs = −0.107, P = 0.2).
Emotion Bias Performance and Relationship with Loneliness and Wisdom
Signal detection sensitivity (d’) and speed for the three task emotions, that is, happy, angry, and sad, each calculated relative to neutral stimuli are shown in Table 2. In rm-ANOVA analyses across emotions, d’ was not different (P > 0.67), but speed was significantly different with the slowest response speeds observed for sad stimuli relative to happy/angry stimuli (F2,278 = 6.70, P = 0.003). Age and gender were not significant covariates of these d’ and speed performance metrics.
Table 2.
Behavioral performance across emotional stimuli for all participants (n = 147), as mean ± SD. All performance metrics for each participant were calculated relative to performance on neutral emotion stimuli. For reference, performance metrics (mean ± SD) on neutral stimuli were: scaled d’ = 0.77 ± 0.18 and speed = 0.28 ± 0.07. Scaled d’ represents d’ as a ratio of max theoretical d’ of 4.65 and units of speed are log10(per second).
| Emotion stimulus | Scaled d’ mean ± SD |
Speed mean ± SD |
|---|---|---|
| Happy | −0.051 ± 0.212 | −0.003 ± 0.015 |
| Angry | −0.043 ± 0.196 | −0.003 ± 0.016 |
| Sad | −0.040 ± 0.205 | −0.006 ± 0.017 |
Consistent with our hypothesis that extent of loneliness would relate to processing of threatening stimuli, we found that when angry emotion distractors were present, attentive response speed negatively correlated with loneliness, that is, individuals across our entire sample reporting greater loneliness had reduced attentive response speed in the presence of angry distractors (rs = −0.179, P = 0.03, Fig. 1C). Notably, this correlation was only observed with loneliness but not wisdom. For wisdom, as hypothesized, we found a relationship with responses when happy stimuli were presented, specifically greater speed of responding was observed for happy emotion bias associated with greater wisdom (rs = 0.196, P = 0.02, Fig. 1D). Exploratory analyses confirmed that the data showed no other loneliness/wisdom cognitive correlates. These cognitive performance metrics also did not correlate with mental health symptom scores for anxiety/depression in our healthy adult sample. Finally, among the five wisdom subscales, response speed on happy trials was significantly correlated with decisiveness (rs = 0.22, P = 0.008) and social advising (rs = 0.19, P = 0.03).
Neural Processing and Relationship with Loneliness and Wisdom
Given that the cognitive results verified our hypotheses that loneliness would relate to angry emotion driven bias and wisdom would relate to positive (i.e., happy) emotion bias, we analyzed the corresponding neural processing of these stimuli and their relationship with loneliness and wisdom, respectively. Again to minimize the effect of age, we processed contrast brain maps for both angry and happy stimuli relative to neutral stimulus processing. Since loneliness, wisdom, and social network score were interrelated factors in the behavioral models across all ages, we controlled for these in the neural robust regression models. That is, the neural model for loneliness versus angry emotion bias controlled for wisdom and social network score. Similarly, the neural model for wisdom versus happy emotion bias processing controlled for loneliness and social network score. These neural models did not include the covariates of demographics or mental health factors as these variables were not significantly related to loneliness or wisdom when considering the entire sample (see Demographic and Behavioral Predictors of Loneliness and Wisdom). Additionally, we focused the neural analyses on spectral activity in the theta, alpha, and beta frequency bands because these provide greater specificity in terms of neural oscillations and also provide greater insight in terms of ERS or desynchronization mechanisms, which cannot be informed by event-related potentials (ERPs) alone (Pfurtscheller 1999; Graimann et al. 2002; Yeung et al. 2004; Mishra et al. 2012). These analyses revealed four independent neural source activations each related to loneliness/wisdom (Supplementary Table 2). To account for multiple comparisons, we then included the independently significant activations related to loneliness/wisdom in multiple linear robust regression models for these constructs.
Only two neural activations each for loneliness/wisdom showed significant relationships in these multiple robust regression analyses (Fig. 2). During angry emotion bias, loneliness displayed a positive association with theta activity in the left transverse temporal ROI (f2 = 0.03, β = 517.95, CI = [17.75, 1018.16], P = 0.04; Fig. 2A); of note, this ROI is in the region of the TPJ. Additionally, loneliness was positively related to angry stimulus-driven beta activity in the left superior parietal ROI (f2 = 0.02, β = 145.86, CI= [23.03, 268.68], P = 0.02; Fig. 2B). In contrast, during happy emotion bias, a positive relationship emerged for wisdom and left transverse temporal theta (f2 = 0.05, β = 184.0, CI = [20.18, 347.82], P = 0.03; Fig. 2C). Wisdom also showed a positive association with happy stimulus-driven alpha activity in the left insula (f2 = 0.04, β = 14.34, CI = [1.20, 27.47], P = 0.03; Fig. 2D). We further confirmed that these neural relationships were emotion-specific, that is, only observed for loneliness versus angry neural processing (but not sad/happy processing), and for wisdom versus happy neural processing (but not angry/sad processing). Additionally, we checked whether these specific neural activity regressions with loneliness/wisdom show any interactions with age and gender. No age/gender interactions were observed in the loneliness neural model, while in the wisdom model, only age showed a significant positive interaction with left transverse temporal theta during happy emotion bias (f2 = 0.04, β = 12.05, CI = [0.37, 23.73], P = 0.04), that is, older individuals showed a stronger positive association between wisdom and happy emotion-driven transverse temporal theta than younger individuals. The channel-level ERPs and ERSPs corresponding to these cortical source activations are shown in Supplementary Figure 1.
Figure 2.
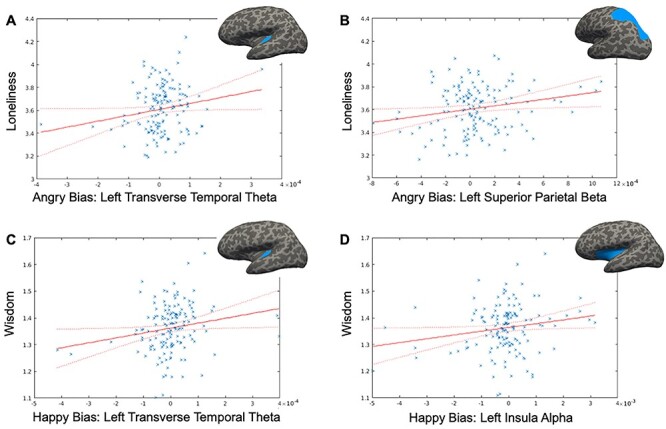
Distinct neural correlates for loneliness and wisdom. A significant relationship was observed for loneliness during angry emotion bias in the left transverse temporal cortex in the theta band (A) and in the left superior parietal cortex in the beta band (B). In contrast, a significant relationship was observed for wisdom during happy emotion bias in the left transverse temporal cortex in the theta band (C) and in the left insula in the alpha band (D). Relevant cortical regions are highlighted in blue on inflated brain surface parcellations. The loneliness robust regression model controlled for social network score and wisdom, and the wisdom model controlled for social network score and loneliness. Neural data 5 SD outliers were removed prior to modeling. Fit lines are shown with 95% CI, y-axes values are log-scaled.
Finally, we analyzed which of the subscales of wisdom (i.e., prosocial, emotional regulation, decisiveness, social advising, insight, and tolerance) relate to the significant neural signatures found during happy emotion bias. Only the tolerance component of wisdom was positively associated with left transverse temporal theta activity (f2 = 0.06, β = 306.73, CI = [90.36, 523.10], P = 0.006). Both social advising and insightfulness components of wisdom showed positive associations with the left insula alpha activity (social advising: f2 = 0.06, β = 24.93, CI = [5.52, 44.34], P = 0.01; insightfulness: f2 = 0.03, β = 26.22, CI = [0.91, 51.52], P = 0.04).
Discussion
In this study of 147 human subjects sampled across the adult lifespan, we simultaneously probed the correlates of loneliness and wisdom in the context of a facial emotion bias task. Here, we first replicated our recent research showing the inverse relationship between loneliness and wisdom (Lee et al. 2019; Jeste, Di Somma, et al. 2020; Nguyen et al. 2020). Furthermore, we found that loneliness and wisdom have distinct cognitive and neural correlates in the context of emotion biasing.
To the best of our knowledge, this is one of the largest sample EEG studies in the literature related to loneliness (Somerville et al. 2006, Cacioppo et al. 2009, 2016, Kanai et al. 2012, Cacioppo, Cacioppo, et al. 2014, 2015, Spithoven et al. 2017, Shao et al. 2019). Notably, prior studies of loneliness have rarely included relevant covarying factors of both social network score and wisdom; our study brings these subjective constructs together under one framework. Behaviorally, we found that social network score as well as wisdom had significant inverse relationships with loneliness across the adult lifespan, replicating prior work (Gow et al. 2013; Lemieux et al. 2013; Medvene et al. 2016; Lee et al. 2019; Jeste, Di Somma, et al. 2020; Nguyen et al. 2020). We did not find age to be a significant factor predictor of loneliness. Depression emerged as a significant predictor only in younger adults but not when considering all ages. Several other studies show a significant correlation between depression and loneliness (Cacioppo, Hughes, et al. 2006; Qualter et al. 2010; Fried et al. 2015; Bhatti and ul Haq 2017; Shao et al. 2019); here, we confirm this relationship only in younger adults, perhaps because our overall lifespan sample consisted of healthy participants with no or low-level depressive symptoms.
Beyond demographic and subjective relationships, in this study, we were interested in how loneliness and wisdom relate to emotional biasing. For probing this, we used a simple cognitive task with superimposed facial emotions that may bias task performance. Such a task has previously shown sensitivity to mental health symptoms, including anxiety and depression (Joormann and Gotlib 2007; Wells and Beevers 2010; Duque and Vázquez 2015; Thai et al. 2016; Kaiser et al. 2018; Dai et al. 2019), and hence is relevant to the study of loneliness, a construct often associated with these symptoms. In line with the social threat model for loneliness (Cacioppo, Hawkley, et al. 2006; Cacioppo, Cacioppo, et al. 2014; Cacioppo et al. 2016; Spithoven et al. 2017), we hypothesized that loneliness would show sensitivity to task trials that presented threatening, that is, angry faces. Indeed, we found that when angry emotion distractors were present, attentive response speeds were significantly negatively correlated with loneliness, suggesting greater emotional dysregulation during this condition in lonelier individuals. The cognitive relationship with loneliness was specific to threatening stimuli and did not emerge for happy/sad stimuli. In contrast, for wisdom, as per our hypothesis, we found a significant positive relationship for responding to happy stimuli, specifically wiser individuals displayed faster response speeds for happy stimuli. Regressions with wisdom subscales showed that decisiveness and social advising (i.e., being able to advise others on their choices) significantly related to faster speed on happy stimuli; in previous work, we have hypothesized these components to be important for cognitive performance (Jeste and Lee 2019).
Guided by our hypotheses that were verified by the cognitive results, we analyzed the neural correlates for loneliness and wisdom during angry and happy emotion biasing, respectively. Uniquely in this study, the neural models controlled for the interrelationships among loneliness, wisdom, and social networks, that is, the loneliness model controlled for wisdom and the wisdom model controlled for loneliness; both models controlled for social network size to ensure that the results are not driven by these covariates.
The neural robust regression for loneliness, controlling for wisdom and social network covariates, showed a positive relationship with left transverse temporal theta activity and left superior parietal beta activity, specifically for angry emotion biasing. Notably, the transverse temporal ROI is in the region of the TPJ that is well known for its role in social-affective processing (Cacioppo et al. 2009, 2016; Zaki et al. 2009; Kanai et al. 2012; Cacioppo, Cacioppo, et al. 2014; Cacioppo, Capitanio, et al. 2014). This region particularly represents theory of mind, which is important in the interpretation of the behavior of others as well as for self-referential processing (Samson et al. 2004; Koush et al. 2019). Recent research shows that activity in left TPJ is enhanced during processing of social interactions (Arioli et al. 2020) and that greater theta band activity in this region represents stronger implicit biases during evaluation of social interactions (Schiller et al. 2019). Research in individuals with interpersonal difficulties versus healthy controls further shows flexible TPJ processing, with TPJ activity showing sensitivity to negative feedback in those with interpersonal problems versus sensitivity to positive feedback in healthy controls (van Schie et al. 2020); that lonelier individuals in our study show heightened TPJ theta processing during implicit biasing by angry emotions is aligned with this evidence base. Furthermore, the superior parietal beta activity elicited to angry stimuli shows evidence for greater attention to these stimuli; the superior parietal region is well known for its role in attention shifting and beta activity is elicited in this region particularly during early processing (Yantis et al. 2002; Thakral and Slotnick 2009; Caspari et al. 2018; Proskovec et al. 2018). Thus, overall, loneliness in this study was associated with enhanced attention to the angry emotional distractors represented by activity in the temporal-parietal region.
Interestingly, the neural model for wisdom during emotion bias from happy stimuli also showed greater theta activity in the left transverse temporal ROI in the region of the TPJ. This result aligns with research showing flexible processing in the TPJ to positive versus negative stimuli in different individuals (Schiller et al. 2019; van Schie et al. 2020); wiser individuals invoke TPJ activity during implicit processing of happy distractors, while lonelier individuals invoke this activity during implicit processing of threatening emotion stimuli. In the wisdom model, we additionally observed significantly enhanced alpha band activity in the left insula during happy emotion biasing. This finding was in line with our hypothesis that the insular region, which is critical to both emotional and cognitive attention, self-awareness, and empathic regulation would be involved in the representation of wisdom (Adolphs 2002; Farb et al. 2007; Lutz et al. 2008; Meeks and Jeste 2009; Posner and Rothbart 2009; Menon and Uddin 2010; Klimecki et al. 2014; Mishra et al. 2020). Prior research additionally shows that greater self-reported empathy, which is the prosocial component of wisdom, is associated with greater insula activity in the presence of positive stimuli (Carr et al. 2003; Hennenlotter et al. 2005; Craig 2009; Salomon et al. 2016; Gogolla 2017); our results showing greater insula activity specifically in positive emotion context for wiser individuals is aligned with this literature. That wisdom correlates to insula activity in the alpha band is in line with studies showing that alpha activity within the insular cortex is a marker for greater visual awareness (Sadaghiani et al. 2010; Salomon et al. 2016). Thus, overall, greater implicit visual awareness to happy stimuli in wiser individuals may explain their faster response speeds in this context.
In conclusion, in this relatively large sample study of healthy human subjects, we were able to simultaneously interrogate the cognitive and neural correlates of both loneliness and wisdom. The main limitation of our study is that it investigated these correlates in the context of a specific emotional attention bias task wherein participants did not explicitly evaluate the emotional stimuli. Other loneliness research has focused on explicit socio-emotional affect evaluation; hence, our specific results and their interpretations are limited in context. This could also be a reason why we did not find any significant activations of medial prefrontal cortex activity that is associated with loneliness and the components of wisdom (Somerville et al. 2006; Meeks and Jeste 2009; Cacioppo et al. 2013; Courtney and Meyer 2020). We also did not find large-scale default mode network activations related to loneliness that have been recently found during resting state (Spreng et al. 2020). Yet, our results align with the broader theoretical and neuroscientific literature that supports the study of loneliness and wisdom. In accordance with the social threat model for loneliness, we found that loneliness most impacted task performance when angry stimuli were presented. Underlying this cognitive processing, left TPJ regional theta activity was enhanced along with attention-related superior parietal beta activity. Wisdom, in contrast, was inversely related to loneliness and was associated with faster speed of responding to happy stimuli. Neural correlates of wisdom were identified as greater left TPJ theta activity, specifically in the context of happy emotion bias along with greater left insula alpha signifying greater alertness to these specific stimuli. Overall, the models used robust regression to minimize outlier effects and controlled for social network score and the inter-relatedness of loneliness and wisdom to reveal construct specific results. Importantly, the specific cognitive and neural associations did not extend to processing of other stimuli, suggesting that emotional stimulus context matters in the evaluation of loneliness and wisdom.
Therefore, our results suggest that loneliness and wisdom relate to contrasting modulations of cognitive processes (reduced versus enhanced response speed biased by angry versus happy emotions) and invoke-related (TPJ) and distinct (superior parietal vs. insula) neural circuits in specific emotional contexts. These identified cognitive and neural processes may serve as targets for future brain plasticity-based treatments (Merzenich et al. 2013; Mishra and Gazzaley 2014). In fact, TPJ is a very common target for neuromodulation (Donaldson et al. 2015; Wang et al. 2016; Bardi, Gheza, et al. 2017; Bardi, Six, et al. 2017; Lega et al. 2020); hence, future studies may focus on neuromodulation-induced plasticity of this region to develop an intervention for loneliness. Recently, there has been much concern for heightened loneliness during the COVID-19 social/physical distancing period, making intervention studies all the more pertinent (Luchetti et al. 2020). Much clinical translational research aims to mitigate loneliness or enhance wisdom (Masi et al. 2011; Hagan et al. 2014; Cohen-Mansfield and Perach 2015; Jeste, Lee, Palmer, et al. 2020; Lee et al. 2020; Treichler et al. 2020). In future work, it will be of interest to investigate whether such interventions can also upregulate the objective, cognitive, and neural correlates identified in this study and thereby establish causal mechanisms.
Notes
We thank several UCSD undergraduate students who assisted with data collection. The BrainE software is copyrighted for commercial use (Regents of the University of California Copyright #SD2018-816) and free for research and educational purposes.
Conflict of Interest: The authors declare no conflict of interest.
Funding
University of California San Diego (UCSD) lab start-up funds (J.M.); T. Denny Sanford Institute for Empathy and Compassion (J.M.); National Institute of Mental Health (grants R01MH094151-01 to D.V.J., K23-MH119375 to E.E.L.); T32 Geriatric Mental Health Program grant (grant T32-MH019934 to D.V.J.); Stein Institute for Research on Aging at the University of California at San Diego.
Supplementary Material
Contributor Information
Gillian Grennan, Department of Psychiatry, University of California, San Diego, La Jolla, 92037 CA, USA; Neural Engineering and Translation Labs, University of California, San Diego, La Jolla, 92037 CA, USA.
Pragathi Priyadharsini Balasubramani, Department of Psychiatry, University of California, San Diego, La Jolla, 92037 CA, USA; Neural Engineering and Translation Labs, University of California, San Diego, La Jolla, 92037 CA, USA.
Fahad Alim, Department of Psychiatry, University of California, San Diego, La Jolla, 92037 CA, USA; Neural Engineering and Translation Labs, University of California, San Diego, La Jolla, 92037 CA, USA.
Mariam Zafar-Khan, Department of Psychiatry, University of California, San Diego, La Jolla, 92037 CA, USA; Neural Engineering and Translation Labs, University of California, San Diego, La Jolla, 92037 CA, USA.
Ellen E Lee, Department of Psychiatry, University of California, San Diego, La Jolla, 92037 CA, USA; Sam and Rose Stein Institute for Research on Aging, University of California, San Diego, La Jolla, 92037 CA, USA; Veterans Affairs San Diego Healthcare System, San Diego, 92161 CA, USA.
Dilip V Jeste, Department of Psychiatry, University of California, San Diego, La Jolla, 92037 CA, USA; Sam and Rose Stein Institute for Research on Aging, University of California, San Diego, La Jolla, 92037 CA, USA; Department of Neurosciences, University of California, San Diego, La Jolla, 92037 CA, USA.
Jyoti Mishra, Department of Psychiatry, University of California, San Diego, La Jolla, 92037 CA, USA; Neural Engineering and Translation Labs, University of California, San Diego, La Jolla, 92037 CA, USA.
References
- Adolphs R. 2002. Neural systems for recognizing emotion. Curr Opin Neurobiol. 12:169–177. [DOI] [PubMed] [Google Scholar]
- Anderson T, Darling D. 1952. Asymptotic theory of certain “goodness of fit” criteria based on stochastic processes. Ann Math Stat. 23:193–212. [Google Scholar]
- Ardelt M. 2000. Intellectual versus wisdom-related knowledge: the case for a different kind of learning in the later years of life. Educ Gerontol. 26:771–789. [Google Scholar]
- Ardelt M, Edwards CA. 2016. Wisdom at the end of life: an analysis of mediating and moderating relations between wisdom and subjective well-being. J Gerontol B Psychol Sci Soc Sci. 71:502–513. [DOI] [PubMed] [Google Scholar]
- Ardelt M, Ferrari M. 2019. Effects of wisdom and religiosity on subjective well-being in old age and young adulthood: exploring the pathways through mastery and purpose in life. Int Psychogeriatrics. 31:477–489. [DOI] [PubMed] [Google Scholar]
- Ardelt M, Jeste DV. 2018. Wisdom and hard times: the ameliorating effect of wisdom on the negative association between adverse life events and well-being. J Gerontol B Psychol Sci Soc Sci. 73:1374–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arevalo-Rodriguez I, Smailagic N, Roquéi Figuls M, Ciapponi A, Sanchez-Perez E, Giannakou A, Pedraza OL, Bonfill Cosp X, Cullum S. 2015. Mini-Mental State Examination (MMSE) for the detection of Alzheimer’s disease and other dementias in people with mild cognitive impairment (MCI). Cochrane Database Syst Rev. 2015(3):CD010783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arioli M, Basso G, Carne I, Poggi P, Canessa N. 2020. Increased pSTS activity and decreased pSTS-mPFC connectivity when processing negative social interactions. Behav Brain Res. 399:113027. [DOI] [PubMed] [Google Scholar]
- Balasubramani PP, Ojeda A, Grennan G, Maric V, Le H, Alim F, Zafar-Khan M, Diaz-Delgado J, Silveira S, Ramanathan D, et al. 2020. Mapping Cognitive Brain Functions at Scale. NeuroImage. 117641. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltes P, Staudinger U. 2000. Wisdom. A metaheuristic (pragmatic) to orchestrate mind and virtue toward excellence. Am Psychol. 55(1):122–136. [DOI] [PubMed] [Google Scholar]
- Bangen KJ, Meeks TW, Jeste DV. 2013. Defining and assessing wisdom: a review of the literature. Am J Geriatr psychiatry Off J Am Assoc Geriatr Psychiatry. 21:1254–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardi L, Gheza D, Brass M. 2017. TPJ-M1 interaction in the control of shared representations: new insights from tDCS and TMS combined. Neuroimage. 146:734–740. [DOI] [PubMed] [Google Scholar]
- Bardi L, Six P, Brass M. 2017. Repetitive TMS of the temporo-parietal junction disrupts participant’s expectations in a spontaneous Theory of Mind task. Soc Cogn Affect Neurosci. 12:1775–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutel ME, Klein EM, Brähler E, Reiner I, Jünger C, Michal M, Wiltink J, Wild PS, Münzel T, Lackner KJ, et al. 2017. Loneliness in the general population: prevalence, determinants and relations to mental health. BMC Psychiatry. 17:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatti AB, ul Haq A. 2017. The pathophysiology of perceived social isolation: effects on health and mortality. Cureus. 9(1):e994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boss L, Kang D-H, Branson S. 2015. Loneliness and cognitive function in the older adult: a systematic review. Int Psychogeriatrics. 27:541–553. [DOI] [PubMed] [Google Scholar]
- Boudreau B, Poulin C. 2009. An examination of the validity of the Family Affluence Scale II (FAS II) in a general adolescent population of Canada. Soc Indic Res. 94:29–42. [Google Scholar]
- BrainSpan: Atlas of the Developing Human Brain . 2020. http://www.brainspan.org/
- Brown EG, Gallagher S, Creaven A-M. 2018. Loneliness and acute stress reactivity: a systematic review of psychophysiological studies. Psychophysiology. 55:e13031. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Cacioppo S, Boomsma DI. 2014. Evolutionary mechanisms for loneliness. Cogn Emot. 28(1):3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Cacioppo S, Cole SW, Capitanio JP, Goossens L, Boomsma DI. 2015. Loneliness across phylogeny and a call for comparative studies and animal models. Perspect Psychol Sci a J Assoc Psychol Sci. 10:202–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC, Ernst JM, Burleson M, Berntson GG, Nouriani B, Spiegel D. 2006. Loneliness within a nomological net: An evolutionary perspective. J Res Pers. 40:1054–1085. [Google Scholar]
- Cacioppo JT, Hughes ME, Waite LJ, Hawkley LC, Thisted RA. 2006. Loneliness as a specific risk factor for depressive symptoms: cross-sectional and longitudinal analyses. Psychol Aging. 21:140–151. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Norris CJ, Decety J, Monteleone G, Nusbaum H. 2009. In the eye of the beholder: individual differences in perceived social isolation predict regional brain activation to social stimuli. J Cogn Neurosci. 21:83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC, Norman GJ, Berntson GG. 2011. Social isolation. Ann N Y Acad Sci. 1231:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo S, Balogh S, Cacioppo JT. 2015. Implicit attention to negative social, in contrast to nonsocial, words in the Stroop task differs between individuals high and low in loneliness: Evidence from event-related brain microstates. Cortex. 70:213–233. [DOI] [PubMed] [Google Scholar]
- Cacioppo S, Bangee M, Balogh S, Cardenas-Iniguez C, Qualter P, Cacioppo JT. 2016. Loneliness and implicit attention to social threat: a high-performance electrical neuroimaging study. Cogn Neurosci. 7:138–159. [DOI] [PubMed] [Google Scholar]
- Cacioppo S, Capitanio JP, Cacioppo JT. 2014. Toward a neurology of loneliness. Psychol Bull. 140(6):1464–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo S, Frum C, Asp E, Weiss RM, Lewis JW, Cacioppo JT. 2013. A quantitative meta-analysis of functional imaging studies of social rejection. Sci Rep. 3:2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr L, Iacoboni M, Dubeaut MC, Mazziotta JC, Lenzi GL. 2003. Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas. Proc Natl Acad Sci U S A. 100:5497–5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspari N, Arsenault JT, Vandenberghe R, Vanduffel W. 2018. Functional similarity of medial superior parietal areas for shift-selective attention signals in humans and monkeys. Cereb Cortex. 28:2085–2099. [DOI] [PubMed] [Google Scholar]
- Cohen-Mansfield J, Perach R. 2015. Interventions for alleviating loneliness among older persons: a critical review. Am J Heal Promot. 29(3):e109–e125. [DOI] [PubMed] [Google Scholar]
- Courtney AL, Meyer ML. 2020. Self-other representation in the social brain reflects social connection. J Neurosci. 40. JN-RM-2826-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. 2009. How do you feel - now? The anterior insula and human awareness. Nat Rev Neurosci. 10:59–70. [DOI] [PubMed] [Google Scholar]
- Dai Q, Hu L, Feng Z. 2019. Attentional bias modification reduces clinical depression and enhances attention toward happiness. J Psychiatr Res. 109:145–155. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. 2004. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 134:9–21. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, et al. 2006. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 31:968–980. [DOI] [PubMed] [Google Scholar]
- Donaldson PH, Rinehart NJ, Enticott PG. 2015. Noninvasive stimulation of the temporoparietal junction: a systematic review. Neurosci Biobehav Rev. 55:547–572. [DOI] [PubMed] [Google Scholar]
- Duque A, Vázquez C. 2015. Double attention bias for positive and negative emotional faces in clinical depression: evidence from an eye-tracking study. J Behav Ther Exp Psychiatry. 46:107–114. [DOI] [PubMed] [Google Scholar]
- Farb NAS, Segal ZV, Mayberg H, Bean J, McKeon D, Fatima Z, Anderson AK. 2007. Attending to the present: mindfulness meditation reveals distinct neural modes of self-reference. Soc Cogn Affect Neurosci. 2:313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried EI, Bockting C, Arjadi R, Borsboom D, Amshoff M, Cramer AOJ, Epskamp S, Tuerlinckx F, Carr D, Stroebe M. 2015. From loss to loneliness: the relationship between bereavement and depressive symptoms. J Abnorm Psychol. 124:256–265. [DOI] [PubMed] [Google Scholar]
- Gogolla N. 2017. The insular cortex. Curr Biol. 27:R580–R586. [DOI] [PubMed] [Google Scholar]
- Gow AJ, Corley J, Starr JM, Deary IJ. 2013. Which social network or support factors are associated with cognitive abilities in old age? Gerontology. 59:454–463. [DOI] [PubMed] [Google Scholar]
- Graimann B, Huggins JE, Levine SP, Pfurtscheller G. 2002. Visualization of significant ERD/ERS patterns in multichannel EEG and ECoG data. Clin Neurophysiol. 113:43–47. [DOI] [PubMed] [Google Scholar]
- Hagan R, Manktelow R, Taylor BJ, Mallett J. 2014. Reducing loneliness amongst older people: a systematic search and narrative review. Aging Ment Heal. 18:683–693. [DOI] [PubMed] [Google Scholar]
- Heeger D, Landy M. 2009. Signal detection theory. In: Goldstein B, editor. Encyclopedia of Perception. SAGE Publications, pp. 887–892. [Google Scholar]
- Hennenlotter A, Schroeder U, Erhard P, Castrop F, Haslinger B, Stoecker D, Lange KW, Ceballos-Baumann AO. 2005. A common neural basis for receptive and expressive communication of pleasant facial affect. Neuroimage. 26:581–591. [DOI] [PubMed] [Google Scholar]
- Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC. 1998. Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr. 22:324–333. [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J, Smith TB, Baker M, Harris T, Stephenson D. 2015. Loneliness and social isolation as risk factors for mortality: a meta-analytic review. Perspect Psychol Sci a J Assoc Psychol Sci. 10:227–237. [DOI] [PubMed] [Google Scholar]
- Insel TR. 2014. Mental disorders in childhood: shifting the focus from behavioral symptoms to neurodevelopmental trajectories. JAMA. 311(17):1727–1728. [DOI] [PubMed] [Google Scholar]
- Jeste D, Di Somma S, Lee E, Nguyen T, Scalcione M, Biaggi A, Daly R, Liu J, Tu X, Ziedonis D, et al.. 2020. Study of loneliness and wisdom in 482 middle-aged and oldest-old adults: a comparison between people in Cilento, Italy and San Diego, USA. Aging & Mental Health. 1:1–11. [DOI] [PMC free article] [PubMed]
- Jeste DV, Lee EE, Cacioppo S. 2020. Battling the modern behavioral epidemic of loneliness: suggestions for research and interventions. JAMA Psychiatry. 77(6):553–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeste DV, Lee EE. 2019. The emerging empirical science of wisdom: definition, measurement, neurobiology, longevity, and interventions. Harv Rev Psychiatry. 27(3):127–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeste DV, Lee EE, Palmer BW, Treichler EBH. 2020. Moving from humanities to sciences: a new model of wisdom fortified by sciences of neurobiology, medicine, and evolution. Psychol Inq. 31:134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, Gotlib IH. 2007. Selective attention to emotional faces following recovery from depression. J Abnorm Psychol. 116:80–85. [DOI] [PubMed] [Google Scholar]
- Kaiser RH, Snyder HR, Goer F, Clegg R, Ironside M, Pizzagalli DA. 2018. Attention bias in rumination and depression: cognitive mechanisms and brain networks. Clin Psychol Sci. 6:765–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai R, Bahrami B, Duchaine B, Janik A, Banissy MJ, Rees G. 2012. Brain structure links loneliness to social perception. Curr Biol. 22:1975–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimecki O, Leiberg S, Ricard M, Singer T. 2014. Differential pattern of functional brain plasticity after compassion and empathy training. Soc Cogn Affect Neurosci. 9:873–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothe C, Medine D, Boulay C, Grivich M, Stenner T. 2019. “Lab Streaming Layer” Copyright. https://labstreaminglayer.readthedocs.io/
- Koush Y, Pichon S, Eickhoff SB, Van De Ville D, Vuilleumier P, Scharnowski F. 2019. Brain networks for engaging oneself in positive-social emotion regulation. Neuroimage. 189:106–115. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JBW. 2001. The PHQ-9. Validity of a brief depression severity measure. J Gen Intern Med. 16:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper JS, Zuidersma M, Oude Voshaar RC, Zuidema SU, Heuvel ER, Stolk RP, Smidt N. 2015. Social relationships and risk of dementia: a systematic review and meta-analysis of longitudinal cohort studies. Ageing Res Rev. 22:39–57. [DOI] [PubMed] [Google Scholar]
- Lara E, Martín-María N, De la Torre-Luque A, Koyanagi A, Vancampfort D, Izquierdo A, Miret M. 2019. Does loneliness contribute to mild cognitive impairment and dementia? A systematic review and meta-analysis of longitudinal studies. Ageing Res Rev. 52:7–16. [DOI] [PubMed] [Google Scholar]
- Layden EA, Cacioppo JT, Cacioppo S, Cappa SF, Dodich A, Falini A, Canessa N. 2017. Perceived social isolation is associated with altered functional connectivity in neural networks associated with tonic alertness and executive control. NeuroImage. 145:58–73. [DOI] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. 2008. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 40:1044–1055. [DOI] [PubMed] [Google Scholar]
- Lee EE, Bangen KJ, Avanzino JA, Hou BC, Ramsey M, Eglit G, Liu J, Tu XM, Paulus M, Jeste DV. 2020. Outcomes of randomized clinical trials of interventions to enhance social, emotional, and spiritual components of wisdom: a systematic review and meta-analysis. JAMA Psychiatry. 77(9):925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EE, Depp C, Palmer BW, Glorioso D, Daly R, Liu J, Tu XM, Kim H-C, Tarr P, Yamada Y, et al. 2019. High prevalence and adverse health effects of loneliness in community-dwelling adults across the lifespan: role of wisdom as a protective factor. Int Psychogeriatrics. 31:1447–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lega C, Santandrea E, Ferrante O, Serpe R, Dolci C, Baldini E, Cattaneo L, Chelazzi L. 2020. Modulating the influence of recent trial history on attentional capture via transcranial magnetic stimulation (TMS) of right TPJ. Cortex. 133:149–160. [DOI] [PubMed] [Google Scholar]
- Lemieux R, Lajoie S, Trainor NE. 2013. Affinity-seeking, social loneliness, and social avoidance among Facebook users. Psychol Rep. 112:545–552. [DOI] [PubMed] [Google Scholar]
- López-Martín S, Albert J, Fernández-Jaén A, Carretié L. 2013. Emotional distraction in boys with ADHD: Neural and behavioral correlates. Brain Cogn. 83:10–20. [DOI] [PubMed] [Google Scholar]
- López-Martín S, Albert J, Fernández-Jaén A, Carretié L. 2015. Emotional response inhibition in children with attention-deficit/hyperactivity disorder: neural and behavioural data. Psychol Med. 45:2057–2071. [DOI] [PubMed] [Google Scholar]
- Lubben J, Blozik E, Gillmann G, Iliffe S, Renteln KW, Beck JC, Stuck AE. 2006. Performance of an abbreviated version of the lubben social network scale among three european community-dwelling older adult populations. Gerontologist. 46:503–513. [DOI] [PubMed] [Google Scholar]
- Lubben JE. 1988. Assessing social networks among elderly populations. Family & Community Health: The Journal of Health Promotion & Maintenance. 11(3):42–52. [Google Scholar]
- Luchetti M, Lee JH, Aschwanden D, Sesker A, Strickhouser JE, Terracciano A, Sutin AR. 2020. The trajectory of loneliness in response to COVID-19. American Psychologist. 75(7):897–908. [DOI] [PMC free article] [PubMed]
- Lutz A, Slagter HA, Dunne JD, Davidson RJ. 2008. Attention regulation and monitoring in meditation. Trends Cogn Sci. 12:163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masi CM, Chen HY, Hawkley LC, Cacioppo JT. 2011. A meta-analysis of interventions to reduce loneliness. Personal Soc Psychol Rev. 15(3):219–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvene LJ, Nilsen KM, Smith R, Ofei-Dodoo S, DiLollo A, Webster N, Graham A, Nance A. 2016. Social networks and links to isolation and loneliness among elderly HCBS clients. Aging Ment Health. 20:485–493. [DOI] [PubMed] [Google Scholar]
- Meeks TW, Jeste DV. 2009. Neurobiology of wisdom: a literature overview. Arch Gen Psychiatry. 66:355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J, Wang X, Wei D, Qiu J. 2020. State loneliness is associated with emotional hypervigilance in daily life: a network analysis. Pers Individ Dif. 165:110154. [Google Scholar]
- Menon V, Uddin LQ. 2010. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 214:655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merzenich M, Nahum M, Vleet T. 2013. Changing brains: applying brain plasticity to advance and recover human ability. Vol. 207. Elsevier, pp. 2–466. [Google Scholar]
- Mishra J, Gazzaley A. 2014. Harnessing the neuroplastic potential of the human brain & the future of cognitive rehabilitation. Front Hum Neurosci. 8:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra J, Martínez A, Schroeder CE, Hillyard SA. 2012. Spatial attention boosts short-latency neural responses in human visual cortex. Neuroimage. 59:1968–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra J, Sagar R, Parveen S, Kumaran S, Modi K, Maric V, Ziegler D, Gazzaley A. 2020. Closed-loop digital meditation for neurocognitive and behavioral development in adolescents with childhood neglect. Transl Psychiatry. 10:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra A, Ojeda A, Mishra J. 2018. BrainE: a digital platform for evaluating, engaging and enhancing brain function. Regents of the University of California Copyright SD2018-816.
- Morlett Paredes A, Lee EE, Chik L, Gupta S, Palmer BW, Palinkas LA, Kim H-C, Jeste DV. 2020. Qualitative study of loneliness in a senior housing community: the importance of wisdom and other coping strategies. Aging Ment Health. 10:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T, Lee E, Daly R, Wu T, Tang Y, Tu X, Van Patten R, Jeste D, Palmer B. 2020. Predictors of loneliness by age decade: study of psychological and environmental factors in 2, 843 community-dwelling Americans aged 20-69 years. J Clin Psychiatry. 81(6):20m13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda A, Klug M, Kreutz-Delgado K, Gramann K, Mishra J. 2019. A Bayesian framework for unifying data cleaning, source separation and imaging of electroencephalographic signals. bioRxiv 559450. [Google Scholar]
- Ojeda A, Kreutz-Delgado K, Mullen T. 2018. Fast and robust Block-Sparse Bayesian learning for EEG source imaging. Neuroimage. 174:449–462. [DOI] [PubMed] [Google Scholar]
- Ovid . 2005. The poems of exile: tristia and the black sea letters. University of California Press. [Google Scholar]
- Pascual-Marqui RD, Michel CM, Lehmann D. 1994. Low resolution electromagnetic tomography: a new method for localizing electrical activity in the brain. Int J Psychophysiol. 18:49–65. [DOI] [PubMed] [Google Scholar]
- Peplaum L, Russell D, Heim M. 1979. The experience of loneliness. In: Frieze I, Bar-Tal D, Carroll J, editors. New approaches to social problems: applications of attribution theory. San Francisco (CA): Jossey-Bass, pp. 53–78. [Google Scholar]
- Perissinotto CM, Stijacic Cenzer I, Covinsky KE. 2012. Loneliness in older persons: a predictor of functional decline and death. Arch Intern Med. 172:1078–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva, FH. 1999. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 110(11):1842–1857. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. 2009. Toward a physical basis of attention and self-regulation. Phys Life Rev. 6:103–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proskovec AL, Heinrichs-Graham E, Wiesman AI, McDermott TJ, Wilson TW. 2018. Oscillatory dynamics in the dorsal and ventral attention networks during the reorienting of attention. Hum Brain Mapp. 39:2177–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qualter P, Brown SL, Munn P, Rotenberg KJ. 2010. Childhood loneliness as a predictor of adolescent depressive symptoms: an 8-year longitudinal study. Eur Child Adolesc Psychiatry. 19:493–501. [DOI] [PubMed] [Google Scholar]
- Rotenberg KJ, Gruman JA, Ariganello M. 2002. Behavioral confirmation of the loneliness stereotype. Basic Appl Soc Psych. 24:81–89. [Google Scholar]
- Russell DW. 1996. UCLA loneliness scale (version 3): reliability, validity, and factor structure. J Pers Assess. 66:20–40. [DOI] [PubMed] [Google Scholar]
- Sadaghiani S, Scheeringa R, Lehongre K, Morillon B, Giraud AL, Kleinschmidt A. 2010. Intrinsic connectivity networks, alpha oscillations, and tonic alertness: a simultaneous electroencephalography/functional magnetic resonance imaging study. J Neurosci. 30:10243–10250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon R, Ronchi R, Dönz J, Bello-Ruiz J, Herbelin B, Martet R, Faivre N, Schaller K, Blanke O. 2016. The insula mediates access to awareness of visual stimuli presented synchronously to the heartbeat. J Neurosci. 36:5115–5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson D, Apperly IA, Chiavarino C, Humphreys GW. 2004. Left temporoparietal junction is necessary for representing someone else’s belief. Nat Neurosci. 7:499–500. [DOI] [PubMed] [Google Scholar]
- Schiller B, Gianotti LRR, Baumgartner T, Knoch D. 2019. Theta resting EEG in the right TPJ is associated with individual differences in implicit intergroup bias. Soc Cogn Affect Neurosci. 14:281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selya AS, Rose JS, Dierker LC, Hedeker D, Mermelstein RJ. 2012. A practical guide to calculating Cohen’s f2, a measure of local effect size, from PROC MIXED. Front Psychol. 3:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao R, Liu HL, Huang CM, Chen YL, Gao M, Lee SH, Lin C, Lee TMC. 2019. Loneliness and depression dissociated on parietal-centered networks in cognitive and resting states. Psychol Med. 50(16):2691–2701. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Heatherton TF, Kelley WM. 2006. Anterior cingulate cortex responds differentially to expectancy violation and social rejection. Nat Neurosci. 9:1007–1008. [DOI] [PubMed] [Google Scholar]
- Spithoven AWM, Bijttebier P, Goossens L. 2017. It is all in their mind: a review on information processing bias in lonely individuals. Clin Psychol Rev. 58:97–114. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Kroenke K, Williams JBW, Löwe B. 2006. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 166:1092–1097. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Dimas E, Mwilambwe-Tshilobo L, Dagher A, Koellinger P, Nave G, Ong A, Kernbach JM, Wiecki TV, Ge T, et al. 2020. The default network of the human brain is associated with perceived social isolation. Nat Commun. 11:6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg R. 1990. Wisdom: its nature, origins, and development. New York: Cambridge University Press. [Google Scholar]
- Thai N, Taber-Thomas BC, Pérez-Edgar KE. 2016. Neural correlates of attention biases, behavioral inhibition, and social anxiety in children: an ERP study. Dev Cogn Neurosci. 19:200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakral PP, Slotnick SD. 2009. The role of parietal cortex during sustained visual spatial attention. Brain Res. 1302:157–166. [DOI] [PubMed] [Google Scholar]
- Thomas ML, Bangen KJ, Palmer BW, Sirkin Martin A, Avanzino JA, Depp CA, Glorioso D, Daly RE, Jeste DV. 2019. A new scale for assessing wisdom based on common domains and a neurobiological model: the San Diego Wisdom Scale (SD-WISE). J Psychiatr Res. 108:40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey B, Nelson C. 2009. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res. 168:242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treichler EBH, Glorioso D, Lee EE, Wu T-C, Tu XM, Daly R, O’Brien C, Smith JL, Jeste DV. 2020. A pragmatic trial of a group intervention in senior housing communities to increase resilience. Int Psychogeriatrics. 32:173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Patten R, Lee EE, Daly R, Twamley E, Tu XM, Jeste DV. 2019. Assessment of 3-dimensional wisdom in schizophrenia: associations with neuropsychological functions and physical and mental health. Schizophr Res. 208:360–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schie CC, Chiu C-D, Rombouts SARB, Heiser WJ, Elzinga BM. 2020. Stuck in a negative me: fMRI study on the role of disturbed self-views in social feedback processing in borderline personality disorder. Psychol Med. 50:625–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Callaghan E, Gooding-Williams G, McAllister C, Kessler K. 2016. Rhythm makes the world go round: an MEG-TMS study on the role of right TPJ theta oscillations in embodied perspective taking. Cortex. 75:68–81. [DOI] [PubMed] [Google Scholar]
- Webster JD, Westerhof GJ, Bohlmeijer ET. 2014. Wisdom and mental health across the lifespan. J Gerontol B Psychol Sci Soc Sci. 69:209–218. [DOI] [PubMed] [Google Scholar]
- Weiss R. 1974. Loneliness: the experience of emotional and social isolation. MIT Press. [Google Scholar]
- Wells TT, Beevers CG. 2010. Biased attention and dysphoria: manipulating selective attention reduces subsequent depressive symptoms. Cogn Emot. 24:719–728. [Google Scholar]
- Yantis S, Schwarzbach J, Serences JT, Carlson RL, Steinmetz MA, Pekar JJ, Courtney SM. 2002. Transient neural activity in human parietal cortex during spatial attention shifts. Nat Neurosci. 5:995–1002. [DOI] [PubMed] [Google Scholar]
- Yeung N, Bogacz R, Holroyd CB, Cohen JD. 2004. Detection of synchronized oscillations in the electroencephalogram: an evaluation of methods. Psychophysiology. 41:822–832. [DOI] [PubMed] [Google Scholar]
- Zaki J, Weber J, Bolger N, Ochsner K. 2009. The neural bases of empathic accuracy. Proc Natl Acad Sci U S A. 106:11382–11387. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


