Abstract
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which has caused the coronavirus diseases 2019 (COVID-19) pandemic, continues to spread rapidly worldwide and is associated with high rates of mortality among older adults, those with comorbidities, and those in poor physiological states. This paper aimed to systematically identify the impact of frailty on overall mortality among older adults with COVID-19. We conducted a systematic review of the literature indexed in 4 databases. A random-effects model with inverse variance-weighted meta-analysis using the odds ratio was used to study the association of frailty levels with clinical outcomes among older adults with COVID-19. Heterogeneity was measured using the I2 statistic and Egger's test. We identified 22 studies that met our inclusion criteria, including 924,520 total patients. Overall, frailty among older adults was associated with high rates of COVID-19-related mortality compared with non-frail older adults (OR [odds ratio]:5.76; 95% confidence interval [95% CI]: 3.85–8.61, I2: 40.5%). Our results show that physical limitations, such as those associated with frailty among older adults, are associated with higher rates of COVID-19-related mortality.
Keywords: Older adults, COVID-19, Frailty, Mortality, Meta-analysis
Introduction
The global pandemic associated with coronavirus disease 2019 (COVID-19) was declared a public health emergency by the World Health Organization in March 2020.1 Since the first case was discovered in Wuhan, China, in late 2019, the pandemic has resulted in more than 102,007,448 cases globally and 2,206,055 deaths, as of January 30, 2021.2 Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative COVID-19 coronavirus, is known to directly invade human extrapulmonary organs and tissues, leading to multiple organ dysfunction.3 Although COVID-19 impacts people of all ages, this disease shows a predisposition for older adults and those with underlying comorbidities.4 The risk of infection, viral load, and poor clinical outcomes, including intensive care unit (ICU) admissions, the need for supplemental oxygen, and mortality, remain high, particularly among those with increased age or who have underlying comorbidities.5
Frailty is defined as an extreme vulnerability to endogenous and exogenous stressors, which exposes an individual to a higher risk of negative health-related outcomes and commonly impacts older adults.6 The factors that contribute to frailty syndrome are regularly assessed in geriatric research, and consist of a combination of deficiencies in strength, balance, motor processing, cognition, nutrition, endurance, and physical activity.7 Frail adults tend to have physical weakness and declining psychological capacity due to multidimensional reductions in physiological function, resulting in adverse health outcomes.8 , 9 Frailty affects over 10% of older adults globally.10 Along with aging, frailty in older adults is typically accompanied by underlying physiological changes that increase the risk of hospitalization and overall mortality.11 , 12
The measurement of frailty using the Clinical Frailty Scale (CFS) has been used to predict falls, delirium, hospitalization, and mortality among older adults.13, 14, 15 Prior studies have indicated significant associations between frailty and poor cancer screening outcomes,16 response to surgery,17 chemotherapy, and overall mortality and morbidity.18 Among COVID-19 patients, a study in Italy found a relatively high number of deaths among hospitalized frail older people.19
Limited evidence exists regarding frailty as a predictor of COVID-19 infection risk or associated outcomes. Given the high prevalence of frailty among older adults, we conducted a systematic review and meta-analysis to study the impacts of frailty on COVID-19 outcomes.
Material and methods
This study was registered in the International Prospective Register of Systematic Review (PROSPERO): CRD42020209962. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis20 guidelines during the conduct of this systematic review.20
Search strategy
To determine relevant studies, we searched CINAHL, Google Scholar, PubMed (MEDLINE), and Web of Science databases from December 1, 2019, to October 14, 2020, with the help of a health science librarian. We later updated the search on March 17, 2021. The medical subheading (MeSH) terms used to develop our search included: “Frail*” AND “older adults” OR “older adults” OR “elderly” OR “older patients” OR “geriatric” AND “COVID 19” OR “coronavirus disease 2019” OR “cov-19” OR “sars-cov-2” OR “coronavirus” OR “Wuhan coronavirus” OR “novel coronavirus” AND “mortality” OR “death” OR “deceased”. We developed the search parameters for one database and later modified the parameters for the other databases (Supplementary Document 1).
Eligibility criteria
To determine the inclusion criteria, the PICOS method (Population, Intervention/issue of interest, Comparison, Outcome, and Study design) was used.21 The following were our eligibility criteria: a) patients with COVID-19 older than 65 years; b) clinical outcomes including mortality, ICU admission, and ventilator use; c) cohort studies, case–control studies, or cross-sectional studies; and d) published in the English language. Studies that were not within the scope of the PICOS criteria or did not provide access to the full text were excluded. Two authors (IDS, ISS) independently screened all relevant abstracts against the inclusion and exclusion criteria. Abstracts were coded as yes or no based on each individual's judgment against the PICOS criteria for full-text abstraction. Discrepancies were resolved through discussion and mutual consensus.
Data extraction
Two authors (IDS, SOB) independently performed the comprehensive abstraction of key data points, including author names, country of study implementation, sample size, death (total number, %, sex), number of patients who required a ventilator or were admitted to the ICU, survival time, demographics, frailty definition, tools used to measure frailty, cutoffs used to define frailty, body mass index, frailty levels, and other relevant components.
Quality assessment
Initially, we assessed the study design of the selected studies using a methodological quality assessment scale to minimize the risk of bias.22 , 23 For each reviewed source, we used the Joana Briggs Institute24 assessment tool24 for cohort studies to assess the level of evidence present. The 12-item JBI Critical Appraisal Checklist for cohort studies, which was updated and released in 2020, was used to assess the overall methodological quality, which classified overall quality as high, moderate, low, and very low.25, 26, 27, 28 Each of the 12 items was scored as 0 (high risk of bias) or 1 (low risk of bias), resulting in a total score ranging from 0 to 12, with 10–12 points categorized as high quality, 7–9 points categorized as moderate quality, 4–6 points categorized as low quality, and 0–3 points categorize as very low quality.
Statistical analysis
We calculated the pooled prevalence of mortality among frail and non-frail older adults with COVID-19 using a random-effects model with inverse variance weighting. We calculated pooled odds ratio (OR) of mortality among frail older adults relative to that of non-frail adults. Funnel plots and forest plots were generated for our analysis. The Egger's test was assessed to measure publication bias due to small sample size.29 , 30 We determined the heterogeneity of each variable assessed by a pooled estimate using I2 with a random-effects model; I2 values of 25%-49% indicated low heterogeneity, 50%-74% indicated moderate heterogeneity, and >75% indicated high heterogeneity.31 P < 0.05 was considered significant. All statistical analyses were conducted using Stata 15.0.
Results
Study selection
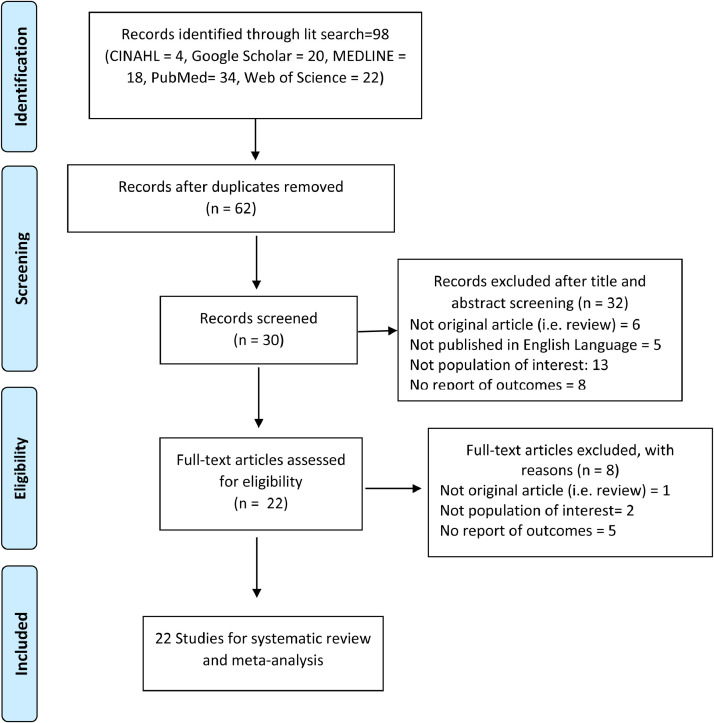
The initial search retrieved 98 articles. Using EndNote software, 36 studies were removed due to duplication. We screened a total of 62 publications during the title and abstract screening, among which 32 were deemed ineligible because they did not meet the scope of the PICOS criteria, as follows: the study included a population with COVID-19 younger than 65 years (n = 13); the study was not an original article (n = 6); the study did not provide outcomes for mortality, ICU admission, or ventilator use among frail vs. non-frail groups (n = 8); and the study was not in the English language (n = 5). A total of 30 full-text sources were screened against the full-text eligibility criteria. A total of 1 additional study was removed because it was not an original article, 2 studies were removed because the population did not include patients with COVID-19, and 5 studies were removed because they did not provide outcome results for mortality among frail vs. non-frail groups.
Finally, 22 sources were included in our final analysis.32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53 The process used to select study sources is presented in Fig. 1 through a PRISMA flow diagram.
Fig. 1.
PRISMA Diagram – process of study selection. From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097. doi:10.1371/journal.pmed10000.
Studies characteristics
Nine studies were conducted in the UK, 2 studies were conducted in Italy, and 1 study each was conducted in Australia, the Netherlands, both the UK and Italy, Belgium, Turkey, Germany, Switzerland, Ireland, Brazil, Sweden, and Europe (11 countries). A total of 924,520 patients who were confirmed as COVID-19-positive across the 22 included studies were included in our final analysis. The majority of participants were women (79%). The ages of our participants ranged from 67.5–86.3 years. Of the 19 that used CFS criteria to define frailty, 16 used the following categorization: CFS 1–3 as non-frail vs. CFS 4–9 as frail. The other 3 studies that used CFS criteria categorized CFS 1–4 as non-frail and CFS 5–9 as frail. Two studies used the frailty phenotype to define frailty, with scores of 0 categorized as non-frail and 1–5 categorized as frail. The last study used the Hospital Frailty Risk Score (HFRS) to define frailty, with HFRS < 5 defined as non-frail and HFRS ≥ 5 defined as frail. The follow-up periods ranged from 30 days to 105 days. A summary of the included studies is provided in Table 1 .
Table 1.
Summary of selected studies on frailty as predictor of mortality among older adults with COVID-19
| No | Author/year | Location | Study design | Total Sample | Men | Age | Study setting |
Follow period (days) | Frailty criteria | Frailty outcome | Frailty Status |
Mortality |
ICU admission |
Ventilators use |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. (%) | No. (%) |
No. (%) |
No. (%) |
||||||||||||||||
| Outpatient | Inpatient | Nonfrail | Frail | Nonfrail | Frail | Nonfrail | Frail | Nonfrail | Frail | ||||||||||
| 1 | (Alsahab et al., 2021) | UK | Cohort study | 4676 | 2114 | 74 | 0 (0) | 4676 (100) | Study period | CFS | CFS 1–3 ‘Non-Frail’ CFS 4–9 ‘Frail’) | 2069 (44) | 3012 (64) | NA | NA | NA | NA | NA | NA |
| 2 | (Aw, Woodrow, Ogliari, & Harwood, 2020) | UK | Cohort study | 677 | 366 | 81.1 | 0 (0) | 667 (100) | 61 | CFS | CFS 1–3 ‘Non-Frail’ CFS 4–9 ‘Frail’) | 97 (14) | 567 (84) | NA | NA | NA | NA | NA | NA |
| 3 | (Blomaard et al., 2021) | Netherlands | Cohort study | 1376 | 830 | 78 | 0 (0) | 1376 (100) | 78 | CFS | CFS 1–3 ‘Non-Frail’ CFS 4–9 ‘Frail’) | 515 (37) | 601 (44) | NA | NA | 120 (23) | 23 (4) | NA | NA |
| 4 | (Carter et al., 2020) | UK | Cohort study | 1564 | 903 | ≥ 65 | 0 (0) | 1564 (100) | 62 | CFS | CFS 1–3 ‘Non-Frail’ CFS 4–9 ‘Frail’) | 91 (6) | 1468 (93) | 7 (8) | 415 (28) | NA | NA | NA | NA |
| 5 | (Chinnadurai et al., 2020) | UK | Cohort study | 215 | 133 | 74 | 0 (0) | 215 (100) | 35 | CFS | CFS 1–4 ‘Non-Frail’ CFS 5–9 ‘Frail’) | 105 (49) | 110 (51) | 17 (16) | 69 (63) | NA | NA | NA | NA |
| 6 | (Cobos-Siles et al., 2020) | Italy | Cohort study | 128 | 73 | 84 | 0 (0) | 128 (100) | 35 | CFS | CFS 1–3 ‘Non-Frail’ CFS 4–9 ‘Frail’) | 39 (30) | 89 (70) | NA | NA | NA | NA | NA | NA |
| 7 | (Darvall et al., 2020) | Australia | Cohort study | 5607 | 3041 | ≥ 65 | 0 (0) | 5607 (100) | Study period | CFS | CFS 1–3 ‘Non-Frail’ CFS 4–9 ‘Frail’) | 3755 (67) | 1852 (33) | NA | NA | 122 (3) | 336 (18) | NA | NA |
| 8 | (Fagard et al., 2021) | Belgium | Cohort study | 105 | 55 | 82 | 0 (0) | 105 (100) | 62 | CFS | CFS 1–4 ‘Non-Frail’ CFS 5–9 ‘Frail’) | 43 | 62 | NA | NA | ||||
| 9 | (Hewitt et al., 2020) | UK and Italy | Cohort study | 1564 | 903 | ≥ 65 | 0 (0) | 1564 (100) | 62 | CFS | CFS 1–3 ‘Non-Frail’ CFS 4–9 ‘Frail’) | 91 (6) | 1468 (93) | 7 (8) | 415 (28) | NA | NA | NA | NA |
| 10 | (Ho et al., 2020) | UK | Cohort study | 502000 | 210019 | ≥ 65 | 0 (0) | 502000 (100) | Study period | Frailty Phenotype | Score 0 ‘Non-Frail’ Score 1-5 ‘Frail’) | 178687 (36) | 186401 (37) | NA | NA | NA | NA | NA | NA |
| 11 | (Kundi et al., 2020) | Turkey | Cohort study | 18234 | 8498 | 74.1 | 0 (0) | 18234 (100) | 104 | HFRS | HFRS <5 ‘Non-Frail’ HFRS ≥5 ‘Frail’) | 5814 (32) | 12420(68) | NA | NA | 975 (17) | 4146 (33) | 650 (11) | 777 (7) |
| 12 | (Labenz et al., 2020) | Germany | Cohort study | 42 | 29 | 67.5 | 0 (0) | 42 (100) | 44 | CFS | CFS 1–3 ‘Non-Frail’ CFS 4–9 ‘Frail’) | 28 (66) | 14 (33) | NA | NA | NA | NA | 6 (21) | 6 (43) |
| 13 | (Marengoni et al., 2021) | Italy | Cohort study | 165 | 100 | 69.3 | 0 (0) | 165 (100) | 41 | CFS | CFS 1–3 ‘Non-Frail’ CFS 4–9 ‘Frail’) | 142 (86) | 20 (12) | 25 (18) | 15 (75) | NA | NA | NA | NA |
| 14 | (Mendes et al., 2020) | Switzerland | Cohort study | 235 | 102 | 86.3 | 0 (0) | 235 (100) | 33 | CFS | CFS 1–3 ‘Non-Frail’ CFS 4–9 ‘Frail’) | 50 (21) | 185 (79) | 5 (10) | 71 (38) | NA | NA | NA | NA |
| 15 | (Moloney et al., 2020) | Ireland | Cohort study | 69 | 40 | 79 | 0 (0) | 69 (100) | 57 | CFS | CFS 1–4 ‘Non-Frail’ CFS 5–9 ‘Frail’) | 25 (36) | 44 (64) | NA | NA | NA | NA | 18 (72) | 25 (57) |
| 16 | (Petermann-Rocha et al., 2020) | UK | Cohort study | 383845 | 172535 | 67.1 | 0 (0) | 383845 (100) | 105 | Frailty Phenotype | Score 0 ‘Non-Frail’ Score 1-5 ‘Frail’) | 170964 (45) | 77668 (20) | NA | NA | NA | NA | NA | NA |
| 17 | (Poco et al., 2021) | Brazil | Cohort study | 711.00 | 405 | 66 | 0 (0) | 711 (100) | 52 | CFS | CFS 1–3 ‘Non-Frail’ CFS 4–9 ‘Frail’) | 530 (75) | 181 (25) | NA | NA | NA | NA | NA | NA |
| 18 | (Osuafor et al., 2020) | UK | Cohort study | 214 | 120 | 80.3 | 0 (0) | 214 (100) | 76 | CFS | CFS 1–3 ‘Non-Frail’ CFS 4–9 ‘Frail’) | 72 (34) | 142 (66) | 15 (21) | 59 (42) | NA | NA | NA | NA |
| 19 | (Owen et al., 2020) | UK | Cohort study | 1071 | 154 | 79.7 | 0 (0) | 285 (100) | 48 | CFS | CFS 1–3 ‘Non-Frail’ CFS 4–9 ‘Frail’) | 90 (8) | 462 (43) | NA | NA | NA | NA | NA | NA |
| 20 | (Sablerolles et al., 2021) | Europe (11 countries) | Cohort study | 1338 | 780 | ≥ 65 | 0 (0) | 1338 (100) | 108 | CFS | CFS 1–3 ‘Non-Frail’ CFS 4–9 ‘Frail’) | 585 (44) | 753 (56) | NA | NA | 166 (28) | 160 (21) | NA | NA |
| 21 | (Tehrani, Killander, Åstrand, Jakobsson, & Gille-Johnson, 2020) | Sweden | Cohort study | 255 | 150 | 81 | 0 (0) | 255 (100) | 56 | CFS | CFS 1–3 ‘Non-Frail’ CFS 4–9 ‘Frail’) | 38 (15) | 115 (45) | 5 (13) | 58 (50) | NA | NA | NA | NA |
| 22 | (Vlachos et al., 2021) | UK | Cohort study | 429 | 234 | ≥ 65 | 0 (0) | 429 (100) | 30 | CFS | CFS 1–3 ‘Non-Frail’ CFS 4–9 ‘Frail’) | 259 (60) | 170 (40) | NA | NA | 62 (24) | 14 (8) | NA | NA |
CFS: Clinical Frailty Scale; HFRS: Hospital Frailty Risk Score; NA: Not available.
Meta-analysis of 13 selected studies
Mortality among frail older adults with COVID-19
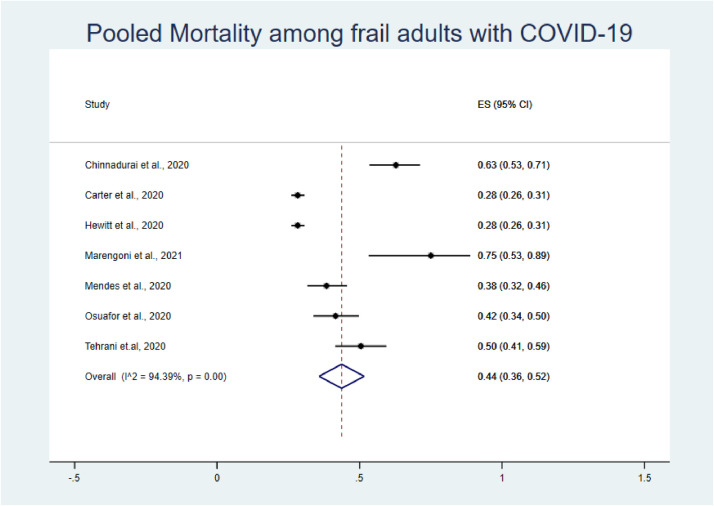
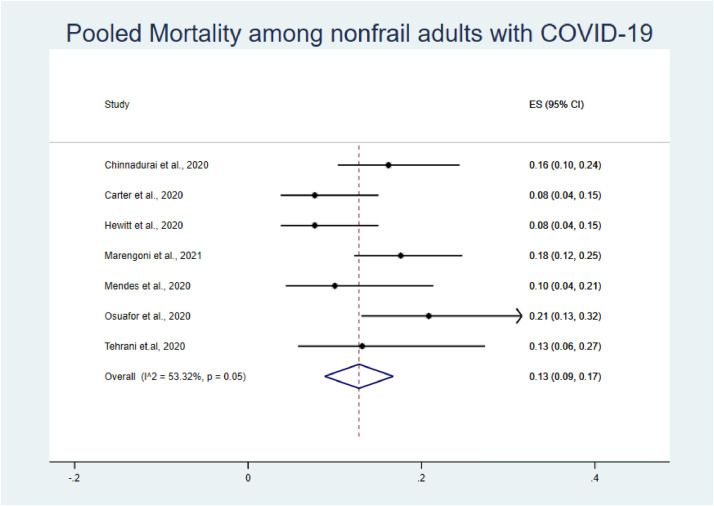
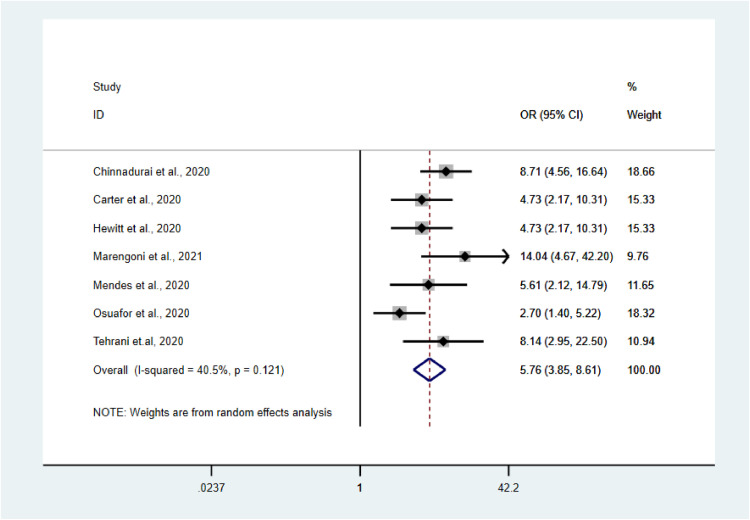
A total of 7 studies were analyzed to estimate the prevalence of mortality among frail and non-frail adults with COVID-19 and the impact of frailty on overall COVID-19-related mortality.35 , 40 , 44 , 45 , 49 , 52 , 54
The pooled prevalence of mortality among frail older adults confirmed as COVID-19-positive was higher than that among non-frail older adults (44% vs. 13%; Fig. 3, Fig. 4). The pooled OR for mortality among frail older adults compared with non-frail older adults was 5.76 (95% confidence interval [CI]: 3.85–8.61; Fig. 2 ). Our analysis showed the presence of low heterogeneity (I2 = 40.5%, p < 0.121). Egger's test was non-significant (t = −0.59, p = 0.583).
Fig. 3.
Forest plot of prevalence of mortality among frail older adults with COVID-19.
Fig. 4.
Forest plot of prevalence of mortality among non-frail older adults with COVID-19.
Fig. 2.
Forest plot of mortality among frail versus non-frail older adults with COVID-19.
Quality assessment for methodology
The JBI tool for cohort studies was used to analyze the 22 articles included in this study. All included studies were assessed with high methodological quality. In general, the strategies used to address incomplete follow-up were responsible for lower scores. One limitation in our study was the observation of asymmetry for all outcomes analyzed, indicating the presence of publication bias due to small sample size, based on the funnel plot visualization (Supplementary Document 2, Figure 5). However, Egger's regression test confirmed that the influence of publication bias was small. A summary of the quality assessments is presented in Table 2 .
Table 2.
Quality assessment of the included studies
| No | JBI checklist question | (Alsahab et al., 2021) | (Aw, Woodrow, Ogliari, & Harwood, 2020) | (Blomaard et al., 2021) | (Carter et al., 2020) | (Chinnadurai et al., 2020) | (Cobos-Siles et al., 2020) | (Darvall et al., 2020) |
|---|---|---|---|---|---|---|---|---|
| 1 | Were the two groups similar and recruited from the same population? | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 2 | Were the exposures measured similarly to assign people? | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 3 | to both exposed and unexposed groups? | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 4 | Was the exposure measured in a valid and reliable way? | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 5 | Were confounding factors identified? | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 6 | Were strategies to deal with confounding factors stated? | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 7 | Were the groups/ participants free of the outcome at the start of the study (or at the moment exposure)? | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 8 | Were the outcomes measured in a valid and reliable way? | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 9 | Was the follow up time reported and sufficient to be long enough for outcomes to occur? | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 10 | Was follow up complete, and if not, were the reasons to loss to follow up described and explored? | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 11 | Were strategies to address incomplete follow up utilized? | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 12 | Was appropriate statistical analysis used? | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Overall Appraisal | Include: 11 | Include: 11 | Include: 11 | Include: 11 | Include: 11 | Include: 11 | Include: 11 | |
| Exclude:1 | Exclude:1 | Exclude:1 | Exclude:1 | Exclude:1 | Exclude:1 | Exclude:1 | ||
| Level of evidence | 3.b cohort study | 3.b cohort study | 3.b cohort study | 3.b cohort study | 3.b cohort study | 3.b cohort study | 3.b cohort study | |
| No | JBI checklist question | (Fagard et al., 2021) | (Hewitt et al., 2020) | (Ho et al., 2020) | (Kundi et al., 2020) | (Labenz et al., 2020) | (Marengoni et al., 2021) | |
| 1 | Were the two groups similar and recruited from the same population? | 1 | 1 | 1 | 1 | 1 | 1 | |
| 2 | Were the exposures measured similarly to assign people? | 1 | 1 | 1 | 1 | 1 | 1 | |
| 3 | to both exposed and unexposed groups? | 1 | 1 | 1 | 1 | 1 | 1 | |
| 4 | Was the exposure measured in a valid and reliable way? | 1 | 1 | 1 | 1 | 1 | 1 | |
| 5 | Were confounding factors identified? | 1 | 1 | 1 | 1 | 1 | 1 | |
| 6 | Were strategies to deal with confounding factors stated? | 1 | 1 | 1 | 1 | 1 | 1 | |
| 7 | Were the groups/ participants free of the outcome at the start of the study (or at the moment exposure)? | 1 | 1 | 1 | 1 | 1 | 1 | |
| 8 | Were the outcomes measured in a valid and reliable way? | 1 | 1 | 1 | 1 | 1 | 1 | |
| 9 | Was the follow up time reported and sufficient to be long enough for outcomes to occur? | 1 | 1 | 1 | 1 | 1 | 1 | |
| 10 | Was follow up complete, and if not, were the reasons to loss to follow up described and explored? | 1 | 1 | 1 | 1 | 1 | 1 | |
| 11 | Were strategies to address incomplete follow up utilized? | 0 | 0 | 0 | 0 | 0 | 0 | |
| 12 | Was appropriate statistical analysis used? | 1 | 1 | 1 | 1 | 1 | 1 | |
| Overall Appraisal | Include: 11 | Include: 11 | Include: 11 | Include: 11 | Include: 11 | Include: 11 | ||
| Exclude:1 | Exclude:1 | Exclude:1 | Exclude:1 | Exclude:1 | Exclude:1 | |||
| Level of evidence | 3.b cohort study | 3.b cohort study | 3.b cohort study | 3.b cohort study | 3.b cohort study | 3.b cohort study | ||
| No | JBI checklist question | (Mendes et al., 2020) | (Moloney et al., 2020) | (Petermann-Rocha et al., 2020) | (Poco et al., 2021) | (Osuafor et al., 2020) | (Owen et al., 2020) | |
| 1 | Were the two groups similar and recruited from the same population? | 1 | 1 | 1 | 1 | 1 | 1 | |
| 2 | Were the exposures measured similarly to assign people? | 1 | 1 | 1 | 1 | 1 | 1 | |
| 3 | to both exposed and unexposed groups? | 1 | 1 | 1 | 1 | 1 | 1 | |
| 4 | Was the exposure measured in a valid and reliable way? | 1 | 1 | 1 | 1 | 1 | 1 | |
| 5 | Were confounding factors identified? | 1 | 1 | 1 | 1 | 1 | 1 | |
| 6 | Were strategies to deal with confounding factors stated? | 1 | 1 | 1 | 1 | 1 | 1 | |
| 7 | Were the groups/ participants free of the outcome at the start of the study (or at the moment exposure)? | 1 | 1 | 1 | 1 | 1 | 1 | |
| 8 | Were the outcomes measured in a valid and reliable way? | 1 | 1 | 1 | 1 | 1 | 1 | |
| 9 | Was the follow up time reported and sufficient to be long enough for outcomes to occur? | 1 | 1 | 1 | 1 | 1 | 1 | |
| 10 | Was follow up complete, and if not, were the reasons to loss to follow up described and explored? | 1 | 1 | 1 | 1 | 1 | 1 | |
| 11 | Were strategies to address incomplete follow up utilized? | 0 | 0 | 0 | 0 | 0 | 0 | |
| 12 | Was appropriate statistical analysis used? | 1 | 1 | 1 | 1 | 1 | 1 | |
| Overall Appraisal | Include: 11 | Include: 11 | Include: 11 | Include: 11 | Include: 11 | Include: 11 | ||
| Exclude:1 | Exclude:1 | Exclude:1 | Exclude:1 | Exclude:1 | Exclude:1 | |||
| Level of evidence | 3.b cohort study | 3.b cohort study | 3.b cohort study | 3.b cohort study | 3.b cohort study | 3.b cohort study | ||
| No | JBI checklist question | (Sablerolles et al., 2021) | (Tehrani, Killander, Åstrand, Jakobsson, & Gille-Johnson, 2020) | (Vlachos et al., 2021) | ||||
| 1 | Were the two groups similar and recruited from the same population? | 1 | 1 | 1 | ||||
| 2 | Were the exposures measured similarly to assign people? | 1 | 1 | 1 | ||||
| 3 | to both exposed and unexposed groups? | 1 | 1 | 1 | ||||
| 4 | Was the exposure measured in a valid and reliable way? | 1 | 1 | 1 | ||||
| 5 | Were confounding factors identified? | 1 | 1 | 1 | ||||
| 6 | Were strategies to deal with confounding factors stated? | 1 | 1 | 1 | ||||
| 7 | Were the groups/ participants free of the outcome at the start of the study (or at the moment exposure)? | 1 | 1 | 1 | ||||
| 8 | Were the outcomes measured in a valid and reliable way? | 1 | 1 | 1 | ||||
| 9 | Was the follow up time reported and sufficient to be long enough for outcomes to occur? | 1 | 1 | 1 | ||||
| 10 | Was follow up complete, and if not, were the reasons to loss to follow up described and explored? | 1 | 1 | 1 | ||||
| 11 | Were strategies to address incomplete follow up utilized? | 0 | 0 | 0 | ||||
| 12 | Was appropriate statistical analysis used? | 1 | 1 | 1 | ||||
| Overall Appraisal | Include: 11 | Include: 11 | Include: 11 | |||||
| Exclude:1 | Exclude:1 | Exclude:1 | ||||||
| Level of evidence | 3.b cohort study | 3.b cohort study | 3.b cohort study | |||||
Discussion
This systematic review and meta-analysis included 924,520 patients from a total of 22 studies, with the aim of assessing the aggregate impact of frailty on clinical outcomes among older adults with COVID-19. We found that the pooled prevalence of mortality among frail older adults with COVID-19 was 44%, which was higher than that of their non-frail counterparts and was associated with an overall increase in mortality odds among frail older adults with COVID-19. This finding emphasizes that in addition to underlying comorbidity profiles, frailty remains an important predictor of overall mortality.
We found that frail older adults had higher rates of mortality associated with COVID-19 compared with their non-frail counterparts. COVID-19 continues to impact people of older age with underlying comorbidities. Those with severe COVID-19 infections and lung manifestations tend to present with shortness of breath, low oxygen saturation, abnormal lung function tests, and abnormal lung computed tomography (CT) imaging that continues to persist weeks to months after the infection subsides. For those who experience a severe disease course, treatment and support with supplemental oxygen and ventilator use can sustain lung function and maintain adequate circulation.55 David Spiegelhalter stated that among people with comorbidities, “getting COVID-19 is like packing a year's worth of risk into a week or two”.56 COVID-19 infection has been associated with the occurrence of cytokine storm, hyper-inflammation, and respiratory distress, which involves the recruitment of cytokines and can initiate downstream processes, including hypercoagulation, thrombosis, and disseminated-intravascular coagulation. These processes can become exaggerated among those who have weakened immune systems, underlying comorbidities, or frailty.
Frailty represents a multidimensional concept associated with declines in multiple aspects, including physicality, functionality, cognition, and sociality.57 Frailty and COVID-19 share similar underlying biological mechanisms, including the role of the renin–angiotensin system (RAS) as an entry mechanism for SARS-CoV-2. In frailty, the RAS plays a role in the regulation of the balance between the pro-inflammatory and anti-inflammatory effects of the angiotensin (Ang) II type 1 receptor and the Ang II type 2 receptor, respectively, and disruption can result in increased inflammation, oxidative stress, and apoptosis, leading to inflammaging.58 Frailty has also been associated with poor post-vaccination immune response and increased rates of influenza-like illness and laboratory-confirmed influenza infections, highlighting similar underlying mechanisms associated with COVID-19-related outcomes among frail individuals. Frail individuals tend to present with sarcopenia, loss of muscle mass, and weak muscle functions, including the respiratory muscles, resulting in a synergistic effect on respiratory function when combined with the pneumonia progression associated with COVID-19.42 , 59
Most of the included studies (19/22) in our analyses used the CFS to predict mortality among older adults with COVID-19, as shown in Table 1. The use of CFS was suggested as a prognostic indicator of survival and predicted functional decline among older adults with COVID-19.12 A continuing debate exists regarding the assessment of frailty, in addition to age and comorbidity burden, when rationing resources during the COVID-19 pandemic.60 The authors stress that admission to acute medical care units and the allocation of resources should consider age, comorbidity status, and frailty, as measured by the CFS, when performing clinical decision-making among patients with COVID-19. One original analyzed age, comorbidities, and frailty to predict death among older adults infected with COVID-19 who required hospitalization and found that CFS was the strongest independent predictor of fatal outcomes among older adults with COVID-19 compared with age and comorbidities.61 The UK National Institute for Health and Care Excellence (NICE) and the guidelines for the German Society of Intensive Care have both endorsed the use of frailty assessment as an important factor for resource allocation.60 We found that frail older adults were more likely to require ICU admission and ventilator use when infected with COVID-19, based on the pooled analysis.
One of the strengths of the current study is that this study represents one of the first meta-analyses aimed at estimating the impacts of frailty on COVID-19 outcomes, including mortality, ICU admission, and ventilator use. An important limitation of our study involves a lack of data on clinical outcomes across all, the use of a gold standard for the measurement of frailty, and outcomes reported across categories of sex, age, and other predictors. Moreover, the screening focused only on articles published in the English language; therefore, some relevant studies published in other languages may have been omitted.
Our study further highlights the need to pay special attention to older adults who are frail or have physical limitations. We believe that this study is the first review to focus on frailty as a predictor of death among older adults infected with COVID-19. Given the multidimensional relationship among age, multimorbidity, and frailty, and the impacts of these factors on biological reserves and the immune system, further studies should provide a comprehensive assessment of the mechanisms underlying poor outcomes among frail older adults with COVID-19.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declarations of Competing Interest
None.
Footnotes
PROSPERO international prospective register of systematic reviews: Registration number CRD42020209962
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.gerinurse.2021.06.003.
Appendix. Supplementary materials
References
- 1.Cucinotta D., Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91:157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meter W. COVID-19 coronavirus pandemic 2020.
- 3.Henry B.M., de Oliveira M.H.S., Benoit S., Plebani M., Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58:1021–1028. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 4.Center for Disease Control Prevention . Division of Population Health ncfcdpahp. CDC; USA: 2020. COVID-19 guidance for older adults. [Google Scholar]
- 5.Magleby R., Westblade L.F., Trzebucki A., et al. Impact of severe acute respiratory syndrome coronavirus 2 viral load on risk of intubation and mortality among hospitalized patients with coronavirus disease 2019. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cesari M., Prince M., Thiyagarajan J.A., et al. Frailty: an emerging public health priority. J Am Med Dir Assoc. 2016;17:188–192. doi: 10.1016/j.jamda.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 7.WHO . World Health Organization; 2015. World Report on Ageing and Health. [Google Scholar]
- 8.Roppolo M., Mulasso A., Gobbens R.J., Mosso C.O., Rabaglietti E. A comparison between uni- and multidimensional frailty measures: prevalence, functional status, and relationships with disability. Clin Interv Aging. 2015;10:1669–1678. doi: 10.2147/CIA.S92328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi J., Ahn A., Kim S., Won C.W. Global prevalence of physical frailty by Fried's criteria in community-dwelling elderly with national population-based surveys. J Am Med Dir Assoc. 2015;16:548–550. doi: 10.1016/j.jamda.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Ma Y., Hou L., Yang X., et al. The association between frailty and severe disease among COVID-19 patients aged over 60 years in China: a prospective cohort study. BMC Med. 2020;18:274. doi: 10.1186/s12916-020-01761-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Adamo H., Yoshikawa T., Ouslander J.G. Coronavirus disease 2019 in geriatrics and long-term care: the ABCDs of COVID-19. J Am Geriatr Soc. 2020;68:912–917. doi: 10.1111/jgs.16445. [DOI] [PubMed] [Google Scholar]
- 12.De Smet R., Mellaerts B., Vandewinckele H., et al. Frailty and mortality in hospitalized older adults with COVID-19: retrospective observational study. J Am Med Dir Assoc. 2020;21:928–932. doi: 10.1016/j.jamda.2020.06.008. e921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hao Q., Sun X., Yang M., Dong B., Dong B., Wei Y. Prediction of mortality in Chinese very old people through the frailty index based on routine laboratory data. Sci Rep. 2019;9:221. doi: 10.1038/s41598-018-36569-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hao Q., Zhou L., Dong B., Yang M., Dong B., Weil Y. The role of frailty in predicting mortality and readmission in older adults in acute care wards: a prospective study. Sci Rep. 2019;9:1207. doi: 10.1038/s41598-018-38072-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salminen M., Viljanen A., Eloranta S., et al. Frailty and mortality: an 18-year follow-up study among Finnish community-dwelling older people. Aging Clin Exp Res. 2019 doi: 10.1007/s40520-019-01383-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walter L.C., Eng C., Covinsky K.E. Screening mammography for frail older women: what are the burdens? J Gen Intern Med. 2001;16:779–784. doi: 10.1111/j.1525-1497.2001.10113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Makary M.A., Segev D.L., Pronovost P.J., et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210:901–908. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 18.Crow R.S., Lohman M.C., Titus A.J., et al. Mortality risk along the frailty spectrum: data from the national health and nutrition examination survey 1999 to 2004. J Am Geriatr Soc. 2018;66:496–502. doi: 10.1111/jgs.15220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abbatecola A.M., Antonelli-Incalzi R. Editorial: COVID-19 spiraling of frailty in older Italian patients. J Nutr Health Aging. 2020;24:453–455. doi: 10.1007/s12603-020-1357-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moher D., Liberati A., Tetzlaff J., Altman D.G., The P.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- 21.Liberati A., Altman D.G., Tetzlaff J., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma L.-L., Wang Y.-Y., Yang Z.-H., Huang D., Weng H., Zeng X.-T. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Milit Med Res. 2020;7:7. doi: 10.1186/s40779-020-00238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Viswanathan M., Patnode C.D., Berkman N.D., et al. 2017.
- 24.JBI. Checklist for cohort studies: critical appraisal tools for use in JBI systematic reviews 2020.
- 25.The University of Adelaide . Global; 2020. Checklist for Cohort Studies: Critical Appraisal Tools for use in JBI Systematic Reviews: JBI. [Google Scholar]
- 26.Buccheri R.K., Sharifi C. Critical appraisal tools and reporting guidelines for evidence-based practice. Worldviews Evid Based Nurs. 2017;14:463–472. doi: 10.1111/wvn.12258. [DOI] [PubMed] [Google Scholar]
- 27.Morgan R.L., Thayer K.A., Bero L., et al. GRADE: assessing the quality of evidence in environmental and occupational health. Environ Int. 2016;92-93:611–616. doi: 10.1016/j.envint.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Institute J.B. The Joanna Briggs Institute Adelaide; 2016. Summary of Findings Tables for Joanna Briggs Institute Systematic Reviews. [Google Scholar]
- 29.Peters J.L., Sutton A.J., Jones D.R., Abrams K.R., Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295:676–680. doi: 10.1001/jama.295.6.676. [DOI] [PubMed] [Google Scholar]
- 30.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huedo-Medina T.B., Sanchez-Meca J., Marin-Martinez F., Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11:193–206. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- 32.Alsahab M., Beishon L., Brown B., et al. Age and frailty are independently associated with increased COVID-19 mortality and increased care needs in survivors: results of an international multi-centre study. Age Ageing. 2021 doi: 10.1093/ageing/afab026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aw D., Woodrow L., Ogliari G., Harwood R. Association of frailty with mortality in older inpatients with COVID-19: a cohort study. Age Ageing. 2020 doi: 10.1093/ageing/afaa184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blomaard L.C., van der Linden C.M.J., van der Bol J.M., et al. Frailty is associated with in-hospital mortality in older hospitalised COVID-19 patients in the Netherlands: the COVID-OLD study. Age Ageing. 2021 doi: 10.1093/ageing/afab018. afab018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carter B., Collins J.T., Barlow-Pay F., et al. Nosocomial COVID-19 infection: examining the risk of mortality. The COPE-nosocomial study (COVID in Older PEople) J Hosp Infect. 2020;106:376–384. doi: 10.1016/j.jhin.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chinnadurai R, Ogedengbe O, Agarwal P, et al. Older age and frailty are the chief predictors of mortality in COVID-19 patients admitted to an acute medical unit in a secondary care setting- a cohort study. BMC Geriatrics. 2020;20:409. doi: 10.1186/s12877-020-01803-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cobos-Siles M., Cubero-Morais P., Arroyo-Jimenez I., et al. Cause-specific death in hospitalized individuals infected with SARS-CoV-2: more than just acute respiratory failure or thromboembolic events. Intern Emerg Med. 2020 doi: 10.1007/s11739-020-02485-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Darvall J.N., Bellomo R., Bailey M., et al. Frailty and outcomes from pneumonia in critical illness: a population-based cohort study. Br J Anaesth. 2020;125:730–738. doi: 10.1016/j.bja.2020.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fagard K., Gielen E., Deschodt M., Devriendt E., Flamaing J. Risk factors for severe COVID-19 disease and death in patients aged 70 and over: a retrospective observational cohort study. Acta Clin Belg. 2021:1–8. doi: 10.1080/17843286.2021.1890452. [DOI] [PubMed] [Google Scholar]
- 40.Hewitt J., Carter B., Vilches-Moraga A., et al. The effect of frailty on survival in patients with COVID-19 (COPE): a multicentre, European, observational cohort study. The Lancet Public Health. 2020;5:e444–e451. doi: 10.1016/S2468-2667(20)30146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ho F.K., Petermann-Rocha F., Gray S.R., et al. Is older age associated with COVID-19 mortality in the absence of other risk factors? General population cohort study of 470,034 participants. PLOS ONE. 2020;15 doi: 10.1371/journal.pone.0241824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kundi H., Cetin E.H.O., Canpolat U., et al. The role of frailty on adverse outcomes among older patients with COVID-19. J Infect. 2020 doi: 10.1016/j.jinf.2020.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Labenz C., Kremer W.M., Schattenberg J.M., et al. Clinical frailty scale for risk stratification in patients with SARS-CoV-2 infection. J Investig Med. 2020;68:1199–1202. doi: 10.1136/jim-2020-001410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marengoni A., Zucchelli A., Vetrano D.L., et al. Beyond chronological age: Frailty and multimorbidity predict in-hospital mortality in patients with coronavirus disease 2019. J Gerontol: Series A. 2021;76:e38–e45. doi: 10.1093/gerona/glaa291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mendes A., Serratrice C., Herrmann F.R., et al. Predictors of in-hospital mortality in older patients with COVID-19: the COVIDAge study. J Am Med Dir Assoc. 2020;21:1546–1554. doi: 10.1016/j.jamda.2020.09.014. e1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moloney E., Eustace J., O'Caoimh R., et al. 2020. Frailty, COVID-19 Disease Severity and Outcome Among Hospitalised Older Adults. [Google Scholar]
- 47.Petermann-Rocha F., Hanlon P., Gray S.R., et al. Comparison of two different frailty measurements and risk of hospitalisation or death from COVID-19: findings from UK Biobank. BMC Med. 2020;18:1–9. doi: 10.1186/s12916-020-01822-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Poco P.C.E., Aliberti M.J.R., Dias M.B., et al. Divergent: age, frailty, and atypical presentations of COVID-19 in hospitalized patients. J Gerontol: Series A. 2021;76:e46–e51. doi: 10.1093/gerona/glaa280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Osuafor CN, Davidson C, Mackett AJ, et al. 2020.
- 50.Owen R.K., Conroy S.P., Taub N., et al. Comparing associations between frailty and mortality in hospitalised older adults with or without COVID-19 infection: a retrospective observational study using electronic health records. Age Ageing. 2020 doi: 10.1093/ageing/afaa167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sablerolles R.S.G., Lafeber M., van Kempen J.A.L., et al. Association between Clinical Frailty Scale score and hospital mortality in adult patients with COVID-19 (COMET): an international, multicentre, retrospective, observational cohort study. Lancet Healthy Longevity. 2021;2:e163–e170. doi: 10.1016/S2666-7568(21)00006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tehrani S., Killander A., Åstrand P., Jakobsson J., Gille-Johnson P. Risk factors for mortality in adult COVID-19 patients; frailty predicts fatal outcome in older patients. Int J Infect Dis. 2020 doi: 10.1016/j.ijid.2020.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vlachos S., Wong A., Metaxa V., et al. Hospital mortality and resource implications of hospitalisation with COVID-19 in London, UK: a prospective cohort study. Crit Care Res Pract. 2021;2021 doi: 10.1155/2021/8832660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chinnadurai R., Ogedengbe O., Agarwal P., et al. Older age and frailty are the chief predictors of mortality in COVID-19 patients admitted to an acute medical unit in a secondary care setting- a cohort study. BMC Geriatr. 2020;20:409. doi: 10.1186/s12877-020-01803-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mo X., Jian W., Su Z., et al. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur Respir J. 2020;55 doi: 10.1183/13993003.01217-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blagosklonny M.V. From causes of aging to death from COVID-19. Aging. 2020;12:10004–10021. doi: 10.18632/aging.103493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sacha J., Sacha M., Soboń J., Borysiuk Z., Feusette P. Is it time to begin a public campaign concerning frailty and pre-frailty? A review article. Front Physiol. 2017;8 doi: 10.3389/fphys.2017.00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xue Q.-L. Frailty as an integrative marker of physiological vulnerability in the era of COVID-19. BMC Med. 2020;18:333. doi: 10.1186/s12916-020-01809-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kundi H., Wadhera R.K., Strom J.B., et al. Association of frailty with 30-day outcomes for acute myocardial infarction, heart failure, and pneumonia among elderly adults. JAMA Cardiol. 2019;4:1084–1091. doi: 10.1001/jamacardio.2019.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nickel C.H., Rueegg M., Pargger H., Bingisser R. Age, comorbidity, frailty status: effects on disposition and resource allocation during the COVID-19 pandemic. Swiss Med Wkly. 2020;150:w20269. doi: 10.4414/smw.2020.20269. [DOI] [PubMed] [Google Scholar]
- 61.Tehrani S., Killander A., Åstrand P., Jakobsson J., Gille-Johnson P. Risk factors for death in adult COVID-19 patients: Frailty predicts fatal outcome in older patients. Int J Infect Dis. 2021;102:415–421. doi: 10.1016/j.ijid.2020.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.