Abstract
Objective
To describe temporal changes in treatment, care, and short-term mortality outcomes of geriatric patients during the first wave of the COVID-19 pandemic.
Design
Observational study.
Setting and Participants
Altogether 1785 patients diagnosed with COVID-19 and 6744 hospitalized for non–COVID-19 causes at 7 geriatric clinics in Stockholm from March 6 to July 31, 2020, were included.
Methods
Across admission month, patient vital signs and pharmacological treatment in relationship to risk for in-hospital death were analyzed using the Poisson regression model. Incidence rates (IRs) and incidence rate ratios (IRRs) of death are presented.
Results
In patients with COVID-19, the IR of mortality were 27%, 17%, 10%, 8%, and 2% from March to July, respectively, after standardization for demographics and vital signs. Compared with patients admitted in March, the risk of in-hospital death decreased by 29% [IRR 0.71, 95% confidence interval (CI) 0.51-0.99] in April, 61% (0.39, 0.26-0.58) in May, 68% (0.32, 0.19-0.55) in June, and 86% (0.14, 0.03-0.58) in July. The proportion of patients admitted for geriatric care with oxygen saturation <90% decreased from 13% to 1%, which partly explains the improvement of COVID-19 patient survival. In non–COVID-19 patients during the pandemic, mortality rates remained relatively stable (IR 1.3%-2.3%). Compared with non–COVID-19 geriatric patients, the IRR of death declined from 11 times higher (IRR 11.7, 95% CI 6.11-22.3) to 1.6 times (2.61, 0.50-13.7) between March and July in patients with COVID-19.
Conclusions and Implications
Mortality risk in geriatric patients from the Stockholm region declined over time throughout the first pandemic wave of COVID-19. The improved survival rate over time was only partly related to improvement in saturation status at the admission of the patients hospitalized later throughout the pandemic. Lower incidence during the later months could have led to less severe hospitalized cases driving down mortality.
Keywords: COVID-19, geriatric, mortality
COVID-19 has affected around 150 million people worldwide. On January 31, 2020, Sweden had its first COVID-19 case, and currently, more than 1 million cases have been reported. The Stockholm region, which accounts for 20% of the Swedish population, was severely affected during the pandemic and has suffered a high mortality rate among older patients.1
Research to understand this new disease began with the first cohorts in Wuhan and has become an unprecedented global effort. To date, thrombosis prophylaxis with low-molecular-weight heparin (LMWH), dexamethasone in patients receiving respiratory support, and more recently, remdesivir, have become treatment options frequently used in hospitalized patients,2, 3, 4, 5, 6 whereas hydroxychloroquine-chloroquine, lopinavir-ritonavir, or convalescent plasma have been progressively abandoned.7, 8, 9
General care for patients with COVID-19 has probably improved in Stockholm, for example, because of lower risk of shortages in personal protective equipment, increased testing capacity, increased capacity for noninvasive ventilation support in geriatrics, and repurposed hospital beds. Medical and nursing expertise and experience have increased throughout the pandemic. Social distancing and seasonal effects might have led to lower viral doses and milder infections.
This study analyzes temporal changes in demographics, severity, treatment, and care in relationship to mortality outcomes of patients treated at geriatric clinics in the Stockholm Region of Sweden during the first wave of the COVID-19 pandemic. Furthermore, we have compared mortality rates between COVID-19 and non–COVID-19 patients hospitalized in geriatric clinics during the same period to account for background trends in mortality outcomes resulting from disrupted health care during the pandemic.
Methods
Study Population
In the present study, we included all patients who were admitted to 7 geriatric clinics in Stockholm, Sweden, from March 6 to August 31, 2020. The geriatric clinics in the Stockholm area enroll patients who are biologically aged with reduced physical and/or cognitive function and multiple comorbidities and in need of geriatric medical care and/or rehabilitation. We excluded patients (1) hospitalized for less than 24 hours (n = 7) or (2) missing information regarding initial vital signs (n = 34), or (3) with admission date after July 31, 2020 (n = 12). A total of 1785 patients hospitalized with the COVID-19 diagnosis were included, together with 6744 patients with non–COVID-19 diagnoses also hospitalized during the same time period (Supplementary Figure 1).
Supplementary Figure 1.
Flowchart.
COVID-19 Diagnosis and Covariates
The COVID-19 diagnosis was based either on a positive reverse transcriptase–polymerase chain reaction (RT-PCR) analysis from nasopharyngeal swabs or for symptomatic patients with a negative RT-PCR but with a typical clinical diagnosis (including a consultation with a specialist in infectious diseases) and a CT scan of the chest with typical COVID-19 findings (ground-glass opacities). Information regarding patient demographics, initial vital signs, medications, diagnoses at discharge, and in-hospital death were collected through the hospital electronic health records. The definition of diagnoses was based on the International Classification of Diseases (ICD)–10 code.10 Diabetes and dementia were further enriched with information on the current prescription of related medications. All diagnoses present in this data set were the diagnoses from the discharge records. They were only used for the description of the patient population and not used as control variables to avoid reverse causation. Medications were defined as medications that patients were prescribed within 24 hours after admission.
Exposures
The main exposure was the month of hospital admission. Five monthly blocks were studied—March, April, May, June, and July—and individual data level was used for analysis. Patients admitted in March served as the reference group because patients in March had the highest incidence rate of in-hospital death.
Because the month for those admitting in March 2020 was not a full month, we also set the secondary exposure as to how many days into the pandemic (between March 6 and July 31) the day of admission to the geriatric clinic for each patient occurred. This number was calculated as the difference between the date of admission for each given patient minus the date of admission of the first patient in the cohort.
Outcome
The main study outcome was in-hospital death. Patients were censored at discharge from hospital, death, or transfer to other departments/clinics, other hospitals, or nursing homes, whichever occurred first.
Statistical Analysis
Continuous variables were displayed as mean ± standard deviation (SD) or median (interquartile range) and categorical variables as proportions. Differences over time were tested with the Jonckheere-Terpstra trend test for continuous data and with linear-by-linear trend test for categorical variables. We evaluated trends in patient admission characteristics, initial vital signs, and use of selected medications within 1-month blocks.
We then evaluated the incidence of in-hospital death using standardized incidence rates with 95% confidence intervals (CIs) via logistic regression models to account for differences in patient characteristics over admission month.11 Stepwise adjustment for explanatory variables included (1) age and sex and (2) initial vital signs.
Via Poisson regression models, we explored whether adjustment for implementation of treatments modified the risk of in-hospital death. To assess the effect of admission month on this outcome, similar stepwise adjustments were performed for (1) age and sex, (2) initial vital signs, and (3) medical treatment. Results were reported as incidence rate ratios (IRRs) and 95% CIs.
The days into the pandemic for each patient were introduced as a cubic spline with knots at 10, 50, 90 percentiles. The model was adjusted for age, sex, initial vital signs, and medications.
Several sensitivity and subgroup analyses were performed to test the robustness of the results. First, to explore the possibility of detection bias, we excluded patients who were transferred to other departments or hospitals (n = 152, 8.5% of all patients). Second, we explored the associations between admission month and in-hospital death in prespecified strata, including sex, age, initial saturation (<90% and ≥90%), and their interactions. In addition, the role of low saturation (<90%) and selected treatment in this association were tested through mediation analyses. The mediating effect was analyzed using the generalized structural equation model.12
Finally, survival trends were also compared against non–COVID-19 patients admitted during the corresponding period, in order to account for mortality changes in the underlying background population.
There are no missing variables to report, and all analyses were performed using R (https://www.r-project.org) and Stata version 16.0 (StataCorp, College Station, TX).
Ethical Statement
The Swedish Ethical Review Authority approved the study (Dnr 2020-02146, and 2020-03345).
Results
Changes in Admission Characteristics and Medications
We identified 1785 patients diagnosed with COVID-19 from 7 geriatric clinics in Stockholm from March 6 to July 31; 1675 patients had a positive COVID-19 RT-PCR (94%), whereas 110 (6%) had negative RT-PCR but were diagnosed with COVID-19 based on typical symptoms and typical findings on a CT scan with no other explanation of the symptoms. Fifty-three percent were women, and the mean age was 83 ± 9 years. Mean initial oxygen saturation was 95 ± 6%, 6% of patients had low saturation (<90%), and the rest had saturation ≥90%. Forty-seven percent of COVID-19 patients were treated with angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, 51% used β-blockers, 29% used calcium-channel blockers, 37% were prescribed statins and 53% used diuretics. LMWHs were used in 45%, non–vitamin K antagonist oral anticoagulants (NOACs) in 30%, warfarin in 7%, antibiotics in 39%, and glucocorticoids in 17% of COVID-19 patients within the first 24 hours of admission. In addition to COVID-19, the most common diagnoses were hypertension (46%), followed by diabetes (36%), atrial fibrillation (31%), heart failure (23%), pulmonary disease (19%), chronic kidney disease (17%), and dementia (16%) (Table 1 ).
Table 1.
Characteristics Among COVID-19 and Non–COVID-19 Patients in Geriatric Clinics During the First Wave of the Pandemic by Admission Month
| COVID-19, n | Overall |
March |
April |
May |
June |
July |
P Value |
|---|---|---|---|---|---|---|---|
| 1785 | 195 | 726 | 524 | 271 | 69 | ||
| Confirmed, n (%) | 1675 (93.8) | 183 (93.8) | 687 (94.6) | 494 (94.3) | 250 (92.3) | 61 (88.4) | .23 |
| Suspected, n (%) | 110 (6.2) | 12 (6.2) | 39 (5.4) | 30 (5.7) | 21 (7.7) | 8 (11.6) | |
| Admission from first patient in the cohort, d, median (IQR) | 54.0 (35.0, 79.0) | 20.0 (15.0, 24.0) | 40.0 (33.0, 47.0) | 69.0 (62.0, 77.0) | 97.0 (91.0, 104.0) | 126.0 (121.0, 133.0) | <.001 |
| Age, y, mean (SD) | 82.8 (8.6) | 83.2 (7.6) | 82.5 (8.7) | 83.1 (8.7) | 83.1 (8.5) | 81.9 (8.1) | .55 |
| Age groups, n (%) | .10 | ||||||
| <80 y | 688 (38.5) | 71 (36.4) | 291 (40.1) | 198 (37.8) | 104 (38.4) | 24 (34.8) | |
| 80-<90 y | 656 (36.8) | 83 (42.6) | 260 (35.8) | 181 (34.5) | 97 (35.8) | 35 (50.7) | |
| ≥90 y | 441 (24.7) | 41 (21.0) | 175 (24.1) | 145 (27.7) | 70 (25.8) | 10 (14.5) | |
| Female, % | 939 (52.6) | 110 (56.4) | 376 (51.8) | 277 (52.9) | 143 (52.8) | 33 (47.8) | .74 |
| Diagnoses, n (%) | |||||||
| Hypertension | 826 (46.3) | 74 (37.9) | 341 (47.0) | 246 (46.9) | 133 (49.1) | 32 (46.4) | .16 |
| Diabetes mellitus | 647 (36.2) | 58 (29.7) | 269 (37.1) | 194 (37.0) | 107 (39.5) | 19 (27.5) | .12 |
| Heart failure | 414 (23.2) | 46 (23.6) | 163 (22.5) | 125 (23.9) | 68 (25.1) | 12 (17.4) | .69 |
| Myocardial infarction | 85 (4.8) | 10 (5.1) | 36 (5.0) | 26 (5.0) | 10 (3.7) | 3 (4.3) | .93 |
| Pulmonary disease | 339 (19.0) | 44 (22.6) | 131 (18.0) | 108 (20.6) | 46 (17.0) | 10 (14.5) | .34 |
| Asthma | 73 (4.1) | 9 (4.6) | 32 (4.4) | 24 (4.6) | 6 (2.2) | 2 (2.9) | .50 |
| Cancer | 155 (8.7) | 17 (8.7) | 64 (8.8) | 39 (7.4) | 26 (9.6) | 9 (13.0) | .56 |
| Stroke | 150 (8.4) | 22 (11.3) | 48 (6.6) | 50 (9.5) | 26 (9.6) | 4 (5.8) | .13 |
| Atrial fibrillation | 556 (31.1) | 59 (30.3) | 205 (28.2) | 184 (35.1) | 83 (30.6) | 25 (36.2) | .11 |
| Dementia | 286 (16.0) | 29 (14.9) | 109 (15.0) | 93 (17.7) | 47 (17.3) | 8 (11.6) | .52 |
| Vital signs at admission, mean (SD) | |||||||
| Temperature, °C | 37.1 (0.7) | 37.1 (0.8) | 37.1 (0.7) | 37.0 (0.6) | 37.0 (0.6) | 37.0 (0.6) | .01 |
| SBP, mm Hg | 131.9 (21.2) | 132.6 (19.5) | 132.1 (21.6) | 132.2 (21.6) | 131.0 (21.3) | 129.4 (18.5) | .77 |
| DBP, mm Hg | 73.1 (11.5) | 72.8 (11.0) | 72.8 (11.9) | 73.7 (11.8) | 72.7 (10.7) | 73.1 (10.8) | .65 |
| Pulse rate, per minute | 79.8 (14.3) | 79.8 (15.8) | 79.7 (13.6) | 79.5 (14.2) | 80.1 (15.2) | 82.6 (13.6) | .53 |
| Oxygen saturation, % | 94.6 (5.5) | 93.2 (8.8) | 94.4 (6.3) | 95.0 (3.4) | 95.4 (3.3) | 95.9 (3.3) | <.001 |
| Saturation <90%, n (%) | 115 (6.4) | 25 (12.8) | 55 (7.6) | 25 (4.8) | 9 (3.3) | 1 (1.4) | <.001 |
| Medications at admission to geriatric hospital, n (%) | |||||||
| ACEI | 415 (23.2) | 47 (24.1) | 176 (24.2) | 120 (22.9) | 60 (22.1) | 12 (17.4) | .73 |
| ARB | 448 (25.1) | 47 (24.1) | 185 (25.5) | 120 (22.9) | 74 (27.3) | 22 (31.9) | .42 |
| β-blockers | 905 (50.7) | 110 (56.4) | 361 (49.7) | 258 (49.2) | 138 (50.9) | 38 (55.1) | .43 |
| CCB | 521 (29.2) | 57 (29.2) | 210 (28.9) | 148 (28.2) | 79 (29.2) | 27 (39.1) | .47 |
| Diuretics | 941 (52.7) | 99 (50.8) | 390 (53.7) | 271 (51.7) | 145 (53.5) | 36 (52.2) | .93 |
| Statins | 656 (36.8) | 73 (37.4) | 262 (36.1) | 193 (36.8) | 101 (37.3) | 27 (39.1) | .98 |
| Warfarin | 127 (7.1) | 19 (9.7) | 49 (6.7) | 36 (6.9) | 15 (5.5) | 7 (10.1) | .37 |
| NOAC | 532 (29.6) | 51 (26.2) | 180 (24.8) | 182 (34.7) | 86 (31.7) | 29 (42.0) | .001 |
| LMWH | 802 (44.6) | 33 (16.9) | 335 (46.1) | 247 (47.1) | 153 (56.5) | 28 (40.6) | <.001 |
| Antiplatelets | 583 (32.7) | 59 (30.3) | 246 (33.9) | 157 (30.0) | 96 (35.4) | 25 (36.2) | .39 |
| NSAID | 56 (3.1) | 5 (2.6) | 28 (3.9) | 16 (3.1) | 6 (2.2) | 1 (1.4) | .58 |
| Glucocorticoid | 301 (16.9) | 37 (19.0) | 107 (14.7) | 93 (17.7) | 49 (18.1) | 15 (21.7) | .32 |
| Antibiotics | 688 (38.5) | 93 (47.7) | 297 (40.9) | 182 (34.7) | 90 (33.2) | 26 (37.7) | .01 |
| Hospital stay, d, median (IQR) | 9.0 (6.0, 14.0) | 10.0 (6.0, 17.0) | 9.0 (6.0, 14.0) | 10.0 (7.0, 15.0) | 9.0 (6.0, 13.0) | 9.0 (6.0, 14.0) | .21 |
| In-hospital death, n (%) |
258 (14.5) |
54 (27.7) |
129 (17.8) |
52 (9.9) |
21 (7.7) |
2 (2.9) |
<.001 |
| Non–COVID-19, n |
6744 |
803 |
1095 |
1253 |
1610 |
1983 |
|
| Age, y, mean (SD) | 83.5 (8.2) | 83.5 (8.4) | 83.5 (8.2) | 83.6 (8.4) | 83.6 (8.2) | 83.4 (8.1) | .88 |
| Age groups, n (%) | .97 | ||||||
| <80 y | 2322 (34.4) | 264 (32.9) | 380 (34.7) | 426 (34.0) | 552 (34.3) | 700 (35.3) | |
| 80-<90 y | 2664 (39.5) | 333 (41.5) | 432 (39.5) | 493 (39.3) | 637 (39.6) | 769 (38.8) | |
| ≥90 y | 1758 (26.1) | 206 (25.7) | 283 (25.8) | 334 (26.7) | 421 (26.1) | 514 (25.9) | |
| Female, n (%) | 3983 (59.1) | 472 (58.8) | 651 (59.5) | 740 (59.1) | 932 (57.9) | 1188 (59.9) | .81 |
| Diagnoses, n (%) | |||||||
| Hypertension | 2824 (41.9) | 320 (39.9) | 503 (45.9) | 530 (42.3) | 647 (40.2) | 824 (41.6) | .03 |
| Diabetes mellitus | 1919 (28.5) | 251 (31.3) | 329 (30.0) | 344 (27.5) | 428 (26.6) | 567 (28.6) | .10 |
| Heart failure | 1621 (24.0) | 235 (29.3) | 246 (22.5) | 316 (25.2) | 372 (23.1) | 452 (22.8) | .01 |
| Myocardial infarction | 356 (5.3) | 47 (5.9) | 67 (6.1) | 60 (4.8) | 73 (4.5) | 109 (5.5) | .33 |
| Pulmonary disease | 1075 (15.9) | 145 (18.1) | 158 (14.4) | 200 (16.0) | 270 (16.8) | 302 (15.2) | .19 |
| Asthma | 138 (2.0) | 17 (2.1) | 28 (2.6) | 30 (2.4) | 30 (1.9) | 33 (1.7) | .42 |
| Cancer | 617 (9.1) | 73 (9.1) | 94 (8.6) | 119 (9.5) | 148 (9.2) | 183 (9.2) | .96 |
| Stroke | 690 (10.2) | 71 (8.8) | 113 (10.3) | 128 (10.2) | 170 (10.6) | 208 (10.5) | .73 |
| Atrial fibrillation | 2178 (32.3) | 265 (33.0) | 358 (32.7) | 443 (35.4) | 507 (31.5) | 605 (30.5) | .06 |
| Dementia | 1042 (15.5) | 114 (14.2) | 162 (14.8) | 210 (16.8) | 254 (15.8) | 302 (15.2) | .53 |
| Vital signs at admission, mean (SD) | |||||||
| Temperature, °C | 36.8 (0.5) | 36.8 (0.5) | 36.8 (0.5) | 36.8 (0.5) | 36.9 (0.5) | 36.8 (0.5) | <.001 |
| SBP, mm Hg | 131.3 (25.0) | 132.1 (21.9) | 132.1 (21.0) | 131.0 (20.9) | 130.7 (34.2) | 131.3 (21.6) | .56 |
| DBP, mm Hg | 72.6 (11.4) | 72.7 (12.0) | 73.0 (11.8) | 72.9 (11.2) | 72.1 (11.5) | 72.5 (11.1) | .29 |
| Pulse rate, per minute | 77.8 (13.7) | 78.8 (13.6) | 78.1 (13.7) | 77.6 (13.5) | 77.7 (13.8) | 77.4 (13.9) | .13 |
| Oxygen saturation, % | 96.0 (3.9) | 95.8 (3.9) | 95.9 (4.1) | 96.1 (3.0) | 95.9 (4.5) | 96.2 (3.7) | .02 |
| Saturation <90%, n (%) | 208 (3.1) | 44 (5.5) | 36 (3.3) | 33 (2.6) | 50 (3.1) | 45 (2.3) | .01 |
| Medications at admission to geriatric hospital, n (%) | |||||||
| ACEI | 1542 (22.9) | 198 (24.7) | 239 (21.8) | 273 (21.8) | 387 (24.0) | 445 (22.4) | .35 |
| ARB | 1640 (24.3) | 184 (22.9) | 240 (21.9) | 322 (25.7) | 380 (23.6) | 514 (25.9) | .07 |
| β-blocker | 3527 (52.3) | 443 (55.2) | 549 (50.1) | 663 (52.9) | 826 (51.3) | 1046 (52.7) | .22 |
| CCB | 1967 (29.2) | 218 (27.1) | 320 (29.2) | 354 (28.3) | 470 (29.2) | 605 (30.5) | .43 |
| Diuretics | 3542 (52.5) | 435 (54.2) | 545 (49.8) | 658 (52.5) | 860 (53.4) | 1044 (52.6) | .32 |
| Statins | 2510 (37.2) | 301 (37.5) | 394 (36.0) | 471 (37.6) | 603 (37.5) | 741 (37.4) | .93 |
| Warfarin | 494 (7.3) | 78 (9.7) | 91 (8.3) | 92 (7.3) | 103 (6.4) | 130 (6.6) | .02 |
| NOAC | 1942 (28.8) | 222 (27.6) | 278 (25.4) | 371 (29.6) | 486 (30.2) | 585 (29.5) | .06 |
| LMWH | 1322 (19.6) | 164 (20.4) | 232 (21.2) | 252 (20.1) | 286 (17.8) | 388 (19.6) | .22 |
| Antiplatelets | 2131 (31.6) | 238 (29.6) | 336 (30.7) | 397 (31.7) | 512 (31.8) | 648 (32.7) | .57 |
| NSAID | 309 (4.6) | 32 (4.0) | 52 (4.7) | 47 (3.8) | 77 (4.8) | 101 (5.1) | .40 |
| Glucocorticoid | 1054 (15.6) | 149 (18.6) | 147 (13.4) | 181 (14.4) | 241 (15.0) | 336 (16.9) | .01 |
| Antibiotics | 2316 (34.3) | 308 (38.4) | 371 (33.9) | 408 (32.6) | 542 (33.7) | 687 (34.6) | .09 |
| Hospital days, median (IQR) | 7.0 (5.0, 9.0) | 7.0 (5.0, 10.0) | 6.0 (4.0, 9.0) | 7.0 (5.0, 9.0) | 7.0 (5.0, 10.0) | 7.0 (5.0, 9.0) | <.001 |
| In-hospital death, n (%) | 102 (1.5) | 13 (1.6) | 26 (2.4) | 16 (1.3) | 22 (1.4) | 25 (1.3) | .13 |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CCB, calcium channel blocker; DBP, diastolic blood pressure; IQR, interquartile range; NSAID, nonsteroidal anti-inflammatory drug; SBP, systolic blood pressure; SD, standard deviation.
Numbers are % or mean (SD) or median (IQR) as appropriate. P values were tested with the Jonckheere-Terpstra trend test for continuous data and with linear-by-linear trend test for categorical variables.
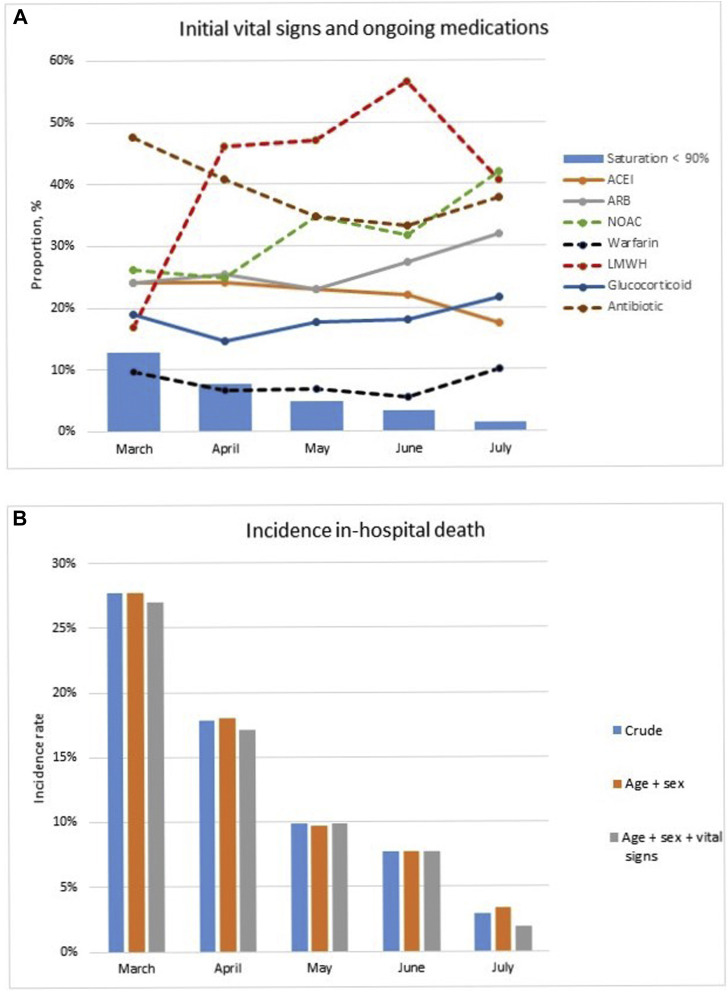
Across the 1-month blocks, the proportion of patients arriving at the hospital with low saturation (<90%) decreased over time from 13% in March to 1% in July, and the use of antibiotics decreased from 48% to 38%, whereas prescription of LMWHs increased from 17% in March to 41% in July and NOACs from 26% to 42%. However, there were no differences in age, sex, and other medication use over time (Table 1, Figure 1 A).
Fig. 1.
Trend of (A) initial vital signs and ongoing medications, and (B) incidence of in-hospital death of COVID-19 patients. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Changes in In-Hospital Death
From March to July, we observed a decreased incidence rate in in-hospital death in the unadjusted model. After standardization for age, gender, and vital signs, the in-hospital death was 27% (March), 17% (April), 10% (May), 8% (June), and 2% (July) (Table 2 , Figure 1B).
Table 2.
Incidence Rate and Incidence Rate Ratio for In-Hospital Death Among COVID-19 and Non–COVID-19 Patients During the Pandemic
| Admission Month | Death, n (%) | Model 1, IR, % (95% CI) | Model 2, IR, % (95% CI) | Model 1, IRR (95% CI) | Model 2, IRR (95% CI) | Model 3, IRR (95% CI) |
|---|---|---|---|---|---|---|
| COVID-19 | ||||||
| March (n = 195) | 54 (27.7) | 27.7 (0.216, 0.338) | 27.0 (0.209, 0.331) | Ref | Ref | Ref |
| April (n = 726) | 129 (17.8) | 18.0 (0.153, 0.207) | 17.1 (0.146, 0.196) | 0.62 (0.45, 0.86)∗ | 0.70 (0.51, 0.98)† | 0.71 (0.51, 0.99)† |
| May (n = 524) | 52 (9.9) | 9.7 (0.073, 0.122) | 9.9 (0.076, 0.121) | 0.31 (0.21, 0.46)‡ | 0.37 (0.25, 0.55)‡ | 0.39 (0.26, 0.58)‡ |
| June (n = 271) | 21 (7.7) | 7.7 (0.046, 0.108) | 7.7 (0.049, 0.104) | 0.25 (0.15, 0.41)‡ | 0.31 (0.18, 0.53)‡ | 0.32 (0.19, 0.55)‡ |
| July (n = 69) | 2 (2.9) | 3.4 (0.000, 0.080) | 1.9 (0.012, 0.026) | 0.11 (0.03, 0.45)∗ | 0.13 (0.03, 0.55)∗ | 0.14 (0.03, 0.58)∗ |
| P trend | <.001 | |||||
| Non–COVID-19 | ||||||
| March (n = 803) | 13 (1.6) | 1.6 (0.007, 0.024) | 1.3 (0.006, 0.020) | 13.3 (7.03, 25.0)‡ | 12.3 (6.44, 23.3)‡ | 11.7 (6.11, 22.3)‡ |
| April (n = 1095) | 26 (2.4) | 2.4 (0.015, 0.033) | 2.3 (0.015, 0.032) | 4.71 (3.07, 7.22)‡ | 4.23 (82.72, 6.56)‡ | 4.04 (2.60, 6.29)‡ |
| May (n = 1253) | 16 (1.3) | 1.3 (0.006, 0.019) | 1.2 (0.006, 0.018) | 4.48 (2.52, 7.97)‡ | 4.72 (82.63, 8.48)‡ | 4.91 (2.68, 9.01)‡ |
| June (n = 1610) | 22 (1.4) | 1.4 (0.008, 0.019) | 1.3 (0.008, 0.019) | 3.53 (1.90, 6.55)‡ | 3.79 (2.03, 7.09)‡ | 3.81 (1.92, 7.53)‡ |
| July (n = 1983) | 25 (1.3) | 1.3 (0.008, 0.018) | 1.3 (0.008, 0.018) | 2.51 (0.57, 11.0) | 2.28 (0.50, 10.5) | 2.61 (0.50, 13.7) |
| P trend | .13 |
Incidence rate (IR).
Model 1 standardized by age and sex.
Model 2 standardized by age and sex and vital signs [temperature, systolic blood pressure (SBP), diastolic blood pressure (DBP), pulse rate, and saturation].
COVID-19 patients, incidence rate ratio (IRR): each admission month vs March.
Non–COVID-19 patients: within each admission month, IRR: COVID-19 vs non–COVID-19.
Model 1 adjusted for age and sex and hospital.
Model 2 adjusted for age and sex and vital signs (temperature, SBP, DBP, pulse rate, and saturation) and hospital.
Model 3 adjusted for age and sex and vital signs (temperature, SBP, DBP, pulse rate and saturation), and medications [use of angiotensin-converting enzyme inhibitors (ACEIs)/angiotensin receptor blockers (ARBs), β-blocking agents, calcium channel blockers, diuretics, statins, LMWH, NOACs, warfarin, antiplatelets, nonsteroidal anti-inflammatory drugs, glucocorticoids, and antibiotics] and hospital.
P < .01.
P < .05.
P < .001.
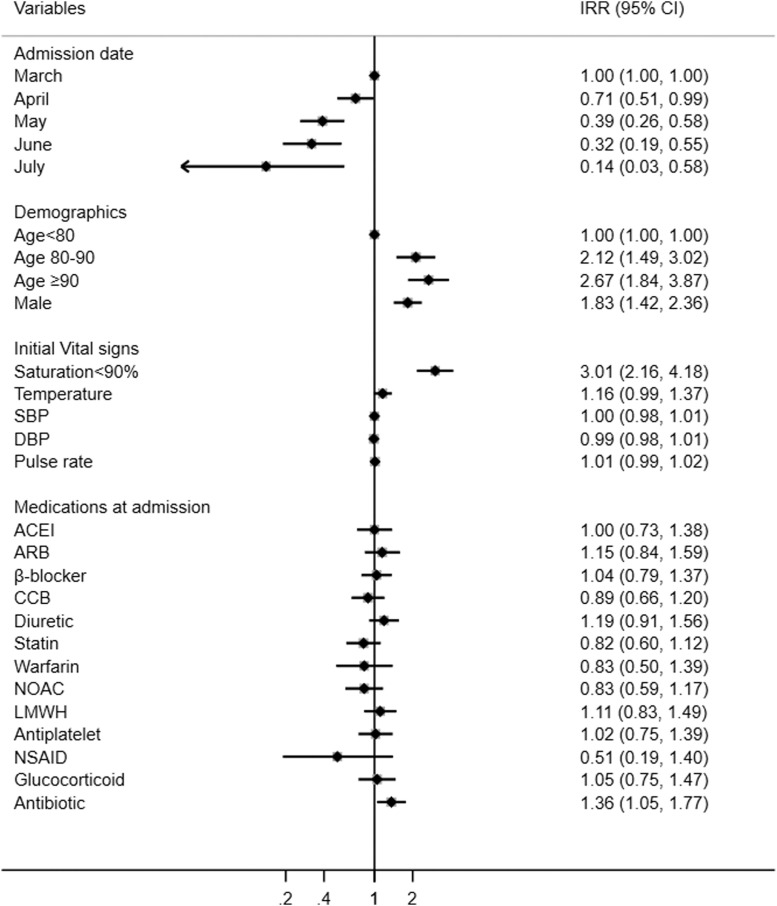
Compared with patients admitted in March, the risk of in-hospital death decreased by 29% (IRR 0.68, 95% CI 0.51-0.99) in April, 61% (0.36, 0.26-0.58) in May, 68% (0.31, 0.19-0.55) in June, and 86% (0.13, 0.03-0.58) in July, after adjusting for age, sex, initial vital signs, and medications (Table 2). Low saturation at admission, older age, male sex, and use of antibiotics were associated with in-hospital death (Figure 2 ).
Fig. 2.
IRR and 95% CI for in-hospital death among COVID-19 patients.
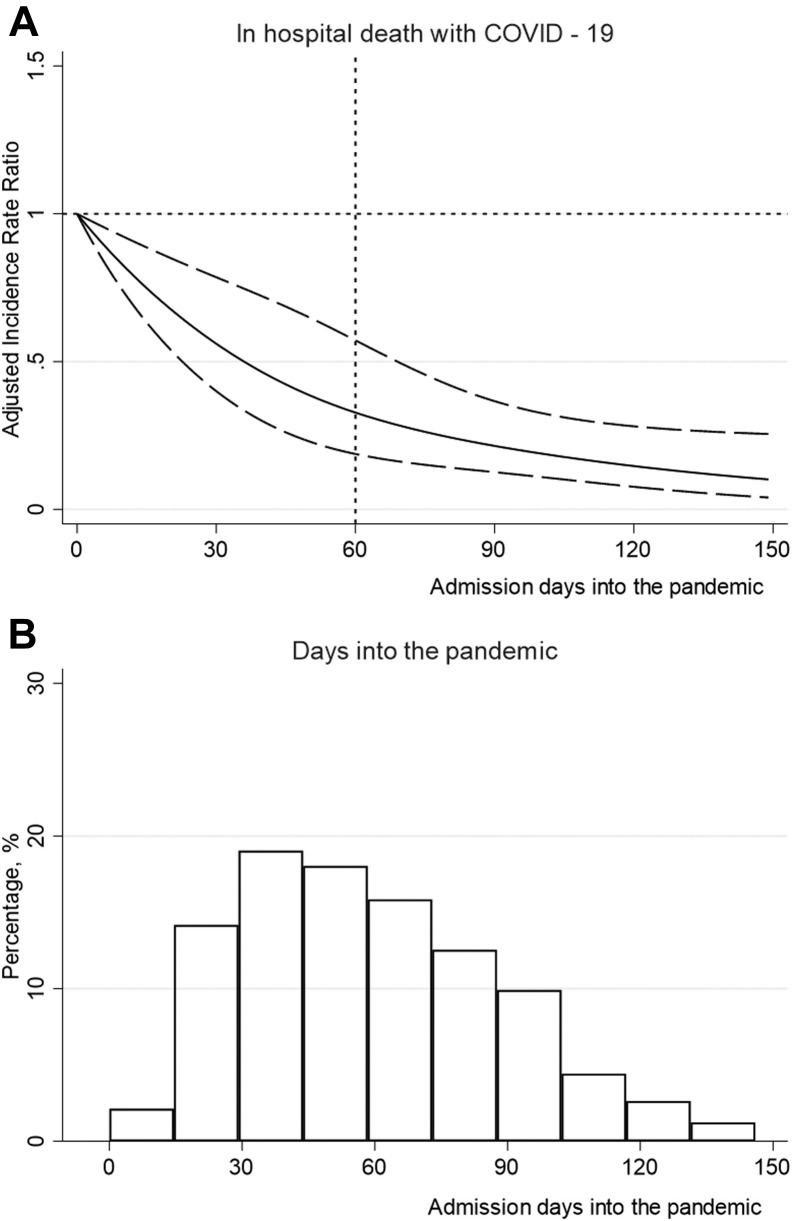
When modeling time as a continuous metric using cubic splines, a declining exponential association was observed between day into the pandemic of the day of admission for each patient and in-hospital death. Patients admitted in the later stage of the first pandemic wave had a lower mortality risk compared with patients admitted in the early stages (Figure 3 ).
Fig. 3.
(A) IRRs for in-hospital death by the days into-pandemic (continuous variable) using cubic splines. Model adjusted for age, sex, vital signs, and medications [use of angiotensin-converting enzyme inhibitors (ACEIs)/angiotensin receptor blockers (ARBs), β-blocking agents, calcium channel blockers, diuretics, statins, LMWH, NOACs, warfarin, anti-platelets, nonsteroidal anti-inflammatory drugs, glucocorticoids, and antibiotics]. Data were reported as IRR (solid line) and 95% CIs (long-dash line). (B) Proportion of participants (n = 1785) across days into pandemic.
Sensitivity, Subgroup, and Mediation Analyses
A similar decreasing trend was observed when we excluded 152 patients who were transferred to other departments or hospitals in sensitivity analyses (Supplementary Table 1).
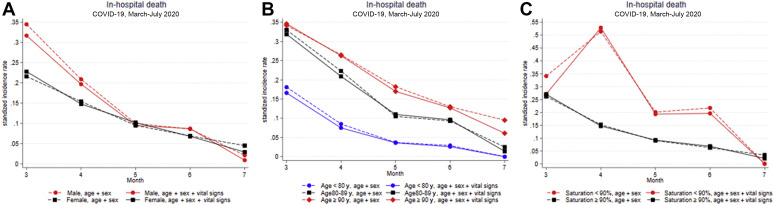
Among sex and age subgroups, men and older patients died more frequently in-hospital but presented a similar decrease in mortality over time. When stratifying for initial saturation (<90%, ≥90%), associations with decreasing mortality over time were observed in patients with normal saturation (≥90%), but no association with decreasing mortality was seen in patients with saturation <90% (Supplementary Table 2, Supplementary Figure 2). The analyses revealed a significant interaction between admission date and saturation at admission (pinteraction = 0.01).
Mediation analyses were performed to elucidate the potential effects of initial vital signs and treatment on the association of admission date with death. Changes in the coefficient of the number of days-into pandemics (continuous) were estimated for models adjusted for saturation at admission <90%, NOACs, and LMWHs separately. In the age, sex, vital signs, and medication adjusted model, low saturation explained on average 8% (95% CI 1%-15%) of the decline in in-hospital death, whereas treatment with NOACs and LMWHs did not show any significant effect on this association (Supplementary Table 3).
Mortality Compared With Non–COVID-19 Older Patients
Baseline characteristics and treatment of non–COVID-19 are shown in Table 1. In 6744 non–COVID-19 older patients, crude mortality rates remained largely stable at 1.3% to 1.6% (standardized incidence rate of mortality 1.2%-1.3%) throughout this first pandemic wave, with the notable exception of April (crude 2.4%, the standardized incidence rate of mortality 2.3%, 95% CI 0.015-0.032). In comparison with non–COVID-19 older patients, the risk of in-hospital death was 11 times higher in March for COVID-19 patients [IRR 11.7 (6.11-22.3)], decreasing to 2.8 times higher in June [3.8 (1.92-7.53)], and 1.6 times higher in July [2.61 (0.5-13.7) (ns)] (Table 2).
Discussion
In this Swedish cohort of geriatric patients hospitalized with COVID-19, patients admitted in the later stages of the pandemic had substantially lower mortality than patients admitted in the early phase. The improved survival rate over time was partly related to improvement in saturation status at admission of the patients hospitalized later throughout the pandemic. However, this improvement in baseline disease severity only explained 8% of the decline in mortality risk.
Our principal finding is an inverse exponential association between admission day during the time period and in-hospital death in older COVID-19 patients hospitalized during the first wave of the pandemic. The earlier in the pandemic the higher the risk of death; thus, patients admitted in June and July had significantly lower mortality than in March and April. Our data agree with several recent reports. Another Swedish study of 15,761 COVID-19 patients (median age of 64 years, 58% men) observed an overall 60-day mortality decrease from 25% in March to 13% in June.13 A German study from 920 hospitals including 10,021 hospitalized COVID-19 patients with median age 72 and 50% men reported a change in mortality from end-February to mid-April of 28% to 19%, respectively.14 In addition, a recent meta-analysis of COVID-19 patients receiving ICU care (10,150 patients from 24 studies) showed that the pooled mortalities were lower in May than in January (25%-60%).15 , 16 The reported mortality rate in our study is similar to the 2 population studies described above,13 , 14 but, as expected, lower than the study on patients in ICU.15 , 16 Interestingly, in non–COVID-patients, mortality rates remained relatively stable throughout the first pandemic wave, with only a slight increase in April. This may be explained by an effect of the COVID-19 situation, when hospital bed shortages may have caused only severely ill non–COVID-patients to be admitted.
Several plausible reasons have been proposed to explain the decreasing trend in mortality over time: first, improved standardized treatment and care may lead to reduced mortality. In April, LMWH treatment became standard following observational publications indicating that anticoagulant therapy with LMWH was associated with a better prognosis.2 However, after adjustments, our study did not demonstrate an association with anticoagulant treatment (including LMWH, NOACs, and warfarin) with mortality. One of the earlier observational studies on LMWH and mortality in COVID-19 also failed to demonstrate a difference in mortality in all patients, showing benefit only for COVID-19 patients with high sepsis-induced coagulopathy score.2 We lacked information to calculate sepsis-induced coagulopathy scores in our data set. Further, corticosteroids became standard care for COVID-19 patients requiring oxygen therapy after June, when the RECOVERY RCT showed survival benefits from corticosteroids.3 In our cohort, which covers data up to July, corticosteroids showed no association with mortality but the Swedish guidelines on dexamethasone arrived in mid-July and by then the number of patients with COVID-19 was quite small. Thus, there might have been insufficient power to demonstrate an effect because of fewer patients in the last month and insufficient follow-up. Ongoing corticosteroid treatment for other ailments did not affect mortality. We recorded treatment when it occurred within 24 hours of hospitalization in the geriatric clinics: patients who were initiated on LMWH or corticosteroids later would be missed so our data may be too crude to pick up the effects of these treatments on mortality.
Second, the high load of new admissions and of patients in hospital care for COVID-19 in March may have contributed to the high initial mortality. In early spring 2020, Stockholm like many metropolitan areas had an exponentially increasing number of COVID-19 patients needing hospital care. The geriatric clinics had an important role in treating, administering, and upholding care guidelines for geriatric patients with COVID-19, and many patients with severe COVID-19 were admitted to the geriatric clinics. One recommendation at that time was to seek hospital care only in clear need. It could be speculated that older patients with initially milder symptoms hesitated to seek hospital care until symptoms became severe. When the pressure of the hospital system eased up later in the pandemic and information on silent hypoxemia became available to the public, other patients with milder symptoms may have also sought care. This is corroborated from our data where the number of patients with milder COVID-19, defined as those with saturation ≥90% at hospitalization, increased over time.
Third, the decline in mortality could perhaps be due to changes in virulence of the SARS-CoV-2 virus over time.17 Increased hygiene and physical distancing may decrease the initial inoculum in those who become infected despite such measures, which could potentially lead to lower mortality. A seasonal effect may be at play, with people spending time and socializing outdoors in the later months of the pandemic. This could have an effect if it for example reduced the initial inoculating dose, or if the population, in general, presented higher vitamin D levels in later months, which has been associated with milder disease course.18 , 19
Fourth, the general spread of the SARS-CoV-2 virus in society has been shown to be clearly correlated to mortality.20 In Stockholm, there was a general virus spread reflected in the high admission rate to hospital, although due to low testing capacity it is impossible to establish knowledge about the true extent of the spread during the first pandemic wave.
We observed a large decrease in the proportion of patients arriving at the geriatric clinics with low saturation (<90%, from 13% to 1%) over time. In addition, low initial saturation was associated with increased in-hospital death, which explained an 8% proportion of the difference in in-hospital mortality in our study. Previous studies showed that higher age, frailty, lower blood oxygen saturation, underlying comorbidities particularly cardiovascular diseases, diabetes, and respiratory diseases are associated with poor prognosis of COVID-19.21 , 22 Low oxygen saturation can be considered as a marker of severe COVID-19 disease.23 , 24 Therefore, low saturation on admission may be a particularly important prognostic marker. Improvements in routines, general care, and health care providers’ competence and confidence in dealing with patients with COVID-19, together with timely hospitalization could explain the improved mortality throughout the study period. However, the mortality for those with low saturation did not improve with time, suggesting that improvements in care primarily benefited those with less severe disease. The reduction in hospital admissions over time as a proxy for reduced societal spread also reduced the number of patients with low saturation as a marker for severe COVID-19. In addition, the decrease in mortality may have at least partially followed the decrease of infection in the community, taking pressure off the health care system and permitting the admission of patients with milder disease: in a pre–peer review retrospective study of hospital admissions in Sweden, 60-day mortality decreased in the first wave following by an increase in the second wave.25 , 26 This suggests that the mortality decrease in the first wave was not only due to improvements in care.
Strengths and limitations must be considered when interpreting the results of the present study. The main strength was the inclusion of a relatively large sample of hospitalized older patients with COVID-19, with rich information of potential risk factors, confounders, as well as access to health records during the hospital stay. One limitation is that we did not have data on discharge outcomes and therefore reported in-hospital death. The mortality rate may thus be underestimated. Second, in our data set, we did not have access to comorbidities, but discharge diagnoses served as a proxy. To avoid reversed causality, these diagnoses were used to describe the population but were not included in the adjustment models. Third, we only had information on the ongoing drug therapy within 24 hours of admission, and thus we could not assess the impact of dose-response of drug therapy on the outcome. Fourth, the majority of patients admitted to geriatric clinics live in their own homes prior to admission. Many of them receive home care. Patients who need care around the clock and are very frail typically live in nursing homes. Most of the older and severely frail patients in nursing homes with COVID-19 were neither sent to emergency hospitals nor to geriatric clinics. These patients received supportive or palliative care in their nursing homes. Furthermore, the most vital older patients may have been treated in the ICU or infectious disease wards instead of geriatric clinics and, therefore, may also not be a part of the study population. Thus, the in-hospital mortality of COVID-19 patients reported here is based on the large older cohort who was admitted to geriatric care in geriatric clinics which did not include a substantial part of the nursing home population. Finally, as in all observational studies, we acknowledge the possibility of residual and unknown confounding, something we tried to tackle in our sensitivity analyses and subgroup analyses.
Conclusions and Implications
Over the first wave of the COVID-19 pandemic in Stockholm from March to July 2020, a reduction in the in-hospital death in older patients with COVID-19 was associated mostly with the duration of the pandemic at admission time. Further research is needed to explain these changes and how mortality developed in later pandemic waves. Studies on mortality changes in other settings, such as nursing homes, are also needed to understand pandemic effects on the whole geriatric population.
Acknowledgments
We thank all patients, caregivers, physicians, reporting units, and coordinators in this study.
Footnotes
H.X. and S.G.-P. contributed equally.
The authors are supported by the regional agreement on medical training and clinical research between the Stockholm county council and the Karolinska Institutet (A.L.F.); Swedish Medical Research Council, Swedish Stroke Association, and FORTE. H.X. is supported by a postdoctoral grant from Strategic Research program in Neuroscience at Karolinska Institutet. S.G.-P. is supported by a stipend from the Swedish Society for Medical Research. The funders played no role in study design or interpretation of results.
The authors declare no conflicts of interest.
Supplementary Data
Supplementary Table 1.
Sensitivity Analyses of Incidence Rates and Incidence Rate Ratios for In-Hospital Death excluding 152 Patients Who Were Transferred to Other Departments or Hospitals
| Admission Date | Death (%) | Model 1, IR, % (95% CI) | Model 2, IR, % (95% CI) | Model 1, IRR (95% CI) | Model 2, IRR (95% CI) | Model 3, IRR (95% CI) |
|---|---|---|---|---|---|---|
| March (n = 173) | 54 (31.2) | 31.0 (0.216, 0.338) | 29.7 (0.209, 0.331) | Ref | Ref | Ref |
| April (n = 669) | 129 (19.3) | 19.6 (0.153, 0.207) | 18.8 (0.146, 0.196) | 0.62 (0.45, 0.85)∗ | 0.68 (0.49, 0.95)† | 0.69 (0.49, 0.97)† |
| May (n = 486) | 52 (10.7) | 10.5 (0.073, 0.122) | 10.5 (0.076, 0.121) | 0.30 (0.21, 0.45)‡ | 0.36 (0.24, 0.53)‡ | 0.37 (0.25, 0.56)‡ |
| June (n = 244) | 21 (8.6) | 8.7 (0.046, 0.108) | 8.3 (0.049, 0.104) | 0.24 (0.15, 0.41)‡ | 0.31 (0.18, 0.52)‡ | 0.32 (0.19, 0.55)‡ |
| July (n = 61) | 2 (3.3) | 3.6 (0.000, 0.080) | 2.0 (0.012, 0.026) | 0.11 (0.03, 0.46)∗ | 0.14 (0.03, 0.57)∗ | 0.14 (0.04, 0.59)∗ |
| P trend | <.001 |
Ref, referent.
Incidence rate (IR).
Model 1 standardized by age and sex.
Model 2 standardized by age and sex and vital signs [temperature, systolic blood pressure (SBP), diastolic blood pressure (DBP), pulse rate, and saturation].
Incidence rate ratio (IRR): each admission month vs March.
Model 1 adjusted for age and sex and hospital.
Model 2 adjusted for age and sex and vital signs (temperature, SBP, DBP, pulse rate, and saturation) and hospital.
Model 3 adjusted for age and sex and vital signs (temperature, SBP, DBP, pulse rate and saturation), and medications [use of angiotensin-converting enzyme inhibitors (ACEIs)/angiotensin receptor blockers (ARBs), β-blocking agents, calcium channel blockers, diuretics, statins, low-molecular-weight heparin, non–vitamin K antagonist oral anticoagulants, warfarin, antiplatelets, nonsteroidal anti-inflammatory drugs, glucocorticoids, and antibiotics] and hospital.
P < .01.
P < .05.
P < .001.
Supplementary Table 2.
Incidence Rates and Incidence Rate Ratios for In-Hospital Death by Sex, Age, and Initial Saturation
| Subgroups | Admission Date | Death, n (%) | Model 1, IR, % | Model 2, IR, % | Model 1, IRR (95% CI) | Model 2, IRR (95% CI) | Model 3, IRR (95% CI) |
|---|---|---|---|---|---|---|---|
| Male | |||||||
| March (n = 85) | 31 (36.5) | 34.5 | 31.7 | Ref | Ref | Ref | |
| April (n = 350) | 72 (20.6) | 20.9 | 19.7 | 0.61 (0.40, 0.94)∗ | 0.74 (0.48, 1.14) | 0.72 (0.46, 1.13) | |
| May (n = 247) | 25 (10.1) | 10.0 | 9.5 | 0.25 (0.15, 0.43)† | 0.30 (0.18, 0.52)† | 0.30 (0.17, 0.53)† | |
| June (n = 128) | 11 (8.6) | 8.6 | 8.7 | 0.21 (0.10, 0.43)† | 0.30 (0.15, 0.61)† | 0.28 (0.13, 0.60)† | |
| July (n = 36) | 1 (2.8) | 2.1 | 0.9 | 0.083 (0.01, 0.61)∗ | 0.11 (0.01, 0.78)∗ | 0.10 (0.01, 0.78)∗ | |
| P trend | <.001 | ||||||
| Female | |||||||
| March (n = 110) | 23 (20.9) | 21.6 | 22.8 | Ref | Ref | Ref | |
| April (n = 376) | 57 (15.2) | 15.4 | 14.8 | 0.64 (0.39, 1.05) | 0.66 (0.39, 1.09) | 0.69 (0.41, 1.15) | |
| May (n = 277) | 27 (9.7) | 9.5 | 10.2 | 0.40 (0.22, 0.70)‡ | 0.45 (0.25, 0.80)‡ | 0.52 (0.28, 0.94)∗ | |
| June (n = 143) | 10 (7.0) | 6.9 | 6.8 | 0.30 (0.14, 0.64)‡ | 0.33 (0.15, 0.70)‡ | 0.35 (0.16, 0.76)‡ | |
| July (n = 33) | 1 (3.0) | 4.5 | 2.9 | 0.15 (0.02, 1.14) | 0.18 (0.02, 1.31) | 0.18 (0.03, 1.39) | |
| P trend | .001 | ||||||
| Age <80 y | |||||||
| March (n = 71) | 14 (19.7) | 18.1 | 16.6 | Ref | Ref | Ref | |
| April (n = 291) | 24 (8.2) | 8.5 | 7.5 | 0.37 (0.19, 0.71)‡ | 0.44 (0.22, 0.90)∗ | 0.39 (0.18, 0.85)∗ | |
| May (n = 198) | 7 (3.5) | 3.7 | 3.6 | 0.13 (0.05, 0.34)† | 0.18 (0.07, 0.47)† | 0.20 (0.07, 0.57) ‡ | |
| June (n = 104) | 3 (2.9) | 2.9 | 2.6 | 0.14 (0.04, 0.50)‡ | 0.20 (0.06, 0.71)∗ | 0.20 (0.05, 0.74)∗ | |
| July (n = 24) | 0 (0.0) | 0 | 0 | — | — | — | |
| P trend | <.001 | ||||||
| Age 80-89 y | |||||||
| March (n = 83) | 26 (31.3) | 33.0 | 31.9 | Ref | Ref | Ref | |
| April (n = 260) | 58 (22.3) | 22.3 | 20.9 | 0.71 (0.44, 1.15) | 0.86 (0.53, 1.40) | 0.86 (0.52, 1.44) | |
| May (n = 181) | 19 (10.5) | 10.5 | 11.0 | 0.31 (0.17, 0.56)† | 0.40 (0.22, 0.74)‡ | 0.41 (0.21, 0.80)‡ | |
| June (n = 97) | 9 (9.3) | 9.3 | 9.6 | 0.26 (0.12, 0.58)† | 0.34 (0.16, 0.75)‡ | 0.36 (0.16, 0.82)∗ | |
| July (n = 35) | 1 (2.9) | 2.5 | 1.4 | 0.09 (0.01, 0.69)∗ | 0.13 (0.02, 0.94)∗ | 0.13 (0.02, 1.01) | |
| P trend | <.001 | ||||||
| Age ≥ 90 y | |||||||
| March (n = 41) | 14 (34.1) | 34.2 | 34.6 | Ref | Ref | Ref | |
| April (n = 175) | 47 (26.9) | 26.5 | 26.3 | 0.76 (0.41, 1.39) | 0.79 (0.43, 1.46) | 0.87 (0.46, 1.66) | |
| May (n = 145) | 26 (17.9) | 18.2 | 17.0 | 0.47 (0.24, 0.91)∗ | 0.53 (0.27, 1.02) | 0.61 (0.31, 1.22) | |
| June (n = 70) | 9 (12.9) | 13.0 | 12.7 | 0.34 (0.14, 0.80)∗ | 0.42 (0.17, 1.01) | 0.45 (0.18, 1.13) | |
| July (n = 10) | 1 (10.0) | 9.5 | 6.1 | 0.36 (0.05, 2.75) | 0.45 (0.06, 3.46) | 0.46 (0.06, 3.57) | |
| P trend | .02 | ||||||
| Saturation < 90% | |||||||
| March (n = 25) | 10 (40) | 34.1 | 27.1 | Ref | Ref | Ref | |
| April (n = 55) | 29 (53) | 51.4 | 52.9 | 1.45 (0.68, 3.08) | 1.46 (0.64, 3.32) | 1.40 (0.59, 3.30) | |
| May (n = 25) | 7 (28) | 20.1 | 19.3 | 0.54 (0.19, 1.51) | 0.54 (0.19, 1.58) | 0.45 (0.13, 1.54) | |
| June (n = 9) | 4 (44) | 21.7 | 19.6 | 1.15 (0.34, 3.90) | 1.46 (0.40, 5.24) | 1.10 (0.24, 5.03) | |
| July (n = 1) | 0 (0) | 0 | 0 | — | — | — | |
| P trend | .26 | ||||||
| Saturation ≥90% | |||||||
| March (n = 170) | 44 (25.9) | 26.2 | 27.0 | Ref | Ref | Ref | |
| April (n = 671) | 100 (14.9) | 15.2 | 14.7 | 0.51 (0.35, 0.73)† | 0.52 (0.36, 0.75)† | 0.54 (0.37, 0.79)‡ | |
| May (n = 499) | 45 (9.0) | 9.0 | 9.2 | 0.27 (0.18, 0.42)† | 0.28 (0.18, 0.43)† | 0.30 (0.19, 0.47)† | |
| June (n = 262) | 17 (6.5) | 6.3 | 6.8 | 0.21 (0.11, 0.37)† | 0.22 (0.12, 0.39)† | 0.23 (0.13, 0.43)† | |
| July (n = 68) | 2 (2.9) | 3.4 | 2.1 | 0.12 (0.03, 0.50)‡ | 0.12 (0.03, 0.50)‡ | 0.12 (0.03, 0.52)‡ | |
| P trend | <.001 | ||||||
Ref, referent.
Incidence rate (IR).
Model 1 standardized by age and sex.
Model 2 standardized by age and sex and vital signs [temperature, systolic blood pressure (SBP), diastolic blood pressure (DBP), pulse rate, and saturation].
Incidence rate ratio (IRR): each admission month vs March.
Model 1 adjusted for age and sex and hospital.
Model 2 adjusted for age and sex and vital signs (temperature, SBP, DBP, pulse rate, and saturation) and hospital.
Model 3 adjusted for age and sex and vital signs (temperature, SBP, DBP, pulse rate and saturation), and medications [use of angiotensin-converting enzyme inhibitors (ACEIs)/angiotensin receptor blockers (ARBs), β-blocking agents, calcium channel blockers, diuretics, statins, low-molecular-weight heparin, non–vitamin K antagonist oral anticoagulants, warfarin, antiplatelets, nonsteroidal anti-inflammatory drugs, glucocorticoids, and antibiotics] and hospital.
P < .05.
P < .001.
P < .01.
Supplementary Table 3.
Mediation Analysis Showing Changes in the Coefficient Relating to the Association of Admission Date With In-Hospital Death
| In-Hospital Death | Coefficient (95% CI) | P | Coefficient Reduction, % | % of Total Effect Mediated | P |
|---|---|---|---|---|---|
| Days into the Pandemic, d | |||||
| Model 1 | −0.75 (−1.06, −0.44) | <.001 | NA | NA | |
| Model 1 + Saturation<90% | −0.63 (−0.94, −0.31) | <.001 | 16 | 8 (1, 15) | .03 |
| Model 1 + NOACs | −0.73 (−1.04, −0.42) | <.001 | 3 | 5 (−4, 15) | .28 |
| Model 1 + LMWH | −0.76 (−1.07, −0.43) | <.001 | 0 | 1 (−24, 22) | .94 |
LMWH, low-molecular-weight heparin; NOACs, non–vitamin K antagonist oral anticoagulants.
Model adjusted for sex, age, vital signs, and medications after separate entry of initial saturation, NOACs, and LMWH into the models. Model 1 is the base model, adjusted for age and sex and vital signs (temperature, systolic blood pressure, diastolic blood pressure, pulse rate), medications [use of angiotensin-converting enzyme inhibitors (ACEIs)/angiotensin receptor blockers (ARBs), β-blocking agents, calcium channel blockers, diuretics, statins, antiplatelets, nonsteroidal anti-inflammatory drugs, glucocorticoids, and antibiotics] and hospital.
Supplementary Figure 2.
Standardized incidence rate and 95% confidence interval of in-hospital death by age, sex, and initial saturation among COVID-19 patients.
References
- 1.Ludvigsson J.F. The first eight months of Sweden's COVID-19 strategy and the key actions and actors that were involved. Acta Paediatr. 2020;109:2459–2471. doi: 10.1111/apa.15582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang N., Bai H., Chen X. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.RECOVERY Collaborative Group. Horby P., Lim W.S., Emberson J.R. Dexamethasone in hospitalized patients with Covid-19—Preliminary report. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beigel J.H., Tomashek K.M., Dodd L.E., ACTT-1 Study Group Members Remdesivir for the Treatment of Covid-19—Final Report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paolisso P., Bergamaschi L., D'Angelo E.C. Preliminary experience with low molecular weight heparin strategy in COVID-19 patients. Front Pharmacol. 2020;11:1124. doi: 10.3389/fphar.2020.01124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y., Zhang D., Du G. Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.RECOVERY Collaborative Group. Horby P., Mafham M., Linsell L. Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;383:2030–2040. doi: 10.1056/NEJMoa2022926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agarwal A., Mukherjee A., Kumar G. Convalescent plasma in the management of moderate covid-19 in adults in India: Open label phase II multicentre randomised controlled trial (PLACID Trial) BMJ. 2020;371:m3939. doi: 10.1136/bmj.m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ludvigsson J.F., Andersson E., Ekbom A. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. doi: 10.1186/1471-2458-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Consonni D., Coviello E., Buzzoni C., Mensi C. A command to calculate age-standardized rates with efficient interval estimation. Stata J. 2012;12:688–701. [Google Scholar]
- 12.Acock A.C. Revised Edition. Stata Press; College Station, TX: 2013. Discovering Structural Equation Modeling Using Stata. [Google Scholar]
- 13.Strålin K., Wahlstörm E., Walther S. Decline in mortality among hospitalised covid-19 patients in Sweden: A nationwide observational study. medRxiv. 2020 doi: 10.1016/j.lanepe.2021.100054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karagiannidis C., Mostert C., Hentschker C. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: An observational study. Lancet Respir Med. 2020;8:853–862. doi: 10.1016/S2213-2600(20)30316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armstrong R.A., Kane A.D., Cook T.M. Decreasing mortality rates in ICU during the COVID-19 pandemic. Anaesthesia. 2021;76(Suppl 3):10. doi: 10.1111/anae.15230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armstrong R.A., Kane A.D., Cook T.M. Outcomes from intensive care in patients with COVID-19: A systematic review and meta-analysis of observational studies. Anaesthesia. 2020;75:1340–1349. doi: 10.1111/anae.15201. [DOI] [PubMed] [Google Scholar]
- 17.Young B.E., Fong S.W., Chan Y.H. Effects of a major deletion in the SARS-CoV-2 genome on the severity of infection and the inflammatory response: An observational cohort study. Lancet. 2020;396:603–611. doi: 10.1016/S0140-6736(20)31757-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Annweiler G., Corvaisier M., Gautier J. Vitamin D supplementation associated to better survival in hospitalized frail elderly COVID-19 patients: The GERIA-COVID Quasi-Experimental Study. Nutrients. 2020;12:3377. doi: 10.3390/nu12113377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pereira M., Dantas Damascena A., Galvão Azevedo L.M. Vitamin D deficiency aggravates COVID-19: Systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2020:1–9. doi: 10.1080/10408398.2020.1841090. [DOI] [PubMed] [Google Scholar]
- 20.Kanu F.A., Smith E.E., Offutt-Powell T. Declines in SARS-CoV-2 transmission, hospitalizations, and mortality after implementation of mitigation measures—Delaware, March-June 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1691–1694. doi: 10.15585/mmwr.mm6945e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang K., Zuo P., Liu Y. Clinical and laboratory predictors of in-hospital mortality in patients with COVID-19: A cohort study in Wuhan, China. Clin Infect Dis. 2020;71:2079–2088. doi: 10.1093/cid/ciaa538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hägg S., Jylhävä J., Wang Y. Age, frailty and comorbidity as prognostic factors for short-term outcomes in patients with COVID-19 in geriatric care. J Am Med Dir Assoc. 2020;21:1555–1559.e2. doi: 10.1016/j.jamda.2020.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan Y., Yang Y., Wang F. Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. BMJ Open Diabetes Res Care. 2020;8:e001343. doi: 10.1136/bmjdrc-2020-001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Velavan T.P., Meyer C.G. Mild versus severe COVID-19: Laboratory markers. Int J Infect Dis. 2020;95:304–307. doi: 10.1016/j.ijid.2020.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strålin K., Wahlström E., Walther S. Second wave mortality among patients hospitalised for COVID-19 in Sweden: A nationwide observational cohort study. medRxiv. 2021. Available at: [DOI] [PMC free article] [PubMed]
- 26.Swedish Board of Health and Welfare. Statistics on hospitalizations of patients with Covid-19. 2021. https://www.socialstyrelsen.se/statistik-och-data/statistik/statistik-om-covid-19/statistik-om-slutenvard-av-patienter-med-covid-19/ Available at: