Abstract
The aim of this study was to evaluate the usage of mometasone furoate nasal spray in the recovery of patients with severe microsmia or anosmia induced by COVID-19.
This was a prospective clinical trial on non-hospitalized adult patients with COVID-19 (>18 years) who had severe microsmia or anosmia within two weeks. The subjects were randomly assigned to the mometasone furoate group (100 mcg twice daily) or sodium chloride group (0.9%); both groups also received olfactory training for 4 weeks. The primary outcome was the improvement of the olfactory score at the end of the study. Visual analog scale (VAS) and the University of Pennsylvania Smell Identification Test (UPSIT) were used to assess primary outcome.
A total of 80 patients were recruited, 77 of them completed the study and were analyzed. There was no statistically significant difference in terms of demographics and baseline clinical characteristics. The olfactory scores (based on VAS) at weekly intervals showed a significant difference between the two groups (P:0.318, <0.001, <0.001, <0.001, respectively). The analyses also showed significant within-group differences from baseline. Nevertheless, the changes were not significant between the two groups (P: 0.444, 0.402, 0.267, 0.329). There was no significant difference between the two groups in terms of the UPSIT results (p > 0.239). However, a significant between-group difference was noted in the severity of loss of smell (P < 0.001).
Compared to olfactory training, mometasone furoate nasal spray combination with olfactory training showed a higher improvement in severe chronic anosmia by COVID-19.
Keywords: Anosmia, COVID-19, Corticosteroids, Olfactory dysfunction
1. Introduction
Olfactory dysfunction is one of the most common and early symptoms of COVID-19 [1]. As a viral infection, COVID-19 may be a more prevalent and severe pathology for loss of smell than other upper respiratory viral infections. Research indicates, for example, that the olfactory function is affected more in patients with COVID-19 than in patients with the common cold [1]. The clinical evolution of COVID-19–associated olfactory loss is still unclear because recovery reports vary considerably [2]. According to recent studies, a significant percentage of patients in the months after recovery from the initial phase of COVID-19 still has shown persistent olfactory dysfunction [3], [4].
Although most patients regain normal olfactory function within 15 days, severe anosmia or hyposmia persists in 7–8% of patients after two months [5], [6]. This frequency of severe olfactory disorders, because of the high prevalence of COVID-19, suggests that a significant number of patients would suffer from long-term olfactory complications. As such, there is a need for effective identification and treatment to improve olfactory function at the earliest time.
To date, there is no evidence-based medical intervention in support of patients with a persistent olfactory disorder caused by COVID-19, although olfactory training is recommended [7]. Some studies have shown that corticosteroids improve olfactory function in some patients with olfactory dysfunction after viral infection [8], [9]. The administration of corticosteroids in COVID-19–induced olfactory dysfunction is still disputable [10], such that many experts have recommended against corticosteroids in early olfactory dysfunction due to lack of sufficient evidence supporting its efficacy/safety [11], [12]. Therefore, we conducted a clinical trial to assess the efficacy of topical corticosteroids (mometasone furoate nasal spray) on olfactory dysfunction in COVID-19 patients.
2. Methods
2.1. Study design
A prospective double-blind randomized clinical trial was conducted to assess the efficacy and safety of intranasal mometasone furoate in the treatment of olfactory dysfunction of COVID-19 patients. The protocol of the study was approved by the Ethical Committee of Mazandaran University of Medical Sciences (Identifier: IR.MAZUMS.REC.1399.877) and registered in the Iranian Registry of Clinical Trials (Code: IRCT20190804044429N6).
2.2. Participants’ characteristics
The study was performed with adult patients aged 18 years or higher referring to the outpatient clinic in Ibne Sina Hospital affiliated with Mazandaran University of Medical Sciences in Sari, northern Iran.
In this trial, patients diagnosed with COVID-19 as per clinical findings and Real-Time Polymerase Chain Reaction (RT-PCR) or lung CT scan results who had symptoms of olfactory dysfunction for two weeks due to COVID-19 but were not hospitalized were assessed; among these, individuals with severe anosmia or microsemia (according to the UPSIT) were recruited for the study.
Exclusion criteria were as follows: pregnant and lactating women, patients with a history of olfactory dysfunction, chronic use of corticosteroids, the presence of anatomical abnormalities of the nose, including history of cancer, rhinosinusitis or surgery, and the presence of nosebleeds and herpes lesions in the mucosa. Moreover, they were excluded if they refused to participate in the study.
2.3. Procedures and data collection
Demographic characteristics of patients, including age, sex, underlying diseases, medications used, laboratory tests, and clinical symptoms of the disease, were recorded at baseline.
Participants in this study were randomly assigned to two groups according to permuted block randomization. In the intervention group, 40 patients received two puffs of topical corticosteroid nasal spray (mometasone furoate 0.05% nasal spray) at an appropriate dose (100 µg) twice daily in each nostril for four weeks along with olfactory training. Another 40 patients, assigned to the control group, received two puffs of topical saline spray in each nostril twice daily together with olfactory training.
According to the study of Whitcroft and Hummel [13], the sniffing of roses, lemons, cloves, and eucalyptus for olfactory training has similar effects to individuals using an olfactory pen containing the associated essential oils twice daily for 20 s. Thus, the eucalyptus olfactory pen with the same manner of administration was employed as olfactory training.
2.4. Outcomes
The primary outcome of the study was the improvement of the olfactory dysfunction score of the patients at the end of the study. It was defined as the number of patients who would return to normosmia state at the end of the study period. Visual analog scale (VAS) and the University of Pennsylvania Smell Identification Test (UPSIT) were two quantitative criteria to measure the patients’ sense of smell. All patients reported their degree of anosmia/hyposmia on VAS from 0 to 10 (0 denoting complete olfactory loss and 10 denoting completely normal olfactory sensation) at baseline, after one week, two weeks, three weeks, and four weeks.
Because the VAS criterion is scored based on individual perception and cannot be an accurate criterion for measuring the smell of participants, we used a more standard and accurate measure, namely, UPSIT. The measure has been validated in Iran. The Iran Smell Identification Test (Iran-SIT), which is the Iranian version of UPSIT, uses odors familiar to the Iranian culture. This is a kit containing 24 different types of odors in eight categories. The test result is reported as a number from 0 to 24, which determines the function of the sense of smell (14) in the range of anosmia (0–9), severe microsmia (10–13) [14] mild microsmia (14–18) and normosmia (19–24). This measure was administered at baseline and the fourth week of the study. Any side effects to corticosteroid nasal spray therapy were assessed and recorded.
2.5. Statistical analysis
Statistical analysis was performed using SPSS 24.0. (IBM Corp., Armonk, NY). Qualitative variables were expressed in percentage and compared using the Chi-square test and Fisher's exact test. All interval variables were tested for normality of distribution using Kolmogorov–Smirnov test. Normally distributed variables were compared using Student’s t test, and variables without normal distribution were compared by nonparametric Mann–Whitney-U test. Wilcoxon signed-rank test and paired t-test were used to compare the results of each group. The data was presented as means and standard deviation or median and interquartile range as appropriate. Only measures with a p < 0.05 were considered statistically significant.
3. Results
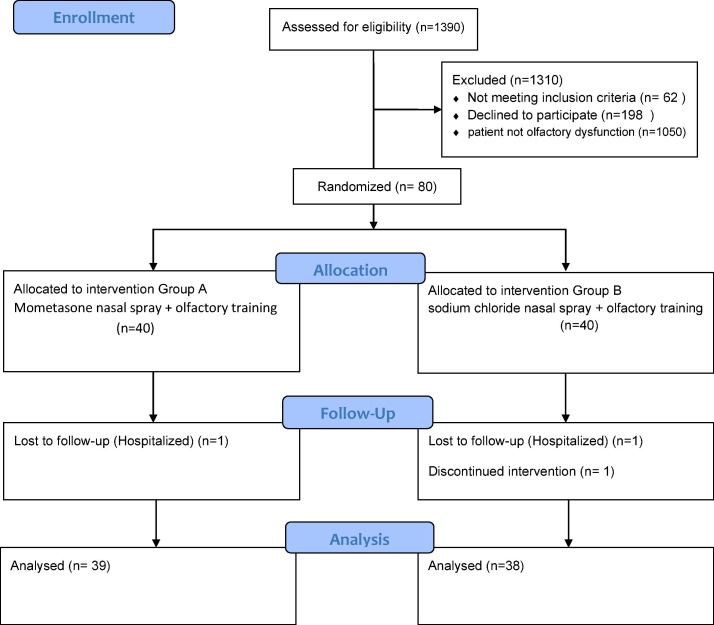
Between February 20 and Jun 30, 20201, 80 patients were included in the study and randomly allocated to two groups. Two patients from the control group and one patient from the intervention group were excluded from the study during the follow-up period, leaving 38 patients in the control group and 39 patients in the intervention group for final analyses [Fig. 1 ].
Fig. 1.
Study diagram.
A total of 77 adult patients were analyzed, of whom 39 were male (50.6%) and 38 were female (49.4%). The median age in the intervention and control groups were 32.0 years (IQR = 12) and 32.5 years (IQR = 10), respectively. There was no statistically significant difference between the two groups in terms of age and sex. The underlying diseases comprised thyroid disease (5.2%), hypertension (2.6%), and diabetes mellitus (1.3%). Fever, myalgia, ageusia, and headache were the most common symptoms in both groups. The clinical characteristics and demographic data are presented in Table 1 .
Table 1.
Demographics and baseline clinical characteristics.
| Intervention | Control | P-Value | |
|---|---|---|---|
| Sex, n (%)Male Female | 20 (51.3) 19 (48.7) | 19 (50) 19 (50) | 0.910 |
| Age,year mean (SD) | 35.4 (9) | 33.2 (8.5) | 0.675 |
| BMImean (SD) | 25.28 (4.22) | 24.22 (4.14) | 0.560 |
| Blood Group N(%)O + A+ | 15 (45.5) 14 (42.4) | 20 (57.1) 4 (11.4) | |
| B+ | 1 (3) | 6 (17.1) | |
| B- | 2 (6.1) | 1 (2.9) | |
| AB | 1 (3) | 2 (5.7) | |
| Medical history | |||
| Smoking, n (%) | 1 (2.6) | 0 | 1.000 |
| Asthma, n (%) | 1 (2.6) | 0 | |
| DM, n (%) | 1 (2.6) | 0 | |
| HTN, n (%) | 1 (2.6) | 1 (2.6) | 1.000 |
| DLP, n (%) | 0 | 1 (2.6) | 1.000 |
| Thyroid disorder, n (%) | 1 (2.6) | 3 (7.9) | 0.358 |
| Sinusitis, n (%) | 4 (10.3) | 1 (5.3) | 0.675 |
| Depression, n (%) | 0 | 1 (2.6) | |
| Baseline clinical symptoms | |||
| Cough, n (%) | 7 (17.9) | 7 (18.4) | 0.957 |
| Sore throat, n (%) | 12 (30.8) | 6 (15.8) | 0.120 |
| Fever, n (%) | 22 (56.4) | 17 (44.7) | 0.306 |
| Chills, n (%) | 13 (33.3) | 13 (34.2) | 0.935 |
| Dyspnea, n (%) | 1 (2.6) | 3 (7.9) | 0.292 |
| Fatigue, n (%) | 14 (35.9) | 14 (36.8) | 0.931 |
| Runny nose, n (%) | 7 (17.9) | 1 (2.6) | 0.056 |
| Loss of appetite, n (%) | 2 (5.1) | 1 (2.6) | 1.000 |
| Phantosmia, n (%) | 0 | 1 (2.6) | 0.494 |
| Chest pain, n (%) | 0 | 1 (2.6) | 0.494 |
| Headache, n (%) | 13 (33.3) | 23 (60.5) | 0.017 |
| Myalgia, n (%) | 25 (64.1) | 20 (52.6) | 0.307 |
| Exacerbation of sinusitis, n (%) | 4 (10.3) | 2 (5.3) | 0.414 |
| Vomiting, n (%) | 1 (2.6) | 2 (5.3) | 0.615 |
| Diarrhea, n (%) | 2 (5.1) | 2 (5.3) | 1.000 |
| Decreased sense of hearing, n (%) | 0 | 1 (2.6) | 0.494 |
| Vertigo, n (%) | 0 | 1 (2.6) | 0.494 |
| Nasal burning, n (%) | 2 (5.1) | 2 (5.3) | 1.000 |
| Nasal congestion, n (%) | 12 (30.8) | 6 (15.8) | 0.120 |
| Ageusia, n (%) | 31 (79.5) | 31 (81.6) | 0.817 |
SD: Standard Deviation, BMI: Body Mass Area, DM: Diabetes Mellitus, HTN: Hypertension, DLP: Dyslipidemia.
Laboratory tests of patients on the first day were normal due to the mild severity of the disease. Baseline laboratory test results are displayed in Table 2 .
Table 2.
Initial assessment laboratory findings.
| Intervention | Control | P-Value | |
|---|---|---|---|
| C-reactive protein (mg/dl), median (IQR) | 5 (14.75) | 6 (9) | 0.753 |
| White Blood Cell*(cell/μl) | 5867.5 (1998.1) | 5797.7 (1425.9) | 0.854 |
| Lymphocyte*(cells/μl) | 1865 (782.6) | 1848.1 (769.3) | 0.923 |
| Neutrophil*(cells/μl) | 3572.3 (1576.1) | 3465.6 (1033.8) | 0.722 |
| Platelet*, cells x103/μl | 211,900 (72145.4) | 214615.4 (76382.6) | 0.867 |
| Hemoglobin*(g/dl) | 12.9 (1.6) | 13 (1.2) | 0.705 |
| Hematocrit* | 38.9 (3.9) | 39 (3.2) | 0.833 |
| Lactate dehydrogenase (U/L)* | 327.5 (82.9) | 325.9 (76) | 0.929 |
| Erythrocyte sedimentation rate (mm/h)* | 21.3 (13.1) | 17.8 (10.4) | 0.200 |
Values shown by mean (minimum–maximum), IQR: Interquartile range, SD: Standard Deviation.
The olfactory scores (VAS) at one week, two weeks, three weeks, and four weeks of the treatment were compared, showing a significant difference between the groups (p: 0.318, <0.001, <0.001, <0.001, respectively). The analyses showed both significant between-group differences at measurement time points and significant within-group differences from baseline (Table 3 ). However, the changes observed during the study intervals were not significant between the two groups (Table 4 ).
Table 3.
Comparison of olfactory scores according to VAS between two groups.
| Intervention | Control | P-value* | P-value** | P-value*** | |
|---|---|---|---|---|---|
| Initial VAS score median (IQR) | 3 (3) | 1.50 (4) | – | – | 0.318 |
| VAS score after 1 weeks, median (IQR) | 6 (2) | 3 (5) | 0.00 | 0.00 | <0.001 |
| VAS score after 2 weeks, median (IQR) | 8 (2) | 5 (4) | 0.00 | 0.00 | <0.001 |
| VAS score after 3 weeks, median (IQR) | 9 (2) | 6 (2.25) | 0.00 | 0.00 | <0.001 |
| VAS score after 4 weeks, median (IQR) | 9 (1) | 7 (2.25) | 0.00 | 0.00 | <0.001 |
VAS: Visual analogue Scale, IQR: Interquartile range.
With-in group A,
With-in group B,
Between groups.
Table 4.
Evaluation of the amount of the changes of VAS scores from baseline between two groups.
| Intervention | Control | P-value | |
|---|---|---|---|
| After 1 weeks, mean (SD) | 2.1 (1.9) | 2.4 (1.7) | 0.444 |
| After 2 weeks, mean (SD) | 4 (2.4) | 4.4 (1.7) | 0.402 |
| After 3 weeks, mean (SD) | 4.8 (2.2) | 5.3 (1.6) | 0.267 |
| After 4 weeks, mean (SD) | 5.2 (2.3) | 5.7 (1.6) | 0.329 |
VAS: Visual analogue Scale, SD: Standard Deviation.
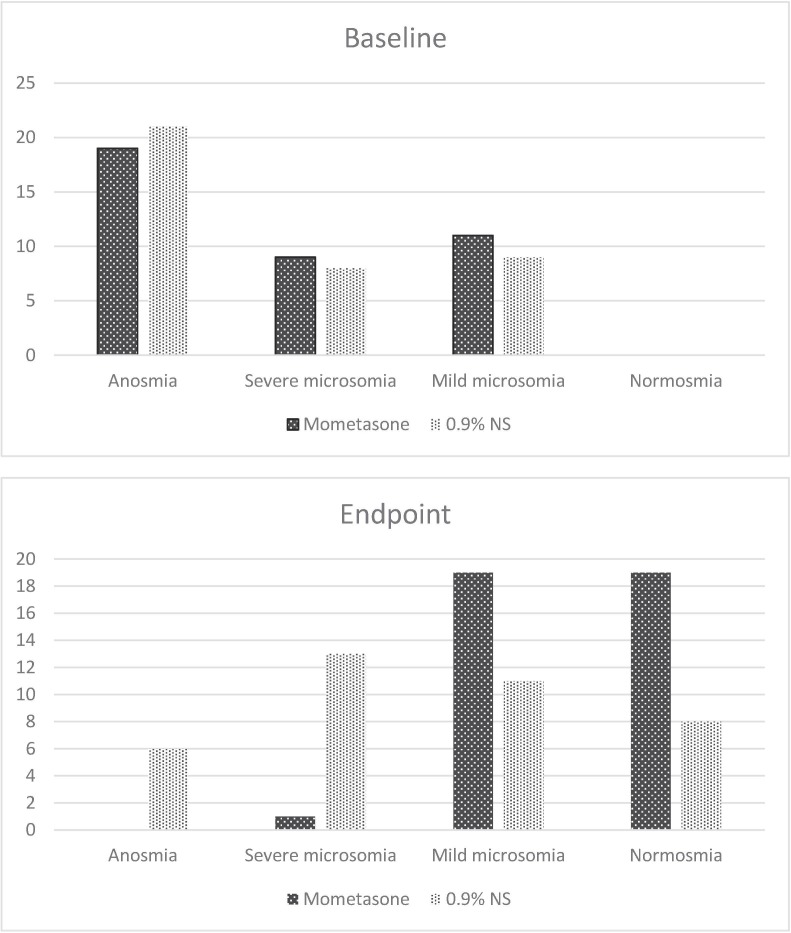
There was no significant difference between the groups according to the olfactory scores on Iran-SIT tests and the observed changes at the study endpoint (P = 0.239, 0.91; Table 5, Table 6 ). However, there was a significant between-group difference concerning the severity of loss of smell. After four weeks, 19 patients in the intervention group regained their normal sense of smell, while the number amounted to 8 in the control group (P < 0.001, Table 7 , Fig. 2 ). No side effects were reported during the study.
Table 5.
Comparison of Smell Test (Iran-SIT*) between two groups.
| Intervention | Control | P-value | |
|---|---|---|---|
| Initial Score of smell test, median (IQR) | 10 (13) | 9 (11.25) | 0.816 |
| End point score of smell test, median (IQR) | 18 (5) | 16 (7.25) | 0.239 |
Iran Smell Identification Test, IQR: Interquartile range.
Table 6.
Evaluation of the amount of Smell Test (Iran-SIT*) changes between two groups.
| Intervention | Control | P-value | |
|---|---|---|---|
| End point score of smell test changes, mean (SD) | 8.1 (5.1) | 7.9 (5) | 0.91 |
Iran Smell Identification Test, SD: Standard Deviation.
Table 7.
Comparison of olfactory dysfunction type between two groups.
| Intervention | Control | P-value | ||
|---|---|---|---|---|
| Type of dysfunction initially, n (%) | Anosmia | 19 (48.7) | 21 (55.3) | 0.841 |
| Severe microsomia | 9 (23.1) | 8 (21.1) | ||
| Mild microsomia | 11 (28.2) | 9 (23.7) | ||
| Type of dysfunction at week 4, n (%) | Anosmia | 0 | 6 (15.8) | <0.001 |
| Severe microsomia | 1 (2.6) | 13 (34.2) | ||
| Mild microsomia | 19 (48.7) | 11 (28.9) | ||
| Normosmia | 19 (48.7) | 8 (21.1) |
Fig. 2.
Olfactory dysfunction at baseline and after four weeks of the study.
4. Discussion
The results of our study indicated that the use of intranasal mometasone furoate spray can be beneficial in accelerating the recovery of olfactory symptoms caused by the COVID-19 infection without any safety concerns. Although both study groups showed improvement in the severity of the symptoms according to VAS during the follow-up period, this improvement was not significant between the two groups. Importantly, while UPSIT scores revealed no differences between groups, the number of patients who showed clinical responses, defined as normosmia or mild microsmia according to baseline scores, were significantly higher in the intervention group.
Recent histopathological studies aimed to understand the pathogenesis of olfactory dysfunction have reported inflammatory neuropathy with high leukocyte infiltration in lamina propria, focal atrophy of the olfactory mucosa and digestion chambers in the neuronal fibers in the acute phase of infection and chronic inflammation, and extensive olfactory epithelial dysfunction in patients with long-term anosmia [15]. This evidence supports the possible role of therapeutic corticosteroids in the prevention and treatment of long-term olfactory dysfunction in COVID-19 patients.
Similarly, in a prospective interventional study, 120 patients who recovered from the COVID-19 infection were assigned to intervention and control groups. Patients in the intervention group used fluticasone intranasal spray and triamcinolone oral paste for anosmia and dysgeusia for five days. The results showed that olfactory and taste dysfunctions were significantly improved in comparison to the control group [16]. While the results of the study were promising, the non-randomized non-blinded design and the short-term follow-up period of the study should be considered in interpreting the results. Moreover, the lack of a reliable tool to evaluate patients, such as UPSIT, and the non-categorization of the severity of the symptoms might have affected the results.
In a randomized controlled trial, 100 patients who had recovered COVID-19 according to RT-PCR negative results were incorporated in order to analyze the efficacy of mometasone furoate nasal spray on anosmia or hyposmia. The patients were assigned to a topical corticosteroid group (100 mcg daily) or a control group for three weeks. The results showed that the use of intranasal corticosteroid offered no benefits over olfactory training, and the median smell scores according to VAS at weekly assessment showed significant recovery rate in each group (P < 0.001) without any significant differences between the two groups [17]. Similar to our study, the authors administered mometasone furoate as local corticosteroid to improve olfactory dysfunction. However, whereas the administration of this medication was determined only by the presence of olfactory dysfunction regardless of symptom onset and severity, we considered patients who had olfactory dysfunction from symptom onset for at least two weeks. One of the most important reasons for considering this period of time was that most of the acute olfactory symptoms were improved within two weeks of the COVID-19 onset without any intervention [6], [18]. Moreover, dissimilar patient settings from home isolation (mild COVID-19) to hospitalization (moderate-severe) might have affected patient outcomes. Consequently, it is not possible to establish whether patients experienced improvement due to the applied intervention and not the normal course of COVID-19 in the study of Abdelalim et al [17]. In contrast, we studied the patients with anosmia or severe microsmia as confirmed by the SIT test, and not only through self-reported olfactory loss. Moreover, the non-blinded nature of the study was another major limitation in interpreting the results. Given the above discussion, it does not seem appropriate to administer local corticosteroids at early stage of COVID-19 for all patients regardless of the duration of olfactory symptoms and severity scores. In addition, using psychophysical tests such as UPSIT rather than VAS alone provides more reliable olfactory symptom assessment, such that it allowed us to discover significant differences in our final evaluation that would have otherwise remained unnoticed (Table 7, Fig. 2).
In another study on corticosteroids by Rashid et al, the efficacy of betamethasone nasal drop on anosmia in COVID-19 patients was evaluated. A total of 276 patients were randomly given either betamethasone nasal drop three times daily or 0.9% sodium chloride solution for a maximum period of one month. The results indicated that the nasal use of betamethasone drop had no significant effect on the anosmia recovery time according to a self-reported evaluation of the smell sense [19]. Moreover, the clinical response of the patients was not accurately determined. Symptom onset, severity, and improvement were not clear and relied merely on patients’ self-reports. Because of these limitations and the non-blinded design of the study, the results should be interpreted cautiously.
In a pilot study, the impact of oral methylprednisolone on the smell loss of 27 patients with COVID-19 was evaluated. Nine patients were assigned to 32 mg oral methylprednisolone along with olfactory training. After 10 weeks, the patients’ smell mean score in the oral corticosteroid group was significantly higher than that of the control group. Besides, most of the reported side effects of the corticosteroid group were mild or transient in comparison to no report of the mere training group [20]. The compliance of the patient to perform olfactory training was reported low. Moreover, the small sample size and non-randomized non-blinded design were the major limitations.
Vaira et al. evaluated the efficacy of systemic prednisone and nasal irrigation with betamethasone in combination with decongestant and mucolytic for 15 days in non-hospitalized patients with severe hyposmia or anosmia induced by COVID-19 after 30 days of symptom onset. Eighteen patients were examined and the Connecticut Chemosensory Clinical Research Center (CCCRC) test score was considered for outcome assessment on days 20 and 40 of the study. The results substantiated the significant improvement of olfactory scores without any reported side effects [21]. Despite proper patient selection as far as symptom onset and reliable scoring test are concerned, the small sample size and non-blinded design of the study should be considered when interpreting the results.
The limitations of our study include the failure to confirm RT-PCR for some patients and the lack of long term follow-up. Clinical trials with larger sample sizes and longer follow-ups can help make firmer conclusions on appropriate treatment choices.
5. Conclusion
The findings of our study, especially SIT results, showed that the combination of mometasone furoate nasal spray and olfactory training for COVID-19–induced olfactory dysfunction could increase the recovery rate more than olfactory training alone. Moreover, the therapeutic regimen was tolerable without any alarming signal.
6. Ethics approval
This study follows the declaration of Helsinki and was approved by the Ethics Committee of Mazandaran University of Medical Sciences (IR.MAZUMS.REC.1399.877).
Funding
This work was supprted by a grant from vice chancellery for research affairs of Mazandaran University of Medical Sciences. (Grant number: IRMAZUMS8435).
CRediT authorship contribution statement
Hossein Kasiri: Data curation, Investigation, Writing - original draft. Nima Rouhani: Data curation, Investigation. Ebrahim Salehifar: Validation, Writing - review & editing. Monireh Ghazaeian: Conceptualization, Methodology, Supervision, Writing - review & editing. Sahar Fallah: Formal analysis.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Huart C., Philpott C., Konstantinidis I., Altundag A., Trecca E., Cassano M., et al. Comparison of COVID-19 and common cold chemosensory dysfunction. Rhinology. 2020 doi: 10.4193/Rhin20.251. [DOI] [PubMed] [Google Scholar]
- 2.Boscolo-Rizzo P., Borsetto D., Fabbris C., Spinato G., Frezza D., Menegaldo A., et al. Evolution of altered sense of smell or taste in patients with mildly symptomatic COVID-19. JAMA Otolaryngol.-Head Neck Surgery. 2020;146(8):729–732. doi: 10.1001/jamaoto.2020.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boscolo-Rizzo P., Guida F., Polesel J., Marcuzzo A.V., Antonucci P., Capriotti V., et al. Self-reported smell and taste recovery in coronavirus disease 2019 patients: a one-year prospective study. Eur. Arch. Oto-Rhino-Laryngol. 2021;2021:1–6. doi: 10.1007/s00405-021-06839-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petrocelli M., Cutrupi S., Salzano G., Maglitto F., Salzano F., Lechien J., et al. Six-month smell and taste recovery rates in coronavirus disease 2019 patients: a prospective psychophysical study. J. Laryngol. Otol. 2021;2021:1–14. doi: 10.1017/S002221512100116X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lechien J.R., Journe F., Hans S., Chiesa-Estomba C.M., Mustin V., Beckers E., et al. Severity of anosmia as an early symptom of COVID-19 infection may predict lasting loss of smell. Front. Med. 2020;7 doi: 10.3389/fmed.2020.582802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaira L.A., Hopkins C., Petrocelli M., Lechien J., Chiesa-Estomba C., Salzano G., et al. Smell and taste recovery in coronavirus disease 2019 patients: a 60-day objective and prospective study. J. Laryngol. Otol. 2020;134(8):703–709. doi: 10.1017/S0022215120001826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whitcroft K.L., Hummel T. Olfactory dysfunction in COVID-19: diagnosis and management. JAMA. 2020;323(24):2512–2514. doi: 10.1001/jama.2020.8391. [DOI] [PubMed] [Google Scholar]
- 8.Heilmann S., Huettenbrink K.-B., Hummel T. Local and systemic administration of corticosteroids in the treatment of olfactory loss. Am. J. Rhinol. 2004;18(1):29–33. [PubMed] [Google Scholar]
- 9.Seo B.S., Lee H.J., Mo J.-H., Lee C.H., Rhee C.-S., Kim J.-W. Treatment of postviral olfactory loss with glucocorticoids, Ginkgo biloba, and mometasone nasal spray. Arch. Otolaryngol.-Head Neck Surgery. 2009;135(10):1000–1004. doi: 10.1001/archoto.2009.141. [DOI] [PubMed] [Google Scholar]
- 10.Kanjanaumporn J., Aeumjaturapat S., Snidvongs K., Seresirikachorn K., Chusakul S. Smell and taste dysfunction in patients with SARS-CoV-2 infection: A review of epidemiology, pathogenesis, prognosis, and treatment options. Asian Pac. J. Allergy Immunol. 2020;38(2):69–77. doi: 10.12932/AP-030520-0826. [DOI] [PubMed] [Google Scholar]
- 11.C. Huart,C.M. Philpott, A. Altundag, A.W. Fjaeldstad, J. Frasnelli, S. Gane, et al., (Eds) Systemic corticosteroids in coronavirus disease 2019 (COVID‐19)‐related smell dysfunction: an international view. International Forum of Allergy & Rhinology, 2021, Wiley Online Library. [DOI] [PMC free article] [PubMed]
- 12.Hopkins C., Alanin M., Philpott C., Harries P., Whitcroft K., Qureishi A., et al. Management of new onset loss of sense of smell during the COVID-19 pandemic-BRS Consensus Guidelines. Clin. Otolaryngol. 2021;46(1):16–22. doi: 10.1111/coa.13636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hummel T., Rissom K., Reden J., Hähner A., Weidenbecher M., Hüttenbrink K.B. Effects of olfactory training in patients with olfactory loss. Laryngoscope. 2009;119(3):496–499. doi: 10.1002/lary.20101. [DOI] [PubMed] [Google Scholar]
- 14.Taherkhani S., Moztarzadeh F., Seraj J.M., Nazari S.S.H., Taherkhani F., Gharehdaghi J., et al. Iran Smell Identification Test (Iran-SIT): a Modified Version of the University of Pennsylvania Smell Identification Test (UPSIT) for Iranian Population. Chemosens. Percept. 2015;8(4):183–191. [Google Scholar]
- 15.Vaira L.A., Hopkins C., Sandison A., Manca A., Machouchas N., Turilli D., et al. Olfactory epithelium histopathological findings in long-term coronavirus disease, 2019 related anosmia. J. Laryngol. Otol. 2020:1–5. doi: 10.1017/S0022215120002455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh C.V., Jain S., Parveen S. The outcome of fluticasone nasal spray on anosmia and triamcinolone oral paste in dysgeusia in COVID-19 patients. Am. J. Otolaryngol. 2021;42(3) doi: 10.1016/j.amjoto.2020.102892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdelalim A.A., Mohamady A.A., Elsayed R.A., Elawady M.A., Ghallab A.F. Corticosteroid nasal spray for recovery of smell sensation in COVID-19 patients: a randomized controlled trial. Am. J. Otolaryngol. 2021;42(2) doi: 10.1016/j.amjoto.2020.102884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hopkins C., Surda P., Whitehead E., Kumar B.N. Early recovery following new onset anosmia during the COVID-19 pandemic–an observational cohort study. J. Otolaryngol.-Head Neck Surgery. 2020;49:1–6. doi: 10.1186/s40463-020-00423-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rashid R.A., Zgair A., Al-Ani R.M. Effect of nasal corticosteroid in the treatment of anosmia due to COVID-19: A randomised double-blind placebo-controlled study. Am. J. Otolaryngol. 2021;103033 doi: 10.1016/j.amjoto.2021.103033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Bon S.-D., Konopnicki D., Pisarski N., Prunier L., Lechien J.R., Horoi M. Efficacy and safety of oral corticosteroids and olfactory training in the management of COVID-19-related loss of smell. Eur. Arch. Otorhinolaryngol. 2021;1–5 doi: 10.1007/s00405-020-06520-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaira L.A., Hopkins C., Petrocelli M., Lechien J.R., Cutrupi S., Salzano G., et al. Efficacy of corticosteroid therapy in the treatment of long-lasting olfactory disorders in COVID-19 patients. Rhinology. 2020 doi: 10.4193/Rhin20.515. [DOI] [PubMed] [Google Scholar]