Abstract
Background
In patients with coronavirus disease (COVID-19) pneumonia, corticosteroids reduce progression to respiratory failure and death. Some patients, however, remain unresponsive to this treatment, or experience a rebound after termination.
Methods
This retrospective cohort study included COVID-19 patients treated with systemic corticosteroids in a Japanese hospital between June 1, 2020, and January 17, 2021. Patients were categorized into three groups: success, rebound, and refractory, and clinical characteristics and outcomes were compared.
Results
A total of 319 COVID-19 patients were admitted to our hospital and 113 patients met inclusion criteria. The success group had 83 patients (73.5%), the rebound group had nine patients (8.0%), and the refractory group had 21 patients (18.6%). Compared with the success group, the rebound group received corticosteroids earlier, for a shorter duration, and stopped them sooner. The median time from symptom onset to rebound was 12 days. There was no rebound after 20 days. Compared with the success group, the hazard ratio for the number of days from corticosteroid onset to an improvement of two points on a seven-point ordinal scale was 0.29 (95% confidence interval [CI], 0.14–0.60, P < .001) for the rebound group versus 0.13 (95% CI, 0.07–0.25, P < .001) for the refractory group.
Conclusions
COVID-19 patients treated with corticosteroids were classified into three response groups: success, rebound, and refractory, between which recovery time and prognosis differed. It was found that corticosteroid administration may prevent rebound phenomena if administered at least two weeks from symptom onset.
Keywords: COVID-19, Steroid, Pneumonia, SARS-CoV-2
1. Introduction
Coronavirus disease (COVID-19), causing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), emerged in the city of Wuhan, China, at the end of 2019 and has since spread worldwide. In a report of 72,314 cases from China, 14% of cases were severe, requiring ventilation in an intensive care unit, and 5% of cases were critical [1]. The availability of data regarding specific treatments for these COVID-19 patients has, however, been limited.
The host immune response is considered to play a key role in causing a cytokine storm syndrome in patients with severe COVID-19 [2]. Therefore, immunomodulatory agents that inhibit an excessive inflammatory response, such as corticosteroids, may be a potential adjunctive therapy for severe COVID-19. The efficacy of corticosteroids has been established in several clinical studies [3,4], and they are widely used in clinical practice to treat COVID-19 patients. In clinical practice, however, cases refractory to corticosteroids, or a rebound phenomenon during steroid reduction or after cessation, have been experienced [3,5].
The purpose of this study was to review COVID-19 patients who had been treated with corticosteroids, to clarify the characteristics of the rebound and refractory cases and examine the optimal timing and duration of corticosteroid administration.
2. Patients and methods
2.1. Patients
This retrospective cohort study was conducted at a tertiary care hospital in Tokyo, Japan, between June 1, 2020, and January 17, 2021. Adult inpatients (≥18 years of age) diagnosed with COVID-19, according to the World Health Organization definition [6], and treated with systemic corticosteroids were included. Patients who were still in-patients after February 5, 2021, were excluded, as well as patients with unclear outcomes. Patients with interstitial lung disease, human immunodeficiency virus (HIV) infection, those taking immunosuppressive agents, and those who were transferred to another hospital during the course of their illness, were excluded.
2.2. Data collection and definitions
Variables extracted from the medical records of eligible patients included the following: sex, age, body mass index (BMI), comorbidities, laboratory test results, clinical status on a seven-point ordinal scale at the start of corticosteroid treatment, type of corticosteroid administered, other therapeutic agents for COVID-19, and clinical outcomes. The clinical status on a seven-point ordinal scale consisted of the following categories [7]: 1, death; 2, hospitalized, receiving invasive mechanical ventilation or extracorporeal membrane oxygenation; 3, hospitalized, receiving noninvasive ventilation or high-flow oxygen devices; 4, hospitalized, requiring low-flow supplemental oxygen; 5, hospitalized, not requiring supplemental oxygen but receiving ongoing medical care (related or not related to COVID-19); 6, hospitalized, requiring neither supplemental oxygen nor ongoing medical care; and 7, not hospitalized. All data were checked by three physicians (RI, SR, and NN).
The success group was defined as consisting of patients who were responsive to initial corticosteroid therapy and discharged without rebound after corticosteroid cessation. The rebound group was defined as follows: 1. patients who were responsive to initial corticosteroid therapy; 2. patients who subsequently showed a clinical deterioration of at least one point on the seven-point ordinal scale, experienced worsened oxygenation, or required additional treatment for COVID-19 within one week after corticosteroid reduction or cessation; and 3. those in whom infection was ruled out by retrospective analysis. The refractory group was defined as consisting of patients who were unresponsive to initial corticosteroid therapy and showed a clinical deterioration of at least one point on the seven-point ordinal scale, experienced worsened oxygenation, or required an additional treatment for COVID-19, 24 h after corticosteroid therapy was initiated.
The study objectives were as follows: 1. to determine the frequency of the rebound phenomenon and the unresponsiveness to corticosteroids in COVID-19 patients; 2. to identify risk factors associated with the development of the rebound phenomenon; 3. to investigate the clinical outcomes of patients with varying responses to corticosteroids; and 4. to determine the optimal timing and duration of corticosteroid treatment.
2.3. Statistical analyses
For descriptive statistics, continuous data are represented in terms of the median (interquartile range [IQR]); and categorical variables are represented in terms of frequencies (percentages). With regard to the analytical statistical process, because the number of events was limited, the least absolute shrinkage and selection operator (LASSO) regression was performed as a multivariable analysis.
First, the LASSO regression was adopted on presumed variables that affected the results of corticosteroid treatment for the success and rebound groups. Based on our knowledge of COVID-19 and available data, variables such as sex, age, BMI, hypertension, white blood cells, C-reactive protein, time of symptom onset to the start of steroid administration, time of symptom onset to the end of steroid administration, and clinical status on a seven-point ordinal scale at the start of corticosteroids, were included. For each of these variables, the estimated odds ratio was determined for the success of corticosteroid therapy.
Second, the number of days from disease onset until a two-point improvement in clinical status was compared between the three response groups. Kaplan–Meier curves were plotted with the right censor defined as patients who died without clinical improvement at two points. Thereafter, Cox proportional-hazards regression was used to determine the hazard ratio for two points of clinical improvement, using the success group as a reference.
All statistical tests were two-tailed, and significance was accepted at P ≤ .05. All analyses were performed using R software (version 4.0.3; R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Patient characteristics
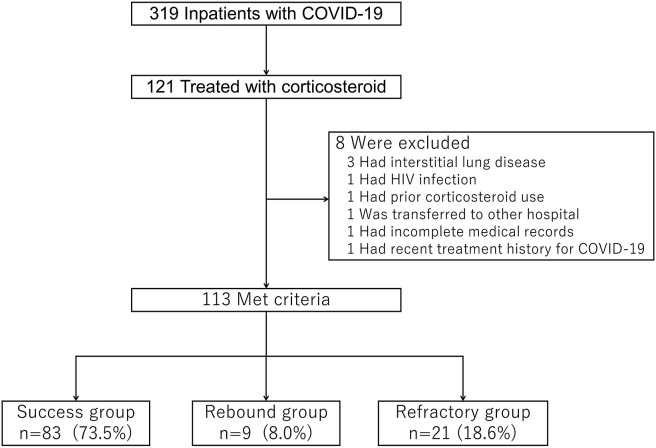
In total, 319 patients diagnosed with COVID-19 were admitted to our hospital during the study period; among them, 121 patients were treated with systemic corticosteroids. After excluding three patients with interstitial lung disease, one patient with HIV infection, one who had prior corticosteroid use, one who was transferred to another hospital, one without available information in the medical records, and one with a recent treatment history for COVID-19, 113 patients met the inclusion criteria. Eighty-three patients (73.5%) were classified into the success group, 9 (8.0%) into the rebound group, and 21 (18.6%) into the refractory group (Fig. 1 ).
Fig. 1.
Flowchart of the study. Patients were categorized into the success, rebound and refractory groups. COVID-19, Coronavirus disease.
Table 1 shows the demographic characteristics of patients, laboratory data, and clinical status on a seven-point ordinal scale when corticosteroid therapy was started for the three groups. In approximately 60% of the total patients, corticosteroids were started when supplemental oxygen was required.
Table 1.
Patient characteristics.
| Success group |
Rebound group |
Refractory group |
|
|---|---|---|---|
| (n = 83, 73.5%) | (n = 9, 8.0%) | (n = 21, 18.6%) | |
| Male, n | 62 (74.7)a | 7 (77.8) | 12 (57.1) |
| Age, year | 59 (49–76) | 73 (64–87) | 79 (61–86) |
| Body Mass Index, kg/m2 | 25.0 (23.1–28.3) | 22.2 (21.2–24.2) | 23.7 (21.4–26.7) |
| Comorbidities | |||
| Cardiovascular disease | 8 (9.6) | 1 (11.1) | 2 (9.5) |
| Cerebrovascular disease | 3 (3.6) | 1 (11.1) | 1 (4.8) |
| Hypertension | 34 (41.0) | 7 (77.8) | 9 (42.9) |
| Diabetes mellitus | 19 (22.9) | 3 (33.3) | 6 (28.6) |
| Laboratory data | |||
| White blood cell count, μl | 5200 (4000–6750) | 5500 (3900–5800) | 5100 (3800–7500) |
| C-reactive protein, mg/dl | 4.22 (1.38–8.86) | 2.30 (0.59–6.88) | 6.64 (3.51–11.02) |
| Lactate dehydrogenase, IU/l | 248 (208–304) | 276 (199–392) | 327 (247–393) |
| Clinical status on a 7-point ordinal scale at the start of corticosteroids | |||
| 2, hospitalized, receiving invasive mechanical ventilation or extracorporeal membraneoxydgenation | |||
| 6 (7.2) | 0 (0) | 2 (9.5) | |
| 3, hospitalized, receiving noninvasive ventilation or high-flow oxygen devices | |||
| 1 (1.2) | 1 (11.1) | 1 (4.8) | |
| 4, hospitalized, requiring low-flow supplemental oxygen | |||
| 51 (61.4) | 4 (44.4) | 14 (66.7) | |
| 5, hospitalized, not requiring supplemental oxygen but receiving ongoing medical care | |||
| 25 (30.1) | 4 (44.4) | 4 (19.0) | |
n (%) or median (interquartile range).
3.2. Treatment
Table 2 summarizes the treatment received by the patients. Approximately 78% of patients were treated with antiviral drugs, such as remdesivir or favipiravir. In terms of non-steroidal immunosuppressive agents, four patients (3.5%) were treated with tocilizumab. Of the total 113 patients, 77 received intravenous dexamethasone, 31 patients were administered 1 mg/kg/day of IV methylprednisolone, and four patients were administered IV methylprednisolone at a dose of 250 mg/day or more for three days.
Table 2.
Treatment.
| Success group |
Rebound group |
Refractory group |
|
|---|---|---|---|
| (n = 83, 73.5%) | (n = 9, 8.0%) | (n = 21, 18.6%) | |
| Antiviral drug | |||
| Remdesivir | 43 (51.8)a | 5 (55.6) | 11 (52.4) |
| Favipiravir | 17 (20.5) | 3 (33.3) | 9 (42.9) |
| Tocilizumab | 2 (2.4) | 0 (0) | 2 (9.5) |
| Antibacterial drug | 68 (81.9) | 7 (77.8) | 19 (90.5) |
| Oxygen administration | 60 (72.3) | 7 (77.8) | 20 (95.2) |
| Invasive mechanical ventilation | |||
| 6 (7.2) | 0 (0) | 9 (42.9) | |
| Extracorporeal membrane oxygenation | |||
| 1 (1.2) | 0 (0) | 1 (4.8) | |
| Corticosteroidb | |||
| Dexamethasone | 60 (72.2) | 6 (66.7) | 11 (52.4) |
| Methylprednisolone | 20 (24.0) | 2 (22.2) | 9 (42.9) |
| Methylprednisolone pulse | 2 (2.4) | 1 (11.1) | 1 (4.8) |
| Prednisolone | 1 (1.2) | 0 (0) | 0 (0) |
| Time of symptom onset to the start of steroid administration, days | |||
| 7 (5–9.5) | 5 (4–6) | 6 (4–7) | |
| Duration of the first steroid administration, days | |||
| 7 (5–9) | 5 (5–9) | 12 (10–27) | |
| Time of symptom onset to the end of the first steroid administration, days | |||
| 13 (11–16.5) | 10 (9–13) | 18 (15–30) | |
| Total duration of steroid administration, days | |||
| 7 (5–9) | 11 (10–12) | 13 (10–27) | |
| Methylprednisolone pulse after corticosteroid refractorya | |||
| 8 (38.1) | |||
n (%).
Dexamethasone, 6.6 mg/day; methylprednisolone, 1 mg/kg/day; methylprednisolone pulse, 250 mg/day or more for three days; prednisolone, 40 mg/day.
3.3. Corticosteroids between success and rebound groups
In the success group, corticosteroids were started at a median of seven days after symptom onset, administered for a median of seven days, and stopped at 13 days after symptom onset. In contrast, in the rebound group, steroids were started at a median of five days after symptom onset and administered for a median of five days (Table 2 and Fig. 2 ). The median (IQR) days from symptom onset to rebound was 12 days (11–13 days), and no cases rebounded 20 days after symptom onset. Compared to the success group, the rebound group tended to be started on corticosteroids earlier in the disease process, received them for a shorter duration, and terminated them earlier in the disease process (Fig. 2). In the LASSO regression analysis, five potential predictors of the rebound phenomenon were identified. These variables were increasing age (odds ratio, 1.02), decreasing BMI (odds ratio, 0.96), hypertension (odds ratio, 1.70), fewer days from symptom onset to starting corticosteroids (odds ratio, 0.94), and fewer days from symptom onset to ending corticosteroids (odds ratio, 0.98).
Fig. 2.
Treatment scheme in the success (A) and rebound (B) groups. Box plots depict time of symptom onset to the start of steroid administration and to rebound. Blackarrows show the duration of the first steroid administration. ∗Median (interquartile range).
3.4. Clinical outcomes
The number of days from corticosteroid initiation to an improvement of two points on the seven-point ordinal scale was shortest in the success group and longest in the refractory group (Fig. 3 ). The hazard ratio was 0.29 (95% confidence interval [CI], 0.14–0.60) in the rebound group compared with that in the success group (P < .001) and 0.13 (95% CI, 0.07–0.25) in the refractory group compared with that in the success group (P < .001). In the rebound group (n = 9), five patients improved on resuming corticosteroids, four patients improved without resuming corticosteroids, and all were discharged. In the refractory group (n = 21), thirteen patients eventually improved, and eight patients died. (Table 3 ).
Fig. 3.
Kaplan–Meier plots of the cumulative probability of improvement of 2 points on the 7-point ordinal scale stratified by the response groups. Dotted line (⋅⋅⋅) = success group, solid line (—) = rebound group, dashed line (–⋅–) = refractory group.
Table 3.
Outcomes.
| Success group |
Rebound group |
Refractory group |
|
|---|---|---|---|
| (n = 83, 73.5%) | (n = 9, 8.0%) | (n = 21, 18.6%) | |
| Time from onset to rebound, days | 12 (11–13)a | ||
| Time from the start of steroids to 2-steps improvement of the clinical status on a 7-point ordinal scale, days | |||
| 7 (6–9) | 14 (10–17) | 15 (13–19)b | |
| Treatment and outcome after rebound or refractory, n | |||
| Improvement without resuming steroids | 4 (44.4) | 9 (42.9) | |
| Improvement after resuming steroids | 5 (55.6) | 0 (0) | |
| Improvement after methylprednisolone pulse | 0 (0) | 3 (14.3) | |
| Improvement after increasing steroids | 0 (0) | 1 (4.8) | |
| Death after methylprednisolone pulse | 0 (0) | 6 (28.6) | |
| Death after increasing steroids | 0 (0) | 1 (4.8) | |
| Death without steroid modification | 0 (0) | 1 (4.8) | |
| Death, n | 0 (0) | 0 (0) | 8 (38.1) |
n (%) or median (interquartile range).
Eight patients who died without any improvement in clinical status on a 7-point ordinal scale were not included.
4. Discussion
In this study, we demonstrated that COVID-19 patients treated with corticosteroids were classified into three response groups: success, rebound, and refractory, between which recovery time and prognosis differed. The rebound groups started and finished corticosteroids earlier, which suggests that rebound could be caused by a shorter treatment duration. In addition, corticosteroids, antivirals, and anticoagulation therapies are considered standard care for patients with moderate to severe COVID-19 in our hospital; immunosuppressive agents, other than corticosteroids, are rarely used. Therefore, we believe that the present results reflect the effects of corticosteroids alone. Furthermore, in Japan, it is recommended that patients with mild COVID-19 be hospitalized; therefore, this study included patients with mild to severe disease.
Long et al. reported that in patients with confirmed COVID-19, the median number of days for both IgG and IgM seroconversion was 13 days after symptom onset [8], around the time when severe or critical disease usually develops [9]. This finding suggests that host immune responses trigger the cytokine storm syndrome, causing severe disease [2,10]. In COVID-19 patients, the release of proinflammatory cytokines, neutrophil recruitment, and T cell activation are considered to be delayed, relative to that of other typical viral infections [11]; thus, it is beneficial to administer corticosteroids for a sufficient period of time to mitigate the cytokine storm.
In our study, the success group was treated with steroids until a median of 13 days after symptom onset, while the rebound group was treated until a median of 10 days after symptom onset. This implies that corticosteroids should be used until 14 days after symptom onset when the cytokine storm has subsided sufficiently. Thus, the rebound group may be managed by extending the duration of corticosteroid administration.
As for the timing of the initiation of corticosteroids, patients in the rebound group started corticosteroids earlier than the success group in our study, suggesting that early initiation of corticosteroids does not contribute to improved prognosis, which is consistent with the results of a previous study [3]. Furthermore, in the RECOVERY trial [3], the benefits of corticosteroids were not exhibited in patients without respiratory failure. Hence, corticosteroids should be started in patients with the onset of respiratory difficulties, not earlier.
Moreover, the refractory group showed a worsened prognosis than the success or rebound groups. For patients who do not respond to initial corticosteroid therapy, immunomodulators and cytokine-storm-targeted therapies besides corticosteroids should be considered to restrain the excessive inflammatory and dysregulated immune responses [10]. Although numerous immunomodulators, including an anti-interleukin-6 receptor antibody and Janus kinase inhibitor have been investigated, further data are needed in the search for a definitive therapy for refractory cases.
In addition, apart from the short-term rebound phenomenon discussed, organizing pneumonia in the later stages is often experienced [12]. The two can be distinguished, and further study is required to clarify the clinical characteristics of the latter.
The present study has some limitations. First, because of the small number of rebound patients, a LASSO regression analysis was conducted instead of a logistic regression analysis, which is the multivariable analysis usually used. Further studies with more cases are required. Second, it was difficult to clarify the cause or pathogenesis of the rebound phenomenon in this study. Further investigation is needed to determine why SARS-CoV-2 causes more prolonged systemic inflammation than other viral infections.
5. Conclusions
In conclusion, the clinical characteristics of the rebound and refractory groups’ response to corticosteroids in patients with COVID-19 was clarified. Patients with the rebound phenomenon tended to receive corticosteroids for a shorter time; thus, steroids should be administered for a sufficient period of time, at least two weeks from symptom onset, to reduce prolonged systemic inflammation.
Guarantor statement
NN takes responsibility for the content of the manuscript including the data analysis.
Author contributions
RI, SR and NN had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. RI, YT and NN contributed substantially to the study design, data analysis and interpretation, and the writing of the manuscript.
Informed consent and patient details
The need for informed consent was waived since it was a retrospective study.
Conflict of Interest
The authors have no conflicts of interest.
Footnotes
The study was approved by the Institutional Review Board of St. Luke's International Hospital (review board number: 20-R213, approval date: February 17, 2021).
References
- 1.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore B.J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020;368:473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 3.The RECOVERY collaborative group Dexamethasone in hospitalized patients with Covid-19 — preliminary report. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The WHO rapid evidence appraisal for COVID-19 therapies (REACT) working group. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. J Am Med Assoc. 2020;324:1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prescott H.C., Rice T.W. Corticosteroids in COVID-19 ARDS: evidence and hope during the pandemic. J Am Med Assoc. 2020;324:1292–1295. doi: 10.1001/jama.2020.16747. [DOI] [PubMed] [Google Scholar]
- 6.The World Health Organization . 2020. Public health surveillance for COVID-19: interim guidance.https://www.who.int/publications/i/item/who-2019-nCoV-surveillanceguidance-2020.8 [Internet] Available at: [Google Scholar]
- 7.Goldman J.D., Lye D.C.B., Hui D.S., Marks K.M., Bruno R., Montejano R. Remdesivir for 5 or 10 days in patients with severe Covid-19. N Engl J Med. 2020;383:1827–1837. doi: 10.1056/NEJMoa2015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Long Q.X., Liu B.Z., Deng H.J., Wu G.C., Deng K., Chen Y.K. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26:845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 9.Hu B., Guo H., Zhou P., Shi Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19:141–154. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chau A.S., Weber A.G., Maria N.I., Narain S., Liu A., Hajizadeh N. The longitudinal immune response to coronavirus disease 2019: chasing the cytokine storm. Arthritis Rheum. 2021;73:23–35. doi: 10.1002/art.41526. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y., Jin C., Wu C.C., Zhao H., Liang T., Liu Z. Organizing pneumonia of COVID-19: time-dependent evolution and outcome in CT findings. PloS One. 2020;15 doi: 10.1371/journal.pone.0240347. [DOI] [PMC free article] [PubMed] [Google Scholar]