Abstract
Background
Severe acute respiratory distress syndrome associated with coronavirus disease-2019 (COVID-19) (CARDS) pneumonitis presents a clinical challenge as regards to the timing of intubation and ambiguity of outcome. There is a lack of clear consensus on when to switch patients from trials of noninvasive therapies to invasive mechanical ventilation. We investigated the effect of the timing of intubation from the time of admission on the clinical outcome of CARDS.
Aim and objective
The aim and objective was to analyze the effect of timing of intubation early (within 48 hours of admission to critical care unit) versus delayed (after 48 hours of admission to critical care unit) on mortality in severe CARDS patients.
Materials and methods
A retrospective observational study performed in a 28-bedded COVID-19 intensive care unit of a tertiary care hospital in Pune, India. All patients admitted between April 1, 2020, and October 15, 2020, with confirmed COVID-19 (RT-PCR positive) requiring mechanical ventilation were included in the study.
Results
The primary outcome was in-hospital mortality. Among 2,230 patients that were admitted to the hospital, 525 required critical care (23.5%), invasive mechanical ventilation was needed in 162 patients and 147 (28%) of critical care admission were included in the study cohort after exclusion. Seventy-five patients (51%) were intubated within 48 hours of critical care admission (early group) and 72 (48.9%) were intubated after 48 hours of critical care admission (delayed group). With regards to the total of 147 included patients; male patients were 74.1% with a median age of 59 years (interquartile range, 51–68 years). Diabetes (44.9%) and hypertension (43.5%) were the most common comorbidities. Higher admission acute physiology and chronic health evaluation II scores and lower absolute lymphocyte count were observed in patients intubated within 48 hours. The early intubated group had a mortality of 60% whereas the same was observed as 77.7% in delayed intubation group, and this difference was statistically significant (p = 0.02).
Conclusion
Current study concludes that early intubation is associated with improved survival rates in severe CARDS patients.
How to cite this article
Zirpe KG, Tiwari AM, Gurav SK, Deshmukh AM, Suryawanshi PB, Wankhede PP, et al. Timing of Invasive Mechanical Ventilation and Mortality among Patients with Severe COVID-19-associated Acute Respiratory Distress Syndrome. Indian J Crit Care Med 2021;25(5):493–498.
Keywords: Acute respiratory distress syndrome, COVID-19, Intubation, Mechanical ventilation, Mortality
Introduction
Coronavirus disease-2019 (COVID-19) that affected countries globally was declared as a pandemic on March 11, 2020, by the authorities of WHO. This pandemic had its roots in China as an epidemic and then spread to other countries rapidly.1 The presentation of this disease varies widely in severity and it mainly affects the respiratory system. The case fatality rate in these patients of COVID-19 is low (<5%) as observed in a single-arm meta-analysis but a sizeable subset of the population (15–18%) do require critical care support.2 Although intubation triggers serve as a classic guide for making carefully weighed decision for appropriate timing of intubation, these triggers themselves are generally the gray area of research in critical care.3 COVID-19 disease presentation is erratic and challenges the clinician with unique respiratory failure.4 At the onset of the disease, there is apparently normal lung mechanics and no clinical clue of derangement in airway resistance or dead space ventilation. Various factors like impaired lung diffusion especially due to the formation of intravascular microthrombi lead to an array of changes in gas exchange leading to a decrease in the partial pressure of oxygen in the blood.5 Hence an unusual feature of COVID-19 pneumonitis is severe hypoxemia with normal lung mechanics.6 This unique pathophysiology of the disease thus tricks the respiratory center that fails to sense hypoxia-related dyspnea. Usually, there is a gross mismatch between the extent of arterial hypoxemia and signs of respiratory distress in these patients.7–10 Thus the term “happy hypoxemia” (severe hypoxia without dyspnea) came to existence in the presentation of such pneumonia during the COVID-19 pandemic.
In contrast to Berlin criteria of acute respiratory distress syndrome (ARDS), the onset of COVID-19-associated ARDS (CARDS) is reported as 8–12 days. CARDS is clinically classified as mild, mild-moderate, and moderate-severe.11 The mainstay of CARDS management in the early stages of presentation is the trial of high-flow nasal oxygen (HFNO) and noninvasive ventilation (NIV).12 The dilemma of the timing of intubation and mechanical ventilation is solved by clinician judgment globally in the pandemic. Patients with high respiratory drive on NIV are at the risk of self-inflicted lung injury (SILI). The pros of early intubation and invasive ventilation focus on the prevention of the same. On the contrary, the perils of invasive ventilation like ventilator-induced lung injury, ventilator-associated pneumonia and ventilator-induced diaphragmatic dysfunction are well known. These factors enforce extended trials of alternate means of oxygenation/ventilation and also delay intubation.13,14 The effect of timing of intubation on the outcome of CARDS is not well understood.15 There is paucity of data and no clear consensus or guidelines regarding timing of intubation and when to switch from NIV to invasive mechanical ventilation. When to intubate remains a topic of research in COVID-19. This retrospective observational study in CARDS patients was conducted to observe the appropriate timing of intubation from intensive care unit (ICU) admission. Our primary hypothesis was that delay in intubation for more than 48 hours since ICU admission may be associated with worse outcomes of mortality. Additionally, we also observed the effect of time from ICU admission to intubation on secondary clinical outcomes such as length of stay and duration of mechanical ventilation.
Materials and Methods
This single-center retrospective observational study was performed in 28-bedded COVID-19 critical care unit of a tertiary care hospital in Pune, Maharashtra, India. The study included all adult patients (>18 years) with CARDS admitted from April 1, 2020, to October 15, 2020. Sample size for the study was not prespecified. Prior intubation at OHC (outside hospital) before shifting and/or written/informed consent for palliative care—such patients were excluded from the study. All decisions about clinical care were made by the COVID-19 treating team in coordination with the Director in Charge intensivist.
Data Collection
Patient data were obtained retrospectively from patient files, nursing charts, and treatment sheets which were accessible as hard copy records or digital archives. Data that were collected included—patients’ age, gender, comorbid conditions, Quick sofa score (qSOFA) score at admission, acute physiology and chronic health evaluation II (APACHE II) score within 24 hours of admission, intubation day from date and time of ICU admission. Similarly, additional laboratory parameters of interest—WBC count, lymphocyte count, D-dimer level, interleukin 6 (IL-6), serum ferritin level, and PaO2/FiO2 ratio were also obtained.
Study Exposure and Outcome
The exposure time from critical care unit admission to endotracheal intubation was categorized as “early” (<48 hours) and “delayed” (>48 hours). The primary outcome was in-hospital mortality after onset of invasive ventilation. Secondary outcome was to observe duration of mechanical ventilation, length of stay. The other parameters observed were secondary infections (culture positive), acute kidney injuries (AKIs), and intervention (e.g., tracheostomy).
Statistical Analyses
All the data was entered into a Microsoft Excel spreadsheet and analyzed. An online statistics calculator was used to analyze the data.16 Values were presented as median [interquartile range (IQR)] or percentages (%) for descriptive data. Categorical and continuous variables were analyzed using Chi-square tests and Kruskal–Wallis test, respectively, for inter-group comparison.
Ethics
Institutional Ethics Committee approval was obtained prior to the study.
Results
Study population
A total of 2,230 COVID-19 (RT-PCR)-positive patients were admitted between April 1, 2020, and October 15, 2020, to the hospital. Amongst these, 525 patients (23.5%) required critical care. A total of 162 patients of the 525 critical patients, were intubated and mechanically ventilated (Flowchart 1).
Flowchart.
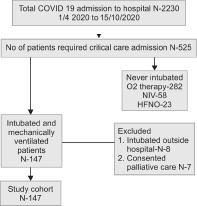
Flow diagram of study participants. COVID-19, coronavirus disease 2019; NIV, noninvasive ventilation; HFNO, high flow nasal oxygen
Patients who consented for palliative care (n = 7) and those intubated prior to transfer from other hospital (n = 8) were excluded from the study population (total n = 15). Total 147 patients (28%) who intubated and ventilated during ICU stay after admission to hospital were included in the study cohort. It was observed that 75 patients (51%) were intubated within 48 hours of admission (early group) and 72 (48.9%) were intubated after 48 hours after ICU admission (delayed group).
The comorbidities demographics of 147 patients categorized by time of ICU admission to intubation are shown in Table 1. The median age of the total study population was 59 years (IQR, 51–68 years) and 74.1% were male. Diabetes (44.9%) and hypertension (43.5%) were most common comorbidities in the study population followed by chronic kidney disease (CKD) (5.4%), ischemic heart disease (IHD) (12.9%), COPD (0.6%), and obesity (2%). Comorbidities like hypothyroidism, chronic medical conditions autoimmune disorders, parkinsonism, old stroke, known malignancy ca rectum, lung cancer, chronic liver disease, and old medical condition with immunocompromised states were included in a separate group as others which comprised 23.8% of the study population.
Table 1.
Demographics and comorbidities of mechanically ventilated patients by time from ICU admission to intubation
| Patient characteristics | Total (n = 147) | Time from ICU admission to intubation | Pb | |
|---|---|---|---|---|
| <48 hrs (n = 75) | >48 hrs (n = 72) | |||
| Age (year) | 59 (51–68) | 58 (50–69) | 59 (52–67) | 0.9 |
| Male | 109 (74.1) | 56 (74.6) | 53 (73.6) | 0.8 |
| Comorbidities | ||||
| Diabetes mellitus | 66 (44.9) | 35 (46.6) | 31 (43.1) | 0.6 |
| Hypertension | 64 (43.5) | 35 (46.6) | 29 (40.3) | 0.4 |
| CKD | 08 (5.4) | 5 (6.6) | 3 (4.2) | 0.5 |
| IHD | 19 (12.9) | 9 (12) | 10 (13.9) | 0.7 |
| COPD | 01 (0.6) | 1 (1.3) | 0 (0) | |
| Obesity | 03 (2.0) | 1 (1.3) | 2 (2.8) | 0.5 |
| Others | 35 (23.8) | 20 (26.7) | 15 (20.8) | 0.4 |
Pb values represent χ2 test or Fisher exact test for differences in proportions for categorical variables and Kruskal–Wallis test for differences in medians for continuous variables; values are expressed as median (interquartile range) or n (%)
Clinical scoring and laboratory characteristics of 147 patients were classified according to early (<48 hours) and delayed intubation (48 hours) from time of ICU admission, which is shown in Table 2. The median APACHE II score of the study recorded within 24 hours of critical care unit admission was 11 (IQR, 8–14). When the two groups were compared with regard to the median APACHE II score; it was significantly higher (p <0.03) in the early group as compared to that of the late group. The median qSOFA score assessed on admission to critical care unit was 1 (IQR, 1–2) and was comparable in both the groups. Apart from the median absolute lymphocyte count (0.8 × 103/mm3) (IQR, 0.4–1.0) that was significantly low (p <0.03) (0.6 × 103/mm3) in those intubated within 48 hours of admission; all other laboratory parameters viz. median WBC count 12.3 × 103mm3 (IQR, 8.6–17.3), median serum ferritin 568 ng/mL (IQR, 249–1080), median D-dimer 582 ng/mL (IQR, 300–2094) and median IL-6 100.6 pg/mL (IQR, 28.8–264.1) were similar in the two groups and differences if at all were not statistically significant. The median PaO2/FiO2 ratio of the study group was 71.8 (IQR, 57.6–89.6) and on further analysis, 16.3% of study patient (24/147) had PaO2/FiO2 ratio from 101 to 200 whereas 83.6% of patients (123/147) had a PaO2/FiO2 ratio of less than or equal to 100. The median PaO2/FiO2 ratio did not significantly differ in the study groups (p = 0.2). The total study population had a median HRCT score 13 (IQR, 10–16); this was similar in both early and late groups (p = 0.2). Secondary infection was 13.3% in early vs 22.2% in late groups (p = 0.6). AKI/renal failure was 21.3% in early group vs 18% in late group (p = 0.1). Number of patients who underwent tracheostomies were 16% in early group vs 25% in late group (p = 0.1). So, the differences in secondary infection, AKI, and intervention tracheostomies were not statistically significant between those intubated within 48 hours (early group) and those intubated after 48 hours of ICU admission (late group), respectively.
Table 2.
Clinical scoring and laboratory characteristics of mechanically ventilated patients by time from ICU admission to intubation
| Clinical scoring | Total (n = 147) | Time from ICU admission to intubation | Pb | |
|---|---|---|---|---|
| <48 hrs (n = 75) | >48 hrs (n = 72) | |||
| APACHE II | 11 (8–14) | 12 (9–15) | 10 (8–13) | 0.03 |
| qSOFA | 1 (1–2) | 1 (1–2) | 1 (1–2) | 0.7 |
| Laboratory characteristics | ||||
| WBC (×103/mm3) | 12.3 (8.6–17.3) | 10.8 (7.9–17.3) | 13.4 (9.6–17.1) | 0.2 |
| Absolute lymphocyte count (×103/mm3) | 0.8 (0.4–1.0) | 0.6 (0.4–1.0) | 0.9 (0.6–1.2) | 0.003 |
| Serum ferritin (ng/mL) (17–464) | 568.0 (249–1080) | 570.8 (290.49–1029.5) | 523.3 (197.7–1356.2) | 0.5 |
| D-dimer (ng/mL) (≤240) | 582 (300–2094) | 560 (300–1410) | 597.5 (297–2584.25) | 0.9 |
| IL-6 (pg/mL) (<6.4) | 100.6 (28.8–264.1) | 75.0 (23.9–214.8) | 152.4 (30.2–291.1) | 0.1 |
| PaO2/FiO2 ratio at intubation | 71.8 (57.6–89.6) | 74.7 (60.0–104.7) | 70.7 (57.3–82.4) | 0.2 |
| HRCT score | 13 (10–16) | 14 (11–17) | 10 (6–16) | 0.2 |
| AKI | 29 (19.7) | 16 (21.3) | 13 (18) | 0.6 |
| Secondary infection | 26 (17.6) | 10 (13.3) | 16 (22.2) | 0.1 |
| Tracheostomies | 30 (20.4) | 12 (16) | 18 (25) | 0.1 |
AKI, acute kidney injury; Pb values represent χ2 test or Fisher exact test for differences in proportions for categorical variables and Kruskal–Wallis test for differences in medians for continuous variables; Values are expressed as median (interquartile range) or n (%)
The analysis of 147 patients intubated and mechanically ventilated was done to note the in-hospital mortality. The observed in-hospital mortality was 68.7% (101 patients of 147) (Table 3). The median time to death after intubation was 6 days (IQR, 2–13 days). Mortality was 60% in those intubated within 48 hours of critical care unit admission (early group) compared to 77.7% in those of delayed group and the difference was found to be statistically significant (p = 0.02).
Table 3.
Hospital mortality, duration of ventilation, and ICU length of stay by time from ICU admission to intubation in a tertiary care center, India
| Outcomes | Total (n = 147) | Time from ICU admission to intubation | Pb | |
|---|---|---|---|---|
| <48 hrs (n = 75) | ≥48 hrs (n = 72) | |||
| Primary outcome | ||||
| Deceased, n (%) | 101 (68.7) | 45 (60) | 56 (77.7) | 0.02 |
| Secondary outcome | ||||
| Duration of ventilation (days), median (IQR) | 7 (3.5–12) | 7 (4–12) | 6 (2–12) | 0.2 |
| ICU length of stay (days), median (IQR) | 15 (9–21) | 14 (9.7–21) | 16 (7–21.7) | 0.9 |
IQR, interquartile range; Pb values represent (Chi-square) χ2 tests for differences in proportions for categorical variables and Kruskal–Wallis test for differences in medians for continuous variables.
As regards to secondary outcome measures; median duration of ventilation was 7 days (IQR, 3.5–12) and median length of stay was 15 days (IQR, 9–21 days). These were similar in both the early and late groups, respectively.
Discussion
COVID-19 presents with unique respiratory failure. The extremes of clinical presentation of COVID-19 vary from asymptomatic infection to severe respiratory distress presents a clinical challenge. Different rates of mechanical ventilation have been reported in publications of COVID-19 across the world.17 In the present study, 23.5% of hospitalized patients required critical care of which 30.8% of them needed invasive mechanical ventilation. In a large epidemiological study from China, out of 344 intensive care patients, 100 of them (29.1%) required invasive mechanical ventilation, so they reported a rate of requirement of invasive ventilation which is similar to the one reported in our study.18 Demographic parameters that attribute to adverse outcomes in COVID-19 patients are increasing age and male sex. This trend was also observed in the present study.19–21
Diabetes (44%) and hypertension (43.5%) were the most commonly reported comorbidities in this study population of CARDS. Therefore, in this study population, comorbid characteristics were not different from the one reported globally as various previous studies also reported diabetes and hypertension as most common comorbidities and its’ association with patients requiring intubation in CARDS.21–23
Clinical scoring systems i.e., APACHE II and qSOFA scores have been evaluated in studies for the severity of illness in COVID-19. Such studies have concluded that qSOFA score had moderate sensitivity and low specificity in COVID-19 patients.24 APACHE II is one of the important predictive factors of mortality in ICU and high scores are associated with nonsurvivors.25 The present study population had a median APACHE II score of 11.
The median values of laboratory parameters viz. WBC count, serum ferritin, D-dimer, and IL-6 although were comparable in the two study groups yet as mentioned in the previous published COVID-19 literature were well above the normal values.26
An interesting observation in this study was that patients intubated early within 48 hours of critical care admission were consistent with lower absolute lymphocyte count along with higher APACHE II score. Baseline lymphopenia in critical COVID-19 patients has been reported in previous studies globally.26–28 These findings highlight that APACHE II score and lymphopenia are linked with a greater risk of severe COVID-19 as well as risk factors for in-hospital death. One retrospective study from China reported that the survivor probability of COVID-19 patients was better in patients with APACHE II score less than 17.29
AKI among critical COVID-19 patients serves as a negative prognostic factor for survival.21,30 Experience in Europe and the USA with critical COVID-19 patients reported that approximately 20–40% of them had AKI; which is similar to that observed in our study where 19.73% of total patients developed renal failure.31
This factor to a certain extent may explain the high mortality in our COVID-19 study population.
The perils of long-time invasive mechanical ventilation like ventilator and hospital-acquired infections are not an exclusion for COVID-19 patients.32 However, although important, many clinicians are evasive about tracheotomy in COVID-19 patients due to various risks involved during and even after the procedure.33
It was observed in this study that a total of 20.41% patients underwent tracheostomies of which fewer belonged to the early intubation group as compared to the delayed group; however, no statistical difference was noted. In a meta-analysis, coinfections in ICU patients have been reported at 14%.34
We observed an overall 17.69% coinfection in this study, where the early intubation group had less patients in comparison to that of the late intubation group, and again, this difference was not statically significant. Therefore, it was observed that tracheotomy patients had a higher probability of secondary bacterial infection which may be one of the adverse events related to the procedure. To establish a robust association and arrive at a valid conclusion, one may require a well-planned study in such patients and delve into other factors as secondary bacterial infection is influenced by an array of factors like prolonged length of stay, intravascular devices, procedures like continuous renal replacement therapy (CRRT), etc.35,36
The positive outcome scenario of mechanically ventilated COVID-19 patients is bleak. Mortality rates ranging from 17 to 62% in such patients were reported globally in small studies.37
In a New York based study, Richardson et al. reported that the mortality rate was 76.4% in the age-group 18–65 years and 97.2% of more than 65 years amongst those required mechanical ventilation.23 In the present study, we found the overall mortality to be 68.7% in patients who received invasive mechanical ventilation. Severity of illness and prognosis is related to PaO2/FiO2 ratio—a ratio of 52.0–74.1 was observed in nonsurvivors.28
A proportion of 83.6% of patients had a PaO2/FiO2 ratio of less than or equal to 100 in our study population. In addition, our analysis revealed those patients intubated ventilated within 48 hours of critical care unit admission showed reduced mortality (60%) as compared to those in the delayed group (77%) admission (p = 0.02). These observations are in agreement with the large multihospital, retrospective cohort study of New York City, which concluded that among mechanically ventilated COVID-19 patients, early intubation after hospital admission was associated with favorable patient outcomes; whereas each day delay in intubation increased the risk of mortality.38 Clinical decision and timing regarding switching from NIV or high-flow oxygen therapy to invasive mechanical ventilation is variable and multifactorial, hence its impact on outcome is debatable. Therefore, Alfonso et al. failed to observe association between time to intubation and mortality.39 In the study by Alfonso et al., 20.5% were COVID-19 patients with PaO2/FiO2 ratio less than 100, whereas our study cohort included 83.6% of severe patients with PaO2/FiO2 ratio less than 100 at time of intubation. The present study clearly defines only two groups with association of time i.e., intubation in less than 48 hours and that after 48 hours which is different than the study by Alfonso et al. who defined study groups of less than 8 hours, 8 to 24 hours and greater than 24 hours. Another interesting observation is that the Alfonso et al. study cohort contained 44.6% female participants, in contrast to 26% in the present study group.39
Kangelaris et al. in their prospective observational study had included non-COVID-19 ARDS patients. Greater mortality was observed by the authors in patients, in whom initial intubation was delayed despite of meeting ARDS criteria.40
In our study, we identified that increased mortality in delayed intubation group was not explained by demographics, comorbidities, and severity of illness measured in 24 hours of ICU admission (APACHE II scoring). In contrast, patient with early intubation had high APACHE II score, and this finding was similar to study by Kangelaris et al., which reported higher average APACHE II score in early intubation group of critically ill non-CARDS patients.40 We infer that timing of intubation could play a key role in overall outcomes and a further large prospective cohort study is deemed necessary to confirm increased mortality observed in late intubation group.
Our analysis also showed that other secondary outcomes like length of stay in critical care units and duration of mechanical ventilation did not show statistically significant differences among the two groups.
In this study, it was observed that high APACHE II score and lower absolute lymphocyte count in the early group was subtly associated with a switch from noninvasive to invasive ventilation, though authors refrain from claiming so due to lack of substantial evidence. Decision for intubation is complex and multifactorial which is usually individualized depending on the clinical condition. Certain unmatched confounders affecting such intubation-related decisions in the study population may not have been accounted for in this study. In the setting of the pandemic, though lung-protective ventilation protocol was followed in all cases, data collection and analysis of the effect of SILI and individual ventilation parameters, tidal volume, compliance, peep, were not done. Further well-planned multicentric prospective trials are required in severe CARDS patients to eventually test the hypothesis that early intubation in high-risk groups improves outcomes and to address the possibilities of unmeasured confounders.
Conclusion
Timing of intubation is a carefully calculated decision taken by a clinician in COVID-19 settings due to its complex presentation and equally varied individual patient response to oxygen therapy. Evidence regarding when to switch from NIV to invasive therapy is limited in absence of lack of clear guidelines in severe CARDS patients. In this study, higher mortality was observed with the delay in intubation. Therefore delaying intubation for severe CARDS patients should be considered with great caution. There is a need to relook new predictor for intubation like APACHE II scores in COVID-19 setting. Well-designed robust studies may provide insights into predictors of improved survival in the coming probable upsurge of pandemic. In this retrospective study, we observed that the timing of intubation may be associated with survival. Our analysis supports that clinicians caring for patients with severe CARDS should not unnecessarily delay intubation.
Orcid
Kapil G Zirpe https://orcid.org/0000-0002-8140-727X
Anand M Tiwari https://orcid.org/0000-0002-9791-8365
Sushma K Gurav https://orcid.org/0000-0001-6875-2071
Abhijeet M Deshmukh https://orcid.org/0000-0001-5602-291X
Prasad B Suryawanshi https://orcid.org/0000-0001-7306-8434
Prajakta P Wankhede https://orcid.org/0000-0002-3620-3390
Upendra S Kapse https://orcid.org/0000-0002-5279-4485
Abhaya P Bhoyar https://orcid.org/0000-0002-0460-3162
Afroz Z Khan https://orcid.org/0000-0003-3170-496X
Ria V Malhotra https://orcid.org/0000-0002-1026-0274
Pranoti H Kusalkar https://orcid.org/0000-0002-6522-9936
Kaustubh J Chavan https://orcid.org/0000-0002-4387-9188
Seema A Naik https://orcid.org/0000-0002-6575-8010
Rahul B Bhalke https://orcid.org/0000-0003-3271-5716
Ninad N Bhosale https://orcid.org/0000-0003-0774-2069
Sonika V Makhija https://orcid.org/0000-0001-7258-5878
Venkata N Kuchimanchi https://orcid.org/0000-0002-9667-2754
Amol S Jadhav https://orcid.org/0000-0001-9741-9884
Kedar R Deshmukh https://orcid.org/0000-0002-1194-7182
Gaurav S Kulkarni https://orcid.org/0000-0001-9131-1805
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.Contini C, Di Nuzzo M, Barp N, Bonazza A, De Giorgio R, Tognon M, et al. The novel zoonotic COVID-19 pandemic: an expected global health concern. J Infect Dev Ctries. 2020;14(3):254–264. doi: 10.3855/jidc.12671. DOI: [DOI] [PubMed] [Google Scholar]
- 2.Sun P, Qie S, Liu Z, Ren J, Li K, Xi J. Clinical characteristics of hospitalized patients with SARS-CoV-2 infection: a single arm meta-analysis. J Med Virol. 2020;92(6):612–617. doi: 10.1002/jmv.25735. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Windisch W, Weber-Carstens S, Kluge S, Rossaint R, Welte T, Karagiannidis C. Invasive and non-invasive ventilation in patients with COVID-19. Dtsch Ärztebl Int. 2020;117(31–32):528. doi: 10.3238/arztebl.2020.0528. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matta SK. Dilemmas in Covid-19 respiratory distress: early vs late intubation; high tidal volume and low PEEP vs traditional approach? J Intensive Crit Care. 2020;6(2):7. doi: 10.36648/2471-8505.6.2.7. DOI: [DOI] [Google Scholar]
- 5.Dhont S, Derom E, Van Braeckel E, Depuydt P, Lambrecht BN. The pathophysiology of ‘happy’ hypoxemia in COVID-19. Respir Res. 2020;21(1):1–9. doi: 10.1186/s12931-020-01462-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Navas-Blanco JR, Dudaryk R. Management of respiratory distress syndrome due to COVID-19 infection. BMC Anesthesiol. 2020;20(1):1–6. doi: 10.1186/s12871-020-01095-7. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie J, Tong Z, Guan X, Du B, Qiu H, Slutsky AS. Critical care crisis and some recommendations during the COVID-19 epidemic in China. Intensive Care Med. 2020:1–4. doi: 10.1007/s00134-020-05979-7. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tobin MJ, Laghi F, Jubran A. Why COVID-19 silent hypoxemia is baffling to physicians. Am J Respir Crit Care Med. 2020;202(3):356–60. doi: 10.1164/rccm.202006-2157CP. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilkerson RG, Adler JD, Shah NG, Brown R. Silent hypoxia: a harbinger of clinical deterioration in patients with COVID-19. Am J Emerg Med. 2020;38(10):2243.e5–2243.e6. doi: 10.1016/j.ajem.2020.05.044. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allali G, Marti C, Grosgurin O, Morélot-Panzini C, Similowski T, Adler D. Dyspnea: the vanished warning symptom of COVID-19 pneumonia. J Med Virol. 2020 doi: 10.1002/jmv.26172. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X, Ma X. Acute respiratory failure in COVID-19: is it “typical” ARDS? Critical Care. 2020;24:1–5. doi: 10.1186/s13054-020-02911-9. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan L, Zhou L, Le Grange JM, Zheng Z, Chen R. Non-invasive ventilation in the treatment of early hypoxemic respiratory failure caused by COVID-19: considering nasal CPAP as the first choice. Crit Care. 2020;24(1):1–2. doi: 10.1186/s13054-020-03054-7. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tobin MJ, Laghi F, Jubran A. Caution about early intubation and mechanical ventilation in COVID-19. Ann Intensive Care. 2020;10(1):1–3. doi: 10.1186/s13613-020-00692-6. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meng L, Qiu H, Wan L, Ai Y, Xue Z, Guo Q, et al. Intubation and ventilation amid the COVID-19 outbreak: Wuhan's experience. Anesthesiology. 2020;132(6):1317–1332. doi: 10.1097/ALN.0000000000003296. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee YH, Choi KJ, Choi SH, Lee SY, Kim KC, Kim EJ, et al. Clinical significance of timing of intubation in critically ill patients with COVID-19: a multi-center retrospective study. J Clin Med. 2020;9(9):2847. doi: 10.3390/jcm9092847. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.https://www.socscistatistics.com/tests/ [(Accessed on 06/12/2020]; at 14.33 IST) (5)
- 17.Wunsch H. Mechanical ventilation in COVID-19: interpreting the current epidemiology. Am J Respir Crit Care Med. 2020;202(1):1–4. doi: 10.1164/rccm.202004-1385ED. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Lu X, Li Y, Chen H, Chen T, Su N, et al. Clinical course and outcomes of 344 intensive care patients with COVID-19. Am J Respir Crit Care Med. 2020;201(11):1430–1434. doi: 10.1164/rccm.202003-0736LE. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, et al. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(6):577–583. doi: 10.1002/jmv.25757. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palaiodimos L, Kokkinidis DG, Li W, Karamanis D, Ognibene J, Arora S, et al. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism. 2020;108:154262. doi: 10.1016/j.metabol.2020.154262. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu C, Chen X, Cai Y, Zhou X, Xu S, Huang H, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan G, Tu C, Zhou F, Liu Z, Wang Y, Song B, et al. Comparison of severity scores for COVID-19 patients with pneumonia: a retrospective study. Eur Respir J. 2020;56(3):2002113. doi: 10.1183/13993003.02113-2020. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrando C, Mellado-Artigas R, Gea A, Arruti E, Aldecoa C, Bordell A, et al. Patient characteristics, clinical course and factors associated to ICU mortality in critically ill patients infected with SARS-CoV-2 in Spain: a prospective, cohort, multicentre study. Rev Esp Anestesiol Reanim. 2020;67(8):425–437. doi: 10.1016/j.redar.2020.07.003. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yao Q, Wang P, Wang X, Qie G, Meng M, Tong X, et al. Retrospective study of risk factors for severe SARS-Cov-2 infections in hospitalized adult patients. Pol Arch Intern Med. 2020;130(5):390–399. doi: 10.20452/pamw.15312. DOI: [DOI] [PubMed] [Google Scholar]
- 27.Fan BE, Chong VC, Chan SS, Lim GH, Lim KG, Tan GB, et al. Hematologic parameters in patients with COVID-19 infection. Am J Hematol. 2020;95(6):E131–E134. doi: 10.1002/ajh.25774. DOI: [DOI] [PubMed] [Google Scholar]
- 28.Yang X, Yu Y, Xu J, Shu H, Liu H, Wu Y, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):P475–P481. doi: 10.1016/S2213-2600(20)30079-5. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zou X, Li S, Fang M, Hu M, Bian Y, Ling J, et al. Acute physiology and chronic health evaluation II score as a predictor of hospital mortality in patients of coronavirus disease 2019. Crit Care Med. 2020;48(8):e657–e665. doi: 10.1097/CCM.0000000000004411. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen YT, Shao SC, Hsu CK, Wu IW, Hung MJ, Chen YC. Incidence of acute kidney injury in COVID-19 infection: a systematic review and meta-analysis. Crit Care. 2020;24(1):1–4. doi: 10.1186/s13054-020-03009-y. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ronco C, Reis T, Husain-Syed F. Management of acute kidney injury in patients with COVID-19. Lancet Respir Med. 2020;8(7):P738–P742. doi: 10.1016/S2213-2600(20)30229-0. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cox MJ, Loman N, Bogaert D, O'grady J. Co-infections: potentially lethal and unexplored in COVID-19. Lancet Microbe. 2020;1(1):e11. doi: 10.1016/S2666-5247(20)30009-4. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piazza C, Filauro M, Dikkers FG, Nouraei SR, Sandu K, Sittel C, et al. Long-term intubation and high rate of tracheostomy in COVID-19 patients might determine an unprecedented increase of airway stenoses: a call to action from the European Laryngological Society. Eur Arch Otorhinolaryngol. 2020:1–7. doi: 10.1007/s00405-020-06112-6. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lansbury L, Lim B, Baskaran V, Lim WS. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. 2020;81(2):266–275. doi: 10.1016/j.jinf.2020.05.046. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gotsman MS, Whitby JL. Respiratory infection following tracheostomy. Thorax. 1964;19(1):89. doi: 10.1136/thx.19.1.89. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang H, Zhang Y, Wu J, Li Y, Zhou X, Li X, et al. Risks and features of secondary infections in severe and critical ill COVID-19 patients. Emerg Microbes Infect. 2020;9(1):1958–1964. doi: 10.1080/22221751.2020.1812437. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chaddha U, Kaul V, Agrawal A. What is the true mortality in the critically ill patients with COVID-19? Indian J Crit Care Med. 2020;24(6):38. doi: 10.5005/jp-journals-10071-23435. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hyman JB, Leibner ES, Tandon P, Egorova NN, Bassily-Marcus A, Kohli-Seth R, et al. Timing of intubation and in-hospital mortality in patients with coronavirus disease 2019. Crit Care Explor. 2020;2(10) doi: 10.1097/CCE.0000000000000254. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hernandez-Romieu AC, Adelman MW, Hockstein MA, Robichaux CJ, Edwards JA, Fazio JC, et al. Timing of intubation and mortality among critically Ill coronavirus disease 2019 patients: a single-center cohort study. Crit Care Med. 2020;48(11):e1045–e1053. doi: 10.1097/CCM.0000000000004600. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kangelaris KN, Ware LB, Wang CY, Janz DR, Hanjing Z, Matthay MA, et al. Timing of intubation and clinical outcomes in adults with ARDS. Crit Care Med. 2016;44(1):120. doi: 10.1097/CCM.0000000000001359. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]


