Abstract
Background/Objectives:
Peripheral neuropathy is a common diabetes complication that can increase fall risk. Regarding fall risk, the impact of pain management using tricyclic antidepressants (TCAs) or gamma-aminobutyric acid (GABA) analogs is unclear because these medications can also cause falls. This study investigates the impact of these drugs on fall and fracture risk in older diabetic peripheral neuropathy patients.
Design:
Historical cohort study with 1-to-1 propensity matching of TCA/GABA-analog users and non-users.
Setting:
Nationally representative 5% Medicare sample between the years 2008 and 2010.
Participants:
After applying all selection criteria, 5,550 patients with prescription and 22,200 patients without prescription of TCAs/GABA-analogs were identified. Both patient groups were then stratified for fall history and matched based on propensity of receiving TCAs/GABA-analogs within each group.
Measurements:
Patients were followed until the first incidence of fall or the first incidence of fracture during the follow-up period (for up to 5 years).
Results:
After matching, users and non-users were largely similar. After covariate adjustment, TCA/GABA-analog use was associated with a statistically significant increase in fall risk (adjusted HR: 1.11, 95% CI 1.03–1.20), but was not associated with fracture risk (adjusted HR: 1.09, 95% CI 0.99–1.19) in the conventional analysis. Treating TCA/GABA-analog use as a time-dependent covariate resulted in statistically significant associations of TCA/GABA-analog use with both fall and fracture risk (HR 1.26, 95% CI 1.17 to 1.36; and HR 1.12, 95% CI 1.02–1.24).
Conclusion:
Among older patients with diabetic peripheral neuropathy, GABA-analogs or TCAs increase fall risk and possibly fracture risk. Use of these medications is therefore a potentially modifiable risk factor for falls and fractures in this population.
Keywords: falls, pain management, neuropathy, diabetes
INTRODUCTION
Mobility is essential for independence in old age. Older patients with diabetes are at particular risk of mobility declines due to diabetic peripheral neuropathy (DPN). Affecting approximately 30% of all diabetes patients, this complication causes impaired sensation, muscular weakness, and neuropathic pain.1, 2 This can result in gait disturbances leading to foot ulceration, falls, and fractures.3–6 Pain management may benefit these patients by preventing falls and fractures associated with neuropathic pain.
However, DPN management is challenging. When our sampling began in 2008, gamma-aminobutyric acid (GABA) analogs, tricyclic antidepressants (TCAs), and serotonin-norepinephrine reuptake inhibitors (SNRIs) were considered first-line pharmaceutical monotherapy for painful DPN.1, 2, 7, 8 However, these drugs have side effects that increase fall risk in older adults. TCAs like amitriptyline act nonspecifically by enhancing serotonergic and noradrenergic mechanisms, while simultaneously blocking histaminic, cholinergic, and α1-adrenergic actions. The anticholinergic side effects of TCAs include drowsiness, blurred vision, dizziness, somnolence, and confusion.9 GABA analogs also increase dizziness and somnolence; however, the mechanism by which this occurs is unknown.10–14 Lastly, SNRIs increase somnolence and dizziness in addition to decreasing bone density, resulting in increased risk of falls and fractures, respectively.15, 16 In the current study, we omitted SNRIs from the analysis for two main reasons. Firstly, there is a relative paucity of contemporary clinical trials indicating the effectiveness of SNRIs for the treatment of neuropathic pain.8 Secondly, we believe that the inclusion of SNRI use in our models would introduce selection bias, as these drugs were not available generically during the study period.
Of these drug classes, only TCAs have been widely tested and demonstrated as contributory to fall and fracture risk in the older population.17–23 However, it is reasonable to hypothesize that GABA analogs also contribute to fall and fracture risk in the older population. Regarding the older population with DPN, none of these drug classes have been examined to determine their associated fall and fracture risk. However, doing so is particularly important in this population, considering these drugs’ poor efficacy. Only one-third of painful diabetic neuropathy patients will achieve pain relief of fifty percent or greater using these drugs.1 Therefore, it is unclear whether their use will 1) decrease neuropathic pain sufficiently to reduce fall and fracture risk, or 2) be associated with increased fall and fracture risk as a result of drug side effects.
In this study, we examine the effect of TCAs and GABA analogs on fall and fracture risk in older patients with DPN using a nationally representative Medicare sample.
METHODS
This study complies with the privacy rules for protected health information as stated by the Health Insurance Portability and Accountability Act of 1996 (HIPAA).
Study Population.
This historical cohort study examines a 5% sample of Medicare beneficiaries from the years 2008 to 2014. From this sample, beneficiaries with diabetes were identified using Chronic Condition Data Warehouse (CCW) chronic conditions categories.24 To identify additional patient characteristics, TCA/GABA-analog use, and study outcomes, this study utilizes Medicare beneficiary summary files, Medicare Provider Analysis and Review (MedPAR) files, Outpatient Standard Analytical Files (OutSAFs), Medicare Carrier files, and Prescription Drug Event (PDE) records.
Cohort Selection.
Figure 1 depicts study cohort selection. After identifying diabetes patients during the years 2008 through 2010 (N=821,034), individuals with DPN were identified using ICD-9 codes 250.60 and 250.62 (N=130,298). Subsequently, individuals who first initiated TCA/GABA-analog use (see Supplementary Table S1) during 2008 to 2010 were identified, and those individuals who began using these drugs prior to diabetes diagnosis were excluded (N=35,106). Date of first TCA/GABA-analog prescription served as the index date. Individuals with comorbid conditions that could alternatively explain TCA/GABA-analog use (epilepsy, migraine, bipolar depression, or lower limb amputation) were excluded, as were those with nursing facility use during the three months prior to initial TCA/GABA-analog prescription. The application of all exclusion criteria resulted in a final cohort of 5,550 DPN patients receiving TCAs/GABA-analogs. Psychiatric comorbidities were identified using ICD-9 codes (see Supplementary Table S2), while nursing facility use was identified using Current Procedural Terminology (CPT) codes 99304–99310, 99315, 99316, or 99318. Nursing facility use was considered exclusionary because fall risk among nursing home residents differs significantly from that community-dwelling elders.25 Comorbid depression and/or anxiety were not considered exclusionary, as TCAs/GABA-analogs are commonly used to treat these disorders and DPN simultaneously.26 These conditions were instead considered as covariates in the propensity model, since they could conceivably influence the likelihood of TCA/GABA-analog prescription.
Figure 1. STROBE diagram for selection of TCA/GABA-analog users and non-users.
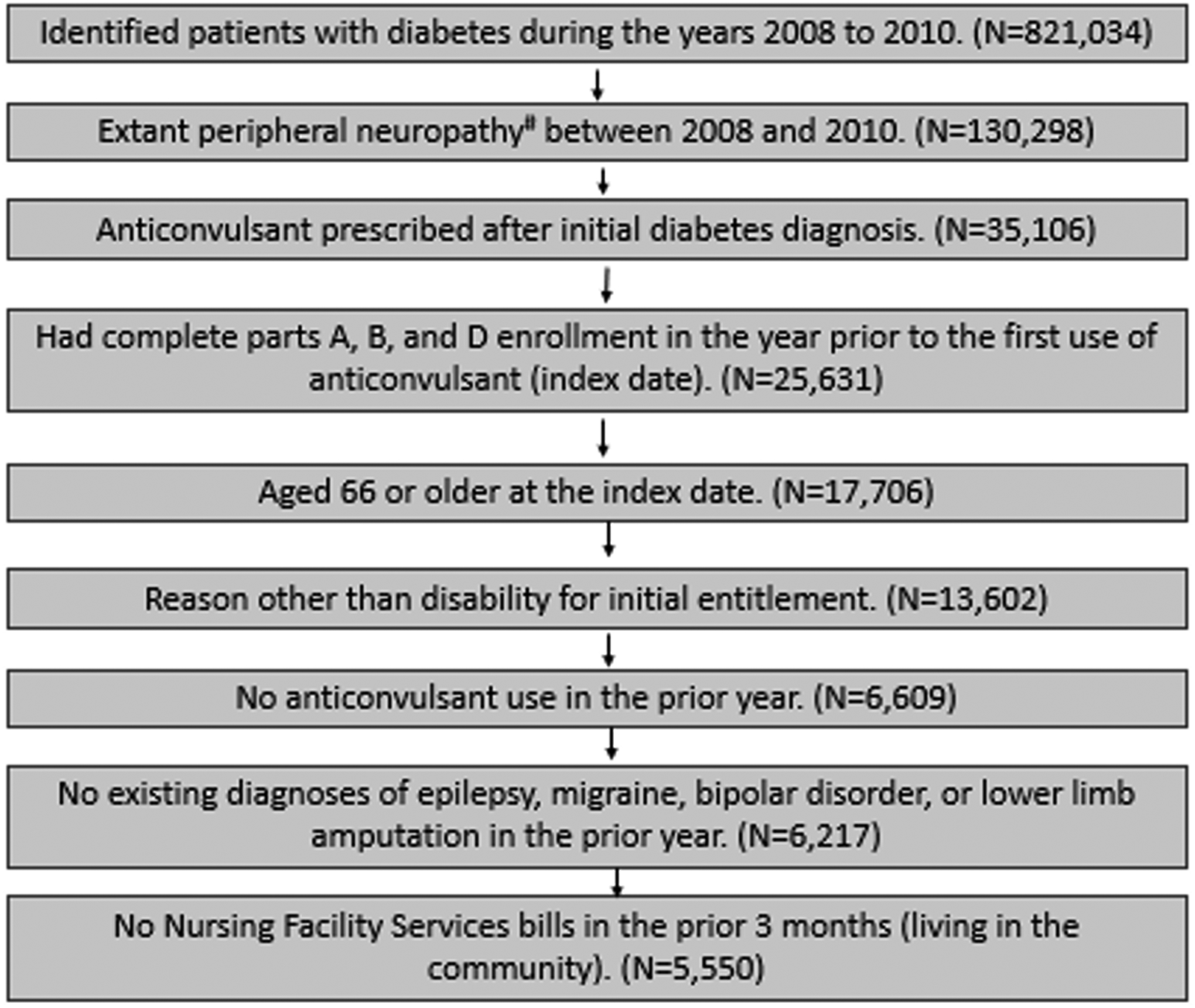
aICD-9 codes for peripheral neuropathy: 250.60 and 250.62. bIn order to keep as many controls as possible, all subjects in the previous step were assigned January 2008 as the index date. Then the selection criteria were applied to identify eligible subjects. Controls needed for that month were then randomly selected from the eligible subjects. This process was repeated 35 more times for all the months from February 2008 to December 2010.
For the non-user group, patients without prescriptions of TCAs/GABA-analogs were identified from the 130,298 individuals with diabetes and peripheral neuropathy in the years 2008 to 2010. From these, we excluded any patients with a PDE record for TCAs/GABA-analogs in the years 2008 to 2010. The resultant 93,345 patients were randomly assigned an index month/year matching the month/year distribution of the TCA/GABA-analog users. The 15th of the month was assigned as the index date for each non-user. Once an index date was assigned, the same inclusion/exclusion criteria for patients receiving TCAs/GABA-analogs were applied to identify eligible individuals without such prescriptions (N=22,200).
Propensity Matching.
To ensure comparability, a propensity score for receiving TCA/GABA-analog prescription was generated using two logistic regression models stratified by fall history, as determined by claims from the previous year listing ICD-9-CM codes for non-sports-related accidental falls. Within each model, we utilized greedy matching techniques based on propensity score to match each user with a non-user.27 Covariates considered for the propensity models were age, gender, ethnicity, insulin or opioid use in the previous year (to approximate severity of diabetes and pain, respectively), uncontrolled or complicated diabetes, anxiety, and depression. Demographic information was obtained from Medicare beneficiary summary files, while insulin and opioid use were both determined using PDE records. Uncontrolled or complicated diabetes, anxiety, and depression were defined based on the presence of 1 inpatient (MedPAR) claim or 2 OutSAF or Carrier claims separated by at least 30 days during the prior year. The ICD-9 codes used can be found in Supplementary Table S2.
Covariates.
To account for coexisting risk factors, our multivariable model adjusts for known risk factors for falls and fractures.28 These covariates were included in the multivariable model instead of the propensity model, because they indicate other reasons why the patient would be prone to falls and fractures. In contrast, variables included in the propensity model are more apt to impact the likelihood of TCA/GABA-analog prescription. The use of sulfonylureas, hypertension medications, or any potentially inappropriate medication (according to Beers criteria, see supplementary table S3)29 were identified using PDE records for the year prior to the index date. Visual impairment and related disorders, hearing impairment and related disorders, urinary incontinence, hypotension, and hypoglycemia occurrence were each identified using either 1 inpatient MedPAR claim or 2 OutSAF or Carrier claims with at least 30 days separation in the year prior to the index date; ICD-9 codes used can be found in Supplementary Table S2. For the multiple chronic conditions variable, we used a list developed by the US Department of Health and Human Services. Of these twenty common conditions, fifteen are common in Medicare population; therefore, CMS has developed algorithms for identifying them in addition to making them available in CCW data. Since 1) hypertension medication was included as one of the covariates, 2) users and non-users were matched on depression, and 3) all patients had diabetes, only the following were considered for the multiple chronic conditions variable: atrial fibrillation, chronic obstructive pulmonary disease, heart failure, ischemic heart disease, osteoporosis, stroke, asthma, hyperlipidemia, and cancer. Given their association with fall risk,28 the chronic conditions arthritis and Alzheimer’s disease or dementia were both considered separately. Additionally, chronic kidney disease was considered separately since kidney function is important for gabapentin excretion.30 All conditions in the combined variable were weighted equally.
Study Outcomes.
The primary outcomes of this study are the first event of fall as any diagnosis and the first event of fracture as a primary diagnosis after the index date (see Supplementary Table S2 for ICD-9-CM codes). Patients were censored at death, loss of Medicare coverage, end of study (5 years after the index date or December 31, 2014), or change in TCA/GABA-analog exposure status. TCA/GABA-analog users were censored upon ‘unexposure’, defined as the end of TCA/GABA-analog supply from the final prescription between 2008 and 2014. Non-users were censored upon exposure to TCAs/GABA-analogs, defined as the date of initial TCA/GABA-analog prescription.
Statistical Analysis.
The balance of covariates between the two groups before and after propensity matching was compared using t-tests and Chi-square tests. The unadjusted event (fall or fracture) rates for both groups were estimated using the Kaplan-Meier method and compared by log-rank test. Frailty models were used to account for the clustering within each propensity-matched pair of patients to estimate the risk of falls/fractures while adjusting for conditions outlined in Table 1.31 To address the potential for selection bias resulting from censoring of patients who switched groups in the conventional analysis, a separate analysis treating TCA/GABA-analog use as a time-dependent covariate was also conducted. In this analysis, users were switched to the non-user group seven days after the end of TCA/GABA-analog supply, to allow adequate washout. Non-users were switched to the user group upon TCA/GABA-analog prescription. All analyses were performed with SAS version 9.4 (SAS Inc., Cary, NC). Hypothesis tests used were two-sided and P values were considered significant at P<.05.
Table 1.
Distribution of baseline characteristics according to TCA/GABA-analog use after propensity score matching.
| Fall risk factor | TCA/GABA-analog user (N=5550), % | Matched non-user (N=5550), % | p valuea |
|---|---|---|---|
| Sulfonylureas use | 44.3 | 41.0 | 0.0005 |
| Any HTN medication | 92.9 | 90.0 | < 0.0001 |
| Any potentially inappropriate medication | 42.7 | 33.2 | < 0.0001 |
| Rheumatoid arthritis/osteoarthritisb | 72.5 | 64.1 | < 0.0001 |
| Alzheimer’s disease/dementiab | 14.4 | 16.9 | 0.0003 |
| Chronic kidney diseaseb | 38.0 | 38.1 | 0.8757 |
| Visual impairment and related disordersc | 27.3 | 27.2 | 0.9152 |
| Hearing impairment and related disordersc | 1.0 | 0.6 | 0.0185 |
| Urinary incontinencec | 2.5 | 2.2 | 0.2357 |
| Hypotensionc | 2.5 | 2.2 | 0.2887 |
| Hypoglycemiac | 4.6 | 4.8 | 0.6863 |
| Other chronic conditionsb | |||
| 0–1 | 13.2 | 15.8 | < 0.0001 |
| 2–3 | 37.7 | 40.0 | |
| 4–5 | 35.4 | 33.4 | |
| 6+ | 13.7 | 10.8 |
Bold face font indicates a statistically significant finding.
Chi-square test.
Obtained from the Chronic Condition Warehouse (CCW). “Other chronic conditions” includes: atrial fibrillation, chronic obstructive pulmonary disease, heart failure, ischemic heart disease, osteoporosis, stroke, asthma, hyperlipidemia, and cancer.
Determined by 1 inpatient bill or 2 outpatient bills with at least 30 days apart in the year prior to the index date.
RESULTS
Our study cohort included 5,550 and 22,200 patients with and without TCA/GABA-analog prescriptions in 2008–2010, respectively. Before propensity matching, several significant differences existed between users and non-users, but afterwards these covariates were comparable (Table 2). It should be noted that the two groups differed widely regarding hypothesized fall risk factors (Table 3). TCA/GABA-analog users were more likely to use sulfonylureas, hypertension medications, and potentially inappropriate medications according to Beers criteria (see Supplementary Table S3). They were also more likely to have osteoarthritis or rheumatoid arthritis or additional chronic diseases, but less likely to have Alzheimer’s disease or dementia. Additionally, users were more likely to have hearing impairment and more chronic conditions.
Table 2.
Balance between TCA/GABA-analog users and non-users before and after propensity score matching using greedy matching techniques, based on characteristics that would likely impact the likelihood of TCA/GABA-analog prescription.
| Fall History | Patient characteristic | Before matching | After matching | ||||
|---|---|---|---|---|---|---|---|
| User | Non-user | p-valuea | User | Non-user | p-valuea | ||
| No | N | 5139 | 20728 | 5139 | 5139 | ||
| Age, mean ± STD | 76.7 ± 6.6 | 77.6 ± 7.1 | <.0001 | 76.7 ± 6.6 | 76.8 ± 6.6 | 0.4592 | |
| N (%) | N (%) | ||||||
| Female gender | 3518 (68.5) | 13120 (63.3) | <.0001 | 3518 (68.5) | 3563 (69.3) | 0.3376 | |
| Race/ethnicity | |||||||
| Non-Hispanic white | 3379 (65.8) | 14721 (71.0) | <.0001 | 3379 (65.8) | 3383 (65.8) | 0.8820 | |
| Black | 626 (12.2) | 2527 (12.2) | 626 (12.2) | 638 (12.4) | |||
| Hispanic | 827 (16.1) | 2462 (11.9) | 827 (16.1) | 829 (16.1) | |||
| Other | 307 (6.0) | 1018 (4.9) | 307 (6.0) | 289 (5.6) | |||
| Uncontrolled/other complications of DM, prior yearb | |||||||
| Controlled, no complications | 3027 (58.9) | 12379 (59.7) | 0.0473 | 3027 (58.9) | 2962 (57.6) | 0.0936 | |
| Controlled, w/ complications | 493 (9.6) | 2178 (10.5) | 493 (9.6) | 563 (11.0) | |||
| Uncontrolled, no complications | 1070 (20.8) | 4063 (19.6) | 1070 (20.8) | 1039 (20.2) | |||
| Uncontrolled, w/ complications | 549 (10.7) | 2108 (10.2) | 549 (10.7) | 575 (11.2) | |||
| Any insulin use, prior year | 1615 (31.4) | 5834 (28.1) | <.0001 | 1615 (31.4) | 1634 (31.8) | 0.6869 | |
| Anxiety, prior yearb | 176 (3.4) | 440 (2.1) | <.0001 | 176 (3.4) | 145 (2.8) | 0.0788 | |
| Depression, prior yearb | 362 (7.0) | 1007 (4.9) | <.0001 | 362 (7.0) | 335 (6.5) | 0.2895 | |
| Using opioids > 90 days, prior year | 765 (14.9) | 1482 (7.1) | <.0001 | 765 (14.9) | 784 (15.3) | 0.6004 | |
| Yes | N | 411 | 4172 | 411 | 411 | ||
| Age, mean ± STD | 78.3 ± 6.9 | 80.8 ± 7.4 | <.0001 | 78.3 ± 6.9 | 78.3 ± 6.9 | 0.9839 | |
| N (%) | N (%) | ||||||
| Female gender | 314 (76.4) | 1071 (72.8) | 0.1390 | 314 (76.4) | 325 (79.1) | 0.3564 | |
| Race/ethnicity | |||||||
| Non-Hispanic white | 302 (73.5) | 1115 (75.7) | 0.0693 | 302 (73.5) | 306 (74.5) | 0.3284 | |
| Black | 29 (7.1) | 135 (9.2) | 29 (7.1) | 40 (9.7) | |||
| Hispanic | 61 (14.8) | 155 (10.5) | 61 (14.8) | 48 (11.7) | |||
| Other | 19 (4.6) | 67 (4.6) | 19 (4.6) | 17 (4.1) | |||
| Uncontrolled/other complications of DM, prior yearb | |||||||
| Controlled, no complications | 213 (51.8) | 791 (53.7) | 0.6316 | 213 (51.8) | 217 (52.8) | 0.6844 | |
| Controlled, w/ complications | 59 (14.4) | 201 (13.7) | 59 (14.4) | 61 (14.8) | |||
| Uncontrolled, no complications | 72 (17.5) | 274 (18.6) | 72 (17.5) | 78 (19.0) | |||
| Uncontrolled, w/ complications | 67 (16.3) | 206 (14.0) | 67 (16.3) | 55 (13.4) | |||
| Any insulin use, prior year | 151 (36.7) | 495 (33.6) | 0.2400 | 151 (36.7) | 140 (34.1) | 0.4224 | |
| Anxiety, prior yearb | 30 (7.3) | 74 (5.0) | 0.0746 | 30 (7.3) | 22 (5.4) | 0.2517 | |
| Depression, prior yearb | 55 (13.4) | 192 (13.0) | 0.8574 | 55 (13.4) | 59 (14.4) | 0.6865 | |
| Using opioids > 90 days, prior year | 110 (26.8) | 173 (11.8) | <.0001 | 110 (26.8) | 105 (25.5) | 0.6915 | |
Bold face font indicates a statistically significant finding.
T-test for age and Chi-square test for others.
Identified by 1 inpatient claim or 2 outpatient claims with at least 30 days apart in the prior year
Table 3.
Associations between fall and fracture risk factors,a including TCA/GABA-analog use, and the likelihood of fall as any diagnosis or fracture as primary diagnosis within 5 years, estimated using both conventional and time-dependent analyses.
| Patient characteristic | Hazard ratio (95% CI) | |||
|---|---|---|---|---|
| Primary analysis | Time-dependent analysisb | |||
| Fracture, primary diagnosis | Fall, any diagnosis | Fracture, primary diagnosis | Fall, any diagnosis | |
| Anticonvulsant user vs. Non-user | 1.09 (0.99, 1.19) | 1.11 (1.03, 1.20) | 1.12 (1.02, 1.24) | 1.26 (1.17, 1.36) |
| Sulfonylureas use, Y vs. N | 1.01 (0.92, 1.11) | 0.98 (0.91, 1.05) | 0.98 (0.91, 1.07) | 0.97 (0.91, 1.04) |
| Any HTN medication, Y vs. N | 0.84 (0.71, 0.99) | 1.06 (0.92, 1.22) | 0.87 (0.75, 1.01) | 1.09 (0.96, 1.24) |
| Any potentially inappropriate medication, Y vs. N | 1.12 (1.02, 1.23) | 1.10 (1.02, 1.19) | 1.12 (1.03, 1.22) | 1.11 (1.04, 1.19) |
| Rheumatoid arthritis/osteoarthritis, Y vs. N | 1.26 (1.14, 1.40) | 1.26 (1.15, 1.37) | 1.27 (1.15, 1.39) | 1.27 (1.17, 1.37) |
| Alzheimer’s disease/dementia, Y vs. N | 1.11 (0.98, 1.25) | 1.39 (1.26, 1.53) | 1.13 (1.01, 1.25) | 1.36 (1.25, 1.48) |
| Chronic kidney disease, Y vs. N | 1.24 (1.13, 1.36) | 1.20 (1.11, 1.30) | 1.22 (1.12, 1.33) | 1.17 (1.09, 1.25) |
| Visual impairment and related disorders, Y vs. N | 1.07 (0.97, 1.18) | 1.02 (0.94, 1.11) | 1.05 (0.96, 1.14) | 1.03 (0.95, 1.10) |
| Hearing impairment and related disorders, Y vs. N | 0.45 (0.22, 0.91) | 0.91 (0.60, 1.40) | 0.71 (0.43, 1.15) | 1.02 (0.72, 1.46) |
| Urinary incontinence, Y vs. N | 1.12 (0.86, 1.47) | 1.16 (0.93, 1.44) | 1.11 (0.87, 1.41) | 1.02 (0.83, 1.25) |
| Hypotension, Y vs. N | 1.09 (0.82, 1.45) | 0.98 (0.77, 1.26) | 1.06 (0.82, 1.37) | 1.05 (0.84, 1.30) |
| Hypoglycemia, Y vs. N | 1.51 (1.25, 1.81) | 1.27 (1.08, 1.50) | 1.38 (1.16, 1.64) | 1.28 (1.11, 1.48) |
| Other chronic conditions | ||||
| 2–3 vs. 0–1 | 1.20 (1.03, 1.40) | 1.18 (1.04, 1.34) | 1.23 (1.07, 1.41) | 1.14 (1.02, 1.27) |
| 4–5 vs. 0–1 | 1.47 (1.26, 1.72) | 1.55 (1.37, 1.76) | 1.46 (1.27, 1.68) | 1.53 (1.37, 1.71) |
| 6+ vs. 0–1 | 1.68 (1.39, 2.03) | 1.89 (1.62, 2.20) | 1.80 (1.52, 2.13) | 1.90 (1.66, 2.18) |
Bold face font indicates a statistically significant finding.
Risk factors examined are those deemed to be potential risk factors for fall and fracture.
In the time-dependent analysis, a user became a non-user when the TCA/GABA-analog supply ended. Similarly, non-users became users when they initiated TCA/GABA-analog treatment. Therefore, compared to the conventional model, users were not censored at the end of supply and non-users were not censored at the initiation of treatment. This allowed us to examine the association with TCAs/GABA-analogs with more data points.
Unadjusted event rates for study outcomes are presented in Figure 2. Overall, TCA/GABA-analog users and non-users exhibited significant differences in both fall (p<.001) and fracture incidence (p=.005), with TCA/GABA-analog users being more likely to experience these events. Additionally, Supplementary Tables S4 and S5 detail the yearly unadjusted risk of fall and fracture, respectively, associated with each covariate. Table 3 lists estimated risks of fall or fracture associated with each covariate in our frailty models after adjusting for other covariates.
Figure 2. Time to the first outcome event for TCA/GABA-analog users (User=Y) and their matched non-users (User=N).
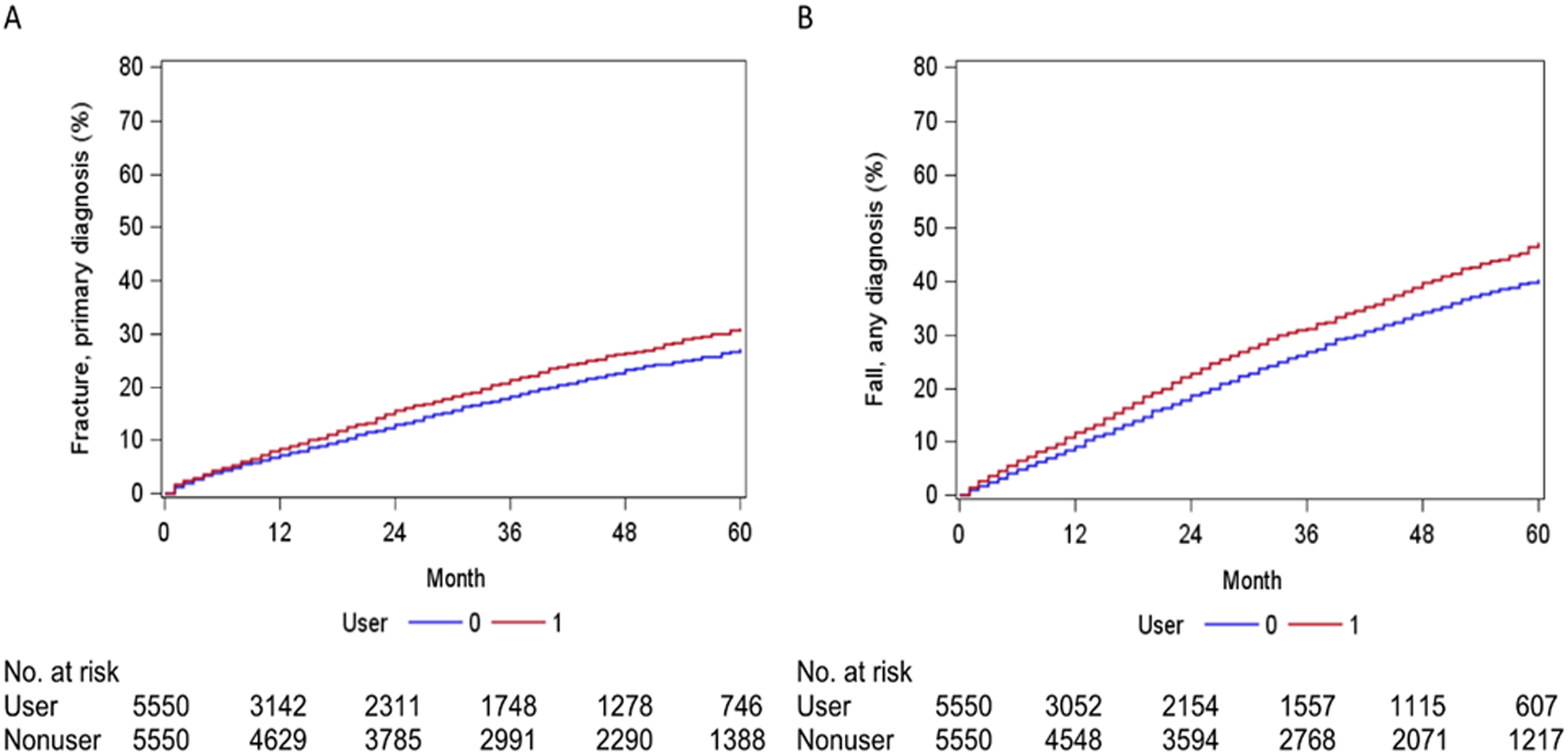
A. Fracture. There was a significant difference between the users and non-users (p=0.005, Log-rank test). B. Fall. There was a significant difference between the users and non-users (p<0. 001, Log-rank test).
In the conventional analysis, TCA/GABA-analog use was associated with increased fall risk (adjusted HR 1.11, 95% CI 1.03–1.20) but not with fracture risk (HR: 1.09, 95% CI 0.99–1.19). However, given the large number of users censored due to end of supply (Supplementary Table S6), we conducted an additional analysis where TCA/GABA-analog use was treated as a time-dependent variable. In this analysis, the numbers of users and nonusers at risk for each outcome during each follow-up year were similar (Supplementary Table S7), and we found a stronger association between TCA/GABA-analog use and the study outcomes (Table 3). The adjusted HR for fall was 1.26, 95% CI 1.17–1.36; for fracture, it was 1.12, 95% CI 1.02–1.24.
Hypoglycemia was primarily associated with increased risk of fracture (adjusted HR 1.38, 95% CI 1.16–1.64), while Alzheimer’s disease and other dementias were primarily associated with increased fall risk (adjusted HR 1.36, 95% CI 1.25–1.48). Arthritic conditions contributed similarly to both fall and fracture risk (adjusted HR 1.27, 95% CI 1.17–1.37, and adjusted HR 1.27, 95% CI 1.15–1.39, respectively), as did the use of potentially inappropriate medication (adjusted HR 1.11, 95% CI 1.04–1.19, and adjusted HR 1.12, 95% CI 1.03–1.22, respectively). Increasing multimorbidity resulted in the greatest risk of falls and fractures. TCA/GABA-analog use did not significantly contribute to either fracture as any diagnosis or fracture hospitalization (adjusted HR 1.09, 95% CI 0.99–1.20, and adjusted HR 1.03, 95% CI 0.87–1.22, respectively, see Supplementary Table S8).
DISCUSSION
Using a large national sample of older DPN patients enrolled in Medicare Part D and matched based on propensity for anticonvulsant prescription, we found that TCA/GABA-analog use is associated with a statistically significant increases in the incidence of both falls and fractures. To the authors’ knowledge, this study represents the first largescale investigation of fall risk in older DPN patients based on TCA/GABA-analog use. Additionally, our use of both conventional and time-dependent analyses illustrates the importance of using time-dependent analysis for this type of study. This importance arises from the fact that time-dependent analysis maximizes the person-time available for analysis by minimizing censorship. Less censorship equates to more data points to analyze, thus resulting in a more accurate analysis.
Our findings of an association between increased fall risk in Alzheimer’s disease and other dementias, arthritic conditions, hypoglycemia, and multimorbidity add external validity to our findings.32–34 It is important to note that the associated 26% increase in fall risk for TCA/GABA-analog users is more than double that which is attributable to use of medications on the Beers list, which are widely recognized as contributory to fall risk.29, 35, 36 Unfortunately, while alternatives exist for many Beers list medications, neuropathic pain treatment alternatives that are not associated with increased fall risk are currently unavailable.29, 37, 38
Our results fall within the range of results observed in previous studies of fall and fracture risk with TCA use. These studies have produced estimates of fall risk associated with TCA use that are highly variable, largely due to differences in study populations. Identified studies finding no increased risk of fall with TCA use either examined a younger population or concerned recurrent falls.39, 40 In contrast, studies in older hospitalized populations suggest that TCA users are up to 2.4 times as likely to fall as non-users.41, 42 In a population more similar to the one examined here, meta-analysis demonstrated a 51% increase in fall risk with TCA use.43 Our estimate is more modest than this value, perhaps due to unmeasured confounders.
Estimates of fracture risk, particularly hip fracture risk, associated with TCA use are consistently increased in the older population.44–46 One study examining a large prospective cohort of older women demonstrated that the risk of any fracture increases by 30% with TCA use.44 A recent meta-analysis demonstrated a 70% increase in risk of hip fracture with TCA use.38 These estimates exceed those obtained in this study. We hypothesize that this may result from the increased fall risk associated with painful DPN.47
Given the known risk of fall associated with TCA/GABA-analog use, it is possible that the associated increase in fall risk in TCA/GABA-analog users was artifactual, given more diligent monitoring and increased reporting of minor falls. This prompted further investigation into fall severity – if the primary diagnoses associated with falls were similar between groups, minor falls were likely to be similarly reported between groups. As illustrated in Supplementary Table S9, primary diagnoses associated with fall were similar between groups, thus supporting the validity of our findings.
Our findings are vulnerable to bias, as Medicare data does not indicate DPN severity. However, we believe it is unlikely that neuropathy severity differed between groups, given the similarity of diabetes severity proxies.
Our study has several limitations. Importantly, one cannot dissect the effects of neuropathy symptoms from those of TCAs/GABA-analogs. Claims data cannot be used to determine whether a fall or fracture resulted from neuropathic pain or TCA/GABA-analog side effects. Additionally, as shown in Supplementary Table S1, the number of TCA users was limited; this precluded individual analyses on TCAs and GABA-analogs to determine which drug, if any, has a preferable safety profile. Observed differences in fall risk factors also raise concern regarding unmeasured confounders.
It is also important to note limitations inherent to studies employing Medicare claims data. For example, the ICD-9 coding used to define DPN status simply indicates that the patient had Type 2 or unspecified diabetes with neurological manifestation; the location of the neuropathy is not indicated. However, distal symmetric polyneuropathy of the feet is the most common initial neurological manifestation of diabetes,48 so our mode of classification is likely to be accurate. Additionally, the terminology in ICD-9 codes reflects clinical judgment rather than a distinct definition. For example, no exact definitions are provided for diabetes control, uncontrolled or complicated diabetes, or diabetes status. Our analysis was also restricted to older DPN patients with complete parts A, B, and D Medicare enrollment, meaning our results may not be applicable to the older DPN patient population as a whole. It is also important to note that falls are underreported and undercoded, meaning that our results most likely underestimate fall incidence.49, 50 One also cannot determine prescription adherence from claims data; it is only possible to determine whether a prescription was filled. Additionally, extreme variability in drug dosing precludes its analysis. Lastly, since data is only available once a patient turns 65, accurate information on diabetes duration is not available, precluding its use as a covariate.
Our findings raise the question of whether the TCAs/GABA-analogs examined should be added to the Beers criteria as potentially inappropriate medications for patients with DPN. Although we believe our findings indicate excessive risk, we believe additional evidence confirming our findings is necessary before adding TCAs/GABA-analogs to the Beers criteria.
In conclusion, most covariates we identified as contributory to fall and fracture risk are chronic conditions that can only be treated symptomatically. However, three of the covariates – Beers list medication use, hypoglycemia, and TCA/GABA-analog use – are potentially avoidable, and can be targeted to reduce fall and fracture risk in older DPN patients. Substitutions for Beers list medications that reduce risk of fall and fracture have been previously identified in the literature.29 Furthermore, hypoglycemia can be prevented by avoiding insulin and insulin secretagogues.51 In contrast, the risk associated with TCA/GABA-analog use cannot be circumvented easily, as the primary alternatives – opioids and duloxetine – are similarly problematic in regards to fall and fracture risk.29, 37 Therefore, our results suggest a significant need in the older DPN patient population – a pain management solution that will not contribute to falls in a patient group that is already predisposed to balance issues.
Supplementary Material
Supplementary Table S1. List of TCAs and GABA-analogs taken by the user group at baseline in this study.
Supplementary Table S3. Summary of drug classes included in the 2003 Beers criteria.
Supplementary Table S2. ICD-9-CM codes used in this study.
Supplementary Table S4. Rate of fall by year associated with risk factors for fall and fracture in the entire study population, estimated by Kaplan-Meier method without adjustment.
Supplementary Table S5. Rate of fracture by year associated with risk factors for fall and fracture in the entire study population, estimated by Kaplan-Meier method without adjustment.
Supplementary Table S6. Number of users and nonusers censored from the conventional analysis due to end (for users) or initiation (for non-users) of TCA/GABA-analog supply.
Supplementary Table S7. Contrast between the number of subjects at risk for each outcome at the conclusion of each follow-up year, when comparing conventional and time-dependent analyses.
Supplementary Table S8. Associations between fall and fracture risk factors, including TCA/GABA-analog use, and the likelihood of fracture and fracture hospitalization within 5 years, estimated using both conventional and time-dependent analyses.
Supplementary Table S9. Top 10 primary diagnoses on the claims with fall in TCA/GABA-analog users versus non-users, grouped by Clinical Classifications Software (CCS).
Impact Statement:
We certify that this work is novel. To the authors’ knowledge, this work represents the first assessment of fall and fracture risk associated with the use of GABA-analogs or TCAs in older patients with diabetic peripheral neuropathy. This has clear clinical implications because use of these medications is a potentially modifiable risk factor for falls and fractures. Therefore, our findings suggest that these medications should only be prescribed after a careful assessment of fall risk, and that they should be promptly discontinued if deemed ineffective.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the following sources of funding: NIH/NIA F30AG058381 (Randolph), NIH/NIA P30AG024832 (Volpi, Kuo), & R01HS020642 (Kuo).
Sponsor’s Role:
Research sponsors played no role in this study’s design, methods, data collection, analysis, or interpretation, nor in the preparation of the manuscript.
Footnotes
Conflict of Interest:
The authors have no conflicts of interest to declare.
Podium presentation at: 2018 Houston Medication Safety Symposium
REFERENCES
- [1].Jensen TS, Backonja MM, Hernández Jiménez S, Tesfaye S, Valensi P, Ziegler D. New perspectives on the management of diabetic peripheral neuropathic pain. Diab Vasc Dis Res. 2006;3: 108–119. [DOI] [PubMed] [Google Scholar]
- [2].Petit WA Jr., Upender RP. Medical evaluation and treatment of diabetic peripheral neuropathy. Clin Podiatr Med Surg. 2003;20: 671–688. [DOI] [PubMed] [Google Scholar]
- [3].Karmakar S, Rashidian H, Chan C, Liu C, Toth C. Investigating the role of neuropathic pain relief in decreasing gait variability in diabetes mellitus patients with neuropathic pain: a randomized, double-blind crossover trial. J Neuroeng Rehabil. 2014;11: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Barak Y, Wagenaar RC, Holt KG. Gait characteristics of elderly people with a history of falls: a dynamic approach. Phys Ther. 2006;86: 1501–1510. [DOI] [PubMed] [Google Scholar]
- [5].Bulat T, Castle SC, Rutledge M, Quigley P. Clinical practice algorithms: medication management to reduce fall risk in the elderly-part 4, anticoagulants, anticonvulsants, anticholinergics/bladder relaxants, and antipsychotics. J Am Acad Nurse Pract. 2008;20: 181–190. [DOI] [PubMed] [Google Scholar]
- [6].Leo RJ. Treatment considerations in neuropathic pain. Curr Treat Options Neurol. 2006;8: 389–400. [DOI] [PubMed] [Google Scholar]
- [7].Gilron I, Watson CP, Cahill CM, Moulin DE. Neuropathic pain: a practical guide for the clinician. CMAJ. 2006;175: 265–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Finnerup NB, Otto M, McQuay HJ, Jensen TS, Sindrup SH. Algorithm for neuropathic pain treatment: an evidence based proposal. Pain. 2005;118: 289–305. [DOI] [PubMed] [Google Scholar]
- [9].Feighner JP. Mechanism of action of antidepressant medications. J Clin Psychiatry. 1999;60 Suppl 4: 4–11; discussion 12–13. [PubMed] [Google Scholar]
- [10].Shoair OA, Nyandege AN, Slattum PW. Medication-related dizziness in the older adult. Otolaryngol Clin North Am. 2011;44: 455–471, x. [DOI] [PubMed] [Google Scholar]
- [11].Rosenstock J, Tuchman M, LaMoreaux L, Sharma U. Pregabalin for the treatment of painful diabetic peripheral neuropathy: a double-blind, placebo-controlled trial. Pain. 2004;110: 628–638. [DOI] [PubMed] [Google Scholar]
- [12].Arezzo JC, Rosenstock J, Lamoreaux L, Pauer L. Efficacy and safety of pregabalin 600 mg/d for treating painful diabetic peripheral neuropathy: a double-blind placebo-controlled trial. BMC Neurol. 2008;8: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tölle T, Freynhagen R, Versavel M, Trostmann U, Young JP. Pregabalin for relief of neuropathic pain associated with diabetic neuropathy: a randomized, double-blind study. Eur J Pain. 2008;12: 203–213. [DOI] [PubMed] [Google Scholar]
- [14].Calandre EP, Rico-Villademoros F, Slim M. Alpha. Expert Rev Neurother. 2016;16: 1263–1277. [DOI] [PubMed] [Google Scholar]
- [15].Goldstein DJ, Lu Y, Detke MJ, Lee TC, Iyengar S. Duloxetine vs. placebo in patients with painful diabetic neuropathy. Pain. 2005;116: 109–118. [DOI] [PubMed] [Google Scholar]
- [16].Rizzoli R, Cooper C, Reginster JY, et al. Antidepressant medications and osteoporosis. Bone. 2012;51: 606–613. [DOI] [PubMed] [Google Scholar]
- [17].Tromp AM, Smit JH, Deeg DJ, Bouter LM, Lips P. Predictors for falls and fractures in the Longitudinal Aging Study Amsterdam. J Bone Miner Res. 1998;13: 1932–1939. [DOI] [PubMed] [Google Scholar]
- [18].Masud T, Frost M, Ryg J, et al. Central nervous system medications and falls risk in men aged 60–75 years: the Study on Male Osteoporosis and Aging (SOMA). Age Ageing. 2013;42: 121–124. [DOI] [PubMed] [Google Scholar]
- [19].Cea-Soriano L, Johansson S, García Rodríguez LA. Risk factors for falls with use of acid-suppressive drugs. Epidemiology. 2013;24: 600–607. [DOI] [PubMed] [Google Scholar]
- [20].Ensrud KE, Blackwell TL, Mangione CM, et al. Central nervous system-active medications and risk for falls in older women. J Am Geriatr Soc. 2002;50: 1629–1637. [DOI] [PubMed] [Google Scholar]
- [21].Faulkner KA, Cauley JA, Studenski SA, et al. Lifestyle predicts falls independent of physical risk factors. Osteoporos Int. 2009;20: 2025–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Koski K, Luukinen H, Laippala P, Kivela SL. Physiological factors and medications as predictors of injurious falls by elderly people: a prospective population-based study. Age Ageing. 1996;25: 29–38. [DOI] [PubMed] [Google Scholar]
- [23].French DD, Campbell R, Spehar A, Cunningham F, Bulat T, Luther SL. Drugs and falls in community-dwelling older people: a national veterans study. Clin Ther. 2006;28: 619–630. [DOI] [PubMed] [Google Scholar]
- [24].Warehouse CCD. Condition Categories. Volume 2017. [Google Scholar]
- [25].Rubenstein LZ, Josephson KR. The epidemiology of falls and syncope. Clin Geriatr Med. 2002;18: 141–158. [DOI] [PubMed] [Google Scholar]
- [26].Nicolson SE, Caplan JP, Williams DE, Stern TA. Comorbid pain, depression, and anxiety: multifaceted pathology allows for multifaceted treatment. Harv Rev Psychiatry. 2009;17: 407–420. [DOI] [PubMed] [Google Scholar]
- [27].Parsons L Reducing bias in a propensity score matched-pair sample using greedy matching techniques. SAS Users Group International Conference, 2001. [Google Scholar]
- [28].Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319: 1701–1707. [DOI] [PubMed] [Google Scholar]
- [29].Fick DM, Cooper JW, Wade WE, Waller JL, Maclean JR, Beers MH. Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts. Arch Intern Med. 2003;163: 2716–2724. [DOI] [PubMed] [Google Scholar]
- [30].Raouf M, Atkinson TJ, Crumb MW, Fudin J. Rational dosing of gabapentin and pregabalin in chronic kidney disease. J Pain Res. 2017;10: 275–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Goodman RA, Posner SF, Huang ES, Parekh AK, Koh HK. Defining and measuring chronic conditions: imperatives for research, policy, program, and practice. Prev Chronic Dis. 2013;10: E66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tinetti ME, Inouye SK, Gill TM, Doucette JT. Shared risk factors for falls, incontinence, and functional dependence. Unifying the approach to geriatric syndromes. JAMA. 1995;273: 1348–1353. [PubMed] [Google Scholar]
- [33].Campbell AJ, Borrie MJ, Spears GF. Risk factors for falls in a community-based prospective study of people 70 years and older. J Gerontol. 1989;44: M112–117. [DOI] [PubMed] [Google Scholar]
- [34].Fried LP, Guralnik JM. Disability in older adults: evidence regarding significance, etiology, and risk. J Am Geriatr Soc. 1997;45: 92–100. [DOI] [PubMed] [Google Scholar]
- [35].Berdot S, Bertrand M, Dartigues JF, et al. Inappropriate medication use and risk of falls--a prospective study in a large community-dwelling elderly cohort. BMC Geriatr. 2009;9: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gallagher PF, Barry PJ, Ryan C, Hartigan I, O’Mahony D. Inappropriate prescribing in an acutely ill population of elderly patients as determined by Beers’ Criteria. Age Ageing. 2008;37: 96–101. [DOI] [PubMed] [Google Scholar]
- [37].Gribbin J, Hubbard R, Gladman J, Smith C, Lewis S. Serotonin-norepinephrine reuptake inhibitor antidepressants and the risk of falls in older people: case-control and case-series analysis of a large UK primary care database. Drugs Aging. 2011;28: 895–902. [DOI] [PubMed] [Google Scholar]
- [38].Oderda LH, Young JR, Asche CV, Pepper GA. Psychotropic-Related Hip Fractures: Meta-Analysis of First-Generation and Second-Generation Antidepressant and Antipsychotic Drugs. Annals of Pharmacotherapy. 2012;46: 917–928. [DOI] [PubMed] [Google Scholar]
- [39].Vestergaard P, Rejnmark L, Mosekilde L. Selective serotonin reuptake inhibitors and other antidepressants and risk of fracture. Calcified Tissue International. 2008;82: 92–101. [DOI] [PubMed] [Google Scholar]
- [40].Marcum ZA, Perera S, Thorpe JM, et al. Antidepressant Use and Recurrent Falls in Community-Dwelling Older Adults: Findings From the Health ABC Study. Annals of Pharmacotherapy. 2016;50: 525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].O’Neil CA, Krauss MJ, Bettale J, et al. Medications and Patient Characteristics Associated With Falling in the Hospital. Journal of Patient Safety. 2018;14: 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Chiu MH, Lee HD, Hwang HF, Wang SC, Lin MR. Medication use and fall-risk assessment for falls in an acute care hospital. Geriatr Gerontol Int. 2015;15: 856–863. [DOI] [PubMed] [Google Scholar]
- [43].Leipzig RM, Cumming RG, Tinetti ME. Drugs and falls in older people: A systematic review and meta-analysis: I. Psychotropic drugs. Journal of the American Geriatrics Society. 1999;47: 30–39. [DOI] [PubMed] [Google Scholar]
- [44].Ensrud KE, Blackwell T, Mangione CM, et al. Central nervous system active medications and risk for fractures in older women. Arch Intern Med. 2003;163: 949–957. [DOI] [PubMed] [Google Scholar]
- [45].Bakken MS, Engeland A, Engesæter LB, Ranhoff AH, Hunskaar S, Ruths S. Increased risk of hip fracture among older people using antidepressant drugs: data from the Norwegian Prescription Database and the Norwegian Hip Fracture Registry. Age Ageing. 2013;42: 514–520. [DOI] [PubMed] [Google Scholar]
- [46].van den Brand MWM, Samson MM, Pouwels S, et al. Use of anti-depressants and the risk of fracture of the hip or femur. Osteoporosis International. 2009;20: 1705–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Macgilchrist C, Paul L, Ellis BM, Howe TE, Kennon B, Godwin J. Lower-limb risk factors for falls in people with diabetes mellitus. Diabet Med. 2010;27: 162–168. [DOI] [PubMed] [Google Scholar]
- [48].Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol. 2012;11: 521–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Roe B, Howell F, Riniotis K, Beech R, Crome P, Ong BN. Older people and falls: health status, quality of life, lifestyle, care networks, prevention and views on service use following a recent fall. J Clin Nurs. 2009;18: 2261–2272. [DOI] [PubMed] [Google Scholar]
- [50].Cummings SR, Nevitt MC, Kidd S. Forgetting falls. The limited accuracy of recall of falls in the elderly. J Am Geriatr Soc. 1988;36: 613–616. [DOI] [PubMed] [Google Scholar]
- [51].Service JF, Vella A. Factitious hypoglycemia. 10 June 2018 edn, 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. List of TCAs and GABA-analogs taken by the user group at baseline in this study.
Supplementary Table S3. Summary of drug classes included in the 2003 Beers criteria.
Supplementary Table S2. ICD-9-CM codes used in this study.
Supplementary Table S4. Rate of fall by year associated with risk factors for fall and fracture in the entire study population, estimated by Kaplan-Meier method without adjustment.
Supplementary Table S5. Rate of fracture by year associated with risk factors for fall and fracture in the entire study population, estimated by Kaplan-Meier method without adjustment.
Supplementary Table S6. Number of users and nonusers censored from the conventional analysis due to end (for users) or initiation (for non-users) of TCA/GABA-analog supply.
Supplementary Table S7. Contrast between the number of subjects at risk for each outcome at the conclusion of each follow-up year, when comparing conventional and time-dependent analyses.
Supplementary Table S8. Associations between fall and fracture risk factors, including TCA/GABA-analog use, and the likelihood of fracture and fracture hospitalization within 5 years, estimated using both conventional and time-dependent analyses.
Supplementary Table S9. Top 10 primary diagnoses on the claims with fall in TCA/GABA-analog users versus non-users, grouped by Clinical Classifications Software (CCS).


