Abstract
The immune system contains a series of checks and balances that maintain tolerance and prevent autoimmunity. Siglecs (Sialic acid-binding immunoglobulin type lectins) are cell surface receptors found on immune cells and inhibit inflammation by recruiting protein tyrosine phosphatases to ITIMs. Islet-resident macrophages (IRM) express Siglec-E, and Siglec-E expression decreases on IRMs as insulitis progresses in the NOD mouse. The sialyltransferase ST8Sia6 generates alpha-2,8-disialic acids that are ligands for Siglec-E in vivo. We hypothesized that engaging Siglec-E through ST8Sia6-generated ligands may inhibit the development of immune-mediated diabetes. Constitutive overexpression of ST8Sia6 in pancreatic beta cells mitigated hyperglycemia in the multiple low-dose streptozotocin (MLD-STZ) model of diabetes, demonstrating that engagement of this immune receptor facilitates tolerance in the setting of inflammation and autoimmune disease.
Introduction:
Sialyltransferases add sialic acid to oligosaccharides present on glycoproteins and glycolipids in the Golgi. Sialylation has several important functions in cell biology, influencing cell fate, adhesion and migration. In terms of immunity, cell surface sialic acid-bearing ligands distinguish self from non-self through interaction with receptors of the immune system known as Siglecs. Siglecs are expressed on hematopoietic cells and generally function to inhibit inflammatory processes via recruitment of the protein tyrosine phosphatases SHP-1 and SHP-2 to their ITIM domains (1). For example, bacteria and viruses utilize sialic acid mimicry to establish infection by modulating the immune system through Siglecs (2). High concentrations of sialic acid promote immune evasion by signaling via Siglecs to inhibit immune cell activation (1). Additionally, hypersialylation is a common feature of tumor cells, and engagement of Siglecs is mechanism that allows tumors to evade immune surveillance (3).
Immune evasion can occur via the engagement of murine Siglec-E, whose closest human ortholog is Siglec-9 (4). Siglec-E is expressed on tissue-resident macrophages, in addition to neutrophils and certain subsets of dendritic cells (5). Its preferential recognition of alpha-2,8-disialic acid motifs is implicated in a variety of immune suppressive functions. For instance, targeting Siglec-E with nanoparticles equipped with alpha-2,8-disialic acids abolished inflammation in vivo using LPS-induced models of sepsis and acute respiratory distress syndrome (6). Furthermore, mice globally deficient in Siglec-E demonstrate increased tumor immune surveillance, emphasizing its function as a suppressor of immune activation (3). Thus, Siglec-E is a potent immune modulator, and its engagement has significant implications in the regulation of innate immunity and by extension, adaptive immunity during inflammation.
ST8Sia6 is a sialyltransferase that catalyzes the addition of alpha-2,8-disialic acids preferentially onto O-linked glycoproteins (7). Here, we definitively demonstrate for the first time that ST8Sia6 produces ligands for Siglec-E in vivo using ST8Sia6 knockout (KO) mice. Therefore, we hypothesized that overexpression of ST8Sia6 may engage Siglec-E to reduce inflammation and disease. Within the pancreatic islet microenvironment, the IRM expresses high levels of Siglec-E, which decreases as insulitis increases in NOD islets. The NOD mouse spontaneously develops robust insulitis, or lymphocytic infiltration of the islets, followed by overt diabetes as beta cell destruction progresses. Although there are many immune cells necessary for disease development, the IRM plays a critical role in antigen presentation and disease initiation (8). IRMs are intimately associated with pancreatic beta cells and present beta cell antigens to infiltrating CD4+ autoreactive T cells. Depletion of IRMs prevents the onset of type 1 diabetes (T1D) (9). However, IRMs also contribute to beta cell health and glucose homeostasis (10). Thus, therapeutically, modulating its pro-inflammatory status may be preferential to overt depletion in T1D. We hypothesized that overexpression of ST8Sia6 in beta cells would produce alpha-2,8-disialic ligands that could engage Siglec-E on IRMs and reduce incidence of diabetes resulting from an inflammatory insult to the beta cell. Utilizing MLD-STZ, whereby beta cell destruction and progression to hyperglycemia is influenced by the innate inflammatory response (11), we found that ST8Sia6 overexpression in beta cells appreciably reduced the severity of hyperglycemia, and importantly preserved beta cell mass as well. Thus, this novel study highlights the potential therapeutic benefit of genetically engineering ST8Sia6, a sialyltransferase that produces preferred ligands for Siglec-E, into cells to protect from deleterious inflammation.
Materials and Methods:
Mice:
C57BL/6J (stock #000664), Ins2-cre (stock #003573), and LNL-tTA mice (stock #008600) were obtained from the Jackson Laboratory. ST8Sia6 KO mice were generated by injecting TALENs into C57BL/6 blastocysts, resulting in a 10-nucleotide deletion in exon 4. This created a frame-shift mutation after amino acid 112, and a truncation that resulted in the loss of the sialyltransferase domain of ST8Sia6. Mice with myc-tagged ST8Sia6 under the control of a tetracycline responsive element (TRE) were generated as performed previously (12). Male mice between 10–14 weeks of age were used for MLD-STZ experiments. Prediabetic NOD (< 250 mg/dL blood glucose) female mice of varying ages were used for assessment of insulitis and Siglec-E expression on IRMs. All animal studies were reviewed and conducted in accordance with the Institutional Animal Care and Use Committee at Mayo Clinic.
Islet Isolation, Cell Culture, and Dissociation:
Pancreata were isolated by perfusing 3 mL of 1.5 mg/mL cold Collagenase P (Millipore Sigma) dissolved in Hank’s Balanced Salt Solution (Corning) through the common bile duct, digesting for 10 minutes at 37˚ C, and separating islets by centrifugation using a 1.077 g/mL Histopaque gradient (Millipore Sigma). For flow cytometry, islets were dissociated into single cells using enzyme-free Cell Dissociation Buffer (Gibco) for 30 minutes at 37˚ C. For cell culture, intact islets were rested overnight in RPMI 1640 (ThermoFisher) before dissociation and probing with recombinant Siglec-E, or before 24 hour incubation with 50 ng/mL IFNγ or 1 mM streptozotocin (STZ).
Flow Cytometry:
Single cells (from dissociated islets or thymi) were washed with FACS buffer, blocked with 1:1 mouse:rat serum on ice for 5 minutes, and stained with antibodies or recombinant Siglec-E on ice for 30 minutes. Directly conjugated fluorescent antibodies (Biolegend) used included CD45 (1:1000, clone 30-F11), CD11c (1:100, clone N418), F4/80 (1:200, clone BM8), CD103 (1:100, clone 2E7), CD11b (1:200, clone M1/70), Ly6C (1:200, clone HK1.4), TCRβ (1:100, clone H57–597), CD19 (1:200, clone 6D5), Siglec-E (1:200, clone M1304A01), Siglec-7 (1:100, clone 6–434), Siglec-9 (1:100, clone K8), CD4 (1:200, clone GK1.5), CD8α (1:200, clone 53–6.7), CD69 (1:200, clone H1.2F3), and H-2Kb (1:200, clone 25-D1.16). Recombinant Siglec-E, Gln20-Phe355 with a C-terminal human IgG1-Fc tag (1:100, Biolegend), was incubated with murine cells alone on ice for 30 minutes, followed by washing and staining with primary and secondary anti-human IgG (1:100, Jackson ImmunoResearch) antibodies on ice for 30 minutes; for human cells, the same process was performed with both recombinant Siglec-7, Gln19-Gly357 with a C-terminal human IgG1-Fc tag (1:100, R&D Systems), and recombinant Siglec-9, Gln18-Gly348 with a C-terminal human IgG1-Fc tag (1:100, R&D Systems). All experiments included subsequent incubation with fixable viability dye (Tonbo) for 10 minutes at room temperature. Stained cells were analyzed using an Attune NxT flow cytometer (ThermoFisher), and data was processed using FlowJo (Tree Star) 10.
Diabetes induction:
STZ (Millipore Sigma) was dissolved in 0.1 M citrate buffer, pH = 4.5 at a dose of 50 mg/kg and immediately injected intraperitoneally within 5 minutes. This was repeated daily for 5 days. Non-fasting blood glucose levels were measured with a Contour Next blood glucose meter and test strips (Bayer). For glucose and insulin tolerance tests, blood was analyzed before, 15, 30, 60, and 90 minutes after intraperitoneal injection of either 1 g/kg glucose, or 0.75 U/kg human insulin (Novolin R). Mice were sacrificed at day 32 for histological analysis, or at humane endpoint.
Immunofluorescence:
Pancreatic sections were stained with antibodies against insulin (1:400, polyclonal/A0564, Agilent), or myc-tag (1:200, clone 9B11, Cell Signaling) overnight at 4˚ C. After washing, slides were incubated with secondary antibody (5 μg/mL, Invitrogen) for 1 hour at room temperature, and imaged under a fluorescent microscope. For analysis of total pancreas sections, blocks were cut at two different depths to obtain unique sections of tissue, and were serially imaged using a Zeiss Axio Observer Z1 microscope and stitched together using ZenPro software (Carl Zeiss Microscopy). Regions of interest were drawn around the entire pancreas based on DAPI staining and beta cells based on insulin staining. % insulin+ area was calculated using ImageJ.
Statistical Analysis:
Statistical tests used are denoted in each figure legend, and were calculated using GraphPad Prism.
Results:
Islet resident macrophages express Siglec-E and cell surface expression is reduced with inflammation
The IRM is an immune cell that occupies the pancreatic islets under homeostatic conditions, and has a crucial role in autoimmune diabetes development (13). Murine IRMs were examined for expression of Siglec-E, Siglec-H and Siglec-F that could potentially counter local inflammation in the islet microenvironment. Only Siglec-E was highly expressed relative to fluorescence minus one controls (Figure 1A, gating strategy in Supplemental Figure 1). Furthermore, expression of Siglec-7 and Siglec-9 (human orthologs of murine Siglec-E), was assessed on IRMs obtained from human non-diabetic cadaveric islets. Interestingly, both Siglec-7 and Siglec-9 were expressed on human IRMs (Supplemental Figure 2A–B).
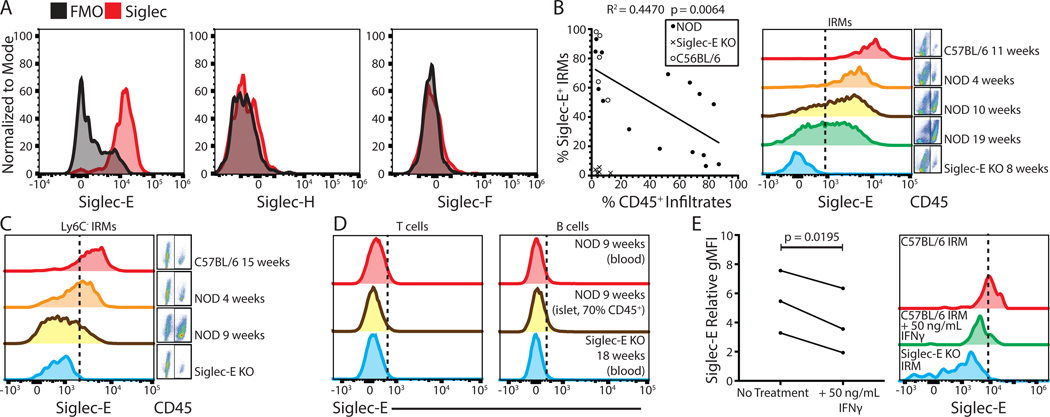
Figure 1. Islet resident macrophages express Siglec-E and cell surface expression is reduced with inflammation.
(A) Examination of Siglec E, H, and F expression on IRMs (defined as live, single CD45+ CD11c+ F4/80+ CD103− cells) compared to fluorescence minus one controls. (B) Correlation of CD45+ infiltrates (insulitis) vs. Siglec-E expression on IRMs in C57BL6/J (n = 5), NOD (n = 15), and Siglec-E KO control mice (n = 5). Linear regression analysis was performed for NOD data points. (C) Siglec-E expression on Ly6C- IRMs. (D) Siglec-E expression analysis on T or B cells (defined as live, single, TCRβ+ or CD19+ cells, respectively). (E) Siglec-E expression on IRMs after 24-hour C57BL/6J islet culture with 50 ng/mL IFNγ (n = 3). A paired T test was performed. All histograms are representative of at least three independent experiments with at least three mice in total.
To examine whether Siglec-E expression is modulated by inflammation, its expression on IRMs during the course of insulitis in prediabetic NOD mice was examined. As insulitis progressed, the frequency of Siglec-E+ IRMs was significantly reduced (Figure 1B). This reduction in Siglec-E was not due to the influx of CD11b+ Ly6C+ inflammatory monocytes, as gating on Ly6C- cells revealed similar results (Figure 1C). Additionally, Siglec-E is not expressed on infiltrating T and B cells (Figure 1D). CD4+ T cells are one of the first immune cells to infiltrate the islet in the NOD model of diabetes, and the diabetogenic T cell response in NOD mice is Th1-skewed, characterized by IFNγ production (14). Interestingly, IFNγ was recently demonstrated to reduce the expression of Siglec-15 in human monocytes (15). Therefore, IFNγ produced by local T cells in the islet microenvironment may downregulate Siglec-E. To address this, intact islets isolated from C57BL/6J mice were cocultured with 50 ng/mL IFNγ for 24 hours. IRM Siglec-E expression was significantly reduced upon IFNγ treatment compared to untreated islets (Figure 1E).
The sialyltransferase ST8Sia6 generates ligands for Siglec-E
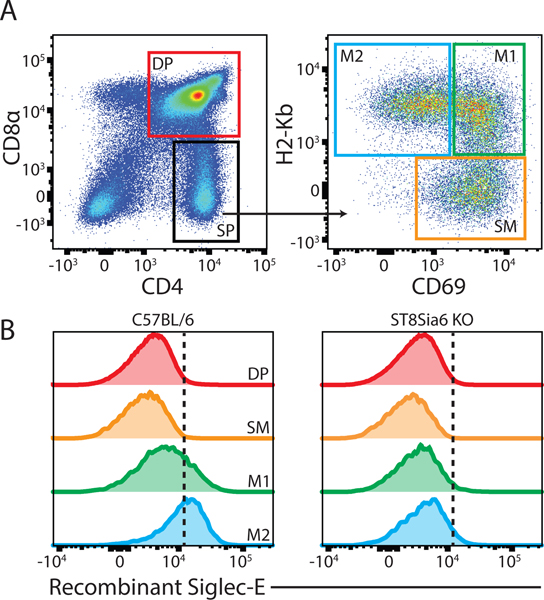
During T cell development, gene expression of ST8Sia6 increases with maturation and correlates with generation of ligands for Siglec-E (16). To demonstrate that ST8Sia6 generates ligands for Siglec-E in vivo, ST8Sia6 KO mice were generated. Following positive selection at the double positive (DP) stage, CD4 single positive (SP) thymocytes mature and transition from CD69+ H2-Kb- semi-mature (SM) to CD69+ H2-Kb+ mature-1 (M1) and finally CD69- H2-Kb+ mature-2 (M2) cells. By probing with recombinant Siglec-E, Siglec-E ligands were significantly reduced in maturing thymocytes at the M2 stage in ST8Sia6 KO mice, definitively demonstrating that ST8Sia6 generates ligands for Siglec-E in vivo (Figure 2A–B, Supplemental Figure 3A). As a control, treatment of wildtype thymocytes with 0.1 U/mL sialidase completely abolished binding of recombinant Siglec-E to M2 cells (Supplemental Figure 3B). This suggests that ST8Sia6 may have implications in immune processes by generating ligands for relevant Siglecs, such as Siglec-E. Therefore, we examined whether ST8Sia6 overexpression could dampen the immune response in a disease model.
Figure 2. The sialyltransferase ST8Sia6 generates ligands for Siglec-E.
(A) Gating strategy and (B) extent of recombinant Siglec-E binding to thymocytes from C57BL/6J vs. ST8Sia6 KO mice during T cell development.
Overexpression of ST8Sia6 in pancreatic beta cells increases ligands for Siglec-E
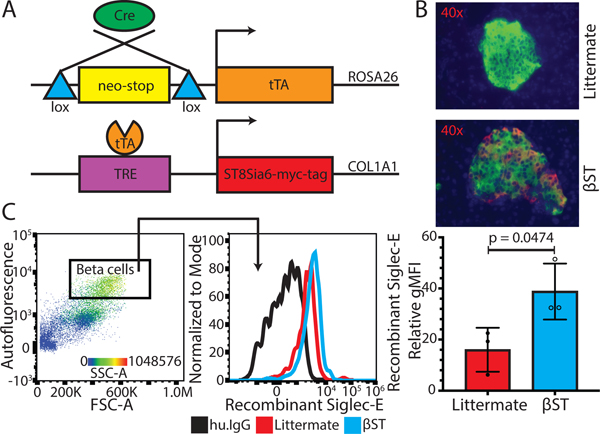
A system was created to spatiotemporally drive overexpression of ST8Sia6 in beta cells. In brief, myc-tagged ST8Sia6 under the control of TRE was knocked into the COL1A1 locus, and interbred with RIP-cre (17) and LNL-tTA (18) mice to generate RIP-cre LNL-tTA ST8Sia6 mice (Figure 3A). When all three alleles are present, ST8Sia6 is overexpressed in pancreatic beta cells in the absence of doxycycline. For simplicity, RIP-cre LNL-tTA ST8Sia6 mice will be referred to as βST, and mice harboring any other combination of 2 or fewer alleles will be referred to as littermates. To confirm ST8Sia6 expression in beta cells, immunofluorescence staining against insulin and myc-tag on pancreatic sections from βST and littermate mice was performed. As expected, ST8Sia6 overexpression was observed in βST islets. In addition, there were no obvious abnormalities in islet morphology and insulitis was absent (Figure 3B). To examine whether overexpression of ST8Sia6 produced ligands that could engage Siglec-E, we probed beta cells from βST and littermate mice with recombinant Siglec-E. Beta cells were identified by flow cytometry as large, autofluorescent, and highly granular. Recombinant Siglec-E bound significantly greater to βST beta cells compared to littermate controls, demonstrating that overexpression of ST8Sia6 led to increased production of ligands for Siglec-E on the beta cell surface (Figure 3C). Human Siglec-7 and Siglec-9 are the closest human orthologs of murine Siglec-E. Therefore, we were interested in whether there was comparable baseline binding to the human beta cell surface, as observed with murine littermate beta cells when probed with recombinant Siglec-E. Human non-diabetic cadaveric beta cells were probed with recombinant Siglec-7 and Siglec-9 and found that only ligands for Siglec-9 were expressed on human beta cells (Supplemental Figure 2A–B).
Figure 3. Overexpression of ST8Sia6 in pancreatic beta cells increases ligands for Siglec-E.
(A) Schematic of gene expression system. In cre-expressing cells, tTA and thus ST8Sia6 expression is induced. (B) Immunofluorescent images of islets stained with anti-insulin (green) and anti-myc-tag (red) antibodies, with DAPI (blue) as counterstain. (C) Extent of recombinant Siglec-E bound to beta cells (defined as live, single CD45−, autofluorescent/BL1+, FSChi, SSChi cells) from littermate (n = 3) vs. βST (RIP-cre LNL-tTA ST8Sia6) mice (n = 3), relative to human IgG secondary antibody bound. An unpaired T test was performed with error bars representing standard deviation.
Beta cells from littermate and βST mice were treated with 0.1 U/mL sialidase. Unexpectedly and unlike thymocytes, this only marginally reduced the ability of recombinant Siglec-E to bind to the beta cell surface. Therefore, the targets of ST8Sia6 may differ depending on cell type. As a detergent must be present for sialidase to hydrolyze glycolipids, sialylated glycolipids were maintained during our live cell analysis. Intriguingly, recombinant Siglec-E bound sialidase-treated βST beta cells significantly more than sialidase-treated littermate beta cells (Supplemental Figure 3C). This suggests ST8Sia6 may target a glycolipid substrate in murine beta cells.
Beta cell ST8Sia6 overexpression mitigates severe hyperglycemia induced by multiple low dose streptozotocin-induced diabetes
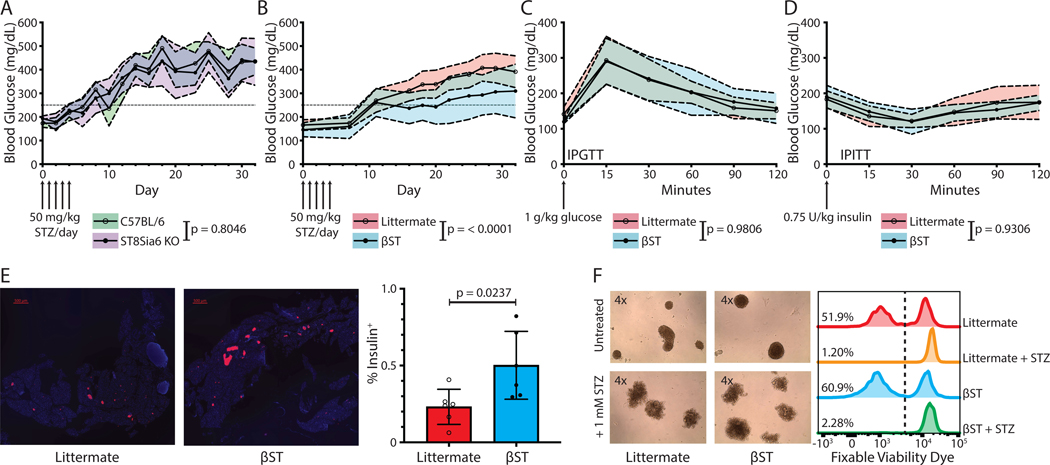
Due to the beta cell’s close contact with Siglec-E+ IRMs in the islet microenvironment, the effects of both ST8Sia6 deficiency and beta cell overexpression of ST8Sia6 in the context of diabetes induced by MLD-STZ was examined. Administration of STZ in low doses induces a DNA damage response, leading to elevated proinflammatory molecules IL-1β and Cxcl10, and subsequent insulitis (11). In contrast to administration of high dose STZ, which causes rapid necrosis of beta cells and severe hyperglycemia, MLD-STZ-induced diabetes occurs progressively in male C57BL/6J mice due to limited apoptosis of beta cells (19). Blood glucose levels in ST8Sia6 KO mice were similar to C57BL/6J controls after MLD-STZ (Figure 4A). In stark contrast, βST mice exhibited significantly lower blood glucose levels compared to littermate controls after administration of MLD-STZ, indicating a protective effect of ST8Sia6 overexpression in beta cells during diabetes development (Figure 4B). The presence of cre did not have a confounding effect on this observation, as comparing cre+ littermates to βST yielded similar results (Supplemental Figure 4). In order to define whether beta cell overexpression of ST8Sia6 impacts glucose homeostasis, an intraperitoneal glucose tolerance test (IPGTT) and intraperitoneal insulin tolerance test (IPITT) comparing βST and littermate mice was performed. There was no statistically significant difference between βST and littermate controls in either IPGTT (Figure 4C) or IPITT (Figure 4D), demonstrating that ectopic expression of ST8Sia6 does not alter glucose homeostasis in βST mice. As βST resistance to hyperglycemia after MLD-STZ challenge could be due to preservation of beta cells, insulin+ cells of total pancreatic sections were examined at experimental endpoint (day 32). There was significantly increased percent insulin+ area in βST pancreatic sections (Figure 4E) compared to littermate controls. Although significantly reduced compared to littermates, an increase in blood glucose after MLD-STZ in βST mice over baseline was observed. As STZ is a beta cell toxin that elicits apoptosis even in low doses, an increase in βST blood glucose after MLD-STZ was not unexpected. To determine if overexpression of ST8Sia6 altered intrinsic effects of STZ on beta cell survival, islets from βST and littermate mice were exposed to 1 mM STZ for 24 hours. Similar disruption in islet morphology and equivalent beta cell death was noted, demonstrating that βST beta cells are not intrinsically resistant to STZ-induced damage and death (Figure 4F). Notably, the difference in blood glucose between βST and littermates diverged at approximately day 16, when an immune response and insulitis would be expected to occur. As overexpression of ST8Sia6 occurs locally within the islets, protection from hyperglycemia may stem from local immune modulation, although the precise mechanism is unclear.
Figure 4. Beta cell ST8Sia6 overexpression mitigates severe hyperglycemia induced by multiple low dose streptozotocin-induced diabetes.
(A) Kinetic analysis of diabetes induction by MLD-STZ in C57BL/6J (n = 9) vs. ST8Sia6 KO (n = 7) mice, or (B) in littermate (n = 16) vs. βST (n = 13) mice. (C) IPGTT (1 g/kg) and (D) IPITT (0.75 U/kg) in littermate vs. βST mice. For IPGTT, littermate n = 7 and βST n = 4. For IPITT, littermate n = 5 and βST n = 6. For figures 4A-D, 2-way ANOVA with Geisser-Greenhouse correction was performed, with shaded regions representing standard deviation. (E) Representative immunofluorescent images of % insulin+ area. The average % insulin+ area of 2 pancreatic sections from littermate (n = 6) vs. βST (n = 6) mice obtained at MLD-STZ endpoint (day 32) were used for quantification. Scale bars represent 500 μm, and an unpaired T test was performed with error bars representing standard deviation. (F) Morphological assessment of islets incubated with 1 mM STZ for 24 hours, with % live/dead analysis of beta cells. Gating on beta cells was performed as in figure 3. Histograms are representative of at least three independent experiments with at least three mice in total.
Discussion:
At this time, we cannot rule out that beta cell ST8Sia6 overexpression has protective effects outside of producing ligands and immunomodulating IRMs via Siglec-E engagement. All autoantigens in T1D are proteins that are linked to the secretory pathway (20). As ST8Sia6 is expressed in the Golgi, sialic acid incorporation into autoantigens may prevent recognition by altering incorporation into MHC and preventing T cell recognition, or allowing polarization of islet-infiltrating T cells to a regulatory phenotype. However, few autoantigens have been identified in the context of MLD-STZ and the role of autoreactive T cells remains controversial in this model, so an influence on innate immunity is more likely.
Beta cell destruction following MLD-STZ was diminished in βST mice, indicating a profound protective effect of ST8Sia6 on cells targeted by an inflammatory insult. Because expression of Siglec-E is reduced as insulitis progresses, the opportunity to engage this receptor with sialylated ligands may be a therapeutic option for people with T1D early in the disease process. Alternatively, altering islets or beta cells before transplantation using gene therapy or physical incorporation of sialic acids into supporting biomaterial or associated nanoparticles presents a unique therapeutic opportunity not currently available in islet transplantation.
Since the first procedure using the Edmonton protocol in 1999, islet transplantation has established itself as a promising therapy for patients with longstanding T1D, and beta cell replacement using replenishable sources like human embryonic stem cell-derived beta cells has the potential to become curative (21). Still, current drug regimens in islet transplantation utilize suppressors of the T cell response and IL-2 production, exposing patients to risk of infection and malignancies (22). However, innate immunity plays a critical role in graft rejection and is required to prime an adaptive response. The incorporation of sialylation locally within the graft may synergize with antigen release and presentation by Siglec-bearing APCs to induce a tolerogenic response to a broad array of native graft-derived antigens. Therefore, engineering sialylated ligands to target Siglecs on graft-infiltrating innate immune cells may offer an innovative local immune therapy option in the context of beta cell replacement.
Future research will determine if inducing ST8Sia6 overexpression only after diabetes onset leads to remission. Finally, backcrossing βST mice to the NOD background, which develops immunity directed against pancreatic beta cells, will determine if protection is still observed using this robust model of spontaneous autoimmunity. In conclusion, Siglec-E is expressed on IRMs and is downregulated in the presence of insulitis, and IFNγ is sufficient to mediate downregulation. Using two novel mouse models, we’ve shown that ST8Sia6 produces ligands for Siglec-E, and that overexpression of ST8Sia6 in pancreatic beta cells significantly increases Siglec-E binding on the beta cell surface. Finally, ST8Sia6 overexpression in beta cells protects against severe hyperglycemia when diabetes is induced using MLD-STZ, initiating a new avenue of research into the role of the Siglec/sialic acid axis as a potential modulator of immune-mediated diabetes.
Supplementary Material
Key points:
Siglec-E is expressed on islet-resident macrophages.
In vivo, ST8Sia6 generates ligands for Siglec-E.
Overexpression of ST8Sia6 preserves beta cells upon diabetes induction.
Acknowledgements:
We thank Barsha Dash and David Friedman for thoughtful discussions, Matthew Brown for assistance in quantifying % insulin+ area, Aleksey Matveyenko for his assistance with the human cadaveric islet studies, Karl Clark and Stephen Ekker for providing TALENs to generate ST8Sia6 KO mice, and the Mayo Clinic Transgenic and Knockout Core for generation of ST8Sia6 KO mice and TRE-ST8Sia6-myc-tag knock-in mice.
This work was supported by National Institutes of Health Grant R21AI138858-01A1 to V.S.S. P.J.B. was supported by the Mayo Clinic Wilbur T. and Grace C. Pobanz Predoctoral Fellowship.
Abbreviations used:
- βST
RIP-cre LNL-tTA ST8Sia6
- IPGTT
Intraperitoneal glucose tolerance test
- IPITT
Intraperitoneal insulin tolerance test
- IRM
Islet resident macrophage
- KO
Knockout
- MLD-STZ
Multiple low dose streptozotocin
- Siglec
Sialic acid-binding immunoglobulin-type lectin
- STZ
Streptozotocin
- T1D
Type 1 diabetes
- TRE
Tetracycline responsive element
References:
- 1.Macauley MS, Crocker PR, and Paulson JC. 2014. Siglec-mediated regulation of immune cell function in disease. Nat. Rev. Immunol. 14: 653–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carlin AF, Uchiyama S, Chang YC, Lewis AL, Nizet V, and Varki A. 2009. Molecular mimicry of host sialylated glycans allows a bacterial pathogen to engage neutrophil Siglec-9 and dampen the innate immune response. Blood 113: 3333–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Läubli H, Pearce OMT, Schwarz F, Siddiqui SS, Deng L, Stanczak MA, Deng L, Verhagen A, Secrest P, Lusk C, Schwartz AG, Varki NM, Bui JD, and Varki A. 2014. Engagement of myelomonocytic Siglecs by tumor-associated ligands modulates the innate immune response to cancer. Proc. Natl. Acad. Sci. U. S. A. 111: 14211–14216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angata T, Hingorani R, Varki NM, and Varki A. 2001. Cloning and Characterization of a Novel Mouse Siglec, mSiglec-F: Differential evolution of the mouse and human (CD33) Siglec-3-related gene clusters. J. Biol. Chem. 276: 45128–45136. [DOI] [PubMed] [Google Scholar]
- 5.Zhang JQ, Biedermann B, Nitschke L, and Crocker PR. 2004. The murine inhibitory receptor mSiglec-E is expressed broadly on cells of the innate immune system whereas mSiglec-F is restricted to eosinophils. Eur. J. Immunol. 34: 1175–1184. [DOI] [PubMed] [Google Scholar]
- 6.Spence S, Greene MK, Fay F, Hams E, Saunders SP, Hamid U, Fitzgerald M, Beck J, Bains BK, Smyth P, Themistou E, Small DM, Schmid D, O’Kane CM, Fitzgerald DC, Abdelghany SM, Johnston JA, Fallon PG, Burrows JF, McAuley DF, Kissenpfennig A, and Scott CJ. 2015. Targeting Siglecs with a sialic acid-decorated nanoparticle abrogates inflammation. Sci. Transl. Med. 7: 303ra140. [DOI] [PubMed] [Google Scholar]
- 7.Takashima S, Ishida HK, Inazu T, Ando T, Ishida H, Kiso M, Tsuji S, and Tsujimoto M. 2002. Molecular cloning and expression of a sixth type of α2,8-sialyltransferase (ST8Sia VI) that sialylates O-glycans. J. Biol. Chem. 277: 24030–24038. [DOI] [PubMed] [Google Scholar]
- 8.Unanue ER 2014. Antigen Presentation in the Autoimmune Diabetes of the NOD Mouse. Annu. Rev. Immunol. 32: 579–608. [DOI] [PubMed] [Google Scholar]
- 9.Carrero JA, McCarthy DP, Ferris ST, Wan X, Hu H, Zinselmeyer BH, Vomund AN, and Unanue ER. 2017. Resident macrophages of pancreatic islets have a seminal role in the initiation of autoimmune diabetes of NOD mice. Proc. Natl. Acad. Sci. U. S. A. 114: E10418–E10427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banaei-Bouchareb L, Gouon-Evans V, Samara-Boustani D, Castellotti MC, Czernichow P, Pollard JW, and Polak M. 2004. Insulin cell mass is altered in Csf1 op /Csf1 op macrophage-deficient mice. J. Leukoc. Biol. 76: 359–367. [DOI] [PubMed] [Google Scholar]
- 11.Horwitz E, Krogvold L, Zhitomirsky S, Swisa A, Fischman M, Lax T, Dahan T, Hurvitz N, Weinberg-Corem N, Klochendler A, Powers AC, Brissova M, Jörns A, Lenzen S, Glaser B, Dahl-Jørgensen K, and Dor Y. 2018. Beta cell DNA damage response promotes islet inflammation in type 1 diabetes. Diabetes 67: 2305–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shapiro MJ, Lehrke MJ, Chung JY, Romero Arocha S, and Shapiro VS. 2019. NKAP Must Associate with HDAC3 to Regulate Hematopoietic Stem Cell Maintenance and Survival. J. Immunol. 202: 2287–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferris ST, Zakharov PN, Wan X, Calderon B, Artyomov MN, Unanue ER, and Carrero JA. 2017. The islet-resident macrophage is in an inflammatory state and senses microbial products in blood. J. Exp. Med. 214: 2369–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carrero JA, Calderon B, Towfic F, Artyomov MN, and Unanue ER. 2013. Defining the Transcriptional and Cellular Landscape of Type 1 Diabetes in the NOD Mouse. PLoS One 9: e59701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, Sun J, Liu LN, Flies DB, Nie X, Toki M, Zhang J, Song C, Zarr M, Zhou X, Han X, Archer KA, O’Neill T, Herbst RS, Boto AN, Sanmamed MF, Langermann S, Rimm DL, and Chen L. 2019. Siglec-15 as an immune suppressor and potential target for normalization cancer immunotherapy. Nat. Med. 25: 656–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu F-C, Shapiro MJ, Chen MW, McWilliams DC, Seaburg LM, Tangen SN, and Shapiro VS. 2014. Immature Recent Thymic Emigrants Are Eliminated by Complement. J. Immunol. 193: 6005–6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Postic C, Shiota M, Niswender KD, Jetton TL, Chen Y, Moates JM, Shelton KD, Lindner J, Cherrington AD, and Magnuson MA. 1999. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic β cell-specific gene knock-outs using Cre recombinase. J. Biol. Chem. 274: 305–315. [DOI] [PubMed] [Google Scholar]
- 18.Wang L, Sharma K, Deng HX, Siddique T, Grisotti G, Liu E, and Roos RP. 2008. Restricted expression of mutant SOD1 in spinal motor neurons and interneurons induces motor neuron pathology. Neurobiol. Dis. 29: 400–408. [DOI] [PubMed] [Google Scholar]
- 19.Like AA, and Rossini AA. 1976. Streptozotocin-induced pancreatic insulitis: New model of diabetes mellitus. Science. 193: 415–417. [DOI] [PubMed] [Google Scholar]
- 20.Arvan P, Pietropaolo M, Ostrov D, and Rhodes CJ. 2012. Islet autoantigens: Structure, function, localization, and regulation. Cold Spring Harb. Perspect. Med. 2: a007658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shapiro AMJ, Lakey JRT, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, and Rajotte RV. 2000. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 343: 230–238. [DOI] [PubMed] [Google Scholar]
- 22.Van Belle T, and Von Herrath M. 2008. Immunosuppression in islet transplantation. J. Clin. Invest. 118: 1625–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.