Abstract
Background
Chronic heat stress (CHS) disrupts hepatic metabolic homeostasis and jeopardizes product quality of pigs. Selenium (Se) may regulate the metabolic state through affect selenoprotein. Thus, we investigate the protective effect of dietary hydroxy-4-methylselenobutanoic acid (HMSeBA) on CHS induced hepatic metabolic disorder in growing pigs, and the corresponding response of selenoprotein.
Methods
Forty crossbreed growing pigs were randomly assigned to five groups: control group raised in the thermoneutral environment (22 ± 2 °C) with basal diet; four CHS groups raised in hyperthermal condition (33 ± 2 °C) with basal diet and supplied with 0.0, 0.2, 0.4, and 0.6 mg Se/kg HMSeBA, respectively. The trial lasted 28 d. The serum biochemical, hepatic metabolism related enzyme, protein and gene expression and 25 selenoproteins in liver tissue were determined by real-time PCR, ELISA and western blot.
Results
CHS significantly increased the rectal temperature, respiration rate, serum aspartate aminotransferase (AST) and low-density lipoprotein cholesterol (LDL-C) of pigs, up-regulated hepatic heat shock protein 70 (HSP70) and induced lower liver weight, glycogen content, hepatic glucokinase and glutathione peroxidase (GSH-Px). The CHS-induced liver metabolic disorder was associated with the aberrant expression of 6 metabolism-related gene and 11 selenoprotein encoding genes, and decreased the protein abundance of GCK, GPX4 and SELENOS. HMSeBA improved anti-oxidative capacity of liver. 0.4 or 0.6 mg Se/kg HMSeBA supplementation recovered the liver weight, glycogen content and rescue of mRNA abundance of genes related to metabolism and protein levels of GCK. HMSeBA supplementation changed expressions of 15 selenoprotein encoding genes, and enhanced protein expression of GPX1, GPX4 and SELENOS in the liver affected by CHS. CHS alone showed no impact while HMSeBA supplementation increased protein levels of p-AMPKα in the liver.
Conclusions
In summary, HMSeBA supplementation beyond nutrient requirement mitigates CHS-induced hepatic metabolic disorder, recovered the liver glycogen content and the processes that are associated with the activation of AMPK signal and regulation of selenoproteins in the liver of growing pigs.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40104-021-00590-2.
Keywords: Chronic heat stress, Hepatic metabolism , HMSeBA, Pigs, Selenoprotein
Background
As global warming intensifies, heat waves become more frequent and longer in duration [1]. Heat stress (HS) has been a common hazard affecting livestock production, which makes billions economic of losses in profit annually to livestock production [2]. The biological response to HS can be divided into acute and chronic phases, the acute phase lasting hours to a few days and the chronic phase lasting several days to weeks [3]. Chronic heat stress (CHS) lead to dysregulation of energy balance and metabolism [4], which caused the decreased quality of livestock products.
Pigs are particularly prone to HS due to thick layers of subcutaneous adipose tissue and lack of functional sweat glands [5]. Previous studies have shown that pigs reared in hyperthermal conditions typically had lower skeletal muscle weight, higher fat tissue mass and poorer meat quality [6, 7], and those responses are associate with the alternation of several metabolic parameters [8, 9]. Liver is a key metabolic organ that regulates the whole-body metabolism in animals and humans. Hepatic injury is a common clinical feature of HS, which leads to elevation of serum aspartate aminotransferase (AST), alanine transaminase (ALT) [10, 11]. It has been reported that CHS significantly reduced liver weight and altered proteomic-associated oxidative response, immune defense and metabolism [12].
Selenium (Se) is an essential micronutrient for humans and animals, and dietary Se supplementation relieves the HS-induced negative effects in intestinal and serum metabolism of pigs through the enhanced antioxidant capacity [13, 14]. Additionally, Se supplementation mitigates HS-induced injury in IPEC-J2 cells [15] and LPS-induced immunological stress in mice [16]. In addition to potentiate antioxidant defenses, Selenium is involved in the regulation of carbohydrate, protein and lipid metabolism [17, 18]. The biological function of Se is mainly mediated by selenoproteins [8]. Currently, 25 selenoprotein encoding genes have been identified in the porcine species [19], and several selenoproteins are involved in metabolic regulations [20]. Although the specific mechanisms are complex and remain unclear, the alternation of GPX1, GPX4, SELENOH, SELENOP, SELENOS, DIO1 and TXNRD1 are associated with gene expression related to glucose metabolism (INSR, IRS1, AKT, PCK2 and GCK), lipogenesis (FOXO1, FAS, ACC1 and SREBP1), and protein synthesis pathway (mTOR, 4E-BP1 and RPS6/S6) [17, 18, 21–25].
Taken together, studies have revealed that HS caused damage to the metabolic homeostasis [26] and Se supplementation alleviates various types of stress on animals [13]. However, the interaction of dietary Se supplementation and CHS on biomarkers related to liver metabolic function remains unclear and intriguing. The pig model offers unique advantages for the study of human nutrition and medicine due to their great similarities in digestive systems, nutrition metabolism, and physiological response to stress [27]. Hydroxy-4-methylselenobutanoic acid (HMSeBA) is a new organic Se with higher bioavailability [28]. Therefore, the pig CHS model was developed to investigate: 1) the protective effect of HMSeBA on alleviating the hepatic metabolic dysfunction induced by CHS, and 2) the possible mechanism that linked the alleviation of HMSeBA to the response of selenoproteins in the liver.
Methods
Animals, experiment design and management
Total of 40 crossbreed castrated boars (Landrace × Yorkshire) × Duroc, aged 14 weeks with average body weight of 49.64 ± 2.48 kg, were randomly divided into 5 treatments with 8 replicates and 1 pig per replicate (n = 8). The control group (CON) raised in a thermoneutral environment (22 °C) and fed on basal diet with no additional Se; the following four treatment groups were subjected to HS (33 ± 2 °C) with basal diet supplemented: 0.0 mg Se/kg (CHS), 0.2 mg Se/kg (CHS + 0.2HMSeBA), 0.4 mg Se/kg (CHS + 0.4HMSeBA), and 0.6 mg Se/kg (CHS + 0.6HMSeBA). The basal diet was formulated according to the National Research Council (2012) and meet the requirements of 50–75 kg class of pigs.
All pigs were housed in individual pen in the artificial climate chamber equipped with a climate control that allows the setting and control of the temperature and relative humidity. The environment temperature was gradually increased and kept at 27 °C on d 1, 28 °C on d 2. Thereafter, the temperature was kept at 33 ± 2 °C, the relative humidity (RH) was 77.05% ± 2.84% and the temperature-humidity index (THI) was 78.05 ± 4.37 in CHS groups while the temperature was maintained at 22 ± 2 °C, RH was 74.42% ± 2.32% and THI was 67.80 ± 0.88 in CON group, until the end of the trial. Pigs were free access to water and feed, and the trial lasted for 4 weeks. The rectal temperature (RT) and respiration rate (RR) of pigs were monitored at 09:00, 13:00 and 16:00 individually and weekly with a mercury thermometer and a mechanical counter as previous described [29].
Blood and tissue collection
At d 28 after an overnight fast, the body weight of all pigs was recorded and six pigs with a body weight close to the average of each group were selected and the blood were collected in anticoagulant-free tubes from the jugular vein, and kept on the ice and centrifuged at 2,500×g for 10 min at 4 °C, then the serum was separated immediately and refrigerated at − 20 °C to analysis. Pigs were sedated by electrical stunning and slaughtered by manual exsanguination, and livers were separated and weighed and the liver index was calculated as the percentage of body weight. Liver samples were collected and rapidly freeze in liquid nitrogen and stored at − 80 °C for biochemical and molecular analyses.
Selenium deposition and glycogen content in liver
The total Se concentration in liver was determined with a hydride generation flame atomic fluorescence spectrometer (AFS-3100, Hai Guang instrument, China) based on the national food safety standard of China (GB 5009.93–2010), and calculated according to protocol described in previous study [30]. The liver glycogen content was assessed with the commercial kits (Jiancheng Bioengineering, China) according to the manufacturer’s instructions.
Serum biochemistry and hormone analyses
Serum alanine transaminase (ALT), aspartate aminotransferase (AST), total bile acid (TBA), total protein (TP), glucose (GLU), total triglycerides (TG), cholesterol (CHO), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C) and non-esterified acid (NEFA) were measured using an automatic biochemistry analyzer (3100, HITACHI, Japan). Serum triiodothyronine (T3), tetraiodothyronine (T4) and fasting insulin (F-insulin) was analyzed with the commercially available radioimmunoassay kits (Beijing North Institute of Biological Technology, China). The procedures followed the manufacturers’ instructions. All measurements were conducted in duplicate.
Antioxidant and metabolic enzyme analyses
Liver homogenates were prepared as previously described by our group [31]. The protein was quantified by the BCA protein assay kit (Jiancheng Bioengineering, China). Glutathione peroxidase (GSH-Px), total superoxide dismutase (T-SOD), total antioxidant capability (T-AOC) and malondialdehyde (MDA) were measured by colorimetric assay using commercial kits (Jiancheng Bioengineering, China). The activity of glucokinase (GCK), phosphoenolpyruvate carboxykinase (PEPCK) and fatty acid synthase (FAS) were measured using a commercial enzyme-linked immunosorbent assay (ELISA) kits (Meimian, China) according to the manufacturer’s instructions. For each measurement, the experiments were performed in triplicate at one occasion.
Real-time PCR analyses
Total RNA of liver was isolated using the Trizol reagent (Invitrogen, USA) according to the manufacturer’s instructions and the reverse transcription was performed using the PrimeScript RT reagent kit (Takara, China). Primers (Supplementary Table 1) for 12 metabolism-related genes, 25 selenoprotein encoding genes and 2 reference genes (β-ACTIN and GAPDH) were designed with Primer Express 3.0 (Applied Biosystems, USA). Quantitative real-time PCR (Q-RT-PCR) was performed on QuantStudio 6 Flex system (Applied Biosystems, USA) using SYBR Premix Ex Taq™ II reagents (No. RR820A, Takara, China) as described previously [22, 31, 32].
Western blot analyses
Liver total protein extracts were prepared using RIPA lysis buffer [50 mmol/L Tris-HCl (pH 7.4), 150 mmol/L NaCl, 0.25% sodium deoxycholate, 1% NP-40, 1 mmol/L EDTA, 10 μL/mL protease inhibitor, 10 μL/mL phosphatase inhibitor 3, 10 μL/mL 100 μmol/L Na3VO4, 10 μL/mL 10 mg/mL PMSF], and measured the protein concentration using a BCA kit (Jiancheng Bioengineering, China). Fixed protein amounts were electrophoresed using 12% SDS-PAGE gel and blotted onto PVDF membrane (Millipore, USA). The membranes were blocked and immunoblotted with primary antibodies against target protein HSP70 (1:5,000; ab5439; Abcam), AMPKα (1:1,000; #5831; Cell Signaling Technology), p-AMPKα (1:1,000; #2535; Cell Signaling Technology), GPX1 (1:1,000; 616,958; Zen BioScience), GPX4 (1:2,000; 513,309, Zen BioScience), SELENOS (1:1,000, 15,591–1-AP, Proteintech Group) and β-ACTIN (1:5,000; MAB1501; Millipore), respectively. Then incubated with corresponding secondary antibodies (horseradish peroxidase-linked goat anti-rabbit or mouse IgG). Autoradiography and chemiluminescence with an enhanced chemiluminescence system (Millipore, USA) was applied to detect and quantify the signal. Image Lab™ software system (Bio-Rad, USA) was used to analyze the densitometric of western blot bands. The ratio of target protein to β-actin protein represented the relative abundance of each target protein.
Statistical analysis
The experiment was the complete random design (CRD) and applied the one-way structure treatment design. Analysis of rectal temperature and respiration rate during the experiment was performed using one-way analysis of variance (ANOVA) on d 0, 7, 14, 21, and 28 of the trial. The effect of different treatment was analysis using PROC MIXED of SAS 9.2 (SAS Institute, 2003). The Tukey test was used to adjust for multiple treatment comparisons using the LSMEAN statement of SAS 9.2 (SAS Institute, 2003) with letter groupings obtained using the SAS pdmix800 macro (Saxton, 1998) [33]. The normality and homogeneity of variances were evaluated by Shapiro-Wilk W test and Levene’s test using the UNIVARIATE and HOVTEST statement, respectively. For different statistical test, significance was declared at P ≤ 0.05 or highly significance at P ≤ 0.01, unless otherwise stated.
Results
Rectal temperature and respiration rate of growing pigs
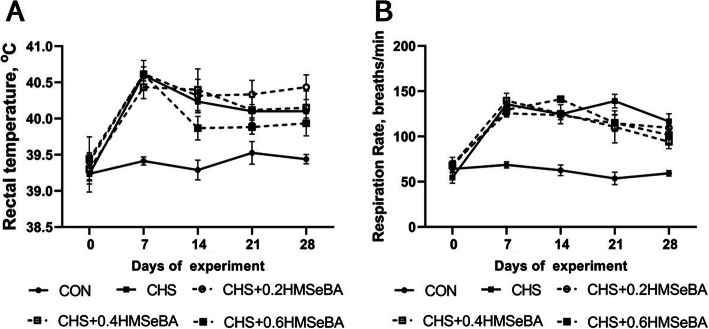
To evaluate the effects of CHS on growing pigs, the rectal temperature and respiration rate were monitored every week. As expected, rectal temperature and respiration rate increased significantly in response to CHS at d 7, 14, 21 and 28 of the trial HMSeBA supplementation exhibited limited effect (P > 0.05) on the rectal temperature and respiration rate of pigs, however pigs received 0.6 mg Se/kg HMSeBA tended to have lower rectal temperature at d 14, 21 and 28, also pigs in three HMSeBA supplementation groups had a relative lower respiration rate at d 21 and 28 (Fig. 1a and b).
Fig. 1.
Effects of chronic heat stress and HMSeBA supplementation on rectal temperature (a) and respiration rate (b) of growing pigs. The P-value of ANOVA analysis on d 0, 7, 14, 21, and 28 of experiment were 0.927, < 0.01, < 0.01, < 0.01, and < 0.01 for rectal temperature and 0.269, < 0.01, < 0.01, < 0.01, and < 0.01 for respiration rate (n = 8)
Effects of CHS and HMSeBA supplementation on liver weight, index, glycogen content and se concentration
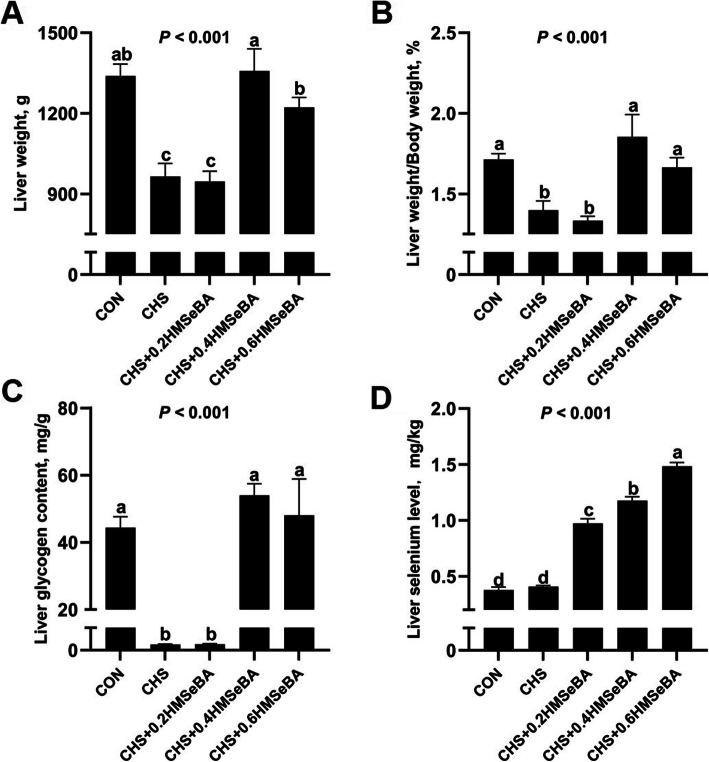
We investigated the effect of CHS on liver mass and Se deposition (Fig. 2). CHS decreased (P < 0.001) the absolute and relative liver weight and glycogen content. Dietary HMSeBA displayed a protective effect and 0.4 and 0.6 mg Se/kg HMSeBA effectively recovered the liver weight, liver weight index and glycogen content to normal level (P > 0.05) (Fig. 2a and b). CHS alone did not affect Se concentration, while dietary HMSeBA supplementation showed a dose-dependent increase in Se deposition in liver (Fig. 2c).
Fig. 2.
Effects of chronic heat stress and HMSeBA supplementation on liver weight (a), liver weight/body weight (b), liver glycogen content (c) and liver Se concentration (d) of growing pigs. The results were expressed as mean ± SEM (n = 6). Different letters denote significant differences (P < 0.05)
Hepatic HSP70 abundance of growing pigs
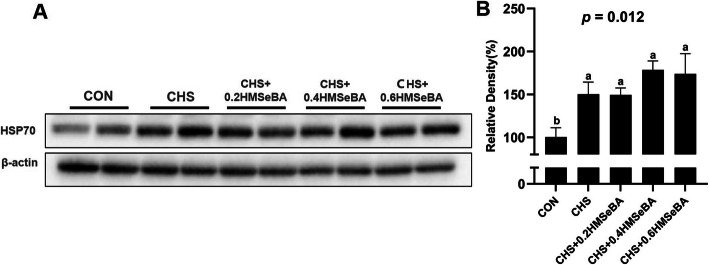
The CHS affected the protein abundance of HSP70 in liver of pigs (Fig. 3). As expected, CHS significantly up-regulated the protein abundance of HSP70 in liver, which indicated pigs were suffered with heat stress. 0.4 and 0.6 mg Se/kg HMSeBA supplementation numerically increased HSP70 abundance compared with CHS groups.
Fig. 3.
Effects of chronic heat stress and HMSeBA supplementation on the protein expression of HSP70 in liver. The results were expressed as mean ± SEM (n = 6). Different letters denote significant differences (P < 0.05)
Effects of CHS and HMSeBA supplementation on hepatic antioxidant variables
We investigated the effect of HMSeBA supplementation on antioxidant measurements in liver of pigs under CHS (Fig. 4). CHS compromised the hepatic antioxidant by decreasing (P < 0.05) GSH-Px (Fig. 4a) and numerically increasing MDA levels (Fig. 4d). Although no statistical difference, CHS decreased (P > 0.05) T-SOD and T-AOC in liver. HMSeBA supplementation exhibited protective effect, which enhanced (P < 0.05) the GSH-Px activity in a dose dependence manner, and effectively decreased (P < 0.05) the MDA level in liver under CHS. Beyond this, HMSeBA supplementation elevated (P > 0.05) T-SOD and T-AOC in values in liver of pigs under CHS.
Fig. 4.
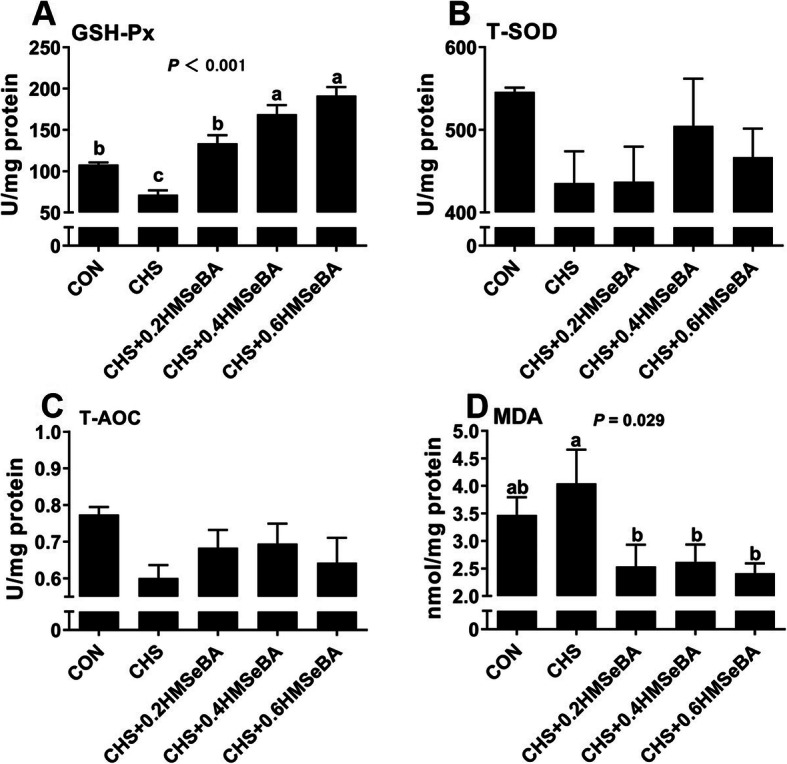
Effects of chronic heat stress and HMSeBA supplementation on GSH-Px (a), T-SOD (b), T-AOC (c) and MDA (d) concentration in liver. The results were expressed as mean ± SEM (n = 6). Different letters denote significant differences (P < 0.05)
Effects of CHS and HMSeBA supplementation on serum biochemical and hormone
We detected the effect of CHS on blood biochemical measures and endocrine (Table 1). Pigs subjected to CHS had higher (P < 0.05) serum TBA, LDL-C, AST and lower (P < 0.05) serum F-insulin and T3, and the serum T4 also tended to decrease (P < 0.1). HMSeBA supplementation moderately ameliorated (P < 0.05) the negative effect of CHS on serum AST and LDL-C. As shown in Table 1, 0.4 and 0.6 mg Se/kg HMSeBA returned (P < 0.05) serum AST activity to control level and 0.6 mg Se/kg HMSeBA reversed (P < 0.05) the serum LDL-C in pigs under CHS condition. Although CHS affected serum TBA, F-insulin, T3 and T4 concentration, dietary HMSeBA supplementation exhibited limited (P > 0.05) impact on those biochemical indicators. CHS or dietary HMSeBA showed no impact (P > 0.05) on serum ALT, GLU, TG, CHO, HDL-C and NEFA.
Table 1.
Effects of chronic heat stress and HMSeBA supplementation on serum biochemical and endocrine parameters of growing pigs
| Parameters | CON | CHS | CHS+ 0.2HMSeBA |
CHS+ 0.4HMSeBA |
CHS+ 0.6HMSeBA |
ANOVA P-value |
|---|---|---|---|---|---|---|
| ALT, U/L | 45.8 ± 3.5 | 48.0 ± 2.6 | 48.5 ± 4.7 | 47.2 ± 5.0 | 42.7 ± 1.6 | 0.807 |
| AST, U/L | 25.4 ± 2.0 | 38.5 ± 4.8 | 41.5 ± 2.7 | 35.0 ± 5.0 | 33.8 ± 3.4 | 0.101 |
| TBA, μmol/L | 25.7 ± 3.0b | 56.1 ± 6.4a | 48.9 ± 6.7a | 56.0 ± 6.9a | 55.6 ± 8.8a | 0.017 |
| F-insulin, μIU/mL | 15.10 ± 2.63a | 10.08 ± 0.98b | 9.94 ± 0.70b | 9.80 ± 0.76b | 9.32 ± 0.69b | 0.036 |
| T3, ng/mL | 0.65 ± 0.08a | 0.45 ± 0.08b | 0.42 ± 0.04b | 0.34 ± 0.03b | 0.39 ± 0.03b | 0.014 |
| T4, ng/mL | 50.72 ± 2.31 | 37.08 ± 5.00 | 39.10 ± 3.25 | 34.89 ± 3.27 | 39.67 ± 1.02 | 0.055 |
| GLU, mmol/L | 5.30 ± 0.44 | 5.54 ± 0.15 | 5.06 ± 0.44 | 5.25 ± 0.42 | 5.67 ± 0.36 | 0.801 |
| TG, mmol/L | 0.52 ± 0.04 | 0.49 ± 0.03 | 0.53 ± 0.03 | 0.51 ± 0.03 | 0.54 ± 0.07 | 0.929 |
| CHO, mmol/L | 3.04 ± 0.11 | 3.28 ± 0.13 | 3.17 ± 0.16 | 3.14 ± 0.17 | 2.98 ± 0.13 | 0.597 |
| LDL-C, mmol/L | 1.04 ± 0.04c | 1.36 ± 0.06a | 1.27 ± 0.06ab | 1.37 ± 0.08a | 1.13 ± 0.05bc | 0.002 |
| HDL-C, mmol/L | 0.80 ± 0.03 | 0.74 ± 0.04 | 0.74 ± 0.03 | 0.68 ± 0.05 | 0.70 ± 0.05 | 0.318 |
| NEFA, mmol/L | 0.20 ± 0.05 | 0.28 ± 0.19 | 0.27 ± 0.15 | 0.55 ± 0.25 | 0.36 ± 0.26 | 0.753 |
ALT alanine transaminase, AST aspartate aminotransferase, TBA total bile acid, F-insulin fasting insulin, GLU glucose, TG total triglycerides, CHO cholesterol, LDL-C low-density lipoprotein cholesterol, HDL-C high-density lipoprotein cholesterol, NEFA non-esterified acid, T3 triiodothyronine, T4 tetraiodothyronine. The results were expressed as mean ± SEM (n = 6). Values within a row with different superscripts differ (P < 0.05)
Effects of CHS and HMSeBA supplementation on hepatic metabolic enzyme activity, metabolism related gene mRNA and protein expression
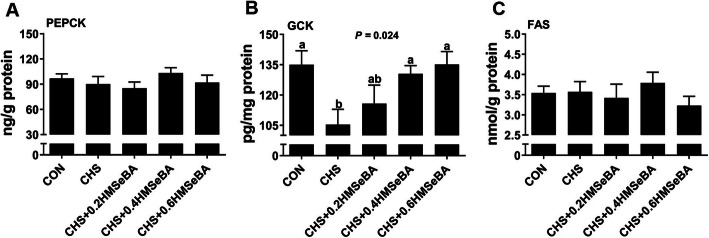
We assessed 3 hepatic enzymes related to liver metabolism (Fig. 5). CHS disturbed hepatic glucose metabolism by decreasing (P < 0.05) the GCK level while had limited impact (P > 0.05) on PEPCK and FAS. HMSeBA supplementation recovered (P < 0.05) the liver GCK levels in a dose dependence manner and 0.4 and 0.6 mg Se/kg HMSeBA recovered the liver GCK levels to normal levels. Dietary HMSeBA had no effect on hepatic PEPCK and FAS level (Fig. 5a and c).
Fig. 5.
Effects of chronic heat stress and HMSeBA supplementation on PEPCK (a), GCK (b) and FAS (c) concentration in liver. The results were expressed as mean ± SEM (n = 6). Different letters denote significant differences (P < 0.05)
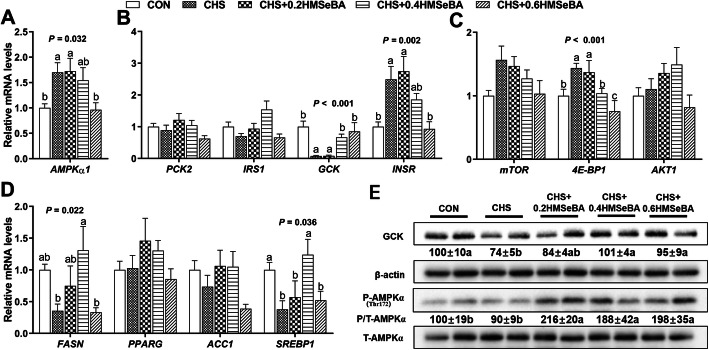
We further investigated the response of mRNA levels of 12 metabolic related genes to HMSeBA in liver of pigs under CHS. The results showed that CHS up-regulated (P < 0.05) the mRNA levels of AMPKα1, 4E-BP1 and INSR (Fig. 6a, b and c), down-regulated (P < 0.05) mRNA levels of GCK, FAS and SREBP1 (Fig. 6c and d) and exhibited no effect (P > 0.05) on expression of mTOR, AKT1, PCK2, IRS1, PPARG and ACC1. HMSeBA supplementation effectively prevented (P < 0.05) the up-regulation of AMPKα1, 4E-BP1 and INSR by CHS in a dose dependent manner. Meanwhile, HMSeBA supplementation reversed (P < 0.05) the down-regulation effect of CHS on GCK, FAS and SREBP1, among them, 0.4 and 0.6 mg Se/kg HMSeBA recovered the mRNA abundance of GCK, and 0.4 mg Se/kg HMSeBA recovered the mRNA abundance of FAS and SREBP1 to normal levels. Other than that, dietary HMSeBA supplementation showed no impact on expressions of mTOR, AKT1, PCK2, IRS1, PPARG and ACC1 in liver of pigs under CHS.
Fig. 6.
Effects of chronic heat stress and HMSeBA supplementation on expression of AMPKα1 (a), genes related to glucose (b), protein (c) and lipid metabolism (d) and protein level of p-AMPKα1 and GCK (e) in liver. The results were expressed as mean ± SEM (n = 6). Different letters denote significant differences (P < 0.05)
We investigated protein expression of GCK in liver, the results showed that CHS inhibited (P < 0.05) the protein expression of GCK, and the decrease of GCK protein level was reversed (P < 0.05) by dietary supplementation with 0.4 and 0.6 mg Se/kg HMSeBA (Fig. 6e). AMPKα is a key protein related to metabolic signal pathway. Although CHS exposure exhibited limited impact (P > 0.05) on the protein expression of p-AMPKα, three levels of dietary HMSeBA supplementation increased (P < 0.05) its proteins abundance (Fig. 6e).
Effects of CHS and HMSeBA supplementation on mRNA and protein expression of selenoproteins
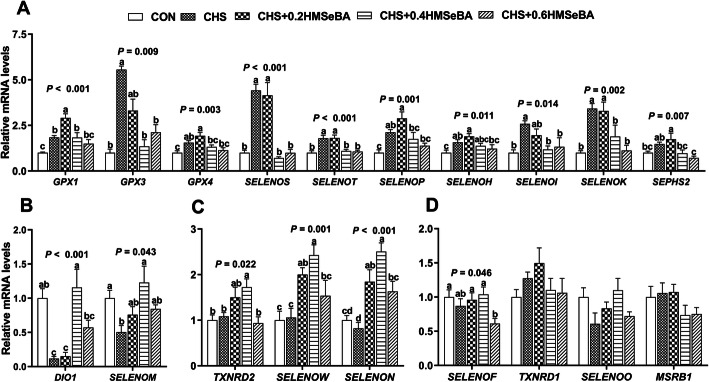
mRNA abundance of 25 selenoprotein encoding genes in liver of pigs were explored (Fig. 7). CHS increased (P < 0.05) mRNA expression of 10 selenoprotein genes (GPX1, GPX3, GPX4, SELENOS, SELENOT, SELENOP, SELENOH, SELENOI, SELENOK, and SEPHS2) (Fig. 7a), decreased (P < 0.05) mRNA expression of DIO1 and SELENOM (Fig. 7b), and exhibited no effect on expression of TXNRD2, SELENOW and SELENON (Fig. 7c). Dietary HMSeBA supplementation exhibited impact on expression of selenoprotein encoding genes in liver of pigs under CHS, which decreased (P < 0.05) expression of GPX3, GPX4, SELENOS, SELENOT, SELENOP, SELENOH, SELENOI, SELENOK, SEPHS2, DIO1, and SELENOM)(Fig. 7a) and increased (P < 0.05) the mRNA abundance of DIO1 and SELENOM (Fig. 7b). CHS did not affect the expression of TXNRD2, SELENOW and SELENON, while dietary 0.2 or 0.4 mg Se/kg HMSeBA supplementation increased (P < 0.05) their expression in liver of pigs under CHS (Fig. 7c). Additionally, CHS or dietary HMSeBA did not affected expression of SELENOF, TXNRD1, SELENO and MSRB1. Taken together, HMSeBA supplementation alleviated the impact of CHS on expression of selenoprotein encoding genes in liver of pigs, 0.4 or 0.6 mg Se/kg dietary HMSeBA supplementation exhibited better recovery effect based on the expression of these selenoprotein encoding genes, which shared similar mRNA profiles compared with that of the control.
Fig. 7.
Effects of chronic heat stress and HMSeBA supplementation on expression of selenoprotein encoding genes in liver. The results were expressed as mean ± SEM (n = 6). Different letters denote significant differences (P < 0.05)
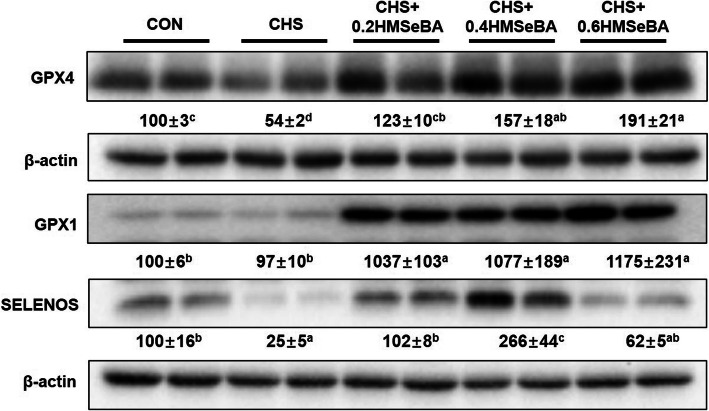
We also investigated protein expression of 3 selenoproteins (Fig. 8). CHS affected expression of selenoproteins, which inhibited (P < 0.05) the protein expression of GPX4 and SELENOS. HMSeBA supplementation inhibited (P < 0.05) this CHS induced reduction and the protein expression of GPX4 was enhanced (P < 0.05) with the increased of HMSeBA supplementation. Also, the decreased SELENOS was reversed (P < 0.05) in CHS + 0.2HMSeBA group and enhanced (P < 0.05) expression in CHS + 0.4HMSeBA group. CHS did not affected the expression of GPX1, while three levels of dietary HMSeBA supplementation greatly increased (P < 0.05) its protein expression in liver of pigs under CHS.
Fig. 8.
Effects of chronic heat stress and HMSeBA supplementation on the protein expression of GPX1, GPX4 and SELENOS in liver. The results were expressed as mean ± SEM (n = 4 or 6). Different letters denote significant differences (P < 0.05)
Discussion
CHS negatively affects animals in various aspects including physiology, oxidative and metabolism balance. In this study, it was hypothesized that HMSeBA supplementation may improve liver antioxidant capacity and mitigate liver metabolic dysfunction induced by CHS. As expected, all pigs housed in the hyperthermal environment showed a significantly higher RT and RR which are typical symptoms of CHS and have been recorded in several studies about the HS animal model [14, 34]. Similar to the results reported previously [14], compared with the CHS group, high dietary HMSeBA supplementation (0.6 mg Se/kg) numerically decreased the RT of pigs during the middle and late period of the trial, which may be related to the reduced total heat production in high Se supplementation group.
Heat shock proteins (HSPs) are ubiquitous and strongly induced by heat shock which is usually categorized according to their molecular weight [35]. Among the HSPs, HSP70 is frequently used to evaluate HS response, which is considered as a cellular thermometer [36]. As expected, in this study the hepatic HSP70 protein abundance was increased in 4 CHS exposure groups, which is consistent with our previous study [15, 16]. HSP70 performs multiple roles which are important for maintaining cell survival during hyperthermia [35]. The up-regulation expression of the HSP70 helps restore unfolded or misfolded proteins to native conformation under HS condition [37]. Se supplementation effectively alleviates the decreased cell viability by HS and increases the protein abundance of HSP70 under HS in IPEC-J2 cells [15]. Similar to the previous study, 0.4 and 0.6 mg Se/kg HMSeBA supplementation numerically elevated the HSP70 protein level in the liver (Fig. 3), and this response may have potential beneficial effect for hepatic cells under CHS.
It is reported that CHS led to hepatic injury accompany the change in the expression of proteins that are mainly involved in oxidative stress response [12]. Consistently, CHS decreased hepatic GSH-Px activity and increased hepatic MDA level (Fig. 4). GSH-Px, T-SOD and T-AOC are important enzymes involved in the cell antioxidant system [16] and MDA is usually used as a biomarker to assess oxidative stress in an organism [38]. These results indicate that CHS weakened the antioxidant systems and led to oxidative stress response in the liver. The hepatic Se deposition and GSH-Px activity was elevated with the increased HMSeBA supplementation, and the MDA content was also decreased under CHS (Figs. 2 and 4). As we know, tissue GPX-Px is always used as an indicator of body selenium status. Therefore, it is not difficult to understand the relationship between the live Se concentration and hepatic GPX-Px activity in current study. In summary, HMSeBA supplementation beyond requirement relieves the hepatic antioxidant damage of pigs suffered from CHS.
In the current study, CHS led to significant decrease in liver weight and index of growing pigs (Fig. 2a and b), which is consistently to the previous report in growing pigs [12]. Meanwhile, the hepatic glycogen was depleted in CHS pigs which implied morbid liver function. It has been reported that CHS induced liver injury occurs in parallel with serum biochemical abnormalities and metabolic dysfunction [4, 34]. It is unexpected that 0.4 and 0.6 mg Se/kg HMSeBA supplementation significantly increased the live glycogen content under CHS. Se ameliorates the chronic liver injury by altering the serum biochemical indices [39]. Although the information about the effects of Se on liver metabolic functions of pigs under CHS is limited, it has been credited that Se has insulin-mimetic and anti-diabetic properties [17], and selenite affects the expression of liver glucose metabolism enzymes in a diabetic rat model [40]. HS affects metabolic function, which decreases serum F-insulin, T3 and T4 concentration and elevates the insulin sensitive [6, 9]. In this study, CHS led to an increase of serum AST, TBA, LDL-C, concentration and a decrease of serum F-insulin, T3, T4 and hepatic GCK level (Table 1; Fig. 5b). Serum ASL is an indicator of liver injury and the increase of serum ASL reflects the magnitude of liver damage [41]. Serum TBA is a highly sensitive marker for liver injury and dysfunction due to minor liver damage cause an increase of serum TBA [42]. LDL-C is the main resource of cholesterol from blood to the liver and liver is the essential organ in cholesterol synthesis and metabolism [43]. GCK is the first and rate-limiting step of glycolysis which catalyzes the conversion of glucose to glucose 6-phosphate in hepatocytes, more importantly, GCK participates in glycogen synthesis [44]. Our results suggest that severe liver injury and functional disruptions of substrates metabolism occurred in the liver. The liver metabolism function under CHS was partially normalized by Se. HMSeBA supplementation partially assuaged these negative reactions of CHS and mostly represented in the decrease of serum AST and LDL-C (Table 1) and an increase of hepatic GCK level (Fig. 5b). Also, the protective effects could be confirmed by the recovery of liver weight, liver index and glycogen content by 0.4 and 0.6 mg Se/kg HMSeBA supplementation (Fig. 2). Therefore, HMSeBA supplementation enhanced the glycogen synthesis in the liver of growing pigs under CHS.
We examined the expression profile of the genes related to hepatic energy homeostasis, protein synthesis, glucose and lipid metabolism. CHS up-regulated the mRNA expression of AMPKα1, 4E-BP1, INSR and down-regulated mRNA expression of FASN, SREBP1 and especially the GCK (Fig. 6). Under energy imbalance or stress, AMPK is activated by phosphorylation to cope with the adverse environments and switch on the catabolic pathway and inhibit anabolic [45]. Dephosphorylated 4E-BP1 binds to elF4E and inhibits protein translation function [46]. FASN and SREBP1 carry an important role in liver lipogenesis [18]. It is not difficult to understand the decreased mRNA expression of FASN and SREBP1 under a negative energy balance in CHS, and appropriative HMSeBA supplementation may affect these two genes through the optimization hepatic energy metabolism. The increased mRNA expression of INSR may interpret the increased insulin sensitivity under HS [9]. Overall, the aberrant responses of these genes in the liver reflect the abnormal metabolic function of pigs under CHS. HMSeBA supplementation moderately recovered the expression of these affected genes, indicating a protective effect.
Se exerts important roles in energy metabolism [7, 18, 21], and the biological functions of Se are mainly mediated by selenoproteins. Expressions of nearly all porcine selenoprotein genes are responsive to dietary Se intakes of pigs in different tissues, and 9 hepatic selenoprotein encoding genes (GPX1, GPX3, GPX4, TXNRD1, SELENOS, SELENOP, SELENOT, SELENOW and SELENOH) of pigs are altered by Se deficiency or excess [18, 22, 23, 47–51]. In this study, selenoprotein encoding genes performed 4 patterns in the liver in response to CHS and Se supplementation.
Firstly, 10 selenoprotein encoding genes were up-regulated by CHS, while all of them were down-regulated by supplementation with 0.6 mg Se/kg HMSeBA, and 8 and 2 of them were down-regulated by 0.4 or 0.2 mg Se/kg HMSeBA (Fig. 7a). Among these affected genes, GPX1, GPX3, GPX4, SELENOP and SELENOH have been shown to be related to insulin signaling, glycolysis, gluconeogenesis, lipogenesis and protein synthesis pathway in pig tissues [18, 22, 23, 52]. The up-regulation of these genes under CHS is consistent with the previous study [53], and is associated with the decreased gene expression of GCK, FASN and SREBP1 in rat liver [19, 20, 54]. The increased SELENOS mRNA level is along with the up-regulation of INSR in rat liver [24]. The function of SELENOT, SELENOI and SELENOK in porcine remains unclear. Currently, SELENOI may be connected to the synthesis of steroid hormones and proteins for the plasma membrane of humans [55]. SELENOK and SELENOT are involved in redox metabolism regulation [56, 57]. Therefore, the abnormal up-regulation of these selenoproteins might be related to the CHS induced hepatic metabolism disorder. Among the above mentioned selenoprotein encoding genes affected by CHS, GPX1, GPX3, GPX4, SELENOS, SELENOP and SELENOH are up-regulated with the increased Se supplementation in liver [18, 19, 22, 47, 50]. The mRNA levels of SELENOK, SELENOT, and SEPHS2 in liver or other organs of pigs or rodents are somewhat resistant to dietary Se deficiency or excess [19, 22, 49]. Additionally, in multiple porcine tissues (pituitary, spleen, and thyroid) both dietary Se deficiency and excess decrease the expression of SELENOI [22]. However, in current study, the expression of these hepatic selenoproteins decreased with the increase of dietary Se content under CHS. Our previous studies in vitro also found the similar phenomenon, HS up-regulates most selenoprotein encoding genes in IPEC-J2 cells [15] and differentiated C2C12 cells [53, 58]. The down-regulation of these genes may be a special hierarchical response of Se to alleviate the CHS induced hepatic damage and promoted normalization of metabolism function.
The second pattern was that CHS down-regulated the mRNA expression of DIO1 and SELENOM in the liver, and HMSeBA supplementation partially increased the mRNA expression of these two genes (Fig. 7b). DIO1 is an oxidoreductase with SeCys residue in the active site and participate in thyroid hormone metabolism [59] and the decrease of mRNA abundance of DIO1 is associated with insulin resistance, lipogenesis and protein synthesis in skeletal muscle or liver of pigs [18]. Se counter thyrotoxicity of Di-(2-ethylhexyl) phthalate (DEHP) via elevating DIO1, and elevates plasma T3 and T4 that was decreased by DEHP in rats [60]. Our results showed that CHS significantly decreased the serum T3 and T4, however, HMSeBA supplementation does not show significant effect under CHS, and this maybe an adaptive response to CHS. The hepatic DIO1 mRNA expression response to CHS and HMSeBA supplementation may reflect a local organ reaction. Additionally, porcine DIO1 transcripts is unaltered by dietary Se deficiency in the liver [22], and DIO1 mRNAs levels decrease less than that of GPX1 mRNA in a Se deficiency rodents, because DIO1 has a relatively high hierarchy of Se status [61]. Therefore, the decreased DIO1 expression in current study is mainly caused by CHS, and the restoration of endocrine and gene expression may be related to the systemic remission of CHS.SELENOM is a thiol-disulfide oxidoreductase and participates in the protection against superoxide and regulation of apoptosis [62]. The expression of porcine splenic SELENOM is up-regulated by a moderately high intake of dietary Se [49]. The down-regulation of this gene may reflect hepatic oxidative injury and metabolism dysfunction induced by CHS.
The third pattern was that CHS had no effects on mRNA expression of TXNRD2, SELENOW and SELENON, while HMSeBA supplementation moderately enhances their mRNA expression and CHS + 0.4 HMSeBA group exhibited a higher profile (Fig. 7c). TXNRD2 has been reported to protect cells from oxidant stress during embryogenesis [63]. SELENOW also has an antioxidant function and overexpression of SELENOW enhance the resistance of hamster ovary and lung cancer cells on H2O2 cytotoxicity [64] SELENON is ubiquitously expressed in muscle, brain, lung and fetal tissue. Although its specific biological function remains unclear, this selenoprotein plays a key role in the proliferation of fibroblast [65]. The expression of porcine splenic SELENON is up-regulated by a high dietary Se supplementation [49], and dietary Se deficiency decreases expression of hepatic SELENOW of pigs [22]. However, mRNA levels of TXNRD2 in tissues or cells of pigs are not sensitive to dietary Se deficiency or excess [22, 49]. Even though the response of these three selenoproteins encoding genes in this study is a litter different from the results of previous studies on pigs and rats, which may reflect that the changed in the synthesis hierarchy of the selenoproteins in HS pig liver, and increased mRNA expression of these genes may contribute to the enhanced antioxidant capacity in the liver. Additionally, the response of SELENOF, TXNRD1, SELENOO and MSRB1 were insensitive to CHS and HMSeBA supplementation (Fig. 7d).
Taken together, the alternation of the selenogenome and associated metabolism genes expression may be a symptom of lack of metabolic homeostasis under CHS. Dietary HMSeBA supplementation (0.4 or 0.6 mg Se/kg) recovered the most profiles of mRNA of selenoproteins in the liver of hyperthermia stressed pigs, which profiles were much similar to that of control pigs. These results suggested that Se alleviates the CHS induced metabolic disorder in the liver of growing pigs mainly by regulating the expression of selenoproteins.
For further determine the relationship between key selenoproteins and metabolism functions, the protein abundance of GPX1, GPX4 and SELENOS were detected. As shown in Fig. 8, CHS decreased the protein expression of GPX4 and SELENOS, and HMSeBA supplementation recovered or further increased the protein abundances of GPX4, SELENOS and GPX1. The selenoprotein response to CHS and dietary Se were inconsistent to the mRNA expression, which may result from the complicated regulation in the transcription, mRNA decay, translation, amino acid properties, and protein degradation [66, 67], and possibly other processes. HS is usually accompanied by severe oxidative stress [13]. GPX1 and GPX4 belong to antioxidant enzyme, the increase of their protein abundance suggests enhancement of hepatic antioxidant capacity in the stressed pigs. SELENOS is an important endoplasmic reticulum transmembrane protein, and overexpression of SELENOS enhances cells’ resistance to oxidative stress [68]. CHS decreased mRNA level, protein abundance (Fig. 6) and activity of hepatic GCK (Fig. 5). GCK catalyzes the conversion of glucose to G-6-P, which modulates glycogen synthesis in liver [44]. The rescued GCK in the liver suggest the mitigation of hepatic metabolic disorder. The protective effect of HMSeBA is mainly through regulation of selenoprotein. In current study, dietary HMSeBA enhanced the protein abundance of GPX1, which is positive correlation with the activity of GCK [20]. In addition, HMSeBA supplementation up-regulated p-AMPKα (Fig. 6e), and the elevation of GPX1 activity increases the hepatic p-AMPKα [18]. AMPK is activated by phosphorylation of the catalytic subunit then regulate the metabolic processes [69]. Therefore, dietary HMSeBA supplementation optimized the hepatic metabolic state under CHS through regulation of selenoproteins and the process is related to the activation of the AMPK signal.
Conclusions
In summary, in present study, CHS causes metabolic disorders in the liver of growing pigs, which is accompanied by alteration of physiological parameters, depleted hepatic glycogen and aberrant expression of selenoprotein encoding genes and selenoprotein. HMSeBA supplementation alleviates the CHS-induced negative effects to the liver with the enhancement of antioxidant capability. More importantly, supplementation with dietary HMSeBA beyond nutrient requirements (0.4 and 0.6 mg/kg) effectively recovers the most profiles of mRNA and proteins of selenoproteins in the liver of pigs suffered from CHS, which profiles are much similar to that of control pigs accompanied by the hepatic glycogen content significantly recovered, thus corresponding hepatic metabolic disorder are alleviated. Especially, the protective effects of HMSeBA are associated with the activation of AMPK signal.
Supplementary Information
Additional file 1: Supplemental Table 1. Primers used for the q-PCR of the target and reference genes.
Acknowledgements
We thank Hua Li, Ruinan Zhang for their technical support in this study.
Abbreviations
- CHS
Chronic heat stress
- Se
selenium
- HMSeBA
Hydroxy-4-methylselenobutanoic acid
- AST
Aspartate aminotransferase
- LDL-C
Low-density lipoprotein cholesterol
- ALT
Alanine transaminase
- TBA
Total bile acid
- TP
Total protein
- GLU
Glucose
- TG
Total triglycerides
- CHO
Cholesterol
- NEFA
Non-esterified acid
- F-insulin
Fasting insulin
- HSP70
Heat shock protein 70
- T-SOD
Total superoxide dismutase
- MDA
Malondialdehyde
- GSH-Px
Glutathione peroxidase
- T-AOC
Total antioxidant capability
- GCK
Glucokinase
- PEPCK
Phosphoenolpyruvate carboxykinase
- FAS
Fatty acid synthase
- AMPKα
AMP-activated protein kinase alpha
- p-AMPKα
Phospho- AMP-activated protein kinase alpha
- PCK2
Phosphoenolpyruvate carboxykinase 2
- IRS1
Insulin receptor substrate 1
- INSR
Insulin receptor
- mTOR
Mammalian target of rapamycin
- 4E-BP1
4 E-binding protein
- AKT1
Serine/threonine-protein kinase
- PPARG
Peroxisome proliferative activated receptor, gamma
- ACC1
Acetyl coenzyme a carboxylase 1
- SREBP1
Sterol-regulatory element binding protein 1
Authors’ contributions
The author contributions are as follows: Y. L., J. T. and H. Z. conceived and designed the experimental plan. Y. L., J. T. and Y. H. were involved in the animal experiments, analysis and data collection. Y. L., J. T. and Y. H. analyzed the data and drafted the original manuscript. G. J., G. L., G. T., X. C., J. C., B. K. and H. Z. made a revision of this manuscript. All authors read and approved the final manuscript.
Funding
This work was supported partly by the National Natural Science Foundation of China (No. 31772643), and the Special Research Funding for Discipline Construction in Sichuan Agricultural University (No. 03570126).
Availability of data and materials
The datasets produced and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The experiment followed the actual law of animal protection and was approved by the Animal Care and Use Committee of the Sichuan Agricultural University (Ethic Approval Code: SCAUAC201808–1).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Footnotes
Yan Liu and Jiayong Tang contributed equally to this work.
References
- 1.Hoegh-Guldberg O, Jacob D, Taylor M, Guillén Bolaños T, Bindi M, Brown S, Camilloni IA, Diedhiou A, Djalante R, Ebi K, Engelbrecht F, Guiot J, Hijioka Y, Mehrotra S, Hope CW, Payne AJ, Pörtner HO, Seneviratne SI, Thomas A, Warren R, Zhou G. The human imperative of stabilizing global climate change at 1.5°C. Science. 2019;365(6459):eaaw6974. doi: 10.1126/science.aaw6974. [DOI] [PubMed] [Google Scholar]
- 2.St-Pierre NR, Cobanov B, Schnitkey G. Economic losses from heat stress by US livestock industries. J Dairy Sci. 2003;86:E52–E77. doi: 10.3168/jds.S0022-0302(03)74040-5. [DOI] [Google Scholar]
- 3.Collier RJ, Renquist BJ, Xiao Y. A 100-year review: stress physiology including heat stress. J Dairy Sci. 2017;100(12):10367–10380. doi: 10.3168/jds.2017-13676. [DOI] [PubMed] [Google Scholar]
- 4.Pearce SC, Gabler NK, Ross JW, Escobar J, Patience JF, Rhoads RP, Baumgard LH. The effects of heat stress and plane of nutrition on metabolism in growing pigs. J Anim Sci. 2013;91(5):2108–2118. doi: 10.2527/jas.2012-5738. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez-Rivas PA, Chauhan SS, Ha M, Fegan N, Dunshea FR, Warner RD. Effects of heat stress on animal physiology, metabolism, and meat quality: a review. Meat Sci. 2020;162:108025. doi: 10.1016/j.meatsci.2019.108025. [DOI] [PubMed] [Google Scholar]
- 6.Seibert JT, Abuajamieh M, Sanz Fernandez MV, Johnson JS, Kvidera SK, Horst EA, Mayorga EJ, Lei S, Patience JF, Ross JW, Rhoads RP, Johnson RC, Lonergan SM, Perfield JW, II, Baumgard LH. Effects of heat stress and insulin sensitizers on pig adipose tissue. J Anim Sci. 2018;96(2):510–520. doi: 10.1093/jas/skx067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu F, Cottrell JJ, Wijesiriwardana U, Kelly FW, Chauhan SS, Pustovit RV, Gonzales-Rivas PA, DiGiacomo K, Leury BJ, Celi P, Dunshea FR. Effects of chromium supplementation on physiology, feed intake, and insulin related metabolism in growing pigs subjected to heat stress. Transl Anim Sci. 2017;1(1):116–125. doi: 10.2527/tas2017.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boddicker RL, Seibert JT, Johnson JS, Pearce SC, Selsby JT, Gabler NK, Lucy MC, Safranski TJ, Rhoads RP, Baumgard LH, Ross JW. Gestational heat stress alters postnatal offspring body composition indices and metabolic parameters in pigs. PLoS One. 2014;9(11):e110859. doi: 10.1371/journal.pone.0110859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Victoria Sanz Fernandez M, Johnson JS, Abuajamieh M, Stoakes SK, Seibert JT, Cox L, et al. Effects of heat stress on carbohydrate and lipid metabolism in growing pigs. Physiol Rep. 2015;3(2):e12315. 10.14814/phy2.12315. [DOI] [PMC free article] [PubMed]
- 10.Roncal-Jimenez CA, Sato Y, Milagres T, Andres Hernando A, García G, Bjornstad P, Dawson JB, Sorensen C, Newman L, Krisher L, Madero M, Glaser J, Gárcía-Trabanino R, Romero EJ, Song Z, Jensen T, Kuwabara M, Rodriguez-Iturbe B, Sanchez-Lozada LG, Lanaspa MA, Johnson RJ. Experimental heat stress nephropathy and liver injury are improved by allopurinol. Am J Physiol Renal Physiol. 2018;315(3):F726–Ff33. doi: 10.1152/ajprenal.00543.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcin JM, Bronstein JA, Cremades S, Courbin P, Cointet F. Acute liver failure is frequent during heat stroke. World J Gastroenterol. 2008;14(1):158–159. doi: 10.3748/wjg.14.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui Y, Hao Y, Li J, Bao W, Li G, Gao Y, et al. Chronic heat stress induces immune response, oxidative stress response, and apoptosis of finishing pig liver: a proteomic approach. Int J Mol Sci. 2016;17(5):393. 10.3390/ijms17050393. [DOI] [PMC free article] [PubMed]
- 13.Liu F, Cottrell JJ, Furness JB, Rivera LR, Kelly FW, Wijesiriwardana U, Pustovit RV, Fothergill LJ, Bravo DM, Celi P, Leury BJ, Gabler NK, Dunshea FR. Selenium and vitamin E together improve intestinal epithelial barrier function and alleviate oxidative stress in heat-stressed pigs. Exp Physiol. 2016;101(7):801–810. doi: 10.1113/EP085746. [DOI] [PubMed] [Google Scholar]
- 14.Liu F, Celi P, Cottrell JJ, Chauhan SS, Leury BJ, Dunshea FR. Effects of a short-term supranutritional selenium supplementation on redox balance, physiology and insulin-related metabolism in heat-stressed pigs. J Anim Physiol Anim Nutr. 2018;102(1):276–285. doi: 10.1111/jpn.12689. [DOI] [PubMed] [Google Scholar]
- 15.Tang JY, Cao L, Jia G, Liu GM, Chen XL, Tian G, Cai J, Shang H, Zhao H. The protective effect of selenium from heat stress-induced porcine small intestinal epithelial cell line (IPEC-J2) injury is associated with regulation expression of selenoproteins. Brit J Nutr. 2019;122(10):1081–1090. doi: 10.1017/S0007114519001910. [DOI] [PubMed] [Google Scholar]
- 16.Tang JY, Wang LQ, Jia G, Liu GM, Chen XL, Tian G, Cai JY, Shang HY, Zhao H. The hydroxy-analogue of selenomethionine alleviated lipopolysaccharide-induced inflammatory responses is associated with recover expression of several selenoprotein encoding genes in the spleens of Kunming mice. RSC Adv. 2019;9(69):40462–40470. doi: 10.1039/C9RA07260H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinto A, Juniper DT, Sanil M, Morgan L, Clark L, Sies H, Rayman MP, Steinbrenner H. Supranutritional selenium induces alterations in molecular targets related to energy metabolism in skeletal muscle and visceral adipose tissue of pigs. J Inorg Biochem. 2012;114:47–54. doi: 10.1016/j.jinorgbio.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Zhao Z, Barcus M, Kim J, Lum KL, Mills C, Lei XG. High dietary selenium intake alters lipid metabolism and protein synthesis in liver and muscle of pigs. J Nutr. 2016;146(9):1625–1633. doi: 10.3945/jn.116.229955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen XD, Zhao ZP, Zhou JC, Lei XG. Evolution, regulation, and function of porcine selenogenome. Free Radic Biol Med. 2018;127:116–123. doi: 10.1016/j.freeradbiomed.2018.04.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan X, Pepper MP, Vatamaniuk MZ, Roneker CA, Li L, Lei XG. Dietary selenium deficiency partially rescues type 2 diabetes-like phenotypes of glutathione peroxidase-1-overexpressing male mice. J Nutr. 2012;142(11):1975–1982. doi: 10.3945/jn.112.164764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou J, Huang KX, Lei XG. Selenium and diabetes--evidence from animal studies. Free Radic Biol Med. 2013;65:1548–1556. doi: 10.1016/j.freeradbiomed.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Zhao H, Zhang Q, Tang J, Li K, Xia XJ, Wang KN, Li K, Lei XG. Prolonged dietary selenium deficiency or excess does not globally affect selenoprotein gene expression and/or protein production in various tissues of pigs. J Nutr. 2012;142(8):1410–1416. doi: 10.3945/jn.112.159020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou JC, Zhao H, Li JG, Xia XJ, Wang KN, Zhang YJ, Liu Y, Zhao Y, Lei XG. Selenoprotein gene expression in thyroid and pituitary of young pigs is not affected by dietary selenium deficiency or excess. J Nutr. 2009;139(6):1061–1066. doi: 10.3945/jn.109.104901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeng MS, Li X, Liu Y, Zhao H, Zhou JC, Li K, Huang JQ, Sun LH, Tang JY, Xia XJ, Wang KN, Lei XG. A high-selenium diet induces insulin resistance in gestating rats and their offspring. Free Radic Biol Med. 2012;52(8):1335–1342. doi: 10.1016/j.freeradbiomed.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang XD, Vatamaniuk MZ, Wang SK, Roneker CA, Simmons RA, Lei XG. Molecular mechanisms for hyperinsulinaemia induced by overproduction of selenium-dependent glutathione peroxidase-1 in mice. Diabetologia. 2008;51(8):1515–1524. doi: 10.1007/s00125-008-1055-3. [DOI] [PubMed] [Google Scholar]
- 26.Cervantes M, Cota M, Arce N, Castillo G, Avelar E, Espinoza S, Morales A. Effect of heat stress on performance and expression of selected amino acid and glucose transporters, HSP90, leptin and ghrelin in growing pigs. J Therm Biol. 2016;59:69–76. doi: 10.1016/j.jtherbio.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 27.Miller ER, Ullrey DE. The pig as a model for human nutrition. Annu Rev Nutr. 1987;7(1):361–382. doi: 10.1146/annurev.nu.07.070187.002045. [DOI] [PubMed] [Google Scholar]
- 28.Chao YM, Yu B, He J, Huang ZQ, Mao XB, Luo JQ, Luo Y, Zheng P, Yu J, Chen D. Effects of different levels of dietary hydroxy-analogue of selenomethionine on growth performance, selenium deposition and antioxidant status of weaned piglets. Arch Anim Nutr. 2019;73(5):374–383. doi: 10.1080/1745039X.2019.1641368. [DOI] [PubMed] [Google Scholar]
- 29.Ou JZ, Cottrell JJ, Ha N, Pillai N, Yao CK, Berean KJ, et al. Potential of in vivo real-time gastric gas profiling: a pilot evaluation of heat-stress and modulating dietary cinnamon effect in an animal model. Sci Rep. 2016;6:33387. doi: 10.1038/srep33387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X, Wu YS, Li BQ, Yang YH, Yang YP. Selenium accumulation characteristics and biofortification potentiality in Turnip (Brassica rapa var. rapa) supplied with selenite or selenate. Front Plant Sci. 2017;8:2207. doi: 10.3389/fpls.2017.02207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang XF, Tang JY, Xu JY, Jia G, Liu GM, Chen XL, Cai J, Shang H, Zhao H. Supranutritional dietary selenium induced hyperinsulinemia and dyslipidemia via affected expression of selenoprotein genes and insulin signal-related genes in broiler. RSC Adv. 2016;6(88):84990–84998. doi: 10.1039/C6RA14932D. [DOI] [Google Scholar]
- 32.Wang LQ, Jing JZ, Yan H, Tang JY, Jia G, Liu GM, et al. Selenium pretreatment alleviated LPS-Induced immunological stress via upregulation of several selenoprotein encoding genes in murine RAW264.7 cells. Biol Trace Elem Res. 2018;186:505–513. doi: 10.1007/s12011-018-1333-y. [DOI] [PubMed] [Google Scholar]
- 33.Saxton AM. Proc Mixed Proc 23rd SAS Users Group International. Cary: SAS Institute; 1998. A macro for converting mean separation output to letter groupings; pp. 1243–1246. [Google Scholar]
- 34.Mendoza SM, Boyd RD, Ferket PR, van Heugten E. Effects of dietary supplementation of the osmolyte betaine on growing pig performance and serological and hematological indices during thermoneutral and heat-stressed conditions. J Anim Sci. 2017;95(11):5040–5053. doi: 10.2527/jas2017.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475(7356):324–332. doi: 10.1038/nature10317. [DOI] [PubMed] [Google Scholar]
- 36.Jacob P, Hirt H, Bendahmane A. The heat-shock protein/chaperone network and multiple stress resistance. Plant Biotechnol J. 2017;15(4):405–414. doi: 10.1111/pbi.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bouchama A, Knochel JP. Heat stroke. N Engl J Med. 2002;346(25):1978–1988. doi: 10.1056/NEJMra011089. [DOI] [PubMed] [Google Scholar]
- 38.Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 2005;15(4):316–328. doi: 10.1016/j.numecd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 39.Hamid M, Abdulrahim Y, Liu D, Qian G, Khan A, Huang K. The hepatoprotective effect of selenium-enriched yeast and gum arabic combination on carbon tetrachloride-induced chronic liver injury in rats. J Food Sci. 2018;83(2):525–534. doi: 10.1111/1750-3841.14030. [DOI] [PubMed] [Google Scholar]
- 40.Becker DJ, Reul B, Ozcelikay AT, Buchet JP, Henquin JC, Brichard SM. Oral selenate improves glucose homeostasis and partly reverses abnormal expression of liver glycolytic and gluconeogenic enzymes in diabetic rats. Diabetologia. 1996;39(1):3–11. doi: 10.1007/BF00400407. [DOI] [PubMed] [Google Scholar]
- 41.Alzeer AH, el-Hazmi MA, Warsy AS, Ansari ZA, Yrkendi MS. Serum enzymes in heat stroke: prognostic implication. Clin Chem. 1997;43(7):1182–1187. doi: 10.1093/clinchem/43.7.1182. [DOI] [PubMed] [Google Scholar]
- 42.Yamazaki M, Miyake M, Sato H, Masutomi N, Tsutsui N, Adam KP, Alexander DC, Lawton KA, Milburn MV, Ryals JA, Wulff JE, Guo L. Perturbation of bile acid homeostasis is an early pathogenesis event of drug induced liver injury in rats. Toxicol Appl Pharmacol. 2013;268(1):79–89. doi: 10.1016/j.taap.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 43.North KE, Göring HH, Cole SA, Diego VP, Almasy L, Laston S, et al. Linkage analysis of LDL cholesterol in American Indian populations: the strong heart family study. J Lipid Res. 2006;47(1):59–66. doi: 10.1194/jlr.M500395-JLR200. [DOI] [PubMed] [Google Scholar]
- 44.Ferre T, Riu E, Bosch F, Valera A. Evidence from transgenic mice that glucokinase is rate limiting for glucose utilization in the liver. FASEB J. 1996;10(10):1213–1218. doi: 10.1096/fasebj.10.10.8751724. [DOI] [PubMed] [Google Scholar]
- 45.Hardie DG, Carling D, Carlson M. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu Rev Biochem. 1998;67(1):821–855. doi: 10.1146/annurev.biochem.67.1.821. [DOI] [PubMed] [Google Scholar]
- 46.Yoshihara T, Naito H, Kakigi R, Ichinoseki-Sekine N, Ogura Y, Sugiura T, Katamoto S. Heat stress activates the Akt/mTOR signalling pathway in rat skeletal muscle. Acta Physiol (Oxf) 2013;207(2):416–426. doi: 10.1111/apha.12040. [DOI] [PubMed] [Google Scholar]
- 47.Li JG, Zhou JC, Zhao H, Lei XG, Xia XJ, Gao G, Wang KN. Enhanced water-holding capacity of meat was associated with increased Sepw1 gene expression in pigs fed selenium-enriched yeast. Meat Sci. 2011;87(2):95–100. doi: 10.1016/j.meatsci.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 48.Liu T, Yang T, Pan T, Liu C, Li S. Effect of low-selenium/high-fat diet on pig peripheral blood lymphocytes: perspectives from Selenoproteins, heat shock proteins, and cytokines. Biol Trace Elem Res. 2018;183(1):102–113. doi: 10.1007/s12011-017-1122-z. [DOI] [PubMed] [Google Scholar]
- 49.Lu Z, Wang P, Teng T, Shi B, Shan A, Lei XG. Effects of Dietary Selenium Deficiency or Excess on Selenoprotein Gene Expression in the Spleen Tissue of Pigs. Animals (Basel). 2019;9(12):1122. 10.3390/ani9121122. [DOI] [PMC free article] [PubMed]
- 50.Zhang K, Zhao Q, Zhan T, Han Y, Tang C, Zhang J. Effect of different selenium sources on growth performance, tissue selenium content, meat quality, and Selenoprotein gene expression in finishing pigs. Biol Trace Elem Res. 2020;196(2):463–471. doi: 10.1007/s12011-019-01949-3. [DOI] [PubMed] [Google Scholar]
- 51.Zhang K, Han Y, Zhao Q, Zhan T, Li Y, Sun W, Li S, Sun D, Si X, Yu X, Qin Y, Tang C, Zhang J. Targeted metabolomics analysis reveals that dietary Supranutritional selenium regulates sugar and Acylcarnitine metabolism homeostasis in pig liver. J Nutr. 2020;150(4):704–711. doi: 10.1093/jn/nxz317. [DOI] [PubMed] [Google Scholar]
- 52.Zhao H, Li K, Tang JY, Zhou JC, Wang KN, Xia XJ, Lei XG. Expression of selenoprotein genes is affected by obesity of pigs fed a high-fat diet. J Nutr. 2015;145(7):1394–1401. doi: 10.3945/jn.115.211318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang JY, He AH, Yan H, Jia G, Liu GM, Chen XL, et al. Damage to the myogenic differentiation of C2C12 cells by heat stress is associated with up-regulation of several selenoproteins. Sci Rep. 2018;8:1–9. doi: 10.1038/s41598-018-29012-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mueller AS, Klomann SD, Wolf NM, Schneider S, Schmidt R, Spielmann J, Stangl G, Eder K, Pallauf J. Redox regulation of protein tyrosine phosphatase 1B by manipulation of dietary selenium affects the triglyceride concentration in rat liver. J Nutr. 2008;138(12):2328–2336. doi: 10.3945/jn.108.089482. [DOI] [PubMed] [Google Scholar]
- 55.Ahmed MY, Al-Khayat A, Al-Murshedi F, Al-Futaisi A, Chioza BA, Pedro Fernandez-Murray J, et al. A mutation of EPT1 (SELENOI) underlies a new disorder of Kennedy pathway phospholipid biosynthesis. Brain. 2017;140(3):547–554. doi: 10.1093/brain/aww318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dikiy A, Novoselov SV, Fomenko DE, Sengupta A, Carlson BA, Cerny RL, Ginalski K, Grishin NV, Hatfield DL, Gladyshev VN. SelT, SelW, SelH, and Rdx12: genomics and molecular insights into the functions of selenoproteins of a novel thioredoxin-like family. Biochemistry. 2007;46(23):6871–6882. doi: 10.1021/bi602462q. [DOI] [PubMed] [Google Scholar]
- 57.Dudkiewicz M, Szczepińska T, Grynberg M, Pawłowski K. A novel protein kinase-like domain in a selenoprotein, widespread in the tree of life. Plos One. 2012;7(2):e32138. doi: 10.1371/journal.pone.0032138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu Y, He A, Tang J, Shah AM, Jia G, Liu G, Tian G, Chen X, Cai J, Kang B, Zhao H. Selenium alleviates the negative effect of heat stress on myogenic differentiation of C2C12 cells with the response of selenogenome. J Therm Biol. 2021;97:102874. doi: 10.1016/j.jtherbio.2021.102874. [DOI] [PubMed] [Google Scholar]
- 59.Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev. 2002;23(1):38–89. doi: 10.1210/edrv.23.1.0455. [DOI] [PubMed] [Google Scholar]
- 60.Zhang P, Guan X, Yang M, Zeng L, Liu C. Roles and potential mechanisms of selenium in countering thyrotoxicity of DEHP. Sci Total Environ. 2018;619:732–739. doi: 10.1016/j.scitotenv.2017.11.169. [DOI] [PubMed] [Google Scholar]
- 61.Sunde RA. Selenoproteins: hierarchy, requirements, and biomarkers. Selenium: Springer; 2011. pp. 137–152. [Google Scholar]
- 62.Ferguson AD, Labunskyy VM, Fomenko DE, Araç D, Chelliah Y, Amezcua CA, et al. NMR structures of the selenoproteins Sep15 and SelM reveal redox activity of a new thioredoxin-like family. J Biol Chem. 2006;281:3536–43. 10.1074/jbc.M511386200. [DOI] [PubMed]
- 63. Conrad M, Jakupoglu C, Moreno SG, Lippl S, Banjac A, Schneider M, et al. Essential role for mitochondrial thioredoxin reductase in hematopoiesis, heart development, and heart function. Mol Cell Biol. 2004;24:9414–23. 10.1128/MCB.24.21.9414-9423.2004. [DOI] [PMC free article] [PubMed]
- 64. Jeong D, Kim TS, Chung YW, Lee BJ, Kim IY. Selenoprotein W is a glutathione-dependent antioxidant in vivo. FEBS Lett. 2002;517:225–8. 10.1016/S0014-5793(02)02628-5. [DOI] [PubMed]
- 65.Deniziak M, Thisse C, Rederstorff M, Hindelang C, Thisse B, Lescure A. Loss of selenoprotein N function causes disruption of muscle architecture in the zebrafish embryo. Exp Cell Res. 2007;313:156–67. 10.1016/j.yexcr.2006.10.005. [DOI] [PubMed]
- 66.Maier T, Güell M, Serrano L. Correlation of mRNA and protein in complex biological samples. FEBS Lett. 2009;583:3966–73. 10.1016/j.febslet.2009.10.036. [DOI] [PubMed]
- 67.Greenbaum D, Colangelo C, Williams K, Gerstein M. Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol. 2003;4:117. 10.1186/gb-2003-4-9-117. [DOI] [PMC free article] [PubMed]
- 68.Gao Y, Feng HC, Walder K, Bolton K, Sunderland T, Bishara N, et al. Regulation of the selenoprotein SelS by glucose deprivation and endoplasmic reticulum stress - SelS is a novel glucose-regulated protein. FEBS Lett. 2004;563:185–90. 10.1016/S0014-5793(04)00296-0. [DOI] [PubMed]
- 69.Steinberg GR. AMPK and the endocrine control of energy metabolism. Mol Cell Endocrinol. 2013;366:125–6. 10.1016/j.mce.2013.01.003. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplemental Table 1. Primers used for the q-PCR of the target and reference genes.
Data Availability Statement
The datasets produced and/or analyzed during the current study are available from the corresponding author on reasonable request.