Abstract
Objective
We reviewed the literature on cerebrospinal fluid (CSF) testing in patients with altered olfactory/gustatory function due to COVID-19 for evidence of viral neuroinvasion.
Methods
We performed a systematic review of Medline and Embase to identify publications that described at least one patient with COVID-19 who had altered olfactory/gustatory function and had CSF testing performed. The search ranged from December 1, 2019 to November 18, 2020.
Results
We identified 51 publications that described 70 patients who met inclusion criteria. Of 51 patients who had CSF SARS-CoV-2 PCR testing, 3 (6%) patients had positive results and 1 (2%) patient had indeterminate results. Cycle threshold (Ct; the number of amplification cycles required for the target gene to exceed the threshold, which is inversely related to viral load) was not provided for the patients with a positive PCR. The patient with indeterminate results had a Ct of 37 initially, then no evidence of SARS-CoV-2 RNA on repeat testing. Of 6 patients who had CSF SARS-CoV-2 antibody testing, 3 (50%) were positive. Testing to distinguish intrathecal antibody synthesis from transudation of antibodies to the CSF via breakdown of the blood-brain barrier was performed in 1/3 (33%) patients; this demonstrated antibody transmission to the CSF via transudation.
Conclusion
Detection of SARS-CoV-2 in CSF via PCR or evaluation for intrathecal antibody synthesis appears to be rare in patients with altered olfactory/gustatory function. While pathology studies are needed, our review suggests it is unlikely that these symptoms are related to viral neuroinvasion.
Keywords: COVID-19, SARS-CoV-2, Anosmia, Ageusia, Taste, Smell, Cerebrospinal fluid
1. Introduction
Although the nasal cavity is believed to be the primary entry point for a number of respiratory viruses, SARS-CoV-2, the virus responsible for COVID-19, has uniquely been cited to cause frequent alteration in olfactory function, often in conjunction with gustatory dysfunction [1], [2]. These symptoms have been reported in 20–85% of patients with COVID-19 [1], [2]. The mechanism for altered olfactory and gustatory function remains unclear, but it has been postulated that this may be the result of viral neuroinvasion [1], [3], [4].
It is feasible to detect SARS-CoV-2 in the cerebrospinal fluid (CSF) via performance of PCR testing (the N2 gene target for SARS-CoV-2 PCR testing was noted to have the most sensitive limit of detection in CSF when compared with detection from nasopharyngeal swab, bronchoalveolar lavage, sputum, plasma or stool) [5]. However, the results of CSF SARS-CoV-2 PCR have not been systematically examined in a cohort of patients with altered olfactory and/or gustatory function. We sought to review CSF results in patients with COVID-19 who had altered olfactory and/or gustatory function to evaluate for evidence of viral neuroinvasion.
2. Methods
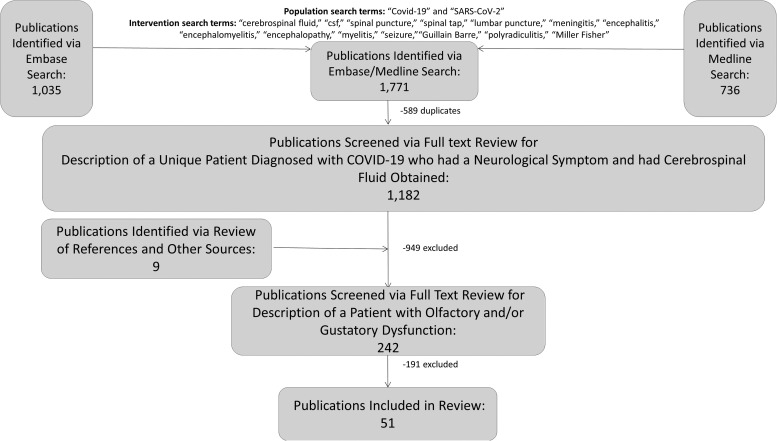
We previously identified publications in English from December 1, 2019 and November 18, 2020 that described a unique patient diagnosed with COVID-19 via SARS-CoV-2 PCR or serology who had a neurological symptom and had CSF obtained via a search of Medline and Embase using the population search terms “COVID-19” or “SARS-CoV-2” and the intervention search terms “cerebrospinal fluid” or “csf” or “spinal puncture” or “spinal tap” or “lumbar puncture” or “meningitis” or “encephalitis” or “encephalomyelitis” or “seizure” or “encephalopathy” or “myelitis” or “Guillain Barre” or “polyradiculitis” or “Miller Fisher.” [6] Publications were excluded if they 1) were not in English or 2) described a patient who had subarachnoid hemorrhage or meningitis/ventriculitis/encephalitis due to an infectious organism other than COVID-19. Two board-certified neurologists (AL and KM) independently performed full-text review of these documents to identify reports of patients who had altered olfactory and/or gustatory function. This search was performed in accordance with PRISMA guidelines ( Fig. 1) [7]. Cases were reviewed and organized based on CSF findings. All laboratory test results were converted to a common unit to facilitate comparison. Data collected for this study will be made available via email request to the corresponding author.
Fig. 1.
Publication selection.
3. Results
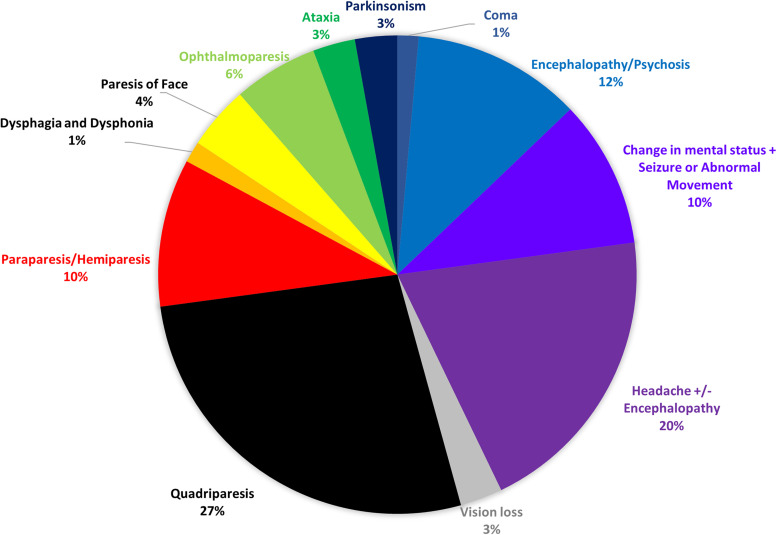
Of 242 publications from December 1, 2019 and November 18, 2020 that described a unique patient diagnosed with COVID-19 via SARS-CoV-2 PCR or serology who had a neurological symptom and had CSF obtained, we identified 51 that met inclusion criteria. After review of the 51 publications, we identified 70 patients reported to have altered olfactory and/or gustatory function (anosmia/cacosmia/hyposmia and/or ageusia/dysgeusia/hypogeusia); 40 (57%) had both olfactory and gustatory dysfunction, 17 (24%) had isolated olfactory dysfunction and 13 (19%) had isolated gustatory dysfunction [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58]. In addition to altered olfactory and/or gustatory function, 39 (56%) had symptoms that localized to the central nervous system and 31 (44%) had symptoms that localized to the peripheral nervous system. The most common symptoms/signs that prompted CSF testing were quadriparesis/paraparesis/hemiparesis (26 patients; 37%) and altered mental status (23 patients; 33%; Fig. 2).
Fig. 2.
Primary neurological symptom/sign prompting evaluation of cerebrospinal fluid.
3.1. CSF pleocytosis
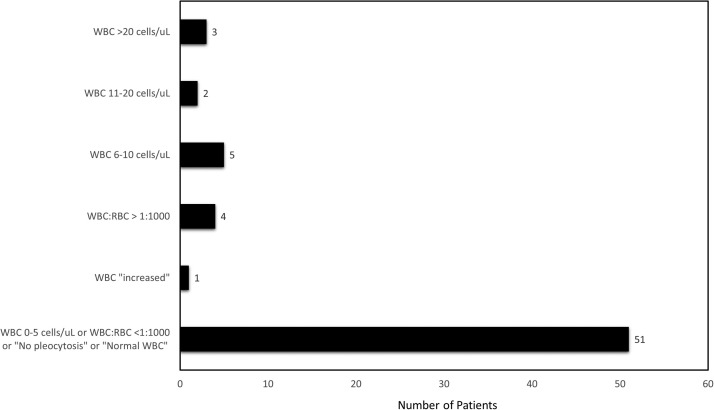
The CSF red blood cell (RBC) count was only reported for 7/70 (10%) patients [21], [30], [32], [41], [44], [45], [50], but the white blood cell (WBC) count was included in the CSF results for 66/70 (94%) patients ( Fig. 3) [8], [10], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [44], [45], [46], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58]. The CSF WBC count ranged from 0 to 37 cells/µL. In 13/66 (20%) patients, the CSF WBC count was 0 cells/µL or the CSF WBC:RBC ratio was <1:1000. The majority of patients (38/66; 58%) had a CSF WBC count of 1–5 cells/µL or were noted to have “no pleocytosis” or “normal WBC.” [10], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [29], [30], [33], [36], [37], [38], [40], [41], [42], [44], [45], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58] There were 11/66 (17%) patients who had a CSF WBC count >5 cells/µL or noted to be “increased.” [8], [12], [17], [28], [31], [32], [33], [34], [35], [39], [46].
Fig. 3.
Cerebrospinal fluid white blood cell count results.
3.2. CSF protein
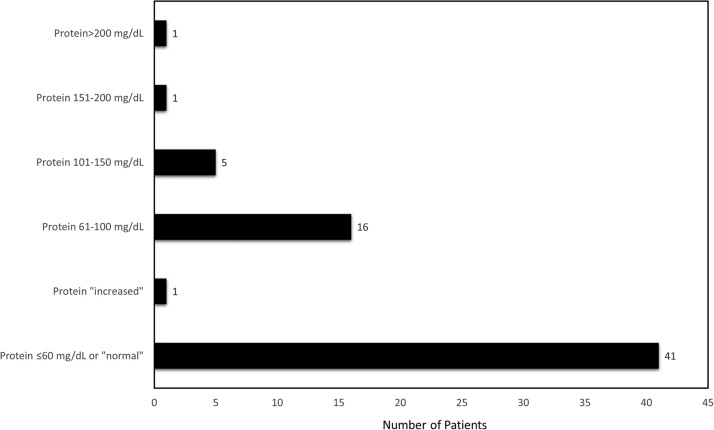
The protein was included in the CSF results for 65/70 (93%) patients ( Fig. 4) [8], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58]. In two cases, we suspect there was an error in the units provided for the CSF protein: Demirci Otluoglu et al. reported a CSF protein of 0.4 mg/L (0.04 mg/dL) and Matos et al. reported a CSF protein of 0.78 mg/dL, but noted it was “mildly elevated;” we presumed these were meant to be 0.4 g/L (40 mg/dL), and 78 mg/dL, respectively [36], [43]. The CSF protein ranged from 12 to 273 mg/dL. The majority of patients (40/65; 62%) had a CSF protein ≤ 60 mg/dL or were noted to have a “normal” protein [8], [11], [14], [16], [20], [21], [23], [24], [26], [27], [29], [30], [33], [34], [36], [37], [38], [39], [40], [41], [44], [46], [48], [49], [52], [53], [54], [55], [56], [58].
Fig. 4.
Cerebrospinal fluid protein.
3.3. CSF SARS-CoV-2 PCR testing
SARS-CoV-2 PCR testing was performed in the CSF for 51/70 (73%) patients [8], [9], [10], [11], [12], [13], [14], [16], [17], [19], [20], [22], [25], [28], [31], [32], [33], [35], [36], [37], [40], [42], [43], [45], [46], [47], [49], [50], [51], [52], [54], [55], [56], [57], [58]. The CSF SARS-CoV-2 PCR resulted positive for 3/51 (6%) patients [8], [36], [42].
Demirci Oluoglu et al. reported a 48-year-old man with no significant past medical history who presented with one week of anosmia, fatigue and myalgia which began three days after onset of cough and headache who had a negative rapid test for COVID-19, but a positive CSF SARS-CoV-2 PCR [36]. The neurological and physical examination were normal. He had a normal serum WBC count and C-reactive protein. The CSF had no cells and was reported to have protein 0.4 mg/L (as noted above, we believe this was meant to be 0.4 g/L (40 mg/dL)) and glucose of 90 mg/dL). MRI revealed hyperintense lesions in the temporal lobe and the upper cervical cord. He was treated with hydroxychloroquine, favipiravir, acyclovir, antibiotics and steroids and remained clinically unchanged at the time of publication.
Lo Monaco et al. reported a 56-year-old man with no significant past medical history who presented with one week of cough followed by fever and encephalopathy who had a positive nasopharyngeal and CSF SARS-CoV-2 PCR [42]. He was minimally arousable, extended his arms to pain and withdrew his legs to pain. He was febrile and tachycardic, but not hypoxic. He had leukopenia (serum WBC count of 3430 cells/µL) and an elevated C-reactive protein of 21.3 mg/L. Chest x-ray and head CT were normal. The CSF had a WBC count of 4 cells/µL, protein of 115 mg/dL and glucose of 82 mg/dL. He was treated with tocilizumab, antibiotics and antivirals and improved rapidly, but reported anosmia.
Novi et al. reported a 64-year-old woman with history of hypertension, monoclonal gammopathy of undetermined significance and hypertension who had two weeks of anosmia, ageusia and an influenza-like illness [8]. She presented a few weeks later with headache, bilateral visual impairment and decreased sensation in her leg and had a negative nasopharyngeal SARS-CoV-2 PCR, but a positive serum SARS-CoV-2 IgG and a positive CSF SARS-CoV-2 PCR. She was irritable, had profound loss of visual acuity and an afferent pupillary defect bilaterally, a right abdominal sensory level and hyperreflexia in the left leg. The CSF had a WBC count of 22 cells/µL, protein of 45.2 mg/dL and oligoclonal bands matched with the serum. MRI showed enhancement of bilateral optic nerves, multiple lesions in the brain and a thoracic lesion. She was treated with steroids and intravenous immunoglobulins and her vision improved. Repeat imaging showed reduction in the number of enhancing lesions.
The cycle threshold (Ct; the number of amplification cycles required for the target gene to exceed the threshold, which is inversely related to viral load) was not provided for any of these patients [8], [36], [42], but it was reported for a man in his 40s with no past medical history described by Eden et al [37]. He presented with ten days of dysgeusia, respiratory symptoms and encephalopathy and had a positive nasopharyngeal SARS-CoV-2 PCR, but an indeterminate CSF SARS-CoV-2 PCR (Ct of 37) two days later. SARS-CoV-2 RNA was not detected when a stored sample was retested. He had a C-reactive protein of 120 mg/L. CSF showed a WBC count < 3 cells/µL, an albumin ratio of 4 (indicative of an intact blood-brain barrier) and an IgG index of 0.31 (indicative of absence of intrathecal IgG synthesis), but elevated neopterin (50 nmol/L; normal <5.8 nmol/L) and β2-microglobulin (7.5 mg/L; normal <1.8 mg/L), suggesting monocytic activation. He was treated with remdesivir and discharged after 12 days. The authors concluded that failure to detect viral RNA when the CSF was retested calls the initial results into question, though retesting of low viral load samples after freezing and thawing can yield inaccurate results. They attributed the elevated CSF biomarkers to indirect effects of systemic infection and inflammation.
3.4. CSF SARS-CoV-2 antibody testing
CSF antibodies to SARS-CoV-2 were tested in 6/70 (9%) patients ( Table 1) [10], [20], [25], [33], [51], [57]. Of these, 3 (50%) patients had CSF antibodies to SARS-CoV-2 [10], [51], [57]. However, only one of these patients had additional testing to determine if the antibodies were synthesized in the CSF or transmitted to the CSF via breakdown in the blood-brain barrier [10]. After calculating the Tibbling-Link, Delpech and transudation indices, Andriuta et al. determined that there was no evidence of intrathecal antibody synthesis.
Table 1.
Cerebrospinal fluid testing for evidence of SARS-CoV-2.
| Test | Number of Patients Tested | Results | Notes |
|---|---|---|---|
| CSF SARS-CoV-2 PCR | 51 | 3 (6%) Positive | |
| 1 (2%) Indeterminate |
|
||
| 47 (92%) Negative | |||
| CSF SARS-CoV-2 Antibodies | 6 | 0 Positive CSF SARS-CoV-2 Antibodies with Evidence of Intrathecal Antibody Synthesis | |
| 2 (33%) Positive CSF SARS-CoV-2 Antibodies but Indeterminate if there was Intrathecal Antibody Synthesis | |||
| 1 (17%) Positive CSF SARS-CoV-2 Antibodies with no Evidence of Intrathecal Antibody Synthesis |
|
||
| 3 (50%) Negative CSF SARS-CoV-2 Antibodies | |||
| CSF Oligoclonal Bands | 15 | 1 (7%) Positive CSF Oligoclonal Bands with Evidence of Intrathecal Antibody Synthesis |
|
| 1 (7%) Positive CSF Oligoclonal Bands but Indeterminate if there was Intrathecal Antibody Synthesis |
|
||
| 5 (33%) Positive CSF Oligoclonal Bands with no Evidence of Intrathecal Antibody Synthesis | |||
| 8 (53%) Negative CSF Oligoclonal Bands | |||
| CSF Immunoglobulins | 6 | 0 Immunoglobulin Results Consistent with Intrathecal Antibody Synthesis | |
| 1 (17%) Immunoglobulin Results Consistent with Possible Intrathecal Antibody Synthesis |
|
||
| 5 (83%) Immunoglobulin Results Not Suggestive of Intrathecal Antibody Synthesis |
|
3.5. CSF oligoclonal bands
CSF oligoclonal bands were tested in 15/70 (21%) patients (Table 1) [8], [11], [20], [24], [25], [26], [35], [39], [40], [41], [45], [47], [50], [55]. There was 1 patient (7%) who had type 2 CSF oligoclonal bands consistent with intrathecal synthesis and 1 patient (7%) who had positive CSF oligoclonal bands, but it was not specified whether they were matched or unmatched in serum [20], [47].
Huber et al. reported a 21-year-old woman with no past medical history who developed anosmia, ageusia, mild respiratory symptoms and myalgia in late March 2020 then presented with ptosis and diplopia in mid-April and was found to have SARS-CoV-2 IgG and IgA antibodies in her serum (but not in her CSF) and type 2 CSF oligoclonal bands [20]. She had elevated acetylcholine receptor antibodies in her serum and a positive intravenous edrophonium chloride test, which led to the diagnosis of myasthenia gravis. She was treated with intravenous immunoglobulins and pyridostigmine and gradually improved. The authors suspected the myasthenia gravis was post-infectious, but noted that they couldn’t rule out co-occurrence of preexisting myasthenia gravis with COVID-19.
Palao et al. reported a 29-year-old woman with history of asthma and rhinoconjunctivitis who developed anosmia, dysgeusia and myalgia followed two weeks later by decreased visual acuity [47]. She was found to have SARS-CoV-2 antibodies in her serum and positive oligoclonal bands in her CSF, with no mention of whether they were matched or unmatched to serum. CSF SARS-CoV-2 PCR was negative. Her MRI demonstrated enhancement of the optic nerve and periventricular demyelinating lesions, only one of which was enhancing, leading to a diagnosis of multiple sclerosis. The authors suspected COVID-19 was a precipitating, rather than causative, factor leading to her presentation, but noted that it is possible the CSF SARS-CoV-2 PCR was a false negative, and that her symptoms were the result of viral neuroinvasion.
3.6. CSF immunoglobulins
In addition to the aforementioned patient reported by Andriuta et al. who had CSF immunoglobulins tested after CSF SARS-CoV-2 antibody detection and was found to have no evidence of intrathecal antibody synthesis, CSF immunoglobulins were tested in 5 other patients (Table 1) [10], [11], [25], [37], [39], [50]. Only one of these patients had results consistent with possible intrathecal antibody synthesis [39].
Ghosh et al. reported a 44-year-old woman with no past medical history who developed hyposmia, hypogeusia, cough, fever and myalgia then presented ten days later in a coma following a seizure [39]. She had a positive nasopharyngeal SARS-CoV-2 PCR and an elevated IgG index (value not provided). Other CSF studies showed a WBC count of 20 cells/µL, protein of 60 mg/dL and negative oligoclonal bands. An MRI showed a frontoparietal hyperintense lesion with surrounding edema and foci of hemorrhage. She was treated with steroids, mannitol, acyclovir and antibiotics, but died shortly after admission.
3.7. CSF autoimmune antibodies
Autoimmune antibodies were tested in the CSF of 9/70 (13%) patients, but were not detected [33], [40], [41], [45], [46], [50], [55], [56].
3.8. Other CSF biomarkers
In addition to the aforementioned patient described by Eden et al. who had elevated CSF neopterin and β2-microglobulin, CSF biomarkers were tested in seven patients [23], [35], [37], [45], [55]. Two of these patients had normal findings, but the remaining five patients had at least one elevated CSF biomarker.
Manganotti et al. demonstrated elevation of CSF IL-8 and IL-1β in the CSF of three patients who presented with Guillain-Barré Syndrome 18–30 days after onset of hyposmia, ageusia, fever and cough, all of whom had a positive nasopharyngeal and negative CSF SARS-CoV-2 PCR, CSF protein <60 mg/dL and CSF WBC count ≤ 2 cells/µL [23]. All three patients had a higher CSF than serum IL-8 (reported normal for both CSF and serum <16.2 pg/mL; 42.6 pg/mL in CSF and 17.8 pg/mL in serum in one patient, 96 pg/mL in CSF and 55 pg/mL in serum in the second patient and 22.7 pg/mL in CSF and 20 pg/mL in serum in the third patient). One of these patients also had elevated CSF and serum IL-6 (reported normal for both CSF and serum <6.4 pg/mL; 9.6 pg/mL in CSF and 113 pg/mL in serum). All three patients improved neurologically.
Delorme et al. reported two patients with mildly elevated CSF IL-6 [35]. The first patient was a 72-year-old man with no past medical history who developed anosmia, fever and cough and had a positive nasopharyngeal SARS-CoV-2 PCR then presented with psychomotor agitation, myoclonus and cerebellar ataxia 15 days after symptom onset. CSF IL-6 was 13 pg/mL (reported normal <6.5 pg/mL; serum level not reported). Additional CSF studies showed a negative SARS-CoV-2 PCR, WBC count of 6 cells/µL, protein of 23 mg/dL and no oligoclonal bands. MRI of the brain was unremarkable. He was treated with intravenous immunoglobulins and was neurologically normal six weeks after onset of COVID-19.
The second patient, who was also reported by Le Guennec et al. was a 69-year-old man with diabetes, hypertension and a seizure in the setting of hyperglycemia who presented with status epilepticus one week after onset of anosmia, ageusia, fever and fatigue who had a positive nasopharyngeal SARS-CoV-2 PCR [45]. He had elevated CSF and serum IL-6 (16 pg/mL in CSF with reported normal <2.5 pg/mL and 28.8 pg/mL in serum with reported normal of <6.5 pg/mL). Additional CSF studies showed RBC count of 18 cell/µL, WBC count of 1 cell/µL, protein of 66 mg/dL, glucose of 189 mg/dL (serum glucose 360 mg/dL), negative SARS-CoV-2 PCR, absence of oligoclonal bands, elevated tau (2000 pg/mL; reported normal 150–450 pg/mL), low β-amyloid (570 pg/mL; reported normal 650–2000 pg/mL) and no IL-10 or interferon-α. MRI showed a hyperintensity in the right orbital prefrontal cortex adjacent to the olfactory bulb which spread to the caudate, prompting the authors to hypothesize his seizures were the result of passage of SARS-CoV-2 through the olfactory pathway. He was treated with steroids and intravenous immunoglobulins and gradually improved, but had persistent dysexecutive syndrome 10 weeks after onset of COVID-19. MRI was repeated after 30 days and was normal.
4. Discussion
A number of mechanisms have been proposed to explain altered olfactory and gustatory function in patients with COVID-19 [1], [4]. First, the virus could infect the olfactory and/or gustatory cortex or associated subcortical tracts [4]. Second, the virus could infect the olfactory, facial, glossopharyngeal or vagus nerve [4]. Third, the virus could have a cytopathic effect on ACE2-expressing taste buds or olfactory epithelium, or on the synthesis of serotonin and dopamine neurotransmitters [4]. Fourth, rhinitis or stomatitis due to the virus could trigger an inflammatory response that results in edema which impairs the functionality of taste buds or the olfactory epithelium temporarily (conductive or obstructive loss of function), but ultimately lead to destruction of normal cilia and sensory neurons and replacement by metaplastic squamous epilethelium [1], [4]. Fifth, the virus could trigger a local immune response resulting in production of antibodies against tongue and olfactory epithelial cell membranes or receptors [4]. Lastly, altered olfactory and gustatory function may be the result of a medication taken to ameliorate symptoms related to the virus, rather than the virus itself [4]. A complete discussion of local immune-mediated mechanisms in the olfactory/gustatory pathways in response to SARS-CoV-2 can be found elsewhere [1], [4], [59].
In this systematic review, we sought to evaluate the CSF of patients with COVID-19 who had altered olfactory and/or gustatory function for evidence of viral neuroinvasion. We identified 70 patients with COVID-19 who had altered olfactory and/or gustatory function and had CSF obtained [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58]. Of the 52 patients who had CSF SARS-CoV-2 PCR testing, only 3 (6%) patients had a positive result and 1 (2%) had an indeterminate result consistent with possible viral neuroinvasion [8], [9], [10], [11], [12], [13], [14], [16], [17], [19], [20], [22], [25], [28], [31], [32], [33], [35], [36], [37], [40], [42], [43], [45], [46], [47], [49], [50], [51], [52], [54], [55], [56], [57], [58]. Evaluation for CSF SARS-CoV-2 antibodies was performed directly or indirectly (via testing for CSF oligoclonal bands or immunoglobulins) for about 25% of patients, but only 1 patient clearly had evidence of intrathecal antibody synthesis (unmatched oligoclonal bands); 4 others had possible intrathecal antibody synthesis, but did not have testing to determine if the antibodies were created intrathecally, or if they, or the cells that secreted them, were transmitted to the CSF via a damaged blood-brain barrier [8], [10], [11], [20], [24], [25], [26], [33], [35], [37], [39], [40], [41], [45], [47], [50], [51], [55], [57]. Although one patient had unmatched oligoclonal bands demonstrative of intrathecal antibody synthesis, the presence of these bands is nonspecific and does not definitively reflect synthesis of SARS-CoV-2 antibodies [20]. Similarly, the fact that 7 patients had one or more elevated CSF biomarkers does not mean virus was present in their CSF; rather, this may reflect neuroinflammation in the setting of systemic infection, as there may be compartment-specific immune responses to COVID-19 [23], [35], [37], [45], [55]. Taken together, these findings suggest that detection of viral neuroinvasion by SARS-CoV-2 in the CSF of patients with altered olfactory and/or gustatory function is rare.
There have also been radiographic studies of this patient population [1], [2], [3], [60]. Saussez et al. performed sinus computed tomography on 16 patients with persistent anosmia after infection with COVID-19 and found that 7 had a completely clear olfactory cleft, 6 had a partially opacified olfactory cleft and 3 had a completely opacified olfactory cleft [2]. Although the MRIs of some patients with SARS-CoV-2-related anosmia revealed a normal olfactory bulb and signal intensity, others have demonstrated inflammation of the olfactory clefts and/or olfactory bulb edema/enhancement/microhemorrhages [1], [3]. Nonetheless, these imaging findings do not clarify the mechanism of altered olfactory and gustatory function in this population.
Neuropathology studies can provide more definitive information about neuroinvasion than evaluation of CSF. Meinhardt et al. evaluated the olfactory mucosa/nerves/tracts in patients with COVID-19 portmortem and found the highest levels of viral RNA for SARS-CoV-2 in the olfactory mucosa directly beneath the cribriform plate for 20 out of 30 patients [61]. Using magnetic resonance microscopy, histopathological evaluation and immunohistochemical analysis, Lee et al. observed multifocal microvascular injury in the olfactory bulbs of postmortem patients with COVID-19 without evidence of viral infection [62]. Additional neuropathology studies, which incorporate both premortem clinical data, neuroimaging and CSF results, are needed.
Our findings are dependent on both publication bias and limitations of our search methodology. There are likely additional patients with COVID-19 who had altered olfactory and/or gustatory function and had CSF obtained that were not included herein because 1) they did not (or could not) report these symptoms or 2) there was no published report of the clinical details of their case during our search period so they were not captured in our search. Additionally, CSF results can change over time, and the CSF from the patients included in this review was not obtained at a specific time point relative to the onset of altered olfactory and/or gustatory function. Lastly, although we excluded patients who had subarachnoid hemorrhage or meningitis/ventriculitis/encephalitis due to an infectious organism other than COVID-19, it is worth noting that we included patients with inflammatory diagnoses which could alter the CSF profile, such as acute disseminated encephalomyelitis.
5. Conclusion
Altered olfactory and/or gustatory function in patients with COVID-19 is common, but the mechanism for these symptoms is uncertain. Our findings suggest that detection of viral neuroinvasion in this patient population via CSF SARS-CoV-2 PCR or evaluation for intrathecal antibody synthesis is rare. Additional research is needed to clarify the pathogenesis of these symptoms in patients with COVID-19.
Disclosures
All authors report no disclosures.
Data
Ariane Lewis takes responsibility for the data and accuracy of data analysis.
CRediT authorship contribution statement
Ariane Lewis was responsible for conception and design, data collection, analysis and interpretation of data, drafting the manuscript and final approval of the manuscript. Kara Melmed was responsible for data collection, critical revision of the manuscript and final approval of the manuscript. Jennifer Frontera, Dimitris Placantonakis, Steven Galetta and Laura Balcer were responsible for critical revision of the manuscript and final approval of the manuscript.
References
- 1.Han A.Y., Mukdad L., Long J.L., Lopez I.A. Anosmia in COVID-19: mechanisms and significance. Chem. Senses. 2020 doi: 10.1093/chemse/bjaa040. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saussez S., Lechien J.R., Hopkins C. Anosmia: an evolution of our understanding of its importance in COVID-19 and what questions remain to be answered. Eur. Arch. Oto Rhino Laryngol. 2021;278:2187–2191. doi: 10.1007/s00405-020-06285-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aragão M., Leal M., Cartaxo Filho O., Fonseca T., Valença M. Anosmia in COVID-19 associated with injury to the olfactory bulbs evident on MRI. AJNR Am. J. Neuroradiol. 2020;41:1703–1706. doi: 10.3174/ajnr.A6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finsterer J., Stollberger C. Causes of hypogeusia/hyposmia in SARS-CoV2 infected patients. J. Med. Virol. 2020;92:1793–1794. doi: 10.1002/jmv.25903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perchetti G.A., Nalla A.K., Huang M.L., Zhu H., Wei Y., Stensland L., Loprieno M.A., Jerome K.R., Greninger A.L. Validation of SARS-CoV-2 detection across multiple specimen types. J. Clin. Virol. 2020;128 doi: 10.1016/j.jcv.2020.104438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewis A., Frontera J., Placantonakis D.G., Lighter J., Galetta S., Balcer L., Melmed K.R. Cerebrospinal fluid in COVID-19: a systematic review of the literature. J. Neurol. Sci. 2021;421 doi: 10.1016/j.jns.2021.117316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J. Clin. Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Novi G., Rossi T., Pedemonte E., Saitta L., Rolla C., Roccatagliata L., Inglese M., Farinini D. Acute disseminated encephalomyelitis after SARS-CoV-2 infection. Neurol. Neuroimmunol. Neuroinflamm. 2020;7 doi: 10.1212/NXI.0000000000000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zanin L., Saraceno G., Panciani P.P., Renisi G., Signorini L., Migliorati K., Fontanella M.M. SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir. 2020;162:1491–1494. doi: 10.1007/s00701-020-04374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andriuta D., Roger P.A., Thibault W., Toublanc B., Sauzay C., Castelain S., Godefroy O., Brochot E. COVID-19 encephalopathy: detection of antibodies against SARS-CoV-2 in CSF. J. Neurol. 2020;267:2810–2811. doi: 10.1007/s00415-020-09975-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Assini A., Benedetti L., Di Maio S., Schirinzi E., Del Sette M. New clinical manifestation of COVID-19 related Guillain-Barrè syndrome highly responsive to intravenous immunoglobulins: two Italian cases. Neurol. Sci. 2020;41:1657–1658. doi: 10.1007/s10072-020-04484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bigaut K., Mallaret M., Baloglu S., Nemoz B., Morand P., Baicry F., Godon A., Voulleminot P., Kremer L., Chanson J.B., de Seze J. Guillain-Barré syndrome related to SARS-CoV-2 infection. Neurol. Neuroimmunol. Neuroinflamm. 2020;7 doi: 10.1212/NXI.0000000000000785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gutiérrez-Ortiz C., Méndez-Guerrero A., Rodrigo-Rey S., San Pedro-Murillo E., Bermejo-Guerrero L., Gordo-Mañas R., de Aragón-Gómez F., Benito-León J. Miller fisher syndrome and polyneuritis cranialis in COVID-19. Neurology. 2020;95:e601–e605. doi: 10.1212/WNL.0000000000009619. [DOI] [PubMed] [Google Scholar]
- 14.Riva N., Russo T., Falzone Y.M., Strollo M., Amadio S., Del Carro U., Locatelli M., Filippi M., Fazio R. Post-infectious Guillain-Barré syndrome related to SARS-CoV-2 infection: a case report. J. Neurol. 2020;267:2492–2494. doi: 10.1007/s00415-020-09907-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scheidl E., Canseco D.D., Hadji-Naumov A., Bereznai B. Guillain-Barré syndrome during SARS-CoV-2 pandemic: a case report and review of recent literature. J. Peripher. Nerv. Syst. JPNS. 2020;25:204–207. doi: 10.1111/jns.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toscano G., Palmerini F., Ravaglia S., Ruiz L., Invernizzi P., Cuzzoni M.G., Franciotta D., Baldanti F., Daturi R., Postorino P., Cavallini A., Micieli G. Guillain-Barré syndrome associated with SARS-CoV-2. New Engl. J. Med. 2020;382:2574–2576. doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chow C.C.N., Magnussen J., Ip J., Su Y. Acute transverse myelitis in COVID-19 infection. BMJ Case Rep. 2020;13:13. doi: 10.1136/bcr-2020-236720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agosti E., Giorgianni A., D’Amore F., Vinacci G., Balbi S., Locatelli D. Is Guillain-Barrè syndrome triggered by SARS-CoV-2? Case report and literature review. Neurol. Sci. 2021;42:607–612. doi: 10.1007/s10072-020-04553-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atakla H.G., Noudohounsi M., Sacca H., Tassiou N.R.A., Noudohounsi W.C., Houinato D.S. Acute Guillain-Barré polyradiculoneuritis indicative of COVID-19 infection: a case report. Pan Afr. Med. J. 2020;35:150. doi: 10.11604/pamj.supp.2020.35.150.25745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huber M., Rogozinski S., Puppe W., Framme C., Höglinger G., Hufendiek K., Wegner F. Postinfectious onset of myasthenia gravis in a COVID-19 patient. Front. Neurol. 2020;11:11. doi: 10.3389/fneur.2020.576153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hutchins K.L., Jansen J.H., Comer A.D., Scheer R.V., Zahn G.S., Capps A.E., Weaver L.M., Koontz N.A. COVID-19-associated bifacial weakness with paresthesia subtype of Guillain-Barré syndrome. AJNR Am. J. Neuroradiol. 2020;41:1707–1711. doi: 10.3174/ajnr.A6654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khaja M., Gomez G.P.R., Santana Y., Hernandez N., Haider A., Lara J., Elkin R. A 44-year-old hispanic man with loss of taste and bilateral facial weakness diagnosed with Guillain-Barré syndrome and Bell’s palsy associated with SARS-CoV-2 infection treated with intravenous immunoglobulin. Am. J. Case Rep. 2020;21 doi: 10.12659/AJCR.927956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manganotti P., Bellavita G., D’Acunto L., Tommasini V., Fabris M., Sartori A., Bonzi L., Buoite Stella A., Pesavento V. Clinical neurophysiology and cerebrospinal liquor analysis to detect Guillain-Barré syndrome and polyneuritis cranialis in COVID-19 patients: a case series. J. Med. Virol. 2021;93:766–774. doi: 10.1002/jmv.26289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masuccio F.G., Barra M., Claudio G., Claudio S. A rare case of acute motor axonal neuropathy and myelitis related to SARS-CoV-2 infection. J. Neurol. 2020:1–4. doi: 10.1007/s00415-020-10219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naddaf E., Laughlin R.S., Klein C.J., Toledano M., Theel E.S., Binnicker M.J., Nagappan V., Abdulrazzak M., Phelan D.M. Guillain-barre syndrome in a patient with evidence of recent SARS-CoV-2 infection. Mayo Clin. Proc. 2020;95:1799–1801. doi: 10.1016/j.mayocp.2020.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zito A., Alfonsi E., Franciotta D., Todisco M., Gastaldi M., Cotta Ramusino M., Ceroni M., Costa A. COVID-19 and Guillain-Barré syndrome: a case report and review of literature. Front. Neurol. 2020;11:909. doi: 10.3389/fneur.2020.00909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Babar A., Lewandowski U., Capin I., Khariton M., Venkataraman A., Okolo N., Sharma D. SARS-CoV-2 encephalitis in a 20-year old healthy female. Pediatr. Infect. Dis. J. 2020;39:e320–e321. doi: 10.1097/INF.0000000000002855. [DOI] [PubMed] [Google Scholar]
- 28.Civardi C., Collini A., Geda D.J., Geda C. Antiganglioside antibodies in Guillain-Barré syndrome associated with SARS-CoV-2 infection. J. Neurol., Neurosurg., Psychiatry. 2020;91:1361–1362. doi: 10.1136/jnnp-2020-324279. [DOI] [PubMed] [Google Scholar]
- 29.Faqihi F., Alharthy A., Memish Z.A., Kutsogiannis D.J., Brindley P.G., Karakitsos D. Peripheral neuropathy in severe COVID-19 resolved with therapeutic plasma exchange. Clin. Case Rep. 2020;8:3234–3239. doi: 10.1002/ccr3.3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lowery M.M., Taimur Malik M., Seemiller J., Tsai C.S. Atypical variant of Guillain Barre syndrome in a patient with COVID-19. J. Crit. Care Med. Univ. Med. Farm. Din. Targu Mures. 2020;6:231–236. doi: 10.2478/jccm-2020-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Casez O., Willaume G., Grand S., Nemoz B., Lupo J., Kahane P., Brion J.P. Teaching neuroimages: SARS-CoV-2-related encephalitis: MRI pattern of olfactory tract involvement. Neurology. 2021;96:645. doi: 10.1212/WNL.0000000000011150. [DOI] [PubMed] [Google Scholar]
- 32.Chaumont H., Etienne P., Roze E., Couratier C., Roger P.M., Lannuzel A. Acute meningoencephalitis in a patient with COVID-19. Rev. Neurol. 2020;176:519–521. doi: 10.1016/j.neurol.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen M.E., Eichel R., Steiner-Birmanns B., Janah A., Ioshpa M., Bar-Shalom R., Paul J.J., Gaber H., Skrahina V., Bornstein N.M., Yahalom G. A case of probable Parkinson’s disease after SARS-CoV-2 infection. Lancet Neurol. 2020;19:804–805. doi: 10.1016/S1474-4422(20)30305-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Oliveira F.A.A., Palmeira D.C.C., Rocha-Filho P.A.S. Headache and pleocytosis in CSF associated with COVID-19: case report. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2020;41:3021–3022. doi: 10.1007/s10072-020-04694-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delorme C., Paccoud O., Kas A., Hesters A., Bombois S., Shambrook P., Boullet A., Doukhi D., Le Guennec L., Godefroy N., Maatoug R., Fossati P., Millet B., Navarro V., Bruneteau G., Demeret S., Pourcher V., CoCo-Neurosciences study group and COVID SMIT PSL study g. Covid-19-related encephalopathy: a case series with brain FDG-PET/CT findings. Eur. J. Neurol. 2020;27:261–2657. doi: 10.1111/ene.14478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Demirci Otluoglu G., Yener U., Demir M.K., Yilmaz B. Encephalomyelitis associated with Covid-19 infection: case report. Br. J. Neurosurg. 2020:1–3. doi: 10.1080/02688697.2020.1787342. [DOI] [PubMed] [Google Scholar]
- 37.Edén A., Kanberg N., Gostner J., Fuchs D., Hagberg L., Andersson L.M., Lindh M., Price R.W., Zetterberg H., Gisslén M. CSF biomarkers in patients with COVID-19 and neurological symptoms: a case series. Neurology. 2021;96:e294–e300. doi: 10.1212/WNL.0000000000010977. [DOI] [PubMed] [Google Scholar]
- 38.Faber I., Brandão P.R.P., Menegatti F., de Carvalho Bispo D.D., Maluf F.B., Cardoso F. Coronavirus disease 2019 and parkinsonism: a Non-post-encephalitic Case. Mov. Disord. Off. J. Mov. Disord. Soc. 2020;35:1721–1722. doi: 10.1002/mds.28277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghosh R., Dubey S., Finsterer J., Chatterjee S., Ray B.K. SARS-CoV-2-associated acute hemorrhagic, necrotizing encephalitis (AHNE) presenting with cognitive impairment in a 44-year-old woman without comorbidities: a case report. Am. J. Case Rep. 2020;21 doi: 10.12659/AJCR.925641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hosseini A.A., Shetty A.K., Sprigg N., Auer D.P., Constantinescu C.S. Delirium as a presenting feature in COVID-19: neuroinvasive infection or autoimmune encephalopathy? Brain Behav., Immun. 2020;88:68–70. doi: 10.1016/j.bbi.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar A., Olivera A., Mueller N., Howard J., Lewis A. Delayed SARS-COV-2 leukoencephalopathy without severe hypoxia. J. Neurol. Sci. 2020;418 doi: 10.1016/j.jns.2020.117146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lo Monaco M.R., Di Giambenedetto S., Martone A.M., De Gaetano Donati K., Landi F. Encephalopathy as neurological involvement of SARS-COV-2 infection. Clin. Infect. Pract. 2020;7 doi: 10.1016/j.clinpr.2020.100034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matos A.R., Quintas-Neves M., Oliveira A.I., et al. COVID-19 associated central nervous system vasculopathy. Can. J. Neurol. Sci. 2021;48:139–140. doi: 10.1017/cjn.2020.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lim S.T., Janaway B., Costello H., Trip A., Price G. Persistent psychotic symptoms following COVID-19 infection. BJPsych Open. 2020;6:105. doi: 10.1192/bjo.2020.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Le Guennec L., Devianne J., Jalin L., Cao A., Galanaud D., Navarro V., Boutolleau D., Rohaut B., Weiss N., Demeret S. Orbitofrontal involvement in a neuroCOVID-19 patient. Epilepsia. 2020;61:e90–e94. doi: 10.1111/epi.16612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muccioli L., Pensato U., Cani I., Guerra L., Provini F., Bordin G., Riccioli L.A., Lodi R., Tinuper P., Bisulli F. COVID-19-related encephalopathy presenting with aphasia resolving following tocilizumab treatment. J. Neuroimmunol. 2020;349 doi: 10.1016/j.jneuroim.2020.577400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Palao M., Fernandez-Diaz E., Gracia-Gil J., Romero-Sanchez C.M., Diaz-Maroto I., Segura T. Multiple sclerosis following SARS-CoV-2 infection. Mult. Scler. Relat. Disord. 2020;45 doi: 10.1016/j.msard.2020.102377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paterson R.W., Brown R.L., Benjamin L., Nortley R., Wiethoff S., Bharucha T., Jayaseelan D.L., Kumar G., Raftopoulos R.E., Zambreanu L., Vivekanandam V., Khoo A., Geraldes R., Chinthapalli K., Boyd E., Tuzlali H., Price G., Christofi G., Morrow J., McNamara P., McLoughlin B., Lim S.T., Mehta P.R., Levee V., Keddie S., Yong W., Trip S.A., Foulkes A., Hotton G., Miller T.D., Everitt A.D., Carswell C., Davies N., Yoong M., Attwell D., Sreedharan J., Silber E., Schott J.M., Chandratheva A., Perry R.J., Simister R., Checkley A., Longley N., Farmer S.F., Carletti F., Houlihan C., Thom M., Lunn M.P., Spillane J., Howard R., Vincent A., Werring D.J., Hoskote C., Jäger H.R., Manji H., Zandi M.S. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143:3104–3120. doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pensato U., Muccioli L., Pasini E., Tappatà M., Ferri L., Volpi L., Licchetta L., Battaglia S., Rossini G., Bon I., Re M.C., Cirillo L., Simonetti L., Gramegna L.L., Michelucci R., Cortelli P., Zini A., Bisulli F. Encephalopathy in COVID-19 presenting with acute aphasia mimicking stroke. Front. Neurol. 2020;11 doi: 10.3389/fneur.2020.587226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perrin P., Collongues N., Baloglu S., Bedo D., Bassand X., Lavaux T., Gautier-Vargas G., Keller N., Kremer S., Fafi-Kremer S., Moulin B., Benotmane I., Caillard S. Cytokine release syndrome-associated encephalopathy in patients with COVID-19. Eur. J. Neurol. 2021;28:248–258. doi: 10.1111/ene.14491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rifino N., Censori B., Agazzi E., Alimonti D., Bonito V., Camera G., Conti M.Z., Foresti C., Frigeni B., Gerevini S., Grimoldi M., La Gioia S., Partziguian T., Quadri S., Riva R., Servalli M.C., Sgarzi M., Storti B., Vedovello M., Venturelli E., Viganò M., Callegaro A., Arosio M., Sessa M. Neurologic manifestations in 1760 COVID-19 patients admitted to Papa Giovanni XXIII Hospital, Bergamo, Italy. J. Neurol. 2020:1–8. doi: 10.1007/s00415-020-10251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Silva M.T.T., Lima M.A., Torezani G., Soares C.N., Dantas C., Brandão C.O., Espíndola O., Siqueira M.M., Araujo A.Q. Isolated intracranial hypertension associated with COVID-19. Cephalalgia Int. J. Headache. 2020;40:1452–1458. doi: 10.1177/0333102420965963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Soto Insuga V., Cantarín-Extremera V., Solís-Muñoz I., Buendía-Martínez S., Atencia-Ballesteros M., Bernardino B., Ruiz Falcó M.L. Pseudotumor cerebri caused by SARS-CoV-2 infection in a boy. J. Pediatr. Neurol. 2020;19:207–209. [Google Scholar]
- 54.Zayet S., Ben Abdallah Y., Royer P.Y., Toko-Tchiundzie L., Gendrin V., Klopfenstein T. Encephalopathy in patients with COVID-19: “causality or coincidence?”. J. Med. Virol. 2021;93:1193. doi: 10.1002/jmv.26027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cao A., Rohaut B., Le Guennec L., Saheb S., Marois C., Altmayer V., Carpentier V.T., Nemlaghi S., Soulie M., Morlon Q., Berthet-Delteil B., Bleibtreu A., Raux M., Weiss N., Demeret S., CoCo-Neurosciences study g. Severe COVID-19-related encephalitis can respond to immunotherapy. Brain. 2020;143:102. doi: 10.1093/brain/awaa337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vandervorst F., Guldolf K., Peeters I., Vanderhasselt T., Michiels K., Berends K.J., Van Laethem J., Pipeleers L., Vincken S., Seynaeve L., Engelborghs S. Encephalitis associated with the SARS-CoV-2 virus: a case report. Interdiscip. Neurosurg. Adv. Tech. Case Manag. 2020;22 doi: 10.1016/j.inat.2020.100821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Helbok R., Beer R., Löscher W., Boesch S., Reindl M., Hornung R., Schiefecker A.J., Deisenhammer F., Pfausler B. Guillain-Barré syndrome in a patient with antibodies against SARS-COV-2. Eur. J. Neurol. 2020;27:1754–1756. doi: 10.1111/ene.14388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lascano A.M., Epiney J.B., Coen M., Serratrice J., Bernard-Valnet R., Lalive P.H., Kuntzer T., Hübers A. SARS-CoV-2 and Guillain-Barré syndrome: AIDP variant with a favourable outcome. Eur. J. Neurol. 2020;27:1751–1753. doi: 10.1111/ene.14368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yazdanpanah N., Saghazadeh A., Rezaei N. Anosmia: a missing link in the neuroimmunology of coronavirus disease 2019 (COVID-19) Rev. Neurosci. 2020;31:691–701. doi: 10.1515/revneuro-2020-0039. [DOI] [PubMed] [Google Scholar]
- 60.Chiu A., Fischbein N., Wintermark M., Zaharchuk G., Yun P.T., Zeineh M. COVID-19-induced anosmia associated with olfactory bulb atrophy. Neuroradiology. 2021;63:147–148. doi: 10.1007/s00234-020-02554-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meinhardt J., Radke J., Dittmayer C., Franz J., Thomas C., Mothes R., Laue M., Schneider J., Brünink S., Greuel S., Lehmann M., Hassan O., Aschman T., Schumann E., Chua R.L., Conrad C., Eils R., Stenzel W., Windgassen M., Rößler L., Goebel H.H., Gelderblom H.R., Martin H., Nitsche A., Schulz-Schaeffer W.J., Hakroush S., Winkler M.S., Tampe B., Scheibe F., Körtvélyessy P., Reinhold D., Siegmund B., Kühl A.A., Elezkurtaj S., Horst D., Oesterhelweg L., Tsokos M., Ingold-Heppner B., Stadelmann C., Drosten C., Corman V.M., Radbruch H., Heppner F.L. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. 2021;24:168–175. doi: 10.1038/s41593-020-00758-5. [DOI] [PubMed] [Google Scholar]
- 62.Lee MH, Perl DP, Nair G, Li Wenxue, Maric D, Murray H, Dodd SJ, Koretesky AP, Watts JA, Cheung V, Masliah E, Horkayne-Szakaly I, Jones R, Stram MN, Moncur J, Hefti M, Folkerth RD, Nath A. Microvascular injury in the brains of patients with Covid-19. N. Engl. J. Med. 2021;384:481–483. doi: 10.1056/NEJMc2033369. [DOI] [PMC free article] [PubMed] [Google Scholar]