Abstract
The obesity epidemic and its associated comorbidities present a looming challenge to health care delivery throughout the world. Obesity is characterized as a sterile inflammatory process within adipose tissues leading to dysregulated secretion of bioactive adipokines such as adiponectin and leptin, as well as systemic metabolic dysfunction. The majority of current obesity research has focused primarily on preclinical animal models in vivo and two-dimensional cell culture models in vitro. Neither of these generalized approaches is optimal due to interspecies variability, insufficient accuracy with respect to predicting human outcomes, and failure to recapitulate the three-dimensional (3D) microenvironment. Consequently, there is a growing demand and need for more sophisticated microphysiological systems to reproduce more physiologically accurate human white and brown/beige adipose depots. To address this research need, human and murine cell lines and primary cultures are being combined with bioscaffolds to create functional 3D environments that are suitable for metabolically active adipose organoids in both static and perfusion bioreactor cultures. The development of these technologies will have considerable impact on the future pace of discovery for novel small molecules and biologics designed to prevent and treat metabolic syndrome and obesity in humans. Furthermore, when these adipose tissue models are integrated with other organ systems they will have applicability to obesity-related disorders such as diabetes, nonalcoholic fatty liver disease, and osteoarthritis.
Impact statement
The current review article summarizes the advances made within the organ-onchip field, as it pertains to adipose tissue models of obesity and obesity-related syndromes, such as diabetes, non-alcoholic fatty liver disease, and osteoarthritis. As humanized 3D adipose-derived constructs become more accessible to the research community, it is anticipated that they will accelerate and enhance the drug discovery pipeline for obesity, diabetes, and metabolic diseases by reducing the preclinical evaluation process and improving predictive accuracy. Such developments, applications, and usages of existing technologies can change the paradigm of personalized medicine and create substantial progress in our approach to modern medicine.
Keywords: ADMET, ASC, BAT, fat-on-a-chip, microphysiological system, WAT
Introduction
Obesity is an epidemic that is currently causing significant economic and societal consequences worldwide. The medical costs associated with obesity and obesity-related conditions are estimated to reach two trillion dollars, which comprise 9% of the world's health expenditures. In addition, the high mortality associated with this disease results in ∼2.8 million deaths annually.1,2 Both the high medical costs and high mortality rates associated with obesity are a result of a multitude of health complications/conditions associated with this disease. As the mean body mass indexes in both adults and children continue to increase, obesity-related conditions, such as diabetes, nonalcoholic fatty liver, and osteoarthritis, are becoming more prevalent in society.3 There is increasing evidence linking each of these diseases to the systemic impact of adipose tissue dysfunction due, in part, to the excessive secretion of adipokines, fibrotic inductive factors, inflammatory cytokines, and lipids.4–9 Industrialization, sedentary lifestyle, easier access to processed foods, and obstacles to accessing healthier foods have all contributed to the global rise of obesity and its associated comorbidities.4
Obesity is associated with a persistent inflammatory condition often characterized as a sterile inflammatory process due to the absence of overt infectious agents. Obesity is accompanied by collagen deposition and fibrosis within adipose depots leading to imbalances in cytokine, free fatty acid (FFA), glucose, insulin, and leptin secretion at the level of individual adipocytes.5 In obese patients, excessive adipose tissue growth and hyperplasia is frequently not accompanied by commensurate levels of angiogenesis and neovascularization, resulting in restricted blood flow to adipocytes and the creation of a hypoxic state that triggers macrophage infiltration of adipose tissues.5 These macrophages then promote a pro-inflammatory state, increasing secretion of interleukin (IL)-6 and tumor necrosis factor (TNF)-α, while also decreasing secretion of adiponectin.6 IL-6 promotes hepatocytes to produce C-reactive protein, a marker of chronic inflammation, which is persistently elevated in obese individuals. Independently, TNF-α impedes the storage capacity of adipose tissue by interfering with carbohydrate metabolism, lipogenesis, adipogenesis, and lipolysis.7 Conversely, adiponectin is responsible for anti-inflammatory effects through M2 macrophage polarization, but its secretion is inhibited by elevated insulin levels seen in the obese.8
Moreover, abundant adipose tissue increases the release of FFAs and leptin. Elevated FFA levels decrease insulin sensitivity resulting in elevated glucose levels. The imbalance of cytokines, FFA, glucose, and insulin induces adipocyte enlargement, pancreatic islet β cell death, atherosclerosis, and fatty liver.9 Macroscopically, these effects result in an array of medical conditions commonly associated with obesity, including increased abdominal fat, high blood pressure, high blood sugar, high serum triglycerides, and low serum high-density lipoprotein. When at least three of these five conditions are present, the diagnosis of metabolic syndrome may be applied.10 Individuals with metabolic syndrome are at increased risk for heart disease, diabetes, and stroke.11 Obesity is also highly associated with an increased risk of cancer of the liver, stomach, gallbladder, pancreas, ovaries, thyroid, breast, colon, and brain.12,13 Obesity-related cancers are responsible for 20% of all deaths from cancer.14 Although the mechanisms by which obesity increases cancer incidence vary, the chronic inflammatory state induced by obesity, most notably the upregulation of IL-6, is pro-oncogenic.15
The primary treatment of obesity is weight loss achieved through a combination of healthy eating behaviors, physical activity, medication, and surgery. The challenges associated with weight loss are numerous and present many risks for patients. For example, increased oxygen demands from exercise can overwhelm the already compromised vascular network of obese individuals, causing further hypoxia and tissue damage.16 Overly aggressive diets can cause gallstones, nutritional deficiencies, and dehydration if the dieter is not careful. Weight loss medications are limited by their cost and side effects. Although surgery has the greatest potential to decrease weight rapidly, it is expensive and its invasive nature carries the risk of further complications (internal bleeding, perforation, long recovery time, etc.). Thus, responding to a public health threat as prevalent, expensive, and complex as obesity will necessitate better tools built using a new generation of advanced models.
Challenges Facing Adipose Tissue Biology and Obesity Research
Combatting the obesity epidemic has proven to be one of the greatest health challenges of the modern era. Obesity's multifactorial causes and complex effects on the body require further research and understanding the problem before novel solutions for treatment and prevention can be developed. However, this can be difficult to achieve from studies using human subjects since interpretation can be complicated by biological variability due to genetic and epigenetic factor heterogeneity. One solution to this problem has been the usage of animal models. Animal models offer greater control of confounders and can look at systemic effects of changes in adipose tissue on other organ systems. Animal models can be loosely divided into monogenic or polygenic models. Monogenic models refer to animals that either lack a gene or have a dysfunctional gene. These models are used to study a particular absent or dysfunctional gene. In contrast, polygenic models refer to animal models that maintain their genetic integrity but are subjected to different factors to induce a desired condition (i.e., obesity). The factors utilized to induce the desired condition can be surgical, chemical, dietary, or environmental.17
Early research into the apparent long-term stability of weight suggests that an axis between body fat and the brain exists to regulate energy intake and expenditure.18 The majority of animal models used to investigate the causes and treatments for obesity have focused on altering this axis to control satiation and limit energy intake.17 However, this strategy is limited by the complicated nature of how organisms regulate their eating behaviors; thus, any pharmacologic solution that attempts to target a single pathway faces a substantial challenge.19 In addition, attempting to increase energy expenditure rather than to limit energy intake is problematic as it is subject to species-dependent translational barriers.20
Limitations of animal models
In obesity research mouse models are the most commonly used nonhuman models. Mice provide researchers several inherent advantages over other animals. These include their short life span and breeding cycle, general ease of use, low upfront and maintenance costs, availability of genetically identical strains, well established protocols for genetic manipulation, and historically generated data.21 However, despite the widespread adoption of mouse models for obesity research, they are suboptimal in several key aspects. One difficulty is inducing obesity in mice. Creating long-standing obesity in mice often requires significant dietary modifications in combination with genetic and chemical modifications. This simultaneous introduction of multiple variables significantly limits the generalizability of mouse-based experiments to human biology.17 On multiple occasions, drug candidates developed from mouse trials showed either no effect on obesity in humans or, in the instances where an effect was seen, resulted in significant adverse side effects that were only seen in humans.22 One particularly notorious example was the appetite suppressant drug, fenfluramine. Fenfluramine was approved for the U.S. market in 1973 after successful mouse testing; however, in 1997, the drug had to be withdrawn from the market after as many as one-third of patients who were taking the drug were diagnosed with pulmonary hypertension and cardiac valvulopathy.23,24
While mouse models have taken hold as the premier economical animal obesity model for early stage research, nonhuman primate (NHP) models represent the other end of the spectrum. NHP models have traditionally served as the bridge between mouse and human models for advanced stage studies due to their close resemblance to human models. Unlike in mice, obesity is easily induced in NHPs, even unintentionally, through carbohydrate heavy diets. In general, the comorbidities that accompany obesity in humans such as hypertension, increased visceral adiposity, dyslipidemia, and secondary insulin resistance can be mirrored in NHP models.25 While NHPs represent a valuable and physiologically relevant model that can serve as an important translational bridge between basic studies performed in rodent models and clinical studies in humans, they are limited by high costs and complicated ethical issues that surround their usage in research.
Advancing knowledge based on adipose structure and function in health and disease
The costs and ethical issues associated with animal testing for new pharmaceuticals have incentivized researchers to utilize in vitro experiments to refine, reduce, and replace animal testing whenever possible. Experiments performed in vitro instead of in vivo are capable of providing faster results, and those using human-derived tissues can yield results with a high likelihood for external validation based on assays and outcomes generated in subsequent clinical trials.26 Much of this obesity-focused in vitro research has centered on the study of intact adipose tissue itself. Of the two major types of adipose tissue found in the body, the most common type is white adipose tissue, a loose connective tissue composed predominantly of white adipose cells which specialize in storing energy as fat. In contrast, the less common brown or beige adipose tissue is composed of brown or beige adipocytes, which consume energy to generate heat.27 In addition to adipocytes, adipose tissue also contains adipose stromal/stem cells, endothelial progenitors, fibroblasts, vascular smooth muscle cells, B and T lymphocytes, and macrophages which support the tissue.28 Recently, studies have begun to apply mathematical models to explain the complexity of adipose depots in three dimensions.29
Adipose tissue also interacts with the body's immunological and endocrine systems in significant ways. These interactions can involve different compartments both within the adipose tissue and within different organs entirely. Depending on the complexity of an experiment, it can become necessary to simulate these interactions among systems when attempting to use adipose tissue in vitro. For example, obese adipose tissue cells are significantly more resistant to insulin and contribute to changes in the overall composition of the tissue. Compartmentalized regions within in vitro models may be necessary for the accurate simulation of vascularization, stromal/stem regional activities, and nutrient/waste movement through adipose tissue.
The signaling and transport of factors through the vascular system involve contributions from multiple organs and tissues. In obesity, the signal and transport of factors through the vascular system are challenged by the decreased blood flow, arising from impairment of the vascular system from vasodilation to vasoconstriction, arterial structural changes, and limitations in microvessel function.16 In addition, the lack of capillaries and blood flow from rapid overgrowth of fat cells can cause adipose tissue to undergo a chronic inflammatory state resulting from hypoxia. This reflects the fact that obesity is a complex disease involving many tissues/cells connected through a vascular network. These components must be integrated into sophisticated in vitro models to ensure accuracy. Recapitulating hypoxic environments is also likely to be an important feature in diseased models.
Obesity is associated with elevated circulating levels of pro-inflammatory cytokines, IL-6, TNF-α, and C-reactive protein, due to increased leptin and FFA release from excess adipose tissue.30 Adipocytes are hypertrophied and hyperplastic in obese tissue, causing microvessels within the adipose tissue to lose the ability to transport oxygen, thereby contributing to a hypoxic state. In turn, hypoxia causes adipocytes to necrose and upregulates expression of inflammatory cytokines while attracting an infiltration of macrophages.31 In addition to the general infiltration of macrophages, decreased adiponectin levels cause resident and recruited macrophages to be induced to the M1 (classical) phenotype with a pro-inflammatory state.16 In addition, excess growth of adipocytes causes cellular stress, especially in the form of an unfolded protein response within the endoplasmic reticulum. This leads to more reactive oxygen species release/stress within the tissue.9 Thus, an accurate simulation of the chronic inflammation component of obesity in in vitro models will require the incorporation of an appropriate immune compartment.
Moreover, excess adipose tissue from obesity is accompanied by a change in the adipokine secretome such that normal endocrine function becomes disrupted.32 These adipokines not only affect the local tissue at which it was released through paracrine actions but also other distant organs using a systemic endocrine response. Therefore, multilevel endocrine functionality in in vitro models also must be intact, as insulin and leptin play a pivotal role in fatty acid synthesis and glucose uptake.33 Leptin's main purpose is to suppress hunger when there is adequate nutrition within the body, but obese patients' resistance to leptin contributes to the need to seek a surplus of energy. In addition, leptin has a reproductive function, as it stimulates the hypothalamus in regulating gonadotrophic releasing hormone, which directly affects luteinizing hormone and follicular stimulating hormone levels.34
In vitro models of obesity must exhibit the behaviors of the disease in all stages to further understand the implications of treatment at different time points. The models should also be able to recapitulate patient data, as obesity symptoms vary from person to person. Finally, in vitro models must recapitulate the adsorption, distribution, metabolism, elimination, toxicity (ADMET) of drugs that are found in vivo, as fat plays a pivotal role in the ADMET of drugs.35 Adipose tissue compromises anywhere from 20% to 50% of the body weight of an adult acting as a storage and release depot. Thus, modeling the human adipose profile could provide more accurate targeted drug delivery, dosing, and decreased toxicity.
Three-Dimensional Cultures—Adipose on Chip Models
Within the “organ-on-chip” field, “chips” are presented as either microfluidic or static multiwell cultures and are seeded with cells that take advantage of cell–matrix and cell–cell interactions and/or fluid flow to more accurately simulate physiologic conditions. These chips are also referred to as microphysiological systems (MPS). Chips used for such purposes are either manufactured as multiwell dishes or by photoetching a pattern of channels and chambers onto silicon wafers.36,37 Both methods are easily adaptable to high throughput applications with sample sizes occurring in the 96-well format or higher. Conventional experimental models culturing animal cell lines or human derived cell lines have been either seeded on plates for two-dimensional (2D) studies or scaffolds for three-dimensional (3D) studies.38,39 Unfortunately, the utility of traditional in vitro pharmaceutical studies has historically been limited by their inability to accurately simulate physiologic conditions. Adipose tissue is exceptionally reactive to the environment around it, as it dynamically stores and releases energy in response to hormonal and energetic cues. As a result, animal cell lines, such as murine 3T3-L1, that undergo adipogenesis during in vitro culture lack the single large lipid droplet that is characteristic of “signet ring-like” cells in vivo.39
Even among in vitro experiments, results from experiments can differ depending on how closely they emulate an in vivo environment. Most models have been found to lack control of major variables, such as fluid volume ratios. Thus, they poorly emulate a physiologic environment. Likewise, in a 2D geometry, cells cannot exhibit many of the behaviors that are normally seen in vivo.38 A common adopted solution to this problem is the replacement of a 2D structure with a 3D structure created using biomaterials such as collagen or silk.32,33,37,38,40,41 While the difference between 2D and 3D in vitro experiments is significant, the applications for such scaffolds also extend to in vivo models where the inclusion of biomaterial scaffolds can limit resorption and increase the engraftment of implanted cells.42,43
Perhaps the most significant issues associated with in vitro models come from their inability to simulate an environment with multiple compartments. This limits the utility of any model using adipose tissue as it is becoming increasingly accepted that adipose tissue has significant endocrine functionality necessary to maintain homeostasis.44 Such constraints prevent in vitro studies from evaluating a drug's interactions with many processes, such as ADMET.35 To create a better model capable of meeting these requirements, multiple organ systems, or outputs from multiple organ systems, must be simulated simultaneously. While this can be accomplished in a variety of ways, organ-on-a-chip systems are among the most promising.
Mature adipocyte models
Culturing mature, primary white adipose cells poses unique challenges. White adipose cells are characterized as being extremely buoyant, which limits their ability to attach to a surface. Traditional methods have utilized biomaterials to address this challenge by taking advantage of buoyancy as property. Harms et al. used a variation on the floating culture model to maintain isolated mature adipocytes from mouse and human tissue.45 By placing the isolated mature adipocyte fraction released by collagenase digestion beneath a transwell insert, the mature adipocytes remained viable for up to 2 weeks. Based on extensive polymerase chain reaction (PCR) and RNA-Seq analyses, the adipocytes continued to express a panel of adipogenic biomarkers. Furthermore, the adipocytes could be transduced with an adenoviral vector expressing the PPAR gamma coactivator 1 (PGC-1) transcription factor to induce expression of biomarkers consistent with a beige/brown phenotype.45 Huber et al. used a photocrosslinkable methacrylated gelatin scaffold to culture mature human adipocytes for periods of up to 2 weeks, which maintained both viability and expression of lineage specific biomarkers such as the lipid vacuole associated protein, perilipin.46 The period of UV light exposure could be adjusted to alter the mechanical properties of the scaffold, thereby mimicking the stiffness of both healthy (less stiff) and obese (more stiff) adipose tissue in vivo.46 Louis et al. achieved similar results using directly mature adipocytes or by differentiating human adipose-derived stromal/stem cell (ASC) and maintaining them for up to 3 weeks in a collagen scaffold.47
Furthermore, they noted more robust adipogenic differentiation in the 3D compared to the 2D culture conditions.47 Additional alternatives have been introduced to allow direct manipulation of adipose tissue fragments.48–50 For example, rather than maintain adipose tissue fragments floating in culture, which only maintains viability for several days to a week, the fragments containing mature adipocytes have been sandwiched in the mature adipocytes between tissue engineered sheets of adipose stromal/stem cells. The interest in adding ASC to sandwich the mature adipocytes resulted from two lines of reasoning. First, the ASC provides a structural extracellular matrix framework that can encompass and support the mature adipocytes. Second, the ASC provides paracrine growth factors that promote/retain adipogenic differentiation and functionality. Published models by the Fried laboratory and others have routinely kept adipose organoids in suspension culture although these only maintain robust longevity routinely for periods of up to 1 week in culture.51 The sandwich approach prevents the adipocyte buoyancy from floating the fragments into the culture media and allows mature adipocytes to remain viable and functional for up to 5 weeks.49
Preadipocyte cell line and primary cell models
An alternative that avoids the buoyancy issue of primary adipocytes has been the use of murine preadipocyte cell lines. These cells can differentiate into mature white adipocytes while remaining attached to a plastic surface. Of these murine cell lines, 3T3-L1 and 3T3-F442A have historically been used for modeling white adipose tissue in vitro in 3D structures.32,35,52,53 However, this alternative comes with its own limitations, which include a prolonged incubation period and the fact that they are derived from a single clone.35,54 It is noteworthy that while murine preadipocyte cell models display a robust in vitro lipolytic response to isoproterenol and glucose uptake response to insulin, these are not necessarily reflective of their human counterparts, which frequently do not exhibit such a pronounced functionality in assays. As a human alternative to these murine cell lines, Simpson-Golabi-Behmel syndrome preadipocyte cells can be used due to their capability of retaining the adipogenic differentiation potential for up to 50 generations.55 In addition, primary human ASC isolated from human adipose tissue, culture expanded as preadipocytes, and cryopreserved for future use can be used in such cultures.56 Well established methods and commercially available media reagents are available to promote the adipogenic differentiation of ASC within a 1- to 2-week period, and these approaches have been validated in both 2D and 3D culture models.57,58 Following exposure to inductive factors, including phosphodiesterase inhibitors capable of elevating cyclic AMP levels and ligands for the glucocorticoid, insulin, and peroxisome proliferator activated receptor γ (PPARγ), human and murine ASCs display a time dependent expression of early (PPARγ, CCAAT enhancer binding protein [C/EBPα]) and late (adiponectin, adipsin, fatty acid binding protein 4, leptin, lipoprotein lipase) genes associated with adipogenesis, as well as vacuolar accumulation of neutral lipid; however, unlike mature adipocytes in the body where lipid bodies coalesce into a single large vacuole occupying >90% of the cell volume, in vitro induced adipocytes frequently display multiple, smaller lipid vacuoles, suggestive of a less mature phenotype. Recent studies by Volz et al. have compared the adipocyte functionality of mature human adipocytes to differentiated primary ASC when encapsulated in collagen scaffolds.59 While both models expressed adipogenic mRNA biomarkers at similar levels, the primary ASC model displayed reduced maturity and increased anabolic indicators relative to mature primary adipocytes. Thus, mature adipocyte models remain an important control for future evaluation of adipose-on-a-chip models.59
Potential of induced pluripotent cell-derived cells in 3D adipose tissue models
Induced pluripotent cells (iPSCs) hold the promise to advance regenerative medicine through disease modeling, drug development, and organ synthesis. They share the ability of embryonic stem cells (ESCs) to differentiate into all three germ layers. Unlike ESCs, iPSCs do not require embryos as their source. Instead, iPSCs can be derived from a patient's own somatic cells, allowing the individual to serve as his or her own stem cell source with a lower risk for transplant rejection for regenerative applications and the potential for patient specific in vitro models. Because of their pluripotentiality, iPSCs hold a unique potential value in allowing investigators to isolate primary adipose-derived cells from patients with common or rare genetic conditions and explore the impact of those genomic mutations or variants not only on adipocytes but also on any cell type in the body.
iPSCs are generated when somatic cells express a panel of transcription factors such as Oct3/4, Sox2, c-Myc, and Klf4.60 This finding was first demonstrated by Takahashi and Yamanaka after screening different ESC transcription factors based on pluripotency induction.60,61 There are now several methods to induce pluripotency, including nonintegrating viral transfections, chemical agents, and nonviral integration approaches. However, the preexisting variations in the somatic cells themselves, the process of transduction, and the continuing passaging of the cells can introduce a risk of mutation.62 While the efficiency is very low—about 0.1%—research demonstrates that these odds can be improved through methods such as protein engineering.63 In addition, iPSCs display pluripotency based on a teratoma assay, which is the standard iPSC validation method. When implanted in vivo, iPSCs form tumors that exhibit properties of all three germ layers,64 based on staining for markers of differentiation (ectoderm, β-III-tubulin; mesoderm, Vimentin; and endoderm, CK AE1-AE3).65 It is noteworthy that a number of iPSC lines have been successfully generated from both human and murine ASCs with relatively high efficiency.66,67 Overall, iPSCs hold considerable promise for advancement of regenerative medicine due to their proliferative properties and telomerase activity, patient and disease specificity, and pluripotency.68
Beige/brown adipose tissue phenotypic models
These same technologies have been adapted for the creation of beige or brown adipose tissue on a chip (BAT-on-a-chip). Klingelhutz et al. demonstrated that spheroids created with murine brown adipose-derived stromal vascular fraction (SVF) cells, but not 2D cultures, displayed robust adipogenic induction of beige/brown biomarkers such as uncoupling protein 1 (UCP1) and cell death activator (CIDEA) based on PCR assays.69 In comparable studies, Vaicik et al. demonstrated expression of UCP1 and CIDEA biomarkers in spheroids prepared from murine SVF cells and encapsulated in a polyethyleneglycol diacrylate hydrogel.70 They noted that the mechanical properties of the hydrogel modulated the expression of beige/brown biomarkers; as the hydrogel stiffened, the level of UCP1 and CIDEA decreased.70 Yang et al. used both rat and human ASCs to create beige/brown differentiated spheroids in a polyethyleneglycol hydrogel.71
In addition to validating differentiation based on PCR detection of biomarkers, they documented altered oxygen consumption rates (OCRs) in response to ATP synthase inhibitors and chemicals known to modify mitochondrial function, consistent with the increased mitochondriogenesis occurring during brown fat adipogenesis.71 Likewise, Tharp et al. used an acrylated hyaluronic acid hydrogel to create spheroids with murine ASC and demonstrated their beige/brown differentiation in 3D cultures, validating this based on both PCR and OCR assays.72,73 In addition, they demonstrated that the in vitro differentiated beige/brown organoids retained their functionality when implanted into live mice for up to 2 weeks based on response to cold exposure, a well characterized physiological feature of BAT.72,73 Finally, Harms et al. have demonstrated the ability to create a beige/brown phenotype following viral transduction of a PGC-1 expression construct.45 In light of the interest in beige/BAT as a target for drug discovery in the context of obesity prevention and treatment, the development of a robust beige/brown adipose 3D model for drug discovery assays holds considerable interest for biotech and pharmaceutical companies.
Organoid and spheroid models
Daquinag et al. used murine 3T3-L1 cells in combination with ferrous nanobeads to develop a spheroid adipocyte model based on magnetic levitation.74 Upon introduction of an endothelial cell line, these constructs displayed evidence of vascularization in vitro.74 Similarly, Klingelhutz et al. have developed hanging drop spheroids created by culturing primary stromal vascular fraction cells from human or murine adipose tissue in low adhesion plates.69 These organoids displayed robust adipogenesis and exhibited functional outputs, such as secretion of adipokines and pro-inflammatory factors, which could be adapted to high-throughput formats suitable for screening of environmental toxins.69
Multicompartment models
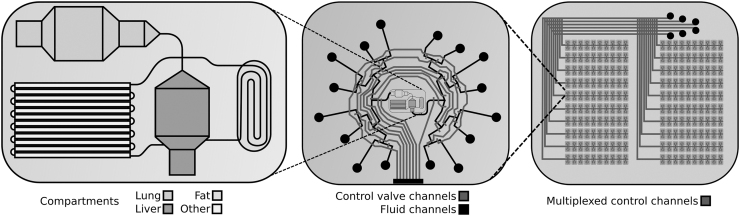
A further advancement of this approach has been achieved by seeding different cell cultures into different chambers on the chip, thereby creating a multicompartment system. Having these systems together on the same chip allows for the simulation of complex processes, such as bioaccumulation of hydrophobic compounds in adipose cell compartments. One of the earliest attempts at creating such a system was reported by Viravaidya and Shuler35 who accomplished this by compartmentalizing different cell cultures into four discrete areas, representing the lung, liver, fat, and other tissues, on a single silicon chip (Fig. 1).35 This study presented the effects of bioaccumulation of several toxins, such as naphthalene, and demonstrated the direct effects of white adipocytes on the toxicity to other organ systems.35 The significance of white adipose tissue's ability to modulate toxicity of compounds is great enough to require that it be accounted for when creating safety profiles for drugs. Likewise, Ahluwalia et al. developed a three-way perfusion circuit joining hepatocyte, endothelial, and human visceral adipocyte compartments.26 These investigators noted a correlation between increased adipocyte differentiation with increased secretion of endothelial-derived pro-inflammatory molecules and hepatic-derived biomarkers, such as albumin. This system has the potential to serve as an in vitro model of visceral or central obesity.26
FIG. 1.
Left: This design represents the microscale compartmentalized cell culture system used in Viravaidya and Shuler35 in which wells and channels were etched onto a silicon chip. The wells were then seeded with cell types corresponding to different organ systems. The result allows for simulation of complex pharmacokinetic mechanisms, such as bioaccumulation.35 Middle: The structure in the middle represents the automated 16-channel microfluidic multiplexer used by Li et al. to dynamically stimulate and interrogate cells of interest.83 The red lines represent control channels, which can be used to automatically control exposure to the fluids loaded into the reservoirs (black circles). Right: Fat-on-a-chip technology allows for multiple instances of an experiment to be run simultaneously with massive parallelization, as seen in Wu et al.84
Other important considerations when attempting to simulate physiologic conditions in vitro are the ratio and spatial arrangement of different cell types. For example, if one cell type is responsible for metabolizing a drug to an active form that then exerts its effect on a second cell type, a smaller ratio of the former to the latter than is found in vivo would show reduced effectiveness of the drug. One method of addressing this problem utilizes allometric scaling principles. These principles use body size to determine the physiologic parameters needed to simulate physiological conditions. However, this approach carries its own issues as organs and cells change in a multitude of ways as they are scaled, which are not necessarily consistent across organs or cells.75 Physiologically based pharmacokinetic (PBPK) modeling is a commonly used alternative that modifies classical pharmacokinetic models to be compatible with multicompartment models.76 Studies evaluating the functional coupling of multiple organ-on-a-chip models in sequence have determined that the metabolism of well characterized compounds (terfenadine, trimethylamine, vitamin D3) in vitro accurately recapitulates clinical in vivo data.77 Nevertheless, while well suited for testing very specific hypotheses, the generalizability of PBPK to organs-on-a-chip may be limited.75 A third method of addressing this issue relies on the proportions of heterogeneity that are unique to the individual donor of the adipose tissue. These characteristics are thought to be conserved within the SVF of the adipose tissue. Recent studies have begun to combine SVF cells isolated from adipose tissue with human-derived bioscaffolds to recreate the heterogeneity and complexity of the intact tissue in vitro.78,79 These fat-on-a-chip cultures are anticipated to be a reflection of the diseased or nondiseased state of the adipose tissue from which the SVF population originated and hold great promise for mimicking diseased adipose tissue responses.
Microfluidic models
Indeed, simulating physiologic conditions suitable for drug testing requires more complex methods of distributing fluids than those achieved by simple diffusion alone. For this purpose microfluidics and/or 3D bioprinting are often used to simulate a circulatory system by circulating medium between compartments.35 In Godwin et al. microfluidics were used to allow both culturing and sampling of primary adipocytes.48 Liu et al. extended this approach by combining primary human preadipocytes alone or in combination with mononuclear cells within a perfusion bioreactor construct.80 The adipocytes could be differentiated in situ over a 2-week period, and the combination cultures displayed increased pro-inflammatory cytokine secretion relative to adipocytes alone, indicating a modulatory effect of the mononuclear cells. In comparable studies, Rogala et al. maintained primary cultures of mature human adipocytes in a microperfusion bioreactor for up to 5 weeks in vitro while maintaining viability and functionality based on fatty acid uptake and lipolytic assays in response to beta-adrenergic agonists.81 The ability to maintain cultures for such extended periods of time while retaining metabolic functionality and viability are features favorable for drug development screening assays.
Microfluidics may also be used to precisely assay secretions or control delivery of hormones and nutrients to discrete regions on the chip.82 While most work with such systems have utilized passively controlled fluid distribution systems, it is possible to build systems with actively controlled valves. Such systems would theoretically be capable of greater parallelization and data resolution by virtue of the ability to precisely deliver or sample fluid at a given time to a single region of a crowded chip (Fig. 1).83,84
However, the usage of microfluidics to move fluid creates new challenges as the direct flow of media exerts potentially damaging shear forces over the large fragile adipocytes.50 This effect has wide ranging consequences affecting cell morphology, proliferation, and differentiation that cannot be eliminated even when using a monolayer culture.85 Adipose cells in vivo are protected from shear stresses by the vasculature, which shields the adipose compartment from the bulk flow of fluids. To simulate this in vitro, Loskill et al. created an endothelial-like barrier that connected the media channels and adipose chambers using micropores, allowing them to maintain functional lipid metabolism for a period of weeks.33 While this study used 3T3-L1 cells to create a white adipose depot, the same laboratory has previously used primary murine ASC in combination with an acrylated hyaluronic acid scaffold to create 3D beige/brown adipose depots in vitro and in vivo.72 This suggests that the microfluidic approach can be adapted to mimic any physiological adipose depot. Another study which used a membrane barrier found it to significantly increase the viability of the cells after several days in culture.54 Static cultures have the advantage of lower risk for shear stress-induced apoptosis, but would need to address the physiological components associated with nutrient delivery through an active and selective vascular barrier to control nutrients to the adipose compartment.
Potential advantages of readouts from fat-on-a-chip models
Metabolic changes experienced by adipose tissues during an experiment may be quantified using various techniques, which may be broadly classified as either destructive or nondestructive. Destructive methods include immunohistochemistry, electron microscopy, flow cytometry, mass spectrometry, or PCR. Conversely, nondestructive methods have usually focused on measuring the products secreted by cells, such as monitoring endocrine products in the supernatant or radiolabeled CO2 production after adding labeled palmitic acid to the culture.27 Newer generations of visual imaging technologies such as two-photon imaging may also be used to nondestructively image tissue function in vitro.86 A significant benefit to nondestructive methods of analysis is that they allow for significantly greater temporal resolution than destructive methods and allow multiple readouts to be correlated on the same chip over time (improving accuracy and reducing the number of samples and thus cost required for each experiment). The goal of such studies is to move toward evaluation methods that provide indications predictive of human clinical responses to drug administration or environmental cues.
Conclusions and Future Directions
New technologies relating to the isolation, characterization, and manipulation of primary human adipose-derived cells and scaffolds have advanced the adipose biology field considerably within the past two decades. There is now ample evidence documenting the superiority of 3D compared to 2D in vitro adipose models as a discovery tool for metabolic and obesity research. For example, adipocytes maintained under conditions mimicking their native biomechanical state are capable of enhanced adipokine secretory function.87 The 3D adipose depots can be generated with either homogeneous or heterogeneous adipose-derived cell populations in native, biological, or synthetic scaffolds. Furthermore, the constructs can be prepared with cells derived from donors in good health, with features consistent with metabolic syndrome, or from individuals with varying levels of severity of diabetes.
Other patient demographics such as age, gender, ethnicity, and so on can also be considered to correlate outcomes as well. The availability of such tools presents novel opportunities for human-based in vitro studies. Using such models, it will be possible to compare the relative response of healthy versus diabetic adipose depots to small molecules targeting metabolic disease processes in a manner that has a greater likelihood to be predictive of responses in a clinical setting. Such studies can be conducted in a static system using traditional cell culture methodologies or in a dynamic perfusion system using microfluidic technologies. In addition, the current 3D adipose depots can be adapted to create a more complex microphysiological system.
First, it will be possible to introduce inflammatory and immune cells into the adipose depot during the preparation process or subsequent to full adipogenic differentiation through circulatory delivery. This modification has the potential to mimic the sterile inflammatory changes now associated with the metabolic syndrome and diabetes. Second, it will be possible to link 3D adipose depots to MPS representing other organs, such as cardiac or skeletal muscle, liver, or pancreas. By creating circulatory microfluidic networks linking multiple human derived metabolic organoids, it will be possible to model the impact of obesity and diabetes on cardiac, hepatic, musculoskeletal, and pancreatic function. The outcomes can be quantified using biochemical assays comparable to those used in a clinical setting. Finally, it will be possible to use adipose 3D constructs alone or in linkage to other organoids to examine the impact of biological aging on metabolic function. With such an approach, it will be feasible to predictably and reliably evaluate rapamycin, metformin, or related agents with respect to their ability to mitigate the effects of chronological aging on the human body. As humanized 3D adipose-derived constructs become more accessible to the research community, it is anticipated that they will accelerate and enhance the drug discovery pipeline for obesity, diabetes, and metabolic diseases by reducing the preclinical evaluation process and improving predictive accuracy. Such developments, applications, and usages of existing technologies can change the paradigm of personalized medicine and create substantial progress in our approach to modern medicine.
Acknowledgments
The authors thank their colleagues at LaCell LLC, Obatala Sciences, Inc., and Axosim, Inc. for their critiques and comments concerning earlier drafts of this article.
Disclosure Statement
T.F., X.W., and J.G. are co-founders, co-owners, and members or employees of Obatala Sciences, Inc.; in addition, X.W. and J.G. are co-founders and co-owners of LaCell LLC and Talaria Antibodies, Inc. A.A. is a current employee of Obatala Sciences, Inc. The remaining co-authors have nothing to disclose.
Funding Information
No federal funding was used to sponsor the writing of this review.
References
- 1. Finkelstein, E.A., Khavjou, O.A., Thompson, H., et al. Obesity and severe obesity forecasts through 2030. Am J Prev Med 42, 563, 2012 [DOI] [PubMed] [Google Scholar]
- 2. Alwan, A. A Global Status Report on Noncommunicable Diseases 2010. ISBN: 9241564229, World Health Organization, 2011 [Google Scholar]
- 3. NCD Risk Factor Collaboration. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 390, 2627, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hruby, A., and Hu, F.B.. The epidemiology of obesity: a big picture. Pharmacoeconomics 33, 673, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marcelin, G., Silveira, A.L.M., Martins, L.B., Ferreira, A.V., and Clement, K.. Deciphering the cellular interplays underlying obesity-induced adipose tissue fibrosis. J Clin Invest 129, 4032, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ellulu, M.S., Patimah, I., Khaza'ai, H., Rahmat, A., and Abed, Y.. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci 13, 851, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cawthorn, W.P., and Sethi, J.K.. TNF-alpha and adipocyte biology. FEBS Lett 582, 117, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vona-Davis, L., and Rose, D.P.. Adipokines as endocrine, paracrine, and autocrine factors in breast cancer risk and progression. Endocr Relat Cancer 14, 189, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Lumeng, C.N., and Saltiel, A.R.. Inflammatory links between obesity and metabolic disease. J Clin Invest 121, 2111, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Prabhakaran, D., and Anand, S.S.. The metabolic syndrome: an emerging risk state for cardiovascular disease. Vasc Med 9, 55, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Kaur, J. A comprehensive review on metabolic syndrome. Cardiol Res Pract 2014, 943162, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12. Azvolinsky, A. The obesity-cancer link: a growing connection. J Natl Cancer Inst 108, pii:, 2016 [DOI] [PubMed] [Google Scholar]
- 13. Strong, A.L., Burow, M.E., Gimble, J.M., and Bunnell, B.A.. Concise review: the obesity cancer paradigm: exploration of the interactions and crosstalk with adipose stem cells. Stem Cells 33, 318, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. De Pergola, G., and Silvestris, F.. Obesity as a major risk factor for cancer. J Obes 2013, 291546, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chang, Q., Bournazou, E., Sansone, P., et al. The IL-6/JAK/Stat3 feed-forward loop drives tumorigenesis and metastasis. Neoplasia 15, 848, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stapleton, P.A., James, M.E., Goodwill, A.G., and Frisbee, J.C.. Obesity and vascular dysfunction. Pathophysiology 15, 79, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lutz, T.A., and Woods, S.C.. Overview of animal models of obesity. Curr Protoc Pharmacol Chapter 5, Unit5.61, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leibel, R.L. Molecular physiology of weight regulation in mice and humans. Int J Obes (Lond) 32 (Suppl 7), S98, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Woods, S.C. The control of food intake: behavioral versus molecular perspectives. Cell Metab 9, 489, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaiyala, K.J., and Schwartz, M.W.. Toward a more complete (and less controversial) understanding of energy expenditure and its role in obesity pathogenesis. Diabetes 60, 17, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barrett, P., Mercer, J.G., and Morgan, P.J.. Preclinical models for obesity research. Dis Model Mech 9, 1245, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vickers, S.P., Jackson, H.C., and Cheetham, S.C.. The utility of animal models to evaluate novel anti-obesity agents. Br J Pharmacol 164, 1248, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brenot, F., Herve, P., Petitpretz, P., Parent, F., Duroux, P., and Simonneau, G.. Primary pulmonary hypertension and fenfluramine use. Br Heart J 70, 537, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Connolly, H.M., Crary, J.L., McGoon, M.D., et al. Valvular heart disease associated with fenfluramine-phentermine. N Engl J Med 337, 581, 1997 [DOI] [PubMed] [Google Scholar]
- 25. Havel, P.J., Kievit, P., Comuzzie, A.G., and Bremer, A.A.. Use and importance of nonhuman primates in metabolic disease research: current state of the field. ILAR J 58, 251, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ahluwalia, A., Misto, A., Vozzi, F., et al. Systemic and vascular inflammation in an in-vitro model of central obesity. PLoS One 13, e0192824, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Abbott, R.D., Borowsky, F.E., Quinn, K.P., Bernstein, D.L., Georgakoudi, I., and Kaplan, D.L.. Non-invasive assessments of adipose tissue metabolism in vitro. Ann Biomed Eng 44, 725, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lindroos, B., Suuronen, R., and Miettinen, S.. The potential of adipose stem cells in regenerative medicine. Stem Cell Rev Rep 7, 269, 2011 [DOI] [PubMed] [Google Scholar]
- 29. Dichamp, J., Barreau, C., Guissard, C., et al. 3D analysis of the whole subcutaneous adipose tissue reveals a complex spatial network of interconnected lobules with heterogeneous browning ability. Sci Rep 9, 6684, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. de Heredia, F.P., Gomez-Martinez, S., and Marcos, A.. Obesity, inflammation and the immune system. Proc Nutr Soc 71, 332, 2012 [DOI] [PubMed] [Google Scholar]
- 31. Ye, J. Adipose tissue vascularization: its role in chronic inflammation. Curr Diab Rep 11, 203, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li, X., and Easley, C.J.. Microfluidic systems for studying dynamic function of adipocytes and adipose tissue. Anal Bioanal Chem 410, 791, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Loskill, P., Sezhian, T., Tharp, K.M., et al. WAT-on-a-chip: a physiologically relevant microfluidic system incorporating white adipose tissue. Lab Chip 17, 1645, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Perez-Perez, A., Sanchez-Jimenez, F., Maymo, J., Duenas, J.L., Varone, C., and Sanchez-Margalet, V.. Role of leptin in female reproduction. Clin Chem Lab Med 53, 15, 2015 [DOI] [PubMed] [Google Scholar]
- 35. Viravaidya, K., and Shuler, M.L.. Incorporation of 3T3-L1 cells to mimic bioaccumulation in a microscale cell culture analog device for toxicity studies. Biotechnol Prog 20, 590, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Brooks, J.C., Judd, R.L., and Easley, C.J.. Culture and sampling of primary adipose tissue in practical microfluidic systems. Methods Mol Biol 1566, 185, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sin, A., Chin, K.C., Jamil, M.F., Kostov, Y., Rao, G., and Shuler, M.L.. The design and fabrication of three-chamber microscale cell culture analog devices with integrated dissolved oxygen sensors. Biotechnol Prog 20, 338, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Wang, R.Y., Abbott, R.D., Zieba, A., Borowsky, F.E., and Kaplan, D.L.. Development of a three-dimensional adipose tissue model for studying embryonic exposures to obesogenic chemicals. Ann Biomed Eng 45, 1807, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Abbott, R.D., Wang, R.Y., Reagan, M.R., et al. The use of silk as a scaffold for mature, sustainable unilocular adipose 3D tissue engineered systems. Adv Healthc Mater 5, 1667, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Abbott, R.D., Kimmerling, E.P., Cairns, D.M., and Kaplan, D.L.. Silk as a biomaterial to support long-term three-dimensional tissue cultures. ACS Appl Mater Interfaces 8, 21861, 2016 [DOI] [PubMed] [Google Scholar]
- 41. Rnjak-Kovacina, J., Wray, L.S., Burke, K.A., et al. Lyophilized silk sponges: a versatile biomaterial platform for soft tissue engineering. ACS Biomater Sci Eng 1, 260, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bellas, E., Lo, T.J., Fournier, E.P., et al. Injectable silk foams for soft tissue regeneration. Adv Healthc Mater 4, 452, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bellas, E., Panilaitis, B.J., Glettig, D.L., et al. Sustained volume retention in vivo with adipocyte and lipoaspirate seeded silk scaffolds. Biomaterials 34, 2960, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rutkowski, J.M., Stern, J.H., and Scherer, P.E.. The cell biology of fat expansion. J Cell Biol 208, 501, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Harms, M.J., Li, Q., Lee, S., et al. Mature human white adipocytes cultured under membranes maintain identity, function, and can transdifferentiate into brown-like adipocytes. Cell Rep 27, 213.e5, 2019 [DOI] [PubMed] [Google Scholar]
- 46. Huber, B., Borchers, K., Tovar, G.E., and Kluger, P.J.. Methacrylated gelatin and mature adipocytes are promising components for adipose tissue engineering. J Biomater Appl 30, 699, 2016 [DOI] [PubMed] [Google Scholar]
- 47. Louis, F., Kitano, S., Mano, J.F., and Matsusaki, M.. 3D collagen microfibers stimulate the functionality of preadipocytes and maintain the phenotype of mature adipocytes for long term cultures. Acta Biomater 84, 194, 2019 [DOI] [PubMed] [Google Scholar]
- 48. Godwin, L.A., Brooks, J.C., Hoepfner, L.D., Wanders, D., Judd, R.L., and Easley, C.J.. A microfluidic interface for the culture and sampling of adiponectin from primary adipocytes. Analyst 140, 1019, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lau, F.H., Vogel, K., Luckett, J.P., et al. Sandwiched white adipose tissue: a microphysiological system of primary human adipose tissue. Tissue Eng Part C Methods 24, 135, 2018 [DOI] [PubMed] [Google Scholar]
- 50. Abbott, R.D., Raja, W.K., Wang, R.Y., et al. Long term perfusion system supporting adipogenesis. Methods 84, 84, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Carswell, K.A., Lee, M.J., and Fried, S.K.. Culture of isolated human adipocytes and isolated adipose tissue. Methods Mol Biol 806, 203, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Park, S.B., Lee, S.Y., Jung, W.H., et al. Development of in vitro three-dimensional co-culture system for metabolic syndrome therapeutic agents. Diabetes Obes Metab 21, 1146, 2019 [DOI] [PubMed] [Google Scholar]
- 53. Aulthouse, A.L., Freeh, E., Newstead, S., and Stockert, A.L.. Part 1: a novel model for three-dimensional culture of 3T3-L1 preadipocytes stimulates spontaneous cell differentiation independent of chemical induction typically required in monolayer. Nutr Metab Insights 12, 1178638819841399, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tanataweethum, N., Zelaya, A., Yang, F., Cohen, R.N., Brey, E.M., and Bhushan, A.. Establishment and characterization of a primary murine adipose tissue-chip. Biotechnol Bioeng 115, 1979, 2018 [DOI] [PubMed] [Google Scholar]
- 55. Fischer-Posovszky, P., Newell, F.S., Wabitsch, M., and Tornqvist, H.E.. Human SGBS cells—a unique tool for studies of human fat cell biology. Obes Facts 1, 184, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gimble, J.M., Katz, A.J., and Bunnell, B.A.. Adipose-derived stem cells for regenerative medicine. Circ Res 100, 1249, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Li, J., Curley, J.L., Floyd, Z.E., Wu, X., Halvorsen, Y.D.C., and Gimble, J.M.. Isolation of human adipose-derived stem cells from lipoaspirates. Methods Mol Biol 1773, 155, 2018 [DOI] [PubMed] [Google Scholar]
- 58. Yu, G., Wu, X., Dietrich, M.A., et al. Yield and characterization of subcutaneous human adipose-derived stem cells by flow cytometric and adipogenic mRNA analyzes. Cytotherapy 12, 538, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Volz, A.C., Omengo, B., Gehrke, S., and Kluger, P.J.. Comparing the use of differentiated adipose-derived stem cells and mature adipocytes to model adipose tissue in vitro. Differentiation 110, 19, 2019 [DOI] [PubMed] [Google Scholar]
- 60. Takahashi, K., and Yamanaka, S.. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663, 2006 [DOI] [PubMed] [Google Scholar]
- 61. Malik, N., and Rao, M.S.. A review of the methods for human iPSC derivation. Methods Mol Biol 997, 23, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yoshihara, M., Hayashizaki, Y., and Murakawa, Y.. Genomic instability of iPSCs: challenges towards their clinical applications. Stem Cell Rev Rep 13, 7, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang, L., Huang, D., Huang, C., et al. Enhanced human somatic cell reprogramming efficiency by fusion of the MYC transactivation domain and OCT4. Stem Cell Res 25, 88, 2017 [DOI] [PubMed] [Google Scholar]
- 64. Nelakanti, R.V., Kooreman, N.G., and Wu, J.C.. Teratoma formation: a tool for monitoring pluripotency in stem cell research. Curr Protoc Stem Cell Biol 32, 4A.8.1, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Montes, R., Romero, T., Cabrera, S., et al. Generation and characterization of the human iPSC line PBMC1-iPS4F1 from adult peripheral blood mononuclear cells. Stem Cell Res 15, 614, 2015 [DOI] [PubMed] [Google Scholar]
- 66. Esteban, M.A., Wang, T., Qin, B., et al. Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell 6, 71, 2010 [DOI] [PubMed] [Google Scholar]
- 67. Sugii, S., Kida, Y., Kawamura, T., et al. Human and mouse adipose-derived cells support feeder-independent induction of pluripotent stem cells. Proc Natl Acad Sci U S A 107, 3558, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Huang, Y., Liang, P., Liu, D., Huang, J., and Songyang, Z.. Telomere regulation in pluripotent stem cells. Protein Cell 5, 194, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Klingelhutz, A.J., Gourronc, F.A., Chaly, A., et al. Scaffold-free generation of uniform adipose spheroids for metabolism research and drug discovery. Sci Rep 8, 523, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Vaicik, M.K., Morse, M., Blagajcevic, A., et al. Hydrogel-based engineering of beige adipose tissue. J Mater Chem B 3, 7903, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yang, J.P., Anderson, A.E., McCartney, A., et al. Metabolically active three-dimensional brown adipose tissue engineered from white adipose-derived stem cells. Tissue Eng Part A 23, 253, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tharp, K.M., Jha, A.K., Kraiczy, J., et al. Matrix-assisted transplantation of functional beige adipose tissue. Diabetes 64, 3713, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tharp, K.M., and Stahl, A.. Bioengineering beige adipose tissue therapeutics. Front Endocrinol (Lausanne) 6, 164, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Daquinag, A.C., Souza, G.R., and Kolonin, M.G.. Adipose tissue engineering in three-dimensional levitation tissue culture system based on magnetic nanoparticles. Tissue Eng Part C Methods 19, 336, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Moraes, C., Labuz, J.M., Leung, B.M., Inoue, M., Chun, T.H., and Takayama, S.. On being the right size: scaling effects in designing a human-on-a-chip. Integr Biol (Camb) 5, 1149, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gelman, A.B.F., and Jiang, J.. Physiological pharmacokinetic analysis using population modeling and informative prior distributions. J Am Stat Assoc 91, 1400, 1996 [Google Scholar]
- 77. Vernetti, L., Gough, A., Baetz, N., et al. Functional coupling of human microphysiology systems: intestine, liver, kidney proximal tubule, blood-brain barrier and skeletal muscle. Sci Rep 7, 42296, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Frazier, T.P., Bowles, A., Lee, S., et al. Serially transplanted nonpericytic CD146(−) adipose stromal/stem cells in silk bioscaffolds regenerate adipose tissue in vivo. Stem Cells 34, 1097, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Muller, S., Ader, I., Creff, J., et al. Human adipose stromal-vascular fraction self-organizes to form vascularized adipose tissue in 3D cultures. Sci Rep 9, 7250, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Liu, Y., Kongsuphol, P., Chiam, S.Y., et al. Adipose-on-a-chip: a dynamic microphysiological in vitro model of the human adipose for immune-metabolic analysis in type II diabetes. Lab Chip 19, 241, 2019 [DOI] [PubMed] [Google Scholar]
- 81. Rogala, J.B.C., Kromidasa, E., Probsta, C., Schneidera, S., Schenke-Layland, K., and Loskilla, P.. WAT's up!? Organ-on-a-chip integrating human mature white adipose tissues for mechanistic research and pharmaceutical applications. bioRxiv 585141, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Brooks, J.C., Ford, K.I., Holder, D.H., Holtan, M.D., and Easley, C.J.. Macro-to-micro interfacing to microfluidic channels using 3D-printed templates: application to time-resolved secretion sampling of endocrine tissue. Analyst 141, 5714, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Li, X., Brooks, J.C., Hu, J., Ford, K.I., and Easley, C.J.. 3D-templated, fully automated microfluidic input/output multiplexer for endocrine tissue culture and secretion sampling. Lab Chip 17, 341, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wu, X., Schneider, N., Platen, A., et al. In situ characterization of the mTORC1 during adipogenesis of human adult stem cells on chip. Proc Natl Acad Sci U S A 113, E4143–E4150, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kim, S.H., Ahn, K., and Park, J.Y.. Responses of human adipose-derived stem cells to interstitial level of extremely low shear flows regarding differentiation, morphology, and proliferation. Lab Chip 17, 2115, 2017 [DOI] [PubMed] [Google Scholar]
- 86. Ward, A., Quinn, K.P., Bellas, E., Georgakoudi, I., and Kaplan, D.L.. Noninvasive metabolic imaging of engineered 3D human adipose tissue in a perfusion bioreactor. PLoS One 8, e55696, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Amos, P.J., Kapur, S.K., Stapor, P.C., et al. Human adipose-derived stromal cells accelerate diabetic wound healing: impact of cell formulation and delivery. Tissue Eng Part A 16, 1595, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]