Abstract
An antimicrobial supramolecular assembly (ASA) is conspicuous in biomedical applications. Among the alternatives to overcome microbial resistance to antibiotics and drugs, ASAs, including antimicrobial peptides (AMPs) and polymers (APs), provide formulations with optimal antimicrobial activity and acceptable toxicity. AMPs and APs have been delivered by a variety of carriers such as nanoparticles, coatings, multilayers, hydrogels, liposomes, nanodisks, lyotropic lipid phases, nanostructured lipid carriers, etc. They have similar mechanisms of action involving adsorption to the cell wall, penetration across the cell membrane, and microbe lysis. APs, however, offer the advantage of cheap synthetic procedures, chemical stability, and improved adsorption (due to multipoint attachment to microbes), as compared to the expensive synthetic routes, poor yield, and subpar in vivo stability seen in AMPs. We review recent advances in polymer−based antimicrobial assemblies involving AMPs and APs.
Keywords: cationic peptides and polymers, structure–function relationship, hydrophobic–hydrophilic balance, mechanism of cell lysis, multidrug−resistant microbes, ESKAPE pathogens, MRSA, quaternized biopolymers, antibiofilm and thromboresistant activity
1. Introduction
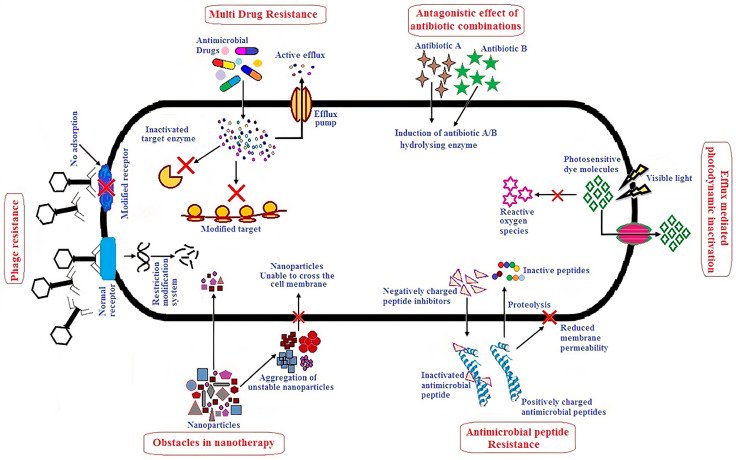
Antibiotic−resistant pathogens have been considered a major menace to humans [1] so that a variety of combinatory anti−pathogenic therapies have emerged [2,3,4]. Antibiotics have been combined with bacteriophages [5], photodynamic light therapy yielding reactive oxygen species (ROS) [6], antimicrobial peptides (AMPs) [7,8,9], nanoparticles (NPs), cationic antimicrobial polymers (APs), and cationic lipids assembled as bilayer disks, vesicles, or micelles [10,11]. In general, alternative/novel therapies against multidrug−resistant (MDR) pathogens have shown promising in vitro results, but overcoming their in vivo drawbacks has remained a central challenge. Figure 1 illustrates some limitations in the way of alternative approaches.
Figure 1.
Alternative approaches to overcome multidrug−resistant (MDR) microbes and their possible shortcomings. Reprinted from [1].
An antimicrobial supramolecular assembly (ASA) has been opening new horizons in terms of allowing for optimal as well as broad antimicrobial activity [1,12,13,14,15,16,17,18,19,20]. Components in ASA materials can be organic, inorganic or hybrid, acting as antibacterial agents themselves and/or as carriers for timed−release of the antibacterial agent(s). ASA formulations have included coatings [21,22,23,24], functionalized surfaces [25,26], NPs [14,17,27,28,29,30,31,32], surfactant and/or lipid dispersions such as vesicles, liposomes, lipid disks [12,13,20,33], hydrogels [34,35,36], wound dressings [37], dentistry materials [38], etc.
Several ASA modes of action have been described in the literature, such as leaching of the antibacterial agent from the material [39], killing upon contact [25,40,41,42,43,44], or preventing microbial adhesion [22,45,46]. Several ASA−delivered AMPs or APs act by penetrating the cell wall, reaching bacterial cell membranes and causing their disruption [25,47].
In this review, recent developments in ASAs employing AMPs or APs were discussed in regards to their structure, activity, and applications.
2. ASA with AMPs
2.1. Structure and Antimicrobial Activity of ASA with AMPs
AMPs have been considered as amphipathic, cationic polymers with less than 50 amino acid residues, often displaying secondary structures such as α−helix [7,8,9,48]. Their main role in the innate immune system as an indispensable line of defense against pathogens in different body parts of mammals, plants, and other animals has been well documented [9]. In humans, AMPs present in oral and nasal mucosae could activate anti−inflammatory cells to sites of damaged tissue [49]. In fish, constant exposure to various types of pathogens has led to an immune system based on AMPs [50]. The cationic character determined AMPs’ interactions with the oppositely charged bacteria cell wall and penetration in the cell membrane. Destabilization of the membrane electrochemical potential allowed AMP insertion in the plasmatic membrane of the bacteria, its rupture and bacterial cell death [7,9,51,52]. Major issues against AMPs applications have been related to AMPs’ toxicity to eukaryotic cells, poor stability in vivo with eventual degradation during transportation to their target cells and organs [32,51,53,54].
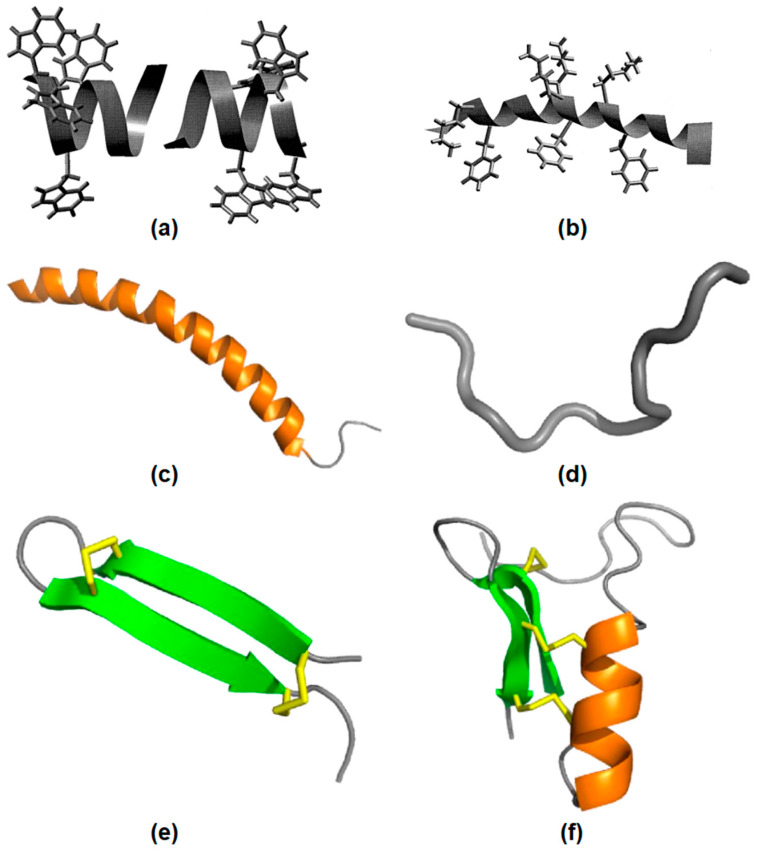
AMPs have been classified according to their origin [8]. When they were extracted from bacteria or fungi, they belong to the nonribosomal synthetized peptides (NRAMP) class. When extracted from eukaryotic cells, they belong to the ribosomal synthetized peptides (RAMP) class. Gramicidin, vancomycin and polymyxin B are examples of NRAMPs, while nisin and melittin are RAMPs [8,55]. AMPs usually have one or more secondary structures such as α−helix, β−sheet, αβ, and non−αβ [56]. A huge structural diversity of AMPs have the common feature of positive charge and amphipathic nature [57]. Figure 2 illustrates AMPs structures. In Figure 2a, gramicidin A incorporated in bilayer membranes can be seen as a peptide dimer traversing the bilayer with four tryptophan side−chains as anchors at the membrane interface. Figure 2b shows the structure of the antimicrobial frog skin peptide magainin as determined by nuclear magnetic resonance (NMR) spectroscopy in the presence of sodium dodecyl sulfate (SDS) micelles with the side−chains of lysine and phenylalanine residues [57]. Figure 2c shows LL−37 peptide adopting a typical α−helical (orange) conformation in the presence of micelles. Figure 2d shows indolicidin in an extended conformation. Figure 2e shows the spider−derived β−hairpin peptide gomesin with β−sheets (green) typically stabilized by disulfide bonds (yellow). Figure 2f shows phormicin with both α−helix and β−sheet secondary structures [58].
Figure 2.
AMPs structural features. (a) The gramicidin A structure in membranes. (b) The magainin structure in micelles, adapted from [57], Copyright 1999, with permission from Elsevier. (c) The LL−37 peptide structure in micelles. (d) The indolicidin structure. (e) The gomesin structure stabilized by disulfide bonds. (f) The insect CSαβ−defensin phormicin, Adapted with permission from [58]. Copyright 2019 Elsevier.
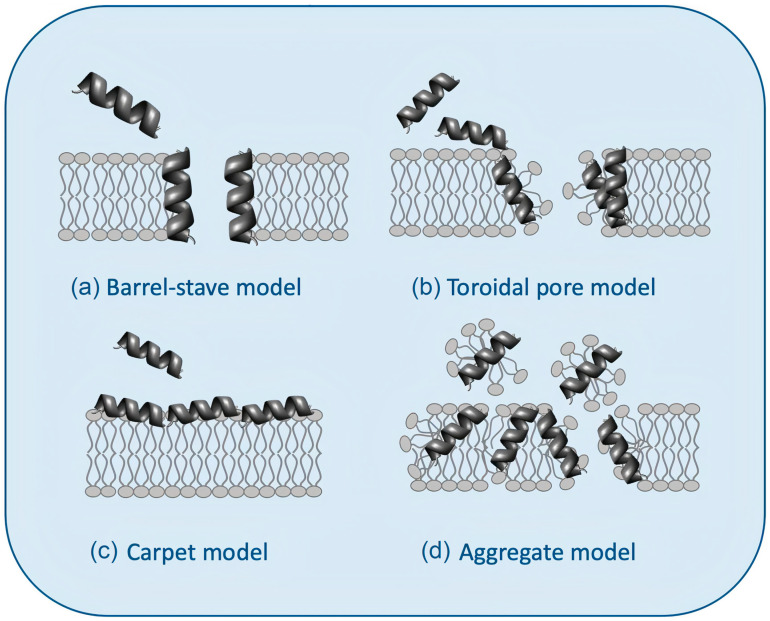
AMPs affect bacteria by inhibiting enzymatic activity, DNA or protein synthesis, or by piercing bacterial cell walls and membranes [7,9,51,52,59]. Figure 3 illustrates possible mechanisms of action for AMPs [60]. Figure 3a shows the barrel−stave model: the AMPs approach the lipid bilayer in parallel orientation but eventually penetrate it perpendicularly, keeping intermolecular peptide interactions. Figure 3b shows the “toroidal pore” model with two stages: at low concentrations (inactive state), peptides remain parallel to the plane of the bilayer; from a critical concentration, peptide molecules reorient perpendicularly penetrating the hydrophobic region of the bilayer (active state) and, together with some lipid molecules, adopt a multi−pore configuration with irreversible rupture of the plasma membrane. Figure 3c shows the carpet model: peptides remain parallel to the lipid bilayer until reaching a threshold concentration above which the membrane becomes unstable and disintegrates, forming micelles, in the so−called aggregate or “detergent−like” model (Figure 3d) [9,52,60,61,62].
Figure 3.
Models for the interaction between antimicrobial peptides (AMPs) and bilayer membranes: (a) the barrel−stave model, (b) the toroidal pore model, (c) the carpet model, and (d) the aggregate or “detergent−like” model was adapted from [60].
The establishment of structure–function relationships for AMPs has been deemed a difficult task [63]. More than 2000 natural or synthetic AMPs with different lengths, sequences, 3−dimensional (3−D) structures and intermolecular interactions have been described. Moreover, AMPs high sensitivity to their environment has been reported from their medium−dependent−conformations. A good example is the behavior of gramicidin D (Gr) in different media [64].
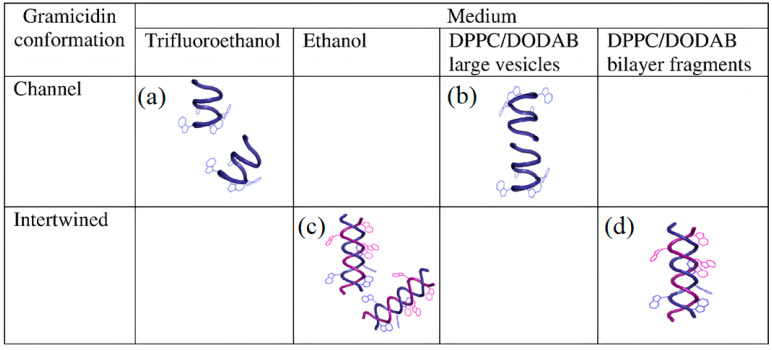
Figure 4 illustrates Gr conformations in different media depicted from Gr circular dichroism (CD) and intrinsic fluorescence spectra [64]. Figure 4a,b shows Gr beta−helix in trifluoroethanol and large lipid vesicles (LV), respectively. Figure 4c,d illustrates the intertwined Gr conformation in ethanol and nanosized lipid bilayer fragments (BF), respectively. The dimeric Gr functional channel has been described as a pore spanning lipid bilayers. This pore has been associated with an ionic imbalance and bacterial cell death. Curiously, Gr channels only have been observed in LV. On the other hand, Gr intertwined dimers in the non−channel conformation only occur at the borders of cationic bilayer fragments, as shown in Figure 4d. Both LVs and BFs shield Gr tryptophans against quenching by acrylamide. However, the Stern–Volmer quenching constant is slightly higher for Gr in BFs than in LVs, confirming that the peptide was more exposed to the water medium in BFs than in LVs [64].
Figure 4.
Medium−dependent gramicidin (Gr) conformation: (a) Gr beta−helix in trifluoroethanol, (b) Gr beta−helix and dimeric channel in large bilayer vesicles (LVs), (c) Gr intertwined beta−helices in ethanol, and (d) Gr intertwined beta−helices in lipid bilayer fragments (BFs). Gr molecules sense a nonpolar medium in the LV bilayer and acquire its functional channel conformation. Gr molecules sense a polar medium in the BF bilayer and become intertwined. The lipids in LVs or BFs are dipalmitoylphosphatidyl choline (DPPC) and dioctadecyl dimethyl ammonium bromide (DODAB) at a 1:1 molar ratio. Reprinted with permission from [64]. Copyright 2012 Elsevier.
2.2. ASA with AMPs for Preserving Activity and Reducing Toxicity
An important issue regarding AMPs performance against bacteria has been the formulation [8]. The Gr behavior in different media can be used to exemplify the importance of the formulation. Furthermore, Gr formulation plays a central role not only on activity but also on toxicity, as discussed below.
Gr extracted from Bacillus brevis contains a group of peptides composed of 80%, 6% and 14% of gramicidin A, B, and C, respectively [64,65]. Due to Gr toxicity against eukaryotic cells, its use over a range of low concentrations has been limited to topical applications avoiding systemic administration [66,67].
In assemblies with dioctadecyldimethylammonium bromide (DODAB) bilayers, both DODAB and Gr interacted with Escherichia coli and Staphylococcus aureus. Thereby DODAB antimicrobial activity against Gram−negative bacteria [42,43] has been combined with Gr activity against Gram−positive bacteria [67]. This combination broadens the spectrum of antimicrobial activity. In addition, the toxicity against yeast eukaryotic cells of the DPDAB/Gr formulation has been tested and yielded improved yeast viability in comparison to the one of Gr alone [67].
Gr has also been formulated in lipid polymer NPs [68]. The insertion of Gr functional channels on DODAB supported bilayers has been achieved thanks to the optimization of the construction onto negatively charged polystyrene sulfate (PSS) NPs. Firstly, PSS NPs have been covered with a positively charged DODAB bilayer, which increased the zeta−average diameter by 8–10 nm, changed the zeta−potential of the NPs from negative to positive, and yielded a narrow size distribution for the PSS/DODAB/Gr NPs [68]. This formulation has been displaying broad and high antimicrobial activity at very small concentrations of the antimicrobials, namely, 0.057 and 0.0057 mM for DODAB and Gr concentrations, respectively. The results emphasized the advantages of highly organized, nanostructured, and lipid polymer cationic NPs to achieve hybrid combinations of antimicrobials with broad−spectrum activity at tiny DODAB and Gr concentrations [68]. Further applications for these Gr formulations using NPs have been envisaged in the biomedical field for treating burns, wounds, ulcers, caries, and pulp infections in dentistry, as antifouling, antimicrobial, and antibiofilm coatings on surfaces or embedded in hydrogels [8,18,35,63,66,69,70].
Since the 1960s, the AMP nisin, a RAMP lantibiotic, has been widely employed as a food preservative to extend the shelf life of dairy products (lantibiotics refers to AMPs produced by bacteria) [52,66]. In contrast to Gr, nisin has been considered nontoxic to eukaryotic cells and effective against food spoiling bacteria, showing stability over a pH and temperature range plus low susceptibility to enzymatic proteolysis [52].
Nisin has also been formulated as films released from polymer/nisin multilayers; whereas nisin/polyacrylic acid (PAA) layers disintegrated in 24 h in water solution, nisin/dextran sulfate (DX) films were stable for 14 days without releasing nisin; both films hampered the spread of Staphylococcus epidermidis biofilms in disk diffusion tests; therapeutic utility proposed for nisin/PAA films was treating burns and wounds due to the quick nisin release, whereas nisin/DX coatings would impart steady sterilization of surfaces over long periods of time [71].
The AMP melittin, the main component of bee venom, has been formulated on a variety of lipid or polymer based−assemblies [55,72]. In model membrane and cell culture studies, certain melittin analogues have been proposed as anticancer, antimicrobial, and low hemolytic activity [73]. The interest for this AMP has been increasing due to possible uses in a variety of cancer treatments [74,75,76,77,78,79] despite the high in vivo cytotoxicity and hemolytic activity in intravenous applications [80]. Apoptosis of cancer cells has been often reported in association with melittin; for example, cancer cell growth was inhibited via the increase of death receptor 3 expression and inactivation of NF−kappa beta in lung cancer cells [76]. A graphene formulation facilitated melittin piercing of the cell wall, causing cell lysis in Gram−negative and −positive bacteria [81].
The acronym ESKAPE pathogens have been employed to encompass E: Enterococcus faecium, S: Staphylococcus aureus or Stenotrophomonas maltophilia, K: Klebsiella pneumoniae or C: Clostridioides difficile, A: Acinetobacter baumannii, P: Pseudomonas aeruginosa, E: Enterobacter spp., or Enterobacteriaceae. These MDR bacteria have been concerning physicians due to very few options left for treating infected patients; AMPs have been considered important for reversing this situation [7,82]. Recently, the synergy between antibiotics and certain AMPs has been described in a murine, sub−cutaneous abscess model caused by ESKAPE pathogens [82]. The bacteria organization on surfaces as single and multispecies biofilms has required several techniques for proper evaluation of unconventional agents, including AMPs in the treatment of biofilm infections [83,84]. Designing and optimizing AMPs will have to consider that the targets reached may not be the same; peptides could be active against several kinds of cells with activity and selectivity resulting from interaction with multiple target cell components; the cellular composition has been affecting the AMP–target cell interaction and also the design of novel AMPs [85].
Various pathogens, such as polymyxin−sensitive Salmonella species, have been able to penetrate macrophages, where they persisted and multiplied; modifications of NPs, liposomes, and mesoporous silica with specific cell ligands have been enabling them with penetration into macrophages and killing of intracellular pathogens [86]. Metal−based NPs, including gold NPs, have been proposed as particularly promising platforms for the intracellular delivery of AMPs, such as polymyxins eliminating intracellular Salmonella Enterica Serovar Typhimurium [87].
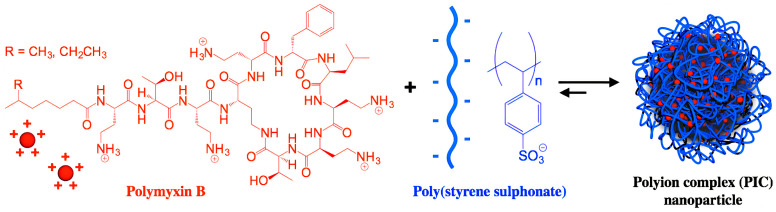
Polymyxin has been deemed of critical medical importance against severe nosocomial multidrug−resistant Gram−negative bacteria causing nosocomial pneumonia. Several polymyxin formulations have been developed for parenteral use (for treatment of cystic fibrosis, pneumonia, bacteremia, and urinary tract infections), inhalation (cystic fibrosis, pneumonia), and topical use (optic and ophthalmic solutions). The most common polymyxin side effects have been dose−dependent nephrotoxicity and neurotoxicity; since polymyxins were essentially not absorbed by the gastrointestinal tract, their encapsulation into suitable carriers improved intestinal permeability, thereby allowing novel formulations administered by the oral route [88]. Figure 5 shows a polymer−based formulation for polymyxin B based on the electrostatic attraction between the cationic peptide and the anionic poly (styrene sulphonate) polymer as reproduced from [89,90]; the antimicrobial activity of the polymyxin AMP was influenced by the degree of polymerization of the poly−ion (DP): a low DP improved antimicrobial activity, while a high DP improved the NPs stability [89].
Figure 5.
Nanoparticles of polymyxin B and poly (styrene sulphonate) active against Pseudomonas aeruginosa. Reprinted with permission from [90]. Copyright 2017 Elsevier.
The relative high burden of methicillin−resistant S. aureus (MRSA) has been a major concern in healthcare; vancomycin, a glycopeptide antibiotic inhibiting cell wall biosynthesis, has remained the drug of choice for treatment of severe MRSA infections for many years. Unfortunately, vancomycin−resistant S. aureus strains have been disclosed in the 1990s; their polygenic molecular basis of resistance was due to stepwise mutations in genes encoding molecules predominantly involved in cell envelope biosynthesis [91]. Resistance has been associated with persistent infections, vancomycin treatment failure, and poor clinical outcomes. S. aureus strains isolated from humans, pigs, and cattle have created intermediate resistance to vancomycin [92]. Vancomycin formulated as nanoplexes of the antibiotic with dextran sulfate sodium salt has recently addressed MRSA infections; the size, polydispersity, and zeta potential of the optimized nanoplexes were 84.6 ± 4.3 nm, 0.449 ± 0.024, and −33.0 ± 4.9 mV, respectively, with 90.4 ± 0.8% complexation efficiency and 62.3 ± 0.2% drug loading; in vivo studies using a BALB/c mouse skin infection model revealed that nanoplexes reduced MRSA burden by 2.3−fold compared to bare vancomycin [93]. Liposomal vancomycin topical formulations have also produced similar results against MRSA, reconfirming the importance of the formulation for fighting drug−resistant microbia [94].
AMPs have been fighting not only bacteria but also other pathogens, such as fungi [95], viruses, and protozoa [15]. Besides, their versatility allowed extensions for treating from skin wounds to cancer. Therefore, future studies with AMPs are necessary, for instance, with the improvement of its stability and its scaling−up projection in the industry [51], to go beyond the combat against antimicrobial resistance. Recent advances in antimicrobial polymers in general [96] or natural and synthetic AMPs, in particular, have been reviewed [50,95,97].
Major applications for AMPs have also been the subject of important review articles such as the use of AMPs for drug design and therapeutics [98,99], natural additives for food preservation [52,100,101], prevention of caries, and pulpal infections due to dental plaques and similar others [102,103].
The most important types of carriers for AMPs were liposomes [64,67,69,70,101], nanostructured lipid carriers [104], lyotropic lipid phases (cubic and hexagonal) [105], lipid nanodisks, and bilayer fragments [10,12], NPs of several types such as biomimetic [20,28,32,68], polymeric [61,89,106], magnetic [107,108], metal−AMPs designed as metallodrugs with nuclease, and protease activity [109] or silver co−spinned with nisin in polymeric nanofibers [110], hydrogels [36,37,111], silver in alginate hydrogels [112], or fabrics [113,114,115].
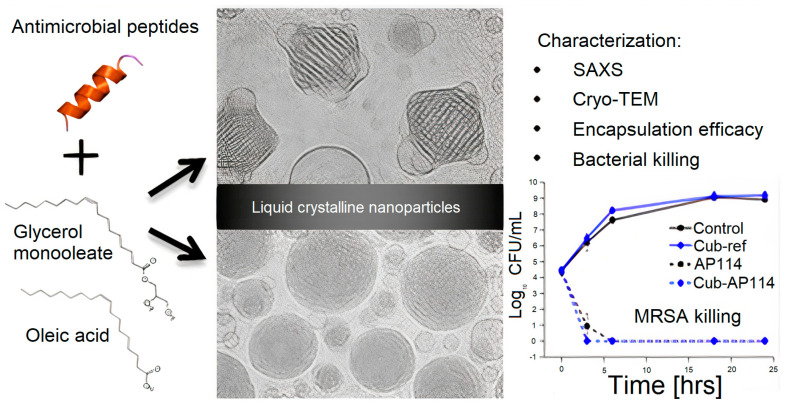
Lipid−based liquid crystals as carriers for antimicrobial peptides emphasized the importance of more fluid lipid phases for the antimicrobial effect [105]. Figure 6 shows lipid−based liquid crystals from cubic and hexagonal phases; these lyotropic liquid crystalline (LC) structures consisting of cubic glycerol monooleate/water and hexagonal glycerol monooleate/oleic acid/water assemblies have been examined as carriers for AMPs. Certain AMPs had their antimicrobial activity preserved, whereas others had their activity reduced by the carriers; LC−structured gels or NPs had the capability of solubilizing both hydrophilic and hydrophobic substances, as well as being biocompatible and biodegradable; depending on AMP nature, LC showed no effect on AMPs antimicrobial activity or a diminished effect on this property.
Figure 6.
Lyotropic liquid−crystalline (LC) nanoparticles (NPs) tested as carriers for AMPs. Reproduced with permission from [105]. Copyright 2016 American Chemical Society.
Importantly, several AMPs formulations have been proposed against multidrug resistance. For example, liposomal AMPs combined with vancomycin exhibited improved activity against intracellular MRSA; after selecting AMPs with high antimicrobial activity, the selected peptides were lipidated, combined with model membranes (liposomes), and tested for intracellular activity against MRSA infecting human embryonic kidney epithelial cells in culture (HEK−293). They possessed good cell penetration to act against the intracellular MRSA; in addition, there was sustained release for the AMPs with a consequent improvement in the bioavailability [116].
Mycobacterium tuberculosis is intrinsically resistant to many antibiotics due to mutations that lead to novel strains. AMPs with metal complexes have been proposed as advantageous combinations since metal complexes associated with known AMPs often present different mechanisms of action with respect to single peptides: the destruction of bacterial plasma membranes as well as hydrolytic or oxidative cleavage of nucleic acids promoted by metal−based compounds followed from their role in the generation of reactive oxygen species able to degrade biomolecules [117]; the formulations complexing metal with AMPs could fight drug resistance against tuberculosis [118]. Adding antimicrobial and antibiofilm activities to AMPs via covalently bound metal−binding motifs improved their activities in certain cases; when combined with meropenem, streptomycin, or chloramphenicol, certain variants showed synergistic effects against E. coli (KpC+ 1812446) biofilms; the addition of motif also improved the survival rate of mice in a systemic infection model and reduced the hemolytic activity of the wild−type AMP [119].
Besides the AMPs, the cationic antimicrobial polymers (APs) represent another extremely promising class of antimicrobial molecules. APs are briefly presented and discussed regarding their outstanding properties in the next section.
3. ASAs with APs
3.1. Structure and Antimicrobial Activity for ASA with APs
Antimicrobial polymers (APs) have been designed to exhibit similar mechanisms of action as AMPs while diminishing AMPs’ disadvantages. APs are not degradable by enzymatic proteolysis as the AMPs, display controllable dose−dependent toxicity towards mammalian cells, have lower manufacturing costs than AMPs do, and can easily become available commercially from their facile production on a large scale following industrial synthetic protocols [48,120,121,122,123,124,125]. Most APs, similar to AMPs, are positively charged in water solution; the electrostatic attraction drives the physical adsorption onto pathogenic microbes as the first step of their mechanism of action. Thereafter, they penetrate cell walls and membranes, leading to various degrees of antimicrobial activity and toxicity that can culminate in cell lysis with leakage of internal contents [14,16,17,26]. The determination of APs specific cytotoxicity against mammalian cells has been a major requirement to establish their utility in vivo; it is important to evaluate whether APs used at a minimal bactericidal concentration (MBC) do not affect mammalian cells in culture [10,126,127]. Certain APs showed dose−dependent cytotoxicity against human epithelial cells, lung fibroblasts, and monocytes [128]. Nevertheless, their high antimicrobial activity at low doses has been the main motivation for the intense and extensive research on APs over the last twenty years [10,13,14,15,17,129]; they have been often described as a promising platform for the development of next−generation antimicrobial agents [120,122]. In addition, their flexible properties, facile synthesis, or modification from natural polymers, such as chitosan, gelatin, dextran, starch, or cellulose, also led to various alternative therapeutic applications [20,130]. Among their important applications, APs have been used as adjuvants for vaccine design and antigen presentation [126,127,129] and for gene and drug delivery [131]; interestingly, aminated microcrystalline cellulose killed melanoma and breast cancer cell lines in culture [131]. Excellent overviews on the synthesis and preparation of cationic polymers are available [48,130,132,133].
Most cationic polymers bear amine functions that can be protonated, such as polyethyleneimine (PEI), poly−L−(lysine) (PLL), chitosan, and poly [2−(N,N−dimethylamino)ethyl methacrylate] (PDMAEMA) [134]. While these polymers have inherent cationic charges, others have been developed by introducing cationic moieties such as aminated cellulose, which, compared to chitosan, represent a novel cationic cellulose derivative with improved mucoadhesive properties as well as sufficient hydration at physiological pH [135]. Chitosan derivatives bearing quaternary ammonium moieties and displaying good antimicrobial activity have also been developed [136].
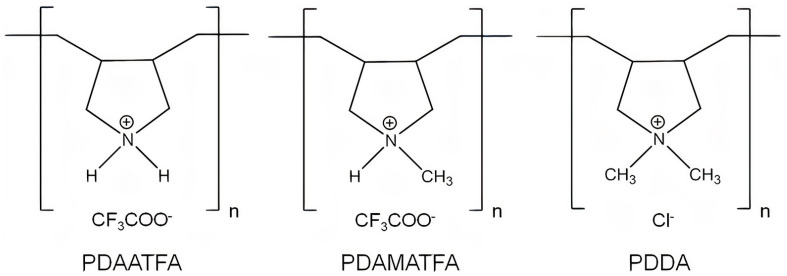
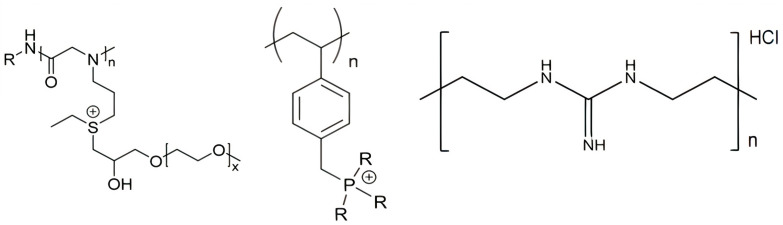
Antimicrobial cationic polymers mainly contain two functional components: the cationic and the hydrophobic groups. The antimicrobial activity is influenced by the type, amount, location, and distribution of these two components; the structure–function relationship for AP could provide some guidelines for developing molecular engineering of antimicrobial cationic polymers with tailor−made structures and functions [137]. For example, the chemical structures of poly (diallyldimethylammonium chloride) (PDDA) derivatives with different hydrophobic–hydrophilic balances such as poly (diallylammonium trifluoroacetate) (PDAATFA), poly (diallylmethylammonium trifluoroacetate) (PDAMATFA), and PDDA itself are shown in Figure 7 [17]; their hydrophobic–hydrophilic balance and their antimicrobial activity against Gram−negative bacteria increase from left to right [17,138].
Figure 7.
Chemical structures of some PDDA derivatives with increasing hydrophobic–hydrophilic balance from left to right. Polymers are poly (diallylammonium trifluoroacetate) (PDAATFA), poly (diallylmethylammonium trifluoroacetate) (PDAMATFA) and poly (diallyldimethylammonium chloride) PDDA. Reproduced from [17].
The activities of the PDDA derivatives on Figure 7 against Gram−positive bacteria or fungus were not so clearly dependent on the hydrophobic–hydrophilic balance of the molecule, possibly due to superimposed effects of AP molecular weight and/or the nature of the molecular composition and the nature of the microbes cell wall for different species; against fungus, no effect of the molecular weight or the hydrophobic–hydrophilic balance were apparent; the fungus was very sensitive to all PDDA derivatives [16,17,138].
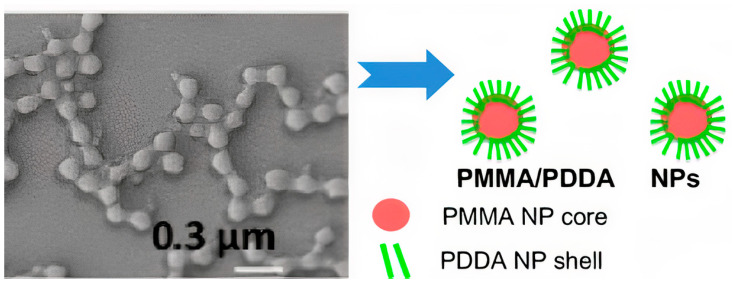
PDDA immobilization in biocompatible poly (methyl methacrylate) (PMMA) NPs diminished its antimicrobial activity to a certain extent; PMMA/PDDA NPs synthesis from emulsion polymerization of the methyl methacrylate (MMA) monomer in the presence of PDDA yielded interesting core−shell NPs; the free PDDA molecules showed lower minimal microbicidal concentrations (MMC) than the immobilized ones [17]. The core−shell nature of PMMA/PDDA NPs was an interesting finding; the hydrophobic, neutral PMMA polymeric core became surrounded by a shell of the hydrophilic, cationic polyelectrolyte PDDA, as shown in Figure 8. Apparently, the cationic NPs were not as efficient as free PDDA to penetrate the microbes’ cell walls and membranes [17,23].
Figure 8.
Scanning electron micrograph of poly (methyl methacrylate) PMMA/poly (diallyl dimethyl ammonium) chloride PDDA antimicrobial nanoparticles (PMMA/PDDA NPs) and schematic representation of their core−shell structure at low ionic strength. Reproduced from [17]. The NPs were obtained from emulsion polymerization of methyl methacrylate (MMA) in the presence of PDDA.
Besides the quaternary ammonium, cationic moieties in antimicrobial polymers could be sulfonium [139,140], guanidinium [125,128,141], or phosphonium [142,143]. Polymeric sulfonium salts exhibited high antibacterial activity against Gram−positive bacteria but were less active against Gram−negative bacteria [139]. It was found that the activity of the polymeric sulfonium salts was much higher than that of the corresponding monomers, particularly against S. aureus. Figure 9 shows some chemical structures for cationic APs with sulfonium, phosphonium, or guanidinium as the cationic moiety.
Figure 9.
From left to right, some cationic polymers with different cationic moieties such as sulfonium [139,140], phosphonium [142], and guanidinium [141,144].
The sulfonium polypeptoid on the left (Figure 9) was obtained via ring−opening polymerization and post−modification strategy with excellent biological performance for the treatment of the infections caused by S. aureus; it contained both sulfonium and oligo (ethylene glycol) (OEG) motifs and displayed high selectivity for the pathogens over mammalian red blood cells [140]. Similar to natural AMPs that contain cationic and amphipathic moieties, several synthetic antimicrobial polymers named polypeptoids have been proposed. They were polymers analogous to AMPs with the advantages of facile synthesis at low cost and excellent stability against degradation in vivo. These peptidomimetic polymers had, for example, an N−substituted glycine backbone similar to the polypeptoid shown on the left in Figure 9 [140].
Various cationic polymers with quaternary ammonium or phosphonium, which possessed high antimicrobial activities in solution, exhibited a significant decrease in their antimicrobial efficiency after crosslinking or solubilization loss [17,145]. The antimicrobial activity of water−insoluble polycations could be preserved as long as the polymeric chains were long and flexible for penetration through the bacterial membranes. In a series of water−insoluble N−alkyl−N,N−dimethyl de−oxy ammonium celluloses, those modified by N,N−dimethyl dodecyl ammonium exhibited antimicrobial activity, while those modified by N,N−dimethyl butyl ammonium did not [146]. PDDA immobilization as the outer shell of PMMA nanoparticles core also reduced antimicrobial activity in comparison to the activity of free PDDA in solution [17]. Another interesting example of phosphonium−modified polymer were some inulin derivatives; inulin is a natural, renewable, biodegradable, and water−soluble carbohydrate recently modified with quaternary phosphonium salt to impart antifungal activity to the molecule. The antifungal activity increased with the alkyl chain length of the grafted quaternary phosphonium salt [147].
3.2. Biomedical Applications for ASA with APs
Synergistic antimicrobial activity against ESKAPE pathogens was reported for combinations of quaternary ammonium and guanidinium homopolymers [148]. Guanidinium polymers were successfully used to target intracellular, multidrug−resistant Staphylococcus aureus [149]. Non−leaching polyacrylate and guanidine−based copolymer NPs with 80–130 nm mean diameter were synthesized by emulsion polymerization with acrylate and glycidyl−methacrylate monomers and modified by oligoguanidine; NPs and their films presented long−term antimicrobial activity [150]. The antimicrobial copolymer of polyhexamethylene guanidine hydrochloride and polypropylene glycol diglycidyl ether adhered onto cotton fabrics both by physical adsorption and covalent binding, resulting in durable antimicrobial properties against Escherichia coli and Staphylococcus aureus; antimicrobial activity remained unchanged even after laundering the fabrics with detergent solution [151]. New chitosan derivatives bearing guanidinium functions were synthesized following different synthesis strategies. N−guanidinium chitosan acetate and N−guanidinium chitosan chloride were synthesized by direct reaction between chitosan and cyanamide in the presence of scandium (III) triflate. The synthesis of N−guanidinium chitosan (N,N′−dicyclohexyl) chloride and N−guanidinium chitosan (N−(3−dimethylaminopropyl)−N’−ethyl hydrochloride) chloride involved the reaction of chitosan with carbodiimides in ionic liquid. All newly guanylated chitosan derivatives displayed high antimicrobial activity in comparison with neat chitosan [152]. Guanidine−based polymers imparting antimicrobial activity to polysaccharides, such as cellulose, starch, and cyclodextrin, have been recently overviewed [153].
The accepted mechanism of action for cationic polymers involves the same membrane disruptive effects observed for AMPs; major events are adsorption into the bacterial cell surface, penetration into the cell wall, and insertion into the cytoplasmic membrane (due to hydrophobic group of the polymer) with membrane disruption, leakage of cytoplasmic contents, and eventually, cell lysis [14,96,121,154,155,156]. Mechanisms of action for APs and AMPs indeed decreased the odds of creating resistant bacteria [7,9,51,52,59].
Antimicrobial biopolymers were an important branch in this field; their exclusive qualities usually include being natural, biodegradable, biocompatible, cheap and extracted from biomass−derived waste, and, some of them, being both antibacterial and antifungal agents [8,157,158]. The interest in these molecules has been growing along with environmental concerns [159]. Some examples of biopolymers are: cellulose, the most abundant one in nature; chitosan, a versatile polymer attainable by treating chitin from the crustacean shell waste generated by the seafood industry; and lignin, a byproduct of the paper industry with great qualities, including antioxidant activity and high thermal stability [158,159,160,161]. Recent studies with hydroxypropyl methylcellulose (HPMC)/lignin and HPMC/lignin/chitosan films had positive results, with antimicrobial effect against both Gram−positive and Gram−negative at 35 and 0–7 °C [158]. Even though most biopolymers came with interesting advantages, some of them have their problems, such as chitosan sensitiveness to temperature and pH [162]. Biopolymers could eventually replace synthetic ones without critical side effects [159].
The biomedical applications for antimicrobial polymers required thromboresistant materials also able to avoid the formation of biofilms [163]. In biomedical devices such as catheters, intravascular grafts, extracorporeal circuits and membrane oxygenators, the adsorption of serum proteins onto these blood−contacting materials may trigger the blood coagulation cascade, whereas the contact with infective pathogens may cause the formation of biofilms and infection.
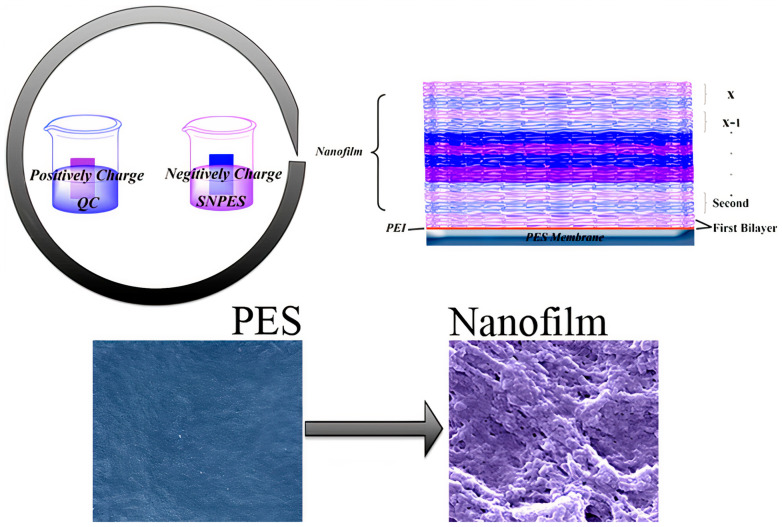
A few examples in the literature deal with the production of materials displaying both thromboresistant and anti−biofilm properties. The sulfonated polymers and sulfated glycosaminoglycan have been widely recognized as heparin−mimetic components since they show similar functionalities as heparin, displaying anticlotting and antithrombotic activities, the stabilization of growth factors, and the promotion of angiogenesis [164,165]. Some combined polymers joining multiple functional groups on one surface, such as a synthetic heparin−mimetic polymer or hydrophilic polymer brushes (e.g., PEG) with antibacterial quaternary compounds (QAC), were described. The layer−by−layer assembly of sulfonic amino poly (ether sulfone) (SNPES) and quaternized chitosan (QC) yielded multilayers. Additionally, when submitted to systematic tests for antithrombotic and antimicrobial activity, the multilayers showed that the heparin−mimetic multilayer−coated membrane suppressed adsorption of bovine serum fibrinogen, platelet adhesion, and activation, prolonged clotting times, and reduced activation of blood complement. Furthermore, the antibacterial test suggested that the multilayer−coated substrates exhibited activity against Escherichia coli and Staphylococcus aureus [166]. Figure 10 illustrates the preparation of these multilayered coatings.
Figure 10.
Scheme of layer−by−layer assembly of sulfonic amino poly (ether sulfone) (SNPES), a heparin−mimetic polymer, and quaternized chitosan (QC), an antimicrobial polymer, onto poly (ether sulfone) (PES) membrane substrates. SNPES and QC are negatively and positively charged, respectively. Reproduced with permission from [166]. Copyright 2015 American Chemical Society.
The layer−by−layer approach has also been useful to impart anti−biofilm property to materials; deposition of PDDA and poly (acrylic acid) (PAA) layers with an outer PAA layer onto polyester fabric prevented S. aureus adhesion to the fabric and allowed removal of bacteria by water rinse [167].
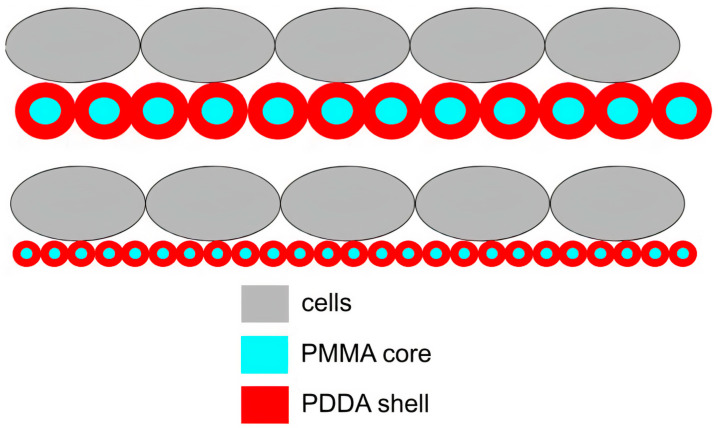
Recent progress in the biomedical applications of polydopamine (PDA) nanostructures, such as drug delivery, photothermal therapy, bone and tissue engineering, cell adhesion, and antimicrobial uses, were recently reviewed [168]. Rough PDA films on various substrates such as reverse osmosis filtration membranes [169], glass, plastic, stainless steel, and gauze showed remarkable antibacterial activity as compared with smooth PDA films [170]. The antifouling and antibacterial properties were attributed to the fact that the PDA film with positively charged amine groups would be responsible for the interaction with bacterial cell walls at high pH, causing cell rupture. Moreover, the rough surface of PDA exhibited higher particle contact with substrates during vigorous shaking and thus exhibited more bactericidal action [170]. Coatings cast onto silicon wafers from PMMA/PDDA nanoparticles also displayed a correlation between contact points between cells and films and the antimicrobial activity [31]. Figure 11 illustrates the compared frequency of contacts between cells and coatings from two coatings cast onto silicon wafers from PMMA/PDDA dispersions as reproduced from reference [31].
Figure 11.
Scheme for the interaction between cells and coatings cast from core−shell nanoparticles. Nanoparticles in the coatings optimized antimicrobial activity due to a higher frequency of multipoint interactions between poly (diallyl dimethyl ammonium) chloride (PDDA) shell (in red) and cells (in grey) than the one for the larger particles. Reproduced from [31].
Another interesting approach recently reviewed was covalently binding or combining by physical adsorption AMPs and functional antimicrobial polymers [171], e.g., chitosan [172] or polydopamine [173]. The conjugation of AMPs into functional polymers broadened the spectrum of antimicrobial activity, including activity against MDR bacteria, reduced toxicity, and offered more functionalities for developing multifunctional biomedical hydrogels, polymer vesicles, or polymer micelles [171]. For example, the peptide anoplin, extracted from wasp venom, was covalently bound to chitosan to create a highly antimicrobial yet selective and nonhemolytic agent [172]. The conjugate displayed greater antibacterial activity when compared to anoplin only, especially against Gram–negative bacteria [172]. Another example of the peptide–polymer conjugate was a thin layer of PDA deposited onto a surface of polydimethylsiloxane (PDMS) to ease the attachment of peptide CWR11, creating a PDMS/PDA/CWR11 slide; CWR11 was attached to PDA through nucleophilic addition via thiol or amine group at either end of the peptide chain or through physical adsorption onto the PDA surface; the attachment of CWR11 conferred the PDA–coated PDMS surfaces a high antimicrobial activity against E. coli, S. aureus, and P. aeruginosa; the antifouling property was also present as determined by seeding fluorescently labeled–P. aeruginosa onto the slides of PMDS/PDA/CWR11; the material might be applied in catheters to prevent catheter–associated urinary tract infections caused by the development of biofilms on its surface [173].
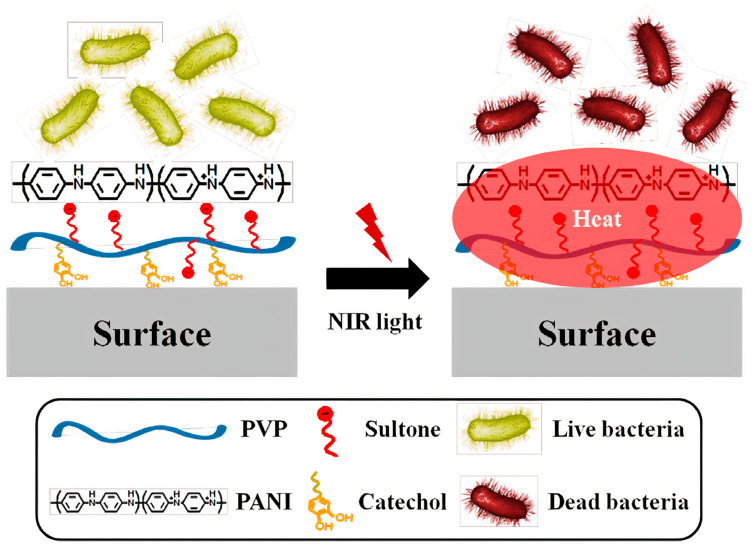
The conducting and hydrophilic polymer polyaniline (PANI) has been considered promising for applications in biomedicine because of its high electrical conductivity and biocompatibility. However, PANI’s low processability and degradability led to its combination with various biopolymers and nanomaterials as blends and nanocomposites, respectively. Biomedical applications of conductive PANI−based nanocomposites were available in antimicrobial therapy, drug delivery, biosensors design, nerve regeneration, and tissue engineering [174,175]. PANI materials have been used in photothermal therapy (PDT) for treating tumors or infections; the incidence of near−infrared radiation (NIR) onto PANI materials led to photothermal ablation of cancer cells or bacteria death [176]. For example, catechol−conjugated poly (vinyl pyrrolidone) sulfobetaine (PVPS) and PANI tightly linked by ionic interaction (PVPS:PANI) has been proposed as a novel photothermal antibacterial agent for surface coating, able to absorb broadband NIR light; the coating released eminent photothermal heat for the rapid killing of surface bacteria [177]. Figure 12 illustrates the photothermal effect of PVPS:PANI coatings on bacteria [177].
Figure 12.
Scheme for the preparation and application of poly (vinyl pyrrolidone) sulfobetaine:poly aniline (PVPS:PANI) coating and near−infrared radiation (NIR) for the photothermolysis of bacteria. Reprinted with permission from [177]. Copyright 2015 American Chemical Society.
Similarly to PVPS: PANI coatings on Figure 12, the photothermal antimicrobial effect was also used to kill bacteria adhered to fabrics of polyethylene (PE) impregnated with poly ethylene imine−poly pyrrole NPs able to absorb the near−infrared light, thereby heating the fabric and killing adsorbed bacteria. Moreover, the fabric became washable, reusable, breathable, biocompatible, and photothermally conversable for active eradication of pathogenic bacteria [178].
A three−dimensional liver scaffold was fabricated from a chitosan/gelatin (CG) solution cross−linked with glutaraldehyde and showed a porous structure similar to the extracellular matrix that facilitated hepatocyte adhesion and proliferation; this CG scaffold had high hepatocyte biocompatibility and mechanical strength but also maintained hepatic functions and viability in in vitro cultures; especially, this liver scaffold revealed high potential for further bioartificial liver design in the near future [179].
Skin traumas such as burns and wounds are susceptible to microorganisms invasion; recent studies succeeded in treating E. coli and S. aureus infected skin with antimicrobial polymers in hydrogels, coatings, nanofibers and nanogels formulations [180,181,182,183,184]. The development of effective wound dressings was essential for speeding up wound healing.
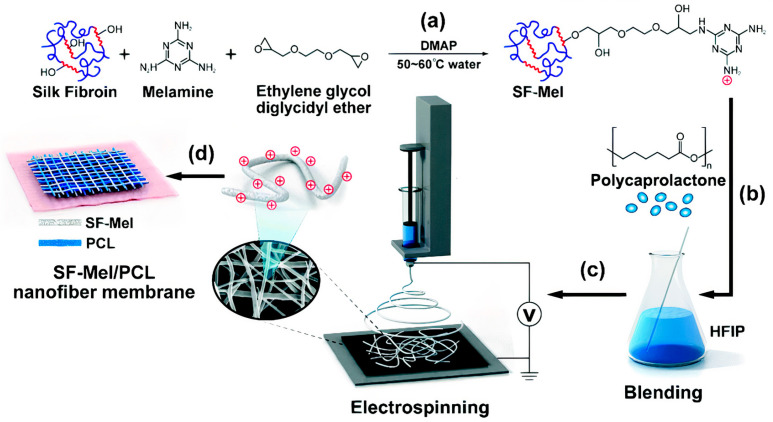
Rectorite, a type of layered silicate, yielded interlayered nanocomposites with positively charged polymers such as quaternized chitin; these composites combined with cellulose fibers created functional sponges with antibacterial and hemostatic properties for wound−healing applications; the in vivo animal tests demonstrated that the sponges rapidly induced hemostasis in a rat tail amputation test, making them superior to the traditional hemostatic materials; in addition, the sponges could substantially promote collagen synthesis and neovascularization, thereby accelerating wound healing 3 days earlier than gauze. This multi−functional biomedical material, fabricated using natural substances, showed great potential to be used for wound healing [185]. Another interesting antimicrobial polymer, melamine−modified silk fibroin (SF–Mel), has been used to produce films with poly caprolactone (PCL) nanofibers via the electrospinning technique. These films were hemocompatible and noncytotoxic, exhibiting broad−spectrum antibacterial activities against both Gram−negative (Escherichia coli) and Gram−positive bacteria (Staphylococcus aureus). In vivo evaluations showed accelerated wound healing by promoting re−epithelialization, collagen deposition, and vascular reconstruction; chemically grafting melamine on the side chains of silk fibroin could improve the antimicrobial properties due to the existence of positively charged amine groups derived from melamine [180]. Figure 13 illustrates the preparation of the PCL/SF–Mel wound dressings [180].
Figure 13.
The film with cationic polymer useful as an antimicrobial wound dressing. (a) Melamine−modified silk fibroin (SF–Mel) was synthesized by covalent conjugation between silk fibroin and melamine. (b) Polycaprolactone (PCL) blended with SF–Mel enhanced the mechanical properties. (c) SF–Mel/PCL nanofiber films were fabricated via electrospinning. (d) Nanofiber membrane comprising SF–Mel/PCL was constructed as a wound dressing for skin repair. Adapted with permission from [180]. Copyright 2019 The Royal Society of Chemistry.
Cryopolimerization of dopamine in the presence of quaternized chitosan (QC) yielded QC/ polydopamine (PDA) cryogel, with PDA concentrations varying from 0.5 to 4.0 mg/mL so that the highest PDA concentrations yielded the best antibacterial and antioxidant activities plus near−infrared photothermal effect; moreover, these cryogels exhibited much better hemostasis than gauze and gelatin sponge in vivo in three different models: a rat liver injury model, a rabbit liver section model, and a pig skin laceration model; there was improved blood cell and platelet adhesion, with quick nonpressing surface hemostasis and wound healing [181]. Tributylammonium alginate (TBAH−Alg) salt was deposited onto modified cationic polyurethane surfaces (CPU) through supramolecular ionic interactions to create a wound dressing. Both CPU and CPU/TBAH−Alg showed large inhibition zones against bacteria in agar diffusion; in vivo experiments in wound models treated with the CPU/TBAH−Alg dressings reduced the persistent inflammatory phase and improved re−epithelialization, collagen deposition, and mature blood vessel formation, showing better results than commercial dressing Tegaderm [182].
3.3. Other Applications for ASA with APs
ASAs with antimicrobial polymers were also involved in the food packaging, fabrics and textile industries, in addition to water treatment [96,157].
In water treatment, the use of organic polyelectrolytes included a myriad of examples of the benefits of polymer use in conventional sedimentation and filtration; however, the influence of polymer chemical structure on performance has been investigated superficially [186]. Organic coagulants and flocculants were usually water−soluble polymers (polyelectrolytes) originated from various natural macromolecular compounds, including polyamines, PDDA, dimethylamine, and polyacrylamides [187]. Among the flocculants, only a few exhibited both antimicrobial and coagulation properties; PDDA was among those able to impart both properties to materials used for water treatment [8,13,14,17,188].
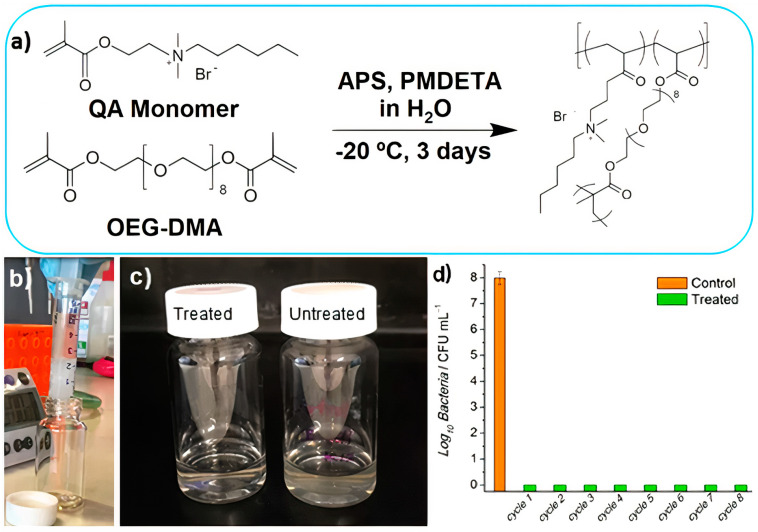
Macroporous antimicrobial polymeric gel (MAPG) containing quaternary ammonium in its chemical structure was synthetized through cryopolymerization; a hydrogel (HG) with the same chemical composition was also prepared for comparison. Firstly, a quaternary ammonium (QA) methacrylate monomer bearing a hydrophobic n−hexyl tail was synthesized [189]. This was an adequate antimicrobial combination of cationic and hydrophobic groups in the monomer to synthesize the polymer: the n−hexyl group was selected as sufficiently hydrophobic to cause membrane disruption, while the cationic moiety implemented adsorption to the bacteria cell wall [189]. The polymerization is shown in Figure 14. First, the QA monomer was synthesized via quaternization reaction between 2−(dimethyl amino) ethyl methacrylate and 1−bromohexane; second, the polymerization of the QA monomer via free−radical polymerization in the presence of a redox radical initiator (i.e., ammonium persulfate), coinitiator N,N,N′,N′′,N′′−pentamethyldiethylene triamine (PMDETA), and a cross−linkable monomer (i.e., oligoethylene glycol dimethacrylate (OEG−DMA)) in water was carried out at subzero temperature (Figure 14a). Filtration of contaminated water using MAPG produced pure water without bacteria (Figure 14b–d) [189]. Cryogelation required only simple mixing of chemicals, hence making the whole process potentially viable for industrial−scale preparation.
Figure 14.
Macroporous antimicrobial polymeric gel (MAPG) for water treatment. (a) MAPG synthesis via free radical polymerization at subzero temperature. (b) Image of macroporous antimicrobial polymeric gel (MAPG) in a syringe and E. coli−contaminated water passing through it. (c) Image of treated water after passing through the syringe. Compared to the untreated, cloudy water due to the presence of bacteria, the treated water was clear. (d) The syringe was subjected to 8 continuous cycles of percolation with E. coli−contaminated water; the recovered water was analyzed via colony−forming unit (CFU) counting. No viable bacteria were detected in the water that passed through the syringe. APS is ammonium persulfate, QA monomer is the quaternary ammonium ethyl methacrylate monomer, PMDETA is the coinitiator N,N,N′,N′′,N′′−pentamethyldiethylenetriamine, and OEG−DMA is oligoethylene glycol dimethacrylate. Adapted from [189].
Polymers, which act as the primary substrate for face masks, could be fine−tuned to impart bio−active and bio−passive properties to the fabrics. The active moieties such as N−halamines, QACs, PEI, benzophenone (BP), polypyrrole, and inorganic groups, such as metals, have been incorporated to yield various antimicrobial polymers suitable for making a reusable facemask [190,191]. Among these, N−halamine and QACs have proven and powerful activity against a broad spectrum of microorganisms. Bath coating, spray coating, and immobilization via carriers have been employed to yield QACs modified antimicrobial fabrics. Direct polymerization of monomers and covalent attachment of QACs was expected to enhance the stability of the coating and the performance. N−halamines had high effectiveness in short contact times in antimicrobial fabrics [192]. However, the real field applicability of N−halamine on face masks has not been explored yet. Natural compounds and antimicrobial peptides are promising molecules due to less ecotoxicity and proven antimicrobial properties. Recently, the inactivation by oxygen singlet of severe acute respiratory syndrome coronavirus 2 (SARS−CoV−2) using light on synthetic conjugated polymers and oligomers was reported; five representative conjugated oligomers and polymers from an array of phenylene ethynylene−based cationic and anionic conjugated materials against SARS−CoV−2 revealed that light activation of the materials at the wavelengths where they absorb gave rise to moderate to very strong inactivation of the virus. Furthermore, no dark inactivation of the virus for three of the five materials/compounds occurred for the quaternary ammonium derivatives. Therefore, the generation of reactive oxygen species definitely inactivated the virus; the incorporation of these materials in wipes, sprays, masks, and clothing and other personal protection equipment would possibly be useful in preventing infections and the spreading of the virus and future outbreaks from similar viruses [193]. A more recent report on the development of a non−woven face mask filter fabricated with a coating of benzalkonium chloride, a quaternary ammonium compound, was able to inactivate more than 99% of SARS−CoV−2 particles in one minute of contact and also methicillin−resistant Staphylococcus aureus and Staphylococcus epidermidis; this would solve the pressing problem of commercial face masks that contained filters not capable of inactivating either SARS−CoV−2 or multidrug−resistant bacteria [194].
4. Conclusions
Antimicrobial polymers, such as APs and AMPs, have been widely explored as materials for biomedical applications. Their chemical structure usually contains both cationic and hydrophobic moieties, exhibiting unlimited potential to fight microbial resistance against available antibiotics. In terms of their potential shortcomings, in vivo AMPs necessitate protection from proteolytic enzymes and rapid degradation, whereas APs still require improvements in terms of their biocompatibility. The similar mechanisms of action found in APs and AMPs involve adsorption to the cell wall, penetration across the cell membrane, and microbe lysis. The synthetic procedures, chemical stability, and improved adsorption of APs—the latter due to their multipoint attachment to microbes—represent significant advantages in comparison to the expensive synthetic pathways for procedure scaling, poor yield, and subpar in vivo stability of AMPs. ASAs with APs and AMPs have also been found useful in water treatment and the production of fabrics and textiles endowed with suitable antimicrobial properties, e.g., face masks and air filters, which have become important and oftentimes crucial defenses in an era of pandemics.
Abbreviations
| NP | Nanoparticle |
| MDR | Multidrug−resistant |
| ROS | Reactive oxygen species |
| AMP | Antimicrobial peptide |
| ASA | Antimicrobial supramolecular assembly |
| PEG | Polyethylene glycol |
| NRAMP | Nonribosomal synthetized peptide |
| RAMP | Ribosomal synthetized peptide |
| DNA | Deoxyribonucleic acid |
| Gr | Gramicidin D |
| LV | Large lipid vesicles |
| BF | Nanosized lipidic bilayer fragments |
| CD | Circular dichroism |
| DPPC | Dipalmitoylphosphatidylcholine |
| DODAB | Dioctadecyldimethylammonium bromide |
| PSS | Polystyrene sulfate |
| DX | Dextran sulfate |
| MRSA | Methicillin−resistant Staphylococcus aureus |
| IDSA | Infectious Diseases Society of America |
| LC | Liquid crystalline |
| AP | Antimicrobial polymer |
| MBC | Minimum bactericidal concentration |
| PEI | Polyethyleneimine |
| PLL | Poly−L−lysine |
| PDMAEMA | Poly [2−(N,N−dimethylamino)ethyl methacrylate] |
| PDDA | Poly (diallyldimethylammonium chloride) |
| PDAATFA | Poly (diallylammonium trifluoroacetate) |
| PDAMATFA | Poly (diallylmethylammonium trifluoroacetate) |
| PMMA | Poly (methyl methacrylate) |
| MMA | Methyl metacrylate |
| OEG | Oligo (ethylene glycol) |
| HPMC | Hydroxypropylmethylcellulose |
| QAC | Antibacterial quaternary compound |
| SNPES | Sulfonic amino poly (ether sulfone) |
| QC | Quaternized chitosan |
| PAA | Poly (acrylic acid) |
| PDA | Polydopamine |
| PDMS | Polydimethylsiloxane |
| PANI | Polyaniline |
| PDT | Photothermal therapy |
| PVPS | Poly (vinylpyrrolidone) sulfobetaine |
| PE | Polyethylene |
| CG | Chitosan/gelatin |
| SF−Mel | Melamine−modified silk fibroin |
| PCL | Polycaprolactone |
| TBAH−Alg | Tributylammonium alginate |
| CPU | Polyurethane surfaces |
| MAPG | Macroporous antimicrobial polymeric gel |
| HG | Hydrogel |
| QA | Quaternary ammonium |
| PMDETA | N,N,N′,N″,N″−pentamethyldiethylenetriamine |
| OEG−DMA | Oligoethylene glycol dimethacrylate |
| BP | Benzophenone |
| SARS−CoV−2 | Severe acute respiratory syndrome coronavirus 2 |
Author Contributions
Conceptualization, A.M.C.-R.; methodology, A.M.C.-R.; validation, A.M.C.-R.; formal analysis, A.M.C.-R. and P.M.A.; investigation, A.M.C.-R. and P.M.A.; resources, A.M.C.-R.; data curation, A.M.C.-R.; writing—original draft preparation, A.M.C.-R. and P.M.A.; writing—review and editing, A.M.C.-R. and P.M.A.; supervision, A.M.C.-R.; project administration, A.M.C.-R.; funding acquisition, A.M.C.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) grant 2019/17685−2 to A.M.C.-R., and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) grants 302758/2019−4 and 302352/2014−7 to A.M.C.−R. P.M.A. was the recipient of an undergraduate fellowship from the Programa Institucional de Bolsas de Iniciação Científica (PIBIC)−CNPq grant 139161/2020−1.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mulani M.S., Kamble E.E., Kumkar S.N., Tawre M.S., Pardesi K.R. Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review. Front. Microbiol. 2019;10:593. doi: 10.3389/fmicb.2019.00539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mandal S.M., Roy A., Ghosh A.K., Hazra T.K., Basak A., Franco O.L. Challenges and Future Prospects of Antibiotic Therapy: From Peptides to Phages Utilization. Front. Pharmacol. 2014;5 doi: 10.3389/fphar.2014.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaur I. Novel Strategies to Combat Antimicrobial Resistance. J. Infect. Dis. Ther. 2016;4:1–6. doi: 10.4172/2332-0877.1000292. [DOI] [Google Scholar]
- 4.León-Buitimea A., Garza-Cárdenas C.R., Garza-Cervantes J.A., Lerma-Escalera J.A., Morones-Ramírez J.R. The Demand for New Antibiotics: Antimicrobial Peptides, Nanoparticles, and Combinatorial Therapies as Future Strategies in Antibacterial Agent Design. Front. Microbiol. 2020;11:1669. doi: 10.3389/fmicb.2020.01669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abedon S.T., Kuhl S.J., Blasdel B.G., Kutter E.M. Phage Treatment of Human Infections. Bacteriophage. 2011;1:66–85. doi: 10.4161/bact.1.2.15845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parasuraman P., R. Y T., Shaji C., Sharan A., Bahkali A.H., Al-Harthi H.F., Syed A., Anju V.T., Dyavaiah M., Siddhardha B. Biogenic Silver Nanoparticles Decorated with Methylene Blue Potentiated the Photodynamic Inactivation of Pseudomonas Aeruginosa and Staphylococcus Aureus. Pharmaceutics. 2020;12:709. doi: 10.3390/pharmaceutics12080709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mukhopadhyay S., Prasad A.S.B., Mehta C.H., Nayak U.Y. Antimicrobial Peptide Polymers: No Escape to ESKAPE Pathogens—A Review. World J. Microbiol. Biotechnol. 2020;36:131. doi: 10.1007/s11274-020-02907-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carmona-Ribeiro A.M., de Melo Carrasco L.D. Novel Formulations for Antimicrobial Peptides. Int. J. Mol. Sci. 2014;15:18040–18083. doi: 10.3390/ijms151018040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lei J., Sun L., Huang S., Zhu C., Li P., He J., Mackey V., Coy D.H., He Q. The Antimicrobial Peptides and Their Potential Clinical Applications. Am. J. Transl. Res. 2019;11:3919–3931. [PMC free article] [PubMed] [Google Scholar]
- 10.Carmona-Ribeiro A.M. Self−Assembled Antimicrobial Nanomaterials. Int. J. Environ. Res. Public. Health. 2018;15:1408. doi: 10.3390/ijerph15071408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mathiazzi B.I., Carmona-Ribeiro A.M. Hybrid Nanoparticles of Poly (Methyl Methacrylate) and Antimicrobial Quaternary Ammonium Surfactants. Pharmaceutics. 2020;12:340. doi: 10.3390/pharmaceutics12040340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vieira D.B., Carmona-Ribeiro A.M. Synthetic Bilayer Fragments for Solubilization of Amphotericin B. J. Colloid Interface Sci. 2001;244:427–431. doi: 10.1006/jcis.2001.7975. [DOI] [Google Scholar]
- 13.Vieira D.B., Carmona-Ribeiro A.M. Cationic Nanoparticles for Delivery of Amphotericin B: Preparation, Characterization and Activity in Vitro. J. Nanobiotechnol. 2008;6:6. doi: 10.1186/1477-3155-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melo L.D., Mamizuka E.M., Carmona-Ribeiro A.M. Antimicrobial Particles from Cationic Lipid and Polyelectrolytes. Langmuir. 2010;26:12300–12306. doi: 10.1021/la101500s. [DOI] [PubMed] [Google Scholar]
- 15.Carmona-Ribeiro A.M., de Melo Carrasco L.D. Cationic Antimicrobial Polymers and Their Assemblies. Int. J. Mol. Sci. 2013;14:9906–9946. doi: 10.3390/ijms14059906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Melo Carrasco L.D., Sampaio J.L.M., Carmona-Ribeiro A.M. Supramolecular Cationic Assemblies against Multidrug−Resistant Microorganisms: Activity and Mechanism of Action. Int. J. Mol. Sci. 2015;16:6337–6352. doi: 10.3390/ijms16036337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanches L.M., Petri D.F.S., de Melo Carrasco L.D., Carmona-Ribeiro A.M. The Antimicrobial Activity of Free and Immobilized Poly (Diallyldimethylammonium) Chloride in Nanoparticles of Poly (Methylmethacrylate) J. Nanobiotechnol. 2015;13:58. doi: 10.1186/s12951-015-0123-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carrasco L.D.D.M., Bertolucci R., Jr., Ribeiro R.T., Sampaio J.L., Carmona-Ribeiro A.M. Cationic Nanostructures against Foodborne Pathogens. Front. Microbiol. 2016;7 doi: 10.3389/fmicb.2016.01804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carrasco L.D.M., Santos H.C.A.S., Sampaio J.L.M., Carmona-Ribeiro A.M. Self−Assembled Antibiotic Nanoparticles Against Intracellular Bacteria. Drug Deliv. Lett. 2017;7:39–47. [Google Scholar]
- 20.Carmona-Ribeiro A.M. Biomimetic Nanomaterials from the Assembly of Polymers, Lipids, and Surfactants. In: Dutta K., editor. Surfactants and Detergents. IntechOpen; London, UK: 2019. [Google Scholar]
- 21.Pereira E.M.A., Kosaka P.M., Rosa H., Vieira D.B., Kawano Y., Petri D.F.S., Carmona-Ribeiro A.M. Hybrid Materials from Intermolecular Associations between Cationic Lipid and Polymers. J. Phys. Chem. B. 2008;112:9301–9310. doi: 10.1021/jp801297t. [DOI] [PubMed] [Google Scholar]
- 22.Cloutier M., Mantovani D., Rosei F. Antibacterial Coatings: Challenges, Perspectives, and Opportunities. Trends Biotechnol. 2015;33:637–652. doi: 10.1016/j.tibtech.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Galvão C.N., Sanches L.M., Mathiazzi B.I., Ribeiro R.T., Petri D.F.S., Carmona-Ribeiro A.M. Antimicrobial Coatings from Hybrid Nanoparticles of Biocompatible and Antimicrobial Polymers. Int. J. Mol. Sci. 2018;19:2965. doi: 10.3390/ijms19102965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krywko-Cendrowska A., di Leone S., Bina M., Yorulmaz-Avsar S., Palivan C.G., Meier W. Recent Advances in Hybrid Biomimetic Polymer−Based Films: From Assembly to Applications. Polymers. 2020;12:1003. doi: 10.3390/polym12051003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tiller J.C., Liao C.-J., Lewis K., Klibanov A.M. Designing Surfaces That Kill Bacteria on Contact. Proc. Natl. Acad. Sci. USA. 2001;98:5981–5985. doi: 10.1073/pnas.111143098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carmona-Ribeiro A.M., Barbassa L., Melo L.D. Antimicrobial Biomimetics. In: George A., editor. Biomimetic Based Applications. Volume 1. IntechOpen; Rijeka, Croatia: 2011. pp. 227–284. [Google Scholar]
- 27.Carmona-Ribeiro A.M., de Moraes Lessa M. Interactions between Bilayer Membranes and Latex. Colloids Surf. A Physicochem. Eng. Asp. 1999;153:355–361. doi: 10.1016/S0927-7757(98)00532-9. [DOI] [Google Scholar]
- 28.Carmona-Ribeiro A.M. Biomimetic Nanoparticles: Preparation, Characterization and Biomedical Applications. Int. J. Nanomed. 2010;5:249–259. doi: 10.2147/IJN.S9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaudhuri R.G., Paria S. Core/Shell Nanoparticles: Classes, Properties, Synthesis Mechanisms, Characterization, and Applications. Chem. Rev. 2011;112:2373–2433. doi: 10.1021/cr100449n. [DOI] [PubMed] [Google Scholar]
- 30.Sheikhpour M., Barani L., Kasaeian A. Biomimetics in Drug Delivery Systems: A Critical Review. J. Control. Release. 2017;253:97–109. doi: 10.1016/j.jconrel.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 31.Ribeiro R.T., Galvão C.N., Betancourt Y.P., Mathiazzi B.I., Carmona-Ribeiro A.M. Microbicidal Dispersions and Coatings from Hybrid Nanoparticles of Poly (Methyl Methacrylate), Poly (Diallyl Dimethyl Ammonium) Chloride, Lipids, and Surfactants. Int. J. Mol. Sci. 2019;20:6150. doi: 10.3390/ijms20246150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carmona-Ribeiro A.M. Biomimetic Lipid Polymer Nanoparticles for Drug Delivery. In: Ferrari E., Soloviev M., editors. Nanoparticles in Biology and Medicine: Methods and Protocols. Springer; New York, NY, USA: 2020. pp. 45–60. Methods in Molecular Biology. [DOI] [PubMed] [Google Scholar]
- 33.Vieira D.B., Carmona-Ribeiro A.M. Cationic Lipids and Surfactants as Antifungal Agents: Mode of Action. J. Antimicrob. Chemother. 2006;58:760–767. doi: 10.1093/jac/dkl312. [DOI] [PubMed] [Google Scholar]
- 34.Kondaveeti S., Bueno P.V.D.A., Carmona-Ribeiro A.M., Esposito F., Lincopan N., Sierakowski M.R., Petri D.F.S. Microbicidal Gentamicin−Alginate Hydrogels. Carbohydr. Polym. 2018;186:159–167. doi: 10.1016/j.carbpol.2018.01.044. [DOI] [PubMed] [Google Scholar]
- 35.Kondaveeti S., Damato T.C., Carmona-Ribeiro A.M., Sierakowski M.R., Petri D.F.S. Sustainable Hydroxypropyl Methylcellulose/Xyloglucan/Gentamicin Films with Antimicrobial Properties. Carbohydr. Polym. 2017;165:285–293. doi: 10.1016/j.carbpol.2017.02.066. [DOI] [PubMed] [Google Scholar]
- 36.Yang K., Han Q., Chen B., Zheng Y., Zhang K., Li Q., Wang J. Antimicrobial Hydrogels: Promising Materials for Medical Application. Int. J. Nanomed. 2018;13:2217–2263. doi: 10.2147/IJN.S154748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghobril C., Grinstaff M.W. The Chemistry and Engineering of Polymeric Hydrogel Adhesives for Wound Closure: A Tutorial. Chem. Soc. Rev. 2015;44:1820–1835. doi: 10.1039/C4CS00332B. [DOI] [PubMed] [Google Scholar]
- 38.Makvandi P., Gu J.T., Zare E.N., Ashtari B., Moeini A., Tay F.R., Niu L. Polymeric and Inorganic Nanoscopical Antimicrobial Fillers in Dentistry. Acta Biomater. 2020;101:69–101. doi: 10.1016/j.actbio.2019.09.025. [DOI] [PubMed] [Google Scholar]
- 39.Campoccia D., Montanaro L., Arciola C.R. A Review of the Biomaterials Technologies for Infection−Resistant Surfaces. Biomaterials. 2013;34:8533–8554. doi: 10.1016/j.biomaterials.2013.07.089. [DOI] [PubMed] [Google Scholar]
- 40.Tapias G.N., Sicchierolli S.M., Mamizuka E.M., Carmona-Ribeiro A.M. Interactions between Cationic Vesicles and Escherichia Coli. Langmuir. 1994;10:3461–3465. doi: 10.1021/la00022a017. [DOI] [Google Scholar]
- 41.Sicchierolli S.M., Mamizuka E.M., Carmona-Ribeiro A.M. Bacteria Flocculation and Death by Cationic Vesicles. Langmuir. 1995;11:2991–2995. doi: 10.1021/la00008a024. [DOI] [Google Scholar]
- 42.Martins L.M.S., Mamizuka E.M., Carmona-Ribeiro A.M. Cationic Vesicles as Bactericides. Langmuir. 1997;13:5583–5587. doi: 10.1021/la970353k. [DOI] [Google Scholar]
- 43.Campanhã M.T.N., Mamizuka E.M., Carmona-Ribeiro A.M. Interactions between Cationic Liposomes and Bacteria: The Physical−Chemistry of the Bactericidal Action. J. Lipid Res. 1999;40:1495–1500. doi: 10.1016/S0022-2275(20)33392-7. [DOI] [PubMed] [Google Scholar]
- 44.Mamizuka E.M., Carmona-Ribeiro A.M. Cationic Liposomes as Antimicrobial Agents. In: Méndez Vila A., editor. Communicating Current Research and Educational Topics and Trends in Applied Microbiology. Volume 2. Formatex; Badajoz, Spain: 2007. pp. 636–647. [Google Scholar]
- 45.Maan A.M.C., Hofman A.H., de Vos W.M., Kamperman M. Recent Developments and Practical Feasibility of Polymer−Based Antifouling Coatings. Adv. Funct. Mater. 2020;30:2000936. doi: 10.1002/adfm.202000936. [DOI] [Google Scholar]
- 46.Dunne W.M. Bacterial Adhesion: Seen Any Good Biofilms Lately? Clin. Microbiol. Rev. 2002;15:155–166. doi: 10.1128/CMR.15.2.155-166.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Green J.-B.D., Fulghum T., Nordhaus M.A. A Review of Immobilized Antimicrobial Agents and Methods for Testing. Biointerphases. 2011;6:MR13–MR28. doi: 10.1116/1.3645195. [DOI] [PubMed] [Google Scholar]
- 48.Kamaruzzaman N.F., Tan L.P., Hamdan R.H., Choong S.S., Wong W.K., Gibson A.J., Chivu A., Pina M.D.F. Antimicrobial Polymers: The Potential Replacement of Existing Antibiotics? Int. J. Mol. Sci. 2019;20:2747. doi: 10.3390/ijms20112747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eckhard L.H., Houri-Haddad Y., Sol A., Zeharia R., Shai Y., Beyth S., Domb A.J., Bachrach G., Beyth N. Sustained Release of Antibacterial Lipopeptides from Biodegradable Polymers against Oral Pathogens. PLoS ONE. 2016;11:e0162537. doi: 10.1371/journal.pone.0162537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shabir U., Ali S., Magray A.R., Ganai B.A., Firdous P., Hassan T., Nazir R. Fish Antimicrobial Peptides (AMP’s) as Essential and Promising Molecular Therapeutic Agents: A Review. Microb. Pathog. 2018;114:50–56. doi: 10.1016/j.micpath.2017.11.039. [DOI] [PubMed] [Google Scholar]
- 51.Teixeira M.C., Carbone C., Sousa M.C., Espina M., Garcia M.L., Sanchez-Lopez E., Souto E.B. Nanomedicines for the Delivery of Antimicrobial Peptides (AMPs) Nanomaterials. 2020;10:560. doi: 10.3390/nano10030560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rai M., Pandit R., Gaikwad S., Kövics G. Antimicrobial Peptides as Natural Bio−Preservative to Enhance the Shelf−Life of Food. J. Food Sci. Technol. 2016;53:3381–3394. doi: 10.1007/s13197-016-2318-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Biswas G.R., Majee S.B. Niosomes in Ocular Drug Delivery. Eur. J. Pharm. Med. Res. 2017;4:813–819. [Google Scholar]
- 54.Faya M., Kalhapure R.S., Kumalo H.M., Waddad A.Y., Omolo C., Govender T. Conjugates and Nano−Delivery of Antimicrobial Peptides for Enhancing Therapeutic Activity. J. Drug Deliv. Sci. Technol. 2018;44:153–171. doi: 10.1016/j.jddst.2017.12.010. [DOI] [Google Scholar]
- 55.Memariani H., Memariani M., Shahidi-Dadras M., Nasiri S., Akhavan M.M., Moravvej H. Melittin: From Honeybees to Superbugs. Appl. Microbiol. Biotechnol. 2019;103:3265–3276. doi: 10.1007/s00253-019-09698-y. [DOI] [PubMed] [Google Scholar]
- 56.Wang G. Improved Methods for Classification, Prediction and Design of Antimicrobial Peptides. Methods Mol. Biol. 2015;1268:43–66. doi: 10.1007/978-1-4939-2285-7_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Epand R.M., Vogel H.J. Diversity of Antimicrobial Peptides and Their Mechanisms of Action. Biochim. Biophys. Acta BBA Biomembr. 1999;1462:11–28. doi: 10.1016/S0005-2736(99)00198-4. [DOI] [PubMed] [Google Scholar]
- 58.Koehbach J., Craik D.J. The Vast Structural Diversity of Antimicrobial Peptides. Trends Pharmacol. Sci. 2019;40:517–528. doi: 10.1016/j.tips.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 59.Wu Q., Patočka J., Kuča K. Insect Antimicrobial Peptides, a Mini Review. Toxins. 2018;10:461. doi: 10.3390/toxins10110461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raheem N., Straus S.K. Mechanisms of Action for Antimicrobial Peptides With Antibacterial and Antibiofilm Functions. Front. Microbiol. 2019;10:2866. doi: 10.3389/fmicb.2019.02866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nordström R. Ph.D. Thesis. Uppsala University; Upsala, Sweden: 2019. Polymeric Nanoparticles as Carriers for Antimicrobial Peptides: Factors Affecting Peptide and Membrane Interactions. [Google Scholar]
- 62.Kumar P., Kizhakkedathu J.N., Straus S.K. Antimicrobial Peptides: Diversity, Mechanism of Action and Strategies to Improve the Activity and Biocompatibility In Vivo. Biomolecules. 2018;8:4. doi: 10.3390/biom8010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li J., Koh J.-J., Liu S., Lakshminarayanan R., Verma C.S., Beuerman R.W. Membrane Active Antimicrobial Peptides: Translating Mechanistic Insights to Design. Front. Neurosci. 2017;11:73. doi: 10.3389/fnins.2017.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carvalho C.A., Olivares-Ortega C., Soto-Arriaza M.A., Carmona-Ribeiro A.M. Interaction of Gramicidin with DPPC/DODAB Bilayer Fragments. Biochim. Biophys. Acta BBA Biomembr. 2012;1818:3064–3071. doi: 10.1016/j.bbamem.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 65.Akaike N. Chapter 8—Gramicidin Perforated Patch. In: Alvarez-Leefmans F.J., Delpire E., editors. Physiology and Pathology of Chloride Transporters and Channels in the Nervous System. Academic Press; San Diego, CA, USA: 2010. pp. 141–147. [Google Scholar]
- 66.Mishra B., Reiling S., Zarena D., Wang G. Host Defense Antimicrobial Peptides as Antibiotics: Design and Application Strategies. Curr. Opin. Chem. Biol. 2017;38:87–96. doi: 10.1016/j.cbpa.2017.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ragioto D.A., Carrasco L.D., Carmona-Ribeiro A.M. Novel Gramicidin Formulations in Cationic Lipid as Broad−Spectrum Microbicidal Agents. Int. J. Nanomed. 2014;9:3183–3192. doi: 10.2147/IJN.S65289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xavier G.R.S., Carmona-Ribeiro A.M. Cationic Biomimetic Particles of Polystyrene/Cationic Bilayer/Gramicidin for Optimal Bactericidal Activity. Nanomaterials. 2017;7:422. doi: 10.3390/nano7120422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Riool M., de Breij A., de Boer L., Kwakman P.H.S., Cordfunke R.A., Cohen O., Malanovic N., Emanuel N., Lohner K., Drijfhout J.W., et al. Controlled Release of LL−37−Derived Synthetic Antimicrobial and Anti−Biofilm Peptides SAAP−145 and SAAP−276 Prevents Experimental Biomaterial−Associated Staphylococcus Aureus Infection. Adv. Funct. Mater. 2017;27 doi: 10.1002/adfm.201606623. [DOI] [Google Scholar]
- 70.Patrick J.W., Gamez R.C., Russell D.H. The Influence of Lipid Bilayer Physicochemical Properties on Gramicidin A Conformer Preferences. Biophys. J. 2016;110:1826–1835. doi: 10.1016/j.bpj.2016.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fael H., Demirel A.L. Nisin/Polyanion Layer−by−Layer Films Exhibiting Different Mechanisms in Antimicrobial Efficacy. RSC Adv. 2020;10:10329–10337. doi: 10.1039/C9RA10135G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang Z., Choi H., Weisshaar J.C. Melittin−Induced Permeabilization, Re−Sealing, and Re−Permeabilization of E. Coli Membranes. Biophys. J. 2018;114:368–379. doi: 10.1016/j.bpj.2017.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jamasbi E., Mularski A., Separovic F. Model Membrane and Cell Studies of Antimicrobial Activity of Melittin Analogues. Curr. Top. Med. Chem. 2016;16:40–45. doi: 10.2174/1568026615666150703115919. [DOI] [PubMed] [Google Scholar]
- 74.Jo M., Park M.H., Kollipara P.S., An B.J., Song H.S., Han S.B., Kim J.H., Song M.J., Hong J.T. Anti−Cancer Effect of Bee Venom Toxin and Melittin in Ovarian Cancer Cells through Induction of Death Receptors and Inhibition of JAK2/STAT3 Pathway. Toxicol. Appl. Pharmacol. 2012;258:72–81. doi: 10.1016/j.taap.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 75.Jeong Y.-J., Choi Y., Shin J.-M., Cho H.-J., Kang J.-H., Park K.-K., Choe J.-Y., Bae Y.-S., Han S.-M., Kim C.-H., et al. Melittin Suppresses EGF−Induced Cell Motility and Invasion by Inhibiting PI3K/Akt/MTOR Signaling Pathway in Breast Cancer Cells. Food Chem. Toxicol. 2014;68:218–225. doi: 10.1016/j.fct.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 76.Choi K.E., Hwang C.J., Gu S.M., Park M.H., Kim J.H., Park J.H., Ahn Y.J., Kim J.Y., Song M.J., Song H.S., et al. Cancer Cell Growth Inhibitory Effect of Bee Venom via Increase of Death Receptor 3 Expression and Inactivation of NF−Kappa B in NSCLC Cells. Toxins. 2014;6:2210–2228. doi: 10.3390/toxins6082210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xiao Y.-F., Jie M.-M., Li B.-S., Hu C.-J., Xie R., Tang B., Yang S.-M. Peptide−Based Treatment: A Promising Cancer Therapy. [(accessed on 7 December 2020)]; doi: 10.1155/2015/761820. Available online: https://www.hindawi.com/journals/jir/2015/761820/ [DOI] [PMC free article] [PubMed]
- 78.Rady I., Siddiqui I.A., Rady M., Mukhtar H. Melittin, a Major Peptide Component of Bee Venom, and Its Conjugates in Cancer Therapy. Cancer Lett. 2017;402:16–31. doi: 10.1016/j.canlet.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mahadevappa R., Ma R., Kwok H.F. Venom Peptides: Improving Specificity in Cancer Therapy. Trends Cancer. 2017;3:611–614. doi: 10.1016/j.trecan.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 80.Światły-Błaszkiewicz A., Mrówczyńska L., Matuszewska E., Lubawy J., Urbański A., Kokot Z.J., Rosiński G., Matysiak J. The Effect of Bee Venom Peptides Melittin, Tertiapin, and Apamin on the Human Erythrocytes Ghosts: A Preliminary Study. Metabolites. 2020;10:191. doi: 10.3390/metabo10050191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lu X., Liu J., Gou L., Li J., Yuan B., Yang K., Ma Y. Designing Melittin−Graphene Hybrid Complexes for Enhanced Antibacterial Activity. Adv. Healthc. Mater. 2019;8:1801521. doi: 10.1002/adhm.201801521. [DOI] [PubMed] [Google Scholar]
- 82.Pletzer D., Mansour S.C., Hancock R.E.W. Synergy between Conventional Antibiotics and Anti−Biofilm Peptides in a Murine, Sub−Cutaneous Abscess Model Caused by Recalcitrant ESKAPE Pathogens. PLoS Pathog. 2018;14:e1007084. doi: 10.1371/journal.ppat.1007084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Magana M., Sereti C., Ioannidis A., Mitchell C.A., Ball A.R., Magiorkinis E., Chatzipanagiotou S., Hamblin M.R., Hadjifrangiskou M., Tegos G.P. Options and Limitations in Clinical Investigation of Bacterial Biofilms. Clin. Microbiol. Rev. 2018;31 doi: 10.1128/CMR.00084-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Talapko J., Škrlec I. The Principles, Mechanisms, and Benefits of Unconventional Agents in the Treatment of Biofilm Infection. Pharmaceuticals. 2020;13:299. doi: 10.3390/ph13100299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Barreto-Santamaría A., Patarroyo M.E., Curtidor H. Designing and Optimizing New Antimicrobial Peptides: All Targets Are Not the Same. Crit. Rev. Clin. Lab. Sci. 2019;56:351–373. doi: 10.1080/10408363.2019.1631249. [DOI] [PubMed] [Google Scholar]
- 86.Kiani M.H., Imran M., Raza A., Shahnaz G. Multi−Functionalized Nanocarriers Targeting Bacterial Reservoirs to Overcome Challenges of Multi Drug−Resistance. DARU J. Pharm. Sci. 2020;28:319–332. doi: 10.1007/s40199-020-00337-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yeom J.-H., Lee B., Kim D., Lee J., Kim S., Bae J., Park Y., Lee K. Gold Nanoparticle−DNA Aptamer Conjugate−Assisted Delivery of Antimicrobial Peptide Effectively Eliminates Intracellular Salmonella Enterica Serovar Typhimurium. Biomaterials. 2016;104:43–51. doi: 10.1016/j.biomaterials.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 88.Dubashynskaya N.V., Skorik Y.A. Polymyxin Delivery Systems: Recent Advances and Challenges. Pharmaceuticals. 2020;13:83. doi: 10.3390/ph13050083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Insua I., Zizmare L., Peacock A.F.A., Krachler A.M., Fernandez-Trillo F. Polymyxin B Containing Polyion Complex (PIC) Nanoparticles: Improving the Antimicrobial Activity by Tailoring the Degree of Polymerisation of the Inert Component. Sci. Rep. 2017;7:9396. doi: 10.1038/s41598-017-09667-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Insua I., Majok S., Peacock A.F.A., Krachler A.M., Fernandez-Trillo F. Preparation and Antimicrobial Evaluation of Polyion Complex (PIC) Nanoparticles Loaded with Polymyxin B. Eur. Polym. J. 2017;87:478–486. doi: 10.1016/j.eurpolymj.2016.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McGuinness W.A., Malachowa N., DeLeo F.R. Vancomycin Resistance in Staphylococcus Aureus. Yale J. Biol. Med. 2017;90:269–281. [PMC free article] [PubMed] [Google Scholar]
- 92.Claeys K.C., Lagnf A.M., Hallesy J.A., Compton M.T., Gravelin A.L., Davis S.L., Rybak M.J. Pneumonia Caused by Methicillin−Resistant Staphylococcus Aureus: Does Vancomycin Heteroresistance Matter? Antimicrob. Agents Chemother. 2016;60:1708–1716. doi: 10.1128/AAC.02388-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hassan D., Omolo C.A., Gannimani R., Waddad A.Y., Mocktar C., Rambharose S., Agrawal N., Govender T. Delivery of Novel Vancomycin Nanoplexes for Combating Methicillin Resistant Staphylococcus Aureus (MRSA) Infections. Int. J. Pharm. 2019;558:143–156. doi: 10.1016/j.ijpharm.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 94.Hajiahmadi F., Alikhani M.Y., Shariatifar H., Arabestani M.R., Ahmadvand D. The Bactericidal Effect of Liposomal Vancomycin as a Topical Combating System against Methicillin−Resistant Staphylococcus Aureus Skin Wound Infection in Mice. Med. J. Islam. Repub. Iran. 2019;33:941–947. doi: 10.47176/mjiri.33.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ciociola T., Giovati L., Conti S., Magliani W., Santinoli C., Polonelli L. Natural and Synthetic Peptides with Antifungal Activity. Future Med. Chem. 2016;8:1413–1433. doi: 10.4155/fmc-2016-0035. [DOI] [PubMed] [Google Scholar]
- 96.Huang K.-S., Yang C.-H., Huang S.-L., Chen C.-Y., Lu Y.-Y., Lin Y.-S. Recent Advances in Antimicrobial Polymers: A Mini−Review. Int. J. Mol. Sci. 2016;17:1578. doi: 10.3390/ijms17091578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Magana M., Pushpanathan M., Santos A.L., Leanse L., Fernandez M., Ioannidis A., Giulianotti M.A., Apidianakis Y., Bradfute S., Ferguson A.L., et al. The Value of Antimicrobial Peptides in the Age of Resistance. Lancet Infect. Dis. 2020;20:e216–e230. doi: 10.1016/S1473-3099(20)30327-3. [DOI] [PubMed] [Google Scholar]
- 98.Gajdács M. The Concept of an Ideal Antibiotic: Implications for Drug Design. Molecules. 2019;24:892. doi: 10.3390/molecules24050892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rafferty J., Nagaraj H., P. McCloskey A., Huwaitat R., Porter S., Albadr A., Laverty G. Peptide Therapeutics and the Pharmaceutical Industry: Barriers Encountered Translating from the Laboratory to Patients. Curr. Med. Chem. 2016;23:4231–4259. doi: 10.2174/0929867323666160909155222. [DOI] [PubMed] [Google Scholar]
- 100.Vukomanović M., Žunič V., Kunej Š., Jančar B., Jeverica S., Podlipec R., Suvorov D. Nano−Engineering the Antimicrobial Spectrum of Lantibiotics: Activity of Nisin against Gram Negative Bacteria. Sci. Rep. 2017;7:4324. doi: 10.1038/s41598-017-04670-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mohan A., McClements D.J., Udenigwe C.C. Encapsulation of Bioactive Whey Peptides in Soy Lecithin−Derived Nanoliposomes: Influence of Peptide Molecular Weight. Food Chem. 2016;213:143–148. doi: 10.1016/j.foodchem.2016.06.075. [DOI] [PubMed] [Google Scholar]
- 102.Mai S., Mauger M.T., Niu L., Barnes J.B., Kao S., Bergeron B.E., Ling J., Tay F.R. Potential Applications of Antimicrobial Peptides and Their Mimics in Combating Caries and Pulpal Infections. Acta Biomater. 2017;49:16–35. doi: 10.1016/j.actbio.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 103.Izadi N., Keikha M., Ghazvini K., Karbalaei M. Oral Antimicrobial Peptides and New Therapeutic Strategies for Plaque−Mediated Diseases. Gene Rep. 2020;21:100811. doi: 10.1016/j.genrep.2020.100811. [DOI] [Google Scholar]
- 104.Lewies A., Wentzel J.F., Jordaan A., Bezuidenhout C., Du Plessis L.H. Interactions of the Antimicrobial Peptide Nisin Z with Conventional Antibiotics and the Use of Nanostructured Lipid Carriers to Enhance Antimicrobial Activity. Int. J. Pharm. 2017;526:244–253. doi: 10.1016/j.ijpharm.2017.04.071. [DOI] [PubMed] [Google Scholar]
- 105.Boge L., Bysell H., Ringstad L., Wennman D., Umerska A., Cassisa V., Eriksson J., Joly-Guillou M.-L., Edwards K., Andersson M. Lipid−Based Liquid Crystals As Carriers for Antimicrobial Peptides: Phase Behavior and Antimicrobial Effect. Langmuir. 2016;32:4217–4228. doi: 10.1021/acs.langmuir.6b00338. [DOI] [PubMed] [Google Scholar]
- 106.Piras A.M., Maisetta G., Sandreschi S., Gazzarri M., Bartoli C., Grassi L., Esin S., Chiellini F., Batoni G. Chitosan Nanoparticles Loaded with the Antimicrobial Peptide Temporin B Exert a Long−Term Antibacterial Activity in Vitro against Clinical Isolates of Staphylococcus Epidermidis. Front. Microbiol. 2015;6:372. doi: 10.3389/fmicb.2015.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Niemirowicz K., Surel U., Wilczewska A.Z., Mystkowska J., Piktel E., Gu X., Namiot Z., Kułakowska A., Savage P.B., Bucki R. Bactericidal Activity and Biocompatibility of Ceragenin−Coated Magnetic Nanoparticles. J. Nanobiotechnol. 2015;13:32. doi: 10.1186/s12951-015-0093-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bueno P.V.A., Hilamatu K.C.P., Carmona-Ribeiro A.M., Petri D.F.S. Magnetically Triggered Release of Amoxicillin from Xanthan/Fe3O4/Albumin Patches. Int. J. Biol. Macromol. 2018;115:792–800. doi: 10.1016/j.ijbiomac.2018.04.119. [DOI] [PubMed] [Google Scholar]
- 109.Agbale C.M., Cardoso M.H., Galyuon I.K., Franco O.L. Designing Metallodrugs with Nuclease and Protease Activity. Metallomics. 2016;8:1159–1169. doi: 10.1039/C6MT00133E. [DOI] [PubMed] [Google Scholar]
- 110.Ahire J.J., Neveling D.P., Dicks L.M.T. Co−Spinning of Silver Nanoparticles with Nisin Increases the Antimicrobial Spectrum of PDLLA: PEO Nanofibers. Curr. Microbiol. 2015;71:24–30. doi: 10.1007/s00284-015-0813-y. [DOI] [PubMed] [Google Scholar]
- 111.Kong E.F., Tsui C., Boyce H., Ibrahim A., Hoag S.W., Karlsson A.J., Meiller T.F., Jabra-Rizk M.A. Development and In Vivo Evaluation of a Novel Histatin−5 Bioadhesive Hydrogel Formulation against Oral Candidiasis. Antimicrob. Agents Chemother. 2016;60:881–889. doi: 10.1128/AAC.02624-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zakia M., Koo J.M., Kim D., Ji K., Huh P., Yoon J., Yoo S.I. Development of Silver Nanoparticle−Based Hydrogel Composites for Antimicrobial Activity. Green Chem. Lett. Rev. 2020;13:34–40. doi: 10.1080/17518253.2020.1725149. [DOI] [Google Scholar]
- 113.Saleem H., Zaidi S.J. Sustainable Use of Nanomaterials in Textiles and Their Environmental Impact. Materials. 2020;13:5134. doi: 10.3390/ma13225134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Deshmukh S.P., Patil S.M., Mullani S.B., Delekar S.D. Silver Nanoparticles as an Effective Disinfectant: A Review. Mater. Sci. Eng. C. 2019;97:954–965. doi: 10.1016/j.msec.2018.12.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Attia N.F., Morsy M.S. Facile Synthesis of Novel Nanocomposite as Antibacterial and Flame Retardant Material for Textile Fabrics. Mater. Chem. Phys. 2016;180:364–372. doi: 10.1016/j.matchemphys.2016.06.019. [DOI] [Google Scholar]
- 116.Faya M., Hazzah H.A., Omolo C.A., Agrawal N., Maji R., Walvekar P., Mocktar C., Nkambule B., Rambharose S., Albericio F., et al. Novel Formulation of Antimicrobial Peptides Enhances Antimicrobial Activity against Methicillin−Resistant Staphylococcus Aureus (MRSA) Amino Acids. 2020;52:1439–1457. doi: 10.1007/s00726-020-02903-7. [DOI] [PubMed] [Google Scholar]
- 117.Yu Z., Cowan J.A. Metal Complexes Promoting Catalytic Cleavage of Nucleic Acids—Biochemical Tools and Therapeutics. Curr. Opin. Chem. Biol. 2018;43:37–42. doi: 10.1016/j.cbpa.2017.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Di Natale C., De Benedictis I., De Benedictis A., Marasco D. Metal–Peptide Complexes as Promising Antibiotics to Fight Emerging Drug Resistance: New Perspectives in Tuberculosis. Antibiotics. 2020;9:337. doi: 10.3390/antibiotics9060337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Agbale C.M., Sarfo J.K., Galyuon I.K., Juliano S.A., Silva G.G.O., Buccini D.F., Cardoso M.H., Torres M.D.T., Angeles-Boza A.M., de la Fuente-Nunez C., et al. Antimicrobial and Antibiofilm Activities of Helical Antimicrobial Peptide Sequences Incorporating Metal−Binding Motifs. Biochemistry. 2019;58:3802–3812. doi: 10.1021/acs.biochem.9b00440. [DOI] [PubMed] [Google Scholar]
- 120.Xing H., Lu M., Yang T., Liu H., Sun Y., Zhao X., Xu H., Yang L., Ding P. Structure−Function Relationships of Nonviral Gene Vectors: Lessons from Antimicrobial Polymers. Acta Biomater. 2019;86:15–40. doi: 10.1016/j.actbio.2018.12.041. [DOI] [PubMed] [Google Scholar]
- 121.Kyzioł A., Khan W., Sebastian V., Kyzioł K. Tackling Microbial Infections and Increasing Resistance Involving Formulations Based on Antimicrobial Polymers. Chem. Eng. J. 2020;385:123888. doi: 10.1016/j.cej.2019.123888. [DOI] [Google Scholar]
- 122.Alfei S., Schito A.M. Positively Charged Polymers as Promising Devices against Multidrug Resistant Gram−Negative Bacteria: A Review. Polymers. 2020;12:1195. doi: 10.3390/polym12051195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Michl T.D., Hibbs B., Hyde L., Postma A., Tran D.T.T., Zhalgasbaikyzy A., Vasilev K., Meagher L., Griesser H.J., Locock K.E.S. Bacterial Membrane Permeability of Antimicrobial Polymethacrylates: Evidence for a Complex Mechanism from Super−Resolution Fluorescence Imaging. Acta Biomater. 2020;108:168–177. doi: 10.1016/j.actbio.2020.03.011. [DOI] [PubMed] [Google Scholar]
- 124.Dong P., Feng J., Li S., Sun T., Shi Q., Xie X. Synthesis, Characterization, and Antimicrobial Evaluation of Random Poly(Ester−Carbonate)s Bearing Pendant Primary Amine in the Main Chain. Polymers. 2020;12:2640. doi: 10.3390/polym12112640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kamenieva T., Tarasyuk O., Derevianko K., Aksenovska O., Shybyryn O., Metelytsia L., Rogalsky S. Antioxidant Activity of Polymeric Biocide Polyhexamethylene Guanidine Hydrochloride. Catal. Petrochem. 2020;30:73–82. doi: 10.15407/kataliz2020.30.073. [DOI] [Google Scholar]
- 126.Pérez-Betancourt Y., Távora B.D.C.L.F., Colombini M., Faquim-Mauro E.L., Carmona-Ribeiro A.M. Simple Nanoparticles from the Assembly of Cationic Polymer and Antigen as Immunoadjuvants. Vaccines. 2020;8:105. doi: 10.3390/vaccines8010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Carmona-Ribeiro A.M., Pérez-Betancourt Y. Cationic Nanostructures for Vaccines Design. Biomimetics. 2020;5:32. doi: 10.3390/biomimetics5030032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Song J., Jung K.J., Yoon S., Lee K., Kim B. Polyhexamethyleneguanidine Phosphate Induces Cytotoxicity through Disruption of Membrane Integrity. Toxicology. 2019;414:35–44. doi: 10.1016/j.tox.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 129.Pérez-Betancourt Y., Távora B.D.C.L.F., Faquim-Mauro E.L., Carmona-Ribeiro A.M. Biocompatible Lipid Polymer Cationic Nanoparticles for Antigen Presentation. Polymers. 2021;13:185. doi: 10.3390/polym13020185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Samal S.K., Dash M., Van Vlierberghe S., Kaplan D.L., Chiellini E., van Blitterswijk C., Moroni L., Dubruel P. Cationic Polymers and Their Therapeutic Potential. Chem. Soc. Rev. 2012;41:7147–7194. doi: 10.1039/c2cs35094g. [DOI] [PubMed] [Google Scholar]
- 131.Nazir F., Iqbal M. Synthesis, Characterization and Cytotoxicity Studies of Aminated Microcrystalline Cellulose Derivatives against Melanoma and Breast Cancer Cell Lines. Polymers. 2020;12:2634. doi: 10.3390/polym12112634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ergene C., Yasuhara K., Palermo E.F. Biomimetic Antimicrobial Polymers: Recent Advances in Molecular Design. Polym. Chem. 2018;9:2407–2427. doi: 10.1039/C8PY00012C. [DOI] [Google Scholar]
- 133.Timofeeva L., Kleshcheva N. Antimicrobial Polymers: Mechanism of Action, Factors of Activity, and Applications. Appl. Microbiol. Biotechnol. 2011;89:475–492. doi: 10.1007/s00253-010-2920-9. [DOI] [PubMed] [Google Scholar]
- 134.Mushtaq S., Ahmad N.M., Mahmood A., Iqbal M. Antibacterial Amphiphilic Copolymers of Dimethylamino Ethyl Methacrylate and Methyl Methacrylate to Control Biofilm Adhesion for Antifouling Applications. Polymers. 2021;13:216. doi: 10.3390/polym13020216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jelkmann M., Menzel C., Baus R.A., Ausserhofer P., Baecker D., Gust R., Bernkop-Schnürch A. Chitosan: The One and Only? Aminated Cellulose as an Innovative Option for Primary Amino Groups Containing Polymers. Biomacromolecules. 2018;19:4059–4067. doi: 10.1021/acs.biomac.8b01069. [DOI] [PubMed] [Google Scholar]
- 136.Martins A.F., Facchi S.P., Follmann H.D.M., Pereira A.G.B., Rubira A.F., Muniz E.C. Antimicrobial Activity of Chitosan Derivatives Containing N−Quaternized Moieties in Its Backbone: A Review. Int. J. Mol. Sci. 2014;15:20800–20832. doi: 10.3390/ijms151120800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yang Y., Cai Z., Huang Z., Tang X., Zhang X. Antimicrobial Cationic Polymers: From Structural Design to Functional Control. Polym. J. 2018;50:33–44. doi: 10.1038/pj.2017.72. [DOI] [Google Scholar]
- 138.Timofeeva L.M., Kleshcheva N.A., Moroz A.F., Didenko L.V. Secondary and Tertiary Polydiallylammonium Salts: Novel Polymers with High Antimicrobial Activity. Biomacromolecules. 2009;10:2976–2986. doi: 10.1021/bm900435v. [DOI] [PubMed] [Google Scholar]
- 139.Kanazawa A., Ikeda T., Endo T. Antibacterial Activity of Polymeric Sulfonium Salts. J. Polym. Sci. Part A Polym. Chem. 1993;31:2873–2876. doi: 10.1002/pola.1993.080311126. [DOI] [Google Scholar]
- 140.Zhang B., Li M., Lin M., Yang X., Sun J. A Convenient Approach for Antibacterial Polypeptoids Featuring Sulfonium and Oligo(Ethylene Glycol) Subunits. Biomater. Sci. 2020;8:6969–6977. doi: 10.1039/D0BM01384F. [DOI] [PubMed] [Google Scholar]
- 141.Garcia I.M., Rodrigues S.B., Leitune V.C.B., Collares F.M., Garcia I.M., Rodrigues S.B., Leitune V.C.B., Collares F.M. Antibacterial, Chemical and Physical Properties of Sealants with Polyhexamethylene Guanidine Hydrochloride. Braz. Oral Res. 2019;33 doi: 10.1590/1807-3107bor-2019.vol33.0019. [DOI] [PubMed] [Google Scholar]
- 142.Cowan M.G., Masuda M., McDanel W.M., Kohno Y., Gin D.L., Noble R.D. Phosphonium−Based Poly(Ionic Liquid) Membranes: The Effect of Cation Alkyl Chain Length on Light Gas Separation Properties and Ionic Conductivity. J. Membr. Sci. 2016;498:408–413. doi: 10.1016/j.memsci.2015.10.019. [DOI] [Google Scholar]
- 143.Kanazawa A., Ikeda T., Endo T. Polymeric Phosphonium Salts as a Novel Class of Cationic Biocides. IV. Synthesis and Antibacterial Activity of Polymers with Phosphonium Salts in the Main Chain. J. Polym. Sci. Part Polym. Chem. 1993;31:3031–3038. doi: 10.1002/pola.1993.080311219. [DOI] [Google Scholar]
- 144.Kim S.-H., Semenya D., Castagnolo D. Antimicrobial Drugs Bearing Guanidine Moieties: A Review. Eur. J. Med. Chem. 2021;216:113293. doi: 10.1016/j.ejmech.2021.113293. [DOI] [PubMed] [Google Scholar]
- 145.Xue Y., Xiao H., Zhang Y. Antimicrobial Polymeric Materials with Quaternary Ammonium and Phosphonium Salts. Int. J. Mol. Sci. 2015;16:3626–3655. doi: 10.3390/ijms16023626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bieser A.M., Tiller J.C. Mechanistic Considerations on Contact−Active Antimicrobial Surfaces with Controlled Functional Group Densities. Macromol. Biosci. 2011;11:526–534. doi: 10.1002/mabi.201000398. [DOI] [PubMed] [Google Scholar]
- 147.Chen Y., Tan W., Li Q., Dong F., Gu G., Guo Z. Synthesis of Inulin Derivatives with Quaternary Phosphonium Salts and Their Antifungal Activity. Int. J. Biol. Macromol. 2018;113:1273–1278. doi: 10.1016/j.ijbiomac.2018.03.055. [DOI] [PubMed] [Google Scholar]
- 148.Leong J., Yang C., Tan J., Tan B.Q., Hor S., Hedrick J.L., Yang Y.Y. Combination of Guanidinium and Quaternary Ammonium Polymers with Distinctive Antimicrobial Mechanisms Achieving a Synergistic Antimicrobial Effect. Biomater. Sci. 2020;8:6920–6929. doi: 10.1039/D0BM00752H. [DOI] [PubMed] [Google Scholar]
- 149.Kuroki A., Tchoupa A.K., Hartlieb M., Peltier R., Locock K.E.S., Unnikrishnan M., Perrier S. Targeting Intracellular, Multi−Drug Resistant Staphylococcus Aureus with Guanidinium Polymers by Elucidating the Structure−Activity Relationship. Biomaterials. 2019;217:119249. doi: 10.1016/j.biomaterials.2019.119249. [DOI] [PubMed] [Google Scholar]
- 150.Li Y., Cai P., Tong Z.-F., Xiao H., Pan Y. Preparation of Copolymer−Based Nanoparticles with Broad−Spectrum Antimicrobial Activity. Polymers. 2017;9:717. doi: 10.3390/polym9120717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Li Z., Chen J., Cao W., Wei D., Zheng A., Guan Y. Permanent Antimicrobial Cotton Fabrics Obtained by Surface Treatment with Modified Guanidine. Carbohydr. Polym. 2018;180:192–199. doi: 10.1016/j.carbpol.2017.09.080. [DOI] [PubMed] [Google Scholar]
- 152.Salama A., Hasanin M., Hesemann P. Synthesis and Antimicrobial Properties of New Chitosan Derivatives Containing Guanidinium Groups. Carbohydr. Polym. 2020;241:116363. doi: 10.1016/j.carbpol.2020.116363. [DOI] [PubMed] [Google Scholar]
- 153.Pan Y., Xia Q., Xiao H. Cationic Polymers with Tailored Structures for Rendering Polysaccharide−Based Materials Antimicrobial: An Overview. Polymers. 2019;11:1283. doi: 10.3390/polym11081283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rauschenbach M., Lawrenson S.B., Taresco V., Pearce A.K., O’Reilly R.K. Antimicrobial Hyperbranched Polymer–Usnic Acid Complexes through a Combined ROP−RAFT Strategy. Macromol. Rapid Commun. 2020;41:2000190. doi: 10.1002/marc.202000190. [DOI] [PubMed] [Google Scholar]
- 155.Olmo J.A.-D., Ruiz-Rubio L., Pérez-Alvarez L., Sáez-Martínez V., Vilas-Vilela J.L. Antibacterial Coatings for Improving the Performance of Biomaterials. Coatings. 2020;10:139. doi: 10.3390/coatings10020139. [DOI] [Google Scholar]
- 156.Murrieta-Rico F.N. Prospects for Further Development of Face Masks to Minimize Pandemics—Functionalization of Textile Materials with Biocide Inorganic Nanoparticles: A Review. IEEE Lat. Am. Trans. 2021;19:1010–1023. [Google Scholar]
- 157.Kumar L., Brice J., Toberer L., Klein-Seetharaman J., Knauss D., Sarkar S.K. Antimicrobial Biopolymer Formation from Sodium Alginate and Algae Extract Using Aminoglycosides. PLoS ONE. 2019;14:e0214411. doi: 10.1371/journal.pone.0214411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Alzagameem A., Klein S.E., Bergs M., Do X.T., Korte I., Dohlen S., Hüwe C., Kreyenschmidt J., Kamm B., Larkins M., et al. Antimicrobial Activity of Lignin and Lignin−Derived Cellulose and Chitosan Composites against Selected Pathogenic and Spoilage Microorganisms. Polymers. 2019;11:670. doi: 10.3390/polym11040670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sivakanthan S., Rajendran S., Gamage A., Madhujith T., Mani S. Antioxidant and Antimicrobial Applications of Biopolymers: A Review. Food Res. Int. 2020;136:109327. doi: 10.1016/j.foodres.2020.109327. [DOI] [PubMed] [Google Scholar]
- 160.Pham T.T., Lindsay A.C., Chen X., Gözaydin G., Yan N., Sperry J. Transferring the Biorenewable Nitrogen Present in Chitin to Several N−Functional Groups. Sustain. Chem. Pharm. 2019;13:100143. doi: 10.1016/j.scp.2019.100143. [DOI] [Google Scholar]
- 161.Sipponen M.H., Lange H., Crestini C., Henn A., Österberg M. Lignin for Nano- and Microscaled Carrier Systems: Applications, Trends, and Challenges. Chemsuschem. 2019;12:2039–2054. doi: 10.1002/cssc.201900480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Karim N., Afroj S., Lloyd K., Oaten L.C., Andreeva D.V., Carr C., Farmery A.D., Kim I.-D., Novoselov K.S. Sustainable Personal Protective Clothing for Healthcare Applications: A Review. ACS Nano. 2020;14:12313–12340. doi: 10.1021/acsnano.0c05537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wo Y., Brisbois E.J., Bartlett R.H., Meyerhoff M.E. Recent Advances in Thromboresistant and Antimicrobial Polymers for Biomedical Applications: Just Say Yes to Nitric Oxide (NO) Biomater. Sci. 2016;4:1161–1183. doi: 10.1039/C6BM00271D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Christman K.L., Vázquez-Dorbatt V., Schopf E., Kolodziej C.M., Li R.C., Broyer R.M., Chen Y., Maynard H.D. Nanoscale Growth Factor Patterns by Immobilization on a Heparin−Mimicking Polymer. J. Am. Chem. Soc. 2008;130:16585–16591. doi: 10.1021/ja803676r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sangaj N., Kyriakakis P., Yang D., Chang C.-W., Arya G., Varghese S. Heparin Mimicking Polymer Promotes Myogenic Differentiation of Muscle Progenitor Cells. Biomacromolecules. 2010;11:3294–3300. doi: 10.1021/bm101041f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wang L., Li H., Chen S., Nie C., Cheng C., Zhao C. Interfacial Self−Assembly of Heparin−Mimetic Multilayer on Membrane Substrate as Effective Antithrombotic, Endothelialization, and Antibacterial Coating. ACS Biomater. Sci. Eng. 2015;1:1183–1193. doi: 10.1021/acsbiomaterials.5b00320. [DOI] [PubMed] [Google Scholar]
- 167.Smith R.J., Moule M.G., Sule P., Smith T., Cirillo J.D., Grunlan J.C. Polyelectrolyte Multilayer Nanocoating Dramatically Reduces Bacterial Adhesion to Polyester Fabric. ACS Biomater. Sci. Eng. 2017;3:1845–1852. doi: 10.1021/acsbiomaterials.7b00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Batul R., Tamanna T., Khaliq A., Yu A. Recent Progress in the Biomedical Applications of Polydopamine Nanostructures. Biomater. Sci. 2017;5:1204–1229. doi: 10.1039/C7BM00187H. [DOI] [PubMed] [Google Scholar]
- 169.Karkhanechi H., Takagi R., Matsuyama H. Biofouling Resistance of Reverse Osmosis Membrane Modified with Polydopamine. Desalination. 2014;336:87–96. doi: 10.1016/j.desal.2013.12.033. [DOI] [Google Scholar]
- 170.Su L., Yu Y., Zhao Y., Liang F., Zhang X. Strong Antibacterial Polydopamine Coatings Prepared by a Shaking−Assisted Method. Sci. Rep. 2016;6:24420. doi: 10.1038/srep24420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sun H., Hong Y., Xi Y., Zou Y., Gao J., Du J. Synthesis, Self−Assembly, and Biomedical Applications of Antimicrobial Peptide−Polymer Conjugates. Biomacromolecules. 2018;19:1701–1720. doi: 10.1021/acs.biomac.8b00208. [DOI] [PubMed] [Google Scholar]
- 172.Sahariah P., Sørensen K.K., Hjálmarsdóttir M.Á., Sigurjónsson Ó.E., Jensen K.J., Másson M., Thygesen M.B. Antimicrobial Peptide Shows Enhanced Activity and Reduced Toxicity upon Grafting to Chitosan Polymers. Chem. Commun. 2015;51:11611–11614. doi: 10.1039/C5CC04010H. [DOI] [PubMed] [Google Scholar]
- 173.Lim K., Chua R.R.Y., Bow H., Tambyah P.A., Hadinoto K., Leong S.S.J. Development of a Catheter Functionalized by a Polydopamine Peptide Coating with Antimicrobial and Antibiofilm Properties. Acta Biomater. 2015;15:127–138. doi: 10.1016/j.actbio.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 174.Zare E.N., Jamaledin R., Naserzadeh P., Afjeh-Dana E., Ashtari B., Hosseinzadeh M., Vecchione R., Wu A., Tay F.R., Borzacchiello A., et al. Metal−Based Nanostructures/PLGA Nanocomposites: Antimicrobial Activity, Cytotoxicity, and Their Biomedical Applications. ACS Appl. Mater. Interfaces. 2020;12:3279–3300. doi: 10.1021/acsami.9b19435. [DOI] [PubMed] [Google Scholar]
- 175.Zare E.N., Makvandi P., Ashtari B., Rossi F., Motahari A., Perale G. Progress in Conductive Polyaniline−Based Nanocomposites for Biomedical Applications: A Review. J. Med. Chem. 2020;63:1–22. doi: 10.1021/acs.jmedchem.9b00803. [DOI] [PubMed] [Google Scholar]
- 176.Korupalli C., Kalluru P., Nuthalapati K., Kuthala N., Thangudu S., Vankayala R. Recent Advances of Polyaniline−Based Biomaterials for Phototherapeutic Treatments of Tumors and Bacterial Infections. Bioengineering. 2020;7:94. doi: 10.3390/bioengineering7030094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kim S.H., Kang E.B., Jeong C.J., Sharker S.M., In I., Park S.Y. Light Controllable Surface Coating for Effective Photothermal Killing of Bacteria. ACS Appl. Mater. Interfaces. 2015;7:15600–15606. doi: 10.1021/acsami.5b04321. [DOI] [PubMed] [Google Scholar]
- 178.Hsiao Y.-C., Jheng P.-R., Nguyen H.T., Chen Y.-H., Manga Y.B., Lu L.-S., Rethi L., Chen C.-H., Huang T.-W., Lin J.-D., et al. Photothermal−Irradiated Polyethyleneimine−Polypyrrole Nanopigment Film−Coated Polyethylene Fabrics for Infrared−Inspired with Pathogenic Evaluation. ACS Appl. Mater. Interfaces. 2021;13:2483–2495. doi: 10.1021/acsami.0c17169. [DOI] [PubMed] [Google Scholar]
- 179.Hou Y.-T., Hsu C.-C. Development of a 3D Porous Chitosan/Gelatin Liver Scaffold for a Bioartificial Liver Device. J. Biosci. Bioeng. 2020;129:741–748. doi: 10.1016/j.jbiosc.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 180.Hu W., Wang Z., Xu Y., Wang X., Xiao Y., Zhang S., Wang J. Remodeling of Inherent Antimicrobial Nanofiber Dressings with Melamine−Modified Fibroin into Neoskin. J. Mater. Chem. B. 2019;7:3412–3423. doi: 10.1039/C9TB00276F. [DOI] [Google Scholar]
- 181.Li M., Zhang Z., Liang Y., He J., Guo B. Multifunctional Tissue−Adhesive Cryogel Wound Dressing for Rapid Nonpressing Surface Hemorrhage and Wound Repair. ACS Appl. Mater. Interfaces. 2020;12:35856–35872. doi: 10.1021/acsami.0c08285. [DOI] [PubMed] [Google Scholar]
- 182.Salekdeh S.S.H., Daemi H., Zare-Gachi M., Rajabi S., Bazgir F., Aghdami N., Nourbakhsh M.S., Baharvand H. Assessment of the Efficacy of Tributylammonium Alginate Surface−Modified Polyurethane as an Antibacterial Elastomeric Wound Dressing for Both Noninfected and Infected Full−Thickness Wounds. ACS Appl. Mater. Interfaces. 2020;12:3393–3406. doi: 10.1021/acsami.9b18437. [DOI] [PubMed] [Google Scholar]
- 183.Milazzo M., Gallone G., Marcello E., Mariniello M.D., Bruschini L., Roy I., Danti S. Biodegradable Polymeric Micro/Nano−Structures with Intrinsic Antifouling/Antimicrobial Properties: Relevance in Damaged Skin and Other Biomedical Applications. J. Funct. Biomater. 2020;11:60. doi: 10.3390/jfb11030060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gu J., Clegg J.R., Heersema L.A., Peppas N.A., Smyth H.D.C. Optimization of Cationic Nanogel PEGylation to Achieve Mammalian Cytocompatibility with Limited Loss of Gram−Negative Bactericidal Activity. Biomacromolecules. 2020;21:1528–1538. doi: 10.1021/acs.biomac.0c00081. [DOI] [PubMed] [Google Scholar]
- 185.Gao H., Zhong Z., Xia H., Hu Q., Ye Q., Wang Y., Chen L., Du Y., Shi X., Zhang L. Construction of Cellulose Nanofibers/Quaternized Chitin/Organic Rectorite Composites and Their Application as Wound Dressing Materials. Biomater. Sci. 2019;7:2571–2581. doi: 10.1039/C9BM00288J. [DOI] [PubMed] [Google Scholar]
- 186.Bolto B., Gregory J. Organic Polyelectrolytes in Water Treatment. Water Res. 2007;41:2301–2324. doi: 10.1016/j.watres.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 187.Abiola O.N. Polymers for Coagulation and Flocculation in Water Treatment. In: Das R., editor. Polymeric Materials for Clean Water. Springer International Publishing; Cham, Switzerland: 2019. pp. 77–92. (Springer Series on Polymer and Composite Materials). [Google Scholar]
- 188.Carmona-Ribeiro A., Carrasco L. Fungicidal Assemblies and Their Mode of Action. OA Biotechnol. 2013;2 doi: 10.13172/2052-0069-2-3-983. [DOI] [Google Scholar]
- 189.Kumar A., Boyer C., Nebhani L., Wong E.H.H. Highly Bactericidal Macroporous Antimicrobial Polymeric Gel for Point−of−Use Water Disinfection. Sci. Rep. 2018;8:7965. doi: 10.1038/s41598-018-26202-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Pullangott G., Kannan U., Gayathri S., Kiran D.V., Maliyekkal S.M. A Comprehensive Review on Antimicrobial Face Masks: An Emerging Weapon in Fighting Pandemics. RSC Adv. 2021;11:6544–6576. doi: 10.1039/D0RA10009A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Majchrzycka K., Okrasa M., Szulc J., Jachowicz A., Gutarowska B. Survival of Microorganisms on Nonwovens Used for the Construction of Filtering Facepiece Respirators. Int. J. Environ. Res. Public. Health. 2019;16:1154. doi: 10.3390/ijerph16071154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Demir B., Cerkez I., Worley S.D., Broughton R.M., Huang T.-S. N−Halamine−Modified Antimicrobial Polypropylene Nonwoven Fabrics for Use against Airborne Bacteria. ACS Appl. Mater. Interfaces. 2015;7:1752–1757. doi: 10.1021/am507329m. [DOI] [PubMed] [Google Scholar]
- 193.Monge F.A., Jagadesan P., Bondu V., Donabedian P.L., Ista L., Chi E.Y., Schanze K.S., Whitten D.G., Kell A.M. Highly Effective Inactivation of SARS−CoV−2 by Conjugated Polymers and Oligomers. ACS Appl. Mater. Interfaces. 2020;12:55688–55695. doi: 10.1021/acsami.0c17445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Martí M., Tuñón-Molina A., Aachmann F.L., Muramoto Y., Noda T., Takayama K., Serrano-Aroca Á. Protective Face Mask Filter Capable of Inactivating SARS−CoV−2, and Methicillin−Resistant Staphylococcus Aureus and Staphylococcus Epidermidis. Polymers. 2021;13:207. doi: 10.3390/polym13020207. [DOI] [PMC free article] [PubMed] [Google Scholar]