Summary
Lysine crotonylation of proteins is a recently identified post‐translational modification (PTM) in plants. However, the function of lysine‐crotonylated proteins in response to abiotic stress in plants has not been reported. In this study, we identified a temperature‐induced lipocalin‐1‐like gene (DgTIL1) from chrysanthemum and showed that it was notably induced in response to cold stress. Overexpression of DgTIL1 enhanced cold tolerance in transgenic chrysanthemum. Ubiquitin membrane yeast two‐hybrid (MYTH) system and bimolecular fluorescence complementation (BIFC) assays showed that DgTIL1 interacts with a nonspecific lipid transfer protein (DgnsLTP), which can promote peroxidase (POD) gene expression and POD activity to reduce the accumulation of reactive oxygen species (ROS) and improve resistance to cold stress in DgnsLTP transgenic chrysanthemum. In addition, we found that DgTIL1 was lysine crotonylated at K72 in response to low temperature in chrysanthemum. Moreover, lysine crotonylation of DgTIL1 prevented DgnsLTP protein degradation in tobacco and chrysanthemum. Inhibition of DgnsLTP degradation by lysine crotonylation of DgTIL1 further enhanced POD expression and POD activity, reduced the accumulation of ROS under cold stress in DgTIL1 transgenic chrysanthemum, thus promoting the cold resistance of chrysanthemum.
Keywords: temperature‐induced lipocalins, nonspecific lipid transfer proteins, cold stress, protein interaction, lysine crotonylation, peroxidase
Introduction
Low temperature markedly impairs the growth and development of plants; however, plants have corresponding defence systems to prevent damage from low temperatures. Cold regulatory proteins (CORs), dehydration‐responsive element (DRE)‐binding protein (DREB) transcription factors and antifreeze molecules maintain the stability of the plasma membrane and reduce the toxicity of reactive oxygen species (ROS) (Krasensky and Jonak, 2012; Miura and Furumoto, 2013). To protect against low temperature stress, post‐translational modifications (PTMs) can regulate cold‐responsive genes, such as the ubiquitination of BT2, which enhances the cold resistance of MdMYB23 in apple (An et al., 2018), and phosphorylation of inducer of CBF expression 1 (ICE1), which regulates the stability of ICE1 and freezing tolerance in Arabidopsis (Li et al., 2017; Zhao et al., 2017). Lysine crotonylation, which was identified in 2011 (Tan et al., 2011), is a novel PTM. According to recent reports in tobacco, papaya, tea plants and rice (Sun et al., 2017; Xu et al., 2017; Liu et al., 2018a,b; Lu et al., 2018; Sun et al., 2019), lysine crotonylation of histones is related to signal transduction and cellular physiology and mainly participates in the process of protein biosynthesis, folding and degradation, chromatin organization, carbon metabolism and photosynthesis. However, the function of lysine‐crotonylated proteins in response to abiotic stress has not been reported.
Temperature‐induced lipocalins (TILs) are members of the lipocalin family, and experiments have shown that TILs are located on the plasma membrane (Charron et al., 2005) and play an important role in increasing the stability of the plasma membrane to improve the cold resistance of Arabidopsis thaliana (Uemura et al., 2006). TIL proteins were first identified in A. thaliana (AtTIL) and wheat (TaTIL), and their transcripts were up‐regulated during cold acclimation as determined by Northern Blot analysis (Charron et al., 2002). In addition, the transcript level of TaTIL was higher, and the protein level of TaTIL was significantly higher in low temperature‐treated wheat than in the control (Kawamura and Uemura, 2003). Furthermore, overexpression of MfTIL1 can also mediate the up‐regulation of the transcript level of cold‐responsive genes, such as the CBF transcription factor and COR15a in Medicago falcate to improve the cold tolerance of plants (He et al., 2005). However, the underlying molecular regulatory mechanism of TIL1 is unclear and information on TILs has not been reported in chrysanthemum.
Nonspecific lipid transfer proteins (nsLTPs), which are localized on the plasma membrane, are widely present in multiple organisms (Debono et al., 2009) and harbour an eight‐cysteine (c) motif backbone, which forms four disulphide bonds that facilitate binding between different lipids and hydrophobic compounds (Kader, 1996; Yeats and Rose, 2008) and protect plants from adverse environmental conditions. Plant nsLTP can respond to cold stress, such as by promoting the overexpression of AtLTP3, which reduces electrolyte leakage induced by cold stress to improve soluble sugar accumulation and the survival rate of A. thaliana (Debono et al., 2009), and inducing the expression of OsLTPL159, which has been shown to regulate the activity of POD enzymes in rice to improve plant cold tolerance (Zhao et al., 2020). However, the TIL‐mediated molecular regulatory mechanism of nsLTP has not yet been studied and the biological function of nsLTPs has not been reported in chrysanthemum.
In our study, we found that DgTIL1 acts as a regulatory gene under low temperature stress and DgTIL1 overexpression improves the cold resistance of chrysanthemum. Ubiquitin membrane yeast two‐hybrid (MYTH) system and bimolecular fluorescence complementation (BIFC) assays showed that DgTIL1 interacts with DgnsLTP and DgnsLTP overexpression can promote POD expression and POD activity to reduce the accumulation of ROS and improve resistance to cold stress in DgnsLTP transgenic chrysanthemum. Lysine crotonylation of DgTIL1 enhances the interaction between DgTIL1 and DgnsLTP in tobacco and prevents DgnsLTP protein degradation in tobacco and chrysanthemum. Finally, inhibition of degradation of DgnsLTP by lysine crotonylation of DgTIL1 further enhances POD activity and minimizes ROS in DgTIL1 transgenic chrysanthemum.
Results
DgTIL1 is responsive to low temperature
To identify cold‐responsive TIL genes in chrysanthemum, we performed transcriptome analyses (accession number GSE117262) using cold‐treated (4°C for 24 h and −4°C for 4 h) and non‐treated (temperature, 25°C) chrysanthemum seedlings. The results showed that 4 TIL genes were significantly induced at the transcription level by cold treatment (Table S1). Among these genes, DgTIL1 (log2fold change = 4.2) (GenBank accession number: MT219513) was notably induced via cold treatment. Therefore, DgTIL1 was chosen for further investigation.
The full‐length cDNA of DgTIL1 contains 558 bp and encodes a predicted protein of 186 amino acids. Alignment of the TILs amino acid sequences of various plants demonstrated that DgTIL1 contains three structurally conserved region (SCR) (Figure S1a).The phylogenetic analysis demonstrated that DgTIL1 is highly homologous with the temperature‐induced lipocalin‐1‐like protein and closely related to TcTIL1 from Tanacetum cinerariifolium (Figure S1b).To determine the subcellular localization of DgTIL1, pSuper1300‐DgTIL1‐GFP and the plasma membrane marker protein PM‐mCherry (CD3‐1007) were coexpressed in the epidermal cells of tobacco leaves, and the results showed that DgTIL1 was localized in the plasma membrane (Figure 1a).
Figure 1.
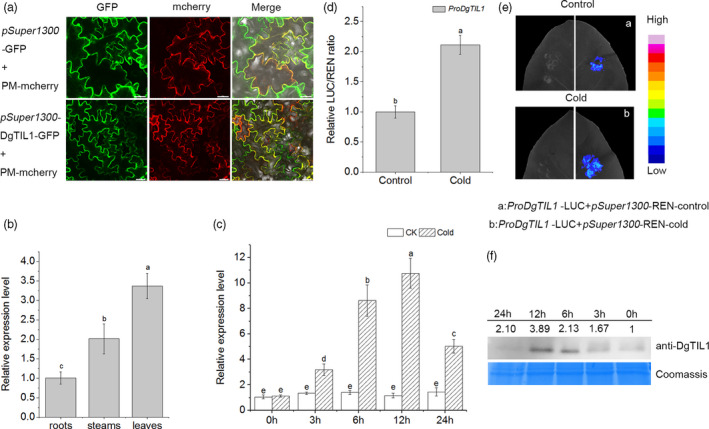
DgTIL1 is responsive to low temperature. (a) Subcellular localization of pSuper1300‐DgTIL1‐GFP in tobacco leaves. pSuper1300‐GFP and CD3‐1007 (an mCherry‐labelled plasma membrane marker) were used as negative controls. Scale bars, 20 μm. (b) Expression of DgTIL1 in the roots, stems, and leaves of WT chrysanthemum at normal temperature using qRT‐PCR (P < 0.05) (Data represent means and standard errors of 3 replicates, 20 plants per replicate). (c) Relative expression levels of the DgTIL1 gene in the WT with low temperature treatment (4°C). CK represents the control under control conditions (25°C day/22°C night). (d and e) DLA assay and LCI assay of the native promoter of ProDgTIL1 after transient expression in tobacco, and the control (25°C day/22°C night) and cold (4°C for 12 h) treatment results were used for comparison. (f) Immunoblot analysis of DgTIL1. Proteins from WT chrysanthemum leaves under low temperature (4°C) treatment were probed with anti‐DgTIL1 (1:1000, from PTM BIO, Hangzhou, China), and Coomassie blue staining was used to demonstrate consistent protein loading.
We measured the expression pattern of DgTIL1 in different tissues and at different times at low temperature in the WT leaves by qRT‐PCR and found that the transcript abundance of DgTIL1 in the leaves was significantly higher (P < 0.05) than that in the roots and stems (Figure 1b). In addition, the expression of DgTIL1 in the leaves reached the highest value (P < 0.05) after 12 h of low temperature treatment (Figure 1c). The promoters (1.3 kbp) of the DgTIL1 and LUC reporter genes were inserted into pSuper1300 to probe the function of the natural promoter. We found that the luciferase (LUC) activity of DgTIL1 natural promoter was enhanced at low temperatures as detected by a dual luciferase reporter gene assay (DLA) (Figure 1d), and the luciferase complementation imaging (LCI) experiments also showed the same results (Figure 1e), indicating that low temperature activates the transcription of DgTIL1.
We performed proteomics sequencing and found that the protein expression of DgTIL1 was 1.20‐times higher in chrysanthemum under low temperature conditions than the control chrysanthemum. The mass spectrometry proteomics data were deposited into the ProteomeXchange consortium via the PRIDE partner repository, with the dataset identifier PXD010297. In addition, a gel blot analysis showed that the protein expression generally gradually increased with increasing low temperature treatment time and the protein abundance peaked at 12 h as evidenced by an anti‐DgTIL1 antibody (Figure 1f). These results revealed that DgTIL1 responds to low temperature.
Overexpression of DgTIL1 improves the cold resistance of chrysanthemum
To verify the role of DgTIL1 in coping with cold stress, the 35S::DgTIL1 overexpression vector was transformed into WT chrysanthemum. Finally, we obtained four independent transgenic chrysanthemum lines and two overexpression lines (35S::DgTIL1‐2 and 35S::DgTIL1‐4) that exhibited relatively high expression, which were used in subsequent experiments. The qRT‐PCR experiment showed that the expression of DgTIL1 in the OE‐2 and OE‐4 lines was significantly (P < 0.05) higher than that in the WT line under the cold treatment (Figure 2a).
Figure 2.
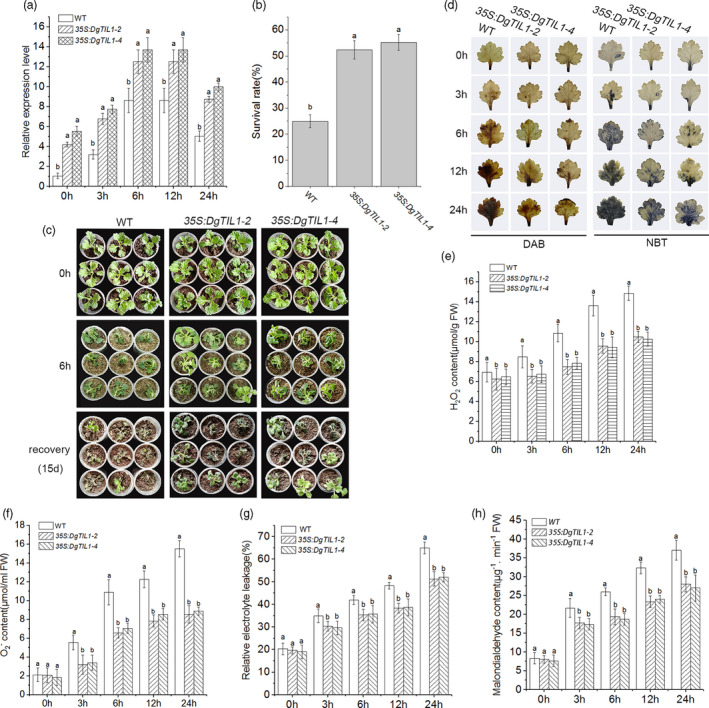
Less ROS products in DgTIL1 transgenic chrysanthemum. (a) Expression levels of DgTIL1 in the WT and transgenic chrysanthemum under control conditions (25°C day/22°C night) and low temperature stress at 4°C (P < 0.05). (b) Survival rates of WT and DgTIL1 transgenic chrysanthemum after 15 days of recovery under control conditions (25°C day/22°C night), with 3 replicates and 50 plants per replicate. (c) Chrysanthemum phenotype changes under low temperature stress at −6°C. (d) DAB and NBT staining of WT and DgTIL1 transgenic chrysanthemum under low temperature stress at 4°C. (e and f) Comparison of accumulation of H2O2 (e) and O2 ‐ (f) in plant lines (WT and 35S transgenic lines) at each time point under 4°C treatment (3 replicates, 50 plants per replicate). (g and h) Analysis of relative electrolyte leakage (g) and malondialdehyde (h) in the WT and DgTIL1 transgenic lines at each time point under 4°C treatment (3 replicates, with 50 plants per replicate).
Then, we further tested the cold resistance of the transgenic lines, and the results showed that the OE‐2 and OE‐4 lines had higher survival rates than the WT line (Figure 2b,c). Moreover, to more intuitively observe H2O2 and O2 ‐ accumulation under cold stress, we stained chrysanthemum leaves sampled from the transgenic and WT lines with diaminobenzidine tetrahydrochloride (DAB) and nitroblue tetrazolium (NBT) (Figure 2d). The results showed fewer brown or blue products of oxidative damage in the transgenic lines than the WT lines, and a quantitative analysis of the H2O2 and O2 ‐ contents showed the same results (Figure 2e,f). Moreover, the electrolyte leakage and malondialdehyde content of DgTIL1 transgenic lines were both lower than those of the WT line (Figure 2g,h), indicating that the DgTIL1 transgenic lines had less membrane damage than the WT line. These results suggested that the DgTIL1 transgenic lines had less ROS accumulation than the WT lines under cold stress, which proved that DgTIL1 overexpression can improve the cold resistance of chrysanthemum.
DgTIL1 interacts with DgnsLTP
We screened potential DgTIL1 interaction factors in chrysanthemum with the ubiquitin MYTH system. According to the results of a BLAST search of positive clones verified by one‐to‐one yeast interaction in GenBank, we identified 10 potential interacting proteins (Table S2). Furthermore, nonspecific lipid transfer protein‐like gene was identified and named DgnsLTP (GenBank accession number MT192757) (Figure S2a).
To further verify the interaction between DgTIL1 and DgnsLTP, a split‐ubiquitin yeast two‐hybrid (Y2H) assay was performed with full‐length DgTIL1 and DgnsLTP (Figure 3a). We found that pBT3‐N‐DgTIL1 interacted with pPR3‐N‐DgnsLTP. In BIFC experiments (Figure 3b), a yellow fluorescence signal was observed in the plasma membrane of cells coexpressing DgTIL1‐YFPn and DgnsLTP‐YFPc, indicating that DgTIL1 interacted with DgnsLTP on the plasma membrane.
Figure 3.
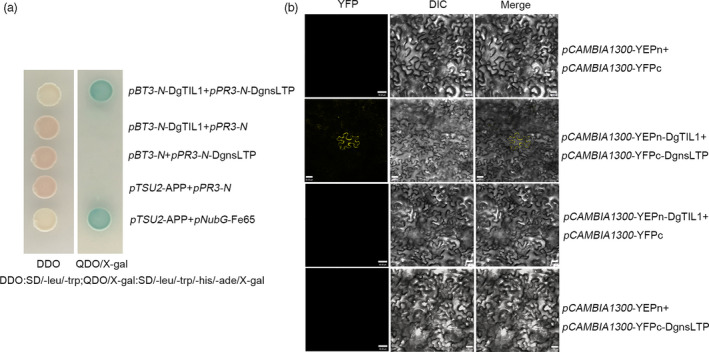
DgTIL1 interacts with DgnsLTP. (a) Split‐ubiquitin Y2H assay validating the interaction between DgTIL1 and DgLTP. pBT3‐N‐DgTIL1 with pPR3‐N, pBT3‐N with pPR3‐N‐DgnsLTP and pTSU2‐APP with pPR3‐N were used as negative controls. DDO:SD/‐leu/‐trp; QDO/X‐gal: SD/‐leu/‐trp/‐his/‐ade/X‐gal. (b) BIFC revealing the interaction of DgTIL1 with DgnsLTP in tobacco. pCAMBIA1300‐35S‐YFPn with pCAMBIA1300‐35S‐YFPc, pCAMBIA1300‐35S‐YFPn‐DgTIL1 with pCAMBIA1300‐35S‐YFPc, and pCAMBIA1300‐35S‐YFPn, with pCAMBIA1300‐35S‐YFPc‐DgnsLTP used as a negative control. Scale bars, 30 μm.
Overexpression of DgnsLTP enhanced tolerance to cold stress
The full‐length cDNA of DgnsLTP contains 537 bp and encodes a predicted protein of 179 amino acids. Alignment of DgnsLTP amino acid sequences in various plants showed that DgnsLTP possesses a conserved eight‐cysteine motif specific to the plant LTP family (Figure S2b). The results of a subcellular localization experiment (Figure 4a) revealed that DgnsLTP is localized on the plasma membrane as evidenced by the overlapping green fluorescence of pSuper1300‐DgnsLTP‐GFP and red fluorescence of a plasma membrane marker (CD3‐1007). The qRT‐PCR revealed that the expression of DgnsLTP was higher (P < 0.05) in the WT chrysanthemum leaves than in other tissues (Figure 4b), and we found the DgnsLTP transcript level was induced by cold treatment (Figure 4c). The promoter (1.5 kbp) of DgnsLTP was assayed by DLA and LCI (Figure 4d,e), and we found that the LUC activity of natural DgnsLTP promoters was increased under cold stress. In addition, Western blot experiments were performed to verify that the DgnsLTP protein in the WT lines was induced by cold treatment using an anti‐DgnsLTP antibody (Figure 4f).
Figure 4.
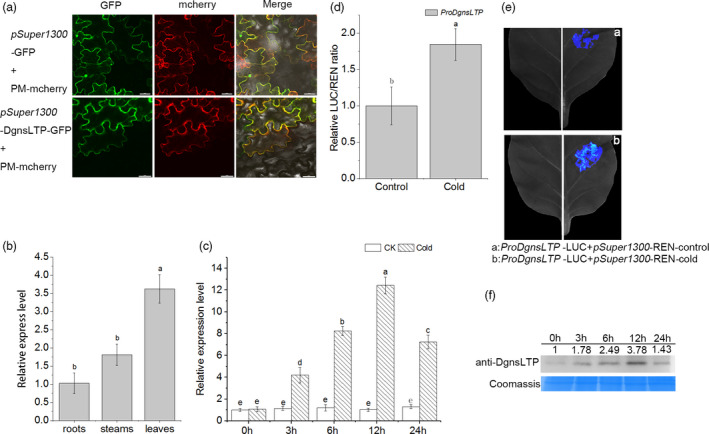
DgnsLTP is responsive to low temperature. (a) pSuper1300‐DgnsLTP‐GFP fusion protein and CD3‐1007 colocalized to the plasma membrane in tobacco cells. Scale bars, μm. (b) Expression of DgnsLTP in the in various tissues of WT chrysanthemum at normal temperature using qRT‐PCR (P < 0.05). (c) Relative expression levels of the DgnsLTP gene in the WT with low temperature treatment (4°C). CK represents the control under control conditions (25°C day/22°C night) (P < 0.05). (d and e) Analysis of native promoter of DgnsLTP with DLA (d) and LCI (e) assay after transient coexpression indicated proteins in tobacco under the control (25°C day/22°C night) and cold (4°C for 12 h) treatment. (f) Proteins of DgnsLTP from WT chrysanthemum leaves under low temperature (4°C) treatment were probed with anti‐DgnsLTP (1:1000, from PTM BIO) using an immunoblot analysis, and Coomassie blue staining was used to demonstrate consistent protein loading.
To determine the function of DgnsLTP under cold stress, two DgnsLTP overexpression lines (OE‐1 and OE‐3) and WT chrysanthemum were treated with or without cold. The cold‐induced transcript level of DgnsLTP in the OE‐1 and OE‐3 lines was higher than that in the WT lines as evaluated by qRT‐PCR assay (Figure 5a). Additionally, the survival rate was higher (Figure 5b,c), and ROS accumulation was lower in the OE‐1 and OE‐3 lines than in the WT lines based on the quantitative analysis of H2O2 and O2 ‐ contents and histochemical staining with DAB and NBT (Figure 5d–f). Moreover, the two transgenic lines had lower relative electrolyte leakage and less malondialdehyde than the WT control line (Figure 5g,h), indicating that DgnsLTP is an active regulatory gene that responds to cold stress in chrysanthemum.
Figure 5.
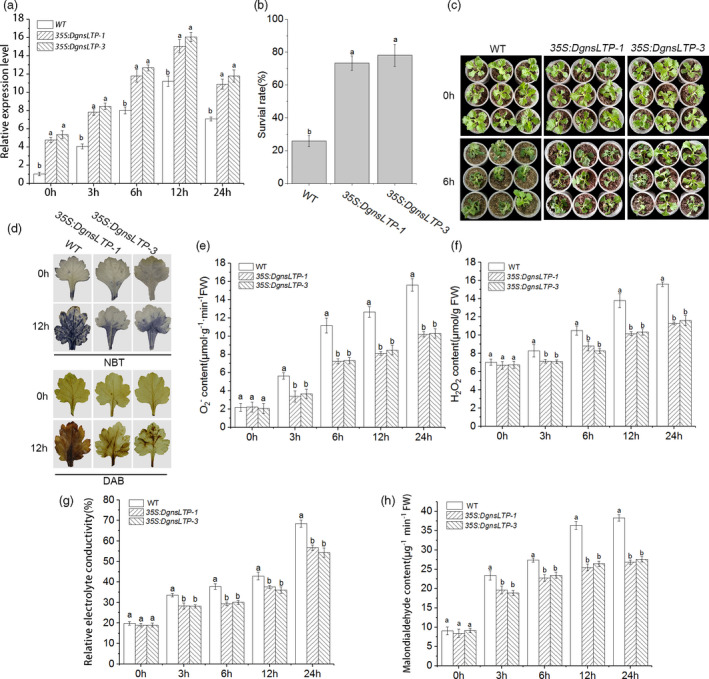
Overexpression of DgnsLTP enhanced cold tolerance. (a) Comparison of the relative expression of DgnsLTP in transgenic chrysanthemum and the WT with increasing low temperature treatment time through qRT‐PCR (P < 0.05). (b) Comparison of the survival rate in the WT and DgnsLTP transgenic lines (3 replicates, 50 plants per replicate) after 15 days of recovery under control conditions (25°C day/22°C night). (c) Phenotype comparison of DgnsLTP transgenic lines and WT. (d) NBT and DAB histochemical staining in the WT and DgnsLTP transgenic lines under low temperature stress (4°C). (e and f) Accumulation of O2 ‐ and H2O2 were evaluated by quantitative analysis, with 3 replicates and 50 plants per replicate. (g and h) Analysis of changes in relative electrolyte leakage (g) and malondialdehyde (h) under low temperature stress.
Cold induces lysine crotonylation of DgTIL1 at the K72 site
We performed lysine crotonylation‐modified TMT‐based quantitative proteome sequencing using cold‐treated (4°C for 24 h and −4°C for 4 h) and non‐treated (temperature, 25°C) chrysanthemum seedlings. We found that the K72 site of the DgTIL1 protein had a high aggregated crotonylated signal intensity (Figure 6a), and the signal intensity of the K72 site of the DgTIL1 protein was 0.76‐times higher than that of the control protein. The mass spectrometry proteomics data have been deposited in the ProteomeXchange consortium via the PRIDE partner repository under the dataset identifier PXD010297. Following crotonylation analysis, the specific antibody anti‐DgTIL1K72 was customized and used to label potential lysine‐crotonylated DgTIL1 proteins in the WT chrysanthemums (Figure 6b). Cold‐induced lysine crotonylation of the DgTIL1 protein in the WT chrysanthemums was successfully detected with the specific anti‐DgTIL1K72 antibody by Western blot analysis, and the level of crotonylation peaked at 12 h.
Figure 6.
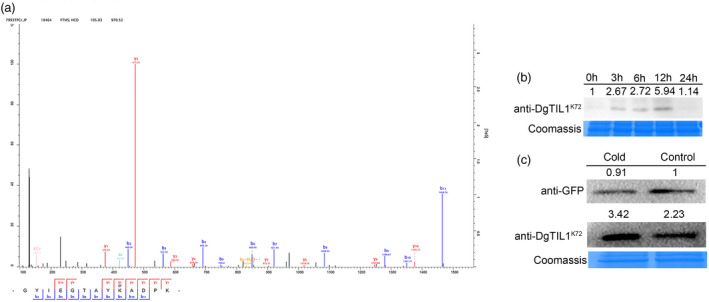
Cold induces lysine crotonylation of DgTIL1 at the K72 site (a) Dissociation mass spectrum revealed that the cold‐induced crotonylation site was the lysine (Lys) at residue 72 (K72) of the DgTIL1 protein that extracted from WT chrysanthemum with affinity purified. (b) Cold‐induced crotonylation of DgTIL1 protein from WT chrysanthemum increased with treatment time (hour) based on a western blot analysis of cold stress (4°C) using a specific anti‐DgTIL1K72 antibody (1:1000, from PTM BIO). (c) Immunoblot analysis of lysine crotonylation of the DgTIL1 protein at the K72 site in tobacco. pSuper1300‐DgTIL1‐GFP fusion protein was extracted from tobacco leaves and analysed with a GFP antibody (1:1000, from PTM BIO) and the specific antibody anti‐DgTIL1K72, and Coomassie blue staining was used to demonstrate consistent protein loading.
In addition, to verify whether lysine crotonylation of the DgTIL1 protein in tobacco leaves was induced under cold stress, the transient expression vector pSuper1300‐DgTIL1‐GFP was constructed to establish transient expression in tobacco leaves. Western blot experiments with anti‐GFP antibody revealed that compared with control conditions, low temperature had almost no effect on the level of the DgTIL1 protein in tobacco leaves. Based on the consistent protein level of DgTIL1, we found that the lysine crotonylation level of DgTIL1 was enhanced using the specific anti‐DgTIL1K72 antibody (Figure 6c), indicating that the DgTIL1 protein also undergoes lysine crotonylation under low temperature conditions in tobacco.
Lysine crotonylation of DgTIL1 enhances the interaction between DgTIL1 and DgnsLTP and stabilizes DgnsLTP
To evaluate whether the degree of crotonylation affects the interaction of DgTIL1 with DgnsLTP, we constructed crotonylation‐deficient and completely crotonylated forms of DgTIL1 by introducing mutations at the Lys (K) 72 site to either Arg (R) or Asn (N) (Figure S3a), which were named DgTIL1K72R and DgTIL1K72N, respectively. We coexpressed pCAMBIA1300‐DgTIL1‐nLUC, pCAMBIA1300‐DgTIL1K72N ‐nLUC or pCAMBIA1300‐DgTIL1K72R ‐nLUC with pCAMBIA1300‐DgnsLTP‐cLUC and performed DLA and LCI assays in tobacco leaves. The REN reporter gene was coexpressed as an internal reference. The LCI assay results (Figure 7a) showed that the relative fluorescence of coexpressed pCAMBIA1300‐DgTIL1‐nLUC and pCAMBIA1300‐DgnsLTP‐cLUC was higher than that of coexpressed pCAMBIA1300‐DgTIL1K72R ‐nLUC and pCAMBIA1300‐DgnsLTP‐cLUC but lower than that of coexpressed pCAMBIA1300‐DgTIL1K72N ‐nLUC and pCAMBIA1300‐DgnsLTP‐cLUC. Additionally, the DLA experiments revealed similar trends (Figure 7b), indicating that the DgTIL1K72N most strongly interacted with DgnsLTP and that the degree of crotonylation affected the interaction of DgTIL1 with DgnsLTP.
Figure 7.
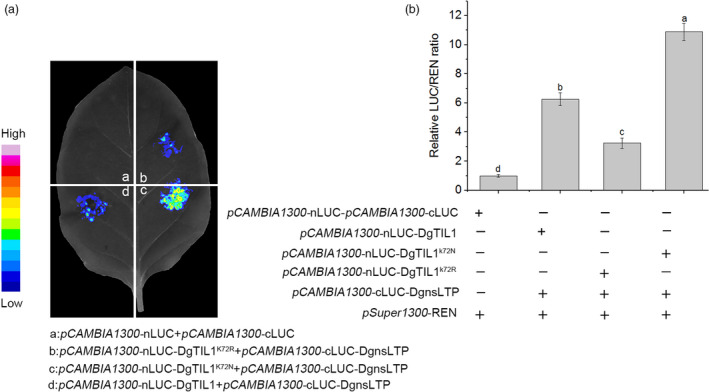
Lysine crotonylation at K72 strengthens the interaction of DgTIL1 with DgnsLTP (a) LCI assay shows the strength of the interaction between DgTIL1 and DgnsLTP. The N‐terminal (nLUC) or C‐terminal (cLUC) fragments of LUC were fused respectively with the indicated protein to transient coexpress. (b) DLA assay showing the interaction strength of relative reporter activity (LUC/REN) of the indicated fusion protein in tobacco, and the value of the empty vector was set to 1.
To investigate whether the stability of DgnsLTP was affected by lysine crotonylation of DgTIL1, pSuper1300‐DgTIL1, pSuper1300‐DgTIL1K72N or pSuper1300‐DgTIL1K72R was coinjected with pSuper1300‐DgnsLTP‐LUC for a transient coexpression assay in tobacco leaves. LUC is a reporter gene, and whether the activity of pSuper1300‐DgnsLTP‐LUC was affected by different degrees of DgTIL1 modification was evaluated by LCI (Figure 8a). The highest fluorescence activity of pSuper1300‐DgnsLTP‐LUC was observed with DgTIL1K72N, followed by DgTIL1, and the lowest fluorescent activity was observed with DgTIL1K72R, which was consistent with the DLA results (Figure 8b). In summary, crotonylation of DgTIL1 was confirmed to affect the stability of DgnsLTP.
Figure 8.
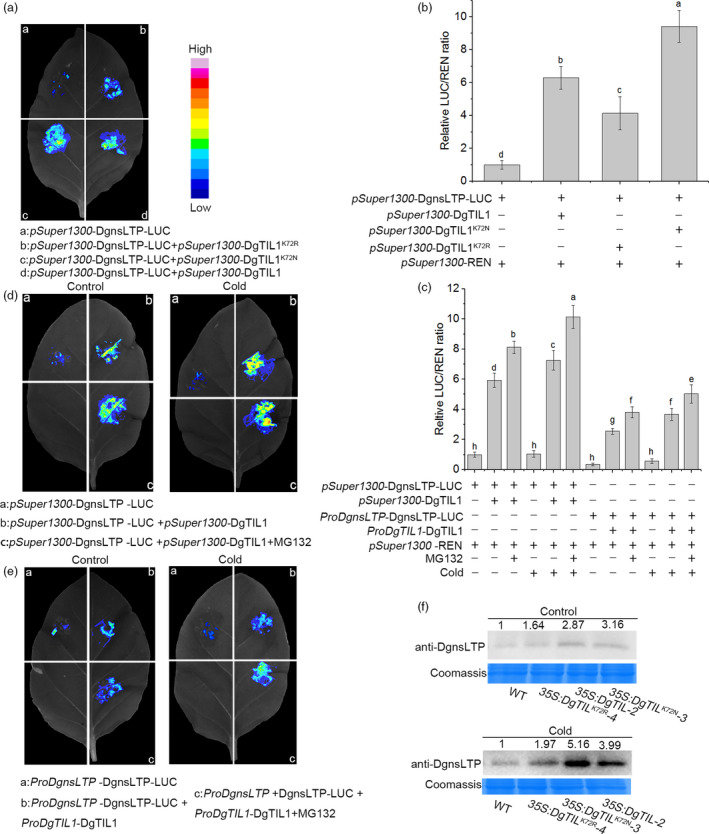
Cold‐induced lysine crotonylation of the DgTIL1 stabilizes DgnsLTP. (a and b) Activity of pSuper1300‐DgnsLTP‐LUC coexpressed with pSuper1300‐DgTIL1,pSuper1300‐DgTIL1K72N and pSuper1300‐DgTIL1K72R in tobacco was detected by LCI assay (a) and DLA assay (b); the fluorescent signal intensity indicates the activity of DgnsLTP under DgTIL1 inhibition, and the value of the empty vector was set to 1. (c, d and e) Comparison of the LUC activity of DgnsLTP‐LUC transiently coexpressed with DgTIL1 between 35S promoter (pSuper1300) and the natural promoter under normal and low temperature treatment in tobacco. MG132 (20 μm) was used as one of the controls, (c) DLA assay, (d) driven by a 35S promoter, (e) driven by a natural promoter. (f) Expression of DgnsLTP protein in the WT and DgnsLTP transgenic chrysanthemum using anti‐DgnsLTP antibody (1:1000, from Sanon Biotech) under control conditions (25°C day/22°C night) and cold (4°C for 12 h) treatment, and Coomassie blue staining was used to demonstrate consistent protein loading.
To further explore the function of lysine crotonylation of DgTIL1 at low temperatures, pSuper1300‐DgTIL1 and pSuper1300‐DgnsLTP‐LUC were coexpressed in tobacco leaves. The relative fluorescence of coexpressed pSuper1300‐DgnsLTP‐LUC and pSuper1300‐DgTIL1 was found to be significantly higher at low temperature than at control conditions (Figure 8c), indicating that lysine crotonylation of DgTIL1 at low temperature further inhibited DgnsLTP degradation. When MG132 was added, the inhibition of DgnsLTP protein degradation was more obvious. Under activation of the natural promoters of DgTIL1 and DgnsLTP, DgTIL1‐mediated lysine crotonylation also prevented the degradation of DgnsLTP, although the relative fluorescence activity of coexpressed ProDgnsLTP‐DgnsLTP‐LUC and ProDgTIL1‐DgTIL1 was lower than that of the 35S promoter. These results implied that DgnsLTP was possibly degraded via the 26S proteasome and that DgTIL1 lysine crotonylation at K72 inhibited this process. The LCI experiment showed the same result in a more intuitive manner (Figure 8d,e).
To further validate our experimental results, an anti‐DgnsLTP antibody was used for Western blotting to measure the protein level of DgnsLTP in DgTIL1, DgTIL1K72N and DgTIL1K72R transgenic chrysanthemum treated with cold stress (Figure 8f; Figure S3b). A comparison of the results under the control and low temperature conditions revealed that the DgTIL1 transgenic lines expressed substantially more DgnsLTP in vivo than the DgTIL1K72R transgenic lines and the WT lines under low temperature but expressed less DgnsLTP than the DgTIL1K72N transgenic lines, indicating that the degree of lysine crotonylation of DgTIL1 can affect the protein expression of DgnsLTP in chrysanthemum.
Inhibition of degradation of DgnsLTP by lysine crotonylation of DgTIL1 further enhances POD activity and minimizes ROS in DgTIL1 transgenic chrysanthemum
To further investigate how lysine crotonylation at K72 affects the function of DgTIL1 in cold tolerance, 35S::DgTIL1K72R and 35S::DgTIL1K72N were constructed and transformed into WT chrysanthemum. The 35S::DgTIL1K72N transgenic lines (35S::DgTIL1K72N‐1 and 35S::DgTIL1K72N‐3), 35S::DgTIL1K72R transgenic lines (35S::DgTIL1K72R‐4 and 35S::DgTIL1K72R‐6) and 35S::DgTIL1 transgenic lines (35S::DgTIL1‐2 and 35S::DgTIL1‐4) were treated with low temperature. The qRT‐PCR revealed that the relative expression of DgTIL1 in the DgTIL1K72R transgenic lines and DgTIL1K72N transgenic lines was significantly (P < 0.05) higher than that in the WT lines under cold treatment, although distinct differences were not observed among the 35S::DgTIL1K72N , 35S::DgTIL1K72R and 35S::DgTIL1 transgenic lines (Figure 9a,b; Figure 2a). Furthermore, the DgTIL1 transgenic lines had higher survival than the DgTIL1K72R transgenic and WT lines but lower survival than the DgTIL1K72N transgenic lines (Figure 9c,d; Figure 2b). Histochemical staining and H2O2 and O2 ‐ content measurements both revealed that all transgenic lines and the WT lines showed an increasing trend with increasing cold treatment time (Figure 9e–g); moreover, the DgTIL1 transgenic lines accumulated less H2O2 and O2 ‐ than the DgTIL1K72R transgenic lines and the WT lines but more H2O2 and O2 ‐ than the DgTIL1K72N transgenic lines. The malondialdehyde and relative electrolyte leakage trends were similar to that of the H2O2 and O2 ‐ results, with a lower relative electrolyte leakage level and less malondialdehyde accumulation observed in the DgTIL1K72N transgenic lines than the DgTIL1 and DgTIL1K72R transgenic lines (Figure 9h–i; Figure 2g–h).
Figure 9.
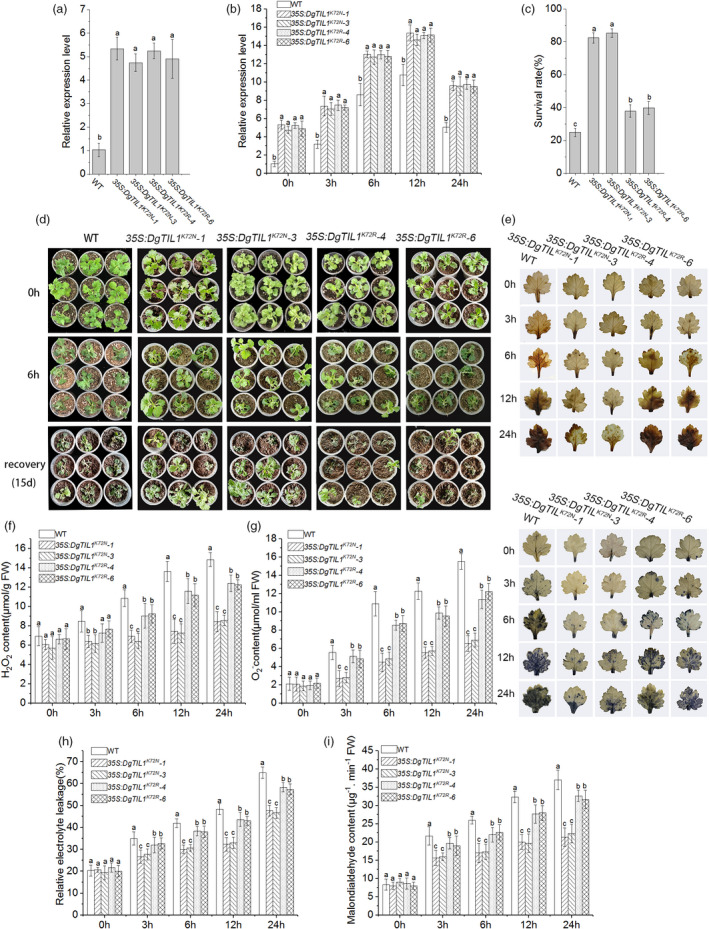
Lysine crotonylation of DgTIL1 at K72 enhances the cold resistance in chrysanthemum. (a and b) Expression levels of DgTIL1 in the WT and transgenic chrysanthemum under control conditions (25°C day/22°C night) (a) and low temperature stress at 4°C (b) using qRT‐PCR (P < 0.05) (3 replicates, 20 plants per replicate). (c) Survival rates of chrysanthemum after 15 days of recovery under control conditions (25°C day/22°C night). (d) Chrysanthemum phenotype changes under low temperature stress at −6°C for 8e and recovery. (e) Histochemical staining was used to reveal the accumulation of H2O2 and O2 ‐ with DAB and NBT in the WT and transgenic lines. (f and g) Analysis of the accumulation of ROS in the WT and transgenic lines as detected by quantitative measurement, (f) content of H2O2, (g) content of O2 ‐ (3 replicates, 50 plants per replicate). (h) Relative electrolyte leakage. (i) Malondialdehyde content (3 replicates, 50 plants per replicate).
At the protein level, immune experiments using anti‐DgTIL1 antibody revealed that the three transgenic chrysanthemum lines (35S::DgTIL1, 35S::DgTIL1K72N and 35S::DgTIL1K72R ) produced more DgTIL1 protein than the WT lines under the control and low temperature conditions (Figure 10a). Additionally, the abundance of the DgTIL1 protein was almost consistent in these transgenic chrysanthemum (Figure 10a; Figure S4a). Therefore, the cold tolerance differences in the DgTIL1, DgTIL1K72N and DgTIL1K72R transgenic chrysanthemums lines are related to the differences in DgnsLTP protein degradation in vivo (Figure 8f).
Figure 10.
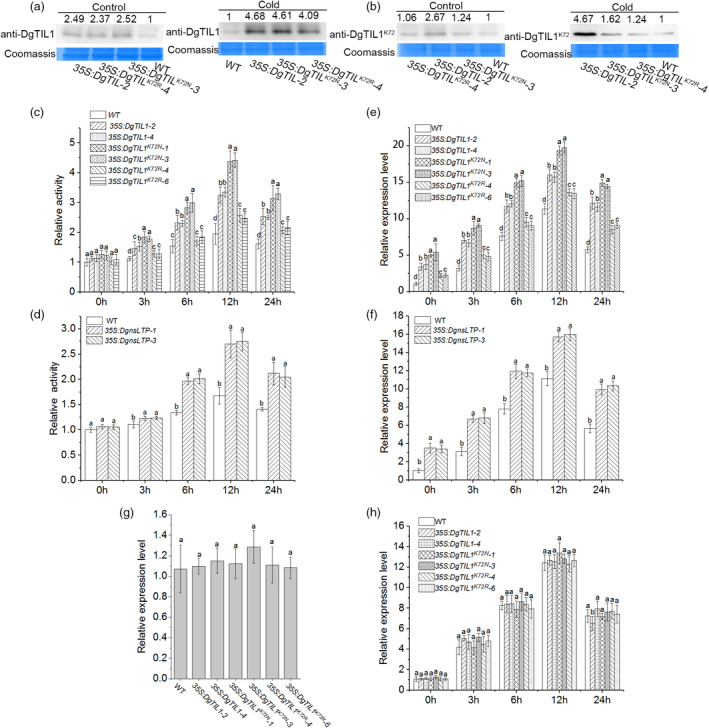
Inhibition of degradation of DgnsLTP by lysine crotonylation of DgTIL1 further enhanced the POD activities (a) Protein abundance of WT and transgenic chrysanthemum as detected by anti‐DgTIL1 antibody under the control and low temperature conditions at 4°C for 12 h. (b) Immunoblot analysis of lysine crotonylation of the DgTIL1 protein in transgenic chrysanthemum and WT chrysanthemum using the anti‐DgTIL1K72 antibody under the control and low temperature conditions at 4°C for 12 h. (c and d) Activity of POD in the WT chrysanthemum, DgTIL1, DgTIL1K72N, DgTIL1K72R transgenic chrysanthemum (c) and DgnsLTP transgenic chrysanthemum (d) under cold stress (4°C), with 3 replicates and 50 plants per replicate. (e and f) Expression level of POD in the WT chrysanthemum, DgTIL1, DgTIL1K72N, DgTIL1K72R transgenic chrysanthemum (e) and DgnsLTP transgenic chrysanthemum (f) under cold stress (4°C), with 3 replicates and 20 plants per replicate. (g and h) DgnsLTP expression level in the WT and transgenic chrysanthemum under control conditions (25°C day/22°C night) (g) and induced by low temperature stress (4°C) (h) (P < 0.05), with 3 replicates and 20 plants per replicate.
At the modification level, the proteins extracted from the three transgenic chrysanthemums were subjected to immunoblot experiments with the anti‐DgTIL1K72 antibody (Figure 10b; Figure S4b). The results showed that the anti‐DgTIL1K72 antibody successfully detected the lysine crotonylation of the DgTIL1 protein under the control and low temperature conditions, although no significant difference was identified among the DgTIL1K72R and DgTIL1K72N transgenic lines and the WT lines.
To explore the mechanism of ROS‐scavenging enzymes, we measured the activity of POD, APX and CAT enzymes in the overexpression and WT lines under the low temperature and control conditions. The results showed that the activity of POD was highest in the DgTIL1K72N transgenic lines that showed less degradation of DgnsLTP protein. Lower DgnsLTP protein degradation was found in the DgTIL1 transgenic lines than the DgTIL1K72R transgenic lines; therefore, the activity of POD in the DgTIL1 transgenic lines was higher than that of the DgTIL1K72R transgenic lines (Figure 10c). Furthermore, the DgnsLTP transgenic lines also showed increased POD activity than the WT lines (Figure 10d), which reduced the ROS toxicity. However, the activity of APX and CAT enzymes in the DgTIL1, DgTIL1K72N , DgTIL1K72R , DgnsLTP transgenic lines and WT lines did not change significantly (Figure S5a‐d). In addition, the expression of POD was consistent with the trend of POD activity (Figure 10e,f), thus indicating that DgnsLTP increases the expression of POD to enhance the cold resistance of the 35S::DgTIL1, 35S::DgTIL1K72N , 35S::DgTIL1K72R and 35S:: DgnsLTP transgenic lines.
A qRT‐PCR assay was performed to verify the effect of the DgTIL1 on DgnsLTP expression in the DgTIL1, DgTIL1K72N and DgTIL1K72R transgenic lines, and almost no differences (P < 0.05) in the expression of DgnsLTP was observed between the transgenic lines and the WT line under the control conditions (Figure 10g) and low temperature conditions (Figure 10h), suggesting that DgTIL1 did not affect the gene expression of DgnsLTP in these transgenic lines.
Overall, the lysine crotonylation modification of DgTIL1 enhanced the ability of DgnsLTP to remove ROS in chrysanthemum and improved the cold resistance of chrysanthemum, with a higher modification level corresponding to less DgnsLTP degradation and stronger cold resistance.
Discussion
To date, TILs have been studied in a variety of plants, including Arabidopsis, wheat (Abo‐Ogiala et al., 2014; Boca et al., 2013; Charron et al., 2002; Chi et al., 2009), M. falcate (He et al., 2005), Narcissus tazetta (Ding et al., 2016) and other plants, and these genes respond to a variety of abiotic stresses. We isolated a temperature‐induced lipocalin‐1‐like gene named DgTIL1 from chrysanthemum. A sequence analysis and phylogenetic analysis revealed that this protein contains three conserved SCR domains that are characteristic of lipoproteins (Flower et al., 2000) and closely homologous to plant temperature‐induced lipocalin‐1‐like proteins (Figure S1a,b). TILs are plasma membrane proteins that can increase the cryostability of the plasma membrane during low temperature treatment (Uemura et al., 2006). TaTIL1 can respond to temperature stress in wheat, and the transcript accumulation of TaTIL1 was nearly 10‐times higher after low temperature stress compared with the control conditions (Charron et al., 2002; Charron, 2005). Furthermore, Arabidopsis AtTIL1 has high homology with wheat TaTIL1 (Chi et al., 2009; Charron, 2005), and the role of AtTIL1 in freezing stress tolerance using AtTIL1 transgenic Arabidopsis has been reported (Charron et al., 2008). AtTIL1 can participate in low temperature stress processes by promoting transcription (Charron et al., 2002), and the protein level of AtTIL1 is also significantly induced by low temperature (Kawamura and Uemura, 2003). In contrast, knockout mutants of AtTIL1 in Arabidopsis under low temperature stress accumulated more peroxidation products and were more sensitive to cold stress than the WT line (Charron et al., 2008; Chi et al., 2009). In addition, overexpression of MfTIL1 can also improve the cold tolerance of plants (He et al., 2005). However, the function of the DgTIL1 gene in response to environmental stress in chrysanthemum remains poorly understood.
In our research, the transcription level of DgTIL1 was up‐regulated and the protein level of DgTIL1 was induced by low temperature in the WT chrysanthemum (Figure 1b,c and f). The DgTIL1 transgenic lines were compared with the WT lines at the transcriptome, protein and physiological levels, and the cold resistance of DgTIL1 transgenic lines was evaluated. After low temperature treatment, the relative expression level of DgTIL1 in transgenic chrysanthemum was significantly up‐regulated compared with that in the WT lines (Figure 2a), and the protein abundance of DgTIL1 in the transgenic lines was also higher than that in the WT lines (Figure 10a; Figure S4a). Moreover, both the relative electrolyte leakage and content of malondialdehyde continued to increase under cold stress in the WT and transgenic chrysanthemum leaves (Figure 2g,h). We speculated that cold stress causes marked accumulation of ROS (such as H2O2 and O2 ‐) in chrysanthemum because high ROS accumulation causes toxicity to plant cells. Electrolyte leakage and malondialdehyde are used as indicators of ROS accumulation levels and can reflect changes in membrane permeability and lipid peroxidation (Farmer and Mueller, 2013; Mittler et al., 2004). The results showed that ROS accumulation increases in chrysanthemum under cold stress (Figure 2d‐f); however, lower relative electrolyte leakage, malondialdehyde contents (Figure 2g,h) and ROS (such as H2O2 and O2 ‐) accumulation (Figure 2d–f) were observed in the DgTIL1 transgenic lines relative to the WT lines, which is consistent with previous reports indicating that TILs can enhance the stability of cell membranes at low temperatures (Chi et al., 2009; Kawamura and Uemura, 2003; Uemura et al., 2006). Maintaining the ROS balance in cells is the key to plant survival. In this process, antioxidants and antioxidative enzymes play a vital role in removing accumulated ROS. POD is a key antioxidant enzyme that eliminates ROS (Gao et al., 2010; Passardi et al., 2004). In our study, the activity of POD was increased in the DgTIL1 transgenic lines compared with the WT lines and the relative expression level of POD was up‐regulated (Figure 10c,e). These changes eliminated ROS toxicity and improved the cold resistance in chrysanthemum.
To better explore the regulated mechanism of DgTIL1 in response to cold stress, we identified a nonspecific nsLTP that interacts with DgTIL1 using the MYTH system (Table S2). nsLTPs are a small family of basic proteins (Stergaard et al., 1993) that can increase the cold resistance of plants. For instance, overexpressing OsLTPL159 in rice can improve cold tolerance (Zhao et al., 2020). In A. thaliana, the promoter of lipid transfer protein 3 (LTP3) is regulated by MYB96 and overexpression of LTP3 can improve the cold tolerance of plants (Guo et al., 2013). In our study, we isolated an nsLTP gene from chrysanthemum, DgnsLTP, which was dramatically induced by low temperature and up‐regulated rapidly in the gene expression level the and in the protein level in the WT chrysanthemum (Figure 4b–c and f). Furthermore, the DgnsLTP‐overexpressing chrysanthemums showed enhanced tolerance to low temperature stress (Figure 5a‐c). In addition, overexpression of DgnsLTP can reduce the accumulation of ROS (H2O2, O2 ‐, relative electrolyte leakage, malondialdehyde) by regulating POD activity and enhancing POD expression (Figure 5d–h; Figure 10d,f). Therefore, DgnsLTP may be an important regulator of cold stress.
In our research, we further evaluated the interactions of DgnsLTP with DgTIL1 on the plasma membrane through Y2H experiments and a BIFC analysis (Figure 3a,b). In addition, we investigated whether the stability of DgnsLTP was influenced by DgTIL1 in tobacco and chrysanthemum. We found that when coexpressing pSuper1300‐DgTIL1 and pSuper1300‐DgnsLTP‐LUC in tobacco, the LUC activity of pSuper1300‐DgnsLTP‐LUC was higher than that without coexpression of pSuper1300‐DgTIL1; moreover, the LUC activity of ProDgnsLTP‐DgnsLTP‐LUC, which is driven by the natural promoter of DgnsLTP, was not as obvious as the results driven by the super promoter. However, the activity was also affected by ProDgTIL1‐DgTIL1 (Figure 8c–e), indicating that DgTIL1 not only interacts with DgnsLTP but also affects the protein stability of DgnsLTP in tobacco. Furthermore, the abundance of the DgnsLTP protein in DgTIL1 transgenic chrysanthemum was higher than that in the WT lines, and more protein accumulated after low temperature induction (Figure 8f; Figure S3b). Therefore, we concluded that DgTIL1 can affect the protein stability of DgnsLTP in chrysanthemum. In addition, we found that the transcript level of DgnsLTP in DgTIL1 transgenic chrysanthemum was not significantly different from that in the WT lines (Figure 10g,h); therefore, we determined that DgTIL1 only affected the protein level of DgnsLTP. Regarding the cold‐stress regulation mechanism, we found that the expression level of POD and activity of POD in the DgTIL1 transgenic lines and DgnsLTP transgenic lines were both induced by low temperature (Figure 10c–f), indicating that the cold‐stress regulation mechanism of these lines is regulated by the POD gene and POD activity. Therefore, we speculated that the enhanced cold resistance of the DgTIL1 transgenic lines was associated with enhanced POD activity and up‐regulated POD expression, which are related to the protein stability of DgnsLTP.
Increasing evidence suggests that PTMs of nonhistone proteins can be involved in abiotic stress processes; for example, ubiquitination and phosphorylation have been widely reported to be involved in low temperature stress (Sun et al., 2017; Tan et al., 2011). These modifications changed the localization and activity status of the modified protein and can also regulate the function of the protein through the protein interaction network (Liu et al., 2018a; Lu et al., 2018; Xu et al., 2017). Evolutionarily conserved lysine crotonylation is a newly discovered type of PTM (Huang et al., 2018). Histone and nonhistone protein lysine crotonylation has been reported from yeast to plants to be involved in transcriptional regulation, photosynthesis, carbon fixation, amino acid metabolism and other biological processes (Liu et al., 2018a; Lu et al., 2018; Tan et al., 2011). In addition, a protein interaction network analysis revealed that nonhistone protein crotonylation can affect not only protein interactions but also enzyme activity through these biological processes (Xu et al., 2017). However, whether nonhistone proteins with lysine crotonylation play a role in cold stress has not been reported. In our research, we found that lysine crotonylation of DgTIL1 occurred at the K72 site under low temperature induction (Figure 6a–c). Additionally, the DgTIL1, DgTIL1K72N and DgTIL1K72R transgenic lines have different cold tolerances and the DgTIL1K72N transgenic lines have a higher survival rate than the DgTIL1 and DgTIL1K72R transgenic lines (Figure 2b; Figure 9c). Moreover, in the DgTIL1K72N transgenic lines, the relative electrolyte leakage, malondialdehyde contents and ROS accumulation (H2O2 and O2 ‐) were significantly lower than those in the DgTIL1 and DgTIL1K72R transgenic lines (Figure 2d–h; Figure 9e–i) and the POD activity and POD expression level were higher than those in the DgTIL1 and DgTIL1K72R transgenic lines (Figure 10c,e). Therefore, the DgTIL1K72N transgenic lines had the highest cold tolerance. However, ROS accumulation (H2O2 and O2 ‐ contents, relative electrolyte leakage, the malondialdehyde content) in the DgTIL1K72R transgenic lines were higher than those in the DgTIL1 and DgTIL1K72N transgenic lines (Figure 2d–h; Figure 9e–i), and POD activity and POD expression in DgTIL1K72R transgenic lines were lower than those in the DgTIL1 and DgTIL1K72N transgenic lines (Figure 10c,e). Therefore, the DgTIL1K72R transgenic lines have lower cold tolerance than the DgTIL1 and DgTIL1K72N transgenic chrysanthemum lines. However, slightly stronger resistance to cold stress is observed in DgTIL1K72R transgenic lines compared with the WT lines, and we speculate that DgTIL1‐mediated cold resistance is only partially mediated by the crotonylation of K72 site. The expression level of DgTIL1 among the DgTIL1, DgTIL1K72R and DgTIL1K72N transgenic lines was nearly consistent (Figure 2a; Figure 9b), and the protein level of DgTIL1 was almost the same (Figure 10a; Figure S4a). Moreover, significant differences in the transcription level of DgnsLTP were not observed between the DgTIL1, DgTIL1K72N and DgTIL1K72R transgenic lines and the WT lines (Figure 10g,h). Therefore, we further clarified that the different cold tolerances are related to lysine crotonylation of DgTIL1. Further experiments showed different interaction strengths between DgTIL1 and DgnsLTP with different degrees of lysine crotonylation of DgTIL1. Coexpressing pCAMBIA1300‐DgTIL1K72N ‐nLUC with pCAMBIA1300‐DgnsLTP‐cLUC yielded the strongest interaction, thus proving that a stronger degree of lysine crotonylation of DgTIL1 corresponds to a stronger interaction with DgnsLTP (Figure 7a,b). Cold stress can induce lysine crotonylation of DgTIL1 in tobacco, preventing the degradation of DgnsLTP (Figure 6c; Figure 8c–e). Moreover, among the coinjection treatments, LUC activity was the highest when pSuper1300‐DgTIL1K72N was coinjected with pSuper1300‐DgnsLTP‐LUC in tobacco (Figure 8a,b), and the inhibition of DgnsLTP protein degradation was more obvious in DgTIL1K72N transgenic chrysanthemum than in the other lines (Figure 8f). The activity of pSuper1300‐DgnsLTP‐LUC regulated by pSuper1300‐DgTIL1K72R in tobacco, and the abundance of the DgnsLTP protein in DgTIL1K72R transgenic chrysanthemum presented opposite results compared with that observed in coexpressing pSuper1300‐DgTIL1K72N with pSuper1300‐DgnsLTP‐LUC in tobacco and in DgTIL1K72N transgenic chrysanthemum (Figure 8a,b and f). This result indicated that a higher degree of crotonylation of DgTIL1 corresponds to lower degradation of the DgnsLTP protein. DgnsLTP further promotes the expression of POD and removes accumulated ROS in chrysanthemum under low temperature stress, thus improving the cold resistance of chrysanthemum. Overall, the above results clarified that a higher degree of crotonylation of DgTIL1 corresponds to stronger protein stability of DgnsLTP, which induced higher POD activity and POD transcription associated with DgnsLTP, thereby increasing the cold resistance of chrysanthemum. Although the pathway of DgnsLTP‐induced POD expression is not clear and requires subsequent verification, these results reveal a new method used by chrysanthemum to resist low temperature stress and provide new insights for the molecular breeding of chrysanthemum.
In our study, a cold stress‐responsive gene, DgTIL1, was identified. Overexpression of DgTIL1 improved the cold resistance of chrysanthemum. The DgnsLTP protein interacts with DgTIL1 and can actively respond to low temperature. Lysine crotonylation of DgTIL1 at the K72 site can improve the stability of the DgnsLTP protein and affect the activity of POD and the expression of POD related to DgnsLTP, thus improving the cold resistance of chrysanthemum (Figure 11).
Figure 11.
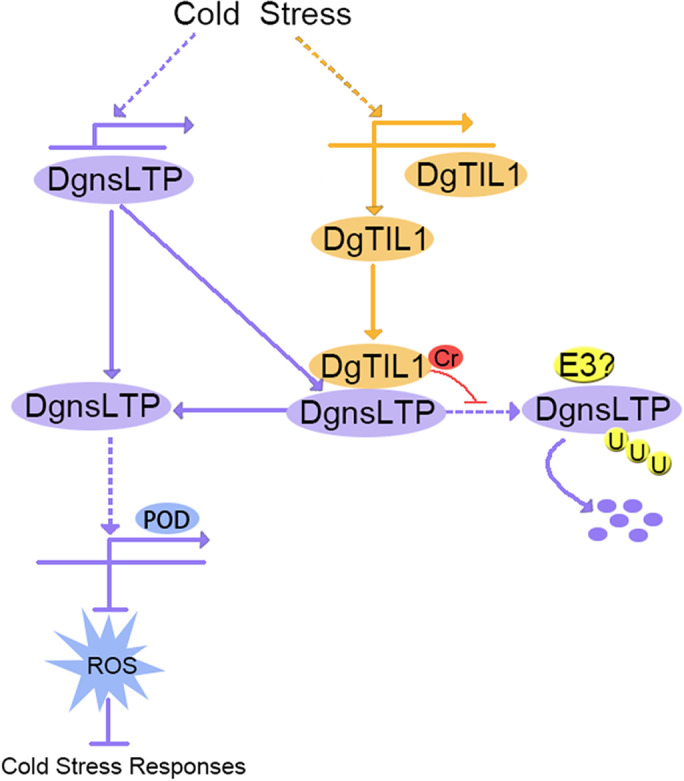
Proposed model for DgTIL1‐mediated DgnsLTP modulation of cold stress. Cold stress induces crotonylation of DgTIL1 at the Lys 72 site, which enhances the interaction between DgTIL1 and DgnsLTP and prevents the degradation of the interacting protein DgnsLTP. These changes allow DgnsLTP to activate POD expression and POD activity and thus improve the cold resistance of chrysanthemum.
Experimental procedures
Plant material preparation and low temperature treatment
Chrysanthemum morifolium var. Jinba was the main plant material using in this study. The top buds of the chrysanthemums were cultured on MS medium for 20 days and then used as material for the genetic transformation experiments. Chrysanthemum seedlings with 7–8 leaves were transplanted into 1:1 mixed peat and perlite and cultivated in biochemical incubators for 3 days (23 ± 2°C, 75% relative humidity), and then low temperature treatments with different time gradients (4°C for 0 h, 3 h, 6 h, 12 h and 24 h, 75% relative humidity) were performed. Subsequently, the seedlings were subjected to total protein and RNA extraction and physiological index measurements. After the freezing treatment (−6°C for 6 h) phenotype changes were observed. The plants were then allowed to recover for 15 days under control conditions (25°C day/22°C night) to detect the survival rates.
RNA extraction and quantitative real‐time polymerase chain reaction (qRT‐PCR) assay
The total RNA of chrysanthemum was extracted via a Spin Column Plant Total RNA Purification kit (Sangon Biotech, Shanghai, China) and prepared using a One‐Step gDNA Removal kit (Transgen Biotech, Beijing, China) based on the manufacturers’ instructions. Then, cDNA was added to the mixture according to the quantitative kit operation system (Transgen Biotech) to conduct qRT‐PCR with a Bio‐Rad CFX96™ detection system. The elongation factor 1α (EF1α) gene was selected as a stable reference gene, and the 2‐ΔΔCT method was used to analyse the results. The primers for gene amplification are shown in Table S3.
Construction of the DgTIL1 and DgnsLTP expression vectors and acquisition of transgenic chrysanthemum
To overexpress DgTIL1, DgTIL1k72N , DgTIL1K72R and DgnsLTP in chrysanthemum (variety ‘Jinba’), the full‐length cDNA was double‐digested with Sall/SpeI and inserted into the pSuper1300 vector, with the specific operation completed by Sanon Biotech. The obtained recombinant plasmids pSuper1300‐DgTIL1, pSuper1300‐DgTIL1k72N , pSuper1300‐DgTIL1K72R and pSuper1300‐DgnsLTP were transformed into chrysanthemum according to previously reported steps to obtain transgenic chrysanthemum (An et al., 1986).
Transient expression assays
Transient expression was performed in the leaves of N. benthamiana at the 5‐6 leaf stage according to a previously reported method (An et al., 2017). The fusion protein was constructed and coinjected into tobacco leaves, and 3 days later (23 ± 2°C, 75% relative humidity), the protein was used for subsequent experiments.
Protein extraction and Western blotting
Proteins from the low temperature treatments with different time gradients (4°C for 0 h, 3 h, 6 h, 12 h and 24 h) were extracted from the samples at each time gradient, which were stored at −80°C and then prepared according to the instructions of the plant total protein extraction kit (BestBio, Shanghai, China). The extracted proteins were measured by a microplate reader (Thermo Fisher Multiskan GO,Waltham, Massachusetts, USA) according to the instructions of the protein quantification kit (BCA) (Transgen Biotech, Beijing, China).
The anti‐DgTIL1 antibody and a specific anti‐DgTIL1K72 antibody were customized by PTM BIO Company (Hangzhou, China), the anti‐GFP antibody was purchased from Transgen Biotech Company, and the anti‐DgnsLTP antibody was customized by Sangon Biotech Company. DgTIL1 and DgnsLTP protein abundances were separately measured by Western blot analysis using the anti‐DgTIL1 antibody and anti‐DgnsLTP antibody. A 15% SDS‐PAGE gel was used for protein separation, 0.22‐µm PVDF membranes were used to transfer the membrane, and the transferred membrane was incubated with the aforementioned primary antibody (1:1000). After washing the membrane in TBST and secondary antibody (Immunoway, USA) incubation (1: 10000), images were finally developed on an imager with a Western blot kit (Transgen Biotech, Beijing, China).
Subcellular localization
Full‐length DgTIL1 and DgnsLTP were cloned into the transient expression vector pSuper300‐GFP at the Sacl/salI site. Then, Agrobacterium tumefaciens GV3101 was cotransformed with the recombinant plasmid, and the gene bacterial fluid and membrane localization marker (CD3‐1007) were cotransformed into tobacco leaves to execute transient expression (An et al., 2017). The results were observed by laser scanning confocal microscopy (LSCM) after 3 days.
Y2H Assay
The Y2H test was conducted in line with previously reported methods (Nan et al., 2012). Full‐length DgTIL1 was added to the pBT3‐N vector using the Sacl/salI digestion site, and full‐length DgnsLTP was added to the pPR3‐N vector using the EcoRI/SalI digestion site. pBT3‐N‐DgTIL1 plasmids were cotransformed with pPR3‐N‐DgnsLTP into strain AH109. pBT3‐N‐DgTIL1 + pPR3‐N, pBT3‐N + pPR3‐N‐DgnsLTP and pTSU2‐APP + pPR3‐N were used as negative controls, and PTSU2‐APP + pNubG‐Fe65 was used as a positive control.
BIFC
Using PCR‐based accurate synthesis (PAS) to design full‐length splicing primers, protective bases were designed on both ends of the primers to synthesize the DgnsLTP and DgTIL1 genes. Correspondingly, the EcoRI‐SalI site of the vector pCAMBIA1300‐35S‐YFPc and the EcoRI‐SalI site of pCAMBIA1300‐35S‐YFPn were linked, and then the recombinant plasmids pCAMBIA1300‐35S‐YFPc‐DgnsLTP and pCAMBIA1300‐35S‐YFPn‐DgTIL1 were obtained. The experiment was performed according to a previously reported method (An et al., 2018).
Construction of expression vectors, luciferase complementation imaging and LUC/REN activity analysis
For construction of the experimental vector used to verify the strength of the interaction, full‐length DgTIL1 and crotonylation‐deficient and lysine complete crotonylation sequences were added to pCAMBIA1300‐nLUC using the Sacl/salI restriction site, full‐length DgnsLTP was added to pCAMBIA1300‐cLUC using the KpnI/SalI restriction site, and the internal reference gene REN was added to pSuper1300 using SalI/KpnI digestion sites, which resulted in the recombinant plasmids pCAMBIA1300‐nLUC‐DgTIL1 and pCAMBIA1300‐cLUC‐DgnsLTP.
To construct the experimental vectors used to measure DgnsLTP‐LUC activity, the full‐length DgnsLTP gene and LUC were added to the same vector, namely pSuper1300, using the SalI/SpeI restriction site and the SalI/KpnI restriction site, respectively. DgTIL1 was added to pSuper1300 using the SalI/KpnI restriction site, which resulted in the recombinant plasmids pSuper1300‐DgnsLTP‐LUC and pSuper1300‐ DgTIL1. The REN reporter gene was coexpressed as an internal reference. To construct a vector with a natural promoter, the natural promoters of DgTIL1 and DgnsLTP (1.5 kb) were cut into the pSuper1300 vector using the BamH1/Kpn1 restriction site. LUC was added to the same vector, pSuper1300, using the SalI/SpeI restriction site, resulting in the recombinant plasmids ProDgTIL1‐LUC and ProDgnsLTP‐LUC. DgTIL1 was used to construct ProDgTIL1‐LUC with the SalI/KpnI restriction site, and the recombinant plasmid ProDgTIL1‐DgTIL1‐LUC was obtained. DgnsLTP was used to construct ProDgnsLTP‐LUC by the SalI/SpeI restriction site, and the recombinant plasmid ProDgnsLTP–DgnsLTP‐LUC was obtained. The above recombinant plasmids were all constructed by Sangon Biotech Company.
For the LCI experiments, the abovementioned related recombinant plasmids were first cotransformed with Agrobacterium GV3101 and then transiently expressed in tobacco leaves. Then, the kit instructions for detecting firefly enzymes were followed, and a live imaging instrument was used to perform fluorescence detection.
To measure double luciferase, samples were collected from the leaves after transient expression and then analysed on a microplate reader according to the instructions of the Dual Luciferase Reporter Gene Assay kit (Beyotime, China).
Determination of hydrogen peroxide (H2O2) and superoxide anion (O2 ‐) and NBT and DAB staining
Measurement of the H2O2 and O2 ‐ contents was performed according to the steps of a quantitative measurement kit (Suzhou Keming Biological Co., Ltd., Suzhou, China), and NBT and DAB were used for histochemical staining according to a previously reported method (Wang et al., 2017).
POD, APX, CAT activity, malondialdehyde content and relative electrolyte leakage
The POD, APX and CAT activities of the samples from the low temperature treatments with different time gradients (4°C for 0 h, 3 h, 6 h, 12 h and 24 h, and 75% relative humidity) were tested according to the kit instructions (Nanjing Jiancheng, China), with three replicates per sample. Analyses of the malondialdehyde content and relative electrolyte leakage followed previously reported methods (Gilmour et al., 1998; Kjellsen et al., 2010).
Conflicts of interest statement
The authors declare no competing interests.
Author contributions
Q.H. designed the experiments, conducted all the data analysis and wrote the manuscript; X.L. and X.Y. performed the experiments, analysed the data and wrote the manuscript; Y.L., P.L., Q.Z. and H.B. performed the experiments and analysed the data; B.J., Y.P., F.Z., L.Z. and Y.J. analysed the data. Q.L. designed the experiment, conceived the project and supervised the study. All authors approved the final version of the manuscript.
Supporting information
Figure S1 Phylogenetic analysis and sequence alignment of the DgTIL1 protein with known homologs in other plants.
Figure S2 Phylogenetic analysis and sequence alignment of the DgnsLTP protein with nsLTP protein from different species.
Figure S3 Analysis of the degradation of DgnsLTP protein in chrysanthemum.
Figure S4 DgTIL1 protein expression and modification.
Figure S5 Comparison of APX and CAT activity in the WT lines and transgenic chrysanthemum.
Table S1 Cold‐responsive TILs genes identified from a cold stress transcriptome analyses.
Table S2 Screening the potential interacting proteins of DgTIL1.
Table S3 Primers used for expression analysis.
Acknowledgements
We thank Dr. Zhizhong Gong (State Key Laboratory of Plant Physiology and Biochemistry, College of Biological Sciences, China Agricultural University) for providing the pSuper1300 and pSuper1300‐GFP plasmids, Dr. Jianmin Zhou (Institute of Genetics and Developmental Biology, Academy of Seed Design, Chinese Academy of Sciences) for providing the pCAMBIA‐35S‐cLUC vector and pCAMBIA‐35S‐nLUC plasmids, and Dr. Nan Ma (Beijing Key Laboratory of Development and Quality Control of Ornamental Crops, Department of Ornamental Horticulture, China Agricultural University) for providing the PM‐mCherry plasmid. This work was supported by grants from the National Natural Science Foundation of China (31971707).
Huang, Q. , Liao, X. , Yang, X. , Luo, Y. , Lin, P. , Zeng, Q. , Bai, H. , Jiang, B. , Pan, Y. , Zhang, F. , Zhang, L. , Jia, Y. and Liu, Q. (2021) Lysine crotonylation of DgTIL1 at K72 modulates cold tolerance by enhancing DgnsLTP stability in chrysanthemum. Plant Biotechnol. J., 10.1111/pbi.13533
References
- Abo‐Ogiala, A. , Carsjens, C. , Diekmann, H. , Fayyaz, P. , Herrfurth, C. , Feussner, I. and Polle, A. (2014) Temperature‐induced lipocalin (til) is translocated under salt stress and protects chloroplasts from ion toxicity. J. Plant Physiol. 171, 250–259. [DOI] [PubMed] [Google Scholar]
- An, J. , Li, R. , Qu, F. , You, C. , Wang, X. , and Hao, Y. (2018) R2R3‐MYB transcription factor MdMYB23 is involved in the cold tolerance and proanthocyanidin accumulation in apple. The Plant Journal, 96 (3), 562–577. 10.1111/tpj.14050 [DOI] [PubMed] [Google Scholar]
- An, G. , Watson, B.D. and Chiang, C.C. (1986) Transformation of tobacco, tomato, potato, and arabidopsis thaliana using a binary Ti vector system. Plant Physiol. 81, 301–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An, J.P. , Yao, J.F. , Wang, X.N. , You, C.X. , Wang, X.F. and Hao, Y.J. (2017) Mdhy5 positively regulates cold tolerance via cbf‐dependent and cbf‐independent pathways in apple. J. Plant Physiol. 218, 275–281. [DOI] [PubMed] [Google Scholar]
- Boca, S. , Koestler, F. , Ksas, B. , Chevalier, A. and Havaux, M. (2013) Arabidopsis lipocalins atchl and attil have distinct but overlapping functions essential for lipid protection and seed longevity. Plant, Cell Environ. 37, 368–381. [DOI] [PubMed] [Google Scholar]
- Charron, J.B.F. , Breton, G. , Badawi, M. and Sarhan, F. (2002) Molecular and structural analyses of a novel temperature stress‐induced lipocalin from wheat and arabidopsis. FEBS Lett. 517, 129–132. [DOI] [PubMed] [Google Scholar]
- Charron, J.B.F. , Ouellet, F. , Houde, M. and Sarhan, F. (2008) The plant apolipoprotein d ortholog protectsarabidopsisagainst oxidative stress. BMC Plant Biol. 8, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi, W.T. , Fung, R.W.M. , Liu, H.C. , Hsu, C.C. and Charng, Y.Y. (2009) Temperature‐induced lipocalin is required for basal and acquired thermotolerance in arabidopsis. Plant, Cell Environ. 32, 917–927. [DOI] [PubMed] [Google Scholar]
- Debono, A. , Yeats, T.H. , Rose, J.K. , Bird, D. , Jetter, R. , Kunst, L. and Samuels, L. (2009) Arabidopsis LTPG is a glycosylphosphatidylinositol‐anchored lipidtransfer protein required for export of lipids to the plant surface. Plant Cell 21, 1230–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, A. , Feng, Y. , Zhu, L. , Xu, S. , Qin, J. and Pan, D. (2016) Cloning and expression analysis of temperature‐induced lipocalin in narcissus tazetta var.chinensis. Acta hort. Sin. 43, 161‐167. [Google Scholar]
- Farmer, E.E. and Mueller, M.J. (2013) Ros‐mediated lipid peroxidation and res‐activated signaling. Annu. Rev. Plant Biol. 64, 429–450. [DOI] [PubMed] [Google Scholar]
- Flower, D.R. , North, A.C.T. and Sansom, C.E. (2000) The lipocalin protein family: structural and sequence overview. Biochem. Biophys. Acta. 1482, 9–24. [DOI] [PubMed] [Google Scholar]
- Frenette Charron, J.‐B. , Ouellet, F. , Pelletier, M. , Danyluk, J. , Chauve, C. and Sarhan, F. (2005) Identification, expression, and evolutionary analyses of plant lipocalins. Plant Physiol. 139, 2017–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, C. , Wang, Y. , Liu, G. , Wang, C. , Jiang, J. and Yang, C. (2010) Cloning of ten peroxidase (pod) genes from tamarix hispida and characterization of their responses to abiotic stress. Plant Mol. Biol. Rep. 28, 77–89. [Google Scholar]
- Gilmour, S.J. , Zarka, D.G. , Stockinger, E.J. , Salazar, M.P. , Houghton, J.M. and Thomashow, M.F. (1998) Low temperature regulation of the Arabidopsis CBF family of AP2 transcriptional activators as an early step in cold‐induced COR gene expression. Plant J. 16, 433–442. [DOI] [PubMed] [Google Scholar]
- Guo, L. , Yang, H. , Zhang, X. and Shuhug, Y. (2013) Lipid transfer protein 3 as a target of MYB96 mediates freezing and drought stress in Arabidopsis. J. Exp. Bot. 64, 1755–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, H. , Wang D. and Zhao Y. (2018) Quantitative Crotonylome Analysis Expands the Roles of p300 in the Regulation of Lysine Crotonylation Pathway. Proteomics 18 (15), 1700230. 10.1002/pmic.201700230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, X. , Sambe, M.A.N. , Zhuo, C. , Qinghua, T. and Zhenfei, G. (2005). A temperature induced lipocalin gene from Medicago falcata (MfTIL1) confers tolerance to cold and oxidative stress. Plant Mol. Biol. 87, 645–654. [DOI] [PubMed] [Google Scholar]
- Kader, J.‐C. (1996). Lipid‐transfer proteins in plants. Ann. Rev. Plant Physiol. Plant Mol. Biol. 47, 627–654. [DOI] [PubMed] [Google Scholar]
- Kawamura, Y. and Uemura, M. (2003) Mass spectrometric approach for identifying putative plasma membrane proteins of arabidopsis leaves associated with cold acclimation. Plant J. 36, 141–154. [DOI] [PubMed] [Google Scholar]
- Kjellsen, T.D. , Shiryaeva, L. , Schröder, W.P. and Strimbeck, G.R. (2010) Proteomics of extreme freezing tolerance in siberian spruce (picea obovata). J. Proteomics. 73, 965–975. [DOI] [PubMed] [Google Scholar]
- Krasensky, J. and Jonak, C. (2012) Drought, salt, and temperature stress‐induced metabolic rearrangements and regulatory networks. J. Exp. Bot. 63, 1593–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H. , Ding, Y. , Shi, Y. , Zhang, X. , Zhang, S. , Gong, Z. and Yang, S. (2017) Mpk3‐ and mpk6‐mediated ice1 phosphorylation negatively regulates ice1 stability and freezing tolerance in arabidopsis. Dev. Cell 43, 630–642. [DOI] [PubMed] [Google Scholar]
- Liu S., Xue C., Fang Y., Chen G., Peng X., Zhou Y., Chen C., Liu G., Gu M., Wang K., Zhang W., Wu Y. and Gong Z. (2018a) Global Involvement of Lysine Crotonylation in Protein Modification and Transcription Regulation in Rice. Molecular & Cellular Proteomics 17(10), 1922–1936. 10.1074/mcp.ra118.000640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, K. , Yuan, C. , Li, H. , Chen, K. , Lu, L. , Shen, C. and Zheng, X. (2018b) A qualitative proteome‐wide lysine crotonylation profiling of papaya (Carica papaya L.). Sci. Rep. 8, 8230. 10.1038/s41598-018-26676-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, Y. , Xu, Q. , Liu, Y. , Yu, Y. , Cheng, Z.Y. , Zhao, Y. and Zhou, D. (2018). Dynamics and functional interplay of histone lysine butyrylation, crotonylation, and acetylation in rice under starvation and submergence. Genome Biol. 19, 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler, R. , Vanderauwera, S. , Gollery, M. and Breusegem, F.V. (2004) Reactive oxygen gene network of plants. Trends Plant Sci. 9, 490–498. [DOI] [PubMed] [Google Scholar]
- Miura, K. and Furumoto, T. (2013) Cold signaling and cold response in plants. Int. J. Mol. Sci. 14, 5312–5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan, Z. , Zhenyi, Q. , Zheng, L. , Bing, M. , Zhengkai, X. , Rentao, S. et al. (2012) Zea mays taxilin protein negatively regulates opaque‐2 transcriptional activity by causing a change in its sub‐cellular distribution. PLoS One 7, e43822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passardi, F. , Longet, D. , Penel, C. and Dunand, C. (2004) The class iii peroxidase multigenic family in rice and its evolution in land plants. Phytochemistry 65, 1879–1893. [DOI] [PubMed] [Google Scholar]
- Stergaard, J. , Vergnolle, C. , Schoentgen, F. and Kader, J.C. (1993) Acyl‐binding/lipid‐transfer proteins from rape seedlings, a novel category of proteins interacting with lipids. Biochem. Biophys. Acta. 1170, 109–117. [DOI] [PubMed] [Google Scholar]
- Sun, H. , Liu, X. , Li, F. , Li, W. , Zhang, J. , Xiao, Z. , Shen, L. , Li, Y. , Wang and F. , Yang, J. (2017) First comprehensive proteome analysis of lysine crotonylation in seedling leaves of Nicotiana tabacum. Scientific Reports 7, 3031. 10.1038/s41598-017-03369-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, J. , Qiu, C. , Qian, W. , Wang, Y. , Sun, L. , Li, Y. and Ding, Z. (2019) Ammonium triggered the response mechanism of lysine crotonylome in tea plants. BMC Genom. 20, 340. 10.1186/s12864-019-5716-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, M. , Luo, H. , Lee, S. , Jin, F. , Yang, J.S. , Montellier, E. , Buchou, T. et al. (2011) Identification of 67 histone marks and histone lysine crotonylation as a new type of histone modification. Cell 146, 1016–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura, M. , Tominaga, Y. , Nakagawara, C. , Shigematsu, S. , Minami, A. and Kawamur, Y. (2006) Responses of the plasma membrane to low temperatures. Physiol. Plant. 126, 81–89. [Google Scholar]
- Wang K., Zhong M., Wu Y., Bai Z., Liang Q., Liu Q., Pan Y., Zhang L., Jiang B., Jia Y. and Liu G. (2017) Overexpression of a chrysanthemum transcription factor gene DgNAC1 improves the salinity tolerance in chrysanthemum. Plant Cell Reports 36 (4), 571–581. 10.1007/s00299-017-2103-6 [DOI] [PubMed] [Google Scholar]
- Xu, W. , Wan, J. , Zhan, J. , Li, X. , He, H. , Shi, Z. and Zhang, H. (2017) Global profiling of crotonylation on non‐histone proteins. Cell Res. 27, 946–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeats, T.H. and Rose, J.K.C. (2008) The biochemistry and biology of extracellular plant lipid‐transfer proteins (ltps). Protein Sci. 17, 191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, J. , Wang, S. , Qin, J. , Sun, C. and Liu, F. (2020) The lipid transfer protein Osltpl159 is involved in cold tolerance at the early seedling stage in rice. Plant Biotechnol. J. 18, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, C. , Wang, P. , Si, T. , Hsu, C.C. , Wang, L. , Zayed, O. , Yu, Z. et al. (2017). Map kinase cascades regulate the cold response by modulating ice1 protein stability. Dev. Cell 43, 618–629.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Phylogenetic analysis and sequence alignment of the DgTIL1 protein with known homologs in other plants.
Figure S2 Phylogenetic analysis and sequence alignment of the DgnsLTP protein with nsLTP protein from different species.
Figure S3 Analysis of the degradation of DgnsLTP protein in chrysanthemum.
Figure S4 DgTIL1 protein expression and modification.
Figure S5 Comparison of APX and CAT activity in the WT lines and transgenic chrysanthemum.
Table S1 Cold‐responsive TILs genes identified from a cold stress transcriptome analyses.
Table S2 Screening the potential interacting proteins of DgTIL1.
Table S3 Primers used for expression analysis.


