Abstract
In late 2019, a new member of the Coronaviridae family, officially designated as “severe acute respiratory syndrome coronavirus 2” (SARS-CoV-2), emerged and spread rapidly. The Coronavirus Disease-19 (COVID-19) outbreak was accompanied by a high rate of morbidity and mortality worldwide and was declared a pandemic by the World Health Organization in March 2020. Within the Coronaviridae family, SARS-CoV-2 is considered to be the third most highly pathogenic virus that infects humans, following the severe acute respiratory syndrome coronavirus (SARS-CoV) and the Middle East respiratory syndrome coronavirus (MERS-CoV). Four major mechanisms are thought to be involved in COVID-19 pathogenesis, including the activation of the renin-angiotensin system (RAS) signaling pathway, oxidative stress and cell death, cytokine storm, and endothelial dysfunction. Following virus entry and RAS activation, acute respiratory distress syndrome develops with an oxidative/nitrosative burst. The DNA damage induced by oxidative stress activates poly ADP-ribose polymerase-1 (PARP-1), viral macrodomain of non-structural protein 3, poly (ADP-ribose) glycohydrolase (PARG), and transient receptor potential melastatin type 2 (TRPM2) channel in a sequential manner which results in cell apoptosis or necrosis. In this review, blockers of angiotensin II receptor and/or PARP, PARG, and TRPM2, including vitamin D3, trehalose, tannins, flufenamic and mefenamic acid, and losartan, have been investigated for inhibiting RAS activation and quenching oxidative burst. Moreover, the application of organic and inorganic nanoparticles, including liposomes, dendrimers, quantum dots, and iron oxides, as therapeutic agents for SARS-CoV-2 were fully reviewed. In the present review, the clinical manifestations of COVID-19 are explained by focusing on molecular mechanisms. Potential therapeutic targets, including the RAS signaling pathway, PARP, PARG, and TRPM2, are also discussed in depth.
Keywords: clinical manifestations, coronavirus, COVID-19, oxidative stress, PARP, PARG, RAS pathway, SARS-CoV-2, TRPM2
1. Introduction
Coronavirus Disease-2019 (COVID-19), caused by the Severe Acute Respiratory Syndrome-CoronaVirus-2 (SARS-CoV-2), is a highly contagious disease. The disease was declared a global public health threat by the World Health Organization on March 11, 2020 [1,2]. The virus SARS-CoV-2 is a member of the Coronaviridae family that presently hosts 46 species. SARS-CoV-2 is the third most highly pathogenic virus that infects humans, following the severe acute respiratory syndrome coronavirus (SARS-CoV) and the Middle East respiratory syndrome coronavirus (MERS-CoV). These three coronaviruses are all of zoonotic origin and responsible for life-threatening disease outbreaks in the 21st century [3]. SARS-CoV-2 is a positive-sense single-stranded ribonucleic acid (RNA) virus. It is the newest member the β-coronavirus genus together with SARS-CoV and MERS-CoV. Based on sequence identity [4], the genomic sequence of SARS-CoV-2 is ~79% similar to that of SARS-CoV. Apart from SARS-CoV, MERS-CoV, and SARS-CoV-2, other coronaviruses such as Gammacoronavirus, Deltacoronavirus, and Alphacoronavirus also infect birds and other mammals [5].
To date, there are several vaccines approved for immunization against SARS-CoV-2 and other vaccines are under clinical trials worldwide. Based on various technologies recruited for vaccine development, vaccines are divided into six major categories including inactivated, recombinant spike protein, viral vector, RNA, live attenuated, and virus-like particle vaccines [6]. The efficacy of all registered vaccines depends on testee age, doses of different vaccines, vaccine type and dissimilarity in vaccination procedure. Above all, mutations that occur in the viral genome of SARS-CoV-2 cause controversy regarding the efficacy of the present approved vaccines [7].
It appears an in-depth understanding of the molecular mechanisms involved in COVID-19 pathogenesis is required for better drug and vaccine design. Accordingly, the molecular mechanisms involved in COVID-19 pathogenesis are presented in the present review. Potential therapeutic targets for treating COVID-19 as well as several approved drugs are reviewed. In addition, potential bioactive molecules that may be applicable for the management of the disease are discussed.
2. Pathogenesis and Therapeutic Targets
2.1. Coronavirus Structure
The genome of coronaviruses (27–32 kb) is a single-stranded positive-sense RNA (+ssRNA). The whole genome sequence of SARS-CoV-2 has been characterized using an RNA-based metagenomic next-generation sequencing approach. The SARS-CoV-2 genome is 29,881 bp in length (GenBank no. MN908947) that encodes 9860 amino acids [8]. The S, E, M, and N genes encode structural proteins, whereas sixteen non-structural proteins (nsp1−16) are encoded by the ORF region [9].
The S glycoprotein is one of the four main structural proteins of SARS-CoV-2, with a size of 180–200 kDa. The S protein is composed of S1 and S2 functional subunits [10]. The S1/S2 protease cleavage site is cleaved by host proteases to activate the protein, which is essential for the fusion of the viral membrane with host cells. The S1 subunit, which consists of the N-terminal domain and the receptor binding domain (RBD), is actively involved in binding to host cell receptors. The S2 subunit mediates viral cell membrane fusion to host cells [9,11].
The initial clinical symptoms of COVID-19 include fever, non-productive cough, nasal congestion, and fatigue. These symptoms are manifested in less than a week after infection [8]. About 75% of patients suffer from severe disease, as seen by computed tomography scan on admission [12]. Pneumonia usually occurs after 10–20 days of the symptomatic infection, which is associated with reduced oxygen saturation, blood gas deviations, and discrete changes in the appearance of the lungs in chest radiographs. Different hematological and inflammatory biomarkers, including lymphopenia, the elevation of C-reactive protein, and pro-inflammatory cytokines, have been used as diagnostic laboratory markers for the diagnosis of COVID-19 [13]. Although SARS-CoV-2 typically causes upper respiratory tract infections, the virus may spread to other tissues such as the central nervous system, cardiovascular system, gastrointestinal system, liver, and kidney, causing damage to those tissues and organs [14,15].
Although scientists discovered that SARS-CoV-2 is genetically similar to SARS-CoV (then named SARS-CoV-1) and MERS-CoV, with about 79% and 50% sequence identity, respectively, the exact mechanism of COVID-19 pathogenesis is still incompletely understood [16]. Homology modeling showed that the RBD structure of the SARS-CoV-2 Spike (S) protein is similar to that of SARS-CoV-1. This suggests that the mechanisms of COVID-19 pathogenesis likely resemble those observed in SARS-CoV-1 infection [16,17].
2.2. Coronavirus Life Cycle
Coronaviruses (CoVs) harbor the largest genome among all RNA viruses. The genome is flanked at 5′ and 3′ ends by untranslated regions (UTRs), containing cis-acting secondary RNA elements necessary for RNA synthesis, viral replication, and packaging. At the 5′ end, two large protein-coding open reading frames (ORFs), called ORF1a, and ORF1b, comprising about two-thirds of the viral genome, encode replication–transcription complex (RTC) subunits [18,19]. ORF1a codes for polyprotein (pp) 1a, ORF1a and ORF1b encode cooperatively pp1ab. Both polypeptides are then proteolytically processed into 16 nsps by viral proteases, harboring a papain-like protease (PL-PRO) domain (nsp3) and a 3-C-like protease (3CLpro) domain (nsp5). These nsps assemble into large RTCs that include RNA-dependent RNA polymerase (RdRp), superfamily 1 helicase (HEL1), RNA-processing enzymes, RNA-modifying enzymes, and RNA proofreading function required for the integrity of the CoV genome [18,20]. Among nsps, nsp1 inhibits host gene expression and antiviral signal transduction. Nsp2 interacts with a host protein complex involved in mitochondrial biogenesis, likely disrupting the intracellular signaling. Additionally, nsp2 interacts with nsp3 to form proteases that cleave ORF1ab. Nsp4 is involved in the early secretory pathway in the formation of replication complexes, nsp5 exhibits a protease activity, and nsp6 generates autophagosomes from the endoplasmic reticulum (ER). Nsp7 and nsp8 form a complex with nsp12 carrying the RdRp activity. Nsp9 in complex with nsp8 is involved in viral RNA synthesis and virulence. Nsp13 exhibits an RNA helicase activity, nsp14 in complex with nsp10 carries an exoribonuclease activity, nsp11 and nsp15 are involved in endoribonuclease activity, and nsp16 exhibits a methyltransferase activity [21]. ORFs encoding the major structural proteins (S, E, M, and N) of CoV particles and a varying number of “accessory proteins” are transcribed from the 3′-terminal one-third of the viral genome to form subgenomic mRNAs (sg mRNAs) [18,20].
Coronaviruses are believed to enter host cells through two routes. The first route involves their fusion with the plasma membrane of the host, which enables direct delivery of the viral genome into the host’s cytosol. The second route involves receptor-mediated endocytosis [22].
Concerning the first route of cell entry, it is generally perceived that viral entry occurs via the interaction of the S protein with the host cell’s angiotensin-converting enzyme 2 (ACE2) receptor. The latter is also known as COVID-19 specific cellular receptor [23,24]. One-step proteolytic cleavage, mediated by host cell proteases in certain SARS-CoV S protein residues (position S2′), facilitates direct membrane fusion between the virus and the host’s plasma membrane [25]. Previous studies have shown that SARS-CoV cell entry also occurs through the ACE2 receptor [26,27]. In contrast, MERS-CoV utilizes a two-step furin-mediated membrane fusion mechanism for host cell entry and involves interaction with the dipeptidyl peptidase-4 (DPP-4, also known as CD26) receptor [28]. Upon entry into the host cells, the viral RNA genome is translated into two polyproteins and structural proteins within the cytoplasm, leading to the assembly of the viral progeny [29]. Ultimately, budding occurs after the transit of the translated structural proteins through the endoplasmic reticulum-Golgi intermediate compartment and interaction with the newly synthesized N-encapsidated RNA genome [30].
With respect to the second route of host cell entry, it has been suggested that SARS-CoV-2 uses clathrin-mediated endocytosis as the main mechanism for cell entry [31]. Similar to SARS-CoV and MERS-CoV, SARS-CoV-2 may employ multiple pathways to gain entry into the cytosol of host cells [32,33].
2.3. The Renin–Angiotensin System (RAS) Signaling Pathway
The role of RAS in maintaining the normal function of the cardiovascular system is well known. Dysfunction of the RAS is associated with many cardiovascular diseases [34]. Upon release into the bloodstream, renin breaks down angiotensinogen into angiotensin I. Angiotensinogen is produced in the liver and is always present in the blood circulation. Angiotensin I (AngI) is inactive and is converted into Angiotensin II (AngII) by angiotensin-converting enzyme (ACE). This enzyme is found in the vascular endothelium of the kidney and lungs [35]. Angiotensin II subsequently binds to angiotensin type-1 and type-2 receptors (AT1R and AT2R). These receptors are found in the heart, blood vessels, kidney, adrenal cortex, lung, circumventricular organs of the brain, basal ganglia, and brainstem. The receptors are involved in the regulation of blood pressure, body fluids, and electrolyte homeostasis [17].
Angiotensin-converting enzyme 2 is an ACE homolog, belonging to the ACE family of dipeptidyl carboxypeptidases which counterbalances the physiological function of ACE. The ACE2 inactivates AngII by catalyzing the elimination of the C-terminal phenylalanine residue to generate Ang (1–7) or changes Ang I to Ang (1–9). The Ang (1–7) peptide potentially exerts vasodilatory action and negatively regulates RAS via the MAS1 oncogene (Mas) receptor [34,36]. Thus, in COVID-19 pathogenesis, ACE2 acts as a double-edged sword, both as a receptor for viral entry and as a negative regulator for severe symptoms of infection and lung injury [37,38].
Earlier studies of SARS-CoV pathogenesis reported that binding of the SARS-CoV S protein to ACE2 causes a decrease in ACE2. This results in Ang (1–7) downregulation and diminished vasodilation. This process causes excessive production of pro-atrophic, pro-fibrotic, pro-inflammatory, and pro-oxidant agents, thereby exacerbating lung injury and enhancing pulmonary vascular permeability (i.e., wet lung) [39].
The lung provides a massive surface area (~100 m2) for viral entry. The alveolar epithelial type II cells (AECII) highly express ACE2 and act as viral reservoirs in the human alveolar compartment [39,40].
2.4. Oxidative Stress and Cell Death
Oxidative stress is triggered by the imbalance between production and clearance of reactive oxygen species (ROS). These metabolic by-products of aerobic metabolism include hydrogen peroxide (H2O2), superoxide radicals (O2−), singlet oxygen (O2), hydroxyl radicals (OH), and peroxynitrite anion (ONOO−) [41]. Both viral infection and RAS activation trigger ROS production, resulting in an oxidative burst. The increased ROS levels are responsible for destructive effects on cellular macromolecules such as lipids, proteins and especially nucleic acids such as deoxyribonucleic acid (DNA) [42].
Oxidative stress-mediated DNA damage is repaired via the base excision repair (BER) pathway. This pathway is primarily recruited among the three excision repair pathways (BER, nucleotide excision repair, and DNA mismatch repair) [43]. Normally, poly ADP-ribose polymerase-1 (PARP-1), a DNA base repair enzyme activated by DNA breaks, triggers the BER pathway for the maintenance of genome stability. Upon activation, PARP-1 rapidly utilizes the substrate nicotinamide adenine dinucleotide (NAD+) to transfer poly ADP-ribose (PAR) to target damaged DNA [44]. The PARP-1 possesses ADP-ribosyl transferase activity and functions as an antiviral agent through ADP-ribosylation of the viral genome (RNA or DNA) and inhibition of viral transcription. However, several virus families, including Togaviridae, Herpesviridae, and Coronaviridae, encode a macrodomain protein that possesses poly(ADP-ribose) glycohydrolase (PARG) activity. The latter hydrolyzes ADP-ribose units from proteins and nucleic acids to promote optimal replication and virulence [45].
Excessive activation of PARP compensates for ADP-ribose hydrolyzation mediated by PARG. Such activity is associated with catalytic consumption of NAD+ followed by adenosine triphosphate (ATP) reduction, leading to depletion of energy and cell death [46]. The DNA damage triggered by PARP and PARG generates a large amount of free ADP-Ribose units which bind to transient receptor potential channel melastatin 2 (TRPM2) through a functional ADP-ribose hydrolase domain in its C terminus [47]. Extracellular calcium concentration, ADP-ribose, and temperature are known to be activators of TRPM2 [47,48]. The TRPM2 present on lysosomal and plasma membranes generates Ca2+ influx that results in high Ca2+ concentration within the cytosol. The overload of cytosolic Ca2+ initiates apoptosis and probably necrosis [49,50]. This is the purported mechanism of severe lung injury in COVID-19 patients [51]. The ability of TRPM2 to respond to oxidative stress made it a promising target for repurposing TRPM2 inhibitor drugs such as fluenamic acid or mefenamic acid, melatonin, and selenium, or for developing new small molecules and therapeutic approaches, such as aptamers [52] and gene editing tools [53,54].
2.5. Cytokine Storm
A well-synchronized immune response is essential for the control and eradication of viral infections. Viruses can be detected by the innate immune system. Innate and subsequently adaptive immune responses are activated as host defense [55]. Following SARS-CoV-2 entry into the host cells, the viral RNA can be sensed by pattern-recognition receptors (PRRs) that generate signals for the production of type I interferons (IFNs) and pro-inflammatory cytokines. These molecular mediators help to recruit immune cells such as macrophages to the infection site. Moreover, eosinophilia was proposed to be beneficial in COVID-19 due to its antiviral effect, as previously demonstrated in viral infections e.g., influenza. While eosinopenia is associated with a higher rate of mortality [56].
Together with resident dendritic cells, macrophages are known as professional antigen-presenting cells (APCs). Antigen presentation primes T cells for cytokine synthesis [57]. Viral genomic double-strand RNA activates interferon regulatory factors and the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway, leading to type I IFN synthesis and promotion of pro-inflammatory cytokine responses [58].
An in-silico study demonstrated that the NSP10, S2, and E mRNA of SARS-CoV-2 can potentially bind to Toll-like receptor-3 (TLR3), TLR9, and TLR7 and activate downstream signaling cascades [59]. The dysregulated and aberrant immune responses subsequently result in hyper-inflammation and generate a cytokine storm. This results in multiple organ failure, pulmonary tissue damage, and reduced lung capacity in patients with severe COVID-19 [55,60].
Patients with COVID-19 demonstrate significant increases in the levels of plasma pro-inflammatory cytokines, including monocyte chemoattractant protein 1 (MCP-1), macrophage inflammatory protein -1α, -1β, interleukin (IL) 1-β, interleukin 1 receptor antagonist (IL1RA), IL7, IL8, IL9, IL10, interferon-inducible protein 10, platelet-derived growth factor subunit B, basic fibroblast growth factor, granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, interferon-gamma (IFNγ), tumor necrosis factor-alpha (TNFα), and vascular endothelial growth factor A (VEGFA) [61,62].
Overall, the viral infection initiates a detrimental cycle of oxidative stress-mediated dysfunction, including PARP and PARG (macrodomain of the virus and host PARG) activities, ADP-ribose increase, TRPM2 activity, apoptosis and/or necrosis, and release of inflammatory mediators and vasodilators [63]. Together, all these events result in endothelial dysfunction and extravasation of immune cells into the alveolar space, producing a ground glass appearance in chest radiographs. The entrapped immune cells release a high amount of cytokines which leads to systemic inflammatory response syndrome, a scenario usually seen in septic shock and poisoning with paraquat (1, 10-dimethyl-4,40-bipyridinium dichloride). The aforementioned events account for the acute respiratory distress syndrome (ARDS) identified in COVID-10 patients [64,65,66]. Activation of TRPM2 increases NLR family pyrin domain containing 3 (NLRP3) activity and IL8 secretion, intensifying inflammation and cytokine storm [54]. In the inflamed lung tissue, IL8 has a chemotactic role in attracting neutrophils to the lung parenchyma to further exert various pathologic effects.
2.6. Endothelial Dysfunction
The earliest and one of the most important indicators of endothelial dysfunction is the chronic reduction of nitric oxide (NO) synthesis and release and/or increased NO degradation by ROS [67]. The reduced bioavailability of NO results in proliferative, pro-oxidative, pro-inflammatory, and pro-thrombotic responses. Various pathological disorders affect endothelial function through changing the molecular mechanisms involved in regulation of NO bioavailability [68].
The optimal environment for the propagation of the coronavirus life cycle is an oxygen-depleted condition. In a hypoxic situation, ROS generation and hypoxia-inducible factor 1-alpha (HIF-1a) activation occur sequentially. These events induce the expression of the furin enzyme, ultimately resulting in cleavage of the spike protein and enabling viral entry into the host cells [69,70].
In the hypoxic milieu, NO release is enhanced by NO synthases through the use of L-arginine amino acid for maintenance of nitroso/redox balance [68,71]. The function of NO synthases is dependent on an adequate amount of tetrahydrobiopterin (BH4) in its active reduced form [72,73]. In the oxidative stress state, extra free radicals interfere with redox homeostasis and adequate BH4 production [74]. It may be concluded that the altered redox homeostasis leads to reduced levels of NO in COVID-19 patients with oxidative burst and RAS activation [75].
Various inflammatory and cardiovascular events occur concomitantly with endothelial dysfunction because of the decreased NO levels. Low levels of NO induce proliferation of vascular smooth muscle cells [76], low-density lipoprotein oxidation [77], as well as the expression of vascular cell adhesion molecule-1 [78] and MCP-1 [79] through inhibition of the NF-κB signaling pathway. Moreover, decreased NO following oxidative burst stimulates the production of matrix metalloproteinases-2 and -9 that contribute to pulmonary damage via degradation of the extracellular matrix [80,81] and induces pro-inflammatory cytokine and chemokine expressions [82]. Platelet aggregation [83], leukocyte adhesion [84], and thrombolysis stimulation [82] are also perceived as manifestations of reduced NO levels that contribute to endothelial dysfunction.
Although the decrease in NO production is prominent in COVID-19 patients, some patients showed a slightly increased level of NO. Nitrous oxide is an important factor in vascular homeostasis because of its role in inhibiting contractile machinery and vasodilatation [85]. In this process, NO produced by endothelial cells spreads to the vascular smooth muscle cells and generates cyclic guanosine-3,5-monophosphate. Subsequently, cyclic guanosine-3,5-monophosphate -dependent protein kinase is activated. The latter contributes to the removal of cytosolic Ca2+, which inhibits the contractile machinery, and ultimately vasodilatation [82,86]. Nitrous oxide release in the peripheral vessels may worsen hemodynamic homeostasis and decrease blood pressure and organ perfusion.
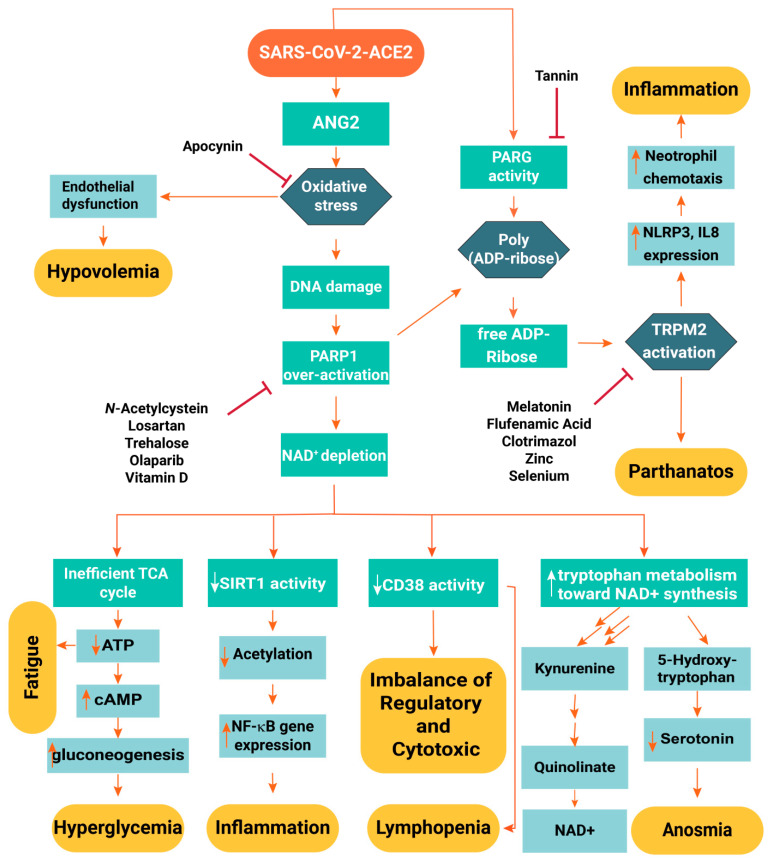
Nitrous oxide may also be released from endothelial cells upon shear stress and ischemia. Such events are enhanced by acetylcholine, bradykinin and serotonin [87]. Endothelial dysfunction causes fluid leakage from the vessels to tissues (increased wet/dry ratio and impaired O2 transfer in lungs). This, in turn, results in hypovolemia and increased viscosity of the intravascular fluid. High viscosity increases the propensity to thrombosis and micro-infarction in different organs. Hypovolemia and hypotension decrease renal perfusion and cause acute tubular necrosis in the kidney. Cytokines excreted by the kidney usually have a molecular mass between 10 and 22 kDa. With reduced glomerular filtration rate, the low excretion and high production of cytokines lead to the development of a cytokine storm. The summary of the pathogenesis and possible therapeutic targets in COVID-19 patients is illustrated in Figure 1.
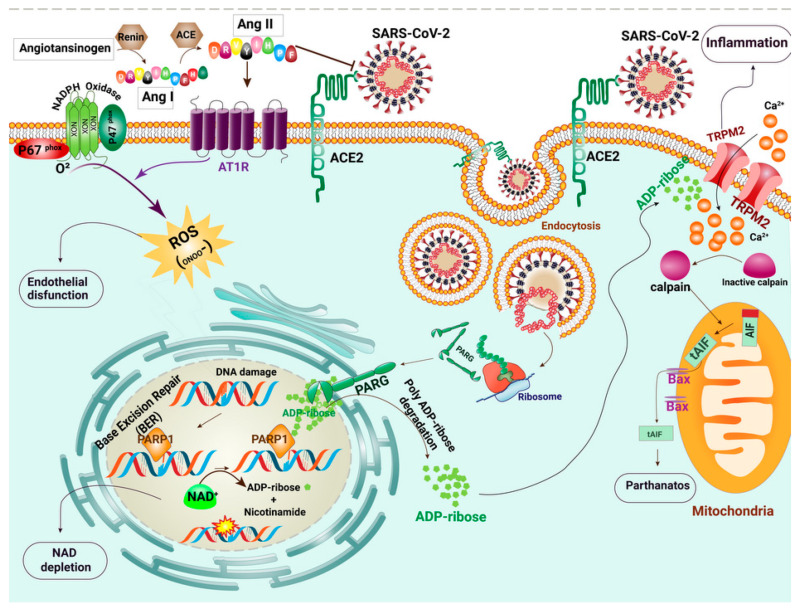
Figure 1.
Schematic of the molecular events that occur in SARS-CoV-2 infected cells. SARS-CoV-2 forms a complex with angiotensin-converting enzyme 2 (ACE2) and enters cells via cell membrane-localized receptors. Receptor occupation by SARS-CoV-2 triggers ACE2 down-regulation and renin-angiotensin system (RAS) over-activation. Renin (REN) mediates the transformation of angiotensinogen (AGT) to angiotensin I (Ang-I). The angiotensin-converting enzyme (ACE) metabolizes Ang I to angiotensin II (Ang II). Because of the reduction in available ACE2, the angiotensin type I receptor (AT1R) is activated by the elevated Ang II. Subsequently, AT1R triggers a signaling cascade via activation of NADPH oxidase and induces intense oxidative stress. The produced ROS causes the breakage of DNA strands. Base excision repair (BER) plays a crucial role in repairing oxidative DNA damage via Poly(ADP-ribose) polymerase (PARP) activity. PARP undergoes auto-modification by transferring ADP-ribose on itself. Poly(ADP-ribose) glycohydrolase (PARG) is a protein encoded by Coronaviridae, which separates ADP-ribose units from PAR. Detrimental PARP and PARG activities lead to the accumulation of ADP ribose in the cytosol. Transient receptor potential channel melastatin 2 (TRPM2) channels are over-activated by direct binding of ADP-ribose and release a large amount of calcium ions (Ca2+) to the cell. The intracellular Ca2+ overload probably results in parthanatos, a form of programmed cell death that is distinct from other cell death processes such as necrosis and apoptosis.
3. Potential Therapeutics for Management of COVID-19
Different players are involved in the molecular mechanisms of COVID-19 pathogenesis. These players include AgII, ACE2, AT1R, NADPH oxidase, PARP, PARG macrodomain (or NSP3), and TRPM2. These players are perceived by clinical scientists as potential targets for therapy using synthetic drugs or natural compounds.
3.1. Vitamin D
Vitamins have been suggested for the treatment of SARS-CoV-2 infection symptoms. Vitamin D is a fat-soluble steroid prohormone with endocrine, paracrine, and autocrine functions [88] with a prescribed daily dose of 2000–5000 international unit (IU). Vitamin D inhibits SARS-CoV-2 through binding to the ACE2 receptor as a mediator of acute lung injury in host cells during the viral infection [89]. The high copy number of ACE2 receptors on the cell surface of human alveolar epithelium considerably facilitates virus internalization and subsequent infection [90]. Experimental administration of the vitamin D agonist, calcitriol, protects the lung from viral injury by modulating components of the RAS pathway, such as ACE I and ACE II, renin, and Ang II [91]. Hence, vitamin D negatively regulates hypertension through direct regulation of RAS activity [92].
Another protective function of vitamin D is based on its immunomodulatory effect via suppression of pro-inflammatory responses and the cytokine storm [93]. Despite the role of vitamin D as a PARP inhibitor, it provides increased amounts of extracellular calcium which facilitates full activation of the TRPM2 channels [94]. Moreover, vitamin D appears to stimulate ACE2 overexpression and may increase viral entry. Nevertheless, the effectiveness of vitamin D in relieving COVID-19 associated symptoms is still controversial and its prescription is limited during the acute phase of COVID-19. Details on the role of vitamin D in COVID-19 pathogenesis are provided in Figure 2.
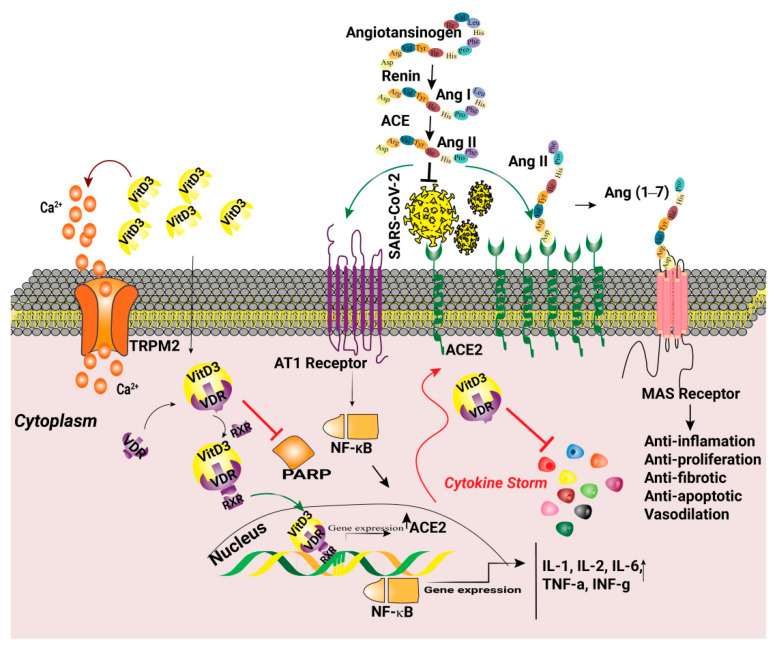
Figure 2.
Overactivation of the renin-angiotensin system (RAS) is a consequence of SARS-CoV-2 infection. Binding S protein to the angiotensin-converting enzyme 2 (ACE2) leads to receptor internalization and downregulation and causes upregulation of AngII. AngII binds to AT1R and activates the NFĸB pathway, causing the release of pro-inflammatory cytokines and inducing oxidative stress. Vitamin D3-VDR-RXR complex increases ACE2 and ACE2 augments the generation of angiotensin 1–7 (Ang (1–7)) by metabolizing Ang II. The Mas receptor (MasR) activated by Ang (1–7) induces beneficial actions such as anti-inflammation, anti-proliferation, anti-fibrotic, anti-apoptotic, and vasodilation. Vitamin D3 (VitD3) is also a poly(ADP-ribose) polymerase (PARP) inhibitor. VitD3 increases extracellular Ca2+ and results in the activation of transient receptor potential channels, melastatin 2 (TRPM2), and parthanatos.
3.2. Thalidomide
Thalidomide, a previously-used nonsteroidal anti-inflammatory drug with the recommended dose of 100 mg QID, was banned in 1961 due to its teratogenic effects. Thalidomide blocks the synthesis of TNF-α, enhances plasma IL-12 levels, peripheral blood CD8+ T cells, and INF-γ production [95]. The anti-inflammatory effects of thalidomide on COVID-19 patients helped to improve survival rate and shorten the hospital stay length [6]. Various antiviral properties, including inhibition of PARP, anti-TNF, and -NADPH oxidase have been assigned to thalidomide [96]. The molecular mechanisms of antiviral activity of thalidomide are shown in Figure 3.
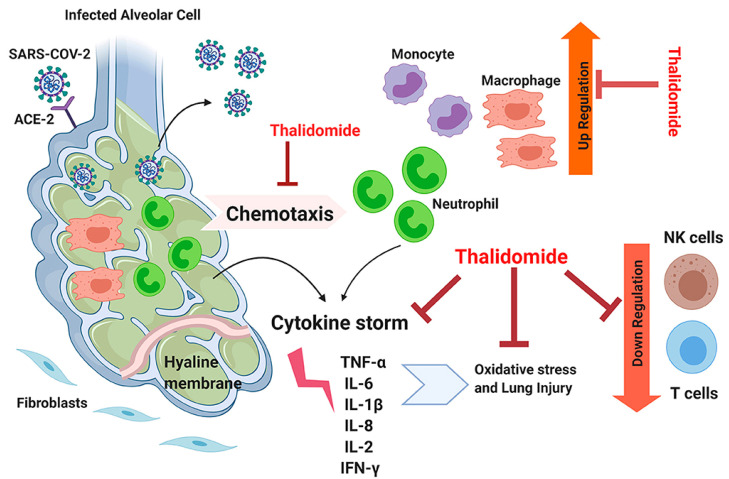
Figure 3.
Schematic of the potential molecular mechanisms of the antiviral activity of thalidomide. The drug potentially blocks the chemotaxis of neutrophils and suppresses the cytokine storm and also reduces the generation of oxidative stress. Thalidomide also stimulates natural killer (NK), and T cell activities, resulting in down-regulation of SARS-CoV-2 activities. Reproduced from [88] with permission from Frontiers. Abbreviations: TNF-α, tumor necrosis factor-alpha; IL, interleukin; ACE-2, angiotensin-converting enzyme-2; IFN-γ, interferon-gamma.
3.3. Trehalose
Trehalose is a non-reducing disaccharide and is usually used as a stabilizer in drug formulations. The average recommended amount of trehalose is 5 “g”per day. Trehalose affects cellular organelles needed for viral replication and impairs viral function via the autophagy system [97]. Because it inhibits PARP1 and PARP2, trehalose may be used as a PARP inhibitor. The drug has been prescribed for SARS-CoV-2 infected patients through a registered clinical trial [98]. Trehalose down-regulates NF-κB signaling through PARP inhibition. It acts as a chemical chaperon and stabilizes the anti-thrombin protein and has an antithrombotic effect that is similar to heparin. Trehalose induces autophagy via the transcription factor EB by inducing rapid and transient lysosomal enlargement and membrane permeabilization [99] (Figure 4).
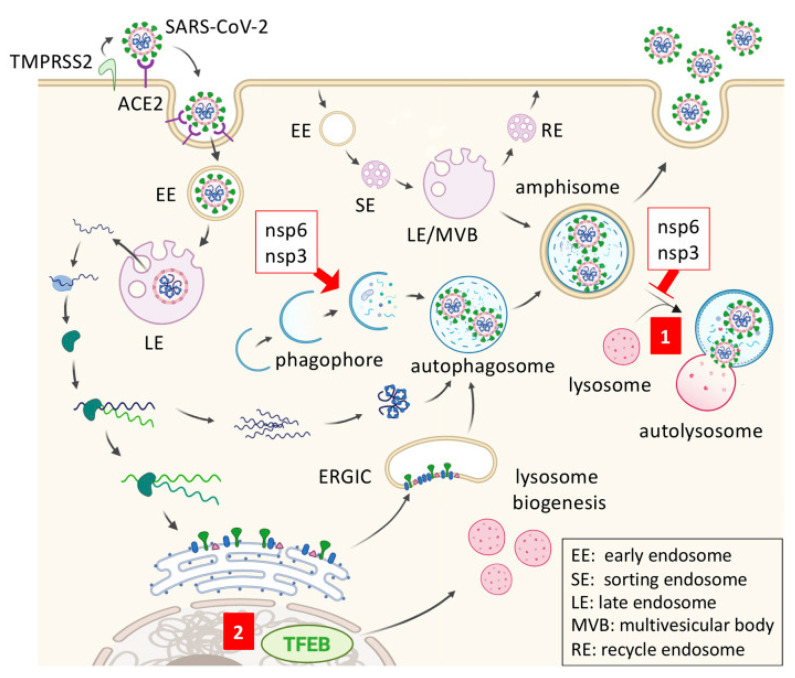
Figure 4.
Schematic of antiviral mechanism of trehalose against SARS-CoV-2. SARS-CoV-2 enters the host cell through the interaction of viral spike protein with ACE2 receptor on the cell surface. Coronavirus nonstructural protein 6 (nsp6) and nsp3 promote autophagosome formation but prevent autophagosome-lysosome fusion. (1) Trehalose may stimulate amphisome-lysosome fusion for virion degradation after the trafficking of viral double-membrane vesicles, (2) Trehalose activates transcription factor EB (TFEB), and up-regulates lysosome biogenesis. Reproduced from [89] with permission from Frontiers.
3.4. N-acetylcysteine
N-acetylcysteine (NAC) has been used for the treatment of toxin and drug poisoning with the recommended dose of 1200 mg BID. This is attributed to its PARP inhibitory effect and antioxidant activity by induction of p53 apoptosis. N-acetylcysteine may be a suitable candidate for treatment and control of SARS-CoV-2 infection symptoms [96]. N-acetylcysteine is a potent scavenger of ROS, hypochlorous acid, and OH [100]. Likewise, NAC has a liver protection effect and may be used as a liver care agent. N-acetylcysteine also protects the lung from fibrosis in acute inflammatory conditions such as COVID-19 [101].
3.5. Tannins
Tannins are water-soluble polyphenolic compounds and are well known as health-promoting components [102] with a recommended dose of 150 mg BID. Studies have reported the anti-radical activities [102] and anti-inflammatory effects of tannins [103,104]. Based on the molecular mechanisms utilized by SARS-CoV-2 in its pathogenesis, COVID-19 may be considered an inflammatory disorder. As natural antioxidants, tannins can reduce disease morbidity and mortality due to their role in redox homeostasis. A recent study demonstrated that tannins block SARS-CoV-2 by binding to the catalytic residues of 3-chymotrypsin-like cysteine protease (3CLPro) of the virus, which regulates its replication [105]. In addition, the inhibitory effect of tannins on the PARG enzyme has also been reported [63]. Gallotannin is a hydrolyzable tannin that has been shown to inhibit cytokine expression [63].
3.6. Flufenamic Acid/Mefenamic Acid and Clotrimazole
Flufenamic acid/mefenamic acid and clotrimazole are potential drugs that inhibit TRPM2 in a non-selective manner and are capable of inhibiting or activating other ion channels [106,107]. Recommended dose of flufenamic acid/mefenamic acid is 250 mg and clotrimazole is 10 mg QID, respectively. Flufenamic suppresses NF-κB nuclear translocation and pro-inflammatory mediator expression. In addition, it induces AMP-activated protein kinase (AMPKα) phosphorylation through the alteration of calcium ion concentrations inside and outside of the mitochondria, ultimately inducing anti-inflammatory action [108].
3.7. NAD+ and Niacin
Almost all molecular mechanisms involved in COVID-19 pathogenesis are originated from NAD+ depletion. Depletion of NAD+ is mediated by aberrant PARP activity. This leads indirectly to the reduction of sirtuin 1 activity. Sirtuin 1 deacetylates nuclear proteins, regulates the expression of cytokines and NF-κB, and eventually modulates inflammation, cell survival, and apoptosis using NAD+ [109]. One of the consequences of NAD consumption in large amounts is the reduction of ATP levels. This, in turn, results in impairment of all cell activities and loss of cellular integrity. In SARS-CoV-2-mediated ARDS, patients are hypovolemic due to a highly reduced level of aldosterone despite RAS activation. The hypovolemia in COVID-19 patients is due to the lack of serotonin; this biomolecule plays important biological roles including stimulation of aldosterone secretion [110]. Serotonin shortage occurs because of the lack of enough tryptophan as the raw material for serotonin production and NAD synthesis during ARDS. The hypoaldosteronism leads to hyponatremia and eventually hypovolemia in a sequential manner. Fatigue and various degrees of mood disorders are other clinical manifestations of COVID-19 patients that are attributed to consequences of NAD, ATP, and serotonin reduction. In this case, administration of NAD alone may worsen the clinical symptoms due to the high activities of PARP and PARG. Concomitant prescription of NAD, niacin, or its precursor L-tryptophan along with a PARP or PARG inhibitor is a promising strategy against SARS-CoV-2 infection [111,112]. The recommended daily dosage of NAD+ is 250 mg and that of niacin is 20 mg.
3.8. Losartan
Losartan, the AgII receptor antagonist, reduces the synthesis of TGF-1β and PARP with the recommended dose of 25 mg BID. Hence, losartan appears to be able to prevent or control chronic fibrotic diseases such as cardiac hypertrophy and asthma in addition to reducing hypertension [113,114,115,116]. Losartan therapy improved paraquat-induced pulmonary fibrosis in an animal study. Losartan acts through the inhibition of TGF-1β mRNA expression and synthesis of collagen to prevent pulmonary fibrosis [64].
In addition to its anti-hypertensive effects, losartan significantly reduces platelet aggregation by ristocetin, hematocrit, and hemoglobin level in newly diagnosed hypertensive patients. This confirms the anti-thrombosis and anti-atherosclerosis function of losartan [117].
Another mechanism of action for losartan resides in its immunomodulatory function. The drug significantly regulates the cytokines IFN-γ, IL6, IL17F, and IL22 s in peripheral blood mononuclear cells of rheumatoid arthritis subjects [118].
A recent animal study provided evidence of the positive effects of losartan on severe acute lung injury (ALI) and ARDS (ALI/ARDS). This may be attributed to the inhibition of the NF-κB and the mitogen-activated protein kinase (MAPK) signaling pathways [119]. Activation of the RAS system induces strong oxidative stresses that form the major pathogenic mechanism in COVID19 [120]. Losartan, being an angiotensin receptor 1 (AT1R) blocker in the RAS pathway, may be useful for COVID-19 patients who experience pneumonia [121,122]. Losartan appears to stimulate ACE2 overexpression and may increase viral entry. However, the level of AgII in COVID-19 patients is high and following losartan intake, up-regulation of ACE2 could trigger the protective arm of the RAS pathway.
Based on existing evidence, TRPM2 targeting may be an appropriate strategy for combating COVID-19. There are several small molecules that inhibit TRPM2, including flufenamic acid/mefenamic acid [123,124], 2-(3-methyl phenyl) aminobenzoic acid [123], N-(pamylcinnamoyl) anthranilic acid [125], econazole, clotrimazole [126], and 2-aminoethoxydiphenyl borate (2-APB) [127,128]. However, none of these agents are TRPM2 channel-specific [129]. Nucleoside analogs, such as adenosine monophosphate and 8-bromoadenosine 5′-diphosphoribose have also been suggested as potential TRPM2 inhibitors [47,130]. Novel TPRM2 channel inhibitors have been synthesized [106] by modification of the ADP ribose (ADPR) analog, including 8-phenyl-2′-deoxy-ADPR. The latter specifically inhibits TRPM2 without interfering with Ca2+ release induced by cyclic ADPR, nicotinic acid adenine dinucleotide phosphate (NAADP), or inositol trisphosphate (IP3) [131]. Melatonin, selenium, and zinc are also TRPM2 inhibitors. The activation of TRPM2 in infected tissues, especially the lungs, causes the influx of extracellular calcium ions into the cytoplasm and promotes apoptosis. Temperature and calcium are TRPM2 stimulators. It is better to limit calcium consumption through nutrition or dietary supplement. Preheating of ventilation air to 37 °C represents the optimum condition for activation of TRPM2 and consequently apoptosis of the infected tissues (such as lungs) that express TRPM2 and contain high levels of ADPR. Because ADPR has a limited activation effect on TRPM2 at 25 °C, it is better to regulate the temperature of the ventilator to 25–30 °C [132]. Because TRPM2 has a pivotal role in COVID-19 pathogenesis, especially in respiratory failure, TRPM2 inhibitors are potential drugs to be valued for combating SARS-CoV-2 infections in human clinical trials.
3.9. Remdesivir
Remdesivir is a 1′-cyano-substituted adenosine analog and an RNA-dependent RNA polymerase (RdRp) blocker with arecommended dose of 100 mg per day. Remdesivir is an efficient antiviral agent because of two mechanisms: (i) bond formation with the active site of RdRp [133], and (ii) inhibition of “proof-reading: via the exoribonuclease of SARS-CoV-2. This results in the prevention of viral nucleic acid synthesis [134]. Remdesivir is typically used for treating Ebola virus infection and is currently considered as a potential drug for the management of COVID-19 [135].
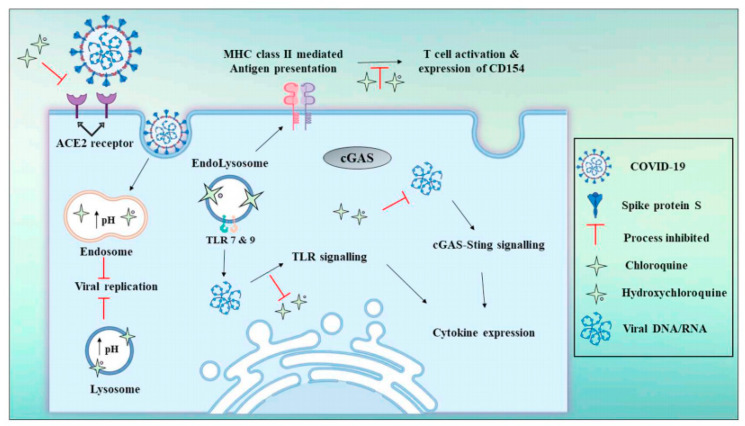
3.10. Chloroquine and Hydroxychloroquine
Chloroquine and hydroxychloroquine, also known as Ivermectin, has been used against SARS-CoV-2 that minimized viral RNA up to 5000 fold in SARS-CoV-2 infected cells [136]. These are antiviral drugs that recently have been adopted for the treatment of COVID-19 patients with pneumonia [137]. The recommended dose of these bioactive compounds is 250–500 mg BID. It has been postulated that these drugs interact directly with the viruses and hinder their attachment to cell surface receptors [138]. Both drugs act on quinone reductase 2 that is related to the UDP-N-acetylglucosamine 2-epimerase structure and the catalysis of sialic acid biosynthesis, being crucial factors for ligand recognition [139]. Chloroquine impairs glycosylation of ACE2, thereby blocking the pre-entry phase of SARS-CoV-2 [138]. In addition, chloroquine impedes SARS-CoV virus replication by increasing the pH of endosomes. Fusion of virus membrane with the host cell membrane occurs at low pH, which facilitates delivery of the SARS-CoV-2 genome into the host cell [140]. An increase in endosomal pH by chloroquine blocks the endocytosis process and disrupts SARS-CoV-2 function. The antiviral mechanisms of hydroxychloroquine and chloroquine are depicted in Figure 5.
Figure 5.
Schematic of the antiviral mechanisms of chloroquine and hydroxychloroquine. These drugs block SARS-CoV-2 invasion as well as reduce the risk of cytokine storm by preventing T cell activation. Reproduced from [141] with permission from Elsevier.
3.11. Monoclonal Antibodies and Recombinant Proteins
Monoclonal neutralizing antibodies provide passive immunity during virus exposure. Palivizumab is a drug in this category that has been applied for the prevention of respiratory syncytial virus (RSV) infection [142]. Anti-inflammatory antibodies such as anti-IL-6 receptor (Tocilizumab) and anti-ITGA4 (Natalizumab) inhibit inflammation and cellular extravasation [101,102]. Another proposed strategy is the administration of recombinant soluble ACE2 receptors to scavenge the virus. Similar strategies have been used in combating HIV infection via the use of soluble CD4 or CD4-mimicking compounds [143].
3.12. Delivery Systems: Role of Nanomedicine
Nanomedicine represents the state-of-art technology for non-invasive diagnosis and targeted treatment of a host of diseases [144]. Because of the unique attributes of nanocarriers in promoting the bioavailability of therapeutics, facilitating cellular uptake, and lowering drug resistance and side effects, nanocarriers are regarded as optimal carriers for diagnosis and treatment of viral infections [145]. To date, a variety of nanocarriers, including lipid-based, polymeric-based, metal-based, and nucleic acid-based carriers have been developed against viral infections [146]. Because multivalent interactions among nanoparticles and viruses are essential for adhesion, recognition, and signaling activation, optimized ligands are required on the nanocarrier surface for effective theranostic applications. Indeed, proper multivalent interactions at the nanoparticle-pathogen interfaces can prevent the pathogens from adhering to the host cell during the early infection stage [147]. Monovalent modifications against viral pathogens are likely to be associated with the rapid development of drug resistance [148]. It is necessary to design and develop nanoscopic antiviral agents that are based on multivalent interactions for effective monitoring of viral particles and inhibiting cell–virus interactions. In the following subsections, potential multivalent theranostic nanoagents that have been experimentally evaluated for the monitoring, diagnosis, and treatment of such viral infections will be discussed.
3.12.1. Organic Nanoparticles
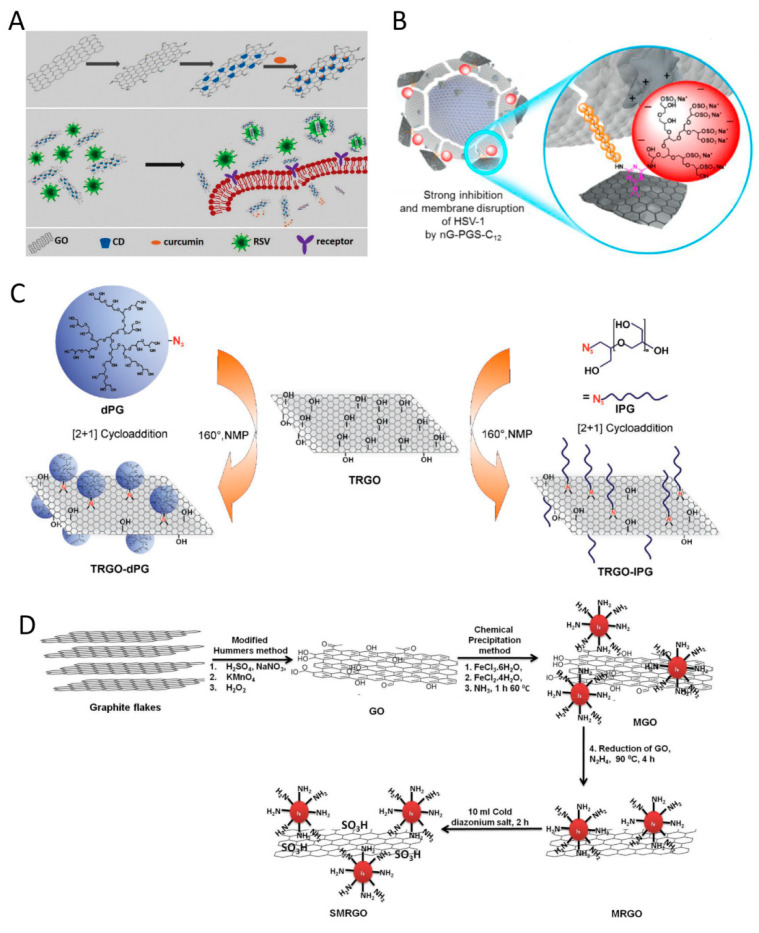
Organic nanoparticles are typically classified as lipid-based nanoparticles, polymer-based nanoparticles, dendrimers, graphene oxide, and cyclodextrins. The predominant advantages of these nanoparticles are their flexibility, easiness of modification, and high stability in biological fluids [149]. Apart from biodegradability and high affinity to specific molecular biomarkers, nanoparticles also exhibit specific characteristics for both diagnosis and treatment [150]. Lipid-based nanoparticles have a high loading capacity for both hydrophilic and hydrophobic drugs, while their outer lipid membrane facilitates cellular uptake of the nanoparticles [151]. The administration of liposomal lactoferrin was useful for the care of patients with SARS-CoV-2 infection [152]. Lactoferrin effectively blocked viral entry through binding to the ACE2 receptors on the host cell membrane. Because of their structure and composition, liposomes are capable of altering the structure of lipids and proteins of viruses [152,153]. Polymers are also useful because of their biocompatibility and easily modifiable features [154,155,156]. Positively charged polymers such as polyethyleneimine, can absorb the viruses with a negative charge, interfere with their genomic structure and inactivate the viruses [157]. Dendrimers can likewise be fabricated in highly-branched 3D structures with the capacity for attachment of multiple functional groups on their surface while encapsulating hydrophobic therapeutics in their core [158]. These exciting properties render nanoparticles suitable for therapeutic applications against tumors, bacterial and viral infections. Because nanoparticles have strong multivalent interactions with viruses, they are highly suitable as antiviral agents for preventing infection of host cells. Cyclodextrin, and its derivatives (α-cyclodextrin, β-cyclodextrin and γ-cyclodextrin) consisting of 6, 7, or 8 α-1,4-linked glucose monomers are the most readily available, commercialized nanoparticles to gain recent attention for antiviral theranostic applications [159]. Interestingly, the hydroxyl groups of cyclodextrin may be replaced to alter its solubility in biological fluids, as well as the binding strength between cyclodextrin and the designated target [160]. Due to the remarkable specific surface area, graphene and its derivatives are promising multivalent 2D platforms with broad theranostic clinical applications [161]. The variety of functional groups on the surface of graphene or graphene oxide enables chemically-selective functionalization with specific target analytes. Graphene oxide can inactivate virus function by direct multivalent interaction of their sharp edges with the virus particles [162]. In addition, the surface of graphene oxide is typically functionalized with other antiviral materials to generate a synergistic effect against the virus. For example, a graphene oxide–based platform has been functionalized with β-cyclodextrin and curcumin to combat RSV (Figure 6A) [163]. This platform inhibits RSV infection of host cells via the prevention of viral attachment, resulting in the inactivation of viral function. Graphene oxide with a high density of sulfates has a high affinity toward viruses. The sulfate groups augment the multivalent interactions between graphene oxide and viruses under physiological conditions, leading to effective antiviral activity (Figure 6B) [164]. Linear polyglycerol azides are similar to the heparan sulfate present in the extracellular matrix. They have better interaction with viruses and have a more potent inhibitory effect when compared to dendritic polyglycerol azides (Figure 6C) [165]. Sulfonates are another category of candidates for functionalization of the graphene oxide surface to suppress the interaction between the viruses and their host cells. After capturing the viruses by the graphene surface, the viral particles may be removed through the use of magnetic nanoparticles, The viruses are subsequently inactivated through exposure to near-infrared radiation (Figure 6D) [166].
Figure 6.
Schematic of various modifications of graphene oxide (GO) for antiviral applications. (A) Functionalized GO with cyclodextrin and curcumin to inhibit Respiratory Syncytial Virus (RSV) infection. Reprinted from [163] with permission from the Royal Society of Chemistry; (B) Functionalized GO with sulfated groups. Nanographene polyglycerol sulfate coverage enables virus interaction with the positively charged domains of glycoproteins on the surface of Herpes Simplex Virus 1 (HSV-1). Derivatives with long alkyl chain segments (≥C12) show the strongest inhibition and disrupt the virus envelope. Reprinted from [164] with permission from the Royal Society of Chemistry; (C) Functionalized reduced GO (rGO) with dendritic polyglycerol (dPG) azide or linear polyglycerol (lPG) azide. Reprinted from [165] with permission from Wiley; (D) Functionalization of GO platform with magnetic nanoparticles to produce MRGO. The MRGO is then sulfonated to yield SMRGO. Reprinted from [166] with permission from American Chemical Society.
Different studies have demonstrated multivalent interactions of organic nanoparticles with immune cells and viral antigens. The results suggest that these nanocarriers are effective immunotherapeutic agents against viral infections, including coronaviruses [159,167,168,169]. Organic nanoparticles have great potential for developing vaccination due to their retention in the systemic circulation. They induce strong immune activity that results in the production of a colossal amount of immunoglobulins [170,171].
3.12.2. Inorganic Nanoparticles
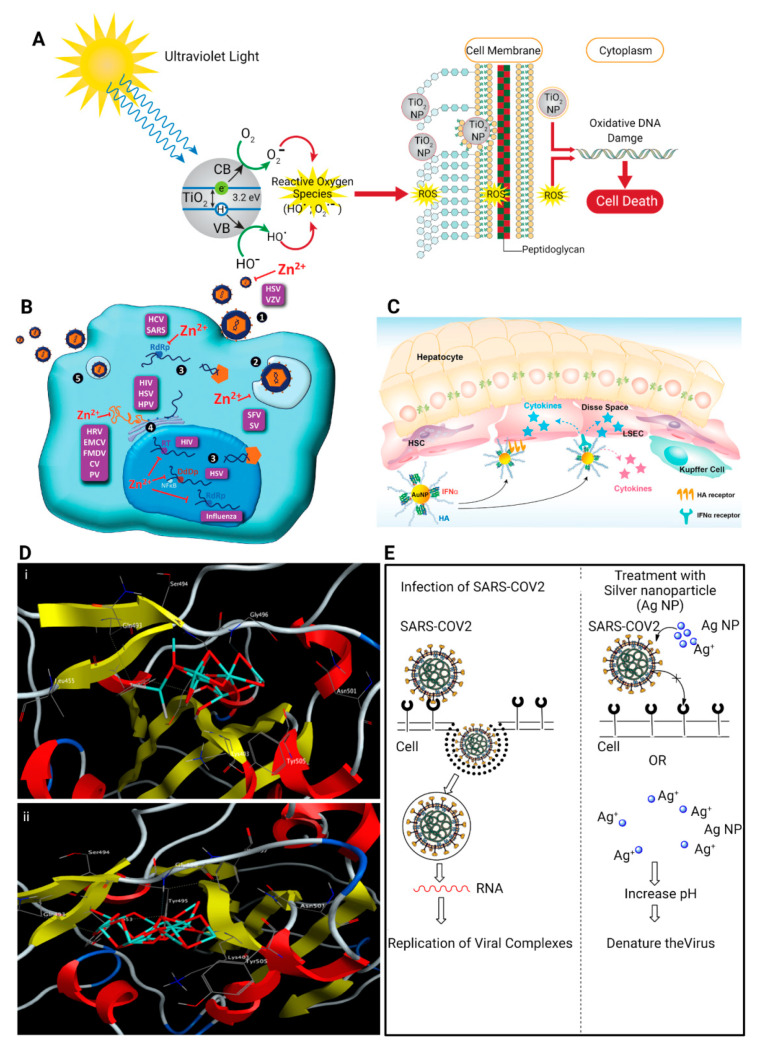
Inorganic nanoparticles such as quantum dots, magnetic nanoparticles, and gold nanoparticles have also been investigated for diagnosis and treatment of various diseases. Inorganic nanoparticles can be non-toxic, hydrophilic, biocompatible, and highly stable when compared with organic materials [172]. Because of their unique chemical, magnetic, and electrical properties, inorganic nanoparticles are highly desirable for diagnostic imaging applications. These applications include luminescence imaging, magnetic resonance imaging, fluorescence imaging, and X-ray computed tomography [173]. The photostability of inorganic nanoparticles enables them to be used for imaging for a prolonged duration. Quantum dots are potential candidates for both diagnosis and inhibition of viruses because of their ease of modification. Quantum dots are capable of binding to the S protein of SARS-CoV-2 to inhibit replication of the viral genome. They also facilitate the monitoring of virus infection via fluorescence biosensing [174]. Iron oxide nanoparticles have been used as agents for clinical trials against SARS-CoV-2. Metal oxide nanoparticles, including TiO2, ZnO, copper oxide (Cu2O), as well as iron oxide with multifunctional properties are applicable in both antiviral diagnosis and therapy [175]. A recent study that investigated the antiviral and photocatalytic process of TiO2 indicates that TiO2 nanoparticles kill viruses via ROS generation. This causes impairment of lipid membranes, damage of DNA structure, and virus inactivation (Figure 7A) [176]. Another study reported that pre-treatment of murine lung epithelial cells with zirconia (ZrO2) nanoparticles resulted in a significant reduction of influenza A virus replication, stimulation of innate immunity, and overexpression of pro-inflammatory cytokines [177]. This mechanism protected the mice against highly pathogenic avian influenza virus, with potential applications against other viral infections. Other studies have reported the potent antiviral potential of zinc (Zn2+), including virus inactivation via inhibition of viral uncoating, viral genome transcription, and viral protein translation (Figure 7B) [178].
Figure 7.
Schematic of the antiviral mechanism of inorganic nanoparticles. (A) The antiviral activity of TiO2 nanoparticles is attributed to the formation of reactive oxygen species. Reprinted from [176] with permission from Elsevier, (B) Antiviral activity of Zn nanoparticles is attributed to the inhibition of viral genome transcription, viral protein translation, and polyprotein processing. Reprinted from [178] with permission from American Society for Nutrients, (C) Strategic illustration of HA-AuNP/IFNR complex for the target-specific systemic treatment of hepatitis C infection. Reprinted from [180] with permission from American Chemical Society, (D) 3D interaction schematic showing (i) Fe2O3 and (ii) Fe3O4 docking interactions with the key amino acids in the receptor- binding domain of the spike protein (S-RBD) of SARS-COV-2. Reprinted from [182] with permission from Elsevier, (E) The proposed antiviral mechanism of Ag nanoparticles against SARS-CoV-2. Reprinted from [183] with permission from Multidisciplinary Digital Publishing Institute.
Gold nanoparticles have been studied extensively for immunotherapy and antiviral applications [179]. They stimulate the immune system and enhance adaptive immune responses through their internalization by APCs. Polyethylene glycol-conjugated IFNα and hyaluronic acid-modified gold nanoparticles (AuNP/IFNα complex) are stable in human serum with antiviral effects against hepatitis C virus infection (Figure 7C) [180]. The antiviral activity of iron oxide nanoparticles against human immunodeficiency virus (HIV) and influenza virus offered theranostic opportunities for combating coronaviruses [181]. Molecular docking analysis of iron oxide nanoparticles indicated interaction of these nanoparticles (Fe2O3 and Fe3O4) with the S protein receptor-binding domain (S1-RBD) of SARS-CoV-2 (Figure 7D) [182].
Silver nanoparticles interact with viral particles via two mechanisms: (i) binding to the outer membrane of the virus, thereby inhibiting the attachment of the virus to the cell receptor; (ii) binding to the nucleic acid (DNA or RNA) of the virus, thereby preventing the replication and transfection of the virus within the cell (Figure 7E) [183]. Silver nanoparticles impair the mitochondrial network and inhibit the influx of antiviral interferon regulatory factors-7 transcription factor into the nucleus of lung epithelial cells [184]. A recent study insinuated that silver nanoparticles may have potential antiviral activity against SARS-CoV-2 [183]. Silica (SiO2) nanoparticles have also gained significant attention for their large surface areas, and intrinsic surface reactivity that enables selective chemical functionalization [185].
Taken together, inorganic nanoparticles are potential theranostic candidates for diagnosis and multivalent interactions with immune cells and antiviral therapy. However, fundamental investigations should be performed to translate them into clinical studies. Table 1 provides an overview of the antiviral mechanisms of various multivalent nanomaterials. Table 2 provides an overview of the various therapeutics for COVID-19 that are currently under clinical trials.
Table 1.
An overview of the antiviral mechanisms of multivalent nanomaterials.
| Nanomaterial | Antiviral Mechanism | Targeted Viruses | References |
|---|---|---|---|
| Liposomes | Alter the structure of lipids and proteins of viruses via their composition (fatty acids, surfactants, alcohol) | DENV, SARS-CoV-2, HIV, Influenza | [186,187] |
| Polymers | Absorb negative charged virus particles via their positive charge and inactivate viruses | HBV, HDV, HSV, Influenza, CoVs | [155,188] |
| Dendrimers | Strong multivalent interactions with viruses via their highly-branched 3D structure and inactivate viruses | HIV, HCV, SARS-CoV-2 | [189,190,191] |
| Cyclodextrin (CD) | Directly inactivate virus and inhibit attachment | HIV, RSV, HSV-2, DENV-2, ZIKV | [159] |
| Graphene oxide (GO) | Inactivate virus function because of the interactions between the sharp edges of the GO layers and the virus particles | RSV, CoVs, HPV | [163,192] |
| Quantum dots (QDs) | Inhibit virus entry at the early stage via interaction with the spike protein of viruses and block genomic replication | SARS-CoV-2, HIV-1 | [174] |
| Gold NPs (AuNPs) | Prevent viral attachment and penetration | DENV, HCV, HPV, CoVs | [193,194] |
| Iron oxide NPs (IONPs) | Interaction with the spike protein receptor binding domain (S1-RBD) and block virus replication | HIV, Influenza A, SARS-CoV-2 | [181,182] |
| Titanium oxide NPs (TiO2NPs) | Generate ROS leading to impair lipid membranes and damage DNA structure of virus particles | CoVs, Influenza A | [195,196] |
| Zirconium oxide NPs (ZrO2NPs) | Stimulation of innate immunity and promote the expression of cytokines | H5N1 influenza virus, Influenza A | [177] |
| Zinc oxide NPs (ZnONPs) |
Releasing of Zn2+, generate ROS and block viral replication and translation | HIV, HSV, HPV, H1N1 influenza | [197] |
| Copper oxide NPs (CuO2NPs) | Releasing of Cu2+, oxidation of viral proteins and degradation of viral genome | HSV | [198,199] |
| Silver NPs (AgNPs) | Iron release (Ag+), increase ROS and oxidation viral proteins, bind to the nucleic acid (DNA or RNA) of the virus | HSV, SARS-CoV-2 | [184,200] |
| Silicon dioxide NPs (SiO2NPs) | Reduce the amount of progeny virus | Influenza virus A | [201] |
Abbreviations. Nanoparicles (NP), Respiratory syncytial virus type (RSV), Dengue virus (DENV), Human immunodeficiency viruses (HIV), Hepatitis B virus (HBV), Hepatitis D virus (HDV), Herpes simplex virus type 1 (HSV), Coronaviruses (CoVs), Zika virus (ZIKV), Human papillomavirus (HPV).
Table 2.
Summary of the drugs for combating COVID-19 that are under clinical trials.
| Therapeutic Agent | Current Status | Rout of Administration | Purpose of Study | Clinical Trial Identification |
|---|---|---|---|---|
| Remdesivir | Phase III | Intravenous | Antiviral drug to manage COVID-19 |
NCT04678739 NCT04647695 NCT04582266 NCT04738045 |
| Chloroquine | Phase I/II/IV | Oral | Antimalarial drug to treat mild Asymptomatic and Symptomatic cases of COVID-19 |
NCT04443270 NCT04328493 NCT04627467 |
| Lopinavir/ ritonavir |
Phase IV/II | Intravenous | Antiviral drug to treat COVID-19 in terminally sick patients with cancer and immune suppression |
NCT04738045 NCT04455958 |
| Azithromycin | Phase III/IV | Oral | Antibiotic drug to treat COVID-19 patients |
NCT04365231 NCT04359316 |
| Hydroxy-chloroquine | Phase II/I | Oral | Antimalarial drug to treat ambulatory patients with mild and severe COVID-19 |
NCT04340544 NCT04351620 |
| Arbidol | Phase IV | Oral | Antiviral drug to treat COVID-19 patients | NCT04350684 |
| Isotretinoin | Phase III | Oral | Retinoid drug to evaluate the safety and efficacy in treatment of COVID-19 |
NCT04663906 NCT04353180 |
| Lenalidomide | Phase IV | Oral | Antiangiogenic drug to treat mild COVID-19 patients | NCT04361643 |
| Chlorpromazine | Phase III | Oral | Antipsychotics drug to manage COVID-19 subjects | NCT04366739 |
| Canakinumab | Phase III | Intravenous | Anti-human-IL-1β monoclonal antibody to study the efficacy in treating COVID-19 patients | NCT04362813 |
| Ruxolitinib | Phase III | Oral | Antiviral drug to assay the efficacy in COVID-19 patients with cytokine storm | NCT04362137 |
| Dexamethasone | Phase IV | Intravenous | Steroid drug to treat COVID-19 patients | NCT04325061 |
| Favipiravir | Phase III | Oral | Antiviral drug to treat COVID-19 patients | NCT04336904 |
| Sildenafil citrate | Phase III | Oral | Phosphodiesterase inhibitor drug to treat COVID-19 patients | NCT04304313 |
4. Future Perspective
Acute respiratory distress syndrome is manifested clinically during viral infections such as COVID-19, septic shock, poisoning, and exposure to chemical warfare agents. The prognosis of ARDS is poor and there is no specific treatment for such a condition. This life-threatening lung injury that allows fluid to leak into the lungs generally begins with massive oxidative/nitrosative stress. The subsequent DNA damage activates PARP, endogenous and viral (macrodomain) PARG, and TRMP2 activity, which results in apoptosis, necrosis, and parthanatosis. SARS-CoV-2 expresses multi-domain non-structural protein 3 (NSP3) as a potent extraneous PARG and likely activates the RAS that provides fuel for oxidative stress in this circuit. This detrimental cycle consumes NAD and decreases antioxidant capacity, enhancing inflammation and cytokine release. Based on the aforementioned scheme of SARS-CoV-2 molecular pathogenesis, there are several therapeutic candidates available for COVID-19 treatment. Renin inhibitors such as aliskiren, which suppresses RAS, may also be recommended for COVID-19 treatment. Poly (ADP-ribose) polymerase inhibitors, such as trehalose, olaparib, losartan, vitamin D, and NAC, are other therapeutic options in SARS-CoV-2 infection. Apocynin is an NADPH oxidase inhibitor and may be used to intercept and break the detrimental circuit.
5. Conclusions
In conclusion, interruption of the aforementioned detrimental infection molecular pathway may help to convert SARS-CoV-2 infection into a mild viral infection with symptoms analogous to the common cold. The aforementioned drugs and supplements have to be scrutinized for their efficacy via registered clinical trials along with conventional multi-drug regimens and anti-viral therapeutic guidelines. A flowchart of SARS-CoV-2 pathogenesis and the antiviral effects of clinical medications for the management of COVID-19 are illustrated in Figure 8.
Figure 8.
Flowchart of the SARS-CoV-2 pathogenesis and the purported antiviral mechanisms of clinical medications used for combating COVID-19.
Acknowledgments
The authors would like to thank Isfahan University of Medical Sciences Vice Chancellor for Research for assistance in COVID 19 projects.
Author Contributions
The manuscript was written with the contributions of all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no specific grant from any funding agency in the public and commercial sectors.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
In this section, please provide details regarding where data.
Conflicts of Interest
There is no conflict of statement.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen H., Guo J., Wang C., Luo F., Yu X., Zhang W., Li J., Zhao D., Xu D., Gong Q., et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: A retrospective review of medical records. Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manski C.F., Molinari F. Estimating the COVID-19 infection rate: Anatomy of an inference problem. J. Econ. 2021;220:181–192. doi: 10.1016/j.jeconom.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y., Liu Q., Guo D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J. Med. Virol. 2020;92:418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan J.F.-W., Kok K.-H., Zhu Z., Chu H., To K.K.-W., Yuan S., Yuen K.-Y. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 2020;9:221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xing K., Tu X.-Y., Liu M., Liang Z.-W., Chen J.-N., Li J.-J., Jiang L.-G., Xing F.-Q., Jiang Y. Efficacy and safety of COVID-19 vaccines: A systematic review. Zhongguo Dang Dai Er Ke Za Zhi. 2021;23:221–228. doi: 10.7499/j.issn.1008-8830.2101133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karpinski T.M., Ozarowski M., Seremak-Mrozikiewicz A., Wolski H., Wlodkowic D. The 2020 race towards SARS-CoV-2 specific vaccines. Theranostics. 2021;11:1690–1702. doi: 10.7150/thno.53691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mo P., Xing Y., Xiao Y., Deng L., Zhao Q., Wang H., Xiong Y., Cheng Z., Gao S., Liang K., et al. Clinical charac-teristics of refractory COVID-19 pneumonia in Wuhan, China. Clin. Infect. Dis. 2020:ciaa270. doi: 10.1093/cid/ciaa270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang M.-Y., Zhao R., Gao L.-J., Gao X.-F., Wang D.-P., Cao J.-M. SARS-CoV-2: Structure, Biology, and Structure-Based Therapeutics Development. Front. Cell. Infect. Microbiol. 2020;10:587269. doi: 10.3389/fcimb.2020.587269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang Y., Yang C., Xu X.F., Xu W., Liu S.W. Structural and functional properties of SARS-CoV-2 spike protein: Potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 2020;41:1141–1149. doi: 10.1038/s41401-020-0485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020;183:1735. doi: 10.1016/j.cell.2020.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdel-Moneim A. COVID-19 Pandemic and Male Fertility: Clinical Manifestations and Pathogenic Mechanisms. Biochemistry (Moscow) 2021;86:1–8. doi: 10.1134/S0006297921040015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zafer M.M., El-Mahallawy H.A., Ashour H.M. Severe COVID-19 and Sepsis: Immune Pathogenesis and Laboratory Markers. Microorganisms. 2021;9:159. doi: 10.3390/microorganisms9010159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soltani S., Tabibzadeh A., Zakeri A., Zakeri A.M., Latifi T., Shabani M., Pouremamali A., Erfani Y., Pakzad I., Malekifar P., et al. COVID-19 associated central nervous system manifestations, mental and neurological symptoms: A systematic review and meta-analysis. Rev. Neurosci. 2021;32:351–361. doi: 10.1515/revneuro-2020-0108. [DOI] [PubMed] [Google Scholar]
- 15.Gavriatopoulou M., Korompoki E., Fotiou D., Ntanasis-Stathopoulos I., Psaltopoulou T., Kastritis E., Terpos E., Dimopoulos M.A. Organ-specific manifestations of COVID-19 infection. Clin. Exp. Med. 2020;20:493–506. doi: 10.1007/s10238-020-00648-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.V’Kovski P., Kratzel A., Steiner S., Stalder H., Thiel V. Coronavirus biology and replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021;19:155–170. doi: 10.1038/s41579-020-00468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakagawa K., Lokugamage K., Makino S. Viral and Cellular mRNA Translation in Coronavirus-Infected Cells. Adv. Appl. Microbiol. 2016;96:165–192. doi: 10.1016/bs.aivir.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim D., Lee J.Y., Yang J.S., Kim J.W., Kim V.N., Chang H. The Architecture of SARS-CoV-2 Transcriptome. Cell. 2020;181:914–921.e910. doi: 10.1016/j.cell.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raj R. Analysis of non-structural proteins, NSPs of SARS-CoV-2 as targets for computational drug designing. Biochem. Biophys. Rep. 2021;25:100847. doi: 10.1016/j.bbrep.2020.100847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pelkmans L., Helenius A. Insider information: What viruses tell us about endocytosis. Curr. Opin. Cell Biol. 2003;15:414–422. doi: 10.1016/S0955-0674(03)00081-4. [DOI] [PubMed] [Google Scholar]
- 23.Wit E.D., Doremalen N.V., Falzarano D., Munster V.J. SARS and MERS: Recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016;14:523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu F., Zhao S., Yu B., Chen Y.-M., Wang W., Song Z.-G., Hu Y., Tao Z.-W., Tian J.-H., Pei Y.-Y., et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belouzard S., Chu V.C., Whittaker G.R. Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites. Proc. Natl. Acad. Sci. USA. 2009;106:5871–5876. doi: 10.1073/pnas.0809524106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian X., Li C., Huang A., Xia S., Lu S., Shi Z., Lu L., Jiang S., Yang Z., Wu Y. Potent binding of 2019 novel coro-navirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg. Microbes Infect. 2020;9:382–385. doi: 10.1080/22221751.2020.1729069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wevers B.A., Hoek L.v.d. Renin–angiotensin system in human coronavirus pathogenesis. Futur. Virol. 2010;5:145–161. doi: 10.2217/fvl.10.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Millet J.K., Whittaker G.R. Host cell entry of Middle East respiratory syndrome coronavirus after two-step, furin-mediated activation of the spike protein. Proc. Natl. Acad. Sci. USA. 2014;111:15214–15219. doi: 10.1073/pnas.1407087111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perlman S., Netland J. Coronaviruses post-SARS: Update on replication and pathogenesis. Nat. Rev. Genet. 2009;7:439–450. doi: 10.1038/nrmicro2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Villamil-Gómez W.E., Sánchez Á., Gelis L., Silvera L.A., Barbosa J., Otero-Nader O., Bonilla-Salgado C.D., Rodríguez-Morales A.J. Fatal human coronavirus 229E (HCoV-229E) and RSV–Related pneumonia in an AIDS patient from Colombia. Travel Med. Infect. Dis. 2020;36:101573. doi: 10.1016/j.tmaid.2020.101573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bayati A., Kumar R., Francis V., McPherson P.S. SARS-CoV-2 uses clathrin-mediated endocytosis to gain access into cells. BioRxiv. 2020 doi: 10.1101/2020.07.13.201509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang N., Shen H.-M. Targeting the Endocytic Pathway and Autophagy Process as a Novel Therapeutic Strategy in COVID-19. Int. J. Biol. Sci. 2020;16:1724–1731. doi: 10.7150/ijbs.45498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hartenian E., Nandakumar D., Lari A., Ly M., Tucker J.M., Glaunsinger B.A. The molecular virology of Corona-viruses. J. Biol. Chem. 2020;295:12910–12934. doi: 10.1074/jbc.REV120.013930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N., Donovan M., Woolf B., Robison K., Jeyaseelan R., et al. A Novel Angiotensin-Converting Enzyme–Related Carboxypeptidase (ACE2) Converts Angiotensin I to Angiotensin 1-9. Circ. Res. 2000;87:E1–E9. doi: 10.1161/01.RES.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 35.Fountain J.H., Lappin S.L. Physiology, Renin Angiotensin System. StatPearls; Treasure Island, FL, USA: 2021. [PubMed] [Google Scholar]
- 36.Fung T.S., Liu D.X. Human Coronavirus: Host-Pathogen Interaction. Annu. Rev. Microbiol. 2019;73:529–557. doi: 10.1146/annurev-micro-020518-115759. [DOI] [PubMed] [Google Scholar]
- 37.Zhao Y., Zhao Z., Wang Y., Zhou Y., Ma Y., Zuo W. Single-Cell RNA Expression Profiling of ACE2, the putative receptor of Wuhan COVID-19. BioRxiv. 2020 doi: 10.1101/2020.01.26.919985e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martins L.D.B.U., Jabour L.G.P.P., Vieira C.C., Nery L.C.C., Dias R.F., Silva A.C.S.E. Renin-angiotensin system (RAS) and immune system profile in specific subgroups with COVID-19. Curr. Med. Chem. 2020;27:1–28. doi: 10.2174/0929867327666200903113117. [DOI] [PubMed] [Google Scholar]
- 39.Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: Molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev. Res. 2020;81:537–540. doi: 10.1002/ddr.21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pizzino G., Irrera N., Cucinotta M., Pallio G., Mannino F., Arcoraci V., Squadrito F., Altavilla D., Bitto A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017;2017:8416763. doi: 10.1155/2017/8416763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu J.Q., Kosten T.R., Zhang X.Y. Free radicals, antioxidant defense systems, and schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2013;46:200–206. doi: 10.1016/j.pnpbp.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 43.Kryston T.B., Georgiev A.B., Pissis P., Georgakilas A.G. Role of oxidative stress and DNA damage in human car-cinogenesis. Mutat. Res. 2011;711:193–201. doi: 10.1016/j.mrfmmm.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 44.Mathews M.T., Berk B. PARP-1 Inhibition Prevents Oxidative and Nitrosative Stress–Induced Endothelial Cell Death via Transactivation of the VEGF Receptor 2. Arter. Thromb. Vasc. Biol. 2008;28:711–717. doi: 10.1161/ATVBAHA.107.156406. [DOI] [PubMed] [Google Scholar]
- 45.Grunewald M.E., Chen Y., Kuny C., Maejima T., Lease R., Ferraris D., Aikawa M., Sullivan C.S., Perlman S., Fehr A.R. The coronavirus macrodomain is required to prevent PARP-mediated inhibition of virus replication and enhancement of IFN expression. PLoS Pathog. 2019;15:e1007756. doi: 10.1371/journal.ppat.1007756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andrabi S.A., Umanah G.K.E., Chang C., Stevens D.A., Karuppagounder S.S., Gagné J.-P., Poirier G.G., Dawson V.L., Dawson T.M. Poly(ADP-ribose) polymerase-dependent energy depletion occurs through inhibition of glycolysis. Proc. Natl. Acad. Sci. USA. 2014;111:10209–10214. doi: 10.1073/pnas.1405158111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lange I., Yamamoto S., Partida-Sanchez S., Mori Y., Fleig A., Penner R. TRPM2 functions as a lysosomal Ca2+-release channel in beta cells. Sci. Signal. 2009;2:ra23. doi: 10.1126/scisignal.2000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perraud A.-L., Fleig A., Dunn C.A., Bagley L.A., Launay P., Schmitz C., Stokes A.J., Zhu Q., Bessman M.J., Penner R., et al. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nat. Cell Biol. 2001;411:595–599. doi: 10.1038/35079100. [DOI] [PubMed] [Google Scholar]
- 49.Ishii M., Shimizu S., Hagiwara T., Wajima T., Miyazaki A., Mori Y., Kiuchi Y. Extracellular-Added ADP-Ribose Increases Intracellular Free Ca2+ Concentration through Ca2+ Release from Stores, but Not Through TRPM2-Mediated Ca2+ Entry, in Rat β-Cell Line RIN-5F. J. Pharmacol. Sci. 2006;101:174–178. doi: 10.1254/jphs.SCJ06001X. [DOI] [PubMed] [Google Scholar]
- 50.Sumoza-Toledo A., Penner R. TRPM2: A multifunctional ion channel for calcium signalling. J. Physiol. 2011;589:1515–1525. doi: 10.1113/jphysiol.2010.201855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gadotti A.C., Lipinski A.L., Vasconcellos F.T., Marqueze L.F., Cunha E.B., Campos A.C., Oliveira C.F., Amaral A.N., Baena C.P., Telles J.P., et al. Susceptibility of the patients infected with Sars-Cov2 to oxidative stress and possible interplay with severity of the disease. Free Radic. Biol. Med. 2021;165:184–190. doi: 10.1016/j.freeradbiomed.2021.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boshtam M., Asgary S., Kouhpayeh S., Shariati L., Khanahmad H. Aptamers Against Pro- and Anti-Inflammatory Cytokines: A Review. Inflammation. 2017;40:340–349. doi: 10.1007/s10753-016-0477-1. [DOI] [PubMed] [Google Scholar]
- 53.Dastjerdeh M.S., Kouhpayeh S., Sabzehei F., Khanahmad H., Salehi M., Mohammadi Z., Shariati L., Hejazi Z., Rabiei P., Manian M. Zinc Finger Nuclease: A New Approach to Overcome Beta-Lactam Antibiotic Resistance. Jundishapur J. Microbiol. 2016;9:29384. doi: 10.5812/jjm.29384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shariati L., Modarressi M.H., Tabatabaiefar M.A., Kouhpayeh S., Hejazi Z., Shahbazi M., Sabzehei F., Salehi M., Khanahmad H. Engineered zinc-finger nuclease to generate site-directed modification in the KLF1 gene for fetal hemoglobin induction. J. Cell. Biochem. 2019;120:8438–8446. doi: 10.1002/jcb.28130. [DOI] [PubMed] [Google Scholar]
- 55.Chen C., Zhang X.R., Ju Z.Y., He W.F. Advances in the research of cytokine storm mechanism induced by Corona Virus Disease 2019 and the corresponding immunotherapies. Zhonghua Shao Shang Za Zhi. 2020;36:E005. doi: 10.3760/cma.j.cn501120-20200224-00088. [DOI] [PubMed] [Google Scholar]
- 56.Dastoli S., Bennardo L., Patruno C., Nistico S.P. Are erythema multiforme and urticaria related to a better outcome of COVID-19? Dermatol. Ther. 2020;33:e13681. doi: 10.1111/dth.13681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Caricchio R., Gallucci M., Dass C., Zhang X., Gallucci S., Fleece D., Bromberg M., Criner G.J. Preliminary predictive criteria for COVID-19 cytokine storm. Ann. Rheum. Dis. 2021;80:88–95. doi: 10.1136/annrheumdis-2020-218323. [DOI] [PubMed] [Google Scholar]
- 58.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J. Infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Choudhury A., Das N.C., Patra R., Mukherjee S. In silico analyses on the comparative sensing of SARS-CoV-2 mRNA by the intracellular TLRs of humans. J. Med. Virol. 2021;93:2476–2486. doi: 10.1002/jmv.26776. [DOI] [PubMed] [Google Scholar]
- 60.Shimabukuro-Vornhagen A., Gödel P., Subklewe M., Stemmler H.J., Schlößer H.A., Schlaak M., Kochanek M., Böll B., Von Bergwelt-Baildon M.S. Cytokine release syndrome. J. Immunother. Cancer. 2018;6:56. doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., Collaboration H.A.S. COVID-19: Con-sider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moore J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020;368:473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 63.Feng X., Koh D.W. Roles of Poly(ADP-Ribose) Glycohydrolase in DNA Damage and Apoptosis. Int. Rev. Cell Mol. Biol. 2013;304:227–281. doi: 10.1016/b978-0-12-407696-9.00005-1. [DOI] [PubMed] [Google Scholar]
- 64.Guo F., Sun Y.B., Su L., Li S., Liu Z.F., Li J., Hu X.T. Losartan attenuates paraquat-induced pulmonary fibrosis in rats. Hum. Exp. Toxicol. 2014;34:497–505. doi: 10.1177/0960327114543840. [DOI] [PubMed] [Google Scholar]
- 65.Henderson L.A., Canna S.W., Schulert G.S., Volpi S., Lee P.Y., Kernan K.F., Caricchio R., Mahmud S., Hazen M.M., Halyabar O., et al. On the Alert for Cytokine Storm: Immunopathology in COVID-19. Arthritis Rheumatol. 2020;72:1059–1063. doi: 10.1002/art.41285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mangalmurti N., Hunter C.A. Cytokine Storms: Understanding COVID-19. Immunity. 2020;53:19–25. doi: 10.1016/j.immuni.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liao J.K. Linking endothelial dysfunction with endothelial cell activation. J. Clin. Investig. 2013;123:540–541. doi: 10.1172/JCI66843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Endemann D.H., Schiffrin E.L. Endothelial dysfunction. J. Am. Soc. Nephrol. 2004;15:1983–1992. doi: 10.1097/01.ASN.0000132474.50966.DA. [DOI] [PubMed] [Google Scholar]
- 69.McMahon S., Grondin F., McDonald P.P., Richard D.E., Dubois C.M. Hypoxia-enhanced expression of the proprotein convertase furin is mediated by hypoxia-inducible factor-1: Impact on the bioactivation of proproteins. J. Biol. Chem. 2005;280:6561–6569. doi: 10.1074/jbc.M413248200. [DOI] [PubMed] [Google Scholar]
- 70.Sega F.V.D., Fortini F., Spadaro S., Ronzoni L., Zucchetti O., Manfrini M., Mikus E., Fogagnolo A., Torsani F., Pavasini R., et al. Time course of endothelial dysfunction markers and mortality in COVID-19 patients: A pilot study. Clin. Transl. Med. 2021;11:e283. doi: 10.1002/ctm2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen X., Touyz R.M., Park J.B., Schiffrin E.L. Antioxidant Effects of Vitamins C and E Are Associated with Altered Activation of Vascular NADPH Oxidase and Superoxide Dismutase in Stroke-Prone SHR. Hypertension. 2001;38:606–611. doi: 10.1161/hy09t1.094005. [DOI] [PubMed] [Google Scholar]
- 72.Annuk M., Zilmer M., Lind L., Linde T., Fellström B. Oxidative Stress and Endothelial Function in Chronic Renal Failure. J. Am. Soc. Nephrol. 2001;12:2747–2752. doi: 10.1681/ASN.V12122747. [DOI] [PubMed] [Google Scholar]
- 73.Heitzer T., Schlinzig T., Krohn K., Meinertz T., Münzel T. Endothelial Dysfunction, Oxidative Stress, and Risk of Cardiovascular Events in Patients With Coronary Artery Disease. Circulation. 2001;104:2673–2678. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- 74.Hasdan G., Benchetrit S., Rashid G., Green J., Bernheim J., Rathaus M. Endothelial dysfunction and hypertension in 5/6 nephrectomized rats are mediated by vascular superoxide. Kidney Int. 2002;61:586–590. doi: 10.1046/j.1523-1755.2002.00166.x. [DOI] [PubMed] [Google Scholar]
- 75.Sims J.T., Krishnan V., Chang C.-Y., Engle S.M., Casalini G., Rodgers G.H., Bivi N., Nickoloff B.J., Konrad R.J., de Bono S., et al. Characterization of the cytokine storm reflects hyperinflammatory endothelial dysfunction in COVID-19. J. Allergy Clin. Immunol. 2021;147:107–111. doi: 10.1016/j.jaci.2020.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Furchgott R.F. The 1996 Albert Lasker Medical Research Awards. The discovery of endothelium-derived relaxing factor and its importance in the identification of nitric oxide. JAMA. 1996;276:1186–1188. doi: 10.1001/jama.1996.03540140074032. [DOI] [PubMed] [Google Scholar]
- 77.Sena C.M., Pereira A.M., Seiça R. Endothelial dysfunction—A major mediator of diabetic vascular disease. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2013;1832:2216–2231. doi: 10.1016/j.bbadis.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 78.De Caterina R., Libby P., Peng H.B., Thannickal V.J., Rajavashisth T.B., Gimbrone M.A., Shin W.S., Liao J.K. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J. Clin. Investig. 1995;96:60–68. doi: 10.1172/JCI118074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zeiher A.M., Fisslthaler B., Schray-Utz B., Busse R. Nitric oxide modulates the expression of monocyte chemoat-tractant protein 1 in cultured human endothelial cells. Circ. Res. 1995;76:980–986. doi: 10.1161/01.RES.76.6.980. [DOI] [PubMed] [Google Scholar]
- 80.Eberhardt W., Beeg T., Beck K.-F., Walpen S., Gauer S., Böhles H., Pfeilschifter J. Nitric oxide modulates expression of matrix metalloproteinase-9 in rat mesangial cells. Kidney Int. 2000;57:59–69. doi: 10.1046/j.1523-1755.2000.00808.x. [DOI] [PubMed] [Google Scholar]
- 81.Uemura S., Matsushita H., Li W., Glassford A.J., Asagami T., Lee K.H., Harrison D.G., Tsao P.S. Diabetes mellitus enhances vascular matrix metalloproteinase activity: Role of oxidative stress. Circ. Res. 2001;88:1291–1298. doi: 10.1161/hh1201.092042. [DOI] [PubMed] [Google Scholar]
- 82.Dhananjayan R., Koundinya K.S.S., Malati T., Kutala V.K. Endothelial Dysfunction in Type 2 Diabetes Mellitus. Indian J. Clin. Biochem. 2016;31:372–379. doi: 10.1007/s12291-015-0516-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Radomski M.W., Palmer R.M., Moncada S. The role of nitric oxide and cGMP in platelet adhesion to vascular en-dothelium. Biochem. Biophys. Res. Commun. 1987;148:1482–1489. doi: 10.1016/S0006-291X(87)80299-1. [DOI] [PubMed] [Google Scholar]
- 84.Kubes P., Suzuki M., Granger D.N. Nitric oxide: An endogenous modulator of leukocyte adhesion. Proc. Natl. Acad. Sci. USA. 1991;88:4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sabioni L., De Lorenzo A., Lamas C., Muccillo F., Castro-Faria-Neto H.C., Estato V., Tibirica E. Systemic micro-vascular endothelial dysfunction and disease severity in COVID-19 patients: Evaluation by laser Doppler perfusion moni-toring and cytokine/chemokine analysis. Microvasc. Res. 2021;134:104119. doi: 10.1016/j.mvr.2020.104119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Verma S., Anderson T.J. Fundamentals of Endothelial Function for the Clinical Cardiologist. Circulation. 2002;105:546–549. doi: 10.1161/hc0502.104540. [DOI] [PubMed] [Google Scholar]
- 87.Fang W., Jiang J., Su L., Shu T., Liu H., Lai S., Ghiladi R.A., Wang J. The role of NO in COVID-19 and potential therapeutic strategies. Free Radic. Biol. Med. 2021;163:153–162. doi: 10.1016/j.freeradbiomed.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dattola A., Silvestri M., Bennardo L., Passante M., Scali E., Patruno C., Nisticò S.P. Role of Vitamins in Skin Health: A Systematic Review. Curr. Nutr. Rep. 2020;9:226–235. doi: 10.1007/s13668-020-00322-4. [DOI] [PubMed] [Google Scholar]
- 89.Quesada-Gomez J.M., Castillo M.E., Bouillon R. Vitamin D Receptor stimulation to reduce Acute Respiratory Distress Syndrome (ARDS) in patients with Coronavirus SARS-CoV-2 infections: Revised Ms SBMB 2020_166. J. Steroid Biochem. Mol. Biol. 2020;202:105719. doi: 10.1016/j.jsbmb.2020.105719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rhodes J.M., Subramanian S., Laird E., Griffin G., Kenny R.A. Perspective: Vitamin D deficiency and COVID-19 severity—Plausibly linked by latitude, ethnicity, impacts on cytokines, ACE2 and thrombosis. J. Intern. Med. 2021;289:97–115. doi: 10.1111/joim.13149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xu J., Yang J., Chen J., Luo Q., Zhang Q., Zhang H. Vitamin D alleviates lipopolysaccharide-induced acute lung injury via regulation of the renin-angiotensin system. Mol. Med. Rep. 2017;16:7432–7438. doi: 10.3892/mmr.2017.7546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pilz S., Tomaschitz A., Ritz E., Pieber T.R. Vitamin D status and arterial hypertension: A systematic review. Nat. Rev. Cardiol. 2009;6:621–630. doi: 10.1038/nrcardio.2009.135. [DOI] [PubMed] [Google Scholar]
- 93.Andersen L.B., Przybyl L., Haase N., von Versen-Höynck F., Qadri F., Jørgensen J.S., Sørensen G.L., Fruekilde P., Poglitsch M., Szijarto I., et al. Vitamin D Depletion Aggravates Hypertension and Target-Organ Damage. J. Am. Heart Assoc. 2015;4:001417. doi: 10.1161/JAHA.114.001417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mabley J.G., Wallace R., Pacher P., Murphy K., Szabo C. Inhibition of poly(adenosine diphosphate-ribose) poly-merase by the active form of vitamin D. Int. J. Mol. Med. 2007;19:947–952. [PMC free article] [PubMed] [Google Scholar]
- 95.Dastan F., Tabarsi P., Marjani M., Moniri A., Hashemian S.M.R., Tavakoli-Ardakani M., Saffaei A. Thalidomide against Coronavirus Disease 2019 (COVID-19): A Medicine with a Thousand Faces. Iran. J. Pharm. Res. 2020;19:1–2. doi: 10.22037/ijpr.2020.113369.14259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang G., Ma H., Wang J., Khan M.F. Contribution of poly(ADP-ribose)polymerase-1 activation and apoptosis in trichloroethene-mediated autoimmunity. Toxicol. Appl. Pharmacol. 2019;362:28–34. doi: 10.1016/j.taap.2018.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Martinon D., Borges V.F., Gomez A.C., Shimada K. Potential Fast COVID-19 Containment With Trehalose. Front. Immunol. 2020;11:1623. doi: 10.3389/fimmu.2020.01623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee H.J., Yoon Y.S., Lee S.J. Mechanism of neuroprotection by trehalose: Controversy surrounding autophagy in-duction. Cell Death Dis. 2018;9:712. doi: 10.1038/s41419-018-0749-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Palmieri M., Pal R., Nelvagal H.R., Lotfi P., Stinnett G.R., Seymour M.L., Chaudhury A., Bajaj L., Bondar V.V., Bremner L. mTORC1-independent TFEB activation via Akt inhibition promotes cellular clearance in neurodegenerative storage diseases. Nat. Commun. 2017;8:1–19. doi: 10.1038/ncomms14338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.De Flora S., Balansky R., La Maestra S. Rationale for the use of N-acetylcysteine in both prevention and adjuvant therapy of COVID-19. FASEB J. 2020;34:13185–13193. doi: 10.1096/fj.202001807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhou N., Yang X., Huang A., Chen Z. The potential mechanism of N-acetylcysteine in treating COVID-19. Curr. Pharm. Biotechnol. 2020;21:1–9. doi: 10.2174/1389201021999201228212043. [DOI] [PubMed] [Google Scholar]
- 102.Amarowicz R., Pegg R.B. Tree Nuts and Peanuts as a Source of Natural Antioxidants in our Daily Diet. Curr. Pharm. Des. 2020;26:1898–1916. doi: 10.2174/1381612826666200318125620. [DOI] [PubMed] [Google Scholar]
- 103.Yeo J., Lee J., Yoon S., Kim W.J. Tannic acid-based nanogel as an efficient anti-inflammatory agent. Biomater. Sci. 2020;8:1148–1159. doi: 10.1039/C9BM01384A. [DOI] [PubMed] [Google Scholar]
- 104.Orlowski P., Żmigrodzka M., Tomaszewska E., Ranoszek-Soliwoda K., Czupryn M., Antos-Bielska M., Szemraj J., Celichowski G., Grobelny J., Krzyzowska M. Tannic acid-modified silver nanoparticles for wound healing: The importance of size. Int. J. Nanomed. 2018;ume 13:991–1007. doi: 10.2147/IJN.S154797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Khalifa I., Zhu W., Mohammed H.H.H., Dutta K., Li C. Tannins inhibit SARS-CoV-2 through binding with catalytic dyad residues of 3CLpro: An in silico approach with 19 structural different hydrolysable tannins. J. Food Biochem. 2020;44:e13432. doi: 10.1111/jfbc.13432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Luo X., Li M., Zhan K., Yang W., Zhang L., Wang K., Yu P., Zhang L. Selective inhibition of TRPM2 channel by two novel synthesized ADPR analogues. Chem. Biol. Drug Des. 2018;91:552–566. doi: 10.1111/cbdd.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mai C., Mankoo H., Wei L., An X., Li C., Li D., Jiang L. TRPM2 channel: A novel target for alleviating ischaemia-reperfusion, chronic cerebral hypo-perfusion and neonatal hypoxic-ischaemic brain damage. J. Cell. Mol. Med. 2020;24:4–12. doi: 10.1111/jcmm.14679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chi Y., Li K., Yan Q., Koizumi S., Shi L., Takahashi S., Zhu Y., Matsue H., Takeda M., Kitamura M., et al. Non-steroidal anti-inflammatory drug flufenamic acid is a potent activator of AMP-activated protein kinase. J. Pharmacol. Exp. Ther. 2011;339:257–266. doi: 10.1124/jpet.111.183020. [DOI] [PubMed] [Google Scholar]
- 109.Kume S., Haneda M., Kanasaki K., Sugimoto T., Araki S., Isshiki K., Isono M., Uzu T., Guarente L., Kashiwagi A., et al. SIRT1 inhibits transforming growth factor beta-induced apoptosis in glomerular mesangial cells via Smad7 deacetyla-tion. J. Biol. Chem. 2007;282:151–158. doi: 10.1074/jbc.M605904200. [DOI] [PubMed] [Google Scholar]
- 110.Matsuoka H., Ishii M., Goto A., Sugimoto T. Role of serotonin type 2 receptors in regulation of aldosterone pro-duction. Am. J. Physiol. 1985;249:E234–E238. doi: 10.1152/ajpendo.1985.249.2.E234. [DOI] [PubMed] [Google Scholar]
- 111.Miller R., Wentzel A., Richards G. COVID-19: NAD+ deficiency may predispose the aged, obese and type2 diabetics to mortality through its effect on SIRT1 activity. Med. Hypotheses. 2020;144:110044. doi: 10.1016/j.mehy.2020.110044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gharote M.A. Role of poly (ADP) ribose polymerase-1 inhibition by nicotinamide as a possible additive treatment to modulate host immune response and prevention of cytokine storm in COVID-19. Indian J. Med Sci. 2020;72:25–28. doi: 10.25259/IJMS_29_2020. [DOI] [Google Scholar]
- 113.Cotton S.A., Herrick A.L., Jayson M.I., Freemont A.J. TGF beta--a role in systemic sclerosis? J. Pathol. 1998;184:4–6. doi: 10.1002/(SICI)1096-9896(199801)184:1<4::AID-PATH968>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 114.McKay S., de Jongste J.C., Saxena P.R., Sharma H.S. Angiotensin II induces hypertrophy of human airway smooth muscle cells: Expression of transcription factors and transforming growth factor-beta1. Am. J. Respir. Cell Mol. Biol. 1998;18:823–833. doi: 10.1165/ajrcmb.18.6.2924. [DOI] [PubMed] [Google Scholar]
- 115.Sanderson N., Factor V., Nagy P., Kopp J., Kondaiah P., Wakefield L., Roberts A.B., Sporn M.B., Thorgeirsson S.S. Hepatic expression of mature transforming growth factor beta 1 in transgenic mice results in multiple tissue lesions. Proc. Natl. Acad. Sci. USA. 1995;92:2572–2576. doi: 10.1073/pnas.92.7.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stula M., Pinto Y.M., Gschwend S., Teisman A.C., Van Gilst W.H., Böhm M., Dietz R., Paul M. Interaction of the Renin-Angiotensin System and the Endothelin System in Cardiac Hypertrophy. J. Cardiovasc. Pharmacol. 1998;31:S403–S405. doi: 10.1097/00005344-199800001-00116. [DOI] [PubMed] [Google Scholar]
- 117.Ünübol M., Yavaşoğlu I., Acar B., Kadıköylü G., Bolaman Z. The effect of losartan on platelet aggregation and hematological parameters in patients with newly diagnosed hypertension. Meandros Med. J. 2015;16:91–96. doi: 10.4274/meandros.2438. [DOI] [Google Scholar]
- 118.Cardoso P.R.G., Matias K.A., Dantas A.T., Marques C.D.L., Pereira M.C., Duarte A., Rego M., Pitta I.D.R., Pitta M. Losartan, but not Enalapril and Valsartan, Inhibits the Expression of IFN-gamma, IL-6, IL-17F and IL-22 in PBMCs from Rheumatoid Arthritis Patients. Open Rheumatol. J. 2018;12:160–170. doi: 10.2174/1874312901812010160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shen L., Mo H., Cai L., Kong T., Zheng W., Ye J., Qi J., Xiao Z. Losartan prevents sepsis-induced acute lung injury and decreases activation of nuclear factor kappaB and mitogen-activated protein kinases. Shock. 2009;31:500–506. doi: 10.1097/SHK.0b013e318189017a. [DOI] [PubMed] [Google Scholar]
- 120.Lanza K., Perez L.G., Costa L.B., Cordeiro T.M., Palmeira V.A., Ribeiro V.T., Simoes E.S.A.C. Covid-19: The ren-in-angiotensin system imbalance hypothesis. Clin. Sci. (Lond.) 2020;134:1259–1264. doi: 10.1042/CS20200492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sun M., Yang J., Sun Y., Su G. Inhibitors of RAS Might Be a Good Choice for the Therapy of COVID-19 Pneumonia. Zhonghua jie he he hu xi za zhi = Chin. J. Tuberc. Respir. Dis. 2020;43:E014. doi: 10.3760/cma.j.issn.1001-0939.2020.0014. [DOI] [PubMed] [Google Scholar]
- 122.Bengtson C.D., Montgomery R.N., Nazir U., Satterwhite L., Kim M.D., Bahr N.C., Castro M., Baumlin N., Salathe M. An Open Label Trial to Assess Safety of Losartan for Treating Worsening Respiratory Illness in COVID-19. Front. Med. 2021;8:152. doi: 10.3389/fmed.2021.630209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen G.-L., Zeng B., Eastmond S., E Elsenussi S., Boa A., Xu S.-Z. Pharmacological comparison of novel synthetic fenamate analogues with econazole and 2-APB on the inhibition of TRPM2 channels. Br. J. Pharmacol. 2012;167:1232–1243. doi: 10.1111/j.1476-5381.2012.02058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hill K., Benham C.D., McNulty S., Randall A.D. Flufenamic acid is a pH-dependent antagonist of TRPM2 channels. Neuropharmacology. 2004;47:450–460. doi: 10.1016/j.neuropharm.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 125.Kraft R., Grimm C., Frenzel H., Harteneck C. Inhibition of TRPM2 cation channels by N -(p -amylcinnamoyl)anthranilic acid. Br. J. Pharmacol. 2006;148:264–273. doi: 10.1038/sj.bjp.0706739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hill K., McNulty S., Randall A.D. Inhibition of TRPM2 channels by the antifungal agents clotrimazole and econazole. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2004;370:227–237. doi: 10.1007/s00210-004-0981-y. [DOI] [PubMed] [Google Scholar]
- 127.Behrendt H.-J., Germann T., Gillen C., Hatt H., Jostock R. Characterization of the mouse cold-menthol receptor TRPM8 and vanilloid receptor type-1 VR1 using a fluorometric imaging plate reader (FLIPR) assay. Br. J. Pharmacol. 2004;141:737–745. doi: 10.1038/sj.bjp.0705652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Togashi K., Inada H., Tominaga M. Inhibition of the transient receptor potential cation channel TRPM2 by 2-aminoethoxydiphenyl borate (2-APB) Br. J. Pharmacol. 2008;153:1324–1330. doi: 10.1038/sj.bjp.0707675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jiang H., Zeng B., Chen G.-L., Bot D., Eastmond S., Elsenussi S.E., Atkin S.L., Boa A., Xu S.-Z. Effect of non-steroidal anti-inflammatory drugs and new fenamate analogues on TRPC4 and TRPC5 channels. Biochem. Pharmacol. 2012;83:923–931. doi: 10.1016/j.bcp.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 130.Kolisek M., Beck A., Fleig A., Penner R. Cyclic ADP-Ribose and Hydrogen Peroxide Synergize with ADP-Ribose in the Activation of TRPM2 Channels. Mol. Cell. 2005;18:61–69. doi: 10.1016/j.molcel.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 131.Moreau C., Kirchberger T., Swarbrick J.M., Bartlett S.J., Fliegert R., Yorgan T., Bauche A., Harneit A., Guse A.H., Potter B.V.L. Structure–Activity Relationship of Adenosine 5′-diphosphoribose at the Transient Receptor Potential Melastatin 2 (TRPM2) Channel: Rational Design of Antagonists. J. Med. Chem. 2013;56:10079–10102. doi: 10.1021/jm401497a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Togashi K., Hara Y., Tominaga T., Higashi T., Konishi Y., Mori Y., Tominaga M. TRPM2 activation by cyclic ADP-ribose at body temperature is involved in insulin secretion. EMBO J. 2006;25:1804–1815. doi: 10.1038/sj.emboj.7601083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cao Y.-C., Deng Q.-X., Dai S.-X. Remdesivir for severe acute respiratory syndrome coronavirus 2 causing COVID-19: An evaluation of the evidence. Travel Med. Infect. Dis. 2020;35:101647. doi: 10.1016/j.tmaid.2020.101647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jean S.-S., Lee P.-I., Hsueh P.-R. Treatment options for COVID-19: The reality and challenges. J. Microbiol. Immunol. Infect. 2020;53:436–443. doi: 10.1016/j.jmii.2020.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Harrison C. Coronavirus puts drug repurposing on the fast track. Nat. Biotechnol. 2020;38:379–381. doi: 10.1038/d41587-020-00003-1. [DOI] [PubMed] [Google Scholar]
- 136.Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. The FDA-approved drug ivermectin inhibits the repli-cation of SARS-CoV-2 in vitro. Antivir. Res. 2020;178:104787. doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Favalli E.G., Ingegnoli F., De Lucia O., Cincinelli G., Cimaz R., Caporali R. COVID-19 infection and rheumatoid arthritis: Faraway, so close! Autoimmun. Rev. 2020;19:102523. doi: 10.1016/j.autrev.2020.102523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Devaux C.A., Rolain J.-M., Colson P., Raoult D. New insights on the antiviral effects of chloroquine against coro-navirus: What to expect for COVID-19? Int. J. Antimicrobe. Agents. 2020;55:105938. doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ibáñez S., Martínez O., Valenzuela F., Silva F., Valenzuela O. Hydroxychloroquine and chloroquine in COVID-19: Should they be used as standard therapy? Clin. Rheumatol. 2020;39:2461–2465. doi: 10.1007/s10067-020-05202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Colson P., Rolain J.-M., Lagier J.-C., Brouqui P., Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int. J. Antimicrob. Agents. 2020;55:105932. doi: 10.1016/j.ijantimicag.2020.105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pandey A., Nikam A.N., Shreya A.B., Mutalik S.P., Gopalan D., Kulkarni S., Padya B.S., Fernandes G., Mutalik S., Prassl R. Potential therapeutic targets for combating SARS-CoV-2: Drug repurposing, clinical trials and recent advancements. Life Sci. 2020;256:117883. doi: 10.1016/j.lfs.2020.117883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Olchanski N., Hansen R.N., Pope E., D’Cruz B., Fergie J., Goldstein M., Krilov L.R., McLaurin K.K., Nabrit-Stephens B., Oster G., et al. Palivizumab Prophylaxis for Respiratory Syncytial Virus: Examining the Evidence Around Value. Open Forum Infect. Dis. 2018;5:ofy031. doi: 10.1093/ofid/ofy031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Haim H., Si Z., Madani N., Wang L., Courter J.R., Princiotto A., Kassa A., Degrace M., McGee-Estrada K., Mefford M., et al. Soluble CD4 and CD4-Mimetic Compounds Inhibit HIV-1 Infection by Induction of a Short-Lived Activated State. PLoS Pathog. 2009;5:e1000360. doi: 10.1371/journal.ppat.1000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Roma-Rodrigues C., Pombo I., Raposo L., Pedrosa P., Fernandes A.R., Baptista P. Nanotheranostics Targeting the Tumor Microenvironment. Front. Bioeng. Biotechnol. 2019;7:197. doi: 10.3389/fbioe.2019.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bidram E., Esmaeili Y., Amini A., Sartorius R., Tay F.R., Shariati L., Makvandi P. Nanobased Platforms for Diagnosis and Treatment of COVID-19: From Benchtop to Bedside. ACS Biomater. Sci. Eng. 2021 doi: 10.1021/acsbiomaterials.1c00318. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Madanayake N.H., Rienzie R., Adassooriya N.M. Nanotheranostics. Springer; Berlin/Heidelberg, Germany: 2019. Nanoparticles in Nanotheranostics Applications; pp. 19–40. [Google Scholar]
- 147.Lanfranco R., Jana P.K., Tunesi L., Cicuta P., Mognetti B.M., Di Michele L., Bruylants G. Kinetics of Nanoparticle–Membrane Adhesion Mediated by Multivalent Interactions. Langmuir. 2019;35:2002–2012. doi: 10.1021/acs.langmuir.8b02707. [DOI] [PubMed] [Google Scholar]
- 148.Wong X.Y., Sena-Torralba A., Álvarez-Diduk R., Muthoosamy K., Merkoçi A. Nanomaterials for Nanotheranostics: Tuning Their Properties According to Disease Needs. ACS Nano. 2020;14:2585–2627. doi: 10.1021/acsnano.9b08133. [DOI] [PubMed] [Google Scholar]
- 149.Palazzolo S., Bayda S., Hadla M., Caligiuri I., Corona G., Toffoli G., Rizzolio F. The Clinical Translation of Organic Nanomaterials for Cancer Therapy: A Focus on Polymeric Nanoparticles, Micelles, Liposomes and Exosomes. Curr. Med. Chem. 2018;25:4224–4268. doi: 10.2174/0929867324666170830113755. [DOI] [PubMed] [Google Scholar]
- 150.Kang K.W., Song M.G. Radionanomedicine. Springer; Berlin/Heidelberg, Germany: 2018. Organic nanomaterials: Liposomes, albumin, dendrimer, polymeric nanoparticles; pp. 105–123. [DOI] [Google Scholar]
- 151.Öncel M.Ö.Ö., Garipcan B., Inci F. Biomimetic Lipid Membranes: Fundamentals, Applications, and Commercialization. Springer; Berlin/Heidelberg, Germany: 2019. Biomedical Applications: Liposomes and Supported Lipid Bilayers for Diagnostics, Theranostics, Imaging, Vaccine Formulation, and Tissue Engineering; pp. 193–212. [Google Scholar]
- 152.Serrano G., Mullor J.L., Sanchez A.V., Kochergina I., Albors A., Serrano J.M., Hueso G., Oroval M., Mir S., Mor I. Liposomal Lactoferrin Effect in Preventing SARS-CoV-2 Binding in HACAT Keratinocytes. Int. J. Res. Health Sci. 2020;8:16–21. doi: 10.5530/ijrhs.8.2.1. [DOI] [Google Scholar]
- 153.Serrano G., Kochergina I., Albors A., Diaz E., Oroval M., Hueso G., Serrano J.M. Liposomal Lactoferrin as Potential Preventative and Cure for COVID-19. Int. J. Res. Health Sci. 2020;8:08–15. doi: 10.5530/ijrhs.8.1.3. [DOI] [Google Scholar]
- 154.Han J., Zhao D., Li D., Wang X., Jin Z., Zhao K. Polymer-Based Nanomaterials and Applications for Vaccines and Drugs. Polymers. 2018;10:31. doi: 10.3390/polym10010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yadav H.K., Almokdad A.A., Shaluf S.I., Debe M.S. Nanocarriers for Drug Delivery. Elsevier; Amsterdam, The Netherlands: 2019. Polymer-Based Nanomaterials for Drug-Delivery Carriers; pp. 531–556. [Google Scholar]
- 156.Jamaledin R., Sartorius R., Di Natale C., Vecchione R., De Berardinis P., Netti P.A. Recombinant Filamentous Bacteriophages Encapsulated in Biodegradable Polymeric Microparticles for Stimulation of Innate and Adaptive Immune Responses. Microorganisms. 2020;8:650. doi: 10.3390/microorganisms8050650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sinclair T., Patil A., Raza B., Reurink D., Hengel S.V.D., Rutjes S., Husman A.D.R., Roesink H., de Vos W. Cationically modified membranes using covalent layer-by-layer assembly for antiviral applications in drinking water. J. Membr. Sci. 2019;570–571:494–503. doi: 10.1016/j.memsci.2018.10.081. [DOI] [Google Scholar]
- 158.Mehta P., Kadam S., Pawar A., Bothiraja C. Dendrimers for pulmonary delivery: Current perspectives and future challenges. New J. Chem. 2019;43:8396–8409. doi: 10.1039/C9NJ01591D. [DOI] [Google Scholar]
- 159.Jones S.T., Cagno V., Janeček M., Ortiz D., Gasilova N., Piret J., Gasbarri M., Constant D.A., Han Y., Vuković L., et al. Modified cyclodextrins as broad-spectrum antivirals. Sci. Adv. 2020;6:eaax9318. doi: 10.1126/sciadv.aax9318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Braga S.S. Cyclodextrins: Emerging Medicines of the New Millennium. Biomolecules. 2019;9:801. doi: 10.3390/biom9120801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Du T., Lu J., Liu L., Dong N., Fang L., Xiao S., Han H. Antiviral Activity of Graphene Oxide–Silver Nanocomposites by Preventing Viral Entry and Activation of the Antiviral Innate Immune Response. ACS Appl. Bio Mater. 2018;1:1286–1293. doi: 10.1021/acsabm.8b00154. [DOI] [PubMed] [Google Scholar]
- 162.Ye S., Shao K., Li Z., Guo N., Zuo Y., Li Q., Lu Z., Chen L., He Q., Han H. Antiviral Activity of Graphene Oxide: How Sharp Edged Structure and Charge Matter. ACS Appl. Mater. Interfaces. 2015;7:21571–21579. doi: 10.1021/acsami.5b06876. [DOI] [PubMed] [Google Scholar]
- 163.Yang X.X., Li C.M., Li Y.F., Wang J., Huang C.Z. Synergistic antiviral effect of curcumin functionalized graphene oxide against respiratory syncytial virus infection. Nanoscale. 2017;9:16086–16092. doi: 10.1039/C7NR06520E. [DOI] [PubMed] [Google Scholar]
- 164.Donskyi I.S., Azab W., Cuellar-Camacho J.L., Guday G., Lippitz A., Unger W.E., Osterrieder K., Adeli M., Haag R. Functionalized nanographene sheets with high antiviral activity through synergistic electrostatic and hydrophobic interactions. Nanoscale. 2019;11:15804–15809. doi: 10.1039/C9NR05273A. [DOI] [PubMed] [Google Scholar]
- 165.Ziem B., Rahn J., Donskyi I., Silberreis K., Cuellar L., Dernedde J., Keil G., Mettenleiter T.C., Haag R. Polyvalent 2D Entry Inhibitors for Pseudorabies and African Swine Fever Virus. Macromol. Biosci. 2017;17:1600499. doi: 10.1002/mabi.201600499. [DOI] [PubMed] [Google Scholar]
- 166.Deokar A.R., Nagvenkar A.P., Kalt I., Shani L., Yeshurun Y., Gedanken A., Sarid R. Graphene-Based “Hot Plate” for the Capture and Destruction of the Herpes Simplex Virus Type 1. Bioconjugate Chem. 2017;28:1115–1122. doi: 10.1021/acs.bioconjchem.7b00030. [DOI] [PubMed] [Google Scholar]
- 167.Dubrovskaya V., Tran K., Ozorowski G., Guenaga J., Wilson R., Bale S., Cottrell C., Turner H.L., Seabright G., O’Dell S., et al. Vaccination with Glycan-Modified HIV NFL Envelope Trimer-Liposomes Elicits Broadly Neutralizing Antibodies to Multiple Sites of Vulnerability. Immunity. 2019;51:915–929.e7. doi: 10.1016/j.immuni.2019.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Di J., Xie F., Xu Y. When liposomes met antibodies: Drug delivery and beyond. Adv. Drug Deliv. Rev. 2020;154-155:151–162. doi: 10.1016/j.addr.2020.09.003. [DOI] [PubMed] [Google Scholar]
- 169.Palmieri V., Papi M. Can graphene take part in the fight against COVID-19? Nano Today. 2020;33:100883. doi: 10.1016/j.nantod.2020.100883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Salomon N., Vascotto F., Selmi A., Vormehr M., Quinkhardt J., Bukur T., Schrörs B., Löewer M., Diken M., Türeci Ö., et al. A liposomal RNA vaccine inducing neoantigen-specific CD4+ T cells augments the antitumor activity of local radiotherapy in mice. OncoImmunology. 2020;9:1771925. doi: 10.1080/2162402X.2020.1771925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Evelyn Roopngam P. Liposome and polymer-based nanomaterials for vaccine applications. Nanomed. J. 2019;6:1–10. [Google Scholar]
- 172.Veeranarayanan S., Maekawa T. External stimulus responsive inorganic nanomaterials for cancer theranostics. Adv. Drug Deliv. Rev. 2019;138:18–40. doi: 10.1016/j.addr.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 173.Bose A., Wong T.W. Nanotechnology-Enabled Drug Delivery for Cancer Therapy. Nanotechnol. Appl. Tissue Eng. 2015:173–193. doi: 10.1016/b978-0-323-32889-0.00011-x. [DOI] [Google Scholar]
- 174.Manivannan S., Ponnuchamy K. Quantum dots as a promising agent to combat COVID-19. Appl. Organomet. Chem. 2020;34:174. doi: 10.1002/aoc.5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.López-Lorente Á.I., Valcárcel M. Comprehensive Analytical Chemistry. Volume 66. Elsevier; Amsterdam, The Netherlands: 2014. Analytical Nanoscience and Nanotechnology; pp. 3–35. [Google Scholar]
- 176.Halbus A.F., Horozov T.S., Paunov V.N. Colloid particle formulations for antimicrobial applications. Adv. Colloid Interface Sci. 2017;249:134–148. doi: 10.1016/j.cis.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 177.Huo C., Xiao J., Xiao K., Zou S., Wang M., Qi P., Liu T., Hu Y. Pre-Treatment with Zirconia Nanoparticles Reduces Inflammation Induced by the Pathogenic H5N1 Influenza Virus. Int. J. Nanomed. 2020;15:661–674. doi: 10.2147/IJN.S221667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Read S.A., Obeid S., Ahlenstiel C., Ahlenstiel G. The Role of Zinc in Antiviral Immunity. Adv. Nutr. 2019;10:696–710. doi: 10.1093/advances/nmz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chen S., Hao X., Liang X., Zhang Q., Zhang C., Zhou G., Shen S., Jia G., Zhang J. Inorganic Nanomaterials as Carriers for Drug Delivery. J. Biomed. Nanotechnol. 2016;12:1–27. doi: 10.1166/jbn.2016.2122. [DOI] [PubMed] [Google Scholar]
- 180.Lee M.-Y., Yang J.-A., Jung H.S., Beack S., Choi J.E., Hur W., Koo H., Kim K., Yoon S.K., Hahn S.K. Hyaluronic acid–gold nanoparticle/interferon α complex for targeted treatment of hepatitis C virus infection. ACS Nano. 2012;6:9522–9531. doi: 10.1021/nn302538y. [DOI] [PubMed] [Google Scholar]
- 181.Kumar R., Nayak M., Sahoo G.C., Pandey K., Sarkar M.C., Ansari Y., Das V., Topno R., Bhawna, Madhukar M., et al. Iron oxide nanoparticles based antiviral activity of H1N1 influenza A virus. J. Infect. Chemother. 2019;25:325–329. doi: 10.1016/j.jiac.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 182.Abo-Zeid Y., Ismail N.S., McLean G.R., Hamdy N.M. A molecular docking study repurposes FDA approved iron oxide nanoparticles to treat and control COVID-19 infection. Eur. J. Pharm. Sci. 2020;153:105465. doi: 10.1016/j.ejps.2020.105465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Salleh A., Naomi R., Utami N.D., Mohammad A.W., Mahmoudi E., Mustafa N., Fauzi M.B. The Potential of Silver Nanoparticles for Antiviral and Antibacterial Applications: A Mechanism of Action. Nanomaterials. 2020;10:1566. doi: 10.3390/nano10081566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Villeret B., Dieu A., Straube M., Solhonne B., Miklavc P., Hamadi S., Le Borgne R., Mailleux A., Norel X., Aerts J., et al. Silver Nanoparticles Impair Retinoic Acid-Inducible Gene I-Mediated Mitochondrial Antiviral Immunity by Blocking the Autophagic Flux in Lung Epithelial Cells. ACS Nano. 2018;12:1188–1202. doi: 10.1021/acsnano.7b06934. [DOI] [PubMed] [Google Scholar]
- 185.Shahabadi N., Khorshidi A., Zhaleh H., Kashanian S. Synthesis, characterization, cytotoxicity and DNA binding studies of Fe3O4@ SiO2 nanoparticles coated by an antiviral drug lamivudine. J. Drug Deliv. Sci. Technol. 2018;46:55–65. doi: 10.1016/j.jddst.2018.04.016. [DOI] [Google Scholar]
- 186.Croci R., Bottaro E., Chan K.W.K., Watanabe S., Pezzullo M., Mastrangelo E., Nastruzzi C. Liposomal Systems as Nanocarriers for the Antiviral Agent Ivermectin. Int. J. Biomater. 2016;2016:1–15. doi: 10.1155/2016/8043983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wang S.X., Michiels J., Ariën K.K., New R., Vanham G., Roitt I. Inhibition of HIV Virus by Neutralizing Vhh Attached to Dual Functional Liposomes Encapsulating Dapivirine. Nanoscale Res. Lett. 2016;11:1–10. doi: 10.1186/s11671-016-1558-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Vaillant A. Nucleic acid polymers: Broad spectrum antiviral activity, antiviral mechanisms and optimization for the treatment of hepatitis B and hepatitis D infection. Antivir. Res. 2016;133:32–40. doi: 10.1016/j.antiviral.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 189.Munoz-Fernandez M.A., Vacas-Córdoba E., Malý M., De La Mata F.J., Gomez R., Pion M. Antiviral mechanism of polyanionic carbosilane dendrimers against HIV-1. Int. J. Nanomed. 2016;11:1281–1294. doi: 10.2147/IJN.S96352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Fröhlich T., Hahn F., Belmudes L., Leidenberger M., Friedrich O., Kappes B., Couté Y., Marschall M., Tsogoeva S.B. Synthesis of artemisinin-derived dimers, trimers and dendrimers: Investigation of their antimalarial and antiviral activities including putative mechanisms of action. Chem. Eur. J. 2018;24:8103–8113. doi: 10.1002/chem.201800729. [DOI] [PubMed] [Google Scholar]
- 191.Paull J.R., Castellarnau A., Luscombe C.A., Fairley J.K., Heery G.P. Astodrimer sodium, dendrimer antiviral, in-hibits replication of SARS-CoV-2 in vitro. Biorxiv. 2020 doi: 10.1101/2020.08.20.260190. [DOI] [Google Scholar]
- 192.Chen Y.-N., Hsueh Y.-H., Hsieh C.-T., Tzou D.-Y., Chang H.-T. Antiviral Activity of Graphene–Silver Nanocomposites against Non-Enveloped and Enveloped Viruses. Int. J. Environ. Res. Public Health. 2016;13:430. doi: 10.3390/ijerph13040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zacheo A., Hodek J., Witt D., Mangiatordi G.F., Ong Q.K., Kocabiyik O., DePalo N., Fanizza E., Laquintana V., Denora N., et al. Multi-sulfonated ligands on gold nanoparticles as virucidal antiviral for Dengue virus. Sci. Rep. 2020;10:1–9. doi: 10.1038/s41598-020-65892-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Peña-González C.E., García-Broncano P., Ottaviani M.F., Cangiotti M., Fattori A., Hierro-Oliva M., González-Martín M.L., Pérez-Serrano J., Gómez R., Muñoz-Fernández M.Á. Dendronized anionic gold nanoparticles: Synthesis, characterization, and antiviral activity. Chem. Eur. J. 2016;22:2987–2999. doi: 10.1002/chem.201504262. [DOI] [PubMed] [Google Scholar]
- 195.Levina A.S., Repkova M.N., Bessudnova E.V., Filippova E.I., Mazurkova N.A., Zarytova V.F. High antiviral effect of TiO2·PL–DNA nanocomposites targeted to conservative regions of (−) RNA and (+) RNA of influenza A virus in cell culture. Beilstein J. Nanotechnol. 2016;7:1166–1173. doi: 10.3762/bjnano.7.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Akhtar S., Shahzad K., Mushtaq S., Ali I., Rafe M.H., Fazal-Ul-Karim S.M. Antibacterial and antiviral potential of colloidal Titanium dioxide (TiO2) nanoparticles suitable for biological applications. Mater. Res. Express. 2019;6:105409. doi: 10.1088/2053-1591/ab3b27. [DOI] [Google Scholar]
- 197.Ghaffari H., Tavakoli A., Moradi A., Tabarraei A., Bokharaei-Salim F., Zahmatkeshan M., Farahmand M., Javanmard D., Kiani S.J., Esghaei M., et al. Inhibition of H1N1 influenza virus infection by zinc oxide nanoparticles: Another emerging application of nanomedicine. J. Biomed. Sci. 2019;26:1–10. doi: 10.1186/s12929-019-0563-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ishida T. Antiviral Activities of Cu2+ Ions in Viral Prevention, Replication, RNA Degradation, and for Antiviral Efficacies of Lytic Virus, ROS-Mediated Virus, Copper Chelation. World Sci. News. 2018;99:148–168. [Google Scholar]
- 199.Tavakoli A., Hashemzadeh M.S. Inhibition of herpes simplex virus type 1 by copper oxide nanoparticles. J. Virol. Methods. 2020;275:113688. doi: 10.1016/j.jviromet.2019.113688. [DOI] [PubMed] [Google Scholar]
- 200.El-Sheekh M.M., Shabaan M.T., Hassan L., Morsi H.H. Antiviral activity of algae biosynthesized silver and gold nanoparticles against Herps Simplex (HSV-1) virus in vitro using cell-line culture technique. Int. J. Environ. Health Res. 2020:1–12. doi: 10.1080/09603123.2020.1789946. [DOI] [PubMed] [Google Scholar]
- 201.Agnihothram S.S., Vermudez S.A., Mullis L., Townsend T.A., Manjanatha M.G., Azevedo M.P. Silicon Dioxide Impedes Antiviral Response and Causes Genotoxic Insult During Calicivirus Replication. J. Nanosci. Nanotechnol. 2016;16:7720–7730. doi: 10.1166/jnn.2016.12828. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
In this section, please provide details regarding where data.