Abstract
Positive valence system (PVS) deficits are increasingly recognized as important treatment targets for depression and anxiety. Emerging behavioral treatments designed to upregulate the PVS show initial promise; however, neural mechanisms underlying these approaches remain unknown. This study investigated neural reward-processing-related changes following Amplification of Positivity (AMP)—a treatment designed to enhance positive thinking, emotions and behaviors through positive activity interventions (Clinicaltrials.gov:NCT02330627).
Individuals with depression and/or anxiety (N=29) were randomized to 10 sessions of AMP (n=16) or waitlist (WL;n=13). Participants completed a monetary incentive delay task during fMRI at baseline and post-assessment. Hypothesis-driven region of interest (ventral striatum, insula, anterior cingulate) and exploratory whole-brain activation and connectivity analyses evaluated pre-to-post changes for AMP vs. WL when anticipating potential monetary gain or loss.
No between-group brain activation changes emerged in regions of interest or whole-brain analyses. Increased neural connectivity from pre-to-post-treatment was observed in AMP vs. WL, including ventral striatum, anterior insula, and anterior cingulate connectivity with prefrontal, limbic, occipital and parietal regions—predominantly during loss anticipation.
This preliminary study is the first to examine neural mechanisms of positive activity interventions in depression and anxiety and suggests that AMP may strengthen brain connectivity in reward processing, attention, and emotion regulation networks.
Keywords: positive affect, clinical trial, reward processing, fMRI, depression, anxiety
Introduction
The positive valance system (PVS) and its role in depression and anxiety treatments is garnering increasing interest (Craske et al., 2016; Insel et al., 2010). The PVS is characterized by positive emotions (e.g., joy, awe) and cognitions (e.g., attentional deployment toward reward-relevant stimuli), and generates approach behaviors toward potentially rewarding stimuli (Fredrickson, 2013). Deficits in the PVS are common in depression and anxiety (Craske et al., 2016; Dillon et al., 2014), yet are not sufficiently addressed by current treatments (Dunn et al., 2020). Emerging behavioral interventions targeting the PVS in samples of adults with depression and/or anxiety disorders have shown to be efficacious at increasing positive affect as well reducing negative affect and depression and anxiety symptoms (Craske et al., 2019; Dunn et al., 2019; Taylor et al., 2017). Theoretical models suggest that brain circuits related to reward processing may be targeted in these treatments (Craske et al., 2016; Layous et al., 2011a), yet this hypothesis has not yet been empirically evaluated and the neural mechanisms of interventions that target the PVS remain poorly understood. Understanding such mechanisms is an important step toward refining interventions and creating more targeted treatments, and is a prominent direction in current mental health research efforts (Insel, 2014). To this end, we evaluated neural reward-processing-related changes following a randomized controlled trial (RCT) of Amplification of Positivity (AMP), a novel intervention that targets the PVS via engagement in positive activities, which demonstrated large and significant symptom improvements and increased positive affect among AMP, compared to WL (Cohen’s d range=|.94–1.57|; Taylor et al., 2017).
Reward processing mechanisms are thought to enable optimal PVS functioning by generating approach behaviors in the context of anticipating and receiving positive (rewarding) outcomes (Berridge et al., 2015) as well as motivation and sustained engagement with the environment. Brain regions involved in reward processing include limbic (e.g., striatum, amygdala) and prefrontal (e.g., anterior cingulate, insula, orbitofrontal cortex) structures (Berridge et al., 2015). These areas evince increased activation (Oldham et al., 2018) and connectivity (Cho et al., 2013; Gu et al., 2019) in the context of potential rewards as well as losses (i.e., removal of rewards; Camara et al., 2009; Cho et al., 2013).
Dysregulation of reward processing networks may be linked to clinical manifestations of PVS deficits. This includes losing the desire to engage in pleasurable activities and/or loss of enjoyment of such activities (i.e., anhedonia; Snaith, 1993), which correspond to the anticipation and consumption phases of reward processing, respectively, as well as expecting to experience less positive affect in anticipation of future positive events (Hoerger et al., 2012). Such deficits are prevalent in depression (Pelizza et al., 2009; Watson et al., 2010) with anhedonia specifically being a core diagnostic feature of depression. Anxiety disorders also often present with PVS deficits (Hopper et al., 2008; Kashdan et al., 2011; Prenoveau et al., 2010); most commonly in Generalized Anxiety Disorder and Social Anxiety Disorder, compared to Panic Disorder and Specific Phobias. Meta-analytic work has suggested that dysregulated corticostriatal connectivity may underlie reward processing deficits in MDD broadly (Ng et al., 2019), and some studies have linked PVS deficits to aberrant brain patterns such as reduced ventral striatum activation during reward anticipation specifically (Chung et al., 2015; Stoy et al., 2012; Ubl et al., 2015). Furthermore, anhedonia has also been related to neural hypoconnectivity of reward circuits during rest (Pornpattananangkul et al., 2019). Importantly, both anhedonia and anxious arousal have been shown to moderate prediction-error-related ventral striatum activation when a reward is expected (Greenberg et al., 2015), which highlights the need for transdiagnostic samples.
AMP (Taylor et al., 2017) leverages positive activity interventions (Layous et al., 2014) to address PVS deficits by increasing exposure and reactivity to pleasurable, engaging and meaningful events, including amplifying positive experiences in-the-moment (e.g., savoring) or memories thereof (e.g., reminiscing; sharing positive events with others). AMP also includes activities to increase awareness of positive outcomes (e.g., gratitude, strengths) and approach motivation (e.g., acts of kindness, make someone else happier). Together, these activities are intended to increase the salience of future opportunities for reward (i.e., positive emotions) and desire for such rewards, thereby targeting reward anticipation processes. Because patients with depression or anxiety tend to focus on negative outcomes (e.g., rumination, worry), AMP strategies also focus on attending to and capitalizing on positive outcomes which necessitates attention redirection in the context of anticipating or experiencing negative outcomes (e.g., loss or punishment). Successful implementation of AMP components in an individual’s daily life and experiencing associated symptom improvement is presumably mediated by changes in reward-processing-related brain networks. In support of that proposition, several studies documented that non-clinical samples demonstrate increased activation in neural reward circuitry while engaging in specific positive activities such as recalling positive autobiographical memories (Speer et al., 2014), social sharing of emotions (Wagner et al., 2015), and engaging in altruistic actions (Moll et al., 2006). To our knowledge the neural mechanisms of positive activity interventions have not been examined in clinical samples of individuals with depression or anxiety.
In the present study we aimed to examine neural changes (activation and connectivity) following AMP in a transdiagnostic sample of patients seeking treatment for depression and/or anxiety (Taylor et al., 2017). As AMP specifically targets the PVS, a monetary incentive delay task shown to reliably probe reward processing circuits (Oldham et al., 2018) was used during fMRI acquisition to examine treatment-related changes. We focused on reward anticipation in the present study because the monetary incentive delay task is well established as probing anticipatory reward processing (Knutson & Greer, 2008) and because neural dysfunction during anticipation of rewards has been linked to PVS dysfunction (Stoy et al., 2012; Ubl et al., 2015).
Positive affect has been shown to be positively related to ventral striatum activation (Wu et al., 2014) and anhedonia has been linked to attenuated striatal activation during reward anticipation (Stoy et al., 2012; Ubl et al., 2015). We therefore hypothesized increased activation in the ventral striatum among individuals who completed AMP relative to waitlist controls during anticipation of potential monetary gains (i.e., rewards). We also examined whether AMP effects generalized to parallel measures of the negative valence system (i.e., neural reactivity to aversive outcomes). Based on evidence that negative affect is related to anterior insula activation during anticipation of losses (Wu et al., 2014), we hypothesized decreased activation in this region post-AMP. We also planned to examine activation in medial prefrontal cortex regions as these have also been implicated in reward processing (Oldman et al., 2018). To complement these region of interest (ROI) analyses, we performed additional exploratory whole-brain analyses evaluating treatment-related neural activation changes among patients who underwent AMP vs. waitlist. Finally, given research suggesting diminished functional connectivity among reward processing regions in depression and anxiety (Jung et al., 2013; Rupprechter et al., 2020), we also explored whether AMP altered brain connectivity.
Methods and Materials
Participants
Participants were treatment seeking adults (age 18–55) with clinically impairing depression and/or anxiety who participated in a randomized controlled trial (NCT02330627) evaluating Amplification of Positivity (AMP; Taylor et al., 2017). Recruitment procedures and sample characteristics were previously described in the outcomes report of this trial. To summarize, 29 participants were randomly allocated to a 10-session AMP group (n=16) or a waitlist (WL) condition (n=13); one participant in the AMP group discontinued treatment after session 7 due to changes in work commitment and therefore did not have a post-treatment MRI scan. One participant in the WL group was excluded from analyses due to having initiated treatment during the wait period. The present analyses therefore included 27 participants (AMP: n=15; WL: n=12). All participants had clinically elevated symptoms of depression (score ≥10 or higher on the Patient Health Questionnaire-9 [PHQ-9]) and/or anxiety (score ≥8 on the Overall Anxiety Severity and Impairment Scale [OASIS]), and 70% of the present sample had anhedonia levels that were more than one standard deviation above a community sample mean (measured via the Snaith Hamilton Pleasure Scale [SHAPS] (Franken et al., 2007). Across participants, 35% were comorbid for depression and anxiety, 23% met criteria for major depression only, and 42% had an anxiety disorder without depression. Additional recruitment details (including CONSORT diagram, Figure S1), and sample characteristics are described in the AMP outcomes report (Taylor et al., 2017) and have been summarized in the Supplemental Materials.
Amplification of Positivity Intervention
Individuals in the AMP group underwent 10 one-hour sessions of individual therapist-delivered treatment. Psychoeducation was provided in an introductory session on emotion science findings regarding the function of positive thoughts, emotions, and behaviors (Fredrickson, 1998, 2001, 2003, 2013; Garland et al., 2010). The core treatment exercises were designed to increase positive thinking, emotions, and/or behavior and were developed based on prior literature on positive affect interventions (Huffman et al., 2014; Huffman et al., 2011; Layous et al., 2014; Layous et al., 2011b; Lyubomirsky et al., 2013; Moskowitz et al., 2012). A summary of the treatment protocol is available in the Supplement (Table S2) and additional details are described in the outcomes report of this trial (Taylor et al., 2017). Waitlist participants completed the pre- and post-assessments at a 10-week interval and were offered AMP after the post-assessment.
Monetary Incentive Delay Task
All participants completed a monetary incentive delay (MID) task while undergoing fMRI acquisition at baseline and post-treatment (or after a 10-week wait period). This task probes neural responses to the anticipation and receipt of reward and loss outcomes and reliably activates a well-delineated neurocircuitry implicated in reward (e.g., ventral striatum) and loss processing (e.g., anterior insula) (Knutson & Greer, 2008; Oldham et al., 2018; Wu et al., 2014). On each trial, participants were presented with a cue indicating potential gains or losses and could either gain or avoid losing money by pressing a button with their index finger when a target was presented. Participants were not excluded based on handedness. Cues signaled the possibility of winning or losing $0.00, $1.00, or $5.00 resulting in six task conditions comprised of 15 trials each, totaling 90 trials. Each trial consisted of an anticipation phase, target presentation, and outcome presentation. The anticipation phase (4000ms) began with the presentation of one of six cue shapes (2000ms) and was followed by a crosshair (2000ms). Then, a white target was briefly presented to which participants were required to quickly respond via button press. Target duration was variable and set to 250ms at the beginning of the task, and then titrated such that participants succeeded on approximately 66% of their target responses. A delay followed (2000ms - duration of target presentation for any given trial). Finally, the outcome was presented (2000ms) notifying participants how much money they had gained or lost for that trial. Trials were separated by a variable inter-trial interval of 2000, 4000, or 6000ms. Prior to entering the scanner, participants were trained and tested for explicit cue comprehension, and shown the cash they could win during the task. All participants had a hit rate of at least 30%, indicating task adherence.
Neuroimaging Acquisition
Anatomical and functional brain images were acquired using a General Electric 3T MR750 Discovery MRI scanner and 8-channel head coil. Participants viewed task stimuli, which were projected onto a screen at the foot of the fMRI bed, via a mirror attached to the head coil. Participants used one button on a response box to respond to the target using their right hand. A 2D EPI pulse sequence acquired T2* blood oxygen level dependent (BOLD) images across 2 runs as 30 axial slices approximately parallel to the AC-PC line, with whole-brain coverage (voxel size=3.5x3.5x3.5mm, 275 image volumes per run, matrix size=64x64, TR=2s, TE=32ms, flip angle=70°, FOV=24mm). A spoiled gradient recalled (SPGR) sequence was used for acquiring anatomical T1-weighted images (172 slices; thickness=1mm; TI=450ms, TR=8ms, TE=3ms; matrix size=192x256; FOV=256cm; flip angle=12°; sagittal plane).
fMRI Data Preprocessing
Analysis of Functional NeuroImages (AFNI; https://afni.nimh.nih.gov/afni/) preprocessing protocols were implemented and included slice-time correction, functional image realignment, EPI/anatomical registration, and non-linear registration to the Talairach template, followed by 6mm spatial smoothing and voxelwise scaling into units of percent signal change. Image volume pairs with frame-wise displacement >.3mm were censored from individual level analyses. Mean frame-wise displacement (head motion) was <0.08mm across all participants.
fMRI Data Analysis
Broadly, our analyses evaluated whether neural changes (pre- to post-treatment) differed for AMP vs. WL in the context of anticipating potential monetary gain or loss. Neural responses to outcome presentation were modeled at the individual level to account for distinct psychological events. At the group level, only anticipation trials were evaluated because the MID task paradigm used in the presented study had a fixed interval (i.e., no jitter) between anticipation and outcome which precluded meaningful separation of BOLD signal in response to anticipation and outcome.
First-level models.
Individual-level general linear models were created to estimate brain activation and connectivity during anticipation, target presentation, and outcome periods. The regressor of interest during the anticipation period (i.e., Reward Condition: low gain, high gain, no gain, low loss, high loss, no loss) was convolved with AFNI’s ‘BLOCK’ function over 4000ms duration. Target and outcome presentation were also modeled by convolving their regressors with AFNI’s ‘BLOCK’ function over 2000ms each. Regressors for target presentation (i.e., Reward Condition) included the same conditions as for the anticipation period and regressors for outcome presentation included Performance (hit, miss) in addition to Reward Condition. Nuisance regressors were added across all individual-level models to account for head motion in the x, y, z, roll, pitch, yaw directions and fourth-degree polynomials to model low-frequency drift. The activation analysis output consisted of beta coefficients at each voxel for each condition during each task period (anticipation, target presentation, outcome presentation). Functional connectivity during the anticipation period was calculated via generalized psychophysiological interaction (gPPI) analysis (McLaren et al., 2012) at the individual-level. Reward processing related ROIs (see second-level analyses below) were used as connectivity seeds. These analyses produced voxel-wise images representing connectivity between the seed region and the rest of the brain for each anticipation condition.
Second-level models.
We used a two-pronged approach to address our study aims: 1) hypothesis-driven analyses evaluated neural activation in reward-processing-related regions of interest (ROIs), and 2) data-driven exploratory analyses evaluated whole-brain activation and connectivity.
ROI analyses.
ROIs were functionally defined based on regions that emerged as important in the context of the PVS and reward processing in prior work (Knutson, Bhanji, et al., 2008; Stoy et al., 2012; Ubl et al., 2015; Wu et al., 2014). Due to the unique composition of the present sample (transdiagnostic depression/anxiety), we generated ROI coordinates via peak activations representing the main effect of Reward Condition during reward anticipation across all participants at baseline (Nikolova et al., 2012), rather than relying on past large-scale studies evaluating neural activation during the MID task in non-patient samples (Oldham et al., 2018) or samples composed of patients with major depressive disorder only (Zhang et al., 2013).
As expected, activation peaks were identified in the left (−9,6,7) and right (12,6,3) ventral striatum and in the left (−30,20,10) and right (33,20,−1) anterior insula. Consistent with medial prefrontal regions shown to be involved in reward anticipation (Oldham et al., 2018; Vassena et al., 2014) an activation peak also emerged in the anterior cingulate cortex (5,31,31; peak was on the right but the cluster and sphere extended bilaterally). Seeds were created by drawing 8mm spheres around peak activation coordinates (Talairach space), and voxel activation values in each seed were averaged for each individual and then extracted for analysis in SPSS v. 26 (IBM; Armonk, NY). Conditions were collapsed to reduce the number of comparisons, resulting in three conditions (i.e., Gain [high/low average], Loss [high/low average, No Incentive [no gain/loss]). We created three condition contrasts (Gain vs. No Incentive, Loss vs. No Incentive, Gain vs. Loss) to directly compare task conditions in the MID task as has been done in prior studies (Oldham et al., 2018), including the work our hypotheses are based on (Wu et al., 2014). Repeated measures general linear models were used to evaluate the following interactions: Group × Time × (Gain vs. No Incentive), Group × Time × (Loss vs. No Incentive), Group × Time × (Gain vs. Loss). Exploratory analyses of the Group × Time and Group × Time × Condition interaction effects were also completed.
Whole-brain analyses.
AFNI’s 3dMVM program was used to build whole-brain ANOVA models and evaluate AMP vs. WL differences in pre to post neural activation and connectivity change for the same condition contrasts as in the ROI analyses. The interactions examined included Group × Time × (Gain vs. No Incentive), Group × Time × (Loss vs. No Incentive), Group × Time × (Gain vs. Loss). We completed additional exploratory analyses evaluating the full Group × Time × Condition model that yielded main effects and interactions between each of the factors. Separate models/analyses evaluated whole-brain activation and whole-brain connectivity for each of the five seeds. Each analysis was corrected for multiple comparisons on the whole-brain level, per the most recent recommendations on cluster correction (Cox, 2017). Cluster thresholds for each analysis were calculated via AFNI’s 3dClustSim using the mixed-model spatial autocorrelation function (-acf) and the NN1 2-sided option and resulted in a cluster extent threshold of k=29 at p<.05. We applied a conservative voxel-wise threshold of p<.005. Resulting clusters were visually inspected to identify their location in the brain and those that were fully situated in the white matter (see Table S3) were not further evaluated as they most likely represent noise. Effects of significant clusters situated in the gray matter were further examined by extracting each participant’s cluster values for post-hoc analyses in SPSS. Two-sample t-tests evaluated group differences at each time point and paired sample t-tests examined change over time within each group; FDR-correction was employed to correct post-hoc analyses for multiple comparisons within each cluster.
Results
Activation
Analyses of functionally defined ROIs (ventral striatum, insula, anterior cingulate) showed robust task effects (i.e., main effect of Condition with grater activation during gain and loss trials compared to no incentive trials) but yielded no significant findings for any of the interactions examined. Whole-brain activation analyses also evinced robust task effects (see Figure S2) but likewise no group differences over time for any contrast of interest.
Connectivity
Multiple connectivity clusters emerged for our contrasts of interest (i.e., Group × Time × [Gain vs. No Incentive], Group × Time × [Loss vs. No Incentive], Group × Time × [Gain vs. Loss)]. These results are described below and listed in Table 1. Selected clusters are displayed in Figures 1–3. Overall, AMP tended to demonstrate an increase in connectivity over time, whereas WL tended to show no change or a decrease in connectivity.
Table 1.
Significant clusters of interest resulting from whole brain analyses
|
Left Ventral Striatum Connectivity Group × Time × Gain vs. Loss |
||||||
| k | F1,26 | x | y | z | BA | Region |
| 42 | 34.6 | 30 | −71 | 28 | 39, 19 | Right Middle Occipital Gyrus |
|
Right Ventral Striatum Connectivity Group × Time × Loss vs. No Incentive |
||||||
| k | F1,26 | x | y | z | BA | Region |
| 31 | 25.6 | −19 | −61 | 17 | 31 | Left Cuneus |
|
Left Anterior Insula Connectivity Group × Time × Gain vs. No Incentive |
||||||
| k | F1,26 | x | y | z | BA | Region |
| 32 | 20.5 | 26 | −12 | 42 | 6 | Right Middle Frontal Gyrus |
| Group × Time × Loss vs. No Incentive | ||||||
| k | F1,26 | x | y | z | BA | Region |
| 35 | 16.7 | −16 | 20 | 3 | - | Left Ventral Striatum |
|
Right Anterior Insula Connectivity Group × Time × Loss vs. No Incentive |
||||||
| k | F1,26 | x | y | z | BA | Region |
| 52 | 21.5 | −12 | −96 | 3 | 18, 17 | Left Lingual Gyrus |
| 33 | 25 | −40 | 20 | 17 | 46 | Left Middle Frontal Gyrus |
| 31 | 21.7 | 30 | −75 | 28 | 31 | Right Middle Occipital Gyrus |
|
Anterior Cingulate Cortex Connectivity Group × Time × Loss vs. No Incentive |
||||||
| k | F1,26 | x | y | z | BA | Region |
| 109 | 32.5 | −16 | 17 | 7 | - | Left Ventral Striatum |
| 72 | 24.4 | 30 | −75 | 21 | 19, 7, 39 | Right Middle Occipital Gyrus |
| 54 | 24.8 | 5 | −68 | −1 | 18, 23 | Bilateral Cuneus |
| 43 | 22.1 | −16 | −71 | 28 | 19, 7 | Left Precuneus |
| 35 | 21.7 | 23 | 24 | 35 | 8 | Right Middle Frontal Gyrus |
| 29 | 21.1 | 12 | −57 | 59 | 7 | Right Precuneus |
BA=Brodmann area; Clusters significant at whole-brain-corrected threshold of p<.05 (see Method for details on cluster threshold); extracted values for bolded clusters are presented in Figures 1–3. A full list of clusters that emerged across all contrasts, including task effects, is available in the Supplement, Table S3.
Figure 1. Ventral Striatum Connectivity.
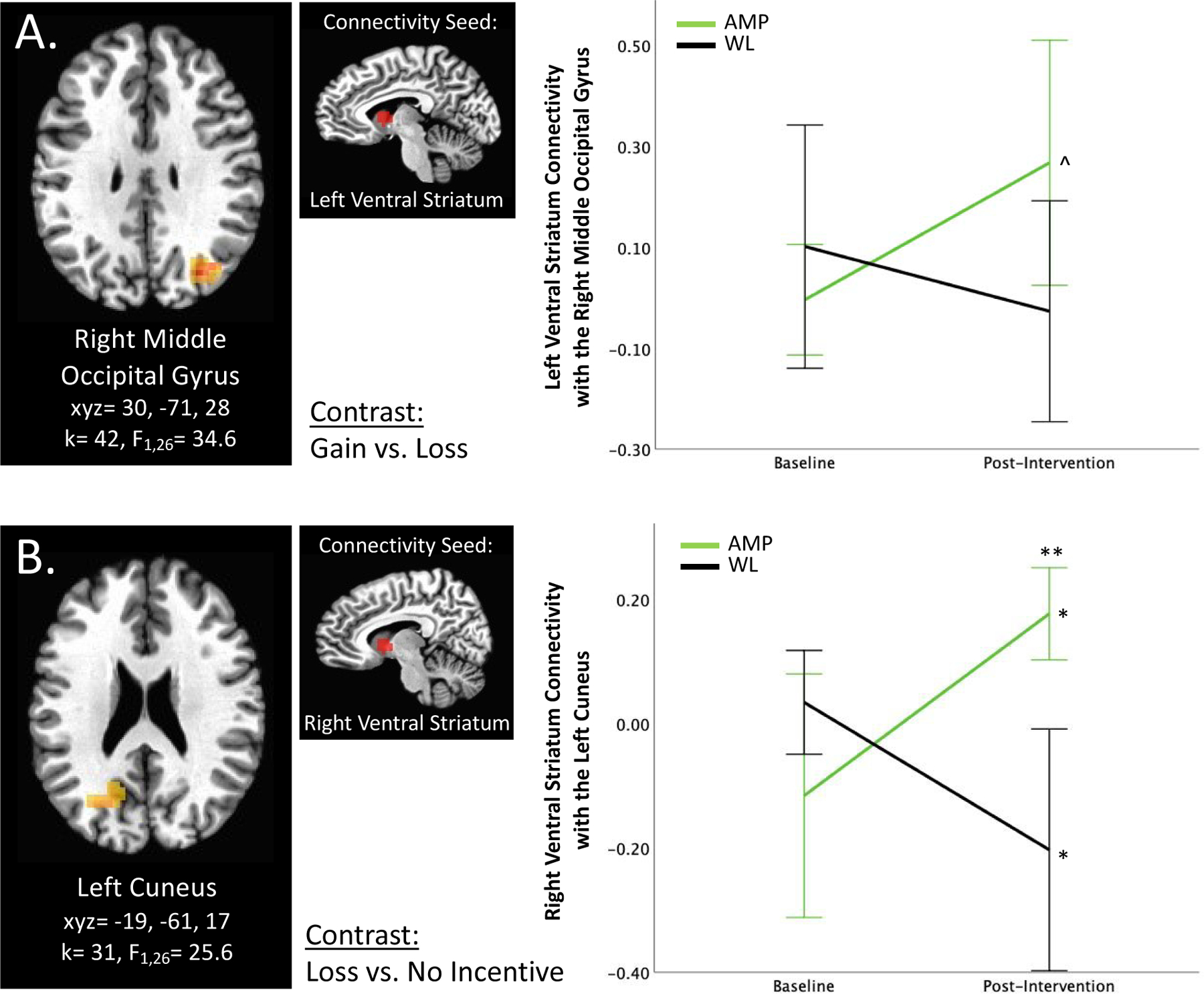
A) Group × Time × Gain vs. Loss interaction predicts left ventral striatum connectivity with the right middle occipital gyrus. B) Group × Time × Loss vs. No Incentive interaction predicts right ventral striatum connectivity with the left cuneus. *p<0.05, ** p<0.01, ^p<0.1, corrected. Error bars represent 95% confidence interval. AMP=treatment group that underwent Amplification of Positivity, WL=waitlist control group that did not undergo an intervention. For this and all figures, brain images represent axial sections (left=left) with threshold set at whole-brain-corrected p<.05.
Figure 3. Anterior Cingulate Cortex Connectivity.
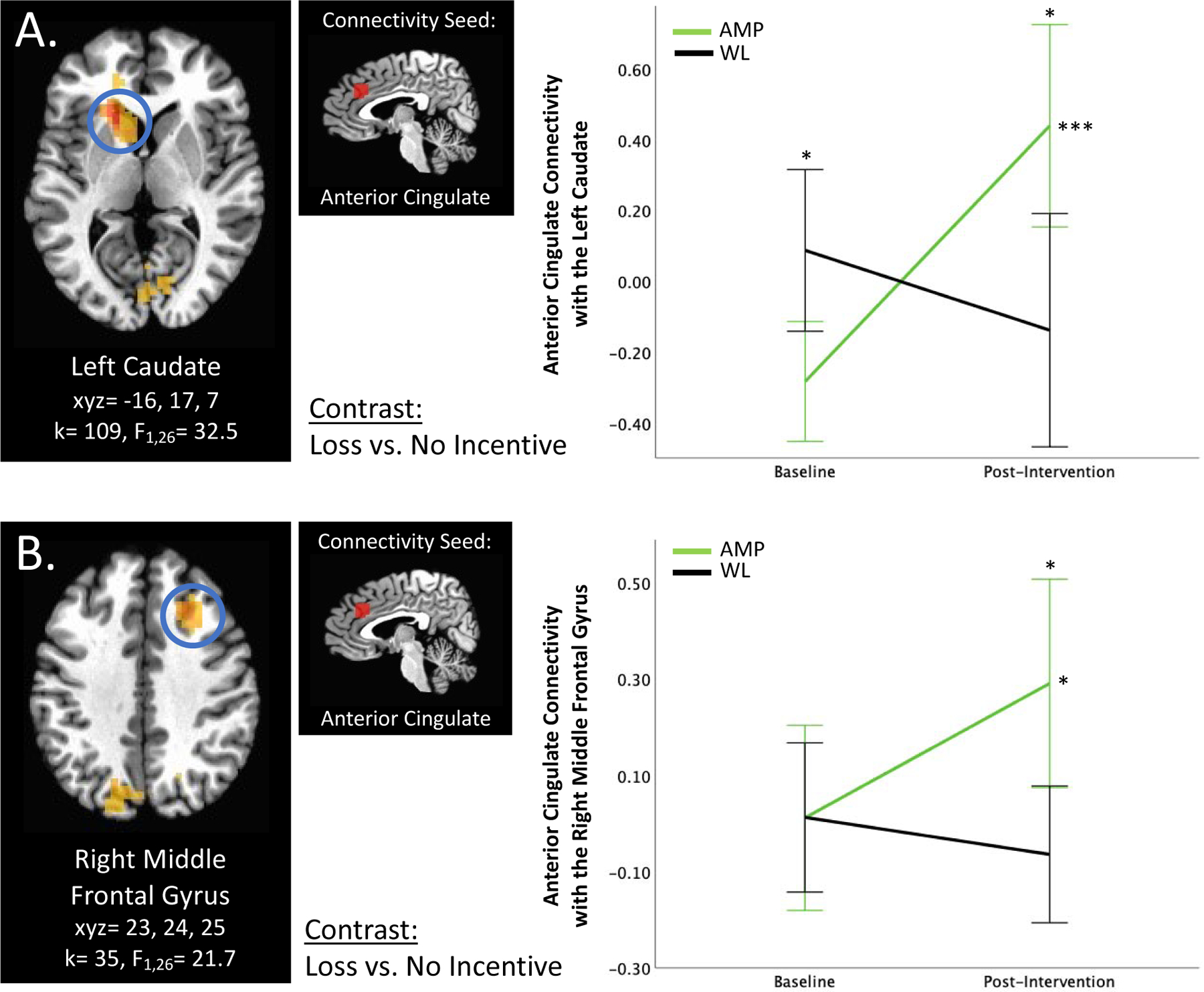
A) Group × Time × Loss vs. No Incentive interaction predicts left anterior cingulate connectivity with the left ventral striatum. B) Group × Time × Loss vs. No Incentive interaction predicts right anterior cingulate connectivity with the right middle frontal gyrus. *p<0.05, ***p<0.001, corrected. Error bars represent 95% confidence interval. AMP=treatment group that underwent Amplification of Positivity, WL=waitlist control group that did not undergo an intervention.
In the omnibus model (i.e., Group × Time × Condition), one cluster emerged for right ventral striatum connectivity with the right precuneus/cuneus (see Table S3); however, post-hoc analyses revealed no significant effects and this cluster is therefore not further discussed. There were no significant connectivity findings for any seed for the Group × Time interaction.
Ventral striatum connectivity.
Group × Time × Gain vs. Loss significantly predicted left ventral striatum connectivity with the right middle occipital gyrus (k=42, F1,26=34.6, xyz=30, −71, 28; Figure 1A). Connectivity between these areas increased in the AMP group over time but did not change in WL; however, post hoc analyses were no longer significant after FDR correction for multiple comparisons. Furthermore, Group × Time × Loss vs. No Incentive significantly predicted right ventral striatum connectivity with the left cuneus (k=31, F1,26=25.6, xyz=−19, −61, 17; Figure 1B). FDR-corrected post-hoc analyses indicated that although there were no connectivity differences between AMP and WL at baseline, AMP had significantly higher connectivity at the post-intervention time point relative to WL (p<0.01). Connectivity significantly increased in AMP (p<0.05) and significantly decreased in WL (p<0.05) over time.
Anterior insula connectivity.
Left anterior insula connectivity analyses revealed two significant clusters across two contrasts. Group × Time × Gain vs. No Incentive significantly predicted left anterior insula connectivity with the right middle frontal gyrus (k=32, F1,26=20.5, xyz=26, −12, 42; Figure 2A). Groups did not differ significantly at baseline but AMP demonstrated higher connectivity relative to WL at post-intervention (p<0.001); connectivity significantly increased in AMP (p<0.05) and significantly decreased in WL (p<0.001). Additionally, Group × Time × Loss vs. No Incentive significantly predicted left anterior insula connectivity with the left ventral striatum (k=35, F1,26=16.7, xyz=−16, 20, 3). Here, AMP vs. WL significantly differed at both baseline (p<0.05, AMP<WL) and post-intervention (p<0.05, AMP>WL), however AMP demonstrated a significant increase in connectivity (p<0.05) whereas WL showed a significant decrease (p<0.05) over time.
Figure 2. Anterior Insula Connectivity.
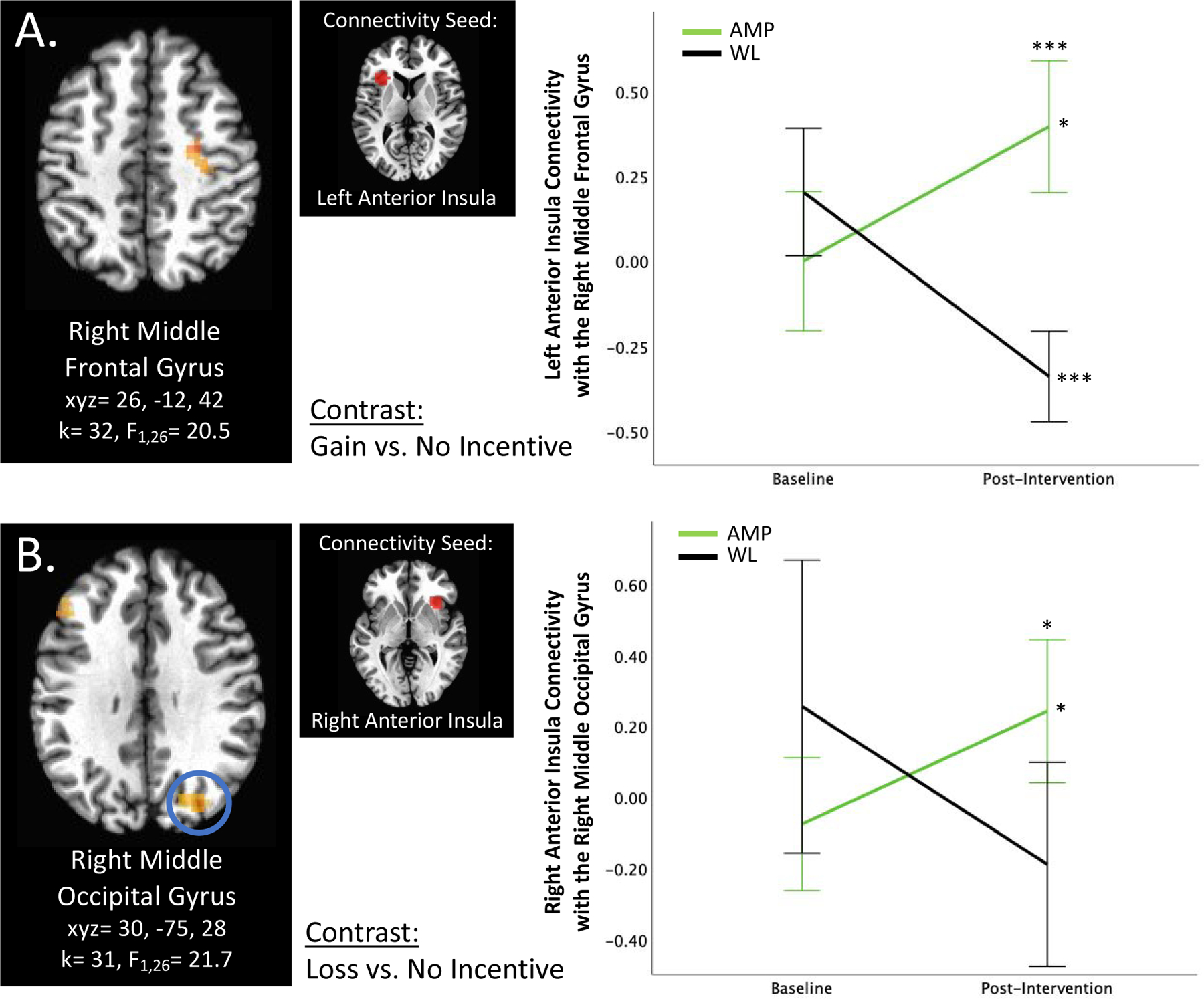
A) Group × Time × Gain vs. No Incentive interaction predicts left anterior insula connectivity with the right middle frontal gyrus. B) Group × Time × Loss vs. No Incentive interaction predicts right anterior insula connectivity with the right middle occipital gyrus. *p<0.05, ***p<0.001, corrected. Error bars represent 95% confidence interval. AMP=treatment group that underwent Amplification of Positivity, WL=waitlist control group that did not undergo an intervention.
Three clusters emerged for right anterior insula connectivity in the Group × Time × Loss vs. No Incentive contrast: the right middle occipital gyrus (k=31, F1,26=21.7, xyz=30, −75, 28; Figure 2B), the left lingual gyrus (k=52, F1,26=21.5, xyz=−12, −96, 3), and the left middle frontal gyrus (k=33, F1,26=25.0, xyz=−40, 20, 17). For all three clusters, post-hoc analyses revealed that AMP vs. WL did not differ significantly at baseline. For the right middle occipital gyrus cluster, AMP but not WL, increased in connectivity from baseline to post-intervention (p<0.05), and connectivity differed significantly between groups at post-intervention (p<0.05, AMP>WL). Groups also differed significantly in connectivity with the left lingual gyrus at post-intervention (p<0.05, AMP>WL), with AMP demonstrating no significant change in connectivity over time and WL a significant decrease in connectivity (p<0.05). AMP showed an increase in right anterior insula connectivity with the left middle frontal gyrus over time but after correcting for multiple comparisons this was no longer significant.
Anterior cingulate cortex connectivity.
Group × Time × Loss vs. No Incentive significantly predicted anterior cingulate cortex connectivity with six clusters: the left ventral striatum (k=109, F1,26=32.5, xyz=−16, 17, 7; Figure 3A), the right middle occipital gyrus (k=72, F1,26=24.4, xyz=30, −75, 21), the bilateral cuneus (k=54, F1,26=24.8, xyz=5, −68, −1), the left precuneus (k=43, F1,26=22.1, xyz=−16, −71, 28), the right middle frontal gyrus (k=35, F1,26=21.7, xyz=23, 24, 35; Figure 3B), and the right precuneus (k=29, F1,26=21.1, xyz=12, −57, 59). Anterior cingulate cortex connectivity with the left ventral striatum was significantly lower in AMP vs. WL at baseline; however, there was a significant increase in connectivity over time in AMP (p<0.001), but not in WL, such that AMP demonstrated significantly higher connectivity at post-intervention (p<0.05). Across the other five anterior cingulate cortex connectivity clusters, post-hoc analyses revealed that AMP did not differ from WL at baseline, that there was no change over time in connectivity among WL, but at least a trend-level increase in connectivity over time among AMP, such that AMP showed higher connectivity compared to WL at post-intervention; after correcting for multiple comparisons, this difference at the post-intervention timepoint remained significant (p<0.05) in the left precuneus, the right middle frontal gyrus, and the right precuneus gyrus.
Discussion
The present study examined potential treatment mechanisms of Amplification of Positivity (AMP), a novel intervention targeting the Positive Valence System (PVS) shown to increase positive affect and reduce symptoms in patients with depression and/or anxiety (Taylor et al., 2017). To this end, we examined neural pre- to post-treatment activation and connectivity changes in response to reward and loss anticipation in individuals who underwent AMP vs. a waitlist control group. Activation changes in reward-processing-related regions of interest did not emerge in our analyses, nor in our exploratory whole-brain activation analyses. However, our exploratory connectivity analyses revealed significant brain connectivity changes in reward processing and emotion regulation networks: connectivity increased among individuals who underwent AMP but not in waitlist controls. To our knowledge, this is the first study to examine neural treatment mechanisms of an integrated positive activity intervention protocol for depression and anxiety. Our findings suggest that improved functioning post-AMP may work through synchronization of reward-processing, attention, and emotion-regulation networks; formal mediation analyses in larger samples are needed to test this hypothesis.
Brain Activation Changes
Participants in our study displayed robust reward circuit activation at baseline. This finding suggests that the current sample may not be characterized by marked neural reward anticipation deficits, which may in part account for the lack of differential change in activation between treatment groups. Direct comparison to healthy controls would be necessary to establish this, however. Although some previous studies demonstrated reduced ventral striatum activation during reward anticipation in depression (e.g., (Ubl et al., 2015), others have failed to identify robust evidence of reward anticipation related neural circuit dysfunction in adult depression (Keren et al., 2018; Knutson, Bhanji, et al., 2008). It is possible that neural dysfunction in transdiagnostic samples of depression and anxiety is more pronounced in other facets of reward processing (e.g., responsiveness; Keren et al., 2018), which may be more sensitive to the effects of positive activity interventions, or that only a subset of patients with depression or anxiety (e.g., those characterized by anhedonia) display reward processing deficits in the context of reward anticipation (Reilly et al., 2020). Although the majority of participants (70%) experienced levels of anhedonia that were one standard deviation or greater from community normative levels, it is possible that the transdiagnostic nature of our sample prevented us from identifying specific reward anticipation deficits. Small samples such as ours are prone to sampling bias and are not sufficiently powered to detect medium or smaller treatment effects. Future research in larger samples is needed to reconcile those possibilities.
Connectivity Changes
Exploratory connectivity analyses revealed treatment-related connectivity changes among regions involved in reward processing (Oldham et al., 2018), including the ventral striatum, insula, anterior cingulate, and prefrontal cortex. Specifically, we observed increased reward-anticipation-related connectivity among individuals who underwent AMP vs. WL. Previous literature demonstrated reward-processing-related hypoconnectivity among patients with depression (Admon et al., 2015; Rupprechter et al., 2020) and although no previous studies to our knowledge have investigated connectivity during reward processing in anxiety, hypoconnectivity of reward circuits during rest has been documented in patients with anxiety disorders (Jung et al., 2013). Resting state hypoconnectivity is also linked to anhedonia (Pornpattananangkul et al., 2019). Several studies documented treatment-related connectivity changes in reward networks. For example, cortico-striatal connectivity increased in the context of drug treatment for depression and was linked to improved functioning (Admon et al., 2017). Furthermore, several studies have demonstrated normalization of connectivity between limbic and prefrontal regions following Cognitive Behavioral Therapy (for a review, see Young et al. 2018) and changes in the reward networks are also thought to underlie treatment related changes following Behavioral Activation for depression (Nagy et al., 2020), but have yet to be established. Based on these previous studies, our findings suggest that AMP may strengthen connectivity deficits in reward processing circuits and that these changes may be related to improved clinical outcomes.
Connectivity findings primarily emerged in the Loss vs. No Incentive contrasts. Changes in reward processing networks in response to loss anticipation are consistent with the boarder literature demonstrating engagement of reward processing networks in response to both gain and loss trials (Camara et al., 2009) and that reward network connectivity is predominantly seen during loss anticipation (Cho et al., 2013). How might AMP—a treatment targeted at upregulating positive affect and reward sensitivity—influence neural processing of potential losses? One possibility is that in this transdiagnostic sample of depression and anxiety loss avoidance is perceived as more salient or rewarding relative to gain acquisition (Alden et al., 2004). It is also possible that the monetary incentive delay task was unable to capture the effect that AMP aims to increase positive emotions by leveraging autobiographical events that are meaningful to the individual—a process that may not generalize to monetary reward expectation. Previous work documented that the specific brain areas recruited for reward appraisal depend on the type of reward, such that the regions recruited in the context of secondary reinforcers including monetary rewards do not completely overlap with regions recruited in the context of primary reinforcers (Sescousse et al., 2013). Evaluating neural changes in responses to positive events that are meaningful to the individual is an important research direction and may shed light on how responsivity to positive events changes in individuals who undergo AMP.
Altogether, our findings provide preliminary evidence that AMP may work by strengthening reward processing networks. This may occur by directly ameliorating dysfunction in those networks or through compensatory processes in other systems that support reward processing. Beyond their involvement in known reward processing networks, the specific connectivity clusters that emerged in our analyses can be separated into three broad functional networks: 1) increased ventral striatum, anterior insula, and anterior cingulate connectivity with occipital and parietal areas may be linked to pre- to post-AMP changes in stimulus driven attention and semantic representation; 2) increased anterior insula connectivity with the ventral striatum and lateral prefrontal cortex may be related to decreased loss aversion and increased readiness to engage with rewards; and 3) increased anterior cingulate connectivity with the ventral striatum and the lateral prefrontal cortex may be linked to improved automatic and voluntary emotion regulation. Each of these broad functional networks is discussed below.
Connectivity Changes with Occipital and Parietal Areas
We found that ventral striatum, anterior insula, and anterior cingulate connectivity with multiple occipital areas (e.g., middle occipital gyrus, cuneus, lingual gyrus) increased pre-to-post-treatment for AMP vs. WL. Aberrations in occipital areas have been documented in depression (Teng et al., 2018; Yue et al., 2013) and anxiety (Liao et al., 2010; Wang et al., 2018), and are predictive of symptom change post-treatment (Marwood et al., 2018). The occipital cortex is primarily implicated in visual processing but has also been shown to be involved in reward anticipation (Hangya et al., 2015; Shuler et al., 2006) and goal-directed and stimulus driven attention (Corbetta et al., 2002). A core component of AMP involves directing one’s attention toward positive experiences (i.e., emotions, thoughts, events) and therefore away from negative experiences (i.e., emotional reactions to an imminent, anticipated, or remembered negative event). Loss anticipation contexts in the MID may involve redirection of attention from anticipating possible monetary loss (negative experience) toward an opportunity to retain a reward (positive experience), thereby facilitating behavioral engagement to achieve desired outcomes. Thus, increased connectivity of reward related regions with occipital areas may map onto pre- to post-AMP shifts in attentional deployment toward the desired anticipated event (i.e., avoidance of loss) and away from the possibility of loss.
We also observed increased anterior cingulate connectivity with the bilateral precuneus during loss anticipation. Parietal regions such as the precuneus are thought to be involved in generating semantic representations of cues in the environment (Messina et al., 2016) and increased connectivity with the anterior cingulate post-AMP may indicate that AMP also works via changes in semantic connections—for example, changes in the perceived meaning of anticipating loss to an opportunity to retain what was gained.
Anterior Insula Connectivity Changes with the Middle Frontal Gyrus and Ventral Striatum
We also found that individuals who completed AMP relative to waitlist had increased pre-to-post anterior insula connectivity with the middle frontal gyrus and ventral striatum. The anterior insula is known to have connections to frontal and parietal areas involved in cognitive processes as well as to limbic areas involved in affective processes (Uddin et al., 2017). These networks have shown aberrant patterns in depression (Hamilton et al., 2012) and anxiety (Etkin et al., 2009). More specifically, the insula is involved in risky decision-making (Von Siebenthal et al., 2017) and loss aversion (Markett et al., 2016). Increased post-AMP connectivity within insula networks in the context of loss anticipation may therefore be associated with decreased loss aversion and contribute to symptom improvement. We also observed one cluster of insula connectivity with the middle frontal gyrus in the context of gain anticipation. The specific region of this middle frontal gyrus cluster overlaps with the premotor cortex involved in motor preparation. Given the insula’s involvement in detecting stimulus salience and initiating switches between default mode and central executive networks (Uddin, 2015), increased connectivity in the context of gain anticipation post-AMP may be related to increased readiness to engage in the task upon detecting a salient stimulus signaling a potentially rewarding outcome (i.e., monetary gain cue).
Anterior Cingulate Connectivity Changes with the Ventral Striatum and with Lateral Prefrontal Cortex
Anterior cingulate connectivity with the ventral striatum and with lateral prefrontal cortex regions during loss anticipation also emerged among AMP from pre- to post-treatment, relative to waitlist. The anterior cingulate is thought to regulate limbic regions such as the ventral striatum which are involved in generating emotional responses (Etkin et al., 2011). It has also been hypothesized to play an important role in cognitive control by engaging the lateral prefrontal cortex to resolve conflict (Carter et al., 2007) including in the context of anticipatory preparation for conflict (Sohn et al., 2007). Furthermore, the anterior cingulate seed region used in the present study is in an area of overlap thought to be related to both voluntary and automatic emotion regulation (Phillips et al., 2008). Taken together, increased anterior cingulate connectivity with the ventral striatum and lateral prefrontal areas among individuals who underwent AMP may mediate improved voluntary and automatic emotion regulation in face of a potential loss. This aligns with theoretical frameworks suggesting that positive activity interventions may work by improving emotion regulation (Quoidbach et al., 2015). CBT (Goldin et al., 2013; Sandman et al., 2020) and behavioral activation (Dichter et al., 2010; Mori et al., 2016) have also demonstrated neural treatment-related changes in limbic and prefrontal circuits that underlie emotion regulation suggesting possible common mechanisms between AMP and other psychosocial treatments.
Limitations
We would like to note several limitations of the present study. First, this study was powered a priori to detect only large treatment effects given the small planned sample size (N=30). This prevented us from being able to detect medium or smaller treatment effects that may be clinically meaningful (Cuijpers et al., 2014). We are also underpowered to examine whether connectivity changes relate to changes in positive affect and symptoms. Relatedly, although the overall trend of increased connectivity among AMP was consistent across clusters, it is important to note that in a subset of clusters at the post-hoc level these increases were no longer significant after correcting for multiple comparisons. It will be important to replicate our findings in larger samples that have enough power to examine the relation between brain and symptoms changes. The interpretations offered in this paper should until then be evaluated with caution. Second, we focused on reward anticipation. Future work could examine other phases of the reward processing cycle. Third, due to inherent limitations of pre-post designs it is unclear if the observed brain connectivity changes preceded clinical change. More frequent assessments of neural functioning and symptoms will be needed to establish the time course of changes. Finally, the present sample was selected based on meeting criteria for depression and/or anxiety disorders rather than dysfunctions in the PVS, such as anhedonia or neural response to reward. Examining neural changes following AMP in a sample with evidence of PVS dysfunction at baseline may shed further insight into specific treatment mechanisms. Overall, replication in larger samples is important and will facilitate examination of the relationship between brain and symptom changes as well as moderation effects with respect to baseline deficits.
Conclusion
Our findings contribute to a growing literature documenting neural mechanisms of treatment change in depression and anxiety disorders (Young et al., 2018) and answer calls for research to examine interventions that target the positive valence system specifically (Craske et al., 2016; Dunn et al., 2020; Layous et al., 2014). Although no significant activation differences emerged in our a priori analyses, exploratory seed-based connectivity analyses revealed increased connectivity in neural networks related to reward-processing, attention, and emotion regulation following AMP. The present study is the first to examine potential neural mechanisms of an intervention that targets the PVS through positive activity interventions and sets the stage for future work to further explore these mechanisms and predictors of response in larger samples. This work may eventually contribute to more fine-tuned, individualized treatments for target populations.
Supplementary Material
Highlights:
Depression and anxiety disorders evince positive valence system (PVS) dysfunction.
Amplification of Positivity (AMP) is a novel intervention targeting the PVS.
We examined the neural mechanisms of AMP during gain and loss anticipation.
There were no between-group brain activation changes.
Connectivity in reward and related brain networks strengthened in AMP vs. waitlist.
Acknowledgments
We would like to thank the many individuals who helped make this research possible: Sarah Pearlstein and Sarah Dowling for conducting diagnostic interviews and overseeing project management; Karalani Cross and Taylor Smith for overseeing project management; and Carl Bolano, Kevin Carlis, Michelle Chang, Joanna Chen, Melody Chen, Christina Cui, Vivi Dang, Angelica Estrada, Alyson Johnson, Sanskruti Kakaria, Sarah Knapp, Stephanie Lee, Mercy Lopez, Gregory Pak, Jasmine Rai, Atiyeh Samadi, Rachel Storer, Aaron Tay, Sarah Tran, Stephanie Zepeda for their help with recruitment, screening, data collection and management. We would also like to thank Richard Reynolds for his assistance in setting up the models used in the individual-level fMRI analyses.
Funding:
This research was supported by a grant awarded to Charles T. Taylor from the University of California, San Diego, National Institute of Health Clinical and Translational Science Awards Program Grant UL1TR001442.
Footnote:
- AMP
Amplification of Positivity
- PVS
Positive Valence System
- WL
waitlist
- MID
monetary incentive delay
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: Maria Kryza-Lacombe, Nana Pearson, and Jillian Lee Wiggins declare that they have no conflicts of interest. Charles T. Taylor declares that in the past 3 years he has been a paid consultant for Homewood Health, and receives payment for editorial work for UpToDate. Murray B. Stein declares that in the past 3 years he has been a paid consultant for Acadia Pharmaceuticals, Aptinyx, Bionomics, Genentech/Roche, GW Pharma, and Janssen, and receives payment for editorial work for UpToDate and the journals Biological Psychiatry and Depression and Anxiety. Sonja Lyubomirsky declares that in the past 3 years she has been a paid lecturer for the University of Arizona Center for Integrative Medicine and the Laureate Institute for Brain Research.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Admon R, Kaiser RH, Dillon DG, Beltzer M, Goer F, Olson DP, … Pizzagalli DA (2017). Dopaminergic enhancement of striatal response to reward in major depression. American Journal of Psychiatry, 174(4), 378–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Admon R, Nickerson LD, Dillon DG, Holmes AJ, Bogdan R, Kumar P, … Pizzagalli DA (2015). Dissociable cortico-striatal connectivity abnormalities in major depression in response to monetary gains and penalties. Psychol Med, 45(1), 121–131. 10.1017/S0033291714001123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alden LE, Mellings TM, & Laposa JM (2004). Framing social information and generalized social phobia. Behaviour research and therapy, 42(5), 585–600. [DOI] [PubMed] [Google Scholar]
- Berridge KC, & Kringelbach ML (2015). Pleasure systems in the brain. Neuron, 86(3), 646–664. 10.1016/j.neuron.2015.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camara E, Rodriguez-Fornells A, & Münte TF (2009). Functional connectivity of reward processing in the brain. Frontiers in Human Neuroscience, 2, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, & van Veen V (2007). Anterior cingulate cortex and conflict detection: an update of theory and data. Cogn Affect Behav Neurosci, 7(4), 367–379. 10.3758/cabn.7.4.367 [DOI] [PubMed] [Google Scholar]
- Cho YT, Fromm S, Guyer AE, Detloff A, Pine DS, Fudge JL, & Ernst M (2013). Nucleus accumbens, thalamus and insula connectivity during incentive anticipation in typical adults and adolescents. Neuroimage, 66, 508–521. 10.1016/j.neuroimage.2012.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung YS, & Barch D (2015). Anhedonia is associated with reduced incentive cue related activation in the basal ganglia. Cognitive, Affective, & Behavioral Neuroscience, 15(4), 749–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, & Shulman GL (2002). Control of goal-directed and stimulus-driven attention in the brain. Nature reviews neuroscience, 3(3), 201–215. [DOI] [PubMed] [Google Scholar]
- Cox RW, Chen G, Glen DR, Reynolds RC, & Taylor PA (2017). FMRI clustering in AFNI: False-positive rates redux. Brain Connect, 7(3), 152–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske MG, Meuret AE, Ritz T, Treanor M, Dour H, & Rosenfield D (2019). Positive affect treatment for depression and anxiety: A randomized clinical trial for a core feature of anhedonia. J Consult Clin Psychol, 87(5), 457–471. 10.1037/ccp0000396 [DOI] [PubMed] [Google Scholar]
- Craske MG, Meuret AE, Ritz T, Treanor M, & Dour HJ (2016). Treatment for Anhedonia: A Neuroscience Driven Approach. Depress Anxiety, 33(10), 927–938. 10.1002/da.22490 [DOI] [PubMed] [Google Scholar]
- Cuijpers P, Turner EH, Koole SL, van Dijke A, & Smit F (2014). What is the threshold for a clinically relevant effect? The case of major depressive disorders. Depress Anxiety, 31(5), 374–378. 10.1002/da.22249 [DOI] [PubMed] [Google Scholar]
- Dichter GS, Felder JN, & Smoski MJ (2010). The effects of Brief Behavioral Activation Therapy for Depression on cognitive control in affective contexts: An fMRI investigation. J Affect Disord, 126(1–2), 236–244. 10.1016/j.jad.2010.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon DG, Rosso IM, Pechtel P, Killgore WD, Rauch SL, & Pizzagalli DA (2014). Peril and pleasure: An RDOC- inspired examination of threat responses and reward processing in anxiety and depression. Depression and Anxiety, 31(3), 233–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn BD, German RE, Khazanov G, Xu C, Hollon SD, & DeRubeis RJ (2020). Changes in positive and negative affect during pharmacological treatment and cognitive therapy for major depressive disorder: A secondary analysis of two randomized controlled trials. Clinical Psychological Science, 8(1), 36–51. [Google Scholar]
- Dunn BD, Widnall E, Reed N, Owens C, Campbell J, & Kuyken W (2019). Bringing light into darkness: A multiple baseline mixed methods case series evaluation of Augmented Depression Therapy (ADepT). Behaviour research and therapy, 120, 103418. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, & Kalisch R (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci, 15(2), 85–93. 10.1016/j.tics.2010.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Schatzberg AF, Menon V, & Greicius MD (2009). Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Archives of general psychiatry, 66(12), 1361–1372. [DOI] [PubMed] [Google Scholar]
- Franken IH, Rassin E, & Muris P (2007). The assessment of anhedonia in clinical and non-clinical populations: further validation of the Snaith–Hamilton Pleasure Scale (SHAPS). Journal of Affective Disorders, 99(1–3), 83–89. [DOI] [PubMed] [Google Scholar]
- Fredrickson BL (1998). What Good Are Positive Emotions? Review of General Psychology, 2(3), 300–319. 10.1037/1089-2680.2.3.300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL (2001). The role of positive emotions in positive psychology - The broaden-and-build theory of positive emotions. American Psychologist, 56(3), 218–226. 10.1037/0003-066x.56.3.218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL (2003). The value of positive emotions - The emerging science of positive psychology in coming to understand why it’s good to feel good. American Scientist, 91(4), 330–335. 10.1511/2003.4.330 [DOI] [Google Scholar]
- Fredrickson BL (2013). Positive Emotions Broaden and Build. Advances in Experimental Social Psychology, Vol 47, 47, 1–53. 10.1016/B978-0-12-407236-7.00001-2 [DOI] [Google Scholar]
- Fredrickson BL (2013). Positive emotions broaden and build. In Advances in experimental social psychology (Vol. 47, pp. 1–53): Elsevier. [Google Scholar]
- Garland EL, Fredrickson B, Kring AM, Johnson DP, Meyer PS, & Penn DL (2010). Upward spirals of positive emotions counter downward spirals of negativity: Insights from the broaden-and-build theory and affective neuroscience on the treatment of emotion dysfunctions and deficits in psychopathology. Clinical psychology review, 30(7), 849–864. 10.1016/j.cpr.2010.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, Ziv M, Jazaieri H, Hahn K, Heimberg R, & Gross JJ (2013). Impact of cognitive behavioral therapy for social anxiety disorder on the neural dynamics of cognitive reappraisal of negative self-beliefs: randomized clinical trial. JAMA Psychiatry, 70(10), 1048–1056. 10.1001/jamapsychiatry.2013.234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg T, Chase HW, Almeida JR, Stiffler R, Zevallos CR, Aslam HA, … Toups M (2015). Moderation of the relationship between reward expectancy and prediction error-related ventral striatal reactivity by anhedonia in unmedicated major depressive disorder: findings from the EMBARC study. American Journal of Psychiatry, 172(9), 881–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu R, Huang W, Camilleri J, Xu P, Wei P, Eickhoff SB, & Feng C (2019). Love is analogous to money in human brain: Coordinate-based and functional connectivity meta-analyses of social and monetary reward anticipation. Neurosci Biobehav Rev, 100, 108–128. 10.1016/j.neubiorev.2019.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, & Gotlib IH (2012). Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of baseline activation and neural response data. American Journal of Psychiatry, 169(7), 693–703. [DOI] [PubMed] [Google Scholar]
- Hangya B, & Kepecs A (2015). Vision: How to train visual cortex to predict reward time. Current Biology, 25(12), R490–R492. [DOI] [PubMed] [Google Scholar]
- Hoerger M, Quirk SW, Chapman BP, & Duberstein PR (2012). Affective forecasting and self-rated symptoms of depression, anxiety, and hypomania: Evidence for a dysphoric forecasting bias. Cognition & Emotion, 26(6), 1098–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper JW, Pitman RK, Su Z, Heyman GM, Lasko NB, Macklin ML, … Elman I (2008). Probing reward function in posttraumatic stress disorder: expectancy and satisfaction with monetary gains and losses. J Psychiatr Res, 42(10), 802–807. 10.1016/j.jpsychires.2007.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman JC, DuBois CM, Healy BC, Boehm JK, Kashdan TB, Celano CM, … Lyubomirsky S (2014). Feasibility and utility of positive psychology exercises for suicidal inpatients. General Hospital Psychiatry, 36(1), 88–94. 10.1016/j.genhosppsych.2013.10.006 [DOI] [PubMed] [Google Scholar]
- Huffman JC, Mastromauro CA, Boehm JK, Seabrook R, Fricchione GL, Denninger JW, & Lyubomirsky S (2011). Development of a positive psychology intervention for patients with acute cardiovascular disease. Heart Int, 6(2), e14. 10.4081/hi.2011.e14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, … Wang P (2010). Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry, 167(7), 748–751. 10.1176/appi.ajp.2010.09091379 [DOI] [PubMed] [Google Scholar]
- Insel TR (2014). The NIMH Research Domain Criteria (RDoC) Project: precision medicine for psychiatry. Am J Psychiatry, 171(4), 395–397. 10.1176/appi.ajp.2014.14020138 [DOI] [PubMed] [Google Scholar]
- Jung WH, Kang DH, Kim E, Shin KS, Jang JH, & Kwon JS (2013). Abnormal corticostriatal-limbic functional connectivity in obsessive-compulsive disorder during reward processing and resting-state. Neuroimage Clin, 3, 27–38. 10.1016/j.nicl.2013.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashdan TB, Weeks JW, & Savostyanova AA (2011). Whether, how, and when social anxiety shapes positive experiences and events: a self-regulatory framework and treatment implications. Clinical Psychology Review, 31(5), 786–799. 10.1016/j.cpr.2011.03.012 [DOI] [PubMed] [Google Scholar]
- Keren H, O’Callaghan G, Vidal-Ribas P, Buzzell GA, Brotman MA, Leibenluft E, … Stringaris A (2018). Reward Processing in Depression: A Conceptual and Meta-Analytic Review Across fMRI and EEG Studies. Am J Psychiatry, 175(11), 1111–1120. 10.1176/appi.ajp.2018.17101124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Bhanji JP, Cooney RE, Atlas LY, & Gotlib IH (2008). Neural responses to monetary incentives in major depression. Biological Psychiatry, 63(7), 686–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, & Greer SM (2008). Anticipatory affect: neural correlates and consequences for choice. Philosophical Transactions of the Royal Society B: Biological Sciences, 363(1511), 3771–3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layous K, Chancellor J, & Lyubomirsky S (2014). Positive activities as protective factors against mental health conditions. Journal of Abnormal Psychology, 123(1), 3. [DOI] [PubMed] [Google Scholar]
- Layous K, Chancellor J, & Lyubomirsky S (2014). Positive Activities as Protective Factors Against Mental Health Conditions. Journal of abnormal psychology, 123(1), 3–12. 10.1037/A0034709 [DOI] [PubMed] [Google Scholar]
- Layous K, Chancellor J, Lyubomirsky S, Wang L, & Doraiswamy PM (2011a). Delivering happiness: translating positive psychology intervention research for treating major and minor depressive disorders. J Altern Complement Med, 17(8), 675–683. 10.1089/acm.2011.0139 [DOI] [PubMed] [Google Scholar]
- Layous K, Chancellor J, Lyubomirsky S, Wang L, & Doraiswamy PM (2011b). Delivering happiness: translating positive psychology intervention research for treating major and minor depressive disorders. Journal of alternative and complementary medicine, 17(8), 675–683. 10.1089/acm.2011.0139 [DOI] [PubMed] [Google Scholar]
- Liao W, Chen H, Feng Y, Mantini D, Gentili C, Pan Z, … Lui S (2010). Selective aberrant functional connectivity of resting state networks in social anxiety disorder. Neuroimage, 52(4), 1549–1558. [DOI] [PubMed] [Google Scholar]
- Lyubomirsky S, & Layous K (2013). How Do Simple Positive Activities Increase Well-Being? Current Directions in Psychological Science, 22(1), 57–62. 10.1177/0963721412469809 [DOI] [Google Scholar]
- Markett S, Heeren G, Montag C, Weber B, & Reuter M (2016). Loss aversion is associated with bilateral insula volume. A voxel based morphometry study. Neuroscience Letters, 619, 172–176. [DOI] [PubMed] [Google Scholar]
- Marwood L, Wise T, Perkins AM, & Cleare AJ (2018). Meta-analyses of the neural mechanisms and predictors of response to psychotherapy in depression and anxiety. Neuroscience & Biobehavioral Reviews, 95, 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu G, & Johnson SC (2012). A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage, 61(4), 1277–1286. 10.1016/j.neuroimage.2012.03.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina I, Sambin M, Beschoner P, & Viviani R (2016). Changing views of emotion regulation and neurobiological models of the mechanism of action of psychotherapy. Cognitive, Affective, & Behavioral Neuroscience, 16(4), 571–587. [DOI] [PubMed] [Google Scholar]
- Moll J, Krueger F, Zahn R, Pardini M, de Oliveira-Souza R, & Grafman J (2006). Human fronto–mesolimbic networks guide decisions about charitable donation. Proceedings of the National Academy of Sciences, 103(42), 15623–15628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori A, Okamoto Y, Okada G, Takagaki K, Jinnin R, Takamura M, … Yamawaki S (2016). Behavioral activation can normalize neural hypoactivation in subthreshold depression during a monetary incentive delay task. J Affect Disord, 189, 254–262. 10.1016/j.jad.2015.09.036 [DOI] [PubMed] [Google Scholar]
- Moskowitz JT, Hult JR, Duncan LG, Cohn MA, Maurer S, Bussolari C, & Acree M (2012). A positive affect intervention for people experiencing health-related stress: development and non-randomized pilot test. J Health Psychol, 17(5), 676–692. 10.1177/1359105311425275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy GA, Cernasov P, Pisoni A, Walsh E, Dichter GS, & Smoski MJ (2020). Reward network modulation as a mechanism of change in behavioral activation. Behavior modification, 44(2), 186–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng TH, Alloy LB, & Smith DV (2019). Meta-analysis of reward processing in major depressive disorder reveals distinct abnormalities within the reward circuit. Translational psychiatry, 9(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolova YS, Bogdan R, Brigidi BD, & Hariri AR (2012). Ventral striatum reactivity to reward and recent life stress interact to predict positive affect. Biol Psychiatry, 72(2), 157–163. 10.1016/j.biopsych.2012.03.014 [DOI] [PubMed] [Google Scholar]
- Oldham S, Murawski C, Fornito A, Youssef G, Yucel M, & Lorenzetti V (2018). The anticipation and outcome phases of reward and loss processing: A neuroimaging meta-analysis of the monetary incentive delay task. Hum Brain Mapp, 39(8), 3398–3418. 10.1002/hbm.24184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelizza L, & Ferrari A (2009). Anhedonia in schizophrenia and major depression: state or trait? Ann Gen Psychiatry, 8, 22. 10.1186/1744-859X-8-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, & Drevets WC (2008). A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular psychiatry, 13(9), 833–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pornpattananangkul N, Leibenluft E, Pine DS, & Stringaris A (2019). Association Between Childhood Anhedonia and Alterations in Large-scale Resting-State Networks and Task-Evoked Activation. JAMA Psychiatry, 76(6), 624–633. 10.1001/jamapsychiatry.2019.0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prenoveau JM, Zinbarg RE, Craske MG, Mineka S, Griffith JW, & Epstein AM (2010). Testing a hierarchical model of anxiety and depression in adolescents: a tri-level model. J Anxiety Disord, 24(3), 334–344. 10.1016/j.janxdis.2010.01.006 [DOI] [PubMed] [Google Scholar]
- Quoidbach J, Mikolajczak M, & Gross JJ (2015). Positive interventions: An emotion regulation perspective. Psychol Bull, 141(3), 655–693. 10.1037/a0038648 [DOI] [PubMed] [Google Scholar]
- Reilly EE, Whitton AE, Pizzagalli DA, Rutherford AV, Stein MB, Paulus MP, & Taylor CT (2020). Diagnostic and dimensional evaluation of implicit reward learning in social anxiety disorder and major depression. Depression and Anxiety. [DOI] [PubMed]
- Rupprechter S, Romaniuk L, Series P, Hirose Y, Hawkins E, Sandu AL, … Steele JD (2020). Blunted medial prefrontal cortico-limbic reward-related effective connectivity and depression. Brain, 143(6), 1946–1956. 10.1093/brain/awaa106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman CF, Young KS, Burklund LJ, Saxbe DE, Lieberman MD, & Craske MG (2020). Changes in functional connectivity with cognitive behavioral therapy for social anxiety disorder predict outcomes at follow-up. Behav Res Ther, 129, 103612. 10.1016/j.brat.2020.103612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sescousse G, Caldú X, Segura B, & Dreher J-C (2013). Processing of primary and secondary rewards: a quantitative meta-analysis and review of human functional neuroimaging studies. Neuroscience & Biobehavioral Reviews, 37(4), 681–696. [DOI] [PubMed] [Google Scholar]
- Shuler MG, & Bear MF (2006). Reward timing in the primary visual cortex. Science, 311(5767), 1606–1609. [DOI] [PubMed] [Google Scholar]
- Snaith P (1993). Anhedonia: a neglected symptom of psychopathology. Psychol Med, 23(4), 957–966. 10.1017/s0033291700026428 [DOI] [PubMed] [Google Scholar]
- Sohn MH, Albert MV, Jung K, Carter CS, & Anderson JR (2007). Anticipation of conflict monitoring in the anterior cingulate cortex and the prefrontal cortex. Proc Natl Acad Sci U S A, 104(25), 10330–10334. 10.1073/pnas.0703225104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speer ME, Bhanji JP, & Delgado MR (2014). Savoring the past: positive memories evoke value representations in the striatum. Neuron, 84(4), 847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoy M, Schlagenhauf F, Sterzer P, Bermpohl F, Hagele C, Suchotzki K, … Strohle A (2012). Hyporeactivity of ventral striatum towards incentive stimuli in unmedicated depressed patients normalizes after treatment with escitalopram. J Psychopharmacol, 26(5), 677–688. 10.1177/0269881111416686 [DOI] [PubMed] [Google Scholar]
- Taylor CT, Lyubomirsky S, & Stein MB (2017). Upregulating the positive affect system in anxiety and depression: Outcomes of a positive activity intervention. Depress Anxiety, 34(3), 267–280. 10.1002/da.22593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng C, Zhou J, Ma H, Tan Y, Wu X, Guan C, … Wang C (2018). Abnormal resting state activity of left middle occipital gyrus and its functional connectivity in female patients with major depressive disorder. BMC Psychiatry, 18(1), 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubl B, Kuehner C, Kirsch P, Ruttorf M, Diener C, & Flor H (2015). Altered neural reward and loss processing and prediction error signalling in depression. Soc Cogn Affect Neurosci, 10(8), 1102–1112. 10.1093/scan/nsu158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ (2015). Salience processing and insular cortical function and dysfunction. Nature reviews neuroscience, 16(1), 55–61. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Nomi JS, Hébert-Seropian B, Ghaziri J, & Boucher O (2017). Structure and function of the human insula. Journal of clinical neurophysiology: official publication of the American Electroencephalographic Society, 34(4), 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassena E, Silvetti M, Boehler CN, Achten E, Fias W, & Verguts T (2014). Overlapping neural systems represent cognitive effort and reward anticipation. PLoS One, 9(3), e91008. 10.1371/journal.pone.0091008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Siebenthal Z, Boucher O, Rouleau I, Lassonde M, Lepore F, & Nguyen DK (2017). Decision-making impairments following insular and medial temporal lobe resection for drug-resistant epilepsy. Social Cognitive and Affective Neuroscience, 12(1), 128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner U, Galli L, Schott BH, Wold A, van der Schalk J, Manstead AS, … Walter H (2015). Beautiful friendship: Social sharing of emotions improves subjective feelings and activates the neural reward circuitry. Social Cognitive and Affective Neuroscience, 10(6), 801–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Cheng B, Luo Q, Qiu L, & Wang S (2018). Gray matter structural alterations in social anxiety disorder: a voxel-based meta-analysis. Frontiers in psychiatry, 9, 449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, & Naragon-Gainey K (2010). On the specificity of positive emotional dysfunction in psychopathology: evidence from the mood and anxiety disorders and schizophrenia/schizotypy. Clinical Psychology Review, 30(7), 839–848. 10.1016/j.cpr.2009.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CC, Samanez-Larkin GR, Katovich K, & Knutson B (2014). Affective traits link to reliable neural markers of incentive anticipation. Neuroimage, 84, 279–289. 10.1016/j.neuroimage.2013.08.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KS, & Craske MG (2018). The cognitive neuroscience of psychological treatment action in depression and anxiety. Current Behavioral Neuroscience Reports, 5(1), 13–25. [Google Scholar]
- Yue Y, Yuan Y, Hou Z, Jiang W, Bai F, & Zhang Z (2013). Abnormal functional connectivity of amygdala in late-onset depression was associated with cognitive deficits. PLoS One, 8(9), e75058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WN, Chang SH, Guo LY, Zhang KL, & Wang J (2013). The neural correlates of reward-related processing in major depressive disorder: a meta-analysis of functional magnetic resonance imaging studies. J Affect Disord, 151(2), 531–539. 10.1016/j.jad.2013.06.039 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


