Abstract
Monitoring one’s safety during low vision navigation demands limited attentional resources which may impair spatial learning of the environment. In studies of younger adults, we have shown that these mobility monitoring demands can be alleviated, and spatial learning subsequently improved, via the presence of a physical guide during navigation. The present study extends work with younger adults to an older adult sample with simulated low vision. We test the effect of physical guidance on improving spatial learning as well as general age-related changes in navigation ability. Participants walked with and without a physical guide on novel real-world paths in an indoor environment and pointed to remembered target locations. They completed concurrent measures of cognitive load on the trials. Results demonstrate an improvement in learning under low vision conditions with a guide compared to walking without a guide. However, our measure of cognitive load did not vary between guidance conditions. We also conducted a cross-age comparison and found support for age-related declines in spatial learning generally and greater effects of physical guidance with increasing age.
Keywords: low vision, navigation, older adults, attentional demands
INTRODUCTION
Spatial learning while navigating is a difficult task that is multi-faceted and cognitively demanding. It becomes even more challenging for low vision individuals—those with uncorrectable severe vision loss—as demonstrated by manipulations of severely degraded acuity and contrast sensitivity (Rand, Creem-Regehr, & Thompson, 2015) and severely restricted peripheral field (Barhorst-Cates, Rand, & Creem-Regehr, 2016). We have argued that the task of navigating with low vision is particularly challenging because of the increased need for mobility monitoring during navigation with severely restricted visual information (Rand et al., 2015). Mobility monitoring refers to the attentional requirements of navigating safely. In navigation with severely degraded vision, attempting to detect and avoid obstacles that cannot easily be seen may detract from the attentional resources that may otherwise be devoted to spatial learning of the environment. Given prior work suggesting increasing age-related attentional demands for navigation (e.g., Gazova et al., 2013) as well as changes in cognitive and visuo-motor processes relating to locomotion with age (e.g., Beauchet et al., 2009), the present study extends a low vision navigation paradigm used with young adults to test the effects of low vision on navigation in an older adult population.
Our prior work has aimed to determine what factors contribute to impairments in learning and memory under severely restricted viewing conditions. On a basic perception level, severely reduced acuity and contrast sensitivity negatively affect object detection and recognition (Bochsler, Legge, Gage, & Kallie, 2013; Bochsler, Legge, Kallie, & Gage, 2012) although absolute distance and size perception remain relatively intact under simulated severely degraded vision (Rand, Tarampi, Creem-Regehr, & Thompson, 2011; Tarampi, Creem-Regehr, & Thompson, 2010). A limitation of this work for generalizing to real world challenges is that it has been conducted using stationary viewing paradigms that limit the viewer’s potential interaction with the space. In contrast, everyday navigational tasks involve locomotion through the space, requiring detection and avoidance of obstacles (Long, Rieser, & Hill, 1990; Pelli, 1987) in addition to spatial learning. Individuals with some types of low vision have greater difficulty with obstacle avoidance (Kuyk, Elliott, Biehl, & Fuhr, 1996; Lovie-Kitchin, Soong, Hassan, & Woods, 2010), which likely contributes to the additional challenge of increased mobility monitoring demands.
Rand et al. (2015) recently argued that the attention needed to maintain safe mobility detracts from the pool of attentional resources available for spatial learning of the environment. They showed that younger adults who navigated with simulated degraded acuity and contrast sensitivity performed worse on a survey-based spatial learning task compared to when they navigated with their normal vision, and that this detriment could be partially explained by increased attentional demands (as measured by an auditory reaction time task to index cognitive load) during low vision trials. Other research has also demonstrated the detrimental effects of cognitive load for spatial learning with limited visual information (Klatzky, Marston, Giudice, Golledge, & Loomis, 2006). Results from Rand et al. (2015) point to mobility monitoring as a specific source of cognitive load that may contribute to impaired learning during low vision navigation. Spatial learning subsequently improved when participants used a physical guide (holding onto an experimenter’s arm) compared to walking without support, suggesting that one contribution to the pull on attentional resources may be the mobility monitoring demands of the task. However, mobility-related guidance appears to have much different effects on learning than other types of guidance. There is a growing literature supporting the finding that navigational guidance systems (such as GPS), impair spatial learning (Gardony, Brunyé, Mahoney, & Taylor, 2013; Gardony, Brunyé, & Taylor, 2015). Research also suggests an advantage in learning when active choices or decisions are required in navigation (Bakdash, Linkenauger, & Proffitt, 2008; Chrastil & Warren, 2012) suggesting that guidance may not be beneficial because it reduces the “desirable difficulties” that enhance learning (Bjork & Bjork, 2011). While at first glance, these results may seem contradictory to Rand et al.’s (2015) claim of facilitation of spatial learning with a guide, they follow a consistent logic about the necessary role of attention in spatial learning. For example, Gardony et al. (2015) argue that navigational aids distract navigators by dividing attention, leading to reduced attentional resources available for encoding spatial locations. Our use of physical guidance also affects attention, but operates at a level of guiding mobility, rather than guiding navigational choices, and thus proposes to free up attentional resources at encoding. In Rand et al. (2015) and the current study, it is important to note that all conditions are “guided” with respect to the planned route for navigation, but what is manipulated is physical guidance, which serves as assistance to avoid obstacles or other potential mobility risks.
The current study extends work on low vision navigation in younger adults to older adults, who may experience even greater difficulty with low vision navigation due to a combination of age-related factors including cognitive, neuropsychological, and motor contributions. Despite the lack of a consensus on what cognitive processes in particular decline in older adulthood, it is commonly observed that greater decrements in performance with age have been associated with more cognitively demanding navigation tasks, such as those that require allocentric learning (Gazova et al., 2013) or the formation and use of a stored representation (a cognitive map) of the environment that is viewer-independent (Iaria, Palermo, Committeri, & Barton, 2009). In contrast, some studies show that older adults’ ability for egocentric navigation, such as following routes and using one’s own body position as a cue for navigating, remains more similar to younger adult performance (e.g., Gazova et al., 2013; but see Head & Isom, 2010 for conflicting evidence), likely because it is less cognitively demanding (Brunyé, Wood, Houck, & Taylor, 2017; Lindberg & Gärling, 1982). Other research suggests that it is not performance with one type of learning versus the other, but task-switching from egocentric to allocentric reference frames that declines with age (Harris, Wiener, & Wolbers, 2012). Age-related impairment may be present for learning of new environments rather than familiar environments (Rosenbaum, Winocur, Binns, & Moscovitch, 2012) and for learning through exploratory navigation more than learning through map reading (Yamamoto & DeGirolamo, 2012). Finally, older adults show deficits in using optic flow and landmark information in path integration, which could influence learning and memory processes in navigation (Harris & Wolbers, 2012). In all, multiple cognitive components of the aging process may contribute to the decline in spatial learning, including changes in both spatial abilities and attentional control (Moffat, Zonderman, & Resnick, 2001).
These age-related changes in the cognitive processes involved in navigation can also be seen when examining underlying neural mechanisms, both those specific to spatial processing and those more generally related to attentional capabilities. Much research links the hippocampus with navigation and points to an overall finding of a decline with age in both hippocampal volume and spatial memory and navigation ability (Chen, Chuah, Sim, & Chee, 2010; Driscoll et al., 2003; Head & Isom, 2010). Consistent with the behavioral work described above, different types of spatial navigation strategies have been linked to differential changes in hippocampal volume with age. Namely, Konishi and Bohbot (2013) found that the tendency to use spatial strategies that relied on flexible spatial memory representations positively correlated with gray matter in the hippocampus and associated areas in older adults; in contrast, the use of “response” strategies such as replicating routes, negatively correlated with these regions. Neural changes in attentional control also support the claim of increasing challenges of navigation with age. The attentional network includes the anterior cingulate cortex, prefrontal cortex, parietal cortex, extrastriate cortex, superior colliculus, thalamus, and basal ganglia, all of which are presumed to work together in a network to amplify task-relevant information and ignore task-irrelevant information. Some of these brain regions underlying attentional control are particularly prone to degeneration in age, such as the prefrontal cortex (Raz, 2000). The observation of greater activity in frontal and parietal regions in older adults during attentionally demanding tasks suggests that attention tasks may require more effort in older adults to be successful (see Grady, 2008, for a review). That is, older adults may have a more difficult time or may have a decreased ability to ignore irrelevant information, seen in the decreased suppression of brain activity. This greater activity (or less suppression) is particularly salient in cognitively demanding tasks (Persson, Lustig, Nelson, & Reuter-Lorenz, 2007; Townsend, Adamo, & Haist, 2006) and many have described it as compensatory, in that it allows older adults to successfully complete attention-demanding tasks (Grady, 2008).
Finally, links between age-related changes in motor processes and increased demands on cognitive resources are also important to consider for their effects on spatial learning while navigating. Declines in mobility become especially apparent in cognitively demanding situations. Postural sway is strongly affected by the addition of higher-cognitive load tasks, especially in older adulthood and particularly for those who are already vulnerable to falls (Shumway-Cook, Woollacott, Kerns, & Baldwin, 1997). In dual-task situations, while actors often prioritize performance on stability tasks at the expense of performance on cognitive tasks, older adults are more strongly affected by these dual-task costs than younger adults. For example, Neider et al. (2011) demonstrated that multi-tasking significantly impaired older, but not younger, adults’ performance on a virtual street-crossing task. Success at multi-tasking with a concurrent cognitive and motor task (i.e., walking and talking) predicts fall risk in older adults (Beauchet et al., 2009), which reveals that simple motor tasks become more complex to perform with age. Furthermore, basic locomotor tasks that are often automatic and easy to perform for younger adults demand more cognitive processing (Berchicci, Lucci, Pesce, Spinelli, & Di Russo, 2012; Lövdén, Schellenbach, Grossman-Hutter, Krüger, & Lindenberger, 2005) and executive control (Niermeyer, Suchy, & Ziemnik, 2017) in older adults, making walking and other common motor activities demand attentional resources, especially when they co-occur with a secondary task. Thus, normal age-related changes in motor control may impact navigation by increasing attentional demands that detract from spatial learning ability. This claim is supported by research on the types of aids that support older-adult wayfinding, showing that assistance with stability support helped older adults in navigation, but only when cognitive load associated with the aid remained low (Schellenbach, Lövdén, Verrel, Krüger, & Lindenberger, 2010).
There is an increasing need to determine the impact of low vision on mobility and navigation in older adults, given the growth of this demographic, and the importance of independent navigation abilities for maintaining an active lifestyle. The National Eye Institute states a population of four million low vision individuals in the U.S. in 2010, with projections to 14 million people by 2050 (https://nei.nih.gov/). Low vision is a term that broadly covers classes of vision loss that are severe and uncorrectable, including loss of acuity, contrast sensitivity, and field of view. The primary goal of the present study is to examine spatial learning during navigation for older adults with simulated degraded acuity and contrast sensitivity. Our simulated low vision population serves as an appropriate age-matched baseline for the typical clinical low-vision population and also allows for more control over visual degradation than testing of a clinical population. Much of the research demonstrating the age-related decline in spatial learning during navigation tasks has used virtual environments, smaller-scale real-world Morris Water Maze types of tasks, or tested spatial memory of familiar environments. The current study adds to this prior work in critical ways by utilizing a novel, real-world, building-scale environment, complete with potential obstacles and distractors that may be found in any real-world setting, and includes the effects of simulated severely degraded vision.
The first aim of this study was to test whether reducing mobility monitoring demands via physical guidance improves spatial learning in older adults with simulated degraded vision. In our task, older adults navigated along real-world paths wearing degraded vision goggles with and without a physical guide. We predicted that the presence of a physical guide would improve spatial learning performance for older adults compared to navigating without a guide (as found originally in Rand et al., 2015 with younger adults) by reducing the demands of mobility monitoring. Our second aim was to conduct a cross-age comparison examining general age-related differences in spatial learning as well as the relative effectiveness of guidance on reducing spatial learning deficits brought on by low vision during navigation. To accomplish this, we compared performance in the current study with previously collected younger adult data (Rand et al., 2015) which had used similar paths and the same design. We predicted that older adults would show a greater overall spatial learning impairment compared to younger adults, and that guidance would improve spatial learning outcomes more for older adults than for younger adults.
METHODS
Participants
We recruited participants from the older adult population in the Salt Lake City community through newspaper ads, flyers at senior centers, and soliciting from senior fairs and older adult continuing education classes at the university. All participants gave informed consent with procedures approved by the University of Utah Institutional Review Board. Participants were paid $20 as compensation for their time. All participants had normal or corrected-to-normal vision (M logMAR = 0.116, M Snellen=20/26.12, M log contrast sensitivity = 1.93), walked without severe impairment, and had normal cognitive functioning measured by the Short Blessed Test (M = 0.4, SD=1.12; Katzman et al., 1983). Fourteen older adults participated in the experiment (8 female)1. The average age was 67 years (range 63 to 71).
Procedure
The experiment took place in the hallways of the Merrill Engineering Building on the University of Utah campus. Upon arrival, participants signed a consent form and filled out demographics information. Participants were tested for normal cognitive functioning using the Short Blessed Test (Katzman et al., 1983). They were also tested for normal or corrected-to-normal vision through both the Lighthouse distance visual acuity chart and the Pelli-Robson contrast sensitivity chart. We used simulated degraded acuity and contrast sensitivity by creating goggles with theatrical lighting filters (Cinegel #3047: Light velvet frost; Rosco Laboratories, Stamford, CT) covering the eye openings. Perceived visual acuity in the goggles for the present participants was an average of 1.55 logMAR (20/708.48 Snellen) and perceived log contrast sensitivity was an average of 0.33. Acuity and contrast values were taken first for each participant while wearing the degraded vision goggles and then for each participant with his or her normal vision.
We then trained participants on a verbal pointing measure (Philbeck, Sargent, Arthur, & Dopkins, 2008) used as a test of their spatial learning. Participants were informed that they would be traveling along pathways through the building, and their task would be to keep track of the locations of specified landmarks encountered along the path. Participants were instructed on how to use the spatial learning response measure, used to assess their knowledge of the landmarks, in the lab and given several practice trials to ensure understanding. The response measure proceeded as follows: At the end of each path, participants remained facing in the final heading direction and pointed to the remembered location of each landmark as if they could draw a line straight from their current location to the location of the landmark. To describe this pointing direction, participants used a two-step verbal response. They were trained to imagine the 360 degrees of space around them as divided into four quadrants (e.g., front-left, front-right, back-left, or back-right). Participants chose one quadrant and then indicated a specific degree from 0–90 to represent the direction within that quadrant to the remembered landmark location. This verbal response was converted into an absolute error value for each landmark. The physical pointing served as a check to ensure that participants were correctly reporting their intended direction. We used this pointing task as our measure of spatial memory because it draws on an individual’s survey knowledge of the previously navigated environment, and has been shown to be at least as accurate as and less variable than other types of physical pointing measures (Philbeck, Sargent, Arthur, & Dopkins, 2008).
Next, they were trained on the secondary, auditory reaction time task (Brunken, Plass, & Leutner, 2003), intended to be a measure of cognitive load (Verwey & Veltman, 1996). Participants listened through wireless headphones to a series of randomly generated tones that occurred every 1–6 seconds. They were instructed to respond to each tone by clicking a cordless mouse as quickly as possible after hearing the tone. Tones were generated and responses were recorded on a laptop carried by a second experimenter throughout the experiment. During the practice, the volume of the tones was adjusted to suit the participant’s comfort. During the experiment, this task was performed concurrently with the spatial learning/navigation task described below (see also Barhorst-Cates et al., 2016; Rand et al., 2015). For the final phase of training, participants completed several full-length practice paths that included stopping at two landmarks, simultaneously completing the auditory reaction time measure, and completing the degree-quadrant pointing task for each landmark at the end of each path.
Once training was complete, participants began the four experimental paths. Each path contained 3 distinct landmarks, so participants encountered 6 landmarks in each of two within-subjects conditions: two paths walking on their own (unguided) and two paths holding onto the experimenter’s arm (guided). The order of the guidance condition was manipulated between subjects and counterbalanced, such that half of participants completed the paths in a guided-unguided-guided-unguided order, and the other half of participants completed the paths in an unguided-guided-unguided-guided order. In the guided condition, the experimenter walked next to the participant on the side opposite of the participant’s dominant hand and instructed the participant to hold onto the experimenter’s arm throughout the path. In the unguided condition, the experimenter walked slightly behind, without touching, the participant on the side opposite his or her dominant hand. Each path had 4 or 5 turns, and ranged from 109–121 meters in length, taking approximately 5 minutes to complete. The paths differed slightly in terms of the numbers of objects located in each of the four quadrants relative to the final heading but there was no difference in pointing error between paths when examined within the same condition (ps > .1). Each of the four paths was unique, such that there was no crossover from one path to another and the participant was not exposed to the same paths or landmarks more than once. Landmarks consisted of normal university building objects, such as a soda machine and a water fountain. Participants walked along the paths following verbal instructions from the experimenter (e.g., walk forward, stop, turn right). The experimenter informed participants that he or she would prevent collisions with obstacles or walls by gently tapping the participant. Participants were encouraged to look around as they normally would. At each landmark, the experimenter stopped the participant and directed his or her attention to the landmark’s location (e.g., “On your right is an eyewash station”). The experimenter and participant began walking again after a three-second pause. Importantly, in both the guided and unguided walking conditions, the experimenter provided continual verbal instruction for landmarks and turns along the route as well as safety-related support to prevent collisions. The goal of this control in verbal instructions across conditions was to allow the presence or absence of the physical guide (arm holding) to be the only manipulated difference between conditions. At the end of each path, participants completed the verbal-pointing response to indicate their memory of the location of each of the three landmarks. Participants were informed during practice that they would be tested for the landmarks in a random order.
After performing the pointing responses, participants reported their level of self-reported safety-related anxiety on the Subjective Units of Distress Scale (SUDS: Bremner et al., 1998), on a scale from 0 to 100, for 1) the path on average, 2) when turning corners, and 3) when encountering people. Finally, participants completed one minute of the auditory reaction time task alone to establish a baseline reaction time for each path. This procedure was repeated for the second path. After completing the first 2 paths, participants sat down to take a break and filled out the Memory Anxiety Questionnaire (Davidson, Dixon, & Hultsch, 1991). They then completed the final 2 paths and filled out the Santa Barbara Sense of Direction Scale (Hegarty, Richardson, Montello, Lovelace, & Subbiah, 2002). At the end of the experiment, they were then debriefed, thanked, and dismissed.
RESULTS
Pointing Error
We identified two outliers in our sample of 14 whose average pointing error fell greater than two standard deviations above the mean. We removed them from the data and the remaining analyses were conducted excluding them. Thus, with the outliers excluded, we had a sample of 12 participants. We conducted a 2x2 repeated measures Analysis of Variance (ANOVA) with Guidance Order as a between subjects factor and Guidance Condition as a within-subjects repeated measures factor. We predicted that participants would show greater absolute pointing error on the unguided paths compared to the guided paths, indicating worse spatial learning on the unguided paths. Our analysis revealed a significant effect of guidance condition on pointing error F(1, 10)= 8.90, p<.02, ηp2=.471. Participants had significantly greater pointing error in the unguided condition (M=36.58, SE=4.90) compared to the guided condition (M=26.71, SE=3.53), suggesting that participants had better spatial learning when they walked with a guide compared to walking on their own. See Figure 1 for a depiction of these results. There was no main effect of guidance order (p = .672) and no guidance condition x guidance order interaction (p = .379). There were no significant correlations between SBSOD score (M = 52.42, SE = 4.77) or Memory Anxiety score (M = 34.58, SE = 2.33) on guided, unguided, or average pointing error (ps > .15).
Fig 1.
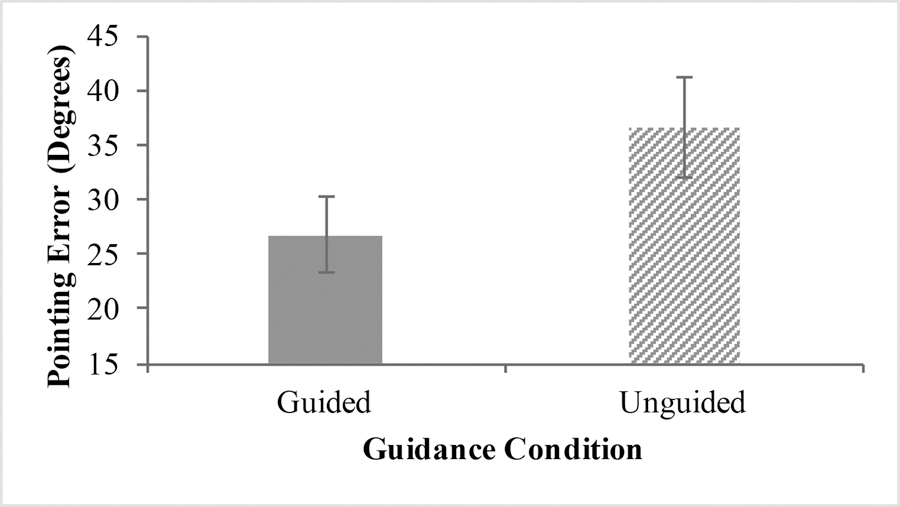
Average Pointing Error between Guidance Conditions
Reaction Time
To test whether guidance modulates attentional resources in older adult low vision navigation, we conducted the same 2x2 repeated measures ANOVA with Guidance Order as a between-subjects factor and Guidance Condition as a within-subjects factor and reaction time as the outcome variable. We predicted that participants would have overall slower reaction times during the unguided trials compared to the guided trials, similar to the effect observed in Rand et al. (2015). Our results did not find support for a difference between the guided (M=.598, SE=.028) and unguided (M=.601, SE=.023) conditions for reaction time (p=.776). There was also no main effect of guidance order (p=.513) and no guidance condition x guidance order interaction (p=.234). Notably, there was no difference in miss rates between guided (M=.077, SD=.08) and unguided (M=.099, SD=.103) conditions (p = .57). This was surprisingly contrary to our predictions, as we suspected older adults would demonstrate an even stronger dual-task cost than younger adults. We present some potential explanations for this lack of an effect in the Discussion.
Anxiety
Finally, to test the effect of guidance on the anxiety component of wayfinding, we ran the same repeated measures ANOVA and looked at differences between conditions on average Subjective Units of Distress ratings. Surprisingly, we did not see a difference in SUDS reports (p=.125) between the guided (M=14.31, SE=3.07) and unguided (M=16.40, SE=3.48) conditions, although a difference in SUDS ratings has typically been found in our younger adult studies when comparing low vision to normal vision, as well as when comparing guidance and no guidance2. Notably, the mean SUDS ratings are low in comparison to values obtained in our previous guidance study with young adults (e.g., Rand et al. (2015) Experiment 2 found guided SUDS mean = 23 and unguided SUDS mean = 30), suggesting overall low levels of anxiety on this task. There was no main effect of guidance order (p=.307) and no guidance condition x guidance order interaction (p=.210).
Comparison to Younger Adults
We compared the current data to data from younger adults in Rand et al. (2015), Experiment 5 who walked 4 similar paths with 3 objects each wearing the same simulated degraded vision goggles. While the participants in the younger adult study did not complete exactly the same paths, they were very similar in that they were approximately the same length and tested the same number of objects. This comparison broadly allowed us to examine the similarities and differences between older and younger adults on this paradigm. Our cross-age sample included 413 younger adults (M age=21.32 years, SE=.7) and the 12 older adults from the present study (M age=66.75 years, SE=.906). For the younger adult group, the average guided pointing error was 19.08° (SE=1.13) and the average unguided pointing error was 22.59° (SE=1.63). For the older adult group, the average guided pointing error was 26.71° (SE=3.5) and the average unguided pointing error was 36.58° (SE=4.7).
We had two aims with the cross-age analysis. First, we aimed to measure age-related differences in spatial learning abilities overall for this large-scale learning task. In order to examine the effect of age on overall spatial learning, we computed an average error score (the average of guided and unguided pointing error). We chose to run a linear regression with age as a continuous predictor rather than treating age as a categorical (younger/older) variable because there was a fair amount of variation even within our younger (range 18 to 37 years) and older (range 63 to 71 years) age groups, and with categorization into groups the statistical power to detect a relationship is greatly reduced. We ran the linear regression model with age predicting the average pointing error and found a significant relationship (B=.234, p <.01). For every one unit increase in age, the average pointing error increased by .234 units. This suggests that overall spatial learning worsens with increasing age, replicating prior research that suggests that older adults have greater difficulty with spatial learning compared to younger adults. See Figure 2 for a graph of these results.
Fig 2.
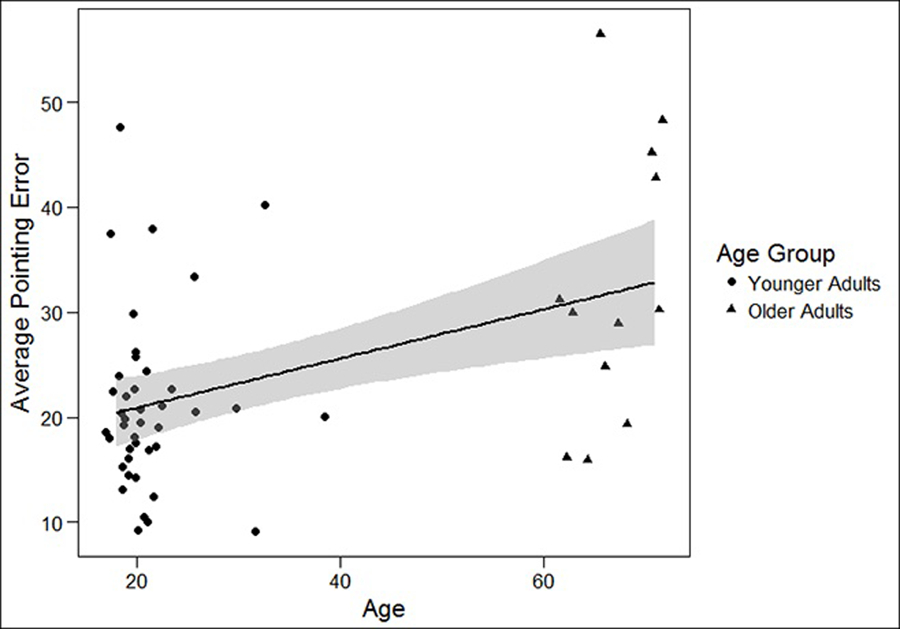
Linear relationship between Age and Average Pointing Error
Our second aim was to examine the differential effect of guidance on improving spatial learning for our different age groups. In order to examine the effect of age on the usefulness of guidance in improving spatial learning, we computed a pointing error difference score (Unguided Error – Guided Error). We ran a regression with age predicting the error difference score and found a significant relationship (B=.143, p<.04). For every one unit increase in age, the difference in pointing error between conditions increased by .143 units. This suggests that the effect of guidance increases with increasing age (i.e., the guide is more helpful in improving spatial learning for older adults compared to younger adults). See Figure 3 for a graph of these results.
Fig 3.
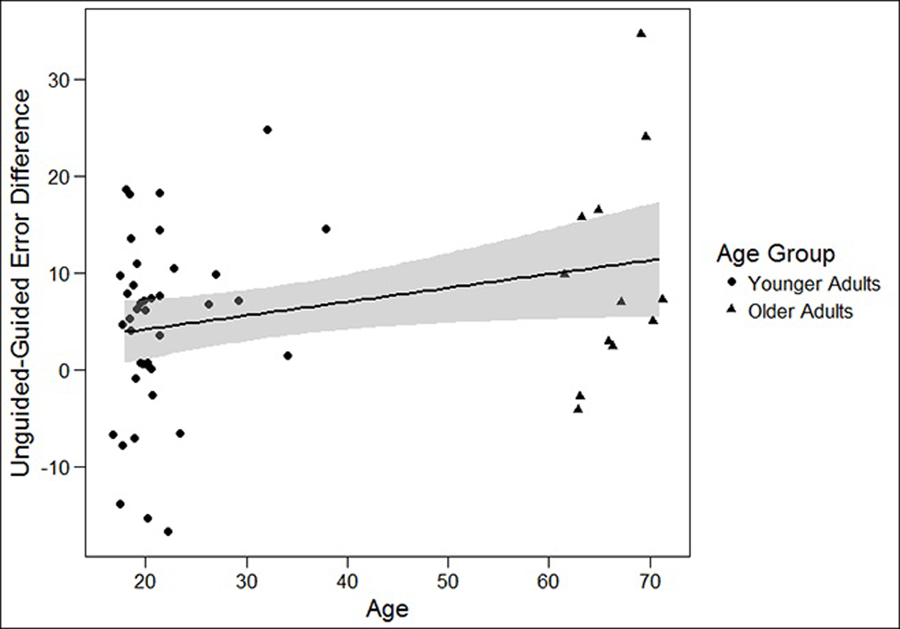
Linear Relationship of Age and Unguided – Guided Error Difference Score
DISCUSSION
In the current study, older adult participants walked paths in an indoor environment following verbal instructions of an experimenter under simulated degraded viewing conditions. They reported their memory for the location of landmarks along the paths and performed a concurrent auditory reaction time task by responding to randomized beeps by clicking a cordless mouse as they walked the paths. We manipulated mobility monitoring demands by including two conditions manipulated within-subjects: a physical guide for the participant to hold onto and a no-guide condition where participants walked on their own without support. Importantly, both conditions involved the same navigational guidance about paths and landmarks, but varied in physical guidance.
Our primary hypothesis was supported: older adults performed better on the spatial learning task when mobility monitoring demands were reduced through physical guidance, despite constant degraded viewing conditions. This finding suggests that mobility monitoring demands can be decreased for older adults during navigation with low vision, and that this may allow the actor to use the resources that would be devoted to maintaining safety and stability on a path instead for spatial learning. We also found that the effect of a physical guide increased in magnitude with age. This is consistent with a fair amount of prior research suggesting that cognitive processing for basic locomotion tasks becomes more demanding in older adulthood. For example, Berchicci et al. (2012) demonstrated that walking itself is more cognitively demanding for older adults while it is largely automatic in younger adults. Mobility monitoring is even more important for older adults than younger adults because of the greater costs of falling in older age. While younger adults are unlikely to be seriously injured from a fall, the stakes are higher as individuals progress into older age (Rubenstein, 2006). We provide evidence that alleviating these locomotion-based demands by having a physical guide results in a greater benefit on a spatial learning task as age increases. In other words, because older adults have more cognitive demands during unsupported locomotion-based navigation than younger adults, the possibility for improvement via the presence of a guide is even greater for older adults.
While we predicted a greater effect of guidance for older adults, we also expected an overall age-related decline in performance on our spatial learning task and found support for this hypothesis. Pointing error increased with increasing age, replicating prior work showing an impairment for older adults on allocentric spatial learning tasks (Gazova et al., 2013). Bates and Wolbers (2014) have attributed this age-related decline in allocentric learning (but not egocentric learning) to underlying brain changes, such as degeneration of the hippocampus with age (Yankner, Lu, & Loerch, 2008). Indeed, a large amount of research connects allocentric learning with the hippocampus. For example, Zaehle et al. (2007) found that allocentric encoding demands more processing than egocentric encoding in a hierarchical manner. While both egocentric and allocentric encoding share some regional processing, allocentric encoding additionally requires the hippocampal formation. Wiener, de Condappa, Harris, and Wolbers (2013) observed that older adults tend to prefer “response” (or route) types of strategies over “place” (or survey-based) strategies even at the cost of success on navigation tasks. Our task could be considered an allocentric learning task in that we ask participants for their survey-based understanding of the object locations. Our task could also be considered an egocentric encoding task with an allocentric recall component. If not completely allocentric during encoding (i.e., participants are instructed to follow along a route, which may encourage an egocentric learning strategy), our task requires a switch from egocentric to allocentric for recall. Research suggests that older adults may show decline in either of these cases (allocentric only— Gazova et al., 2013; task-switching—Harris, Wiener, & Wolbers, 2012), consistent with the current results.
Although the primary effect of guidance as reducing spatial pointing error does point to the demands of mobility monitoring on this task, we did not find the expected difference in reaction time on the auditory task (measure of cognitive load) between guidance conditions that would be supportive of a difference in available attentional resources. Older adults performed just as well on the auditory task when they were unguided as when they were guided, inconsistent with what Rand et al. (2015) observed in younger adults, where the presence of a guide did improve reaction time on this task. Furthermore, the overall mean reaction time was not different between younger and older adults when compared with the data from Rand et al. (2015)4. Further consideration of these lack of differences is needed. The similar RT across the two age groups suggests that the older adults were high functioning overall in that they showed similar speed of processing as the younger adults. Our sample of older adults may not yet have experienced deficits in processing speed but have started to experience deficits in their spatial learning ability.
For the lack of guidance effect on the auditory task, there are several possible explanations. It is possible that older adult participants were prioritizing the auditory reaction time task above the spatial learning task. Although participants were given the explicit goal to primarily remember target locations as they walked, the immediacy of the task to respond to the tones may have led them to devote additional resources to the auditory task at the expense of the learning task. This interpretation would suggest that guidance did affect attentional resources but that the outcome was a decrement in performance on the primary spatial memory task and not the RT task. This could be one explanation for the relatively greater increase in pointing error seen in older versus younger adults in the no-guidance condition. Another possibility is that physical guidance affects older and younger adults differently with respect to attentional resources. Because older adults are likely already monitoring their mobility in everyday situations, the introduction of the low vision viewing condition may not have changed their locomotion activity as much as in a younger population. Consistent with this idea, Bates and Wolbers (2014) found in a navigation task that involved integration of visual and self-motion cues that older adults weighted visual information less than optimally, compared to younger adults. We did not conduct a guidance manipulation under normal vision with older adults, so it is possible that similar beneficial effects of guidance would have resulted without low vision as well. Finally, it is possible that additional participants would have led to the ability to detect a difference in RT between conditions5. Despite these open questions about the underlying causes of the lack of RT effect, we cannot ignore the strong effect of guidance on reducing pointing error seen in this study, suggesting that physical guidance does facilitate spatial learning, even if it is not explained by the RT measure of cognitive load.
This research extends prior work documenting age-related declines in navigation abilities in two important ways. First, while much research on navigation in aging populations has used virtual environments with either stationary viewing in a single large room-sized space or treadmill-locomotion paradigms, we used a novel real-world environment extending to environmental space (Montello, 1993), a space that requires active locomotion to experience completely. Many navigation tasks in the real world are carried out in this category of spatial environments, and it is important to identify age-related changes in spatial processing in this context. Second, in addition to examining general age-related effects, we also examined navigation in the context of severely degraded vision, considering the possibility that mobility monitoring costs would increase with age because of corresponding increases in the cognitive demands of locomotion itself. Given our lack of a significant difference in the secondary reaction time task with the decrease of mobility monitoring demands, future work should look at other indicators of cognitive load such as changes in gait (Springer et al., 2006). Future work should also examine the interacting effects of age and degraded vision across different types of aids and environments, such as smaller-scale spaces (e.g., a single room) or large-scale unstructured spaces (e.g., a hotel or museum lobby).
Our findings contribute to a growing understanding of active learning during navigation under low vision, supporting the role of both visual information and attentional resources on spatial learning in large-scale spaces (Barhorst-Cates et al., 2016; Klatzky et al, 2006; Rand et al., 2015). While there is limited low vision work on environmental-scale navigation as tested here, other related work in large room-sized spaces shows selective impairments in judging room size and orientation after walking within the space under simulated severe blur (Legge, Gage, Baek, & Bochsler, 2016). An important question is how these types of findings with simulated severely degraded vision generalize to people with clinical low vision diagnoses. For judgments of obstacles (ramps and steps), Bochsler et al. (2013) found qualitatively similar effects of distance and target type for people with low vision and normal vision with simulated reduced acuity. Likewise, Fortenbaugh, Hicks, Hao, and Turano (2007) found similar effects of peripheral field loss in clinical patients and restricted normally sighted participants, with respect to distance compression in spatial memory. However, recent work with locomotion in large rooms showed that real vision loss had little effect on the ability to judge room size and update spatial positions along simple paths (Legge, Granquist, Baek, & Gage, 2016). Given multiple factors that can influence navigation and spatial learning in clinical low vision patients, such as amount of visual experience (onset of vision loss) and type of visual deficit (Rieser, Hill, Talor, Bradfield, & Rosen, 1992), more research in large-scale spatial cognition is needed that directly tests low vision populations. Our work provides a basis for this future work by validating a spatial learning paradigm that can be used across the adult lifespan, and demonstrating a significant role of cognitive processing in what might be assumed to be only a visually-defined problem.
CONCLUSION
The research described here shows that the spatial learning by older adults in low vision navigation situations can be improved by minimizing physical risks to learners in the environment. While we replicate the phenomenon that older adults appear to have poorer spatial learning abilities than younger adults generally, our research suggests that they may have greater potential for improvement with the assistance of a physical aid. With the increasing prevalence of navigational aids used in wayfinding, our work shows that it is also important to consider the physical safety demands of the environment and their effects on spatial learning while navigating. This research has implications for understanding and improving low vision navigation and should be further studied in a clinical low vision population.
ACKNOWLEDGEMENTS
This research is supported by the National Eye Institute of the National Institutes of Health under award number R01EY017835. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
COMPLIANCE WITH ETHICAL STANDARDS
The authors declare that they have no conflicts of interest. All procedures in this study were in accordance with the ethical standards of the University of Utah Institutional Review Board and the Declaration of Helsinki. All participants provided informed consent.
A different group of 14 older adults was tested on a pilot study to affirm older adults’ abilities to do the task safely and without fatigue. The pilot study used paths similar to those used in Rand et al. (2015) Experiment 5, and compared older adults’ performance on a spatial learning task when they were navigating with their normal vision compared to when they were navigating with simulated degraded acuity and contrast sensitivity, all while guided. The results generally replicated the young adult findings, although the mean difference in pointing error between degraded vision (M=30.6, SE=3.37) and normal vision (M=25.49, SE=2.66) conditions was only marginally significant, likely because of the guidance provided in both conditions. After establishing that our methodological approach worked well with the pilot group, the current study focused on the difference in impairment between guided and unguided conditions, all with simulated degraded vision.
Power analyses based on Barhorst-Cates et al. (2016), which showed a large within-subjects difference in SUDS anxiety reports comparing 60 to 10 degree restricted field-of-view, indicated a sample size of 7 participants necessary to detect a difference at α = .05, power = .80, Cohen’s d = 1.29. The lower effect size (d = .61) calculated based on Rand et al. (2015) Experiment 2 indicated n=23 necessary to detect a difference at α = .05, power = .80. Given the smaller effect size for guidance vs. no guidance, it is possible that the current study would have detected a difference with a larger sample size, but the small observed mean difference (2.31) makes this unlikely.
Age data was obtained from 41 of 46 participants from Rand et al., (2015).
Regression analyses indicated that age did not predict the Guided-Unguided RT difference score (p=.486) or the Guided-Unguided average RT value (p=.869).
Rand et al. (2015) Experiment 5 was run with larger n=48 participants because of the intent to run a mediation analysis. Other work using this measure (Barhorst-Cates et al., 2016) has found an RT difference with n= 28.
References
- Bakdash JZ, Linkenauger SA, & Proffitt D (2008). Comparing decision-making and control for learning a virtual environment: Backseat drivers learn where they are going. Paper presented at the Proceedings of the human factors and ergonomics society annual meeting. [Google Scholar]
- Barhorst-Cates EM, Rand KM, & Creem-Regehr SH (2016). The effects of restricted peripheral field of view on spatial learning while navigating. PLoS ONE, 11(10), e0163785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates SL, & Wolbers T (2014). How cognitive aging affects multisensory integration of navigational cues. Neurobiology of aging, 35(12), 2761–2769. [DOI] [PubMed] [Google Scholar]
- Beauchet O, Annweiler C, Dubost V, Allali G, Kressig R, Bridenbaugh S, … Herrmann FR (2009). Stops walking when talking: a predictor of falls in older adults? European Journal of Neurology, 16(7), 786–795. [DOI] [PubMed] [Google Scholar]
- Berchicci M, Lucci G, Pesce C, Spinelli D, & Di Russo F (2012). Prefrontal hyperactivity in older people during motor planning. NeuroImage, 62(3), 1750–1760. [DOI] [PubMed] [Google Scholar]
- Bjork EL, & Bjork RA (2011). Making things hard on yourself, but in a good way: Creating desirable difficulties to enhance learning. Psychology and the real world: Essays illustrating fundamental contributions to society, 56–64.
- Bochsler TM, Legge GE, Gage R, & Kallie CS (2013). Recognition of ramps and steps by people with low vision. Investigative Ophthalmology & Visual Science, 54(1), 288–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochsler TM, Legge GE, Kallie CS, & Gage R (2012). Seeing Steps and Ramps with Simulated Low Acuity: Impact of Texture and Locomotion. Optometry and vision science : official publication of the American Academy of Optometry, 89(9), E1299–E1307. doi: 10.1097/OPX.0b013e318264f2bd [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS, & Mazure CM (1998). Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS). Journal of Traumatic Stress, 11, 125–136. [DOI] [PubMed] [Google Scholar]
- Brunken R, Plass JL, & Leutner D (2003). Direct measurement of cognitive load in multimedia learning. Educational Psychologist, 38(1), 53–61. [Google Scholar]
- Brunyé TT, Wood MD, Houck LA, & Taylor HA (2017). The path more travelled: Time pressure increases reliance on familiar route-based strategies during navigation. The Quarterly Journal of Experimental Psychology, 70(8), 1439–1452. [DOI] [PubMed] [Google Scholar]
- Chen KH, Chuah LY, Sim SK, & Chee MW (2010). Hippocampal region-specific contributions to memory performance in normal elderly. Brain and Cognition, 72(3), 400–407. [DOI] [PubMed] [Google Scholar]
- Chrastil ER, & Warren WH (2012). Active and passive contributions to spatial learning. Psychonomic Bulletin & Review, 19(1), 1–23. [DOI] [PubMed] [Google Scholar]
- Davidson HA, Dixon RA, & Hultsch DF (1991). Memory anxiety and memory performance in adulthood. Applied Cognitive Psychology, 5(5), 423–433. [Google Scholar]
- Driscoll I, Hamilton DA, Petropoulos H, Yeo RA, Brooks WM, Baumgartner RN, & Sutherland RJ (2003). The aging hippocampus: cognitive, biochemical and structural findings. Cerebral Cortex, 13(12), 1344–1351. [DOI] [PubMed] [Google Scholar]
- Fortenbaugh FC, Hicks JC, Hao L, & Turano KA (2007). Losing sight of the bigger picture: Periperhal field loss compresses representations of space. Vision Research, 47, 2506–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardony AL, Brunyé TT, Mahoney CR, & Taylor HA (2013). How navigational aids impair spatial memory: Evidence for divided attention. Spatial Cognition & Computation, 13(4), 319–350. [Google Scholar]
- Gardony AL, Brunyé TT, & Taylor HA (2015). Navigational aids and spatial memory impairment: the role of divided attention. Spatial Cognition & Computation, 15(4), 246–284. [Google Scholar]
- Gazova I, Laczó J, Rubinova E, Mokrisova I, Hyncicova E, Andel R, … Hort J (2013). Spatial navigation in young versus older adults. Frontiers in Aging Neuroscience, 5(94). doi: 10.3389/fnagi.2013.00094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL (2008). Cognitive neuroscience of aging. Annals New York Academy of Sciences, 1124(1), 127–144. [DOI] [PubMed] [Google Scholar]
- Harris MA, Wiener J, & Wolbers T (2012). Aging specifically impairs switching to an allocentric navigational strategy. Frontiers in Aging Neuroscience, 4(29). doi: 10.3389/fnagi.2012.00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris MA, & Wolbers T (2012). Ageing effects on path integration and landmark navigation. Hippocampus, 22(8), 1770–1780. [DOI] [PubMed] [Google Scholar]
- Head D, & Isom M (2010). Age effects on wayfinding and route learning skills. Behavioural Brain Research, 209(1), 49–58. [DOI] [PubMed] [Google Scholar]
- Hegarty M, Richardson AE, Montello DR, Lovelace K, & Subbiah I (2002). Development of a self-report measure of environmental spatial ability. Intelligence, 30, 425–447. [Google Scholar]
- Iaria G, Palermo L, Committeri G, & Barton JJ (2009). Age differences in the formation and use of cognitive maps. Behavioural Brain Research, 196(2), 187–191. [DOI] [PubMed] [Google Scholar]
- Katzman R, Brown T, Fuld P, Peck A, Schechter R, & Schimmel H (1983). Validation of a short Orientation-Memory-Concentration Test of cognitive impairment. American Journal of Psychiatry, 140(6), 734–739. [DOI] [PubMed] [Google Scholar]
- Konishi K, & Bohbot VD (2013). Spatial navigational strategies correlate with gray matter in the hippocampus of healthy older adults tested in a virtual maze. Frontiers in Aging Neuroscience, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuyk T, Elliott JL, Biehl J, & Fuhr PS (1996). Environmental variables and mobility performance in adults with low vision. Journal of the American Optometric Association, 67(7), 403–409. [PubMed] [Google Scholar]
- Legge GE, Gage R, Baek Y, & Bochsler TM (2016). Indoor Spatial Updating with Reduced Visual Information. PLoS ONE, 11(3), e0150708. doi:doi: 10.137/journalpone.0150708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legge GE, Granquist C, Baek Y, & Gage R (2016). Indoor Spatial Updating With Impaired VisionIndoor Spatial Updating With Impaired Vision. Investigative Ophthalmology & Visual Science, 57(15), 6757–6765. doi: 10.1167/iovs.16-20226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg E, & Gärling T (1982). Acquisition of locational information about reference points during locomotion: The role of central information processing. Scandinavian Journal of Psychology, 23(1), 207–218. [DOI] [PubMed] [Google Scholar]
- Long RG, Rieser JJ, & Hill EW (1990). Mobility in individuals with moderate visual impairments. Journal of Visual Impairment & Blindness [Google Scholar]
- Lövdén M, Schellenbach M, Grossman-Hutter B, Krüger A, & Lindenberger U (2005). Environmental topography and postural control demands shape aging-associated decrements in spatial navigation performance. Psychology and Aging, 20(4), 683. [DOI] [PubMed] [Google Scholar]
- Lovie-Kitchin JE, Soong GP, Hassan SE, & Woods RL (2010). Visual field size criteria for mobility rehabilitation referral. Optometry & Vision Science, 87(12), E948–E957. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Zonderman AB, & Resnick SM (2001). Age differences in spatial memory in a virtual environment navigation task. Neurobiology of aging, 22(5), 787–796. [DOI] [PubMed] [Google Scholar]
- Montello DR (1993). Scale and Multiple Psychologies of Space. Spatial Information Theory: A Theoretical Basis for GIS. Proceedings of COSIT ‘93. Lecture Notes in Computer Science vol1 716., pp. 312–321. [Google Scholar]
- Neider MB, Gaspar JG, McCarley JS, Crowell JA, Kaczmarski H, & Kramer AF (2011). Walking and talking: dual-task effects on street crossing behavior in older adults. Psychology and Aging, 26(2), 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niermeyer MA, Suchy Y, & Ziemnik RE (2017). Motor sequencing in older adulthood: relationships with executive functioning and effects of complexity. The Clinical Neuropsychologist, 31(3), 598–618. [DOI] [PubMed] [Google Scholar]
- Pelli DG (1987). The Visual Requirements of Mobility. Low Vision: Principles and Applications, pp. 134–146.
- Persson J, Lustig C, Nelson JK, & Reuter-Lorenz PA (2007). Age differences in deactivation: a link to cognitive control? Journal of Cognitive Neuroscience, 19(6), 1021–1032. [DOI] [PubMed] [Google Scholar]
- Philbeck J, Sargent J, Arthur J, & Dopkins S (2008). Large manual pointing errors, but accurate verbal reports, for indications of target azimuth. Perception, 37, 511–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand KM, Creem-Regehr SH, & Thompson WB (2015). Spatial learning while navigating with severely degraded viewing: The role of attention and mobility monitoring. Journal of Experimental Psychology: Human Perception & Performance, 41(3), 649–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand KM, Tarampi MR, Creem-Regehr SH, & Thompson WB (2011). The importance of a visual horizon for distance judgments under severely degraded vision. Perception, 40, 143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N (2000). Aging of the brain and its impact on cognitive performance: Integration of structural and functional findings
- Rieser JJ, Hill EW, Talor CR, Bradfield A, & Rosen S (1992). Visual experience, visual field size, and the development of nonvisual sensitivity to the spatial structure of outdoor neighborhoods explored by walking. Journal of Experimental Psychology: General, 121(2), 210–221. [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, Winocur G, Binns M, & Moscovitch M (2012). Remote spatial memory in aging: all is not lost. Frontiers in Aging Neuroscience, 4(25). doi: 10.3389/fnagi.2012.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein LZ (2006). Falls in older people: epidemiology, risk factors and strategies for prevention. Age and ageing, 35(suppl 2), ii37–ii41. [DOI] [PubMed] [Google Scholar]
- Schellenbach M, Lövdén M, Verrel J, Krüger A, & Lindenberger U (2010). Sensorimotor-cognitive couplings in the context of assistive spatial navigation for older adults. GeroPsych: The Journal of Gerontopsychology and Geriatric Psychiatry, 23(2), 69. [Google Scholar]
- Shumway-Cook A, Woollacott M, Kerns KA, & Baldwin M (1997). The effects of two types of cognitive tasks on postural stability in older adults with and without a history of falls. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 52(4), M232–M240. [DOI] [PubMed] [Google Scholar]
- Springer S, Giladi N, Peretz C, Yogev G, Simon ES, & Hausdorff JM (2006). Dual-tasking effects on gait variability: The role of aging, falls, and executive function. Movement Disorders, 21(7), 950–957. [DOI] [PubMed] [Google Scholar]
- Tarampi MR, Creem-Regehr SH, & Thompson WB (2010). Intact spatial updating with severely degraded vision. Attention, Perception, & Psychophysics, 72(1), 23–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend J, Adamo M, & Haist F (2006). Changing channels: an fMRI study of aging and cross-modal attention shifts. NeuroImage, 31(4), 1682–1692. [DOI] [PubMed] [Google Scholar]
- Verwey WB, & Veltman HA (1996). Detecting short periods of elevated workload: A comparison of nine workload assessment techniques. Journal of Experimental Psychology: Applied, 2(3), 270–285. [Google Scholar]
- Wiener JM, de Condappa O, Harris MA, & Wolbers T (2013). Maladaptive bias for extrahippocampal navigation strategies in aging humans. Journal of Neuroscience, 33(14), 6012–6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N, & DeGirolamo G (2012). Differential effects of aging on spatial learning through exploratory navigation and map reading. Frontiers in Aging Neuroscience, 4(14). doi: 10.3389/fnagi.2012.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yankner BA, Lu T, & Loerch P (2008). The aging brain. Annu. Rev. pathmechdis. Mech. Dis, 3, 41–66. [DOI] [PubMed] [Google Scholar]
- Zaehle T, Jordan K, Wüstenberg T, Baudewig J, Dechent P, & Mast FW (2007). The neural basis of the egocentric and allocentric spatial frame of reference. Brain Research, 1137, 92–103. [DOI] [PubMed] [Google Scholar]


