Abstract
The consumption of pharmaceuticals and personal care products (PPCPs) for controlling and preventing the COVID-19 would have sharply increased during the pandemic. To evaluate their post-pandemic environmental impacts, five categories of drugs were detected in lakes and WWTP-river-estuary system near hospitals of Jinyintan, Huoshenshan and Leishenshan in the three regions (J, H and L) (Regions J, H and L) in Wuhan, China. The total amount of PPCPs (ranging from 2.61 to 1122 ng/L in water and 0.11 to 164 ng/g dry weight in sediments) were comparable to historical reports in Yangtze River basin, whereas the detection frequency and concentrations of ribavirin and azithromycin were higher than those of historical studies. The distribution of concerned drugs varied with space, season, media and water types: sampling sites located at WWTPs-river-estuary system around two hospitals (Regions L and J) usually had relatively high waterborne contamination levels, most of which declined in autumn; lakes had relatively low waterborne contamination levels in summer but increased in autumn. The potential risks of detected PPCPs were further evaluated using the multiple-level ecological risk assessment (MLERA): sulfamethoxazole and azithromycin were found to pose potential risks to aquatic organisms according to a semi-probabilistic approach and classified as priority pollutants based on an optimized risk assessment. In general, the COVID-19 pandemic did not cause serious pollution in lakes and WWTPs-river-estuary system in Wuhan City. However, the increased occurrence of certain drugs and their potential ecological risks need further attention. A strict source control policy and an advanced monitoring and risk warning system for emergency response and long-term risk control of PPCPs is urgent.
Keywords: PPCPs, COVID-19 pandemic, Risk assessment, Wastewater treatment plants, Antiviral drugs, Antibiotics
Graphical abstract
1. Introduction
Since December 2019, the whole world has encountered a serious and challenging period due to the outbreak of coronavirus disease 2019 (COVID-19). The pandemic of COVID-19 has a substantial impact on the world in diverse ways (Ahmed et al., 2020; Kargar et al., 2020). In line with the menace to human health, another substantial issue is the environmental impacts. As reviewed by Shakil et al. (2020), the impacts of COVID-19 pandemic by and on environmental factors can been summarized into four research clusters including the environmental degradation, air pollution, climate/metrological factors and temperature. While many studies have reported dropped remarkably pollution levels in air, noise and water quality due to governmental restrictions on human and industrial activities and even lockdown of cities (Bao and Zhang, 2020; Wang and Su, 2020; Wang et al., 2021; Yunus et al., 2020; Zambrano-Monserrate et al., 2020; Zheng et al., 2020), the negative consequences due to the increasing amount of anti-epidemic chemicals should not be neglected.
For example, the sharply increased drug use for controlling and preventing COVID-19 would probably cause environmental pollution and risks for the ecology. According to the diagnosis and treatment program of COVID-19 recommend by the National Health Commission of the People's Republic of China, antiviral drugs such as ribavirin and antibiotics such as moxifloxacin hydrochloride are widely used in the treatment of COVID-19 (NHCC, 2020). Glucocorticoids such as methyl prednisone and dexamethasone are also used in therapy for the severe COVID-19 patients (Chen et al., 2020; Li et al., 2020). In addition, due to the limitation of hospital beds at the beginning, a great majority of COVID-19 cases with mild symptoms received therapy at home. Even some patients of ordinary influenza also took the above mentioned or similar drugs because of panic since many antiviral and antibiotics were sold as over-the-counter (OTC) drugs in China. Therefore, the consumption of these drugs would have sharply increased during this period. Consequently, the unconsumed drugs could be dumped into sewers and lead to elevated loads in the environment (Castillo-Zacarías et al., 2020; Reinstadler et al., 2021).
These drugs all can be cataloged as pharmaceuticals and personal care products (PPCPs). Since the treatment processes used in the wastewater treatment plants (WWTPs) are not designed for removing these substances, PPCPs could be discharged to receiving waters along with the effluents. The risk of PPCPs have during the past years been a major concern and intensively studied in the field of environmental toxicology (Nkoom et al., 2018; Wu et al., 2014; Wu et al., 2021). The aquatic environment is probably susceptible to PPCPs, because these substances have been reported to be bio-accumulative in non-target aquatic organisms at different trophic levels and specifically interact with certain target in the organisms at very low concentrations (Gaffney et al., 2015; Kumar et al., 2020; Zhang et al., 2020). Hence, it is urgent to evaluate the pollution levels and risks of the anti-epidemic pharmaceuticals in the aquatic environment post COVID-19 pandemic. Therefore, in the present study, the aims were to (1) investigate the occurrence, spatial and seasonal distributions of PPCPs in the surface water and sediments from lakes and WWTP-river-estuary system around hospitals in Wuhan, China and (2) evaluate the post-pandemic ecological risk of the detected PPCPs in the aquatic environment.
2. Materials and methods
2.1. Study area and sample collection
Wuhan (E113°41′–E115°05′, N29°58′–N31°22′), the capital of Hubei province, China, has a population of 14.19 million. A total of 19 sampling sites were designed near hospitals of Jinyintan, Huoshenshan and Leishenshan in the three regions (J, H and L), including six lakes (L1, L6–7, L12–13 and L19), three sewage discharge outlets and rivers within 1 km and 2 km from the sewage outlets (R2–4, R8–10 and R14–16), three estuaries (E5, E11 and E17) of Yangtze River and Xunsi River (R18) (Fig. 1 ). The detailed information of sampling sites is shown in Table S1.
Fig. 1.
Map showing the sampling sites in the lakes and WWTP-river-estuary system in Wuhan City.
To investigate the occurrence, spatial and seasonal distributions of PPCPs in the surface water and sediments in Wuhan City after COVID-19 epidemic, two sampling events were respectively performed in June (summer) and October (autumn) 2020 because the COVID-19 epidemic was well under control and the three Hospitals had been all closed before May 2020. Thirty-eight surface water samples (20 cm) were obtained with a 5-L glass sampler. Qisodium ethylenediamine tetraacetate (Na2EDTA) (0.25 g) and ascorbic acid (150 mg) were added in 1-L water samples for antibiotics and ribavirin analysis. Thirty-seven surficial sediments (0–5 cm) were collected using a Van Veen grab, and all samples were immediately kept on ice in the dark. Water samples were passed through 0.45 μm glass fiber filters and the filtered water samples were stored in the dark at 4 °C before extraction within 24 h. The sediments were freeze-dried, homogenized, filtered through a 100-mesh sieve, and stored at −20 °C.
2.2. Sample pretreatment and instrumental analysis
Water samples were extracted by using solid-phase extraction (SPE) method, while sediment samples were extracted by using ultrasonic-assisted extraction method, followed by clean-up step with SPE according to the previously published methods (Liu et al., 2015; Xu et al., 2019; Zhou et al., 2019). Antibiotics, glucocorticoids and ribavirin in the extracts were quantified by ultra-high-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) (Waters Acquity UPLC coupled to Waters Xevo TQD) coupled to a Colochem 5600 A electrochemical detector (ESA) equipped with a BEH-C18 column (2.1 mm × 100 mm, 1.7 μm) (Waters, USA). The present study detected 72 drugs (1 antiviral drug, 9 macrolides, 10 fluoroquinolones and 12 sulfonamides and 40 glucocorticoids) from 5 categories (Tables S2–S5), which were widely used against COVID-19 (NHCC, 2020; Chen et al., 2020) or frequently detected in aquatic environment (Li et al., 2019; Liu et al., 2015). Details for the pretreatment, analysis and also the quality control are given in the Supporting Information.
2.3. Mass loadings of PPCPs from WWTPs
Since effluents from WWTPs is a major source of PPCPs dumping to aquatic environment (Reinstadler et al., 2021), the mass loading of the determined drugs from outlets of the three WWTPs was estimated during two periods (April to June and July to October) according to the following equation: Mass loadings (kg) = Concentration (g/L) × wastewater loadings (L/d) × T (d) / 1000. April was selected as the beginning of post-pandemic time because most COVID-19 patients have been cured and discharged from hospital and people began to return to work from then on. The measured concentrations of drugs in June and October were as used as their concentrations at the two periods, respectively. The wastewater treatment ability of the three WWTPs is 10 × 107 L/d (WWTP-J), 5 × 107 L/d (WWTP-H) and 30 × 107 L/d (WWTP-L) as indicated on their official websites.
2.4. Ecological risk assessment
2.4.1. Tier-1: a primary screening
A primary risk assessment of ribavirin, antibiotics and glucocorticoids in water was performed using the deterministic quotient approach. The deterministic risk quotients (RQs) of PPCPs was calculated by the following equations:
| (1) |
here, C is the detected environmental concentration or median concentration of a single chemical in the water samples. PNEC is the predicted no-effect concentration of each chemical for aquatic organisms. PNEC is obtained from the most sensitive toxicity data with assessment factors (AFs) of 10 or 1000 based on test endpoints of EC10, EC50 or LC50 (Godoy et al., 2018; Liu et al., 2020). The EC10, LC50 and EC50 of individual chemicals were obtained from references, experimental data in this study or ECOSAR Program developed by the US EPA's Office of Chemical Safety and Pollution Prevention (Table S6). The commonly used ecological risk ranking criterion was following: RQ ≥ 1, high risk; 0.1 ≤ RQ < 1, medium risk; 0.01 ≤ RQ < 0.1, low risk; and RQ < 0.01, insignificant (Hernando et al., 2006; Sanchez-Bayo et al., 2002).
2.4.2. Tier-2: a semi-probabilistic assessment
Although the RQ approach can preliminarily reflect relative risks posed by pollutions, it cannot indicate at what degree the pollutions might actually affect the aquatic creatures. Thus, a semi-probabilistic assessment was carried out depending on the framework for ecotoxicological risk assessment (US EPA, 1998). Briefly, to calculate the frequency of exceeding PNEC, the measured environmental concentration of individual PPCPs at each sampling site was compared to its PNEC values. Concentration of pollution exceeding PNEC poses a potential risk, while concentration lower than PNEC is regarded as insignificant risk to aquatic creatures. Therefore, the frequency of exceeding PNEC can be used to prioritize the pollutants (Johnson et al., 2018). The frequency of exceeding PNEC (F) of a target chemical can be calculated as the number of sampling sites with concentration exceeding PNEC divided by the total number of sampling sites (Eq. (2)). The results suggest the proportion of sites which showed a possibility of potential risk (Ohe et al., 2011).
| (2) |
where F is the frequency of exceeding PNEC, n is the number of sampling sites with concentration exceeding PNEC and, N is the total number of sites.
2.4.3. Tier-3: an optimized risk assessment
An optimized risk assessment was conducted in line with the NORMAN Network (NORMAN Association, 2013; Tousova et al., 2017). Prioritization index (PI) was adopted to further highlight the priority of PPCPs which needed the greatest concern in lakes and WWTP-river-estuary system in Wuhan. PI was calculated by the following equation:
| (3) |
here, RQm represents the risk quotient of a single chemical based on its median concentrations and PNEC, F represents the PNEC exceeding frequency.
3. Results and discussion
3.1. Occurrence of PPCPs in water and sediments from lakes and WWTP-river-estuary system in Wuhan City
The detection frequencies and concentrations of PPCPs in water and sediments are shown in Table 1 . Twenty-seven among the 72 quantified pharmaceutical compounds, including 1 antiviral drug, 9 sulfonamides, 3 fluoroquinolones, 4 macrolides and 10 glucocorticoids, were detected in water samples, and twenty-six pharmaceutical compounds (1 antiviral drug, 7 sulfonamides, 8 fluoroquinolones, 4 macrolides and 6 glucocorticoids) in sediment samples. Among the detected PPCPs, the detection frequency of sulfadimidine, sulfamethoxazole, azithromycin, erythromycin and clarithromycin were higher than 78.9% in water samples and ribavirin up to 100% in sediments in both summer and autumn, indicating that these drugs were commonly pollutants in Wuhan city.
Table 1.
Occurrence of ribavirin, antibiotics and glucocorticoids in water (ng/L) and sediments (ng/g dry weight) from Wuhan, China.
| Analyte | Water in summer (n = 19) |
Water in autumn (n = 19) |
Sediment in summer (n = 18) |
Sediment in autumn (n = 19) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DFa (%) | Min | Max | Median | DF (%) | Min | Max | Median | DF (%) | Min | Max | Median | DF (%) | Min | Max | Median | ||
| Anti-virus drug | Ribavirin | 89.5 | 1.04 | 52.2 | 4.36 | 57.9 | 1.11 | 2.26 | 1.55 | 100 | 0.13 | 2.46 | 0.86 | 100 | 0.10 | 10.4 | 1.39 |
| Sulfonamides antibiotics | Sulfadimidine | 94.7 | 0.12 | 8.91 | 0.69 | 94.7 | 0.97 | 2.21 | 1.45 | 11.1 | 0.11 | 0.13 | 0.12 | 26.3 | 0.18 | 0.22 | 0.19 |
| Sulfamethoxazole | 84.2 | 1.12 | 16.6 | 8.48 | 94.7 | 1.42 | 11.7 | 2.63 | 11.1 | 0.18 | 0.19 | 0.18 | 10.5 | 0.11 | 0.11 | 0.11 | |
| Sulfadiazine | 63.2 | 0.10 | 2.56 | 0.49 | 57.9 | 0.84 | 9.14 | 1.48 | – | – | – | – | – | – | – | – | |
| Sulfamonomethoxine | 52.6 | 0.53 | 8.81 | 1.46 | 47.4 | 1.23 | 17.8 | 3.07 | 33.3 | 0.22 | 0.29 | 0.25 | 31.6 | 0.31 | 0.39 | 0.32 | |
| Sulfachloropyridazine | 42.1 | 0.59 | 8.15 | 1.42 | 42.1 | 0.90 | 6.00 | 3.50 | – | – | – | – | 26.3 | 0.09 | 0.10 | 0.09 | |
| Sulfaquinoxaline | 10.5 | 0.17 | 0.58 | 0.38 | 21.1 | 1.08 | 1.08 | 1.08 | – | – | – | – | 5.3 | 0.09 | 0.09 | 0.09 | |
| Sulfadimethoxine | 5.3 | 0.18 | 0.18 | 0.18 | – | – | – | – | – | – | – | – | – | – | – | – | |
| Sulfamethizole | 5.3 | 1.36 | 1.36 | 1.36 | – | – | – | – | – | – | – | – | 10.5 | 0.01 | 0.13 | 0.07 | |
| Sulfamerazine | 5.3 | 0.11 | 0.11 | 0.11 | – | – | – | – | – | – | – | – | – | – | – | – | |
| Sulfisoxazole | – | – | – | – | – | – | – | – | 33.3 | 0.28 | 0.35 | 0.31 | – | – | – | – | |
| ΣSulfonamides | – | 0.33 | 24.9 | 16.5 | – | 2.39 | 32.5 | 10.0 | – | 0.11 | 0.78 | 0.29 | – | 0.01 | 0.70 | 0.39 | |
| Fluoroquinolones antibiotics | Norfloxacin | 10.5 | 0.49 | 4.51 | 2.50 | 11.1 | 0.62 | 2.65 | 1.63 | 21.1 | 1.84 | 25.2 | 6.27 | ||||
| Ofloxacin | 5.3 | 21.0 | 21.0 | 21.0 | 10.5 | 39.0 | 172 | 106 | 50.0 | 0.15 | 17.0 | 0.37 | 89.5 | 0.04 | 125 | 0.73 | |
| Moxifloxacin hydrochloride | – | – | – | – | – | – | – | – | 33.3 | 0.87 | 2.80 | 1.49 | 31.6 | 0.53 | 2.26 | 0.76 | |
| Enoxacin | – | – | – | – | – | – | – | – | 27.8 | 0.11 | 8.48 | 0.54 | 10.5 | 2.62 | 3.47 | 3.04 | |
| Enrofloxacin | – | – | – | – | – | – | – | – | 22.2 | 0.10 | 5.45 | 1.10 | 31.6 | 0.02 | 7.70 | 0.06 | |
| Ciprofloxacin | – | – | – | – | – | – | – | – | 16.7 | 0.11 | 0.38 | 0.12 | 31.6 | 0.17 | 0.78 | 0.24 | |
| ΣFluoroquinolones | – | 0.49 | 21.0 | 4.51 | – | 39.0 | 172 | 106 | – | 0.11 | 19.2 | 1.64 | – | 0.08 | 152 | 1.40 | |
| Macrolides antibiotics | Erythromycin | 100 | 0.06 | 5.75 | 0.49 | 78.9 | 0.91 | 12.9 | 1.15 | 61.1 | 0.10 | 8.51 | 0.38 | 73.7 | 0.14 | 0.37 | 0.16 |
| Azithromycin | 94.7 | 3.14 | 935 | 43.9 | 84.2 | 1.98 | 920 | 17.4 | 83.3 | 2.70 | 41.1 | 12.6 | 52.6 | 0.43 | 39.2 | 4.87 | |
| Clarithromycin | 89.5 | 0.18 | 266 | 5.93 | 10.5 | 1.65 | 1.99 | 1.82 | 5.6 | 2.10 | 2.10 | 2.10 | – | – | – | – | |
| Tilmicosin | 42.1 | 1.26 | 7.30 | 2.60 | 10.5 | 4.80 | 11.3 | 8.03 | 66.7 | 0.12 | 14.5 | 2.88 | – | – | – | – | |
| Tylosin | 5.3 | 2.14 | 2.14 | 2.14 | – | – | – | – | – | – | – | – | – | – | – | – | |
| Spiramycin | – | – | – | – | – | – | – | – | – | – | – | – | 42.1 | 0.95 | 1.05 | 0.97 | |
| ΣMacrolides | – | 0.06 | 1100 | 29.6 | – | 1.07 | 937 | 16.1 | – | 0.19 | 45.5 | 14.0 | – | 0.14 | 40.6 | 1.56 | |
| Glucocorticoids | Triamcinolone acetonide | 21.1 | 0.28 | 0.89 | 0.35 | 10.5 | 1.13 | 2.24 | 1.68 | – | – | – | – | – | – | – | – |
| Hydrocortisone | 10.5 | 0.64 | 2.03 | 1.34 | 10.5 | 0.90 | 2.18 | 1.54 | – | – | – | – | 10.5 | 0.01 | 0.02 | 0.01 | |
| Budesonide | 10.5 | 6.65 | 15.5 | 11.1 | – | – | – | – | – | – | – | – | – | – | – | – | |
| Beclomethasone | 5.3 | 32.5 | 32.5 | 32.5 | – | – | – | – | – | – | – | – | – | – | – | – | |
| Hydrocortisone 17-valerate | 5.3 | 1.17 | 1.17 | 1.17 | – | – | – | – | – | – | – | – | – | – | – | – | |
| Prednicarbate | 5.3 | 0.27 | 0.27 | 0.27 | – | – | – | – | – | – | – | – | – | – | – | – | |
| Mometasone furoate | 5.3 | 10.8 | 10.8 | 10.8 | – | – | – | – | – | – | – | – | – | – | – | – | |
| Cortisone | – | – | – | – | 26.3 | 0.47 | 3.69 | 0.91 | – | – | – | – | – | – | – | – | |
| Fludroxycortide | – | – | – | – | 10.5 | 2.86 | 5.36 | 4.11 | – | – | – | – | – | – | – | – | |
| Fluoromethalone | – | – | – | – | 10.5 | 0.66 | 0.67 | 0.67 | – | – | – | – | 10.5 | 0.10 | 0.11 | 0.10 | |
| Prednisone 21-acetate | – | – | – | – | – | – | – | – | 100 | 0.22 | 0.88 | 0.48 | 5.3 | 0.01 | 0.01 | 0.01 | |
| Hydrocortisone 21-acetate | – | – | – | – | – | – | – | – | – | – | – | – | 15.8 | 0.02 | 0.03 | 0.03 | |
| Prednisolone | – | – | – | – | – | – | – | – | – | – | – | – | 5.3 | 0.08 | 0.08 | 0.08 | |
| ΣGlucocorticoids | – | 0.28 | 32.5 | 2.03 | – | 0.47 | 5.72 | 2.99 | – | 0.22 | 0.88 | 0.48 | – | 0.01 | 0.12 | 0.03 | |
DF: detection frequency.
The concentration of each drug in surface water was lower than 1000 ng/L and usually varied from ND (not detected) to hundreds ng/L, which was similar to most of previous studies on PPCPs in the world (Arikan et al., 2008; Bu et al., 2013; Kim et al., 2007; Simazaki et al., 2015). The total concentrations of PPCPs ranged from 2.61 to 1122 ng/L (median: 45.2 ng/L) in water and 0.11 to 164 ng/g dry weight (dw) (median: 12.0 ng/g dw) in sediments from lakes and effluents-rivers-estuary system in Wuhan City, which were comparable to historical reports of Yangtze River basin of China (Zhou et al., 2019; Xu et al., 2019). Thus, the COVID-19 pandemic did not aggravate the PPCPs pollution in aquatic environment in Wuhan City. In this study, antibiotics were the mainly PPCPs category, which was consistent with previous studies in China (Bu et al., 2013; Xu et al., 2019). For sulfonamides, the concentrations of sulfamethoxazole in water in Wuhan City were comparable to those in central and lower Yangtze River (ND– 18.5 ng/L) (Wu et al., 2014), but the highest level of sulfadiazine in water was nearly two orders of magnitudes lower than those in the Pearl Rivers (726 ng/L) (Yang et al., 2011). For fluoroquinolones, the maximum concentration of ofloxacin in water (172 ng/L) were comparable to those in lakes along the middle and lower reaches of the Yangtze River Basin (106 ng/L) (Li et al., 2019), and ofloxacin, norfloxacin and enrofloxacin in sediments similar to those (235 ng/g, 14.5 ng/g and 2.45 ng/g) in the streams near livestock farms in China (Zhou et al., 2013). However, for macrolides, the detection frequency as well as concentrations of azithromycin were much higher than those in studies before COVID-19 pandemic, where azithromycin was detetected with frequency of 11.9% (up to 4.3 ng/L) in the water and 7.96% (up to 6.68 ng/g dw) in the sediments from the lower-middle reaches of the Yangtze River (Zhou et al., 2019).
3.2. Spatial and seasonal variation of PPCPs in surface water and sediments from lakes and river-estuary system in Wuhan
3.2.1. Surface water
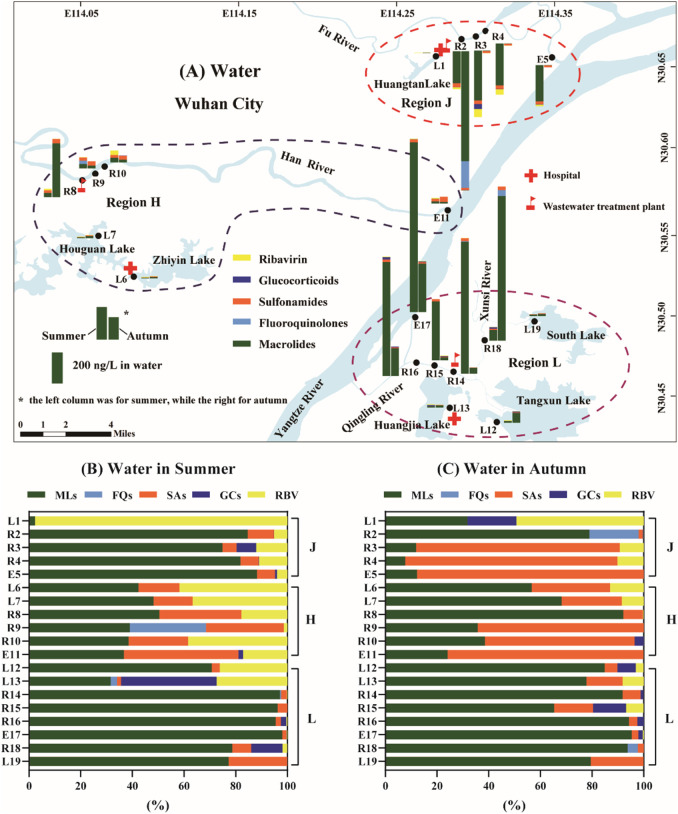
In summer, the total concentrations of PPCPs in water samples of lakes were much lower than those in WWTP-river-estuary system (Fig. 2A), because the sewage from the hospitals and residents living were passed though wastewater treatment plants and discharged into rivers instead of lakes. This result was consistent with previous studies that the contents of antibiotics in lakes were lower than those in Yangtze River (Wu et al., 2014). Among the five categories of drugs, macrolides were dominant in 17 out of 19 of water samples in summer with contribution rates ranging from 38.5% to 97.7% (Fig. 2B). The dominance of the macrolides has been observed in urban rivers of Japanese (Murata et al., 2011) and in the Yangtze River of China (Li et al., 2019; Liu et al., 2020; Wu et al., 2014). The spatial distribution pattern of total PPCPs concentration in WWTP-rivers system in the Region L was significantly higher than those in the Region H and Region J (P < 0.01) (Fig. S1). Region H has the lowest concentrations of total PPCPs, which may be because it has the lower population density, and that Huoshen Hospital has been closed in 15th, April 2020. Jinyintan Hospital is the first to receive COVID-19 patients, and still be responsible for the remaining patients after Leishen and Huoshen Hospital closed. Therefore, the concentrations of PPCPs in the rivers of Region J was higher than that in the Region H. Although Leishen hospital in Region L was also closed, the concentrations of anti-epidemic drugs at R14–R16 were the highest among the three regions. There may be several reasons: (i) the population density in the Region L has higher than that in the Region J and Region H; (ii) Qingling River received additional wastewater from other WWTPs, and it had poor dilution capacity compared with Fu River and Han River. In addition, the water from South lake and Tangxun Lake was also delivered via Xunsi River and Qingling River, then pumped into underground culvert, and discharged into Yangtze River through a pumping station located near E17. Therefore, the estuary of Qingling River (E17) has the highest concentrations of PPCPs among the three estuaries (E5, E11 and E17), and even among all sampling sites.
Fig. 2.
Occurrence of five categories of PPCPs in surface water from Wuhan City. (A) Spatial and seasonal distributions of PPCPs in three regions. (B) The composition of PPCPs in surface water in summer. (C) The composition of PPCPs in surface water in autumn. RBV: ribavirin; FQs: fuoroquinolones; SAs: sulfonamides; MLs: macrolides; GC: glucocorticoids.
In autumn, the total amount of waterborne drugs in most of the sampling sites located at rivers and their estuaries were lower than those in summer, which were mainly due to the decreases of macrolides (Fig. 2A and C). The three highest concentrations of PPCPs in water were located at R2, R8 and R18, but their concentrations in autumn were 3 to 11 times higher than those in summer. The sampling sites of R2 and R8 were nearby the drain outlets, whereas R18 located at Xunsi River, where small drain outlets were also observed. These results also supported previous reports that wastewater effluents were a major source of antiviral and antibiotics to aquatic environment in rivers-estuary system (Reinstadler et al., 2021).
In addition, in most sampling sites of lakes (5 out of 7), the total amounts of waterborne drugs were increased to varying degrees. One possible explanation is that during the lockdown, human activities that may cause pollution have also been prohibited. Furthermore, the amount of precipitation in Wuhan city in June of rainy season was more than 3 times than that in October of dry season, which caused dilution of the PPCPs concentrations. Improved water quality has been reported in several studies after the pandemic (Yunus et al., 2020). However, in autumn, the production and life have almost recovered as before the pandemic, and the increased human activities may be responsible for the increased drugs concentrations in lakes post pandemic.
3.2.2. Sediment
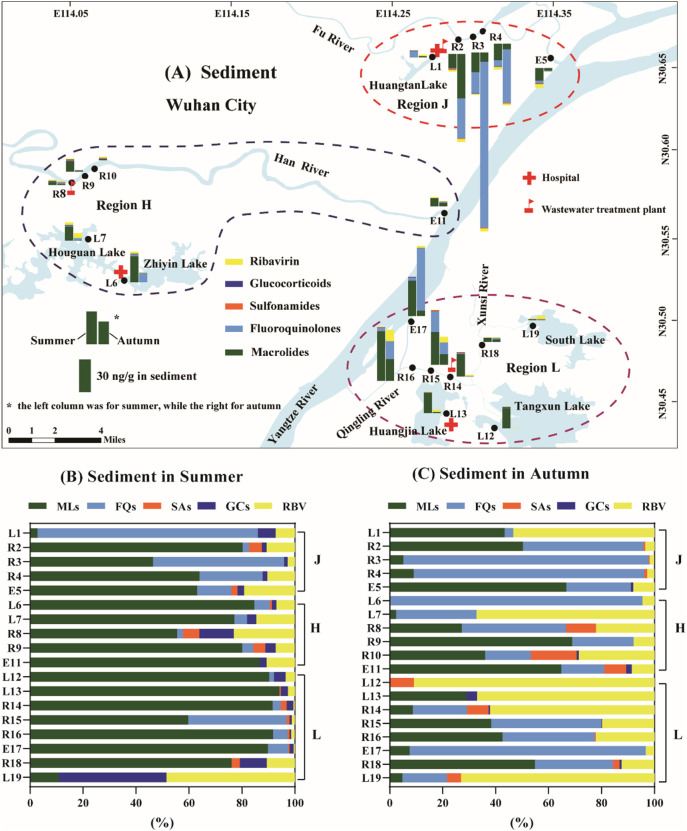
In summer, the total amount of PPCPs in sediments from river-estuary system in Region J (R2–R4 and E5) and Region L (R14– R16 and E17) were higher than those in Region H (R8–R9 and E11) (Fig. 3A). Among the five categories of drugs, macrolides were still dominant in all sampling sites located at rivers and their estuary in summer, followed by fluoroquinolones and ribavirin (Fig. 3B). It should be noted that the concentrations of macrolides and total PPCPs in sediments in summer were both statistically related to those in water (P < 0.01) (Fig. S2), suggesting the pollution of PPCPs in sediments could be sourced from those in water.
Fig. 3.
Occurrence of five categories of PPCPs in sediments from Wuhan City.
(A) Spatial and seasonal distributions of PPCPs in three regions. (B) The composition of PPCPs in sediments in summer. (C) The composition of PPCPs in sediments in summer. RBV: ribavirin; FQs: fuoroquinolones; SAs: sulfonamides; MLs: macrolides; GC: glucocorticoids.
In autumn, the total amount of PPCPs were decreased in sediments from a majority of sampling sites in rivers and estuaries in Region H and Region L. These spatial and seasonal variance of PPCPs in sediments were similar those in water phase, suggesting that the source of drugs in sediments may due to their adsorption and sedimentation in water. In contrast, the total amounts of PPCPs in sediments from R2– R4 and E17 in autumn were higher than those in summer. It is noted that the levels of fluoroquinolones in sediments in autumn are obviously higher than those in summer and became the dominant among the five categories in most rivers and their estuary (Fig. 3C). Furthermore, the concentrations of fluoroquinolones in sediments in autumn were statistically related to those in summer (P < 0.01) (Fig. S3). These results indicated that fluoroquinolones could be accumulated in the sediments, which could be attributed to their low biodegradation, hydrolysis and photodegradation in water (Jiao et al., 2008; Kümmerer, 2009) or their high organic carbon-water partition coefficients (Kd) resulting in their accumulation to sediment (Rabølle and Spliid, 2000). Previous studies have also reported that fluoroquinolones had relative high contribution rates comparing with macrolides and sulfonamides, because of their high partitioning and low biodegradation rates in sediment (Liu et al., 2020; Ying et al., 2013).
3.3. Mass loadings of drugs from WWTPs
As we have estimated, the usage of anti-epidemic pharmaceuticals according to the reported number of cases and recommended prescription, the total usage of seven recommended pharmaceuticals (lopinavir/ritonavir, ribavilin, albido, moxifloxacin hydrochloride, chloroquine phosphate, methyl prednisolone) could be about 2472 kg in Wuhan during the COVID-19 pandemic, with ribavilin at the top place (Table S7). However, this does not include the usage by COVID-19 patients as well as ordinary influenza patients received therapy at home, which could be much more both in quantity and in variety. The residuals of these chemicals can be dumped to the sewers and discharged into rivers along with effluents from WWTPs. Moreover, these drugs will surely be continuously used post pandemic not only for human health, but also for animal husbandry. The total amounts of the determined drugs discharged into rivers were estimated to be 40.0 kg, 2.57 kg and 8.50 kg from WWTP-J, WWTP-H and WWTP-L, respectively (Table 2 ). While the mass loading of most drugs was declined in the second period, fluoroquinolones and macrolides from WWTP-J as well as sulfonamides and macrolides from WWTP-H were increased in the second period, indicating increased use of these drugs post pandemic. The widely usage and continuously inputs of these drugs could cause seriously pollution in water, and then could induce potential risk to the aquatic environment.
Table 2.
Estimated mass loading of determined drugs from three WWTPs in Wuhan post COVID-19 pandemic.
| Drugs | WWTP-J (kg) |
WWTP-H (kg) |
WWTP-L (kg) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| March–June | July–October | Total | March–June | July–October | Total | March–June | July–October | Total | |
| Antiviral drug | 0.34 | 0.04 | 0.38 | 0.04 | 0.01 | 0.05 | 0.01 | 0.00 | 0.01 |
| Sulfonamides | 0.68 | 0.54 | 1.22 | 0.08 | 0.18 | 0.26 | 0.17 | 0.04 | 0.21 |
| 0.20 | 6.36 | 6.56 | 0.00 | 0.00 | 0.00 | 0.06 | 0.00 | 0.06 | |
| Macrolides | 5.46 | 26.3 | 31.7 | 0.12 | 2.14 | 2.26 | 7.75 | 0.46 | 8.22 |
| Glucocorticoids | 0.01 | 0.08 | 0.09 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.01 |
| Total (kg) | 6.69 | 33.3 | 40.0 | 0.25 | 2.32 | 2.57 | 8.00 | 0.50 | 8.50 |
3.4. Ecological risk assessment
The potential ecological risk for the detected PPCPs in surface water from the lakes and WWTP-river-estuary system of Wuhan City was assessed using the multiple-level ecological risk assessment (MLERA) in accordance with previously published methods (Liu et al., 2020). Due to lack of toxicity data for benthons and risk assessment methods for the sediment, this study only evaluated the potential risks upon waterborne exposure by the target drugs.
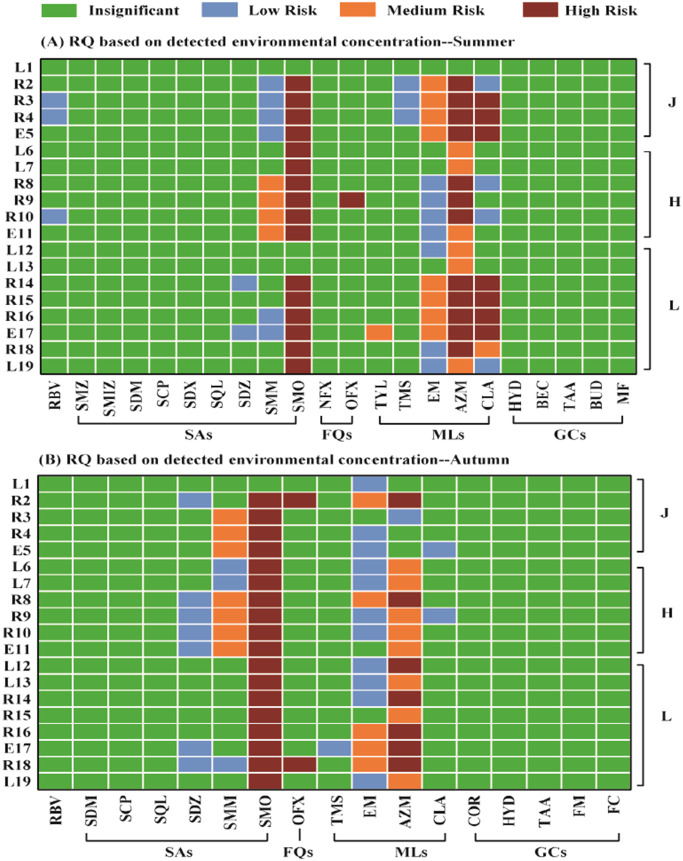
The RQ based on the measured environmental concentration is a useful method which can be indicated whether the relative risk is high or low in water samples. As shown in Fig. 4 , the RQs for glucocorticoids were all less than 0.01 in either summer or autumn indicating insignificant risks. Ribavirin was found to low ecological risk in 3 water samples in summer, and insignificant in autumn. However, certain antibiotics posed medium to high risk in the aquatic environment of Wuhan city, especially in WWTPs-river-estuary system. For sulfonamides, sulfamethoxazole posed high risk in 16 out of 19 sampling sites in summer and 18 out of 19 in autumn, while sulfamonomethoxine was found to medium risk in 4 sampling sites in summer and 7 sites in autumn. For fluoroquinolones, ofloxacin was occasionally appeared with high risk, occurring at site R9 in summer and sites R2 and R18 in autumn. For macrolides, azithromycin posed high risks in 12 out of 13 river-estuary sampling sites in summer and 6 sites in autumn, while azithromycin posed medium risks in 5 out of 6 lakes. In summer, clarithromycin posed high risks in 7 out of 19 sampling sites, and erythromycin posed medium risks in 8 out of 19 sites, which were all occurring in WWTPs-river-estuary system of Region J and Region L. It is noticed that the sampling sites facing high risk were mostly occurring at WWTPs- river-estuary system, and the ecological risk of antibiotics in the rivers were higher than those in the lakes. Previous studies in the Yangtze River Basin before COVID-19 pandemic also reported medium to high risks of sulfamethoxazole, sulfamonomethoxine and ofloxacin to aquatic organisms, but no risk of azithromycin, erythromycin and clarithromycin (Li et al., 2019; Guo et al., 2019; Zhou et al., 2019).
Fig. 4.
RQs of detected PPCPs to the most sensitive species across sampling sites.
The risk quotients (RQ) based on the measured concentration of PPCPs in surface water at an individual location of Wuhan City in summer (A) and autumn (B). SAs: sulfonamides; FQs: fuoroquinolones; MLs: macrolides; GCs: glucocorticoids; RBV: ribavirin; SMZ: sulfamerazine; SMIZ: sulfamethizole; SDM: sulfadimidine; SCP: sulfachloropyridazine; SDX: sulfadimethoxine; SQL: sulfaquinoxaline; SDZ: sulfadiazine; SMM: sulfamonomethoxine; SMO: sulfamethoxazole; NFX: norfloxacin; TMS: tilmicosin; OFX: ofloxacin; TYL: tylosin; EM: erythromycin; AZM: azithromycin; CLA: clarithromycin; HYD: hydrocortisone; BEC: beclomethasone; TAA: triamcinolone acetonide acetate; BUD: budesonide; MF: mometasone furoate; COR: cortisone; FM: fluoromethalone; FC: fludroxycortide.
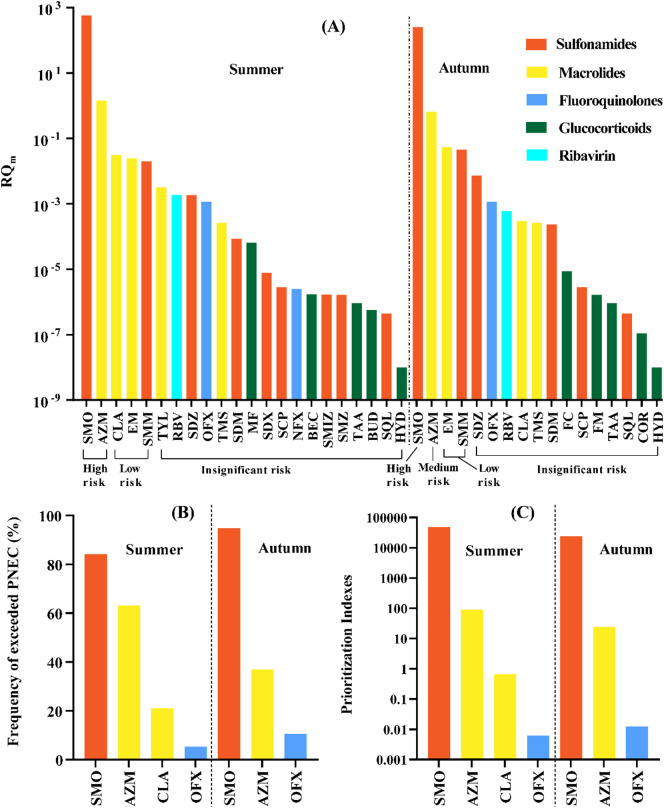
The RQ values based on the median concentrations of detected PPCPs were ranked in descending order (Fig. 5A). Among 26 PPCPs that posted potential ecological risk in the aquatic environment (RQ ≥ 0.01) were all antibiotics. Sulfamethoxazole was found to pose greatest risk to the aquatic organisms, with the RQ of 579 in summer and 252 in autumn, due to its toxic potency to Caenorhabditis elegans (Yu et al., 2011). Azithromycin posed high risk to aquatic systems in summer, but dropt to medium risk in autumn. Clarithromycin, erythromycin and sulfamonomethoxine could also have low ecological risk. In this study, a number of antibiotics were detected at relatively high detection frequencies and concentrations. However, only sulfamethoxazole and azithromycin were considered to pose high risks to aquatic organisms in surface water of Wuhan city. This was consistent with previous reports that, among 18 antibiotics, only tetracycline and sulfamethoxazole were found to pose high risks in river system of China (Liu et al., 2020).
Fig. 5.
The RQm (A), frequency of exceeded PNEC (B) and prioritization indexes (C) based on the median concentration of PPCPs in surface water of Wuhan City.
The RQm based on median concentration could be distort by the detection frequency, so the potential risks of PPCPs were further evaluated using a semi-probabilistic approach and optimized risk assessment. A semi-probabilistic approach screened sulfamethoxazole and azithromycin which were found to have potential risks (Fig. 5B). A possible threat was found in ofloxacin that posed no risk to aquatic species according to the RQ method. To make priority drugs more sensible, the exceeding PNEC value was compare with the detection frequency. For instance, the exceeding PNEC values of sulfamethoxazole were the same as detection frequencies, both of which were 84% in summer and 95% in autumn. So aquatic species could be at ecological risk once sulfamethoxazole was found in surface water. In optimized risk assessment method, both frequency and concentration were considered. As shown in Fig. 5C, there were 4 prioritized PPCPs in summer and 3 PPCPs in autumn based on PI in descending order, which were similar to those in semi-probabilistic approach. Sulfamethoxazole and azithromycin were classified as priority drugs in accordance with the optimized risk assessment, with PI of 48,793 for sulfamethoxazole and 90 for azithromycin in summer. Liu et al. (2020) also reported in Chinese surface waters that sulfamethoxazole was categorized as priority pollution, with PI of 5666 and 94% frequency of exceeding PNEC. It is noticed that the ecological risk of ofloxacin in water of Wuhan city was found to insignificant using the optimized method and the RQ method based on median concentration, but it could not be completely ignored due to the 11% frequency of exceeding PNEC. Over all, in the present study, the environmental risks of certain antibiotics were ranked higher than other drugs to aquatic species, which was consisted with those in stream and drinking water of United Sates (Kumar and Xagoraraki, 2010). The potential ecological risks of sulfamethoxazole and azithromycin is need to take more attention and controll seriously in wastewater effluents and receiving rivers in the future.
4. Conclusions
Above all, the total concentrations of PPCPs, ranging from 2.61 to 1122 ng/L in water and 0.11 to 164 ng/g in sediments, were comparable or lower than those reported in Yangtze River basin before COVID-19 pandemic. However, ribavirin and azithromycin had higher detection frequency and concentrations in aquatic environment of Wuhan than historically reported. Ribavirin and azithromycin, as well as many other antiviral and antibiotics, are sold as over-the-counter (OTC) drugs in China, thus our results may indicate that a strict usage control of these drugs is needed. The spatial distribution revealed relative high environmental loads of PPCPs in wastewater receiving rivers when compared with those in lakes. The order of environmental loads of PPCPs among the three regions was: Region L > Region J > Region H. Although the waterborne concentrations of most concerned drugs showed decline in autumn when compared to summer, those of azithromycin were notably increased especially in river-estuary locations receiving effluents. The ecological risk assessment using MLERA indicated that ribavirin and glucocorticoids posed low to insignificant risks, but 6 antibiotics (sulfamethoxazole, sulfamonomethoxine, ofloxacin, azithromycin, erythromycin and clarithromycin) posed medium to high risks in the aquatic environment of Wuhan city. Sulfamethoxazole and azithromycin were classified as priority pollutions in accordance with the optimized risk assessment. At the beginning of 2021, the second round of COVID-19 storm is coming around China as well as all over the world. A mature monitoring and risk warning system concerning PPCPs basing on both emergency response and long-term risk control is extremely urgent. Only in this way, can we be prepared for the challenges in the future.
CRediT authorship contribution statement
Xiangping Chen: Investigation, Data curation, Methodology. Lei Lei: Investigation, Data curation, Methodology. Sitian Liu: Investigation, Data curation. Jian Han: Investigation, Software. Ruiwen Li: Investigation, Software. Jun Men: Formal analysis, Validation. Lin Li: Formal analysis, Supervision. Lin Wei: Formal analysis, Supervision. Yaqi Sheng: Conceptualization, Data curation. Lihua Yang: Conceptualization, Investigation, Software, Formal analysis, Writing – original draft. Bingsheng Zhou: Conceptualization, Funding acquisition, Supervision. Lizhong Zhu: Conceptualization, Funding acquisition, Project administration, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by grants from the consulting research project of Chinese Academy of Engineering (2020-ZD-15), the State Key Laboratory of Freshwater Ecology and Biotechnology (2019FBZ03). We would like to thank the Analysis and Testing Center of Institute of Hydrobiology for assistance in analysis of PPCPs.
Editor: Daqiang Yin
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2021.148352.
Appendix A. Supplementary data
Supplementary material
References
- Ahmed W., Tscharke B., Bertsch P.M., Bibby K., Bivins A., Choi P. Sars-Cov-2 RNA monitoring in wastewater as an early warning system for COVID-19 transmission in the community: a temporal case study. Sci. Total Environ. 2020;761 doi: 10.1016/j.scitotenv.2020.144216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arikan O.A., Rice C., Codling E. Occurrence of antibiotics and hormones in a major agricultural watershed. Desalination. 2008;226:121–133. [Google Scholar]
- Bao R., Zhang A. Does lockdown reduce air pollution? Evidence from 44 cities in northern China. Sci. Total Environ. 2020;731 doi: 10.1016/j.scitotenv.2020.139052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu Q., Wang B., Huang J., Deng S., Yu G. Pharmaceuticals and personal care products in the aquatic environment in China: a review. J. Hazard. Mater. 2013;262:189–211. doi: 10.1016/j.jhazmat.2013.08.040. [DOI] [PubMed] [Google Scholar]
- Castillo-Zacarías C., Barocio M.E., Hidalgo-Vázquez E., Sosa-Hernández J.E., Parra-Arroyo L., López-Pacheco I.Y., et al. Antidepressant drugs as emerging contaminants: occurrence in urban and non-urban waters and analytical methods for their detection. Sci. Total Environ. 2020;757 doi: 10.1016/j.scitotenv.2020.143722. [DOI] [PubMed] [Google Scholar]
- Chen H., Xie J., Su N., Wang J., Sun Q., Li S., et al. Corticosteroid therapy is associated with improved outcome in critically ill COVID-19 patients with hyperinflammatory phenotype. Chest. 2020;159:1793–1802. doi: 10.1016/j.chest.2020.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffney V.D., Almeida C.M.M., Rodrigues A., Ferreira E., Benoliel M.J., Cardoso V.V. Occurrence of pharmaceuticals in a water supply system and related human health risk assessment. Water Res. 2015;72:199–208. doi: 10.1016/j.watres.2014.10.027. [DOI] [PubMed] [Google Scholar]
- Godoy A.A., Domingues I., Nogueira A.J.A., Kummrow F. Ecotoxicological effects, water quality standards and risk assessment for the anti-diabetic metformin. Environ. Pollut. 2018;243:534–542. doi: 10.1016/j.envpol.2018.09.031. [DOI] [PubMed] [Google Scholar]
- Guo X., Feng C., Gu E., Tian C., Shen Z. Spatial distribution, source apportionment and risk assessment of antibiotics in the surface water and sediments of the Yangtze estuary. Sci. Total Environ. 2019;671:548–557. doi: 10.1016/j.scitotenv.2019.03.393. [DOI] [PubMed] [Google Scholar]
- Hernando M.D., Mezcua M., Fernandez-Alba A.R., Barcelo D. Environmental risk assessment of pharmaceutical residues in wastewater effluents, surface waters and sediments. Talanta. 2006;69:334–342. doi: 10.1016/j.talanta.2005.09.037. [DOI] [PubMed] [Google Scholar]
- Jiao S., Zheng S., Yin D., Wang L., Chen L. Aqueous photolysis of tetracycline and toxicity of photolytic products to luminescent bacteria. Chemosphere. 2008;73(3):377–382. doi: 10.1016/j.chemosphere.2008.05.042. [DOI] [PubMed] [Google Scholar]
- Johnson A.C., Jürgens M.D., Su C., Zhang M., Zhang Y., Shi Y. Which commonly monitored high risk chemical in the Bohai Region, Yangtze and Pearl Rivers of China poses the greatest threat to aquatic wildlife? Environ. Toxicol. Chem. 2018;37:1115–1121. doi: 10.1002/etc.4042. [DOI] [PubMed] [Google Scholar]
- Kargar S., Pourmehdi M., Paydar M.M. Reverse logistics network design for medical waste management in the epidemic outbreak of the novel coronavirus (COVID-19) Sci. Total Environ. 2020;746 doi: 10.1016/j.scitotenv.2020.141183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.D., Cho J., Kim I.S., Vanderford B.J., Snyder S.A. Occurrence and removal of pharmaceuticals and endocrine disruptors in South Korean surface, drinking, and waste waters. Water Res. 2007;41:1013–1021. doi: 10.1016/j.watres.2006.06.034. [DOI] [PubMed] [Google Scholar]
- Kumar A., Xagoraraki I. Pharmaceuticals, personal care products and endocrine-disrupting chemicals in U.S. surface and finished drinking waters: a proposed ranking system. Sci. Total Environ. 2010;408:5972–5989. doi: 10.1016/j.scitotenv.2010.08.048. [DOI] [PubMed] [Google Scholar]
- Kumar V., Singh S.B., Singh S. COVID-19: environment concern and impact of Indian medicinal system. J. Environ. Chem. Eng. 2020;8 doi: 10.1016/j.jece.2020.104144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kümmerer K. Antibiotics in the aquatic environment – a review – part I. Chemosphere. 2009;75:417–434. doi: 10.1016/j.chemosphere.2008.11.086. [DOI] [PubMed] [Google Scholar]
- Li L., Liu D., Zhang Q., Song K., Zhou X.H., Tang Z., et al. Occurrence and ecological risk assessment of selected antibiotics in the freshwater lakes along the middle and lower reaches of Yangtze River basin. J. Environ. Manag. 2019;249 doi: 10.1016/j.jenvman.2019.109396. [DOI] [PubMed] [Google Scholar]
- Li Y., Shi K., Qi F., Yu Z., Chen C., Pan J., et al. Thalidomide combined with short-term low-dose glucocorticoid therapy for the treatment of severe COVID-19: a case-series study. Int. J. Infect. Dis. 2020;103:507–513. doi: 10.1016/j.ijid.2020.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., Zhang D., Xiao X., Cui L., Zhang H. Occurrence of quinotone antibiotics and their impacts on aquatic environment in typical river-estuary system of Jiaozhou Bay, China. Ecotoxicol. Environ. Saf. 2020;190 doi: 10.1016/j.ecoenv.2019.109993. [DOI] [PubMed] [Google Scholar]
- Liu S., Chen H., Zhou G.J., Liu S.S., Yue W.Z., Yu S., Sun K.F., Cheng H.F., Ying G.G., Xu X.R. Occurrence, source analysis and risk assessment of androgens, glucocorticoids and progestagens in the Hailing Bay region, South China Sea. Sci. Total Environ. 2015;536:99–107. doi: 10.1016/j.scitotenv.2015.07.028. [DOI] [PubMed] [Google Scholar]
- Murata A., Takada H., Mutoh K., Hosoda H., Harada A., Nakada N. Nationwide monitoring of selected antibiotics: distribution and sources of sulfonamides, trimethoprim, and macrolides in japanese rivers. Sci. Total Environ. 2011;409:5305–5312. doi: 10.1016/j.scitotenv.2011.09.014. [DOI] [PubMed] [Google Scholar]
- National Health Commission of the People'’s Republic of China (NHCC) 2020. http://www.nhc.gov.cn/yzygj/s7653p/202001/4294563ed35b43209b31739bd0785e67 [DOI] [PMC free article] [PubMed]
- Nkoom M., Lu G.H., Liu J.C. Occurrence and ecological risk assessment of pharmaceuticals and personal care products in Taihu Lake, China: a review. Environ. Sci. Process. Imp. 2018;20:1640–1648. doi: 10.1039/c8em00327k. [DOI] [PubMed] [Google Scholar]
- NORMAN Association . 2013. NORMAN Prioritisation Framework for Emerging Substances. ISBN: 978-2-9545254-0-2. [Google Scholar]
- Ohe P.C.V.D., Dulio V., Slobodnik J., Deckere E.D., Kühne R., Ebert R.U., Ginebreda A., Cooman W.D., Schüürmann G., Brack W. A new risk assessment approach for the prioritization of 500 classical and emerging organic microcontaminants as potential river basin specific pollutants under the European Water Framework Directive. Sci. Total Environ. 2011;409:2064–2077. doi: 10.1016/j.scitotenv.2011.01.054. [DOI] [PubMed] [Google Scholar]
- Rabølle M., Spliid N.H. Sorption and mobility of metronidazole, olaquindox, oxytetracycline and tylosin in soil. Chemosphere. 2000;40:715–722. doi: 10.1016/s0045-6535(99)00442-7. [DOI] [PubMed] [Google Scholar]
- Reinstadler V., Ausweger V., Grabher A.-L., Kreidl M., Huber S., Grander J., et al. Monitoring drug consumption in Innsbruck during coronavirus disease 2019 (COVID-19) lockdown by wastewater analysis. Sci. Total Environ. 2021;757 doi: 10.1016/j.scitotenv.2020.144006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Bayo F., Baskaran S., Kennedy I.R. Ecological relative risk (EcoRR): another approach for risk assessment of pesticides in agriculture. Agric. Ecosyst. Environ. 2002;91:37–57. [Google Scholar]
- Shakil M.H., Munim Z.H., Tasnia M., Sarowar S. COVID-19 and the environment: a critical review and research agenda. Sci. Total Environ. 2020;745 doi: 10.1016/j.scitotenv.2020.141022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simazaki D., Kubota R., Suzuki T., Akiba M., Nishimura T., Kunikane S. Occurrence of selected pharmaceuticals at drinking water purification plants in Japan and implications for human health. Water Res. 2015;76:187–200. doi: 10.1016/j.watres.2015.02.059. [DOI] [PubMed] [Google Scholar]
- Tousova Z., Oswald P., Slobodnik J., Blaha L., Muz Me, Hu M. European demonstration program on the effect-based and chemical identification and monitoring of organic pollutants in European surface waters. Sci. Total Environ. 2017;601–602:1849–1868. doi: 10.1016/j.scitotenv.2017.06.032. [DOI] [PubMed] [Google Scholar]
- US EPA . United States Environmental Protection Agency; Washington, DC: 1998. Guidelines for Ecological Risk Assessment. [Google Scholar]
- Wang Q., Su M. A preliminary assessment of the impact of COVID-19 on environment — a case study of China. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Zhang Y., Ma J., Zhu S., Shen J., Wang P., et al. Responses of decline in air pollution and recovery associated with COVID-19 lockdown in the Pearl River delta. Sci. Total Environ. 2021;756 doi: 10.1016/j.scitotenv.2020.143868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Huang X., Witter J.D., Spongberg A.L., Wang K., Wang D., et al. Occurrence of pharmaceuticals and personal care products and associated environmental risks in the central and lower Yangtze River, China. Ecotoxicol. Environ. Saf. 2014;106:19–26. doi: 10.1016/j.ecoenv.2014.04.029. [DOI] [PubMed] [Google Scholar]
- Wu D., Sui Q., Yu X., Zhao W., Li Q., Fatta-Kassinos D., et al. Identification of indicator ppcps in landfill leachates and livestock wastewaters using multi-residue analysis of 70 PPCPs: analytical method development and application in Yangtze River delta, China. Sci. Total Environ. 2021;753 doi: 10.1016/j.scitotenv.2020.141653. [DOI] [PubMed] [Google Scholar]
- Xu M., Huang H., Li N., Li F., Wang D., Luo Q. Occurrence and ecological risk of pharmaceuticals and personal care products (PPCPs) and pesticides in typical surface watersheds, China. Ecotoxicol. Environ. Saf. 2019;175:289–298. doi: 10.1016/j.ecoenv.2019.01.131. [DOI] [PubMed] [Google Scholar]
- Yang J.F., Ying G.G., Zhao J.L., Tao R., Su H.C., Liu Y.S. Spatial and seasonal distribution of selected antibiotics in surface waters of the Pearl Rivers, China. J. Environ. Sci. Health B Pest. Food Contam. Agric. Wastes. 2011;46:272–280. doi: 10.1080/03601234.2011.540540. [DOI] [PubMed] [Google Scholar]
- Ying G.G., Zhao J.L., Zhou L.J., Liu S. In: Comprehensive Analytical Chemistry. Petrovic M., Barcelo D., Pérez S., editors. vol. 62. Elsevier; 2013. Chapter 14 - fate and occurrence of pharmaceuticals in the aquatic environment (surface water and sediment) pp. 453–557. [Google Scholar]
- Yu Z.Y., Jiang L., Yin D.Q. Behavior toxicity to Caenorhabditis elegans transferredto the progeny after exposure to sulfamethoxazole at environmentally relevant concentrations. J. Environ. Sci. 2011;23:294–300. doi: 10.1016/s1001-0742(10)60436-6. [DOI] [PubMed] [Google Scholar]
- Yunus A.P., Masago Y., Hijioka Y. COVID-19 and surface water quality: improved lake water quality during the lockdown. Sci. Total Environ. 2020;731 doi: 10.1016/j.scitotenv.2020.139012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrano-Monserrate M.A., Ruano M.A., Sanchez-Alcalde L. Indirect effects of COVID-19 on the environment. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Chen H., Jing L., Teng Y. Ecotoxicological risk assessment and source apportionment of antibiotics in the waters and sediments of a peri-urban river. Sci. Total Environ. 2020;731 doi: 10.1016/j.scitotenv.2020.139128. [DOI] [PubMed] [Google Scholar]
- Zheng H., Kong S., Chen N., Yan Y., Liu D., Zhu B., et al. Significant changes in the chemical compositions and sources of PM2.5 in Wuhan since the city lockdown as COVID-19. Sci. Total Environ. 2020;739 doi: 10.1016/j.scitotenv.2020.140000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L.J., Ying G.G., Liu S., Zhang R.Q., Lai H.J., Chen Z.F., et al. Excretion masses and environmental occurrence of antibiotics in typical swine and dairy cattle farms in China. Sci. Total Environ. 2013;444:183–195. doi: 10.1016/j.scitotenv.2012.11.087. [DOI] [PubMed] [Google Scholar]
- Zhou L.J., Li J., Zhang Y.D., Kong L.Y., Jin M., Yang X.D., et al. Trends in the occurrence and risk assessment of antibiotics in shallow lakes in the lower-middle reaches of the Yangtze River basin, China. Ecotoxicol. Environ. Safe. 2019;183 doi: 10.1016/j.ecoenv.2019.109511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material