Abstract
The Severe Acute Respiratory Syndrome Coronavirus 2 (SARSCoV- 2) emerged in Wuhan City, China. The SARS-CoV-2 crossed borders and quickly transformed into a “Public health emergency of international concern”. Countries around the globe are in the race to achieve herd immunity. We describe the steps taken by Saudi Arabia to achieve this goal.
1. Introduction
The emergence of the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) was in the Wuhan City, China [1,2]. The SARS-CoV-2 crossed borders and quickly transformed into a “Public health emergency of international concern”. The SARS-CoV-2 virus incites an important disease called Coronavirus Disease 2019 (COVID-19) where its symptoms fall into a spectrum of severity ranging from mild disease to severe illness [3]. Typical symptoms of COVID-19 include fever, shortness of breath and cough, whereas less common symptoms involve sudden onset of ageusia and/or anosmia, diarrhea, nausea and vomiting [4]. Despite the immediate response to this public health emergency, the SARS-CoV-2 virus was moving at a fast speed. By March 2020, the World Helath Organization (WHO) declared COVID-19 a pandemic and the disease later went on to claim 2,684,471 lives as of March 17, 2021 and caused 116 million cases globally. The number of cases in the Kingdom of Saudi Arabia (KSA) had reached 385,424 cases on March 22nd, 2021 and 6613 mortalities.
With modest insight on combating this pandemic, few medications have been trialed – both old and new- and only a small number of medications has shown to be potentially effective against the virus [5]. By the end of 2020, and with the hastened efforts of researchers worldwide, a few vaccines were approved to fight back the pandemic and protect at-risk groups from severe morbidity and mortality.
In the Arabian Gulf Countries, the first cases of COVID-19 were travel-associated cases [6,7] and the first case in KSA was reported on March 2nd, 2020 [6]. Early on, KSA implemented multiple interventions to decrease the number of cases and slow the spread of SARS-CoV-2 infection into the country [6,8,9].
These strategies included: cancellation of social events, mass gathering events, citywide festivals, religious gatherings, cultural celebrations and scientific conferences [6]. All international flights were suspended on March 15, 2020 and KSA adopted quarantines of returning travelers in ministry of health operated or supervised hotels across the country [7].
2. Vaccines
The BNT162b2 mRNA (Pfizer-BioNtech) vaccine was the first vaccine to be approved for use in KSA in mid-December 2020. In addition to the initial 500,000 doses that were delivered during the month of December and were administered to vaccinnees by the end of January 2021. An additional inflow of 10 million doses are expected to be delivered between February and September 2021.
The chimpanzee adenovirus vectored vaccine ChAdOx1 nCoV-19 (AZD1222) of the Oxford-AstraZeneca was the second vaccine that was approved for use in KSA as of February 2021. During the month of February, 3 million doses of Oxford-AstraZeneca (AZD1222) reached the Kingdom, with an additional 7 million doses awaiting transportation during March 2021. Due to the flexibility in storage and handling of the Oxford-AstraZeneca (AZD1222) vaccine, it is easy to distribute the vaccine to all regions of the Kingdom and be stored in central warehouses prior distribution (a pull model of supply chain).
There is a large number of required logistics for the distribution of BNT162b2 mRNA (Pfizer-BionTech) COVID-19 vaccine including cold chain maintenance. KSA had addressed many issues related to vaccine delivery including: supply chain, governance, operations, digital communication, clinical care and education, quality assurance, and costumer experience. Integrated processes ensure that supply is being carefully accounted for. Every spillage, breakage, damaged, or contaminated vials must be reported through the incident reporting and ticketing systems for further investigation. When the above procedure cannot be followed or there are any concerns about the safety of personnel this must be escalated to the Nominated Responsible Person at the designated site. In turn, they should contact the senior clinical person in the project management organization. For the development of any vaccine-related adverse events, the ministry of health (MOH) partnered with the Saudi Food and Drug Administration (SFDA) to have an electronic reporting system. These events are reviewed by a panel of experts to characterize the type of the adverse event and the relation to the administered vaccine.
3. Planning and coordination
KSA is very proactive in the preparation for the availability of COVID-19 vaccine. There had been multiple activities including establishing COVID-19 vaccine deployment and vaccination coordination mechanism. The activation of the national immunization technical advisory group and the establishment of the COVID-19 vaccine committee. These bodies reviewed all vaccines in the pipeline and worked diligently to establish a chain of reporting and management structure.
4. Strategy and regulatory process plan
4.1. Vaccine allocation and distribution throughout the regions in a timely fashion
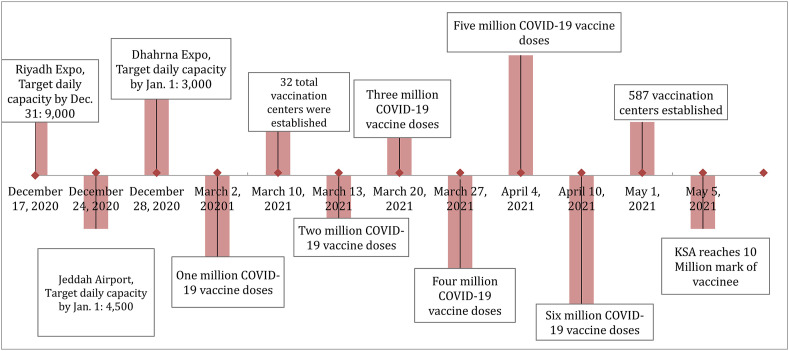
In the beginning of this program, there was a challenge in the distribution of limited vaccine supply, however, to overcome this challenge; a distribution plan was constructed based on the size of the population of each city, prioritizing cities of high population (Riyadh, Jeddah, Dammam, Madinah, and Makkah). Additionally, vaccine availability was taken into consideration and vaccine allocation has been performed accordingly. Moving forward, larger quantities of the vaccine was provided so a ramp up plan was proposed to achieve herd immunity and eventually limit the spread of the virus and the progression of COVID-19. The plan was to expand vaccination operations to include dedicated vaccine centers (mega sites, Expos, old airport terminals, etc.) and leverage pre-existing medical facilities (Primary Healthcare Centers, field hospitals, public hospitals) (Fig. 1 ). Program expansion also included in-hospital deployment program (inpatient, specialized centers like long-term care), non-MOH health systems, university hospitals and private sector. As of February 28th, 2021, 122 sites have been activated throughout the regions, and 783,720 doses have been administered (504,004 and 279,716 first and second doses respectively). The initial plan was to administer 6.5 Million doses in the first quarter of 2021 and 10.5 Million in the second quarter for a total of 17 Million doses (accounting for the coverage of 8.5 Million people). In parallel, a plan is being put in place to prepare for capacity to vaccinate up to 17.4 Million adults (70% of the adult population of Saud Arabia) by the thord quarter of 2021 contingent on vaccine supply.
Fig. 1.
The Timeline of the launch of and mile stones of COVID-19 Vaccines in the Kingdom of Saudi Arabia.
4.2. Identification of target populations
As the global and local demands for approved COVID-19 vaccines are escalating, with the scarcity of clinical trials confirming the optinal medical management, it becomes crucial to establish an efficient distribution plan for vaccines in KSA, where populations are prioritized, and waste is eliminated. In this context, vaccine distribution in KSA is based on a phased approach. Three identified phases and priority population in each phase were identified (Table 1 ). The three phases take into consideration the estimated size of targeted population, avoidance of any threat to the integrity of the health care system, assurance of continuity of essential services, reduction of severe morbidity and mortality rates associated to COVID-19, diminishing transmission rate of infection to the populations at greatest risk in the community, generation of herd immunity by expanding vaccination to include everyone.
Table 1.
Prioritization of COVID-19 vaccination in Saudi Arabia.
| Priority | Description | Estimated size (Millions of populations) | Estimated period of time for vaccination strategy |
|---|---|---|---|
| Priority 1 |
|
3 | By July 2021 |
| Priority 2 |
|
10 | By July 2021 |
| Priority 3 | The rest of the population | 15 | By September 2021 |
BMI: Body mass index in kg/m2.
Phase one is targeting healthcare workers at higher risk for exposure to COVID-19 infection, individuals in security and military sectors, and all individuals at increased risk for sever disease (those above 65 of age and individuals with > 2 chronic medical conditions. Phase two is targeting other front-liners (healthcare workers, security, and military) and essential workers in the public and private sector (education, communications, transportation, energy, etc.) and those with one chronic medical condition. Phase three is directed to the rest of the population. All these phases include citizens and residents at no cost to any individual. These priorities are put in place as it is expected that the supply would not be enough to provide coverage for all the population as it is in other countries [10]. Of course, these guidelines are preliminary and are subjective to further refinement based on the supply, availability of vaccines, and any new scientific data. The recommendations were based also on the epidemiology of COVID-19 in KSA [11,12], ethical principles, and vaccination implementation. The available seroprevalence of COVID-19 studies indicate pockets of infection among certain population and certain provinces [11,12].
As of February 2021, the vaccine supply increased rapidly, allowing the vaccination to be expanded to additional critical populations and to the general public. Therefore, it is important to note that the recommendations on the phased population groups to receive initial doses of vaccine are according to vaccine availability, depending on each vaccine's characteristics, vaccine supply, disease epidemiology, and local community factors.
Allocation of COVID-19 vaccines depends on vaccine-specific storage and transport conditions and delivery schedules. Due to the requirements to have −70C of the Pfizer-Biotech vaccine, KSA designated large exhibition centers in key cities for the administration of the vaccine (Fig. 1). For the first month, the vaccination activities were limited to five central mega centers with ultra-cold storage and transport capabilities.
Prior to the start of the vaccination program, there had been a campaign. The campaign prioritized frontline healthcare workers namely staff working in emergency rooms, fever clinics, drive-through testing centers, intensive care units, isolation wards in addition to public health, and infection control professionals. From the higher risk groups, older individuals (>65 years) were the highest priority including those living in nursing homes. The next priority population includes those with chronic medical conditions that increase the risk of severe COVID-19, immune-suppressed individuals. The decision to include solid-organ transplant, hematological malignancies, and cancer, was supported by professional national societies.
No effort was spared to assure the highest level of vaccine equity. The government announced the launch of a public registration system that prioritizes vaccination appointments based on preset conditions. Citizens, expatriates, and visitors are all eligible to register and receive the vaccine free of charge. It was necessary to arrange for proxy registration for those who cannot access the system for any reason. It's worth mentioning that refugees are awarded special residency permits that enable them to use government services including vaccination services.
4.3. Estimating size of targeted populations
The size of the population being considered for each category was based on the total number of residents and citizens of KSA with a target to vaccinate 28 (87%) out of 32 million (adult population). The size of vaccine in each category was based on the available epidemiology of COVID-19 in KSA, seroprevalence of SARS-CoV-2 antibodies, high-risk groups for complication and data from the MOH. The risk of hospitalization increased from 2.5 times to 5X in individuals with 1 condition and those with ≥ 3 conditions, respectively [10].
4.4. Assuring equity in distribution
Two important guiding principles were utilized during the early phase of the COVID-19 vaccine distribution: equity and risk-mitigation. A Royal decree had been issued that the vaccine should be provided to all Saudi citizens and residents, including illegal residents, equally and at no cost to the individual. This point is particularly important to address as COVID-19 has affected minorities disproportionately [13]. All efforts are also taken to ensure the distribution of the vaccine in rural and urban communities as COVID-19 also tends to have unique contribution in these different settings [14]. In addition, risk mitigation was based on the risk factors that had been identified among those infected with COVID-19 internationally and nationally [[15], [16], [17]].
4.4.1. Standardization and quality management
In its aspiration toward a standardized error-free process of immunization, the MOH has regulated a blueprint, that is comprised of carefully written Standard Operation Procedures (SOPs) and demonstrates thoroughly all activities and interactions which may be encountered by all stakeholders. This blueprint is being used all stakeholders to initiate, execute, control, and close any phase of the immunization campaign.
The quality team of the MOH aimed to cover all aspects of the program in the blueprint. Governance with all the stakeholders, Governing Committees-roles and responsibilities, and the Ticketing system. Operations are in charge of operating different vaccine administration sites including mega site, premium outlets, Drive-throughs, hospitals and primary care clinics (PHCs). Clinical care and Education included vaccine handling and storing, procedure for safe administration of COVID-19 vaccine, management of clinical waste, observing adverse events response (AER), management, and reporting, clinical audit, distribution of training materials, and maintenance of training records. Quality assurance included origination and control of the SOPs, control of documents and records, internal quality management audits, quality management review and risk assessment. Digital team included online and/or offline vaccine recording, reporting, and end-of-day reconciliation. Moreover, communication, supply chain and customer experience were also covered by SOPs of the blueprint. Therefore, maintaining optimum quality throughout the vaccination operation program.
4.4.2. Workforce and training competency
The vaccination program started by preparing the regulatory clinical team through training and education, in order to prepare for COVID-19 vaccine application and distribution throughout the regions in a timely fashion. Therefore, training the vaccination managers, both at regional and site levels, by conducting an in-person and virtual COVID-19 vaccination clinical and digital training programs. These were achieved with contribution of all the regional leads and supervisors, introducing powerful managerial and planning skills, including effective monitoring of the vaccination system, confirming infection control surveillance, efficient work and ensuring strong continuous communication among the clinical staff. Healthcare workers, including vaccinators are provided with an electronic COVID-19 vaccination training platform that has been developed (Vaccine-specific), which provides training material, SOP's and an assessment that vaccinators need to submit with a passing grade to earn the eligibility to vaccinate. Verification of the Saudi Commission for Health Specialty (SCFHS) registration documents is needed, in order to certify those who passed the vaccine-specific test. The training platform covers the requirements of robust skills in relation to the correct handling and administration of the new vaccine, storage, and equipment managing. Also, there had been the developement of tools such as audit checklist, adverse reaction reports and incident reports. These are implemented to help monitor and investigate any vaccination related events. It is necessary that vaccinators learn great interpersonal communication skills because they are in direct contact with the community and are the key in building the society trust for vaccination. Therefore, it is important to ensure proper education and update of the immunization supervisors and healthcare workers on differential aspects of COVID-19 vaccination programs and provide these teams with required tool to do their responsibilities in a successful and safe manner.
4.4.3. Establishment of vaccination operation center
KSA was among the first countries in the world to roll-out the vaccination program using Pfizer-BioNTech on the 17th of December 2020. This was achieved through the COVID-19 Vaccination Operation Program (VOP). Developing this program during the COVID-19 pandemic required diligent preparation, management, and resources, to ensure the alignment of all stakeholders and to overcome unexpected challenges, and to guarantee positive reaction of the population at large, thus increasing vaccination acceptance and demand. The program was created to manage and monitor the daily operations of the vaccination activities, which helps in monitoring vaccine-administration, tracking and solving of any developing issues. Moreover, utilizing the ticketing system which is created mainly to enable quality assurance team to efficiently manage tickets progress and deliver a consistent customer service experience.
4.5. Overcoming encountered vaccine related obstacles
Constant monitoring throughout COVID-19 vaccination program is crucial for early detection of vaccine related obstacles and problems to ensure successful implementation of the program.
4.6. Adverse reactions related to the vaccine
The first obstacle is the potential developement of COVID-19 vaccine related adverse events that require close observing in order to ensure vaccinees' health and wellbeing post vaccination. These adverse events can range from dizziness and fever to vaso-vagal attacks and anaphylactic reactions which could be fatal if not managed properly. For that, the Saudi MOH Emergency Medical Protocol for Management of Anaphylactic Reaction was published and the use of SFDA platform for adverse events reporting (Tayaqath; meaning be vigilent) was mandated to determine the frequency and severity of vaccine related adverse events. Furthermore, this platform has been integrated with Sehaty (my health App) to properly manage those who experienced hypersensitivity and anaphylaxis in the first dose and pintend to receive the second dose.
4.6.1. Vaccine related incidents
In order to overcome the second obstacle, an incidents reporting system (IRS) tool was developed to monitor vaccine related technical incidents. This includes a number of incidents types like spillage, breakage, fire, shortage of supply, needlestick injuries and other types of incidents that can occur at vaccination sites. This tool helps MOH in monitoring vaccine related incidents, taking actions and solving critical issues. For instance, syringe quality is important for safe and proper vaccination. Therefore, it is the responsibility of MOH in collaboration with the supply chain partner: National Unified Procurement Company (NUPCO) to provide top-quality products and provide alternatives in case of reoccurring incidents. In addition, some incidents, like in case of finding particulate matter in the vaccine vial, require the intervention of other entities. This will initiate a cascade of events starting with raising the issue to SFDA to arrange vials collection for investigation and testing and ends with reports being shared to identify the cause of such an incident.
4.7. Knowledge, attitude, and experience toward COVID-19 vaccines in general population of Saudi Arabia
Since the start of the vaccination program, the population have expressed different opinions toward the vaccine. Two previous surveys of the acceptance of the vaccines for COVID-19 showed a rate of 45% in 3101 participants and in 65% among 992 participants [18,19]. The acceptance of COVID-19 among healthcare workers (HCWs) was 70% especially among male HCWs and those who believe in vaccine safety [20]. Among HCWs, 24.4% were willing to receive the ChAdOx1 COVID-19 vaccine, and 20.9% were willing to receive the mRNA BNT162b2 vaccine [21]. In the early phase of the enrollment of the mRNA vaccine in KSA, 352 (33.27%) of 1058 HCWs were enrolled to receive or received the vaccine [22].
4.8. The way forward
Giving the increasing number of COVID-19 around the globe, the need for herd immunity is with no doubt an important issue to tackle. KSA had put a very active process to provide immunization to all residents. The MOH had made the vaccine available at all healthcare sectors including the MOH facilities, institutions, private hospitals and clinics as well as large public pharmacies. This active process is reflected by the fact that the SFDA approved the Pfizer-BioNTech COVID-19 vaccine as early as the scientific publication of the results of the Phase III clinical trial. The mass vaccination campaigns are already scaled up to all citizens and residents free of charge. The breakdown of the population who received vaccination by age group is shown in Table 2 as retrieved via the national vaccine registry (NVR) system. In addition, the numbers of HCWs and non-healthcare workers who were registered or vaccinated are shown in Table 3 . The first one million vaccinees was completed in more than 2 months, the second million was accomplished in 10 days and the third million in 7 days. The registration for the vaccine had been automated through MOH Sehhaty (my health) App thus facilitate ease and timely access. Daily MOH briefings and updates include strong messages for the public to accept the vaccine and share histories of those who were hesitant to take the vaccine and unfortunately developed COVID-19 with poor clinical outcome. The coverage of the vaccination in KSA is being escalated and currently about 13 million (43%) had been vaccinated. On the other hand, the total number of COVID-19 cases in the country had reached 442,071 with 7264 (1.6%) death. However, it is too early to see the effect of the vaccine on the total cases and death. KSA is taking proactive steps to have all the population be vaccinated including the use of mobile Apps to show vaccination status before entering public and Governmental buildings.
Table 2.
The breakdown of the population who received vaccination by age group, data were retrieved via the national vaccine registry (NVR) system.
| age group | Estimated Population | AstraZeneca-Oxford vaccine | Pfizer-BioNTech Vaccine |
|---|---|---|---|
| 17–25 | 4,987,675 | 641,239 | 854,875 |
| 26–45 | 13,603,824 | 2,924,283 | 2,984,716 |
| 46–50 | 2,460,559 | 391,728 | 471,358 |
| 51–64 | 3,676,997 | 574,683 | 998,795 |
| >65 | 1,099,151 | 126,923 | 384,596 |
Table 3.
The number of the healthcare workers versus non-healthcare workers registered and vaccinated. The data retrieved via NVR (number of vaccination) and Sehaty App (number of registration).
| Registered | Vaccinated | |
|---|---|---|
| Healthcare workers | 481,876 | 383,821 |
| Non-healthcare workers | 14,388,646 | 11,645,461 |
Financial support and sponsorship
Nil.
CRediT authorship contribution statement
Abdullah Assiri: Conceptualization, Writing – original draft. Jaffar A. Al-Tawfiq: Conceptualization, Writing – original draft. Monira Alkhalifa: Formal analysis, Writing – original draft. Hessa Al Duhailan: Formal analysis, Writing – original draft. Sara Al Qahtani: Formal analysis, Writing – original draft. Reema Abu Dawas: Formal analysis, Writing – original draft. Abdul Aziz El Seoudi: Formal analysis, Writing – original draft. Najd Alomran: Formal analysis, Writing – original draft. Omar Abu Omar: Formal analysis, Writing – original draft. Nawaf Alotaibi: Formal analysis, Writing – original draft. Sami S. Almudarra: Formal analysis, Writing – original draft. Khalid Alabdulkarim: Formal analysis, Writing – original draft. Saleh Alqahtani: Formal analysis, Writing – original draft. Hani Jokhdar: Conceptualization, Writing – original draft, All authors finalized and approved the final draft of the manuscript.
Declaration of competing interest
There are no conflicts of interest.
References
- 1.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;6736:1–9. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta O.P., Bhandari P., Raut A., Kacimi S.E.O., Huy N.T. Coronavirus disease (COVID-19): comprehensive review of clinical presentation. Front Public Heal. 2021;8 doi: 10.3389/fpubh.2020.582932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel A., Charani E., Ariyanayagam D., Abdulaal A., Denny S.J., Mughal N., et al. New-onset anosmia and ageusia in adult patients diagnosed with SARS-CoV-2 infection. Clin Microbiol Infect. 2020;26:1236–1241. doi: 10.1016/j.cmi.2020.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fiorino S., Tateo F., De Biase D., Giuseppe Gallo C., Emilio Orlandi P., Corazza I., et al. 2021. SARS-CoV-2 PANDEMIC: a narrative review ON lessons and viewpoints arising from both the history OF medicine and from the biological behaviour OF other well-known viruses. Preprints. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Tawfiq J.A., Memish Z.A. COVID-19 in the eastern mediterranean region and Saudi Arabia: prevention and therapeutic strategies. Int J Antimicrob Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Tawfiq J.A., Sattar A., Al-Khadra H., Al-Qahtani S., Al-Mulhim M., Al-Omoush O., et al. Incidence of COVID-19 among returning travelers in quarantine facilities: a longitudinal study and lessons learned. Trav Med Infect Dis. 2020;38 doi: 10.1016/j.tmaid.2020.101901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Tawfiq J.A., Leonardi R., Fasoli G., Rigamonti D. Prevalence and fatality rates of COVID-19: what are the reasons for the wide variations worldwide? Trav Med Infect Dis. 2020;35:101711. doi: 10.1016/j.tmaid.2020.101711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi H., Han X., Jiang N., Cao Y., Alwalid O., Gu J., et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dooling K., Marin M., Wallace M., McClung N., Chamberland M., Lee G.M., et al. The advisory committee on immunization practices' updated interim recommendation for allocation of COVID-19 vaccine — United States, december 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1657–1660. doi: 10.15585/mmwr.mm695152e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alserehi H.A., Alqunaibet A.M., Al-Tawfiq J.A., Alharbi N.K., Alshukairi A.N., Alanazi K.H., et al. Seroprevalence of SARS-CoV-2 (COVID-19) among healthcare workers in Saudi Arabia: comparing case and control hospitals. Diagn Microbiol Infect Dis. 2020 doi: 10.1016/j.diagmicrobio.2020.115273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banjar A., Al-Tawfiq J.A., Alruwaily A., Alserehi H., Al-Qunaibet A., Alaswad R., et al. Seroprevalence of antibodies to SARS-CoV-2 among blood donors in the early month of the pandemic in Saudi Arabia. Int J Infect Dis. 2021;104 doi: 10.1016/j.ijid.2021.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tirupathi R., Muradova V., Shekhar R., Salim S.A., Al-Tawfiq J.A., Palabindala V. COVID-19 disparity among racial and ethnic minorities in the US: a cross sectional analysis. Trav Med Infect Dis. 2020;38:101904. doi: 10.1016/j.tmaid.2020.101904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bohrn M.A., Benenson R., Bush C.M., Bell T., Black C., Doan B., et al. Demographics and clinical characteristics of adult patients hospitalized due to COVID-19 in a rural/suburban integrated health system in southcentral Pennsylvania, March through may 2020. Open Forum Infect Dis. 2021 doi: 10.1093/ofid/ofab132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.AlJishi J.M., Alhajjaj A.H., Alkhabbaz F.L., AlAbduljabar T.H., Alsaif A., Alsaif H., et al. Clinical characteristics of asymptomatic and symptomatic COVID-19 patients in the Eastern Province of Saudi Arabia. J Infect Public Health. 2021;14:6–11. doi: 10.1016/j.jiph.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al Mutair A., Alhumaid S., Alhuqbani W.N., Zaidi A.R.Z., Alkoraisi S., Al-Subaie M.F., et al. Clinical, epidemiological, and laboratory characteristics of mild-to-moderate COVID-19 patients in Saudi Arabia: an observational cohort study. Eur J Med Res. 2020;25:61. doi: 10.1186/s40001-020-00462-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Omari A., Alhuqbani W.N., Zaidi A.R.Z., Al-Subaie M.F., AlHindi A.M., Abogosh A.K., et al. Clinical characteristics of non-intensive care unit COVID-19 patients in Saudi Arabia: a descriptive cross-sectional study. J Infect Public Health. 2020;13:1639–1644. doi: 10.1016/j.jiph.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magadmi R.M., Kamel F.O. Beliefs and barriers associated with COVID-19 vaccination among the general population in Saudi Arabia. Res Sq. 2020 doi: 10.21203/rs.3.rs-48955/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Mohaithef M., Padhi B.K. p>Determinants of COVID-19 vaccine acceptance in Saudi Arabia: a web-based national survey</p>. J Multidiscip Healthc. 2020;13:1657–1663. doi: 10.2147/JMDH.S276771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barry M., Temsah M.-H., Alhuzaimi A., Alamro N., Al-Eyadhy A., Aljamaan F., et al. COVID-19 vaccine confidence and hesitancy among healthcare workers: a cross-sectional survey from a MERS-CoV experienced nation. MedRxiv. 2020 doi: 10.1101/2020.12.09.20246447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Temsah M.H., Barry M., Aljamaan F., Alhuzaimi A., Al-Eyadhy A., Saddik B., et al. Adenovirus and RNA-based COVID-19 vaccines: perceptions and acceptance among healthcare workers. MedRxiv. 2020 doi: 10.1101/2020.12.22.20248657. 2020.12.22.20248657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barry M., Temsah M.-H., Aljamaan F., Saddik B., Al-Eyadhy A., Alenezi S., et al. COVID-19 vaccine uptake among healthcare workers in the fourth country to authorize BNT162b2 during the first month of rollout. MedRxiv. 2021 doi: 10.1101/2021.01.29.21250749. 2021.01.29.21250749. [DOI] [PMC free article] [PubMed] [Google Scholar]