Abstract
Purpose
With the spread of transjugular intrahepatic portosystemic shunts (TIPS), portosystemic shunt surgery (PSSS) has decreased and leaves more complex patients with great demands for accurate preoperative planning. The aim was to evaluate the role of imaging for predicting the most suitable PSSS approach.
Material and methods
Forty-four patients who underwent PSSS (2002 to 2013) were examined by contrast-enhanced CT (n = 33) and/or MRI (n = 15) prior to surgery. Imaging was analyzed independently by two observers (O1 and O2) with different levels of experience (O1 > O2). They recommended two shunting techniques (vessels and anastomotic variant) for each patient and ranked them according to their appropriateness and complexity. Findings were compared with the actually performed shunt procedure and its outcome.
Results
The first two choices taken together covered the performed PSSS regarding vessels in 88%/100% (CT/MRI, O1) and 76%/73% (O2); and vessels + anastomosis in 79%/73% (O1) and 67%/60% (O2). The prediction of complex surgical procedures (resection of interposing structures, additional thrombectomy, use of a collateral vessel, and use of a graft interposition) was confirmed in 87%, resulting in 80% sensitivity and 96% specificity. Larger shunt vessel distances were associated with therapy failure (p = 0.030) and a vessel distance of ≥ 20 mm was identified as optimal cutoff, in which a graft interposition was used. There was no significant difference between MRI and CT in predicting the intraoperative decisions (p = 0.294 to 1.000).
Conclusion
Preoperative imaging and an experienced radiologist can guide surgeons in PSSS. CT and MRI provide the information necessary to identify technically feasible variants and complicating factors.
Electronic supplementary material
The online version of this article (10.1007/s00261-020-02599-z) contains supplementary material, which is available to authorized users.
Keywords: Portosystemic shunt surgery, Magnetic resonance imaging, Computed tomography, Portal hypertension
Introduction
Transjugular intrahepatic portosystemic shunt (TIPS) has become the most common intervention used today to treat intrahepatic portal hypertension, PHT [1]. Before even being proved to be better than portosystemic shunt surgery (PSSS), TIPS quickly spread worldwide [2]. Moreover, there are various conditions in which TIPS is not feasible or even recommendable [3]. TIPS is usually not used in the treatment of pre- and posthepatic PHT [4]. Specifically, TIPS is not suitable for patients with extrahepatic veno-occlusive disease, in which the vascular connection between splenic and portal circulation is interrupted, or for patients with advanced hepatic cirrhosis, because of the high risk of hepatic encephalopathy [5]. Contraindications to the placement of a TIPS include severe cardiac disease (e.g., congestive heart failure), multiple hepatic cysts, systemic infection/sepsis, or unrelieved biliary obstruction [6]. Nevertheless, these patients need to be treated, and PSSS is indicated to prevent variceal bleeding, spontaneous bacterial peritonitis, hepatorenal syndrome, and progressive liver failure [3, 7–14].
Due to the widespread use of TIPS, PSSS is becoming less common and experience with this operation is diminishing. There are many surgical portosystemic (PS) shunt options to consider in the preoperative planning (e.g., portacaval, mesocaval, splenorenal). The different PSSS techniques modify hepatopetal blood flow in different ways and can be categorized into nonselective, partially selective, and selective types. Nonselective PS shunt techniques establish complete drainage of portal as well as mesenterial blood flow into the inferior vena cava. Partially selective shunt techniques maintain the PS pressure gradient and thus ensure residual blood flow in the portal vein. Selective techniques separate the downgradient via esophageal collaterals from the portal hypertensive system and therefore have the smallest effects on portal perfusion. Interestingly, there are no long-term differences in terms of recurrent bleeding, hepatic encephalopathy, or overall survival between the different shunt techniques [15, 16]. This is in part attributable to a loss of selectivity over the years [17]. Since there is no “standard” shunt, the anatomy of the individual must be evaluated in each case to select the most appropriate shunting procedure. Careful planning, which shunting vessels and anastomosis (e.g., end-to-side, side-to-side) to choose, is necessary to avoid shunt failure or hepatic encephalopathy caused by (too) high shunt volume. Another shunt technique besides the more common shunt variants already mentioned is the mesoportal (Meso-Rex) shunt, which actually is a bypass rather than a true PS shunt. Meso-Rex shunts are of special interest in children and younger patients with extrahepatic portal occlusion. They maintain liver function and hepatopetal flow, therefore mostly avoiding hypersplenism, coagulopathies, and hyperammonemia while at the same time allowing normal neurological development to continue [18–21].
Nonetheless, PSSS is invasive and is associated with the intra- and perioperative risks of major abdominal vascular surgery—its major disadvantage in comparison to TIPS. Therefore, precise preoperative imaging and planning are of utmost importance.
The purpose of this study was to evaluate whether CT and MRI are sufficiently accurate and reliable for the preoperative planning of PSSS in patients who are not suitable for a TIPS procedure. With decreasing surgeon experience caused by the widespread of TIPS, a radiologist may be in an important position to assist the surgical team with pre-surgical planning to help guide patient management.
Material and methods
Patient population
Sixty-five patients who underwent PSSS between March 2002 and September 2013 were retrospectively identified from the surgical database of our hospital. Surgical reports, histology reports, cross-sectional imaging datasets, laboratory results, and medical records were obtained to generate the endpoint parameter. The patients underwent PSSS after having been classified as unsuitable for TIPS procedure in an interdisciplinary discussion. The main reason for not choosing TIPS was prehepatic PHT with chronic extrahepatic portal vein occlusion in 31 patients (70%). In seven pediatric patients with patent portal venous flow, PSSS was preferred due to the still incomplete body growth. In two patients, PSSS was indicated because of chronic TIPS occlusion. One patient was treated with PSSS during a hemicolectomy for cecum carcinoma to avoid a further intervention. Another patient was unsuitable for TIPS because of a previously performed right hepatic trisectionectomy because of a Klatskin’s tumor. In the other two patients, PSSS was preferred because of vascular anomalies (intrahepatic portal vein dysplasia and a mesenteric arteriovenous malformation). Nineteen of the initially identified patients were excluded because of incomplete medical records or lack of preoperative imaging. Two patients underwent an atypical shunt procedure, which was not part of the retrospective imaging-based evaluation. These two patients also had to be excluded. Forty-four patients were finally included (Fig. 1).
Fig. 1.
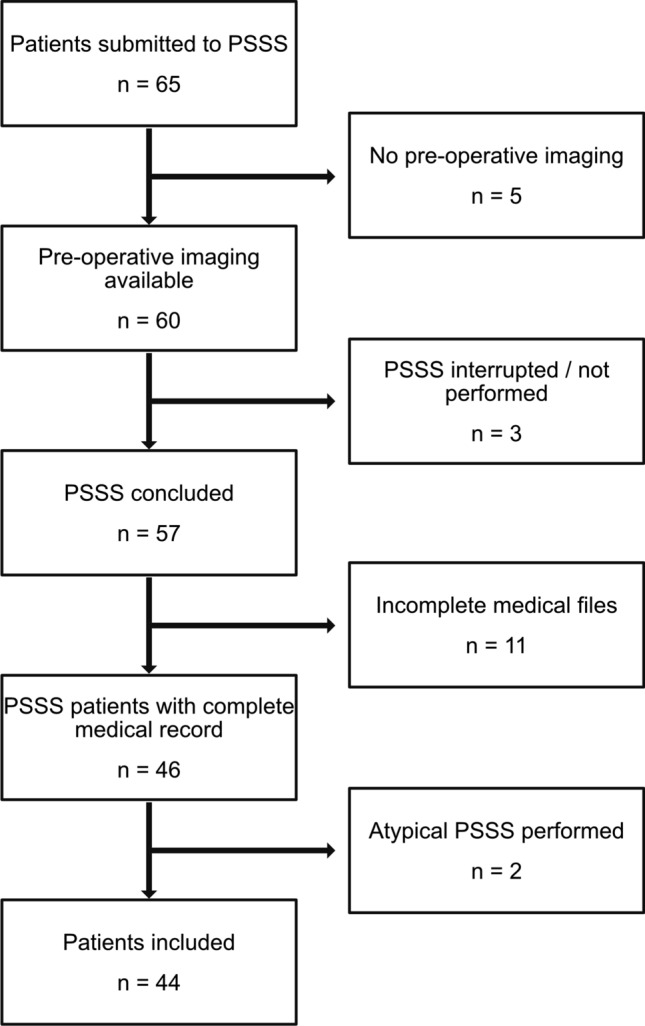
Flowchart of retrospective enrollment
Our Institutional Review Board approved the study protocol including adult and pediatric patients, waiving informed consent (Application Number EA1/148/14) because of the retrospective study design.
Imaging (CT and MRI)
Multiphasic contrast-enhanced CT [with iopromide (Ultravist 370®, Bayer Schering Pharma) or with iobitridol (Xenetic 350®-Guerbet GmbH)] was performed on a 16- or 64-slice CT scanner (Light Speed Power 16 or VCT 64; GE Medical Solutions, Fairfield, CT) using a triple-phase acquisition technique with arterial (~ 15-s delay), portal venous (~ 40-s delay), and equilibrium phase (~ 80-s delay) acquisition. The primary slice thickness was 0.625 mm. Tube voltage was 120 kV, tube current was modulated automatically based on a noise index of 15 and a maximally allowed current of 350 mA. Maximum intensity projections (MIPs) were reconstructed for each contrast phase.
Contrast-enhanced MRI was performed with gadoterate meglumine (Dotarem®-Guerbet GmbH) or gadobutrol (Gadovist®-Bayer Schering Pharma) at 1.5 T (Siemens Magnetom Avanto, Siemens Healthcare, Erlangen, Germany) using an eight-channel body phased-array surface coil. Due to the long observation period, the MRI protocols vary within the examined collective. All MRI protocols included multiphase dynamic contrast-enhanced T1-weighted sequences with fat saturation in axial (arterial, portal venous, venous, and delayed phase) and coronal orientation (venous phase).
Planning of portosystemic shunt surgery
Preoperative images of all 44 patients—contrast-enhanced CT (n = 33) and/or MRI datasets (n = 15; n = 4 patients with both CT and MRI)—were analyzed on a dedicated PACS viewing workstation (Centricity, GE Healthcare, General Electric, Milwaukee, USA) by 2 radiologists with different levels of experience [Observer 1 (O1; T.D.): 12 years and Observer 2 (O2; J.S.): 4 years of experience in abdominal imaging]. Both observers were blinded to the surgical technique and outcomes.
The two observers evaluated patency and diameters of the portal venous system. Maximum diameters of the possible shunt vessels were measured at the suggested connection (down- and upstream) sites by the radiologists. The shortest distances between the possible shunt vessels [inferior vena cava (IVC), portal vein (PV), left and right portal vein branches, superior mesenteric vein (SMV), splenic vein (SV), and left renal vein (RV)] were measured.
Structures intervening between possible shunting vessels were identified in consensus by O1 and O2 as factors increasing surgical complexity (need for tissue resection before shunt creation). Other factors contributing to complexity included large vessel distance (> 20 mm) (necessity of graft interposition), thrombosis adjacent to connecting vessels (requires additional thrombectomy), and unavoidable use of a collateral vein instead of (occluded) major vein for establishing the shunt.
An algorithm for deciding about the surgical shunt technique based on clinical factors (e.g., age, indication, urgency of vascular decompression, vascular beds to relieve) has been proposed by the surgical team and is shown in Table 1. This algorithm takes the physiological properties of each shunt into account and was used in our retrospective analysis as a guideline rather than an absolute standard in assessing the recommendations made by our two observers.
Table 1.
Institutional surgical algorithm that represents a synopsis of local surgeon preferences
| Indication | Preferred shunt |
|---|---|
| Acute esophageal bleeding | Portacaval (end-to-side) |
| Extrahepatic portal thrombosis | Mesocaval, splenorenal (distal or side-to-side) |
| Extrahepatic portal thrombosis in children | Meso-Rex |
| Budd–Chiari syndrome | Portacaval or mesocaval |
| Ascites | Splenorenal (not distal) |
| Possible liver transplantation candidate | Portacaval or mesocaval |
Based on the information available in this retrospective analysis, each of the two observers made a ranked recommendation (first and second choice) of two shunt techniques including the most appropriate anastomosis technique:
- Splenorenal
- Anastomosis proximal end-to-side (Linton), distal end-to-side (Warren) or side-to-side (Cooley).
- Portacaval
- Anastomosis end-to-side or side-to-side.
- Mesocaval
- Anastomosis side-to-side.
Meso-Rex shunt.
Each technique proposed in our retrospective analysis was classified as “standard” or “complex”. If any factor of complexity was present, the procedure was classified as complex. During the decision process, the two observers were blinded to the later chosen surgical procedure. The imaging-based recommendations made by the two observers were compared with the shunt procedures actually accomplished by the surgeons treating the patients included in our analysis.
The success of PSSS was evaluated during postoperative hospitalization until discharge (< 30 days). PSSS failure was defined as early shunt occlusion or occurrence of major complications (rebleeding, organ failure, death).
To correlate the outcome of PSSS with vessel diameters, we calculated the shunt vessel ratio using the following formula:
The shunt vessel ratio, diameter of the smaller shunt vessel, and distance of the connected vessels were correlated with PSSS outcome.
Statistical analysis
Statistical analysis was performed using SPSS Statistics Version 22 (IBM, Armonk, NY, USA). The χ2 test was used to assess differences in the accuracy of CT and MRI in preoperative planning. Cohen’s κ was used to evaluate inter-rater reliability. Continuous variables from two independent samples were evaluated using the Mann–Whitney U test. Receiver operating characteristic curve analysis was used to investigate binary classifiers. p values < 0.05 were considered statistically significant.
Results
The collective consisted of 19 (43%) pediatric patients (0–21 years) with 3 infants (< 2 years), 8 children (2 to 12 years), and 8 adolescents (12 to 21 years) [22]. The mean age in adult patients (n = 25) was 44 years. Both genders were equally represented (22 each) in the study population. Table 2 summarizes diagnoses underlying PHT, signs and clinical findings on admission, and major indications for surgery. The average MELD score (applicable to patients ≥ 12 years) was 12 (range 7 to 24). For patients below 12 years of age, the average PELD score was 1.7 (range 0 to 10.9). In the overall collective, the average serum albumin level was 3.42 g/dl (range 2.0–5.1) [23].
Table 2.
Characteristics of the study patients
| n | % | |
|---|---|---|
| Major underlying causes of PHT | ||
| Extrahepatic portal vein thrombosis | 31 | 70 |
| Associated with liver cirrhosis | 7 | |
| Other causes (e.g., clotting disorder) | 24 | |
| Liver cirrhosis | 16 | 36 |
| Metabolic/toxic | 13 | |
| Viral hepatitis | 2 | |
| Autoimmune hepatitis | 1 | |
| Wilson’s disease | 1 | 2 |
| Rendu–Osler–Weber disease | 1 | 2 |
| Budd–Chiari syndrome | 1 | 2 |
| Post-hemihepatectomy lymph fistula | 1 | 2 |
| Signs and clinical findings in PHT | ||
| Esophagogastric varices | 40 | 91 |
| Splenomegaly | 31 | 70 |
| Ascites | 19 | 43 |
| Advanced stage symptomsa | 3 | 7 |
| Complications of PHT and indication for PSSS | ||
| Varices with previous bleeding episode | 25 | 57 |
| Acute variceal bleeding | 2 | |
| Splenomegaly and secondary thrombocytopenia | 19 | 43 |
| Excessive ascites | 5 | 11 |
PHT portal hypertension, PSSS portosystemic shunt surgery
aHepatorenal syndrome, hepatic encephalopathy, spontaneous bacterial peritonitis
Eighteen patients had portacaval PSSS (Fig. 2), in which end-to-side was the most commonly used anastomosis. Splenorenal (Fig. 3) and mesocaval (Fig. 4) PSSS were performed in 15 and 11 cases, respectively (Table 3). A Meso-Rex shunt was not chosen by the surgeons in our evaluated cases. However, as it was part of the available options to the radiologists reading, it is still mentioned in Table 3. All PSSS procedures included in this study were performed or at least supervised by the same surgeon in charge.
Fig. 2.
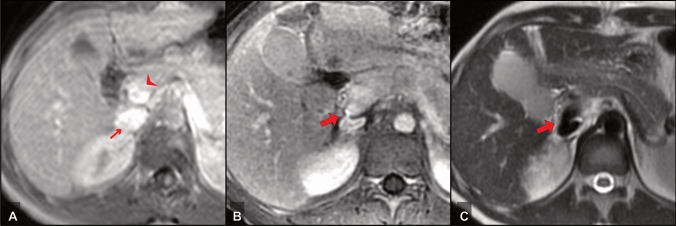
A 17-year-old female PHT patient with Wilson’s disease and recurrent variceal bleeding—PSSS procedure: portacaval side-to-side; a preoperative MRI, post-contrast T1-w, b postoperative MRI, post-contrast T1-w and c postoperative MRI, T2w. Small arrow: IVC; arrowhead: portal vein; bold arrow: portacaval anastomosis
Fig. 3.
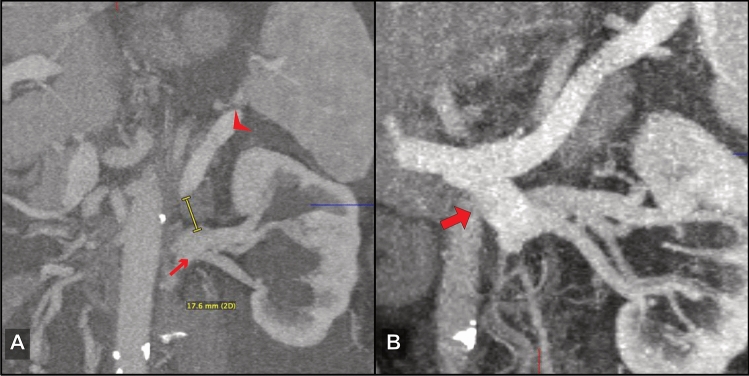
A 71-year-old male PHT patient with excessive ascites after extended right hemihepatectomy (diagnosis: intrahepatic cholangiocarcinoma)—PSSS: splenorenal side-to-side; a preoperative CT, oblique MIP reconstruction and b postoperative CT, oblique MIP reconstruction. Small arrow: left renal vein; arrowhead: splenic vein; bold arrow: splenorenal anastomosis
Fig. 4.
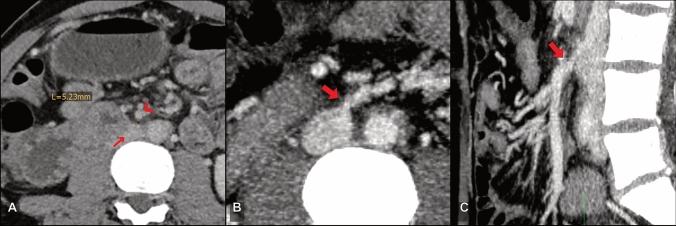
A 49-year-old male PHT patient with liver cirrhosis, extrahepatic portal vein thrombosis, and advanced symptoms—PSSS procedure: mesocaval; a preoperative CT, b postoperative CT, axial MIP reconstruction and c postoperative CT, sagittal MIP reconstruction. Small arrow: IVC; arrowhead: SMV; bold arrow: mesocaval anastomosis
Table 3.
Types of portosystemic shunts performed in the study population
| Shunt | n | % |
|---|---|---|
| Splenorenal | 15 | 34 |
| Side-to-side (Cooley) | 10 | |
| Distal (Warren) | 3 | |
| Proximal (Linton) | 2 | |
| Portacaval | 18 | 41 |
| End-to-side | 12 | |
| Side-to-side | 6 | |
| Mesocaval | 11 | 25 |
| Side-to-side | 11 | |
| Meso-Rex | 0 | 0 |
In terms of the vessels (splenorenal, portacaval, mesocaval, and Meso-Rex) used for PSSS, accuracy of the two observers was 73%/80% (CT/MRI) for O1 and 52%/60% for O2 if only their first choices were taken into consideration. Inter-rater reliability for the first choices was fair (Cohen’s κ = 0.271, p = 0.006). If the shunt technique actually used matched the first or second choice, accuracy increased to 88%/100% (O1) and 76%/73% (O2). Evaluation of recommended shunt vessels and the way they should be anastomosed (e.g., end-to-side, side-to-side, proximal, distal) showed an accuracy of 64%/53% (O1) and 36%/33% (O2) if only the first choice was considered. Inter-rater reliability for the first choices of vessels and anastomosis was fair (Cohen’s κ = 0.257, p < 0.001). If the shunt vessel and anastomosis technique matched the proposed first two choices, accuracy increased to 79%/72% (O1) and 67%/60% (O2). There was no significant difference in accuracy between MRI- and CT-based recommendations for either observer (p > 0.05, Table 4). PSSS procedures and radiological recommendations for each patient are displayed in Supplementary Data.
Table 4.
Accuracy in predicting shunt procedure including anastomotic variants
| O1 | O2 | |
|---|---|---|
| Accuracy of shunt procedures recommended on the basis of CT and/or MRI | ||
| 1st option | ||
| CT | 73% | 52% |
| MRI | 80% | 60% |
| p value | 0.728 | 0.756 |
| 1st + 2nd option | ||
| CT | 88% | 76% |
| MRI | 100% | 73% |
| p value | 0.294 | 1.000 |
| Accuracy of suggested shunts and proposed anastomotic variant | ||
| 1st option | ||
| CT | 64% | 36% |
| MRI | 53% | 33% |
| p value | 0.538 | 1.000 |
| 1st + 2nd option | ||
| CT | 79% | 67% |
| MRI | 73% | 60% |
| p value | 0.720 | 0.749 |
Surgeons actually chose a vessel connection which was not included among the first 2 choices by O1 in 5 cases (11%) and by O2 in 12 cases (26%).
In 15 patients, surgeons chose a PSSS technique that was classified as complex by the radiologist. In 13 (87%) of these patients, complexity of the procedure was confirmed by the operative report. The retrospective imaging-based evaluation had 80% sensitivity and 96% specificity in predicting complex surgical procedures identified in consensus by the two radiologists. In two patients, resection of interposing structures was deemed necessary by the radiologists. In one of these cases, segmental liver resection (segment 1 according to the Couinaud classification) was performed (Fig. 5a). In the second case, embolization of an intervening arteriovenous malformation was performed in preparation for PSSS. In a third patient, a segmental liver resection (segment 1) that was not suggested by the radiology observers was performed. Partial thrombosis of the connecting vessels was identified by the radiologists in 4 patients, and thrombectomy was performed in 3 (75%) of these patients (Fig. 5b). Use of a collateral vessel was proposed and also performed in one patient (100%). In 9 patients, the distance between the connecting vessels was > 20 mm, and graft interposition was recommended by the observers. 8 (89%) of these patients actually received a graft (Fig. 5c, d).
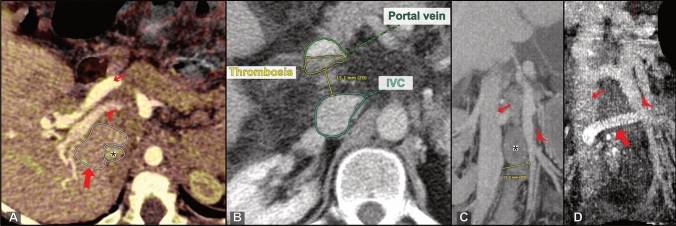
Fig. 5.
Factors of complexity. a Oblique axial CT (fused portal venous phase and venous phase) shows an intervening caudate lobe (PSSS procedure: portacaval end-to-side with subsegmental liver resection); bold arrow: caudate lobe; arrowhead: portal vein; small arrow: hepatic artery; asterisk: IVC. b Axial CT with partial thrombosis of the extrahepatic portal vein (PSSS procedure: portacaval side-to-side after thrombectomy). c Oblique coronal CT MIP shows a large distance of 29 mm between IVC (small arrow) and superior mesenteric vein (arrowhead). In this patient, an allograft was interposed as seen in d (PSSS procedure: mesocaval with graft interposition). d Postoperative oblique coronal CT MIP reconstruction shows the interposed graft (bold arrow) connecting the SMV (arrowhead) and IVC (small arrow)
Overall, graft interposition (splenorenal n = 3; portacaval n = 2; mesocaval n = 5) was performed in 10 patients of our study population. ROC analysis of vessel distances and graft use showed an AUC of 0.950 (p < 0.001, Fig. 6). According to the Youden index, the optimal cutoff was 20 mm (Youden index 0.771). Only one patient with a distance of > 20 mm did not receive a graft interposition, and this patient developed early shunt occlusion.
Fig. 6.
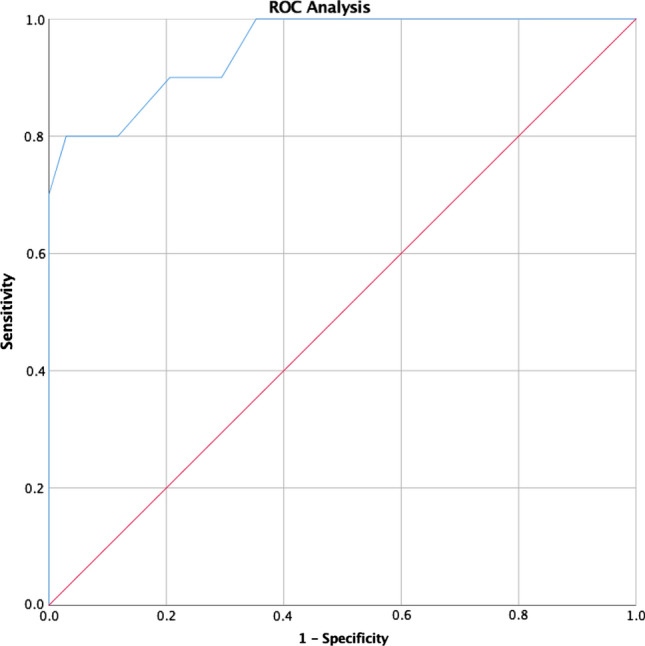
ROC analysis—distance of connected vessels and need for graft interposition; AUC 0.950 (p < 0.001); max. Youden index 0.771 at 20 mm
PSSS was successful in 38 patients (82%), and their shunts were perfused until discharge. Eight patients (18%) showed therapy failure/early shunt occlusion (< 30 days after shunt surgery). Three of these patients (shunt indication: acute variceal bleeding) died during hospitalization.
Analysis of PSSS outcome revealed a significant correlation between shunt vessel distance and early occlusion. The larger the distance, the higher the risk of early shunt occlusion (p = 0.030). There was no significant correlation between successful PSSS and the diameter of smaller connecting vessels or the shunt vessel ratio (p > 0.05, Table 5).
Table 5.
Analysis of correlation of shunt vessel diameter and distance with early success and failure/shunt occlusion (< 30 days)
| Outcome of PSSS during hospitalization (<30 days) | |||||||
|---|---|---|---|---|---|---|---|
| Success (n = 36) | Failure (n = 8) | Significance | |||||
| Mean | SD | Range | Mean | SD | Range | ||
| Distance between vessels (mm) | 12.67 | 11.69 | 56.00 | 20.38 | 10.87 | 33.00 | p = 0.030 |
| Small shunt vessel diameter (mm) | 10.17 | 4.39 | 21.00 | 10.25 | 3.01 | 9.00 | p = 0.709 |
| Shunt vessel ratio | 0.734 | 0.567 | 2.742 | 0.500 | 0.156 | 0.475 | p = 0.482 |
Discussion
Despite the success story of TIPS [2], PSSS can have benefits in the treatment of advanced PHT [5]. We retrospectively analyzed 44 patients who underwent PSSS at our center because TIPS was no adequate treatment option. Longer-term results available for the different shunting techniques suggest that preoperative diagnostic workup should focus on vascular anatomy [15]. This is the rationale for the study presented here, which, to the best of our knowledge, is the first study focusing on the role of preoperative imaging for predicting the most suitable approach of PSSS. Our analysis shows that contrast-enhanced CT and MRI are suitable for the imaging-based planning of surgical shunt creation and at the same time allow identification of complicating circumstances. The results also show that interpretation of the complex imaging findings and the consecutive planning of the surgical approach require an experienced radiologist and may also require a multidisciplinary discussion to choose the most appropriate surgical management.
With its higher periprocedural morbidity due to advanced liver disease, PSSS requires adequate preoperative planning to avoid extensive surgical exploration [24]. With the high-resolution cross-sectional imaging modalities now available, adequate planning of the most suitable shunt should be possible even in patients with advanced PHT. Adequate evaluation of portal venous anatomy for reliable imaging-based surgical planning has been shown in patients undergoing liver transplantation and pancreatic surgery [25, 26]. While it has been shown that precise puncture guidance in TIPS procedures is possible with contrast-enhanced CT and the option of 3D reconstruction [27, 28], similar data for the imaging-based planning of PSSS are not available, and our study was conducted to fill this gap. Besides choosing between TIPS and PSSS, the combination of both is a feasible alternative for complex cases with extrahepatic disease and should be kept in mind in the pre-interventional planning [29]. In our collective, the main reason to prefer PSSS to TIPS in adults was a chronic extrahepatic portal vein occlusion. However, TIPS as a possibility should not be excluded per se in these patients, since TIPS can also help in the treatment of extrahepatic portal vein occlusion [30].
Our results show that an experienced reader can (retrospectively) predict which surgical technique was going to be used by an experienced surgeon with high accuracy in both CT (88%) and MRI (100%). A less-experienced reader achieves lower accuracies of about 75% with both modalities, which is still adequate, though, the fair inter-rater reliability of their first choices underlines the need for an experienced radiologist in the interpretation of the complex preoperative imaging. Moreover, our findings suggest that there are no significant differences in the preoperative accuracy of MRI and CT. These findings support an earlier study reporting the good performance of CT venography in the evaluation of portosystemic collateral vessels [31]. In only 10% of the patients included in our retrospective analysis did the surgeon choose a vessel connection not recommended by the experienced reader. The high incidence of therapy failure (40%) in these cases indicates that deviation from preoperative imaging findings could lead to higher rates of shunt failure and consecutively increased morbidity. Similarly, it has been shown that preoperative CT assessment improves intraoperative management of portosystemic shunts during liver transplantation [32].
In addition to providing information on vascular anatomy, MRI and CT identify complicating factors that require additional surgical techniques. Caudate lobe enlargement is common in cirrhosis of different etiologies and is frequently found in patients with PHT requiring PSSS [33]. Therefore, a hypertrophic caudate lobe has to be considered as a complicating factor in the planning of potential portacaval shunt surgery. Our study shows that imaging reliably identifies interfering structures, which can thus be considered in preoperative planning. Large distances between connecting vessels sometimes require the use of interposition grafts. General recommendations regarding the vein distance above which graft interposition should be performed do not exist. Our analysis identified a vessel distance of 20 mm as the optimal cutoff for use of an interposition graft by experienced surgeons. In addition to its use as a criterion for deciding about the need for an interposition graft, vessel distance was identified as the only risk factor for early shunt failure/occlusion in our cohort. In contrast, the diameters of the veins connected for shunt creation did not correlate with early shunt occlusion. The observed incidence of surgical failure/shunt occlusion (17%) in our patients is attributable to the wide range of shunt indications and a high percentage of advanced and acute cases with poor hepatic reserve compared to other studies [34, 35].
Our study has some limitations. Despite the retrospective nature and the small sample size, only patients who underwent surgery were included in our analysis. This is attributable to the fact that PSSS is increasingly being replaced by TIPS. The surgeons performing PSSS were also aware of the preoperative imaging findings; however, there was no documentation how the imaging findings influenced the surgeons’ choice of shunt procedure. Practice patterns and surgeons’ experience are likely variable between institutions, so that the results of the study may not be applicable to other sides. There are various confound factors that could influence the prediction. The surgeons’ personal preferences may have played a role in the choice of PSSS variants, and these may have changed over the 11-year study period. The more experienced radiologist could have been better at the prediction because he knew the surgeons and their preferred techniques more intimately. To minimize these effects, we evaluated the first two radiological choices instead of only using a single recommendation. Our results could pave the way for a prospective study, which is needed, to answer the question if pre-surgical imaging provides adequate and important information and impacts patient outcomes.
In conclusion, preoperative cross-sectional imaging and an experienced radiologist can guide the surgeon in PSSS. Imaging is not only inevitable to provide accurate planning in general but rather to reliably exclude unfavorable shunt variants. Optimized CT and MRI examinations provide the information necessary to identify technically feasible alternatives and complicating factors (which may require an interposition graft, resection of intervening structures, or prior thrombectomy).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- IVC
Inferior vena cava
- O1
Observer one (more experienced)
- O2
Observer two (less experienced)
- PHT
Portal hypertension
- PSSS
Portosystemic shunt surgery
- PV
Portal vein
- SMV
Superior mesenteric vein
- TIPS
Transjugular intrahepatic portosystemic shunt
Authors’ contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by UF, SG-K, and MSM. The first draft of the manuscript was written by UF and all authors commented on previous versions of the manuscript. All authors approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Compliance with ethical standards
Conflicts of interest
Research grants: Siemens, b.e. imaging, Guerbet. Travel costs and honoraria: Siemens, Canon, Novartis, IPSEN, b.e. imaging, Bayer, Parexel.
Footnotes
The original version of this article was revised due to a retrospective open access order.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Uli Fehrenbach and Safak Gül-Klein contributed equally to this work.
Change history
6/5/2021
The following OA funding note is addressed above reference section: Open Access funding enabled and organized by Projekt DEAL.
Change history
6/22/2021
A Correction to this paper has been published: 10.1007/s00261-021-03153-1
References
- 1.Knechtle SJ, D’Alessandro AM, Armbrust MJ, Musat A, Kalayoglu M (1999) Surgical portosystemic shunts for treatment of portal hypertensive bleeding: outcome and effect on liver function. Surgery 126 (4):708–711; discussion 711–703 [PubMed]
- 2.Rosemurgy AS, Molloy DL, Thometz DP, Villadolid DV, Cowgill SM, Zervos EE (2007) TIPS in Florida: is its application a result of evidence-based medicine? J Am Coll Surg 204 (5):794–801; discussion 801–792. 10.1016/j.jamcollsurg.2007.01.014 [DOI] [PubMed]
- 3.Puhl G, Gul S, Neuhaus P. Portosystemic shunt surgery between TIPS and liver transplantation. Chirurg. 2011;82(10):898–905. doi: 10.1007/s00104-011-2100-1. [DOI] [PubMed] [Google Scholar]
- 4.Pal S. Current role of surgery in portal hypertension. Indian J Surg. 2012;74(1):55–66. doi: 10.1007/s12262-011-0381-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brand M, Prodehl L, Ede CJ (2018) Surgical portosystemic shunts versus transjugular intrahepatic portosystemic shunt for variceal haemorrhage in people with cirrhosis. Cochrane Database Syst Rev 10:Cd001023. 10.1002/14651858.cd001023.pub3 [DOI] [PMC free article] [PubMed]
- 6.Fidelman N, Kwan SW, LaBerge JM, Gordon RL, Ring EJ, Kerlan RK., Jr The transjugular intrahepatic portosystemic shunt: an update. AJR American journal of roentgenology. 2012;199(4):746–755. doi: 10.2214/ajr.12.9101. [DOI] [PubMed] [Google Scholar]
- 7.Bosch J, Berzigotti A, Garcia-Pagan JC, Abraldes JG. The management of portal hypertension: rational basis, available treatments and future options. J Hepatol. 2008;48(Suppl 1):S68–S92. doi: 10.1016/j.jhep.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 8.Bari K, Garcia-Tsao G. Treatment of portal hypertension. World journal of gastroenterology. 2012;18(11):1166–1175. doi: 10.3748/wjg.v18.i11.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Procopet B, Tantau M, Bureau C. Are there any alternative methods to hepatic venous pressure gradient in portal hypertension assessment? Journal of gastrointestinal and liver diseases : JGLD. 2013;22(1):73–78. [PubMed] [Google Scholar]
- 10.Wright AS, Rikkers LF. Current management of portal hypertension. J Gastrointest Surg. 2005;9(7):992–1005. doi: 10.1016/j.gassur.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 11.de Franchis R. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2010;53(4):762–768. doi: 10.1016/j.jhep.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Kim DH, Park JY. Prevention and management of variceal hemorrhage. Int J Hepatol. 2013;2013:434609. doi: 10.1155/2013/434609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fortune B, Garcia-Tsao G. Current Management Strategies for Acute Esophageal Variceal Hemorrhage. Curr Hepatol Rep. 2014;13(1):35–42. doi: 10.1007/s11901-014-0221-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarin SK, Kumar A, Angus PW, Baijal SS, Baik SK, Bayraktar Y, et al. Diagnosis and management of acute variceal bleeding: Asian Pacific Association for Study of the Liver recommendations. Hepatol Int. 2011;5(2):607–624. doi: 10.1007/s12072-010-9236-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knechtle SJ. Portal hypertension: from Eck’s fistula to TIPS. Ann Surg. 2003;238(6 Suppl):S49–S55. doi: 10.1097/01.sla.0000097526.74747.90. [DOI] [PubMed] [Google Scholar]
- 16.Henderson JM, Nagle A, Curtas S, Geisinger M, Barnes D. Surgical shunts and TIPS for variceal decompression in the 1990s. Surgery. 2000;128(4):540–547. doi: 10.1067/msy.2000.108209. [DOI] [PubMed] [Google Scholar]
- 17.Wolff M, Hirner A. Surgical treatment of portal hypertension. Zentralbl Chir. 2005;130(3):238–245. doi: 10.1055/s-2005-836545. [DOI] [PubMed] [Google Scholar]
- 18.Bambini DA, Superina R, Almond PS, Whitington PF, Alonso E (2000) Experience with the Rex shunt (mesenterico-left portal bypass) in children with extrahepatic portal hypertension. J Pediatr Surg 35 (1):13–18; discussion 18–19. 10.1016/s0022-3468(00)80005-6 [DOI] [PubMed]
- 19.Superina R, Bambini DA, Lokar J, Rigsby C, Whitington PF. Correction of extrahepatic portal vein thrombosis by the mesenteric to left portal vein bypass. Ann Surg. 2006;243(4):515–521. doi: 10.1097/01.sla.0000205827.73706.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scholz S, Sharif K. Surgery for portal hypertension in children. Curr Gastroenterol Rep. 2011;13(3):279–285. doi: 10.1007/s11894-011-0186-8. [DOI] [PubMed] [Google Scholar]
- 21.Lautz TB, Keys LA, Melvin JC, Ito J, Superina RA. Advantages of the meso-Rex bypass compared with portosystemic shunts in the management of extrahepatic portal vein obstruction in children. J Am Coll Surg. 2013;216(1):83–89. doi: 10.1016/j.jamcollsurg.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 22.Hardin AP, Hackell JM. Age Limit of Pediatrics. Pediatrics. 2017;140(3):e20172151. doi: 10.1542/peds.2017-2151. [DOI] [PubMed] [Google Scholar]
- 23.Ronald J, Wang Q, Choi SS, Suhocki PV, Hall MD, Smith TP, et al. Albumin-bilirubin grade versus MELD score for predicting survival after transjugular intrahepatic portosystemic shunt (TIPS) creation. Diagn Interv Imaging. 2018;99(3):163–168. doi: 10.1016/j.diii.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 24.Garbuzenko DV. Current approaches to the management of patients with liver cirrhosis who have acute esophageal variceal bleeding. Curr Med Res Opin. 2016;32(3):467–475. doi: 10.1185/03007995.2015.1124846. [DOI] [PubMed] [Google Scholar]
- 25.Grieser C, Denecke T, Steffen IG, Avgenaki M, Frohling V, Mogl M, et al. Multidetector computed tomography for preoperative assessment of hepatic vasculature and prediction of splenic artery steal syndrome in patients with liver cirrhosis before transplantation. European radiology. 2010;20(1):108–117. doi: 10.1007/s00330-009-1535-y. [DOI] [PubMed] [Google Scholar]
- 26.Grieser C, Steffen IG, Grajewski L, Stelter L, Streitparth F, Schnapauff D et al. (2010) Preoperative multidetector row computed tomography for evaluation and assessment of resection criteria in patients with pancreatic masses. Acta radiologica (Stockholm, Sweden : 1987) 51 (10):1067–1077. 10.3109/02841851.2010.520023 [DOI] [PubMed]
- 27.Qin JP, Tang SH, Jiang MD, He QW, Chen HB, Yao X, et al. Contrast enhanced computed tomography and reconstruction of hepatic vascular system for transjugular intrahepatic portal systemic shunt puncture path planning. World journal of gastroenterology. 2015;21(32):9623–9629. doi: 10.3748/wjg.v21.i32.9623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo X, Wang X, Yu J, Zhu Y, Xi X, Ma H, et al. Transjugular intrahepatic portosystemic shunt creation: three-dimensional roadmap versus CO(2) wedged hepatic venography. European radiology. 2018;28(8):3215–3220. doi: 10.1007/s00330-018-5316-3. [DOI] [PubMed] [Google Scholar]
- 29.David A, Metairie S, Perret C, Schnee M, Archambeaud I, Frampas E, et al. Minilaparotomy-assisted transmesenteric approach for portal vein recanalization and transjugular intrahepatic portosystemic shunt in a non-cirrhotic patient with portal cavernoma. Diagn Interv Imaging. 2019 doi: 10.1016/j.diii.2019.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Luo J, Li M, Zhang Y, Wang H, Huang M, Li Z, et al. Percutaneous transhepatic intrahepatic portosystemic shunt for variceal bleeding with chronic portal vein occlusion after splenectomy. European radiology. 2018;28(9):3661–3668. doi: 10.1007/s00330-018-5360-z. [DOI] [PubMed] [Google Scholar]
- 31.Kang HK, Jeong YY, Choi JH, Choi S, Chung TW, Seo JJ et al. (2002) Three-dimensional multi-detector row CT portal venography in the evaluation of portosystemic collateral vessels in liver cirrhosis. Radiographics : a review publication of the Radiological Society of North America, Inc 22 (5):1053–1061. 10.1148/radiographics.22.5.g02se011053 [DOI] [PubMed]
- 32.Aucejo FN, Hashimoto K, Quintini C, Kelly D, Vogt D, Winans C, et al. Triple-phase computed tomography and intraoperative flow measurements improve the management of portosystemic shunts during liver transplantation. Liver Transpl. 2008;14(1):96–99. doi: 10.1002/lt.21377. [DOI] [PubMed] [Google Scholar]
- 33.Ozaki K, Matsui O, Kobayashi S, Minami T, Kitao A, Gabata T. Morphometric changes in liver cirrhosis: aetiological differences correlated with progression. Br J Radiol. 2016;89(1059):20150896. doi: 10.1259/bjr.20150896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosemurgy AS, Zervos EE, Bloomston M, Durkin AJ, Clark WC, Goff S (2003) Post-shunt resource consumption favors small-diameter prosthetic H-graft portacaval shunt over TIPS for patients with poor hepatic reserve. Ann Surg 237 (6):820–825; discussion 825–827. 10.1097/01.sla.0000072102.38993.05 [DOI] [PMC free article] [PubMed]
- 35.Helton WS, Maves R, Wicks K, Johansen K. Transjugular intrahepatic portasystemic shunt vs surgical shunt in good-risk cirrhotic patients: a case-control comparison. Arch Surg. 2001;136(1):17–20. doi: 10.1001/archsurg.136.1.17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.