Abstract
Objective.
To test whether change in motor evoked potential (ΔMEP) induced by continuous theta-burst stimulation (cTBS) of motor cortex (M1) distinguishes adults with autism spectrum disorder (ASD) from neurotypicals, and to explore the contribution of two common polymorphisms related to neuroplasticity.
Methods.
44 adult neurotypical (NT) participants (age 21–65, 34 males) and 19 adults with ASD (age 21–58, 17 males) prospectively underwent M1 cTBS. Their data were combined with previously obtained results from 35 NT and 35 ASD adults.
Results.
ΔMEP at 15 minutes post-cTBS (T15) was a significant predictor of diagnosis (p=0.04) in the present sample (n=63). T15 remained a significant predictor in a larger sample (n=91) and when partially imputed based on T10–T20 from a yet-greater sample (N=133). T15 also remained a significant predictor of diagnosis among brain-derived neurotrophic factor (BDNF) Met+ and apolipoprotein E (APOE) ε4− subjects (p’s<0.05), but not among Met− or ε4+ subjects (p’s>0.19).
Conclusions.
ΔMEP at T15 post-cTBS is a significant biomarker for adults with ASD, and its utility is modulated by BDNF and APOE polymorphisms.
Significance.
M1 cTBS response is a physiologic biomarker for adults with ASD in large samples, and controlling for BDNF and APOE polymorphisms can improve its diagnostic utility.
Keywords: transcranial magnetic stimulation, continuous theta-burst stimulation, biomarker, autism spectrum disorder, BDNF, APOE
1. Introduction
Autism spectrum disorder (ASD) refers to a group of complex neurodevelopmental disorders characterized by: (1) persistent deficiencies in social communication and social interaction, and (2) limited interests and repetitive behavior (American Psychiatric Association, 2013). ASD has an estimated prevalence of 14.5 per 1,000 in the U.S. (Christensen et al., 2018) and often results in significant impairments in activities of daily living, both in children and adults (Haertl et al., 2013). Because of the large heterogeneity of the clinical endophenotype in ASD and symptom manifestation over a range of ages and to different degrees, the clinical diagnosis of ASD can be challenging and is typically based on behavioral interviews and subjective clinical impression. Thus, an objective neurophysiologic biomarker that can facilitate ASD diagnosis is highly desirable, especially to improve diagnostic accuracy and to enable metrics of target engagement and clinical/behavioral outcomes in therapeutic interventions.
Data from rodent ASD models and studies on genetic syndromes with high prevalence of ASD symptoms in humans indicate aberrant mechanisms of synaptic plasticity in ASD pathophysiology, including use-dependent changes in synaptic strength (Bhakar et al., 2012; Bourgeron, 2009; Gipson and Johnston, 2012; Krueger and Bear, 2011; Peça et al., 2011; Percy, 2011) and abnormalities in long-term potentiation (LTP) and long-term depression (LTD) of excitatory synaptic strength (Dani et al., 2005; Gogolla et al., 2009; Huber et al., 2002; Narita et al., 2002; Rinaldi et al., 2008a, 2008b, 2007; Tordjman et al., 2007).
Plasticity mechanisms similar to LTP and LTD can be studied noninvasively in humans using transcranial magnetic stimulation (TMS) (Barker, 2017; Barker et al., 1985; Thickbroom, 2007; Ziemann, 2004). TMS is a neurophysiological technique based on the principle of electromagnetic induction that enables triggering or modulation of neural activity in the brain (Hallett, 2007) and is considered safe when applied following the recommended guidelines (Rossi et al., 2009; Rossini et al., 2015). Delivering a single TMS pulse (spTMS) to primary motor cortex (M1) can induce a motor evoked potential (MEP) in the target muscle. TMS has been used in various forms including spTMS, paired-pulse TMS (ppTMS), and repetitive TMS (rTMS) at specific intensities, frequencies, and patterns of stimulation to study, modify, or restore activity in corticospinal pathways, as well as various brain regions and networks (see Valero-Cabré et al. 2017 for a review).
Several spTMS studies have found no significant difference in baseline M1 excitability in ASD (Enticott et al., 2012; Enticott et al., 2013; Minio-Paluello et al., 2009; Théoret et al., 2005). PpTMS studies have found no alteration in short-interval intracortical inhibition (SICI) (Jung et al., 2013; Théoret et al., 2005) or intracortical facilitation (ICF) in ASD (Enticott et al., 2010; Peter G Enticott et al., 2013; Théoret et al., 2005). The findings of long-interval intracortical inhibition (LICI) in ASD have been mixed, with some ASD individuals exhibiting abnormal intracortical inhibition while others have responses similar to those of neurotypical (NT) individuals (Enticott et al., 2010; Enticott et al., 2013; Oberman et al., 2010).
A form of rTMS referred to as theta-burst stimulation (TBS) of M1 (Huang et al., 2005) consists of 50Hz bursts of triplet TMS pulses repeated at 5 Hz for a total of 600 pulses, in one of two protocols: (1) intermittent theta-burst stimulation (iTBS) with a 2-sec on, 8-sec off pattern for 190 sec that typically induces MEP facilitation by ~35% for up to 60 min; (2) continuous theta-burst stimulation (cTBS) for 40 sec that typically induces MEP suppression by ~25% for up to 50 min (Wischnewski and Schutter, 2015). MEP facilitation and suppression by iTBS and cTBS protocols are considered to involve mechanisms similar to LTP and LTD, respectively (Huang et al. 2005; Huerta and Volpe 2009). The return of post-TBS MEP amplitudes to their baseline levels is considered a neurophysiologic index of the efficacy of the mechanisms of cortical plasticity (Oberman et al., 2010, 2016, 2014, 2012; Pascual-Leone et al., 2011, 2005; Suppa et al., 2016; Tremblay et al., 2015). TBS aftereffects involve mechanisms of gamma-aminobutyric acid- (GABA-)ergic and glutamatergic plasticity (Benali et al., 2011; Huang et al., 2008, 2007; Stagg et al., 2009; Trippe et al., 2009).
Studies by Oberman and colleagues (Oberman et al., 2016, 2012) found greater and longer-lasting TBS-induced changes in MEP amplitude in adults with ASD compared to NT adults, indicating an exaggerated, hyperplastic response to TBS in ASD. Recently, we found that children and adolescents with high-functioning ASD (HF-ASD) had abnormally greater facilitatory responses to cTBS relative to typically developing children (Jannati et al., 2020). Moreover, cTBS measures of plasticity showed a maturational trajectory in children and adolescents with HF-ASD, in which the extent of, or the maximum, cTBS-induced suppression of MEPs increased linearly with age (Jannati et al., 2020; Oberman et al., 2014).
These results collectively support the utility of cTBS cortical plasticity measures as biomarkers for individuals with ASD across the lifespan (Jannati et al. 2020). In recent years, however, several studies have documented large inter- and intra-individual variability in M1 cTBS responses among healthy adults (Corp et al., 2020; Goldsworthy et al., 2014; Hamada et al., 2013; Hordacre et al., 2017; Jannati et al., 2019, 2017; Nettekoven et al., 2015; Vallence et al., 2015). Such variability may limit the biomarker utility of cTBS for differentiating individuals with ASD from their NT counterparts. Careful consideration of possible sources of within- and across-individual variability is thus important.
Here we address these issues by comparing M1 cTBS aftereffects in a relatively large sample of NT participants (n=44) and a group of ASD participants (n=19), and then validating the obtained results by combining individual participant data from the present sample with the corresponding values from 70 additional subjects from two separate datasets reported previously (Oberman et al., 2012). The total sample (N=133) included 79 NT and 54 ASD subjects across three datasets and two centers. Moreover, we stratified the participants with available DNA data in the present sample for two single-nucleotide polymorphism (SNPs) identified as important contributors to variability of rTMS and other measures of neuroplasticity: the Val66Met SNP in the brain-derived neurotrophic factor (BDNF) gene (Antal et al., 2010; Chang et al., 2014; Cheeran et al., 2008; Di Lazzaro et al., 2015; Fried et al., 2017; Jannati et al., 2019, 2017; Lee et al., 2013) and the presence of the ε4 allele in the apolipoprotein E (APOE) gene (Jannati et al., 2019; Mahley and Rall Jr, 2000; Nichol et al., 2009; Peña-Gomez et al., 2012; White et al., 2001; Wolk et al., 2010). We then calculated the standard measures of biomarker utility for each comparison of cTBS responses.
2. Methods
2.1. Participants
63 adults (age range 21–65, 12 females) participated in this study. The local Institutional Review Board approved the study in accordance with the Declaration of Helsinki. All participants provided written informed consent prior to enrollment and received monetary compensation for their participation. All participants were screened for TMS contraindications (Rossi et al., 2009), and all had a normal neurological examination. The present sample (Dataset 1) consisted of two groups: (1) high-functioning adults with non-syndromic ASD (n = 19); (2) neurotypical age- and gender-matched control participants (n = 44). Local community advertisements and autism associations and clinics were used for participant recruitment. Participants in the ASD group were required to provide documentation of a clinical diagnosis made by a psychiatrist or clinical psychologist, met the ASD criteria in the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) (American Psychiatric Association, 2013), and were independently assessed with the Autism Diagnostic Observation Schedule (ADOS; mean score = 8.68; SD = 4.35). Participants in the NT group had no neurological or psychological disorder. Screening for TMS contraindications was based on the safety recommendations of the International Federation of Clinical Neurophysiology (IFCN) (Rossi et al., 2009). Detailed demographic characteristics of the participants are presented in Table 1 and comparisons of those characteristics between ASD and NT groups are presented in Table 2. Racial categories were defined according to the National Institutes of Health guidelines for inclusion of minorities as subjects in clinical research (NIH Office of Extramural Research, 2001). Handedness in the present sample was determined by asking participants their hand preference. Individual handedness data were not available for the other two datasets.
Table 1.
Participants’ demographics, neuropsychological measures, and single-nucleotide polymorphisms.
| Group | Age | Sex | Race | Education* (yr) | Handedness | BDNF† | APOE‡ | IQ | Verbal KN | Nonverbal FR | ADOS | AQ | Neuroactive medication(s) | Neurological / Psychiatric Comorbidities |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
ASD (n = 19) |
||||||||||||||
| 21 | M | Other | – | L | Val/Val | ε3/ε4 | 94 | 9 | 9 | 7 | – | Risperidone, Methylphenidate | – | |
| 21 | M | Asian | 13 | R | Val/Met | ε3/ε3 | 124 | 16 | 12 | 15 | 26 | Escitalopram | Anxiety, Depression | |
| 25 | M | White | 12 | R | Val/Val | ε3/ε3 | 79 | 8 | 5 | 8 | – | Amphetamine/Dextroamphetamine | ADHD | |
| 27 | M | White | 15 | R | Val/Met | ε3/ε3 | 94 | 8 | 6 | 10 | 33 | Risperidone, Bupropion | Anxiety, Depression | |
| 28 | M | White | 20+ | R | Val/Met | ε3/ε4 | 121 | 16 | 11 | 7 | 20 | – | Dysthymia | |
| 31 | M | White | – | R | Val/Val | ε3/ε3 | 79 | 9 | 4 | 17 | – | Buspirone, Escitalopram | Anxiety, Depression | |
| 32 | M | White | 13 | R | – | – | 70 | 9 | 1 | 4 | 16 | Aripiprazole, Hydroxyzine, Fluvoxamine, Methimazole | Anxiety, Depression | |
| 33 | F | White | 20 | R | Val/Val | ε3/ε4 | 118 | 17 | 9 | 5 | 35 | Sumatriptan | Depression, Migraines | |
| 34 | M | White | 19 | R | Val/Met | ε3/ε4 | 118 | 13 | 13 | 4 | 35 | – | – | |
| 37 | M | Asian | 18 | R | Val/Met | ε3/ε3 | 115 | 15 | 10 | 16 | 21 | – | ADHD, Depression | |
| 41 | M | White | 17 | R | Val/Met | ε3/ε3 | 109 | 10 | 13 | 13 | 26 | Acetyl-L-carnitine | Depression, Essential tremor | |
| 41 | M | White | 16 | R | Val/Met | ε3/ε3 | 88 | 4 | 12 | 8 | 17 | – | – | |
| 42 | M | White | 15 | R | Val/Met | ε3/ε3 | 124 | 14 | 14 | 3 | 28 | – | Anxiety, Depression | |
| 49 | M | White | 19 | R | Val/Val | ε3/ε4 | 112 | 17 | 7 | 12 | – | Aripiprazole, Zolpidem, Melatonin, Lisdexamfetamine | Anxiety, Depression | |
| 50 | M | White | 12 | R | Val/Met | ε3/ε3 | 103 | 12 | 9 | 8 | 34 | Citalopram | Anxiety, Depression, Fainting/Dizzy spells | |
| 50 | M | – | 16 | R | Val/Val | ε3/ε3 | 112 | 12 | 12 | 10 | 32 | Sertraline | Social Anxiety Disorder | |
| 52 | M | White | – | L | Val/Val | ε3/ε4 | 118 | 19 | 7 | 3 | 43 | – | ADHD, Anxiety | |
| 52 | M | White | 11 | R | – | – | 100 | 9 | 11 | 5 | 36 | – | Anxiety, Migraine | |
| 58 | F | White | 16 | R | Val/Val | – | 121 | 18 | 9 | 10 | 36 | Desipramine, Frovatriptan, Modafinil | Depression, Migraines | |
|
NT (n = 44) |
||||||||||||||
| 21 | M | White | 15 | R | Val/Val | ε3/ε4 | 109 | 14 | 9 | – | 12 | – | – | |
| 21 | M | Multiracial | 16 | R | – | – | 112 | 11 | 13 | – | 7 | – | – | |
| 21 | M | Asian | 16 | R | – | – | 94 | 8 | 10 | – | 17 | – | – | |
| 22 | M | Asian | 17 | R | Val/Met | ε3/ε4 | 103 | 9 | 12 | – | 16 | – | – | |
| 22 | M | White | 17 | R | Val/Val | ε2/ε3 | 112 | 10 | 14 | – | 7 | – | – | |
| 22 | F | White | 16 | R | Val/Val | ε3/ε3 | 100 | 10 | 10 | – | 6 | – | – | |
| 22 | M | White | 16 | R | Val/Val | ε3/ε3 | 118 | 17 | 9 | – | 18 | – | – | |
| 23 | M | Multiracial | 16 | R | Val/Val | ε3/ε3 | 106 | 11 | 11 | – | 20 | – | – | |
| 23 | M | White | 17 | R | Val/Val | ε2/ε3 | 127 | 16 | 13 | – | 11 | – | – | |
| 23 | M | White | 17 | R | Val/Met | ε3/ε3 | 118 | 13 | 13 | – | 22 | – | – | |
| 23 | M | Black | 14 | R | Val/Val | ε2/ε3 | 103 | 9 | 12 | – | 8 | – | – | |
| 23 | M | Asian | 17 | R | Val/Val | ε3/ε3 | 115 | 11 | 14 | – | 19 | – | – | |
| 23 | F | Other | 16 | R | Val/Met | ε3/ε3 | 118 | 14 | 12 | – | 10 | Sumatriptan | Migraine | |
| 23 | F | Asian | 16 | L | Met/Met | ε4/ε4 | 103 | 14 | 7 | – | 19 | – | – | |
| 24 | M | Asian | 16 | R | Val/Met | ε3/ε3 | 100 | 10 | 10 | – | 14 | – | – | |
| 24 | M | Multiracial | 17 | R | Val/Met | ε2/ε3 | 115 | 12 | 13 | – | 12 | – | – | |
| 24 | M | White | 13 | R | Val/Met | ε3/ε4 | 118 | 12 | 14 | – | 17 | Cannabis | Anxiety, Depression | |
| 24 | F | White | 17 | R | Val/Met | ε3/ε3 | 106 | 15 | 7 | – | 14 | – | Fainting/Dizzy spells | |
| 25 | M | White | 16 | R | Val/Met | ε3/ε3 | 130 | 17 | 13 | – | 16 | – | – | |
| 25 | M | Asian | 18 | R | Val/Val | ε3/ε3 | 91 | 7 | 10 | – | 17 | – | – | |
| 25 | F | Asian | 17 | R | Val/Val | ε3/ε4 | 94 | 8 | 10 | – | 28 | – | – | |
| 25 | F | Other | 20 | R | – | – | 106 | 12 | 10 | – | 10 | – | – | |
| 26 | M | Other | 19 | R | Val/Val | ε3/ε4 | 121 | 17 | 10 | – | 23 | – | – | |
| 28 | F | White | – | R | Val/Val | ε3/ε3 | 100 | 10 | 10 | – | 16 | – | – | |
| 28 | M | White | – | R | Val/Val | ε3/ε3 | 115 | 13 | 12 | – | 19 | – | Headaches, Fainting/Dizzy spells | |
| 28 | M | Multiracial | 18 | R | Val/Val | ε3/ε3 | 100 | 9 | 11 | – | 13 | – | – | |
| 29 | F | White | 18 | R | – | – | 118 | 12 | 14 | – | 9 | – | – | |
| 30 | M | White | 20 | L | Val/Met | ε3/ε4 | 97 | 10 | 9 | – | 13 | – | – | |
| 30 | M | White | 18 | R | Val/Met | ε3/ε3 | 109 | 10 | 13 | – | 11 | – | – | |
| 32 | M | White | 19 | R | Val/Val | ε3/ε3 | 100 | 9 | 11 | – | 14 | – | Anxiety, Depression | |
| 32 | M | Asian | 20+ | R | Val/Met | ε3/ε4 | 103 | 8 | 13 | – | 11 | – | – | |
| 45 | M | White | 16 | R | Val/Val | ε2/ε3 | 94 | 9 | 9 | – | 14 | – | – | |
| 46 | M | White | 13 | R | Val/Val | ε3/ε4 | 88 | 9 | 7 | – | 21 | – | – | |
| 47 | M | Black | 18 | R | Val/Met | ε3/ε3 | 88 | 9 | 7 | – | 15 | – | – | |
| 47 | M | White | 20+ | R | Val/Val | ε3/ε3 | 133 | 17 | 14 | – | 11 | – | Migraine | |
| 47 | M | Black | 18 | R | Val/Val | ε3/ε4 | 118 | 16 | 10 | – | 11 | – | – | |
| 49 | M | Asian | 16 | R | Val/Met | ε3/ε3 | 112 | 12 | 12 | – | 22 | – | – | |
| 50 | F | Black | 12 | R | – | – | 97 | 10 | 9 | – | 10 | – | – | |
| 51 | M | White | 16 | R | Val/Val | ε3/ε3 | 115 | 14 | 11 | – | 21 | – | – | |
| 53 | M | White | 16 | R | Val/Val | ε3/ε4 | 106 | 13 | 9 | – | 11 | – | – | |
| 56 | F | Black | 14 | R | Val/Val | ε3/ε4 | 97 | 9 | 10 | – | 9 | – | – | |
| 56 | M | White | 16 | R | Val/Met | ε3/ε3 | 109 | 13 | 10 | – | 16 | – | – | |
| 62 | M | White | 16 | R | Val/Met | ε3/ε4 | 118 | 12 | 14 | – | 25 | – | – | |
| 65 | M | Asian | 20+ | R | Val/Val | ε3/ε4 | 124 | 15 | 13 | – | 14 | – | – | |
Participants in each group are sorted by age. Racial categories were defined according to the National Institutes of Health policy and guidelines on the inclusion of minorities as subjects in clinical research (NIH Office of Extramural Research, 2001). IQ scores were estimated using the Abbreviated Battery of Stanford-Binet IV intelligence scale. APOE, apolipoprotein E; AQ, autism-spectrum quotient; ASD, autism spectrum disorder; BDNF, brain-derived neurotrophic factor; IQ, intelligence quotient; Met, metionine; NT, neurotypical; Val, Valine. Values marked by “–” were either not reported by the participant or not available.
Education data were available for 16 and 42 participants in the ASD and NT groups, respectively.
BDNF data were available for 17 and 40 participants in the ASD and NT groups, respectively.
APOE data were available for 16 and 40 participants in the ASD and NT groups, respectively.
Table 2.
Participants’ demographics, single-nucleotide polymorphisms, neuropsychological scores, and baseline neurophysiological measures.
| All (N = 63) | NT (n = 44) | ASD (n = 19) | p | NT Met− (n = 23)‡ | ASD Met− (n = 8)‡ | p | NT Met+ (n = 16)‡ | ASD Met+ (n = 9)‡ | p | NT ε4− (n = 26)§ | ASD ε4− (n = 10)§ | p | NT ε4+ (n = 13)§ | ASD ε4+ (n = 6)§ | p | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (yr, mean ± SD) | 34.57 ± 12.77 | 33.05 ± 13.15 | 38.11 ± 11.38 | 0.150 | 33.96 ± 13.69 | 39.88 ± 13.96 | 0.303 | 32.94 ± 3.31 | 35.67 ± 3.02 | 0.589 | 31.74 ± 2.14 | 36.50 ± 3.21 | 0.246 | 37.69 ± 4.57 | 36.17 ± 4.92 | 0.843 |
| Sex (M : F) | 51 : 12 | 34 : 10 | 17 : 2 | 0.318 | 19 : 4 | 6 : 2 | 0.634 | 13 : 3 | 9 : 0 | 0.280 | 22 : 4 | 10 : 0 | 0.559 | 10 : 3 | 5 : 1 | 1.000 |
| Race (White : non-White) | 37 : 26 | 22 : 22 | 15 : 4 | 0.050 | 13 : 10 | 6 : 2 | 0.433 | 8 : 8 | 7 : 2 | 0.229 | 16 : 10 | 7 : 3 | 0.716 | 5 : 8 | 5 : 1 | 0.141 |
| Education (yr, mean ± SD) † | 16.52 ± 2.1 | 16.79 ± 2.09 | 15.81 ± 3.06 | 0.171 | 16.76 ± 2.07 | 16.60 ± 3.13 | 0.888 | 16.94 ± 1.95 | 16.22 ± 2.86 | 0.465 | 16.63 ± 1.56 | 14.89 ± 2.15 | 0.015 | 17.23 ± 2.65 | 19.75 ± 0.96 | 0.087 |
| BDNF (Met− : Met+) ‡ | 31 : 25 | 23 : 16 | 8 : 9 | 0.560 | 23 : 0 | 8 : 0 | N/A | 16 : 0 | 9 : 0 | N/A | 16 : 10 | 3 : 7 | 0.139 | 7 : 6 | 4 : 2 | 1.000 |
| APOE (ε4− : ε4+) § | 36 : 19 | 26 : 13 | 10 : 6 | 0.765 | 16 : 7 | 3 : 4 | 0.372 | 10 : 6 | 7 : 2 | 0.661 | 26 : 0 | 10 : 0 | N/A | 13 : 0 | 6 : 0 | N/A |
| Handedness (Right : Left) | 59 : 4 | 42 : 2 | 17 : 2 | 0.578 | 24 : 0 | 6 : 2 | 0.056 | 14 : 2 | 9 : 0 | 0.520 | 27 : 0 | 10 : 0 | 1.000 | 11 : 2 | 4 : 2 | 0.557 |
| IQ (mean ± SD) | 107.29 ± 13.03 | 108.18 ± 11.12 | 105.21 ± 16.82 | 0.411 | 107.63 ± 12.13 | 104.13 ± 17.56 | 0.533 | 109.19 ± 10.30 | 110.67 ± 13.17 | 0.758 | 108.11 ± 12.02 | 102.70 ± 17.00 | 0.286 | 108.54 ± 10.16 | 113.50 ± 9.99 | 0.334 |
| AQ (mean ± SD) ¶ | 18.42 ± 8.67 | 14.75 ± 5.13 | 29.20 ± 7.98 | < 0.001 | 14.91 ± 5.58 | 36.50 ± 4.65 | < 0.001 | 15.81 ± 4.34 | 26.67 ± 6.48 | < 0.001 | 14.88 ± 4.59 | 27.13 ± 5.96 | < 0.001 | 16.08 ± 6.03 | 33.25 ± 9.60 | 0.001 |
| RMT (% MSO, mean ± SD) | 35.65 ± 7.42 | 36.00 ± 8.29 | 34.84 ± 4.97 | 0.574 | 35.04 ± 7.28 | 34.88 ± 6.49 | 0.954 | 36.69 ± 10.21 | 34.22 ± 3.99 | 0.497 | 34.88 ± 8.89 | 33.40 ± 5.19 | 0.625 | 37.38 ± 7.77 | 34.67 ± 3.44 | 0.429 |
| AMT (% MSO, mean ± SD) | 26.52 ± 5.32 | 26.09 ± 5.61 | 27.52 ± 4.55 | 0.329 | 25.35 ± 4.60 | 28.00 ± 6.04 | 0.206 | 26.56 ± 6.81 | 26.33 ± 3.12 | 0.925 | 26.15 ± 5.94 | 26.40 ± 4.40 | 0.906 | 25.23 ± 4.90 | 26.67 ± 3.67 | 0.533 |
| Baseline MEP amplitude (mV, mean ± SD) | 1.34 ± 1.33 | 1.27 ± 1.31 | 1.51 ± 1.39 | 0.523 | 1.45 ± 1.58 | 1.54 ± 1.71 | 0.885 | 1.19 ± 1.03 | 1.57 ± 1.29 | 0.422 | 1.37 ± 1.58 | 1.86 ± 1.69 | 0.418 | 1.28 ± 0.88 | 0.83 ± 0.68 | 0.287 |
Racial categories were defined according to the National Institutes of Health guidelines (NIH Office of Extramural Research, 2001). Comparisons of proportions were conducted with Fisher’s exact test. Education and single-nucleotide polymorphisms were not statistically compared between the subgroups because the data were not available for the total sample. N/A indicates that a statistical comparison between the two subgroups was not applicable because the measure of interest itself was the basis for subdividing the sample. Significant p-values are in bold font. The p-values were not adjusted for multiple comparisons. AMT, active motor threshold; APOE, apolipoprotein E; APOE ε4+, ε2/ε4 or ε3/ε4 genotype; APOE ε4−, ε2/ε3 or ε3/ε3; AQ, autism-spectrum quotient; BDNF, brain-derived neurotrophic factor; BDNF Met−, Val66Val; BDNF Met+, Val66Met; IQ, intelligence quotient; MEP, motor evoked potential; Met, metionine; MSO, maximum stimulator output; N/A, not applicable; RMT, resting motor threshold; SD, standard deviation; Val, valine.
Education data were available for 16 and 42 participants in the ASD and NT groups, respectively.
BDNF data were available for 17 and 39 participants in the ASD and NT groups, respectively.
APOE data were available for 16 and 39 participants in the ASD and NT groups, respectively.
AQ scores were available for 15 and 44 participants in the ASD and NT groups, respectively.
The two other datasets, reported previously (Oberman et al., 2012), included Dataset 2 collected in Boston, Massachusetts (n=40; 20 ASD subjects; age 18–64, 4 females) and Dataset 3 collected in Barcelona, Spain (n=30; 15 ASD subjects; age 29–52, 2 females).
2.2. Neuropsychological testing
The Abbreviated Battery of Stanford–Binet IV intelligence scale (Thorndike et al., 1986) and the Autism-Spectrum Quotient (AQ) (Baron-Cohen et al., 2001) were completed for both groups in Dataset 1. All participants in the ASD group had an Abbreviated IQ ≥ 70 (Table 1). AQ scores were used to quantify the situation of each participant on the continuum from normality to autism and to rule out clinically significant levels of autistic traits in the NT group (Baron-Cohen et al., 2001). The Autism Diagnostic Observation Schedule (ADOS) Module 4 was conducted by a research-reliable investigator (L.O.) to assess the current social-communicative behavior in the ASD group (Lord et al., 2000). Corresponding neuropsychological details of Datasets 2 and 3 were reported previously (Oberman et al., 2012). Individual age data were available for all three datasets, whereas individual IQ scores were only available for Datasets 1 and 2. ASD and NT groups in each of the three datasets were age-, gender-, and IQ-matched.
2.3. Genetic testing
Saliva samples from 56 participants in Dataset 1, including 17 participants (89.5%) in the ASD group and 39 participants (88.6%) in the NT group, were assessed for single-nucleotide polymorphisms (SNPs) in the brain-derived neurotrophic factor (BDNF), Val66Met, and APOE genes. Four NT and two ASD participants did not consent to providing DNA samples, and one NT sample was deemed unusable.
The Oragene Discover OGR-250 Kit (DNA Genotek Inc., Ottawa, ON, Canada) was used to extract genomic DNA from saliva samples using standard methodology and the prepIT•L2P reagent (DNA Genotek Inc., 2015). Each sample’s quality was assessed using PicoGreen fluorometry for double-stranded DNA quantification, Nanodrop spectrophotometry as to estimate purity using A260/A280 ratios, and agarose gel electrophoresis to visualize DNA integrity. A TaqMan single tube genotyping assay, using polymerase chain reaction (PCR) amplification and a pair of fluorescent dye detectors targeting the SNP, was used to analyze the rs6265 SNP of the BDNF gene, and the rs429358 and rs7412 SNPs of the APOE gene.
2.4. Transcranial magnetic stimulation
Participants sat in a comfortable chair with the right arm and hand in a natural pronated ~90° angle on a pillow, were instructed to keep their right hand relaxed and to keep their eyes open during the session. Participants were also monitored for drowsiness during TMS. Live electromyogram (EMG) was monitored to ensure hand relaxation throughout the session.
TMS procedures were conducted according to the IFCN-recommended guidelines (Rossi et al., 2009; Rossini et al., 2015). All TMS pulses were applied with a MagPro X100 stimulator, using the ‘normal’ settings, attached to a MC-B70 Butterfly Coil (outer diameter: 97mm; MagVenture A/S, Farum, Denmark), generating biphasic pulses with induced current in the antero-posterior–postero-anterior (AP-PA) direction in the brain. The intensity of single pulses was set at 120% of individual resting motor threshold (RMT) whereas cTBS was applied at 80% of individual active motor threshold (AMT). The coil was held tangential to the scalp, with the handle pointing posteriorly and at 45° angle relative to the mid-sagittal line. To achieve consistent targeting, neuronavigation was performed with Polaris infrared-optical tracking (Northern Digital Inc., Waterloo, ON, Canada) and Brainsight software for frameless stereotaxy (Rogue Research Inc., Montreal, QC, Canada). To ensure the consistency of the coil position and orientation, the participant’s brain MRI or an MRI template was used to register the participant’s head using pre-defined cranial landmarks and 12 additional samples from the scalp (Ruohonen and Karhu, 2010).
A PowerLab 4/25 data-acquisition device and LabChart software (AD Instruments, Colorado Springs, CO, USA) were used to record surface EMG from the right FDI, using the belly-tendon electrode montage. The TMS and EMG systems were synchronized via triggered pulses issued by the TMS device. EMG signal was digitized at 1 kHz, epoched from 100 ms pre-trigger to 500 ms post-trigger, amplified within ±10 mV range, and band-pass filtered (0.3–1000 Hz).
At the beginning of each TMS session, the motor hotspot, defined as the optimal spot for eliciting maximal motor evoked potentials (MEPs) from the right FDI was localized, and the RMT, defined as the lowest stimulation intensity that elicited MEPs ≥ 50 μV on at least five of ten trials, was measured. To assess baseline cortico-motor reactivity, three blocks of 30 single TMS pulses were applied to M1, with a 5–min inter-block interval and at a random 4–6 s interval between successive pulses. Individual MEPs within each block that were > 2.5 SD from the mean were excluded from further analyses. There were never more than three MEPs excluded from each block. It has been estimated that at least 20 pulses are required to obtain a reliable estimate of the MEP amplitude at a given point in time (Chang et al., 2016; Goldsworthy et al., 2016).
The peak-to-peak amplitudes of MEPs in the three pre-cTBS blocks were averaged to calculate the baseline MEP amplitude. The AMT was then measured as the lowest stimulation intensity that evoked MEPs ≥ 200 μV on at least five of ten trials while the participant slightly contracted the FDI. To control the effects of voluntary hand movements on cTBS aftereffects (Iezzi et al., 2008), there was a 5-minute break between the AMT measurement and the delivery of cTBS, during which participants were asked to maintain hand relaxation. cTBS was applied as triplet pulses at 50 Hz, delivered as 200 bursts repeated every 200 ms for 40 s (for a total of 600 pulses). To assess post-cTBS cortico-motor reactivity, 30 single TMS pulses were applied at 5, 10, 15, 20, 30, 40, 50, and 60 min following the cTBS (T5–T60). Each time point of interest was in the middle of each post-cTBS block.
Datasets 2 and 3, reported previously (Oberman et al., 2012), were collected with Magstim Super Rapid stimulators and monophasic pulses inducing posterior-anterior (PA) currents in the brain. TMS procedures for Datasets 2 and 3 were identical to those employed in the present study, except T15 data were not collected in Dataset 2 and individual T5, T10, T20 and T50 data were not collected in Dataset 3.
2.5. Statistical analyses
Research Electronic Data Capture (REDCap) electronic data capture tools hosted at Beth Israel Deaconess Medical Center (BIDMC) (Harris et al., 2019, 2009) were used for study data collection and management. Statistical analyses were conducted with Stata 16.1 (StataCorp, College Station, TX, USA) and MATLAB R2016b (The MathWorks, Natick, MA, USA) software.
RMT and AMT were expressed as percentage of maximum stimulator output (MSO), and the baseline MEP amplitude was calculated as the average of baseline MEP amplitude in 3 blocks of 30 single TMS pulses. The aftereffects of cTBS were calculated as the percent change from baseline (%Δ) by calculating the average amplitude of 30 MEPs at T5–T60 for each participant. Significant deviations from normal distribution in MEP values were found with the Shapiro–Wilk test. Thus, each post-cTBS MEP amplitude was first baseline-corrected in each participant. A natural log-transformation was then applied to the baseline-corrected MEP amplitudes at each post-cTBS time point (ΔMEP) (Nielsen, 1996a, 1996b; Pasqualetti and Ferreri, 2011). The same transformations were done on the raw MEP values in Datasets 2 and 3.
Grand-average ΔMEPs were calculated separately for each time-point in each group. In Dataset 1, ΔMEP at T5–T20 for one NT subject and ΔMEP at T15 in another NT subject were not obtained due to technical difficulties. The missing ΔMEPs in Dataset 1 were imputed using multiple regression on Age and IQ score by sampling from conditional distribution with bootstrap (Gelman et al., 2014; Royston, 2004). The uncollected ΔMEPs at T15 in Dataset 2 were imputed as the average of ΔMEPs at T10 and T20 for each subject.
For the purpose of investigating the utility of cTBS aftereffects for differentiating ASD and NT individuals, assessing the differential responses of the two groups (relative to each other) is more important than assessing whether cTBS induced a significant effect in either group. For this reason, and to avoid multiple comparisons that would reduce the power of our contrasting analyses, we focused our analyses on the separability of cTBS responses between the two groups rather than the significance of cTBS-induced modulation within each group.
Pairwise comparisons were conducted to assess whether demographic, neuropsychological, genetic, and neurophysiological measures (RMT, AMT, and baseline MEP amplitude) were significantly different between ASD and NT groups (Table 2). Continuous variables were compared using Student’s t-tests, while proportions were compared using Fisher’s Exact tests. All analyses were two-tailed, and α level was set to 0.05.
To compare cTBS aftereffects between ASD and NT groups, ΔMEPs were entered into a 2 (Diagnosis) × 8 (Time) repeated-measures analysis of variance (ANOVA). However, as the maximum modulation of MEP amplitudes typically occurs within the first 20 minutes after cTBS (Table 4 in Wischnewski and Schutter 2015), planned pairwise comparisons between ΔMEPs at T5–T20 in the two groups were conducted using Student’s t-tests. When indicated, false discovery rate (FDR) was controlled by adjusting the p-values for multiple comparisons using the Benjamini-Hochberg method (Benjamini and Hochberg, 1995). T15 was selected post hoc (see Results) as the time-point at which cTBS plasticity measures were most altered in ASD relative to NT controls. To assess the predictive utility of cTBS aftereffects, logistic-regression analyses were conducted with Diagnosis (ASD vs. NT) as dependent variable (DV) and ΔMEP at T15 as the main independent variable (IV).
Table 4.
The logistic regression models within BDNF and APOE subgroups.
| LR χ2model | Pmodel | βIV | z IV | PIV | βcovariate | z covariate | Pcovariate | AUROC | PPV | NPV | % Dx | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BDNF Met − (n = 31; 23 NT, 8 ASD) | ||||||||||||
| T15 ΔMEP | 0.50 | 0.480 | –1.0 | –0.69 | 0.490 | – | – | – | 0.571 | – | 73.33% | 73.33% |
| T15 ΔMEP plus Education† | 0.11 | 0.950 | 0.52 | 0.32 | 0.750 | –0.002 | –0.01 | 0.992 | 0.570 | – | 80.00% | 80.00% |
| T15 ΔMEP plus Race | 1.26 | 0.533 | –1.09 | –0.75 | 0.452 | 0.79 | 0.84 | 0.399 | 0.622 | – | 73.33% | 73.33% |
| BDNF Met + (n = 25; 16 NT, 9 ASD) | ||||||||||||
| T15 ΔMEP | 5.20 | 0.023 | –2.12 | –1.98 | 0.048 | –0.50 | –1.04 | 0.296 | 0.689 | 100.00% | 78.95% | 83.33% |
| T15 ΔMEP plus Education† | 5.25 | 0.073 | –2.09 | –1.92 | 0.055 | –.05 | –.21 | 0.833 | 0.711 | 100.00% | 83.33% | 87.50% |
| T15 ΔMEP plus Race | 5.90 | 0.052 | –1.84 | –1.68 | 0.093 | 0.86 | 0.83 | 0.408 | 0.682 | 85.71% | 82.35% | 83.33% |
| APOE ε– (n = 36; 26 NT, 10 ASD) | ||||||||||||
| T15 ΔMEP | 5.46 | 0.019 | –2.11 | –2.09 | 0.037 | – | – | – | 0.688 | 100.00% | 77.42% | 79.41% |
| T15 ΔMEP plus Education† | 9.48 | 0.009 | –2.03 | –1.85 | 0.064 | –0.63 | –2.04 | 0.041 | 0.828 | 83.33% | 84.00% | 83.87% |
| T15 ΔMEP plus Race | 5.48 | 0.065 | –2.15 | –2.06 | 0.040 | –0.12 | –0.14 | 0.892 | 0.671 | 100.00% | 77.42% | 79.41% |
| APOE ε+ (n = 19; 13 NT, 6 ASD) | ||||||||||||
| T15 ΔMEP | 1.67 | 0.197 | –2.18 | –1.21 | 0.228 | – | – | – | 0.705 | 100.00% | 72.22% | 73.68% |
| T15 ΔMEP plus Education† | 4.42 | 0.110 | –1.68 | –0.86 | 0.388 | 0.63 | 1.51 | 0.131 | 0.789 | – | 73.33% | 64.71% |
| T15 ΔMEP plus Race | 4.81 | 0.091 | –1.85 | –1.06 | 0.288 | 2.02 | 1.61 | 0.108 | 0.808 | 66.67% | 84.62% | 78.95% |
| APOE ε+ (n = 19; 13 NT, 6 ASD) | ||||||||||||
| T30 ΔMEP | 3.69 | 0.055 | –2.63 | –1.62 | 0.106 | – | – | – | 0.795 | 100.00% | 76.47% | 78.95% |
| T30 ΔMEP plus Education† | 5.63 | 0.060 | –1.92 | –1.30 | 0.193 | 0.63 | 1.40 | 0.163 | 0.827 | 50.00% | 84.62% | 76.47% |
| T30 ΔMEP plus Race | 8.19 | 0.017 | –2.93 | –1.73 | 0.083 | 2.60 | 1.87 | 0.062 | 0.859 | 80.00% | 1.20% | 84.21% |
Models in each genetic subgroup are ranked based on their % Dx. Diagnosis was 0 for NT and 1 for ASD. IV refers to ΔMEP at T15 or T30, as specificed. Race was 0 for White and 1 for non-White. Racial categories were defined according to the National Institutes of Health guidelines (NIH Office of Extramural Research, 2001). Significant p-values are in bold font. In each subgroup, the highest % Dx value of a significant model is in bold font. % Dx, percentage correctly classified; APOE, apolipoprotein E; ASD, autism spectrum disorder; AUROC, area under the receiver operating characteristic curve; BDNF, brain-derived neurotrophic factor; IV, independent variable; LR, likelihood ratio; ΔMEP, baseline-corrected, natural log-transformed amplitude of the motor evoked potential; Met, metionine; NPV, negative predictive value; NT, neurotypical; PPV, positivie predictive value; Tn, n minutes post-cTBS.
APOE data were available for 16 and 39 participants in the ASD and NT groups, respectively.
Education data were available for 16 and 42 participants in the ASD and NT groups, respectively.
BDNF data were available for 17 and 39 participants in the ASD and NT groups, respectively.
Studies have found TMS measures can be influenced by demographic variables such as age (Corp et al., 2020; Freitas et al., 2011), sex (Cahn et al., 2003; De Gennaro et al., 2004; Huber et al., 2003), genetic polymorphisms (Antal et al., 2010; Chang et al., 2014; Cheeran et al., 2008; Di Lazzaro et al., 2015; Peña-Gomez et al., 2012), and baseline neurophysiological measures including rMT, aMT, and baseline MEP amplitude (Corp et al., 2020, In Press; Fried et al., 2017; Jannati et al., 2017). Race and education can sometimes act as, albeit imperfect, proxy variables for socioeconomic status and possibly other/unknown covariates in studies with health-related outcomes (Geronimus et al., 1996; Krieger, 1992, 1990; Krieger and Gordon, 1999; Soobader et al., 2001). To control for potential confounders, demographic (age, sex, race, and education), neuropsychological (IQ), genetic (BDNF and APOE SNPs, when available), and baseline neurophysiological measures (RMT, AMT, baseline MEP amplitude) were added, one at a time, as covariates to the logistic-regression model. For each model, area under the receiver operating characteristic curve (AUROC), positive and negative predictive values (PPV and NPV) and percentage correctly classified (Dx) were calculated.
To validate the utility of T15 ΔMEP in a larger sample, we conducted an overall logistic regression with Diagnosis as DV and ΔMEP at T15 as IV. Dataset was included as a covariate to control for differences in TMS equipment and pulse waveform and other potential differences in subject composition across the datasets. The logistic-regression analyses involving T15 were conducted both with and without including the imputed T15 values in Dataset 2. We also controlled for Age and IQ (when available) as additional covariates. As exploratory analyses, we checked whether post-cTBS time points other than T15 were also significant predictors of Diagnosis in the overall regression models. The analyses referring to n = 91 consisted of participants in Datasets 1 and 3, for whom T15 ΔMEP was obtained.
Based on previous studies that found a developmental trajectory in cTBS response in children and adolescents with ASD (Jannati et al., 2020; Oberman et al., 2014), we assessed the changes in cTBS response across the adult lifespan (age 21–65) by evaluating the correlation between age and ΔMEP at T15 separately for each group across the two datasets in which the age data were available (Datasets 1 and 3; n=91).
To control for influences of BDNF and APOE genotypes on cTBS aftereffects, ΔMEPs of ASD and NT groups in Dataset 1 were compared separately for BDNF Met−, BDNF Met+, APOE ε4−, and APOE ε4+ participants. For APOE ε4+ participants, T30 was selected post hoc (see Results) as the time-point at which cTBS aftereffects were most altered in the ASD group relative to NT controls. Thus, the logistic-regression analyses to predict Diagnosis among APOE ε4+ participants were conducted with ΔMEP at T30 as the IV.
To assess whether BDNF or APOE genotype influenced the type of cTBS response, we classified participants in Dataset 1 as inhibitor, facilitator, or non-responder if their ΔMEP at T15 was equivalent to ≤ 90% (≤ −0.105), ≥ 110% (≥ 0.095), or between 90 and 110%, (−0.105, 0.095), respectively (Nettekoven et al., 2015). We then compared the proportion of participants with different types of cTBS response between the ASD and NT groups and their corresponding BDNF and APOE subgroups. Unless explicitly specified, all three types of responses were included in the comparisons of proportions between different groups/subgroups.
3. Results
Table 1 details demographics, BDNF and APOE SNPs, single-nucleotide polymorphisms, and neuropsychological measures for individual participants. Group means ± SD for those measures and their comparisons in the ASD and NT groups as well as in their BDNF and APOE subgroups are presented in Table 2. Education data were available for 16 (84.2%) and 42 (95.4%) participants in the ASD and NT groups, respectively. BDNF data were available for 17 (89.5%) and 40 (90.9%) participants in the ASD and NT groups, respectively. APOE data were available for 16 (84.2%) and 40 participants (90.9%) in the ASD and NT groups, respectively. Considering the high percentage of participants in the two groups for whom education and genetic data were available, we decided it would be beneficial to control for those covariates even in the presence of incomplete data.
3.1. Demographics and neuropsychogical testing
There was no significant group difference in age, sex, handedness, or education (p’s > 0.1), but the proportion of White participants was significantly higher in the ASD group than in the NT group (p = 0.050). Therefore, to control for potential confounding effect of Race, we repeated the main-group logistic regression analysis among White participants.
In the neuropsychological measures, the IQ scores were comparable between the two groups (p = 0.41), indicating that ASD participants did not significantly differ from NT controls in terms of overall cognitive function. As expected, participants with ASD had significantly higher AQ scores than their NT counterparts in the whole sample and in all BDNF and APOE subgroups (p’s ≤ 0.001).
3.2. Genetic analyses
Among 56 participants with available BDNF results, the proportions of BDNF Val/Val and Val/Met genotypes were 55.4% and 44.6%, whereas among 55 participants with available APOE results, the proportions of APOE ε4− and ε4+ genotypes were 65.5% and 34.6%, respectively. The two groups were comparable in BDNF Met− : Met+ and APOE ε4− : ε4+ ratios (p’s > 0.5).
ASD and NT participants in each BDNF and APOE subgroup were comparable in terms of demographics and IQ scores (p’s > 0.05; Table 2). APOE ε4− participants were more educated in the NT group than in the ASD group (p = 0.015).
3.3. Measures of corticospinal excitability and plasticity
All participants tolerated TMS procedures with no complications or unexpected side effects. As detailed in Table 2, RMT, AMT, and baseline MEP amplitudes were comparable between the ASD and NT groups (p’s > 0.3), and their BDNF and APOE subgroups (p’s > 0.2). These results indicate the ASD participants did not differ significantly from NT controls in baseline corticospinal excitability.
The repeated-measures ANOVA indicated the ΔMEPs at T5–T60 did not vary significantly by Diagnosis, F(1,61) = 0.64, p = 0.428, η2p = 0.01, Time, F(7,422) = 1.82, p = 0.082, η2p = 0.03, or their interaction, F(7,422) = 1.37, p = 0.218, η2p = 0.02. Planned t-tests at T5–T20 found ASD subjects had significantly greater inhibition of MEPs at T15 (p = 0.029), which did not survive FDR adjustment (PFDR = 0.116). In the NT group, 29.5%, 13.6%, and 56.8% of participants were inhibitors, non-responders, and facilitators, respectively, whereas the corresponding proportions in the ASD group were 57.9%, 10.5%, and 31.6%. There was no significant overall difference in the type of cTBS response between the two groups (p = 0.103). When the 8 non-responders were excluded from the two groups, the proportion of inhibitors was significantly greater in the ASD group than in the NT group (p = 0.044).
ΔMEPs at T5–T10 were not significantly different between the two groups (p’s > 0.2). Figure 1 shows the cTBS aftereffects in ASD and NT groups and the ROC curve associated with the logistic regression.
Figure 1.
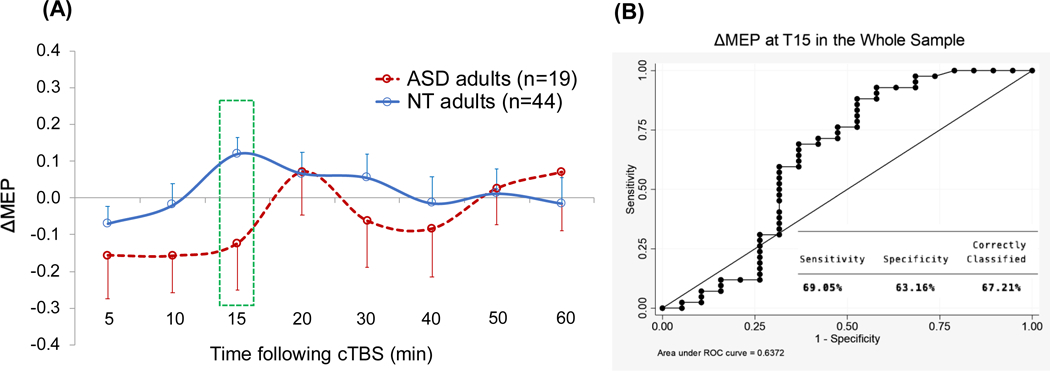
(A) Grand-average change from baseline in MEP amplitude recorded from the right FDI muscle at 5–60 min following cTBS of the left motor cortex in ASD and NT groups. Error bars represent standard error of the mean. (B) The receiver operating characteristic curve associated with the logistic regression analysis with Diagnosis (ASD vs. NT) as IV and ΔMEP at T15 as DV. ASD, autism spectrum disorder; cTBS, continuous theta-burst stimulation; ΔMEP, natural log-transformed, baseline-corrected amplitude of motor evoked potential; DV, dependent variable; FDI, first dorsal interosseous; IV, independent variable; NT, neurotypical; Tn, n minutes post-cTBS.
Logistic regression analysis found that T15 ΔMEP was a significant predictor of Diagnosis, β = −1.55, p = 0.038, indicating a more negative ΔMEP at T15 was predictive of ASD diagnosis, AUROC = 0.64. As detailed in Table 3, follow-up analyses found that T15 ΔMEP remained a significant predictor of Diagnosis after controlling for IQ, Handedness, BDNF, APOE, RMT, AMT, or Baseline MEP (p’s < 0.046) but not after controlling for Age, Sex, or Education (p’s > 0.052). However, none of the added covariates was a significant predictor (p’s > 0.08), and the predictive powers of all the logistic-regression models were comparable (AUROC range 0.64–0.70). Controlling for Education or Race resulted in the most predictive models (Dx > 80%), whereas controlling for other covariates resulted in 72–78% correct classification (Table 3). T15 ΔMEP remained a significant predictor of Diagnosis among White participants (p = 0.039; AUROC = 0.70).
Table 3.
Changes in the logistic regression model predicting diagnosis after adding covariates.
| LR χ2model | Pmodel | βIV | z IV | PIV | βcovariate | z covariate | Pcovariate | AUROC | PPV | NPV | % Dx | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T15 ΔMEP | 4.79 | 0.029 | –1.55 | –2.07 | 0.038 | – | – | – | 0.637 | 100.00% | 72.41% | 73.77% |
| T15 ΔMEP plus Education† | 4.94 | 0.084 | –1.32 | –1.75 | 0.081 | –0.18 | –1.32 | 0.187 | 0.644 | 100.00% | 78.43% | 80.36% |
| T15 ΔMEP plus Race | 8.13 | 0.017 | –1.31 | –1.74 | 0.081 | 1.15 | 1.75 | 0.080 | 0.704 | 88.89% | 78.85% | 80.33% |
| T15 ΔMEP plus BDNF ‡ | 6.04 | 0.049 | –1.76 | –2.11 | 0.034 | 0.53 | 0.85 | 0.397 | 0.635 | 100.00% | 75.51% | 77.78% |
| T15 ΔMEP plus APOE * | 7.16 | 0.028 | –2.13 | –2.41 | 0.016 | 0.20 | 0.31 | 0.756 | 0.699 | 100.00% | 75.51% | 77.36% |
| T15 ΔMEP plus Handedness | 5.54 | 0.063 | –1.58 | –2.09 | 0.036 | –0.94 | –0.88 | 0.379 | 0.647 | 100.00% | 75.00% | 77.05% |
| T15 ΔMEP plus IQ | 5.85 | 0.054 | –1.63 | –2.12 | 0.034 | –0.02 | –1.03 | 0.304 | 0.691 | 83.33% | 74.55% | 75.41% |
| T15 ΔMEP plus Age | 6.02 | 0.049 | –1.45 | –1.93 | 0.053 | 0.02 | 1.11 | 0.266 | 0.668 | 71.43% | 74.07% | 73.77% |
| T15 ΔMEP plus Sex | 5.41 | 0.067 | –1.42 | –1.86 | 0.063 | –0.65 | –0.76 | 0.447 | 0.664 | 100.00% | 72.41% | 73.77% |
| T15 ΔMEP plus Baseline MEP | 4.80 | 0.091 | –1.54 | –2.01 | 0.044 | 0.02 | 0.11 | 0.915 | 0.639 | 100.00% | 72.41% | 73.77% |
| T15 ΔMEP plus RMT | 4.79 | 0.091 | –1.54 | –2.00 | 0.045 | –0.001 | –0.03 | 0.976 | 0.639 | 100.00% | 72.41% | 73.77% |
| T15 ΔMEP plus AMT | 6.65 | 0.036 | –1.78 | –2.27 | 0.023 | 0.08 | 1.35 | 0.177 | 0.677 | 66.67% | 72.73% | 72.13% |
Models including a covariate are ranked based on their % Dx. Diagnosis was 0 for NT and 1 for ASD. IV refers to T15 ΔMEP. Sex was 0 for males and 1 for females. Race was 0 for White and 1 for non-White. Racial categories were defined according to the National Institutes of Health guidelines (NIH Office of Extramural Research, 2001). Handedness was for 0 for right-handed and 1 for left-handed. BDNF was 0 for Met− and 1 for Met+. APOE was 0 for ε4− and 1 for ε4+. Significant p-values are in bold font. % Dx, percentage correctly classified; AMT, active motor threshold; APOE, apolipoprotein E; ASD, autism spectrum disorder; AUROC, area under the receiver operating characteristic curve; BDNF, brain-derived neurotrophic factor; IQ, intelligence quotient; IV, independent variable; LR, likelihood ratio; ΔMEP, baseline-corrected, natural log-transformed amplitude of the motor evoked potential; Met, metionine; NPV, negative predictive value; NT, neurotypical; PPV, positivie predictive value; RMT, resting motor threshold; T15, 15 minutes post-cTBS.
APOE data were available for 16 and 39 participants in the ASD and NT groups, respectively.
Education data were available for 16 and 42 participants in the ASD and NT groups, respectively.
BDNF data were available for 17 and 39 participants in the ASD and NT groups, respectively.
Across all three datasets (N = 133), while controlling for Dataset and Age, T15 ΔMEP (partially imputed based on T10 and T20) was a significant predictor of Diagnosis (β = −1.25, p = 0.004; AUROC = 0.70). Across Datasets 1 and 3 in which T15 ΔMEP values were obtained (n = 91), while controlling for Dataset and Age, T15 ΔMEP was also a significant predictor of Diagnosis (β = −1.54, p = 0.025; AUROC = 0.71). Among subjects in Datasets 1 and 2 for whom individual IQ scores were available (n= 89), while controlling for Dataset, Age, and IQ, T15 ΔMEP remained a significant predictor of Diagnosis (β = −1.42, p = 0.016, AUROC = 0.76). The effects of Dataset, Age, or IQ were not significant in any of the models above (p’s > 0.09).
Across all three datasets (N = 133), among post-cTBS time points other than T15 and while controlling for Dataset and Age, ΔMEPs at T30 (β = −1.28, p = 0.002; AUROC = 0.73) and T40 (β = −1.17, p = 0.002; AUROC = 0.72) were significant predictors of Diagnosis but ΔMEPs at other time points were not (p’s > 0.07). Among subjects in Datasets 1 and 2 for whom individual IQ scores were available (n= 89), while controlling for Dataset, Age, and IQ, none of the post-cTBS time points other than T15 was a significant predictor of Diagnosis (p’s > 0.053).
ΔMEP at T15 was negatively correlated with Age among NT subjects across datasets 1 and 3 (n = 57; r = −0.32, p = 0.016) but not among ASD subjects in the same datasets (n = 34; r = −0.23, p = 0.189).
3.4. BDNF and APOE influences in cTBS measures of plasticity
Among 53 participants in Dataset 1, after controlling for both BDNF and APOE SNPs as covariates, T15 ΔMEP remained a significant predictor of Diagnosis (p = 0.018; AUROC = 0.69; Dx = 79.3%), whereas neither BDNF nor APOE status was a significant predictor (p’s > 0.2).
Table 4 presents results of the logistic regression analyses predicting Diagnosis with T15 ΔMEP as the IV within BDNF and APOE subgroups. Because controlling for Education or Race in the overall logistic regression resulted in the models with the highest Dx values, Education and Race were added, one at a time, as covariates to each model within genetic subgroups. For APOE ε4+ participants, T30 ΔMEP was chosen post hoc as the time point at which cTBS aftereffects in ASD participants showed the largest alteration compared to NT participants, and similar logistic regression analyses as above were conducted with T30 ΔMEP as the IV. The analyses found T15 ΔMEP was a significant predictor of Diagnosis among 25 BDNF Met+ participants (16 NT, 9 ASD; p = 0.048; AUROC = 0.69; Dx = 83.3%) and among 36 APOE ε4− participants (26 NT, 10 ASD; p = 0.037; AUROC = 0.69; Dx = 79.4%), but not among 31 BDNF Met− participants (23 NT, 8 ASD; p = 0.490; AUROC = 0.57; Dx = 73.3%). Figures 2–5 show the comparisons of cTBS aftereffects in BDNF and APOE subgroups of ASD and NT participants and the ROC curves associated with the significant logistic-regression models.
Figure 2.
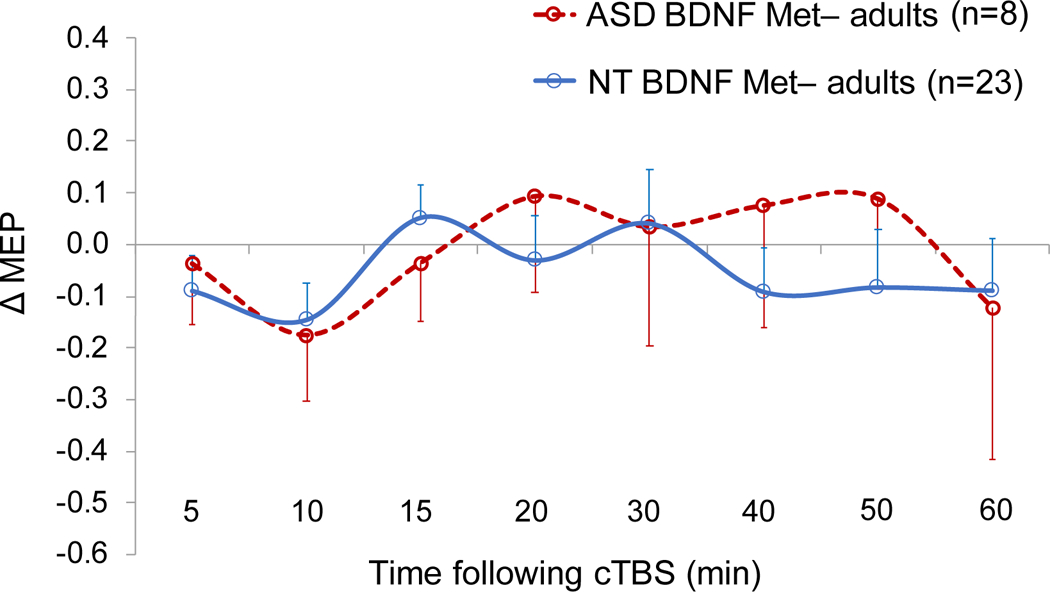
Grand-average change from baseline in MEP amplitude recorded from the right FDI muscle at 5–60 min following cTBS of the left motor cortex in ASD and NT subjects with BDNF Met− genotype. Error bars represent standard error of the mean. ASD, autism spectrum disorder; BDNF, brain-derived neurotrophic factor; cTBS, continuous theta-burst stimulation; ΔMEP, natural log-transformed, baseline-corrected amplitude of motor evoked potential; FDI, first dorsal interosseous; Met, metionine; NT, neurotypical.
Figure 5.
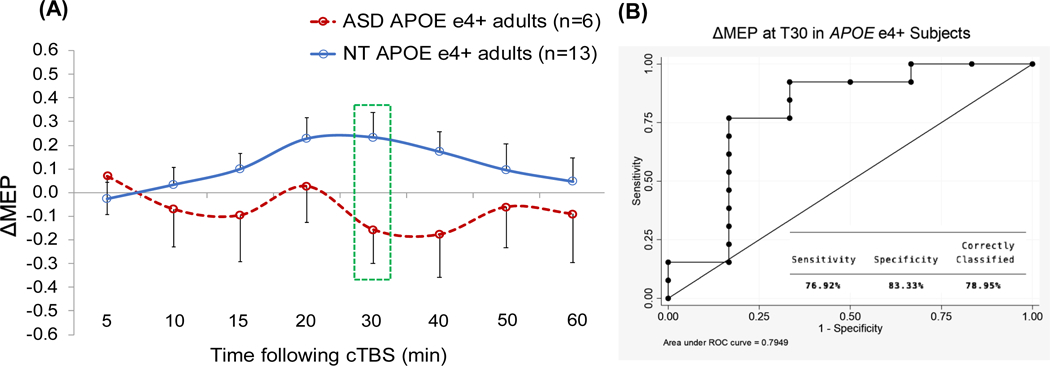
(A) Grand-average change from baseline in MEP amplitude recorded from the right FDI muscle at 5–60 min following cTBS of the left motor cortex in ASD and NT subjects with APOE ε4+ genotype. Error bars represent standard error of the mean. (B) The receiver operating characteristic curve associated with the logistic regression analysis with Diagnosis (ASD vs. NT) as IV and ΔMEP at T30 as DV. APOE, apolipoprotein E; ASD, autism spectrum disorder; cTBS, continuous theta-burst stimulation; ΔMEP, natural log-transformed, baseline-corrected amplitude of motor evoked potential; DV, dependent variable; FDI, first dorsal interosseous; IV, independent variable; NT, neurotypical; Tn, n minutes post-cTBS.
In the analysis of APOE ε4− participants, when Education was added as a covariate, it was a significant predictor (p = 0.041), whereas T15 ΔMEP only showed a trend (p = 0.064), even though the model itself remained significant, p = 0.009, AUROC = 0.83; Dx = 83.9%. It is possible that the loss of significant effect of T15 ΔMEP was due to the listwise deletion of a few participants for whom Education data were not available (Table 2).
Among APOE ε4+ participants, T15 ΔMEP was not a significant predictor of Diagnosis, with or without controlling for Education or Race (p’s > 0.2). Choosing T30 ΔMEP as the IV and controlling for Race resulted in a significant model, p = 0.017, AUROC = 0.86; Dx = 84.2%, and both T30 ΔMEP and Race had non-significant effects (p’s < 0.1) (Table 4).
Among either APOE ε4− or ε4+ participants, there was no significant difference between ASD and NT groups in the type of cTBS response (p = 0.780), whereas among BDNF Met+ participants, the proportion of inhibitors was significantly greater in the ASD group than in the NT group (p = 0.006). Among either APOE ε4− participants or APOE ε4+ participants, there was no significant difference between ASD and NT groups in the type of cTBS response (p = 0.257 and p = 0.185, respectively).
4. Discussion
The present study compared cTBS measures of brain plasticity in adults with HF-ASD and their age-, gender-, and IQ-matched NT controls. We found, among 63 participants including 19 participants with ASD, cTBS-induced MEP suppression at 15 minutes post-stimulation (T15) was a significant predictor of diagnosis with moderate (72–80%) classification accuracy, depending on the covariate included in the model. The discriminatory power of T15 ΔMEP was not driven by demographic, neuropsychological, or genetic differences between ASD and NT groups. Moreover, we found T15 ΔMEP remained a significant predictor of ASD diagnosis when the present data were combined with the results of two previously reported cohorts, for a total of 133 subjects including 54 ASD subjects. In exploratory analyses, ΔMEPs at T30 and T40 were the only other post-cTBS time point that were significant predictors of ASD diagnosis when controlling for Dataset and Age. In combined analyses, T15 ΔMEP had the highest discriminatory power among all post-cTBS time points.
A novel contribution of the present study was to control for influences of BDNF and APOE SNPs on cTBS aftereffects, and to assess the discriminatory power of cTBS responses between ASD and NT individuals within the more-homogenous SNP subgroups of either BDNF or APOE genes. Stratifying the study sample by either BDNF or APOE SNP status made a noticeable difference in the discriminatory power of cTBS aftereffects. While T15 ΔMEP was not a significant predictor of diagnosis among BDNF Met− participants, it was a stronger predictor among BDNF Met+ and APOE ε4− participants, with a classification accuracy of up to 88% and 84%, respectively. The overall pattern of cTBS aftereffects among APOE ε4+ was distinct from that among the other genetic subgroups, with ΔMEP at T30 showing the greatest alteration in cTBS response in ASD participants, predicting diagnosis with an accuracy of up to 84%.
4.1. Overall cTBS measures of plasticity in ASD and NT adults
The main finding of the present study is that, despite large inter-individual variability in both groups, M1 cTBS responses were still able to differentiate individuals with ASD from their NT counterparts in a sample that was larger than most other TBS studies (Wischnewski and Schutter, 2015). The lack of a significant overall cTBS-induced suppression of MEPs in the NT group is consistent with the results of several recent studies in healthy adults (Goldsworthy et al., 2014; Hamada et al., 2013; Hordacre et al., 2017; Jannati et al., 2019, 2017; Nettekoven et al., 2015; Vallence et al., 2015). The present overall cTBS results in the NT group further confirm the large interindividual variability in TBS aftereffects (Corp et al., 2020).
As in previous studies investigating brain plasticity mechanisms with rTMS (Fried et al., 2017, 2016; Jannati et al., 2020, 2019, 2017; Oberman et al., 2010, 2016, 2014, 2012), we focused on the primary motor cortex in the left hemisphere. It is unlikely, however, that the observed differences in cTBS metrics of cortical plasticity between the two groups result from an ASD-related pathophysiological process specific to motor cortex. A structured neurological exam or medical history review found no evidence of gross or fine motor abnormalities in our ASD participants. Moreover, consistent with previous studies (Enticott et al., 2010; Peter G Enticott et al., 2013; Peter G. Enticott et al., 2013; Minio-Paluello et al., 2009; Oberman et al., 2016, 2012; Théoret et al., 2005), baseline neurophysiological measures including RMT, AMT, and baseline MEP amplitude were comparable in the two groups, indicating that baseline M1 excitability, cortico-motor reactivity, and the function of the corticospinal pathway are not necessarily affected in ASD.
The greater and longer-lasting inhibitory response to cTBS is likely due to ASD-related abnormalities in the efficacy of plasticity mechanisms in the cortex. These include both classic LTD-like synaptic plasticity as well as nonsynaptic plasticity mechanisms, including biochemical and genetic changes (Tang et al., 2017). Although abnormalities in motor domain are not among the core symptoms of ASD, motor deficits among individuals with ASD have been reported, including alterations in motor learning (Sharer et al., 2016). Moreover, there is evidence to suggest abnormalities in motor function may precede higher-level social and communication impairments, including inferring others’ intentions, gesturing, and imitation (Casartelli et al., 2016; Mostofsky and Ewen, 2011). It remains unclear whether these aberrant TMS-derived physiological responses are causal or a consequence of ASD pathology. It also remains to be investigated whether and to what extent these findings translate to non-motor cortical regions. Such investigations would require combining TMS and electroencephalography (EEG) or other neuroimaging techniques (Pascual-Leone et al., 2011; Thut et al., 2005; Thut and Pascual-Leone, 2010a, 2010b; Tremblay et al., 2019).
The present results build upon and expand the main finding of previous cTBS studies in adults with ASD (Oberman et al., 2016, 2012) that identified abnormal plastic regulation in the form of prolonged cTBS aftereffects in adults with ASD compared to NT adults. Maximum sensitivity and selectivity of cTBS measures of plasticity found in the present study were comparable with those found previously, 0.75 and 0.85, respectively (Oberman et al., 2012). One noticeable difference was the substantially earlier return to baseline of cTBS-modulated MEP amplitudes in the present study (by T20) compared to those found previously, i.e., T40–T60 in the ASD group (Oberman et al., 2012). This difference in pattern of cTBS aftereffects may be attributed to interindividual variability of cTBS responses, differences across datasets in proportions of BDNF, APOE, and other relevant SNPs, demographics, neuropsychological characteristics, or neuroactive medications (i.e., any drug that can influence neural activity in the central nervous system, whether as a stimulant or suppressant) in the ASD group. Moreover, the inter-study variability in the combined analyses likely resulted in the lower diagnostic utility of later time points compared to T15.
The present results can also be considered in the context of studies on aberrant TBS responses in children and adolescents with HF-ASD (Jannati et al., 2020; Oberman et al., 2014). Those studies showed a paradoxical, facilitatory response to cTBS in approximately one-third of children and adolescents with HF-ASD (Oberman et al., 2014) or at the group level relative to typically developing children (Jannati et al., 2020). Moreover, the extent of (Oberman et al., 2014) or the maximum inhibitory response to cTBS (Jannati et al., 2020) showed a maturational trajectory, increasing linearly up to the age of 16. Among the adult subjects in the present study (aged 21–65), the NT group showed a maturational trajectory, with increasingly greater inhibitory cTBS response across the lifespan, whereas the ASD group did not show such a relationship. Assuming that the lack of significant correlations is not due to low power, these findings considered together with the greater and/or longer-lasting cTBS aftereffects observed in ASD adults (Oberman et al. 2012, 2016; present study) suggest a gradual change in the pattern of alteration in cTBS-derived plasticity measures in ASD across the lifespan relative to NT individuals. Individuals with ASD seem more likely to show a facilitatory response to cTBS in childhood and early adolescence. As ASD individuals grow older, they become more likely to show a greater inhibitory response to cTBS that may plateau by the age of 21. NT individuals show a more-inhibitory, but stable, response in childhood and adolescence, relative to individuals with ASD. In adulthood, NT individuals continue to show an increasingly inhibitory response to cTBS, perhaps due to the effects of normal aging on excitatory:inhibitory ratio in the cortex, which may be aberrant in ASD (Ben-Ari et al., 2012).
Two points are worth mentioning in comparing the present results to our previous cTBS results among healthy adults (Jannati et al., 2017). First, in that study (which included 21 of the 44 NT participants in the present study), we found T10 was the time point with the greatest explanatory power of interindividual variability in cTBS responses in healthy adults, whereas in the present study T15 was the strongest predictor of diagnosis, with little discriminability between ASD and NT groups at T10 (Figures 1–5). These contrasting results indicate that not only the most suitable post-TBS time point(s) to differentiate a given clinical population such as those with ASD from healthy individuals can differ from those in other clinical populations (e.g., McClintock et al. 2011; Tremblay et al. 2015; Fried et al. 2016), they can also differ from the time points that best characterize the gamut of TBS responses in healthy adults (Jannati et al., 2017) and across the adulthood (Freitas et al., 2011).
Second, we previously found AMT was a significant predictor of cTBS response in healthy adults (Jannati et al., 2017), whereas in the present study neither AMT nor RMT or baseline MEP amplitude were significant predictors of ASD diagnosis. These results suggest, while baseline neurophysiological measures can influence TBS responses among healthy individuals (Corp et al., 2020), they may not necessarily be a good differentiator of ASD and NT populations.
Interestingly, the finding that cTBS aftereffects retained their diagnostic utility across datasets that were obtained with two different types of TMS device (Magstim vs. MagPro) and pulse waveforms (monophasic vs. biphasic) suggest the utility of cTBS response as a physiologic biomarker is not necessarily susceptible to differences in TMS equipment and pulse-waveform characteristics (Davila-Pérez et al., 2018; Di Lazzaro and Rothwell, 2014).
4.2. The roles of BDNF and APOE polymorphisms in cTBS aftereffects
Factors contributing to inter- and intra-individual variability in TBS responses include the activation state of intracortical networks (Hamada et al., 2013), functional connectivity in the motor system (Nettekoven et al., 2015, 2014), state-dependent factors (Suppa et al., 2016), and SNPs that influence neuroplasticity, including BDNF (Antal et al., 2010; Chang et al., 2014; Cheeran et al., 2008; Di Lazzaro et al., 2015; Fried et al., 2017; Jannati et al., 2019, 2017; Lee et al., 2013) and APOE (Jannati et al., 2019; Mahley and Rall Jr, 2000; Nichol et al., 2009; Peña-Gomez et al., 2012; White et al., 2001; Wolk et al., 2010).
The prevalence of BDNF Met+ in the American population (AMR) population is estimated at ~28.2 % (1000 Genomes Project Consortium et al., 2015), whereas the prevalence of APOE ε4+ is estimated at ~14.5% (Eisenberg et al., 2010), indicating approximate prevalence of 61.4%, 10.4%, 24.1%, and 4.1% for Met−/ε4−, Met−/ε4+, Met+/ε4−, and Met+/ε4+ subgroups, respectively. The current results suggest, after adjusting for the relevant demographics (Table 4), predictiveness of cTBS aftereffects may be greater among Met+/ε4− and Met+/ε4+ subgroups, followed by the Met− subgroups.
Compared to the logistic regressions based on continuous measures of cTBS response, subdividing the proportion of participants based on their type of cTBS response (inhibitor, non-responder, or facilitator) resulted in a less-sensitive measure, as it was only able to differentiate ASD and NT participants among BDNF Met+ participants but not in the other subgroups.
The present results on the influence of BDNF and APOE SNPs on cTBS aftereffects have two main implications: (1) The existence and pattern of abnormality in cTBS responses in ASD adults are substantially modulated by both BDNF and APOE SNPs (Figures 2–5); (2) Limiting the analyses to each SNP subgroup of BDNF or APOE noticeably improves the predictive power of cTBS aftereffects in most, but not all, SNP subgroups. These two findings indicate that using the same logistic-regression model for differentiating all individuals with ASD from their NT counterparts is not optimal. Instead, we propose a hierarchical decision tree based on BDNF and APOE SNPs and perhaps other characteristics of a given individual with ASD (Tables 3 and 4). For example, if an individual with ASD is BDNF Met−, the next best step may be to compare his/her cTBS response with the cTBS responses of NT individuals who have a similar APOE ε4 status, while also controlling for potentially important covariates. The post-cTBS time-point of interest in each step of analysis and estimations of the PPV and NPV of the cTBS biomarker may need to be chosen and adjusted accordingly, i.e., based on the specific SNP subgroup to which that individual belongs and perhaps demographic covariates such as race and/or level of education (Tables 3 and 4).
4.3. Additional considerations
A number of factors may limit the generalizability of the present findings. First, because our ASD sample was relatively small (n=19), it is likely there were heterogeneities among individuals with HF-ASD in demographics, clinical endophenotype, symptom severity, behavioral interventions and neuroactive medications, as well as underlying structural and functional brain differences that were not adequately represented in our sample. Such sampling errors could have reduced the representativeness of cTBS response in our ASD sample. We attempted to address these limitations by validating the present results in a relatively large, multi-site cohort including two previously obtained datasets. Such efforts are necessary for better assessment of the biomarker utility of cTBS, as well as enabling more-robust indices of target engagement and therapeutic response to experimental pharmacotherapy (e.g., Lemonnier et al. 2017) and potential rTMS treatments for ASD (Cole et al., 2019). Larger samples of TMS data from healthy individuals (e.g., Corp et al. 2020) and clinical populations may also allow for evaluating multivariate models that take into account potentially important covariates such as demographic, neuropsychological, and neurophysiological measures as well as multiple relevant SNPs at the same time, enabling more-encompassing predictive models for individuals with ASD and those with other neuropsychiatric disorders.
Second, the representativeness of our ASD sample was limited in two ways: (1) because of lack of established feasibility and tolerability of rTMS in low-functioning individuals with ASD, all of our ASD participants were high-functioning; (2) because of the potential risk of rTMS-induced seizure, however small (Lerner et al., 2019), we excluded ASD participants with history of epilepsy. The prevalence of history of epilepsy in ASD can be as high as 26% (Viscidi et al., 2013). Moreover, a history of epilepsy is associated with more severe ASD symptoms, history of developmental regression, and poorer adaptive and language functioning (Viscidi et al., 2013). Thus, it is possible that if our ASD group included low-functioning individuals and/or those with a history of epilepsy, the overall pattern of cTBS responses in the ASD group would have been substantially different from present results. One option to study TMS measures of plasticity in individuals with ASD and current or history of epilepsy, while further reducing the potential risk of TMS-induced seizure, is to use TMS protocols such as paired associative stimulation (PAS) (Stefan et al. 2000; Ziemann 2004; see Suppa et al. 2017 for a recent review) that involve applying spTMS to the brain but still enable measuring TMS indices of plasticity in ASD (Jung et al., 2013).
Third, we were only able to stratify our sample by either BDNF or APOE SNP, as our sample size did not allow for robust assessment of the biomarker utility of cTBS aftereffects while stratifying simultaneously for both SNPs. Due to potential interactions between the effects of BDNF and APOE SNPs on rTMS plasticity metrics, the discriminatory power of cTBS aftereffects may differ substantially among those four SNP subgroups, even though, to our knowledge, such interactions have not been reported.
Fourth, a potentially important limitation of the present study was the absence of a sham control. Even though including a sham-TMS condition in purely neurophysiological studies such as the present work may seem not as critical as in studies with behavioral or psychological outcomes, it may nevertheless help assess possible natural fluctuations in corticospinal excitability that are not related to the rTMS intervention. For example, studies have found increased corticospinal excitability over time (Julkunen et al., 2012) or as a result of receiving successive single TMS pulses over the duration of a study visit (Pellicciari et al., 2016). Assuming these potential effects are similar between the ASD and NT groups, they would be canceled out when comparing their cTBS aftereffects, but still the inclusion of a sham-TMS condition would enable a more-robust assessment of post-cTBS changes in MEP amplitude that are not associated with the cTBS itself.
Fifth, another factor that could have influenced the difference in cTBS responses between ASD and NT groups is the potential effects of the various neuroactive medications received by all but three of the ASD participants. Due to the relatively small sample size and the disparate mechanisms of those neuroactive medications, controlling for them as a covariate when comparing the cTBS responses between the two groups was not be statistically feasible. Future studies with larger cohorts may benefit from dividing ASD participants based on the type of neuroactive medications they receive and controlling for those medications as a covariate in multivariate models. A further valuable control would be to assess the same cTBS measures among non-ASD participants who receive the same or similar medications for other reasons.
Lastly, another limitation of our study was the lack of data on other SNPs that influence rTMS measures of brain plasticity, e.g., the catechol-O-methyltransferase (COMT) Val158Met SNP (Lee et al., 2014) that can interact with the effect of BDNF polymorphism on rTMS responses (Witte et al., 2012).
For cTBS measures of plasticity to ultimately become a valid and reliable physiologic biomarker for ASD, more consistent results are needed. Beyond controlling for relevant demographics, medications, and genetic variations known to influence neuroplasticity, possible future directions in this regard include assessing the effect of a range of stimulation intensities, including higher intensities than what has been used conventionally for TBS, conducting repeated stimulation sessions, and controlling for response to realistic sham-TMS, which itself may vary across individuals. Other future directions include combining TMS-EMG plasticity metrics with electroencephalography (EEG) and functional magnetic resonance imaging (fMRI) measures of TBS-induced plasticity, including modulation of TMS-evoked potentials and changes in resting-state functional connectivity (Eldaief et al., 2011; Farzan et al., 2016; Halko et al., 2014; Pascual-Leone et al., 2011).
4.4. Conclusions
The results of the present study show the utility of cTBS measures of M1 plasticity as a biomarker for adults with ASD, and the importance of controlling for BDNF and APOE SNPs in comparing those measures between ASD and NT individuals. The validation of the main findings from the present cohort in a larger multi-site cohort indicates the promise of TBS measures of plasticity as physiologic biomarkers for individuals with ASD.
Figure 3.
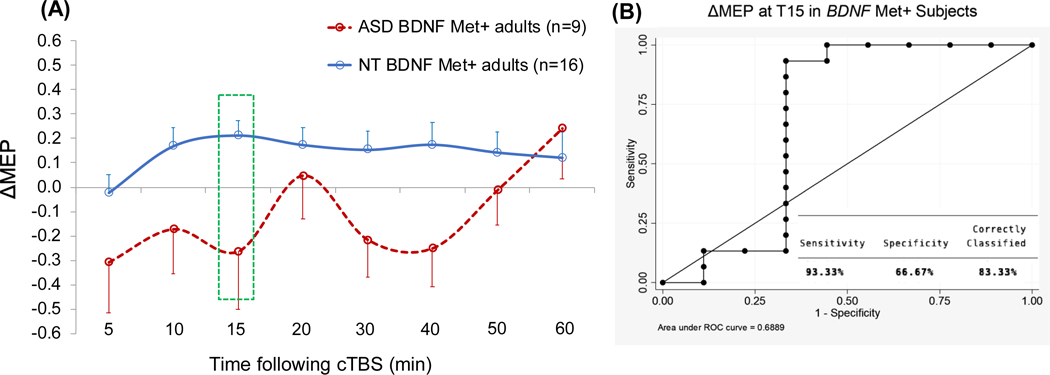
(A) Grand-average change from baseline in MEP amplitude recorded from the right FDI muscle at 5–60 min following cTBS of the left motor cortex in ASD and NT subjects with BDNF Met+ genotype. Error bars represent standard error of the mean. (B) The receiver operating characteristic curve associated with the logistic regression analysis with Diagnosis (ASD vs. NT) as IV and ΔMEP at T15 as DV. ASD, autism spectrum disorder; BDNF, brain-derived neurotrophic factor; cTBS, continuous theta-burst stimulation; ΔMEP, natural log-transformed, baseline-corrected amplitude of motor evoked potential; DV, dependent variable; FDI, first dorsal interosseous; IV, independent variable; Met, metionine; NT, neurotypical; Tn, n minutes post-cTBS.
Figure 4.
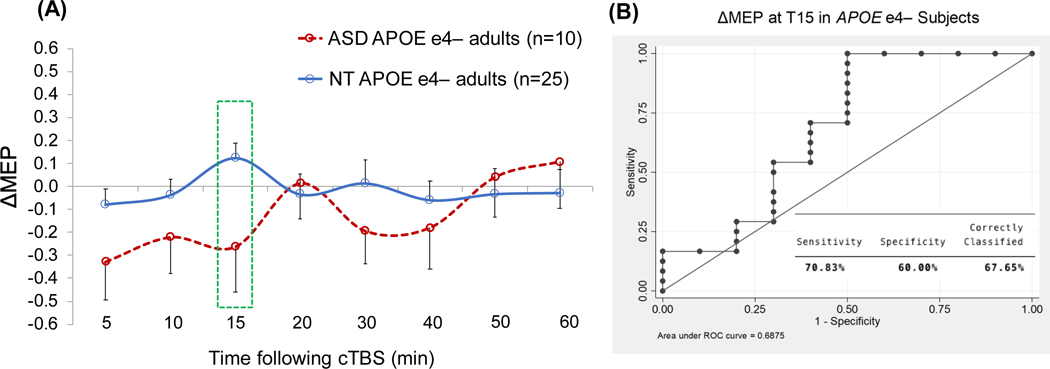
(A) Grand-average change from baseline in MEP amplitude recorded from the right FDI muscle at 5–60 min following cTBS of the left motor cortex in ASD and NT subjects with APOE ε4− genotype. Error bars represent standard error of the mean. (B) The receiver operating characteristic curve associated with the logistic regression analysis with Diagnosis (ASD vs. NT) as IV and ΔMEP at T15 as DV. APOE, apolipoprotein E; ASD, autism spectrum disorder; cTBS, continuous theta-burst stimulation; ΔMEP, natural log-transformed, baseline-corrected amplitude of motor evoked potential; DV, dependent variable; FDI, first dorsal interosseous; IV, independent variable; NT, neurotypical; Tn, n minutes post-cTBS.
Highlights.
cTBS aftereffects are different in a large sample of individuals with ASD compared to neurotypical controls.
T15 cTBS aftereffect allows to distinguish individuals with ASD diagnosis from neurotypical controls.
Diagnostic utility of cTBS aftereffects for ASD is modulated by BDNF and APOE SNPs.
Acknowledgement
We thank Stephanie Changeau, Aaron Boes, and Simon Laganiere (BIDMC) for assistance with neurological examinations, and Ann Connor and Joanna Macone (BIDMC) for regulatory oversight and compliance, and for assistance with evaluation of participants’ health and medical history.
This study was primarily funded by the National Institutes of Health (NIH R01 MH100186). A.P.-L. is further partly supported by the National Institutes of Health (R24AG06142, and P01 AG031720), the National Science Foundation, and the Barcelona Brain Health Initiative (La Caixa and Institute Guttmann), as well as sponsored research agreements with Neuroelectrics for home-based transcranial current stimulation and EGI for high-density EEG in cognitive disability. A.J. was further supported by postdoctoral fellowships from the Natural Sciences and Engineering Research Council of Canada (NSERC 454617) and the Canadian Institutes of Health Research (CIHR 41791). L.O. was further supported by the Simons Foundation Autism Research Initiative (SFARI) and the Nancy Lurie Marks Family Foundation. A.R. was further supported by the NIH (R01 NS088583), The Boston Children’s Hospital Translational Research Program, Autism Speaks, Massachusetts Life Sciences, The Assimon Family, Brainsway, CRE Medical, Eisai, Neuroelectrics, Roche, Sage Therapeutics, and Takeda Medical. The content is solely the responsibility of the authors and does not necessarily represent the official views of the involved institutions or granting agencies.
Footnotes
Conflicts of Interest Statement
A.P.-L. is a co-founder of Linus Health and TI Solutions AG; serves on the scientific advisory boards for Starlab Neuroscience, Magstim Inc., and MedRhythms; and is listed as an inventor on several issued and pending patents on the real-time integration of noninvasive brain stimulation with electroencephalography and magnetic resonance imaging. A.R. is a founder and advisor for Neuromotion and PrevEp, and serves on the scientific advisory board or has consulted for Cavion, Epihunter, Gamify, Neural Dynamics, NeuroRex, Praxis, Roche, Otsuka, and is listed as an inventor on a patent related to integration of TMS and EEG. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1000 Genomes Project Consortium, Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, et al. A global reference for human genetic variation. Nature 2015;526:68–74. 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub; 2013. [Google Scholar]
- Antal A, Chaieb L, Moliadze V, Monte-Silva K, Poreisz C, Thirugnanasambandam N, et al. Brain-derived neurotrophic factor (BDNF) gene polymorphisms shape cortical plasticity in humans. Brain Stimulation 2010;3:230–7. 10.1016/j.brs.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Barker AT. Transcranial Magnetic Stimulation - past, present and future. Brain Stimulation 2017;10:540. 10.1016/j.brs.2017.01.573. [DOI] [Google Scholar]
- Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. The Lancet 1985;325:1106–7. 10.1016/S0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism-spectrum quotient (AQ): Evidence from asperger syndrome/high-functioning autism, malesand females, scientists and mathematicians. Journal of Autism and Developmental Disorders 2001;31:5–17. [DOI] [PubMed] [Google Scholar]
- Benali A, Trippe J, Weiler E, Mix A, Petrasch-Parwez E, Girzalsky W, et al. Theta-Burst Transcranial Magnetic Stimulation Alters Cortical Inhibition. Journal of Neuroscience 2011;31:1193–203. 10.1523/JNEUROSCI.1379-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y, Khalilov I, Kahle KT, Cherubini E. The GABA excitatory/inhibitory shift in brain maturation and neurological disorders. Neuroscientist 2012;18:467–86. 10.1177/1073858412438697. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B (Methodological) 1995;57:289–300. [Google Scholar]
- Bhakar AL, Dölen G, Bear MF. The pathophysiology of fragile X (and what it teaches us about synapses). Annu Rev Neurosci 2012;35:417–43. 10.1146/annurev-neuro-060909-153138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeron T A synaptic trek to autism. Curr Opin Neurobiol 2009;19:231–4. 10.1016/j.conb.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Cahn SD, Herzog AG, Pascual-Leone A. Paired-pulse transcranial magnetic stimulation: effects of hemispheric laterality, gender, and handedness in normal controls. Journal of Clinical Neurophysiology 2003;20:371–4. [DOI] [PubMed] [Google Scholar]
- Casartelli L, Molteni M, Ronconi L. So close yet so far: Motor anomalies impacting on social functioning in autism spectrum disorder. Neurosci Biobehav Rev 2016;63:98–105. 10.1016/j.neubiorev.2016.02.001. [DOI] [PubMed] [Google Scholar]
- Chang WH, Bang OY, Shin Y-I, Lee A, Pascual-Leone A, Kim Y-H. BDNF Polymorphism and Differential rTMS Effects on Motor Recovery of Stroke Patients. Brain Stimulation 2014;7:553–8. 10.1016/j.brs.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Chang WH, Fried PJ, Saxena S, Jannati A, Gomes-Osman J, Kim Y-H, et al. Optimal number of pulses as outcome measures of neuronavigated transcranial magnetic stimulation. Clin Neurophysiol 2016;127:2892–7. 10.1016/j.clinph.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeran BJ, Talelli P, Mori F, Koch G, Suppa A, Edwards M, et al. A common polymorphism in the brain-derived neurotrophic factor gene ( BDNF ) modulates human cortical plasticity and the response to rTMS. The Journal of Physiology 2008;586:5717–25. 10.1113/jphysiol.2008.159905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen DL, Braun KVN, Baio J, Bilder D, Charles J, Constantino JN, et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years — Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2012. MMWR Surveill Summ 2018;65:1–23. 10.15585/mmwr.ss6513a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole EJ, Enticott PG, Oberman LM, Gwynette MF, Casanova MF, Jackson SLJ, et al. The Potential of Repetitive Transcranial Magnetic Stimulation for Autism Spectrum Disorder: A Consensus Statement. Biol Psychiatry 2019;85:e21–2. 10.1016/j.biopsych.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corp DT, Bereznicki HG, Clark GM, Youssef GJ, Fried PJ, Jannati A, et al. Large-scale analysis of interindividual variability in single and paired-pulse TMS data: Results from the ‘Big TMS Data Collaboration.’ Clinical Neurophysiology In Press. [DOI] [PubMed]
- Corp DT, Bereznicki HGK, Clark GM, Youssef GJ, Fried PJ, Jannati A, et al. Large-scale analysis of interindividual variability in theta-burst stimulation data: Results from the “Big TMS Data Collaboration.” Brain Stimul 2020. 10.1016/j.brs.2020.07.018. [DOI] [PMC free article] [PubMed]
- Dani VS, Chang Q, Maffei A, Turrigiano GG, Jaenisch R, Nelson SB. Reduced cortical activity due to a shift in the balance between excitation and inhibition in a mouse model of Rett syndrome. Proc Natl Acad Sci USA 2005;102:12560–5. 10.1073/pnas.0506071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davila-Pérez P, Jannati A, Fried PJ, Cudeiro Mazaira J, Pascual-Leone A. The Effects of Waveform and Current Direction on the Efficacy and Test–Retest Reliability of Transcranial Magnetic Stimulation. Neuroscience 2018;393:97–109. 10.1016/j.neuroscience.2018.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gennaro L, Bertini M, Pauri F, Cristiani R, Curcio G, Ferrara M, et al. Callosal effects of transcranial magnetic stimulation (TMS): the influence of gender and stimulus parameters. Neuroscience Research 2004;48:129–37. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Pellegrino G, Di Pino G, Corbetto M, Ranieri F, Brunelli N, et al. Val66Met BDNF Gene Polymorphism Influences Human Motor Cortex Plasticity in Acute Stroke. Brain Stimulation 2015;8:92–6. 10.1016/j.brs.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Rothwell JC. Corticospinal activity evoked and modulated by non-invasive stimulation of the intact human motor cortex: Corticospinal activity and the human motor cortex. The Journal of Physiology 2014;592:4115–28. 10.1113/jphysiol.2014.274316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DNA Genotek Inc. Laboratory protocol for manual purification of DNA from 0.5 mL of sample 2015. http://www.dnagenotek.com/US/pdf/PD-PR-006.pdf.
- Eisenberg DTA, Kuzawa CW, Hayes MG. Worldwide allele frequencies of the human apolipoprotein E gene: Climate, local adaptations, and evolutionary history. Am J Phys Anthropol 2010;143:100–11. 10.1002/ajpa.21298. [DOI] [PubMed] [Google Scholar]
- Eldaief MC, Halko MA, Buckner RL, Pascual-Leone A. Transcranial magnetic stimulation modulates the brain’s intrinsic activity in a frequency-dependent manner. Proc Natl Acad Sci USA 2011;108:21229–34. 10.1073/pnas.1113103109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enticott Peter G, Kennedy HA, Rinehart NJ, Bradshaw JL, Tonge BJ, Daskalakis ZJ, et al. Interpersonal motor resonance in autism spectrum disorder: evidence against a global “mirror system” deficit. Front Hum Neurosci 2013;7:218. 10.3389/fnhum.2013.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enticott Peter G, Kennedy HA, Rinehart NJ, Tonge BJ, Bradshaw JL, Fitzgerald PB. GABAergic activity in autism spectrum disorders: an investigation of cortical inhibition via transcranial magnetic stimulation. Neuropharmacology 2013;68:202–9. [DOI] [PubMed] [Google Scholar]
- Enticott PG, Kennedy HA, Rinehart NJ, Tonge BJ, Bradshaw JL, Taffe JR, et al. Mirror neuron activity associated with social impairments but not age in autism spectrum disorder. Biol Psychiatry 2012;71:427–33. 10.1016/j.biopsych.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Enticott PG, Rinehart NJ, Tonge BJ, Bradshaw JL, Fitzgerald PB. A preliminary transcranial magnetic stimulation study of cortical inhibition and excitability in high-functioning autism and Asperger disorder. Dev Med Child Neurol 2010;52:e179–183. 10.1111/j.1469-8749.2010.03665.x. [DOI] [PubMed] [Google Scholar]
- Farzan F, Vernet M, Shafi MMD, Rotenberg A, Daskalakis ZJ, Pascual-Leone A. Characterizing and Modulating Brain Circuitry through Transcranial Magnetic Stimulation Combined with Electroencephalography. Front Neural Circuits 2016;10:73. 10.3389/fncir.2016.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas C, Perez J, Knobel M, Tormos JM, Oberman L, Eldaief M, et al. Changes in Cortical Plasticity Across the Lifespan. Frontiers in Aging Neuroscience 2011;3. 10.3389/fnagi.2011.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried PJ, Jannati A, Davila-Pérez P, Pascual-Leone A. Reproducibility of Single-Pulse, Paired-Pulse, and Intermittent Theta-Burst TMS Measures in Healthy Aging, Type-2 Diabetes, and Alzheimer’s Disease. Frontiers in Aging Neuroscience 2017;9. 10.3389/fnagi.2017.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried PJ, Schilberg L, Brem A-K, Saxena S, Wong B, Cypess AM, et al. Humans with Type-2 Diabetes Show Abnormal Long-Term Potentiation-Like Cortical Plasticity Associated with Verbal Learning Deficits. Journal of Alzheimer’s Disease 2016;55:89–100. 10.3233/JAD-160505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman A, Carlin JB, Stern HS, Rubin DB. Bayesian data analysis (Vol. 2). Boca Raton, FL: Chapman; 2014. [Google Scholar]
- Geronimus AT, Bound J, Neidert LJ. On the validity of using census geocode characteristics to proxy individual socioeconomic characteristics. Journal of the American Statistical Association 1996;91:529–37. [Google Scholar]
- Gipson TT, Johnston MV. Plasticity and mTOR: towards restoration of impaired synaptic plasticity in mTOR-related neurogenetic disorders. Neural Plast 2012;2012:486402. 10.1155/2012/486402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogolla N, Leblanc JJ, Quast KB, Südhof TC, Fagiolini M, Hensch TK. Common circuit defect of excitatory-inhibitory balance in mouse models of autism. J Neurodev Disord 2009;1:172–81. 10.1007/s11689-009-9023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsworthy MR, Hordacre B, Ridding MC. Minimum number of trials required for within- and between-session reliability of TMS measures of corticospinal excitability. Neuroscience 2016;320:205–9. 10.1016/j.neuroscience.2016.02.012. [DOI] [PubMed] [Google Scholar]
- Goldsworthy MR, Müller-Dahlhaus F, Ridding MC, Ziemann U. Inter-subject Variability of LTD-like Plasticity in Human Motor Cortex: A Matter of Preceding Motor Activation. Brain Stimulation 2014;7:864–70. 10.1016/j.brs.2014.08.004. [DOI] [PubMed] [Google Scholar]
- Haertl K, Callahan D, Markovics J, Sheppard SS. Perspectives of Adults Living With Autism Spectrum Disorder: Psychosocial and Occupational Implications. Occupational Therapy in Mental Health 2013;29:27–41. 10.1080/0164212X.2012.760303. [DOI] [Google Scholar]
- Halko MA, Farzan F, Eldaief MC, Schmahmann JD, Pascual-Leone A. Intermittent Theta-Burst Stimulation of the Lateral Cerebellum Increases Functional Connectivity of the Default Network. Journal of Neuroscience 2014;34:12049–56. 10.1523/JNEUROSCI.1776-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M Transcranial Magnetic Stimulation: A Primer. Neuron 2007;55:187–99. 10.1016/j.neuron.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Hamada M, Murase N, Hasan A, Balaratnam M, Rothwell JC. The Role of Interneuron Networks in Driving Human Motor Cortical Plasticity. Cerebral Cortex 2013;23:1593–605. 10.1093/cercor/bhs147. [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: Building an international community of software platform partners. Journal of Biomedical Informatics 2019;95:103208. 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics 2009;42:377–81. 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hordacre B, Goldsworthy MR, Vallence A-M, Darvishi S, Moezzi B, Hamada M, et al. Variability in neural excitability and plasticity induction in the human cortex: a brain stimulation study. Brain Stimulation: Basic, Translational, and Clinical Research in Neuromodulation 2017;10:588–95. [DOI] [PubMed] [Google Scholar]
- Huang Y-Z, Chen R-S, Rothwell JC, Wen H-Y. The after-effect of human theta burst stimulation is NMDA receptor dependent. Clinical Neurophysiology 2007;118:1028–32. 10.1016/j.clinph.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Huang Y-Z, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta Burst Stimulation of the Human Motor Cortex. Neuron 2005;45:201–6. 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Huang Y-Z, Rothwell JC, Edwards MJ, Chen R-S. Effect of Physiological Activity on an NMDA-Dependent Form of Cortical Plasticity in Human. Cerebral Cortex 2008;18:563–70. 10.1093/cercor/bhm087. [DOI] [PubMed] [Google Scholar]
- Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci USA 2002;99:7746–50. 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber TJ, Schneider U, Rollnik J. Gender differences in the effect of repetitive transcranial magnetic stimulation in schizophrenia. Psychiatry Research 2003;120:103–5. [DOI] [PubMed] [Google Scholar]
- Huerta PT, Volpe BT. Transcranial magnetic stimulation, synaptic plasticity and network oscillations. Journal of NeuroEngineering and Rehabilitation 2009;6:7. 10.1186/1743-0003-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jannati A, Block G, Oberman LM, Rotenberg A, Pascual-Leone A. Interindividual variability in response to continuous theta-burst stimulation in healthy adults. Clinical Neurophysiology 2017;128:2268–78. 10.1016/j.clinph.2017.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jannati A, Block G, Ryan MA, Kaye HL, Kayarian FB, Bashir S, et al. Continuous theta-burst stimulation in children with high-functioning autism spectrum disorder and typically developing children. Front Integr Neurosci 2020;14:13. 10.3389/fnint.2020.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jannati A, Fried PJ, Block G, Oberman LM, Rotenberg A, Pascual-Leone A. Test–retest reliability of the effects of continuous theta-burst stimulation. Front Neurosci 2019;13:447. 10.3389/fnins.2019.00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jannati A, Ryan MA, Kaye HL, Tsuboyama M, Rotenberg A. Biomarkers obtained by transcranial magnetic stimulation in neurodevelopmental disorders. Journal of Clinical Neurophysiology In Press. [DOI] [PMC free article] [PubMed]
- Julkunen P, Säisänen L, Hukkanen T, Danner N, Könönen M. Does second-scale intertrial interval affect motor evoked potentials induced by single-pulse transcranial magnetic stimulation? Brain Stimul 2012;5:526–32. 10.1016/j.brs.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Jung NH, Janzarik WG, Delvendahl I, Münchau A, Biscaldi M, Mainberger F, et al. Impaired induction of long-term potentiation-like plasticity in patients with high-functioning autism and Asperger syndrome: Long-term Potentiation-like Plasticity in ASD. Developmental Medicine & Child Neurology 2013;55:83–9. 10.1111/dmcn.12012. [DOI] [PubMed] [Google Scholar]
- Krieger N Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health 1992;82:703–10. 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger N Social class and the black/white crossover in the age-specific incidence of breast cancer: a study linking census-derived data to population-based registry records. Am J Epidemiol 1990;131:804–14. 10.1093/oxfordjournals.aje.a115571. [DOI] [PubMed] [Google Scholar]
- Krieger N, Gordon D. Re: “Use of census-based aggregate variables to proxy for socioeconomic group: evidence from national samples.” Am J Epidemiol 1999;150:892–6. 10.1093/oxfordjournals.aje.a010095. [DOI] [PubMed] [Google Scholar]
- Krueger DD, Bear MF. Toward fulfilling the promise of molecular medicine in fragile X syndrome. Annu Rev Med 2011;62:411–29. 10.1146/annurev-med-061109-134644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Kim SE, Kim WS, Lee J, Yoo HK, Park K-D, et al. Interaction of Motor Training and Intermittent Theta Burst Stimulation in Modulating Motor Cortical Plasticity: Influence of BDNF Val66Met Polymorphism. PLoS ONE 2013;8:e57690. 10.1371/journal.pone.0057690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NJ, Ahn HJ, Jung K-I, Ohn SH, Hong J, Kim YJ, et al. Reduction of Continuous Theta Burst Stimulation-Induced Motor Plasticity in Healthy Elderly With COMT Val158Met Polymorphism. Annals of Rehabilitation Medicine 2014;38:658. 10.5535/arm.2014.38.5.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemonnier E, Villeneuve N, Sonie S, Serret S, Rosier A, Roue M, et al. Effects of bumetanide on neurobehavioral function in children and adolescents with autism spectrum disorders. Transl Psychiatry 2017;7:e1056. 10.1038/tp.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner AJ, Wassermann EM, Tamir DI. Seizures from transcranial magnetic stimulation 2012–2016: Results of a survey of active laboratories and clinics. Clin Neurophysiol 2019;130:1409–16. 10.1016/j.clinph.2019.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, et al. The Autism Diagnostic Observation Schedule—Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders 2000;30:205–23. [PubMed] [Google Scholar]
- Mahley RW, Rall SC Jr. Apolipoprotein E: far more than a lipid transport protein. Annual Review of Genomics and Human Genetics 2000;1:507–37. [DOI] [PubMed] [Google Scholar]
- McClintock SM, Freitas C, Oberman LM, Lisanby SH, Pascual-Leone A. Transcranial magnetic stimulation: a neuroscientific probe of cortical function in schizophrenia. Biol Psychiatry 2011;70:19–27. 10.1016/j.biopsych.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minio-Paluello I, Baron-Cohen S, Avenanti A, Walsh V, Aglioti SM. Absence of embodied empathy during pain observation in Asperger syndrome. Biol Psychiatry 2009;65:55–62. 10.1016/j.biopsych.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Ewen JB. Altered connectivity and action model formation in autism is autism. Neuroscientist 2011;17:437–48. 10.1177/1073858410392381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita N, Kato M, Tazoe M, Miyazaki K, Narita M, Okado N. Increased monoamine concentration in the brain and blood of fetal thalidomide- and valproic acid-exposed rat: putative animal models for autism. Pediatr Res 2002;52:576–9. 10.1203/00006450-200210000-00018. [DOI] [PubMed] [Google Scholar]
- Nettekoven C, Volz LJ, Kutscha M, Pool E-M, Rehme AK, Eickhoff SB, et al. Dose-Dependent Effects of Theta Burst rTMS on Cortical Excitability and Resting-State Connectivity of the Human Motor System. Journal of Neuroscience 2014;34:6849–59. 10.1523/JNEUROSCI.4993-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettekoven C, Volz LJ, Leimbach M, Pool E-M, Rehme AK, Eickhoff SB, et al. Inter-individual variability in cortical excitability and motor network connectivity following multiple blocks of rTMS. NeuroImage 2015;118:209–18. 10.1016/j.neuroimage.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol K, Deeny SP, Seif J, Camaclang K, Cotman CW. Exercise improves cognition and hippocampal plasticity in APOE ε4 mice. Alzheimer’s & Dementia 2009;5:287–94. 10.1016/j.jalz.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen JF. Improvement of amplitude variability of motor evoked potentials in multiple sclerosis patients and in healthy subjects. Electroencephalography and Clinical Neurophysiology/Electromyography and Motor Control 1996a;101:404–11. [PubMed] [Google Scholar]
- Nielsen JF. Logarithmic distribution of amplitudes of compound muscle action potentials evoked by transcranial magnetic stimulation. Journal of Clinical Neurophysiology 1996b;13:423–34. [DOI] [PubMed] [Google Scholar]
- NIH Office of Extramural Research. NIH Policy and Guidelines on The Inclusion of Women and Minorities as Subjects in Clinical Research 2001. https://grants.nih.gov/grants/funding/women_min/guidelines_amended_10_2001.htm (accessed February 1, 2016).
- Oberman L, Ifert-Miller F, Najib U, Bashir S, Woollacott I, Gonzalez-Heydrich J, et al. Transcranial magnetic stimulation provides means to assess cortical plasticity and excitability in humans with fragile X syndrome and autism spectrum disorder. Frontiers in Synaptic Neuroscience 2010;2:26. 10.3389/fnsyn.2010.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberman LM, Eldaief M, Fecteau S, Ifert-Miller F, Tormos JM, Pascual-Leone A. Abnormal modulation of corticospinal excitability in adults with Asperger’s syndrome. European Journal of Neuroscience 2012;36:2782–8. 10.1111/j.1460-9568.2012.08172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberman LM, Ifert-Miller F, Najib U, Bashir S, Gonzalez-Heydrich J, Picker J, et al. Abnormal Mechanisms of Plasticity and Metaplasticity in Autism Spectrum Disorders and Fragile X Syndrome. Journal of Child and Adolescent Psychopharmacology 2016;26:617–24. 10.1089/cap.2015.0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberman LM, Pascual-Leone A, Rotenberg A. Modulation of corticospinal excitability by transcranial magnetic stimulation in children and adolescents with autism spectrum disorder. Frontiers in Human Neuroscience 2014;8:627. 10.3389/fnhum.2014.00627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A, Amedi A, Fregni F, Merabet LB The plastic human brain cortex. Annual Review of Neuroscience 2005;28:377–401. 10.1146/annurev.neuro.27.070203.144216. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Freitas C, Oberman L, Horvath JC, Halko M, Eldaief M, et al. Characterizing Brain Cortical Plasticity and Network Dynamics Across the Age-Span in Health and Disease with TMS-EEG and TMS-fMRI. Brain Topography 2011;24:302–15. 10.1007/s10548-011-0196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasqualetti P, Ferreri F. Amplitude values of motor evoked potentials: statistical properties and neurophysiological implications. Clinical Neurophysiology, vol. 122, 2011, p. S44–5. 10.1016/s1388-2457(11)60148-x. [DOI] [Google Scholar]
- Peça J, Ting J, Feng G. SnapShot: Autism and the synapse. Cell 2011;147:706, 706.e1. 10.1016/j.cell.2011.10.015. [DOI] [PubMed] [Google Scholar]
- Pellicciari MC, Miniussi C, Ferrari C, Koch G, Bortoletto M. Ongoing cumulative effects of single TMS pulses on corticospinal excitability: An intra- and inter-block investigation. Clinical Neurophysiology 2016;127:621–8. 10.1016/j.clinph.2015.03.002. [DOI] [PubMed] [Google Scholar]
- Peña-Gomez C, Solé-Padullés C, Clemente IC, Junqué C, Bargalló N, Bosch B, et al. APOE Status Modulates the Changes in Network Connectivity Induced by Brain Stimulation in Non-Demented Elders. PLoS ONE 2012;7:e51833. 10.1371/journal.pone.0051833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percy AK. Rett syndrome: exploring the autism link. Arch Neurol 2011;68:985–9. 10.1001/archneurol.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi T, Kulangara K, Antoniello K, Markram H. Elevated NMDA receptor levels and enhanced postsynaptic long-term potentiation induced by prenatal exposure to valproic acid. Proc Natl Acad Sci USA 2007;104:13501–6. 10.1073/pnas.0704391104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi T, Perrodin C, Markram H. Hyper-connectivity and hyper-plasticity in the medial prefrontal cortex in the valproic Acid animal model of autism. Front Neural Circuits 2008a;2:4. 10.3389/neuro.04.004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi T, Silberberg G, Markram H. Hyperconnectivity of local neocortical microcircuitry induced by prenatal exposure to valproic acid. Cereb Cortex 2008b;18:763–70. 10.1093/cercor/bhm117. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 2009;120:2008–39. 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol 2015;126:1071–107. 10.1016/j.clinph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royston P Multiple imputation of missing values. The Stata Journal 2004;4:227–41. [Google Scholar]
- Ruohonen J, Karhu J. Navigated transcranial magnetic stimulation. Neurophysiologie Clinique/Clinical Neurophysiology 2010;40:7–17. 10.1016/j.neucli.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Sharer EA, Mostofsky SH, Pascual-Leone A, Oberman LM. Isolating Visual and Proprioceptive Components of Motor Sequence Learning in ASD. Autism Res 2016;9:563–9. 10.1002/aur.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soobader M, LeClere FB, Hadden W, Maury B. Using aggregate geographic data to proxy individual socioeconomic status: does size matter? Am J Public Health 2001;91:632–6. 10.2105/ajph.91.4.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Wylezinska M, Matthews PM, Johansen-Berg H, Jezzard P, Rothwell JC, et al. Neurochemical Effects of Theta Burst Stimulation as Assessed by Magnetic Resonance Spectroscopy. Journal of Neurophysiology 2009;101:2872–7. 10.1152/jn.91060.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain 2000;123:572–84. 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- Suppa A, Huang Y-Z, Funke K, Ridding MC, Cheeran B, Di Lazzaro V, et al. Ten Years of Theta Burst Stimulation in Humans: Established Knowledge, Unknowns and Prospects. Brain Stimul 2016;9:323–35. 10.1016/j.brs.2016.01.006. [DOI] [PubMed] [Google Scholar]
- Suppa A, Quartarone A, Siebner H, Chen R, Di Lazzaro V, Del Giudice P, et al. The associative brain at work: Evidence from paired associative stimulation studies in humans. Clinical Neurophysiology 2017;128:2140–64. 10.1016/j.clinph.2017.08.003. [DOI] [PubMed] [Google Scholar]
- Tang A, Thickbroom G, Rodger J. Repetitive Transcranial Magnetic Stimulation of the Brain: Mechanisms from Animal and Experimental Models. Neuroscientist 2017;23:82–94. 10.1177/1073858415618897. [DOI] [PubMed] [Google Scholar]
- Théoret H, Halligan E, Kobayashi M, Fregni F, Tager-Flusberg H, Pascual-Leone A. Impaired motor facilitation during action observation in individuals with autism spectrum disorder. Curr Biol 2005;15:R84–85. 10.1016/j.cub.2005.01.022. [DOI] [PubMed] [Google Scholar]
- Thickbroom GW. Transcranial magnetic stimulation and synaptic plasticity: experimental framework and human models. Exp Brain Res 2007;180:583–93. 10.1007/s00221-007-0991-3. [DOI] [PubMed] [Google Scholar]
- Thorndike RL, Hagen EP, Sattler JM. Stanford-Binet intelligence scale. Riverside Publishing Company; 1986. [Google Scholar]
- Thut G, Ives JR, Kampmann F, Pastor MA, Pascual-Leone A. A new device and protocol for combining TMS and online recordings of EEG and evoked potentials. Journal of Neuroscience Methods 2005;141:207–17. 10.1016/j.jneumeth.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Thut G, Pascual-Leone A. A review of combined TMS-EEG studies to characterize lasting effects of repetitive TMS and assess their usefulness in cognitive and clinical neuroscience. Brain Topogr 2010a;22:219–32. 10.1007/s10548-009-0115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut G, Pascual-Leone A. Integrating TMS with EEG: How and what for? Brain Topogr 2010b;22:215–8. 10.1007/s10548-009-0128-z. [DOI] [PubMed] [Google Scholar]
- Tordjman S, Drapier D, Bonnot O, Graignic R, Fortes S, Cohen D, et al. Animal models relevant to schizophrenia and autism: validity and limitations. Behav Genet 2007;37:61–78. 10.1007/s10519-006-9120-5. [DOI] [PubMed] [Google Scholar]
- Tremblay S, Rogasch NC, Premoli I, Blumberger DM, Casarotto S, Chen R, et al. Clinical utility and prospective of TMS–EEG. Clinical Neurophysiology 2019;130:802–44. 10.1016/j.clinph.2019.01.001. [DOI] [PubMed] [Google Scholar]
- Tremblay S, Vernet M, Bashir S, Pascual-Leone A, Théoret H. Theta burst stimulation to characterize changes in brain plasticity following mild traumatic brain injury: A proof-of-principle study. Restorative Neurology and Neuroscience 2015;33:611–20. 10.3233/RNN-140459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trippe J, Mix A, Aydin-Abidin S, Funke K, Benali A. Theta burst and conventional low-frequency rTMS differentially affect GABAergic neurotransmission in the rat cortex. Experimental Brain Research 2009;199:411–21. 10.1007/s00221-009-1961-8. [DOI] [PubMed] [Google Scholar]
- Valero-Cabré A, Amengual JL, Stengel C, Pascual-Leone A, Coubard OA. Transcranial magnetic stimulation in basic and clinical neuroscience: A comprehensive review of fundamental principles and novel insights. Neurosci Biobehav Rev 2017;83:381–404. 10.1016/j.neubiorev.2017.10.006. [DOI] [PubMed] [Google Scholar]
- Vallence A-M, Goldsworthy MR, Hodyl NA, Semmler JG, Pitcher JB, Ridding MC. Inter- and intra-subject variability of motor cortex plasticity following continuous theta-burst stimulation. Neuroscience 2015;304:266–78. 10.1016/j.neuroscience.2015.07.043. [DOI] [PubMed] [Google Scholar]
- Viscidi EW, Triche EW, Pescosolido MF, McLean RL, Joseph RM, Spence SJ, et al. Clinical characteristics of children with autism spectrum disorder and co-occurring epilepsy. PLoS ONE 2013;8:e67797. 10.1371/journal.pone.0067797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White F, Nicoll JAR, Roses AD, Horsburgh K. Impaired Neuronal Plasticity in Transgenic Mice Expressing Human Apolipoprotein E4 Compared to E3 in a Model of Entorhinal Cortex Lesion. Neurobiology of Disease 2001;8:611–25. 10.1006/nbdi.2001.0401. [DOI] [PubMed] [Google Scholar]
- Wischnewski M, Schutter DJLG. Efficacy and Time Course of Theta Burst Stimulation in Healthy Humans. Brain Stimulation 2015;8:685–92. 10.1016/j.brs.2015.03.004. [DOI] [PubMed] [Google Scholar]
- Witte AV, Kurten J, Jansen S, Schirmacher A, Brand E, Sommer J, et al. Interaction of BDNF and COMT Polymorphisms on Paired-Associative Stimulation-Induced Cortical Plasticity. Journal of Neuroscience 2012;32:4553–61. 10.1523/JNEUROSCI.6010-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk DA, Dickerson BC, the Alzheimer’s Disease Neuroimaging Initiative, Weiner M, Aiello M, Aisen P, et al. Apolipoprotein E (APOE) genotype has dissociable effects on memory and attentional-executive network function in Alzheimer’s disease. Proceedings of the National Academy of Sciences 2010;107:10256–61. 10.1073/pnas.1001412107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U TMS induced plasticity in human cortex. Rev Neurosci 2004;15:253–66. 10.1515/revneuro.2004.15.4.253. [DOI] [PubMed] [Google Scholar]


