Abstract
To assess plasma phosphorylated tau181 (p-tau181) as a progression biomarker in Alzheimer’s disease (AD), we examined longitudinal plasma p-tau181 of 1184 participants (403 cognitively normal (CN), 560 patients with mild cognitive impairment (MCI), and 221 with AD dementia) from Alzheimer’s Disease Neuroimaging Initiative (ADNI). The plasma p-tau level was increased at baseline for MCI and AD dementia (mean: CN, 15.4 pg/mL; MCI, 18.4 pg/mL; AD dementia, 23.7 pg/mL; P < 0.001) and increased significantly over time at preclinical (Aβ-positive CN), prodromal (Aβ-positive MCI), and dementia (Aβ-positive dementia) stage of AD. A longitudinal increase of plasma p-tau181 was associated with abnormal cerebrospinal fluid biomarker levels (low Aβ42, high phosphorylated tau, and high total tau, all P < 0.001), amyloid accumulation (P < 0.001) and hypometabolism (P = 0.002) on positron emission tomography, atrophy in structure imaging (small hippocampal (P = 0.030), middle temporal (P = 0.008), and whole brain (P = 0.027) volume, and large ventricular volume (P = 0.008)), and deteriorated cognitive performance (global cognition and memory, language, executive function, and visuospatial function, all P < 0.050) at baseline. Furthermore, longitudinal plasma p-tau181 correlated with concurrent changes of nearly all these AD-related hallmarks and faster increase in plasma p-tau181 correlated with faster worsening cognition in all diagnostic groups. Importantly, most associations remained significant in Aβ-positive group and became non-significant in Aβ-negative group. Longitudinal analyses of plasma p-tau181 suggest its potential as a noninvasive biomarker to track disease progression in AD and to monitor effects of disease-modifying therapeutics in clinical trials.
Subject terms: Neuroscience, Biomarkers
Introduction
The deposition of extracellular β-amyloid (Aβ) and accumulation of intracellular misfolded phosphorylated tau (p-tau) protein are the major hallmarks of Alzheimer’s disease (AD). Since introduced in the International Working Group (IWG)-2 criteria and the National Institute of Aging and Alzheimer Association (NIA-AA) Research Framework for the diagnosis of AD, biomarkers reflecting the two pathological features are increasingly utilized in clinical assessment of AD and in clinical trials for participants’ recruitment and outcome measures [1,2,]. At present, there are mainly two types of biomarkers for AD, namely, neuroimaging and fluid biomarkers. Nevertheless, invasiveness, limited availability, high costs, and potential side effects hamper their clinical utility and make it difficult to biologically monitor the clinical change. Thus, there is a great need for cost-effective and easily accessible biomarkers to track disease progression.
Blood immunoassays have been developed for measurement of blood tau phosphorylated at threonine 181 (p-tau181) [3–5]. Recent researches have shown promising results for plasma p-tau181 as a diagnostic biomarker of AD [6,7,]. However, it remains unclear whether plasma p-tau181 could serve as the progression biomarker for AD. Previous analyses showed that high plasma P-tau181 at baseline was associated with subsequent hippocampal atrophy, cognitive decline, and development of AD dementia from cognitively normal (CN) and mild cognitive impairment (MCI) subjects [6,7,]. Nevertheless, studies investigating longitudinal plasma p-tau181 are lacking.
In the present study, we aimed to identify the potential of plasma p-tau181 in tracking AD progression using longitudinal data. The study involved 1184 people from the Alzheimer disease neuroimaging initiative (ADNI) who were CN, or diagnosed with MCI or AD dementia. We hypothesized that plasma p-tau181 levels would increase over time with disease progression and were longitudinally associated with biochemical, imaging, and cognitive measures in the course of the disease.
Methods
Study design
The data used in this study was downloaded from the online repository of Alzheimer’s Disease Neuroimaging Initiative (ADNI) (http://adni.loni.usc.edu/) in July 2020. The ADNI was launched in 2003 as a public-private partnership with the primary goal of testing whether serial magnetic resonance imaging, PET, and various clinical, biologic, and neuropsychological markers can be combined to measure progression of mild cognitive impairment and early AD dementia. Regional ethical committees of all institutions approved the ADNI study. Written informed consent was obtained from all participants or authorized representatives.
Participants
Inclusion and exclusion criteria of ADNI participants have been described previously [8]. We selected all the 1190 participants with available plasma p-tau181 recruited across 3 funding cycles (ADNI-1, ADNI-GO, and ADNI-2). Diagnostic status including CN, mild cognitive impairment (MCI), or AD dementia were based on the cognitive assessments. The CN participants reported a Mini-Mental State Examination (MMSE) score of 24 or higher and a Clinical Dementia Rating Scale (CDR) score of zero. The MCI participants reported an MMSE score of 24 or higher, objective memory loss tested by delayed recall of the Wechsler Memory Scale Logical Memory II, a CDR score of 0.5, preserved activities of daily living, and absence of dementia. The individuals considered as AD dementia fulfilled the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer Disease and Related Disorders Association criteria for probable AD [9], reported an MMSE score between 20 and 26, and a CDR score from 0.5 to 1.0.
Plasma phosphorylated tau 181
Plasma p-tau181 levels were measured by the Single Molecule array (Simoa) technique, using an in-house assay developed in the Clinical Neurochemistry Laboratory, University of Gothenburg, Sweden [7]. A combination of two monoclonal antibodies (Tau12 and AT270) was used to measure N-terminal to mid domain forms of P-tau181. The quantification range was from 1.0 to 128.0 pg/mL after dilution correction. The study using this assay reported within-run variations of 4.6% to 7.4% and between-run variations of 9.0% to 9.9% for the high-concentration internal quality control (iQC) samples (mean = 18.7 pg/mL, range = 16.7–20.0 pg/mL), and within-run variations of 4.6% to 12.7% and between-run variations of 12.0% to 12.7% for the low-concentration iQC samples (mean = 5.4 pg/mL, range = 4.3–6.4 pg/mL. More details of the assay can be found in the study by Karikari et al. [7].
Biomarkers in cerebrospinal fluid
CSF was collected by lumbar punctures (LPs) in a standardized procedure as described in the ADNI procedures manual (http://adni.loni.usc.edu/). Samples were properly centrifuged, aliquoted to 500 μL in polypropylene tubes, frozen within 1 h after collection, shipped overnight on dry ice to the ADNI Biomarker Core laboratory, and stored at -80 °C. Aβ42, t-tau, and p-tau181 were measured with the corresponding Elecsys immunoassays on the Elecsys cobas e 601 analyzer as previously described [10,11,]. Meanwhile, CSF Aβ42, p-tau181, and t-tau were respectively used to define amyloid pathology (A), tau pathology (T), neurodegeneration (N) to stratify participants according to the ATN framework [2] and published cut point (CSF Aβ42 < 977 pg/mL, p-tau18 > 27 pg/mL, and t-tau>300 pg/mL) [12] were used in the definition of biomarker positivity and A/T/N status. Using two large multicentre longitudinal studies (ADNI and BioFINDER), the CSF biomarkers showed satisfying performance in predicting clinical deterioration and cut-off values for single tau biomarkers were also derived and validated [12].
Neuroimaging
Structural brain magnetic resonance imaging (MRI) was performed using 3.0 T scanners with T1-weighted scans in volumetric magnetization-prepared rapid gradient echo (MP-RAGE) sequence. FreeSurfer, version 5.1 (FreeSurfer) was used to quantify the regional volumes according to the 2010 Desikan–Killany atlas [13]. Data of hippocampal, entorhinal, middle temporal, ventricular, and whole brain volume were used and were adjusted for total intracranial volume in our analyses.
Amyloid PET imaging in the ADNI was performed using florbetapir (18F-AV-45). The imaging data obtained from the ADNI dataset had been fully preprocessed using a standardized pipeline [14] and details of florbetapir image acquisition were stated elsewhere (adni-info.org). Briefly, florbetapir image data were obtained 50 to 70 min postinjection, and images were averaged, spatially aligned, interpolated to a standard voxel size, and smoothed to a common resolution of 8 mm full width at half maximum [15]. The MP-RAGE image of each participant from the nearest available visit was segmented and parcellated with Freesurfer (version 5.3.0) to define regions of interest (ROIs) in native space. Then, the PET images were coregistered to the corresponding MP-RAGE using SPM (version 5). The intensity-normalized standard uptake value ratio (SUVR) value for each ROI was acquired by dividing tracer uptake in these regions by the value in whole cerebellum (the predefined reference region). To estimate the global florbetapir SUVr, values from four cortical gray matter regions (frontal, anterior cingulate, precuneus, and parietal cortex) were averaged. Florbetapir-PET results were considered positive if global SUVRs were at least 1.11 as recommended by ADNI [16]. Fluorodeoxyglucose (FDG)-PET data were also obtained and reconstructed according to a standardized protocol. Briefly, FDG image data were obtained 30–60 min postinjection and underwent the same preprocessing procedure as florbetapir-PET (frames were averaged, spatially aligned, interpolated to a standard voxel size, and smoothed to a common resolution of 8 mm full width at half maximum) [15]. An FDG composite score of each scan was calculated as the mean uptake in left and right angular, temporal, and posterior cingulate regions relative to the mean of a pons and cerebellar vermis reference region.
Determination of amyloid status
CSF Aβ42 was first used to determine amyloid status. To maximize the participants, for participants who did not have CSF assessment at baseline, CSF Aβ42 measurement within 12 months of baseline plasma p-tau181 measurement would be considered. If CSF measurement was not available, then amyloid status would be determined using PET for those who had florbetapir-PET at baseline or within 12 months of baseline plasma.
Measurement of cognition
Global cognition was evaluated using MMSE, the 11-item version of the Alzheimer Disease Assessment Scale-Cognitive Subscale (ADAS-Cog), and the CDR Scale Sum of Boxes (CDR-SB). Specific cognitive domains were also investigated using composite measures developed by ADNI for memory, executive function (EF), language, and visuospatial (VS) function [17,18,].
Statistical analysis
Baseline characteristics between diagnostic groups were compared using tests appropriate for the distribution of each variable including ANOVA, Kruskal–Wallis, chi-square, or Fisher’s exact test.
Linear mixed-effect (LME) models were used to estimate the longitudinal changes of plasma p-tau181 levels in groups of different clinical diagnoses (stratified by Aβ status or not) and of different ATN classification. All LME models included an group × time interaction item with random intercepts and slopes and were adjusted for age, gender, APOE ε4 counts, and education years for comparisons among groups.
Cross-sectional associations of plasma p-tau181 with CSF biomarkers’ levels, neuroimaging measures, and cognitive measures were examined using linear regressions, adjusted for age, gender, APOE ε4 counts, and education years. The same models were applied for the association between rates of plasma p-tau181 and other AD-related hallmarks at baseline. Pearson correlations were computed to investigate concurrent longitudinal changes between plasma p-tau181 and other variables with individual rates extracted from similar LME models. Rates of change for each participant were derived from these models by summing the fixed and the individual-specific random effects terms. Baseline and change rates of all variables except for the plasma p-tau181 were scaled to have zero mean and unit variance so that the effect sizes could be directly comparable between association analyses. P values corrected for multiple comparisons underwent Benjamini–Hochberg procedure. Association and correlation analyses were repeated in different diagnostic groups and in different amyloid status.
All statistical analyses were performed using the R statistical software (version 3.5.1). Two-sided P values less than 0.05 were considered statistically significant.
Results
All ADNI participants were aged between 55 and 90 years, had acquired at least 6 years of education, and showed no significant neurological disorders other than AD. We included 1184 participants in our study after exclusion of six individuals with extreme plasma p-tau levels. The mean (SD, standard deviation) age of all the included participants was 74.3 (7.59) years; 45.7% were women; 97.8% had more than 12 years of education; 43.8% had at least one APOE ε4 allele.
The demographic, CSF biomarkers, neuroimaging, and cognition characteristics of the included participants by clinical diagnosis are shown in Table 1 and differed among diagnostic groups. Of the 1184 participants included, 403 (34.0%) participants were CN, 560 (47.8%) were MCI, and 221 (18.7%) were Dementia. The mean MMSE scores were 29.0, 28.1, and 22.1 respectively for the three clinical groups. Baseline plasma p-tau181 level differed significantly between diagnostic groups (CN, 15.1 pg/mL; MCI, 18.3 pg/mL; Dementia 24.1 pg/mL). A total of 3732 plasma p-tau181 samples were obtained up to 8 years after the baseline. Participants baseline characteristics by diagnosis and Aβ status, and by ATN profiles were presented in Appendix eTables 1–2.
Table 1.
Baseline participant demographics.
| CN (N = 403) | MCI (N = 560) | Dementia (N = 221) | |
|---|---|---|---|
| Age | 74.9(0.33) | 72.9(0.34) | 75.3(0.53) |
| Gender | 216(52.9) | 238(42.4) | 90(40.7) |
| Education | 16.5(0.13) | 16.1(0.12) | 15.8(0.19) |
| APOE | |||
| APOE ε4−/−, No. (%) | 295(43.9) | 305(45.4) | 72(10.7) |
| APOE ε4−/+, No. (%) | 105(25.2) | 207(49.6) | 105(25.2) |
| APOE ε4+/+, No. (%) | 8(7.9) | 49(48.5) | 44(43.6) |
| Plasma p-tau | 15.4(10.5) | 18.4(11.1) | 23.7(8.85) |
| CSF biomarkers | |||
| Aβ42 | 1364.1(634.3) | 1077.8(565.0) | 721.0(436.0) |
| p-tau | 22.3(9.42) | 26.2(14.2) | 36.8(16.1) |
| t-tau | 242.9(91.06) | 274.1(127.0) | 375.1(153.2) |
| PET imaging | |||
| Aβ-PET | 1.11(0.17) | 1.22(0.22) | 1.38(0.22) |
| FDG-PET | 1.31(0.11) | 1.26(0.13) | 1.06(0.15) |
| Structure imaging (volume)* | |||
| Hippocampal | 7395.5(937.1) | 7016.8(1093.9) | 5756.1(1054.5) |
| Entorhinal | 3800.4(624.4) | 3631.1(716.4) | 2820.7(710.5) |
| Mid temporal | 20305.6(2695.6) | 20273.9(2719.1) | 17283.1(3212.3) |
| Ventricular | 34159.5(18634.2) | 38337.5(22241.7) | 50289.3(23003.5) |
| Whole brain | 1033302.8(105601.4) | 1049783.5(106082.5) | 986043.3(109077.5) |
| Cognitive measures | |||
| MMSE | 29.0(1.23) | 28.1(1.71) | 22.1(3.53) |
| CDR-SB | 0.072(0.27) | 1.50(1.03) | 5.34(2.46) |
| ADAS-Cog | 5.78(3.05) | 9.11(4.39) | 21.9(8.22) |
| Memory | 1.08(0.61) | 0.38(0.70) | -1.00(0.59) |
| EF | 0.85(0.82) | 0.35(0.88) | -0.95(0.99) |
| Language | 0.83(0.72) | 0.32(0.76) | -0.87(1.04) |
| VS | 0.21(0.59) | 0.0058(0.71) | -0.64(1.00) |
| Plasma p-tau, No. of samples | |||
| Month | |||
| 0 | 403 | 560 | 221 |
| 12 | 290 | 491 | 151 |
| 24 | 307 | 428 | 66 |
| 36 | 98 | 358 | 24 |
| 48 | 115 | 184 | 13 |
| 60 | 2 | 5 | 0 |
| 72 | 3 | 2 | 0 |
| 84 | 3 | 1 | 0 |
| 96 | 2 | 0 | 0 |
*Structure imaging measures reported here are unadjusted by total intracranial volume.
CN cognitively normal, CSF cerebrospinal fluid, EF executive function, FDG fluorodeoxyglucose, MCI mild cognitive impairment, PET positron emission tomography, p-tau phosphorylated tau, t-tau total tau, MMSE mini-mental state examination, VS visuospatial.
Longitudinal plasma p-tau181 and baseline diagnosis
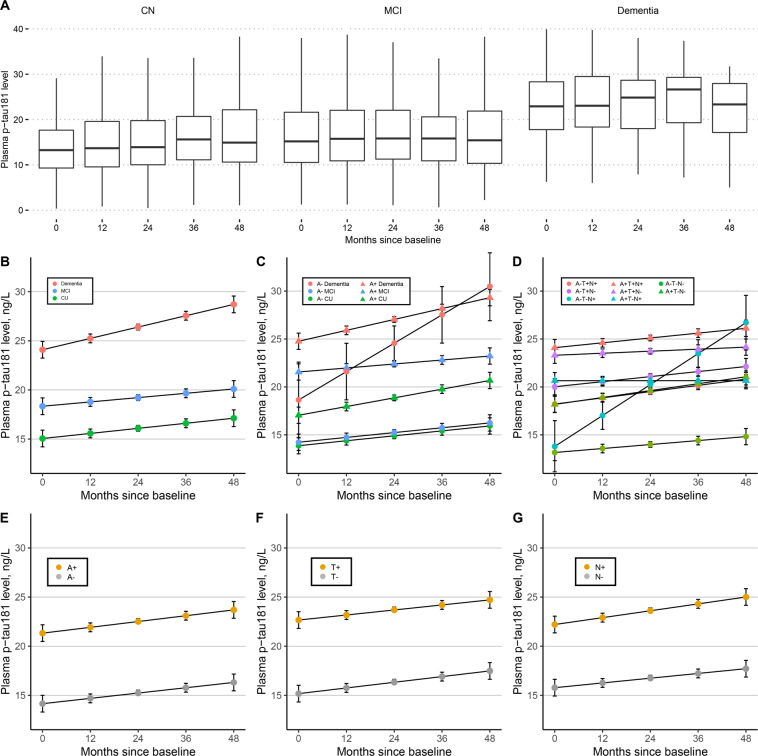
Results from LME models reported that all the three groups showed significant increase in plasma p-tau181 level over time (CN, 0.52 pg/mL per year; MCI, 0.45 pg/mL per year; Dementia 1.08 pg/mL per year) (Fig. 1A, B). Although no significant difference of increase rates was detected between diagnostic groups, the baseline levels differed significantly between groups (Appendix eTable 3). These results were same for the participants with Aβ-positive status (Fig. 1C, Appendix eTables 4-5). In contrast, there were no significant baseline difference between CN, MCI and dementia groups with Aβ-negative status.
Fig. 1. Plasma p-tau181 levels by diagnostic group, Aβ-status, and ATN classification.
(A) Observed data in different diagnostic groups; Estimated plasma p-tau181 trajectories by diagnosis (B), by diagnosis and Aβ status (C), by ATN classification (D); Estimated plasma p-tau181 trajectories by A status (E), by T status (F), and by N status (G). Aβ β-amyloid; A amyloid; T tau pathology; N neurodegeneration; p-tau phosphorylated tau; CN cognitive normal; MCI mild cognitive impairment.
Intercepts and rates were also compared within diagnostic groups, stratified by Aβ status (Appendix eTable 5). Participants with Aβ-positive status all had significantly higher baseline levels than those with Aβ-negative status within each diagnostic group. However, there were no slope differences between Aβ-negative and Aβ-positive subjects within each diagnostic group.
Longitudinal plasma p-tau181 in groups stratified by Aβ, Tau, and Neurodegeneration
Baseline plasma p-tau181 level was higher for participants with Alzheimer’s pathologic change (A + T-N-) compared to participants with normal AD biomarkers (A-T-N-) (P < 0.001), and for participants with Alzheimer’s disease (A + T + N- or A + T + N + ) compared to those with Alzheimer’s pathologic change (P = 0.009 or P < 0.001) and to those with normal AD biomarkers (both P < 0.001) (Fig. 1D, Appendix eTables 6 and 7). However, no differences in change rates were identified between these ATN groups. Additionally, participants with A + , T + , and N + respectively showed significantly elevated plasma p-tau181 levels at baseline compared to those with A- (P < 0.001), T- (P < 0.001), and N- (P < 0.001) (Fig. 1E–G). Similarly, there was no slope difference between the positive biomarker group and the negative biomarker group (Appendix eTable 8).
Association of plasma p-tau181 with CSF biomarkers, neuroimaging, and cognition
Table 2 show associations of baseline and longitudinal plasma p-tau181 levels with CSF Aβ42, p-tau181, and t-tau levels; hippocampal, entorhinal, middle temporal, ventricular, and whole brain volume; MMSE, CDR-SB, and ADAS-Cog score; memory, executive function, language, and visual-spatial composite irrespective of the diagnostic groups.
Table 2.
Associations of baseline AD-related hallmarks with baseline and longitudinal changes of plasma p-tau181.
| Baseline plasma p-tau181 | Longitudinal plasma p-tau181 | |||||||
|---|---|---|---|---|---|---|---|---|
| β | s.e. | P value | R2 | β | s.e. | P value | R2 | |
| CSF biomarkers | ||||||||
| Aβ42 | −0.348 | 0.0472 | <0.001 (<0.001) | 0.312 | −2.934 | 0.681 | <0.001 (<0.001) | 0.285 |
| p-tau | 0.434 | 0.0499 | <0.001 (<0.001) | 0.232 | 3.152 | 0.727 | <0.001 (<0.001) | 0.185 |
| t-tau | 0.381 | 0.0509 | <0.001 (<0.001) | 0.202 | 2.610 | 0.736 | <0.001 (<0.001) | 0.165 |
| PET imaging | ||||||||
| Aβ-PET | 0.494 | 0.0438 | <0.001 (<0.001) | 0.304 | 4.339 | 0.673 | <0.001 (<0.001) | 0.249 |
| FDG-PET | −0.343 | 0.0476 | <0.001 (<0.001) | 0.141 | −2.514 | 0.741 | <0.001 (0.0023) | 0.108 |
| Structure imaging (volume) | ||||||||
| Hippocampal | −0.281 | 0.0450 | <0.001 (<0.001) | 0.277 | −1.607 | 0.714 | 0.025 (0.030) | 0.252 |
| Entorhinal | −0.110 | 0.0484 | <0.001 (<0.001) | 0.092 | −1.389 | 0.792 | 0.080 (0.085) | 0.086 |
| Middle temporal | −0.061 | 0.0484 | <0.001 (<0.001) | 0.091 | −2.236 | 0.773 | 0.0039 (0.0083) | 0.132 |
| Ventricular | 0.251 | 0.0448 | <0.001 (<0.001) | 0.261 | 1.963 | 0.662 | 0.0031 (0.0075) | 0.246 |
| Whole brain | −0.042 | 0.0495 | <0.001 (<0.001) | 0.050 | −1.490 | 0.635 | 0.019 (0.027) | 0.019 |
| Cognitive measures | ||||||||
| MMSE | −0.261 | 0.0453 | <0.001 (<0.001) | 0.133 | −1.047 | 0.671 | 0.12 (0.12) | 0.110 |
| CDR-SB | 0.311 | 0.0449 | <0.001 (<0.001) | 0.147 | 1.891 | 0.668 | 0.0047 (0.0089) | 0.118 |
| ADAS-Cog | 0.366 | 0.0442 | <0.001 (<0.001) | 0.174 | 1.770 | 0.664 | 0.0078 (0.012) | 0.131 |
| Memory composite | −0.408 | 0.0416 | <0.001 (<0.001) | 0.268 | −1.773 | 0.631 | 0.0050 (0.0085) | 0.212 |
| EF composite | −0.293 | 0.0438 | <0.001 (<0.001) | 0.191 | −2.558 | 0.648 | <0.001 (<0.001) | 0.172 |
| Language composite | −0.265 | 0.0441 | <0.001 (<0.001) | 0.177 | −1.458 | 0.653 | 0.026 (0.029) | 0.154 |
| VS composite | −0.238 | 0.0470 | <0.001 (<0.001) | 0.065 | −1.601 | 0.694 | 0.021 (0.027) | 0.048 |
Baseline levels of all AD-related hallmarks were normalized to have zero mean and unit variance so that effect sizes were directly comparable.
P values in parentheses were corrected for multiple comparisons by Benjamini–Hochberg procedure.
Aβ β-amyloid, AD Alzheimer’s disease, ADAS-Cog Alzheimer Disease Assessment Scale-Cognitive Subscale, CDR-SB Clinical Dementia Rating Scale Sum of Boxes, CSF cerebrospinal fluid, EF executive function, FDG fluorodeoxyglucose, MCI mild cognitive impairment, PET positron emission tomography, p-tau phosphorylated tau, t-tau total tau, MMSE mini-mental state examination, VS visuospatial.
All the variables were cross-sectionally associated with the plasma p-tau181 level at baseline and the associations remained significant after correction for multiple comparisons. All the associations remained significant in the Aβ positive group with exception of CSF Aβ42, while only CSF p-tau was significantly associated with plasma p-tau181 at baseline in the Aβ negative group after corrections for multiple comparisons (Appendix eTable 9).
Except for the entorhinal volume and MMSE score, all the variables were also associated with more rapid change of plasma p-tau181 levels regardless of P value corrections. Of the CSF biomarkers, p-tau had the strongest association with longitudinal plasma p-tau181 change. Of the imaging measures, Aβ-PET and middle temporal volume had the largest associations with plasma p-tau slope. CDR-SB, ADAS-Cog, memory composite, and EF composite had the largest associations among the cognitive measures. After dividing participants into different amyloid status, we observed that no associations were significant (all corrected P > 0.05) in the Aβ negative group (Appendix eTable 10). In contrast, the associations remained significant for all CSF biomarkers, both PET imaging measures, ventricular volume, whole brain volume, CDR-SB, and EF composite and entorhinal volume, ADAS-Cog, memory composite, language composite, and VS composite had marginally significant associations with longitudinal plasma p-tau181.
Association of longitudinal plasma p-tau181 with CSF biomarkers, neuroimaging, and cognition in different diagnostic groups
We reported associations in each diagnostic group in Appendix eTable 11. Among CN participants, only Aβ PET was associated with the longitudinal plasma p-tau181 after P value correction. In MCI group, faster plasma p-tau181 increase was associated with lower CSF Aβ level, greater cerebral Aβ deposition, lower uptake in FDG-PET, larger ventricular volume, higher CDR-SB score, and lower memory, executive function, and language composites. Among participants with AD dementia, the association remained significant only for CSF p-tau181, CSF t-tau, and Aβ PET.
Variability in plasma p-tau181 rates
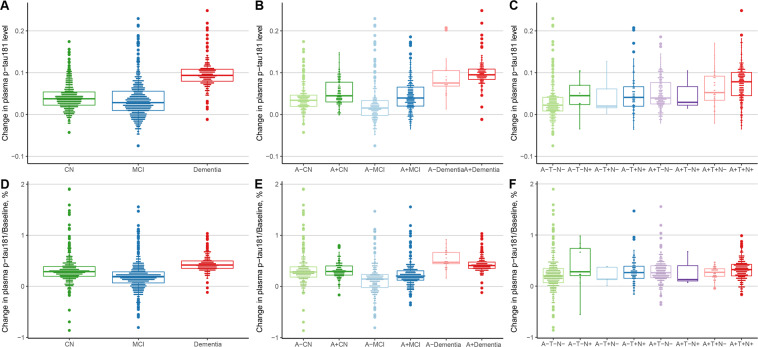
Figure 2 shows the variability in participant-specific rates of plasma p-tau181. When compared between clinical diagnoses, variability of slopes was highest in the CN group (SD, 0.27% of baseline levels vs 0.26% in patients in the MCI group and 0.16% in the dementia group). When stratifying by diagnosis and Aβ status, variability was higher for Aβ-negative groups compared with Aβ-positive groups (SD, 0.16% of baseline in the Aβ-positive CN group vs 0.31% in the Aβ-negative CN group; 0.21% in the Aβ-positive MCI group vs 0.32% in the Aβ-negative MCI group; and 0.15% of baseline levels in the Aβ-positive AD group vs 0.17% in the Aβ-negative AD group). When stratified by ATN classification, slope variability in groups of Alzheimer’s pathological change and Alzheimer’s disease had lower variability compared with the group of normal AD biomarker (0.23% of baseline in A + T-N-, 0.12% of baseline in A + T + N-, and 0.20% of baseline in A + T + N + vs 0.35% in A-T-N-).
Fig. 2. Distribution of participant-specific plasma p-tau181 slope.
Participant-specific plasma p-tau181 change rate by diagnosis (A), by diagnosis and Aβ status (B), and by ATN classification (C); Variability of participant-specific plasma p-tau181 change rate by diagnosis (D), by diagnosis and Aβ status (E), and by ATN classification (F). Abbreviation: Aβ β-amyloid; A amyloid; T tau pathology; N neurodegeneration; p-tau phosphorylated tau; CN cognitive normal; MCI mild cognitive impairment.
Concurrent changes in plasma p-tau181 levels and other measures
In the whole sample, longitudinal plasma p-tau181 showed significant associations with concurrent changes of multiple AD-related hallmarks including CSF p-tau, t-tau, Aβ-PET, FDG-PET, hippocampal volume, middle temporal volume, ventricular volume, whole brain volume, MMSE, CDR-SB, ADAS-Cog, memory composite, EF composite, language composite, and visuospatial composite (Table 3). The longitudinal memory composite showed the strongest correlation with concurrent plasma p-tau181 change compared to other AD-related hallmarks.
Table 3.
Correlations of concurrent changes in plasma p-tau181 and other measures.
| ρ | P value | |
|---|---|---|
| CSF biomarkers | ||
| Aβ42 | 0.0519 | 0.10 (0.11) |
| p-tau | 0.1393 | <0.001 (0.031) |
| t-tau | 0.1391 | <0.001 (0.028) |
| PET imaging | ||
| Aβ-PET | 0.1034 | <0.001 (0.0011) |
| FDG-PET | −0.0671 | 0.024 (0.027) |
| Structure imaging (volume) | ||
| Hippocampal | −0.0699 | 0.017 (0.021) |
| Entorhinal | −0.0014 | 0.96 (0.96) |
| Middle temporal | −0.1078 | <0.001 (<0.001) |
| Ventricular | 0.1299 | <0.001 (<0.001) |
| Whole brain | −0.1342 | <0.001 (<0.001) |
| Cognitive measures | ||
| MMSE | -0.0885 | 0.0023 (0.0036) |
| CDR-SB | 0.0716 | 0.014 (0.018) |
| ADAS-Cog | 0.1114 | <0.001 (0.009) |
| Memory composite | −0.1434 | <0.001 (0.0017) |
| EF composite | −0.1293 | <0.001 (<0.001) |
| Language composite | −0.0925 | 0.0014 (0.0024) |
| VS | −0.0835 | 0.0041 (0.0058) |
P values in parentheses were corrected for multiple comparisons by Benjamini-Hochberg procedure. Aβ β-amyloid, AD Alzheimer’s disease, ADAS-Cog Alzheimer Disease Assessment Scale-Cognitive Subscale, CDR-SB Clinical Dementia Rating Scale Sum of Boxes; CSF cerebrospinal fluid, EF executive function, FDG fluorodeoxyglucose, MCI mild cognitive impairment, PET positron emission tomography, p-tau phosphorylated tau; t-tau total tau; MMSE mini-mental state examination; VS visuospatial.
After stratifying participants by amyloid status, we noted that all the 15 significant associations identified in the whole sample remained in the Aβ positive group except for CDR-SB and entorhinal volume and became non-significant in the Aβ negative group except for whole brain volume and memory composite (Appendix eTable 12).
Greater increase of plasma p-tau181 also correlated with the accelerated decrease of memory composite in all three diagnostic groups except that the association in MCI became marginally significant after correction for multiple comparisons (Appendix eTable 13). Greater rates of plasma p-tau181 changes were also associated with accelerated loss of ventricular volume and whole brain volume in CN controls and patients with MCI, and with accelerated increase of CSF p-tau181 and t-tau in CN controls and patients with dementia. There were also associations with Aβ PET in CN group, with executive function in MCI group, and with language and visuospatial function in dementia group.
Discussion
The findings in the present study supported our primary hypothesis that plasma p-tau181 levels increase over time with disease progression. Compared with CN groups, plasma p-tau181 levels was increased at baseline in patients with MCI and dementia. Besides, increasing levels over time were identified at preclinical (Aβ-positive cognitively normal), prodromal (Aβ-positive MCI), and dementia (Aβ-positive dementia) stage of Alzheimer’s disease. The secondary hypothesis that plasma p-tau181 were longitudinally associated with other AD-associated measures throughout the course of the disease was also supported. Associations between longitudinal plasma p-tau181 and biochemical, imaging, and cognitive measures at baseline were extensively found and were strongest in patients with MCI. Besides, change of plasma p-tau181 correlated with change of cognitive measures in all diagnostic groups, with change of imaging measures in CN and MCI groups, and with change of CSF biomarkers in MCI and dementia groups. Importantly, all the significant associations identified in the whole sample were driven by the amyloid positive group. Taken together, our analysis of longitudinal plasma p-tau181 suggest that the plasma p-tau181 is a dynamic biomarker along the course of the disease and may be used to track disease progression in AD.
Plasma p-tau181 may outperform other biomarkers in monitoring clinical change of AD. Although MRI was recognized to show neurodegeneration in AD [19], the presence of characteristic imaging features used in clinical settings such as hippocampus atrophy and their changes only merge at the later stage of the disease. Fluid biomarkers such as plasma neurofilament lights were also identified in the previous study that was capable of tracing disease progression [20]. However, these molecules were not as specific as p-tau181 to the AD pathology [21–23]. Other neurological comorbidity may interfere with the NLF levels to index AD severity for NLF levels would rise as long as the neuronal injury appear [20]. Thus, the plasma p-tau181 is competent as an indicator for AD progression. It is suggested that, once validated to regulatory standards, this easily-accessible biomarker would prompt the development of novel disease-modifying medicine and, possibly, offer guidance in clinical decisions once such treatments become available. Besides, considering a rather close relation between the memory composite and the plasma p-tau181, it is promising that the memory function can be estimated objectively in future memory clinics. Additionally, the possible dual function of diagnosis and progression indication makes it a desirable biomarker for clinical use in primary care. For these purposes, we recommend that measurement of plasma p-tau181 should be included in future observational and therapeutic trials for AD to further investigate and validate its utility in clinical practice.
A recent work on the longitudinal plasma p-tau181 discovered that the level of plasma p-tau181 plateaued within the last four years prior to death in patients with AD neuropathology [24]. This appeared to be contradictory with our findings that plasma p-tau181 still increase at the stage of dementia. The difference may attribute to the distinct clinical severity of included subjects. While the participants included in the literature reported a mean MMSE score of 17.7 at baseline, the mean MMSE score was 22.1 for individuals with AD dementia in our study. Thus, it might be the case that the level would keep increasing until the stage of mild dementia and then plateau at the end stage of AD course. In addition, we found no difference of change rate between different diagnostic groups for plasma p-tau181. It seemed that the level of the blood biomarker increased in a linear fashion from the asymptomatic stage to mild dementia. Meanwhile, a positive association was clearly illustrated between the change rate of plasma p-tau181 and baseline AD-related outcomes such as Aβ-PET and ADAS-Cog score, which suggested that the rate of plasma p-tau181 change would be accelerated as the disease progressed. One explanation would be insufficient sample size or follow up time to detect the minor signal as annual change of plasma p-tau181 was less than 1 pg/mL. We also noticed that participants in A-T-N- and A-T-N + showed elevating levels of plasma p-tau181. The analysis of longitudinal trajectories of plasma p-tau181 by Moscoso A might explained it as they found earliest elevations of plasma p-tau181 levels occurred before PET and CSF biomarkers of amyloid reached their respective abnormality thresholds [25]. Small sample sizes of certain groups (e.g. n = 13 in A-T-N + ) might also had an impact on analytical results, giving an inaccurate estimate.
Our study replicated some previous findings on plasma p-tau181 [6,7,] and comprehensively revealed its associations with other AD-related biological and clinical outcomes for the first time. Besides, by first using CSF Aβ42 (higher sensitivity and better linked to disease state compared to amyloid-PET [1,26,]) as a main approach and then including florbetapir-PET and data within 12 months of baseline as an auxiliary approach to determine amyloid positivity, we maximized the participants in AD continuum as well as the whole sample size, generating a sufficiently large, prospective cohort. However, there are also several limitations to the present study. First, the participants enrolled in this research did not represent the totality of AD population. As mentioned above, the clinical severity is mild in dementia group according to the global cognitive measures. Thus, the analysis results cannot be extended to the population with moderate to severe dementia and future studies covering participants at advanced stage of AD would be necessary to examine the potential of plasma p-tau as progression biomarker. Second, ADNI has a relatively pure AD population by mainly including amnestic patients. Reproducibility of findings with different phenotypes of AD and different participants from other cohorts would be beneficial. Third, for tau pathology, only CSF tau biomarkers not tau PET were investigated. As tau PET was introduced in 2015 at the phase of ADNI-3, the baseline interval between plasma p-tau181 and tau PET was quite long (more than 6 years). It would be valuable for future researches with different study designs to include PET data and to analyze the association between different modalities of tau biomarkers. Forth, there were high drop-out rates in each diagnostic group after 5 years since baseline that lead to a rather short-term follow-up data. This might affect the estimates of longitudinal plasma p-tau181 change and its association with other variables.
In conclusion, our findings suggest that plasma p-tau181 level can be used as a noninvasive biomarker to track disease progression in AD and therefore may be candidate tool to monitor effects in trials of disease-modifying therapeutics.
Supplementary information
Acknowledgements
Data used in preparation for this paper were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (http://adni.loni.usc.edu/). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in the analysis or in the writing of this paper. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf. We express appreciation to contributors of Alzheimer’s Disease Neuroimaging Initiative (ADNI) database. Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC; Johnson & Johnson Pharmaceutical Research & Development LLC; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Author contributions
JTY and QD were responsible for the conception and design of the study. SDC, YYH, XNS, YG, and JTY acquisition, analysis of data, drafting of the manuscript, and preparing the figures. LT and QD coedited the article.
Funding
This study was supported by grants from the National Natural Science Foundation of China (91849126), the National Key R&D Program of China (2018YFC1314700), Shanghai Municipal Science and Technology Major Project (No.2018SHZDZX01) and ZJLAB, Tianqiao and Chrissy Chen Institute, and the State Key Laboratory of Neurobiology and Frontiers Center for Brain Science of Ministry of Education, Fudan University.
Data availability
Data used in the presented study were originally from the online repository of Alzheimer’s Disease Neuroimaging Initiative (ADNI) (http://adni.loni.usc.edu/). The data generated during processing and analyzing are available from the authors upon request.
Code availability
All scripts used in analyses are available from the authors upon request.
Conflict of interest
The authors declare no competing interest.
Ethics approval
Regional ethical committees of all institutions approved the ADNI study.
Footnotes
#Data used in preparation for this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in the analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-ontent/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Patient consent:
Written informed consent was obtained from all participants or authorized representatives.
Data availability statement: The data are available to interested researchers on request.
These authors contributed equally: Shi-Dong Chen, Yu-Yuan Huang
Contributor Information
Qiang Dong, Email: dong_qiang@fudan.edu.cn.
Jin-Tai Yu, Email: jintai_yu@fudan.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41398-021-01476-7.
References
- 1.Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 2014;13:614–29. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- 2.Jack CR, Jr., Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–62. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mielke MM, Hagen CE, Xu J, Chai X, Vemuri P, Lowe VJ, et al. Plasma phospho-tau181 increases with Alzheimer’s disease clinical severity and is associated with tau- and amyloid-positron emission tomography. Alzheimers Dement. 2018;14:989–97. doi: 10.1016/j.jalz.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang CC, Chiu MJ, Chen TF, Chang HL, Liu BH, Yang SY. Assay of plasma phosphorylated tau protein (Threonine 181) and Total Tau Protein in early-stage Alzheimer’s disease. J Alzheimers Dis. 2018;61:1323–32. doi: 10.3233/JAD-170810. [DOI] [PubMed] [Google Scholar]
- 5.Tatebe H, Kasai T, Ohmichi T, Kishi Y, Kakeya T, Waragai M, et al. Quantification of plasma phosphorylated tau to use as a biomarker for brain Alzheimer pathology: pilot case-control studies including patients with Alzheimer’s disease and down syndrome. Mol Neurodegener. 2017;12:63. doi: 10.1186/s13024-017-0206-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janelidze S, Mattsson N, Palmqvist S, Smith R, Beach TG, Serrano GE, et al. Plasma P-tau181 in Alzheimer’s disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat Med. 2020;26:379–86. doi: 10.1038/s41591-020-0755-1. [DOI] [PubMed] [Google Scholar]
- 7.Karikari TK, Pascoal TA, Ashton NJ, Janelidze S, Benedet AL, Rodriguez JL, et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 2020;19:422–33. doi: 10.1016/S1474-4422(20)30071-5. [DOI] [PubMed] [Google Scholar]
- 8.Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, et al. Alzheimer’s Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010;74:201–9. doi: 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–44. doi: 10.1212/WNL.34.7.939. [DOI] [PubMed] [Google Scholar]
- 10.Bittner T, Zetterberg H, Teunissen CE, Ostlund RE, Jr., Militello M, Andreasson U, et al. Technical performance of a novel, fully automated electrochemiluminescence immunoassay for the quantitation of beta-amyloid (1-42) in human cerebrospinal fluid. Alzheimers Dement. 2016;12:517–26. doi: 10.1016/j.jalz.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Hansson O, Seibyl J, Stomrud E, Zetterberg H, Trojanowski JQ, Bittner T, et al. CSF biomarkers of Alzheimer’s disease concord with amyloid-beta PET and predict clinical progression: a study of fully automated immunoassays in BioFINDER and ADNI cohorts. Alzheimers Dement. 2018;14:1470–81. doi: 10.1016/j.jalz.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blennow K, Shaw LM, Stomrud E, Mattsson N, Toledo JB, Buck K, et al. Predicting clinical decline and conversion to Alzheimer’s disease or dementia using novel Elecsys Abeta(1-42), pTau and tTau CSF immunoassays. Sci. Rep. 2019;9:19024. doi: 10.1038/s41598-019-54204-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jack CR, Jr., Bernstein MA, Fox NC, Thompson P, Alexander G, Harvey D, et al. The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27:685–91. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo T, Landau SM, Jagust WJ, Alzheimer’s Disease Neuroimaging, I. Detecting earlier stages of amyloid deposition using PET in cognitively normal elderly adults. Neurology. 2020;94:e1512–e1524. doi: 10.1212/WNL.0000000000009216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Landau SM, Mintun MA, Joshi AD, Koeppe RA, Petersen RC, Aisen PS, et al. Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Ann Neurol. 2012;72:578–86. doi: 10.1002/ana.23650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer PF, Pichet Binette A, Gonneaud J, Breitner JCS, Villeneuve S. Characterization of Alzheimer disease biomarker discrepancies using cerebrospinal fluid phosphorylated Tau and AV1451 positron emission tomography. JAMA Neurol. 2020;77:508–16. doi: 10.1001/jamaneurol.2019.4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gibbons LE, Carle AC, Mackin RS, Harvey D, Mukherjee S, Insel P, et al. A composite score for executive functioning, validated in Alzheimer’s Disease Neuroimaging Initiative (ADNI) participants with baseline mild cognitive impairment. Brain Imaging Behav. 2012;6:517–27. doi: 10.1007/s11682-012-9176-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crane PK, Carle A, Gibbons LE, Insel P, Mackin RS, Gross A, et al. Development and assessment of a composite score for memory in the Alzheimer’s Disease Neuroimaging Initiative (ADNI) Brain Imaging Behav. 2012;6:502–16. doi: 10.1007/s11682-012-9186-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jack CR, Jr., Barnes J, Bernstein MA, Borowski BJ, Brewer J, Clegg S, et al. Magnetic resonance imaging in Alzheimer’s Disease Neuroimaging Initiative 2. Alzheimers Dement. 2015;11:740–56. doi: 10.1016/j.jalz.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mattsson N, Cullen NC, Andreasson U, Zetterberg H, Blennow K. Association between longitudinal plasma neurofilament light and neurodegeneration in patients with Alzheimer disease. JAMA Neurol. 2019;76:791–9. doi: 10.1001/jamaneurol.2019.0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shahim P, Gren M, Liman V, Andreasson U, Norgren N, Tegner Y, et al. Serum neurofilament light protein predicts clinical outcome in traumatic brain injury. Sci Rep. 2016;6:36791. doi: 10.1038/srep36791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Marchis GM, Katan M, Barro C, Fladt J, Traenka C, Seiffge DJ, et al. Serum neurofilament light chain in patients with acute cerebrovascular events. Eur J Neurol. 2018;25:562–8. doi: 10.1111/ene.13554. [DOI] [PubMed] [Google Scholar]
- 23.Disanto G, Barro C, Benkert P, Naegelin Y, Schädelin S, Giardiello A, et al. Serum Neurofilament light: a biomarker of neuronal damage in multiple sclerosis. Ann Neurol. 2017;81:857–70. doi: 10.1002/ana.24954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lantero Rodriguez, J, et al. Plasma p-tau181 accurately predicts Alzheimer’s disease pathology at least 8 years prior to post-mortem and improves the clinical characterisation of cognitive decline. Acta Neuropathol. (2020). [DOI] [PMC free article] [PubMed]
- 25.Moscoso A, Grothe MJ, Ashton NJ, Karikari TK, Rodriguez JL, Snellman A, et al. Time course of phosphorylated-tau181 in blood across the Alzheimer’s disease spectrum. Brain. 2021;144:325–39. doi: 10.1093/brain/awaa399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dubois B, Hampel H, Feldman HH, Scheltens P, Aisen P, Andrieu S, et al. Preclinical Alzheimer’s disease: definition, natural history, and diagnostic criteria. Alzheimers Dement. 2016;12:292–323. doi: 10.1016/j.jalz.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used in the presented study were originally from the online repository of Alzheimer’s Disease Neuroimaging Initiative (ADNI) (http://adni.loni.usc.edu/). The data generated during processing and analyzing are available from the authors upon request.
All scripts used in analyses are available from the authors upon request.