Abstract
The pervasive use of opioid compounds for pain relief is rooted in their utility as one of the most effective therapeutic strategies for providing analgesia. While the detrimental side effects of these compounds have significantly contributed to the current Opioid Epidemic, opioids still provide millions of patients with reprieve from the relentless and agonizing experience of pain. The human experience of pain has long recognized the perceived unpleasantness entangled with a unique sensation that is immediate and identifiable from the first-person subjective vantage point as “painful”. From this phenomenological perspective, how is it that opioids interfere with pain perception? Evidence from human lesion, neuroimaging, and preclinical functional neuroanatomy approaches is sculpting the view that opioids predominately alleviate the affective or inferential appraisal of nociceptive neural information. Thus, opioids weaken pain-associated unpleasantness rather than modulate perceived sensory qualities. Here, we discuss the historical theories of pain to demonstrate how modern neuroscience is revisiting these ideas to deconstruct the brain mechanisms driving the emergence of aversive pain perceptions. We further detail how targeting opioidergic signaling within affective or emotional brain circuits remains a strong avenue for developing targeted pharmacological and gene-therapy analgesic treatments that might reduce the dependence on current clinical opioid options.
Keywords: Emotion, cingulate cortex, periaqueductal gray, amygdala, nucleus accumbens, valence, mu opioid receptor
1 |. A framework for tackling the dual Pain and Opioid Epidemics
The staggering incidence of untreated pain is a worldwide crisis. On top of this, the implementation of opiates as a frontline therapeutic strategy has heavily contributed to the current opioid abuse and overdose epidemic in the United States. Improved strategies for treating chronic pain with safer side effect profiles carry an enormous potential payoff for pain patients (Volkow & Collins, 2017). Currently, the clinical management of chronic pain remains a significant challenge given the heterogeneity of underlying causes, and the limited efficacy and/or significant detrimental side effects of many current medications. The cognate receptors for both exogenous opioids and other current pharmacological pain treatments are located not only in the known pain pathways but also in brain reward circuits and brainstem breathing pattern generators (Corder et al., 2018) Therefore, comprehensive strategies that provide substantive relief across pain types, and with reduced abuse and death liabilities, are needed (Davis et al., 2020).
Chronic pain is not merely a persistent sensory disorder, but a debilitating disease of affective dysfunction that negatively impacts the mental state, professional goals, and personal relationships of millions of pain patients (Gilam et al., 2020). Pain and general emotional processes share many common neural circuitries within the brain, which is unsurprising given the strong emotional qualities that define the perception of pain. This is, of course, not a new conceptual theory, but the predominant view of pain since Aristotle and Cicero, who considered pain emotions, or pathe, the key motivational factor of the perception (Schmitter, 2010). This view was again strongly reiterated in 1968 by Ronald Melzack and Kenneth Casey, stating: “To consider only the sensory features of pain, and ignore its motivational and affective properties, is to look at only part of the problem, and not even the most important part at that” (Melzack & Casey, 1968).
One of the primary barriers that medical researchers have faced while attempting to prevent and alleviate pain is the approach of biological isolation, which fails to integrate discoveries made at different levels of description of pain experience. Interdisciplinary teams of systems neuroscience researchers consisting of basic- and physician- scientists could accelerate the identification of meaningful new circuit-level targets to mitigate the affective component of pain (Rudebeck et al., 2019). Such research avenues could start with the human model to better understand mechanisms of pain perception and analgesia, and then transfer to the bench. This is motivated, in part, by the observations that: (1) the clinical problem of pain is a biopsychosocial disease that is best represented in the human, and (2) many of the pain treatments developed in animals have failed to show efficacy in humans (Yekkirala et al., 2017). The fact that many pain treatments developed in rodents have failed in humans should not be construed to mean that rodent models have little value. On the contrary, failure is seldom explained by an established difference between how pain information is processed in human versus rodents. Often the design of the clinical trial does not correspond to the basic science discovery, e.g., preclinical analgesia described in terms of mitigated reflexive behaviors. Thus, using a reverse-translational approach, we can more readily make sense of experimental data from rodents if designed a priori with human data guiding its rationale (Garner, 2014).
The brain is a complex and dynamic system, which until very recently, has been largely impregnable for dissecting the specific and functional neural circuits underlying nociceptive processing. Given the recent explosion of neuroscience technologies for reading and re-writing neural circuit activities at the level of individual cells and circuits, we must now use such advancements to identify creative approaches to manage the affective dimension of pain. Leveraging these tools for translational neuroscience should accelerate our basic understanding of how neural circuits are wired and function together at a resolution currently unachievable in human research—genetically and functionally-identifiable individual neurons and interregional circuits—which can then be applied toward clinical practice (Woolf, 2020)( Figure 1). Here, we will discuss the role of nociceptive brain circuits that encode pain perception, in particular the affective component, that are embedded in emotional and opioidergic brain pathways.
Figure 1. A roadmap for reverse-translational pipelines to interrogate the neural circuitry underlying pain affect and opioid analgesia.
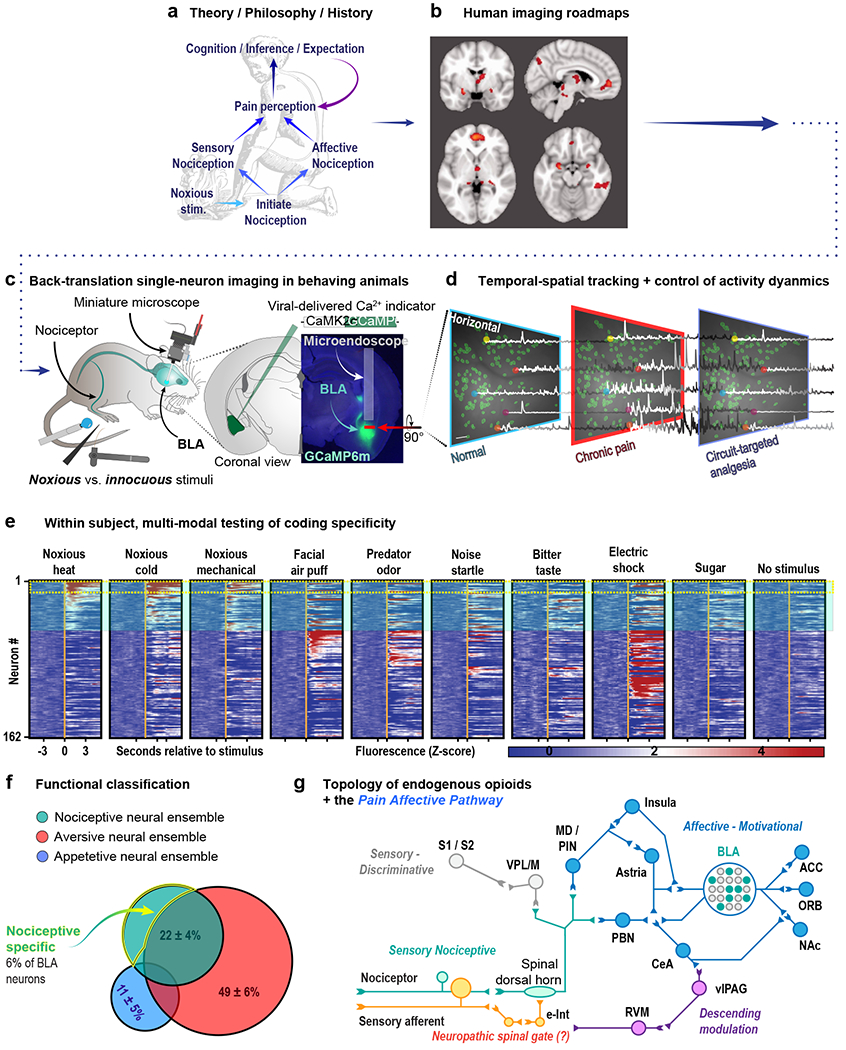
(a) The fundamental groundwork laid forth by historical philosophical and clinical work inform modern investigation of the neural control of pain affect. (b) Human imaging and lesion studies qualitatively and broadly address questions of neuroanatomical function in pain affect. Evidence from historical and modern clinical pain research can provide a starting point from which to identify and interrogate specific neural circuitry at a resolution unachievable with conventional clinical methods. (c) Modern calcium imaging techniques allow for tracking of thousands of individual neurons in awake, behaving rodents at single-cell resolution. This approach has led to, for example, the identification of basolateral amygdala neurons that encode the aversiveness of noxious stimuli (Corder et al., 2019). (d) Endoscopy and two-photon microscopy allow for the simultaneous investigation of genetically defined populations of neurons at high spatial and temporal resolution time-locked to behaviorally significant events (e.g. the application of a noxious stimulus). (e) These techniques reveal the signaling dynamics of individual neurons embedded within neural microcircuitry to be tracked over multiple days in response to numerous stimuli. (f) The resulting information creates a picture of both the role of the circuit as a whole as well as the identification of functional subpopulations within the circuit (g) Ultimately, combining behavioral studies of pain affect informed by clinical findings with circuit-based, single-neuron imaging, and opioidergic cell-type-specific genetic approaches will allow for the most efficient mapping of pain affective pathways embedded within the endogenous opioid system.
1.1 |. Chronic pain as a disease within brain emotion and cognitive circuits
Pain is often considered a symptom of something: a hand on a stove-top, arthritic inflammation, or a malignant pancreatic tumor. It is now realized that some types of chronic pain are a disease of the nervous system, as opposed to a physiologic response to injury and noxious stimuli. Certainly, chronic pain impacts multiple physiologic systems beyond the CNS, including the immune and endocrine systems(Chapman et al., 2008; Massart et al., 2016; Tennant, 2013; Vines et al., 2003). Indeed, a purely somatosensory account of pain is inconsistent with observations of the variable relationship between injury and pain, in which: (1) non-noxious stimuli produce pain; (2) the locus of pain and the actual tissue damage are sometimes inconsistent; (3) pain can persist long after tissue has healed; (4) the nature and location of pain can change over time; and (5) treatments that address purely somatosensory origins of chronic pain have been, to date, inadequate.
The concept of chronic pain as a disease of the CNS (Sullivan et al., 2013), arising from reclassifications in clinical medicine, posits that many chronic pain conditions–fibromyalgia, complex regional pain syndrome, headache, irritable bowel syndrome, pelvic pain, temporomandibular dysfunction, and low back pain–are all associated with multiple somatic symptoms but share a core emotional unpleasantness. Patients often are labeled with a particular painful condition based on somatic-sensory phenotypes. However, the affective and emotional aspects are considered less consequential, and the broader dysfunction of the patients’ CNS circuits responsible for affective processing is often overlooked. Further, the co-morbid psychiatric disorders (e.g., catastrophizing, anxiety, depression) associated with chronic pain that further amplify the pain experience must also be better taken into account during chronic pain treatment. Consequently, psychologists and psychiatrists specialized in pain are beginning to put pain emotion at the forefront of treatment (Gilam et al., 2020) and the advancement of neurotechnologies is allowing for new preclinical views of nociceptive circuit function with unprecedented spatial and temporal resolution (National Institute of Heath, 2019).
2 |. Building a neuroscience model of pain affect
The perception of pain arises from the integration of sensory-discriminative and affective-cognitive neural information. An enduring challenge in pain treatment is the need to decipher the functional architecture of cells and circuits that transform inert nociceptive information into an unpleasant pain perception; however, this picture is starting to come into focus. Despite the nervous system’s immense complexity, fundamental aspects of subcortical organization are stereotypic among individuals of the same species and are maintained across mammals. For example, the amygdala is evolutionarily conserved in both mice and humans, as well as in many other reptilian and mammalian species, and likely serves similar functions for threat detection, aversive learning, and the valence coding of nociception. This preserved functional role of some cortical and subcortical nociceptive neural circuits provides the basis for employing translationally relevant animal studies. In this way, preclinical models powerfully provide the ability to dissect and model different dimensions of the human pain-experience at a resolution not possible in humans: the individual neuron or neural circuit (Woolf, 2020) (Figure 1).
Functional measurement at the individual neuron scale, as well as cell type-resolution, is critical for inferring the types of information a neuron encodes. What does it mean for a neuron to represent something in the environment? What information is encoded or decoded in these neural responses? These questions are fundamental to our understanding of pain and the endogenous inhibitory signaling, such as opioid peptides and receptors, that modulate our affective experience of pain. Human studies employing imaging approaches can only make correlative conclusions on functional contributions to pain experience. Indeed, methods such as functional magnetic resonance imaging (fMRI) provide a general understanding of which brain regions are active during painful stimuli. However, if nociceptive processing is encoded by a small number of cells within a voxel of many thousands of neurons, then fMRI might fail to observe important cellular targets and misinterpret the function of the heterogeneous neurons whose activities are averaged out. For example, amygdalar BOLD activity is observed in very few pain fMRI studies (Tracey et al., 2019). In contrast, animal single-neuron resolution imaging studies find that ~24% of neurons in the basolateral subnucelus are nociceptive responsive (Corder et al., 2019). These unconditioned nociceptive negative valence-tuned neurons are anatomically intermixed in a “salt-and-pepper” architecture alongside neurons encoding the opposite appetitive valence. Furthermore, only ~6% of BLA neurons are purely nociceptive responsive and are consistently active during every exposure to a noxious stimulus regardless of the noxious stimulus sensory modality (Corder et al., 2019). Similar observations of intermingled unconditioned valence-coding neurons in the primate amygdala suggest that this circuit motif may exist in humans as well (W. Zhang et al., 2013). Thus, it is likely that fMRI is unable to provide the resolution to discern if the neuronal activity within a larger brain region is “pain-specific,” a common but potential incorrect conclusion of many low-resolution imaging studies. Nonetheless, human studies do provide critical roadmaps that can be “back translated” to animal investigations.
Emerging preclinical work provides unprecedented access to nociception-encoding neural populations and circuits through endoscopic brain imaging combined with direct neural circuit manipulation, thereby linking nociceptive neural activities to pain-like nocifensive and affective-motivational behaviors. However, the most obvious shortcoming of animal models of pain and analgesia is the difficulty in determining the subjective state of non-verbal animals. In human subjects, perception of pain or analgesia can be somewhat reliably determined with verbal reports. Although, we might argue that it is unimportant to fully resolve the mechanisms of subjective experience or an emergent consciousness phenomenon. Rather, reductionist approaches for identifying translational therapy targets based on functionalism—be it at the molecular, cellular, or circuit level—can be more than sufficient to generate novel therapies for mitigating pain. If there is a sufficient and rapid back-and-forth discussion or involvement of human and preclinical researchers, evolutionarily conserved target homologs can be identified and translational progress can continue. To do so, pain researchers should move beyond broad, imprecise words describing pain experiences and adopt operationalized neuroscience terminologies that can be applied to both humans and non-verbal species, such that these terms can be linked explicitly to brain activity states (Box 1).
Box 1. Pain-related terminology and implementation.
Melzack and Casey (1968) proposed that pain perception consists of three distinct dimensions: (1) a sensory-discriminative dimension, which conveys the intensity, location, and modality of the painful stimulus; (2) an affective-motivational dimension, which comprises the unpleasantness of pain independent of sensory modality; and (3) a cognitive-evaluative dimension (Melzack & Casey, 1968). The endurance of the multidimensional model, and especially of the sensory and affective dimensions, is echoed in the recently updated IASP description of pain as an “an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage” (Raja et al., 2020). While pain is often conceptualized as a unitary (albeit multifaceted) experience, genetic conditions and injuries can result in specific deficits that demonstrate separation of its dimensions (see Table 1). Key among them is the phenomenon of pain asymbolia, in which pain is perceived in a manner divorced from its unpleasantness – that is, patients identify an experience as “painful”, but it does not bother them or motivate them to take steps to relieve it (Berthier et al., 1988).
Collectively, the deficits outlined in Table 1 indicate distinct but overlapping neural mechanisms for the construction of these dimensions. Translational medicine does not require the description of such mechanisms at the level of subjectivity; rather, it is adequate to use concepts at the level of nociception where we can interfere with the physical processing of neural information. In order to identify mechanisms in preclinical models of pain, however, it is first critical to draw a few key distinctions.
Nociception, derived from the Latin for ‘harm’, refers to the neural information that is encoded and transmitted by any neuron in the peripheral and central nervous system that is activated by a noxious or harmful stimulus. Any additional neural processing, action potential spiking, or neurotransmissions that continue beyond those that explicitly contribute to the immediate perception is not categorized as nociceptive.
Pain comprises both the representations of somatosensory information (e.g., location, modality, sharpness, dullness), as well as the assignment of emotional valence, resulting from nociception. Pain thus requires a conscious perception, underscored by a hierarchal neural process of evaluation of nociception information.
Nociception can elicit reflexive and complex motor responses, in the absence of conscious pain perception–for example, Baliki and Apkarian comment that we non-volitionally shift body weight positions while sitting in a chair for a long period (a complex set of motor commands, involving many muscles) due to some level of nociception that initiates this protective behavior (Baliki & Apkarian, 2015). Importantly, pain can occur in the absence of nociceptive input, and according to the IASP definition, an experience only needs to “resemble” that associated with nociception to qualify as painful (Raja et al., 2020). For example, central neuropathic pain associated with multiple sclerosis, tumors, strokes, or other conditions can occur independent of peripheral nociceptors (Murphy et al., 2017). Tangentially, intense emotional distress, which is often described as painful, does not require nociception, but it involves many of the same brain regions as physical pain (Eisenberger et al., 2003; Meerwijk et al., 2013; Onoda et al., 2009) Additionally, social feedback and empathy, reinforcement history, expectation, and a multitude of other factors can shape the intensity and emotional significance of pain for an individual. Although these factors are not nociceptive they can directly alter nociception in numerous brain circuits.
Another concept is that of pain-related suffering. The intense unpleasant affect of pain imposes the goal of escaping with an extremely high priority. The resulting disruption of ongoing goal-oriented behaviors or neural programs to attend to the noxious cause or affected tissue is a small inconvenience, but generally patients do not ascribe suffering to acute pain perceptions. Counter to this useful function of acute pain, chronic pain has no survival function and cannot be easily remedied by any behavioral actions that the organism is designed to execute as nociceptive processes continue to percolate through the nervous system. This inescapable neural processing places a significant neuroeconomic demand on systems designed to compute conflict resolution, such as the cingulate cortex. “Suffering” is therefore an inability to rectify or cease ongoing signals that disrupt other ongoing survival-directed behaviors, and arises secondary to nociception, not parallel. When strategies fail and are exhausted while nociceptive affective-motivational signals persistently enter inferential or conflict-resolution circuits, a comorbid sense of helplessness can emerge.
The last important distinction is that between the “affective dimension of pain” and “pain affect”. Preclinical pain research into pain affect must clearly separate “affective-motivational”, or “nocifensive”, behaviors from co-morbid changes in avoidance or reward-consummatory behaviors. In its original definition related to pain put forth by Melzack, “affect”, or the “affective component of pain”, refers to the immediate, in-the-moment unpleasantness that forces the organism into action (Melzack & Casey, 1968). This should not be conflated with alternative uses of “pain affect”, “pain-induced negative affect”, or “pain-depressed behaviors”, which have recently been applied as an alternative for “mood” or co-morbid psychiatric disorders, such as anxiety, depression, or well-being metrics, including feeding and nest-building (Massaly et al., 2019; Negus et al., 2010; Schwartz et al., 2014).
Behaviors indicative of each of these elements emerge over different time scales, reflecting the differences in their function (Figure 2; see Table 1 for specific examples of associated behaviors). In mice, brief noxious stimuli rapidly elicit several distinct behavioral responses: (1) Withdrawal reflexes: rapid reflexive retraction or digit splaying of the paw, (2) Affective-motivational responses: directed licking and biting of the paw (termed ‘attending’), extended lifting or guarding of the paw, and/or escape responses characterized by hyperlocomotion, rearing or jumping away from the noxious stimulus. Functionally, nociception serves as an instructive signal that immediately generates reflexive behavior (e.g., removing the hand from the hot stove, grimacing, yelling “ouch!”), and rapidly gives rise to the perception of pain – both its sensory and affective dimension. Our group’s best attempt, thus far, to quantify affective-motivational behavior focuses on the cascade of stereotyped behaviors that immediately follow a withdrawal response. Paw or tail withdrawal reflexes are classically measured in studies of hypersensitivity and involve simple spinal cord and brainstem circuits (as these behaviors are observed in decerebrated rodents). In contrast, affective-motivational responses are complex behaviors requiring processing by limbic and cortical circuits in the brain, the appearance of which indicates the subject’s motivation and arousal to make the aversive sensations cease, by licking the affected tissue (thus engaging spinal gate mechanisms), protecting the tissue, or seeking an escape route (Blanchard & Blanchard, 1969; Bolles, 1970; Bolles & Fanselow, 1980; Darwin, 1872; Estes, 1948; Estes & Skinner, 1941; Fanselow, 1982; Koch, 2004; Mogil, 2009; Rescola, 1968; Rescola & Lolordo, 1965; Skinner, 1938; Wiech & Tracey, 2013; Woolf, 1984). Behaviors suppressed by or exacerbated by pain affect arise later; the involvement of inferential and conflict-resolution circuits result from consequent mechanisms working on significantly slower timescale well beyond nociceptive processes, and reflect comorbid plasticity in non-nociceptive circuits. This approach at stringing together behavioral motifs highlights the necessity of nociceptive processing in affective circuits to flexibly construct these reactive behaviors.
2.1 |. Measuring pain affect in preclinical models by linking neural activities to volitional behaviors
Undeniably, brains are not functionally designed to represent the “truth” of the world. Neurons in the brain do not interact with the environment, but represent the relevant information to move the body, and keep the organism alive and propagating. Primordial species did not require perception; it was sufficient to move its mass of cells to avoid dying. With evolutionary pressures driving the expanded complexity of brains for deploying future planning and decision-making processes, we might then observe the emergence of perception—new integrative neural circuit motifs that can directly compute past, present, and future states for inferential predictions that enable appropriate avoidance or approach actions. The Latin word “emovere,” where e- means ‘out’ and movere means ‘move’, forms the basis of the English word “emotion” and derives the term “motivation.” Thus, definitions of emotion and motivation are rooted in terms describing bodies in action. In this light, we can employ behavior as a readout of internal states, such as pain affect. However, it is a complex and difficult, but not impossible, task to assign a function to a specific neuron, circuit, or network for pain perception that is established from a behavioral readout.
In humans, experimental pain can be measured by simple self-reflective or self-evaluative reports, along with visual analog scales and detailed questionnaires. Recently, in an attempt to identify visualized biomarkers of pain, machine-learning classifiers applied to fMRI and EEG datasets have decoded unique patterns of activity that correlate with noxious stimulus events or self-reports (Davis et al., 2020; Tayeb et al., 2020; Wager et al., 2013). However, basic pain neurobiology research has a clear need for better assays and endpoints of acute and chronic pain with superior predictive validity for translational target identification and compound screening (Mogil, 2019). A key issue with current assays, such as the von Frey mechanical sensitivity assay, is the experimenter (Bove, 2006). The experimenter provides a subjective determination of a reflexive-paw withdrawal as a “pain-like” response or not. Critically, reflexes are not a primary endpoint for assessing human chronic pain; this severely restricts the translational utility of reflexive assays like von Frey. Equally, experimenter presence, (i.e., a relatively large mammal next to or underneath the rodent), is itself a looming threat and a cue of imminent noxious stimuli. This repeated threat or stressor can greatly alter nociceptive processing, but is rarely addressed in data analysis (Hawranko et al., 1994; Sorge et al., 2014). Thus, the measurement of pain perception in non-verbal animals has much room for improvement.
A few recent attempts to automate nociceptive behavior for withdrawal metrics (Abdus-Saboor et al., 2019; J. Jones et al., 2020) and facial grimace (Baptista-de-Souza et al., 2020; Tuttle et al., 2018) have pushed the field forward, but still rely on anthropomorphic, supervised labeling of video frames for network training. Indeed, rodents are not small humans, and humans remain poor rodent psychologists. Thus, unsupervised deep-learning of video-recorded behaviors derived from pose-tracking systems (e.g. DeepLabCut and Social LEAP Estimates Animal Poses (SLEAP)) can improve upon this behavioral classification system, allowing for the discovery of a modular structure of rodent pain-affective behavior (Mathis et al., 2018; Pereira et al., 2019). These generative statistical models, including Motion Sequencing (MoSeq) and Behavioral Segmentation of Open-field In DeepLabCut (B-SOiD), parse behavior into units of action based upon underlying statistical structure without relying upon explicit human labeling (Hsu & Yttri, 2019; Wiltschko et al., 2015).
In line with ethological theory, pain affective behavior, like nearly all behavior, is constructed of repeated, stereotyped, and objectively quantifiable behavioral motifs. Indeed, our group’s best attempt to quantify affective-motivational behavior focuses on the cascade of stereotyped behaviors that follow a withdrawal response and on the spontaneous behaviors that emerge within the days and weeks following the onset of pain (Corder et al., 2017, 2019; Manglik et al., 2016)(Figure 2). By quantifying only unconditioned responses, one can begin to dissect the discrete networks processing cues and outcomes by linking the activity of nociceptive neurons to behavior. Thus, pain researchers can separate the sensory-discriminative from affective-motivational dimensions of pain perception and more objectively determine the animal’s in-the-moment behavioral responses to noxious events. To achieve this, there is an array of behavioral paradigms that have been developed to assay pain-related behavior in rodents, which we have outlined in Table 1.
Figure 2. Time scales of pain-related behaviors in rodents.
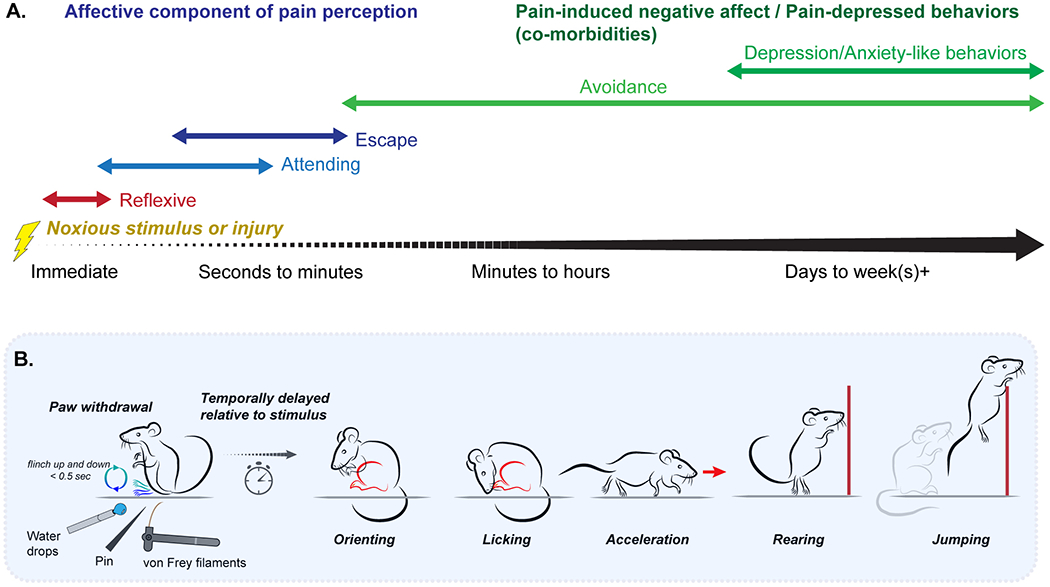
(a) Spontaneous behavior in preclinical models of acute and chronic pain reflective of the sensory-discriminative and affective-motivational dimensions, and pain affect, arise at different timescales. Application of noxious stimuli immediately trigger reflexive behaviors (red), including withdrawal and vocalization, followed by a series of affective-motivational behaviors (blue) aimed at reducing ongoing pain. The ongoing negative affect associated with pain can lead to the emergence of enduring behaviors associated with pain affect (purple), either directly initiated by motivational processes to avoid further pain, such as contextual avoidance and gait alteration; or that arise as a consequence of ongoing negative affect, including anxiety- and depression-like behaviors, that disrupt normal behavior. (b) Our group categorizes pain-related behavior across a number of identifiable and distinct categories following application of an acutely-painful stimulus: paw withdrawal, orienting, licking, acceleration, rearing, and jumping.
Table 1.
Behavioral measures used to quantify pain in preclinical studies, including reflexive behaviors; spontaneous or evoked affective-motivational, nocifensive behaviors; Avoidance and conditioned behaviors; and pain affect-suppressed (well-being) or -evoked (anxiety- and depression-like) behaviors.
A current preclinical methodology for purportedly evaluating the affective component of pain is a modified Conditioned Place Preference (CPP) paradigm, first developed by Sufka (Sufka, 1994) and refined by Porreca and colleagues (T. King et al., 2009). While this assay has moved the basic pain research field beyond mere reflexive withdrawal behaviors, it may not be the most appropriate tool for assessing the in-the-moment, rapid neural processes underlying pain perception, given the premise of CPP is a learning and memory task. As such, CPP is limited in its description of “spontaneous” pain affect because it conflates the associative learning process with the affective state being associated to the environment. Broadly, many brain regions show activity to both conditioned and unconditioned stimuli (Baeg et al., 2009; Hyman et al., 2017; Monosov, 2017), which makes it nearly impossible to tell on the post-conditioning test day which process (or both) was blocked by the administered analgesic. Moreover, using reward-seeking relief from chronic pain as an indirect readout of aversiveness of the resting state relies on the assumption that motivational-reward processes and cognitive flexibility remain unchanged in the chronic pain state. However, chronic pain causes dysfunction in reward-related processes, necessitating careful interpretation (Berryman et al., 2013; Moriarty et al., 2011, 2017; Schwartz et al., 2014). Thus, the CPP assay can assess the effectiveness of an intervention to motivate behavioral change, but this is fundamentally different from the affective state resulting from nociception.
Facial grimace is another measure of immediate, unconditioned reactions to noxious stimuli that might provide insight to pain affect intensity. Indeed, humans and animals partly communicate through facial expressions. Darwin asserted that the ability to communicate one’s emotional experience through facial expressions, including pain and pleasure, is a fundamental aspect of the social world (Ekman, 2009). As a first step toward understanding this important question, Mogil and colleagues developed the original Mouse Grimace Scale that relied on human-scored single video-frames on a 0-2 scale (Langford et al., 2010). This process was later semi-automated by a supervised neural network trained on human-selected video frames from mice exposed to noxious stimuli. In a refined methodology from Gogolla and colleagues, the team used a machine-learning algorithm to classify facial features of not only pain, but disgust, pleasure, fear and malaise, and to then link those facial behaviors to noxious-responsive ensembles in the insular cortex via 2-photon calcium imaging in awake, behaving mice (Dolensek et al., 2020). This is an elegant demonstration of how combining machine deep-learning for facial-feature detection or bodily pose-estimation with single-neuron imaging and optogenetic manipulations can accelerate the decoding of neuronal circuit functions and mechanisms that generate internal pain affective experiences, and their outward behavioral responses.
2.2 |. Distributed neural circuits for sensory vs. affective-motivational dimensions of pain
Regardless of the peripheral (e.g., TRPV1+ nociceptors, PIEZO2+ mechanoreceptors) or spinal (e.g., somatostatin+, PKCγ+ interneurons) processes that generate ascending nociception, and regardless of the sensory perceptive qualities (e.g. burning, stabbing, throbbing), all pain perceptions are heavily weighted with a professed aversion. This shared affective feature among pain types suggests the existence of a common neural circuit in the brain for transforming nociception into negative valence information. We hypothesize that this valence transformation process likely plays a role in forming the emotional aspects of pain perception. An intriguing aside worth mentioning is that in some more rare cases, ascending nociception can be shunted to appetitive valence circuits and perceived as pleasurable, as in the case of masochism (Dunkley et al., 2020). Masochism could reflect a learned response at the neural level, in which nociception no longer elicits an unconditioned protective behavior, but rather serves as a conditioned response signal. This was first observed in Pavlov’s early conditioning experiments, where dogs were trained to expect a food reward following an electric shock; the shock became a predictive hedonic signal and the dogs would salivate upon delivery of a noxious event (Trow et al., 1929). Thus, while the lower-level sensory and spinal processing of nociception is understood in great detail, far less detail is known about the dynamic circuit architecture that gives rise to pain’s affective qualities.
Though pain is often perceived as a unitary experience, dissociation of the sensory and affective dimensions can occur following central nervous system damage (Table 2). Frontal lobotomy patients can exhibit pain relief alongside a lowered pain threshold (H. E. King et al., 1950), and anterior cingulotomy patients often identify sensations as intense, yet not unpleasant (Foltz & White, 1962). Intriguingly, the famous Patient H.M., whose medial left temporal lobe was removed to halt intractable epilepsy, could discriminate the intensity of thermal stimuli that evoked pain in healthy controls—but H.M. did not label any of the stimuli “painful” or protectively guard his arm (Hebben et al., 1985). Meanwhile, peripheral nervous system damage that eliminates nociceptive input appears to eliminate both the sensory and affective dimensions of pain. For example, patients with loss-of-function mutations in the sodium channel Nav1.7 (Cox et al., 2006) or a gain-of-function mutation in Nav1.9 (Leipold et al., 2013) exhibit a congenital insensitivity to pain and do not identify noxious stimuli as painful despite intact senses of touch. Unlike Patient H.M., such patients with congenital insensitivity to pain will accumulate injuries due to the lack of nociception that can generate reflexive withdrawals. Without nociceptive input, neither sensory nor affective information can be deciphered, and as a result these patients simply do not have a concept of pain.
Table 2.
The effect of lesions or damage within the nociceptive system on human pain behavior (protective withdrawal/reaction), aversive perception (unpleasantness), and self-report categorization of the experience as “pain.”
| Condition/Treatment | Syndrome | Withdrawal | Unpleasantness | Self-report experiencing “Pain” |
|---|---|---|---|---|
| SCN9A or SCN11A mutation (loss of function for sodium channel Nav1.7 or gain of function for Nav1.9) | Congenital insensitivity to pain | No | No | No sensation or experience of “pain” |
| Damage to basolateral amygdala and/or posterior Insula | Pain asymbolia | Yes | No | No “pain” but sensory qualities perceived |
| Cingulotomy (lesion of the cingulum bundle) | Pain dissociation | Yes | Yes | “Pain” but “less bothersome” |
| Morphine | Pain dissociation | Yes | Yes | “Pain” but “less bothersome” |
Both the sensory and affective dimensions of pain play vital adaptive roles in allowing animals to recognize, escape, and avoid tissue damage. While the sensory dimension of pain provides qualitative information to identify harmful stimuli, the affective dimension drives behaviors to halt ongoing damage (e.g., escape behaviors) and avoid future damage (e.g., avoidance, guarding, and rest). Both types of behaviors are useful during acute pain, where damage has or may occur imminently. In the case of chronic pain, where pain may persist in the absence of tissue damage or following healing, pain affect-driven behaviors are devoid of adaptive benefit and instead interfere with daily life.
Several lines of evidence point to the affective dimension of pain as the major driver of chronic pain-related impairment. First, reported pain intensity does not increase with the onset of chronic pain (Hashmi et al., 2013) and pain threshold does not correlate with reported disability level (Coronado et al., 2015). Second, as pain transitions from the acute to chronic phases, there is a corresponding shift in brain activities from sensory areas to limbic areas (Hashmi et al., 2013). Common gray matter alterations to the functional salience and attention networks are also evident following the induction of several chronic pain conditions. In contrast, those in sensory-motor brain regions are condition-specific (Cauda et al., 2014). Finally, pain catastrophizing – characterized by excessive focus on pain, feelings of helplessness, and magnification of the threat posed by ongoing or future pain – plays a critical role in the distress associated with chronic pain. An increased propensity towards pain catastrophizing predicts the likelihood of chronic post-surgical pain (Theunissen et al., 2012). Excessive focus on pain makes it challenging for patients to divert their attention to other activities, thereby interfering with normal body movements. As a result, high pain catastrophization scores correlate with high pain-related disability in chronic pain patients (Ramírez-Maestre et al., 2017; Severeijns et al., 2001). Unsurprisingly, reductions in pain catastrophizing is a treatment goal for behavioral therapy in chronic pain, and reduction in pain catastrophizing is associated with concurrent reductions in reported disability, pain behavior, and symptoms of depression (Smeets et al., 2006; Spinhoven et al., 2004)
2.3 |. The amygdala as a key entry point to deconstructing the brain’s pain affective network
Recently, we identified one of the first nociception-encoding neural circuits in a deep-brain limbic structure, the basolateral amygdala (BLA), by leveraging in vivo calcium imaging with single-neuron resolution in freely behaving mice (Corder et al., 2019). This represented the first comprehensive demonstration and direct comparative mapping of unconditioned nociceptive and aversive responses in the BLA by imaging the activity of over 17,000 neurons across an unprecedented 49-day imaging period that covered the acute-to-chronic pain transition. Combining this imaging with genetically-modified, activity-dependent Cre-recombinase mice (TRAP, Targeted Recombination in Active Populations) mice that permit the chemogenetic control of nociceptive-responsive neurons in the BLA, we determined that this circuit encodes the negative valence of pain without altering the detection of noxious stimuli, withdrawal reflexes, anxiety, or reward.
While learning and adaptability are crucial, many circuits subsume hard-wired ethological functions, including nociception. The amygdala, particularly the BLA, is well studied for its role in conditioned learning, e.g. fear conditioning where a conditioned neutral stimulus predicts punishment, such as an electric shock. For decades, electric foot shock has been the primary punisher for studies on associative fear learning and active avoidance (Jean-Richard-Dit-Bressel et al., 2018). It is assumed that electric shocks activate some generalizable unconditioned stimulus (US) network in the BLA (Gore et al., 2015). Prior to the recent development of genetically-encoded calcium indicator (GECI)-based imaging technologies, in vivo electrophysiological probes could not evaluate the neural representation of noxious electric shocks due to the passing current. As such, most BLA studies have focused on the conditioned cue response network (CR+) that predict the aversive shocks. Using miniature microendoscope in vivo Ca2+ imaging approaches, we found that electric foot shocks do in fact activate a US network in the BLA that is distinguishable from appetitive and CR+ representations (Grewe et al., 2017), which is supported by optogenetic manipulations (Gore et al., 2015). However, these electric shock US ensembles do not engage the same nociceptive ensemble that encodes natural, noxious stimuli representations (e.g. noxious heat, cold, and mechanical pin prick). Not only could we decode the activity patterns distinguishing nociceptive versus shock stimuli with high fidelity, but we also found that most individual BLA neurons never displayed nociceptive-related activity during electric shocks. Surprisingly, we observed more co-active neurons than expected by chance between electric shocks and facial air puffs or isopentalamine odorant, than between shocks and the nociceptive stimuli. This demonstrates that experimental shocks may not be processed in a similar biological mechanism as evolved nociceptive processes. Depending on the current, voltage, and frequency of the electric shock, as well as the cutaneous bodily location it is applied to (glabrous vs. hairy skin, or finger-tips vs. trunk), there is likely to be activation of a multitude of functionally heterogeneous somatosensory primary afferents, each with their own activation threshold properties, which in turn could transduce competing neural information into the spinal cord “pain gate” (Melzack & Wall, 1965; Millan, 1999). This undoubtedly produces an unnatural pain-like percept, or even numerous sensations that are self-reported as perceived itch, light touch, stinging, pressure, and tickle (Ekman, 2009; Tashiro & Higashiyama, 1981). Thus, the identity and function of these non-nociceptive, shock-encoding BLA neurons remains unclear. This is not to say that electric shocks cannot be perceived as aversive or induce reactive or goal-directed behaviors. Indeed, rodents show increased escape, as well as audible and ultrasonic vocalizations to shocks (Fanselow, 1982; Han et al., 2015). However, we suggest the use of more natural stimuli, or optogenetic activation of specific peripheral sensory neuron populations (Beaudry et al., 2017), over electric shocks when identifying the specific functions of individual circuit elements relating to translational efforts for pain, fear, anxiety, or other psychiatry disorders.
In total, the BLA nociceptive ensemble provides a stable, hard-wired set of target neurons that are responsive to both acutely nociceptive and formerly-innocuous stimuli during chronic neuropathic pain states. While we did find that this same set of neurons can differentially encode noxious stimulus modality and intensity within high-dimensional neural activity codes, in the end, for translating this BLA circuit-level target for pain therapies, the neural coding patterns are not as important as the circuit itself. If future therapies can act to turn off this circuit signaling on-demand, then this could represent a strategy to alleviate the unpleasant emotion of pain perception, regardless of the cause of the nociception (e.g., arthritis, cancer, fibromyalgia, etc.).
While it is not resolved how and to what specific functions this BLA nociceptive circuit executes as it integrates with other cortical and subcortical structures for pain perception, future work can use the BLA nociceptive ensemble as a critical genetic entry point into deconstructing the functional and anatomical topology of the pain affective neural networks.
2.3.1 |. Comparing the roles of basolateral and central amygdala in pain affective-motivational processes
Outside of the BLA nociceptive ensemble, there are decades of rich research into pain affective processing brain regions, which include the parabrachial nucleus, central amygdala, bed nucleus of the stria terminalis, and insula, among others. Pain affect (to reiterate, a term that encompasses many different sub-dimensions) likely emerges from the totality of this larger, distributed brain network. However, we found that the BLA nociceptive ensemble is a critical bottle-neck circuit for pain affect, at least in the affective-motivational sub-dimensions that are quantifiable with our behavior measures. In addition to the multiple cortical and striatal connections, a major projection target of the BLA is the central amygdala (CeA; Neugebauer, 2015), known for executing learned locomotor freezing and flight responses to threats (Fadok et al., 2017; Terburg et al., 2018). In addition to the BLA nociceptive ensemble, there is a possible parallel or co-requisite circuit for encoding physiologic or emotional pain-related information that ascends from the spinal cord dorsal horn through the lateral parabrachial nucleus (PBN) to the lateral capsule of the CeA (Gauriau & Bernard, 2002). Importantly, the PBN→CeA circuit is not selective for nociception, as it is activated by several forms of non-nociceptive information, including itch, nausea, hunger, and bacterial infection (Campos et al., 2018; Carter et al., 2013; Palmiter, 2018). However, inhibition of this pathway reportedly reduces the immediate reactive locomotor response during an electric foot shock (Han et al., 2015) and optogenetic stimulation produces robust escape-like behaviors (Chiang et al., 2020). In contrast, inhibition of the BLA nociceptive ensemble does not eliminate the immediate reactive or reflexive nociceptive response, but does reduce the temporally-delayed, non-stereotyped affective-motivational behaviors that continue beyond active nociceptive input. Furthermore, the BLA integrates with higher-order corticostriatal circuits (Burgos-Robles et al., 2017; Janak & Tye, 2015; Ji et al., 2010; Ramirez et al., 2015), whereas the CeA largely influences descending nociception-control circuits, such as the ventrolateral periaqueductal gray (Fox & Sorenson, 1994; Oliveira & Prado, 2001; Ozawa et al., 2017; Tovote et al., 2016; Zhu & Pan, 2005). Interestingly, Patient H.M’s temporal lobe surgery removed the BLA but left the CeA largely intact (Annese et al., 2014). H.M.’s experience suggests a dissociative role between the BLA and CeA over the conscious qualitative experience of pain negative affect.
Parallel CeA circuits for affective processing of nociception likely exist (Veinante et al., 2013), but studies may be confounded by our limited linguistic ability to ascribe function to these different amygdalar nuclei. Instead, it is proposed that the functional role of PBN→CeA serves as an autonomic alarm system (Palmiter, 2018), which can be triggered by bottom-up sensory or top-down predictive signals. In this view, the CeA coordinates with hypothalamic and brainstem endogenous antinociceptive systems to generate arousal, endogenous analgesia, and motivation while also integrating abstract affective information from the BLA nociceptive ensemble (Balleine & Killcross, 2006). Thus, the CeA might compute danger information, including nociception, as a digital go/no-go reflexive motivational signal. Conversely, the BLA uses a high-dimensional analog process to assign valence as well as each stimulus’ sensory modality and intensity involved in the affective aspects of pain perception (Kyriazi et al., 2018). Currently, the BLA nociceptive ensemble is the only known stable set of neurons in the brain responsive to noxious stimuli that uses a unique combinatorial activity-code to selectively encode nociception during both acute and chronic pain. Adopting a longitudinal tracking and intervention approach similar to ours that takes advantage of long-term, cross-day imaging and activity manipulation to study the PBN→CeA circuit should greatly accelerate the discovery of the global circuits for pain.
3 |. The overlapping topology of the endogenous opioid and nociceptive systems
Despite the ongoing Opioid Epidemic, opioid drugs are still the mainstay of treatment for some types of severe and chronic pain, likely due to their selective targeting of pain affect. Morphine was first shown to reduce pain affect ratings without altering pain sensation ratings in neuropathic pain patients, independent of the central or peripheral origin of the pain (Kupers et al., 1991). Further work in rodents demonstrated selective involvement of endogenous opioid signaling in affective pain relief by both opioidergic and non-opioidergic analgesics (Gomtsian et al., 2018; Navratilova et al., 2015; Navratilova et al., 2019) In both cases, affective pain relief was obtained without alterations of paw withdrawal thresholds. Similarly, changes in affective-motivational behavior, such as wheel running, or paw attending-escape behavior, can be observed with opioid “low doses” (Cobos et al., 2012) or partial agonists (Manglik et al., 2016). Thus, at doses relevant to alleviate pain affective-motivational behaviors, the peripheral and sensory circuits that are sensitive to exogenous opioid drugs do not play a factor in the analgesic phenotype. These rodent observations appear to be a closer proxy for the analgesic effect of opioids in humans, again suggesting that it is beneficial for preclinical and translational studies to move beyond reflexive measures for determining analgesic efficacy. This is further supported by our work that genetically and pharmacologically blocked peripheral opioid receptors in TRPV1+ nociceptors, and found no changes in morphine analgesia, measured by reflexive or affective-motivational assays (Corder et al., 2017). Collectively, these studies suggest that pain affect relief may be achieved by targeting endogenous opioid circuits in the CNS.
Exogenous opioid compounds (e.g., morphine, heroin, fentanyl, oxycodone) exploit the evolutionarily designed endogenous pain-modifying circuits expressing the inhibitory Gαi/o-protein coupled receptor, the mu opioid receptor (MOR; Corder et al., 2018). MOR is expressed throughout the ascending and descending nociceptive circuits, as well as in appetitive-motivational circuits (e.g., ventral tegmental area (VTA) and nucleus accumbens) that contribute to reward and addiction-like effects (Veinante et al., 2013). MOR is also the cognate molecular target of the endogenous opioid peptides, endorphins and enkephalins, including the well-studied β-endorphin and met- and leu-enkephalin, as well as the often-overlooked endomorphin, hepta-/octa-peptides and α/β-neoendorphin (J. Zhao et al., 2012). In the brain and spinal cord, opioid peptides appear to be released from large-dense core vesicles in response to strong, repetitive neural signals, in order to fine-tune the flow of information between nociceptive neurons, resulting in endogenous analgesia, or pain relief derived from internal signaling mechanisms. With the opioid receptors and peptides identified over 50 years ago, the precise anatomic location and specific cell-type identify of receptor-expressing and peptide-releasing neurons in the CNS that are specifically relevant to nociception remains a vast arena for continued exploration, and an exciting avenue for therapy development aimed to leverage these neural circuits for modulating pain-processes.
Brain regions involved in canonical pain pathways, such as the ACC and periaqueductal gray, overlap widely with MOR expression and are strongly connected to the broader emotional-limbic circuitries, including the amygdala (Figure 3). Thus, the global MOR network is positioned to modulate the activity of many key parts of the limbic system that controls emotional behavioral responses generally, as well as responses to noxious events that direct attention and engage protective behaviors. While this review focuses on circuits within the brain, MOR is also widely expressed in nociceptors, the spinal cord, and the dorsal root ganglia (DRG). These peripheral and spinal areas are also able to modulate incoming sensory information and alter perception. We have elected to focus on the role of MOR brain circuits responsible for encoding the affect, expectation, and attention surrounding the experience of pain because of the importance of these behaviors in the human experience of chronic pain, their potential as targets for treatment, and their role in driving the Opioid Epidemic. Below, we highlight discoveries on the role of opioid signaling in affective and learning-related processes relevant for pain perception.
Figure 3.
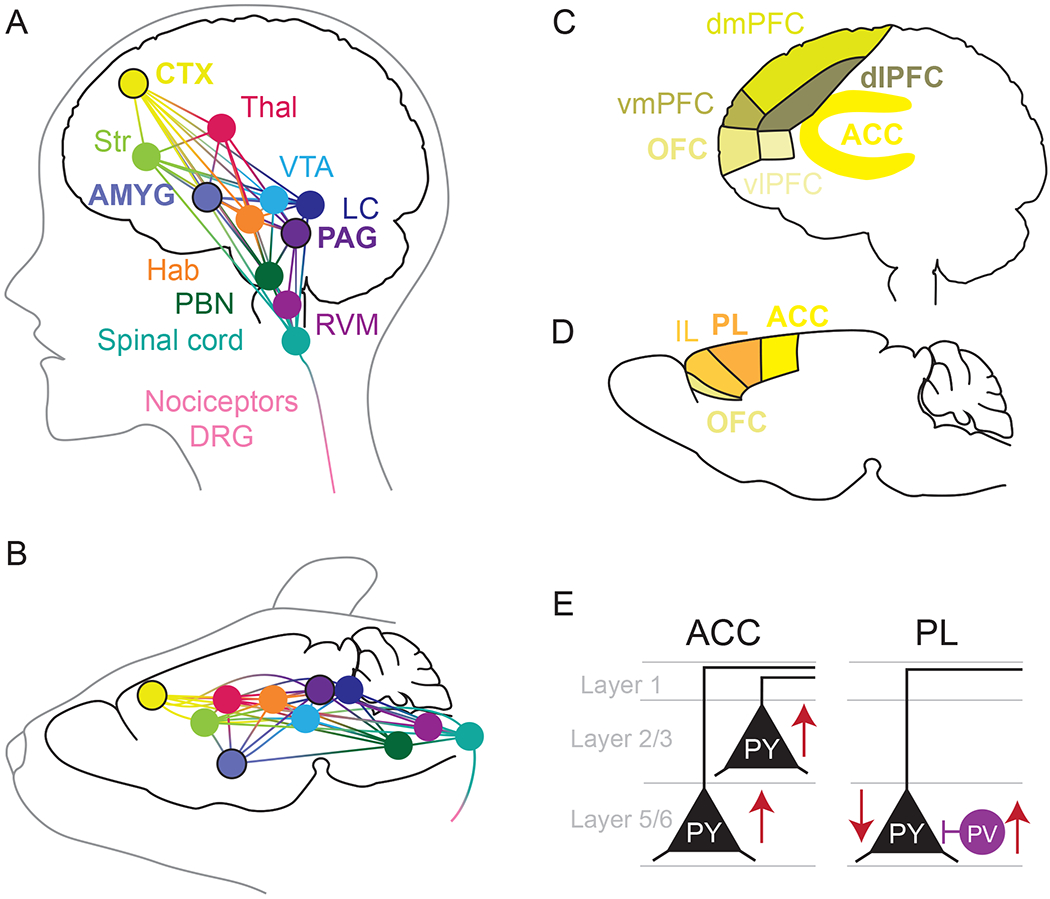
Neuroanatomical substrates of pain processing and affect. Overlapping and highly integrated circuits in humans (A) and rodents (B) both contain mu opioid receptors and their endogenous ligands and are involved in the processing of pain. This includes nociceptors, dorsal root ganglia (DRG), and the spinal cord, as well as several brain regions. In this review, we focus on the role of the amygdala (AMYG), cortex (CTX), and periaquductal gray (PAG), which are highly involved in the pain-affective network, attention towards pain, and pain expectation. These brain regions connect to other regions, including the parabrachial nucleus (PBN), rostral ventromedial medulla (RVM), habenula (Hab), locus coeruleus (LC), ventral tegmental area (VTA), striatum (Str) which incorporates the nucleus accumbens, and thalamus (Thal), illustrating the complexity of the endogenous opioid-pain network. The nomenclature for the frontal cortex in humans (C) and rodents (D) is complicated by differences in complexity, structure, and connectivity. The human literature has found that the anterior cingulate cortex (ACC), orbitofrontal cortex (OFC), dorsal lateral prefrotal cortex (dlPFC) are highly involved in the affective processing of pain. Other areas of the frontal cortex that we do not focus on here include the dorsomedial prefrontal cortex (dmPFC), the ventromedial prefrontal cortex (vmPFC), and the ventrolateral prefrontal cortex (vlPFC). The primate ACC is most analogous the rodent ACC, prelimbic cortex (PL), and infralimbic cortex (IL). While rodents have an OFC region it’s connectivity differs from that of the primate OFC. E) Single neuron electrophysiology studies of chronic pain models have focused on the ACC and PL rodent cortex, finding that pyramidal neurons of the ACC in layer 2/3 display increased excitability or potentiated excitatory synaptic transmission; some studies indicate no change in L5 ACC, while others show increased excitability as well. On the other hand, PL L5 pyramidal neurons are less excitable in models of chronic pain, perhaps driven by increase parvalbumin interneuron synaptic input.
4 |. Integrative cortical regions mediating opioid affective analgesia
The prefrontal frontal cortex (PFC) integrates many different modes of information - sensory, visual, auditory, emotional - to direct attention towards acute pain and to guide action. In this way, acute pain acts as a protective signal, facilitating the prioritization of responses to perceived external or internal threats. However, the persistence of chronic pain can impair many cognitive processes, including attentional inflexibility. Such pain-induced cognitive impairments are likely worsened by pain catastrophizing, the vicious cycle of negative mental focus on actual or anticipated pain that dominates the attention of chronic pain patients (Darnall et al., 2017). These cognitive symptoms are often comorbid with depression (Gelonch et al., 2017; Kummer et al., 2020), suggesting an underlying biological mechanism involving integration with affective circuits. The forced attention upon the negative valence, or unpleasantness, of pain perception prevents engagement in other goal-directed actions and is a key feature of what makes the experience of pain “bad.” In Section 4, we focus on PFC regions containing endogenous MOR-rich pathways that influence the affective and cognitive components on pain (A. K. Jones et al., 1991). Functional imaging in humans has identified that acutely noxious stimuli activate the frontal cortex and that the excitability of the frontal cortex increases during the transition from acute to chronic pain (Borras et al., 2004; J. K. Zubieta et al., 2001). Similarly, studies with non-human primates and rodents demonstrate that the cortex is activated acutely by noxious stimuli, and its activity is changed over the course of chronic pain (Zhuo, 2019). These facets of the chronic pain experience represent under-targeted mechanisms that could help bring relief to chronic pain patients and curb the Opioid Epidemic.
While rodent models are advantageous for the study of neural circuits and specific cell types, it is challenging to translate findings from rodent cortex, as the primate cortex is more complex. The term “prefrontal cortex” is used to describe similar, but not analogous, brain regions in the primate and rodent, which has led to lively debate about whether rodents possess a prefrontal cortex (Figure 3). Prefrontal cortex has historically been defined by projections from the mediodorsal thalamus (Brown & Bowman, 2002; Rose & Woolsey, 1948), and in primates, the prefrontal cortex often refers to the granular dorsolateral prefrontal cortex (dlPFC), the orbital areas of the frontal cortex (orbitofrontal cortex, OFC), the agranular anterior cingulate cortex (ACC), and the dorsomedial, and ventromedial cortices (Carlén, 2017; Laubach et al., 2018). Recent reviews suggest that the “rodent prefrontal cortex” corresponds most directly to the primate ACC (Laubach et al., 2018; van Heukelum et al., 2020). In rodents, the prefrontal cortex refers to the ACC, the prelimbic cortex (PL), and the infralimbic (IL) cortex, also known as Brodman’s Areas 24, 32, and 25, respectively. While area 32 is granular in primates, it is agranular in rodents, along with areas 24 and 25. Another key distinction is that rodent OFC is not analogous to primate OFC; while primate OFC is part of the prefrontal cortex, rodent OFC is differentially connected (Laubach et al., 2018). Although rodents lack a granular section of lateral PFC, present in primates as the dlPFC, the rodent prefrontal cortex can serve similar behavioral functions (Laubach et al., 2018). Mapping approaches drawing on functional connectivity and circuit projections to define brain regions, rather than by cytoarchitecture alone, suggest these cortical regions are somewhat similar (Heilbronner et al., 2016; Mars et al., 2018; Schaeffer et al., 2020). While more work needs to be done to reconcile the rodent and primate definitions of the prefrontal cortex, it is clear that several cortical regions are involved in the affective processing of pain and can be modulated by the endogenous opioid system. Here, we focus on the human and rodent ACC and the human and non-human primate OFC and dlPFC.
4.1 |. ACC directs attention towards the negative affect of pain
Like many cortical regions, the ACC is engaged by multimodal input and has been implicated in a variety of behaviors. In the context of pain negative affect, the ACC is involved in directing attention and anticipating the presentation of noxious stimuli. Pain alerts one of a threat and elicits orientation towards the threatening stimulus. Following this orientation, nociceptive signals are rapidly evaluated and contextualized by recurrent neural circuits for threat while an appropriate response is employed - escape or withdrawal. Additionally, learning and memory systems are engaged to avoid similar future threats. Indeed, the ACC displays increased activity during acute pain and persistent increased activity in chronic pain and models of chronic pain (Barthas et al., 2015; Laneri et al., 2017; Onoda et al., 2009; Sellmeijer et al., 2018; Singer et al., 2004; Zhuo, 2019; J. K. Zubieta et al., 2001). Further, behavioral similarities following ACC lesion and opioid treatment suggests overlapping roles in mediating the negative affective component of pain.
Much can be learned about the ACC by reviewing early descriptions of patients whose intractable chronic pain was treated with surgical cingulotomy lesions (Foltz & White, 1962; Hassenbusch et al., 1990; Rainville et al., 1997; Santo et al., 1990) (Table 2). These patients did not report a change in pain perception, intensity discriminations, or reactions to momentary harmful stimuli. Rather, their attitude toward pain was changed, dissociating the negative valence from the experience of pain. Here, pain became a sensation and not a threat. Cingulotomy results in difficulty sustaining attention and focus, and impairs attention/executive measures of response intention, generation, and persistence (Cohen et al., 1999). The major overall effect of cingulotomy is the attenuation of the patients’ continual response, emotionally and behaviorally, to their ever-present pain (i.e., reduced rumination on pain). The major overall effect of cingulotomy is the attenuation of the patients’ continual response, emotionally and behaviorally, to their ever-present pain, i.e., reduced rumination. The function of the ACC in the affective perception of pain is further revealed by its involvement in cognitive tasks that require attention (Corbetta et al., 1991; Devinsky et al., 1995; George et al., 1994; Stolyarova et al., 2019) as well as the influence of ACC by valence information (Feroz et al., 2017; Reed et al., 2018). Microelectrode exploration of the ACC of human patients undergoing bilateral cingulotomy for treatment of depression and obsessive compulsive disorder found that some ACC neurons responded specifically to noxious stimuli - with a bias toward excitation - and were also responsive to the anticipation of noxious stimuli (Hutchison et al., 1999). These cingulotomy studies point to the role of the ACC in generating attention toward pain, which contributes to the affective facet of chronic pain.
Similar to cingulotomy, exogenous opioids separate the sensation of pain from its negative valence and draw of attention. Indeed, reports from patients treated with ACC lesions and opiates suggest the relief from the affective symptoms of chronic pain is therapeutically meaningful. Acute pain engages MORs in the dorsal anterior cingulate and lateral prefrontal cortex, and the affective perception of pain is correlated with MOR availability in the ACC, among other brain regions (J. K. Zubieta et al., 2001). Together, this suggests that MOR signaling within the ACC contributes to the human perception of pain affect. However, the precise neural circuit mechanisms within the ACC that facilitate pain indifference from cingulotomy, or opioids remains unclear. The similarity between the affective mechanisms of morphine and cingulotomy gives us a clue to this function: the inhibition or lesion of MOR-expressing cortical circuits reduces the integration of negative valence information within the ACC, resulting in reduced aversive arousal.
To gain further insight into the mechanisms through which ACC MOR signaling exerts affective analgesic effects, we must turn to the preclinical literature. Similar to the human literature, lesion of the ACC results in a dissociation of anxiodepressive negative affect behavior from sensory behavior in a model of chronic pain (Barthas et al., 2015). Intra-ACC MOR activation induces conditioned place preference in rodent models of chronic pain (Gomtsian et al., 2018; Edita Navratilova et al., 2015, 2020; C. Qu et al., 2011). Similarly, low dose morphine does not alter mechanical sensitivity in a model of neuropathic pain, but does reduce the affective aversive quality of mechanical stimulation (LaGraize et al., 2006). Pharmacologic blockade of MORs in the ACC blunts the emotional behaviors associated with the opioid analgesic morphine, but not sensory-mechanical behaviors (Edita Navratilova et al., 2015). In chronic pain-naïve rodent models, lesion of anterior ACC decreases learned aversion driven by a noxious inflammatory pain model (Gao et al., 2004; Johansen et al., 2001; Johansen & Fields, 2004; Kung et al., 2003; Pastoriza et al., 1996). Further, ACC lesion does not alter mechanical paw withdrawal thresholds in a model of neuropathic pain, but reduces the aversiveness of such stimulation (LaGraize et al., 2004). In comparison, the medial cingulate cortex, immediately caudal to the ACC, can influence descending nociceptive hypersensitivity following injury through claustrum-insular pathways (Tan et al., 2017). Together, these ACC behavioral studies suggest that the affective behavioral effect of opioids are partly attributable to ACC MORs, and possibly in the anterior Cg1 subregion.
Anticipation of pain, acute noxious stimuli, and chronic pain activate the ACC (Apkarian et al., 2005; Cao et al., 2016; Davis et al., 1997; Hsieh et al., 1999; Lenz et al., 1998; Porro et al., 2002) (Figure 4). Indeed, the pyramidal neurons of layer 2/3 ACC become hyperexcitable in rodent models of chronic pain manifesting as long term potentiation of excitatory synapses through pre- and postsynaptic mechanisms (Koga et al., 2015, 2017; X.-Y. Li et al., 2010; Sellmeijer et al., 2018; H. Xu et al., 2008; Zhuo, 2019). Layer 5 of the ACC also appears to have increase excitability in chronic pain models (Blom et al., 2014; Santello & Nevian, 2015). Further, optogenetic inhibition of the ACC in rodent chronic pain models reverses the negative affective but not sensory behavioral effects (Sellmeijer et al., 2018) The circuit effects of ACC change excitability are potentially far reaching, as the ACC projects to many regions, including other cortical regions, BLA, striatum, hypothalamus, thalamus, brainstem, and spinal cord. Direct projections from the ACC to the spinal cord contribute to mechanical threshold sensitization and could contribute to the development of chronic pain (Chen et al., 2018; Tsuda et al., 2017). While these studies suggest that hyperexcitability of the ACC is a key feature of chronic pain, the activation of the ACC with deep brain stimulation is also associated with relief from chronic pain (Davis et al., 2000). These seemingly opposing findings suggest that subpopulations of neurons within the ACC and neighboring prefrontal cortical subregions could have opposing pain-related activity. Indeed, glutamatergic neurons in Cg2 of midcingulate cortex are inhibited by painful stimulation, project to inhibitory neurons of the zona incerta, lose excitability during chronic pain, and their activation is analgesic. Conversely, glutamatergic neurons in Cg2 of midcingulate cortex become hyperactive in chronic pain and their inhibition is analgesic (Hu et al., 2019).
Figure 4. Conserved brain structures involved in pain and analgesia expectation.
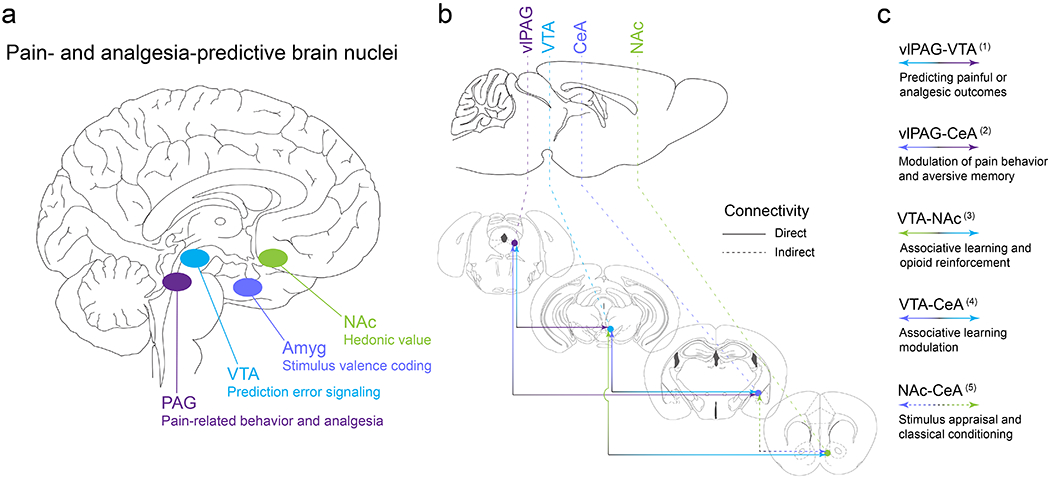
(a) Deep brain stimulation and imaging modalities have revealed nuclei in the human brain that underlie endogenous opioid-mediated analgesia as well as expectancy signals that contribute to pain and the relief of pain. This work provides an excellent foundation for back-translational approaches to identify, target, and manipulate the endogenous opioid circuits that mediate pain relief directly or through learning mechanisms (i.e. placebo analgesia). Here we highlight several critical brain areas, all of which are modulated by endogenous opioid peptides: periaqueductal gray (PAG), ventral tegmental area (VTA), amygdala (Amyg), and nucleus accumbens (NAc). (b-c) The rodent brain shares the same pain-responsive structures. Preclinical models of pain and analgesia have further revealed functional connectivity between nodes and their subnuclei in the distributed pain- and analgesia-predictive neural circuitry. Reciprocal, direct connectivity has been documented between the ventrolateral PAG (vlPAG) and the VTA, the vlPAG and the central nucleus of the amygdala (CeA), the VTA and CeA, as well as the VTA and NAc. Indirect connectivity has been noted for the CeA and NAc. Here we provide broad functional definitions for these neural connections based on ample preclinical studies in animals. (1) vlPAG-VTA (Geisler et al., 2007; Ntamati et al., 2018; Omelchenko & Sesack, 2010; St Laurent et al., 2020; Suckow et al., 2013; Waung et al., 2019); (2) vlPAG-CeA (C. Li et al., 2016; Ozawa et al., 2017; Tovote et al., 2016; Wright & McDannald, 2019); (3) VTA-NAc (Beier et al., 2015; Corre et al., 2018; Klawonn & Malenka, 2018; S. Peciña et al., 2006; Pignatelli & Bonci, 2018; Xia et al., 2011); (4) VTA-CeA (Dedic et al., 2018; Fudge et al., 2017; H. J. Lee et al., 2011; Zhou et al., 2019); (5) NAc-CeA (Ahn & Phillips, 2002; Cardinal et al., 2002; Hall et al., 2001; Zahm et al., 1999).
While ACC pyramidal neurons display increased excitability in chronic pain models, the neighboring prelimbic (PL) pyramidal neurons display decreased excitability in chronic pain models (Figure 3). Layer 5 PFC pyramidal neurons show reduced excitatory input and reduced intrinsic excitability in chronic pain (Cheriyan & Sheets, 2018; C. J. Kelly et al., 2016; Metz et al., 2009). PFC projection neurons, typically layer 5 pyramidal cells, made hypoactive during chronic pain, likely project to the NAc, a key valence structure (Baliki et al., 2012; Michelle Lee et al., 2015). Neighboring prelimbic (PL) neurons also display increased firing rates following the presentation of an acute noxious stimulus in rodents (Dale et al., 2018). Contrastingly, basal and noxious event-evoked firing rates of PL neurons are deceased in chronic pain (Dale et al., 2018). Consistent with this finding, enhancing basal PFC activity with low-frequency optogenetic stimulation diminishes hypersensitivity in mechanical, thermal, and valence/affective nocifensive behaviors during chronic pain (Dale et al., 2018). Indeed, exogenous activation of PFC principle neurons is analgesic and anxiolytic in naïve and chronic pain rodent models (Ji & Neugebauer, 2011; Jurik et al., 2015; G.-Q. Wang et al., 2015; S. Zhang et al., 1997), while optogenetic inhibition of PFC is anxiogenic (G.-Q. Wang et al., 2015).
Conversely, PFC GABAergic interneurons neurons are activated during a persistent visceral nociception model (Jurik et al., 2015), while MORs expressed in parvalbumin interneurons allow for the disinhibition of PL pyramidal neurons and the facilitation of reward-related behavior (Jiang et al., 2019). Injury increases the excitability of PL PFC layer 5 parvalbumin neurons in males and decreases the excitability of PL layer 2/3 somatostatin neurons in females (A. F. Jones & Sheets, 2020), highlighting the need to consider the contribution of sex differences to nociceptive processes in the cortex and remain an important point for future work. Furthermore, chronic neuropathic pain results in a loss of parvalbumin interneurons and concurrently impairs performance in a set-shifting assay in male mice (Shiers et al., 2018). Optogenetic inhibition and activation of GABAergic PL neurons results in decreased and increased pain responses, respectively, as well as imbuing positive and negative valence to context in conditioned place preference and escape/avoidance behavioral assays (Z. Zhang et al., 2015). These findings collectively suggest that principle PL neurons are disinhibited by GABAergic interneurons to promote cortical-driven antinociceptive behaviors, likely driven by the activation of MOR on GABAergic interneurons. In a model of chronic pain, PL pyramidal neurons are inhibited by parvalbumin-expressing GABAergic interneurons, counteracting the decrease in pyramidal neuron activity in this model, due to enhanced excitatory drive onto PV interneurons (Z. Zhang et al., 2015). Indeed, MOR activation by morphine can bi-directionally modulate the dendritic complexity of somatostatin and parvalbumin interneurons, with increased activation increasing complexity (X. Wang et al., 2019).
Overall, the human phenomenology of lesions or cortical opioid action suggests that the ACC’s role in pain experience is not simply to encode the affective component of pain. Rather, the ACC integrates, and temporally-smooths, the nociceptive valence information within working memory to facilitate attention, decision-making, and motor planning, which logarithmically shapes the perception of pain (akin to smooth, continuous visual perception, despite eye saccades) (Fuchs et al., 2014; Heilbronner & Hayden, 2016; Xiao & Zhang, 2018). Importantly, all the behavioral tests of affect rely upon the ability of animals to attend to their context and associate it with negative valence. Future animal studies should strive to dissociate negative valence from cognitive processes to better tease apart the function of the ACC and frontal cortex (Box 2).
Box 2. Improving the study of negative valence directed attention in preclinical models of chronic pain.
A core feature ACC cingulotomy in human patients is pain dissociation, an ability to perceive pain, but find it less bothersome. The separation of the sensory perception of pain from the negative valance of pain is a key outcome of opioid treatment for pain. Better understanding the underlying mechanism of this behavior could be critical to identifying non-addictive treatments for chronic pain that preserve the protective sensory facet of acute pain. Current approaches for studying the negative affect component of pain in rodents draw from the fields of affective and addiction neuroscience. Classic depressive-like tests, such as the forced swim test, novelty-suppressed feeding, and sucrose splash tests (Barthas et al., 2015), allow for insight into the how chronic pain alters the overall mood of the subject, which is a central debilitating feature of chronic pain, but does not provide insight into how pain is perceived. Conditioned place preference assays, which assess preference for a context associated with an experimental condition (e.g., stimulation of region, or local infusion of an opioid agonist) or analgesic, provide insight into the rewarding valence of pain relief. However, this technique requires several days of conditioning, and doesn’t provide direct insight into how much pain draws attention from other tasks. While learning and memory assays, such as the set shifting assay, can assess this – and have deficits in chronic pain (Shiers et al., 2018) – this behavioral focus hasn’t received a lot of attention in the pain field as a whole. Going forward, behavioral tasks should assess how acute pain holds attention – e.g., orientation towards and time attending to acute noxious stimuli – and how chronic pain alters everyday life – e.g., spontaneous activity in the home cage. To best capture these behaviors, machine learning approaches represent promising improved methods to quantify movement during sensory assays, facial grimace, and other fine details that could provide insight into how attention is directed during chronic pain (J. Jones et al., 2020; Mogil et al., 2020). Ideally, these measures could allow for automated, closed loop assessment in odor and sound-proof arenas, removing the traditional confounds of experimenter presence, subjective assessment of movement-based outcomes, and social cues from other animals in the testing.
4.2 |. OFC and dlPFC MORs direct attention to pain-related affect
Human studies have implicated the orbitofrontal cortex (OFC) and the dorsolateral prefrontal cortex (dlPFC) in the integration of cognitive, affective, and sensory aspects of nociception (Ong et al., 2019). Human imaging has revealed that medial OFC is activated by reward and lateral OFC is activated by punishment in a decision making task, and changes in OFC functional activity correlated with the magnitude of valence (O’Doherty et al., 2001). Damage to the OFC increases impulsivity, alters emotional behavior, and is involved in decision making based upon representation of value. Preclinical work suggests that the OFC plays a role in opioid-mediated analgesia. Ventrolateral OFC infusion of morphine reduces mechanical and thermal allodynia and hypersensitivity in naive and chronic pain states (X. Huang et al., 2001; M. Zhao et al., 2007), and MOR activation in the ventrolateral OFC mediates the antinociceptive effect of morphine on rats experiencing formalin-induced hypersensitivity (Xie et al., 2004). The effect of morphine is reversed by naloxone (X. Huang et al., 2001), and blocked by a GABAA receptor antagonist (C.-L. Qu et al., 2006), suggesting an opioid-induced decrease in GABAergic signaling underlies the behavioral effects of opiates in the OFC. Together, human and preclinical research reveals that MORs expressed on cortical GABAergic interneurons are positioned to disinhibit subsets of pyramidal neurons during chronic pain, allowing for MOR to reduce attention to pain, attenuating affective and sensory measures of nocifensive and attention-requiring behaviors, including cognitive flexibility impairments (Shiers et al., 2018).
In contrast, lesion of the dlPFC suggests that its influence is biased towards learning (i.e., decision making based on the task and context feedback) (Anderson et al., 1999; Berlin et al., 2004; Fellows, 2011; Hornak et al., 2003; Rolls et al., 1994). The dlPFC is involved in spatial discrimination of pain, as well as attention, encoding of value, working memory, decision making, and emotional regulation of these processes (Oshiro et al., 2009; Seminowicz & Moayedi, 2017). Intriguingly, when subjects were given the ability to control the level of noxious events during a trial the dlPFC appeared to downregulate nociceptive brain activity in the insula, while uncontrollable noxious trials resulted in increased connectivity between the PFC and insula (Bräscher et al., 2016). Furthermore, repetitive transcranial magnetic stimulation (rTMS) of dlPFC induces analgesia that is blocked by naloxone pretreatment (J. J. Taylor et al., 2013). This suggests that the dlPFC plays a role in the assignment of emotional weight to pain perception, which is tunable via endogenous opioid signaling.
The experience of pain is modified by expectations, which builds from the importance of attention in modulating pain perception. The PFC integrates contextual information to form a representation of pain expectation, demonstrated by the ability of PFC dopamine to tune hindbrain-projecting PFC neurons to noxious stimuli, and the activation of this population to induce avoidance and defensive behavior (Vander Weele et al., 2018). This information allows for the top-down modulation of pain via expectation and placebo processes (Coulombe et al., 2016; J. Huang et al., 2019). Indeed, placebo-induced analgesia occurs alongside decreased ACC activation, and pharmacological blockade of opioid receptors or the modulation of pyramidal neuron activity in the PFC prevents placebo analgesia (I.-S. Lee et al., 2015; L. Xu et al., 2018). In Section 5, we detail the role of opioid signaling in predictive coding and placebo circuits.
5 |. Modulation of pain experience and relief expectation by endogenous opioids
Nocifensive behaviors are a first line of defense engaged by animals presented with noxious, painful stimuli to prevent tissue damage. Those behaviors reveal the motivational nature of pain (Fields, 2006): to protect, escape, and cope. Moreover, learning to avoid or engage with stimuli that are either painful or soothing, respectively, demonstrates the power of teaching signals generated by aversive motivational states (Johansen & Fields, 2004). Such actions are mediated, at least in part, by endogenous opioid-enriched sites in the brain. Interestingly, the same endogenous opioid neural circuits that contribute to anti-nociception and pain coping can be activated or recruited by non-pharmacological means to promote analgesia. Thus, these neural circuits can facilitate pain relief by capitalizing on the motivation to heal, Pavlovian conditioning, or through homeostatic processes that direct neural signaling towards mitigation of competing internal need states (e.g., stress abatement, hunger, thirst, etc.). The periaqueductal gray (PAG), nucleus accumbens (NAc), and central amygdala (CeA) are important brain areas that can drive pain relief according to learned associations or ongoing behavioral states even in the absence of exogenous opioid drug consumption.
5.1 |. The periaqueductal gray mediates passive and learned analgesia via endogenous opioid peptides
The ventrolateral aspect of the PAG (vlPAG) was among the first brain regions recognized for its important role in facilitating analgesia in humans. This function was revealed by electrical stimulation and pharmacological studies. Critically, the research groups of Liebeskind (Akil et al., 1976) and Linchitz (Hosobuchi et al., 1977) demonstrated that vlPAG stimulation-induced anti-nociception required endogenous MOR activation as administration of naloxone prevented the anti-nociceptive effects, in rats and humans respectively. Indeed, direct infusion of morphine into the PAG produces robust analgesia (Yaksh & Rudy, 1978). Furthermore, it was revealed that the vlPAG is a hub of enkephalin peptide signaling necessary for analgesia (Hughes et al., 1975; Simantov et al., 1977). Despite these important findings, intracranial stimulation remains a highly invasive procedure with variable results. Therefore, engaging endogenous opioid circuits for pain relief without pharmacological or surgical intervention is a goal of ongoing pain research.
Pain relief expectation cues are sufficient to trigger endogenous opioid signaling (Amanzio & Benedetti, 1999). Neuroimaging has been fruitful in revealing behavioral mechanisms that engage this endogenous opioid mechanism. Human brain imaging (fMRI) and positron emission tomography (PET) studies have found that pain relief anticipation drives activity in anti-nociceptive neural circuitry and involves MOR activity (Eippert, Bingel, et al., 2009; Pecina & Zubieta, 2018; Scott et al., 2008). Anticipatory neural signaling is a key feature of placebo analgesia, defined as reduced pain experience resulting from the belief that a treatment, inert or otherwise, will provide pain relief (Laska & Sunshine, 1973). In some cases, the placebo intervention produces analgesia just as effectively as an active treatment, such as lidocaine (Hashmi et al., 2012). This effect can be conditioned through Pavlovian associations or through various learning mechanisms, including instructional, observational, or experiential, provided that the cues signaling pain relief are relatively stable and unchanging (Colloca et al., 2019).
Activity in the vlPAG tracks with reported placebo analgesia, suggesting that this brain area is engaged by internal processes in a manner similar to exogenous electrical stimulation and mediates pain relief through MOR activation (Amanzio & Benedetti, 1999; Levine et al., 1978; Scott et al., 2008; Wager et al., 2007; J.-K. Zubieta et al., 2005). Likewise, conditioned analgesia resulting from re-exposure to the pain relief context can be blocked by naloxone (Berna et al., 2018). In concert with vlPAG activation, placebo analgesia also robustly activates the dlPFC in an anticipatory manner that likely precedes vlPAG activity (Wager et al., 2004). Moreover, those individuals displaying larger placebo analgesic effects also demonstrated higher levels of dlPFC activation (Lui et al., 2010). Remarkably, it was recently demonstrated that activation of PAG and cortical areas during pain relief correspond to decreases in functional activity of dorsal horn neurons (Eippert, Finsterbusch, et al., 2009; Sprenger et al., 2015). Thus, top-down endogenous opioid-enriched circuits modulate pain experience and can be triggered by conditioned or learned associations between an intervention and its purported analgesic properties (Fields, 2004).
Placebo analgesia can be modeled in laboratory animals (Keller et al., 2018a), which demonstrates that this form of pain relief is conserved across mammalian species and provides a robust experimental platform that bridges preclinical and clinical pain research. However, these initial studies should be regarded with caution as a number of limitations are inherent to current approaches to modeling placebo analgesia in rodents (Box 3). Because pain perception can be shaped by precise learned expectancies and contextual cues (Büchel et al., 2014), the underlying neural circuitry will encode pain-relief prediction signals during placebo analgesia training that become more robust with experience (Colloca et al., 2010). In this way, placebo analgesia-active vlPAG neural ensembles will shunt top-down negative pain affect processing allowing for coping mechanisms to be effectively engaged. For example, blockade of MORs specifically in the vlPAG attenuated conditional hypoalgesia during a Pavlovian conditioning paradigm (Bellgowan & Helmstetter, 1998). Overall, the phenomenon of placebo analgesia suggests a more fundamental role of motivational and learning processes in pain relief, with more work needed to produce an adequate model and thereby elucidate the vlPAG cell types circuits involved in placebo analgesia.
Box 3. Limitations of rodent models of placebo analgesia.
Preclinical efforts to model placebo analgesia, the experience of pain relief from otherwise inert treatments, has only just begun in earnest in the past two decades. The state of animal models of placebo analgesia is discussed at greater length previously (Keller et al., 2018b). The bulk of these studies rely on classical or Pavlovian conditioning to generate a drug-context association typically followed by a test for acute anti-nociception (e.g., hotplate assay) (Bryant et al., 2009; Jian-You Guo et al., 2011; J.-Y. Guo et al., 2010; Valone et al., 1998; R.-R. Zhang et al., 2013). However, mixed results have been found across rodent species with drug-pairing protocols producing either no placebo analgesia (McNabb et al., 2014) or heightened thermal nociception (Krank et al., 1981). The strength of the drug conditioning procedure is tested by omitting the active drug treatment, such as morphine (10 mg/kg), in lieu of the inert vehicle, the placebo. Increased latency to perform nocifensive behavior is the principal readout of the success of placebo analgesia in rodent models to date. Although these studies appear face valid, they are limited by several important experimental factors:
Placebo analgesia testing in rodents largely relies on reflexive pain assays. While informative, these assays do not readily reflect the self-report procedures used in clinical placebo analgesia experiments. Therefore, in addition to latency measures, placebo analgesia paradigms in rodents should incorporate analysis of affective pain behaviors (e.g., paw licking) as reported data.
Single or repeated administration of opioid analgesic can induce locomotor sensitization (Vanderschuren et al., 2001; Vezina & Leyton, 2009). This heightened locomotion may make assessment of anti-nociception difficult, especially under paradigms that rely on precise stimulus application to the paw (e.g., with von Frey filaments).
Repeated opioid administration can result in a paradoxical state of heightened nociception or hyperalgesia (Marion Lee et al., 2011) that would confound detection of placebo analgesia.
While the extant work shows that exogenous opioid-related placebo analgesia can be blocked by the mu opioid receptor naloxone (J.-Y. Guo et al., 2010), suggesting a role for endogenous opioid signaling, it is likely that repeated opioid analgesic treatment alters endogenous opioid circuits. Thus, drug-induced plasticity will hamper interpretations of the role of endogenous opioids in placebo analgesia.
To address these limitations and improve our understanding of endogenous opioid signaling mechanisms in placebo analgesia, new models must be developed that omit exogenous opioid conditioning while retaining the critical aspects of learning that generate placebo analgesia: expectation and experience. Indeed, some efforts have been made to develop a non-pharmacological placebo analgesia. To do this, hotplates set to different temperatures, one less noxious and one more noxious, are paired with distinct contextual cues. After conditioning to the less noxious hotplate-context pairing, rodents were found to exhibit decreased nocifensive behavior on test day when the temperature was raised to a more noxious temperature in the same context (I.-S. Lee et al., 2015; L. Xu et al., 2018). Critically, these studies revealed that pain expectancy-based placebo analgesia involved endogenous opioid signaling, as naloxone treatment blocked the expression of placebo analgesia on test day.
5.2 |. Endogenous opioid signaling in limbic nuclei shapes pain experience and expectation
At the population level, pain relief expectation is the greatest predictor of long-term chronic pain outcomes and illustrates the importance of expectancy in modulating pain experience (Cormier et al., 2016). This relies on pain relief signals that are stable, reproducible, and comparable to past experiences, suggesting that prediction error contributes to the encoding of anticipatory signals of placebo analgesia (Büchel et al., 2014; M. Peciña et al., 2014) and involves endogenous opioid release in limbic nuclei (Scott et al., 2008). Thus, within the motivation-decision framework postulated by Howard Fields (Fields, 2006), pain relief experience or expectation is driven by negative reinforcement processes, whereby the mitigation of potential tissue damage, either through direct action or indirect observation, is encoded as a teaching signal that will spare an individual from pain in the future. Although this conceptual framework continues to evolve some studies are already beginning to discern by what means cognitive pain modulation occurs and the roles endogenous opioids may play. In the brain, a number of limbic, subcortical regions across the neuraxis contribute to prediction error coding that enables anticipation of significant events (Schultz & Dickinson, 2000) including the ventral tegmental area (VTA), nucleus NAc, CeA, as well as the PAG (L. Becerra et al., 2001) (Figure 4). To inform and facilitate future research into endogenous opioid-mediated analgesic processes among limbic nuclei, we present a number of foundational studies below.
Pain relief expectation cues are associated with increased dopamine release in the NAc of humans (Scott et al., 2008). Likewise, pain offset is associated with increased neural activity in the NAc (Lino Becerra & Borsook, 2008). In rodents, electrical stimulation of the VTA or dopamine D2 receptor agonist application to the NAc are sufficient to reduce nociception (Sotres-Bayón et al., 2001; B. K. Taylor et al., 2003). In agreement with this finding, activation of VTA dopamine neurons, as indicated by increased cFos, has been observed following intra-popliteal fossa administration of lidocaine to block incision injury pain in rats (Edita Navratilova et al., 2012). Likewise, placebo analgesia occurs alongside increased tyrosine hydroxylase expression in the VTA, also suggesting engagement of VTA dopamine neurons (I.-S. Lee et al., 2015). Within the NAc, and more generally the mesolimbic pathway, little is known regarding the role of endogenous MOR signaling in facilitating or modulating pain processing in the dopamine system. However, increasing work utilizing exogenous agonists has begun to shed light on the complexities of endogenous opioid signaling in the mesolimbic dopamine pathway. For example, while MOR activity appears to have uniform effects across the medial shell subregion with respect to driving reward wanting (S. Peciña & Berridge, 2013), the NAc has a clear hedonic “hotspot” in the rostrodorsal region which mediates reward liking that is positively modulated by mu, delta, and kappa opioid receptor activation (Castro & Berridge, 2014). In contrast, MOR in the caudal medial shell “coldspot” reduces hedonic liking of sucrose (S. Peciña & Berridge, 2013). Surprisingly, microinjection of DAMGO, a MOR peptide agonist, into the NAc caudal coldspot can elicit a defensive treading, escape behavior. This effect of MOR-mediated aversion indicates that mu agonists are not themselves intrinsically pleasurable or positively hedonic, but rather their action on specific affective-motivational circuits can lead to either approach or avoidance behaviors (Richard et al., 2013). Likewise, outside the hedonic “hotspot” kappa opioid receptor activation attenuates reward liking (S. Peciña & Berridge, 2013) that becomes pathologically active in chronic pain states (Massaly et al., 2016, 2019; Vergara et al., 2020). These results suggest that opioid peptides in the NAc can tune pain relief reward signals arising from the VTA in a region-specific manner and thereby differentially engage recurrent circuit targets of the NAc, such as the CeA.
While the mesolimbic system plays a central role in negative reinforcement and motivation, the CeA provides crucial modulatory control that serves to translate learned or conditioned responses into motivationally salient decisions (Mahler & Berridge, 2009). For example, the CeA encodes expectation signals, that when violated, either through omission or over-expectation (Haney et al., 2010), initiate extinction learning (Iordanova et al., 2016). Moreover, the CeA demonstrates endogenous MOR signaling in human imaging studies as a preparatory mechanism to facilitate placebo analgesic responses (Wager et al., 2007). Some CeA interneurons are enkephalinergic, and thereby promote negative reinforcement processes by inhibiting the projection neurons of the CeA (Paretkar & Dimitrov, 2019), which can become hyperexcitable in acute and chronic pain states (Neugebauer et al., 2020; Neugebauer & Li, 2003). This effect manifests differentially among genetically-defined CeA populations. Notably, it was found that PKCδ neurons, a subset of which expresses enkephalin (Hua et al., 2020), demonstrate pain-related sensitization while somatostatin neurons were silenced (Wilson et al., 2019). Pain relief prediction signals likely activate CeA interneurons to suppress efferent noxious signals (i.e., PKCδ neurons) and promote antinociceptive signals (i.e., somatostatin neurons) to areas such as the VTA and PAG, thereby limiting pain-cue learned associations (Beier et al., 2015; J.-N. Li & Sheets, 2018). Utilization of prediction error coding to facilitate learning by the amygdala and PAG has been demonstrated in the context of fear conditioning (LeDoux et al., 1988). For example, muscimol-induced inactivation of the PAG resulted in deficits in acquisition of conditioned fear learning (Johansen et al., 2010). Furthermore, CeA to vlPAG projections have been specifically implicated in mediating early instructive signals encoded during fear conditioning, which are blocked by optogenetic inhibition of this pathway (Ozawa et al., 2017) (Figure 4). Despite these impactful studies, much remains unknown regarding the precise role of endogenous opioid signaling in the CeA during placebo analgesia and pain relief processes.
In total, capitalizing on learning mechanisms that may involve endogenous opioid signaling represents a novel therapeutic avenue for alleviating the need for exogenous opioid drugs. Identifying the neural populations, their connectivity, and activity patterns will provide new insights into our ability to safely and effectively recruit the brain’s intrinsic pain relief neural circuits.
6 |. Future approaches for treating chronic pain by harnessing endogenous opioid systems
Emerging preclinical work is providing unprecedented access to nociception-encoding neural cell-types and circuits through rodent and viral genetics, deep-brain endoscopic brain imaging, and cell-type circuit manipulation, all of which allows for the direct and causal linking of neural nociceptive codes to the emergence of pain-related behaviors. Classically, pain research has focused on the sensory and reflexive components of pain, often leading to unsuccessful avenues of treatment in humans. Indeed, this approach is limited in its description of pain-related behavior in the affective-motivational domain. By studying pain affect and its neural substrates, we now know that the BLA encodes diverse noxious stimuli activity of which is necessary to evoke pain-related negative affect (Corder et al., 2019). As highlighted herein, pain affective circuitry extends beyond the BLA to the frontal cortex and integrates with brain regions that encode prediction error signals (e.g., PAG, CeA, NAc) to instruct our future behavior in the face of noxious stimuli and provide anticipatory relief when pain is unavoidable.
Timely advances in rodent and viral genetic manipulations have significantly furthered our ability to map nociceptive and pain affective neural circuits. Resources, such as the Allen Institute Mouse Brain Atlas (Lein et al., 2007) and the recent molecular map of the mouse brain (Ortiz et al., 2020), provide valuable foundational tools for pain neuroscientists to develop and utilize cutting edge molecular approaches. For example, rabies virus (RABV) enables transsynaptic retrograde tracing of neurons projecting to a brain region of interest (R. M. Kelly & Strick, 2000). Due to the multisynaptic labeling provided by RABV, circuit architecture can be inferred. Indeed, injection of green fluorescent protein-tagged RABV into the planar skin of the mouse paw revealed that sensory neurons received descending inputs from the rostroventral medulla (RVM) (Y. Zhang et al., 2015). Similarly, a distributed circuit connecting the trigeminal nucleus to the ACC has been revealed by RABV-based circuit mapping (Iwata et al., 2011). When combined with recently developed mouse genetic models (e.g. Penk-cre, Oprm1-mCherry, Oprm1-cre, and Pomc-cre lines) (Bailly et al., 2020; Mambretti et al., 2016), RABV and viral genetic tracing will reveal diverse endogenous opioid circuit elements involved in pain and its relief.
Once identified, gaining genetic access to pain affective or pain relief expectation neural circuits represents a potential next generation of pain treatment approaches. An excessive pain affect signal in the frontal cortex could act as an attractor-state, disrupting ACC function and perpetuating a lasting negative internal state during chronic pain. Because the cortex is one of the most superficial regions in the pain affect network, it is a convenient target for translation treatments that do not violate the skin or require diffusely-acting pharmacological agents, such as transcranial approaches such TMS or focused ultrasound (fUS) chemogenetics. TMS targeted to frontal cortical regions for the treatment of chronic pain and depression suggests that such a strategy could recruit endogenous opioid signaling (DosSantos et al., 2014; Downar & Daskalakis, 2013; Lamusuo et al., 2017; J. J. Taylor et al., 2013). At the neural circuit level, fUS is now being explored as a potential vehicle for delivery of pharmacological agents to specific areas of the brain by creating blood brain barrier permeability at sites of interest (Burgess et al., 2015). In this way, novel ligands that normally lack brain penetrance can be targeted to identified endogenous opioid circuits to modulate pain affect or pain relief in real time, but in a non-cell type-specific manner.
Gene therapy-based approaches, in current development for chemogenetic control of peripheral nociceptor populations, could offer unique cell type-specific access to CNS neurons critical to pain affect. Preclinically, chemogenetics and optogenetics, which involve ectopic expression of novel receptors that respond selectively to exogenous ligand or light, respectively, have promoted neural circuit dissection for a breadth of behaviors (Whissell et al., 2016). Chemogenetic receptors and ligands (e.g., DREADDs, PSAMs, DARTs) (Atasoy & Sternson, 2018) as well as optogenetic receptors (e.g., channelrhodopsin) (Kim et al., 2017) are already widely used in neuroscience research and enable restricted expression of receptors or ligands (i.e., DARTs) to desired cell populations. In the clinic, mimicking morphine analgesia by engineering endogenous intra-ACC enkephalin-release with on-demand opto- or chemo- genetic control, could restore cognitive capacity and limit chronic pain affect, for example. Also of note, engineered MOR-like substrates are already under development that respond to specific wavelengths of light (i.e., opto-MORs and UV-light-sensitive caged-opioids) (Banghart et al., 2018; Siuda et al., 2015). However, the translation of these approaches to human patients remains limited due to safety testing and verification of viral payload expression (Keifer et al., 2020).
Over past decades, a large number of studies, in various human and animal models, has provided invaluable knowledge about the neurobiological elements underlying opioid analgesia and some brain nociceptive processes. However, until recently the restricted resolution of current neurotechnologies has limited the discovery of nociceptive-specific circuits, outside of the spinal cord, to relatively few brain regions. Now, numerous transformative tools like those described herein, leveraging elegant combinations of molecular genetics, optics, electrical engineering and other multidisciplinary fields, are beginning to assist in more unbiased investigations of brain circuits with high spatial and temporal resolution. Together, basic neuroscientists and physician-scientists, working with increased cooperation and effort toward reverse-translation experimental designs, have an unprecedented opportunity to repave the direction of translational pain research and lay the groundwork for new treatment targets for opioid analgesia and pain affective processes.
Analgesic drugs, including medicinal opioids, bathe the body in chemicals, acting on widely expressed molecular targets that promote addictive and fatal side effects (Yekkirala et al., 2017). Indeed, the cellular diversity within defined neural circuits reveals a major methodological shortcoming of current pharmacotherapeutic strategies. Pain patients desperately need options with high precision, minimal side effect profiles, and on-demand availability. Looking ahead, we propose that some of our next generation chronic pain therapies should not target receptors, enzymes, or other molecular players, but rather the brain’s neural circuits that underlie the negative affective perception of pain. This strategy would have the ethical prowess and advantage of not permitting opioid activity within subcortical reward networks that promote addiction, or fatal effects such as respiratory depression. Clinically, our efforts might have a two-pronged utility: reduce the suffering of chronic pain patients, while simultaneously mitigating the current Opioid Epidemic.
Supplementary Material
Acknowledgments:
The authors are supported by the following funding mechanisms: National Institute of General Medical Sciences, DP2GM140923 (GC), National Institute on Drug Abuse, R00DA043609 (GC), Brain and Behavior Research Foundation Young Investigator (GC), Alkermes Pathways Research (GC), Whitehall Foundation (GC), National Institute on Mental Health, T32-MH01465 (NMM).
Footnotes
Conflict of Interest Statement: The authors declare no conflicts of interest.
DATA AVAILABILITY STATEMENT: This document contains no primary scientific data.
References
- Abdus-Saboor I, Fried NT, Lay M, Burdge J, Swanson K, Fischer R, Jones J, Dong P, Cai W, Guo X, Tao Y-X, Bethea J, Ma M, Dong X, Ding L, & Luo W (2019). Development of a Mouse Pain Scale Using Sub-second Behavioral Mapping and Statistical Modeling. Cell Reports, 28(6), 1623–1634.e4. 10.1016/j.celrep.2019.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn S, & Phillips AG (2002). Modulation by central and basolateral amygdalar nuclei of dopaminergic correlates of feeding to satiety in the rat nucleus accumbens and medial prefrontal cortex. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 22(24), 10958–10965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akil H, Mayer DJ, & Liebeskind JC (1976). Antagonism of stimulation-produced analgesia by naloxone, a narcotic antagonist. Science (New York, N.Y.), 191(4230), 961–962. 10.1126/science.1251210 [DOI] [PubMed] [Google Scholar]
- Amanzio M, & Benedetti F (1999). Neuropharmacological dissection of placebo analgesia: Expectation-activated opioid systems versus conditioning-activated specific subsystems. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 19(1), 484–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SW, Bechara A, Damasio H, Tranel D, & Damasio AR (1999). Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nature Neuroscience, 2(11), 1032–1037. 10.1038/14833 [DOI] [PubMed] [Google Scholar]
- Annese J, Schenker-Ahmed NM, Bartsch H, Maechler P, Sheh C, Thomas N, Kayano J, Ghatan A, Bresler N, Frosch MP, Klaming R, & Corkin S (2014). Postmortem examination of patient H.M.’s brain based on histological sectioning and digital 3D reconstruction. Nature Communications, 5, 3122. 10.1038/ncomms4122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV, Bushnell MC, Treede R-D, & Zubieta J-K (2005). Human brain mechanisms of pain perception and regulation in health and disease. European Journal of Pain, 9(4), 463–463. 10.1016/j.ejpain.2004.11.001 [DOI] [PubMed] [Google Scholar]
- Atasoy D, & Sternson SM (2018). Chemogenetic Tools for Causal Cellular and Neuronal Biology. Physiological Reviews, 98(1), 391–418. 10.1152/physrev.00009.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeg EH, Jackson ME, Jedema HP, & Bradberry CW (2009). Orbitofrontal and anterior cingulate cortex neurons selectively process cocaine-associated environmental cues in the rhesus monkey. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 29(37), 11619–11627. 10.1523/JNEUROSCI.3206-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly J, Del Rossi N, Runtz L, Li J-J, Park D, Scherrer G, Tanti A, Birling M-C, Darcq E, & Kieffer BL (2020). Targeting Morphine-Responsive Neurons: Generation of a Knock-In Mouse Line Expressing Cre Recombinase from the Mu-Opioid Receptor Gene Locus. ENeuro, 7(3). 10.1523/ENEURO.0433-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, & Apkarian AV (2015). Nociception, Pain, Negative Moods, and Behavior Selection. Neuron, 87(3), 474–491. 10.1016/j.neuron.2015.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Petre B, Torbey S, Herrmann KM, Huang L, Schnitzer TJ, Fields HL, & Apkarian AV (2012). Corticostriatal functional connectivity predicts transition to chronic back pain. Nature Neuroscience, 15(8), 1117–1119. 10.1038/nn.3153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, & Killcross S (2006). Parallel incentive processing: An integrated view of amygdala function. Trends in Neurosciences, 29(5), 272–279. 10.1016/j.tins.2006.03.002 [DOI] [PubMed] [Google Scholar]
- Banghart MR, He XJ, & Sabatini BL (2018). A Caged Enkephalin Optimized for Simultaneously Probing Mu and Delta Opioid Receptors. ACS Chemical Neuroscience, 9(4), 684–690. 10.1021/acschemneuro.7b00485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptista-de-Souza D, Tavares-Ferreira D, Megat S, Sankaranarayanan I, Shiers S, Flores CM, Ghosh S, Luiz Nunes-de-Souza R, Canto-de-Souza A, & Price TJ (2020). Sex differences in the role of atypical PKC within the basolateral nucleus of the amygdala in a mouse hyperalgesic priming model. Neurobiology of Pain (Cambridge, Mass.), 8, 100049. 10.1016/j.ynpai.2020.100049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthas F, Sellmeijer J, Hugel S, Waltisperger E, Barrot M, & Yalcin I (2015). The anterior cingulate cortex is a critical hub for pain-induced depression. Biological Psychiatry, 77(3), 236–245. 10.1016/j.biopsych.2014.08.004 [DOI] [PubMed] [Google Scholar]
- Beaudry H, Daou I, Ase AR, Ribeiro-da-Silva A, & Séguéla P (2017). Distinct behavioral responses evoked by selective optogenetic stimulation of the major TRPV1+ and MrgD+ subsets of C-fibers. Pain, 158(12), 2329–2339. 10.1097/j.pain.0000000000001016 [DOI] [PubMed] [Google Scholar]
- Becerra L, Breiter HC, Wise R, Gonzalez RG, & Borsook D (2001). Reward circuitry activation by noxious thermal stimuli. Neuron, 32(5), 927–946. 10.1016/s0896-6273(01)00533-5 [DOI] [PubMed] [Google Scholar]
- Becerra Lino, & Borsook D. (2008). Signal valence in the nucleus accumbens to pain onset and offset. European Journal of Pain (London, England), 12(7), 866–869. 10.1016/j.ejpain.2007.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier KT, Steinberg EE, DeLoach KE, Xie S, Miyamichi K, Schwarz L, Gao XJ, Kremer EJ, Malenka RC, & Luo L (2015). Circuit Architecture of VTA Dopamine Neurons Revealed by Systematic Input-Output Mapping. Cell, 162(3), 622–634. 10.1016/j.cell.2015.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellgowan PS, & Helmstetter FJ (1998). The role of mu and kappa opioid receptors within the periaqueductal gray in the expression of conditional hypoalgesia. Brain Research, 791(1–2), 83–89. 10.1016/s0006-8993(98)00057-2 [DOI] [PubMed] [Google Scholar]
- Berlin HA, Rolls ET, & Kischka U (2004). Impulsivity, time perception, emotion and reinforcement sensitivity in patients with orbitofrontal cortex lesions. Brain: A Journal of Neurology, 127(Pt 5), 1108–1126. 10.1093/brain/awh135 [DOI] [PubMed] [Google Scholar]
- Berna C, Leknes S, Ahmad AH, Mhuircheartaigh RN, Goodwin GM, & Tracey I (2018). Opioid-Independent and Opioid-Mediated Modes of Pain Modulation. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 38(42), 9047–9058. 10.1523/JNEUROSCI.0854-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryman C, Stanton TR, Jane Bowering K, Tabor A, McFarlane A, & Lorimer Moseley G (2013). Evidence for working memory deficits in chronic pain: A systematic review and meta-analysis. Pain, 154(8), 1181–1196. 10.1016/j.pain.2013.03.002 [DOI] [PubMed] [Google Scholar]
- Berthier M, Starkstein S, & Leiguarda R (1988). Asymbolia for pain: A sensory-limbic disconnection syndrome. Annals of Neurology, 24(1), 41–49. 10.1002/ana.410240109 [DOI] [PubMed] [Google Scholar]
- Blanchard R, & Blanchard D (1969). Passive and active reactions to fear-eliciting stimuli. J Comp Physiol Psychol, 68, 129–135. [DOI] [PubMed] [Google Scholar]
- Blom SM, Pfister J-P, Santello M, Senn W, & Nevian T (2014). Nerve Injury-Induced Neuropathic Pain Causes Disinhibition of the Anterior Cingulate Cortex. Journal of Neuroscience, 34(17), 5754–5764. 10.1523/JNEUROSCI.3667-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolles R (1970). Species-specific defense reactions and avoidance learning. Psychol Rev, 77, 32–48. [Google Scholar]
- Bolles R, & Fanselow M (1980). A perceptual-defensive-recuperative model of fear and pain. Behav Brain Sci, 3, 291–323. [Google Scholar]
- Borras MC, Becerra L, Ploghaus A, Gostic JM, DaSilva A, Gonzalez RG, & Borsook D (2004). FMRI measurement of CNS responses to naloxone infusion and subsequent mild noxious thermal stimuli in healthy volunteers. Journal of Neurophysiology, 91(6), 2723–2733. 10.1152/jn.00249.2003 [DOI] [PubMed] [Google Scholar]
- Bove G (2006). Mechanical sensory threshold testing using nylon monofilaments: The pain field’s “tin standard.” Pain, 124(1–2), 13–17. 10.1016/j.pain.2006.06.020 [DOI] [PubMed] [Google Scholar]
- Bräscher A-K, Becker S, Hoeppli M-E, & Schweinhardt P (2016). Different Brain Circuitries Mediating Controllable and Uncontrollable Pain. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 36(18), 5013–5025. 10.1523/JNEUROSCI.1954-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown VJ, & Bowman EM (2002). Rodent models of prefrontal cortical function. Trends in Neurosciences, 25(7), 340–343. 10.1016/S0166-2236(02)02164-1 [DOI] [PubMed] [Google Scholar]
- Bryant CD, Roberts KW, Culbertson CS, Le A, Evans CJ, & Fanselow MS (2009). Pavlovian conditioning of multiple opioid-like responses in mice. Drug and Alcohol Dependence, 103(1–2), 74–83. 10.1016/j.drugalcdep.2009.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büchel C, Geuter S, Sprenger C, & Eippert F (2014). Placebo analgesia: A predictive coding perspective. Neuron, 81(6), 1223–1239. 10.1016/j.neuron.2014.02.042 [DOI] [PubMed] [Google Scholar]
- Burgess A, Shah K, Hough O, & Hynynen K (2015). Focused ultrasound-mediated drug delivery through the blood-brain barrier. Expert Review of Neurotherapeutics, 15(5), 477–491. 10.1586/14737175.2015.1028369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos-Robles A, Kimchi EY, Izadmehr EM, Porzenheim MJ, Ramos-Guasp WA, Nieh EH, Felix-Ortiz AC, Namburi P, Leppla CA, Presbrey KN, Anandalingam KK, Pagan-Rivera PA, Anahtar M, Beyeler A, & Tye KM (2017). Amygdala inputs to prefrontal cortex guide behavior amid conflicting cues of reward and punishment. Nature Neuroscience, 20(6), 824–835. 10.1038/nn.4553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos CA, Bowen AJ, Roman CW, & Palmiter RD (2018). Encoding of danger by parabrachial CGRP neurons. Nature, 555(7698), 617–622. 10.1038/nature25511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B, Wang J, Zhang X, Yang X, Poon DC-H, Jelfs B, Chan RHM, Wu JC-Y, & Li Y (2016). Impairment of decision making and disruption of synchrony between basolateral amygdala and anterior cingulate cortex in the maternally separated rat. Neurobiology of Learning and Memory, 136, 74–85. 10.1016/j.nlm.2016.09.015 [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Lachenal G, Halkerston KM, Rudarakanchana N, Hall J, Morrison CH, Howes SR, Robbins TW, & Everitt BJ (2002). Effects of selective excitotoxic lesions of the nucleus accumbens core, anterior cingulate cortex, and central nucleus of the amygdala on autoshaping performance in rats. Behavioral Neuroscience, 116(4), 553–567. 10.1037//0735-7044.116.4.553 [DOI] [PubMed] [Google Scholar]
- Carlén M (2017). What constitutes the prefrontal cortex? Science, 358(6362), 478–482. 10.1126/science.aan8868 [DOI] [PubMed] [Google Scholar]
- Carter ME, Soden ME, Zweifel LS, & Palmiter RD (2013). Genetic identification of a neural circuit that suppresses appetite. Nature, 503(7474), 111–114. 10.1038/nature12596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro DC, & Berridge KC (2014). Opioid hedonic hotspot in nucleus accumbens shell: Mu, delta, and kappa maps for enhancement of sweetness “liking” and “wanting.” The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 34(12), 4239–4250. 10.1523/JNEUROSCI.4458-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauda F, Palermo S, Costa T, Torta R, Duca S, Vercelli U, Geminiani G, & Torta DME (2014). Gray matter alterations in chronic pain: A network-oriented meta-analytic approach. NeuroImage: Clinical, 4, 676–686. 10.1016/j.nicl.2014.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman CR, Tuckett RP, & Song CW (2008). Pain and stress in a systems perspective: Reciprocal neural, endocrine, and immune interactions. The Journal of Pain: Official Journal of the American Pain Society, 9(2), 122–145. 10.1016/j.jpain.2007.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Taniguchi W, Chen Q-Y, Tozaki-Saitoh H, Song Q, Liu R-H, Koga K, Matsuda T, Kaito-Sugimura Y, Wang J, Li Z-H, Lu Y-C, Inoue K, Tsuda M, Li Y-Q, Nakatsuka T, & Zhuo M (2018). Top-down descending facilitation of spinal sensory excitatory transmission from the anterior cingulate cortex. Nature Communications, 9(1), 1886. 10.1038/s41467-018-04309-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheriyan J, & Sheets PL (2018). Altered Excitability and Local Connectivity of mPFC-PAG Neurons in a Mouse Model of Neuropathic Pain. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 38(20), 4829–4839. 10.1523/JNEUROSCI.2731-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang MC, Nguyen EK, Canto-Bustos M, Papale AE, Oswald A-MM, & Ross SE (2020). Divergent Neural Pathways Emanating from the Lateral Parabrachial Nucleus Mediate Distinct Components of the Pain Response. Neuron, 106(6), 927–939.e5. 10.1016/j.neuron.2020.03.014 [DOI] [PubMed] [Google Scholar]
- Cobos EJ, Ghasemlou N, Araldi D, Segal D, Duong K, & Woolf CJ (2012). Inflammation-induced decrease in voluntary wheel running in mice: A nonreflexive test for evaluating inflammatory pain and analgesia. Pain, 153(4), 876–884. 10.1016/j.pain.2012.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RA, Kaplan RF, Moser DJ, Jenkins MA, & Wilkinson H (1999). Impairments of attention after cingulotomy. Neurology, 53(4), 819–824. 10.1212/wnl.53.4.819 [DOI] [PubMed] [Google Scholar]
- Colloca L, Petrovic P, Wager TD, Ingvar M, & Benedetti F (2010). How the number of learning trials affects placebo and nocebo responses. Pain, 151(2), 430–439. 10.1016/j.pain.2010.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colloca L, Schenk LA, Nathan DE, Robinson OJ, & Grillon C (2019). When Expectancies Are Violated: A Functional Magnetic Resonance Imaging Study. Clinical Pharmacology and Therapeutics, 106(6), 1246–1252. 10.1002/cpt.1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, Dobmeyer S, Shulman GL, & Petersen SE (1991). Selective and divided attention during visual discriminations of shape, color, and speed: Functional anatomy by positron emission tomography. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 11(8), 2383–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder G, Ahanonu B, Grewe BF, Wang D, Schnitzer MJ, & Scherrer G (2019). An amygdalar neural ensemble that encodes the unpleasantness of pain. Science (New York, N.Y.), 363(6424), 276–281. 10.1126/science.aap8586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder G, Castro DC, Bruchas MR, & Scherrer G (2018). Endogenous and Exogenous Opioids in Pain. Annual Review of Neuroscience, 41, 453–473. 10.1146/annurev-neuro-080317-061522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder G, Tawfik VL, Wang D, Sypek EI, Low SA, Dickinson JR, Sotoudeh C, Clark JD, Barres BA, Bohlen CJ, & Scherrer G (2017). Loss of μ opioid receptor signaling in nociceptors, but not microglia, abrogates morphine tolerance without disrupting analgesia. Nature Medicine, 23(2), 164–173. 10.1038/nm.4262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormier S, Lavigne GL, Choinière M, & Rainville P (2016). Expectations predict chronic pain treatment outcomes. Pain, 157(2), 329–338. 10.1097/j.pain.0000000000000379 [DOI] [PubMed] [Google Scholar]
- Coronado RA, Bialosky JE, Bishop MD, Riley JL, Robinson ME, Michener LA, & George SZ (2015). The Comparative Effects of Spinal and Peripheral Thrust Manipulation and Exercise on Pain Sensitivity and the Relation to Clinical Outcome: A Mechanistic Trial Using a Shoulder Pain Model. Journal of Orthopaedic & Sports Physical Therapy, 45(4), 252–264. 10.2519/jospt.2015.5745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corre J, van Zessen R, Loureiro M, Patriarchi T, Tian L, Pascoli V, & Lüscher C (2018). Dopamine neurons projecting to medial shell of the nucleus accumbens drive heroin reinforcement. ELife, 7. 10.7554/eLife.39945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombe M-A, Erpelding N, Kucyi A, & Davis KD (2016). Intrinsic functional connectivity of periaqueductal gray subregions in humans. Human Brain Mapping, 37(4), 1514–1530. 10.1002/hbm.23117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JJ, Reimann F, Nicholas AK, Thornton G, Roberts E, Springell K, Karbani G, Jafri H, Mannan J, Raashid Y, Al-Gazali L, Hamamy H, Valente EM, Gorman S, Williams R, McHale DP, Wood JN, Gribble FM, & Woods CG (2006). An SCN9A channelopathy causes congenital inability to experience pain. Nature, 444(7121), 894–898. 10.1038/nature05413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale J, Zhou H, Zhang Q, Martinez E, Hu S, Liu K, Urien L, Chen Z, & Wang J (2018). Scaling Up Cortical Control Inhibits Pain. Cell Reports, 23(5), 1301–1313. 10.1016/j.celrep.2018.03.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnall BD, Sturgeon JA, Cook KF, Taub CJ, Roy A, Burns JW, Sullivan M, & Mackey SC (2017). Development and Validation of a Daily Pain Catastrophizing Scale. The Journal of Pain : Official Journal of the American Pain Society, 18(9), 1139–1149. 10.1016/j.jpain.2017.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin C (1872). The expression of the emotions in man and animals (Murray J, Ed.). Albemarle. [Google Scholar]
- Davis KD, Aghaeepour N, Ahn AH, Angst MS, Borsook D, Brenton A, Burczynski ME, Crean C, Edwards R, Gaudilliere B, Hergenroeder GW, Iadarola MJ, Iyengar S, Jiang Y, Kong J-T, Mackey S, Saab CY, Sang CN, Scholz J, … Pelleymounter MA (2020). Discovery and validation of biomarkers to aid the development of safe and effective pain therapeutics: Challenges and opportunities. Nature Reviews Neurology, 16(7), 381–400. 10.1038/s41582-020-0362-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KD, Lozano AM, & Dostrovsky JO (2000). Activation of the anterior cingulate cortex by thalamic stimulation in patients with chronic pain: A positron emission tomography study. J. Neurosurg, 92, 6. [DOI] [PubMed] [Google Scholar]
- Davis KD, Taylor SJ, Crawley AP, Wood ML, & Mikulis DJ (1997). Functional MRI of Pain- and Attention-Related Activations in the Human Cingulate Cortex. Journal of Neurophysiology, 77(6), 3370–3380. 10.1152/jn.1997.77.6.3370 [DOI] [PubMed] [Google Scholar]
- Dedic N, Kühne C, Jakovcevski M, Hartmann J, Genewsky AJ, Gomes KS, Anderzhanova E, Pöhlmann ML, Chang S, Kolarz A, Vogl AM, Dine J, Metzger MW, Schmid B, Almada RC, Ressler KJ, Wotjak CT, Grinevich V, Chen A, … Deussing JM (2018). Chronic CRH depletion from GABAergic, long-range projection neurons in the extended amygdala reduces dopamine release and increases anxiety. Nature Neuroscience, 21(6), 803–807. 10.1038/s41593-018-0151-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, & Vogt BA (1995). Contributions of anterior cingulate cortex to behaviour. Brain: A Journal of Neurology, 118 ( Pt 1), 279–306. 10.1093/brain/118.1.279 [DOI] [PubMed] [Google Scholar]
- Dolensek N, Gehrlach DA, Klein AS, & Gogolla N (2020). Facial expressions of emotion states and their neuronal correlates in mice. Science, 368(6486), 89–94. 10.1126/science.aaz9468 [DOI] [PubMed] [Google Scholar]
- DosSantos MF, Martikainen IK, Nascimento TD, Love TM, DeBoer MD, Schambra HM, Bikson M, Zubieta J-K, & DaSilva AF (2014). Building up analgesia in humans via the endogenous μ-opioid system by combining placebo and active tDCS: A preliminary report. PloS One, 9(7), e102350. 10.1371/journal.pone.0102350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downar J, & Daskalakis ZJ (2013). New targets for rTMS in depression: A review of convergent evidence. Brain Stimulation, 6(3), 231–240. 10.1016/j.brs.2012.08.006 [DOI] [PubMed] [Google Scholar]
- Dunkley CR, Henshaw CD, Henshaw SK, & Brotto LA (2020). Physical Pain as Pleasure: A Theoretical Perspective. The Journal of Sex Research, 57(4), 421–437. 10.1080/00224499.2019.1605328 [DOI] [PubMed] [Google Scholar]
- Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, & Büchel C (2009). Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron, 63(4), 533–543. 10.1016/j.neuron.2009.07.014 [DOI] [PubMed] [Google Scholar]
- Eippert F, Finsterbusch J, Bingel U, & Büchel C (2009). Direct evidence for spinal cord involvement in placebo analgesia. Science (New York, N.Y.), 326(5951), 404. 10.1126/science.1180142 [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, & Williams KD (2003). Does rejection hurt? An FMRI study of social exclusion. Science (New York, N.Y.), 302(5643), 290–292. 10.1126/science.1089134 [DOI] [PubMed] [Google Scholar]
- Ekman P (2009). Darwin’s contributions to our understanding of emotional expressions. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 364(1535), 3449–3451. 10.1098/rstb.2009.0189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes W (1948). Discriminative conditioning; effects of a Pavlovian conditioned stimulus upon a subsequently established operant response. J Exp Psychol, 38, 173–177. [DOI] [PubMed] [Google Scholar]
- Estes W, & Skinner B (1941). Some quantitative properties of anxiety. J Exp Psychol, 29, 390–400. [Google Scholar]
- Fadok JP, Krabbe S, Markovic M, Courtin J, Xu C, Massi L, Botta P, Bylund K, Müller C, Kovacevic A, Tovote P, & Lüthi A (2017). A competitive inhibitory circuit for selection of active and passive fear responses. Nature, 542(7639), 96–100. 10.1038/nature21047 [DOI] [PubMed] [Google Scholar]
- Fanselow MS (1982). The postshock activity burst. Animal Learning and Behavior, 10(4), 448–454. [Google Scholar]
- Fellows LK (2011). Orbitofrontal contributions to value-based decision making: Evidence from humans with frontal lobe damage. Annals of the New York Academy of Sciences, 1239, 51–58. 10.1111/j.1749-6632.2011.06229.x [DOI] [PubMed] [Google Scholar]
- Fields H (2004). State-dependent opioid control of pain. Nature Reviews. Neuroscience, 5(7), 565–575. 10.1038/nrn1431 [DOI] [PubMed] [Google Scholar]
- Fields H (2006). A Motivation-Decision Model of Pain: The Role of Opioids. In Proceedings of the 11th World Congress on Pain (pp. 449–459). IASP Press. [Google Scholar]
- Foltz EL, & White LE (1962). Pain “Relief” by Frontal Cingulumotomy. Journal of Neurosurgery, 19(2), 89–100. 10.3171/jns.1962.19.2.0089 [DOI] [PubMed] [Google Scholar]
- Fox RJ, & Sorenson CA (1994). Bilateral lesions of the amygdala attenuate analgesia induced by diverse environmental challenges. Brain Research, 648(2), 215–221. 10.1016/0006-8993(94)91120-7 [DOI] [PubMed] [Google Scholar]
- Fuchs PN, Peng YB, Boyette-Davis JA, & Uhelski ML (2014). The anterior cingulate cortex and pain processing. Frontiers in Integrative Neuroscience, 8, 35. 10.3389/fnint.2014.00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudge JL, Kelly EA, Pal R, Bedont JL, Park L, & Ho B (2017). Beyond the Classic VTA: Extended Amygdala Projections to DA-Striatal Paths in the Primate. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 42(8), 1563–1576. 10.1038/npp.2017.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo MS, Karas AZ, Pritchett-Corning K, Garner Guy Mulder JP, & Gaskill BN (2020). Tell-tale TINT: Does the Time to Incorporate into Nest Test Evaluate Postsurgical Pain or Welfare in Mice? Journal of the American Association for Laboratory Animal Science: JAALAS, 59(1), 37–45. 10.30802/AALAS-JAALAS-19-000044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y-J, Ren W-H, Zhang Y-Q, & Zhao Z-Q (2004). Contributions of the anterior cingulate cortex and amygdala to pain- and fear-conditioned place avoidance in rats. Pain, 110(1), 343–353. 10.1016/j.pain.2004.04.030 [DOI] [PubMed] [Google Scholar]
- Garner JP (2014). The significance of meaning: Why do over 90% of behavioral neuroscience results fail to translate to humans, and what can we do to fix it? ILAR Journal, 55(3), 438–456. 10.1093/ilar/ilu047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauriau C, & Bernard J-F (2002). Pain pathways and parabrachial circuits in the rat. Experimental Physiology, 87(2), 251–258. 10.1113/eph8702357 [DOI] [PubMed] [Google Scholar]
- Geisler S, Derst C, Veh RW, & Zahm DS (2007). Glutamatergic afferents of the ventral tegmental area in the rat. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 27(21), 5730–5743. 10.1523/JNEUROSCI.0012-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelonch O, Garolera M, Valls J, Rosselló L, & Pifarré J (2017). Cognitive complaints in women with fibromyalgia: Are they due to depression or to objective cognitive dysfunction? Journal of Clinical and Experimental Neuropsychology, 39(10), 1013–1025. 10.1080/13803395.2017.1301391 [DOI] [PubMed] [Google Scholar]
- George MS, Ketter TA, Parekh PI, Rosinsky N, Ring H, Casey BJ, Trimble MR, Horwitz B, Herscovitch P, & Post RM (1994). Regional brain activity when selecting a response despite interference: An H2 (15) O PET study of the stroop and an emotional stroop. Human Brain Mapping, 1(3), 194–209. 10.1002/hbm.460010305 [DOI] [PubMed] [Google Scholar]
- Gilam G, Gross JJ, Wager TD, Keefe FJ, & Mackey SC (2020). What Is the Relationship between Pain and Emotion? Bridging Constructs and Communities. Neuron, 0(0). 10.1016/j.neuron.2020.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomtsian L, Bannister K, Eyde N, Robles D, Dickenson AH, Porreca F, & Navratilova E (2018). Morphine effects within the rodent anterior cingulate cortex and rostral ventromedial medulla reveal separable modulation of affective and sensory qualities of acute or chronic pain. Pain, 159(12), 2512–2521. 10.1097/j.pain.0000000000001355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore F, Schwartz EC, Brangers BC, Aladi S, Stujenske JM, Likhtik E, Russo MJ, Gordon JA, Salzman CD, & Axel R (2015). Neural Representations of Unconditioned Stimuli in Basolateral Amygdala Mediate Innate and Learned Responses. Cell, 162(1), 134–145. 10.1016/j.cell.2015.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewe BF, Gründemann J, Kitch LJ, Lecoq JA, Parker JG, Marshall JD, Larkin MC, Jercog PE, Grenier F, Li JZ, Lüthi A, & Schnitzer MJ (2017). Neural ensemble dynamics underlying a long-term associative memory. Nature, 543(7647), 670–675. 10.1038/nature21682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Jian-You, Yuan X-Y, Sui F, Zhang W-C., Wang J-Y, Luo F, & Luo J (2011). Placebo analgesia affects the behavioral despair tests and hormonal secretions in mice. Psychopharmacology, 217(1), 83–90. 10.1007/s00213-011-2259-7 [DOI] [PubMed] [Google Scholar]
- Guo J-Y, Wang J-Y, & Luo F (2010). Dissection of placebo analgesia in mice: The conditions for activation of opioid and non-opioid systems. Journal of Psychopharmacology (Oxford, England), 24(10), 1561–1567. 10.1177/0269881109104848 [DOI] [PubMed] [Google Scholar]
- Hall J, Parkinson JA, Connor TM, Dickinson A, & Everitt BJ (2001). Involvement of the central nucleus of the amygdala and nucleus accumbens core in mediating Pavlovian influences on instrumental behaviour. The European Journal of Neuroscience, 13(10), 1984–1992. 10.1046/j.0953-816x.2001.01577.x [DOI] [PubMed] [Google Scholar]
- Han S, Soleiman MT, Soden ME, Zweifel LS, & Palmiter RD (2015). Elucidating an Affective Pain Circuit that Creates a Threat Memory. Cell, 162(2), 363–374. 10.1016/j.cell.2015.05.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney RZ, Calu DJ, Takahashi YK, Hughes BW, & Schoenbaum G (2010). Inactivation of the central but not the basolateral nucleus of the amygdala disrupts learning in response to overexpectation of reward. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 30(8), 2911–2917. 10.1523/JNEUROSCI.0054-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashmi JA, Baliki MN, Huang L, Baria AT, Torbey S, Hermann KM, Schnitzer TJ, & Apkarian AV (2013). Shape shifting pain: Chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain, 136(9), 2751–2768. 10.1093/brain/awt211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashmi JA, Baliki MN, Huang L, Parks EL, Chanda ML, Schnitzer T, & Apkarian AV (2012). Lidocaine patch (5%) is no more potent than placebo in treating chronic back pain when tested in a randomised double blind placebo controlled brain imaging study. Molecular Pain, 8, 29. 10.1186/1744-8069-8-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassenbusch SJ, Pillay PK, & Barnett GH (1990). Radiofrequency Cingulotomy for Intractable Cancer Pain Using Stereotaxis Guided by Magnetic Resonance Imaging. Neurosurgery, 27(2), 220–223. 10.1227/00006123-199008000-00008 [DOI] [PubMed] [Google Scholar]
- Hawranko AA, Monroe PJ, & Smith DJ (1994). Repetitive exposure to the hot-plate test produces stress induced analgesia and alters beta-endorphin neuronal transmission within the periaqueductal gray of the rat. Brain Research, 667(2), 283–286. 10.1016/0006-8993(94)91508-3 [DOI] [PubMed] [Google Scholar]
- Hebben N, Corkin S, Eichenbaum H, & Shedlack K (1985). Diminished ability to interpret and report internal states after bilateral medial temporal resection: Case H.M. Behavioral Neuroscience, 99(6), 1031–1039. 10.1037/0735-7044.99.6.1031 [DOI] [PubMed] [Google Scholar]
- Heilbronner SR, & Hayden BY (2016). Dorsal Anterior Cingulate Cortex: A Bottom-Up View. Annual Review of Neuroscience, 39(1), 149–170. 10.1146/annurev-neuro-070815-013952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronner SR, Rodriguez-Romaguera J, Quirk GJ, Groenewegen HJ, & Haber SN (2016). Circuit-Based Corticostriatal Homologies Between Rat and Primate. Biological Psychiatry, 80(7), 509–521. 10.1016/j.biopsych.2016.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornak J, Bramham J, Rolls ET, Morris RG, O’Doherty J, Bullock PR, & Polkey CE (2003). Changes in emotion after circumscribed surgical lesions of the orbitofrontal and cingulate cortices. Brain: A Journal of Neurology, 126(Pt 7), 1691–1712. 10.1093/brain/awg168 [DOI] [PubMed] [Google Scholar]
- Hosobuchi Y, Adams JE, & Linchitz R (1977). Pain relief by electrical stimulation of the central gray matter in humans and its reversal by naloxone. Science (New York, N.Y.), 197(4299), 183–186. 10.1126/science.301658 [DOI] [PubMed] [Google Scholar]
- Hsieh JC, Stone-Elander S, & Ingvar M (1999). Anticipatory coping of pain expressed in the human anterior cingulate cortex: A positron emission tomography study. Neuroscience Letters, 262(1), 61–64. 10.1016/s0304-3940(99)00060-9 [DOI] [PubMed] [Google Scholar]
- Hsu AI, & Yttri EA (2019). B-SOiD: An Open Source Unsupervised Algorithm for Discovery of Spontaneous Behaviors [Preprint]. Neuroscience. 10.1101/770271 [DOI] [Google Scholar]
- Hu T-T, Wang R-R, Du Y, Guo F, Wu Y-X, Wang Y, Wang S, Li X-Y, Zhang S-H, & Chen Z (2019). Activation of the Intrinsic Pain Inhibitory Circuit from the Midcingulate Cg2 to Zona Incerta Alleviates Neuropathic Pain. The Journal of Neuroscience, 39(46), 9130–9144. 10.1523/JNEUROSCI.1683-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua T, Chen B, Lu D, Sakurai K, Zhao S, Han B-X, Kim J, Yin L, Chen Y, Lu J, & Wang F (2020). General anesthetics activate a potent central pain-suppression circuit in the amygdala. Nature Neuroscience. 10.1038/s41593-020-0632-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Gadotti VM, Chen L, Souza IA, Huang S, Wang D, Ramakrishnan C, Deisseroth K, Zhang Z, & Zamponi GW (2019). A neuronal circuit for activating descending modulation of neuropathic pain. Nature Neuroscience, 22(10), 1659–1668. 10.1038/s41593-019-0481-5 [DOI] [PubMed] [Google Scholar]
- Huang X, Tang JS, Yuan B, & Jia H (2001). Morphine applied to the ventrolateral orbital cortex produces a naloxone-reversible antinociception in the rat. Neuroscience Letters, 299(3), 189–192. 10.1016/s0304-3940(01)01497-5 [DOI] [PubMed] [Google Scholar]
- Hughes J, Smith TW, Kosterlitz HW, Fothergill LA, Morgan BA, & Morris HR (1975). Identification of two related pentapeptides from the brain with potent opiate agonist activity. Nature, 258(5536), 577–580. 10.1038/258577a0 [DOI] [PubMed] [Google Scholar]
- Hutchison WD, Davis KD, Lozano AM, Tasker RR, & Dostrovsky JO (1999). Pain-related neurons in the human cingulate cortex. Nature Neuroscience, 2(5), 403–405. 10.1038/8065 [DOI] [PubMed] [Google Scholar]
- Hyman JM, Holroyd CB, & Seamans JK (2017). A Novel Neural Prediction Error Found in Anterior Cingulate Cortex Ensembles. Neuron, 95(2), 447–456.e3. 10.1016/j.neuron.2017.06.021 [DOI] [PubMed] [Google Scholar]
- Iordanova MD, Deroche MLD, Esber GR, & Schoenbaum G (2016). Neural correlates of two different types of extinction learning in the amygdala central nucleus. Nature Communications, 7, 12330. 10.1038/ncomms12330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata K, Miyachi S, Imanishi M, Tsuboi Y, Kitagawa J, Teramoto K, Hitomi S, Shinoda M, Kondo M, & Takada M (2011). Ascending multisynaptic pathways from the trigeminal ganglion to the anterior cingulate cortex. Experimental Neurology, 227(1), 69–78. 10.1016/j.expneurol.2010.09.013 [DOI] [PubMed] [Google Scholar]
- Janak PH, & Tye KM (2015). From circuits to behaviour in the amygdala. Nature, 517(7534), 284–292. 10.1038/nature14188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean-Richard-Dit-Bressel P, Killcross S, & McNally GP (2018). Behavioral and neurobiological mechanisms of punishment: Implications for psychiatric disorders. Neuropsychopharmacology, 43(8), 1639–1650. 10.1038/s41386-018-0047-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G, & Neugebauer V (2011). Pain-related deactivation of medial prefrontal cortical neurons involves mGluR1 and GABA(A) receptors. Journal of Neurophysiology, 106(5), 2642–2652. 10.1152/jn.00461.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G, Sun H, Fu Y, Li Z, Pais-Vieira M, Galhardo V, & Neugebauer V (2010). Cognitive impairment in pain through amygdala-driven prefrontal cortical deactivation. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 30(15), 5451–5464. 10.1523/JNEUROSCI.0225-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Wang X, Le Q, Liu P, Liu C, Wang Z, He G, Zheng P, Wang F, & Ma L (2019). Morphine coordinates SST and PV interneurons in the prelimbic cortex to disinhibit pyramidal neurons and enhance reward. Molecular Psychiatry. 10.1038/s41380-019-0480-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirkof P, Fleischmann T, Cesarovic N, Rettich A, Vogel J, & Arras M (2013). Assessment of postsurgical distress and pain in laboratory mice by nest complexity scoring. Laboratory Animals, 47(3), 153–161. 10.1177/0023677213475603 [DOI] [PubMed] [Google Scholar]
- Johansen JP, & Fields HL (2004). Glutamatergic activation of anterior cingulate cortex produces an aversive teaching signal. Nature Neuroscience, 7(4), 398–403. 10.1038/nn1207 [DOI] [PubMed] [Google Scholar]
- Johansen JP, Fields HL, & Manning BH (2001). The affective component of pain in rodents: Direct evidence for a contribution of the anterior cingulate cortex. Proceedings of the National Academy of Sciences, 98(14), 8077–8082. 10.1073/pnas.141218998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JP, Tarpley JW, LeDoux JE, & Blair HT (2010). Neural substrates for expectation-modulated fear learning in the amygdala and periaqueductal gray. Nature Neuroscience, 13(8), 979–986. 10.1038/nn.2594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AF, & Sheets PL (2020). Sex-Specific Disruption of Distinct mPFC Inhibitory Neurons in Spared-Nerve Injury Model of Neuropathic Pain. Cell Reports, 31(10), 107729. 10.1016/j.celrep.2020.107729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AK, Qi LY, Fujirawa T, Luthra SK, Ashburner J, Bloomfield P, Cunningham VJ, Itoh M, Fukuda H, & Jones T (1991). In vivo distribution of opioid receptors in man in relation to the cortical projections of the medial and lateral pain systems measured with positron emission tomography. Neuroscience Letters, 126(1), 25–28. 10.1016/0304-3940(91)90362-w [DOI] [PubMed] [Google Scholar]
- Jones J, Foster W, Twomey C, Burdge J, Ahmed O, Wojick JA, Corder G, Plotkin JB, & Abdus-Saboor I (2020). A machine-vision approach for automated pain measurement at millisecond timescales [Preprint]. Neuroscience. 10.1101/2020.02.18.955070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurik A, Auffenberg E, Klein S, Deussing JM, Schmid RM, Wotjak CT, & Thoeringer CK (2015). Roles of prefrontal cortex and paraventricular thalamus in affective and mechanical components of visceral nociception. Pain, 156(12), 2479–2491. 10.1097/j.pain.0000000000000318 [DOI] [PubMed] [Google Scholar]
- Kandasamy R, Lee AT, & Morgan MM (2017). Depression of home cage wheel running: A reliable and clinically relevant method to assess migraine pain in rats. The Journal of Headache and Pain, 18(1), 5. 10.1186/s10194-017-0721-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keifer O, Kambara K, Lau A, Makinson S, & Bertrand D (2020). Chemogenetics a robust approach to pharmacology and gene therapy. Biochemical Pharmacology, 175, 113889. 10.1016/j.bcp.2020.113889 [DOI] [PubMed] [Google Scholar]
- Keller A, Akintola T, & Colloca L (2018a). Placebo Analgesia in Rodents: Current and Future Research. International Review of Neurobiology, 138, 1–15. 10.1016/bs.irn.2018.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A, Akintola T, & Colloca L (2018b). Placebo Analgesia in Rodents: Current and Future Research. International Review of Neurobiology, 138, 1–15. 10.1016/bs.irn.2018.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly CJ, Huang M, Meltzer H, & Martina M (2016). Reduced Glutamatergic Currents and Dendritic Branching of Layer 5 Pyramidal Cells Contribute to Medial Prefrontal Cortex Deactivation in a Rat Model of Neuropathic Pain. Frontiers in Cellular Neuroscience, 10, 133. 10.3389/fncel.2016.00133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RM, & Strick PL (2000). Rabies as a transneuronal tracer of circuits in the central nervous system. Journal of Neuroscience Methods, 103(1), 63–71. 10.1016/s0165-0270(00)00296-x [DOI] [PubMed] [Google Scholar]
- Kim CK, Adhikari A, & Deisseroth K (2017). Integration of optogenetics with complementary methodologies in systems neuroscience. Nature Reviews. Neuroscience, 18(4), 222–235. 10.1038/nrn.2017.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King HE, Clausen J, & Scarff JE (1950). Cutaneous thresholds for pain before and after unilateral prefrontal lobotomy; preliminary report. The Journal of Nervous and Mental Disease, 112(2), 93–96. [PubMed] [Google Scholar]
- King T, Vera-Portocarrero L, Gutierrez T, Vanderah TW, Dussor G, Lai J, Fields HL, & Porreca F (2009). Unmasking the tonic-aversive state in neuropathic pain. Nature Neuroscience, 12(11), 1364–1366. 10.1038/nn.2407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klawonn AM, & Malenka RC (2018). Nucleus Accumbens Modulation in Reward and Aversion. Cold Spring Harbor Symposia on Quantitative Biology, 83, 119–129. 10.1101/sqb.2018.83.037457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C (2004). What are the neuronal correlates of consciousness? In The Quest for Consciousness. Roberts and Company Publishers. [Google Scholar]
- Koga K, Descalzi G, Chen T, Ko H-G, Lu J, Li S, Son J, Kim T, Kwak C, Huganir RL, Zhao M-G, Kaang B-K, Collingridge GL, & Zhuo M (2015). Coexistence of two forms of LTP in ACC provides a synaptic mechanism for the interactions between anxiety and chronic pain. Neuron, 85(2), 377–389. 10.1016/j.neuron.2014.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga K, Yao I, Setou M, & Zhuo M (2017). SCRAPPER Selectively Contributes to Spontaneous Release and Presynaptic Long-Term Potentiation in the Anterior Cingulate Cortex. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 37(14), 3887–3895. 10.1523/JNEUROSCI.0023-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krank MD, Hinson RE, & Siegel S (1981). Conditional hyperalgesia is elicited by environmental signals of morphine. Behavioral and Neural Biology, 32(2), 148–157. 10.1016/s0163-1047(81)90411-8 [DOI] [PubMed] [Google Scholar]
- Kummer KK, Mitrić M, Kalpachidou T, & Kress M (2020). The Medial Prefrontal Cortex as a Central Hub for Mental Comorbidities Associated with Chronic Pain. International Journal of Molecular Sciences, 21(10). 10.3390/ijms21103440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung J-C, Su N-M, Fan R-J, Chai S-C, & Shyu B-C (2003). Contribution of the anterior cingulate cortex to laser-pain conditioning in rats. Brain Research, 970(1–2), 58–72. 10.1016/s0006-8993(02)04276-2 [DOI] [PubMed] [Google Scholar]
- Kupers RC, Konings H, Adriaensen H, & Gybels JM (1991). Morphine differentially affects the sensory and affective pain ratings in neurogenic and idiopathic forms of pain: Pain, 47(1), 5–12. 10.1016/0304-3959(91)90004-H [DOI] [PubMed] [Google Scholar]
- Kyriazi P, Headley DB, & Pare D (2018). Multi-dimensional Coding by Basolateral Amygdala Neurons. Neuron, 99(6), 1315–1328.e5. 10.1016/j.neuron.2018.07.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaGraize SC, Borzan J, Peng YB, & Fuchs PN (2006). Selective regulation of pain affect following activation of the opioid anterior cingulate cortex system. Experimental Neurology, 197(1), 22–30. 10.1016/j.expneurol.2005.05.008 [DOI] [PubMed] [Google Scholar]
- LaGraize SC, Labuda CJ, Rutledge MA, Jackson RL, & Fuchs PN (2004). Differential effect of anterior cingulate cortex lesion on mechanical hypersensitivity and escape/avoidance behavior in an animal model of neuropathic pain. Experimental Neurology, 188(1), 139–148. 10.1016/j.expneurol.2004.04.003 [DOI] [PubMed] [Google Scholar]
- Lamusuo S, Hirvonen J, Lindholm P, Martikainen IK, Hagelberg N, Parkkola R, Taiminen T, Hietala J, Helin S, Virtanen A, Pertovaara A, & Jääskeläinen SK (2017). Neurotransmitters behind pain relief with transcranial magnetic stimulation—Positron emission tomography evidence for release of endogenous opioids. European Journal of Pain (London, England), 21(9), 1505–1515. 10.1002/ejp.1052 [DOI] [PubMed] [Google Scholar]
- Laneri D, Krach S, Paulus FM, Kanske P, Schuster V, Sommer J, & Müller-Pinzler L (2017). Mindfulness meditation regulates anterior insula activity during empathy for social pain. Human Brain Mapping, 38(8), 4034–4046. 10.1002/hbm.23646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford DJ, Bailey AL, Chanda ML, Clarke SE, Drummond TE, Echols S, Glick S, Ingrao J, Klassen-Ross T, LaCroix-Fralish ML, Matsumiya L, Sorge RE, Sotocinal SG, Tabaka JM, Wong D, van den Maagdenberg AMJM, Ferrari MD, Craig KD, & Mogil JS (2010). Coding of facial expressions of pain in the laboratory mouse. Nature Methods, 7(6), 447–449. 10.1038/nmeth.1455 [DOI] [PubMed] [Google Scholar]
- Laska E, & Sunshine A (1973). Anticipation of analgesia. A placebo effect. Headache, 13(1), 1–11. 10.1111/j.1526-4610.1973.hed1301001.x [DOI] [PubMed] [Google Scholar]
- Laubach M, Amarante LM, Swanson K, & White SR (2018). What, If Anything, Is Rodent Prefrontal Cortex? ENeuro, 5(5). 10.1523/ENEURO.0315-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, & Reis DJ (1988). Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 8(7), 2517–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Wheeler DS, & Holland PC (2011). Interactions between amygdala central nucleus and the ventral tegmental area in the acquisition of conditioned cue-directed behavior in rats. The European Journal of Neuroscience, 33(10), 1876–1884. 10.1111/j.1460-9568.2011.07680.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I-S, Lee B, Park H-J, Olausson H, Enck P, & Chae Y (2015). A new animal model of placebo analgesia: Involvement of the dopaminergic system in reward learning. Scientific Reports, 5(1), 17140. 10.1038/srep17140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Marion, Silverman SM, Hansen H, Patel VB, & Manchikanti L (2011). A comprehensive review of opioid-induced hyperalgesia. Pain Physician, 14(2), 145–161. [PubMed] [Google Scholar]
- Lee Michelle, Manders TR., Eberle SE, Su C, D’amour J, Yang R, Lin HY, Deisseroth K, Froemke RC, & Wang J (2015). Activation of Corticostriatal Circuitry Relieves Chronic Neuropathic Pain. Journal of Neuroscience, 35(13), 5247–5259. 10.1523/JNEUROSCI.3494-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen L, Chen T-M, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, … Jones AR (2007). Genome-wide atlas of gene expression in the adult mouse brain. Nature, 445(7124), 168–176. 10.1038/nature05453 [DOI] [PubMed] [Google Scholar]
- Leipold E, Liebmann L, Korenke GC, Heinrich T, Gießelmann S, Baets J, Ebbinghaus M, Goral RO, Stödberg T, Hennings JC, Bergmann M, Altmüller J, Thiele H, Wetzel A, Nürnberg P, Timmerman V, De Jonghe P, Blum R, Schaible H-G, … Kurth I (2013). A de novo gain-of-function mutation in SCN11A causes loss of pain perception. Nature Genetics, 45(11), 1399–1404. 10.1038/ng.2767 [DOI] [PubMed] [Google Scholar]
- Lenz FA, Rios M, Zirh A, Chau D, Krauss G, & Lesser RP (1998). Painful Stimuli Evoke Potentials Recorded Over the Human Anterior Cingulate Gyrus. Journal of Neurophysiology, 79(4), 2231–2234. 10.1152/jn.1998.79.4.2231 [DOI] [PubMed] [Google Scholar]
- Levine JD, Gordon NC, & Fields HL (1978). The mechanism of placebo analgesia. Lancet (London, England), 2(8091), 654–657. 10.1016/s0140-6736(78)92762-9 [DOI] [PubMed] [Google Scholar]
- Li C, Sugam JA, Lowery-Gionta EG, McElligott ZA, McCall NM, Lopez AJ, McKlveen JM, Pleil KE, & Kash TL (2016). Mu Opioid Receptor Modulation of Dopamine Neurons in the Periaqueductal Gray/Dorsal Raphe: A Role in Regulation of Pain. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 41(8), 2122–2132. 10.1038/npp.2016.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J-N, & Sheets PL (2018). The central amygdala to periaqueductal gray pathway comprises intrinsically distinct neurons differentially affected in a model of inflammatory pain. The Journal of Physiology, 596(24), 6289–6305. 10.1113/JP276935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X-Y, Ko H-G, Chen T, Descalzi G, Koga K, Wang H, Kim SS, Shang Y, Kwak C, Park S-W, Shim J, Lee K, Collingridge GL, Kaang B-K, & Zhuo M (2010). Alleviating neuropathic pain hypersensitivity by inhibiting PKMzeta in the anterior cingulate cortex. Science (New York, N.Y.), 330(6009), 1400–1404. 10.1126/science.1191792 [DOI] [PubMed] [Google Scholar]
- Lui F, Colloca L, Duzzi D, Anchisi D, Benedetti F, & Porro CA (2010). Neural bases of conditioned placebo analgesia. Pain, 151(3), 816–824. 10.1016/j.pain.2010.09.021 [DOI] [PubMed] [Google Scholar]
- Mahler SV, & Berridge KC (2009). Which cue to “want?” Central amygdala opioid activation enhances and focuses incentive salience on a prepotent reward cue. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 29(20), 6500–6513. 10.1523/JNEUROSCI.3875-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mambretti EM, Kistner K, Mayer S, Massotte D, Kieffer BL, Hoffmann C, Reeh PW, Brack A, Asan E, & Rittner HL (2016). Functional and structural characterization of axonal opioid receptors as targets for analgesia. Molecular Pain, 12. 10.1177/1744806916628734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manglik A, Lin H, Aryal DK, McCorvy JD, Dengler D, Corder G, Levit A, Kling RC, Bernat V, Hübner H, Huang X-P, Sassano MF, Giguère PM, Löber S, Da Duan null, Scherrer G, Kobilka BK, Gmeiner P, Roth BL, & Shoichet BK. (2016). Structure-based discovery of opioid analgesics with reduced side effects. Nature, 537(7619), 185–190. 10.1038/nature19112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars RB, Sotiropoulos SN, Passingham RE, Sallet J, Verhagen L, Khrapitchev AA, Sibson N, & Jbabdi S (2018). Whole brain comparative anatomy using connectivity blueprints. ELife, 7. 10.7554/eLife.35237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaly N, Copits BA, Wilson-Poe AR, Hipólito L, Markovic T, Yoon HJ, Liu S, Walicki MC, Bhatti DL, Sirohi S, Klaas A, Walker BM, Neve R, Cahill CM, Shoghi KI, Gereau RW, McCall JG, Al-Hasani R, Bruchas MR, & Morón JA (2019). Pain-Induced Negative Affect Is Mediated via Recruitment of The Nucleus Accumbens Kappa Opioid System. Neuron, 102(3), 564–573.e6. 10.1016/j.neuron.2019.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaly N, Morón JA, & Al-Hasani R (2016). A Trigger for Opioid Misuse: Chronic Pain and Stress Dysregulate the Mesolimbic Pathway and Kappa Opioid System. Frontiers in Neuroscience, 10, 480. 10.3389/fnins.2016.00480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massart R, Dymov S, Millecamps M, Suderman M, Gregoire S, Koenigs K, Alvarado S, Tajerian M, Stone LS, & Szyf M (2016). Overlapping signatures of chronic pain in the DNA methylation landscape of prefrontal cortex and peripheral T cells. Scientific Reports, 6, 19615. 10.1038/srep19615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis A, Mamidanna P, Cury KM, Abe T, Murthy VN, Mathis MW, & Bethge M (2018). DeepLabCut: Markerless pose estimation of user-defined body parts with deep learning. Nature Neuroscience, 21(9), 1281–1289. 10.1038/s41593-018-0209-y [DOI] [PubMed] [Google Scholar]
- McNabb CT, White MM, Harris AL, & Fuchs PN (2014). The elusive rat model of conditioned placebo analgesia. Pain, 155(10), 2022–2032. 10.1016/j.pain.2014.07.004 [DOI] [PubMed] [Google Scholar]
- Meerwijk EL, Ford JM, & Weiss SJ (2013). Brain regions associated with psychological pain: Implications for a neural network and its relationship to physical pain. Brain Imaging and Behavior, 7(1), 1–14. 10.1007/s11682-012-9179-y [DOI] [PubMed] [Google Scholar]
- Melzack R, & Casey K (1968). Sensory,Motivational,and Central Control Determinants of Pain. In Kenshalo DR (Ed.), The Skin Senses. Charles C. Thomas. https://www.researchgate.net/publication/225039227_SensoryMotivationaland_Central_Control_Determinants_of_Pain [Google Scholar]
- Melzack R, & Wall PD (1965). Pain Mechanisms: A New Theory. Science, 150(3699), 971–978. 10.1126/science.150.3699.971 [DOI] [PubMed] [Google Scholar]
- Merskey H, Albe Fessard D, Bonica JJ, Carmon A, Dubner R, Kerr FWL, Lindblom U, Mumford JM, Nathan PW, Noordenbos W, Pagni CA, Renaer MJ, Sterbach RA, & Sunderland S. (1979). Pain terms: A list with definitions and notes on usage. Recommended by the IASP Subcommittee on Taxonomy. Pain, 6(3), 249. [PubMed] [Google Scholar]
- Metz AE, Yau H-J, Centeno MV, Apkarian AV, & Martina M (2009). Morphological and functional reorganization of rat medial prefrontal cortex in neuropathic pain. Proceedings of the National Academy of Sciences of the United States of America, 106(7), 2423–2428. 10.1073/pnas.0809897106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ (1999). The induction of pain: An integrative review. Progress in Neurobiology, 57(1), 1–164. 10.1016/S0301-0082(98)00048-3 [DOI] [PubMed] [Google Scholar]
- Mogil JS (2009). Animal models of pain: Progress and challenges. Nature Reviews. Neuroscience, 10(4), 283–294. 10.1038/nrn2606 [DOI] [PubMed] [Google Scholar]
- Mogil JS (2019). The Measurement of Pain in the Laboratory Rodent. In Wood JN (Ed.), The Oxford Handbook of the Neurobiology of Pain. Oxford University Press. 10.1093/oxfordhb/9780190860509.013.21 [DOI] [Google Scholar]
- Mogil JS, Pang DSJ, Silva Dutra GG, & Chambers CT (2020). The development and use of facial grimace scales for pain measurement in animals. Neuroscience and Biobehavioral Reviews, 116, 480–493. 10.1016/j.neubiorev.2020.07.013 [DOI] [PubMed] [Google Scholar]
- Monosov IE (2017). Anterior cingulate is a source of valence-specific information about value and uncertainty. Nature Communications, 8(1), 134. 10.1038/s41467-017-00072-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty O, McGuire BE, & Finn DP (2011). The effect of pain on cognitive function: A review of clinical and preclinical research. Progress in Neurobiology, 93(3), 385–404. 10.1016/j.pneurobio.2011.01.002 [DOI] [PubMed] [Google Scholar]
- Moriarty O, Ruane N, O’Gorman D, Maharaj CH, Mitchell C, Sarma KM, Finn DP, & McGuire BE (2017). Cognitive Impairment in Patients with Chronic Neuropathic or Radicular Pain: An Interaction of Pain and Age. Frontiers in Behavioral Neuroscience, 11, 100. 10.3389/fnbeh.2017.00100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KL, Bethea JR, & Fischer R (2017). Neuropathic Pain in Multiple Sclerosis—Current Therapeutic Intervention and Future Treatment Perspectives. In Zagon IS & McLaughlin PJ (Eds.), Multiple Sclerosis: Perspectives in Treatment and Pathogenesis. Codon Publications. http://www.ncbi.nlm.nih.gov/books/NBK470151/ [PubMed] [Google Scholar]
- National Institute of Heath. (2019). The Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Initiative 2.0: From cells to circuits, toward cures. https://braininitiative.nih.gov/strategic-planning/acd-working-groups/brain-initiative-20-cells-circuits-toward-cures
- Navratilova E, Xie JY, Meske D, Qu C, Morimura K, Okun A, Arakawa N, Ossipov M, Fields HL, & Porreca F (2015). Endogenous Opioid Activity in the Anterior Cingulate Cortex Is Required for Relief of Pain. Journal of Neuroscience, 35(18), 7264–7271. 10.1523/JNEUROSCI.3862-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navratilova Edita, Ji G, Phelps C, Qu C, Hein M, Yakhnitsa V, Neugebauer V, & Porreca F (2019). Kappa opioid signaling in the central nucleus of the amygdala promotes disinhibition and aversiveness of chronic neuropathic pain: PAIN, 160(4), 824–832. 10.1097/j.pain.0000000000001458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navratilova Edita, Nation K, Remeniuk B, Neugebauer V, Bannister K., Dickenson AH, & Porreca F (2020). Selective modulation of tonic aversive qualities of neuropathic pain by morphine in the central nucleus of the amygdala requires endogenous opioid signaling in the anterior cingulate cortex. Pain, 161(3), 609–618. 10.1097/j.pain.0000000000001748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navratilova Edita, Xie JY, Meske D, Qu C, Morimura K, Okun A, Arakawa N, Ossipov M, Fields HL, & Porreca F (2015). Endogenous Opioid Activity in the Anterior Cingulate Cortex Is Required for Relief of Pain. Journal of Neuroscience, 35(18), 7264–7271. 10.1523/JNEUROSCI.3862-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navratilova Edita, Xie JY, Okun A, Qu C, Eyde N, Ci S, Ossipov MH, King T, Fields HL, & Porreca F (2012). Pain relief produces negative reinforcement through activation of mesolimbic reward-valuation circuitry. Proceedings of the National Academy of Sciences of the United States of America, 109(50), 20709–20713. 10.1073/pnas.1214605109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Bilsky EJ, Do Carmo GP, & Stevenson GW (2010). Rationale and methods for assessment of pain-depressed behavior in preclinical assays of pain and analgesia. Methods in Molecular Biology (Clifton, N.J.), 617, 79–91. 10.1007/978-1-60327-323-7_7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V (2015). Amygdala pain mechanisms. Handbook of Experimental Pharmacology, 227, 261–284. 10.1007/978-3-662-46450-2_13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V, & Li W (2003). Differential sensitization of amygdala neurons to afferent inputs in a model of arthritic pain. Journal of Neurophysiology, 89(2), 716–727. 10.1152/jn.00799.2002. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Mazzitelli M, Cragg B, Ji G, Navratilova E, & Porreca F (2020). Amygdala, neuropeptides, and chronic pain-related affective behaviors. Neuropharmacology, 170, 108052. 10.1016/j.neuropharm.2020.108052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntamati NR, Creed M, Achargui R, & Lüscher C (2018). Periaqueductal efferents to dopamine and GABA neurons of the VTA. PloS One, 13(1), e0190297. 10.1371/journal.pone.0190297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, & Andrews C (2001). Abstract reward and punishment representations in the human orbitofrontal cortex. Nature Neuroscience, 4(1), 95–102. 10.1038/82959 [DOI] [PubMed] [Google Scholar]
- Oliveira MA, & Prado WA (2001). Role of PAG in the antinociception evoked from the medial or central amygdala in rats. Brain Research Bulletin, 54(1), 55–63. 10.1016/s0361-9230(00)00420-2 [DOI] [PubMed] [Google Scholar]
- Omelchenko N, & Sesack SR (2010). Periaqueductal gray afferents synapse onto dopamine and GABA neurons in the rat ventral tegmental area. Journal of Neuroscience Research, 88(5), 981–991. 10.1002/jnr.22265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong W-Y, Stohler CS, & Herr DR (2019). Role of the Prefrontal Cortex in Pain Processing. Molecular Neurobiology, 56(2), 1137–1166. 10.1007/s12035-018-1130-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoda K, Okamoto Y, Nakashima K, Nittono H, Ura M, & Yamawaki S (2009). Decreased ventral anterior cingulate cortex activity is associated with reduced social pain during emotional support. Social Neuroscience, 4(5), 443–454. 10.1080/17470910902955884 [DOI] [PubMed] [Google Scholar]
- Ortiz C, Navarro JF, Jurek A, Märtin A, Lundeberg J, & Meletis K (2020). Molecular atlas of the adult mouse brain. Science Advances, 6(26), eabb3446. 10.1126/sciadv.abb3446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshiro Y, Quevedo AS, McHaffie JG, Kraft RA, & Coghill RC (2009). Brain mechanisms supporting discrimination of sensory features of pain: A new model. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 29(47), 14924–14931. 10.1523/JNEUROSCI.5538-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa T, Ycu EA, Kumar A, Yeh L-F, Ahmed T, Koivumaa J, & Johansen JP (2017). A feedback neural circuit for calibrating aversive memory strength. Nature Neuroscience, 20(1), 90–97. 10.1038/nn.4439 [DOI] [PubMed] [Google Scholar]
- Palmiter RD (2018). The Parabrachial Nucleus: CGRP Neurons Function as a General Alarm. Trends in Neurosciences, 41(5), 280–293. 10.1016/j.tins.2018.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paretkar T, & Dimitrov E (2019). Activation of enkephalinergic (Enk) interneurons in the central amygdala (CeA) buffers the behavioral effects of persistent pain. Neurobiology of Disease, 124, 364–372. 10.1016/j.nbd.2018.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastoriza LN, Morrow TJ, & Casey KL (1996). Medial frontal cortex lesions selectively attenuate the hot plate response: Possible nocifensive apraxia in the rat. Pain, 64(1), 11–17. 10.1016/0304-3959(95)00070-4 [DOI] [PubMed] [Google Scholar]
- Peciña M, Stohler CS, & Zubieta J-K (2014). Neurobiology of placebo effects: Expectations or learning? Social Cognitive and Affective Neuroscience, 9(7), 1013–1021. 10.1093/scan/nst079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecina M, & Zubieta J-K (2018). Expectancy Modulation of Opioid Neurotransmission. International Review of Neurobiology, 138, 17–37. 10.1016/bs.irn.2018.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peciña S, & Berridge KC (2013). Dopamine or opioid stimulation of nucleus accumbens similarly amplify cue-triggered “wanting” for reward: Entire core and medial shell mapped as substrates for PIT enhancement. The European Journal of Neuroscience, 37(9), 1529–1540. 10.1111/ejn.12174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peciña S, Smith KS, & Berridge KC (2006). Hedonic hot spots in the brain. The Neuroscientist: A Review Journal Bringing Neurobiology, Neurology and Psychiatry, 12(6), 500–511. 10.1177/1073858406293154 [DOI] [PubMed] [Google Scholar]
- Pereira TD, Aldarondo DE, Willmore L, Kislin M, Wang SS-H, Murthy M, & Shaevitz JW (2019). Fast animal pose estimation using deep neural networks. Nature Methods, 16(1), 117–125. 10.1038/s41592-018-0234-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignatelli M, & Bonci A (2018). Spiraling Connectivity of NAc-VTA Circuitry. Neuron, 97(2), 261–262. 10.1016/j.neuron.2017.12.046 [DOI] [PubMed] [Google Scholar]
- Porro CA, Baraldi P, Pagnoni G, Serafini M, Facchin P, Maieron M, & Nichelli P (2002). Does anticipation of pain affect cortical nociceptive systems? The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 22(8), 3206–3214. https://doi.org/20026310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu C, King T, Okun A, Lai J, Fields HL, & Porreca F (2011). Lesion of the Rostral Anterior Cingulate Cortex Eliminates the Aversiveness of Spontaneous Neuropathic Pain Following Partial or Complete Axotomy. Pain, 152(7), 1641–1648. 10.1016/j.pain.2011.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu C-L, Tang J-S, & Jia H (2006). Involvement of GABAergic modulation of antinociception induced by morphine microinjected into the ventrolateral orbital cortex. Brain Research, 1073–1074, 281–289. 10.1016/j.brainres.2005.12.067 [DOI] [PubMed] [Google Scholar]
- Rainville P, Duncan GH, Price DD, Carrier B, & Bushnell MC (1997). Pain Affect Encoded in Human Anterior Cingulate But Not Somatosensory Cortex. Science, 277(5328), 968–971. 10.1126/science.277.5328.968 [DOI] [PubMed] [Google Scholar]
- Ramirez F, Moscarello JM, LeDoux JE, & Sears RM (2015). Active avoidance requires a serial basal amygdala to nucleus accumbens shell circuit. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 35(8), 3470–3477. 10.1523/JNEUROSCI.1331-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Maestre C, Esteve R, Ruiz-Párraga G, Gómez-Pérez L, & López-Martínez AE (2017). The Key Role of Pain Catastrophizing in the Disability of Patients with Acute Back Pain. International Journal of Behavioral Medicine, 24(2), 239–248. 10.1007/s12529-016-9600-9 [DOI] [PubMed] [Google Scholar]
- Rescola R (1968). Pavlovian conditioned fear in Sidman avoidance learning. J Comp Physiol Psychol, 65(1), 55–60. [DOI] [PubMed] [Google Scholar]
- Rescola R, & Lolordo V (1965). Inhibition of avoidance behavior. J Comp Physiol Psychol, 59, 406–412. [DOI] [PubMed] [Google Scholar]
- Richard JM, Castro DC, Difeliceantonio AG, Robinson MJF, & Berridge KC (2013). Mapping brain circuits of reward and motivation: In the footsteps of Ann Kelley. Neuroscience and Biobehavioral Reviews, 37(9 Pt A), 1919–1931. 10.1016/j.neubiorev.2012.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Hornak J, Wade D, & McGrath J (1994). Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. Journal of Neurology, Neurosurgery, and Psychiatry, 57(12), 1518–1524. 10.1136/jnnp.57.12.1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE, & Woolsey CN (1948). Structure and relations of limbic cortex and anterior thalamic nuclei in rabbit and cat. The Journal of Comparative Neurology, 89(3), 279–347. 10.1002/cne.900890307 [DOI] [PubMed] [Google Scholar]
- Rudebeck PH, Rich EL, & Mayberg HS (2019). From bed to bench side: Reverse translation to optimize neuromodulation for mood disorders. Proceedings of the National Academy of Sciences, 116(52), 26288. 10.1073/pnas.1902287116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santello M, & Nevian T (2015). Dysfunction of cortical dendritic integration in neuropathic pain reversed by serotoninergic neuromodulation. Neuron, 86(1), 233–246. 10.1016/j.neuron.2015.03.003 [DOI] [PubMed] [Google Scholar]
- Santo JL, Arias LM, Barolat G, Schwartzman RJ, & Grossman K (1990). Bilateral cingulumotomy in the treatment of reflex sympathetic dystrophy. Pain, 41(1), 55–59. 10.1016/0304-3959(90)91109-V [DOI] [PubMed] [Google Scholar]
- Schaeffer DJ, Hori Y, Gilbert KM, Gati JS, Menon RS, & Everling S (2020). Divergence of rodent and primate medial frontal cortex functional connectivity. Proceedings of the National Academy of Sciences. 10.1073/pnas.2003181117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitter AM (2010). 17th and 18th Century Theories of Emotions. In Zalta EN (Ed.), The Stanford Encyclopedia of Philosophy (Winter 2016). https://plato.stanford.edu/entries/emotions-17th18th/LD1Background.html [Google Scholar]
- Schultz W, & Dickinson A (2000). Neuronal coding of prediction errors. Annual Review of Neuroscience, 23, 473–500. 10.1146/annurev.neuro.23.1.473 [DOI] [PubMed] [Google Scholar]
- Schwartz N, Temkin P, Jurado S, Lim BK, Heifets BD, Polepalli JS, & Malenka RC (2014). Decreased motivation during chronic pain requires long-term depression in the nucleus accumbens. Science (New York, N.Y.), 345(6196), 535–542. 10.1126/science.1253994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, & Zubieta J-K (2008). Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Archives of General Psychiatry, 65(2), 220–231. 10.1001/archgenpsychiatry.2007.34 [DOI] [PubMed] [Google Scholar]
- Sellmeijer J, Mathis V, Hugel S, Li X-H, Song Q, Chen Q-Y, Barthas F, Lutz P-E, Karatas M, Luthi A, Veinante P, Aertsen A, Barrot M, Zhuo M, & Yalcin I (2018). Hyperactivity of Anterior Cingulate Cortex Areas 24a/24b Drives Chronic Pain-Induced Anxiodepressive-like Consequences. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 38(12), 3102–3115. 10.1523/JNEUROSCI.3195-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminowicz DA, & Moayedi M (2017). The Dorsolateral Prefrontal Cortex in Acute and Chronic Pain. The Journal of Pain: Official Journal of the American Pain Society, 18(9), 1027–1035. 10.1016/j.jpain.2017.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severeijns R, Vlaeyen JWS, van den Hout MA, & Weber WEJ (2001). Pain Catastrophizing Predicts Pain Intensity, Disability, and Psychological Distress Independent of the Level of Physical Impairment: The Clinical Journal of Pain, 17(2), 165–172. 10.1097/00002508-200106000-00009 [DOI] [PubMed] [Google Scholar]
- Shiers S, Pradhan G, Mwirigi J, Mejia G, Ahmad A, Kroener S, & Price T (2018). Neuropathic Pain Creates an Enduring Prefrontal Cortex Dysfunction Corrected by the Type II Diabetic Drug Metformin But Not by Gabapentin. Journal of Neuroscience, 38(33), 7337–7350. 10.1523/JNEUROSCI.0713-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simantov R, Kuhar MJ, Uhl GR, & Snyder SH (1977). Opioid peptide enkephalin: Immunohistochemical mapping in rat central nervous system. Proceedings of the National Academy of Sciences of the United States of America, 74(5), 2167–2171. 10.1073/pnas.74.5.2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, & Frith CD (2004). Empathy for pain involves the affective but not sensory components of pain. Science (New York, N.Y.), 303(5661), 1157–1162. 10.1126/science.1093535 [DOI] [PubMed] [Google Scholar]
- Siuda ER, Copits BA, Schmidt MJ, Baird MA, Al-Hasani R, Planer WJ, Funderburk SC, McCall JG, Gereau RW, & Bruchas MR (2015). Spatiotemporal control of opioid signaling and behavior. Neuron, 86(4), 923–935. 10.1016/j.neuron.2015.03.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner B (1938). The behavior of organisms: An experimental analysis. Appleton-CenturyCrofts, New York. [Google Scholar]
- Smeets RJEM, Vlaeyen JWS, Kester ADM, & Knottnerus JA (2006). Reduction of Pain Catastrophizing Mediates the Outcome of Both Physical and Cognitive-Behavioral Treatment in Chronic Low Back Pain. The Journal of Pain, 7(4), 261–271. 10.1016/j.jpain.2005.10.011 [DOI] [PubMed] [Google Scholar]
- Sotres-Bayón F, Torres-López E, López-Avila A, del Angel R, & Pellicer F (2001). Lesion and electrical stimulation of the ventral tegmental area modify persistent nociceptive behavior in the rat. Brain Research, 898(2), 342–349. 10.1016/s0006-8993(01)02213-2 [DOI] [PubMed] [Google Scholar]
- Spinhoven P, Kuile M, Kole-Snijders AMJ, Mansfeld MH, Ouden D-J, & Vlaeyen JWS (2004). Catastrophizing and internal pain control as mediators of outcome in the multidisciplinary treatment of chronic low back pain. European Journal of Pain, 8(3), 211–219. 10.1016/j.ejpain.2003.08.003 [DOI] [PubMed] [Google Scholar]
- Sprenger C, Finsterbusch J, & Büchel C (2015). Spinal cord-midbrain functional connectivity is related to perceived pain intensity: A combined spino-cortical FMRI study. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 35(10), 4248–4257. 10.1523/JNEUROSCI.4897-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Laurent R, Martinez Damonte V, Tsuda AC, & Kauer JA (2020). Periaqueductal Gray and Rostromedial Tegmental Inhibitory Afferents to VTA Have Distinct Synaptic Plasticity and Opiate Sensitivity. Neuron, 106(4), 624–636.e4. 10.1016/j.neuron.2020.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson GW, Mercer H, Cormier J, Dunbar C, Benoit L, Adams C, Jezierski J, Luginbuhl A, & Bilsky EJ (2011). Monosodium iodoacetate-induced osteoarthritis produces pain-depressed wheel running in rats: Implications for preclinical behavioral assessment of chronic pain. Pharmacology, Biochemistry, and Behavior, 98(1), 35–42. 10.1016/j.pbb.2010.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolyarova A, Rakhshan M, Hart EE, O’Dell TJ, Peters M. a. K., Lau H, Soltani A, & Izquierdo A (2019). Contributions of anterior cingulate cortex and basolateral amygdala to decision confidence and learning under uncertainty. Nature Communications, 10(1), 4704. 10.1038/s41467-019-12725-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suckow SK, Deichsel EL, Ingram SL, Morgan MM, & Aicher SA (2013). Columnar distribution of catecholaminergic neurons in the ventrolateral periaqueductal gray and their relationship to efferent pathways. Synapse (New York, N.Y.), 67(2), 94–108. 10.1002/syn.21624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sufka KJ (1994). Conditioned place preference paradigm: A novel approach for analgesic drug assessment against chronic pain: Pain, 58(3), 355–366. 10.1016/0304-3959(94)90130-9 [DOI] [PubMed] [Google Scholar]
- Sullivan MD, Cahana A, Derbyshire S, & Loeser JD (2013). What Does It Mean to Call Chronic Pain a Brain Disease? The Journal of Pain, 14(4), 317–322. 10.1016/j.jpain.2012.02.012 [DOI] [PubMed] [Google Scholar]
- Tan LL, Pelzer P, Heinl C, Tang W, Gangadharan V, Flor H, Sprengel R, Kuner T, & Kuner R (2017). A pathway from midcingulate cortex to posterior insula gates nociceptive hypersensitivity. Nature Neuroscience, 20(11), 1591–1601. 10.1038/nn.4645 [DOI] [PubMed] [Google Scholar]
- Tashiro T, & Higashiyama A (1981). The perceptual properties of electrocutaneous stimulation: Sensory quality, subjective intensity, and intensity-duration relation. Perception & Psychophysics, 30(6), 579–586. 10.3758/bf03202013 [DOI] [PubMed] [Google Scholar]
- Tayeb Z, Bose R, Dragomir A, Osborn LE, Thakor NV, & Cheng G (2020). Decoding of Pain Perception using EEG Signals for a Real-Time Reflex System in Prostheses: A Case Study. Scientific Reports, 10(1), 5606. 10.1038/s41598-020-62525-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor BK, Joshi C, & Uppal H (2003). Stimulation of dopamine D2 receptors in the nucleus accumbens inhibits inflammatory pain. Brain Research, 987(2), 135–143. 10.1016/s0006-8993(03)03318-3 [DOI] [PubMed] [Google Scholar]
- Taylor JJ, Borckardt JJ, Canterberry M, Li X, Hanlon CA, Brown TR, & George MS (2013). Naloxone-reversible modulation of pain circuitry by left prefrontal rTMS. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 38(7), 1189–1197. 10.1038/npp.2013.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant F (2013). The physiologic effects of pain on the endocrine system. Pain and Therapy, 2(2), 75–86. 10.1007/s40122-013-0015-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terburg D, Scheggia D, Triana Del Rio R, Klumpers F, Ciobanu AC, Morgan B, Montoya ER, Bos PA, Giobellina G, van den Burg EH, de Gelder B, Stein DJ, Stoop R, & van Honk J (2018). The Basolateral Amygdala Is Essential for Rapid Escape: A Human and Rodent Study. Cell, 175(3), 723–735.e16. 10.1016/j.cell.2018.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theunissen M, Peters ML, Bruce J, Gramke H-F, & Marcus MA (2012). Preoperative Anxiety and Catastrophizing: A Systematic Review and Meta-analysis of the Association With Chronic Postsurgical Pain. The Clinical Journal oF Pain, 28(9), 819–841. 10.1097/AJP.0b013e31824549d6 [DOI] [PubMed] [Google Scholar]
- Tovote P, Esposito MS, Botta P, Chaudun F, Fadok JP, Markovic M, Wolff SBE, Ramakrishnan C, Fenno L, Deisseroth K, Herry C, Arber S, & Lüthi A (2016). Midbrain circuits for defensive behaviour. Nature, 534(7606), 206–212. 10.1038/nature17996 [DOI] [PubMed] [Google Scholar]
- Tracey I, Woolf CJ, & Andrews NA (2019). Composite Pain Biomarker Signatures for Objective Assessment and Effective Treatment. Neuron, 101(5), 783–800. 10.1016/j.neuron.2019.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trow Wm. C., Pavlov IP, Gantt WH, Volborth G, & Cannon WB. (1929). Lectures on Conditioned Reflexes. The Journal of Philosophy, 26(10), 275. 10.2307/2013906 [DOI] [Google Scholar]
- Tsuda M, Koga K, Chen T, & Zhuo M (2017). Neuronal and microglial mechanisms for neuropathic pain in the spinal dorsal horn and anterior cingulate cortex. Journal of Neurochemistry, 141(4), 486–498. 10.1111/jnc.14001 [DOI] [PubMed] [Google Scholar]
- Tuttle AH, Molinaro MJ, Jethwa JF, Sotocinal SG, Prieto JC, Styner MA, Mogil JS, & Zylka MJ (2018). A deep neural network to assess spontaneous pain from mouse facial expressions. Molecular Pain, 14, 1744806918763658. 10.1177/1744806918763658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valone JM, Randall CK, Kraemer PJ, & Bardo MT (1998). Olfactory cues and morphine-induced conditioned analgesia in rats. Pharmacology, Biochemistry, and Behavior, 60(1), 115–118. 10.1016/s0091-3057(97)00554-6 [DOI] [PubMed] [Google Scholar]
- van Heukelum S, Mars RB, Guthrie M, Buitelaar JK, Beckmann CF, Tiesinga PHE, Vogt BA, Glennon JC, & Havenith MN (2020). Where is Cingulate Cortex? A Cross-Species View. Trends in Neurosciences, 43(5), 285–299. 10.1016/j.tins.2020.03.007 [DOI] [PubMed] [Google Scholar]
- Vander Weele CM, Siciliano CA, Matthews GA, Namburi P, Izadmehr EM, Espinel IC, Nieh EH, Schut EHS, Padilla-Coreano N, Burgos-Robles A, Chang C-J, Kimchi EY, Beyeler A, Wichmann R, Wildes CP, & Tye KM (2018). Dopamine enhances signal-to-noise ratio in cortical-brainstem encoding of aversive stimuli. Nature, 563(7731), 397–401. 10.1038/s41586-018-0682-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, De Vries TJ, Wardeh G, Hogenboom FA, & Schoffelmeer AN (2001). A single exposure to morphine induces long-lasting behavioural and neurochemical sensitization in rats. The European Journal of Neuroscience, 14(9), 1533–1538. 10.1046/j.0953-816x.2001.01775.x [DOI] [PubMed] [Google Scholar]
- Veinante P, Yalcin I, & Barrot M (2013). The amygdala between sensation and affect: A role in pain. Journal of Molecular Psychiatry, 1(1), 9. 10.1186/2049-9256-1-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara F, Sardi NF, Pescador AC, Guaita GO, Jark Stern CA, Chichorro JG, & Fischer L (2020). Contribution of mesolimbic dopamine and kappa opioid systems to the transition from acute to chronic pain. Neuropharmacology, 178, 108226. 10.1016/j.neuropharm.2020.108226 [DOI] [PubMed] [Google Scholar]
- Vezina P, & Leyton M (2009). Conditioned cues and the expression of stimulant sensitization in animals and humans. Neuropharmacology, 56 Suppl 1, 160–168. 10.1016/j.neuropharm.2008.06.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vines SW, Gupta S, Whiteside T, Dostal-Johnson D, & Hummler-Davis A (2003). The relationship between chronic pain, immune function, depression, and health behaviors. Biological Research for Nursing, 5(1), 18–29. 10.1177/1099800403005001002 [DOI] [PubMed] [Google Scholar]
- Volkow ND, & Collins FS (2017). The Role of Science in Addressing the Opioid Crisis. New England Journal of Medicine, 377(4), 391–394. 10.1056/NEJMsr1706626 [DOI] [PubMed] [Google Scholar]
- Wager TD, Atlas LY, Lindquist MA, Roy M, Woo C-W, & Kross E (2013). An fMRI-Based Neurologic Signature of Physical Pain. New England Journal of Medicine, 368(15), 1388–1397. 10.1056/NEJMoa1204471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, & Cohen JD (2004). Placebo-induced changes in FMRI in the anticipation and experience of pain. Science (New York, N.Y.), 303(5661), 1162–1167. 10.1126/science.1093065 [DOI] [PubMed] [Google Scholar]
- Wager TD, Scott DJ, & Zubieta J-K (2007). Placebo effects on human mu-opioid activity during pain. Proceedings of the National Academy of Sciences of the United States of America, 104(26), 11056–11061. 10.1073/pnas.0702413104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G-Q, Cen C, Li C, Cao S, Wang N, Zhou Z, Liu X-M, Xu Y, Tian N-X, Zhang Y, Wang J, Wang L-P, & Wang Y (2015). Deactivation of excitatory neurons in the prelimbic cortex via Cdk5 promotes pain sensation and anxiety. Nature Communications, 6, 7660. 10.1038/ncomms8660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Goffer Y, Xu D, Tukey DS, Shamir DB, Eberle SE, Zou AH, Blanck TJJ, & Ziff EB (2011). A single subanesthetic dose of ketamine relieves depression-like behaviors induced by neuropathic pain in rats. Anesthesiology, 115(4), 812–821. 10.1097/ALN.0b013e31822f16ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Liu P, Ma L, & Wang F (2019). Opioid signal transduction regulates the dendritic morphology of somatostatin and parvalbumin interneurons in the medial prefrontal cortex. Neuroreport, 30(8), 592–599. 10.1097/WNR.0000000000001254 [DOI] [PubMed] [Google Scholar]
- Waung MW, Margolis EB, Charbit AR, & Fields HL (2019). A Midbrain Circuit that Mediates Headache Aversiveness in Rats. Cell Reports, 28(11), 2739–2747.e4. 10.1016/j.celrep.2019.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whissell PD, Tohyama S, & Martin LJ (2016). The Use of DREADDs to Deconstruct Behavior. Frontiers in Genetics, 7, 70. 10.3389/fgene.2016.00070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiech K, & Tracey I (2013). Pain, decisions, and actions: A motivational perspective. Frontiers in Neuroscience, 7(7 APR), 1–12. 10.3389/fnins.2013.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TD, Valdivia S, Khan A, Ahn H-S, Adke AP, Martinez Gonzalez S, Sugimura YK, & Carrasquillo Y (2019). Dual and Opposing Functions of the Central Amygdala in the Modulation of Pain. Cell Reports, 29(2), 332–346.e5. 10.1016/j.celrep.2019.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltschko AB, Johnson MJ, Iurilli G, Peterson RE, Katon JM, Pashkovski SL, Abraira VE, Adams RP, & Datta SR (2015). Mapping Sub-Second Structure in Mouse Behavior. Neuron, 88(6), 1121–1135. 10.1016/j.neuron.2015.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ (1984). Long term alterations in the excitability of the flexion reflex produced by peripheral tissue injury in the chronic decerebrate rat. Pain, 18(4), 325–343. 10.1016/0304-3959(84)90045-9 [DOI] [PubMed] [Google Scholar]
- Woolf CJ (2020). Capturing Novel Non-opioid Pain Targets. Biological Psychiatry, 87(1), 74–81. 10.1016/j.biopsych.2019.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KM, & McDannald MA (2019). Ventrolateral periaqueductal gray neurons prioritize threat probability over fear output. ELife, 8. 10.7554/eLife.45013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Driscoll JR, Wilbrecht L, Margolis EB, Fields HL, & Hjelmstad GO (2011). Nucleus accumbens medium spiny neurons target non-dopaminergic neurons in the ventral tegmental area. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 31(21), 7811–7816. 10.1523/JNEUROSCI.1504-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, & Zhang Y-Q (2018). A new perspective on the anterior cingulate cortex and affective pain. Neuroscience & Biobehavioral Reviews, 90, 200–211. 10.1016/J.NEUBIOREV.2018.03.022 [DOI] [PubMed] [Google Scholar]
- Xie YF, Wang J, Huo FQ, Jia H, & Tang JS (2004). Mu but not delta and kappa opioid receptor involvement in ventrolateral orbital cortex opioid-evoked antinociception in formalin test rats. Neuroscience, 126(3), 717–726. 10.1016/j.neuroscience.2004.04.013 [DOI] [PubMed] [Google Scholar]
- Xu H, Wu L-J, Wang H, Zhang X, Vadakkan KI, Kim SS, Steenland HW, & Zhuo M (2008). Presynaptic and Postsynaptic Amplifications of Neuropathic Pain in the Anterior Cingulate Cortex. Journal of Neuroscience, 28(29), 7445–7453. 10.1523/JNEUROSCI.1812-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Wan Y, Ma L, Zheng J, Han B, Liu F-Y, Yi M, & Wan Y (2018). A Context-Based Analgesia Model in Rats: Involvement of Prefrontal Cortex. Neuroscience Bulletin, 34(6), 1047. 10.1007/s12264-018-0279-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh TL, & Rudy TA (1978). Narcotic analgestics: CNS sites and mechanisms of action as revealed by intracerebral injection techniques. Pain, 4(4), 299–359. 10.1016/0304-3959(77)90145-2 [DOI] [PubMed] [Google Scholar]
- Yekkirala AS, Roberson DP, Bean BP, & Woolf CJ (2017). Breaking barriers to novel analgesic drug development. Nature Reviews. Drug Discovery, 16(8), 545–564. 10.1038/nrd.2017.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm DS, Jensen SL, Williams ES, & Martin JR (1999). Direct comparison of projections from the central amygdaloid region and nucleus accumbens shell. The European Journal of Neuroscience, 11(4), 1119–1126. 10.1046/j.1460-9568.1999.00524.x [DOI] [PubMed] [Google Scholar]
- Zhang R-R, Zhang W-C, Wang J-Y, & Guo J-Y (2013). The opioid placebo analgesia is mediated exclusively through μ-opioid receptor in rat. The International Journal of Neuropsychopharmacology, 16(4), 849–856. 10.1017/S1461145712000673 [DOI] [PubMed] [Google Scholar]
- Zhang S, Tang JS, Yuan B, & Jia H (1997). Involvement of the frontal ventrolateral orbital cortex in descending inhibition of nociception mediated by the periaqueductal gray in rats. Neuroscience Letters, 224(2), 142–146. 10.1016/s0304-3940(97)13478-4 [DOI] [PubMed] [Google Scholar]
- Zhang W, Schneider DM, Belova MA, Morrison SE, Paton JJ, & Salzman CD (2013). Functional Circuits and Anatomical Distribution of Response Properties in the Primate Amygdala. Journal of Neuroscience, 33(2), 722–733. 10.1523/JNEUROSCI.2970-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhao S, Rodriguez E, Takatoh J, Han B-X, Zhou X, & Wang F (2015). Identifying local and descending inputs for primary sensory neurons. The Journal of Clinical Investigation, 125(10), 3782–3794. 10.1172/JCI81156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Gadotti VM, Chen L, Souza IA, Stemkowski PL, & Zamponi GW (2015). Role of Prelimbic GABAergic Circuits in Sensory and Emotional Aspects of Neuropathic Pain. Cell Reports, 12(5), 752–759. 10.1016/j.celrep.2015.07.001 [DOI] [PubMed] [Google Scholar]
- Zhao J, Xin X, Xie G, Palmer PP, & Huang Y (2012). Molecular and cellular mechanisms of the age-dependency of opioid analgesia and tolerance. Molecular Pain, 8, 38. 10.1186/1744-8069-8-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Wang JY, Jia H, & Tang JS (2007). Roles of different subtypes of opioid receptors in mediating the ventrolateral orbital cortex opioid-induced inhibition of mirror-neuropathic pain in the rat. Neuroscience, 144(4), 1486–1494. 10.1016/j.neuroscience.2006.11.009 [DOI] [PubMed] [Google Scholar]
- Zhou Z, Liu X, Chen S, Zhang Z, Liu Y, Montardy Q, Tang Y, Wei P, Liu N, Li L, Song R, Lai J, He X, Chen C, Bi G, Feng G, Xu F, & Wang L (2019). A VTA GABAergic Neural Circuit Mediates Visually Evoked Innate Defensive Responses. Neuron, 103(3), 473–488.e6. 10.1016/j.neuron.2019.05.027 [DOI] [PubMed] [Google Scholar]
- Zhu W, & Pan ZZ (2005). Mu-opioid-mediated inhibition of glutamate synaptic transmission in rat central amygdala neurons. Neuroscience, 133(1), 97–103. 10.1016/j.neuroscience.2005.02.004 [DOI] [PubMed] [Google Scholar]
- Zhuo M (2019). Long-term cortical synaptic changes contribute to chronic pain and emotional disorders. Neuroscience Letters, 702, 66–70. 10.1016/j.neulet.2018.11.048 [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, & Stohler CS (2001). Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science (New York, N.Y.), 293(5528), 311–315. 10.1126/science.1060952 [DOI] [PubMed] [Google Scholar]
- Zubieta J-K, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, Nichols TE, & Stohler CS (2005). Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 25(34), 7754–7762. 10.1523/JNEUROSCI.0439-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


