Abstract
Digital health tools can provide convenient delivery of evidence-based treatments. The DynamiCare Health smartphone app delivers a contingency management intervention for substance use disorder consisting of remote self-testing for alcohol (breath) and drugs (saliva) with remote test validation and delivery of financial incentives for negative test results. This study examined feasibility, engagement (duration and consistency of app utilization), and impact on usual care treatment participation when a community substance use treatment program implemented this digital therapy among its patients. The study randomly assigned patients with alcohol use disorder (N=61) to receive either DynamiCare along with treatment-as-usual (TAU; N=29) or TAU only (N=32) during a 90-day evaluation period. Mean duration between first and last app use was 64 (±35) days, with mean earnings of $248 (±$209, out of $600 maximum). Among those with any app use (n=25), compliance was 68% and 74%, respectively for requested breath and saliva samples. Overall, two thirds of patients (66%) assigned to the app used it for at least 57 days and with high rates of self-testing compliance. Those completing the assessment (n=13; 45% of sample) endorsed high satisfaction ratings. DynamiCare versus TAU participants were more likely to be retained in usual care treatment at 90 days (24% vs 3%; (χ2(1, 61)=5.9,p <0.05), but sustained app utilization was associated with a wide range of usual care treatment participation. These data suggest that DynamiCare Health is feasible and potentially beneficial as a complement to community substance use treatment programs.
Keywords: Technology, Digital health, Mobile app, DynamiCare Health, Alcohol use disorder, Feasibility
1. Introduction
Digital therapeutics, including smartphone applications (apps), can deliver with fidelity evidence-based behavioral interventions as a supplement to enhance standard care therapy for substance use disorder. Contingency management (CM) is among the most effective evidence-based interventions currently available to treat substance use disorders. CM is an approach in which immediate, tangible financial rewards are delivered as a result of demonstrated abstinence from alcohol and/or illicit drugs. Six meta-analyses (Benishek, 2014; Dutra et al., 2008; Getty et al., 2019; Griffith, 2000; Lussier, 2006; Prendergast, 2006) have been conducted that demonstrate the effectiveness of this approach. However, CM is in fact infrequently used by community treatment programs (Herbeck, 2008). Practical barriers to adoption include the need for frequent, valid, random drug testing, a lack of training for staff, difficulty managing the rewards on an individual basis, and lack of funds to pay for rewards (Carroll, 2014).
The advent of modern digital technology has now made it possible to automate delivery of this highly effective intervention (Dallery et al., 2019). For example, Dallery et al. (2017) describe a computerized CM program for smoking cessation, called Mōtiv8, in which a computer webcam allowed the smoker to record a video of themselves using a home carbon monoxide detector, thus obviating the need for manual, clinic-based testing. Research staff validated the results from the video and reward payments were made using PayPal©. In that study, CM patients had abstinence rates three times higher than the controls at the end of treatment. Another intervention targeting illicit drug use is the Therapeutic Education System (TES), developed for computer-based delivery of a treatment that combined abstinence incentives and cognitive behavioral skills training therapy (community reinforcement approach). The study delivered skills training in self-administered modules accessed remotely on a computer and calculation of reward amounts was automated by the computer program. However, abstinence incentives required in-person sample collection, drug testing, and distribution of rewards. Compared to a treatment-as-usual control, the 12-week TES intervention reduced dropouts by 28% and increased abstinence by 62% (Campbell et al., 2014) among a large diverse sample of persons entering outpatient psychosocial treatment for substance use disorder (SUD), with strongest results seen for patients whose primary problem substance was either cocaine or alcohol (Cochran et al., 2015). The TES intervention has now been translated for delivery on smartphones in a product called reSET© (https://www.resetforrecovery.com; Voelker, 2019).
In the treatment of alcohol use disorder (AUD), use of CM has been hampered by the short duration over which alcohol can be detected after drinking. Thus, while breath alcohol devices provide valid data and are easy to use, testing needs to be done frequently, and ideally on a random schedule to provide valid data on recent drinking. A self-testing strategy including frequent random sample collection may be ideal for solving this dilemma. In one small (N = 30) 4-week randomized controlled trial (Alessi & Petry, 2013) with heavy drinkers not enrolled in treatment, participants used cell phones to self-record videos of breath alcohol (BrAC) tests and sent videos to researchers for validation. The random testing schedule was implemented with manual text messages, and the study made payments via mailed checks. In addition to being well-received by participants, the CM procedure with incentives for abstinence was associated with a higher rate of negative samples submitted (87% vs 67%; p = 0.001) and with a substantial reduction in number of drinking days (21.8 days for controls vs. 10.8 with CM; p = 0.001) relative to control participants who received only modest incentives for video submission. Researchers conducted another 21-day randomized demonstration trial among treatment-seeking AUD patients (N = 40) using more advanced smartphone technology that allowed BrAC sample collection, verification, and payment all to be managed remotely. Rate of negative sample submission was 85% for those receiving contingent financial incentives versus 38% for a noncontingent control group (Koffarnus et al., 2018).
A recent meta-analysis summarized existing data evaluating smartphone-delivered CM; the meta-analysis determined that digital CM to reduce smoking and alcohol use had superior outcomes compared to control conditions (Getty et al., 2019). Thus, ample evidence exists that CM procedures can be automated and are effective in promoting treatment aims. However, the ability of remotely delivered CM to enter mainstream SUD therapy will require additional development and translation to enhance convenience of remote delivery and address other barriers to use of CM. DynamiCare Health developed a new phone app with remote delivery of CM, which can incorporate abstinence incentives for both alcohol and other drugs to allow treatment for community samples that may include multiple substances. This app has the unique advantage of automating the entire CM process including sample submission and validation, incentive reward scheduling, and payout calculation and delivery. Alcohol and drug testing are conducted remotely by the patients themselves using breath alcohol devices and oral fluid test strips for drugs; videos of the self-testing process and outcome are taken by the smartphone and integrated into the app, then validated remotely by DynamiCare staff. Random testing schedules and payment calculations for negative tests are accomplished by the background program, and payment is automatically loaded onto smart debit cards provided to the patient. This automated delivery greatly enhances convenience of the intervention and addresses one of the major barriers to use of CM—the need for busy clinicians to manage complex implementation. The current pilot study provides important information on feasibility and clinical benefit of the DynamiCare Health smartphone-based CM program when used as a supplement to standard care among a real-world sample of patients with AUD enrolled in a community-based SUD treatment program. This information provides a critical foundation for subsequent development and testing of this innovative digital therapeutic either in community treatment or other SUD samples.
2. Methods
2.1. Study sample
Participants in the study were 61 patients enrolled at one of three outpatient SUD clinics that Gosnold Treatment Centers operated in the Boston/Cape Cod region (Centerville, Falmouth, or Stoughton locations). Eligible participants at treatment intake 1) were >18 years old; 2) met DSM-5 criteria for current (past year) AUD of at least moderate severity; 3) identified alcohol as their primary substance; 4) recently used alcohol (past 7 days) or, having been in treatment 14 days or fewer, had used alcohol within 7 days prior to treatment entry; 5) used an Android or iOS smartphone with acceptable capability; 6) were willing to participate in self-administered home testing; 7) spoke and read the English language adequately enough to understand smartphone commands and responses; and 8) were willing to be randomly assigned to receive treatment with or without the smartphone app as an added feature. Participants could not be currently suicidal or actively psychotic, based on clinician judgement.
2.2. Recruitment
The study used an open advertisement recruitment strategy that included displaying posters, distributing flyers, having multiple staff members making announcements during groups, and having counselors tell their patients about the study during individual sessions. This approach did not lend itself to recording how many people heard about the study and why they did or did not choose to participate. However, two sub-groups of patients (DUI program participants and ASAM Level 2.5 partial hospital program patients) were less likely to participate, compared to ASAM Level I outpatients. Research staff interviewed 103 potential participants who expressed interest in the study and screened them for initial eligibility. If they remained interested after receiving written materials and a video to review, and met study inclusion criteria, they met with research staff who answered any questions they had and obtained written informed consent. Of the 103 who were screened, 61 were eligible. Two were ineligible due to lack of appropriate cell phone access, 31 (74% of ineligible) had an absence of alcohol use in the specified window, and 9 were ineligible for other reasons. The Sterling Institutional Review Board (Atlanta, GA) approved all study procedures. Overall, the study recruited 44 of 61 eligible participants within 20 days of completing intake for treatment entry, comprising 69% of treatment and 75% of control participants. The study recruited the remainder during a treatment episode or while waiting for an appropriate (drug education) treatment group to start.
The study performed a baseline assessment at the time of consent, which recorded demographic data, and information about recent substance use and severity using the self-administered Brief Addiction Monitor (BAM; Cacciola et al., 2013). In addition, the study collected urine samples and tested them for a comprehensive panel of 12 drug categories including the alcohol metabolite ethyl glucuronide (EtG).
2.3. Study design
The study randomly assigned participants following completion of baseline assessment to either treatment-as-usual (TAU; N = 32) alone or TAU combined with access to the DynamiCare app (N = 29) for a 90-day planned duration. The study stratified randomization on two variables 1) reported use of drugs in addition to alcohol and 2) recent treatment in an inpatient medically supervised withdrawal program.
2.4. Treatment-as-usual (TAU)
The Gosnold program offered a variety of services that included physical and mental health services as well as several levels of substance use treatment. The study recruited the majority of participants (n = 43; 70%) from intensive outpatient group therapy in the Structured Outpatient Addiction Program (SOAP; ASAM Level 2.1). The program offered SOAP groups 3 days per week; insurance typically authorized 12 or 20 sessions, with no constraints on time to complete. Six participants were attending a court-ordered Drivers Alcohol Education (DAE) program in which 16 sessions were offered on a weekly basis. New DAE groups started when the program accumulated a sufficient cohort of patients. Six participants began the study while in a short-term partial hospital program (PHP; ASAM Level 2.5) designed for dual diagnosis patients with additional mental health disorders. Six were enrolled in individual counseling (ASAM Level 1) with flexible scheduling and no time frame imposed pending periodic insurance reauthorization. Several persons participated sequentially in more than one type of standard care treatment including brief inpatient medically supervised withdrawal following relapses and post-treatment recovery coaching. Treatment types were well-balanced across the two groups. All programs conducted drug and alcohol testing per the clinic standard, and no contingencies were involved.
2.5. Digital therapy intervention
DynamiCare Health is an iOS/Android app that automates CM to help the patient focus attention on behavioral goals (i.e., abstinence) for alcohol or other substance use. During the 3-month time period in which app use was available, participants were periodically prompted to self-conduct a breathalyzer and/or saliva test (Figure 1). Participants used the smartphone’s camera to take a photo/video of the test process and submitted the video to DynamiCare Health where customer service staff reviewed validity of the testing procedure and results. If the sample was negative for breath alcohol and/or a panel of drugs, monetary rewards were immediately loaded onto a debit card that could be accessed by the participant and spent on all but a limited number of shops and venues (liquor stores excluded). The app displays results in real time for each test and provides a continually updated record of testing compliance, rewards earned, balance still available to be spent, and the reserve amount available for future earnings. Costs associated with the intervention include the breathalyzer tests, saliva tests, DynamiCare staff review of tests, and patient incentives. The app uses a HIPAA-compliant platform that protects patient information with encryption in-transit and at-rest and limits patient data visibility to only those with authorized access.
Figure 1.

DynamiCare Health App procedures. App screens are shown to illustrate the steps in substance use self-testing and reward delivery.
Remote BrAC testing employed the BACtrack® Mobile Pro, a police-grade, digital, Bluetooth-connected device that has good sensitivity at low BrAC levels; the study used results of this test for delivery of CM rewards. Remote oral fluid testing employed the Alere T-Cube® 9-panel test that provides reliable immunoassay testing for 9 drug categories: cocaine, opiates, amphetamine, methamphetamine, THC, benzodiazepines, methadone, buprenorphine, and oxycodone. The breathalyzer submits alcohol results directly to the smartphone app via Bluetooth. The participant submitted videos of the saliva test procedure and test results.
The study modeled test scheduling parameters on the successful methods of Alessi and Petry (2013). For the substance testing, 75% of tests were breathalyzer and 25% were oral fluids. Testing occurred on a random schedule 3–10 times per week (1–3 times per day) with 2–7 breathalyzer and 1–3 saliva tests. Testing started at an average of 7 per week with a missing or positive sample triggering more frequent testing to a maximum of 10 per week and consistent negative samples triggering less frequent testing to a minimum of 3 tests per week. There was a higher probability of testing in the evening (vs. morning or afternoon), of multiple tests in evenings, and on weekends and holidays, given findings that drinking is elevated at these times in AUD (Lapham et al., 2009). At sign-up, DynamiCare staff systematically reviewed sleep schedules, work patterns, and other expectable routines that could impose appropriate constraints on random testing with each participant. The study constructed testing schedules accordingly and the study could modify them upon changes in participants’ schedules. Participants received an app notification when a test was due and the result was expected within 60 minutes (for breathalyzer) or 90 minutes (for saliva) of receiving the notification. Participants received feedback from the app, thanking them for submissions, noting any problems with the video or test procedures that needed correcting, congratulating them for demonstrated abstinence, and indicating the amount earned and total earned to date. DynamiCare staff were available to assist with any technical issues regarding app operation, video submission, and transfers to the debit card.
DynamiCare customer service checked submitted videos for quality (clear lighting and focus) and validity (self-testing and results adequately displayed). Incentive awards required two conditions: 1) breath alcohol reading ≤0.01g/dl and (if requested for that test) 2) a saliva drug test that was negative for all 9 nonprescribed substances tested. Verified negative tests resulted in an electronic display of virtual “coins” indicating the cash value award for the current negative test. The value of each coin ranged from $0 to $50, with an expected average value of $1 per coin. Earnings started at 2 coins for the first successful test sample and progressively escalated by 1 coin to reach a maximum value of 10 coins (worth $10) after 8 consecutive negative tests. The study used a “reset” procedure such that missed samples resulted in a return to 1 coin for the next negative test. However, in an attempt to encourage submission of samples that might be positive, each positive sample only set the next reward value back by 1 coin, with a minimum of 2 coins always available for the next negative sample following a positive.
Earnings were automatically loaded to the spendable Next Step® debit card (True Link Financial, Inc.) with the typical time from submission of a test video to provision of the reward on the debit card and notice to the participant being less than two hours. The card incorporates multiple protections from risky spending, e.g., blocking cash withdrawals and spending at identified liquor stores and bars, and so on. Under the rewards schedule, participants who were continuously negative could earn a maximum of $560 to $600 over the 90-day study, depending on their testing frequency and results.
At study start, research staff assisted eligible volunteers who were assigned to the DynamiCare group in viewing an online instructional video introducing the program and procedures, and signed patients up for their personal debit card to which CM rewards would be posted. Before beginning off-site procedures, participants practiced proficiency using the alcohol and drug testing devices and self-recorded video transmission. Research staff reviewed the testing schedule, available earnings, and how to access and spend from their debit card account. Participants were then issued the requisite equipment to take home, which included a debit card, pocket-size breathalyzer, and saliva test kits. Study staff instructed them to keep the equipment with them from the time they woke up until they went to bed each day. The study terminated access to the app at the next contact after 90 days had elapsed from randomization.
2.6. Application utilization and satisfaction assessment
The study measured two objective measures of feasibility and engagement—duration of app usage and percent of requested tests completed. The study defined duration of app usage as days elapsed between first and last use of the app for submission of drug or alcohol tests during the 90-day intervention period. We also report the number of tests and percent of requested tests that were completed between first and last use of the app, which could vary across individual participants. The study also asked participants in the DynamiCare group to complete a satisfaction rating after one month (Patient Satisfaction Survey). Using 7-point Likert scales (with higher scores indicating greater satisfaction), participants rated how well they liked the program, whether it helped them to remain abstinent from drugs and alcohol, and whether they would recommend the app to a friend or family member. Participants also indicated which features of the DynamiCare program were helpful and which features of their overall treatment program (counseling, self-help, DynamiCare) were most important. Additionally, the study sought feedback on functionality of the program features (e.g., video submission, debit card procedures, quality of instructions, and training provided) and technical support required.
2.7. Outcome assessments
The study scheduled four research assessment visits for both groups at monthly intervals after randomization with the last visit (month 4) one month after the planned intervention had ended. The BAM included questions about drug and alcohol use in the past 30 days (Cacciola et al., 2013). In addition, the study collected a urine sample at baseline and each study visit. In-person assessment employed DrugConfirm™ (Confirm Biosciences, San Diego, CA) 12-panel urine test cups. This test provides reliable immunoassay testing for all drugs included in the 9-panel test as well as EtG, barbiturates, and methylenedioxymethamphetamine (MDMA). In-person interviews lasted approximately 30–45 minutes each. Participants could earn $100 for completion of all assessments with available pay amount increasing with successive assessments to motivate continued participation.
2.7.1. App utilization and satisfaction.
Descriptive data on app utilization is reported for the DynamiCare group. Measures include 1) duration of app use (days between first and last submitted drug or alcohol test capped at 90 days), 2) percent of requested breath and saliva tests completed during the period of use as defined for each participant, and 3) percent of submitted tests negative for substances (reported separately for breath and saliva tests). The study compared data to a priori benchmarks for feasibility/engagement that had been set using successful sample submission rates from Alessi & Petry (2013) and defined in the study protocol as: ≥67% of participants submitting ≥67% of requested videos on time. We report satisfaction data descriptively for those app users who completed assessments (n = 13, 45% of sample).
2.7.2. Monthly research assessments.
The originally planned primary outcome in the DynamiCare group versus TAU-only group was number of alcohol and drug negative tests submitted at the 1-, 2-, 3- and 4-month assessment time points. A clinically meaningful secondary outcome available for both groups from clinic records was duration of participation in TAU. The study determined substance use treatment duration by days elapsed between date of study randomization to the date of last TAU substance use treatment session during the 90-day evaluation period. The study terminated duration if a gap between treatment sessions of greater than 30 days occurred and was capped at 90 days. Data from clinic records indicated that 13 participants (6 in DynamiCare; 7 in TAU) had no substance use treatment services recorded during their study participation, though they may have received psychiatric and/or medical (ambulatory medical care or inpatient medical) services during that time. Because these services were potentially unrelated to substance use disorder treatment, these individuals were coded as 0 days for substance use treatment duration.
2.8. Statistical analysis
The study compared basic participant characteristics (see Table 1) between the TAU and DynamiCare groups using chi-square for categorical variables (e.g., recruitment location, sex) and t-tests for continuous variables (i.e., age). Only treatment site differed significantly between the groups (Table 1; χ2(1, 61) = 6.1,p <0.05). Comparison of days in treatment for TAU patients at Centerville, where the majority were recruited (n = 17; M= 22.5, SD = 23.0), versus other sites (n =17; M= 21.9, SD = 19.7) showed no statistically significant difference (t(30) = .08,p = 0.94). Therefore, the study did not include site as a covariate in subsequent analyses. Data on app utilization and satisfaction are descriptive only (as these did not apply to TAU patients). Study staff compared research assessment completion rates across groups using chi-square. Urinalysis outcomes from research assessments are presented descriptively but are not compared statistically due to high rates of missing assessment data. The study compared mean days in treatment for DynamiCare versus usual care participants using an independent samples t-test and calculated the effect size using Cohen’s d. The study also formed a two-group categorical distribution based on duration of usual care treatment at 90 days versus shorter durations and compared across groups using chi-square. The study assessed association between app use and usual care treatment durations within the DynamiCare group using Pearson’s r correlation.
Table 1.
Participant characteristics.
| Variable | Control (N = 32) | Treatment (N = 29) | Total (N = 61) | |||
|---|---|---|---|---|---|---|
| Treatment information Location, count (%)* | ||||||
| Centerville | 17 | (53) | 24 | (83) | 41 | (67) |
| Falmouth/Stoughton | 15 | (47) | 5 | (17) | 20 | (33) |
| Program, count (%) | ||||||
| SOAP | 23 | (72) | 20 | (69) | 43 | (70) |
| PHP/DAE/Individual Counseling | 9 | (28) | 9 | (31) | 18 | (30) |
| Prior withdrawal treatment, count (%) | 10 | (31) | 13 | (45) | 23 | (38) |
| Prior SUD treatment, count (%) | 18 | (56) | 21 | (74) | 39 | (64) |
| Drug use other than alcohol† | ||||||
| Past year other drug use, count (%) | 14 | (44) | 14 | (48) | 28 | (46) |
| Opioid use, count (%) | 7 | (22) | 8 | (28) | 15 | (25) |
| Stimulant use, count (%) | 8 | (25) | 7 | (24) | 15 | (25) |
| Benzodiazepine use, count (%) | 9 | (28) | 6 | (21) | 15 | (25) |
| Cannabis use, count (%) | 16 | (50) | 15 | (52) | 31 | (51) |
| Nicotine use, count (%) | 15 | (47) | 15 | (52) | 30 | (49) |
| Demographics | ||||||
| Age in years, mean (SD) | 40.1 | (12.2) | 39.0 | (10.7) | 39.6 | (11.4) |
| Gender, count (%) | ||||||
| Men | 18 | (56) | 19 | (66) | 37 | (61) |
| Women | 14 | (44) | 10 | (34) | 24 | (39) |
| Race/Ethnicity, count (%) | ||||||
| White and Non-Hispanic or Latinx | 27 | (84) | 26 | (90) | 53 | (87) |
| Other Race or Hispanic/Latinx1 | 5 | (16) | 3 | (10) | 8 | (13) |
| Marital status, count (%) | ||||||
| Single | 20 | (63) | 17 | (59) | 37 | (61) |
| Married | 8 | (25) | 6 | (21) | 14 | (23) |
| Divorced | 4 | (13) | 6 | (21) | 10 | (16) |
| Living with, count (%) | ||||||
| Other relatives/Parents | 11 | (34) | 11 | (38) | 22 | (36) |
| Spouse/partner | 12 | (38) | 7 | (25) | 19 | (31) |
| Alone | 9 | (28) | 11 | (38) | 20 | (33) |
| Education achieved, count (%) | ||||||
| High school/GED or less | 21 | (66) | 15 | (52) | 36 | (59) |
| Greater than high school | 11 | (34) | 14 | (48) | 25 | (41) |
| Household income, count (%) | ||||||
| $0-$50,000 | 23 | (72) | 20 | (69) | 43 | (70) |
| $50,000+ | 9 | (28) | 9 | (31) | 18 | (30) |
| Insurance, count (%) | ||||||
| Medicaid/Medicare | 17 | (53) | 14 | (48) | 31 | (51) |
| Private | 12 | (38) | 11 | (38) | 23 | (38) |
| Self-pay/other | 3 | (9) | 4 | (14) | 7 | (11) |
| Employment status, count (%) | ||||||
| Full time/Part time | 18 | (56) | 18 | (62) | 36 | (59) |
| Unemployed/Homemaker/Disabled | 14 | (44) | 11 | (38) | 25 | (41) |
Significant difference between Treatment and Control;
Includes 2 participants who identified as Black or African American, 2 American Indian, Native American or Alaska Native, 1 more than one race, 2 Hispanic or Latinx, and 1 indicating other; SOAP = Structured Outpatient Addiction Program, PHP = Partial Hospital Program, DAE = Drivers Alcohol Education, SUD = Substance Use Disorder,
self-report on BAM
3. Results
3.1. Study sample characteristics
As shown in Table 1, study groups did not differ significantly on any of the demographic or drug use variables. The community treatment sample was primarily white/not Hispanic (87%), 61% men with an average age of 39.6 years (SD = 11.4). The majority (61%) were single, virtually all were high school graduates or above in educational attainment, 59% were employed and 89% had Medicare or Medicaid or private insurance. Prior to entering the outpatient program, 38% had undergone medically supervised withdrawal, while 46% reported using drugs in addition to alcohol during the 30 days prior to treatment entry. The study detected ethyl glucuronide in only 5 participants at study intake (8.5% of the 59 participants with study intake test results) while a positive test for any other nonprescribed drug was detected in 49% of the samples, with a positive test for more than one non-prescribed drug detected in 34% of the samples. THC and sedatives (benzodiazepines or barbiturates) were the most common drugs detected, present in 20% and 17% of samples, respectively. Non–prescribed stimulants were rare (3.4% of samples) and the study did not detect any illicit opioids at baseline.
3.2. App utilization and satisfaction data
For the majority (n = 21; 72%) of the 29 participants assigned to DynamiCare treatment, time between first and last use of the app for sample submission was 57 days or more during the 90-day treatment period (mean = 84 days, SD = 9), whereas smaller subgroups had app use durations of 7–23 days (n = 4; 14%), two days (n =1), and not at all (n = 3). The study defined a long use group (n = 21) as those submitting samples for 57 days or more, while the study defined a per protocol group (n = 25) as those who used the app for 7 days or more. Mean number of days between first and last app use for the intent-to-treat DynamiCare sample (N= 29) was 64 (SD = 35) out of 90 possible days. Table 2 displays utilization data for the per protocol group (n = 25) whose mean duration of use was 74 days and mean rate of compliance with requested testing during the period of app utilization was 68% for breath tests and 74% for saliva tests. Examination of individual data revealed that two individuals within the per protocol sample qualified for long app utilization durations but had poor compliance (<25% of requested tests submitted). Thus, overall, 66% (19/29) of those assigned to the app displayed persistent and compliant app utilization for 57 days or more.
Table 2.
DynamiCare app utilization (n = 25).§
| App Use Days† | Dollars Earned | Breath Testing | Saliva Testing | ||||
|---|---|---|---|---|---|---|---|
| # Samples Requested | % Submitted | % of Submitted Negative* | # Samples Requested | % Submitted | % of Submitted Negative* | ||
| 74 (26) | 287 (202) | 56 (30) | 68 (32) | 92 (14) | 22 (12) | 74 (27) | 95 (7) |
includes participants with 7 days or more between first and last app use for sample submission; all tabled values are mean (SD)
Mean days from first to last use during 90-day evaluation period
Negative for alcohol (breathalyzer test) and/or all illicit (non-prescribed) drugs (saliva test)
Table 2 also shows that the overwhelming majority of tests submitted were negative for drugs and alcohol. Participants in the intent-to-treat sample (N = 29) earned an average of $248 (±$209) in incentives, while those in the per protocol group (n = 25) earned an average of $287 during the 90-day study; 62% of the full DynamiCare group earned over $100, and 38% earned more than $400.
Among DynamiCare participants, 45% completed a utilization satisfaction survey at 1 month postrandomization. Ninety-two percent of respondents rated the overall experience with the app as extremely or mostly positive. All felt that the app helped them to stay away from drugs and alcohol (either extremely, mostly, or somewhat) during their treatment episode and would recommend the program to a friend or relative with similar goals. Table 3 shows that features of the app endorsed as being helpful were “surprise testing kept me on my toes” (92%), “earning money for staying abstinent” (77%), “monitoring my own behavior” (69%), and “making recovery enjoyable” (69%). Only two reports of minor technical problems with the app were noted and resolved by DynamiCare staff. Three individuals reported contesting the results of a breath alcohol test. When asked to select the component(s) of their overall treatment experience that were most important, 100% of respondents endorsed DynamiCare Health, 77% indicated self-help groups, and 62% indicated counseling.
Table 3.
Endorsement of helpful app features.†
| What features of the app program where helpful? | % Endorsing (n = 13) |
|---|---|
| Surprise testing kept me on my toes | 92 |
| Earning money for staying abstinent | 77 |
| Monitoring my own behavior | 69 |
| Making recovery enjoyable | 69 |
| Being in charge of my own test | 54 |
Responses obtained at 1 month postrandomization. The response rate for the satisfaction survey was 45%.
3.3. Usual care treatment outcomes
Participants assigned to use DynamiCare had greater mean days in usual care treatment compared to the TAU group, but these mean differences were not statistically significant (mean of 29.8 versus 22.2 days; t(59) = 1.01,p = .32; Cohen’s d= 0.26). Twenty-four percent of DynamiCare versus 3% of usual care remained in treatment at 90 days. The differences in proportion of participants retained at 90 days was significantly different (χ2(1, 61) = 5.9, p <0.05).
Figure 2 shows that durations of usual treatment participation and of app use were not well correlated (r = 0.26; p = 0.17). Long durations of app use were associated with widely varying durations of usual care treatment participation. However, only a single participant with a long duration of usual care treatment participation had relatively brief (19 days) app use.
Figure 2.
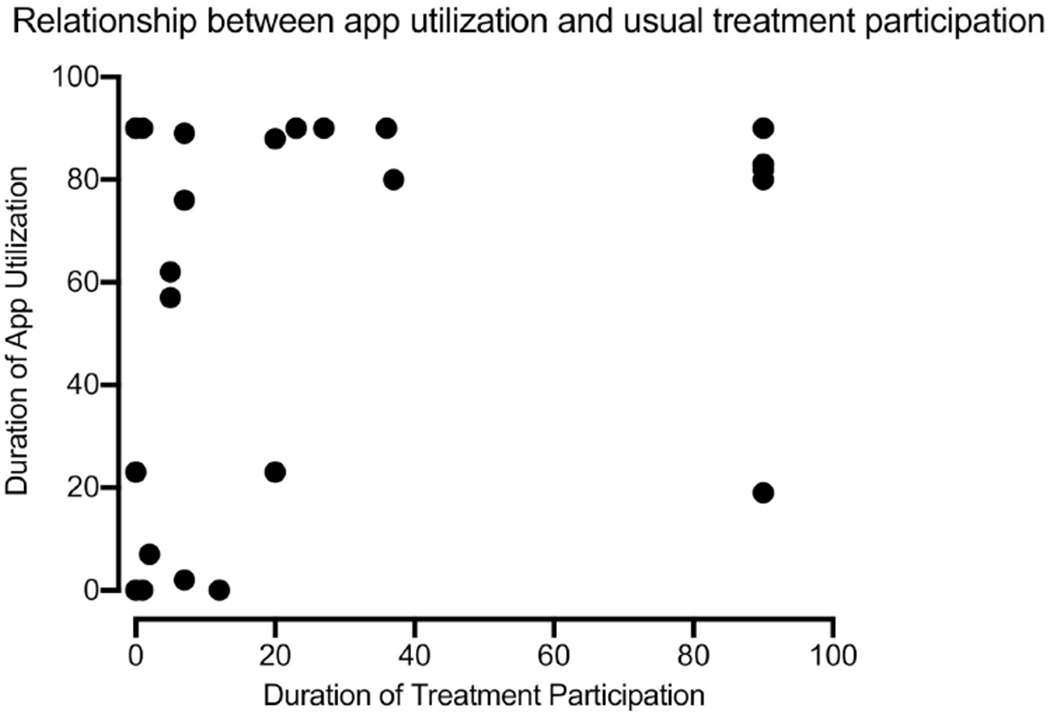
Relationship between app utilization and usual care treatment participation. App utilization was defined as days from study randomization to last submitted breath or saliva sample truncated at 90 days. Treatment participation was defined as days from study randomization to last substance use treatment session attended prior to a gap of ≥30 days between sessions with duration capped at 90 days from randomization in absence of 30-day gaps.
3.4. Research assessment data
Table 4 shows drug testing results for the two study groups. Research assessment completion rates at the 1-, 2-, 3- and 4-month time points were 41%, 45%, 45%, and 34% for the DynamiCare group (41% overall); and 28%, 28%, 19%, and 16% for the TAU group (23% overall). Chi-square analysis indicates the proportion of completed assessments was significantly greater in the DynamiCare treatment group relative to the TAU group (χ2(1, 244) = 9.9, p <.01). Among the 77 cumulative urine samples collected at follow-ups, only 18 (23%) tested positive for one or more non-prescribed drugs. Four samples (5.2%) positive for ethyl glucuronide were submitted by 3 participants in the TAU group. Thirteen samples (16.9%) submitted by 7 participants were positive for non–prescribed THC (recreational cannabis is legal in Massachusetts). Only 6 samples (7.8%) were positive for another non–prescribed drug. The overall rate of testing-confirmed recent abstinence from alcohol and non-prescribed drugs in the intent to treat sample (considering missing samples as not confirmed abstinence) was 33% for DynamiCare versus 16% for the TAU group. Among individual participants, there were 12 DynamiCare (41%) versus 7 TAU (22%) participants with two or more confirmed abstinence results at research assessment visits.
Table 4.
Urine drug testing results at monthly research assessments.†
| DynamiCare (N = 29) | TAU (N =32) | |||||||
|---|---|---|---|---|---|---|---|---|
| Research Assessment | Research Assessment | |||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| Abstinent n (%) | 10 (35) | 9 (31) | 11 (38) | 8 (28) | 7 (22) | 7 (22) | 4 (13) | 3 (9) |
| Non-abstinent n (%) | 2 (7) | 4 (14) | 2 (7) | 2 (7) | 2 (6) | 2 (6) | 2 (6) | 2 (6) |
| Missing n (%) | 17 (59) | 16 (55) | 16 (55) | 19 (66) | 23 (72) | 23 (72) | 26 (81) | 27 (84) |
Abstinent results include those that were negative, as well as those results that were consistent with the participant’s prescriptions or cannabis card.
4. Discussion
This study showed that 66% of participants (19/29) randomized to use DynamiCare Health as a supplement to usual care treatment exhibited both substantial durations of utilization (first to last app use for drug or alcohol sample submission) and high levels of compliance with testing requests. The observed rate of engagement is consistent with a priori benchmarks of successful engagement based on data from Alessi and Petry (2013) and is also generally consistent with outcomes in the large body of research on efficacy of abstinence incentives when added to usual care treatment (e.g., Campbell et al., 2014; Silverman et al., 2004). The app was able to engage a majority of participants over time once they initiated use of the app. In addition, those app users who completed a satisfaction survey (n = 13; 45% of assigned sample) uniformly rated the DynamiCare Health app as a positive experience, endorsing its utility in helping them stay away from drugs and alcohol, and indicating their willingness to recommend this app to family or friends struggling with substance use. A minority of participants’ reasons for poor engagement are not readily discernible from the data, but problems with cell phone operation, software technology, self-testing procedures, or privacy concerns were unlikely to limit engagement with the app, given that few were noted among our sample. Technical support was nevertheless available and useful to solve occasional problems with login, blue tooth connectivity, and video uploading. Such support is likely to be needed for real-world implementation of any digital therapeutic. Importantly, this study did not find mobile phone ownership to be a major limiting factor in initial study engagement. Inexpensive mobile phones are available via federal assistance programs that would suffice for this technology. Together, the utilization and satisfaction data support the feasibility of implementing the DynamiCare Health app for patients with primary alcohol use enrolled in a real-world substance use community treatment program.
Some preliminary data were obtained in this pilot study supporting better usual care outcomes for the DynamiCare Health group versus the TAU group. In particular, DynamiCare participants had significantly higher usual treatment retention rates at 90 days compared to the usual care only group. The study also observed higher rates of participation and confirmed abstinence at research assessments in the DynamiCare group (33%) relative to TAU (16%), but overall compliance with assessments was quite low, particularly in the control group, which hinders interpretation of these outcome assessments since the study counted missing samples as positive. Our data are nonetheless consistent with findings of a recent meta-analysis (Getty et al., 2019), which concluded that mobile-phone delivered CM was associated with better outcomes compared to control conditions for reducing both tobacco and alcohol use among adults not in treatment for SUDs.
App utilization was not well correlated with usual care treatment engagement among participants who received DynamiCare (Figure 2). In particular, a number of those with little usual care treatment participation continued to use the app for long periods. This finding suggests that the app may have future utility not only as an adjunct in treatment-compliant patients, where it could help to bridge the gap in time between visits to an on-site SUD treatment service during which many patients may experience risk, but also as a support by providing added motivation and accountability for treatment nonadherent patients who may be at high risk for early dropout. Given engagement with the app among those with little treatment participation, future research may also evaluate whether DynamiCare is useful as a substitute for in-person treatment in individuals who opt not to utilize formal SUD treatment services, who drop out prematurely, or among those with limited treatment access. The COVID-19 pandemic has demonstrated the utility of telehealth and other remote service delivery options to prevent abrupt disruption of efficacious treatments and to allow for remote monitoring of drug and alcohol use during crisis situations (Lin, Fernandez and Bonar, 2020).
Limitations of the current study should be considered when evaluating feasibility of DynamiCare Health in community treatment samples. Although the study showed good rates of sustained engagement in the breath and saliva self-testing program, most samples submitted were negative for alcohol and drugs, leaving the status of unsubmitted samples unknown. While there may be a variety of reasons for missing samples, these missing samples may indicate lapses to substance use and as such could be used clinically as an early indicator of relapse or other life difficulties to trigger more intensive interventions. The close monitoring afforded by digital health tools seems ideal for such adaptive treatment strategies. In addition, the current study was conducted in a small and demographically homogeneous sample of persons with primary alcohol use, which could limit the generalizability of our results. Further, a limited subgroup of those randomized to use the app provided satisfaction data, which could introduce potential bias. Variability also existed in usual care treatment modalities among the study participants. However, this finding reflects the real-world population of substance use treatment seekers and the variety of treatment options they encounter in clinical settings. High rates of engagement with DynamiCare across multiple treatment modalities, across persons who use multiple substances, and those with co-morbid mental health conditions speaks to its potential utility across diverse clinical samples. Another useful perspective on engagement potential, beyond sustained utilization among those who initiated app use in a research study, would be gained from rates of app initiation among clinical samples offered use of the app as a recommended treatment component outside the context of a research study, where additional considerations may influence participation decisions. A final limitation of this study is that the research team conducted it with a specific set of CM testing and payment parameters, which could be adjusted and examined in future research.
The research team designed this pilot efficacy trial to provide clinically useful information when an infrequently used evidence-based behavioral intervention is added to usual care with convenient remote delivery via phone app. This comparison has practical and public health importance. Future research could consider including attention control procedures in standard care groups to gain insight on mechanisms of improved outcomes. A limitation of the treatment outcome data is that participation in assessment interviews was low overall and unequal across groups. Future research on digital therapeutics should aim for improved research assessment completion rates to minimize bias in reported results. Increasing incentives for assessment completion and conducting assessments remotely using mobile phone technology among both treatment and control groups may improve completion rates. Finally, further investigation in more diverse populations and treatment settings, including persons who are out-of-treatment, could also provide insight into the target population(s) for which this app might prove useful and efficacious.
Overall, this study highlights the feasibility and potential clinical benefit of implementing the DynamiCare Health smartphone app in a population of persons with primary alcohol use enrolled in community treatment. We demonstrated high rates of engagement and better retention than usual care. With its unique features of substance use self-testing and automated delivery of abstinence incentives, DynamiCare delivers an evidence-based intervention, CM, with fidelity and in a conveniently automated manner. The app effectively addresses an important implementation barrier—the need for clinicians to manage a CM program—and could provide a unique and versatile addition to current mobile health interventions for SUD.
HIGHLIGHTS.
DynamiCare Health delivers contingency management via a mobile phone app
App features alcohol & drug self-testing and remote delivery of monetary incentives
66% used the app with good compliance over 90 days
App was shown to be feasible as an adjunct to usual care SUD treatment
Role of the funding source:
This work was funded by the NIH/NIAAA Small Business Innovative Research Grant 1R43AA026234-01. The funding source was not involved in the study design, research, nor the preparation of the article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: We have no conflicts of interest to disclose.
References
- Alessi SM, & Petry NM (2013). A randomized study of cellphone technology to reinforce alcohol abstinence in the natural environment. Addiction (Abingdon, England), 108(5), 900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benishek LA, Dugosh KL, Kirby KC, Matejkowski J, Clements NT, Seymour BL, & Festinger DS (2014). Prize-based contingency management for the treatment of substance abusers: a meta-analysis. Addiction, 109, 1426–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciola JS, Alterman AI, DePhilippis D, Drapkin ML, Valadez C Jr, Fala NC, … & McKay JR (2013). Development and initial evaluation of the Brief Addiction Monitor (BAM). Journal of Substance Abuse Treatment, 44(3), 256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell AN, Nunes EV, Matthews AG, Stitzer M, Miele GM, Polsky D, & Wahle A (2014). Internet-delivered treatment for substance abuse: a multisite randomized controlled trial. American Journal of Psychiatry, 171(6), 683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM. Lost in translation? Moving contingency management and cognitive behavioral therapy into clinical practice. (2014). Ann N Y Acad Sci. 1327(1).94–111. doi: 10.1111/nyas.12501. Epub 2014 September 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran G, Stitzer M, Campbell AN, Hu MC, Vandrey R, & Nunes EV (2015). Web-based treatment for substance use disorders: Differential effects by primary substance. Addictive Behaviors, 45, 191–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallery J, Raiff BR, Grabinski MJ, & Marsch LA (2019). Technology-Based Contingency Management in the Treatment of Substance Use Disorders. Perspect Behav Sci. 42(3):445–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallery J, Raiff BR, Kim SJ, Marsch LA, Stitzer M, & Grabinski MJ (2017). Nationwide access to an internet-based contingency management intervention to promote smoking cessation: a randomized controlled trial. Addiction, 112(5):875–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, & Otto MW (2008). A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry, 165(2): 179–87. doi: 10.1176/appi.ajp.2007.06111851. Epub 2008 January 15. [DOI] [PubMed] [Google Scholar]
- Getty CA, Morande A, Lynskey M, Weaver T, & Metrebian N (2019). Mobile telephone-delivered contingency management interventions promoting behaviour change in individuals with substance use disorders: a meta-analysis. Addiction, 114(11), 1915–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith JD, Rowan-Szal GA, Roark RR, & Simpson DD (2000). Contingency management in outpatient methadone treatment: a meta-analysis. Drug Alcohol Depend, 58(l-2):55–66. doi: 10.1016/s0376-8716(99)00068-x. [DOI] [PubMed] [Google Scholar]
- Herbeck DM, Hser YI, Teruya C (2008). Empirically supported substance abuse treatment approaches: a survey of treatment providers’ perspectives and practices. Addict Behav, 33(5):699–712. doi: 10.1016/j.addbeh.2007.12.003. Epub 2007 Dec 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffarnus MN, Bickel WK, & Kablinger AS (2018). Remote alcohol monitoring to facilitate incentive-based treatment for alcohol use disorder: a randomized trial. Alcoholism: Clinical and Experimental Research, 42(12), 2423–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapham S, Forman R, Alexander M, Illeperuma A, Bohn MJ (2009). The effects of extended-release naltrexone on holiday drinking in alcohol-dependent patients. J Subst Abuse Treat, 36(1): 1 –6. [DOI] [PubMed] [Google Scholar]
- Lin LA, Fernandez AC, & Bonar EE (2020). Telehealth for substance-using populations in the age of coronavirus disease 2019: Recommendations to enhance adoption. JAMA Pyschiatry. Published online July 01, 2020. doi: 10.1001/jamapsychiatry.2020.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lussier JP, Heil SH, Mongeon JA, Badger GJ, & Higgins ST (2006). A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction (Abingdon, England), 101(2), 192–203. [DOI] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, Roll J (2006). Contingency management for treatment of substance use disorders: a meta-analysis. Addiction, 101(11): 1546–60. doi: 10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- Silverman K, Robles E, Mudric T, Bigelow GE, & Stitzer ML (2004). A randomized trial of long-term reinforcement of cocaine abstinence in methadone-maintained patients who inject drugs. Journal of Consulting mid Clinical Psychology, 72, 839–854. [DOI] [PubMed] [Google Scholar]
- Voelker R (2019). App aids treatment retention for opioid use disorder. The Journal of the American Medical Association, 321(5), 444. 10.1001/jama.2018.21932 [DOI] [PubMed] [Google Scholar]


