Abstract
Background:
Maintenance treatments with medications for opioid use disorder (MOUD) are highly effective at reducing overdose risk while patients remain in care. However, few patients initiate medication and retention remains a critical challenge across settings. Much remains to be learned about individual and structural factors that influence successful retention, especially among populations dispensed MOUD in outpatient settings.
Methods:
We examined individual and structural characteristics associated with MOUD treatment retention among a national sample of adults seeking MOUD treatment in outpatient substance use treatment settings using the 2017 Treatment Episode Dataset-Discharges (TEDS-D). The study assessed predictors of retention in MOUD using multivariate logistic regression and accelerated time failure models.
Results:
Of 130,300 episodes of MOUD treatment in outpatient settings, 36% involved a duration of care greater than six months. The strongest risk factors for treatment discontinuation by six months included being of younger age, ages 18–29 ((OR):0.52 [95%CI:0.50–0.54]) or 30–39 (OR:0.57 [95%CI:0.55–0.59); being homeless (OR: 0.70 [95%CI:0.66–0.73]); co-using methamphetamine (OR:0.48 [95%CI:0.45–0.51]); and being referred to treatment by a criminal justice source (OR:0.55 [95%CI:0.52–0.59) or by a school, employer, or community source (OR:0.71 [95%CI:0.66–0.76).
Conclusions:
Improving retention in treatment is a pivotal stage in the OUD cascade of care and is critical to reducing overdose deaths. Efforts should prioritize interventions to improve retention among patients who are both prescribed and dispended MOUD, especially youth, people experiencing homelessness, polysubstance users, and people referred to care by the justice system who have especially short stays in care.
Keywords: Medication treatment, Opioid use disorder, Retention, Discontinuation, Overdose
1. Introduction
Opioid use continues to be a leading cause of morbidity and mortality across the United States (Scholl et al., 2019). Increasing access to treatment involving medications for opioid use disorder (MOUD)—methadone, buprenorphine, and extended-release naltrexone—is a critical component to addressing the ongoing opioid crisis. The opioid use disorder (OUD) cascade of care, a framework preferred by federal health agencies, emphasizes two stages along the cascade: MOUD initiation and MOUD retention (Blanco & Volkow, 2019; Arthur Robin Williams, Nunes, et al., 2019).
Maintenance with MOUD is the gold standard treatment for OUD, and results in better outcomes than short-term regimens or tapers (Calsyn et al., 2006; Fiellin et al., 2014; H. E. Jones et al., 2008; Magura & Rosenblum, 2001; Martin et al., 2018; SAMHSA, 2019a). The protective effect of MOUD is greatly diminished upon discontinuation: multiple studies have found maintaining abstinence to be rare (Kornør & Waal, 2005), while risk of overdose and other adverse events increases substantially, especially in the first 2–4 weeks following cessation (Clausen et al., 2008; Cousins et al., 2011; Krawczyk et al., 2020; Arthur Robin Williams, Samples, et al., 2019). As a result, the national quality forum (NQF) endorsed a minimum of 180 days of MOUD as a quality measure for OUD treatment continuity (National Quality Forum, 2017).
Despite these recommendations, barriers to access and retention in evidence-based treatment with MOUD remain an ongoing and critical challenge. First, only a minority of patients receive any treatment with MOUD (Andrilla et al., 2019; Mojtabai et al., 2019; Stein et al., 2018). Second, among those who do begin MOUD, sufficient retention to confer long-term protection is rare: Most patients who begin buprenorphine discontinue care within the first few weeks or months (Meinhofer et al., 2019; Morgan et al., 2019; Saloner et al., 2017). Studies of extended-release naltrexone indicate even greater odds of discontinuation (Jarvis et al., 2018; Lincoln et al., 2018; Morgan et al., 2018). While methadone has consistently yielded superior retention rates (Hser et al., 2014; Timko et al., 2016), early methadone discontinuation remains a commonly reported problem (Deck & Carlson, 2005; Reisinger et al., 2009).
Challenges to retention are multifaceted. Such challenges include the chronic, relapsing nature of addiction; patient ambivalence; medication stigma; and programmatic, regulatory, and logistical hurdles (Andraka-Christou, 2016; Bentzley et al., 2015; Gryczynski et al., 2014; Reisinger et al., 2009; Rosenblum et al., 2011; Truong et al., 2019). Still, there is much to be learned about individual and structural factors that influence successful retention to guide interventions to improve duration of care as the opioid epidemic evolves. In particular, more research is needed to understand risk factors for discontinuation in programs that dispense MOUD. Currently, most MOUD retention studies examine trends in persons who receive prescribed MOUD, using prescription claims or pharmacy data of patients treated in office-based settings (Saloner et al., 2017; Samples et al., 2018). Such studies miss a critical group of patients who access MOUD through specialty substance treatment programs that dispense medications, rather than prescribe. Such patients, often the most complex clinical cases, differ substantially from those prescribed buprenorphine in office-based settings (Fingerhood et al., 2014). To date, however, studies of retention in populations dispensed MOUD have largely focused on fairly limited clinical samples (Cox et al., 2013; Kelly et al., 2011; Proctor et al., 2015), In this study, we aimed to expand this literature by examining sociodemographic and clinical characteristics associated with MOUD treatment retention among a national sample of patients seeking OUD treatment in outpatient substance use treatment settings.
2. Material and methods
2.1. Data sources
We utilized data from the 2017 Treatment Episode Dataset-Discharges (TEDS-D), a Substance Abuse and Mental Health Services Administration–administered database compiling information on discharges from publicly licensed or funded specialty (i.e. nonprivate physician office) substance use treatment facilities (Batts et al., 2014). TEDS-D captures the majority of admissions to specialty substance use treatment in the United States from all 50 states,a the District of Columbia, and Puerto Rico (SAMHSA, 2019b).
We restricted data to discharges of adults 18+ treated in outpatient settings with MOUD. Outpatient settings include opioid treatment programs licensed by the Drug Enforcement Agency to dispense all medications including methadone, or other outpatient programs (intensive and nonintensive) that may deliver buprenorphine or naltrexone as part of care. We excluded episodes that took place in detoxification, inpatient, or residential treatment settings, which generally do not offer MOUD maintenance (Huhn et al., 2020). The study defined retention in treatment using the TEDS “length of stay in treatment (days)” variable. Number of days in treatment is computed using the date of admission and the date of last contact. One day is added to all outpatient discharges, so that the first day and last day of outpatient treatment are counted. Length of treatment is reported using a continuous measure of up to 30 days, and thereafter in categories of 31–45, 46–60, 61–90, 91–120, 120–180, 181–365, and 366+ days. For consistency, we recoded the first 30 days of treatment into categories of 1–15 and 16–30 days. The dataset reported discharge reason as either treatment completed, dropped out of treatment, terminated by facility, transferred to another program or facility, incarcerated, death, or other reason.
2.2. Modeling treatment retention
Our primary outcome of interest was retention beyond six months, based on NQF treatment cascade metrics for MOUD continuity (National Quality Forum, 2017; Arthur Robin Williams et al., 2018). We analyzed predictors of treatment retention using two approaches: First, we used multivariate logistic regression to assess predictors of attending in MOUD treatment longer than six months (181+ days). The study included all treatment episodes, regardless of discharge reason. We complemented this with a sensitivity analysis in which the discharge reason “transferred to another program or facility” was equivalent to remaining in MOUD.
Given that there is no gold standard for ideal length of treatment in MOUD, we also tested a second approach using multivariate accelerated time failure (ATF) models to assess treatment retention in a time-to-event framework (Wei, 1992). This approach allowed us to assess risk factors for shorter treatment retention without restricting the model to a preset standard of six months. Unlike the more commonly known Cox regression model, the ATF model does not depend on the major assumption of proportional hazards over time. In addition, ATFs provide a more intuitive interpretation for studying length of care by generating a time ratio of the relative time to failure (treatment discontinuation), with ratios lower than one indicating a shorter time to discontinuation associated with that characteristic. We used a loglogistic distribution for the ATF model as it resulted in the lowest Akaike information criterion (Akaike, 1974). The study censored treatment episodes if a patient’s reason for discharge was “transferred to another program or facility” or at a maximum of six months follow-up.
To assess whether predictors of retention changed if we used a different length of treatment cutoff, we repeated regression analyses using retention past twelve months as the binary outcome and accelerated time failure analyses using twelve months as the censoring follow-up cut-off. Due to a large proportion of episodes with missing covariates (28%), we repeated multivariable analyses using multiple imputation using chained equations (I. R. White et al., 2011) as a sensitivity analysis. Research staff conducted all analyses in Stata 15 (StataCorp, 2017).
2.3. Predictors of MOUD treatment retention
We first examined sociodemographic predictors of MOUD retention, including age, race/ethnicity, education, employment, housing, and veteran status (Table 1), which research has shown play a role in substance use treatment access and outcomes (N. Krawczyk, Feder, et al., 2017; Proctor et al., 2015; Saloner & Cook, 2013). We also included an indicator for prior month arrest, as justice involvement often influences conditions of treatment (Fox et al., 2014). Given geographic variation in access to MOUD and MOUD practices (Rosenblatt et al., 2015; Rosenblum et al., 2011; Sigmon, 2014), we included a dummy variable for each U.S. state in our database, along with Puerto Rico and the District of Columbia.
Table 1:
Characteristics of patients in MOUD treatment (N=130,300).1
| Prevalence (N(%)) | |
|---|---|
| Sex | |
| Male | 77,220 (59.26%) |
| Female | 53,062 (40.72%) |
| Missing | 18 (0.01%) |
| Age Group | |
| 18–29 | 39,327 (30.18%) |
| 30–39 | 43,038 (33.03%) |
| 40–49 | 23,637 (18.14%) |
| 50+ | 24,298 (18.65%) |
| Race/Ethnicity | |
| Non-Hispanic White | 86,031 (66.03%) |
| Non-Hispanic Black | 15,493 (11.89%) |
| Non-Hispanic Other | 6,639 (5.10%) |
| Hispanic (any race) | 21,541 (16.53%) |
| Missing | 596 (0.46%) |
| Education | |
| Eight or less | 6,523 (5.01%) |
| Nine to eleven | 28,081 (21.55%) |
| Twelve | 62,160 (47.71%) |
| Thirteen or more | 31,950 (24.52%) |
| Missing | 1,586 (1.22%) |
| Employment | |
| Employed | 30,447 (23.37%) |
| Not Employed | 97,465 (74.80%) |
| Missing | 2,388 (1.83%) |
| Housing | |
| Independent Housing | 10,0075 (76.80%) |
| Dependent Housing | 17,145 (13.16%) |
| Homeless | 10,642 (8.17%) |
| Missing | 2,438 (1.87%) |
| Veteran | |
| Not Veteran | 12,0678 (92.62%) |
| Veteran | 3,226 (2.48%) |
| Missing | 6,396 (4.91%) |
| Prior Month Arrest | |
| No Arrest | 11,4621 (87.97%) |
| Arrest | 5,807 (4.46%) |
| Missing | 9,872 (7.58%) |
| Age of First Use | |
| Up to 17 | 30,911 (23.72%) |
| 18–24 | 51,477 (39.51%) |
| 25+ | 46,615 (35.78%) |
| Missing | 1,297 (1.00%) |
| Frequency of Use in Prior Month | |
| No use | 25,824 (19.82%) |
| Some use | 20,362 (15.63%) |
| Daily use | 69,983 (53.71%) |
| Missing | 14,131 (10.84%) |
| Primary Opioid Use | |
| Primary Prescription Opioids | 27,900 (21.41%) |
| Primary Heroin | 10,2400 (78.59%) |
| Comorbid Psychiatric Problem | |
| No Psych Problem | 74,492 (57.17%) |
| Psych Problem | 46,657 (35.81%) |
| Missing | 9,151 (7.02%) |
| Alcohol | 13,087 (10.04%) |
| Marijuana | 22,983 (17.64%) |
| Benzos | 8,497 (6.52%) |
| Cocaine | 28,956 (22.22%) |
| Methamphetamine | 12,067 (9.26%) |
| Prior Tx | |
| No Prior Tx | 32,762 (25.14) |
| Prior Tx | 95,773 (73.50%) |
| Missing | 1,765 (1.35%) |
| Referral Source | |
| Individual/Self | 94,103 (72.22%) |
| Criminal justice | 9,062 (6.95%) |
| Health/SUD provider | 18,212 (13.98%) |
| School/employer/community provider | 8,058 (6.18%) |
| Missing | 865 (0.66%) |
| Health Insurance (N=33,939)2 | |
| No Insurance | 6,835 (20.14%) |
| Private/commercial | 1,991 (5.87%) |
| Medicaid | 22,839 (67.29) |
| Medicare | 2,274 (6.70%) |
Table Notes:
Number of episodes included per state is displayed in Table 3
Estimates excludes states that reported insurance on less than 50% of episodes: Arizona, California, Connecticut, Florida, Illinois, Michigan, Minnesota, New Mexico, New York, North Carolina, Ohio, Rhode Island, Vermont, Virginia, Washington, Wisconsin.
We then examined clinical and treatment predictors of MOUD retention, including substance use behaviors prior to admission, age of first use, frequency of use, primary use of heroin vs. other opioids, and psychiatric comorbidity (Noa Krawczyk et al., 2017; Nielsen et al., 2015). We also examined the role of reporting a secondary or tertiary substance problem involving alcohol, cannabis, benzodiazepine, cocaine, or methamphetamine (Proctor et al., 2015; Samples et al., 2018). We examined two additional variables that we hypothesized may impact treatment retention: having had prior substance use treatment and referral source, which has been shown to heavily influence treatment regimen and outcomes (N. Krawczyk, Picher, et al., 2017; Sahker et al., 2015). Last, we examined the role of insurance, as certain types of insurance limit care duration (Polsky et al., 2019; Reif et al., 2017). As only certain states report insurance status to TEDS, we conducted a subanalysis of this risk factor excluding sixteen statesb for which this variable was missing in most records.
2.4. Reasons for discharge among high risk groups
To explore potential drivers of MOUD discontinuation, we plotted the distribution of discharge reasons across MOUD episodes discharged prior to six months. We then explored such distribution across specific groups with predictors strongly associated with lower retention, defined as an odds ratio of six-month retention <0.75.
3. Results
3.1. Sample characteristics
A total of 301,080 treatment discharges were recorded in the TEDS-D 2017 for adults seeking OUD treatment in outpatient programs. Of these, 43.3% (130,300) of episodes included MOUD. A survival curve of the distribution of length of treatment across all MOUD episodes is displayed in Figure 1. Only 36% of episodes involved durations of care greater than six months. Across the entire sample (regardless of length of treatment), the most common reason for discharge was dropout (38.1%), followed by transfer (31.8%), treatment completion (12.1%), termination by facility (8.6%), other (4.5%), incarcerated (3.8%), and death (1.1%).
Figure 1:
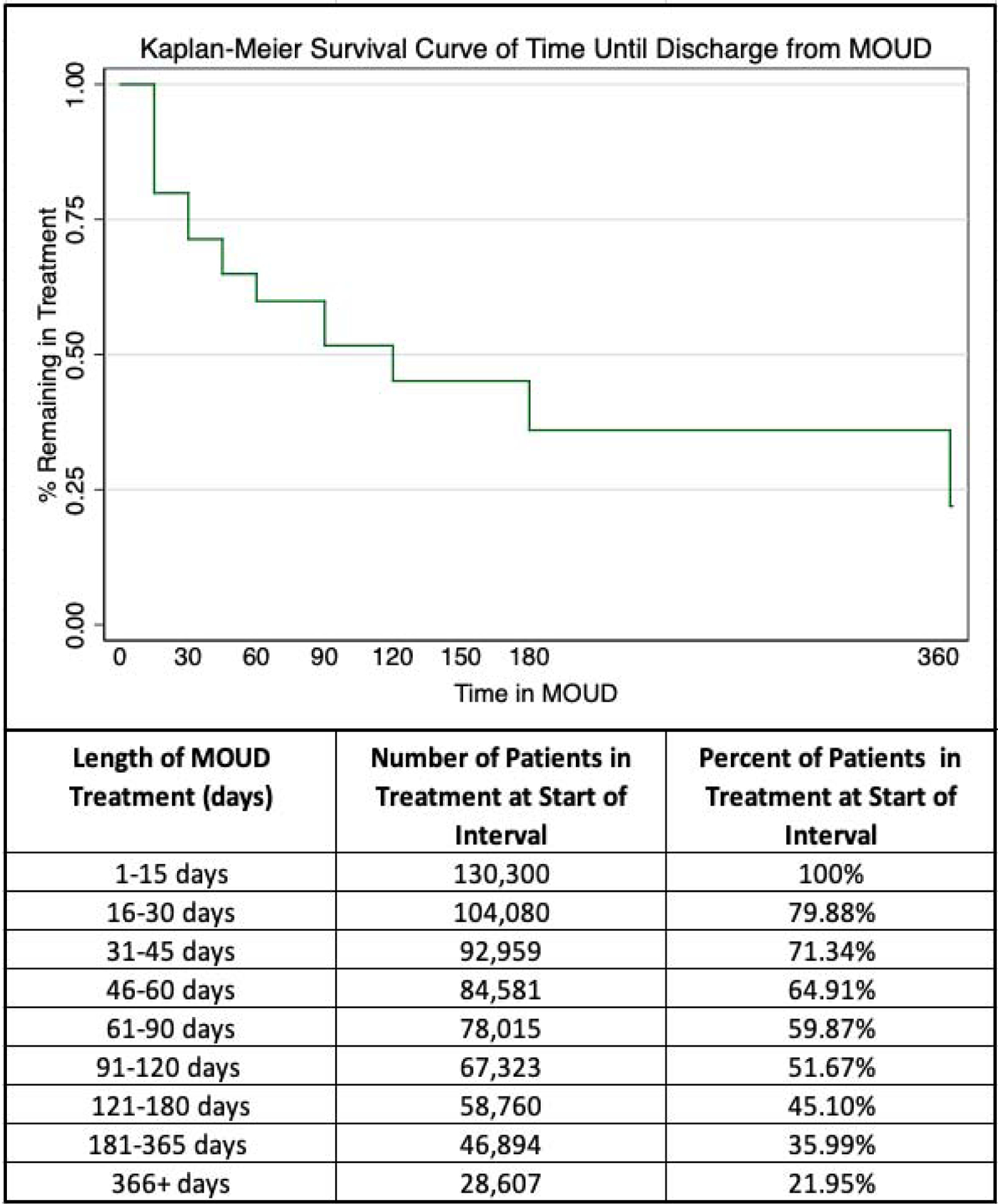
Length of treatment across all medication for opioid use disorder (MOUD) episodes.
Table 1 contains sociodemographic and clinical characteristics of MOUD patients. Patients were primarily male (59%), ages 18–39 (63%), and non-Hispanic White (66%). The majority had 12+ years of education (72%) and were unemployed (75%). Nearly 77% indicated living in independent housing. Few were veterans (2%) or had prior month arrests (4%).
Most patients started using opioids after age 18 (75%) and 54% reported daily use of opioids. Seventy-nine percent reported primary use of heroin, and 36% reported a comorbid psychiatric problem. The most commonly reported secondary substance used was cocaine (22%), followed by cannabis (18%), alcohol (10%), methamphetamine (9%) ,and benzodiazepines (7%). Nearly three quarters reported having prior substance use treatment. Seventy-two percent were self-referred/referred by another individual, 14% by a health care provider, 7% by a criminal justice entity, and 6% by school/employer/community referral. Of patient episodes in states that reported health insurance (N=33,939), 67% had Medicaid, 7% had Medicare, 6% had private insurance, and 20% were uninsured.
A total of 28% of episodes were missing at least one or more of the above variables. With the exception of Health Insurance, the variables with the largest proportion of missing data included frequency of use (10.8%), past month arrest (7.6%), comorbid psychiatric problem (7.0%), and veteran status (4.9%). All other variables were missing for 2% of episodes or less.
3.2. Predictors of treatment retention
Table 2 presents odds ratios and time ratios for retention in MOUD. Predictors most strongly associated with shorter retention in MOUD were generally consistent across both models (see Table 2 for time ratios). Sociodemographic factors most strongly associated with lower odds of six-month retention included being aged 18–29 (adjusted odds ratio (OR):0.52 [95%CI:0.50–0.54]) or 30–39 (OR:0.57 [95%CI:0.55–0.59), compared to 50+; and being homeless (OR: 0.70 [95%CI:0.66–0.73]).
Table 2:
Odds ratios and time ratios for retention greater than six months in MOUD.
| Odds Ratio (OR) | 95% Confidence Interval | Time Ratio (TR) | 95% Confidence Interval | |
|---|---|---|---|---|
| Sociodemographic Characteristicsa | ||||
| Female | 1.10 | [1.07,1.13] | 1.09 | [1.07,1.12] |
| Age Group | ||||
| 50+ (ref) | 1 | – | 1 | – |
| 40–49 | 0.79 | [0.75,0.82] | 0.84 | [0.81,0.87] |
| 30–39 | 0.57 | [0.55,0.59] | 0.68 | [0.66,0.71] |
| 18–29 | 0.52 | [0.50,0.54] | 0.64 | [0.62,0.66] |
| Race/Ethnicity | ||||
| Non-Hispanic White (ref) | 1 | – | 1 | – |
| Non-Hispanic Black | 1.09 | [1.04,1.14] | 0.96 | [0.94,1.01] |
| Non-Hispanic Otherb | 0.91 | [0.86,0.97] | 0.9 | [0.86,0.94] |
| Hispanic (any race) | 1.18 | [1.14,1.22] | 1.07 | [1.04,1.10] |
| Education (years) | ||||
| Eight or less (ref) | 1 | – | 1 | – |
| Nine to eleven | 0.98 | [0.92,1.04] | 1.01 | [0.97,1.06] |
| Twelve | 0.9 | [0.85,0.96] | 0.99 | [0.95,1.03] |
| Thirteen or more | 0.95 | [0.89,1.01] | 1.03 | [0.99,1.09] |
| Not Employed | 0.83 | [0.80,0.85] | 0.89 | [0.86,0.91] |
| Housing | ||||
| Independent Housing (ref) | 1 | – | 1 | – |
| Dependent Housing | 0.87 | [0.83,0.90] | 0.95 | [0.92,0.98] |
| Homeless | 0.7 | [0.66,0.73] | 0.77 | [0.75,0.80] |
| Veteran | 1.02 | [0.94,110] | 1.03 | [0.96,1.09] |
| Prior Month Arrest | 0.75 | [0.71,0.80] | 0.83 | [0.79,0.87] |
| Clinical and Treatment Characteristicsc | ||||
| Age of First Use | ||||
| Up to 17 | 1 | – | 1 | – |
| 18–24 | 1.01 | [0.97,1.05] | 1 | [0.97,1.03] |
| 25+ | 0.9 | [0.87,0.94] | 0.93 | [0.91,0.96] |
| Frequency of Use in Month Prior to Tx | ||||
| No use | 1 | – | 1 | – |
| Some use | 0.89 | [0.85,0.93] | 0.89 | [0.86,0.92] |
| Daily use | 0.91 | [0.88,0.95] | 0.87 | [0.84,0.89] |
| Primary Heroin | 0.86 | [0.83,0.90] | 0.97 | [0.94,1.00] |
| Comorbid Psychiatric Problem | 0.95 | [0.92,0.99] | 0.97 | [0.95,0.99] |
| Cum orb id Alcohol | 0.87 | [0.83,0.92] | 0.9 | [0.87,0.93] |
| Comorbid Cannabis | 0.92 | [0.88,0,95] | 0.95 | [0.92,0.98] |
| Comorbid Benzos | 0.97 | [0.91,1.03] | 1.02 | [0.98,1.06] |
| Comorbid Cocaine | 0.83 | [0.80,0.86] | 0.87 | [0.85,0.90] |
| Comorbid Methamphetamine | 0.48 | [0.45,0.51] | 0.64 | [0.61,0.66] |
| Prior Treatment | 1.09 | [1.05,1.13] | 1.08 | [1.05,1.11] |
| Referral Source to Treatment | ||||
| Individual/Self | 1 | 1 | _ | |
| Criminal justice | 0.55 | [0.52,0.59] | 0.77 | [0.74,0.80] |
| Health/Substance Use provider | 0.77 | [0.74,0.81] | 0.82 | [0.79,0.84] |
| School/Employer/Community provider | 0.71 | [0.66,0.76] | 0.85 | [0.81,0.88] |
| Health Insurance (N = 33,939)d | ||||
| No Insurance | 1 | – | 1 | – |
| Private/commercial | 0.89 | [0.77,1.02] | 0.95 | [0.88,1.03] |
| Medicaid | 0.96 | [0.88,1.04] | 1.08 | [1.04,1.14] |
| Medicare | 1.11 | [0.97,1.28] | 1.05 | [0.97,1.14] |
Bold indicated statistically significant at p<0.05 level.
Estimates are adjusted for all sociodemographic characteristics, including state
Other race includes Asian, Asian/Pacific Islander, Native Hawaiian or Other Pacific Islander, American Indian, Alaska Native, other single race, or two or more races
Estimates are adjusted for all sociodemographic and clinical characteristics
Estimates exclude states that reported insurance on less than 50% of episodes: Arizona, California, Connecticut, Florida, Illinois, Michigan, Minnesota, New Mexico, New York, North Carolina, Ohio, Rhode Island, Vermont, Virginia, Washington, Wisconsin. Estimates are adjusted for all sociodemographic and clinical characteristics
Clinical and treatment factors most strongly associated with lower odds of six-month retention were comorbid methamphetamine use (OR:0.48 [95%CI:0.45–0.51]); and referral to treatment from a criminal justice source (OR:0.55 [95%CI:0.52–0.59), or by a school/employer/community source (OR:0.71 [95%CI:0.66–0.76) compared to self/other individual.
The study also found striking differences in retention based on state of residence. We present the proportion of episodes that had treatment lengths greater than six months by state/territory in Table 3. Regions with the highest proportion of episodes with length of greater than six months included Puerto Rico (70.4%), the District of Columbia (68.66%), and Arizona (58.02%). On the other hand, those with the lowest proportion of episodes longer than six months included North Carolina (4.3%), Kentucky (5.4%), and Illinois (10.0%).
Table 3:
MOUD treatment episodes by state and proportion of episodes greater than six months.
| State | Number of Treatment Episodes | Proportion of Treatment Episodes with Length > 6 Months |
|---|---|---|
| NORTH CAROLINA | 9,541 | 4.32% |
| KENTUCKY | 5,263 | 5.38% |
| ILLINOIS | 1,331 | 9.99% |
| NEW MEXICO | 16 | 12.50% |
| MARYLAND | 6,000 | 15.87% |
| SOUTH CAROLINA | 509 | 17.68% |
| IDAHO | 35 | 20.00% |
| NEW HAMPSHIRE | 195 | 20.00% |
| DELAWARE | 262 | 20.61% |
| MISSOURI | 1,306 | 28.41% |
| PENNSYLVANIA | 1,540 | 29.22% |
| IOWA | 388 | 29.97% |
| INDIANA | 388 | 31.44% |
| MISSISSIPPI | 25 | 32.00% |
| UTAH | 903 | 33.22% |
| SOUTH DAKOTA | 36 | 33.33% |
| WISCONSIN | 9 | 33.33% |
| MINNESOTA | 3,638 | 34.06% |
| NEBRASKA | 17 | 35.29% |
| MICHIGAN | 6,717 | 35.43% |
| NEW JERSEY | 10,322 | 36.49% |
| ALASKA | 154 | 37.01% |
| OHIO | 2,427 | 38.48% |
| ARKANSAS | 221 | 38.91% |
| VERMONT | 1,990 | 38.94% |
| ALABAMA | 279 | 39.78% |
| MASSACHUSETTS | 3,550 | 39.92% |
| NEW YORK | 25,292 | 40.74% |
| WYOMING | 22 | 40.91% |
| MAINE | 2,122 | 41.89% |
| NEVADA | 26 | 42.31% |
| CALIFORNIA | 31,239 | 44.45% |
| WASHINGTON | 149 | 46.31% |
| LOUISIANA | 15 | 46.67% |
| FLORIDA | 586 | 47.78% |
| COLORADO | 1,487 | 47.81% |
| HAWAII | 22 | 50.00% |
| RHODE ISLAND | 2,311 | 51.10% |
| CONNECTICUT | 7,287 | 52.01% |
| TEXAS | 546 | 54.21% |
| TENNESSEE | 7 | 57.14% |
| VIRGINIA | 26 | 57.69% |
| ARIZONA | 1,558 | 58.02% |
| DISTRICT OF COLUMBIA | 67 | 68.66% |
| PUERTO RICO | 527 | 70.40% |
3.3. Supplementary analyses
First, logistic regression models that treated transferred discharges as continuing MOUD rather than discontinuing overall yielded qualitatively similar results (see Appendix Table 1), with slightly attenuated odds ratios for most variables. Second, analyses performed using 12-month retention as the primary outcome identified the same predictors to be most strongly associated with retention in care, with somewhat attenuated odds and time ratios (Appendix Table 2). Analyses using Multiple Imputation for missing variables did not qualitatively change findings.
3.4. Discharge reasons among high risk groups
Figure 2 presents the distribution of reasons for discharge by six months for all episodes and specifically for those with predictors associated with highest risk of discontinuation by six months (OR < 0.75). These predictors included being < 40 years of age, experiencing homelessness, having co-used methamphetamine, and referred either by a criminal justice source or a school/employer/community source. Drop out was the primary reason for discharge prior to six months across all episodes (38.9%) and accounted for the largest proportion of discharges among those under 40 (37.1%), homeless (43.4%), and justice-referred (29.9%) and referred by a school, employer, or community referrals (35.8%). Transfers accounted for another 35.9% of all episodes discharged prior to six months and were especially prevalent among those with methamphetamine co-use (46.6%). Only 9.2% of episodes had a discharge reason prior to six months noted as completed, but this outcome was more common among those referred by the criminal justice system (16.3%) and by a school/employer/community referral (11.0%). Termination by the facility accounted for 7.8% of discharges overall, and was reported more often for episodes with criminal justice referral (11.5%). Incarceration, death, and other reason together accounted for 8.1% of discharge reasons across episodes.
Figure 2:
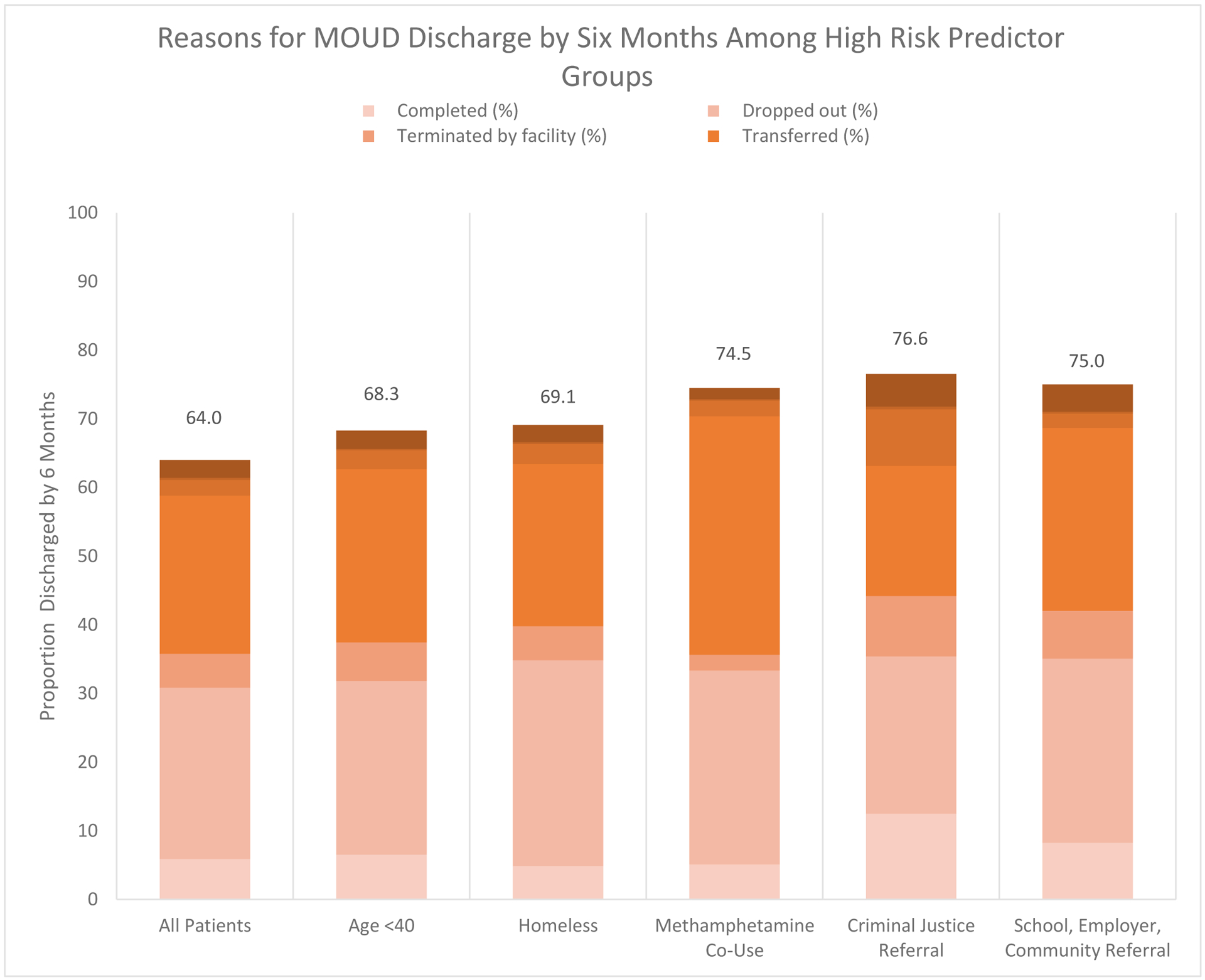
Reasons for MOUD discharge by six months among high risk predictor groups.
4. Discussion
The current study aimed to explore patterns and predictors of MOUD retention among a national sample seeking OUD care in outpatient specialty treatment programs. While highly vulnerable to overdose (Krawczyk et al., 2020), this segment of patients is missing from population-based studies on MOUD retention that use pharmacy claims data. We found that only 36% of MOUD patients had lengths of treatment greater than six months. This substantial gap in MOUD continuity, which has also been noted in studies of office-based buprenorphine patients (Meinhofer et al., 2019; Morgan et al., 2019; Saloner et al., 2017), is concerning given MOUD maintenance is critical for reducing overdose death (Bentzley et al., 2015; Krawczyk et al., 2020; Arthur Robin Williams, Samples, et al., 2019).
Our findings highlight that variation in treatment retention was strongly related to patient characteristics. Of demographic characteristics, being younger than 40 was the strongest predictor of shorter retention, corroborating prior studies and suggesting a great need for intervention development (Proctor et al., 2015; Samples et al., 2018). Targeted interventions for younger patients could include mobile technologies, involving family/peer supports, and contingency management (Schuman-Olivier et al., 2014). Patients experiencing homelessness also had shorter treatment retention, highlighting the need for specific services such as care coordination (Paudyal et al., 2017). Expanding models that prioritize housing for those with behavioral health conditions such as Housing First (Appel et al., 2012), low-threshold MOUD through more flexible programs (Greenfield et al., 1996; Krawczyk et al., 2019) and alternative payment models (such as bundled payments for social services and transportation) (Arthur R. Williams & Bisaga, 2016) may help to facilitate retention.
Geographic location was also a strong predictor of treatment retention, with the proportion of episodes that lasted longer than six months varying drastically across states/territories. There was no explicit variation by state size, Medicaid expansion status, or region, as states with both high and low proportions of patients retained past six months had a variety of such characteristics Puerto Rico had a particularly high retention rate, with nearly three quarters of treatment episodes lasting longer than six months. This retention rate should be subject to further inquiry for potential models of care delivery that may encourage and facilitate longer treatment adherence and continuity. On the other hand, findings that in multiple states less than a quarter, and in some cases, less than 5 percent, of MOUD episodes reached the minimum recommended six months raises questions about whether MOUD is largely being used as a taper rather than for maintenance (Calsyn et al., 2006). These findings emphasize the importance of both national efforts as well as local strategies to investigate structural drivers for low MOUD retention and help to identify levers to improve length and successful continuity in care.
Of all clinical factors examined, concomitant methamphetamine was the strongest predictor of shorter treatment retention. Previous studies have increasingly pointed to polysubstance use as a risk factor for early discontinuation of MOUD (Levine et al., 2015; Samples et al., 2018). However, we found that alcohol, cannabis, and benzodiazepines had minimal or no impact on treatment retention. Methamphetamine findings corroborate a recent clinical study of buprenorphine patients in which methamphetamine use was strongly associated with treatment discontinuation (Tsui et al., 2020). This finding is concerning given the growing prevalence of methamphetamine in opioid overdose deaths (Kariisa et al., 2019) and the growing number of treatment admissions involving both heroin and methamphetamine (C. M. Jones et al., 2019). In light of these trends, current findings suggest an urgent need to focus on developing specific interventions to encourage retention for psychostimulant users in MOUD treatment.
We also found that source of referral was strongly associated with MOUD retention: Patients referred by criminal justice or by a school/employer/community provider had significantly shorter episodes than those who self-referred/were referred by another individual. These differences indicate that programs affiliated with criminal justice entities, employers, or schools may require or impose time limits for MOUD that are lower than the recommended six month minimum, or may lead to a taper regimen rather than maintenance. Alternatively, this may reflect a lower treatment need or desire for maintenance treatment among justice or other special programs-referred patients relative to those who choose to engage in care on their own. Still, findings of low duration of care among justice-referred individuals are especially troubling given high rates of overdose (Krawczyk et al., 2020; Merrall et al., 2010) and already extremely limited access to MOUD among this group (N. Krawczyk, Picher, et al., 2017; Noa Krawczyk et al., 2020).
When exploring reasons for discharge prior to six months, we found that most clients dropped out or transferred to other programs. Many studies have examined contributors of early dropout from MOUD, including relapse, interruptions in treatment due to justice involvement, and conflicts with program requirements (Bentzley et al., 2015; Reisinger et al., 2009; Truong et al., 2019). The challenges of attending treatment may be exacerbated for patients attending daily or near-daily specialty treatment programs that dispense MOUD (Harris & McElrath, 2012; Magura & Rosenblum, 2001). Stigma toward medications from patients and families, peers, and providers may also continue to influence early cessation of MOUD (Allen & Harocopos, 2016; Andraka-Christou, 2016; Noa Krawczyk et al., 2018; W. L. White, 2011). Early cessation may explain many episodes whose discharge reason was “treatment completion,” given OUD is a chronic disease requiring long-term care, and few succeed on such short MOUD treatment regimens (Fiellin et al., 2014). The notion of completing treatment in such a short period of time may reflect stigma related to long-term treatment and provider, program, and patient preferences toward MOUD tapers over maintenance. While this study could not to determine whether patients who transferred continued MOUD elsewhere, prior literature has identified risks that can occur during transfer from one provider to another, including overdose (Bogdanowicz et al., 2018). In some cases, patients may be transferred to non-MOUD treatment programs, which may place them at increased overdose risk (Krawczyk et al., 2020). Given high-risk groups such as those with methamphetamine co-use were very likely to be transferred prior to six months, more work is needed to understand the nature of transfers and how to minimize disruptions in MOUD continuity.
Last, our results highlight that the risk factors discussed were not unique in their association with discontinuation prior to six months but rather to a shorter duration of treatment overall. This was exemplified by modeling these relationships using the ATF model as well as using a twelve-month retention cut-off point rather than a six-month cut-off. For example, we found that patients referred to treatment by the justice system had lengths of care three quarters that of those who were self-referred, but had only about half their odds of staying in treatment past six months. The relative duration of care is important given the ideal length of MOUD is still under study and guidelines generally recommend longer care for better outcomes (SAMHSA, 2019a).
4.1. Limitations
This study has multiple limitations. First, data were limited to administrative records of treatment episodes with a discharge date in 2017. Therefore, we were not able to assess treatment length of patients who continued indefinitely in treatment as they were not included in this database. We also could not assess whether patients whose discharge reason was noted as transferred continued MOUD. Second, we only had access to categorical time intervals for length of treatment episodes up to the category of 366+ days, and therefore could not assess treatment retention in a fully continuous manner. Third, we did not have access to clinical information about the nature of MOUD treatment. For example, while methadone is the most common MOUD delivered in specialty outpatient settings (Center for Behavioral Health Statistics, n.d.), we could not distinguish what type of medication patients received (methadone, buprenorphine, or naltrexone), nor the dose of the medication; two important variables known to be strongly associated with retention (Bao et al., 2009; Hser et al., 2014; Timko et al., 2016.) We also could not assess how often medication was dispensed, taken home or prescribed, or the frequency of attendance during each episode of care. Last, we had no information regarding clinical outcomes such as ongoing drug use, which could impact treatment outcomes and retention.
5. Conclusions
Findings highlight that most patients receiving MOUD in outpatient specialty settings do not continue treatment for the minimum recommended time period of six months (National Quality Forum, 2017), similar to what has been found in studies of office-based buprenorphine patients. Findings also show that younger patients, those who are homeless, and those who use methamphetamine are at particular risk of shorter treatment retention. In addition, treatment discontinuation is particularly high in the Midwest and South, and among patients referred to treatment by criminal justice and school/employer/community sources. Given the risks of MOUD discontinuation, especially in an era of such high lethality of the drug supply, improving retention in treatment should be an urgent priority for programs to reduce overdose deaths. Interventions need to focus on incentivizing retention and reducing barriers to care continuation among the most vulnerable groups. Public health efforts to address the opioid crisis should therefore move beyond measuring availability of MOUD alone and prioritize retention as a pivotal stage in the OUD cascade of care (Arthur Robin Williams et al., 2018).
Highlights.
64% of patients in medication treatment for opioid use discontinue prior to six months
Opioid patients who co-use methamphetamine have lower retention in medication treatment
Medication for opioid use disorder treatment retention varies substantially across states
Younger and homeless opioid patients have lower retention in medication treatments
Appendix
Appendix Table 1:
Odds ratios for retention greater than six months in MOUD, assuming transfers continued in MOUD past six months.
| Odds Ratio (OR) | 95% Confidence Interval | |
|---|---|---|
| Sociodemographic Characteristics1 | ||
| Female | 1.13 | [1.11,1.17] |
| Age Group | ||
| 50+ (ref) | 1 | – |
| 40–49 | 0.83 | [0.80,0.87] |
| 30–39 | 0.67 | [0.64,0.70] |
| 18–29 | 0.63 | [0.61,0.66] |
| Race/Ethnicity | ||
| Non-Hispanic White (ref) | 1 | – |
| Non-Hispanic Black | 0.98 | [0.94,1.02] |
| Non-Hispanic Other2 | 0.9 | [0.85,0.95] |
| Hispanic (any race) | 1.05 | [1.02,1.09] |
| Education (years) | ||
| Eight or less (ref) | 1 | – |
| Nine to eleven | 1.03 | [0.97,1.10] |
| Twelve | 0.99 | [0.93,1.05] |
| Thirteen or more | 1.07 | [1.00,1.14] |
| Not Employed | 0.89 | [0.86,0.92] |
| Housing | ||
| Independent Housing (ref) | 1 | – |
| Dependent Housing | 0.93 | [0.89,0.96] |
| Homeless | 0.78 | [0.74,0.81] |
| Veteran | 1 | [0.92,1.08] |
| Prior Month Arrest | 0.8 | [0.75,0.85] |
| Clinical and Treatment Characteristics3 | ||
| Age of First Use | ||
| Up to 17 | 1 | – |
| 18–24 | 1 | [0.96,1.04] |
| 25+ | 0.91 | [0.88,0.95] |
| Frequency of Use in Month Prior to Tx | ||
| No use | 1 | – |
| Some use | 0.96 | [0.91,1.00] |
| Daily use | 0.92 | [0.89,0.96] |
| Primary Heroin | 0.97 | [0.93,1.01] |
| Comorbid Psychiatric Problem | 0.98 | [0.95,1.01] |
| Comorbid Alcohol | 0.89 | [0.85,0.93] |
| Comorbid Cannabis | 0.92 | [0.89,0.96] |
| Comorbid Benzos | 1.08 | [1.02,1.15] |
| Comorbid Cocaine | 0.85 | [0.82,0.88] |
| Comorbid Methamphetamine | 0.67 | [0.63,0.70] |
| Prior Treatment | 1.02 | [0.99,1.06] |
| Referral Source to Treatment | ||
| Individual/Self | 1 | |
| Criminal justice | 0.61 | [0.57,0.65] |
| Health/Substance Use provider | 0.78 | [0.75,0.81] |
| School/Employer/Community provider | 0.8 | [0.75,0.85] |
Appendix Table 2:
Time ratios and odds ratios for retention greater than twelve months in MOUD.
| Odds Ratio (OR) | 95% Confidence Interval | Time Ratio (TR) | 95% Confidence Interval | |
|---|---|---|---|---|
| Sociodemographic Characteristics1 | ||||
| Female | 1.11 | [1.07,1.15] | 1.11 | [1.09,1.13] |
| Age Group | ||||
| 50+ (ref) | 1 | – | 1 | – |
| 40–49 | 0.78 | [0.75,0.82] | 0.82 | [0.80,0.85] |
| 30–39 | 0.52 | [0.49,0.54] | 0.65 | [0.63,0.67] |
| 18–29 | 0.5 | [0.48,0.52] | 0.61 | [0.59,0.63] |
| Race/Ethnicity | ||||
| Non-Hispanic White (ref) | 1 | – | 1 | – |
| Non-Hispanic Black | 1.18 | [1.12,1.24] | 0.98 | [0.95,1.02] |
| Non-Hispanic Other2 | 0.95 | [0.88,1.02] | 0.89 | [0.85,0.94] |
| Hispanic (any race) | 1.29 | [1.24,1.35] | 1.09 | [1.06,1.13] |
| Education (years) | ||||
| Eight or less (ref) | 1 | – | 1 | – |
| Nine to eleven | 0.97 | [0.91,1.04] | 1.01 | [0.96,1.07] |
| Twelve | 0.88 | [0.83,0.95] | 0.98 | [0.94,1.03] |
| Thirteen or more | 0.91 | [0.85,0.98] | 1.03 | [0.98,1.08] |
| Not Employed | 0.81 | [0.78,0.84] | 0.87 | [0.85,0.89] |
| Housing | ||||
| Independent Housing (ref) | 1 | – | 1 | – |
| Dependent Housing | 0.85 | [0.81,0.89] | 0.93 | [0.90,0.96] |
| Homeless | 0.66 | [0.62,0.70] | 0.75 | [0.72,0.77] |
| Veteran | 1.18 | [1.08,1.30] | 1.05 | [0.98,1.12] |
| Prior Month Arrest | 0.77 | [0.71,0.83] | 0.81 | [0.77,0.85] |
| Clinical and Treatment Characteristics3 | ||||
| Age of First Use | ||||
| Up to 17 | 1 | – | 1 | – |
| 18–24 | 0.96 | [0.92,1.01] | 1 | [0.97,1.02] |
| 25+ | 0.85 | [0.81,0.89] | 0.92 | [0.89,0.95] |
| Frequency of Use in Month Prior toTx | ||||
| No use | 1 | – | 1 | – |
| Some use | 0.95 | [0.89,1.00] | 0.89 | [0.86,0.92] |
| Daily use | 0.96 | [0.91,1.00] | 0.86 | [0.84,0.89] |
| Primary Heroin | 0.81 | [0.78,0.85] | 0.95 | [0.92,0.98] |
| Comorbid Psychiatric Problem | 0.96 | [0.92,1.00] | 0.97 | [0.94,0.99] |
| Comorbid Alcohol | 0.83 | [0.78,0.88] | 0.88 | [0.85,0.92] |
| Comorbid Cannabis | 0.86 | [0.82,0.91] | 0.93 | [0.90,0.96] |
| Comorbid Benzos | 0.86 | [0.79,0.92] | 1 | [0.96,1.05] |
| Comorbid Cocaine | 0.81 | [0.77,0.84] | 0.86 | [0.84,0.88] |
| Comorbid Methamphetamine | 0.38 | [0.35,0.41] | 0.58 | [0.55,0.60] |
| Prior Treatment | 1.14 | [1.09,1.19] | 1.09 | [1.06,1.12] |
| Referral Source to Treatment | ||||
| Individual/Self | 1 | 1 | – | |
| Criminal justice | 0.41 | [0.38,0.45] | 0.72 | [0.69,0.76] |
| Health/Substance Use provider | 0.8 | [0.76,0.84] | 0.8 | [0.78,0.83] |
| School/Employer/Community provider Health Insurance (N=33,939)4 | 0.61 | [0.56,0.66] | 0.81 | [0.78,0.85] |
| No Insurance | 1 | – | 1 | – |
| Private/commercial | 1.15 | [0.98,1.34] | 0.98 | [0.90,1.07] |
| Medicaid | 0.86 | [0.78,0.95] | 1.09 | [1.04,1.14] |
| Medicare | 0.99 | [0.84,1.18] | 1.06 | [0.97,1.15] |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
In 2017, Georgia, Oregon, and West Virginia did not report data to TEDS
The following states were excluded for reporting health insurance for none or less than half of admissions in 2017: Arizona, California, Connecticut, Florida, Illinois, Michigan, Minnesota, New Mexico, New York, North Carolina, Ohio, Rhode Island, Vermont, Virginia, Washington, Wisconsin
References
- Akaike H (1974). A New Look at the Statistical Model Identification (pp. 215–222). 10.1007/978-1-4612-1694-0_16 [DOI] [Google Scholar]
- Allen B, & Harocopos A (2016). Non-prescribed buprenorphine in New York City: motivations for use, practices of diversion, and experiences of stigma. Journal of Substance Abuse Treatment, 70, 81–86. [DOI] [PubMed] [Google Scholar]
- Andraka-Christou B (2016). Social & Legal Perspectives on Underuse of Medication-Assisted Treatment for Opioid Dependence.
- Andrilla CHA, Moore TE, Patterson DG, & Larson EH (2019). Geographic Distribution of Providers With a DEA Waiver to Prescribe Buprenorphine for the Treatment of Opioid Use Disorder: A 5-Year Update. The Journal of Rural Health, 35(1), 108–112. 10.1111/jrh.12307 [DOI] [PubMed] [Google Scholar]
- Appel PW, Tsemberis S, Joseph H, Stefancic A, & Lambert-Wacey D (2012). Housing first for severely mentally ill homeless methadone patients. Journal of Addictive Diseases, 31(3), 270–277. 10.1080/10550887.2012.694602 [DOI] [PubMed] [Google Scholar]
- Bao Y, Liu Z, Epstein DH, Du C, Shi J, & Lu L (2009). A Meta-Analysis of Retention in Methadone Maintenance by Dose and Dosing Strategy. The American Journal of Drug and Alcohol Abuse, 35(1), 28–33. 10.1080/00952990802342899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batts K, Pemberton M, Bose J, Weimer B, Henderson L, Penne M, & Strashny A (2014). Comparing and evaluating substance use treatment utilization estimates from the National Survey on Drug Use and Health and other data sources. CBHSQ Data Review, 1–120. [PubMed] [Google Scholar]
- Bentzley BS, Barth KS, Back SE, & Book SW (2015). Discontinuation of buprenorphine maintenance therapy: perspectives and outcomes. Journal of Substance Abuse Treatment, 52, 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco C, & Volkow N (2019). Management of opioid use disorder in the USA: present status and future directions. Lancet (London, England). https://www.sciencedirect.com/science/article/pii/S0140673618330782 [DOI] [PubMed] [Google Scholar]
- Bogdanowicz KM, Stewart R, Chang CK, Shetty H, Khondoker M, Day E, Hayes RD, & Strang J (2018). Excess overdose mortality immediately following transfer of patients and their care as well as after cessation of opioid substitution therapy. Addiction, 113(5), 946–951. 10.1111/add.14114 [DOI] [PubMed] [Google Scholar]
- Calsyn DA, Malcy JA, & Saxon AJ (2006). Slow tapering from methadone maintenance in a program encouraging indefinite maintenance. Journal of Substance Abuse Treatment, 30(2), 159–163. 10.1016/j.jsat.2005.11.007 [DOI] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics, S. (n.d.). CBHSQ Data Review: Comparing and Evaluating Substance Use Treatment Utilization Estimates from the National Survey on Drug Use and Health and Other Data Sources. Retrieved October 31, 2019, from http://www.samhsa.gov/data/. [PubMed]
- Clausen T, Anchersen K, & Waal H (2008). Mortality prior to, during and after opioid maintenance treatment (OMT): a national prospective cross-registry study. Drug and Alcohol Dependence, 94(1–3), 151–157. 10.1016/j.drugalcdep.2007.11.003 [DOI] [PubMed] [Google Scholar]
- Cousins G, Teljeur C, Motterlini N, McCowan C, Dimitrov BD, & Fahey T (2011). Risk of drug-related mortality during periods of transition in methadone maintenance treatment: a cohort study. Journal of Substance Abuse Treatment, 41(3), 252–260. 10.1016/j.jsat.2011.05.001 [DOI] [PubMed] [Google Scholar]
- Cox J, Allard R, Maurais E, Haley N, & Small C (2013). Predictors of methadone program non-retention for opioid analgesic dependent patients. Journal of Substance Abuse Treatment, 44(1), 52–60. 10.1016/j.jsat.2012.03.002 [DOI] [PubMed] [Google Scholar]
- Deck D, & Carlson MJ (2005). Retention in Publicly Funded Methadone Maintenance Treatment in Two Western States. Journal of Behavioral Health Services & Research, 32(1), 43–60. [DOI] [PubMed] [Google Scholar]
- Fiellin DA, Schottenfeld RS, Cutter CJ, Moore BA, Barry DT, & O’Connor PG (2014). Primary care–based buprenorphine taper vs maintenance therapy for prescription opioid dependence: a randomized clinical trial. JAMA Internal Medicine, 174(12), 1947–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingerhood MI, King VL, Brooner RK, & Rastegar DA (2014). A Comparison of Characteristics and Outcomes of Opioid-Dependent Patients Initiating Office-Based Buprenorphine or Methadone Maintenance Treatment. Substance Abuse, 35(2), 122–126. 10.1080/08897077.2013.819828 [DOI] [PubMed] [Google Scholar]
- Fox AD, Anderson MR, Bartlett G, Valverde J, Starrels JL, & Cunningham CO (2014). Health Outcomes and Retention in Care Following Release from Prison for Patients of an Urban Post-incarceration Transitions Clinic. Journal of Health Care for the Poor and Underserved, 25(3), 1139–1152. 10.1353/hpu.2014.0139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield L, Brady JV, Besteman KJ, & De Smet A (1996). Patient retention in mobile and fixed-site methadone maintenance treatment. Drug and Alcohol Dependence, 42(2), 125–131. 10.1016/0376-8716(96)01273-2 [DOI] [PubMed] [Google Scholar]
- Gryczynski J, Mitchell SG, Jaffe JH, O’Grady KE, Olsen YK, & Schwartz RP (2014). Leaving buprenorphine treatment: Patients’ reasons for cessation of care. Journal of Substance Abuse Treatment, 46(3), 356–361. 10.1016/j.jsat.2013.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J, & McElrath K (2012). Methadone as social control: institutionalized stigma and the prospect of recovery. Qualitative Health Research, 22(6), 810–824. 10.1177/1049732311432718 [doi] [DOI] [PubMed] [Google Scholar]
- Hser Y-I, Saxon AJ, Huang D, Hasson A, Thomas C, Hillhouse M, Jacobs P, Teruya C, McLaughlin P, Wiest K, Cohen A, & Ling W (2014). Treatment retention among patients randomized to buprenorphine/naloxone compared to methadone in a multi-site trial. Addiction, 109(1), 79–87. 10.1111/add.12333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhn AS, Hobelmann JG, Strickland JC, Oyler GA, Bergeria CL, Umbricht A, & Dunn KE (2020). Differences in Availability and Use of Medications for Opioid Use Disorder in Residential Treatment Settings in the United States. JAMA Network Open. 10.1001/jamanetworkopen.2019.20843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis BP, Holtyn AF, Subramaniam S, Tompkins DA, Oga EA, Bigelow GE, & Silverman K (2018). Extended-release injectable naltrexone for opioid use disorder: a systematic review. Addiction, 113(7), 1188–1209). 10.1111/add.14180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM, Underwood N, & Compton W (2019). Increases in Methamphetamine Use among Heroin Treatment Admissions in the United States, 2008–2017. Addiction, add.14812. 10.1111/add.14812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HE, O’grady KE, Malfi D, & Tuten M (2008). Methadone maintenance vs. methadone taper during pregnancy: maternal and neonatal outcomes. American Journal on Addictions, 17(5), 372–386. [DOI] [PubMed] [Google Scholar]
- Kariisa M, Scholl L, Wilson N, Seth P, & Hoots B (2019). Drug Overdose Deaths Involving Cocaine and Psychostimulants with Abuse Potential—United States, 2003–2017. MMWR. Morbidity and Mortality Weekly Report, 68(17), 388–395. 10.15585/mmwr.mm6817a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SM, O’Grady KE, Mitchell SG, Brown BS, & Schwartz RP (2011). Predictors of methadone treatment retention from a multi-site study: A survival analysis. Drug and Alcohol Dependence, 117(2–3), 170–175. 10.1016/j.drugalcdep.2011.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornør H, & Waal H (2005). From opioid maintenance to abstinence: A literature review. Drug and Alcohol Review, 24(3), 267–274. 10.1080/09595230500170241 [DOI] [PubMed] [Google Scholar]
- Krawczyk N;, Schneider KE, Eisenberg M, Richards TM, Ferris L, Mojtabai R, Stuart EA, Lyons BC, Jackson K, Weiner JP, & Saloner B (2020). Opioid overdose death following criminal justice involvement: Linking statewide corrections and hospital databases to detect individuals at highest risk. Drug and Alcohol Dependence. [DOI] [PubMed] [Google Scholar]
- Krawczyk N, Buresh M, Gordon MS, Blue T, Fingerhood MI, & Agus D (2019). Expanding Low-Threshold Buprenorphine to Justice-Involved Individuals through Mobile Treatment: Addressing a Critical Care Gap. Journal of Substance Abuse Treatment, 103, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk N, Feder KA, Fingerhood MI, & Saloner B (2017). Racial and ethnic differences in opioid agonist treatment for opioid use disorder in a U.S. national sample. Drug and Alcohol Dependence, 178, 512–518. 10.1016/j.drugalcdep.2017.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk N, Mojtabai R, Stuart E, Fingerhood M, Agus D, Lyons BC, Weiner J, & Saloner B (2020). Opioid agonist treatment and fatal overdose risk in a statewide population receiving opioid use disorder services. Addiction. 10.1111/add.14991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk N, Picher CE, Feder KA, & Saloner B (2017). Only One in Twenty Justice-Referred Adults In Specialty Treatment for Opioid Use Receive Methadone or Buprenorphine. Health Affairs (Project Hope), 36(12), 2046–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk Noa, Feder KA, Saloner B, Crum RM, Kealhofer M, & Mojtabai R (2017). The association of psychiatric comorbidity with treatment completion among clients admitted to substance use treatment programs in a U.S. national sample. Drug and Alcohol Dependence, 175, 157–163. 10.1016/j.drugalcdep.2017.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk Noa, Mojtabai R, Stuart EA, Fingerhood MI, Agus D, Lyons BC, Weiner JP, & Saloner B (2020). Opioid agonist treatment is highly protective against overdose death among a U.S. statewide population of justice-involved adults. 10.1080/00952990.2020.1828440 [DOI]
- Krawczyk Noa, Negron T, Nieto M, Agus D, & Fingerhood MI (2018). Overcoming medication stigma in peer recovery: a new paradigm. Substance Abuse. 10.1080/08897077.2018.1439798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AR, Lundahl LH, Ledgerwood DM, Lisieski M, Rhodes GL, & Greenwald MK (2015). Gender-Specific Predictors of Retention and Opioid Abstinence During Methadone Maintenance Treatment. Journal of Substance Abuse Treatment, 54, 37–43. 10.1016/j.jsat.2015.01.009 [DOI] [PubMed] [Google Scholar]
- Lincoln T, Johnson BD, McCarthy P, & Alexander E (2018). Extended-release naltrexone for opioid use disorder started during or following incarceration. Journal of Substance Abuse Treatment, 85, 97–100. 10.1016/j.jsat.2017.04.002 [DOI] [PubMed] [Google Scholar]
- Magura S, & Rosenblum A (2001). Leaving methadone treatment: lessons learned, lessons forgotten, lessons ignored. The Mount Sinai Journal of Medicine, New York, 68(1), 62–74. http://www.ncbi.nlm.nih.gov/pubmed/11135508 [PubMed] [Google Scholar]
- Martin SA, Chiodo LM, Bosse JD, & Wilson A (2018). The Next Stage of Buprenorphine Care for Opioid Use Disorder: A Narrative Review (in press). Annals of Internal Medicine. http://annals.org/aim/fullarticle/2708164 [DOI] [PubMed] [Google Scholar]
- Meinhofer A, Williams A, Johnson P, Schackman B, & Bao Y (2019). Prescribing decisions at buprenorphine treatment initiation: Do they matter for treatment discontinuation and adverse opioid-related events? Journal of Substance Abuse Treatment, 105, 37–43. https://www.sciencedirect.com/science/article/pii/S0740547219301928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrall ELC, Kariminia A, Binswanger IA, Hobbs MS, Farrell M, Marsden J, Hutchinson SJ, & Bird SM (2010). Meta-analysis of drug-related deaths soon after release from prison. Addiction, 105(9), 1545–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojtabai R, Mauro C, Wall MM, Barry CL, & Olfson M (2019). Medication Treatment For Opioid Use Disorders In Substance Use Treatment Facilities. Health Affairs, 38(1), 14–23. 10.1377/hlthaff.2018.05162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JR, Schackman BR, Leff JA, Linas BP, & Walley AY (2018). Injectable naltrexone, oral naltrexone, and buprenorphine utilization and discontinuation among individuals treated for opioid use disorder in a United States commercially insured population. Journal of Substance Abuse Treatment, 85, 90–96. 10.1016/J.JSAT.2017.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JR, Schackman BR, Weinstein ZM, Walley AY, & Linas BP (2019). Overdose following initiation of naltrexone and buprenorphine medication treatment for opioid use disorder in a United States commercially insured cohort. Drug and Alcohol Dependence. 10.1016/J.DRUGALCDEP.2019.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Quality Forum. (2017). Behavioral Health 2016–2017 Final Report. https://www.qualityforum.org/Publications/2017/08/Behavioral_Health_2016-2017_Final_Report.aspx
- Nielsen S, Hillhouse M, Mooney L, Ang A, & Ling W (2015). Buprenorphine pharmacotherapy and behavioral treatment: comparison of outcomes among prescription opioid users, heroin users and combination users. Journal of Substance Abuse Treatment, 48(1), 70–76. 10.1016/j.jsat.2014.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paudyal V, MacLure K, Buchanan C, Wilson L, Macleod J, & Stewart D (2017). ‘When you are homeless, you are not thinking about your medication, but your food, shelter or heat for the night’: behavioural determinants of homeless patients’ adherence to prescribed medicines. Public Health, 148, 1–8. 10.1016/j.puhe.2017.03.002 [DOI] [PubMed] [Google Scholar]
- Polsky D, Arsenault S, & Azocar F (2019). Private Coverage of Methadone in Outpatient Treatment Programs. Psychiatric Services, appi.ps.2019003. 10.1176/appi.ps.201900373 [DOI] [PubMed] [Google Scholar]
- Proctor SL, Copeland AL, Kopak AM, Hoffmann NG, Herschman PL, & Polukhina N (2015). Predictors of Patient Retention in Methadone Maintenance Treatment. Psychology of Addictive Behaviors, 29(4), 906–917. 10.1037/adb0000090 [DOI] [PubMed] [Google Scholar]
- Reif S, Creedon TB, Horgan CM, Stewart MT, & Garnick DW (2017). Commercial Health Plan Coverage of Selected Treatments for Opioid Use Disorders from 2003 to 2014. Journal of Psychoactive Drugs, 49(2), 102–110. 10.1080/02791072.2017.1300360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisinger HS, Schwartz RP, Mitchell SG, Peterson JA, Kelly SM, O’Grady KE, Marrari EA, Brown BS, & Agar MH (2009). Premature Discharge from Methadone Treatment: Patient Perspectives. Journal of Psychoactive Drugs, 41(3), 285–296. 10.1080/02791072.2009.10400539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt RA, Andrilla CH, Catlin M, & Larson EH (2015). Geographic and specialty distribution of US physicians trained to treat opioid use disorder. Annals of Family Medicine, 13(1), 23–26. 10.1370/afm.1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum A, Cleland CM, Fong C, Kayman DJ, Tempalski B, & Parrino M (2011). Distance traveled and cross-state commuting to opioid treatment programs in the United States. Journal of Environmental and Public Health, 2011, 948789. 10.1155/2011/948789 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahker E, Toussaint MN, Ramirez M, Ali SR, & Arndt S (2015). Evaluating racial disparity in referral source and successful completion of substance abuse treatment. Addictive Behaviors, 48, 25–29. [DOI] [PubMed] [Google Scholar]
- Saloner B, & Cook BL (2013). Blacks and Hispanics are less likely than whites to complete addiction treatment, largely due to socioeconomic factors. Health Affairs (Project Hope), 32(1), 135–145. 10.1377/hlthaff.2011.0983 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saloner B, Daubresse M, & Caleb AG (2017). Patterns of Buprenorphine-Naloxone Treatment for Opioid Use Disorder in a Multistate Population. Medical Care, 55(7), 669–676. 10.1097/MLR.0000000000000727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA. (2019a). TIP 63: Medications for Opioid Use Disorder. https://store.samhsa.gov/product/TIP-63-Medications-for-Opioid-Use-Disorder-Full-Document-Including-Executive-Summary-and-Parts-1-5-/SMA19-5063FULLDOC
- SAMHSA. (2019b). Treatment Episode Data Set (TEDS): 2007–2017. https://doi.org/HHS Publication No. (SMA) 17–4360.
- Samples H, Williams AR, Olfson M, & Crystal S (2018). Risk factors for discontinuation of buprenorphine treatment for opioid use disorders in a multi-state sample of Medicaid enrollees. Journal of Substance Abuse Treatment, 95, 9–17. 10.1016/j.jsat.2018.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl L, Seth P, Kariisa M, Wilson N, & Baldwin G (2019). Drug and Opioid-Involved Overdose Deaths — United States, 2013–2017. MMWR. Morbidity and Mortality Weekly Report, 67(5152). 10.15585/mmwr.mm6751521e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuman-Olivier Z, Weiss RD, Hoeppner BB, Borodovsky J, & Albanese MJ (2014). Emerging adult age status predicts poor buprenorphine treatment retention. Journal of Substance Abuse Treatment, 47(3), 202–212. 10.1016/j.jsat.2014.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmon SC (2014). Access to treatment for opioid dependence in rural America: challenges and future directions. JAMA Psychiatry, 71(4), 359–360. [DOI] [PubMed] [Google Scholar]
- StataCorp. (2017). Stata Statistical Software: Release 15. StataCorp LP. [Google Scholar]
- Stein BD, Dick AW, Sorbero M, Gordon AJ, Burns RM, Leslie DL, & Pacula RL (2018). A population-based examination of trends and disparities in medication treatment for opioid use disorders among Medicaid enrollees. Substance Abuse, 39(4), 419–425. 10.1080/08897077.2018.1449166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timko C, Schultz NR, Cucciare MA, Vittorio L, & Garrison-Diehn C (2016). Retention in medication-assisted treatment for opiate dependence: A systematic review. Journal of Addictive Diseases, 35(1), 22–35. 10.1080/10550887.2016.1100960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong C, Krawczyk N, Dejman M, Marshall-Shah S, Tormohlen K, Agus D, & Bass J (2019). Challenges on the road to recovery: Exploring attitudes and experiences of clients in a community-based buprenorphine program in Baltimore City. Addictive Behaviors, 93, 14–19. 10.1016/J.ADDBEH.2019.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui JI, Mayfield J, Speaker EC, Yakup S, Ries R, Funai H, Leroux BG, & Merrill JO (2020). Association between methamphetamine use and retention among patients with opioid use disorders treated with buprenorphine. Journal of Substance Abuse Treatment, 109, 80–85. 10.1016/j.jsat.2019.10.005 [DOI] [PubMed] [Google Scholar]
- Wei LJ (1992). The accelerated failure time model: A useful alternative to the cox regression model in survival analysis. Statistics in Medicine, 11(14–15), 1871–1879. 10.1002/sim.4780111409 [DOI] [PubMed] [Google Scholar]
- White IR, Royston P, & Wood AM (2011). Multiple imputation using chained equations: issues and guidance for practice. Statistics in Medicine, 30(4), 377–399. [DOI] [PubMed] [Google Scholar]
- White WL (2011). Narcotics Anonymous and the Pharmacotherapeutic Treatment of Opioid Addiction in the United States. 61
- Williams Arthur R., & Bisaga A (2016). From AIDS to opioids - How to combat an epidemic. New England Journal of Medicine, 375 (9), 813–815. Massachussetts Medical Society. 10.1056/NEJMp1604223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams Arthur Robin, Nunes EV, Bisaga A, Levin FR, & Olfson M (2019). Development of a Cascade of Care for responding to the opioid epidemic. The American Journal of Drug and Alcohol Abuse, 45(1), 1–10. 10.1080/00952990.2018.1546862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams Arthur Robin, Nunes EV, Bisaga A, Pincus HA, Johnson KA, Campbell AN, Remien RH, Crystal S, Friedmann PD, Levin FR, & Olfson M (2018). Developing an opioid use disorder treatment cascade: A review of quality measures. Journal of Substance Abuse Treatment, 91, 57–68. 10.1016/J.JSAT.2018.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams Arthur Robin, Samples H, Crystal S, & Olfson M (2019). Acute Care, Prescription Opioid Use, and Overdose Following Discontinuation of Long-Term Buprenorphine Treatment for Opioid Use Disorder. American Journal of Psychiatry. 10.1176/appi.ajp.2019.19060612 [DOI] [PMC free article] [PubMed] [Google Scholar]


