Abstract
Introduction
The epidemiology of severe lower respiratory tract infections (LRTI) is constantly changing. We aimed to describe it using the BioFire® FilmArray® Pneumonia plus (PNplus) Panel.
Methods
In a sub-study of the PROGRESS trial, sputum samples of 90 patients with sepsis and LRTI were retrospectively studied. The primary endpoint was the comparative detection rate of pathogens between conventional microbiology and PNplus Panel; secondary endpoints were microbiology and the association with the inflammatory host response.
Results
Fifty-six patients with community-acquired pneumonia without risk factors for multidrug-resistant (MDR) pathogens and another 34 patients with risk factors for MDR were studied; median pneumonia severity index (PSI) was 113 (88–135). PNplus detection rate was 72.2% compared to 10% by conventional microbiology (p < 0.001); Streptococcus pneumoniae was the most common pathogen. PSI and procalcitonin were greater among patients with bacterial pathogens than viral pathogens. Median procalcitonin was 0.49 ng/ml and 0.18 ng/ml among patients with ≥ 105 and < 105 copies/ml of detected bacteria, respectively (p = 0.004). Resistance reached 14.4%.
Conclusion
PNplus detects severe pneumonia pathogens at a greater rate than conventional microbiology. High levels of inflammation accompany bacterial detection.
Trial Registration
PROGRESS, ClinicalTrials.gov NCT03333304, 06/11/2017.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-021-00459-x.
Keywords: FilmArray®, Syndromic testing, Pneumonia, Procalcitonin, Lower respiratory tract infection, S. pneumoniae
Key Summary Points
| Data about etiology of severe lower respiratory tract infections are sparse. |
| BioFire® FilmArray® Pneumonia plus Panel detects pathogens at a high rate. |
| Streptococcus pneumoniae is the commonest pathogen of community-acquired pneumonia. |
| High serum procalcitonin levels reflect true virulence of detected pathogens. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.14579211.
Introduction
Community-acquired pneumonia (CAP) is a major cause of morbidity and mortality. Mortality is mainly associated with progression into sepsis; this is reaching almost 28% among patients with CAP [1, 2]. The ideal strategy for the management of pneumonia should rely on the early identification of the pathogen and the administration of targeted antimicrobial therapy. However, this is hampered by conventional microbiology that is positive in less than half of the patients. This was underscored by the results of the EPIC study (Etiology of Pneumonia in the Community). In the EPIC study [3], extensive microbiology and serology workups were done to detect the pathogen of 2320 patients with CAP; molecular methods were also used. Despite the rigorous efforts, the culprit pathogens were detected in only 38% of patients. This strongly supports utilization of the syndromic diagnostic approach for the diagnosis of the pathogen and for acquiring early information on resistance.
PROGRESS (PROcalcitonin-Guided antimicrobial therapy to REduce long-term Sequelae of infectionS) is a prospective randomized clinical trial aiming to validate the impact of early stop of antibiotics guided by procalcitonin (PCT) in the prevention of infections by multidrug-resistant microorganisms (MDRO) and Clostridiodes difficile [4]. Enrolled patients had sepsis due to either lower respiratory tract infections (LRTI) or acute pyelonephritis (AP) or primary bloodstream infection (BSI). The current manuscript is a subgroup analysis of participants with LRTI where the diagnostic performance of syndromic molecular testing using BioFire® FilmArray® Pneumonia plus Panel was studied.
Methods
Study Design and Participants
This is a substudy of patients with sepsis due to LRTI enrolled in the PROGRESS trial [4]. PROGRESS was a prospective, multicenter, randomized trial designed to evaluate a procalcitonin (PCT)-guided antibiotic stopping rule compared to standard-of-care treatment, for the incidence of infection-associated adverse events in patients with sepsis (ClinicalTrials.gov NCT03333304, 06/11/2017). The study was conducted according to the Declaration of Helsinki. From November 2017 to January 2019, the trial recruited patients with sepsis hospitalized in seven internal medicine departments in Greece. Enrolled patients were adults (≥ 18 years) with LRTI, AP, or BSI. All enrolled patients had sepsis according to the Sepsis-3 definitions, i.e., presented in the emergency department with a Sequential Organ Failure Assessment (SOFA) score of at least two or had an increase of baseline SOFA score of at least two points while hospitalized [5]. Only patients with CAP participated in this sub-study. LRTI was defined as a new or evolving infiltrate on chest X-ray accompanied by at least two clinical findings compatible with LRTI (new onset or worsening of cough; dyspnea; auscultatory findings consistent with pulmonary consolidation) and at least one finding consistent with impaired respiratory function (PCT ≥ 0.25 μg/l; hypoxemia pO2 ≤ 60 mmHg; oxygen saturation ≤ 90% in room air; respiratory rate ≥ 20 breaths/min) [6, 7]. Patients without any previous intake of antimicrobials and without any contact with the hospital environment during the last 90 days were classified into CAP without risk factors for MDR; patients under chronic dialysis or residents in long-term care facilities or with hospitalization or previous antimicrobial intake during the last 3 months or previous isolation of an MDR pathogen were classified into CAP with risk factors for MDR [6–8]. Main exclusion criteria were infections requiring prolonged antibiotic therapies; parasite infections; tuberculosis; cystic fibrosis; severe immunosuppression; pregnancy/lactation. Written informed consent was obtained from patients before enrollment. PROGRESS was approved by the National Organization for Medicines of Greece (approval number, IS-62/17), the National Ethics Committee of Greece (approval number, 62/17), and by the local ethics committees of all participating hospitals. Data were captured after review of all medical and nursing charts by a physician team blinded to the allocation group.
Blood samples were collected for PCT measurements within the first 24 h and on day 5 of treatment, using the VIDAS® assay with a lower detection limit of 0.05 μg/l (bioMérieux, Marcy l’Etoile, France). Standard-of-care microbiology investigation was done before allocation of participants into the study arm and prior to initiation of antimicrobials. This comprised bacterial cultures of blood, sputum, and pleural fluid; urine analysis for antigen detection of Legionella pneumophila and Streptococcus pneumoniae (BinaxNOW, Abbott); antibody titer testing for Mycoplasma pneumoniae, Chlamydia pneumoniae, and Legionella pneumoniae; and nasopharyngeal sample analysis by routine real-time PCR assays for the detection of influenza A and B virus and parainfluenza virus.
Following collection, sputum samples were transported within less than 30 min to the central lab for Gram stain and culture. An amount of high-quality sputum samples was frozen at − 80 °C and the rest was cultured after lysis with Sputasol on blood, chocolate, and Sabouraud agar plates. Each bacterial strain grown was identified by Analytical Profile Index (API, bioMérieux, Marcy l’Etoile, France), and tested for susceptibilities by Etest (bioMérieux, Marcy l’Etoile, France). Susceptibility to colistin was tested according to Clinical & Laboratory Standards Institute (CLSI) standards by broth microdilution technique in plates with twofold dilutions from 0.06 to 64 mg/l with cation-adjusted Mueller–Hinton broth (BDBBLTM Mueller Hinton II Broth Cation-Adjusted; Becton Dickinson). Antimicrobial susceptibility results were interpreted according to the European Committee on Antimicrobial Susceptibility Testing recommendations (EUCAST 2019, version 9.0) [9]. Sputum samples were retrospectively analyzed at the end of the study by the PNplus Panel (BioFire Diagnostics, LLC, Salt Lake City, UT, USA). PNplus Panel is a multiplex PCR system that can detect eight viruses (influenza A and B virus, parainfluenza virus, respiratory syncytial virus, rhinovirus or enterovirus, human metapneumovirus, coronavirus), three atypical pathogens (C. pneumoniae, L. pneumophila, M. pneumoniae), 15 bacteria (Acinetobacter calcoaceticus-baumannii complex, Enterobacter cloacae, Escherichia coli, Klebsiella oxytoca, Klebsiella pneumoniae, Klebsiella aerogenes, Moraxella catarrhalis, Proteus spp., Serratia marcescens, Haemophilus influenzae, Pseudomonas aeruginosa, Staphylococcus aureus, S. pneumoniae, Streptococcus pyogenes, Streptococcus agalactiae), and seven resistance genes (CTX-M, KPC, OXA-48-like, NDM, VIM, IMP, mecA/C/MREJ). The reported sensitivity and specificity of the method by using sputum samples are 96.3% and 97.2%, respectively [10]. Testing was performed by technicians blinded to clinical data.
Study Endpoints
The primary endpoint was the comparison of the detection rate of pathogens between conventional microbiology and PNplus Panel. Secondary endpoints were (i) the detailed etiology of severe LRTI; (ii) the association of the PNplus Panel with the inflammatory host response; and (iii) the detection of antimicrobial resistance.
Statistical Analysis
Categorical data were presented as frequencies and confidence intervals (CI); continuous variables with normal distribution as mean with standard deviation (SD) and with non-normal distribution as median with upper and lower quartile (Q1–Q3). Fisher’s exact test was used for comparison of categorical data whereas Student’s t test and analysis of variance (ANOVA) or non-parametric Mann–Whitney and Kruskal–Wallis test were used for the comparison of continuous data, as appropriate. Bonferroni adjustment was applied for multiple comparisons. Any two-sided P value lower than 0.05 was considered statistically significant. Statistical analysis was performed using the software SPSS version 25.0.
Results
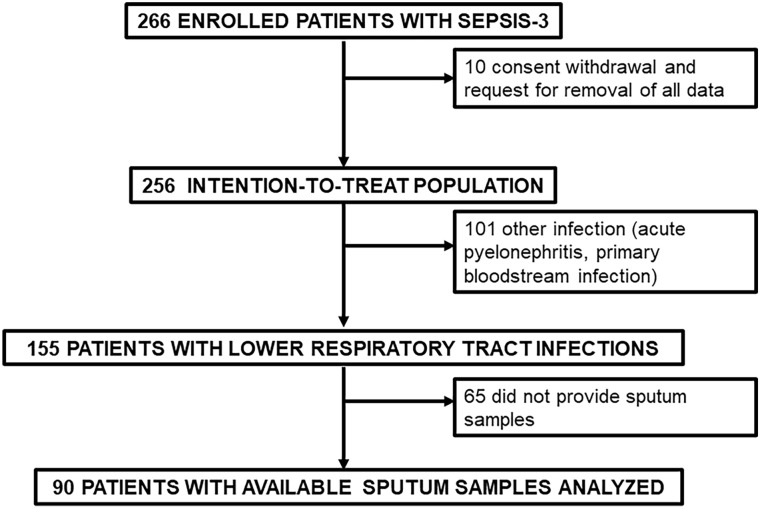
The study flowchart is demonstrated in Fig. 1. More precisely, of the 90 patients enrolled in the sub-study, 56 patients (62.2%) had CAP without risk factors for MDR and 34 patients (37.8%) had CAP with risk factors for MDR. Median pneumonia severity index was 113 (Q1–Q3, 87.8–135.3). Mortality after 28 days was 25.6% (23/90). Baseline demographics are shown in Table 1.
Fig. 1.
Study flowchart
Table 1.
Baseline characteristics of patients
| Characteristics | Cohort (N = 90) |
|---|---|
| Male gender, no. (%) | 56 (62.2) |
| Age (years), mean ± SD | 75.8 ± 12.3 |
| APACHE II, mean ± SD | 13.0 ± 4.6 |
| SOFA score, mean ± SD | 4.3 ± 2.4 |
| Pneumonia severity index, median (Q1–Q3) | 113 (87.8–135.3) |
| Charlson’s comorbidity index, mean ± SD | 5.2 ± 2.0 |
| Septic shock, no. (%) | 5 (5.6) |
| Length of hospital stay, median (Q1–Q3) | 7 (6–11) |
| Laboratory findings | |
| Procalcitonin (μg/l), median (Q1–Q3) | 0.46 (0.15–2.68) |
| C-reactive protein (mg/l), median (Q1–Q3) | 99.6 (35.2–201.0) |
| White blood cells (/mm3), mean ± SD | 11,088 ± 4910 |
| Type of community-acquired pneumonia, no. (%) | |
| Without risk factors for multidrug-resistant pathogens | 56 (62.2) |
| With risk factors for multidrug-resistant pathogens | 34 (37.8) |
| Comorbidities and risk factors, no. (%) | |
| Type 2 diabetes mellitus | 30 (33.3) |
| Chronic heart failure | 17 (18.9) |
| Chronic renal disease | 15 (16.7) |
| Chronic obstructive pulmonary disease | 20 (22.2) |
| Solid tumor malignancy | 9 (10.0) |
| Cerebrovascular disease | 13 (14.4) |
| Degenerative neurological disease | 7 (7.8) |
| Dementia | 23 (25.6) |
| Coronary heart disease | 18 (20.0) |
| Residency in long-term healthcare facilities | 11 (12.2) |
| Previous hospitalization last 3 months | 19 (21.1) |
| Antibiotic intake last 3 months | 40 (44.4) |
| Administered antimicrobials, no. (%) | |
| β-lactamase inhibitors | 16 (17.8) |
| Ceftriaxone | 34 (37.8) |
| Piperacillin/tazobactam | 53 (58.9) |
| Carbapenem | 12 (13.3) |
| Levofloxacin/moxifloxacin | 14 (15.6) |
| Linezolid | 11 (12.2) |
| Clarithromycin | 29 (32.2) |
| Azithromycin | 23 (25.6) |
| Clindamycin | 6 (6.7) |
| Oseltamivir | 33 (36.7) |
| Outcome, no. (%) | |
| In-hospital mortality | 22 (24.4) |
| 28-day mortality | 23 (25.6) |
APACHE Acute Physiology And Chronic Health Evaluation, Q quartile, SD standard deviation, SOFA Sequential Organ Failure Assessment
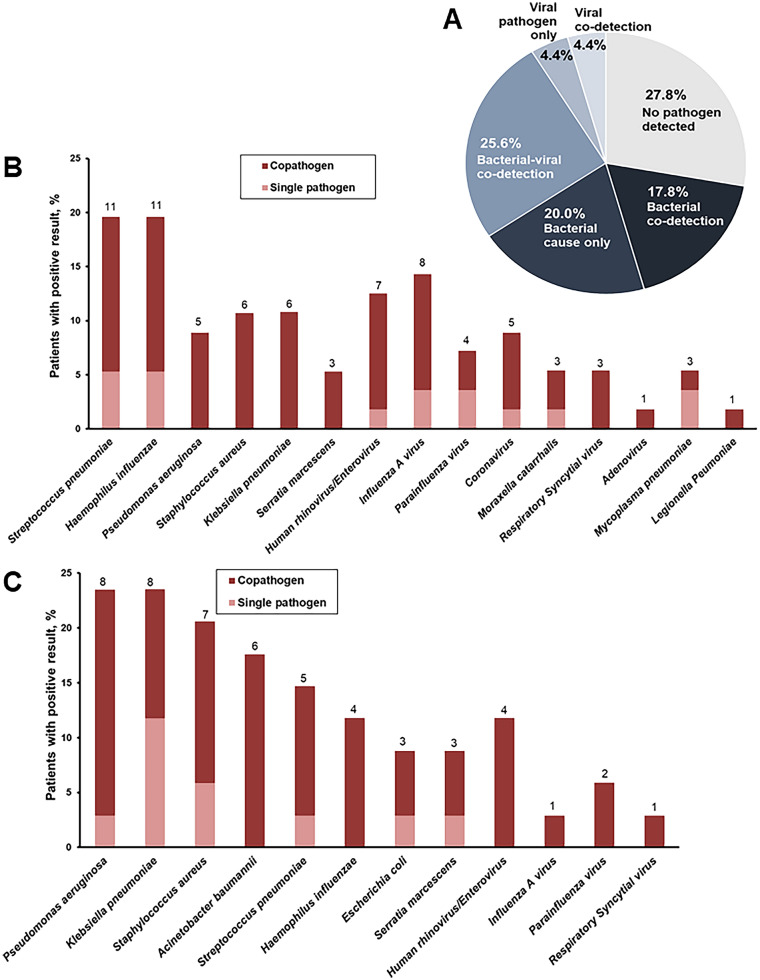
The primary endpoint was the rate of positive microbiology using conventional techniques and the PNplus Panel. When conventional microbiology was used, at least one pathogen was detected in nine patients (10%; 95% confidence intervals [CI], 5.4–17.9%); the PNplus assay detected at least one pathogen in 65 patients (72.2%; 95% CI, 62.2–84.4%; P < 0.001). All pathogens detected by conventional microbiology were also detected using PNplus. Pathogens detected by conventional microbiology were Acinetobacter baumannii (4/9), P. aeruginosa (3/9), E. coli (1/9), and S. aureus (1/9). When PNplus was used, it was found that bacteria were the most common pathogens, whereas co-detection of bacterial and viral pathogens was found in 23 patients (25.6%). No pathogen was detected in 25 patients (27.8%) (Fig. 2a). S. pneumoniae (11/56; 19.6%) and H. influenzae (11/56; 19.6%) were the most common pathogens of CAP without risk factors for MDR (Fig. 2b) whereas P. aeruginosa was the most common pathogen of CAP with risk factors for MDR (8/34; 23.5%) (Fig. 2c). Differences in the detection of pathogen between patients with CAP with and without risk factors for MDR are presented in Table 2.
Fig. 2.
Pathogen detection among hospitalized patients with sepsis and severe lower respiratory tract infections. a Proportions of viral, viral–viral, bacterial–viral, bacterial, and no pathogen detected are provided in the pie chart for the total study cohort. Numbers and percentages of patients with community-acquired pneumonia b without and c with risk factors for multidrug-resistant pathogens, in whom a specific pathogen (single pathogen is indicated in light pink color; co-pathogens are indicated in deeper color) was detected are provided in the bar chart. Pathogens detected in a single patient are represented in their respective bars
Table 2.
Differences in pathogen detection between patients with community-acquired pneumonia (CAP) without and with risk factors for multidrug-resistant (MDR) pathogens
| Detection by PNplus Panel, no. (%) | CAP-MDR risk factors (−) (N = 56) | CAP-MDR risk factors (+) (N = 34) | P value |
|---|---|---|---|
| At least one pathogen | 39 (69.6) | 26 (76.5) | 0.628 |
| At least one resistance gene | 2 (3.6) | 10 (29.4) | < 0.001 |
| At least one virus | 23 (41.1) | 7 (20.3) | 0.065 |
| S. pneumoniae | 11 (19.6) | 6 (17.6) | > 0.99 |
| H. influenzae | 11 (19.6) | 5 (14.7) | 0.777 |
| S. aureus | 6 (10.7) | 7 (20.6) | 0.226 |
| P. aeruginosa | 5 (8.9) | 8 (23.5) | 0.069 |
| K. pneumoniae | 3 (5.4) | 8 (23.5) | 0.018 |
| A. baumannii | 2 (3.6) | 7 (20.6) | 0.024 |
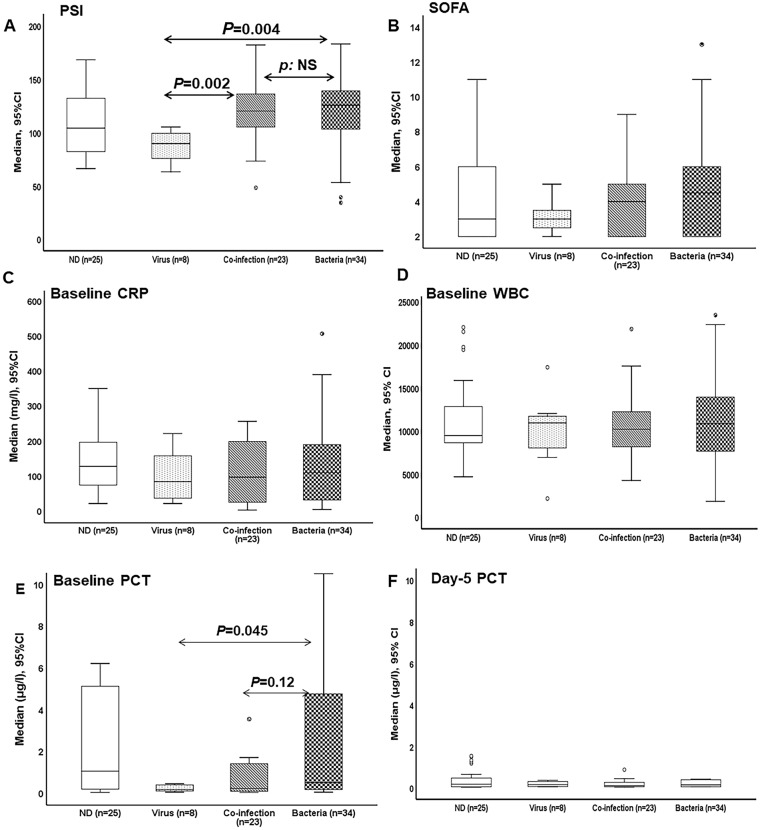
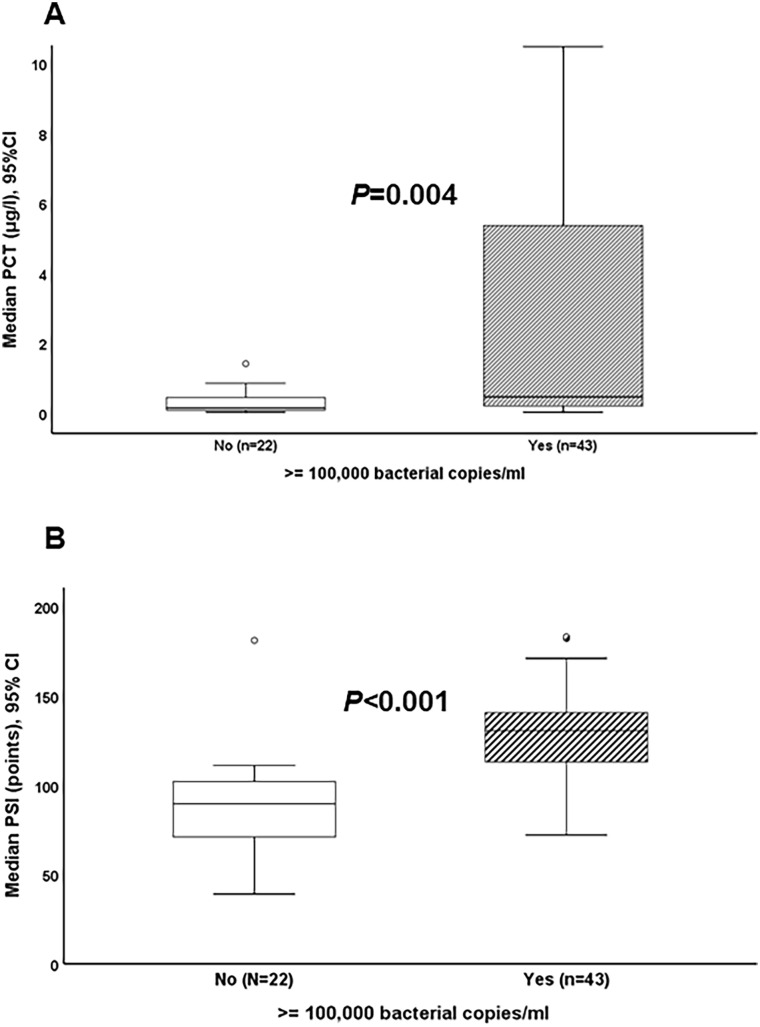
The analysis of the association of the findings of the PNplus Panel with the inflammatory response of the host showed that the pneumonia severity index (PSI) was largely different among patients with LRTI of viral etiology and patients with LRTI of bacterial etiology or of bacterial and viral co-infection (Fig. 3a). Sequential Organ Failure Assessment (SOFA) score, serum C-reactive protein (CRP), and white blood cells at baseline did not differ (Figs. 3b–d). Median baseline PCT was 0.52 μg/l in patients with bacterial pathogens compared to 0.19 μg/l in patients with viral pathogens (P = 0.045) (Fig. 3e). Median PCT remained low after 5 days of treatment and levels did not differ among the groups (Fig. 3f). When analysis was limited to patients with detected bacterial pathogens by PNplus, it was found that median PCT was 0.49 μg/l among patients with ≥ 105 copies/ml of any bacterial pathogen compared to 0.18 μg/l for patients with bacterial pathogen detected at < 105 copies/ml (P = 0.004) (Fig. 4a). Median PSI was 130.5 among patients with ≥ 105 copies/ml of any bacterial pathogen compared to 89.5 for patients with bacterial pathogens detected at < 105 copies/ml (P < 0.001) (Fig. 4b). In patients with CAP without risk factors for MDR and baseline PCT values ≥ 0.25 μg/l, no pathogen was detected in 35.5% of cases (11/31), a bacterial cause was detected in 54.8% (17/31), and a viral cause was detected in 9.7% (3/31). In patients with CAP with risk factors for MDR and baseline PCT values ≥ 0.25 μg/l, no pathogen was detected in 28.6% (6/21), a bacterial cause in 71.4 (15/21), and a viral cause in none of these patients.
Fig. 3.
Markers of severity by detected pathogen. Comparison of a Pneumonia Severity Index (PSI), b Sequential Organ Failure Assessment (SOFA) score, c C-reactive protein (CRP), d total white blood cell count (WBC), and e procalcitonin (PCT) between patients with no pathogen detected (ND), viral pathogen, bacterial pathogen, and bacterial–viral co-infection are provided. P values are provided. Comparisons are done by the Mann–Whitney test. CI confidence interval
Fig. 4.
Markers of severity by bacterial load. Comparison of a serum procalcitonin (PCT) and of b Pneumonia Severity Index (PSI) between patients with ≥ 105 and < 105 copies/ml of a bacterial pathogen in PNplus Panel are provided. P values are provided. Comparisons are done by the Mann–Whitney test. CI confidence interval
At least one resistance gene was detected in 10 (29.4%; 95% CI, 16.8–46.2) patients with CAP with risk factors for MDR compared to 2 (3.6%; 95% CI, 1.0–12.1) patients with CAP without such risk factors (P < 0.001) (Table 2). The most common detected gene was blaCTX-M (6/90, 6.6%), followed by mecA/C (5/90, 5.5%) and blaKPC (4/90, 4.4%). Conventional microbiology detected resistance in only 4 of these 12 samples (33.3%) (P = 0.060).
Discussion
In this study, using the PNplus Panel, we showed that bacteria are the most common pathogens of severe LRTI. S. pneumoniae dominates in cases of CAP without risk factors for MDR and P. aeruginosa in CAP with risk factors for MDR. PNplus detected pathogens at significantly greater rate than conventional microbiology.
EPIC is the largest epidemiological study conducted so far in CAP; samples of blood, urine, and respiratory secretions were collected for culture, serologic testing, antigen detection, and molecular diagnostic testing from 2320 adults with radiographic evidence of pneumonia. Analysis revealed that 24% of cases were of viral etiology, 11% of bacterial etiology, and that 3% of cases were co-infected by bacteria and viruses; no pathogen was detected in 62% of cases [3]. The comparison of the study population of the PROGRESS study and of the EPIC study revealing that participants of the EPIC study had sepsis and that they were older with more comorbidities highlights that the distribution of pathogens is different in severe LRTIs than in non-severe LRTI (see supplementary material).
A subsequent study using a novel serotype-specific urinary antigen detection assay in 1736 samples coming from the EPIC trial identified additional 76 (4.4%) cases of pneumococcal CAP [11]. Similar results were provided by the Global Initiative for Methicillin-resistant S. aureus Pneumonia (GLIMP) point-prevalence international study among 2564 patients hospitalized with CAP in 2015; S. pneumoniae was detected in 8.2% followed by P. aeruginosa (4.1%) and K. pneumoniae (3.45), whereas in 35.3% no pathogen was detected [12]. Many patients of the PROGRESS trial were found with bacterial and viral co-infection. Although similar rates of co-infection have also been reported by others [13], it is difficult to interpret if these viruses represent true pathogens or if they are bystanders that initiate an initial inflammatory reaction leading to vulnerability for one subsequent bacterial infection. Unfortunately, we were not able to obtain serial sputum samples to conclude if viruses were detected only early during the disease course as remnants of a prior viral infection leading to a secondary bacterial infection or their isolation in sputum really represents co-infection, which is certainly a limitation of the current study.
PCT may be a useful biomarker to discriminate viral from bacterial LRTI. In the EPIC study, PCT at concentrations greater than 0.1 ng/ml could indicate bacterial pathogens with 80.9% sensitivity and 51.6% specificity [14]. In our study, serum PCT levels were significantly elevated in patients with bacterial LRTI compared to those with viral pathogens. PCT levels were even more pronounced in patients with high bacterial load in sputum, which was helpful in understanding and interpreting the semiquantitative values of PNplus. High bacterial load in sputum may be also helpful in distinguishing true bacterial pathogens from bystanders in cases of isolation of more than one species of bacteria in sputum. Clinical improvement in spite of inappropriate empirical antimicrobial administration in cases of bacterial co-detection may be an indirect index that these isolates are not true pathogens. In our study, this was the case only in 3 of 27 patients with more than one bacterial species detected; thus, even this retrospective evaluation was unable to exclude true pathogenicity of co-detected bacteria.
Low levels of PCT could prevent inappropriate antibiotic prescriptions. A large number of clinical trials and meta-analyses so far demonstrated that PCT was effective in reducing antibiotic prescription, length of therapy, and antimicrobial-associated adverse events [15–17]. A combination of PCT guidance with molecular syndromic testing may be a powerful weapon of antimicrobial stewardship programs to combat emergence of resistance, as implied by retrospective data and data from randomized clinical trials [18–20]. Three main limitations of our study should be acknowledged: (a) the retrospective nature of processing respiratory samples with PNplus did not give us the opportunity to investigate any effect of the reporting of these results to the attending physicians on their antibiotic prescription policy and any potential reduction in use of antimicrobials or neuraminidase inhibitors; (b) the lack of longitudinal samplings; and (c) the low success rate of conventional microbiology which over-reports the diagnostic sensitivity of BioFire® FilmArray®. The only explanations for this low success rate are the high number of patients with viral infection and the high number of patients with low counts of bacterial pathogens.
Conclusions
PNplus Panel is more sensitive than conventional microbiology for the detection of pathogens in cases of severe LRTI. High serum PCT and PSI correlate with detection of bacterial pathogens. More research is needed to investigate the potential benefit of a combination strategy of PCT guidance and BioFire® FilmArray® PNplus in antimicrobial stewardship programs.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the patients, families, clinical, laboratory, and research staff who contributed to the study.
Funding
The PROGRESS trial was supported by grants by the Hellenic Institute for the Study of Sepsis and by bioMérieux, Lyon, France. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication. The Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Evdoxia Kyriazopoulou participated in data analysis, drafted the manuscript, had full access to all of the study data and takes responsibility for their integrity and the accuracy of the analysis. Athanasios Karageorgos, Panagiotis Koufargyris, Georgia Damoraki and Asimina Safarika performed laboratory experiments, and revised the manuscript for important intellectual content. Lydia Liaskou-Antoniou, Vasileios Lekakis, Maria Saridaki and George Adamis recruited patients in this study, collected data, and revised the manuscript for important intellectual content. All authors gave final approval for the version to be published. Evangelos J. Giamarellos-Bourboulis conceptualized the study design, participated in data analysis and drafting the manuscript, had full access to all of the study data and takes responsibility for their integrity and the accuracy of the analysis.
Disclosures
E.J. Giamarellos-Bourboulis has received honoraria from AbbVie USA, Abbott CH, Angelini Italy, Biotest Germany, InflaRx GmbH, MSD Greece, ThermoFisher Brahms GmbH, XBiotech Inc. and Swedish Orphan Biovitrum; independent educational grants from AbbVie, Abbott, Astellas Pharma Europe, AxisShield, bioMérieux Inc, InflaRx GmbH, ThermoFisher Brahms GmbH, and XBiotech Inc.; and funding from the FrameWork 7 program HemoSpec (granted to the National and Kapodistrian University of Athens), the Horizon2020 Marie-Curie Project European Sepsis Academy (granted to the National and Kapodistrian University of Athens), and the Horizon 2020 European Grant ImmunoSep (granted to the Hellenic Institute for the Study of Sepsis). E. Kyriazopoulou, A. Karageorgos, L. Liaskou-Antoniou, P. Koufargyris, A. Safarika, G. Damoraki, V. Lekakis, M. Saridaki and G. Adamis have nothing to disclose.
Compliance with Ethics Guidelines
PROGRESS was approved by the National Organization for Medicines of Greece (approval number, IS-62/17), the National Ethics Committee of Greece (approval number, 62/17), and by the local ethics committees of all participating hospitals (“Attikon” University Hospital of Athens, “G. Gennimatas” General Hospital of Athens, “Thriasio” General Hospital of Athens, “Sismanogleio” General Hospital of Athens, “Sotiria” General Hospital of Athens, “Tzaneio” General Hospital of Piraeus) and was conducted according to the Declaration of Helsinki. All patients provided written informed consent before trial enrollment. Full protocol of the PROGRESS trial is provided in the supplementary material.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Peyrani P, Mandell L, Torres A, Tillotson GS. The burden of community-acquired bacterial pneumonia in the era of antibiotic resistance. Expert Rev Respir Med. 2019;13:139–152. doi: 10.1080/17476348.2019.1562339. [DOI] [PubMed] [Google Scholar]
- 2.Kara S, Akçay MŞ, Ekici Ünsal Z, Bozkurt Yılmaz HE, Habeşoğlu MA. Comparative analysis of the patients with community-acquired pneumonia (CAP) and health care-associated pneumonia (HCAP) requiring hospitalization. Tuberk Toraks. 2019;67:108–115. doi: 10.5578/tt.68421. [DOI] [PubMed] [Google Scholar]
- 3.Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among US adults. N Engl J Med. 2015;373:415–427. doi: 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kyriazopoulou E, Liaskou-Antoniou L, Adamis G, et al. Procalcitonin to reduce long-term infection-associated adverse events in sepsis: a randomized trial. Am J Respir Crit Care Med. 2021;203:202–210. doi: 10.1164/rccm.202004-1201OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Müller B, Harbarth S, Stolz D, et al. Diagnostic and prognostic accuracy of clinical and laboratory parameters in community-acquired pneumonia. BMC Infect Dis. 2007;7:10. doi: 10.1186/1471-2334-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:1. doi: 10.1093/cid/ciw504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45–e67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.European Committee on Antimicrobial Susceptibility Testing. Clinical breakpoints for bacteria. Version 9.0. 2019. http://www.eucast.org/clinical_breakpoints/. Accessed 31 Oct 2020.
- 10.BIOFIRE® FILMARRAY® Pneumonia plus Panel. 2021. https://www.biomerieux-diagnostics.com/biofire-filmarray-pneumonia-panel. Accessed 6 May 2021.
- 11.Wunderink RG, Self WH, Anderson EJ, et al. Pneumococcal community-acquired pneumonia detected by serotype-specific urinary antigen detection assays. Clin Infect Dis. 2018;66:1504–1510. doi: 10.1093/cid/cix1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carugati M, Aliberti S, Sotgiu G, et al. Bacterial etiology of community-acquired pneumonia in immunocompetent hospitalized patients and appropriateness of empirical treatment recommendations: an international point-prevalence study. Eur J Clin Microbiol Infect Dis. 2020;39:1513–1525. doi: 10.1007/s10096-020-03870-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zakharenkov IA, Rachina SA, Dekhnich NN, et al. Etiology of severe community-acquired pneumonia in adults: results of the first Russian multicenter study. Ter Arkh. 2020;92:36–42. doi: 10.26442/00403660.2020.01.000491. [DOI] [PubMed] [Google Scholar]
- 14.Self WH, Balk RA, Grijalva CG, et al. Procalcitonin as a marker of etiology in adults hospitalized with community-acquired pneumonia. Clin Infect Dis. 2017;65:183–190. doi: 10.1093/cid/cix317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schuetz P, Christ-Crain M, Thomann R, et al. Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA. 2009;302:1059–1066. doi: 10.1001/jama.2009.1297. [DOI] [PubMed] [Google Scholar]
- 16.Christ-Crain M, Stolz D, Bingisser R, et al. Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia: a randomized trial. Am J Respir Crit Care Med. 2006;174:84–93. doi: 10.1164/rccm.200512-1922OC. [DOI] [PubMed] [Google Scholar]
- 17.Schuetz P, Wirz Y, Sager R, et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: a patient level meta-analysis. Lancet Infect Dis. 2018;18:95–107. doi: 10.1016/S1473-3099(17)30592-3. [DOI] [PubMed] [Google Scholar]
- 18.Timbrook T, Maxam M, Bosso J. Antibiotic discontinuation rates associated with positive respiratory viral panel and low procalcitonin results in proven or suspected respiratory infections. Infect Dis Ther. 2015;4:297–306. doi: 10.1007/s40121-015-0087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shengchen D, Gu X, Fan G, et al. Evaluation of a molecular point-of-care testing for viral and atypical pathogens on intravenous antibiotic duration in hospitalized adults with lower respiratory tract infection: a randomized clinical trial. Clin Microbiol Infect. 2019;25:1415–1421. doi: 10.1016/j.cmi.2019.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brendish NJ, Malachira AK, Armstrong L, et al. Routine molecular point-of-care testing for respiratory viruses in adults presenting to hospital with acute respiratory illness (ResPOC): a pragmatic, open-label, randomised controlled trial. Lancet Respir Med. 2017;5:401–411. doi: 10.1016/S2213-2600(17)30120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.