Abstract
Supratentorial sensory perception, including pain, is subserved by the trigeminal nerve, in particular, by the branches of its ophthalmic division, which provide an extensive innervation of the dura mater and of the major brain blood vessels. In addition, contrary to previous assumptions, studies on awake patients during surgery have demonstrated that the mechanical stimulation of the pia mater and small cerebral vessels can also produce pain. The trigeminovascular system, located at the interface between the nervous and vascular systems, is therefore perfectly positioned to detect sensory inputs and influence blood flow regulation. Despite the fact that it remains only partially understood, the trigeminovascular system is most probably involved in several pathologies, including very frequent ones such as migraine, or other severe conditions, such as subarachnoid haemorrhage. The incomplete knowledge about the exact roles of the trigeminal system in headache, blood flow regulation, blood barrier permeability and trigemino‐cardiac reflex warrants for an increased investigation of the anatomy and physiology of the trigeminal system. This translational review aims at presenting comprehensive information about the dural and brain afferents of the trigeminovascular system, in order to improve the understanding of trigeminal cranial sensory perception and to spark a new field of exploration for headache and other brain diseases.
Keywords: anatomy, brain innervation, cranial sensitivity, dura mater innervation, migraine, trigeminal system, trigemino‐cardiac reflex, trigeminovascular system
New synthesis about the cranial sensitivity. Is the extensive trigeminal innervation underestimated?
1. INTRODUCTION
Supratentorial sensory perception is subserved by the trigeminal nerve, which provides extensive innervation of the dura mater and of the major brain blood vessels. The exact pathogenesis of headache is still unresolved and debated, yet a peripheral trigeminal sensitization from extracranial, dural and pial arteries seems to play an important role (Olesen et al., 2009). In addition, the trigeminal system acts as a powerful vasodilator, decreasing cerebrovascular resistance in normal and pathological conditions (Goadsby et al., 1997; Kumada et al., 1977; Lambert et al., 1984) and it also controls the permeability of the blood vessels wall. The increase in permeability of blood vessels causing plasma extravasation concerns capillaries and postcapillary vessels (Moskowitz et al., 1988). Finally, stimulation of the peripheral or central trigeminal pathways may cause a severe bradycardia that can lead to asystole and sudden death (Chowdhury, Mendelowith, et al., 2015; Schaller, 2004; Schaller et al., 1999).
These various conditions rely upon a complex autonomic (sympathetic and parasympathetic) and trigeminal network (Arbab et al., 1986; Hara & Weir, 1986; Macfarlane & Moskowitz, 1995; Mayberg et al., 1984; Nielsen & Owman, 1967), which is only partially understood, despite extensive research spanning over three centuries (Arnold, 1831; Bolay et al., 2002; Fontaine et al., 2018; Joo et al., 2014; Olesen et al., 2009; Penfield & McNaughton, 1940). Current anatomical knowledge about cephalic innervation is mainly based on experimental animals studies (Arbab et al., 1986; Edvinsson, 2011; Liu‐Chen, Mayberg, et al., 1983, 1983; Mayberg et al., 1984; Schueler et al., 2014; Strassman et al., 2004), human anatomical studies (Davidson et al., 2012; Lee, Hwang, et al., 2017; Lee, Shin, et al., 2017; Penfield & McNaughton, 1940) and more recently on observations reported on awake patients during surgery (Fontaine et al., 2018; Ray & Wolff, 1940).
2. METHODS
A PubMed search was performed, using the phrases “Trigeminovascular system”, “cranial sensitivity”, “Trigeminal system”, “Dura mater innervation”, “brain innervation”, “Trigemino‐cardiac reflex” and “Trigeminal nerve”, for all years up to 2019.
Systematic reviews, experimental animal studies, human anatomical studies, observations reported during awake neurosurgery, radiological and immunohistochemical studies were used. The references of these articles were examined for additional sources, and we ended up examining 87 articles for this review.
3. TRIGEMINAL NERVE—DURAL AND BRAIN INNERVATION
Beyond the well‐known implication of the trigeminal nerve in face tactile and pain perception (Figure 1), historical observations have also demonstrated its importance for mediating headache (Penfield & McNaughton, 1940). In the early part of the 20th century, relief of headache had indeed been a frequent finding following trigeminal rhizotomy, gasserian alcohol injection or bulbar tractotomy, but only occurred when the ophthalmic division (V1) was injured, as discussed by Mayberg et al. (1984). Similarly, headache induced by intravenous injection of histamine was only abolished by section of the V1, but not by section of the maxillar (V2) or mandibular (V3) divisions, neither by inferior cervical/stellate ganglionectomy nor glossopharyngeal roots section. Trigeminal nerve somato‐sensory receptors are exclusively nociceptors. They are free nerve ending located within the leptomeninges as well as in cerebral arteries and venous sinuses (Noseda & Burstein, 2013), and are activated by strong mechanical stimuli, heat and various chemical substances (Mayberg et al., 1984). The absence of intracranial tactile and proprioception receptors is further confirmed by the composition of trigeminal fibres, which mainly contain small myelinated A‐delta (1–6 µm) and unmyelinated C‐fibres (0.1–0.4 µm), classically connected to nociceptors. A very few A‐beta fibres innervating the dura mater have nevertheless been described, but their significance remains unexplained (Ramachandran, 2018).
FIGURE 1.
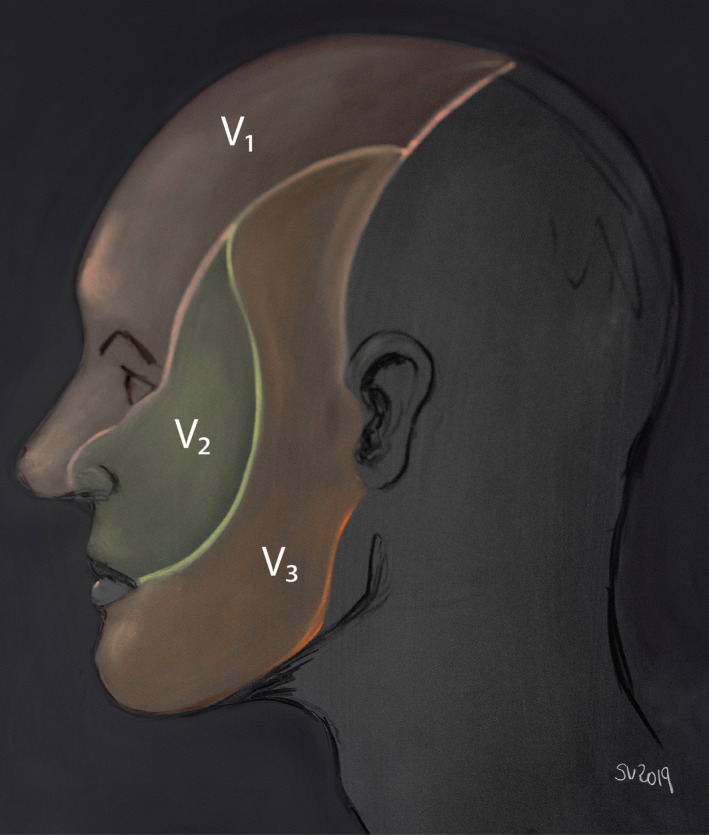
Skin territory of the three divisions of the trigeminal nerve. The ophthalmic nerve (V1) supplies the eye bulb including cornea, upper eyelid, back of the nose and skin of the forehead. The maxillary nerve (V2) innervates the cheek, lover eyelid, lateral part of the nose, upper teeth and gingiva and hard palate. Finally, the mandibular nerve (V3) supplies the mandibular gums and teeth, skin in the temporal region, part of the auricle and lower lip
Below we review both dura mater and cerebral innervation.
3.1. Trigeminal nerve and dura mater innervation
The first anatomical studies of human cranial dura mater innervation were published in 1831 by Arnold and 1856 by Von Luschka (Arnold, 1831; Von Luschka, 1856). In 1929, Dowgjallo and Grzybowski localized the origin of meningeal nerve fibres in the trigeminal ganglion (Dowgjallo, 1929; Grzybowski, 1931; Schueler et al., 2014), and Penfield and McNaughton (1940) specified that dural sensitivity was supported by the three divisions of the trigeminal nerve. It is now well established that these trigeminal divisions innervate the supratentorial dura mater, whereas the dural innervation of the posterior fossa is supplied by the recurrent meningeal branches of the vagus and glossopharyngeal nerves, as well as by the sympathetic trunk (Kemp et al., 2012; Lee, Shin, et al., 2017; Penfield & McNaughton, 1940). Within the dura, trigeminal and autonomic meningeal fibres are intermingled, but can be distinguished using neuropeptide‐specific staining. For instance, calcitonin gene‐related peptide (CGRP) is not present, and substance P is only somewhat present in the autonomic nerves, whereas highly associated with the sensitive fibres of meninges (Edvinsson, 2011, 2017; Strassman et al., 2004). CGRP‐like immunoreactivity has revealed a wide network of dural fibres, especially dense along borders of the dural sinuses, and more particularly along the caudal border of the transverse sinus, 2–4 mm from the midline (Strassman et al., 2004). The higher arborization of axons is usually located in the initial part of their trajectory, before they follow a relatively straight course across the dura, with little or no branching; axons, for instance, cross the distal branches of the middle meningeal artery (MMA) without altering their course (Strassman et al., 2004). Using silver nitrate preparation, dural nerves can be traced up to the reunion of the three trigeminal divisions, but usually not within the trigeminal ganglion (Penfield & McNaughton, 1940). Despite many interindividual variations, these supratentorial dural nerves follow three main routes to reach the trigeminal nerve (Figure 2):
a rostral route, originating within the anterior cranial fossa, and following the anterior and posterior ethmoidal nerves to connect to the V1;
a caudal route, for the transverse sinus, torcula, tentorium, and posterior part of the falx, which follows the tentorial nerve to reach the V1;
and a lateral route, which innervates the middle cranial fossa and follows the meningeal branches of V2 and V3, running along with branches of the MMA (Fontaine et al., 2018; Strassman et al., 2004).
FIGURE 2.
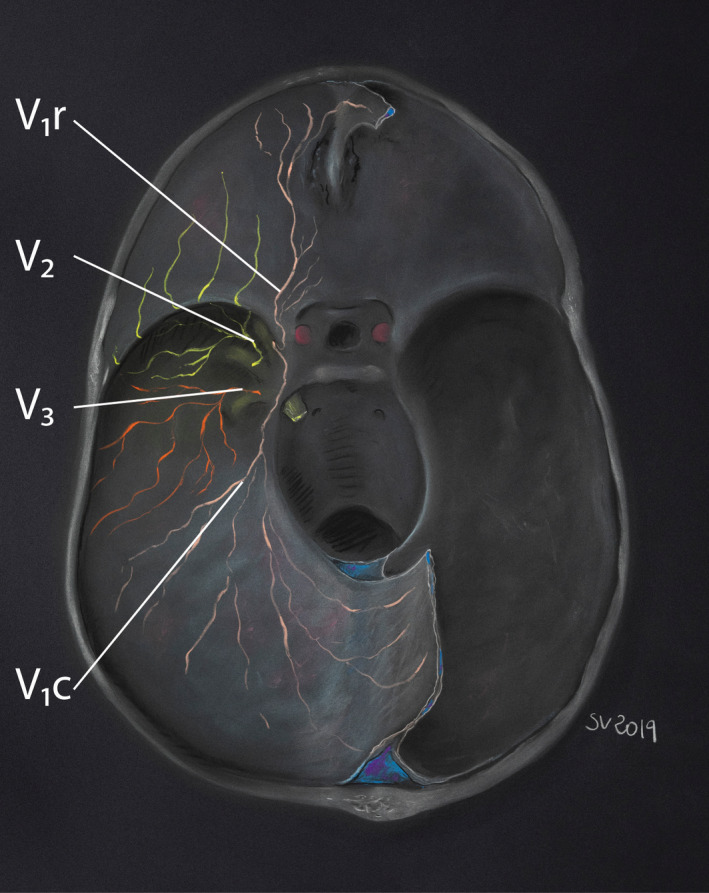
Dural territory of the three divisions of the trigeminal nerve. (after Penfield & McNaughton, 1940; Lee, Hwang, et al., 2017; Lee, Shin, et al., 2017; Gray's Anatomy, 40th ed., Standring, Intracranial region, p. 433, 2008). The supratentorial dural innervation depends on three main routes: a rostral route(V1 r), which innervates the anterior cranial fossa and anterior part of the falx, and following the anterior and posterior ethmoidal nerves to connect to the V1; a caudal route(V 1c), for the transverse sinus, torcula, tentorium and posterior part of the falx, and which follows the tentorial nerve to reach the V1; and a lateral route (V2 and V3), which innervates the middle cranial fossa and follows the meningeal branches of V2 and V3
The rostral and caudal dural routes mainly involve several branches of the Ophthalmic division (V1) of the trigeminal nerve (Lee, Shin, et al., 2017; Penfield & McNaughton, 1940):
The anterior and posterior ethmoidal nerves originate from fine nervous fasciculi within the dura surrounding the cribiform plate, and are responsible for the anterior cranial fossa innervation (Penfield & McNaughton, 1940). In addition, the anterior ethmoidal nerves collect tiny branches arriving from either sides of the superior sagittal sinus and joining the anterior part of the falx and the crista galli.
The tentorial nerve (former nervus tentoria of Arnold) is another constant afferent to V1. It gets afferent branches from a rich plexus in the torcular region (Penfield & McNaughton, 1940) and has a large meningeal territory which includes: the tentorium cerebelli, the posterior two thirds of the falx cerebri, the posterior part of the superior sagittal sinus, the superior wall of the transverse sinus and probably the straight and inferior sagittal sinuses. On the convexity, it innervates the distal MMA and occasionally the dura mater of the parieto‐occipital region (Lee, Shin, et al., 2017). According to Penfield, the tentorial nerve follows the dural sheath of the trochlear nerve and joins the upper border of the V1 within the lateral wall of the cavernous sinus, about 1 cm distal to the trigeminal ganglion (Kemp et al., 2012; Penfield & McNaughton, 1940).
Occasionally V1 also receives one or more nerves which follow the anterior branch of the MMA (Penfield & McNaughton, 1940).
By contrast to these V1 rostral and caudal routes, the maxillary (V2) and mandibular (V3) divisions of the trigeminal nerve are mainly involved in the lateral dural route. They receive meningeal branches (former nervus medius and nervus spinosus; Kemp et al., 2012, respectively) which mainly innervate the middle cerebral fossa and the lesser wing of the sphenoid bone, and sometimes the anterior part of the falx cerebri (Fontaine et al., 2018; Lee, Hwang, et al., 2017; Penfield & McNaughton, 1940); these meningeal branches get dural nerve fibres in the area of the pterion or close to the intersection of the MMA and the sphenoparietal sinus; they then follow the frontal or sometimes more posterior branches of the MMA (Lee, Hwang, et al., 2017). Animal studies using anterograde and/or retrograde neural tracing support the hypothesis that meningeal branches of V2 and V3 also receive afferents from pericranial temporal, parietal, and occipital periosteum and adjacent deep layers of the temporal and upper neck muscles. These rami enter the skull through emissary canals and fissures to join the meningeal branches of V2 and V3 (Burstein et al., 2017; Kerr, 1961; Kosaras et al., 2009; Schueler et al., 2013, 2014).
Finally, these intracranial lateral meningeal branches run along the middle cerebral fossa and join the V2 or V3 partitions of the TG, or the angles between V1 and V2, or V2 and V3 divisions (Penfield & McNaughton, 1940). According to Penfield, a trigeminal branch may go outside the cranial cavity through the foramen spinosum with the MMA and join the V3 division (Penfield & McNaughton, 1940).
Awake patients during surgery show that dura mater of the skull vault is generally insensitive, but in areas with high density of trigeminal fibres, mechanical stimulation of the middle cerebral fossa dura adjacent to MMA branches induces ipsilateral temporal, retroorbital and frontal pain (Kemp et al., 2012; Mayberg et al., 1984). Stimulation of the floor of the anterior fossa produces ipsilateral retro‐orbital pain (Mayberg et al., 1984), whereas stimulation along the falx cerebri produces ipsilateral temporoparietal and frontal pain. Pain decreases after injection of procaine into the caudal falx, adjacent to the posterior confluence of sinuses, suggesting the innervation by the neighbouring tentorial nerves (Feindel et al., 1960; Fontaine et al., 2018; Kemp et al., 2012; Mayberg et al., 1984). Stimulation of the superior aspect of the tentorium, torcular or straight sinus produces ipsilateral forehead and periorbital pain (Kemp et al., 2012; Mayberg et al., 1984). Similarly, the walls of the sinuses, the posterior third of the falx and the entry points of large veins within sinuses are extremely sensitive and correspond to dural areas having the densest trigeminal innervation (Feindel et al., 1960; Fontaine et al., 2018; Kemp et al., 2012; Macfarlane & Moskowitz, 1995).
3.2. Trigeminal nerve and brain innervation
Despite the fact that autonomic nerves were first described along intracranial arteries in 1664 by Willis (1664), innervation of the cerebral blood vessels is still poorly understood with regards to both the nociceptive sensitivity and the neurogenic control of the cerebral circulation. Cranial blood vessels are innervated by sympathetic (Edvinsson et al., 1972; Macfarlane & Moskowitz, 1995; Nielsen & Owman, 1967), parasympathetic (Hara & Weir, 1986; Macfarlane & Moskowitz, 1995) and sensory nerves (trigeminal afferent fibres) (Arbab et al., 1986; Macfarlane & Moskowitz, 1995; Mayberg et al., 1984) with a considerable overlap (Macfarlane & Moskowitz, 1995), yet the exact function of each of these nerves in physiological or pathological situations is still up for debate and subject of controversy (Goadsby et al., 1997). Inasmuch as autonomous (sympathetic and parasympathetic), and trigeminal innervations may be of importance for cerebral blood flow control and pain perception, all three will be reviewed below.
The sympathetic innervation of the cerebral blood vessels derives from the superior cervical sympathetic ganglion, with small contributions from the middle cervical sympathetic and stellate (fusion of the lower cervical sympathetic and first thoracic) ganglions (Macfarlane & Moskowitz, 1995). In addition to its trophic effects on blood vessels (Goadsby et al., 1997; Macfarlane & Moskowitz, 1995), the sympathetic system promotes vasoconstriction, thus protecting vessels and the surrounding brain against acute hypertension. In a study of rat cerebral vascular innervation (Arbab et al., 1986), injection of WGA‐HRP into the superior cervical ganglion revealed a strictly ipsilateral dense meshwork of nerve fibres along the internal carotid, middle cerebral and anterior cerebral arteries, and a sparser distribution to the anterior communicating, posterior cerebral and basilar arteries (Arbab et al., 1986). The injection of WGA‐HRP into the first cervical spinal ganglion labelled fibres on the ipsilateral vertebral and caudal basilar artery, while injections into the second cervical spinal ganglion labelled nerve fibres on the ipsilateral vertebral, internal carotid outside the cerebral arterial circle and the basilar artery up to its bifurcation in posterior cerebral arteries (Arbab et al., 1986). The stellate ganglion also innervates the cerebral vasculature, especially the vertebral and basilar arteries (Arbab et al., 1986; Kajikawa, 1968).
The parasympathetic innervation, which promotes vasodilation of the cerebral blood vessels, originates from multiple sources according to Macfarlane and Moskowitz (1995), including the sphenopalatine and otic ganglions and “small aggregates of ganglion cells within the cavernous plexus, vidian and lingual nerves”. Although the density of parasympathetic fibres is greatest in the proximal vessels, innervation also exists in vessels as small as 20 µm (Macfarlane & Moskowitz, 1995).
The trigeminal nociceptive innervation of the proximal arteries of the brain was reported in 1940 by Ray and Wolff (1940), who described pain perception in the trigeminal territory of humans during intraoperative mechanical, electrical or heat stimulation. This nociceptive perception has constantly been defined as sharp, intense, brief, ipsilateral and reversible upon discontinuation of the stimulus (Fontaine et al., 2018; Mayberg et al., 1984; Penfield & McNaughton, 1940; Ray & Wolff, 1940). No tactile or proprioceptive sensation has been reported, but nausea, vomiting and restlessness sometimes accompany the headaches (Fontaine et al., 2018).
In cats, rats and monkeys, histochemistry studies suggest that the supratentorial vascular structures contain afferent projections from V1 (Edvinsson, 2011; Feindel et al., 1960; Mayberg et al., 1984; Olesen et al., 2009; Ray & Wolff, 1940; Steiger et al., 1982). The trigeminovascular system consists of bipolar neurons, with cell bodies within the trigeminal ganglion, and peripheral and central fibres connecting to cranial blood vessels and the spinal division of the trigeminal nucleus located in brainstem and upper cervical cord respectively (May & Goadsby, 1999). The trigeminovascular system has been proposed to explain the pathogenesis of headaches (Mayberg et al., 1984) and has been explored in several models; Arbab et al. (1986) investigated the projection of nerve fibres related to cerebral vessels by injecting WGA‐HRP into the rat trigeminal ganglion, revealing a sparse meshwork of trigeminal fibres located close to the smooth muscle layer of the vessel wall. These trigeminal afferents contain several neuropeptides (substance P, CGRP, neurokinin A). CGRP plays an important role in migraine attacks (Edvinsson, 2017). The role of substance P and neurokinin A for migraine is still unclear (Edvinsson, 2011, 2017; Edvinsson et al., 1981; Yamamoto et al., 1983). Fibbers then group in bundles, located within the adventitia and between adventitia and media (Liu‐Chen et al., 1986; Liu‐Chen, Mayberg, et al., 1983, 1983; Macfarlane & Moskowitz, 1995). Most of the fibres range from 2 to 4.5 µm in diameter and are occasionally larger than 5 µm (Penfield & McNaughton, 1940; Strassman et al., 2004). Fibbers are mainly found on the ipsilateral internal carotid artery, arterial circle of Willis and neighbouring segments of its branches (middle cerebral, anterior cerebral, posterior communicating and posterior cerebral arteries) and rostral part of the basilar artery (Arbab et al., 1986; Mayberg et al., 1984). Fewer fibres are present on the contralateral anterior cerebral artery (Arbab et al., 1986). The density of trigeminal fibres thus appears to be greater along proximal arteries of the arterial circle, but decreases over the convexity of the hemispheres (Macfarlane & Moskowitz, 1995), and is minimal for vessels supplying the basal ganglia (Macfarlane & Moskowitz, 1995).
Innervation of smaller arteries was suggested by Liu‐Chen et al. who showed trigeminal projections from pial arteries and “pia arachnoid” using immunohistochemistry in the cat. They showed that the level of substance P in “pia arachnoid” is comparable to the one of peripheral structures innervated by the trigeminal nerve, but decreases 50% or more after ipsilateral lesion of the trigeminal ganglion (Liu‐Chen, Han, et al., 1983; Liu‐Chen, Mayberg, et al., 1983, 1983). These experimental findings support Fontaine's awake neurosurgery experience: contrary to what had been accepted for a long time, pain occurred not only after stimulation of major vessels (arterial circle) but also 25% of the time during pia mater and small cerebral vessels stimulation (Fontaine et al., 2018). This dramatically extends the trigeminal territory for brain innervation, although it requires further investigation to precisely define how distally the vascular system and pia are innervated.
Despite the fact that trigeminovascular fibres are supposed to reach the V1 of the trigeminal ganglion, direct anatomical evidence for such connections is lacking and their existence is still debated. Penfield & McNaughton (1940) discussed the hypothesis stating that “trigeminal fibres join the carotid artery at its intracavernous segment, at a point where the carotid plexus lies adjacent to the medial aspect of the ganglion”, but did not find fibres connecting the trigeminal nerve to cerebral arteries. They nevertheless mentioned a branch of nervus intermedius of the facial nerve, reaching the middle cerebral artery through the greater superficial petrosal nerve and suggested that the greater superficial petrosal nerve may carry sensory fibres (Penfield & McNaughton, 1940). In their fibre degeneration experiments in the monkey, Ruskell and Simons (1987) generally observed two or three small branches (but up to 6) joining the adventitia of the internal carotid artery to the V1, via the plexus of autonomic nerves of the cavernous sinus and starting at about 1.5 mm from the trigeminal ganglion. Interestingly, these fibres were distinct from the neighbouring autonomic branches. Indeed, “the branches contained unmyelinated fibre bundles and numerous myelinated fibres of various diameters which permitted them to be distinguished from neighbouring autonomic branches of the plexus whose myelinated fibres, by comparison, were infrequent and of nearly uniformly small diameter.” (Ruskell & Simons, 1987). They also observed a strong recurrent branch, which follows the abducens nerve up to the pons to innervate the basilar artery and the caudal arterial circle (Ruskell & Simons, 1987).
3.2.1. Trigeminal vasoactive innervation
More surprisingly, the trigeminovascular system may also play a non‐perceptive role by modulating cerebral blood flow: contrary to sympathetic fibres, the trigeminovascular system may serve as a powerful vasodilator decreasing cerebrovascular resistance (Goadsby et al., 1997; Kumada et al., 1977; Lambert et al., 1984). The trigeminal endings around cranial blood vessels indeed contain vasodilator neuropeptides, including CGRP, substance P and Neurokinin A (Macfarlane & Moskowitz, 1995). The vasodilatory effect of CGRP is mediated by its action on CGRP receptors on smooth muscles of the vessels, resulting in activation of adenylyl cyclase and enhancing the levels of cyclic AMP (Ramachandran, 2018). According to Sakas et al. (1989), the increase in blood flow in cortical grey matter observed during severe hypertension is not totally passive, but is also mediated by trigeminovascular fibres via a peripheral mechanism involving vasoactive neuropeptides release from trigeminal axonal endings, not dependent on central neurotransmission. An attenuation of 20–30% of such blood flow increase is induced by trigeminal ganglionectomy in cats, but not by trigeminal root section (rhizotomy), and is limited to regions adjacent to pial arteries receiving innervation from the trigeminal nerve. Similarly, deep grey matter nuclei that have minimal trigeminal innervation do not manifest significant blood flow drop, suggesting a major implication of the trigeminal nerve in vasodilatation (Sakas et al., 1989). Goadsby et al. (1997) also suggested a highly functional and somatotopic organization in the blood flow control by the trigeminal system; in cats, stimulation of the superior sagittal sinus, innervated by V1, increases cerebral blood flow with little effect on bulk carotid flow, whereas the stimulation of the trigeminal ganglion increases both bulk carotid and cerebral blood flow.
Additionally to regulating blood flow changes, the trigeminal system also controls the permeability of the arterial wall. Substance P is the prime mediator in causing plasma protein leakage by its action on the NK‐1 receptors expressed by microvessels (Ramachandran, 2018). The removal of trigeminal ganglia induces an ipsilateral decrease in extravasation of intravenously administered iodinated albumin (Moskowitz et al., 1988). Thus, the activation of the trigeminal system could contribute to pathophysiological conditions associated with severe hypertension, such as haemorrhage or enema (Moskowitz et al., 1988), and new strategies to block or stimulate the activity of the trigeminal nerve by neuromodulation to limit or promote the blood flow and the vascular permeability are emerging (Sakas et al., 1989). Significant inhibition of plasma protein extravasation following administration of classic antimigraine drugs (ergots and sumatriptan) have been shown in preclinical studies (Ramachandran, 2018).
4. THE TRIGEMINOVASCULAR SYSTEM IN PATHOLOGICAL CONDITIONS
Due to its anatomical location and physiological roles, the trigeminovascular system is a good candidate for several diseases involving the neurovascular vasculo‐nervous interface, some of them being summarized in this section, and most of them sharing the presence of a sterile inflammation.
4.1. Neurogenic inflammation, trigeminal sensitization and headache
A local sterile inflammation of the tissues surrounding trigeminal endings can be the result of two—partially intermingled—processes (Ramachandran, 2018):
a direct way, which directly induces a local inflammation after various stimuli, such as stress, hormonal changes or cortical spread depression (CSD; Boes & Levy, 2012; Dalkara et al., 2006; Pietrobon & Moskowitz, 2014; Ramachandran, 2018; Theoharides et al., 2005, 1995; Zaitsu et al., 2007). For instance, CSD encountered in migraine with aura is a slowly propagating wave of neuronal and glial depolarization, probably ignited by local elevations of extracellular K+, which leads to hyperactive cortical circuits (Figure 3; Pietrobon & Moskowitz, 2013; Zhang et al., 2010, 2011). Propagation of CSD opens PANX1 mega channels in stressed neurons and locally releases various proinflammatory‐signalling molecules, which in turn induce glial synthesis of prostaglandins and cytokins.
a hypothetic indirect trigeminally mediated way, which further increases this local inflammation: released inflammation mediators activate the trigeminal receptors located on the pia and the pial arteries, and produce a nervous signal running ortho‐ and antidromically; orthodromically, this nociceptive message reaches the ganglion of the trigeminal nerve and continues to the central nervous system. The antidromical activation of the trigeminal nerve (axon reflex) is supposed to follow the trigeminovascular pathway, especially along the MMA and to release vasoactive proinflammatory peptides, such as CGRP. Nevertheless, the antidromic activation hypothesis depends on the existence of a pathway of collateral dural and meningeal branches of same afferents, which have not been found as yet
FIGURE 3.
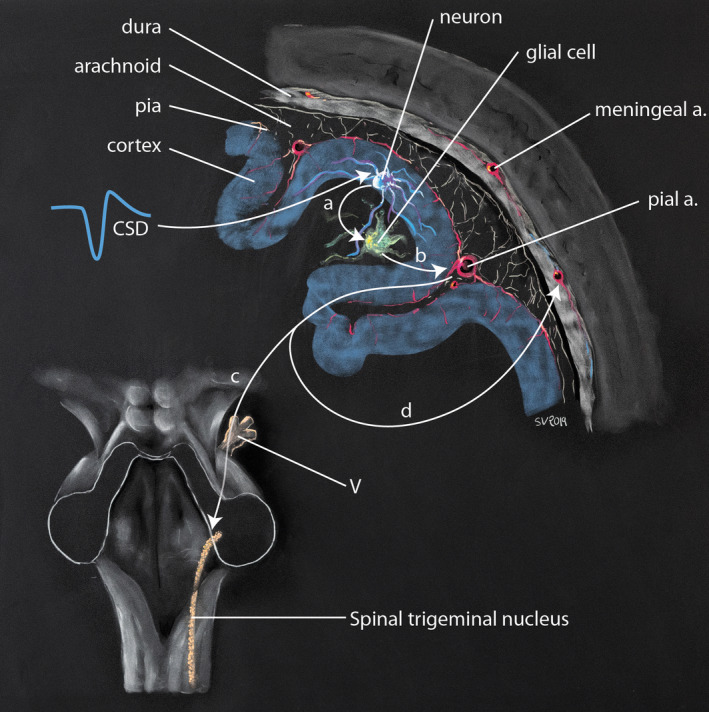
Proposed mechanism of activation of the trigeminovascular in migraine following cortical spreading depression (after Pietrobon & Moskowitz, 2014). During cortical spreading depression (CSD), neurons locally release various proinflammatory signalling molecules (a), which in turn induce glial synthesis of prostaglandins and cytokins (b) (direct pathway). These molecules activate the trigeminal receptors located on the pia and pial arteries, and produce a nervous signal running ortho and antidromically; orthodromically (c), this signal reaches the ganglion of the trigeminal nerve and transmits the nociceptive message to the central nervous system. The CSD‐induced antidromical activation of the trigeminal nerve (d) is supposed to follow the trigeminovascular pathway, especially along the middle meningeal artery and to release vasoactive proinflammatory peptides in the dura mater (indirect pathway). This produces vasodilatation, plasma extravasation and local activation of dural mast cells, the later producing a long‐lasting activation and sensitization of dural nociceptors
Both processes promote inflammation, gathering vasodilation (CGRP action on CGRP receptors on the smooth muscle), plasma protein extravasation (SP action on NK‐1 receptors on the endothelium) and mast cell degranulation around brain vessels. This modified local milieu is hypothesized to induce sensitization of trigeminal high‐threshold mechanoreceptors, which could exaggerate painful perception of usually innocuous mechanical stimuli such as coughing, sudden jerks or movement (Ramachandran, 2018). This would explain the ability of oral non‐steroid anti‐inflammatory drugs in aborting acute migraine headaches and avoiding the development of persistent pain (Edvinsson et al., 2019; Ramachandran, 2018). Olesen et al. (2009) also suggest that activation of peripheral nociceptors induces a wider facilitation of transmission of nociceptive information through the spinal trigeminal nucleus but also through the thalamus and the cortex. A hyperexcitability of the trigeminal system is confirmed by molecular changes observed in nociceptive neurons during migraine (Burstein et al., 2011). Burstein et al. consider the following assumption: if the pain is not stopped within 10–20 min after the headache starts, a first set of neurons, located within the trigeminal ganglion, undergoes molecular changes and become hypersensitive to changes in intracranial pressure, explaining why migraine headache increases by bending over and sneezing (peripheral sensitization). If the pain is not stopped within 60–120 min, a second set of neurons located in the spinal trigeminal nucleus undergoes molecular changes that convert them into an independent headache generator (sensitization of second order neurons in spinal nucleus). The activation of other brain structures can then result in head cutaneous allodynia (Burstein et al., 2011, 2015). Nevertheless, it is not clear whether these neurons become spontaneously active or whether they need the peripheral drive of active afferent input. Given these consecutive stages suspected in a migraine attack, the success rate of pain relief may increase dramatically if medication is taken before the establishment of central sensitization. Clinical effects of the triptans medication can be explained by a disruption of nociceptive transmission between peripheral and central trigeminovascular neurons, rather than directly inhibiting peripheral or central neurons (Burstein et al., 2011).
These three phenomena (direct inflammation, trigeminal release of inflammatory mediators and trigeminal sensitization) will cross‐cooperate to further increase pain. They offer several entry points in this vicious circle converging towards a common biological mechanism (neuroinflammation). Neuroinflammation thus appears as the keystone for the pathophysiology of primary and secondary headaches (Levy et al., 2009), both possibly constituting a continuum (Schankin & Straube, 2012). For instance, tumour headache, just like post‐craniotomy headache, is more frequent in patients with pre‐existing migraine headache history (Schankin & Straube, 2012; Taylor, 2014).
4.2. Trigeminovascular system, unruptured and ruptured intracranial aneurysms
Unruptured intracranial aneurysms are relatively common, present in about 1–2% of the population (Brown & Broderick, 2014). Although chronic headache is rarely described in unruptured intracranial aneurysm, some authors consider headache as a possible symptom, more frequent for aneurysms greater than 1 centimetre than for smaller aneurysms (Friedman et al., 2001; Gilard et al., 2016; Link et al., 2008). The headache could be related to the stimulation of the trigeminovascular system by the distention, dissection or thrombosis of the aneurysm wall (Link et al., 2008).
The acute headache observed a few days before an aneurysm rupture, also called “sentinel headache”, has been described in up to 43% of patients with aneurysmal subarachnoid haemorrhage (SAH; Gilard et al., 2016; Linn et al., 1994; Polmear, 2003). The sentinel headache could be mediated by the perivascular trigeminal innervation due to aneurysm wall distension, or caused by a minimum fissuration, with « intra‐saccular bleeding by way of vasa vasorum rupture, leading to local inflammatory reaction» according to Gilard et al. (2016) This hypothesis may explain the lack of subarachnoidal bleeding in CT scans or lumbar puncture in these cases.
Finally, ruptured intracranial aneurysm is characterized by sudden headache accompanied by vomiting and constitutes a serious event leading to 50% of mortality and more or less heavy disability among the survivors (van Gijn et al., 2007). The extreme head pain during SAH may be explained by a spatial and temporal summation secondary to a long‐lasting and large area of stimulation of the trigeminal peripheral nociceptors (Olesen et al., 2009), and by the release of vasodilator neuropeptides such as CGRP and substance P (Goadsby et al., 1997). Macfarlane and Moskowitz (1995) raised the possibility that the trigeminovascular system may also prevent excessive vasoconstriction of large arteries by releasing vasodilating neuropeptides after SAH. They observed an unexplained axonal degeneration of perivascular nerves about 7 days after SAH, a time window which coincides with the development of delayed vasospasm (Macfarlane & Moskowitz, 1995).
Dagistan et al. investigated the effects of surgical cervical sympathectomy on the neurogenic inflammatory neuropeptides shortly after SAH induction in rats. They found that the sympathectomy reduced the increases in markers of neuronal activation and dural neurogenic inflammation, including c‐fos, CGRP, Substance P and VIP evoked by SAH, and suggest that cervical sympathectomy treatment may prevent early brain injury by modulating SAH‐induced neurogenic inflammatory neuropeptides (Dagistan et al., 2019).
4.3. Trigeminovascular system and trigemino‐cardiac reflex
The trigemino‐cardiac reflex (TCR) is a brainstem reflex defined as the sudden onset of parasympathetic bradycardia up to asystole, hypotension, apnoea and gastric hypermotility, following stimulation of any of the sensory branches of the trigeminal nerve (Chowdhury, Mendelowith, et al., 2015; Leon‐Ariza et al., 2018; Schaller, 2004, 2005a), or during central stimulation of the trigeminal pathway (trigeminal depressor response; Chowdhury, Mendelowith, et al., 2015; Kumada et al., 1977). Its first description was probably the one provided by Penfield and McNaughton (1940), who reported asystole following bilateral stimulation of the falx cerebri, an area densely innervated by V1 branches. TCR was later observed in several surgical procedures involving one of the trigeminal branches (e.g. transsphenoidal surgery, trigeminal rhizolysis, microvascular trigeminal decompression, cerebellopontine angle surgery, skull base surgery; Abou‐Zeid et al., 2009; Chowdhury, Ahuja, et al., 2015; Koerbel et al., 2005; Rath et al., 2007; Schaller et al., 1999, 2007; Schaller, 2005b) and during embolization of arterio‐venous malformations or intracranial dural fistulas (Khatibi et al., 2017; Lv et al., 2007, 2010; Wang et al., 2016).
The understanding of this reflex and its role in severe pathological conditions remain unclear (Chowdhury, Mendelowith, et al., 2015; Schaller, 2004; Schaller et al., 1999); for instance, severe haemodynamic compromise during SAH was assigned so far to heart dysfunction following hyperactivation of sympathetic nervous system (Zaroff et al., 2006). Nevertheless, sudden subarachnoid bleeding may also be regarded as the starting point of TCR, resulting in subarachnoid bleeding‐related asystole.
5. PERSPECTIVES, CHALLENGES AND FUTURES RESEARCHES
Pathophysiology of primary, secondary headache and facial pain remains only partially understood, even though these conditions are the most common neurological disorders and cause many disabilities. The trigeminal system is now well known to be the anatomical substrate of various cephalalgias, and increasing evidence indicates that the headache phase depends on nociceptive input from terminal sensory trigeminal nerve, even if the origin of the migraine cascade may have taken place in the cortex or deep brain structures.
Trigeminal inputs originate from extracranial, dural or pial blood vessels, probably more from arteries than veins. Thus, thanks to the extensive trigeminal innervation of the dura mater and the brain (with trigeminal afferents fibres surrounding major cerebral blood vessels, small cerebral vessels and also possibly the pia matter), the trigeminovascular system is well placed to detect perturbations, trigger pain activation and also influence blood flow regulation.
Much has been learned about headache mechanisms during the last decade, nevertheless more research is clearly needed, especially in neuroanatomy, to answer the following questions:
How do trigeminal fibres reach the cerebral arteries in human?
What is the extent of brain surface coverage by the trigeminal network?
What is the exact involvement of the trigeminal system in primary and secondary headache, blood flow regulation and blood–brain barrier?
Could the sensitization of the trigeminal system and the frequent chronic aspect of headache be the consequence of silent nociceptors, which are activated by inflammatory promoters and fire long after disappearance of the stimulus?
Answering these questions will require more investigations in the anatomy and physiology of the trigeminal system, to better understand the pathophysiology and improve therapeutics in numerous diseases such as headache, facial pain or vasospasm.
6. CONCLUSION
The trigeminal system has a dramatically broader somatosensory territory compared to previously known classical involvement in facial sensitivity touch, pain and proprioception. Many evidences indeed plead for an extensive trigeminal innervation of the dura mater, large but also smaller cerebral blood vessels and possibly pia matter. Thanks to its location (interface between the nervous and vascular systems), the trigeminovascular system can detect intra‐ and extracranial stimuli, transmit‐related somatosensory information to the central nervous system and also influence cerebral perfusion and blood–brain barrier permeability—hence, its importance in physiological and pathological conditions, some of them being very frequent (migraine) or severe (rupture of vascular malformations and SAH).
AUTHOR CONTRIBUTIONS
Louis‐Marie Terrier, MD: Literature search, study concept and design, writing. Nouchine Hadjikhani, MD, PhD: Writing and critical revision of the manuscript. Stéphane Velut, MD, PhD: Drawing (Figures 1, 2 and 3) and critical revision of the manuscript. Caroline Magnain, PhD: Study concept, future perspective in imaging. Aymeric Amelot, MD, PhD: Writing, critical revision of the manuscript. Florian Bernard, MD: writing, critical revision of the manuscript. Lilla Zöllei, PhD: Study concept and design, supervision, future perspective, critical revision of the manuscript. Christophe Destrieux, MD, PhD: Study concept and design, supervision, future perspective, critical revision of the manuscript.
ACKNOWLEDGMENTS
We thank the French Academy of Medicine, the French Society of Neurosurgery and the Foundation of « Gueules Cassées » for their financial support.
REFERENCES
- Abou‐Zeid, A.H. , Davis, J.R.E. , Kearney, T. & Gnanalingham, K.K. (2009) Transient asystole during endoscopic transsphenoidal surgery for acromegaly: An example of trigeminocardiac reflex. Pituitary, 12(4), 373–374. 10.1007/s11102-008-0118-2 [DOI] [PubMed] [Google Scholar]
- Arbab, M.A. , Wiklund, L. & Svendgaard, N.A. (1986) Origin and distribution of cerebral vascular innervation from superior cervical, trigeminal and spinal ganglia investigated with retrograde and anterograde WGA‐HRP tracing in the rat. Neuroscience, 19(3), 695–708. [DOI] [PubMed] [Google Scholar]
- Arnold, F. (1831) Der Kopfteil des Vegetativen Nerven systems beim Menschen. Heidelberg: K. Groos. [Google Scholar]
- Boes, T. & Levy, D. (2012) Influence of sex, estrous cycle, and estrogen on intracranial dural mast cells. Cephalalgia, 32(12), 924–931. 10.1177/0333102412454947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolay, H. , Reuter, U. , Dunn, A.K. , Huang, Z. , Boas, D.A. & Moskowitz, M.A. (2002) Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nature Medicine, 8(2), 136–142. 10.1038/nm0202-136 [DOI] [PubMed] [Google Scholar]
- Brown, R.D. & Broderick, J.P. (2014) Unruptured intracranial aneurysms: Epidemiology, natural history, management options, and familial screening. The Lancet Neurology, 13(4), 393–404. 10.1016/S1474-4422(14)70015-8 [DOI] [PubMed] [Google Scholar]
- Burstein, R. , Blake, P. , Schain, A. & Perry, C. (2017) Extracranial origin of headache. Current Opinion in Neurology, 30(3), 263–271. 10.1097/WCO.0000000000000437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein, R. , Jakubowski, M. & Rauch, S.D. (2011) The science of migraine. Journal of Vestibular Research: Equilibrium & Orientation, 21(6), 305–314. 10.3233/VES-2012-0433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein, R. , Noseda, R. & Borsook, D. (2015) Migraine: Multiple processes, complex pathophysiology. The Journal of Neuroscience, 35(17), 6619–6629. 10.1523/JNEUROSCI.0373-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury, T. , Ahuja, N. & Schaller, B. (2015) Severe bradycardia during neurosurgical procedure: Depth of anesthesia matters and leads to a new surrogate model of the trigeminocardiac reflex: a case report. Medicine, 94(49), e2118. 10.1097/MD.0000000000002118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury, T. , Mendelowith, D. , Golanov, E. , Spiriev, T. , Arasho, B. , Sandu, N. et al. (2015) Trigeminocardiac reflex: The current clinical and physiological knowledge. Journal of Neurosurgical Anesthesiology, 27(2), 136–147. 10.1097/ANA.0000000000000065 [DOI] [PubMed] [Google Scholar]
- Dagistan, Y. , Kilinc, E. & Balci, C.N. (2019) Cervical sympathectomy modulates the neurogenic inflammatory neuropeptides following experimental subarachnoid hemorrhage in rats. Brain Research, 1722, 146366. 10.1016/j.brainres.2019.146366 [DOI] [PubMed] [Google Scholar]
- Dalkara, T. , Zervas, N.T. & Moskowitz, M.A. (2006) From spreading depression to the trigeminovascular system. Neurological Sciences, 27(Suppl 2), S86–90. 10.1007/s10072-006-0577-z [DOI] [PubMed] [Google Scholar]
- Davidson, J.R. , Mack, J. , Gutnikova, A. , Varatharaj, A. , Darby, S. & Squier, W. (2012) Developmental changes in human dural innervation. Child’s Nervous System, 28(5), 665–671. 10.1007/s00381-012-1727-7 [DOI] [PubMed] [Google Scholar]
- Dowgjallo, N. (1929) Über die Nerven der harten Hirnhaut des Menschen und einiger. Zeitschrift für Anatomie und Entwicklungsgeschichte, 89, 453–466. 10.1007/BF02117630 [DOI] [Google Scholar]
- Edvinsson, L. (2011) Tracing neural connections to pain pathways with relevance to primary headaches. Cephalalgia, 31(6), 737–747. 10.1177/0333102411398152 [DOI] [PubMed] [Google Scholar]
- Edvinsson, L. (2017) The trigeminovascular pathway: Role of CGRP and CGRP receptors in migraine. Headache, 57(Suppl. 2), 47–55. 10.1111/head.13081 [DOI] [PubMed] [Google Scholar]
- Edvinsson, L. , Haanes, K.A. & Warfvinge, K. (2019) Does inflammation have a role in migraine? Nature Reviews Neurology, 15(8), 483–490. 10.1038/s41582-019-0216-y [DOI] [PubMed] [Google Scholar]
- Edvinsson, L. , McCulloch, J. & Uddman, R. (1981) Substance P: Immunohistochemical localization and effect upon cat pial arteries in vitro and in situ. The Journal of Physiology, 318, 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvinsson, L. , Owman, C. , Rosengren, E. & West, K.A. (1972) Concentration of noradrenaline in pial vessels, choroid plexus, and iris during two weeks after sympathetic ganglionectomy or decentralization. Acta Physiologica Scandinavica, 85(2), 201–206. 10.1111/j.1748-1716.1972.tb05251.x [DOI] [PubMed] [Google Scholar]
- Feindel, W. , Penfield, W. & McNAUGHTON, F. (1960) The tentorial nerves and Iocalization of intracranial pain in man. Neurology, 10, 555–563. [DOI] [PubMed] [Google Scholar]
- Fontaine, D. , Almairac, F. , Santucci, S. , Fernandez, C. , Dallel, R. , Pallud, J. & et al. (2018) Dural and pial pain‐sensitive structures in humans: New inputs from awake craniotomies. Brain, 141(4), 1040–1048. 10.1093/brain/awy005 [DOI] [PubMed] [Google Scholar]
- Friedman, J.A. , Piepgras, D.G. , Pichelmann, M.A. , Hansen, K.K. , Brown, R.D. & Wiebers, D.O. (2001) Small cerebral aneurysms presenting with symptoms other than rupture. Neurology, 57(7), 1212–1216. [DOI] [PubMed] [Google Scholar]
- Gilard, V. , Grangeon, L. , Guegan‐Massardier, E. , Sallansonnet‐Froment, M. , Maltête, D. , Derrey, S. et al. (2016) Headache changes prior to aneurysmal rupture: A symptom of unruptured aneurysm? Neuro‐Chirurgie, 62(5), 241–244. 10.1016/j.neuchi.2016.03.004 [DOI] [PubMed] [Google Scholar]
- Goadsby, P.J. , Knight, Y.E. , Hoskin, K.L. & Butler, P. (1997) Stimulation of an intracranial trigeminally‐innervated structure selectively increases cerebral blood flow. Brain Research, 751(2), 247–252. [DOI] [PubMed] [Google Scholar]
- Grzybowski, J. (1931). L’innervation de la dure – mére cranienne chez l’homme. pp. 387–428.
- Hara, H. & Weir, B. (1986) Pathway of acetylcholinesterase containing nerves to the major cerebral arteries in rats. The Journal of Comparative Neurology, 250(2), 245–252. 10.1002/cne.902500211 [DOI] [PubMed] [Google Scholar]
- Joo, W. , Yoshioka, F. , Funaki, T. , Mizokami, K. & Rhoton, A.L. (2014) Microsurgical anatomy of the trigeminal nerve. Clinical Anatomy (New York, N.Y.), 27(1), 61–88. 10.1002/ca.22330. [DOI] [PubMed] [Google Scholar]
- Kajikawa, H. (1968) Fluorescence histochemical studies on the distribution of adrenergic nerve fibers to intracranial blood vessels. Nihon Geka Hokan. Archiv Fur Japanische Chirurgie, 37(4), 473–484. [PubMed] [Google Scholar]
- Kemp, W.J. , Tubbs, R.S. & Cohen‐Gadol, A.A. (2012) The innervation of the cranial dura mater: Neurosurgical case correlates and a review of the literature. World Neurosurgery, 78(5), 505–510. 10.1016/j.wneu.2011.10.045 [DOI] [PubMed] [Google Scholar]
- Kerr, R.W. (1961) A mechanism to account for frontal headache in cases of posterior‐fossa tumors. Journal of Neurosurgery, 18, 605–609. 10.3171/jns.1961.18.5.0605 [DOI] [PubMed] [Google Scholar]
- Khatibi, K. , Choudhri, O. , Connolly, I.D. , McTaggart, R.A. & Do, H.M. (2017) Asystole during onyx embolization of a pediatric arteriovenous malformation: A severe case of the trigeminocardiac reflex. World Neurosurgery, 98, 884.e1–884.e5. 10.1016/j.wneu.2016.07.025 [DOI] [PubMed] [Google Scholar]
- Koerbel, A. , Gharabaghi, A. , Samii, A. , Gerganov, V. , von Gösseln, H. , Tatagiba, M. et al. (2005) Trigeminocardiac reflex during skull base surgery: Mechanism and management. Acta Neurochirurgica, 147(7), 727–733.discussion 732–733. 10.1007/s00701-005-0535-1 [DOI] [PubMed] [Google Scholar]
- Kosaras, B. , Jakubowski, M. , Kainz, V. & Burstein, R. (2009) Sensory innervation of the calvarial bones of the mouse. The Journal of Comparative Neurology, 515(3), 331–348. 10.1002/cne.22049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumada, M. , Dampney, R.A. & Reis, D.J. (1977) The trigeminal depressor response: A novel vasodepressor response originating from the trigeminal system. Brain Research, 119(2), 305–326. [DOI] [PubMed] [Google Scholar]
- Lambert, G.A. , Bogduk, N. , Goadsby, P.J. , Duckworth, J.W. & Lance, J.W. (1984) Decreased carotid arterial resistance in cats in response to trigeminal stimulation. Journal of Neurosurgery, 61(2), 307–315. 10.3171/jns.1984.61.2.0307 [DOI] [PubMed] [Google Scholar]
- Lee, S.‐H. , Hwang, S.‐J. , Koh, K.‐S. , Song, W.‐C. & Han, S.‐D. (2017) Macroscopic innervation of the dura mater covering the middle cranial fossa in humans correlated to neurovascular headache. Frontiers in Neuroanatomy, 11, 127. 10.3389/fnana.2017.00127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S.‐H. , Shin, K.‐J. , Koh, K.‐S. & Song, W.‐C. (2017) Visualization of the tentorial innervation of human dura mater. Journal of Anatomy, 231(5), 683–689. 10.1111/joa.12659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon‐Ariza, D.S. , Leon‐Ariza, J.S. , Nangiana, J. , Vargas Grau, G. , Leon‐Sarmiento, F.E. & Quiñones‐Hinojosa, A. (2018) Evidences in neurological surgery and a cutting edge classification of the trigeminocardiac reflex: A systematic review. World Neurosurgery, 117, 4–10. 10.1016/j.wneu.2018.05.208 [DOI] [PubMed] [Google Scholar]
- Levy, D. , Strassman, A.M. & Burstein, R. (2009) A critical view on the role of migraine triggers in the genesis of migraine pain. Headache, 49(6), 953–957. 10.1111/j.1526-4610.2009.01444.x [DOI] [PubMed] [Google Scholar]
- Link, A.S. , Kuris, A. & Edvinsson, L. (2008) Treatment of migraine attacks based on the interaction with the trigemino‐cerebrovascular system. The Journal of Headache and Pain, 9(1), 5–12. 10.1007/s10194-008-0011-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn, F.H. , Wijdicks, E.F. , van der Graaf, Y. , Weerdesteyn‐van Vliet, F.A. , Bartelds, A.I. & van Gijn, J. (1994) Prospective study of sentinel headache in aneurysmal subarachnoid haemorrhage. Lancet (London, England), 344(8922), 590–593. [DOI] [PubMed] [Google Scholar]
- Liu‐Chen, L.Y. , Han, D.H. & Moskowitz, M.A. (1983) Pia arachnoid contains substance P originating from trigeminal neurons. Neuroscience, 9(4), 803–808. [DOI] [PubMed] [Google Scholar]
- Liu‐Chen, L.Y. , Liszczak, T.M. , King, J.C. & Moskowitz, M.A. (1986) Immunoelectron microscopic study of substance P‐containing fibers in feline cerebral arteries. Brain Research, 369(1–2), 12–20. [DOI] [PubMed] [Google Scholar]
- Liu‐Chen, L.Y. , Mayberg, M.R. & Moskowitz, M.A. (1983) Immunohistochemical evidence for a substance P‐containing trigeminovascular pathway to pial arteries in cats. Brain Research, 268(1), 162–166. [DOI] [PubMed] [Google Scholar]
- Lv, X. , Li, Y. , Jiang, C. & Wu, Z. (2010) The incidence of trigeminocardiac reflex in endovascular treatment of dural arteriovenous fistula with onyx. Interventional Neuroradiology: Journal of Peritherapeutic Neuroradiology, Surgical Procedures and Related Neurosciences, 16(1), 59–63. 10.1177/159101991001600107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv, X. , Li, Y. , Lv, M. , Liu, A. , Zhang, J. & Wu, Z. (2007) Trigeminocardiac reflex in embolization of intracranial dural arteriovenous fistula. American Journal of Neuroradiology, 28(9), 1769–1770. 10.3174/ajnr.A0675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane, R. & Moskowitz, M.A. (1995) The innervation of pial blood vessels and their role in cerebrovascular regulation. In: Caplan, L.R. (Ed.) Brain Ischemia. London: Springer, pp. 247–259. 10.1007/978-1-4471-2073-5_25 [DOI] [Google Scholar]
- May, A. & Goadsby, P.J. (1999) The trigeminovascular system in humans: Pathophysiologic implications for primary headache syndromes of the neural influences on the cerebral circulation. Journal of Cerebral Blood Flow and Metabolism, 19(2), 115–127. 10.1097/00004647-199902000-00001 [DOI] [PubMed] [Google Scholar]
- Mayberg, M.R. , Zervas, N.T. & Moskowitz, M.A. (1984) Trigeminal projections to supratentorial pial and dural blood vessels in cats demonstrated by horseradish peroxidase histochemistry. The Journal of Comparative Neurology, 223(1), 46–56. 10.1002/cne.902230105 [DOI] [PubMed] [Google Scholar]
- Moskowitz, M.A. , Wei, E.P. , Saito, K. & Kontos, H.A. (1988) Trigeminalectomy modifies pial arteriolar responses to hypertension or norepinephrine. The American Journal of Physiology, 255(1 Pt 2), H1–6. 10.1152/ajpheart.1988.255.1.H1 [DOI] [PubMed] [Google Scholar]
- Nielsen, K.C. & Owman, C. (1967) Adrenergic innervation of pial arteries related to the circle of Willis in the cat. Brain Research, 6(4), 773–776. [DOI] [PubMed] [Google Scholar]
- Noseda, R. & Burstein, R. (2013) Migraine pathophysiology: Anatomy of the trigeminovascular pathway and associated neurological symptoms, cortical spreading depression, sensitization, and modulation of pain. Pain, 154(Suppl 1), S44–53. 10.1016/j.pain.2013.07.021 [DOI] [PubMed] [Google Scholar]
- Olesen, J. , Burstein, R. , Ashina, M. & Tfelt‐Hansen, P. (2009) Origin of pain in migraine: Evidence for peripheral sensitisation. The Lancet Neurology, 8(7), 679–690. 10.1016/S1474-4422(09)70090-0 [DOI] [PubMed] [Google Scholar]
- Penfield, W. & McNaughton, F. (1940) Dural headache and innervation of the dura mater. Archives of Neurology & Psychiatry, 44(1), 43–75. 10.1001/archneurpsyc.1940.02280070051003 [DOI] [Google Scholar]
- Pietrobon, D. & Moskowitz, M.A. (2013) Pathophysiology of migraine. Annual Review of Physiology, 75, 365–391. 10.1146/annurev-physiol-030212-183717 [DOI] [PubMed] [Google Scholar]
- Pietrobon, D. & Moskowitz, M.A. (2014) Chaos and commotion in the wake of cortical spreading depression and spreading depolarizations. Nature Reviews Neuroscience, 15(6), 379–393. 10.1038/nrn3770 [DOI] [PubMed] [Google Scholar]
- Polmear, A. (2003) Sentinel headaches in aneurysmal subarachnoid haemorrhage: What is the true incidence? A systematic review. Cephalalgia, 23(10), 935–941. 10.1046/j.1468-2982.2003.00596.x [DOI] [PubMed] [Google Scholar]
- Ramachandran, R. (2018) Neurogenic inflammation and its role in migraine. Seminars in Immunopathology, 40(3), 301–314. 10.1007/s00281-018-0676-y [DOI] [PubMed] [Google Scholar]
- Rath, G.P. , Dash, H.H. , Prabhakar, H. & Pandia, M.P. (2007) Cardiorespiratory arrest during trigeminal rhizolysis. Anaesthesia, 62(9), 971–972. 10.1111/j.1365-2044.2007.05243.x [DOI] [PubMed] [Google Scholar]
- Ray, B.S. & Wolff, H.G. (1940) Experimental studies on headache: Pain‐sensitive structures of the head and their significance in headache. Archives of Surgery, 41(4), 813–856. 10.1001/archsurg.1940.01210040002001 [DOI] [Google Scholar]
- Ruskell, G.L. & Simons, T. (1987) Trigeminal nerve pathways to the cerebral arteries in monkeys. Journal of Anatomy, 155, 23–37. [PMC free article] [PubMed] [Google Scholar]
- Sakas, D.E. , Moskowitz, M.A. , Wei, E.P. , Kontos, H.A. , Kano, M. & Ogilvy, C.S. (1989) Trigeminovascular fibers increase blood flow in cortical gray matter by axon reflex‐like mechanisms during acute severe hypertension or seizures. Proceedings of the National Academy of Sciences of the United States of America, 86(4), 1401–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller, B. (2004) Trigeminocardiac reflex. A clinical phenomenon or a new physiological entity? Journal of Neurology, 251(6), 658–665. 10.1007/s00415-004-0458-4 [DOI] [PubMed] [Google Scholar]
- Schaller, B. (2005a) Trigemino‐cardiac reflex during microvascular trigeminal decompression in cases of trigeminal neuralgia. Journal of Neurosurgical Anesthesiology, 17(1), 45–48. [PubMed] [Google Scholar]
- Schaller, B. (2005b) Trigemino‐cardiac reflex during transsphenoidal surgery for pituitary adenomas. Clinical Neurology and Neurosurgery, 107(6), 468–474. 10.1016/j.clineuro.2004.12.004 [DOI] [PubMed] [Google Scholar]
- Schaller, B. , Probst, R. , Strebel, S. & Gratzl, O. (1999) Trigeminocardiac reflex during surgery in the cerebellopontine angle. Journal of Neurosurgery, 90(2), 215–220. 10.3171/jns.1999.90.2.0215 [DOI] [PubMed] [Google Scholar]
- Schaller, B.J. , Weigel, D. , Filis, A. & Buchfelder, M. (2007) Trigemino‐cardiac reflex during transsphenoidal surgery for pituitary adenomas: Methodological description of a prospective skull base study protocol. Brain Research, 1149, 69–75. 10.1016/j.brainres.2005.08.060 [DOI] [PubMed] [Google Scholar]
- Schankin, C.J. & Straube, A. (2012) Secondary headaches: Secondary or still primary? The Journal of Headache and Pain, 13(4), 263–270. 10.1007/s10194-012-0443-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schueler, M. , Messlinger, K. , Dux, M. , Neuhuber, W.L. & De Col, R. (2013) Extracranial projections of meningeal afferents and their impact on meningeal nociception and headache. Pain, 154(9), 1622–1631. 10.1016/j.pain.2013.04.040 [DOI] [PubMed] [Google Scholar]
- Schueler, M. , Neuhuber, W.L. , De Col, R. & Messlinger, K. (2014) Innervation of rat and human dura mater and pericranial tissues in the parieto‐temporal region by meningeal afferents. Headache, 54(6), 996–1009. 10.1111/head.12371 [DOI] [PubMed] [Google Scholar]
- Steiger, H.J. , Tew, J.M. & Keller, J.T. (1982) The sensory representation of the dura mater in the trigeminal ganglion of the cat. Neuroscience Letters, 31(3), 231–236. [DOI] [PubMed] [Google Scholar]
- Strassman, A.M. , Weissner, W. , Williams, M. , Ali, S. & Levy, D. (2004) Axon diameters and intradural trajectories of the dural innervation in the rat. The Journal of Comparative Neurology, 473(3), 364–376. 10.1002/cne.20106 [DOI] [PubMed] [Google Scholar]
- Taylor, L.P. (2014) Mechanism of brain tumor headache. Headache, 54(4), 772–775. 10.1111/head.12317 [DOI] [PubMed] [Google Scholar]
- Theoharides, T.C. , Donelan, J. , Kandere‐Grzybowska, K. & Konstantinidou, A. (2005) The role of mast cells in migraine pathophysiology. Brain Research Reviews, 49(1), 65–76. 10.1016/j.brainresrev.2004.11.006 [DOI] [PubMed] [Google Scholar]
- Theoharides, T.C. , Spanos, C. , Pang, X. , Alferes, L. , Ligris, K. , Letourneau, R. et al. (1995) Stress‐induced intracranial mast cell degranulation: A corticotropin‐releasing hormone‐mediated effect. Endocrinology, 136(12), 5745–5750. 10.1210/endo.136.12.7588332 [DOI] [PubMed] [Google Scholar]
- van Gijn, J. , Kerr, R.S. & Rinkel, G.J.E. (2007) Subarachnoid haemorrhage. Lancet (London, England), 369(9558), 306–318. 10.1016/S0140-6736(07)60153-6 [DOI] [PubMed] [Google Scholar]
- Von Luschka, H. (1856) Die Nerven der Harten Hirnhaut. Tübingen: H. Laupp. [Google Scholar]
- Wang, J. , Wu, H.‐C. , Wang, W.‐W. , Zhao, H.‐S. , Dao, R.‐N. , Liu, W.‐M. et al. (2016) Trigeminal cardiac reflex caused by onyx embolization of intracranial dural arteriovenous fistula. Turkish Neurosurgery, 26(3), 325–330. 10.5137/1019-5149.JTN.8008-13.1 [DOI] [PubMed] [Google Scholar]
- Willis, T. (1664). Cerebri Anatome. London: Martyn & Alleftry. [Google Scholar]
- Yamamoto, K. , Matsuyama, T. , Shiosaka, S. , Inagaki, S. , Senba, E. , Shimizu, Y. et al. (1983) Overall distribution of substance P‐containing nerves in the wall of the cerebral arteries of the guinea pig and its origins. The Journal of Comparative Neurology, 215(4), 421–426. 10.1002/cne.902150406 [DOI] [PubMed] [Google Scholar]
- Zaitsu, M. , Narita, S.‐I. , Lambert, K.C. , Grady, J.J. , Estes, D.M. , Curran, E.M. et al. (2007) Estradiol activates mast cells via a non‐genomic estrogen receptor‐alpha and calcium influx. Molecular Immunology, 44(8), 1977–1985. 10.1016/j.molimm.2006.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaroff, J.G. , Pawlikowska, L. , Miss, J.C. , Yarlagadda, S. , Ha, C. , Achrol, A. et al. (2006) Adrenoceptor polymorphisms and the risk of cardiac injury and dysfunction after subarachnoid hemorrhage. Stroke, 37(7), 1680–1685. 10.1161/01.STR.0000226461.52423.dd [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. , Levy, D. , Kainz, V. , Noseda, R. , Jakubowski, M. & Burstein, R. (2011) Activation of central trigeminovascular neurons by cortical spreading depression. Annals of Neurology, 69(5), 855–865. 10.1002/ana.22329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. , Levy, D. , Noseda, R. , Kainz, V. , Jakubowski, M. & Burstein, R. (2010) Activation of meningeal nociceptors by cortical spreading depression: Implications for migraine with aura. The Journal of Neuroscience, 30(26), 8807–8814. 10.1523/JNEUROSCI.0511-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]


