Abstract
During cardiac excitation contraction coupling, the arrival of an action potential at the ventricular myocardium triggers voltage-dependent L-type Ca2+ (CaV1.2) channels in individual myocytes to open briefly. The level of this Ca2+ influx tunes the amplitude of Ca2+-induced Ca2+ release from ryanodine receptors (RyR2) on the junctional sarcoplasmic reticulum and thus the magnitude of the elevation in intracellular Ca2+ concentration and ultimately the downstream contraction. The number and activity of functional CaV1.2 channels at the t-tubule dyads dictates the amplitude of the Ca2+ influx. Trafficking of these channels and their auxiliary subunits to the cell surface is thus tightly controlled and regulated to ensure adequate sarcolemmal expression to sustain this critical process. To that end, recent discoveries have revealed the existence of internal reservoirs of preformed CaV1.2 channels that can be rapidly mobilized to enhance sarcolemmal expression in times of acute stress when hemodynamic and metabolic demand increases. In this review, we provide an overview of the current thinking on CaV1.2 channel trafficking dynamics in the heart. We highlight the numerous points of control including the biosynthetic pathway, the endosomal recycling pathway, ubiquitination, and lysosomal and proteasomal degradation pathways, and discuss the effects of β-adrenergic and angiotensin receptor signaling cascades on this process.
Keywords: L-type calcium channels, ion channel trafficking, t-tubule, caveolae, calcium signaling, β-adrenergic receptor, angiotensin II
1. Introduction
Voltage-gated, L-type CaV1.2 channels play an essential role in cardiac excitation-contraction (EC) coupling and can regulate cardiac gene expression. The number and activity of voltage-gated, L-type CaV1.2 channels localized to specialized dyadic regions of the t-tubule sarcolemma, adjacent to ryanodine receptor (RyR2) clusters, dictates the degree of Ca2+ influx into cardiomyocytes and is thus a major determinant of the magnitude of ventricular contraction. On the other hand, CaV1.2 channels localized to caveolae are thought to play a critical role in regulation of gene expression in a process known as excitation–transcription coupling. Targeting of CaV1.2 channels to the appropriate membrane compartment is thus critical for their proper physiological function. The number of CaV1.2 channels at the sarcolemma at any given time is governed by the relative amount of channel insertions achieved with anterograde trafficking via the secretory and recycling pathways, versus endocytosis via retrograde trafficking pathways. Endocytosed channels can refuel recycling endosome pathways to provide a rapidly mobilizable pool of channels, or they can be targeted for degradation in lysosomes or the proteasome. In this review, we summarize the current literature on CaV1.2 channel trafficking and its regulation. We highlight recent data indicating that G-protein coupled receptor (GPCR)-signaling can positively (in the case of β-adrenergic receptors) or negatively (in the case of Angiotensin type 1 receptors; AT1R) influence the surface abundance of CaV1.2 channels, providing a means to tune cardiac EC-coupling by altering channel expression. We begin with a brief summary of the structure and function of these multimeric channels.
2. CaV1.2 Channel Structure and Function
2.1. CaVα Subunits
L-type Ca2+ channels (LTCC) are a family of voltage-gated Ca2+ channels (CaV1.1–1.4) that allow Ca2+ influx into excitable cells in response to depolarization. Their structure and function is reviewed extensively elsewhere [1,2,3]. Here we focus on trafficking and regulation of CaV1.2, a multimeric protein complex composed of a pore-forming α1 subunit encoded by cacna1c, and associated auxiliary subunits CaVβ, CaVα2δ and sometimes, CaVγ [1]. CaVα1C subunits are widely expressed in brain, smooth muscle, and pancreas, and they form the core of the most prevalent L-type Ca2+ channel in the cardiac muscle of the heart [4,5]. Underscoring their fundamental importance in cardiac function, cacna1c knockout is embryonic lethal in mice [6]. The typical structure of the CaV1.2 channel complex is depicted in Figure 1. When expressed alone, CaVα1C subunits are not efficiently inserted into the membrane resulting in very low current density [7,8,9]. The full functional identity of the channel becomes evident once associated with its auxiliary subunits.
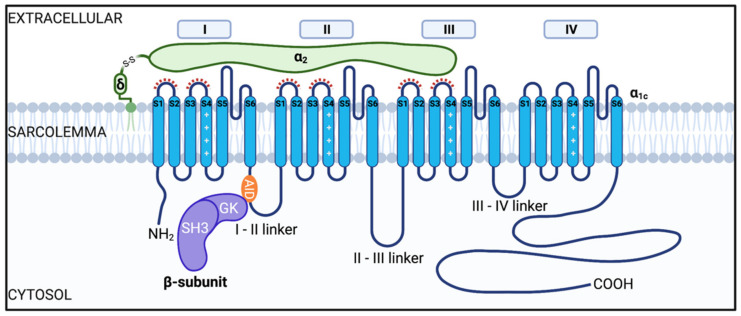
Figure 1.
Illustration of the cardiac voltage gated L-type CaV1.2 channel complex. The channel consists of a pore-forming CaVα1C subunit and auxiliary subunits CaVβ and CaVα2δ. The CaVα1c is composed of four homologous repeat domains (I-IV), each having six transmembrane spanning segments (S1–S6, shown in blue). S1–S4 comprise the voltage sensing domain (VSD) and S5–S6 form the pore domain (PD). CaVβ subunits (depicted in purple) are composed of a SH3, HOOK, and GK domain. Interaction of CaVβ with the CaVα1c occurs between the GK domain on CaVβ subunits and the alpha interaction domain (AID) on the I-II linker (orange). CaVα2δ subunits are proposed to interact with the extracellular loops of domains I-III as highlighted by the red dashed lines [10,11,12,13].
2.2. CaVβ Subunits
CaVβ subunits are cytosolic proteins with Src homology 3 (SH3), HOOK, and guanylate kinase (GK) domains forming the core of the protein responsible for the majority of functional properties of the β subunits [14,15,16,17]. There are four main CaVβ subunits (CaVβ1-4) encoded by separate genes with several splice variants. CaVβ2b is the most highly expressed isoform in the heart [9]. Accordingly, CaVβ2−/− mice suffer embryonic lethality and disrupted cardiac phenotype [18]. In heterologous and native systems interactions between CaVβ and CaVα1c subunits lead to alterations in channel activation and inactivation as well as robust increases in surface expression and current density, likely due to an increased open probability and/or enhanced cell membrane localization of the channel complex [9,19,20,21,22,23]. This 1:1 stochiometric interaction between the subunits is thought to occur within the α-interaction domain (AID), located within the I-II pore loop of CaVα1c subunits [21,24], and small region of the GK domain on CaVβ, referred to as the AID-binding pocket (ABP) [16]. Mutations within this region interfere with CaVβ and CaVα1c interactions and result in diminished membrane targeting of CaVα1c [21]. Furthermore, deletion mutants of regions within the CaVα1c C-terminus result in reduced plasma membrane localized channels, with many channels remaining stuck in internal membranes [25].
2.3. CaVα2δ Subunits
CaVα2δ, is comprised of two subunits α2 and δ which are encoded by the same gene and bound together by disulfide bonds. The N-terminal α2 region is extracellular, with the δ region anchored to the outer leaflet of the plasma membrane via a GPI anchor [26]. Of the several isoforms, CaVα2δ1-3 have been found in the heart [27,28]. Within all the isoforms there is a Von Willebrand factor-A (VWA) domain in the extracellular region, and in CaVα2δ1 and CaVα2δ2 this consists of a metal-ion-dependent adhesion site (MIDAS) which is thought to interact directly with the extracellular loops of CaVα1c [11,29]. When this MIDAS site is mutated, there is a diminished trafficking response and retention of the subunits within the ER [29]. As well as promoting trafficking of the channel complex, association of CaVα2δ with CaVα1c also enhances the voltage-dependent activation by increasing the voltage sensitivity of the VSDs, allowing for calcium influx at more physiological membrane potentials [10].
2.4. CaVγ Subunits
The final auxiliary subunit, γ, is composed of four transmembrane spanning segments with intracellular N- and C-termini. This is the least studied auxiliary subunit of cardiac CaV1.2 channels but of the eight isoforms of γ subunits, four have been shown to be expressed in cardiac tissue and to associate with the CaV1.2 complex (CaVγ4, CaVγ6, CaVγ7 and CaVγ8). In HEK293 cells coexpression of CaVα1c with the various CaVγ subunits results in altered activation and inactivation kinetics of the whole cell Ca2+ current (ICa) [30]. However, the specific role of endogenous CaVγ subunits in native heart tissue and their effects on channel trafficking remain unknown.
3. Trafficking of CaV1.2 Channels
3.1. Anterograde Transport of CaV1.2 to the Sarcolemma
CaVα1c, like all proteins destined for the cell membrane, is synthesized by membrane-bound ribosomes on the rough endoplasmic reticulum (rER) [31]. This is the first step in the classical secretory pathway. Briefly, free ribosomes initially pick-up the mRNA for the channel in the cytosol and begin translation but this is paused once the ribosome translates a signal sequence, A.K.A. the leader sequence. This is a short, ~20 amino acid long chain of hydrophobic amino acids near the N-terminus of the polypeptide, that once translated, finds and binds a signal recognition particle (SRP) that initiates targeting of the entire complex to the rER membrane. This idea was originally proposed by Blobel and Sabatini in their ‘signal hypothesis’ [32]. Recognition and binding of the signal sequence and ribosome by the SRP puts a halt to translation until the SRP binds to an SRP receptor. Once situated on the rER membrane, the SRP is released and the ribosome and polypeptide are handed off to a protein translocation complex (Sec61) where translation resumes. The Sec61 translocon complex forms an ER membrane spanning channel that acts as a conduit for entry of the growing polypeptide chain into the ER [33]. Since CaVα1c is a transmembrane protein bound for the plasma membrane, it is directly inserted into the ER membrane as it forms, with the N and C-termini located within the cytosol, the extracellular loops in the ER lumen, and the hydrophobic transmembrane regions spanning the ER membrane. CaVα2δ and CaVγ are similarly produced while cytosolic proteins like CaVβ subunits lack signal sequences and are thus translated on free ribosomes and released directly into the cytosol. Whether the entire channel complex assembles in the ER membrane remains unclear but there is a school of thought suggesting that binding of at least CaVβ to CaVα1c is necessary to release the channels from the ER and promote forward trafficking (discussed in more detail below). Upon their exit, the channels move in vesicles onwards to the Golgi apparatus, subsequently exiting from trans-Golgi complex in vesicles that are propelled by kinesin motor proteins along microtubule highways to their t-tubular, caveolar, or surface sarcolemmal destinations (see Figure 2).
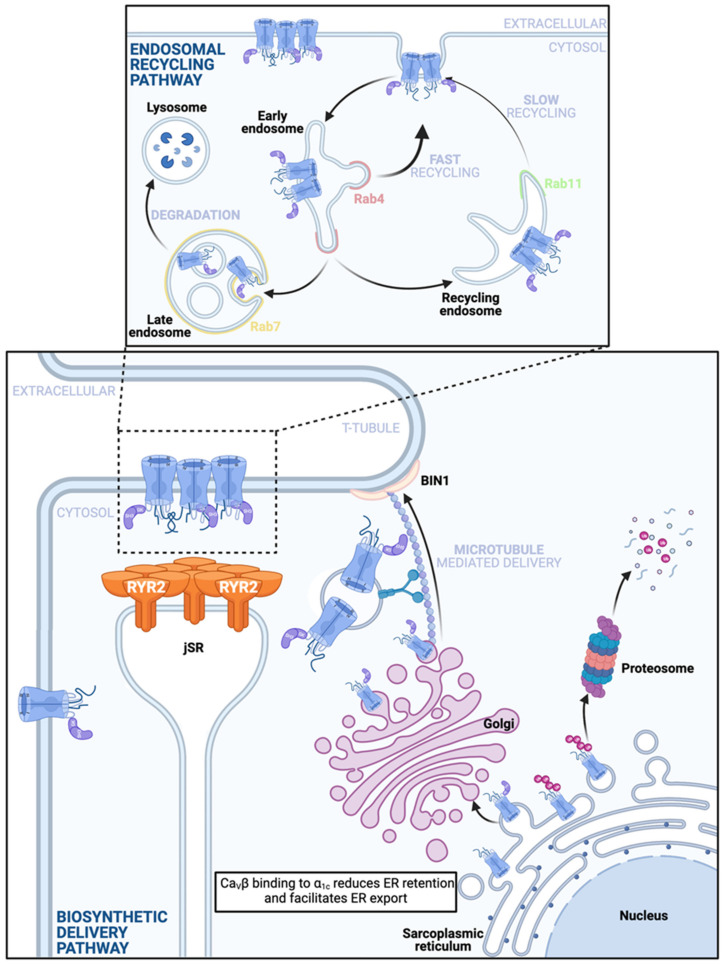
Figure 2.
Anterograde CaV1.2 channel delivery pathways. Bottom: A depiction of the ‘Biosynthetic Delivery Pathway’ that commences when CaVα1c are translated directly into the rER membrane. These pore-forming subunits have several ER retention motifs and just one identified ER export motif. Binding of the CaVβ subunit to the CaVα1c while in the ER membrane is thought to lessen the influence of the retention signals and favor channel export, whereby the channels subsequently exit the ER in vesicles to the Golgi complex. Absence of the CaVβ subunit may render the channels more vulnerable to ubiquitination on the ER membrane, precipitating proteosomal degradation. Note this vulnerability has been reported in neuronal CaV1.2 and has not yet been confirmed in the cardiac channels. Single or clustered channels exit the trans-Golgi network (TGN) and are carried along microtubules by motor proteins to BIN1-anchored delivery hubs on the t-tubule sarcolemma to assume positions in dyadic and caveolar regions. It is unclear exactly when and where in the anterograde trafficking pathway CaVα2δ joins the complex. Top: Stable sarcolemmal CaV1.2 channel expression is maintained in the face of ongoing channel internalization by the endosomal recycling pathway. Endocytosed channels enter the early endosome and are sorted, a subpopulation is sent to Rab7 positive late endosomes and may subsequently undergo lysosomal degradation. The remainder of the channels are recycled back to the membrane either through a fast, direct pathway from the early endosome along a Rab4-dependent fast recycling pathway, or through a slower Rab11-dependent recycling pathway from the recycling endosome. If some channels are degraded, it is possible that endosomal reservoirs of channels take their place and supplement this pathway to maintain stable membrane expression.
3.2. Role of the CaVβ-Subunit in CaV1.2 Trafficking
Until very recently, the prevailing thought was that CaVβ subunit interactions with α1c were indispensable for channel trafficking to the cardiomyocyte surface membrane where the channels fulfill a duality of functions at the t-tubules in triggering Ca2+ induced Ca2+ release from RyR2, and contributing to loading of the SR [34], and play a further role at the caveolae in activation of calcineurin-NFAT signaling pathways [35]. The role of the CaVβ-subunit in membrane targeting of the channels has been extensively reviewed elsewhere [36,37] and so we mention it only briefly here to highlight some more recent advances [38,39]. In heterologous expression systems and in neurons, interactions between β-subunits and α1 are fundamentally required for channel trafficking to the plasma membrane [7,19,24,40,41,42,43,44]. Expression of the α1-subunit alone in the absence of the β-subunit, yields dramatically reduced surface expression and little to no currents, in contrast to the robust surface expression and currents in cells in which the β-subunit is co-expressed [19,23,43,45,46,47]. This effect is abrogated by mutations in the AID or the ABP suggesting that it requires CaVβ binding to the AID on the I-II loop of CaVα1 [48,49,50]. It was initially suggested that this interaction shielded an ER retention motif on the I-II loop and thus promoted enhanced exit of the channel from the ER and on through the anterograde trafficking pathway to the surface membrane [51]. However, this idea was later refuted in work from the Colecraft lab that used chimeric channel constructs in which various intracellular portions of CaVα1c were substituted into another calcium channel (the α1g of CaV3.1) known to exhibit CaVβ-independent surface expression [23]. In this paradigm-shifting study, it was revealed that the I-II loop actually contains an ER-export signal whereas all the other intracellular loops and the N- and C-termini contain ER-retention signals. It is thus thought that binding of the CaVβ to the AID induces a conformational change in the channel that weakens the ER retention influences and instead shifts the balance toward ER export and trafficking to the surface. Furthermore, in heterologous expression systems and hippocampal neurons, binding of CaVβ to CaVα1c in the ER, reportedly protects the channel from ubiquitination and proteosomal degradation [52].
In cardiomyocytes the cellular architecture is vastly different from that of heterologous cells, but until recently, the same principals and reliance on the CaVβ-subunit for surface trafficking were thought to apply, in part fueled by studies showing that short hairpin RNA-mediated knockdown of CaVβ2 reduced ICa in adult rat ventricular myocytes by ~60% [53], and others showing that CaVβ2−/− mice succumbed to embryonic lethality at around E10.5 due to heart failure caused by reduced L-type calcium current [18]. Marx and colleagues have cast doubt on the reliance of cardiac CaVα1c on CaVβ for trafficking in a study where they generated transgenic mice that expressed WT CaVα1c and could be doxycycline-induced to additionally express dihydropyridine (DHP)-resistant CaVα1c with a triple alanine mutation in their AID that significantly decreases the affinity of CaVβ-binding [44]. The AID mutant channels did not co-immunoprecipitate (co-IP) with CaVβ but did display t-tubule localization and generated whole-cell Ca2+ currents that could be distinguished from WT channels with addition of the DHP nisoldipine. This study raises questions about the role of CaVβ in CaVα1c trafficking in cardiomyocytes, however, the interpretation of these results is complicated by the presence of WT channels. Work from our lab reveals that CaV1.2 channels often insert into the sarcolemma as large, multi-channel clusters [54]. In addition, we and others have shown that CaV1 channels can functionally interact within clusters, such that the conformational change in one channel that occurs upon gating, can be transmitted to adjacent interacting channels, triggering their coordinate opening [55,56,57,58,59,60,61,62,63,64]. With this in mind, it seems possible that the conformational change induced by CaVβ subunits binding to WT channels, could be conferred to β-less AID mutant channels in the cluster and that this allows those channels to escape the ER and traffic to the sarcolemma. This was partially addressed by Marx et al. with experiments performed in a heterologous expression system, where they expressed WT channels and DHP-insensitive WT or DHP-insensitive AID mutant channels. Addition of nisoldipine resulted in remaining ICa in the DHP-insensitive WT case and almost eliminated ICa in the DHP-insensitive AID mutant channels. The conclusion was that CaVβ-less channels could not hitch a ride with WT channels in tsA cells. To unequivocally dispel the CaVβ-CaVα1c trafficking hypothesis, a transgenic mouse that expresses only AID mutant channels would be the gold standard. Nevertheless, this study also revealed that CaVβ-less channels are refractory to β-adrenergic stimulation and formed the prelude to the ground-breaking discovery that binding of the small Ras-like G protein Rad to CaVβ on the CaV1.2 channel complex, partially inhibits channel activity until Rad it is phosphorylated by PKA, causing it to dislodge from CaVβ and revealing the enhanced open probability attributed to adrenergic regulation of these channels [39].
3.3. CaV1.2 Recycling
Membrane protein expression is maintained in the face of ongoing endocytosis by both delivery via the biosynthetic pathway and from the endosomal recycling pathway (see Figure 2). Rab GTPases choreograph the trafficking of vesicular cargo from the membrane, through endocytosis, sorting, and subsequent recycling or degradation [65]. The >60-member family of Rab proteins is the largest family in the superfamily of Ras small GTPases. These proteins regulate the tethering or docking of vesicles to different membrane and endomembrane compartments, and may play roles in transport of vesicles along different cytoskeletal filaments by interacting with specific motor proteins [66]. For example, insulin reportedly stimulates Rab4 activity and association with the motor protein kinesin II (KIF3) to facilitate microtubule-mediated delivery and plasma membrane insertion of the GLUT4 glucose transporter in 3T3-L1 adipocytes [67]. Rab4 is known to facilitate so-called ‘fast recycling’ (t1/2 = 1–5 min) of vesicular cargo from early endosomes back to the plasma membrane [68,69]. Rab11 is another Rab protein which is known to facilitate ‘slow recycling’ (t1/2 = 12–30 min) of vesicular cargo from recycling endosomes back to the plasma membrane [68,69], and reportedly interacts with actin-filament associated myosin V motor proteins [70]. In the heart, the role of Rab protein interactions with cytoskeletal elements in CaV1.2 channel recycling has not yet been investigated but our recent work suggests the presence of three endosomal reservoirs of CaV1.2 channels located on: (1) Rab4-positive early endosomes, (2) Rab11-positive recycling endosomes, and (3) Rab7-positive late endo/lysosomes [54]. This recent work further provided the first reports of CaV1.2 channel trafficking and recycling in live adult mouse ventricular myocytes [54]. This was achieved by transducing cardiomyocytes in vivo via retro-orbital injection with a cardiotropic AAV9-packaged, fluorescent protein-tagged CaVβ2 (AAV9-CaVβ2-paGFP) which essentially served as a fluorescent ‘biosensor’ to enable monitoring of the localization and dynamics of a portion of the endogenous channels. Total internal reflection fluorescence (TIRF) imaging using an ~150 nm evanescent field of excitation light, allowed examination of channel dynamics in the surface sarcolemma and initial portion of the t-tubular membrane in isolated, transduced cardiomyocytes, and revealed ongoing, rapid insertion and removal of channels [54,59] that likely reflects delivery from both the biosynthetic and recycling pathways, and counterbalancing endocytosis. Often these insertion and removal events involved entire clusters of channels although smaller discrete events that may represent removal of individual channels were also observed. The arrival and departure of channel clusters implies that CaV1.2 channels can cluster in intracellular compartments. This idea was corroborated by immunostaining experiments that revealed clusters of channels on endosomes, and others lined-up in vesicles along microtubules. In agreement with the previously reported ~3 h lifetime of plasma membrane CaV1.2 channels [19], 2 h cytoskeletal disruption with nocodazole, latrunculin A, or a combination thereof, produced no change in ICa density measured with whole-cell patch clamp electrophysiology, or in the overall expression of sarcolemmal channels assessed with super-resolution microscopy of immunostained channels. While this study did not explicitly examine the role of the cytoskeleton in ongoing (unstimulated) CaV1.2 channel insertion and endocytosis, a previous study in tsA-201 cells using TIRF-fluorescence recovery after photobleaching (TIRF-FRAP) revealed a significant deficit in channel delivery to the membrane when microtubules, actin, or both were pharmacologically disrupted, and intracellular dynamics of vesicular CaV1.2 was largely halted by these treatments [58]. With regards to the dependence of CaV1.2 endosomal recycling on the cytoskeleton, in HL-1 cells Rab11a-mediated recycling of CaV1.2 channels occurs along actin filaments whereas Rab4-dependent fast recycling is thought to occur along microtubules [71]. Thus, a model of CaV1.2 channel recycling is beginning to emerge as illustrated in Figure 2.
3.4. Endocytosis and Retrograde Transport of CaV1.2
There remains a distinct absence of studies on CaV1.2 channel endocytosis in cardiomyocytes. As already discussed, channel removal from the sarcolemma has been visualized in these cells [54,59] but the mechanisms underlying it, and focused study of channel removal/endocytosis from distinct compartments of the membrane such as the t-tubules, crest, or caveolae, has not been explored. It is known that the endosomal recycling pathway is replenished by endocytosed channels, as nicely illustrated by the apparent increase in plasma membrane localized CaV1.2, and concominant reduction of the Rab11-positive pool of endosomal CaV1.2 channels when dynamin-dependent endocytosis was pharmacologically inhibited with dynasore in HL-1 cells [71]. Cell surface biotinylation experiments performed on adult ventricular myocytes also suggest that dynasore treatment increases the surface expression of CaV1.2 [72]. However, dynasore is known to have off-target effects and can inhibit both dynamin-dependent and dynamin-independent endocytosis [73]. Like other membrane proteins, endocytosis of CaV1.2 channels, may occur via a dynamin-dependent mechanism which may be further subclassified into either clathrin-dependent or clathrin-independent, or it can occur though dynamin-independent mechanisms [74,75]. In neurons, depolarization and activity dependent endocytosis of CaV1.2 channels has been reported to occur and is thought to impart a level of protection against Ca2+ overload and associated neurotoxicity [76,77,78]. More in depth investigations are required to determine whether a similar cardioprotective mechanism occurs in cardiac muscle cells and to fully explore the mechanisms of endocytosis in these cells.
3.5. CaV1.2 Degradation
Upon endocytosis, CaV1.2 channels enter early endosomes (also known as sorting endosomes) where they are sorted and designated for either recycling or degradation [79]. Sorting signals, which enable recognition that a given cargo should be routed to the degradation pathway, include the presence of covalently attached ubiquitin [80]. Ubiquitination is a post-translational modification that involves addition of small 7 kDa ubiquitin to target proteins in a process that involves a series of enzymes called E1, E2, and E3 [80]. E1 activates ubiquitin in an ATP-dependent manner, and E2 then transfers it to E3-ubiquitin ligases which then covalently attach one or more ubiquitins to a lysine residue on their target protein. Ubiquitination of membrane proteins can stimulate their endocytosis, and/or function as a sorting signal recognized in the early endosome and result in targeting to late endosomes and lysosomes for degradation [81]. Ubiquitination can also occur to newly formed CaV1.2 channels on the ER membrane and when it occurs there, channels are targeted to the proteasome for degradation. Figure 2 (top panel) shows an illustration of the endosomal degradation pathway, highlighting the role of Rab7 which regulates movement of vesicular cargo to late endosomes and lysosomes for degradation. Rab11b is also illustrated there in the recycling endosome where, it reportedly plays a role in targeting CaV1.2 channels out of the recycling endosome and toward degradation in mouse neonatal cardiomyocytes [82]. This is in contrast to the role that we and others have reported for its close family member Rab11a in facilitating slow recycling of CaV1.2 in HL-1 cells [71] and adult mouse ventricular myocytes [54].
Nedd4 ubiquitin ligases are expressed in the heart and have been reported to ubiquitinate various cardiac ion channels including NaV1.5 and hERG [80]. Nedd4-1 reportedly plays a role in reducing total and surface membrane expression of CaV1.2 α1c in transfected tsA-201 cells by promoting channel degradation [83]. Furthermore, co-expression of an adaptor protein called lipopolysaccharide-induced tumor necrosis factor (LITAF) was found to enhance α1c ubiquitination levels [84]. In rabbit cardiomyocytes, overexpression of LITAF has been found to decrease ICa, reduce Ca2+ transient amplitude, and lower total α1c transcriptional expression [84]. An earlier study concluded that Nedd4-1 effects on CaV1.2 were not direct as they could not detect ubiquitination of any of the channel subunits in transfected tsA-201 cells but did observe a significant reduction of ICa, and surface biotinylation assays and western blots revealed reduced surface and total cellular channel expression [83]. Brefeldin-A, an inhibitor of ER-Golgi trafficking, abrogated the Nedd4-1 effects suggesting that the enzyme was acting to promote sorting of newly synthesized channels for degradation, even before their forward traffic to the membrane. Furthermore, Nedd4-1 dependent degradation of CaV1.2 was prevented by MG132 an inhibitor of the proteasome, while lysosomal inhibitors also impacted the regulatory effects on CaV1.2, implying that Nedd4-1 promoted channel sorting toward both of these degradation pathways. Interestingly, the Nedd4-1 effects on channel degradation were dependent on co-expression of CaVβ subunits. Recall that binding of CaVβ to CaVα1c in the ER, is thought to protect neuronal CaV1.2 channels from ubiquitination and proteosomal degradation [52], so the dependence of this Nedd4-1 effect on CaVβ implies interference with its binding or chaperoning of α1c. A similar dependence on CaVβ was also noted in the more recent Moshal et al. study [84].
An elegant study from the Colecraft lab recently capitalized on the importance of CaVβ subunits for stable membrane expression of CaV1.2 in their report on the creation of a potent engineered CaV1.2 channel inhibitor which targets the catalytic domain of Nedd4-2 E3-ubiquitin ligase to CaVβ subunits using a nanobody [85]. Adenoviral transduction of adult guinea pig cardiomyocytes with this cleverly named ‘CaV-aβlator’ led to complete eradication of ICa, due to removal of CaV1.2 from the dyad and routing to Rab7 positive late endosomes for degradation. Thus, despite the doubt cast on whether CaVβ plays an essential role in CaV1.2 forward trafficking, it seems to play an important role in regulating its degradation and in this way controlling its membrane availability. Moreover, given that CaV-aβlator was targeted toward the CaVβ subunit, yet ubiquitination was detected on both CaVβ and CaVα1c, leading to channel redistribution from the dyad to Rab7 positive late endosomes, this work raises the intriguing question, does CaVβ binding to CaVα1c at the sarcolemma confer a protection from ubiquitination in an analogous manner to that described in the ER of neurons [52]? This question remains unanswered.
4. CaV1.2 Localization and Targeting
4.1. Dyads
To fulfill their function as a channel for Ca2+ flux from the extracellular milieu into the cytosol, CaV1.2 channels need to make their way to the sarcolemma. In the heart, the sarcolemma is a complex system of periodically arranged t-tubules approximately coincident with the z-lines, as well as the region on the surface of the cell between t-tubules known as the surface sarcolemma or crest [86,87]. T-tubules plunge 2–9 µm deep into the myocytes [87] and bring the membrane into close (~12 nm) proximity of junctional sarcoplasmic reticulum (jSR) localized ryanodine receptors (RyR2) at sites known as dyads or couplons. At some locations, the surface sarcolemma also comes into close apposition with the jSR and these non-t-tubular couplons are estimated to represent 22–25% of the total cellular couplon content [88]. Several reports have weighed in on the proportion of total CaV1.2 expressed at dyads with Scriven et al. estimating that 75% of CaV1.2 channel clusters reside at these specialized initiation sites of EC-coupling [89], while Pásek et al. found that approximately 80 % of CaV1.2 is localized to the t-tubule membranes [90]. Dyadic CaV1.2 channels are essential components of cardiac EC-coupling, this is demonstrated during heart failure when the architecture of cardiomyocytes is remodeled, and t-tubules become fractured, displaced, disorganized, and separated from the jSR [72] creating orphaned RYR2 [91], and resulting in reduced Ca2+ transient amplitude, contractile dysfunction, and promotion of arrhythmia promoting dyssynchronous Ca2+ release events [92,93,94,95]. β2-adrenergic receptors (β2-ARs) preferentially signal on the t-tubule membrane [96]. In contrast, β1-adrenergic receptors (β1-ARs) are located across the entire sarcolemma and produce less restrictive, global signals [96,97,98]. Regulation of CaV1.2 channels by β-ARs is the most important regulatory pathway for tuning of cardiac EC-coupling to meet metabolic and hemodynamic demands.
Given the importance of t-tubule localized CaV1.2 channels for the fundamental process and tuning of EC-coupling, it is vital that an efficient trafficking route exists to deliver and maintain a functional CaV1.2 channel population at these sites. It has been determined that targeted transport of CaV1.2 channels to the t-tubule membrane is conferred by Bridging integrator 1 (BIN1) [72], a member of the membrane-curvature mediating BAR (Bin1-Amphiphysin-Rvs) domain superfamily that is also involved in biogenesis and maintenance of the t-tubule network [99,100,101]. BIN1 has been shown to anchor microtubules at the t-tubule membrane, providing a delivery ‘hub’ for CaV1.2 channels as they exit the TGN and travel in vesicles along these cellular highways in the anterograde trafficking pathway [72]. A multitude of evidence supports this idea as discussed below. Firstly, immunolabeling of CaV1.2 and BIN1 in ventricular myocytes has revealed the two proteins colocalize along the t-tubules, biochemical studies have indicated they co-IP, while transfection-mediated overexpression of BIN1 in atrial HL-1 and non-cardiac HeLa cell-lines co-transfected with CaV1.2 results in enhanced formation of membrane invaginations and surface expression of CaV1.2 [72]. Lentiviral transduction of human embryonic stem cell-derived cardiomyocytes (hESC-CMs) with BIN1 facilitates development of the t-tubule network and CaV1.2 clustering, cooperative gating, and overall activity (Po) [101]. Dynamic imaging of BIN1 and α-tubulin in HeLa cells has revealed tethering of microtubules at BIN1-positive sites [72]. In the more architecturally complex adult ventricular myocytes, vesicular CaV1.2 has been shown to decorate cardiomyocyte and microtubules [54,72]. BIN1 knockdown in the heart either with siRNA [72] or shRNA [102], a cardiac specific knockout mouse model [99], or pathologically during heart failure [102], results in reduced CaV1.2 channel expression at the cell surface and t-tubules [102]. Further evidence of a role for microtubules and BIN1 in CaV1.2 delivery to the surface is provided by the reported blunting of cell surface accumulation of CaV1.2 in cells where both dynamin-mediated endocytosis and microtubule-dependent delivery were pharmacologically disrupted with dynasore and nocodazole over 18–24 h [72]. This dependence on microtubules for maintenance of surface CaV1.2 channel expression appears absent in the less specialized atrial HL-1 cell-line lacking in t-tubules wherein 18 hr incubation in nocodazole left CaV1.2 channel ICa density unaltered [71].
The precise nature of the molecular interaction between BIN1 and microtubules has not yet been fully elucidated but may involve the cytoplasmic linker protein CLIP-170 [103,104]. Localization of phosphorylated CLIP-170 is most evident in the intercalated disks of adult mouse cardiomyocytes [105] but close inspection of the images in the aforementioned article reveals a robust periodic staining pattern that appears to coincide with the z-lines and so conceivable that CLIP-170 could be an intermediate between BIN1 and microtubules in these cells. However, in skeletal muscle this CLIP-170—BIN1 interaction appears to at least partially rely on the BAR domain of BIN1, as a point mutation in this region strongly reduced co-IP of BIN1 with CLIP-170 [103]. This is somewhat in conflict with a previous report that truncated BIN1, named BIN1-BAR (as it retains the BAR-domain important for the membrane curvature-mediating effects of BIN1, but lacks the coiled-coil and SRC homology 3 (SH3) domains,) fails to recruit CaV1.2 channels to the surface membrane [72], the inference being that the BAR domain in the cardiac isoforms of BIN1 is neither necessary nor sufficient for the CaV1.2 trafficking and targeting effects. It should be noted that these BIN1-BAR experiments were performed in HL-1 cells which, as already discussed above, may have a less robust reliance on microtubules for anterograde trafficking of CaV1.2. It remains to be seen if BIN1 and CLIP-170 co-localize and co-IP in ventricular myocytes.
BIN1 has been reported to bind to another cytoskeletal highway, actin via its BAR domain [106]. The SH3 domain of BIN1 has further been found to interact with neuronal Wiskott–Aldrich syndrome protein (N-WASP) [107], and there is evidence that the cardiac-specific isoform of BIN1 (lacking exon 7, 11 and 14–16 but containing exons 13 and 17, A.K.A. BIN1 + 13 + 17), can bind to and activate N-WASP to promote actin polymerization by Arp2/3 complexes [99,108]. BIN1 + 13 + 17 also associates with F-actin and α-actinin, and these interactions likely stabilize t-tubules by anchoring them to the z-lines via α-actinin [99]. Indeed, actin stabilization with cytochalasin-D is well-known to preserve the t-tubule network in cultured cardiomyocytes [109,110]. Additionally, BIN1 + 13 + 17 is thought to be the main cardiac isoform responsible for generating micro-folds on the t-tubule membrane [99]. It has been speculated that these microfolds may limit the lateral diffusion of CaV1.2 channels in the membrane and thus facilitating channel clustering. Indeed, in a conference abstract we have yet to develop into a full manuscript [111], we reported that CaV1.2 channel clusters are ~42% smaller in cardiac-specific BIN1 heterozygous knockout (BIN1+/−) mouse ventricular myocytes that have a less dense population of t-tubule micro-folds than WT counterparts [99]. Pharmacological disruption of actin with latrunculin-A reportedly decreases the amount of membrane in ventricular myocyte t-tubules after 24 h in culture, compared to controls, suggesting that actin organizes and supports the highly folded regions of the t-tubules [99]. This finding appears contrary to a previous report that inhibition of actin polymerization with latrunculin-B enhances membrane tubule formation by F-BAR and BAR-proteins [112]. Two explanations were presented for this enhanced tubulation: firstly, actin disruption removes the stabilizing membrane scaffold and permits enhanced membrane deformation; second, they suggested an indirect antagonistic effect of the disruption of actin on dynamin such that endocytic vesicles that normally bud-off the membrane to internalize proteins and maintain homeostatic membrane protein populations, no longer underwent efficient fission and instead promoted elongation of membrane tubules. This cell biological study was not performed on cardiomyocytes but on more architecturally primitive COS-7 cells and did not specifically test the effect of actin disruption on BIN1-induced membrane tubulation, but focused on other BAR-domain proteins. In our recent work, we found that short-term (2 h) actin disruption with latrunculin-A did not appreciably change CaV1.2 clustering, membrane expression, or current density under basal, unstimulated conditions [54].
Another class of cardiac ion channel, Cx43 hemichannels are known to undergo targeted delivery to the specialized cardiomyocyte membrane region of the intercalated disk in a manner that involves actin ‘rest-stops’ [113]. These are sites of pause for Cx43-containing vesicles that may have traveled from the TGN along a microtubule and are then handed-off to actin to form a pool of sub-membrane channels. Mobilized channels are ultimately handed-back to microtubules to be delivered to their final destination. These actin rest-stops may serve as sorting nexuses to redirect microtubule-mediated delivery to specialized sub-domains, and/or as pick-up locations to add accessory proteins, and/or as intracellular reservoirs to store ready-made channels which can be rapidly inserted into the membrane when demand arises. It remains to be determined whether actin rest stops play a role in CaV1.2 channel trafficking in the heart but recent evidence from our group suggests that the mobilization of an internal pool of endosomal channels in adult mouse ventricular myocytes is affected by actin disruption pointing to a role for actin in the endosomal recycling pathway [54]. In HL-1 cells, actin disruption reportedly leads to impaired recycling of CaV1.2 channels via the Rab11a pathway from recycling endosomes to the membrane [71]. A noteworthy point to consider when examining the role of actin in cardiomyocytes is that somewhat oddly, cytochalasin-D, a drug that is widely used as a means to disrupt actin polymerization, appears to have a stabilizing effect on actin in these cells, explaining why it preserves cell shape and prevents loss of t-tubules in culture [109,110]. Latrunculin-A on the other hand seems to act in the expected manner to disrupt the actin cytoskeleton [99,109]. Stabilization of actin with cytochalasin-D reportedly facilitates CaV1.2 trafficking in cultured cardiomyocytes and prevents the peri-nuclear accumulation of the channels seen in cultured cells without cytochalasin-D supplementation [109]. This implies a role for actin in CaV1.2 trafficking through the biosynthetic and anterograde trafficking pathway. However, actin is also known to play a role in stabilizing and guiding microtubules, so it may also be the case that stabilization of actin and preservation of cell shape and architecture, simply facilitates forward trafficking along microtubules [114,115,116]. Given the preponderance of data supporting the role of microtubules in forward trafficking of CaV1.2, it seems likely that a substantial portion of the cytochalasin-D effect could be explained by this supporting role of actin.
4.2. Caveolae
Caveolae represent another specialized sarcolemmal compartment where a subset of CaV1.2 channels are known to reside [117]. It has been estimated that as much as 15% of sarcolemmal CaV1.2 reside in caveolae [35]. At ~50–100 nm in diameter [118], these flask shaped, cholesterol and sphingolipid rich invaginations of the sarcolemma are much smaller than the ~200–300 nm mean diameter t-tubules [119], the contrast in size between the two can be appreciated on freeze-fracture electron micrographs of rabbit ventricular myocytes [120]. Caveolae decorate the surface and t-tubular sarcolemma of cardiomyocytes although they are notably absent from dyadic regions [120]. This is perhaps a geometric phenomenon as the dyadic cleft is only ~12–15 nm wide to allow close proximity between the t-tubular CaV1.2 and jSR localized RYR2, thus the physical restrictions of that narrow space intrinsically exclude 50–100 nm diameter caveolae. The precise role of caveolar CaV1.2 channels in the heart remains unclear with some studies suggesting their involvement in EC-coupling [121], others finding no role in EC-coupling but supporting a role in ET-coupling [35], and others still finding no role in either physiological processes [122]. In terms of channel regulation, β2-AR are known to specifically associate with CaV1.2 and Cav-3 in caveolae [117], and intact caveolae are necessary for β2-AR-mediated regulation of CaV1.2 channels, but not for their regulation by β1-ARs. How the channels are targeted to these microdomains remains unknown. The fact that caveolae are sub-diffraction limit structures may have hampered their study thus far but the increased resolution afforded by ‘super-resolution’ light microscopy techniques may help provide answers as to how CaV1.2 channels are localized to these specialized domains, and the role they play there.
5. CaV1.2 Lifetime
Studies of the lifetime of CaV1.2 channels in the t-tubule sarcolemma and the caveolar compartments would give us information about the dynamics of channel turnover and may reveal differences in delivery and removal mechanisms during health and disease, or circumstances that promote channel insertion or endocytosis. However, there are no reports of CaV1.2 channel lifetime in adult cardiomyocytes, likely due to a lack of live cell fluorescent markers of the channel and the resistance of these cells to chemical transfection methods. Existing measurements of CaV1.2 channel lifetime have all been performed in immortalized cell-lines, and none examine specific sarcolemmal sub-populations of channels. Nonetheless, there are some interesting insights to be gained from studies in heterologous expression systems. Pulse-chase experiments monitoring the percentage of ‘pulsed’ channels remaining in the membrane fraction up to 10 h post-chase, performed on transiently transfected human embryonic kidney cells (HEK293) expressing the pore forming α1c and auxiliary β2a subunits suggest the channels have a half-life of ~3 h [19]. A different study in HEK293T cells tracked pulse-chased channels over a 25 h period after the chase and reported a half-life of α1c of ~25 h, after an initial rapid degradation in the first 4 h post-chase [123]. The difference in these results is likely because the first study was examining the membrane fraction while the second examined total cellular CaV1.2. Altogether, these results suggest that membrane CaV1.2 turns over more rapidly than cellular CaV1.2. In agreement with that, a more recent study found that while endogenous ICa current density in HL-1 cells was maintained at a stable level for >18 h despite disruption of microtubule-based channel delivery with nocodazole, internalization of transfected CaV1.2-HA channels labeled with a 10 min pulse of anti-HA DyLight 488, was seen to occur a much faster rate with a time constant of internalization of 7–8 min [71]. A similar 9–10 min time constant was obtained in tsA201 cells using an approach in which photoactivatable GFP tagged CaV1.2 was photoactivated and its presence at the membrane measured over the subsequent 100 min [124]. Conrad et al. posited that if the membrane population can remain at a stable expression level for >18 h despite ongoing internalization of channels at this rapid rate, then there must also be an ongoing insertion of channels to counterbalance this [71]. They used a clever ‘double pulse-chase’ protocol to visualize this dynamic insertion of channels wherein initial surface CaV1.2-HA were labeled with anti-HA DyLight 488, and 20 min later, a second pulse of anti-HA DyLight 561 revealed robust staining reflecting the presence of channels that were newly inserted during the intervening 20 min. The authors further concluded that surface membrane CaV1.2 channel population in HL-1 cells is constantly and dynamically maintained by endosomal recycling. This endosomal recycling pathway was studied in more detail by our group in live adult mouse ventricular myocytes, where we found that a pool of readily insertable CaV1.2 form a reservoir of channels, which is replenished by channel internalization, and can be rapidly mobilized to the t-tubule sarcolemma in times of acute stress [54,59].
6. GPCR Regulation of CaV1.2 Trafficking
6.1. Stimulated Insertion
The presence of several internal pools of ready-made CaV1.2 channels, invites the thought that perhaps there is some redundancy in these reservoirs under steady-state basal conditions, and that additional channels from the pools could be rapidly mobilized to the sarcolemma to increase channel and current density in times of high demand. Our lab recently reported that activation of β-ARs with isoproterenol (ISO; 100 nM) in isolated adult mouse ventricular myocytes, triggers PKA-dependent augmentation of sarcolemmal insertion of CaV1.2 channels [54,59]. We visualized this dynamic process using the aforementioned AAV9-CaVβ2-paGFP biosensor approach, and found that the stimulated insertions occurred extremely rapidly, with a τ = 4.12 s at physiological temperature [54]. Immunostaining experiments revealed that these inserted channels were mobilized from subsarcolemmal pools of Rab4 positive endosomes and Rab11 positive recycling endosomes (see illustration of the pathway in Figure 3). This manifested as significantly reduced colocalization between these specific endosomal markers and CaV1.2 after ISO, suggesting that the CaV1.2 cargo of these endosomes had been delivered to the sarcolemma. The role of the Rab4-dependent fast recycling pathway, and the Rab11-dependent slow recycling pathway was confirmed by experiments in transiently transfected tsA-201 cells using dominant negative (GDP-locked) and constitutively active (GTP-locked) Rab4 and Rab11 to study the impact on ISO-induced increase in membrane CaV1.2 channel expression. Furthermore, super-resolution microscopy of the t-tubule and sarcolemmal crest regions revealed that stimulated channel insertions occurred predominantly at the t-tubule membrane. This has implications for EC-coupling as more channels in the t-tubule dyad regions could enhance EC-coupling. Indeed we found that the larger superclusters of CaV1.2 channels observed in response to ISO promoted enhanced cooperative gating behaviors [59]. This gating behavior is a known property of CaV1.2 channels in which physically interacting channels within a cluster can communicate with one another via Ca2+-calmodulin dependent associations between their C-terminal tails [56]. The opening of the highest activity channel in the cluster drives the other attached channels leading to amplification of Ca2+ influx [55]. This raises an interesting point when one considers that ISO-stimulated insertion of channels is a PKA-dependent phenomenon, wherein super-clustering and enhanced sarcolemmal expression of CaV1.2 is prevented by pharmacological inhibition of PKA with PKAi or H-89 [59]. It is unknown whether PKA-phosphorylation of CaV1.2 channels can occur while they are localized on endosomal membranes but there is a PKA-anchoring protein called D-AKAP2, that associates with endosomes in cardiomyocytes and displays an ISO-stimulated enhanced colocalization with Rab11 positive endosomes, that could potentially support that [54]. Notably, D-AKAP2 has been shown to regulate transferrin receptor recycling through interactions with Rab4 and Rab11 [125], and a human functional polymorphism in D-AKAP2 (1646V), is known to lower heart rate variability [126], suggestive of a heart that cannot respond well to stressors. PKA-phosphorylation of the CaV1.2 channel complex leads to enhanced open probability (Po) of these channels, and increased longer-lived mode 2 openings, that generates enhanced Ca2+ influx and the positive inotropic response downstream of β-AR activation during the fight-or-flight response [39,127,128,129]. Thus, it is possible that just a small number of high Po, phosphorylated channels inserted into the membrane from the endosomal pool could have a disproportionately large effect on ICa and EC-coupling. This idea that β-AR signaling-mediated regulation of CaV1.2 channel recycling could by itself, generate the stereotypical augmentation of ICa seen in cardiomyocytes during flight-or-flight, is supported by functional patch clamp data in which disruption of endosomal channel insertion with cytoskeletal disruptors, abrogates the left-ward shift in voltage-dependent activation and enhanced ICa response to ISO despite preserved ISO-stimulated cAMP production and robust adrenergic signaling [54]. The idea that an increase in the number of functional channels in the sarcolemma could at least partially underlie the augmented ICa associated with β-AR signaling was previously suggested by Bean et al. in a 1984 study on frog ventricular heart cells where fluctuation analysis of ICa recordings revealed an ISO-stimulated increase in the number of functional channels per cell [130], although the same group later clarified that an increase in the number of functional channels did not necessarily mean there were more channels in the membrane, but could instead reflect previously quiescent channels that became more compelled to open in the presence of ISO [131]. Nonetheless, receptor stimulation of ion channel insertion from intracellular pools has been reported in several other cells and tissues including: (1) neurons where β2-AR signaling via Gαs/adenylyl cyclase/cAMP/PKA stimulates Rab11-dependent insertion of AMPA receptors from recycling endosomes into the plasma membrane of dendritic spines [132]; (2) kidney, where V2R vasopressin receptor signaling through Gαs/adenylyl cyclase/cAMP/PKA stimulates PKA-dependent insertion of aquaporin 2 (AQP2) from Rab11-positive recycling endosomes into the apical membrane [133,134]; and (3) heart, where two other cardiac ion channels, namely KATP and KCNQ1 are mobilized from Rab11-positive endosomal pools to the sarcolemma in response to acute stress [135,136,137].
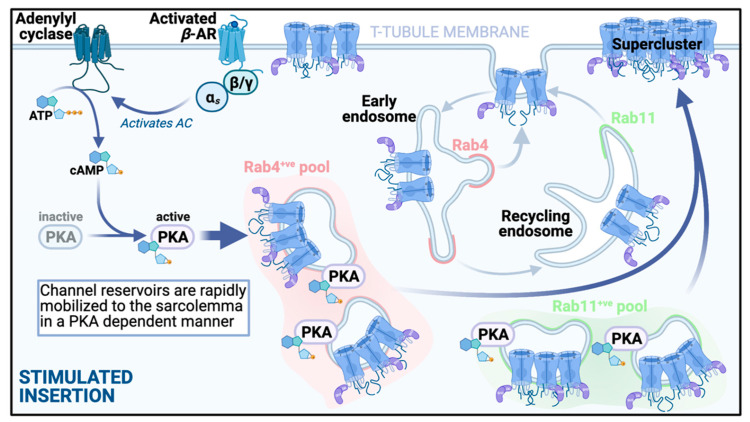
Figure 3.
β-adrenergic receptor stimulated insertion. An illustration of the stimulated insertion pathway that ensues upon acute activation of Gs-coupled β-ARs. Reservoirs of preformed channels are present in subsarcolemmal pools. Activation of the β-AR/AC/cAMP/PKA pathway triggers rapid mobilization of these individual and clustered channels to the t-tubule membrane, along Rab4 and Rab11 dependent trafficking pathways. As a result of this enhanced insertion, there is an increase in the number of functional channels at the sarcolemmal. Large superclusters of CaV1.2 channels form on the t-tubule membrane. These channels exhibit more cooperative interactions and generate enhanced Ca2+ influx to amplify EC-coupling [59].
6.2. Stimulated Endocytosis
The endosomal reservoir of channels, which is constantly replenished by ongoing channel internalization, may similarly have the capacity to accommodate more channels if there is a need to reduce sarcolemmal channel density. This may occur if an analogous Ca2+ overload-preventative channel internalization system as has been described in neurons, also exists in cardiomyocytes [76,77,78]. Prolonged activation of AT1R receptors with angiotensin II has been reported to stimulate endocytosis/internalization of CaV1.2 channels in adult rat cardiomyocytes [138]. This process is seen to occur over about an hour and involves β-arrestin1 recruitment to t-tubular CaV1.2 channels. A preferential internalization of t-tubule localized channels occurs over the period of around an hour and leads to reduced ICa and reduced amplitude Ca2+ transients and cell-shortening. It remains to be determined what endosomal pool these channels are targeted toward and whether they can be quickly recycled back to the membrane again after the angiotensin II stimulus is removed, or if they are targeted for degradation. Results from Hermosilla et al. suggest that a subpopulation of the endocytosed channels may be redistributed to surface membrane locations as indicated by enhanced immunostaining of CaV1.2 on the surface membrane while t-tubular signal faded. This stimulated endocytosis pathway is represented in Figure 4.
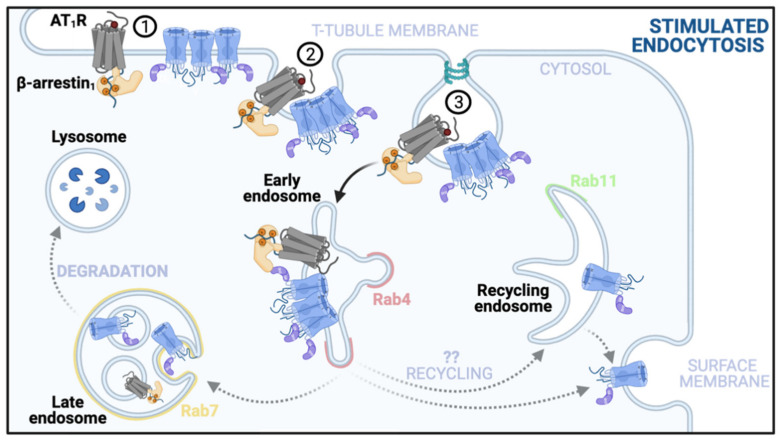
Figure 4.
Angiotensin type 1 receptor stimulated endocytosis. Prolonged exposure to angiotensin II, over the course of an hour, leads to: (1) recruitment of β-arrestin1 to t-tubule localized CaV1.2 channel-AT1R receptor complexes; (2) clathrin-coated pit formation, and (3) endocytosis. This reduces CaV1.2 channel expression and ICa. The identity of the cellular compartment to which the channels are targeted upon internalization are unknown as represented by the grey dotted arrows. Their fate may lie in lysosomal degradation or they may be stored in intracellular reservoirs, or finally, as indicated by the study by Altier et al., some channels may be recycled to other membrane regions, including the surface membrane which appeared to grow more intensely positive for CaV1.2 immunostaining as t-tubular and dyadic expression dropped [138].
Given the findings that β-AR stimulation can trigger rapid CaV1.2 channel insertion into the t-tubule membrane [54,59], an obvious question to explore is whether subsequent phosphatase mediated dephosphorylation of CaV1.2 can trigger internalization. This has been reported to occur in dendritic spines where, as already discussed above, a pool of intracellular GluA1-containing AMPARs is thought to transition to a readily insertable state upon phosphorylation AMPA receptors by PKA and/or calcium/calmodulin-dependent protein kinase II (CaMKII), or protein kinase C (PKC) [139,140,141]. The resulting plasma membrane insertion of the receptors, facilitates long term potentiation (LTP) by increasing channel open probability (Po) and homeostatic scaling up. Conversely, dephosphorylation by calcineurin (CaN) leads to receptor internalization, long term depression and homeostatic scaling down [142]. Whether a similar system exists in cardiomyocytes will make for an interesting future study.
7. Conclusions
CaV1.2 channel expression on the cardiomyocyte sarcolemma is tightly controlled and regulated. They are constantly being internalized, sorted, recycled, degraded, but at steady-state this complex system reaches an equilibrium where a constant level of expression is maintained. Regulatory mechanisms have emerged in recent years that suggest the balance between channel insertion and internalization can be tilted by GPCR-mediated regulatory pathways. Rapid channel insertion from endosomal pools provides extra CaV1.2 channels to the t-tubule membrane during fight-or-flight to fuel the harder-working myocytes with more Ca2+ influx capacity to generate a positive inotropic effect and meet the enhanced hemodynamic and metabolic demands associated with this response. Furthermore, prolonged AT1R signaling triggers channel endocytosis and reduced expression generating a negative inotropic effect. We await further detailed characterization of the kinetics of CaV1.2 channel trafficking, and of when in the anterograde pathway the auxiliary subunits join the complex. Moreover, the specific mechanism of targeting and transport of these channels to non-t-tubular locations including caveolae and the surface membrane remains to be determined. An interesting avenue for future research in those topics is that of the role of the junctional tethering protein junctophilin 2 (JPH2) in targeting CaV1.2 to microdomains within the sarcolemma. A recent study reported that JPH2 recruits CaV1.2 to lipid rafts on the T-tubules wherein overexpression of JPH2 in cultured rat cardiomyocytes led to increased channel density at the t-tubule and surface membrane that was not accompanied by an increase in total cellular CaV1.2 protein, implying JPH2 increased channel trafficking rather than altered channel biosynthesis [143]. Future studies should also parse out the mechanistic details of trafficking alterations that may explain how failing hearts redistribute CaV1.2 from the t-tubular sarcolemma to the surface membrane [144,145,146], and others that mediate the enhanced CaV1.2 sarcolemmal expression observed with aging [147,148,149].
It will be important for the field moving forward to have a definitive measure of CaV1.2 channel lifetime in cardiomyocytes and a full picture of how channels are targeted to caveolae, t-tubules, or the surface sarcolemma, and indeed how these targeting mechanisms become altered in disease. The recently developed Retention Using Selective Hooks (RUSH) system [150] may make more accurate measurements and visualization of CaV1.2 channel trafficking feasible. This system allows the user to trap channels at certain points along the biosynthetic or recycling pathways, release them at will, and observe the kinetics and direction of the trafficking of the channels as they travel to their destinations in the cell. The RUSH system utilizes various protein ‘hooks’ fused to streptavidin which reversibly anchor and retain streptavidin-binding peptide (SBP) fused proteins of interest in a cellular compartment. The addition of biotin outcompetes the SBP/streptavidin interaction and severs the connection between the hook and bait allowing the protein to be released into the secretory pathway. This system has recently been used to study glutamate receptor [151], KCNQ1/KCNE1 [152], and to study altered transport kinetics during pathological conditions [153]. One could envisage that this system could be used to determine when and where the various auxiliary subunits of the channel join the α1 subunit.
In addition, future studies should examine size and mobility of the endosomal pool of channels and how it may be altered by aging or disease. Furthermore, as already mentioned above, we eagerly anticipate future determination of the molecular mechanisms underlying PKA-triggered mobilization of the endosomal channel reservoirs and whether the inverse effect is seen with phosphatase-mediated channel dephosphorylation. In addition, the question still remains open as to whether the triggered insertion of CaV1.2 channels downstream of β-adrenergic receptor stimulation, is accompanied by an increase in RyR2 expression on the other side of the dyad. There is some evidence that this might be the case as cardiomyocyte stimulation with isoproterenol reportedly enhances phosphorylated RyR2 clustering in dyadic regions [154]. A coordinated stimulated enhancement of both CaV1.2 and RyR2 in dyadic regions during fight-or-flight could facilitate an even larger inotropic response.
This relationship between CaV1.2 and RyR2 expression raises a final intriguing idea that should be explored. Recent work has suggested that the functional expression of ion channel complexes at the cardiomyocyte sarcolemma is regulated by a ‘microtranslatome’ whereby mRNA transcripts of NaV1.5 and hERG associate with each other during translation to coordinate and regulate the balance of expression of these channels [155]. A fine balance between the depolarizing NaV1.5 and repolarizing hERG channels is critical to maintain action potential production of the correct duration and to avoid arrhythmias. The idea of co-translational regulation of CaV1.2 is an interesting one considering that gain-of-function mutations in CaV1.2 can lead to long QT8 (Timothy syndrome) [5] and that aberrant interactions between adjacent CaV1.2 channels can also facilitate arrhythmogenic activity [56,156,157]. Could there be association between CaV1.2 channel transcripts that regulates its functional expression or balances it against a repolarizing channel? Could CaV1.2 channel subunit transcripts associate and undergo co-translation that facilitates ER export? These are all open questions in this still developing field of CaV1.2 trafficking regulation.
Acknowledgments
All figures for this manuscript were created with BioRender.com (accessed on 30 May 2021).
Author Contributions
Conceptualization, R.E.D.; writing—original draft preparation, R.E.D. and M.W.; writing—review and editing, R.E.D. and M.W. All authors have read and agreed to the published version of the manuscript.
Funding
Research in the Dixon lab is supported by NIH NIA grant R01AG063796 to R.E.D.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zamponi G.W., Striessnig J., Koschak A., Dolphin A.C. The Physiology, Pathology, and Pharmacology of Voltage-Gated Calcium Channels and Their Future Therapeutic Potential. Pharmacol. Rev. 2015;67:821–870. doi: 10.1124/pr.114.009654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Catterall W.A. Voltage-gated calcium channels. Cold Spring Harb. Perspect. Biol. 2011;3:a003947. doi: 10.1101/cshperspect.a003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Catterall W.A., Lenaeus M.J., Gamal El-Din T.M. Structure and Pharmacology of Voltage-Gated Sodium and Calcium Channels. Annu. Rev. Pharmacol. Toxicol. 2020;60:133–154. doi: 10.1146/annurev-pharmtox-010818-021757. [DOI] [PubMed] [Google Scholar]
- 4.Ertel E.A., Campbell K.P., Harpold M.M., Hofmann F., Mori Y., Perez-Reyes E., Schwartz A., Snutch T.P., Tanabe T., Birnbaumer L., et al. Nomenclature of Voltage-Gated Calcium Channels. Neuron. 2000;25:533–535. doi: 10.1016/S0896-6273(00)81057-0. [DOI] [PubMed] [Google Scholar]
- 5.Splawski I., Timothy K.W., Sharpe L.M., Decher N., Kumar P., Bloise R., Napolitano C., Schwartz P.J., Joseph R.M., Condouris K., et al. CaV1.2 Calcium Channel Dysfunction Causes a Multisystem Disorder Including Arrhythmia and Autism. Cell. 2004;119:19–31. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Seisenberger C., Specht V., Welling A., Platzer J., Pfeifer A., Kuhbandner S., Striessnig J., Klugbauer N., Feil R., Hofmann F. Functional embryonic cardiomyocytes after disruption of the L-type α1C (CaV1.2) calcium channel gene in the mouse. J. Biol. Chem. 2000;275:39193–39199. doi: 10.1074/jbc.M006467200. [DOI] [PubMed] [Google Scholar]
- 7.Gao T., Chien A.J., Hosey M.M. Complexes of the α1C and β Subunits Generate the Necessary Signal for Membrane Targeting of Class C L-type Calcium Channels. J. Biol. Chem. 1999;274:2137–2144. doi: 10.1074/jbc.274.4.2137. [DOI] [PubMed] [Google Scholar]
- 8.Pérez-García M.T., Kamp T.J., Marbán E. Functional properties of cardiac L-type calcium channels transiently expressed in HEK293 cells. Roles of α1 and β subunits. J. Gen. Physiol. 1995;105:289–305. doi: 10.1085/jgp.105.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hullin R., Khan I.F., Wirtz S., Mohacsi P., Varadi G., Schwartz A., Herzig S. Cardiac L-type calcium channel beta-subunits expressed in human heart have differential effects on single channel characteristics. J. Biol. Chem. 2003;278:21623–21630. doi: 10.1074/jbc.M211164200. [DOI] [PubMed] [Google Scholar]
- 10.Savalli N., Pantazis A., Sigg D., Weiss J.N., Neely A., Olcese R. The α2δ-1 subunit remodels CaV1.2 voltage sensors and allows Ca2+ influx at physiological membrane potentials. J. Gen. Physiol. 2016;148:147–159. doi: 10.1085/jgp.201611586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gurnett C.A., Felix R., Campbell K.P. Extracellular Interaction of the Voltage-dependent Ca2+ Channel α2δ and α1 Subunits. J. Biol. Chem. 1997;272:18508–18512. doi: 10.1074/jbc.272.29.18508. [DOI] [PubMed] [Google Scholar]
- 12.Walsh C.P., Davies A., Butcher A.J., Dolphin A.C., Kitmitto A. Three-dimensional Structure of CaV3.1: Comparison with the cardiac L-type voltage-gated calcium channel monomer architecture. J. Biol. Chem. 2009;284:22310–22321. doi: 10.1074/jbc.M109.017152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walsh C.P., Davies A., Nieto-Rostro M., Dolphin A.C., Kitmitto A. Labelling of the 3D structure of the cardiac L-type voltage-gated calcium channel. Channels. 2009;3:387–392. doi: 10.4161/chan.3.6.10225. [DOI] [PubMed] [Google Scholar]
- 14.De Waard M., Pragnell M., Campbell K.P. Ca2+ channel regulation by a conserved beta subunit domain. Neuron. 1994;13:495–503. doi: 10.1016/0896-6273(94)90363-8. [DOI] [PubMed] [Google Scholar]
- 15.Opatowsky Y., Chomsky-Hecht O., Kang M.G., Campbell K.P., Hirsch J.A. The voltage-dependent calcium channel β subunit contains two stable interacting domains. J. Biol. Chem. 2003;278:52323–52332. doi: 10.1074/jbc.M303564200. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y.-h., Li M.-h., Zhang Y., He L.-l., Yamada Y., Fitzmaurice A., Shen Y., Zhang H., Tong L., Yang J. Structural basis of the α1–β subunit interaction of voltage-gated Ca2+ channels. Nature. 2004;429:675–680. doi: 10.1038/nature02641. [DOI] [PubMed] [Google Scholar]
- 17.Richards M.W., Leroy J., Pratt W.S., Dolphin A.C. The HOOK-Domain Between the SH3- and the GK-Domains of CaVβ Subunits Contains Key Determinants Controlling Calcium Channel Inactivation. Channels. 2007;1:92–101. doi: 10.4161/chan.4145. [DOI] [PubMed] [Google Scholar]
- 18.Weissgerber P., Held B., Bloch W., Kaestner L., Chien K.R., Fleischmann B.K., Lipp P., Flockerzi V., Freichel M. Reduced Cardiac L-Type Ca2+ Current in CaVβ2−/− Embryos Impairs Cardiac Development and Contraction with Secondary Defects in Vascular Maturation. Circ. Res. 2006;99:749–757. doi: 10.1161/01.RES.0000243978.15182.c1. [DOI] [PubMed] [Google Scholar]
- 19.Chien A.J., Zhao X., Shirokov R.E., Puri T.S., Chang C.F., Sun D., Rios E., Hosey M.M. Roles of a membrane-localized β subunit in the formation and targeting of functional L-type Ca2+ channels. J. Biol. Chem. 1995;270:30036–30044. doi: 10.1074/jbc.270.50.30036. [DOI] [PubMed] [Google Scholar]
- 20.Brice N.L., Berrow N.S., Campbell V., Page K.M., Brickley K., Tedder I., Dolphin A.C. Importance of the different β subunits in the membrane expression of the α1A and α2 calcium channel subunits: Studies using a depolarization-sensitive α1A antibody. Eur. J. Neurosci. 1997;9:749–759. doi: 10.1111/j.1460-9568.1997.tb01423.x. [DOI] [PubMed] [Google Scholar]
- 21.Gerster U., Neuhuber B., Groschner K., Striessnig J., Flucher B.E. Current modulation and membrane targeting of the calcium channel α1C subunit are independent functions of the β subunit. J. Physiol. 1999;517:353–368. doi: 10.1111/j.1469-7793.1999.0353t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei S.-k., Colecraft H.M., DeMaria C.D., Peterson B.Z., Zhang R., Kohout T.A., Rogers T.B., Yue D.T. Ca2+ Channel Modulation by Recombinant Auxiliary β Subunits Expressed in Young Adult Heart Cells. Circ. Res. 2000;86:175–184. doi: 10.1161/01.RES.86.2.175. [DOI] [PubMed] [Google Scholar]
- 23.Fang K., Colecraft H.M. Mechanism of auxiliary β-subunit-mediated membrane targeting of L-type (CaV1.2) channels. J. Physiol. 2011;589:4437–4455. doi: 10.1113/jphysiol.2011.214247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dalton S., Takahashi S.X., Miriyala J., Colecraft H.M. A single CaVβ can reconstitute both trafficking and macroscopic conductance of voltage-dependent calcium channels. J. Physiol. 2005;567:757–769. doi: 10.1113/jphysiol.2005.093195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao T., Bünemann M., Gerhardstein B.L., Ma H., Hosey M.M. Role of the C terminus of the α1C(CaV1.2) Subunit in Membrane Targeting of Cardiac L-type Calcium Channels*. J. Biol. Chem. 2000;275:25436–25444. doi: 10.1074/jbc.M003465200. [DOI] [PubMed] [Google Scholar]
- 26.Davies A., Kadurin I., Alvarez-Laviada A., Douglas L., Nieto-Rostro M., Bauer C.S., Pratt W.S., Dolphin A.C. The α2δ subunits of voltage-gated calcium channels form GPI-anchored proteins, a posttranslational modification essential for function. Proc. Natl. Acad. Sci. USA. 2010;107:1654–1659. doi: 10.1073/pnas.0908735107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klugbauer N., Lacinová L., Marais E., Hobom M., Hofmann F. Molecular Diversity of the Calcium Channel α2δ Subunit. J. Neurosci. 1999;19:684–691. doi: 10.1523/JNEUROSCI.19-02-00684.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Angelotti T., Hofmann F. Tissue-specific expression of splice variants of the mouse voltage-gated calcium channel α2δ subunit. FEBS Lett. 1996;397:331–337. doi: 10.1016/S0014-5793(96)01205-7. [DOI] [PubMed] [Google Scholar]
- 29.Cantí C., Nieto-Rostro M., Foucault I., Heblich F., Wratten J., Richards M.W., Hendrich J., Douglas L., Page K.M., Davies A., et al. The metal-ion-dependent adhesion site in the Von Willebrand factor-A domain of α2δ subunits is key to trafficking voltage-gated Ca2+ channels. Proc. Natl. Acad. Sci. USA. 2005;102:11230–11235. doi: 10.1073/pnas.0504183102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang L., Katchman A., Morrow J.P., Doshi D., Marx S.O. Cardiac L-type calcium channel (CaV1.2) associates with gamma subunits. FASEB J. 2011;25:928–936. doi: 10.1096/fj.10-172353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green W.N. Ion channel assembly: Creating structures that function. J. Gen. Physiol. 1999;113:163–170. doi: 10.1085/jgp.113.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blobel G., Sabatini D. Dissociation of mammalian polyribosomes into subunits by puromycin. Proc. Natl. Acad. Sci. USA. 1971;68:390–394. doi: 10.1073/pnas.68.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gorlich D., Rapoport T.A. Protein translocation into proteoliposomes reconstituted from purified components of the endoplasmic reticulum membrane. Cell. 1993;75:615–630. doi: 10.1016/0092-8674(93)90483-7. [DOI] [PubMed] [Google Scholar]
- 34.Fabiato A. Simulated calcium current can both cause calcium loading in and trigger calcium release from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J. Gen. Physiol. 1985;85:291–320. doi: 10.1085/jgp.85.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Makarewich C.A., Correll R.N., Gao H., Zhang H.Y., Yang B.H., Berretta R.M., Rizzo V., Molkentin J.D., Houser S.R. A Caveolae-Targeted L-Type Ca2+ Channel Antagonist Inhibits Hypertrophic Signaling Without Reducing Cardiac Contractility. Circ. Res. 2012;110:669–674. doi: 10.1161/CIRCRESAHA.111.264028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buraei Z., Yang J. Structure and function of the beta subunit of voltage-gated Ca2+ channels. Biochim. Biophys. Acta. 2013;1828:1530–1540. doi: 10.1016/j.bbamem.2012.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buraei Z., Yang J. The ss subunit of voltage-gated Ca2+ channels. Physiol. Rev. 2010;90:1461–1506. doi: 10.1152/physrev.00057.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Papa A., Kushner J., Hennessey J.A., Katchman A.N., Zakharov S.I., Chen B.X., Yang L., Lu R., Leong S., Diaz J., et al. Adrenergic CaV1.2 Activation via Rad Phosphorylation Converges at α1C I-II Loop. Circ. Res. 2021;128:76–88. doi: 10.1161/CIRCRESAHA.120.317839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu G., Papa A., Katchman A.N., Zakharov S.I., Roybal D., Hennessey J.A., Kushner J., Yang L., Chen B.X., Kushnir A., et al. Mechanism of adrenergic CaV1.2 stimulation revealed by proximity proteomics. Nature. 2020;577:695–700. doi: 10.1038/s41586-020-1947-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Obermair G.J., Schlick B., Di Biase V., Subramanyam P., Gebhart M., Baumgartner S., Flucher B.E. Reciprocal interactions regulate targeting of calcium channel β subunits and membrane expression of α1 subunits in cultured hippocampal neurons. J. Biol. Chem. 2010;285:5776–5791. doi: 10.1074/jbc.M109.044271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dolphin A.C. β subunits of voltage-gated calcium channels. J. Bioenerg. Biomembr. 2003;35:599–620. doi: 10.1023/B:JOBB.0000008026.37790.5a. [DOI] [PubMed] [Google Scholar]
- 42.Kanevsky N., Dascal N. Regulation of maximal open probability is a separable function of CaVβ subunit in L-type Ca2+ channel, dependent on NH2 terminus of α1C (CaV1.2α) J. Gen. Physiol. 2006;128:15–36. doi: 10.1085/jgp.200609485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Josephson I.R., Varadi G. The beta subunit increases Ca2+ currents and gating charge movements of human cardiac L-type Ca2+ channels. Biophys. J. 1996;70:1285–1293. doi: 10.1016/S0006-3495(96)79685-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang L., Katchman A., Kushner J.S., Kushnir A., Zakharov S.I., Chen B.X., Shuja Z., Subramanyam P., Liu G., Papa A., et al. Cardiac CaV1.2 channels require beta subunits for beta-adrenergic-mediated modulation but not trafficking. J. Clin. Investig. 2018 doi: 10.1172/JCI123878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mori Y., Friedrich T., Kim M.S., Mikami A., Nakai J., Ruth P., Bosse E., Hofmann F., Flockerzi V., Furuichi T., et al. Primary structure and functional expression from complementary DNA of a brain calcium channel. Nature. 1991;350:398–402. doi: 10.1038/350398a0. [DOI] [PubMed] [Google Scholar]
- 46.Kamp T.J., Perez-Garcia M.T., Marban E. Enhancement of ionic current and charge movement by coexpression of calcium channel beta 1A subunit with α1C subunit in a human embryonic kidney cell line. J. Physiol. 1996;492:89–96. doi: 10.1113/jphysiol.1996.sp021291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamaguchi H., Okuda M., Mikala G., Fukasawa K., Varadi G. Cloning of the β2a subunit of the voltage-dependent calcium channel from human heart: Cooperative effect of α2δ and β2a on the membrane expression of the α1C subunit. Biochem. Biophys. Res. Commun. 2000;267:156–163. doi: 10.1006/bbrc.1999.1926. [DOI] [PubMed] [Google Scholar]
- 48.Pragnell M., De Waard M., Mori Y., Tanabe T., Snutch T.P., Campbell K.P. Calcium channel β-subunit binds to a conserved motif in the I-II cytoplasmic linker of the alpha 1-subunit. Nature. 1994;368:67–70. doi: 10.1038/368067a0. [DOI] [PubMed] [Google Scholar]
- 49.He L.L., Zhang Y., Chen Y.H., Yamada Y., Yang J. Functional modularity of the β-subunit of voltage-gated Ca2+ channels. Biophys. J. 2007;93:834–845. doi: 10.1529/biophysj.106.101691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Petegem F., Duderstadt K.E., Clark K.A., Wang M., Minor D.L., Jr. Alanine-scanning mutagenesis defines a conserved energetic hotspot in the CaVα1 AID-CaVβ interaction site that is critical for channel modulation. Structure. 2008;16:280–294. doi: 10.1016/j.str.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bichet D., Cornet V., Geib S., Carlier E., Volsen S., Hoshi T., Mori Y., De Waard M. The I-II loop of the Ca2+ channel alpha1 subunit contains an endoplasmic reticulum retention signal antagonized by the beta subunit. Neuron. 2000;25:177–190. doi: 10.1016/S0896-6273(00)80881-8. [DOI] [PubMed] [Google Scholar]
- 52.Altier C., Garcia-Caballero A., Simms B., You H., Chen L., Walcher J., Tedford H.W., Hermosilla T., Zamponi G.W. The CaVβ subunit prevents RFP2-mediated ubiquitination and proteasomal degradation of L-type channels. Nat. Neurosci. 2011;14:173–180. doi: 10.1038/nn.2712. [DOI] [PubMed] [Google Scholar]
- 53.Cingolani E., Ramirez Correa G.A., Kizana E., Murata M., Cho H.C., Marban E. Gene therapy to inhibit the calcium channel beta subunit: Physiological consequences and pathophysiological effects in models of cardiac hypertrophy. Circ. Res. 2007;101:166–175. doi: 10.1161/CIRCRESAHA.107.155721. [DOI] [PubMed] [Google Scholar]
- 54.Del Villar S.G., Voelker T.L., Westhoff M., Reddy G.R., Spooner H.C., Navedo M.F., Dickson E.J., Dixon R.E. β-Adrenergic control of sarcolemmal CaV1.2 abundance by small GTPase Rab proteins. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2017937118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dixon R.E., Yuan C., Cheng E.P., Navedo M.F., Santana L.F. Ca2+ signaling amplification by oligomerization of L-type Cav1.2 channels. Proc. Natl. Acad. Sci. USA. 2012;109:1749–1754. doi: 10.1073/pnas.1116731109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dixon R.E., Moreno C.M., Yuan C., Opitz-Araya X., Binder M.D., Navedo M.F., Santana L.F. Graded Ca2+/calmodulin-dependent coupling of voltage-gated CaV1.2 channels. eLife. 2015;4:e05608. doi: 10.7554/eLife.05608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moreno C.M., Dixon R.E., Tajada S., Yuan C., Opitz-Araya X., Binder M.D., Santana L.F. Ca2+ entry into neurons is facilitated by cooperative gating of clustered CaV1.3 channels. eLife. 2016;5 doi: 10.7554/eLife.15744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ghosh D., Nieves-Cintron M., Tajada S., Brust-Mascher I., Horne M.C., Hell J.W., Dixon R.E., Santana L.F., Navedo M.F. Dynamic L-type CaV1.2 channel trafficking facilitates CaV1.2 clustering and cooperative gating. Biochim. Biophys. Acta. 2018;1865:1341–1355. doi: 10.1016/j.bbamcr.2018.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ito D.W., Hannigan K.I., Ghosh D., Xu B., Del Villar S.G., Xiang Y.K., Dickson E.J., Navedo M.F., Dixon R.E. beta-adrenergic-mediated dynamic augmentation of sarcolemmal CaV1.2 clustering and co-operativity in ventricular myocytes. J. Physiol. 2019;597:2139–2162. doi: 10.1113/JP277283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Navedo M.F., Amberg G.C., Votaw V.S., Santana L.F. Constitutively active L-type Ca2+ channels. Proc. Natl. Acad. Sci. USA. 2005;102:11112–11117. doi: 10.1073/pnas.0500360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Navedo M.F., Amberg G.C., Nieves M., Molkentin J.D., Santana L.F. Mechanisms Underlying Heterogeneous Ca2+ Sparklet Activity in Arterial Smooth Muscle. J. Gen. Physiol. 2006;127:611–622. doi: 10.1085/jgp.200609519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Navedo M.F., Cheng E.P., Yuan C., Votaw S., Molkentin J.D., Scott J.D., Santana L.F. Increased coupled gating of L-type Ca2+ channels during hypertension and Timothy syndrome. Circ. Res. 2010;106:748–756. doi: 10.1161/CIRCRESAHA.109.213363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Santana L.F., Navedo M.F. Natural inequalities: Why some L-type Ca2+ channels work harder than others. J. Gen. Physiol. 2010;136:143–147. doi: 10.1085/jgp.200910391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hymel L., Striessnig J., Glossmann H., Schindler H. Purified skeletal muscle 1,4-dihydropyridine receptor forms phosphorylation-dependent oligomeric calcium channels in planar bilayers. Proc. Nat. Acad. Sci. USA. 1988;85:4290–4294. doi: 10.1073/pnas.85.12.4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 66.Jordens I., Marsman M., Kuijl C., Neefjes J. Rab proteins, connecting transport and vesicle fusion. Traffic. 2005;6:1070–1077. doi: 10.1111/j.1600-0854.2005.00336.x. [DOI] [PubMed] [Google Scholar]
- 67.Imamura T., Huang J., Usui I., Satoh H., Bever J., Olefsky J.M. Insulin-induced GLUT4 translocation involves protein kinase C-lambda-mediated functional coupling between Rab4 and the motor protein kinesin. Mol. Cell. Biol. 2003;23:4892–4900. doi: 10.1128/MCB.23.14.4892-4900.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maxfield F.R., McGraw T.E. Endocytic recycling. Nat. Rev. Mol. Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 69.Hopkins C.R., Trowbridge I.S. Internalization and processing of transferrin and the transferrin receptor in human carcinoma A431 cells. J. Cell Biol. 1983;97:508–521. doi: 10.1083/jcb.97.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lapierre L.A., Kumar R., Hales C.M., Navarre J., Bhartur S.G., Burnette J.O., Provance D.W., Jr., Mercer J.A., Bahler M., Goldenring J.R. Myosin vb is associated with plasma membrane recycling systems. Mol. Biol. Cell. 2001;12:1843–1857. doi: 10.1091/mbc.12.6.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Conrad R., Stölting G., Hendriks J., Ruello G., Kortzak D., Jordan N., Gensch T., Hidalgo P. Rapid Turnover of the Cardiac L-Type CaV1.2 Channel by Endocytic Recycling Regulates Its Cell Surface Availability. iScience. 2018;7:1–15. doi: 10.1016/j.isci.2018.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hong T.T., Smyth J.W., Gao D., Chu K.Y., Vogan J.M., Fong T.S., Jensen B.C., Colecraft H.M., Shaw R.M. BIN1 localizes the L-type calcium channel to cardiac T-tubules. PLoS Biol. 2010;8:e1000312. doi: 10.1371/journal.pbio.1000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Park R.J., Shen H., Liu L., Liu X., Ferguson S.M., De Camilli P. Dynamin triple knockout cells reveal off target effects of commonly used dynamin inhibitors. J. Cell Sci. 2013;126:5305–5312. doi: 10.1242/jcs.138578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Balse E., Steele D.F., Abriel H., Coulombe A., Fedida D., Hatem S.N. Dynamic of ion channel expression at the plasma membrane of cardiomyocytes. Physiol. Rev. 2012;92:1317–1358. doi: 10.1152/physrev.00041.2011. [DOI] [PubMed] [Google Scholar]
- 75.Hilgemann D.W., Lin M.J., Fine M., Deisl C. On the existence of endocytosis driven by membrane phase separations. Biochim. Biophys. Acta Biomembr. 2020;1862:183007. doi: 10.1016/j.bbamem.2019.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Green E.M., Barrett C.F., Bultynck G., Shamah S.M., Dolmetsch R.E. The tumor suppressor eIF3e mediates calcium-dependent internalization of the L-type calcium channel CaV1.2. Neuron. 2007;55:615–632. doi: 10.1016/j.neuron.2007.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hall D.D., Dai S., Tseng P.Y., Malik Z., Nguyen M., Matt L., Schnizler K., Shephard A., Mohapatra D.P., Tsuruta F., et al. Competition between alpha-actinin and Ca2+-calmodulin controls surface retention of the L-type Ca2+ channel CaV1.2. Neuron. 2013;78:483–497. doi: 10.1016/j.neuron.2013.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tseng P.Y., Henderson P.B., Hergarden A.C., Patriarchi T., Coleman A.M., Lillya M.W., Montagut-Bordas C., Lee B., Hell J.W., Horne M.C. alpha-Actinin Promotes Surface Localization and Current Density of the Ca2+ Channel CaV1.2 by Binding to the IQ Region of the alpha1 Subunit. Biochemistry. 2017;56:3669–3681. doi: 10.1021/acs.biochem.7b00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jovic M., Sharma M., Rahajeng J., Caplan S. The early endosome: A busy sorting station for proteins at the crossroads. Histol. Histopathol. 2010;25:99–112. doi: 10.14670/HH-25.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rougier J.S., Albesa M., Abriel H. Ubiquitylation and SUMOylation of cardiac ion channels. J. Cardiovasc. Pharmacol. 2010;56:22–28. doi: 10.1097/FJC.0b013e3181daaff9. [DOI] [PubMed] [Google Scholar]
- 81.Mukhopadhyay D., Riezman H. Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science. 2007;315:201–205. doi: 10.1126/science.1127085. [DOI] [PubMed] [Google Scholar]
- 82.Best J.M., Foell J.D., Buss C.R., Delisle B.P., Balijepalli R.C., January C.T., Kamp T.J. Small GTPase Rab11b regulates degradation of surface membrane L-type CaV1.2 channels. Am. J. Physiol. Cell Physiol. 2011;300:C1023–C1033. doi: 10.1152/ajpcell.00288.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rougier J.S., Albesa M., Abriel H., Viard P. Neuronal precursor cell-expressed developmentally down-regulated 4-1 (NEDD4-1) controls the sorting of newly synthesized CaV1.2 calcium channels. J. Biol. Chem. 2011;286:8829–8838. doi: 10.1074/jbc.M110.166520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moshal K.S., Roder K., Kabakov A.Y., Werdich A.A., Chiang D.Y., Turan N.N., Xie A., Kim T.Y., Cooper L.L., Lu Y., et al. LITAF (Lipopolysaccharide-Induced Tumor Necrosis Factor) Regulates Cardiac L-Type Calcium Channels by Modulating NEDD (Neural Precursor Cell Expressed Developmentally Downregulated Protein) 4-1 Ubiquitin Ligase. Circ. Genom. Precis. Med. 2019;12:407–420. doi: 10.1161/CIRCGEN.119.002641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morgenstern T.J., Park J., Fan Q.R., Colecraft H.M. A potent voltage-gated calcium channel inhibitor engineered from a nanobody targeted to auxiliary CaVbeta subunits. eLife. 2019;8 doi: 10.7554/eLife.49253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Soeller C., Cannell M.B. Examination of the transverse tubular system in living cardiac rat myocytes by 2-photon microscopy and digital image-processing techniques. Circ. Res. 1999;84:266–275. doi: 10.1161/01.RES.84.3.266. [DOI] [PubMed] [Google Scholar]
- 87.Hong T., Shaw R.M. Cardiac T-Tubule Microanatomy and Function. Physiol. Rev. 2017;97:227–252. doi: 10.1152/physrev.00037.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bers D.M. Excitation-Contraction Coupling and Cardiac Contractile Force. Springer Science & Business Media; Dordrecht, The Netherlands: 2001. p. 427. [Google Scholar]
- 89.Scriven D.R., Asghari P., Schulson M.N., Moore E.D. Analysis of CaV1.2 and ryanodine receptor clusters in rat ventricular myocytes. Biophys. J. 2010;99:3923–3929. doi: 10.1016/j.bpj.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pasek M., Brette F., Nelson A., Pearce C., Qaiser A., Christe G., Orchard C.H. Quantification of t-tubule area and protein distribution in rat cardiac ventricular myocytes. Prog. Biophys. Mol. Biol. 2008;96:244–257. doi: 10.1016/j.pbiomolbio.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 91.Song L.S., Sobie E.A., McCulle S., Lederer W.J., Balke C.W., Cheng H. Orphaned ryanodine receptors in the failing heart. Proc. Natl. Acad. Sci. USA. 2006;103:4305–4310. doi: 10.1073/pnas.0509324103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gomez A.M., Valdivia H.H., Cheng H., Lederer M.R., Santana L.F., Cannell M.B., McCune S.A., Altschuld R.A., Lederer W.J. Defective excitation-contraction coupling in experimental cardiac hypertrophy and heart failure. Science. 1997;276:800–806. doi: 10.1126/science.276.5313.800. [DOI] [PubMed] [Google Scholar]
- 93.Cheng H., Lederer M.R., Lederer W.J., Cannell M.B. Calcium sparks and [Ca2+]i waves in cardiac myocytes. Am. J. Physiol. 1996;270:C148–C159. doi: 10.1152/ajpcell.1996.270.1.C148. [DOI] [PubMed] [Google Scholar]
- 94.Lu F., Pu W.T. The architecture and function of cardiac dyads. Biophys. Rev. 2020;12:1007–1017. doi: 10.1007/s12551-020-00729-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Louch W.E., Mork H.K., Sexton J., Stromme T.A., Laake P., Sjaastad I., Sejersted O.M. T-tubule disorganization and reduced synchrony of Ca2+ release in murine cardiomyocytes following myocardial infarction. J. Physiol. 2006;574:519–533. doi: 10.1113/jphysiol.2006.107227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nikolaev V.O., Moshkov A., Lyon A.R., Miragoli M., Novak P., Paur H., Lohse M.J., Korchev Y.E., Harding S.E., Gorelik J. Beta2-adrenergic receptor redistribution in heart failure changes cAMP compartmentation. Science. 2010;327:1653–1657. doi: 10.1126/science.1185988. [DOI] [PubMed] [Google Scholar]
- 97.Harvey R.D., Hell J.W. CaV1.2 signaling complexes in the heart. J. Mol. Cell Cardiol. 2013;58:143–152. doi: 10.1016/j.yjmcc.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen-Izu Y., Xiao R.P., Izu L.T., Cheng H., Kuschel M., Spurgeon H., Lakatta E.G. Gi-dependent localization of β2-adrenergic receptor signaling to L-type Ca2+ channels. Biophys. J. 2000;79:2547–2556. doi: 10.1016/S0006-3495(00)76495-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hong T., Yang H., Zhang S.S., Cho H.C., Kalashnikova M., Sun B., Zhang H., Bhargava A., Grabe M., Olgin J., et al. Cardiac BIN1 folds T-tubule membrane, controlling ion flux and limiting arrhythmia. Nat. Med. 2014;20:624–632. doi: 10.1038/nm.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Caldwell J.L., Smith C.E.R., Taylor R.F., Kitmitto A., Eisner D.A., Dibb K.M., Trafford A.W. Dependence of Cardiac Transverse Tubules on the BAR Domain Protein Amphiphysin II (BIN-1) Circ. Res. 2014;115:986–996. doi: 10.1161/CIRCRESAHA.116.303448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.De La Mata A., Tajada S., O’Dwyer S., Matsumoto C., Dixon R.E., Hariharan N., Moreno C.M., Santana L.F. BIN1 Induces the Formation of T-Tubules and Adult-Like Ca2+ Release Units in Developing Cardiomyocytes. Stem Cells. 2019;37:54–64. doi: 10.1002/stem.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hong T.T., Smyth J.W., Chu K.Y., Vogan J.M., Fong T.S., Jensen B.C., Fang K., Halushka M.K., Russell S.D., Colecraft H., et al. BIN1 is reduced and CaV1.2 trafficking is impaired in human failing cardiomyocytes. Heart Rhythm. 2012;9:812–820. doi: 10.1016/j.hrthm.2011.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.D’Alessandro M., Hnia K., Gache V., Koch C., Gavriilidis C., Rodriguez D., Nicot A.S., Romero N.B., Schwab Y., Gomes E., et al. Amphiphysin 2 Orchestrates Nucleus Positioning and Shape by Linking the Nuclear Envelope to the Actin and Microtubule Cytoskeleton. Dev. Cell. 2015;35:186–198. doi: 10.1016/j.devcel.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 104.Meunier B., Quaranta M., Daviet L., Hatzoglou A., Leprince C. The membrane-tubulating potential of amphiphysin 2/BIN1 is dependent on the microtubule-binding cytoplasmic linker protein 170 (CLIP-170) Eur. J. Cell Biol. 2009;88:91–102. doi: 10.1016/j.ejcb.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 105.Yashirogi S., Nagao T., Nishida Y., Takahashi Y., Qaqorh T., Yazawa I., Katayama T., Kioka H., Matsui T.S., Saito S., et al. AMPK regulates cell shape of cardiomyocytes by modulating turnover of microtubules through CLIP-170. EMBO Rep. 2021;22:e50949. doi: 10.15252/embr.202050949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Drager N.M., Nachman E., Winterhoff M., Bruhmann S., Shah P., Katsinelos T., Boulant S., Teleman A.A., Faix J., Jahn T.R. Bin1 directly remodels actin dynamics through its BAR domain. EMBO Rep. 2017;18:2051–2066. doi: 10.15252/embr.201744137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yamada H., Padilla-Parra S., Park S.J., Itoh T., Chaineau M., Monaldi I., Cremona O., Benfenati F., De Camilli P., Coppey-Moisan M., et al. Dynamic interaction of amphiphysin with N-WASP regulates actin assembly. J. Biol. Chem. 2009;284:34244–34256. doi: 10.1074/jbc.M109.064204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Takenawa T., Suetsugu S. The WASP-WAVE protein network: Connecting the membrane to the cytoskeleton. Nat. Rev. Mol. Cell Biol. 2007;8:37–48. doi: 10.1038/nrm2069. [DOI] [PubMed] [Google Scholar]
- 109.Leach R.N., Desai J.C., Orchard C.H. Effect of cytoskeleton disruptors on L-type Ca channel distribution in rat ventricular myocytes. Cell Calcium. 2005;38:515–526. doi: 10.1016/j.ceca.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 110.Tian Q., Pahlavan S., Oleinikow K., Jung J., Ruppenthal S., Scholz A., Schumann C., Kraegeloh A., Oberhofer M., Lipp P., et al. Functional and morphological preservation of adult ventricular myocytes in culture by sub-micromolar cytochalasin D supplement. J. Mol. Cell Cardiol. 2012;52:113–124. doi: 10.1016/j.yjmcc.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 111.Dixon R.E., Hong T.T., Shaw R.M., Santana L.F. Bin1 Regulates CaV1.2 Channel Clustering in Ventricular Myocytes. Biophys. J. 2016;110:441a–442a. doi: 10.1016/j.bpj.2015.11.2378. [DOI] [Google Scholar]
- 112.Itoh T., Erdmann K.S., Roux A., Habermann B., Werner H., De Camilli P. Dynamin and the actin cytoskeleton cooperatively regulate plasma membrane invagination by BAR and F-BAR proteins. Dev. Cell. 2005;9:791–804. doi: 10.1016/j.devcel.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 113.Smyth J.W., Vogan J.M., Buch P.J., Zhang S.S., Fong T.S., Hong T.T., Shaw R.M. Actin cytoskeleton rest stops regulate anterograde traffic of connexin 43 vesicles to the plasma membrane. Circ. Res. 2012;110:978–989. doi: 10.1161/CIRCRESAHA.111.257964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wittmann T., Bokoch G.M., Waterman-Storer C.M. Regulation of leading edge microtubule and actin dynamics downstream of Rac1. J. Cell Biol. 2003;161:845–851. doi: 10.1083/jcb.200303082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bartolini F., Ramalingam N., Gundersen G.G. Actin-capping protein promotes microtubule stability by antagonizing the actin activity of mDia1. Mol. Biol. Cell. 2012;23:4032–4040. doi: 10.1091/mbc.e12-05-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Basheer W.A., Shaw R.M. Connexin 43 and CaV1.2 Ion Channel Trafficking in Healthy and Diseased Myocardium. Circ. Arrhythm. Electrophysiol. 2016;9:e001357. doi: 10.1161/CIRCEP.115.001357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Balijepalli R.C., Foell J.D., Hall D.D., Hell J.W., Kamp T.J. Localization of cardiac L-type Ca2+ channels to a caveolar macromolecular signaling complex is required for β2-adrenergic regulation. Proc. Natl. Acad. Sci. USA. 2006;103:7500–7505. doi: 10.1073/pnas.0503465103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Balijepalli R.C., Kamp T.J. Caveolae, ion channels and cardiac arrhythmias. Prog. Biophys. Mol. Biol. 2008;98:149–160. doi: 10.1016/j.pbiomolbio.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Brette F., Orchard C. T-tubule function in mammalian cardiac myocytes. Circ. Res. 2003;92:1182–1192. doi: 10.1161/01.RES.0000074908.17214.FD. [DOI] [PubMed] [Google Scholar]
- 120.Levin K.R., Page E. Quantitative studies on plasmalemmal folds and caveolae of rabbit ventricular myocardial cells. Circ. Res. 1980;46:244–255. doi: 10.1161/01.RES.46.2.244. [DOI] [PubMed] [Google Scholar]
- 121.Calaghan S., White E. Caveolae modulate excitation-contraction coupling and beta2-adrenergic signalling in adult rat ventricular myocytes. Cardiovasc. Res. 2006;69:816–824. doi: 10.1016/j.cardiores.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 122.Correll R.N., Makarewich C.A., Zhang H.Y., Zhang C., Sargent M.A., York A.J., Berretta R.M., Chen X.W., Houser S.R., Molkentin J.D. Caveolae-localized L-type Ca2+ channels do not contribute to function or hypertrophic signalling in themouse heart. Cardiovasc. Res. 2017;113:749–759. doi: 10.1093/cvr/cvx046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Catalucci D., Zhang D.H., DeSantiago J., Aimond F., Barbara G., Chemin J., Bonci D., Picht E., Rusconi F., Dalton N.D., et al. Akt regulates L-type Ca2+ channel activity by modulating CaVα1 protein stability. J. Cell Biol. 2009;184:923–933. doi: 10.1083/jcb.200805063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sato D., Hernandez-Hernandez G., Matsumoto C., Tajada S., Moreno C.M., Dixon R.E., O’Dwyer S., Navedo M.F., Trimmer J.S., Clancy C.E., et al. A stochastic model of ion channel cluster formation in the plasma membrane. J. Gen. Physiol. 2019 doi: 10.1085/jgp.201912327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Eggers C.T., Schafer J.C., Goldenring J.R., Taylor S.S. D-AKAP2 interacts with Rab4 and Rab11 through its RGS domains and regulates transferrin receptor recycling. J. Biol. Chem. 2009;284:32869–32880. doi: 10.1074/jbc.M109.022582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Neumann S.A., Tingley W.G., Conklin B.R., Shrader C.J., Peet E., Muldoon M.F., Jennings J.R., Ferrell R.E., Manuck S.B. AKAP10 (I646V) functional polymorphism predicts heart rate and heart rate variability in apparently healthy, middle-aged European-Americans. Psychophysiology. 2009;46:466–472. doi: 10.1111/j.1469-8986.2009.00802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Reuter H., Scholz H. The regulation of the calcium conductance of cardiac muscle by adrenaline. J. Physiol. 1977;264:49–62. doi: 10.1113/jphysiol.1977.sp011657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yue D.T., Herzig S., Marban E. Beta-adrenergic stimulation of calcium channels occurs by potentiation of high-activity gating modes. Proc. Natl. Acad. Sci. USA. 1990;87:753–757. doi: 10.1073/pnas.87.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sperelakis N., Schneider J.A. A metabolic control mechanism for calcium ion influx that may protect the ventricular myocardial cell. Am. J. Cardiol. 1976;37:1079–1085. doi: 10.1016/0002-9149(76)90428-8. [DOI] [PubMed] [Google Scholar]
- 130.Bean B.P., Nowycky M.C., Tsien R.W. Beta-adrenergic modulation of calcium channels in frog ventricular heart cells. Nature. 1984;307:371–375. doi: 10.1038/307371a0. [DOI] [PubMed] [Google Scholar]
- 131.Tsien R.W., Bean B.P., Hess P., Lansman J.B., Nilius B., Nowycky M.C. Mechanisms of calcium channel modulation by beta-adrenergic agents and dihydropyridine calcium agonists. J. Mol. Cell Cardiol. 1986;18:691–710. doi: 10.1016/S0022-2828(86)80941-5. [DOI] [PubMed] [Google Scholar]
- 132.Diering G.H., Huganir R.L. The AMPA Receptor Code of Synaptic Plasticity. Neuron. 2018;100:314–329. doi: 10.1016/j.neuron.2018.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nedvetsky P.I., Stefan E., Frische S., Santamaria K., Wiesner B., Valenti G., Hammer J.A., 3rd, Nielsen S., Goldenring J.R., Rosenthal W., et al. A Role of myosin Vb and Rab11-FIP2 in the aquaporin-2 shuttle. Traffic. 2007;8:110–123. doi: 10.1111/j.1600-0854.2006.00508.x. [DOI] [PubMed] [Google Scholar]
- 134.Fushimi K., Sasaki S., Marumo F. Phosphorylation of serine 256 is required for cAMP-dependent regulatory exocytosis of the aquaporin-2 water channel. J. Biol. Chem. 1997;272:14800–14804. doi: 10.1074/jbc.272.23.14800. [DOI] [PubMed] [Google Scholar]
- 135.Bao L., Hadjiolova K., Coetzee W.A., Rindler M.J. Endosomal KATP channels as a reservoir after myocardial ischemia: A role for SUR2 subunits. Am. J. Physiol. Heart Circ. Physiol. 2011;300:H262–H270. doi: 10.1152/ajpheart.00857.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Seebohm G., Strutz-Seebohm N., Birkin R., Dell G., Bucci C., Spinosa M.R., Baltaev R., Mack A.F., Korniychuk G., Choudhury A., et al. Regulation of endocytic recycling of KCNQ1/KCNE1 potassium channels. Circ. Res. 2007;100:686–692. doi: 10.1161/01.RES.0000260250.83824.8f. [DOI] [PubMed] [Google Scholar]
- 137.Wang Y., Zankov D.P., Jiang M., Zhang M., Henderson S.C., Tseng G.N. [Ca2+]i elevation and oxidative stress induce KCNQ1 protein translocation from the cytosol to the cell surface and increase slow delayed rectifier (IKs) in cardiac myocytes. J. Biol. Chem. 2013;288:35358–35371. doi: 10.1074/jbc.M113.504746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hermosilla T., Encina M., Morales D., Moreno C., Conejeros C., Alfaro-Valdes H.M., Lagos-Meza F., Simon F., Altier C., Varela D. Prolonged AT1R activation induces CaV1.2 channel internalization in rat cardiomyocytes. Sci. Rep. 2017;7:10131. doi: 10.1038/s41598-017-10474-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Diering G.H., Heo S., Hussain N.K., Liu B., Huganir R.L. Extensive phosphorylation of AMPA receptors in neurons. Proc. Natl. Acad. Sci. USA. 2016;113:E4920–E4927. doi: 10.1073/pnas.1610631113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Makino Y., Johnson R.C., Yu Y., Takamiya K., Huganir R.L. Enhanced synaptic plasticity in mice with phosphomimetic mutation of the GluA1 AMPA receptor. Proc. Natl. Acad. Sci. USA. 2011;108:8450–8455. doi: 10.1073/pnas.1105261108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Olivito L., Saccone P., Perri V., Bachman J.L., Fragapane P., Mele A., Huganir R.L., De Leonibus E. Phosphorylation of the AMPA receptor GluA1 subunit regulates memory load capacity. Brain Struct. Funct. 2016;221:591–603. doi: 10.1007/s00429-014-0927-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lee H.K., Kameyama K., Huganir R.L., Bear M.F. NMDA induces long-term synaptic depression and dephosphorylation of the GluR1 subunit of AMPA receptors in hippocampus. Neuron. 1998;21:1151–1162. doi: 10.1016/S0896-6273(00)80632-7. [DOI] [PubMed] [Google Scholar]
- 143.Poulet C., Sanchez-Alonso J., Swiatlowska P., Mouy F., Lucarelli C., Alvarez-Laviada A., Gross P., Terracciano C., Houser S., Gorelik J. Junctophilin-2 tethers T-tubules and recruits functional L-type calcium channels to lipid rafts in adult cardiomyocytes. Cardiovasc. Res. 2021;117:149–161. doi: 10.1093/cvr/cvaa033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sanchez-Alonso J.L., Loucks A., Schobesberger S., van Cromvoirt A.M., Poulet C., Chowdhury R.A., Trayanova N., Gorelik J. Nanoscale regulation of L-type calcium channels differentiates between ischemic and dilated cardiomyopathies. EBioMedicine. 2020;57:102845. doi: 10.1016/j.ebiom.2020.102845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bryant S.M., Kong C.H., Watson J., Cannell M.B., James A.F., Orchard C.H. Altered distribution of ICa impairs Ca release at the t-tubules of ventricular myocytes from failing hearts. J. Mol. Cell Cardiol. 2015;86:23–31. doi: 10.1016/j.yjmcc.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sanchez-Alonso J.L., Bhargava A., O’Hara T., Glukhov A.V., Schobesberger S., Bhogal N., Sikkel M.B., Mansfield C., Korchev Y.E., Lyon A.R., et al. Microdomain-Specific Modulation of L-Type Calcium Channels Leads to Triggered Ventricular Arrhythmia in Heart Failure. Circ. Res. 2016;119:944–955. doi: 10.1161/CIRCRESAHA.116.308698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lakatta E.G. Cardiovascular regulatory mechanisms in advanced age. Physiol. Rev. 1993;73:413–467. doi: 10.1152/physrev.1993.73.2.413. [DOI] [PubMed] [Google Scholar]
- 148.Josephson I.R., Guia A., Stern M.D., Lakatta E.G. Alterations in properties of L-type Ca channels in aging rat heart. J. Mol. Cell Cardiol. 2002;34:297–308. doi: 10.1006/jmcc.2001.1512. [DOI] [PubMed] [Google Scholar]
- 149.Hatch F., Lancaster M.K., Jones S.A. Aging is a primary risk factor for cardiac arrhythmias: Disruption of intracellular Ca2+ regulation as a key suspect. Expert Rev. Cardiovasc. Ther. 2011;9:1059–1067. doi: 10.1586/erc.11.112. [DOI] [PubMed] [Google Scholar]
- 150.Boncompain G., Divoux S., Gareil N., de Forges H., Lescure A., Latreche L., Mercanti V., Jollivet F., Raposo G., Perez F. Synchronization of secretory protein traffic in populations of cells. Nat. Methods. 2012;9:493–498. doi: 10.1038/nmeth.1928. [DOI] [PubMed] [Google Scholar]
- 151.Evans A.J., Gurung S., Wilkinson K.A., Stephens D.J., Henley J.M. Assembly, Secretory Pathway Trafficking, and Surface Delivery of Kainate Receptors Is Regulated by Neuronal Activity. Cell Rep. 2017;19:2613–2626. doi: 10.1016/j.celrep.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wilson Z.T., Jiang M., Geng J., Kaur S., Workman S.W., Hao J., Bernas T., Tseng G.N. Delayed KCNQ1/KCNE1 assembly on the cell surface helps IKs fulfill its function as a repolarization reserve in the heart. J. Physiol. 2021 doi: 10.1113/JP281773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kutchukian C., Vivas O., Casas M., Jones J.G., Tiscione S.A., Simo S., Ory D.S., Dixon R.E., Dickson E.J. NPC1 regulates the distribution of phosphatidylinositol 4-kinases at Golgi and lysosomal membranes. EMBO J. 2021:e105990. doi: 10.15252/embj.2020105990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fu Y., Shaw S.A., Naami R., Vuong C.L., Basheer W.A., Guo X., Hong T. Isoproterenol Promotes Rapid Ryanodine Receptor Movement to Bridging Integrator 1 (BIN1)-Organized Dyads. Circulation. 2016;133:388–397. doi: 10.1161/CIRCULATIONAHA.115.018535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Eichel C.A., Rios-Perez E.B., Liu F., Jameson M.B., Jones D.K., Knickelbine J.J., Robertson G.A. A microtranslatome coordinately regulates sodium and potassium currents in the human heart. eLife. 2019;8 doi: 10.7554/eLife.52654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dixon R.E., Cheng E.P., Mercado J.L., Santana L.F. L-type Ca2+ channel function during Timothy syndrome. Trends Cardiovasc. Med. 2012;22:72–76. doi: 10.1016/j.tcm.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sato D., Dixon R.E., Santana L.F., Navedo M.F. A model for cooperative gating of L-type Ca2+ channels and its effects on cardiac alternans dynamics. PLoS Comput. Biol. 2018;14:e1005906. doi: 10.1371/journal.pcbi.1005906. [DOI] [PMC free article] [PubMed] [Google Scholar]