Abstract
The innate and adaptive immune systems play an essential role in host defense against pathogens. Various signal transduction pathways monitor and balance the immune system since an imbalance may promote pathological states such as allergy, inflammation, and cancer. Mast cells have a central role in the regulation of the innate/adaptive immune system and are involved in the pathogenesis of many inflammatory and allergic diseases by releasing inflammatory mediators such as histamines, proteases, chemotactic factors, and cytokines. Although various signaling pathways are associated with mast cell activation, our discovery and characterization of the pLysRS-Ap4A signaling pathway in these cells provided an additional important step towards a full understanding of the intracellular mechanisms involved in mast cell activation. In the present review, we will discuss in depth this signaling pathway’s contribution to host defense and the pathological state.
Keywords: allergic disease, mast cell activation, host defense, pathological condition, pLysRS-Ap4A signaling
1. Mast Cells (MCs) and Their Activation Mechanisms
Mast cells (MCs) are a subset of leukocytes and are derived from hematopoietic progenitor cells. In 1879, Paul Ehrlich characterized the mast cell by the presence of large granules in the cytoplasm and identified them as “mastzellen”, meaning “feeding cells”. Mast cells (MCs) are sentinel cells of connective tissue that, upon activation by an allergen, can trigger an immediate inflammatory response. An immature and undifferentiated form of mast cells circulates in blood and migrates to tissues of the body that are in close proximity with the external environment, such as skin, airways and intestine. Maturation of mast cells (MCs) occurs in these vascular tissues aided by cytokines, stem cell factors secreted by fibroblasts and endothelial cells [1].
There are several mechanisms of mast cell activation, but the most important signaling pathway is the antigen–allergen interaction with the immunoglobulin E (IgE) antibody in the tissues and the subsequent IgE binding to the high-affinity receptor Fc epsilon RI (FcεRI). This IgE-FcεRI cross-linking triggers multiple transduction pathways allowing the mast cells (MCs) to secrete their various allergic mediators (histamines, heparins, proteoglycans, neutral proteases, and cytokines) to the external environment. Another type of immunoglobulin and Fc receptor triggering also assists the host defense in response to Interferon (IFN) during the condition of an autoimmune disease, an IgG-FcγRI cross-linking causes the human mast cell (MC) activation [2]. Their Toll-like receptors (TLRs) are surface pathogen recognition receptors (PRRs) that can directly activate mast cells and help in controlling bacterial infections [3]. In addition to microbial challenges, an anti-viral response mechanism in rat peritoneal mast cells (MCs) has been described in which viral dsRNA binding to TLR3 can trigger the release of IFN-α and β, various chemokines and cytokines such as CCL3, CXCL8, TNF, and IL-1β, and a pro-inflammatory lipid mediator, cysLTs [4].
Mast cells (MCs) are one of the major effector cells in vascular connective tissues with a key role in the regulation of innate and adaptive immunity. They are involved in the pathogenesis of many inflammatory and allergic diseases, such as immediate hypersensitivity autoimmune diseases [5], experimental allergic encephalomyelopathy [6], rheumatoid arthritis [7], as well as in delayed-type hypersensitivity [8], tumor growth, angiogenesis [9] and congestive heart failure [10]. Under physiological conditions, the host defense mechanism of mast cells against the invading pathogens is due to the increased IgE-FcεRI cross-linking. The hypersensitivity of mast cells will switch the host defense to a pathological condition ranging from simple nasal allergies caused by pollen or fungal spores to mild dermatological eczema/erythema and to severe asthma or hypoxia, leading to life threatening anaphylactic allergic reactions [11].
2. The pLysRS Signaling Pathway in IgE-FcεRI Activated Mast Cells (MCs)
The FcεRI receptor is found on various immune cells such as mast cells (MCs) and basophils, and is a multimeric protein that comprises an external IgE-binding α subunit, a transmembrane β subunit and a dimer of disulfide-linked γ subunits [12]. Cross-linking of FcεRI with an IgE bound antigen results in the triggering of a series of complex signal transduction pathways as a part of the allergic response of mast cells (MCs). The immunoreceptor tyrosine-based activation motifs (ITAMs), located on the γ subunit of FcεRI, provide a docking site for Syk after being tyrosine-phosphorylated by Lyn and Src kinases. The enzymatically activated Syk sends a downstream signal and phosphorylates several proteins, including phospholipases Cγ (PLCγ), phosphoinositide 3-kinases (PI3Ks), regulatory subunits and several other SH2 domain-containing leukocyte protein (SLP) family proteins [13]. Various small GTPases, such as Rac, Ras and Rho, are also activated; thus, causing the stimulation of the ERK, JNK and p38 MAP kinase pathways [14]. Here, MAP kinases play a crucial role in regulating the translocation of a variety of transcription factors to the nucleus from the cytosol, thereby controlling the degranulation process. It was identified from our previous works that IgE-antigen stimulation induces serine 207 phosphorylation of Lysine-tRNA synthetase (LysRS) by this MAPK-dependent stimulation, allowing the pLysRS to be released from the multi-synthetase complex (MSC) [15].
3. pLysRS Transcriptional Regulation of MITF, USF2 via the Ap4A–HINT Pathway
LysRS is a member of the aminoacyl-tRNA synthetase (aaRSs) family, and has been shown to be involved not only in the translation step of protein synthesis, but also as a transcriptional regulation factor of MITF and USF2 [16,17]. In mammalian cells, LysRS forms a cytosolic aminoacyl-tRNA MSC along with another eight aaRSs and three nonanalytic components, p18, p43 and p38 [18]. When mast cells (MCs) are in the quiescent state, LysRS is bound to the cytoplasmic MSC via its interactions with p38. In the activated mast cell (MC), a series of MAPK signaling cascades lead to phosphorylation of LysRS at Ser207 to give LysRSPS207, which is essential for Ap4A production, and the pLysRS being released from the MSC. Mutation of serine at LysRSS207 to alanine (LysRSS207A), which mimics the dephosphorylation state, significantly reduced Ap4A production. In contrast, over expression of the phosphomimetic mutant LysRSS207D (serine mutated to aspartate) increased the concentration of Ap4A; thus, enhancing the transcription of MITF target genes in mast cells (MCs) [15]. In both prokaryotes and eukaryotes, Ap4A provides an important signal for gene expression, and pLysRS’s involvement in Ap4A production through a zinc-dependent chemical reaction has been known for decades [19,20]. The identification of the pLysRS-Ap4A–MITF signaling pathway by our group demonstrated that Ap4A acts as a secondary messenger whereby, as a result of the IgE-antigen stimulation, Ap4A binds to the histidine triad nucleotide-binding protein 1 (HINT1), causing the release of MITF from the complex. We identified by Biacore analysis that specific binding of HINT1 to Ap4A, but not to other ApnA molecules (Ap3A or Ap5A), affected the MITF–HINT-1 protein complex [17]. A similar mechanism was identified in USF2, another transcription factor, whereby USF2 is regulated by the interaction of HINT1 with Ap4A [16]. Thus, pLysRS regulates the MITF and USF2 transcriptional activity via Ap4A as illustrated in Figure 1.
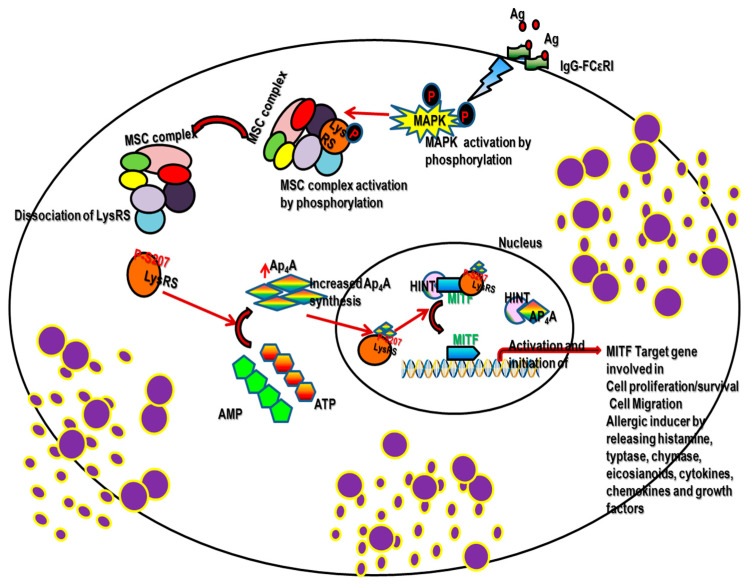
Figure 1.
Demonstrates the schematic diagram of pLysRS-Ap4A signaling in mast cell activation. Our pioneering work demonstrated, following physiological stimulation in the mast cell, the activated ERK-mediated phosphorylation of 207 serine lysyl-tRNA synthetase (LysRS), which is one of the components of the multi-synthetase–cytosolic complex. This confirmational change in the LysRS enzyme causes its dissociation from its complex and catalysis in the synthesis of Ap4A. This Ap4A acts as a 2nd messenger and binds specifically to Hint1 in the nucleus. This change in confirmation causes its dissociation from the transcription factor MITF, which is now able to activate its target genes of MITF that are involved in cell proliferation, migration, allergic inducer, etc.
4. Role of pLysRS-Ap4A Mediated Signaling under Pathological Conditions
The location of mast cells at the host–environment interface generates inflammatory reactions by releasing pro-inflammatory mediators during the immune activation [21] and a disordered homeostasis causes multiple pathological conditions and could result in mast cell activation syndrome [22]. It has been identified from many studies that mast cells provide the first line of defense against the invading pathogens through multiple mechanisms. Activated mast cells (MCs) functionally impact the other immune cells’ activities by their effective signaling mechanism; then, control the pathological conditions caused by autoinflammatory cells, cancerous cells or by external invaders such as bacteria and viruses.
5. Autoimmune Disease
Previous studies on multiple sclerosis and rheumatoid arthritis recognized the significant role played by mast cells (MCs) in the initiation and propagation of these autoimmune diseases. The presence of various mast cell (MC) mediators, such as tryptase, in the cerebrospinal fluid of multiple sclerosis patients [23] supports the inflammatory response by activating peripheral mononuclear cells to produce various factors, including TNF, IL-6, and IL-1; then, stimulates the protease-activated receptor (PAR) causing microvascular leakage [24]. Although various cytokines and mediators of mast cells (MCs) were identified in the regions of inflammation in autoimmune conditions, the necessity of LysRS-Ap4A signaling molecules remains unclear. Cyclic GMP–AMP (cGAMP) synthetase (cGAS) belongs to the family of intracellular PRRs. It identifies cytosolic soluble stranded nucleic acids (dsDNAs) and generates cGAMP, which in turn binds to the stimulator of the interferon genes (STING). This cGAS–STING pathway produces type 1 IFNs and NF-κB necessary for the induction of the pro-inflammatory response, and a tight regulation is needed for the suppression of autoimmune diseases [25]. Recently, LysRSAp4A were proposed as pharmacological targets to control the STING signaling pathway for the treatment of autoinflammatory diseases. Of the two mechanisms identified, one showed that LysRS interacts with RNA–DNA hybrids, causing the delay in recognition by cGAS essential for cGAMP production. In the other mechanism, LysRS-dependent Ap4A production causes the attenuation of STING signaling; thus, regulating the autoimmunity disease [26].
6. Tumor Microenvironment
The tumor microenvironment plays a pivotal role in cancer progression and metastasis. As a result of chronic inflammation, the tumor microenvironment frequently contains an abundance of growth factors, cytokines, chemokines, prostaglandins, and reactive oxygen species and also immune cells such as mast cells (MCs), tumor-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs), as well as adaptive immune cells, including T and B lymphocytes and dendritic cells (DCs) which can promote tumor metastasis.
In recent years, the involvement of cytosolic aaRSs in tumorigenesis and metastasis, apart from their canonical (protein translation) role [27], has been the subject of much research. Of the aaRSs, LysRS is highly expressed in various tissues such as the colon, lung, gastric, breast and thyroid cancer cells, and has noncanonical functions in the immune response and tumor metastasis [28]. The activation of the pLysRS-Ap4A-regulated MITF pathway is the main hallmark in both cancer and immune cells. An early report from our group described how activated serine 207 phosphorylated lysyl-tRNA synthetase (p-s207 LysRS) is released from the cytoplasmic MSC; then, relocates into the nucleus, promoting the transcriptional activity of MITF in stimulated cultured mast cells and cardiomyocytes. The activation of MITF is mediated by an increased Ap4A level through the release of HINT1 suppression [16,17,29,30,31,32]. Since LysRS is found in all tissues, the activation of the p-LysRS-Ap4A pathway occurs in cancer cells also. The HINT1 (tumor suppressor protein)-deficient mice have an increased susceptibility for both spontaneous tumors and tumor proliferation [33]. Hence, this pathway should be tightly regulated. Ap4A regulates MITF activation through HINT1 polymerization. The polymerization of HINT1 may block the MITF–HINT1 interaction that releases MITF for the increased transcription in the MITF downstream genes (c-Met, c-Kit). Thus, the second messenger Ap4A activates the MITF downstream target by mediating HINT1 polymerization that is involved in cell proliferation and migration [34]. Phosphorylation of S207 on LysRS by ERKs results in translocation to the nucleus, and is associated with an improved mean disease-free survival (DFS) in patients with EGFR mutations [35] compared to WT EGFR patients. This was also followed by the participation of membranous LysRS and nuclear LysRS in invasive cell dissemination—an important step in cancerous cell progression was identified in colon cancer spheroids [36].
DCs are involved in acquired immune activity by migrating to lymph nodes for antigen presentation to naive T or B cells [37]. The highly conserved function of antigen presentation can be precisely impaired by low levels of intracellular Ap4A. Our previous study shows that DCs may functionally benefit from the increased intracellular Ap4A concentration by knocking out Ap4A hydrolase, thereby enhancing the antigen cross-presentation by DCs [38], which aids in antigen recognition by immune cells during cancer conditions. The above evidence clearly indicates that p-LysRS-Ap4A-regulated MITF signaling pays a major role in tumor metastasis (Figure 2).
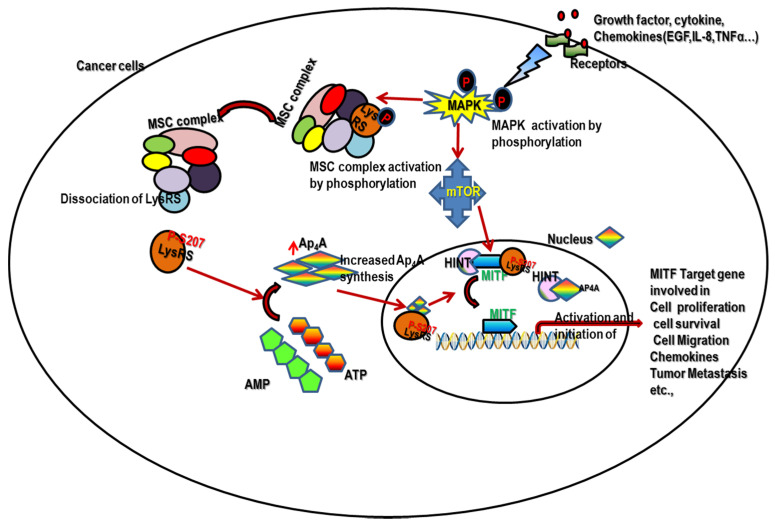
Figure 2.
Represents the schematic diagram of pLysRS-Ap4A signaling activation during cancer metastasis. During chronic inflammation, the immune cells, such as mast cells (MCs), tumor-associated macrophages (TAMs), etc., release an abundance of growth factors, cytokines, chemokines, prostaglandins, and reactive oxygen species in the tumor microenvironment which can promote tumor metastasis. The released growth factors, chemokines and cytokines cause the activation of the pLysRS-Ap4A-regulated MITF pathway. Thus, it promotes cancer cell survival, proliferation, migration and metastasis. Hence, the pLysRS-Ap4A-regulated MITF pathway is the main hallmark in both cancer and immune cells.
7. Conclusions
The delineation of a new signaling mechanism could provide new insights and a better understanding of a disease condition. The discovery and characterization of the p-LysRS-Ap4A signaling pathway has attracted the attention of many researchers. pLysRS has several roles besides its function as a key enzyme involved in translation. In the immune system, it may function both as an extracellular cytokine-like molecule and a signal transduction protein in a signal transduction pathway, ultimately regulating gene expression. Apart from its canonical mechanism, the p-LysRS-Ap4A-regulated MITF pathway is involved not only in allergic disease but also in cancer metastasis. It seems that much more research is needed in order to understand the complex regulation of the pLysRS function and its involvement in such various unrelated pathological processes as described above. Such understanding could help in designing potential drug targets for various pathologies.
Acknowledgments
We would like to thank Gillian for editing our manuscript.
Abbreviations
| aaRSs | Aminoacyl-tRNA synthetase |
| Ap3A or Ap5A | Diadenosine tetraphosphate or diadenosine pentaphosphate |
| Ap4A | Diadenosine tetraphosphate |
| B cell | B cell lymphocytes |
| CCL3 | Chemokine CC motif ligand 3, |
| cGAMP | Cyclic GMP–AMP |
| cGAS | Cyclic GMP–AMP(cGAMP) synthetase |
| c-Kit | CD117, also called KIT or c-Kit receptor |
| c-Met | Mesenchymal–epithelial transition factor |
| CXCL8 | Chemokine CXC motif ligand 8 |
| DCs | Dendritic cells |
| EGFR | Epidermal growth factor receptor |
| ERK | Extracellular signal-related kinase |
| HINT1 | Histidine triad nucleotide-binding protein 1 |
| IFN-α, β | Interferon alpha, beta |
| IFNγ | Interferon gamma γ |
| IgE | Immunoglobulin E |
| IgE-FcεR | Immunoglobulin E FC epsilon RI |
| IgG-FcγRI | Immunoglobulin G FC gamma RI |
| IL-1 | Interleukin-1 |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| ITAM | Immunoreceptor tyrosine-based activation motifs |
| JNK | Janus kinase |
| Lyn | Lck/Yes novel tyrosine kinase |
| LysRS | Lysine-tRNA synthetase |
| LysRSS207A | Lys phosphorylation at serine 207 serine-to-alanine mutation |
| LysRSS207D | Lys phosphorylation at serine 207 serine-to-aspartate mutation |
| MAPK | Mitogen-activated protein kinase |
| MCs | Mast cells |
| MDSCs | Myeloid-derived suppressor cell |
| MITF | Microphthalmia-associated transcription factor |
| MSC | Multisynthetase complex |
| NF-κB | Nuclear factor kappa B |
| p38 MAP kinase | p38 mitogen-activated protein kinase |
| PAR | Protease-activated receptor |
| PI3Ks | Phosphoinositide 3-kinases |
| PLCγ | Phospholipases Cγ |
| pLysRS | Phosphorylated lysine-tRNA synthetase |
| PRRs | Pattern recognition receptors |
| Rac | Rho family GTPase |
| Ras | Rat sarcoma |
| Rho | Ras homologous protein |
| SLP | SH2-domain-containing leukocyte protein |
| Src | v-src sarcoma (Schmidt-Ruppin A-2) viral oncogene homolog (avian) |
| STING | Stimulator of interferon genes |
| SyK | Spleen tyrosine kinase |
| T cell | Thymus lymphocytes |
| TAMs | Tumor-associated macrophages |
| TNF | Tumor necrosis factor |
| USF2 | Upstream transcription factor 2 |
Author Contributions
S.G. and L.B.P. contributed equally to the design of the article and interpreting the relevant literature. E.R. revised it for its important intellectual content and approved the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The present study was supported by the Israel Science Foundation, 115/2013, and Hebrew-University-National Research Foundation of Singapore HUJ-CREATE (R182-005-172-281) to E.R.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no competing interests.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Urb M., Sheppard D.C. The Role of Mast Cells in the Defence against Pathogens. PLoS Pathog. 2012;8:e1002619. doi: 10.1371/journal.ppat.1002619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woolhiser M., Brockow K., Metcalfe D.D. Activation of human mast cells by aggregated IgG through FcγRI: Additive effects of C3a. Clin. Immunol. 2004;110:172–180. doi: 10.1016/j.clim.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Supajatura V., Ushio H., Nakao A., Akira S., Okumura K., Ra C., Ogawa H. Differential responses of mast cell Toll-like receptors 2 and 4 in allergy and innate immunity. J. Clin. Investig. 2002;109:1351–1359. doi: 10.1172/JCI0214704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Witczak P., Brzezińska-Błaszczyk E., Agier J. The Response of Tissue Mast Cells to TLR3 Ligand Poly(I:C) Treatment. J. Immunol. Res. 2020;2020:1–13. doi: 10.1155/2020/2140694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu Y., Chen G. Mast Cell and Autoimmune Diseases. Mediat. Inflamm. 2015;2015:1–8. doi: 10.1155/2015/246126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenner T., Soffer V., Shalit M., Levi-Schaffer F. Mast cells in experimental allergic encephalomyelitis: Characterization, distribution in the CNS and in vitro activation by myelin basic protein and neuropeptides. J. Neurol. Sci. 1994;122:210–213. doi: 10.1016/0022-510X(94)90300-X. [DOI] [PubMed] [Google Scholar]
- 7.Min H.K., Kim K.-W., Lee S.-H., Kim H.-R. Roles of mast cells in rheumatoid arthritis. Korean J. Intern. Med. 2020;35:12–24. doi: 10.3904/kjim.2019.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Constantinescu C.S., Farooqi N., O’Brien K., Gran B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS) Br. J. Pharmacol. 2011;164:1079–1106. doi: 10.1111/j.1476-5381.2011.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ribatti D., Crivellato E. Mast cells, angiogenesis, and tumour growth. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2012;1822:2–8. doi: 10.1016/j.bbadis.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Hermans M.A.W., Van Lennep J.E.R., Van Daele P.L.A., Bot I. Mast Cells in Cardiovascular Disease: From Bench to Bedside. Int. J. Mol. Sci. 2019;20:3395. doi: 10.3390/ijms20143395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malak A., Mohamed M., Hassan A. Type I Hypersensitivity Reaction. StatPearls Publishing, Treasure Island (FL); Hong Kong, China: May 4, 2021. [(accessed on 25 May 2021)]. Available online: https://www.ncbi.nlm.nih.gov/books/NBK560561/ [Google Scholar]
- 12.Blank U., Ra C., Miller L., White K., Metzger H., Kinet J.-P. Complete structure and expression in transfected cells of high affinity IgE receptor. Nat. Cell Biol. 1989;337:187–189. doi: 10.1038/337187a0. [DOI] [PubMed] [Google Scholar]
- 13.Mócsai A., Ruland J., Tybulewicz V.L.J. The SYK tyrosine kinase: A crucial player in diverse biological functions. Nat. Rev. Immunol. 2010;10:387–402. doi: 10.1038/nri2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siraganian R.P., De Castro R.O., Barbu E.A., Zhang J. Mast cell signaling: The role of protein tyrosine kinase Syk, its activation and screening methods for new pathway participants. FEBS Lett. 2010;584:4933–4940. doi: 10.1016/j.febslet.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yannay-Cohen N., Carmi-Levy I., Kay G., Yang C.M., Han J.M., Kemeny D.M., Kim S., Nechushtan H., Razin E. LysRS Serves as a Key Signaling Molecule in the Immune Response by Regulating Gene Expression. Mol. Cell. 2009;34:603–611. doi: 10.1016/j.molcel.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 16.Lee Y.N., Razin E. Nonconventional Involvement of LysRS in the Molecular Mechanism of USF2 Transcriptional Activity in FcεRI-Activated Mast Cells. Mol. Cell. Biol. 2005;25:8904–8912. doi: 10.1128/MCB.25.20.8904-8912.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee Y.-N., Nechushtan H., Figov N., Razin E. The Function of Lysyl-tRNA Synthetase and Ap4A as Signaling Regulators of MITF Activity in FcϵRI-Activated Mast Cells. Immunity. 2004;20:145–151. doi: 10.1016/S1074-7613(04)00020-2. [DOI] [PubMed] [Google Scholar]
- 18.Khan K., Baleanu-Gogonea C., Willard B., Gogonea V., Fox P.L. 3-Dimensional architecture of the human multi-tRNA synthetase complex. Nucleic Acids Res. 2020;48:8740–8754. doi: 10.1093/nar/gkaa569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brevet A., Plateau P., Cirakoğlu B., Pailliez J.P., Blanquet S. Zinc-dependent synthesis of 5’,5’-diadenosine tetraphosphate by sheep liver lysyl- and phenylalanyl-tRNA synthetases. J. Biol. Chem. 1982;257:14613–14615. doi: 10.1016/S0021-9258(18)33321-0. [DOI] [PubMed] [Google Scholar]
- 20.Hilderman R.H., Ortwerth B.J. A preferential role for lysyl-tRNA4 in the synthesis of diadenosine 5’,5’’’-P1P4-tetraphosphate by an arginyl-tRNA synthetase-lysyl-tRNA synthetase complex from rat liver. Biochemistry. 1987;26:1586–1591. doi: 10.1021/bi00380a015. [DOI] [PubMed] [Google Scholar]
- 21.Abraham S.N., John A.S. Mast cell-orchestrated immunity to pathogens. Nat. Rev. Immunol. 2010;10:440–452. doi: 10.1038/nri2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akin C. Mast cell activation syndromes. J. Allergy Clin. Immunol. 2017;140:349–355. doi: 10.1016/j.jaci.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 23.Rozniecki J.J., Hauser S.L., Stein M., Lincoln R., Theoharides T.C. Elevated mast cell tryptase in cerebrospinal fluid of multiple sclerosis patients. Ann. Neurol. 1995;37:63–66. doi: 10.1002/ana.410370112. [DOI] [PubMed] [Google Scholar]
- 24.Malamud V., Vaaknin A., Abramsky O., Mor M., Burgess L.E., Ben-Yehudah A., Lorberboum-Galski H. Tryptase activates peripheral blood mononuclear cells causing the synthesis and release of TNF-α, IL-6 and IL-1β: Possible relevance to multiple sclerosis. J. Neuroimmunol. 2003;138:115–122. doi: 10.1016/S0165-5728(03)00090-0. [DOI] [PubMed] [Google Scholar]
- 25.Kumar V. A STING to inflammation and autoimmunity. J. Leukoc. Biol. 2019;106:171–185. doi: 10.1002/JLB.4MIR1018-397RR. [DOI] [PubMed] [Google Scholar]
- 26.Guerra J., Valadao A.-L., Vlachakis D., Polak K., Vila I.K., Taffoni C., Prabakaran T., Marriott A.S., Kaczmarek R., Houel A., et al. Lysyl-tRNA synthetase produces diadenosine tetraphosphate to curb STING-dependent inflammation. Sci. Adv. 2020;6:eaax3333. doi: 10.1126/sciadv.aax3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim S., You S., Hwang D. Aminoacyl-tRNA synthetases and tumorigenesis: More than housekeeping. Nat. Rev. Cancer. 2011;11:708–718. doi: 10.1038/nrc3124. [DOI] [PubMed] [Google Scholar]
- 28.Young H.J., Lee J.W., Kim S. Function of membranous lysyl-tRNA synthetase and its implication for tumorigenesis. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2016;1864:1707–1713. doi: 10.1016/j.bbapap.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 29.Carmi-Levy I., Yannay-Cohen N., Kay G., Razin E., Nechushtan H. Diadenosine Tetraphosphate Hydrolase Is Part of the Transcriptional Regulation Network in Immunologically Activated Mast Cells. Mol. Cell. Biol. 2008;28:5777–5784. doi: 10.1128/MCB.00106-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carmi-Levy I., Motzik A., Ofir-Birin Y., Yagil Z., Yang C.M., Kemeny D.M., Han J.M., Kim S., Kay G., Nechushtan H., et al. Importin Beta Plays an Essential Role in the Regulation of the LysRS-Ap4A Pathway in Immunologically Activated Mast Cells. Mol. Cell. Biol. 2011;31:2111–2121. doi: 10.1128/MCB.01159-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huete F., Guzmán-Aránguez A., Ortín J., Hoyle C.H.V., Pintor J. Effects of diadenosine tetraphosphate on FGF9-induced chloride flux changes in achondroplastic chondrocytes. Purinergic Signal. 2011;7:243–249. doi: 10.1007/s11302-011-9234-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tshori S., Razin E., Nechushtan H. Amino-Acyl tRNA Synthetases Generate Dinucleotide Polyphosphates as Second Messengers: Functional Implications. Aminoacyl-tRNA Synth. Biol. Med. 2014;344:189–206. doi: 10.1007/128_2013_426. [DOI] [PubMed] [Google Scholar]
- 33.Genovese G., Ghosh P., Li H., Rettino A., Sioletic S., Cittadini A., Sgambato A. The tumor suppressor HINT1 regulates MITF and β-catenin transcriptional activity in melanoma cells. Cell Cycle. 2012;11:2206–2215. doi: 10.4161/cc.20765. [DOI] [PubMed] [Google Scholar]
- 34.Yu J., Liu Z., Liang Y., Luo F., Zhang J., Tian C., Motzik A., Zheng M., Kang J., Zhong G., et al. Second messenger Ap4A polymerizes target protein HINT1 to transduce signals in FcεRI-activated mast cells. Nat. Commun. 2019;10:1–12. doi: 10.1038/s41467-019-12710-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boulos S., Park M.C., Zeibak M., Foo S.Y., Jeon Y.K., Kim Y.T., Motzik A., Tshori S., Hamburger T., Kim S., et al. Cor-rection: Serine 207 phosphorylated lysyl-tRNA synthetase predicts disease-free survival of non-small-cell lung carcinoma. Oncotarget. 2017;8:36250. doi: 10.18632/oncotarget.18053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nam S.H., Kim D., Lee D., Lee H.-M., Song D.-G., Jung J.W., Kim J.E., Kim H.-J., Kwon N.H., Jo E.-K., et al. Lysyl-tRNA synthetase–expressing colon spheroids induce M2 macrophage polarization to promote metastasis. J. Clin. Investig. 2018;128:5034–5055. doi: 10.1172/JCI99806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Germain R.N., Robey E.A., Cahalan M.D. A Decade of Imaging Cellular Motility and Interaction Dynamics in the Immune System. Science. 2012;336:1676–1681. doi: 10.1126/science.1221063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.La Shu S., Paruchuru L.B., Tay N.Q., Chua Y.L., Foo A.S.Y., Yang C.M., Liong K.H., Koh E.G.L., Lee A., Nechushtan H., et al. Ap4A Regulates Directional Mobility and Antigen Presentation in Dendritic Cells. Iscience. 2019;16:524–534. doi: 10.1016/j.isci.2019.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.