Abstract
Although the epidemic caused by SARS-CoV-2 callings for international attention to develop new effective therapeutics, no specific protocol is yet available, leaving patients to rely on general and supportive therapies. A range of respiratory diseases, including pulmonary fibrosis, have been associated with higher iron levels that may promote the course of viral infection. Recent studies have demonstrated that some natural components could act as the first barrier against viral injury by affecting iron metabolism. Moreover, a few recent studies have proposed the combination of protease inhibitors for therapeutic use against SARS-CoV-2 infection, highlighting the role of viral protease in virus infectivity. In this regard, this review focuses on the analysis, through literature and docking studies, of a number of natural products able to counteract SARS-CoV-2 infection, acting both as iron chelators and protease inhibitors.
Keywords: SARS-Cov-2, COVID-19, iron chelation, protease inhibition, natural products
1. Introduction
Coronaviruses, named for the crown-like spikes on their surface, are a family consisting of enveloped single-stranded and positive-strand RNA viruses, possessing a helical nucleocapsid. They are known to cause acute and chronic respiratory and central nervous system diseases in animals and humans [1]. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) primarily affects the tissues expressing angiotensin-converting enzyme 2 (ACE2) receptor, including the lungs, heart, kidney and endothelium, leading to systemic manifestations [2]. Furthermore, the role of Neurolipin-1, abundantly expressed in the respiratory and olfactory epithelium, has been recently investigated as a significant enhancer of SARS-CoV-2 infectivity by promoting the interaction of the virus with ACE2 receptor [3]. The ongoing epidemic outbreak caused by the coronavirus disease 2019 (COVID-19) calls for international attention to develop effective therapeutics including selective vaccines. Nevertheless, no specific therapeutic is yet available, leaving patients to rely on general and supportive therapies, such as oxygen supply, glucocorticoid, and human serum albumin [4]. Although some therapies, such as antiviral drugs, Chloroquine, and recombinant monoclonal antibodies, are showing a promising efficacy, additional therapeutic options should be explored and new therapeutic targets should be considered when taking into account the increasing number of SARS-CoV-2 cases [5]. Among factors capable of affecting viral infections, iron plays a critical role and represents a double-edged sword that acts both on favoring viral progression and exacerbating inflammatory processes; on the other hand, the inhibition of proteases, crucial to impede the virus-host cell fusion, could be highlighted by exploiting the activity of unconventional drugs, such as natural products.
Dysregulated iron homeostasis is one of the potential causes of diffuse endothelial inflammation with systemic involvement, resulting in oxidative stress and inflammatory response [6]. A range of respiratory diseases, including acute respiratory distress syndrome (ARDS) and pulmonary fibrosis, have been associated with higher iron levels that may promote the course of viral infection [7,8,9]. Iron dependence on viral replication and the modulation of host iron metabolism exerted by virus infection highlight the importance of cellular iron homeostasis in the viral life cycle and lead to the development of iron chelation strategies in treating viral infections. Currently, few iron chelators have been approved by the U.S. Food and Drug Administration for clinical use, such as Deferoxamine and Deferasirox. With a strong and selective affinity with iron ions, these drugs can bind free iron and remove it from iron-storing proteins [10,11]. Recent studies have demonstrated that some natural components of the human innate immunity could act as a first barrier against viral injury and, in this regard, increasing interest has been shown in the possible preventive role of lactoferrin as adjunct treatment [12,13]. Lactoferrin is a glycoprotein of human secretion, belonging to a non-specific defensive system, known to play a pivotal role against viral infections and able to regulate iron metabolism. The main capability of Lactoferrin is to reversibly chelate two Fe3+ per molecule with high affinity, binding iron until pH values of 3.0 [14]. Through sequestering free iron and restoring iron homeostasis, Lactoferrin reduces oxidative stress and inflammation, principally associated with the cytokines storm and COVID-19 pathology [15,16,17].
Proteases are the enzymes involved in proteolysis, a protein catabolism by hydrolysis of peptide bonds. Proteolytic processes are necessary for normal physiological functions in the body, such as digestion, angiogenesis, and bone remodeling [18]. In enveloped viruses, post-translational proteolytic activation is a crucial step for the fusion with the host and thus for the infectivity of the virus. Both membrane receptors and proteolytic activation are indispensable for effective virus spread in the infected host, determining the level of the pathogenicity [19]. Proteases have been identified in a wide range of viruses, without any correlation to the envelope presence: cysteine proteases are present in adenoviruses, while the family of aspartyl proteases has been found in human immunodeficiency virus of type 1 (HIV1). Furthermore, the proteases present in various viruses specifically belong to the family of serine protease, as in hepatitis C virus (HCV), herpesvirus, and in SARS-CoV-2 [20]. Several protease inhibitors have been considered as drugs of choice to counteract the infection of viruses whose entry into the host cell is strictly related to proteases activity, including SARS-CoV-2 [21,22,23]. Few recent studies have proposed the combination of existing drugs involving anti-HIV drugs (lopinavir/ritonavir, lamivudine, tenofovir) for therapeutic use against SARS-CoV-2 infection: the consequent recognition of proteases as a new attractive target led to a deep investigation of alternative compounds able to target viral serine protease [24,25]. With this purpose, a recent in-silico study reported the potential of a few phytoconstituents (including glycyrrhizin, tryptanthrine, rhein, and berberin) to target the main protease involved in COVID-19 activity; the obtained results showed a high degree of interaction with the viral protease accompanied by low binding energy, thus leading to favorable drug-like properties exerted by natural compounds [26].
Starting from these assumptions, it is possible to suggest that a number of natural products, whose effect as iron chelators and/or viral protease inhibitors has been proven (Table 1), could show the capability to control and reduce the inflammatory condition and the relative oxidative stress raised after SARS-CoV-2 infection, furthermore acting by preventing virus replication.
2. Iron and SARS-CoV-2
Iron is an essential element that plays a pivotal role in many cellular processes that are necessary for life, including oxygen transportation, oxygen sensing, electron transfer, energy metabolism, and DNA synthesis [27]. Iron is required for viral replication and other processes including ATP generation, cell survival, ferroptosis, and DNA/RNA synthesis and repair [28]. Low intracellular iron levels are sufficient to support coronavirus replication, whereas iron deficiency interferes with viral transcription, translation, assembly, and exocytosis [29]. The main site of iron storage (in its ferric state) is Ferritin, that can carry up to 4500 iron molecules in its core [30,31]. Systemic inflammations are generally associated with increased serum ferritin levels: indeed, during strong inflammation state, cytokines stimulate ferritin and the hepcidin synthesis, the main regulator of the tissue iron store [32]. Regarding this, a high level of ferritin has been reported in patients with COVID-19 disease [33,34,35]: on one hand, SARS-CoV-2 attacks one of the beta chains of the hemoglobin, which leads to the dissociation of iron from heme and the consequent increased free iron and ferritin levels in the body [11,34,36,37,38]; on the other hand, one of the causes has been associated with the inflammation induced by COVID-19 infection, with a remarkable overexpression of IL-6, IL-1β, and IFN-γ, leading to the increase of the hepcidin level [6,39,40]. Hepcidin, as key iron regulatory hormone, sequesters iron in the enterocytes and macrophages, enhancing intracellular levels of ferritin and preventing iron efflux from store cells through the inhibition of the iron-exporting protein ferroportin [41]. Ehsani recently supposed a similarity between the hepcidin protein and the distant amino acid sequence of the SARS-CoV-2 spike glycoprotein cytoplasmic tail, highlighting the potential route of investigation of factors that interplay between cytokine-mediated inflammatory processes, respiratory infections, and systemic iron regulation (Table 2) [42].
3. Protease Inhibition and SARS-CoV-2
As reported in the literature, infections by SARS coronaviruses are dependent not only on the host ACE2 receptor but also on the priming of the virus’s spike (S) protein by the Transmembrane Serine Protease 2 (TMPRSS2). The cleavage of the S protein is the necessary step that leads to the membrane fusion of virus and host and the consequent cell entry [43,44]. Although further clinical data are needed to prove it, several studies suggested the use of protease inhibitors for the prevention of COVID-19, recommending the administration of antiretroviral drugs, such as the lopinavir/ritonavir combination for the initial clinical management of patients with SARS-CoV-2 infection [25,45,46]. In addition, it has been shown that nafamostat mesylate and camostat mesylate, both potent inhibitors of TMPRSS2, can be used to block SARS-CoV-2 cell entry as promising prophylactic options for the clinical manifestation of COVID-19 infection in critically ill patients, especially in cases with possible coagulopathies [25,43,47,48,49,50]. With the purpose to focus on SARS-CoV-2 protease for the development of new therapeutical strategies, a recent computational study highlighted the possibility to discover and identify new lead compounds able to target main protease (Mpro) of SARS-CoV-2 that represent a key coronaviruses enzyme crucial for replication and transcription [51].
4. Materials and Methods
The docking studies were performed according to the protocol used in a paper previously published [52]. All compounds were built and their energy minimized with “Flare preparation ligand” [52]. The protease coordinates were downloaded from the protein databank with the code 6Y2E that possess a resolution of 1.75 angstrom. According to the literature, the binding site was identified in a specific region named TAEDMLN. The catalytic domain has been located between residue 41 and residue 145, specifically from amino acids 45 to 51, right charges were calculated with “Flare protein preparation”. Docking calculation were performed with Flare with the “Accurate but Slow” setting that provides the dG score which provides an accurate estimation of the free energy of protein-ligand binding. The best docking poses were refined by ligand-protein complex energy minimization using Flare. Finally, to improve the stability of each complex, short (5ns of production) molecular dynamic runs were performed at a constant temperature followed by a quick minimization of all the atoms involved in the binding site.
5. α-Lipoic Acid
Alpha-lipoic acid (ALA), also known as 1,2-dithiolane-3-pentanoic acid and thioctic acid, is a natural substance existing in almost all types of prokaryotic and eukaryotic cells (Figure 1). The ability as antioxidant and metal chelator of ALA allows the reduction of the oxidized forms of several antioxidant agents, such as glutathione (GSH) and vitamins E and C [53]. The efficacy of ALA as iron chelator has been demonstrated in a combined treatment with ferric ammonium citrate (FAC), simulating an iron overload condition both in vitro and in vivo. Administration of ALA in mesenchymal stem cells leads to a decrease of reactive oxygen species (ROS) levels and a restoring of mitochondrial membrane potential and integrity, following the treatment with FAC. Augmented levels of GSH have been associated with the direct antioxidant effect of ALA, leading to enhanced antioxidant defenses for the cells. Consequently, through the increase of intracellular GSH content, ALA prevents the nuclear factor erythroid 2–related factor 2 (NRF2) pathway activation, leading to the reduction of heme oxygenase 1 (HO-1) expression. The same result has been confirmed in an in vivo model of zebrafish, with significant reduction of heme oxygenase 1b (HMOX1b), mitochondrial superoxide dismutase (mtSOD), and ferroportin 1 (FPN1) expression after treatment with ALA in the presence of an iron overload [54,55]. Following different in vivo experiments, Zhao et al. demonstrated the capacity of ALA as iron chelator to prevent the light-induced retinal degeneration in a mouse model of AMD (age-related macular degeneration) through systemic administrations [56]. Abnormally high levels of iron in the brain have been demonstrated in a number of neurodegenerative disorders, such as Alzheimer’s disease and Parkinson’s disease (PD). The neuroprotective effect of ALA was tested in a PD model induced by 6-hydroxydopamine (6-OHDA), showing significant reduction of ROS and an improvement of iron metabolism levels, confirming the therapeutic potential of ALA for the treatment of neurodegenerative diseases associated with iron metabolism dysfunction and oxidative stress [57].
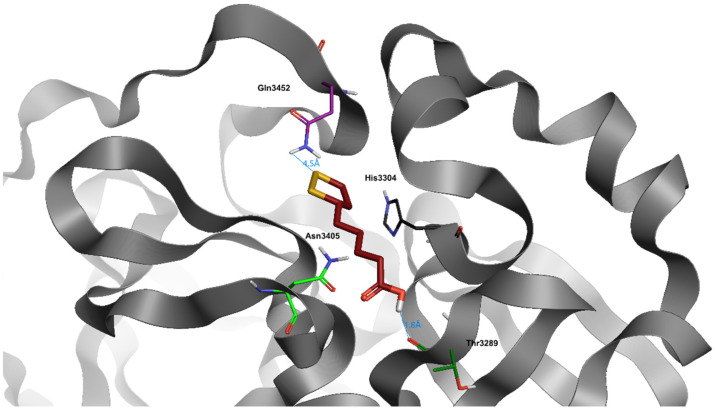
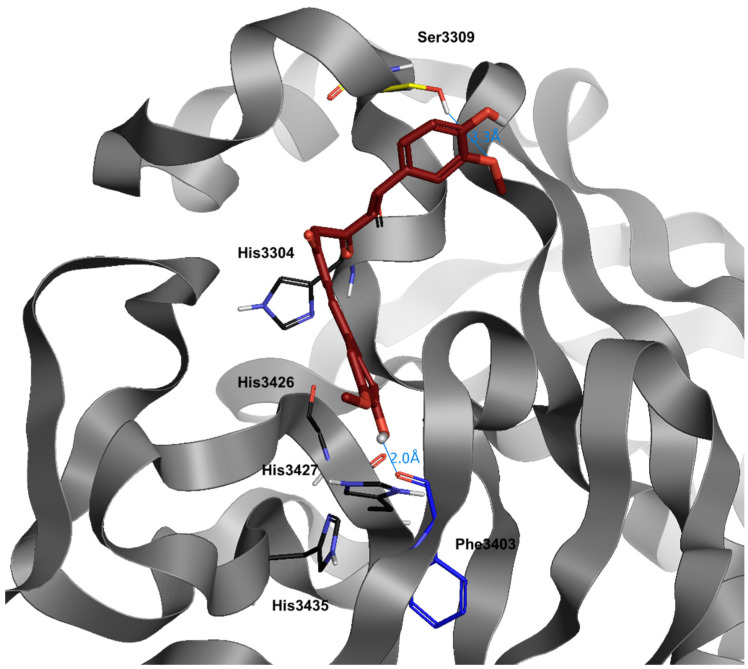
Figure 1.
Docking position of α-lipoic acid (dark red) in the main protease of SARS-CoV-2 virus binding site. Water and hydrogen were omitted for clarity.
Alpha-lipoic acid shows an excellent dG (−8006) (Table 3). Naturally, the small steric footprint of this compound allows it to enter into the bonding site with ease.
It also establishes stable hydrogen bridges throughout the dynamics time with Thr3289, through the carboxylic group of the molecule, and with Gln3452 via an NH-S bond.
6. Quercetin
Quercetin is the most abundant dietary flavonoid, especially enriched in onions, cranberry, blueberry, tea, and apples (Figure 2) [58]. Many polyphenol compounds, including quercetin, are potent iron chelators. In common with most polyphenols, quercetin is found almost exclusively in foods as glycoside conjugates but can be converted rapidly into the aglycone in the intestinal lumen via the actions of glycosidases [59]. Quercetin is able to exert a significant decrease of non-heme iron absorption in duodenum through different mechanisms: increasing iron uptake and retention by the duodenal mucosa; reducing tissue iron pool and expression of non-heme iron transporters in enterocytes; increasing hepcidin expression, leading to iron depletion [60]. Oral administration of quercetin, in an in vivo model of rat, led to the downregulation of divalent metal transporter-1 (DMT1) and FPN mRNA levels; conversely, following the same experiment in an in vitro model with Caco-2 cells, quercetin did not affect DMT1 and FPN mRNA or protein expression [61]. It has been shown that treatment with quercetin in an in vivo model of iron overload did not demonstrate significant differences compared to treatment with Deferoxamine, leading to the reduction of serum and tissue iron and enhancing the inflammatory condition by reducing IL-6 and increasing IL-10 expression [62]. A wide range of studies reported the potent ability of quercetin as protease inhibitor when used against virus infection: Bachmetov et al. demonstrated a direct inhibitory effect of quercetin on HCV NS3 serine protease catalytic activity, while Yao et al. showed that quercetin potently inhibited Enterovirus 71 (EV71) 3C-protease activity, blocking EV71 replication [63,64]. The same anti-replication activity has been investigated in computational studies on Middle East respiratory syndrome-coronavirus (MERS-CoV) and SARS-CoV, where results proved a strong interaction between quercetin and the catalytic site of viral 3C-like protease [65,66,67]. It has also been reported that co-administration of quercetin, as a promising inhibitor of crucial viral enzymes (reverse transcriptase, integrase and protease), and Vitamin C could be suggested for prophylaxis in a high-risk population and for the treatment of COVID-19 patients as support therapy: the synergistic antiviral action could be due to the overlap of multiple properties of the different compounds, such as antioxidant and immunomodulatory properties, and to the capability of ascorbate to recycle quercetin, increasing its efficacy [68].
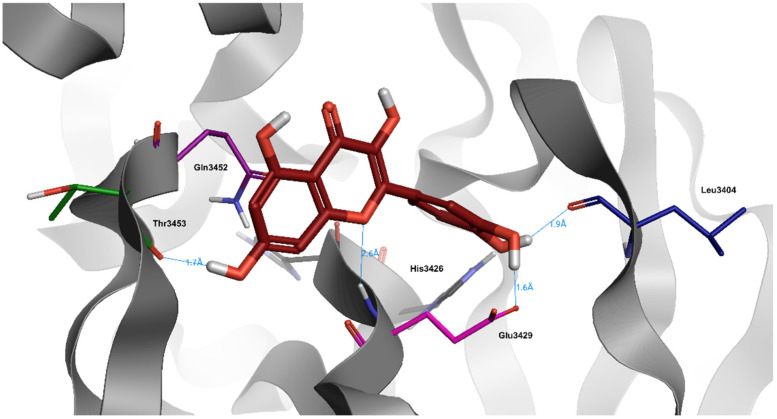
Figure 2.
Docking position of quercetin (dark red) in the main protease of SARS-CoV-2 virus binding site. Water and hydrogen were omitted for clarity.
Quercetin has a very particular structure with a high number of phenolic groups, and it is capable of generating hydrogen bonds with Glu3429, His3426, and Leu3404, also proven by its docking score (−8.0) (Table 3). The tri-hydroxy-chromium-4-onic portion is always water exposed throughout the dynamics, probably due to its extensive positive charge.
7. Caffeic Acid
Caffeic acid (CA) is a phenolic compound produced by the secondary metabolism of plants and is the major hydroxycinnamic acid present in the human diet (Figure 3). Several studies have determined the ability of CA and its metabolites to bind metal ions, and iron in particular, and to affect the redox reactions mediated by these metals, such as Fenton reaction [69,70,71]. Moreover, antiviral activity of CA and related compounds with the caffeoyl moiety, such as rosmarinic acid, has been reported. Although the mechanism of action has not yet been determined, evidence suggests that iron chelators may target the extracellular attachment between the virion glycoprotein B and the heparan sulfate proteoglycans on the cell surface. The viruses most affected by the CA-iron complexes, that can utilize heparan sulfate proteoglycans for cellular attachment, are herpes simplex viruses (HSV1 and HVS2), influenza A and human immunodeficiency virus (HIV) [72]. It has been showed that the activity of CA on HIV is not related only to iron complexes formation: Wang et al. demonstrated that CA derivatives possess inhibitory activities towards HIV proteases, in addition to the known inhibitory activity on HIV integrase, suggesting the use of CA as lead compound to develop new potential anti-HIV drugs [73]. The inhibitory effect of CA phenethyl ester derivatives on viral protease have also been studied on HCV, where CA compounds showed the capability to decrease NS3 protease expression, leading to the reduction of virus replication [74]. Recent papers reported the noteworthy inhibition on the main SARS-CoV-2 protease Mpro exerted by CA derivatives: both studies showed that CA derivatives bind to the substrate-binding pocket of SARS-CoV-2 Mpro possessing more efficacy and binding energies than nelfinavir, an already claimed N3 protease inhibitor [75,76].
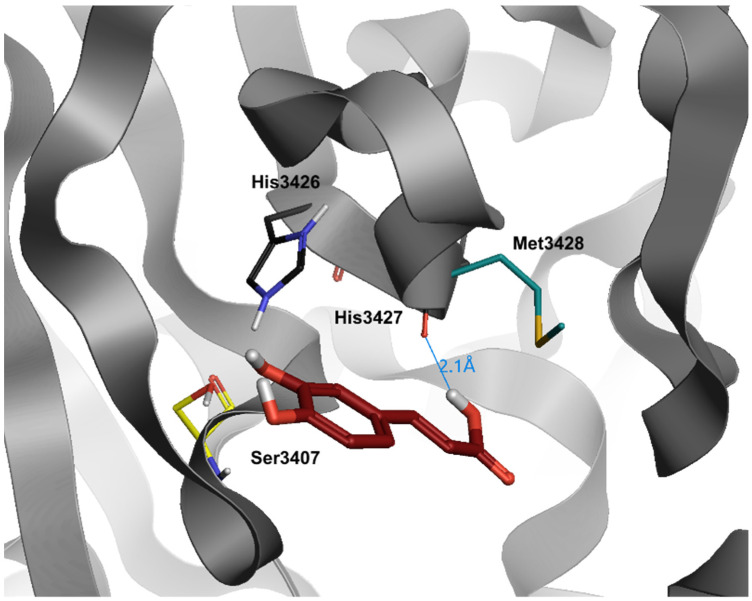
Figure 3.
Docking position of caffeic acid (dark red) in the main protease of SARS-CoV-2 virus binding site. Water and hydrogen were omitted for clarity.
Partially similar to quercetin, but simplified in the structure, caffeic acid maintains the possibility to enter deepest in the bond pocket, but, as opposed to the previous molecule, it loses the ability to establish a high number of hydrogen bonds (dG = −6.3) (Table 3). In fact, the only hydrogen bond occurs with the carboxylic portion towards the His3427 in its portion of the backbone. In addition, the layout of caffeic acid, during dynamics simulations, is more changeable compared to other compounds.
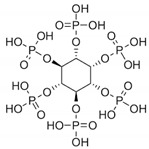
8. Phytic Acid
Phytic acid (myo-inositol hexaphosphate, IP6) has been recognized as a potent antioxidant and inhibitor of iron-catalyzed hydroxyl radical formation under in vitro and in vivo conditions (Figure 4). IP6, a common content of cereals and legumes, has been generally considered as an antinutrient because of its ability to chelate divalent minerals and reduce their absorption and is also considered as beneficial because of the same property [77,78]. Phytic acid was shown to inhibit radical OH formation and decrease lipid peroxidation catalyzed by iron and ascorbic acid in human erythrocytes [79]. Xu et al. reported that IP6, in a cell model of Parkinson’s disease, protected dopaminergic neurons against 1-methyl-4-phenylpyridinium (MPP+) induced apoptosis, in the presence of an iron-excess condition [80]. IP6 was also tested to ameliorate the pulmonary inflammation and fibrosis raised after intratracheal instillation of asbestos in rats: since iron-dependent enzymes are necessary for collagen secretion, such as prolyl hydroxylase and lysine hydroxylase, IP6 showed the important ability, as iron chelator, to control the fibrosis raised during pulmonary toxicity. Furthermore, IP6 was suggested to limit lymphocyte functions that contribute to pulmonary fibrosis [81]. Other studies demonstrated the antioxidant activity of IP6 as iron chelator in the inhibition of lipid peroxidation. IP6 was capable of inhibiting linoleic acid autoxidation and Fe2+/ascorbate-induced peroxidation, as well as Fe2+/ascorbate-induced lipid peroxidation in Caco-2 cells [82]. Moreover, Miyamoto et al. demonstrated the strong iron ion-chelating ability both of IP6 and its hydrolysis products (IP2, IP3, IP4, and IP5), able to prevent iron ion-induced lipid peroxidation. Results showed that subproducts of IP6, containing three or more phosphate groups, maintained the ability to inhibit lipid peroxidation, although their effectiveness decreased with dephosphorylation [83].
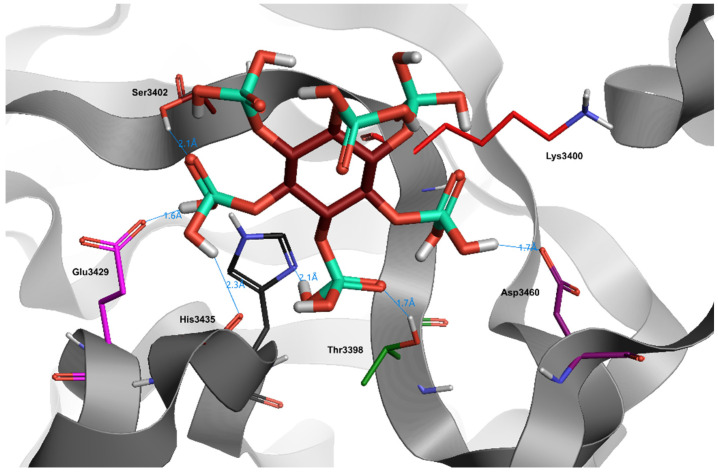
Figure 4.
Docking position of phytic acid (dark red) in the main protease of SARS-CoV-2 virus binding site. Water and hydrogen were omitted for clarity.
Despite the numerous phosphate groups and the consequent high total binding capacity, the sterically complex structure of Phytic Acid impedes a facilitated insertion into the receptor pocket, proved by a low docking score (−1.5) (Table 3). This result is justified by the excessive water exposure of the molecule. In fact, although Phytic Acid is capable of forming hydrogen bonds with a single aminoacidic binding site (Glu3429) and with other external sites (His3435, Tyr3398, Asp3460, Ser3402), the obtained bonds result unstable, due to the interference given by the surrounding waters.
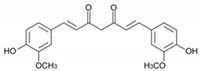
9. Curcumin
Curcumin (diferuloylmethane, or (E, E)-1,7-bis (4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) is a natural yellow colored product extracted from the Indian herb turmeric (Figure 5) [84]. A wide range of beneficial pharmacological effects has been proved for curcumin, including anti-inflammatory, antioxidant, antiviral, and antitumorigenic effects [85,86]. Curcumin has been reported to modulate proteins of iron metabolism in cells and in tissues, confirming the properties of an iron chelator. Because of its polyphenol structure, curcumin forms complexes with a number of different metal ions and especially with iron [87]. By inhibiting the Fenton reaction and other iron-catalyzed pathways of oxidative stress, curcumin is able to act as a chemopreventive agent, reducing oxidative injury to critical cellular targets, including DNA, lipids, and protein [88]. Jiao et al. demonstrated how curcumin affects the iron homeostasis, using an in vivo model of mice with low levels of body iron, describing different ways to cause iron depletion. Curcumin caused a dramatic reduction on hematological parameters of iron metabolism, such as hemoglobin, hematocrit, serum iron, and transferrin saturation. Moreover, curcumin positively affected the activity of iron regulatory proteins (IRPs); under iron-deficient conditions, these proteins are activated, leading to translational ferritin repression. Conversely, under iron overload conditions, IRPs are inactivated, thereby increasing the translation of ferritin mRNA [89]. In mice that had received the combination of high dietary iron and curcumin, IRPs activity was reported as significantly increased [90]. It has been reported that a high dose of curcumin can upregulate hepcidin and its regulators, such as bone morphogenic protein (BMP-6) Sekelsky Mothers Against DPP (SMAD) and transferrin receptor 2 (TfR2), in a mouse model of aplastic anemia with iron overload. The treatment with curcumin protected hematopoiesis from immune and iron overload-induced apoptosis, exerting an iron chelation effect in vivo more effective than Deferoxamine [91]. Since 1990s, numerous studies have focused on the antiviral properties of curcumin, investigating its activity as protease inhibitor and subsequently finding notable results on HIV protease inhibition and the collateral decrease of HIV replication [92,93,94]. Other works performed on flaviviruses (Dengue virus and Zika virus) found an allosteric inhibition of NS2B-NS3 proteases, exerted by curcumin by its binding to a cavity with no overlap with the active site, suggesting the use of curcumin as lead compound to design new small molecule allosteric inhibitors [95]. According to the study performed on the anti-SARS-CoV activity of a wide range of phytocompounds, Wen et al. demonstrated a significant inhibitory effect of curcumin on SARS-CoV 3CL protease activity, which is essential for virus replication, providing promising evidence for curcumin as a potential anti-SARS-CoV agent [96,97]. For this purpose, in recent years, several molecular docking studies have been performed that suggest curcumin would be effective at inhibiting SARS-CoV-2 replication through a range of ways, including the inhibition of the main viral protease [98,99,100,101]. Data obtained suggested that the chemical derivatives of curcumin could present a significant activity against COVID-19 disease by inhibiting the SARS CoV-2 main protease enzyme [99,101,102,103].
Figure 5.
Docking position of curcumin (dark red) in the main protease of SARS-CoV-2 virus binding site. Water and hydrogen were omitted for clarity.
Curcumin has a great bonding capacity in the pocket of the viral protease: its high rotational capacity structure allows it to adapt to the receptor pocket in an optimal way, as demonstrated by its dG (−8.1) (Table 3). Curcumin’s best docking positions are achieved with Ser3309, due to the methoxylic moiety (in the apical portion of the protease) and with Phe3403, due to the opposite phenolic moiety.
Table 1.
Effect of natural products on COVID-19 related diseases.
| Natural Product | Effect | Reference |
|---|---|---|
| Lactoferrin | Reduction of oxidative stress and inflammation through iron chelation properties |
[17] |
| Glycyrrhizin | Inhibition of SARS-CoV-2 protease Mpro | [26] |
| Tryptanthrine | Inhibition of SARS-CoV-2 protease Mpro | [26] |
| Rhein | Inhibition of SARS-CoV-2 protease Mpro | [26] |
| Berberin | Inhibition of SARS-CoV-2 protease Mpro | [26] |
| Quercetin | Inhibition of SARS-CoV-2 protease 3CLpro | [66] |
| Caffeic acid | Inhibition of SARS-CoV-2 protease Mpro | [76] |
| Curcumin | Inhibition of SARS-CoV-2 protease 3CLpro | [97] |
Table 2.
Iron involvement in COVID-19 disease.
| Type of Study | Iron Metabolism | Reference |
|---|---|---|
| Clinical | Anemic patients had increased levels of inflammation markers; Hyperferritinemia; Correlation between increased ferritin levels and cytokine mRNA over-expression |
[33] |
| Meta-analysis | Elevated levels of serum ferritin have been found in non-survivors compared with survivors |
[34] |
| Meta-analysis | The ferritin level was significantly increased in severe patients compared with non-severe patients |
[35] |
| Clinical | High ferritin levels | [36] |
| Clinical | High ferritin levels | [37] |
| Meta-analysis | SARS-CoV-2 attacks one of the beta chains of the hemoglobin, causing dissociation of iron from the porphyrins and its release into the circulation |
[38] |
| Clinical | Iron overload and Hepcidin overexpression | [40] |
| Computational | Similarity between hepcidin and the coronavirus spike glycoprotein | [42] |
Table 3.
Natural products and their structures with docking score (dG).
| Name | Structure | dG |
|---|---|---|
| α-Lipoic Acid |
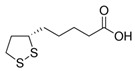
|
−8.0 |
| Quercetin |
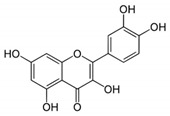
|
−8.0 |
| Caffeic Acid |
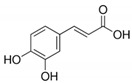
|
−6.3 |
| Phytic Acid |
|
−1.5 |
| Curcumin |
|
−8.1 |
10. Conclusions
During the COVID-19 disease, increased iron levels in the body generate reactive oxygen species which cause oxidative stress and damage to the lungs, leading to subsequent lung fibrosis and the decline in the lung function [7]. Evidence shows that iron overload increases viral replication, playing an important role in the severity of the infection [27]. Through their iron chelation effect, substances like Deferoxamine and Lactoferrin were shown to reduce iron availability in the serum and body tissue, preventing lung injury and fibrosis following COVID-19 infection [11,12]. In parallel, recent results nominate compounds with a potential as protease inhibition as new candidates to face SARS-CoV-2 infection [104,105]. In our review, we exposed and summarized the effects of some important natural products with an amply demonstrated activity as iron chelators and protease inhibitors, suggesting their involvement for new possible therapeutical strategies towards the pathological contexts related to COVID 19 infection.
Acknowledgments
Giuseppe Carota performed this work within the project PON “Ricerca ed Innovazione 2014–2020 dd 2008 del 22-10-2019”.
Author Contributions
Conceptualization, D.T., G.L.V., S.R., G.C., F.P. and A.N.; validation, G.L.V.; writing—original draft preparation, D.T., G.L.V., S.R., G.C., F.P., A.N.; supervision, G.L.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Weiss S.R., Navas-Martin S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol. Mol. Biol. Rev. 2005;69:635–664. doi: 10.1128/MMBR.69.4.635-664.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cantuti-Castelvetri L., Ojha R., Pedro L.D., Djannatian M., Franz J., Kuivanen S., van der Meer F., Kallio K., Kaya T., Anastasina M., et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 2020;370:856–860. doi: 10.1126/science.abd2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Working Group of Novel Coronavirus P.U.M.C.H. Diagnosis and clinical management of 2019 novel coronavirus infection: An operational recommendation of Peking Union Medical College Hospital (V2.0) Zhonghua Nei Ke Za Zhi. 2020;59:186–188. doi: 10.3760/cma.j.issn.0578-1426.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Ali M.J., Hanif M., Haider M.A., Ahmed M.U., Sundas F., Hirani A., Khan I.A., Anis K., Karim A.H. Treatment Options for COVID-19: A Review. Front. Med. 2020;7:480. doi: 10.3389/fmed.2020.00480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cavezzi A., Troiani E., Corrao S. COVID-19: Hemoglobin, iron, and hypoxia beyond inflammation. A narrative review. Clin. Pract. 2020;10:1271. doi: 10.4081/cp.2020.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ali M.K., Kim R.Y., Brown A.C., Donovan C., Vanka K.S., Mayall J.R., Liu G., Pillar A.L., Jones-Freeman B., Xenaki D., et al. Critical role for iron accumulation in the pathogenesis of fibrotic lung disease. J. Pathol. 2020;251:49–62. doi: 10.1002/path.5401. [DOI] [PubMed] [Google Scholar]
- 8.Khiroya H., Turner A.M. The role of iron in pulmonary pathology. Multidiscip. Respir. Med. 2015;10:34. doi: 10.1186/s40248-015-0031-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghio A.J., Carter J.D., Richards J.H., Richer L.D., Grissom C.K., Elstad M.R. Iron and iron-related proteins in the lower respiratory tract of patients with acute respiratory distress syndrome. Crit. Care Med. 2003;31:395–400. doi: 10.1097/01.CCM.0000050284.35609.97. [DOI] [PubMed] [Google Scholar]
- 10.Cappellini M.D. Exjade(R) (deferasirox, ICL670) in the treatment of chronic iron overload associated with blood transfusion. Clin. Risk. Manag. 2007;3:291–299. doi: 10.2147/tcrm.2007.3.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abobaker A. Can iron chelation as an adjunct treatment of COVID-19 improve the clinical outcome? Eur. J. Clin. Pharm. 2020;76:1619–1620. doi: 10.1007/s00228-020-02942-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang R., Ng T.B., Sun W.Z. Lactoferrin as potential preventative and adjunct treatment for COVID-19. Int. J. Antimicrob. Agents. 2020;56:106118. doi: 10.1016/j.ijantimicag.2020.106118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mann J.K., Ndung’u T. The potential of lactoferrin, ovotransferrin and lysozyme as antiviral and immune-modulating agents in COVID-19. Future Virol. 2020;15:609–624. doi: 10.2217/fvl-2020-0170. [DOI] [Google Scholar]
- 14.Rosa L., Cutone A., Lepanto M.S., Paesano R., Valenti P. Lactoferrin: A Natural Glycoprotein Involved in Iron and Inflammatory Homeostasis. Int. J. Mol. Sci. 2017;18:1985. doi: 10.3390/ijms18091985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campione E., Cosio T., Rosa L., Lanna C., Di Girolamo S., Gaziano R., Valenti P., Bianchi L. Lactoferrin as Protective Natural Barrier of Respiratory and Intestinal Mucosa against Coronavirus Infection and Inflammation. Int. J. Mol. Sci. 2020;21:4903. doi: 10.3390/ijms21144903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parisi G.F., Carota G., Castruccio Castracani C., Spampinato M., Manti S., Papale M., Di Rosa M., Barbagallo I., Leonardi S. Nutraceuticals in the Prevention of Viral Infections, including COVID-19, among the Pediatric Population: A Review of the Literature. Int. J. Mol. Sci. 2021;22:2465. doi: 10.3390/ijms22052465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Habib H.M., Ibrahim S., Zaim A., Ibrahim W.H. The role of iron in the pathogenesis of COVID-19 and possible treatment with lactoferrin and other iron chelators. Biomed. Pharm. 2021;136:111228. doi: 10.1016/j.biopha.2021.111228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jedinak A., Maliar T., Grancai D., Nagy M. Inhibition activities of natural products on serine proteases. Phytother. Res. 2006;20:214–217. doi: 10.1002/ptr.1836. [DOI] [PubMed] [Google Scholar]
- 19.Kido H., Niwa Y., Beppu Y., Towatari T. Cellular proteases involved in the pathogenicity of enveloped animal viruses, human immunodeficiency virus, influenza virus A and Sendai virus. Adv. Enzym. Regul. 1996;36:325–347. doi: 10.1016/0065-2571(95)00016-X. [DOI] [PubMed] [Google Scholar]
- 20.Wruck W., Adjaye J. SARS-CoV-2 receptor ACE2 is co-expressed with genes related to transmembrane serine proteases, viral entry, immunity and cellular stress. Sci. Rep. 2020;10:21415. doi: 10.1038/s41598-020-78402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chandwani A., Shuter J. Lopinavir/ritonavir in the treatment of HIV-1 infection: A review. Clin. Risk Manag. 2008;4:1023–1033. doi: 10.2147/tcrm.s3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patick A.K., Potts K.E. Protease inhibitors as antiviral agents. Clin. Microbiol. Rev. 1998;11:614–627. doi: 10.1128/CMR.11.4.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson J., Schiffer C., Lee S.K., Swanstrom R. Viral protease inhibitors. Handb. Exp. Pharm. 2009;189:85–110. doi: 10.1007/978-3-540-79086-0_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV) Biosci. Trends. 2020;14:69–71. doi: 10.5582/bst.2020.01020. [DOI] [PubMed] [Google Scholar]
- 25.Sagawa T., Inoue K.I., Takano H. Use of protease inhibitors for the prevention of COVID-19. Prev. Med. 2020;141:106280. doi: 10.1016/j.ypmed.2020.106280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Narkhede R.R., Pise A.V., Cheke R.S., Shinde S.D. Recognition of Natural Products as Potential Inhibitors of COVID-19 Main Protease (Mpro): In-Silico Evidences. Nat. Prod. Bioprospect. 2020;10:297–306. doi: 10.1007/s13659-020-00253-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edeas M., Saleh J., Peyssonnaux C. Iron: Innocent bystander or vicious culprit in COVID-19 pathogenesis? Int. J. Infect. Dis. 2020;97:303–305. doi: 10.1016/j.ijid.2020.05.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khodour Y., Kaguni L.S., Stiban J. Iron-sulfur clusters in nucleic acid metabolism: Varying roles of ancient cofactors. Enzymes. 2019;45:225–256. doi: 10.1016/bs.enz.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Liu W., Zhang S., Nekhai S., Liu S. Depriving Iron Supply to the Virus Represents a Promising Adjuvant Therapeutic against Viral Survival. Curr. Clin. Microbiol. Rep. 2020;7:1–7. doi: 10.1007/s40588-020-00140-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pretorius E., Kell D.B. Diagnostic morphology: Biophysical indicators for iron-driven inflammatory diseases. Integr. Biol. 2014;6:486–510. doi: 10.1039/C4IB00025K. [DOI] [PubMed] [Google Scholar]
- 31.Perricone C., Bartoloni E., Bursi R., Cafaro G., Guidelli G.M., Shoenfeld Y., Gerli R. COVID-19 as part of the hyperferritinemic syndromes: The role of iron depletion therapy. Immunol. Res. 2020;68:213–224. doi: 10.1007/s12026-020-09145-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garrick M.D., Ghio A.J. Iron chelation may harm patients with COVID-19. Eur. J. Clin. Pharm. 2021;77:265–266. doi: 10.1007/s00228-020-02987-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sonnweber T., Boehm A., Sahanic S., Pizzini A., Aichner M., Sonnweber B., Kurz K., Koppelstatter S., Haschka D., Petzer V., et al. Persisting alterations of iron homeostasis in COVID-19 are associated with non-resolving lung pathologies and poor patients’ performance: A prospective observational cohort study. Respir. Res. 2020;21:276. doi: 10.1186/s12931-020-01546-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taneri P.E., Gomez-Ochoa S.A., Llanaj E., Raguindin P.F., Rojas L.Z., Roa-Diaz Z.M., Salvador D., Jr., Groothof D., Minder B., Kopp-Heim D., et al. Anemia and iron metabolism in COVID-19: A systematic review and meta-analysis. Eur. J. Epidemiol. 2020;35:763–773. doi: 10.1007/s10654-020-00678-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng L., Li H., Li L., Liu C., Yan S., Chen H., Li Y. Ferritin in the coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. J. Clin. Lab. Anal. 2020;34:e23618. doi: 10.1002/jcla.23618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu W.L.H. COVID-19: Attacks the 1-Beta Chain of Hemoglobin and Captures the Porphyrin to Inhibit Heme Metabolism. ChemRxiv. 2020 doi: 10.26434/chemrxiv.11938173.v8. [DOI] [Google Scholar]
- 39.Abobaker A. Reply: Iron chelation may harm patients with COVID-19. Eur. J. Clin. Pharm. 2021;77:267–268. doi: 10.1007/s00228-020-02988-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Banchini F., Vallisa D., Maniscalco P., Capelli P. Iron overload and Hepcidin overexpression could play a key role in COVID infection, and may explain vulnerability in elderly, diabetics, and obese patients. Acta Biomed. 2020;91:e2020013. doi: 10.23750/abm.v91i3.9826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Daher R., Manceau H., Karim Z. Iron metabolism and the role of the iron-regulating hormone hepcidin in health and disease. Presse Med. 2017;46:e272–e278. doi: 10.1016/j.lpm.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 42.Ehsani S. COVID-19 and iron dysregulation: Distant sequence similarity between hepcidin and the novel coronavirus spike glycoprotein. Biol. Direct. 2020;15:19. doi: 10.1186/s13062-020-00275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–280.e278. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ragia G., Manolopoulos V.G. Inhibition of SARS-CoV-2 entry through the ACE2/TMPRSS2 pathway: A promising approach for uncovering early COVID-19 drug therapies. Eur. J. Clin. Pharm. 2020;76:1623–1630. doi: 10.1007/s00228-020-02963-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bhatnagar T., Murhekar M.V., Soneja M., Gupta N., Giri S., Wig N., Gangakhedkar R. Lopinavir/ritonavir combination therapy amongst symptomatic coronavirus disease 2019 patients in India: Protocol for restricted public health emergency use. Indian J. Med. Res. 2020;151:184–189. doi: 10.4103/ijmr.IJMR_502_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song Y., Peng W., Tang D., Dai Y. Protease Inhibitor Use in COVID-19. Sn Compr. Clin. Med. 2020;2:1–8. doi: 10.1007/s42399-020-00448-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Doi K., Ikeda M., Hayase N., Moriya K., Morimura N., Group C.-U.S. Nafamostat mesylate treatment in combination with favipiravir for patients critically ill with Covid-19: A case series. Crit. Care. 2020;24:392. doi: 10.1186/s13054-020-03078-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamamoto M., Kiso M., Sakai-Tagawa Y., Iwatsuki-Horimoto K., Imai M., Takeda M., Kinoshita N., Ohmagari N., Gohda J., Semba K., et al. The Anticoagulant Nafamostat Potently Inhibits SARS-CoV-2 S Protein-Mediated Fusion in a Cell Fusion Assay System and Viral Infection In Vitro in a Cell-Type-Dependent Manner. Viruses. 2020;12:629. doi: 10.3390/v12060629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoffmann M., Schroeder S., Kleine-Weber H., Muller M.A., Drosten C., Pohlmann S. Nafamostat Mesylate Blocks Activation of SARS-CoV-2: New Treatment Option for COVID-19. Antimicrob. Agents Chemother. 2020;64 doi: 10.1128/AAC.00754-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baughn L.B., Sharma N., Elhaik E., Sekulic A., Bryce A.H., Fonseca R. Targeting TMPRSS2 in SARS-CoV-2 Infection. Mayo Clin. Proc. 2020;95:1989–1999. doi: 10.1016/j.mayocp.2020.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jin Z., Du X., Xu Y., Deng Y., Liu M., Zhao Y., Zhang B., Li X., Zhang L., Peng C., et al. Structure of M(pro) from SARS-CoV-2 and discovery of its inhibitors. Nature. 2020;582:289–293. doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- 52.Cheeseright T., Mackey M., Rose S., Vinter A. Molecular field extrema as descriptors of biological activity: Definition and validation. J. Chem. Inf. Model. 2006;46:665–676. doi: 10.1021/ci050357s. [DOI] [PubMed] [Google Scholar]
- 53.Biewenga G.P., Haenen G.R., Bast A. The pharmacology of the antioxidant lipoic acid. Gen. Pharm. 1997;29:315–331. doi: 10.1016/S0306-3623(96)00474-0. [DOI] [PubMed] [Google Scholar]
- 54.Camiolo G., Tibullo D., Giallongo C., Romano A., Parrinello N.L., Musumeci G., Di Rosa M., Vicario N., Brundo M.V., Amenta F., et al. alpha-Lipoic Acid Reduces Iron-induced Toxicity and Oxidative Stress in a Model of Iron Overload. Int. J. Mol. Sci. 2019;20:609. doi: 10.3390/ijms20030609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rochette L., Ghibu S., Richard C., Zeller M., Cottin Y., Vergely C. Direct and indirect antioxidant properties of alpha-lipoic acid and therapeutic potential. Mol. Nutr. Food Res. 2013;57:114–125. doi: 10.1002/mnfr.201200608. [DOI] [PubMed] [Google Scholar]
- 56.Zhao L., Wang C., Song D., Li Y., Song Y., Su G., Dunaief J.L. Systemic administration of the antioxidant/iron chelator alpha-lipoic acid protects against light-induced photoreceptor degeneration in the mouse retina. Investig. Ophthalmol. Vis. Sci. 2014;55:5979–5988. doi: 10.1167/iovs.14-15025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tai S., Zheng Q., Zhai S., Cai T., Xu L., Yang L., Jiao L., Zhang C. Alpha-Lipoic Acid Mediates Clearance of Iron Accumulation by Regulating Iron Metabolism in a Parkinson’s Disease Model Induced by 6-OHDA. Front. Neurosci. 2020;14:612. doi: 10.3389/fnins.2020.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leopoldini M., Russo N., Chiodo S., Toscano M. Iron chelation by the powerful antioxidant flavonoid quercetin. J. Agric. Food Chem. 2006;54:6343–6351. doi: 10.1021/jf060986h. [DOI] [PubMed] [Google Scholar]
- 59.Day A.J., Gee J.M., DuPont M.S., Johnson I.T., Williamson G. Absorption of quercetin-3-glucoside and quercetin-4′-glucoside in the rat small intestine: The role of lactase phlorizin hydrolase and the sodium-dependent glucose transporter. Biochem. Pharm. 2003;65:1199–1206. doi: 10.1016/S0006-2952(03)00039-X. [DOI] [PubMed] [Google Scholar]
- 60.Lesjak M., Balesaria S., Skinner V., Debnam E.S., Srai S.K.S. Quercetin inhibits intestinal non-haem iron absorption by regulating iron metabolism genes in the tissues. Eur. J. Nutr. 2019;58:743–753. doi: 10.1007/s00394-018-1680-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lesjak M., Hoque R., Balesaria S., Skinner V., Debnam E.S., Srai S.K., Sharp P.A. Quercetin inhibits intestinal iron absorption and ferroportin transporter expression in vivo and in vitro. PLoS ONE. 2014;9:e102900. doi: 10.1371/journal.pone.0102900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.El-Sheikh A.A., Ameen S.H., AbdEl-Fatah S.S. Ameliorating Iron Overload in Intestinal Tissue of Adult Male Rats: Quercetin vs Deferoxamine. J. Toxicol. 2018;2018:8023840. doi: 10.1155/2018/8023840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bachmetov L., Gal-Tanamy M., Shapira A., Vorobeychik M., Giterman-Galam T., Sathiyamoorthy P., Golan-Goldhirsh A., Benhar I., Tur-Kaspa R., Zemel R. Suppression of hepatitis C virus by the flavonoid quercetin is mediated by inhibition of NS3 protease activity. J. Viral. Hepat. 2012;19:e81–e88. doi: 10.1111/j.1365-2893.2011.01507.x. [DOI] [PubMed] [Google Scholar]
- 64.Yao C., Xi C., Hu K., Gao W., Cai X., Qin J., Lv S., Du C., Wei Y. Inhibition of enterovirus 71 replication and viral 3C protease by quercetin. Virol. J. 2018;15:116. doi: 10.1186/s12985-018-1023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jo S., Kim H., Kim S., Shin D.H., Kim M.S. Characteristics of flavonoids as potent MERS-CoV 3C-like protease inhibitors. Chem. Biol. Drug Des. 2019;94:2023–2030. doi: 10.1111/cbdd.13604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jo S., Kim S., Shin D.H., Kim M.S. Inhibition of SARS-CoV 3CL protease by flavonoids. J. Enzym. Inhib. Med. Chem. 2020;35:145–151. doi: 10.1080/14756366.2019.1690480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mehrbod P., Hudy D., Shyntum D., Markowski J., Los M.J., Ghavami S. Quercetin as a Natural Therapeutic Candidate for the Treatment of Influenza Virus. Biomolecules. 2020;11:10. doi: 10.3390/biom11010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Colunga Biancatelli R.M.L., Berrill M., Catravas J.D., Marik P.E. Quercetin and Vitamin C: An Experimental, Synergistic Therapy for the Prevention and Treatment of SARS-CoV-2 Related Disease (COVID-19) Front. Immunol. 2020;11:1451. doi: 10.3389/fimmu.2020.01451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hynes M.J., O’Coinceanainn M. The kinetics and mechanisms of reactions of iron(III) with caffeic acid, chlorogenic acid, sinapic acid, ferulic acid and naringin. J. Inorg. Biochem. 2004;98:1457–1464. doi: 10.1016/j.jinorgbio.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 70.Yamanaka N., Oda O., Nagao S. Prooxidant activity of caffeic acid, dietary non-flavonoid phenolic acid, on Cu2+-induced low density lipoprotein oxidation. FEBS Lett. 1997;405:186–190. doi: 10.1016/S0014-5793(97)00185-3. [DOI] [PubMed] [Google Scholar]
- 71.Genaro-Mattos T.C., Mauricio A.Q., Rettori D., Alonso A., Hermes-Lima M. Antioxidant Activity of Caffeic Acid against Iron-Induced Free Radical Generation—A Chemical Approach. PLoS ONE. 2015;10:e0129963. doi: 10.1371/journal.pone.0129963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Langland J., Jacobs B., Wagner C.E., Ruiz G., Cahill T.M. Antiviral activity of metal chelates of caffeic acid and similar compounds towards herpes simplex, VSV-Ebola pseudotyped and vaccinia viruses. Antivir. Res. 2018;160:143–150. doi: 10.1016/j.antiviral.2018.10.021. [DOI] [PubMed] [Google Scholar]
- 73.Wang X., Wei Y., Tian W.Y., Sakharkar M.K., Liu Q., Yang X., Zhou Y.Z., Mou C.L., Cai G.L., Yang J. Characterization of Nine Compounds Isolated from the Acid Hydrolysate of Lonicera fulvotomentosa Hsu et S.C. Cheng and Evaluation of Their In Vitro Activity towards HIV Protease. Molecules. 2019;24:4526. doi: 10.3390/molecules24244526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shen H., Yamashita A., Nakakoshi M., Yokoe H., Sudo M., Kasai H., Tanaka T., Fujimoto Y., Ikeda M., Kato N., et al. Inhibitory effects of caffeic acid phenethyl ester derivatives on replication of hepatitis C virus. PLoS ONE. 2013;8:e82299. doi: 10.1371/journal.pone.0082299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kumar V., Dhanjal J.K., Kaul S.C., Wadhwa R., Sundar D. Withanone and caffeic acid phenethyl ester are predicted to interact with main protease (M(pro)) of SARS-CoV-2 and inhibit its activity. J. Biomol. Struct. Dyn. 2020;39:1–13. doi: 10.1080/07391102.2020.1772108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Adem S., Eyupoglu V., Sarfraz I., Rasul A., Zahoor A.F., Ali M., Abdalla M., Ibrahim I.M., Elfiky A.A. Caffeic acid derivatives (CAFDs) as inhibitors of SARS-CoV-2: CAFDs-based functional foods as a potential alternative approach to combat COVID-19. Phytomedicine. 2020:153310. doi: 10.1016/j.phymed.2020.153310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Petry N., Egli I., Zeder C., Walczyk T., Hurrell R. Polyphenols and phytic acid contribute to the low iron bioavailability from common beans in young women. J. Nutr. 2010;140:1977–1982. doi: 10.3945/jn.110.125369. [DOI] [PubMed] [Google Scholar]
- 78.Nielsen A.V., Tetens I., Meyer A.S. Potential of phytase-mediated iron release from cereal-based foods: A quantitative view. Nutrients. 2013;5:3074–3098. doi: 10.3390/nu5083074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Graf E., Empson K.L., Eaton J.W. Phytic acid. A natural antioxidant. J. Biol. Chem. 1987;262:11647–11650. doi: 10.1016/S0021-9258(18)60858-0. [DOI] [PubMed] [Google Scholar]
- 80.Xu Q., Kanthasamy A.G., Reddy M.B. Neuroprotective effect of the natural iron chelator, phytic acid in a cell culture model of Parkinson’s disease. Toxicology. 2008;245:101–108. doi: 10.1016/j.tox.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 81.Kamp D.W., Israbian V.A., Yeldandi A.V., Panos R.J., Graceffa P., Weitzman S.A. Phytic acid, an iron chelator, attenuates pulmonary inflammation and fibrosis in rats after intratracheal instillation of asbestos. Toxicol. Pathol. 1995;23:689–695. doi: 10.1177/019262339502300606. [DOI] [PubMed] [Google Scholar]
- 82.Zajdel A., Wilczok A., Weglarz L., Dzierzewicz Z. Phytic acid inhibits lipid peroxidation in vitro. BioMed Res. Int. 2013;2013:147307. doi: 10.1155/2013/147307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miyamoto S., Kuwata G., Imai M., Nagao A., Terao J. Protective effect of phytic acid hydrolysis products on iron-induced lipid peroxidation of liposomal membranes. Lipids. 2000;35:1411–1413. doi: 10.1007/s11745-000-0659-y. [DOI] [PubMed] [Google Scholar]
- 84.Messner D.J., Surrago C., Fiordalisi C., Chung W.Y., Kowdley K.V. Isolation and characterization of iron chelators from turmeric (Curcuma longa): Selective metal binding by curcuminoids. Biometals. 2017;30:699–708. doi: 10.1007/s10534-017-0038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ruby A.J., Kuttan G., Babu K.D., Rajasekharan K.N., Kuttan R. Anti-tumour and antioxidant activity of natural curcuminoids. Cancer Lett. 1995;94:79–83. doi: 10.1016/0304-3835(95)03827-J. [DOI] [PubMed] [Google Scholar]
- 86.Badria F.A., Ibrahim A.S., Badria A.F., Elmarakby A.A. Curcumin Attenuates Iron Accumulation and Oxidative Stress in the Liver and Spleen of Chronic Iron-Overloaded Rats. PLoS ONE. 2015;10:e0134156. doi: 10.1371/journal.pone.0134156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jiao Y., Wilkinson J.t., Christine Pietsch E., Buss J.L., Wang W., Planalp R., Torti F.M., Torti S.V. Iron chelation in the biological activity of curcumin. Free Radic. Biol. Med. 2006;40:1152–1160. doi: 10.1016/j.freeradbiomed.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 88.Rainey N.E., Moustapha A., Saric A., Nicolas G., Sureau F., Petit P.X. Iron chelation by curcumin suppresses both curcumin-induced autophagy and cell death together with iron overload neoplastic transformation. Cell Death Discov. 2019;5:150. doi: 10.1038/s41420-019-0234-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wallander M.L., Leibold E.A., Eisenstein R.S. Molecular control of vertebrate iron homeostasis by iron regulatory proteins. Biochim. Biophys. Acta. 2006;1763:668–689. doi: 10.1016/j.bbamcr.2006.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jiao Y., Wilkinson J.T., Di X., Wang W., Hatcher H., Kock N.D., D’Agostino R., Jr., Knovich M.A., Torti F.M., Torti S.V. Curcumin, a cancer chemopreventive and chemotherapeutic agent, is a biologically active iron chelator. Blood. 2009;113:462–469. doi: 10.1182/blood-2008-05-155952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dijiong W., Xiaowen W., Linlong X., Wenbin L., Huijin H., Baodong Y., Yuhong Z. Iron chelation effect of curcumin and baicalein on aplastic anemia mouse model with iron overload. Iran. J. Basic Med. Sci. 2019;22:660–668. doi: 10.22038/ijbms.2019.30840.7440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sui Z., Salto R., Li J., Craik C., Ortiz de Montellano P.R. Inhibition of the HIV-1 and HIV-2 proteases by curcumin and curcumin boron complexes. Bioorg. Med. Chem. 1993;1:415–422. doi: 10.1016/S0968-0896(00)82152-5. [DOI] [PubMed] [Google Scholar]
- 93.Prasad S., Tyagi A.K. Curcumin and its analogues: A potential natural compound against HIV infection and AIDS. Food Funct. 2015;6:3412–3419. doi: 10.1039/C5FO00485C. [DOI] [PubMed] [Google Scholar]
- 94.Vajragupta O., Boonchoong P., Morris G.M., Olson A.J. Active site binding modes of curcumin in HIV-1 protease and integrase. Bioorg. Med. Chem. Lett. 2005;15:3364–3368. doi: 10.1016/j.bmcl.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 95.Lim L., Dang M., Roy A., Kang J., Song J. Curcumin Allosterically Inhibits the Dengue NS2B-NS3 Protease by Disrupting Its Active Conformation. ACS Omega. 2020;5:25677–25686. doi: 10.1021/acsomega.0c00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wen C.C., Kuo Y.H., Jan J.T., Liang P.H., Wang S.Y., Liu H.G., Lee C.K., Chang S.T., Kuo C.J., Lee S.S., et al. Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus. J. Med. Chem. 2007;50:4087–4095. doi: 10.1021/jm070295s. [DOI] [PubMed] [Google Scholar]
- 97.Liu Z., Ying Y. The Inhibitory Effect of Curcumin on Virus-Induced Cytokine Storm and Its Potential Use in the Associated Severe Pneumonia. Front. Cell Dev. Biol. 2020;8:479. doi: 10.3389/fcell.2020.00479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Khaerunnisa S., Kurniawan H., Awaluddin R., Suhartati S., Soetjipto S. Potential Inhibitor of COVID-19 Main Protease (Mpro) from Several Medicinal Plant Compounds by Molecular Docking Study. Preprints. 2020 doi: 10.20944/preprints202003.0226.v1. [DOI] [Google Scholar]
- 99.Das S., Sarmah S., Lyndem S., Singha Roy A. An investigation into the identification of potential inhibitors of SARS-CoV-2 main protease using molecular docking study. J. Biomol. Struct. Dyn. 2020;39:3347–3357. doi: 10.1080/07391102.2020.1763201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jennings M.R., Parks R.J. Curcumin as an Antiviral Agent. Viruses. 2020;12:1242. doi: 10.3390/v12111242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rajagopal K., Varakumar P., Baliwada A., Byran G. Activity of phytochemical constituents of Curcuma longa (turmeric) and Andrographis paniculata against coronavirus (COVID-19): An in silico approach. Futur J. Pharm. Sci. 2020;6:104. doi: 10.1186/s43094-020-00126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zahedipour F., Hosseini S.A., Sathyapalan T., Majeed M., Jamialahmadi T., Al-Rasadi K., Banach M., Sahebkar A. Potential effects of curcumin in the treatment of COVID-19 infection. Phytother. Res. 2020;34:2911–2920. doi: 10.1002/ptr.6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Manoharan Y., Haridas V., Vasanthakumar K.C., Muthu S., Thavoorullah F.F., Shetty P. Curcumin: A Wonder Drug as a Preventive Measure for COVID19 Management. Indian J. Clin. Biochem. 2020;35:373–375. doi: 10.1007/s12291-020-00902-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McKee D.L., Sternberg A., Stange U., Laufer S., Naujokat C. Candidate drugs against SARS-CoV-2 and COVID-19. Pharm. Res. 2020;157:104859. doi: 10.1016/j.phrs.2020.104859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Keretsu S., Bhujbal S.P., Cho S.J. Rational approach toward COVID-19 main protease inhibitors via molecular docking, molecular dynamics simulation and free energy calculation. Sci. Rep. 2020;10:17716. doi: 10.1038/s41598-020-74468-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.