Abstract
Ambient air pollution impairs lung development in children, particularly in industrialized areas. The air quality in Zabrze, a city located in the Upper Silesian Industrial Region of Poland, is among the worst in Europe. We compared lung function and the frequency of respiratory or allergic symptoms between children living in Zabrze and those living in Gdynia, a city on the Baltic coast, which has the best long-term air quality in Poland. We enrolled children aged 9–15 years from both cities who were able to perform a spirometry. The following spirometry variables were measured for all participants: forced vital capacity (FVC), forced expiratory volume during the first second of expiration (FEV1), FEV1/FVC index, and peak expiratory flow (PEF). The frequencies of respiratory or allergic symptoms were taken from a survey completed by the participants’ parents. In total, 258 children from Gdynia and 512 children from Zabrze were examined. The mean values of FVC, FEV1, and PEF were significantly greater among children in Gdynia than those reported in Zabrze (p ≤ 0.032), and the frequencies of seasonal rhinorrhea (p = 0.015) or coughing episodes (p = 0.022) were significantly higher in Zabrze than in Gdynia. In conclusion, lung function was significantly impaired in children living in Zabrze, an area which is associated with poor air quality. Strategies to improve air quality in the Silesia region are urgently needed.
Keywords: air quality, air pollution, spirometry, lung function, children
1. Introduction
Ambient air pollution has many detrimental health effects, causing about seven million premature deaths annually worldwide [1]. Children are particularly susceptible to the effects of ambient air pollution because their respiratory systems are immature; moreover, they are exposed to air pollution to a greater extent than adults due to having higher minute ventilation and spending more time outdoors [2]. Many studies using spirometry measures have shown that increased concentrations of different air pollutants slow down lung development in children [3,4,5,6,7,8]. In particular, children living in urbanized, industrialized areas can suffer from the effects of air pollution on lung function [9,10,11,12,13,14]. These effects are likely due to various types of air pollution, with increased concentrations of many pollutants, such as particulate matter (PM), ozone (O3), sulfur dioxide (SO2), and nitrogen dioxide (NO2) [15,16]. In addition, air pollution in children may cause cardiovascular diseases, such as hypertension, by affecting the microvasculature (arteriole constriction) [17,18].
The air quality in Silesia, an industrialized region of Poland, is among the worst in Europe. This densely populated area is responsible for most of the coal production in Poland and has numerous coal-fired power plants, steelworks, and mineral mines. Of the 50 most polluted cities in the European Union, 36 are in Poland, of which most are located in the Silesia region [19,20]. Likely due to the high level of pollution, the region of Silesia is characterized by the shortest urban life expectancy and the highest incidence of premature births, genetic birth defects, and spontaneous miscarriages in Poland [21].
Although the poor air quality in Silesia is well known, no study to date has assessed its influence on lung development in children. In this study, we compared lung function in preadolescent children living in Silesia with that of children living on the Baltic coast, which has the best long-term air quality in Poland. We used objective spirometry measures of lung function and gathered data on the frequency of respiratory and allergic symptoms.
2. Materials and Methods
2.1. Study Design and Participants
This was a cross-sectional study carried out from 16 May 2019 to 12 June 2019, among children aged 9–15 years who were capable of completing a spirometry. Children were recruited from 6 primary schools in Gdynia, Poland, and 10 primary schools in Zabrze, Poland. The study was approved by the Bioethics Committee of the Military Institute of Medicine, Warsaw, and all children and their parents agreed to take part in the study.
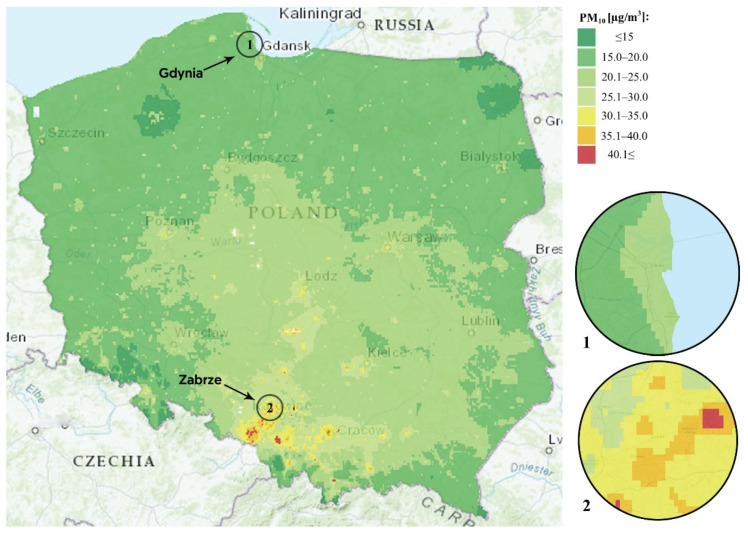
We chose to compare Gdynia and Zabrze because these two cities differ substantially in terms of long-term air quality. Gdynia, located in the north of Poland on the Baltic coast, has good overall air quality, whereas Zabrze, located in the south of Poland in the region of Silesia, has poor overall air quality. According to the spatial modeling data of the Chief Inspectorate of Environmental Protection, the annual concentrations of PM2.5, PM10, SO2, and NO2 are higher in the whole of the Zabrze area compared to the Gdynia area, and hence the long-term air quality in any of the schools in Gdynia should be better than those in any of the schools in Zabrze. The annual mean concentrations of PM10 for these cities are shown in Figure 1.
Figure 1.
Spatial modeling of PM10 pollution in Poland. Inserts show Zabrze (1) and Gdynia (2).
We estimated whole-life exposure to air pollution for participants in Zabrze and Gdynia. Whole-life exposure to PM10, SO2, NO2, and O3 was defined as the mean of the daily concentrations for the period between the year of the participant’s birth and the start of the study (15 May 2019). Data from all available measuring stations were used—i.e., four stations in Gdynia and one station in Zabrze. The whole-life exposures were estimated to check whether the differences in air pollution between the cities remained over many years.
2.2. Spirometry
All spirometry examinations were conducted by trained staff with the use of an AioCare portable spirometer connected to a smartphone application (HealthUp, Warsaw, Poland) [22]. The examinations were conducted in compliance with the standards of the American Thoracic Society and the European Respiratory Society [23]. Only examinations of quality grade A or B were included in the analyses. The following spirometry variables were measured for all participants: forced vital capacity (FVC), forced expiratory volume during the first second of expiration (FEV1), FEV1/FVC index, and peak expiratory flow (PEF).
2.3. Survey on Respiratory Symptoms
We asked the participants’ parents to fill out a survey to gather data on the distance from their house to a major road (<100 m, 100–200 m, 200–500 m, >500 m), the use of a coal- or wood-burning stove in the house, the presence of a heating furnace in an in-house living area, the smoking status of the parents, the physical activity of the child, and the existence of an asthma diagnosis. Additionally, we asked parents about the following allergic or respiratory symptoms in their children: all-year rhinorrhea; seasonal rhinorrhea; acute allergic reactions (food, exercise, insect sting); dyspnea episodes; coughing episodes; wheezing episodes; skin changes (eczema, urticaria, edema, erythema, pruritus); allergies (any); allergic skin reactions; recurring bronchitis with coughing, wheezing, or dyspnea; and wheezing (ever).
2.4. Statistical Analysis
Descriptive statistics were chosen based on data distribution. Continuous variables were compared between the cities with the t-test or Mann–Whitney test. The chi-squared test with continuity correction was used to compare categorical variables. Linear regression models were used to compare spirometry variables between Zabrze and Gdynia, adjusting for age, sex, weight, height, distance to a major road, the presence of coal- or wood-burning stove, the presence of a heating furnace in an in-house living area, and parents’ smoking status. A p < 0.05 was considered statistically significant. R software (Version. 3.6.2, R Foundation for Statistical Computing, Vienna, Austria) was used for all analyses.
3. Results
3.1. Whole-Life Exposure to Air Pollution
The whole-life exposure to PM10, NO2, and SO2 was higher in Zabrze than in Gdynia, but the whole-life exposure to O3 was higher in Gdynia (Table 1).
Table 1.
Air pollution in Gdynia and Zabrze—whole-life exposure by age group.
| Age Group | Pollutant | |||||||
|---|---|---|---|---|---|---|---|---|
| PM10, µg/m3 | SO2, µg/m3 | NO2, µg/m3 | O3, µg/m3 | |||||
| Gdynia | Zabrze | Gdynia | Zabrze | Gdynia | Zabrze | Gdynia | Zabrze | |
| 9-year-olds | 21.96 | 50.06 | 3.81 | 18.18 | 16.34 | 25.16 | 52.40 | 42.11 |
| 10-year-olds | 21.73 | 50.05 | 3.71 | 17.68 | 16.37 | 24.86 | 52.44 | 41.24 |
| 11-year-olds | 18.83 | 49.30 | 3.55 | 16.85 | 16.51 | 24.42 | 51.79 | 40.97 |
| 12-year-olds | 18.41 | 49.74 | 3.49 | 16.97 | 16.79 | 24.60 | 51.28 | 41.80 |
| 13-year-olds | 18.06 | 50.31 | 3.42 | 17.08 | 16.87 | 24.65 | 50.78 | 42.80 |
| 14-year-olds | 17.59 | 50.68 | 3.41 | 16.91 | 16.99 | 24.64 | 50.98 | 42.81 |
| 15-year-olds | 17.16 | 50.34 | 3.30 | 16.07 | 16.44 | 24.28 | 50.99 | 43.37 |
Values show means for the whole lifespan depending on age.
3.2. Cohort Characteristics
There were 258 children from Gdynia and 512 children from Zabrze who had spirometry examinations of grade A or B. The cohorts from both cities were similar in age, sex ratio, weight, and height (Table 2). A greater percentage of children in Zabrze than in Gdynia lived near a major road or had a coal- or wood-burning stove or a heating furnace in a living area. (Table 2). Detailed cohort characteristics are shown in Table 2.
Table 2.
Cohort characteristics.
| Variable | Gdynia (n = 258) | Zabrze (n = 512) | p-Value |
|---|---|---|---|
| Age (years), mean ± SD | 11.11 ± 1.32 | 11.23 ± 1.44 | 0.254 |
| Girls, n (%) | 123 (47.67) | 264 (51.56) | 0.308 |
| Height (cm), mean ± SD | 152.23 ± 10.41 | 151.54 ± 11.08 | 0.406 |
| Weight (kg), mean ± SD | 45.45 ± 11.60 | 45.87 ± 13.43 | 0.673 |
| BMI (kg/m2), mean ± SD | 19.38 ± 3.32 | 19.70 ± 4.17 | 0.289 |
| Distance to a major road, n (%) | <0.001 | ||
| <100 m | 56 (24.24) | 173 (37.94) | |
| 100–200 m | 64 (27.70) | 123 (26.97) | |
| 200–500 m | 59 (25.54) | 101 (22.15) | |
| >500 m | 52 (22.51) | 59 (12.94) | |
| Coal- or wood-burning stove, n (%) | 0 | 11 (2.21) | 0.040 |
| Heating furnace in a living area, n (%) | 17 (6.80) | 63 (12.70) | 0.014 |
| Smoking parent, n (%) | 59 (23.51) | 144 (28.97) | 0.112 |
| Physical activity, n (%) | 0.011 | ||
| Not active | 2 (0.78) | 5 (1.01) | |
| Irregular | 150 (59.76) | 238 (48.18) | |
| Regular | 99 (39.44) | 251 (50.81) |
3.3. Spirometry Variables and Respiratory or Allergic Symptoms
The mean values for FVC, FEV1, and PEF were significantly higher among children in Gdynia than among children in Zabrze (p ≤ 0.032), but the mean FEV1/FVC value was significantly greater among children in Zabrze (p < 0.001, Table 3). Results of regression models for all of these spirometry variables were presented in the Supplemental Materials (Supplementary Tables S1–S4).
Table 3.
Spirometry variables among schoolchildren in Gdynia and Zabrze.
| Variable | Gdynia | Zabrze | p-Value * |
|---|---|---|---|
| FVC, mean ± SD (L) | 2.42 ± 0.77 | 2.21 ± 0.73 | <0.001 |
| FEV1, mean ± SD (L) | 2.17 ± 0.60 | 2.07 ± 0.67 | 0.032 |
| FEV1/FVC, mean ± SD | 0.91 ± 0.08 | 0.94 ± 0.07 | <0.001 |
| PEF, mean ± SD, (L/min) | 259.34 ± 83.90 | 244.64 ± 81.64 | 0.018 |
FEV1, forced expiratory volume during the first second of expiration; FVC, forced vital capacity; PEF, peak expiratory flow. * p-values for comparisons between Gdynia and Zabrze are from linear regression models adjusted for age, sex, weight, height, distance to a major road, indoor particulate matter emission sources, and smoking status of parents. Full models are shown in Supplemental Materials.
The frequencies of seasonal rhinorrhea (p = 0.015) or coughing episodes (p = 0.022) were significantly higher in Zabrze than in Gdynia (Table 4). The frequency of allergic skin reactions tended to be higher in Gdynia (p = 0.067, Table 4). Other respiratory or allergic symptoms had similar frequencies in both cities (Table 4).
Table 4.
Allergic and respiratory symptoms in schoolchildren in Gdynia and Zabrze.
| Variable | Gdynia | Zabrze | p-Value |
|---|---|---|---|
| All-year rhinorrhea, n (%) | 15 (6.10) | 27 (5.92) | 0.925 |
| Seasonal rhinorrhea, n (%) | 99 (39.60) | 241 (48.98) | 0.015 |
| Acute allergic reactions, n (%) | 66 (26.29) | 1107 (21.49) | 0.140 |
| Dyspnea episodes, n (%) | 26 (10.53) | 58 (11.88) | 0.584 |
| Coughing episodes, n (%) | 56 (22.40) | 148 (30.39) | 0.022 |
| Wheezing episodes, n (%) | 23 (9.27) | 52 (10.68) | 0.552 |
| Skin changes, n (%) | 108 (43.20) | 194 (39.59) | 0.345 |
| Allergies (any), n (%) | 81 (32.66) | 134 (27.52) | 0.147 |
| Allergic skin reactions, n (%) | 110 (44.18) | 185 (37.23) | 0.067 |
| Recurring bronchitis, n (%) | 46 (18.33) | 99 (19.88) | 0.612 |
| Wheezing (ever), n (%) | 32 (12.80) | 69 (14.02) | 0.646 |
| Asthma diagnosis, n (%) | 16 (6.56) | 37 (7.65) | 0.594 |
4. Discussion
This study showed that preadolescent children living in Zabrze, in which the air quality is among the worst in Europe, had significantly impaired lung function, as indicated by their lower FVC, FEV1, and PEF compared to their peers from Gdynia on the Baltic coast. Moreover, children in Zabrze suffered from seasonal rhinorrhea or coughing episodes more often than children in Gdynia. There were no significant differences with respect to the frequencies of other allergic or respiratory symptoms.
The whole-life exposure to PM10, SO2, and NO2 was higher in Zabrze than in Gdynia, which was likely industry-related. These findings are in agreement with historical data on air pollution in Silesia and on the Polish coast. Previous evidence has shown that the air pollutants measured in our study have detrimental effects on respiratory health by inducing oxidative damage, inflammation, compensatory proliferation, and airway remodeling and fibrosis [24,25,26]. Consequently, PM10, SO2, and NO2 all slow down lung development in children, as shown by the reduced FVC or FEV1 with increasing concentrations of these pollutants in ambient air [27,28,29].
Our study indicates that the mixed, chronic air pollution in Silesia may be associated with impaired development in preadolescent children. We observed that the FVC in children from Zabrze was more than 200 mL lower than in their peers from the coastal area (~10%). Similarly, FEV1 was lower by about 100 mL (~5%). These findings are in line with those of previously published works. Gehring et al., in a study among 6–8-year-old children, found that FVC and FEV1 both decreased with increasing concentrations of PM2.5, NO2, and NOX in ambient air [30]. Likewise, Asgari et al. reported decreased FVC and FEV1 among children living in urbanized areas of Iran, with negative correlations between these two spirometry measures and the concentrations of PM, SO2, and NO2 in ambient air [10]. Another study carried out among children aged 9–13 years who lived in an industrialized area showed decreased FVC and FEV1 owing to increased NOX concentrations [9]. Similarly, FEV1 and FVC were decreased in children living in urbanized areas of China compared to their peers from rural areas, which was also attributed to the difference in air pollution [31]. Rusconi et al. reported that children exposed to oil refinery pollution, characterized by increased concentrations of SO2 and NO2, had reduced FEV1 and other spirometry measures compared to children living in an area with less air pollution [32]. We observed that the FEV1/FVC ratios were significantly higher in children from Zabrze than in those from Gdynia. This difference was likely because FVC was reduced by a greater extent than FEV1 in children from Zabrze. This explanation is supported by the finding that PEF, which is a measure of airway obstruction, was significantly lower in children from Zabrze than in children from Gdynia. Previous studies have shown that PEF may decrease due to air pollution in asthmatic children [33], but a similar effect among healthy children was reported only in some studies [34] and not in others [35].
We also found that children from Zabrze reported more frequent episodes of coughing or seasonal rhinorrhea compared to children from Gdynia. Air pollution has been linked to an increased risk of respiratory infections [36], which could explain the increased frequency of coughing episodes in Zabrze. Likewise, the increased frequency of seasonal rhinorrhea could be related to the poor air quality in Zabrze, because air pollution plays a role in the development of allergic rhinitis [37]. Although the frequencies of bronchitis, wheezing, and asthma were similar in both cities, one might expect the lifetime risk of asthma to be higher in Zabrze than in Gdynia [38]. Similarly, reduced FVC in children in Zabrze could increase the risk of restrictive lung diseases later in life. Living in Zabrze was not significantly associated with the risk of allergic skin symptoms. This finding agrees with previous evidence that the association between air pollution and skin allergies is less well-established than the association between air pollution and respiratory symptoms [39].
The limitations of our work need to be mentioned. First, the study was cross-sectional, with measurements taken during one season. A longitudinal study could assess whether the differences in lung function are independent of the season. In addition, a longitudinal study would be able to directly investigate lung development in cohorts of children from both cities. Moreover, the concentrations of air pollutants on the days when the spirometry tests were completed were higher in Zabrze than in Gdynia (Supplementary Table S5). Therefore, a further study should assess whether lung function in children from Zabrze remains lower than in their peers from Gdynia when the air pollution on spirometry days is equal. Ideally, spirometry should be repeated in children from Zabrze and Gdynia at the same time and place (e.g., summer camp), but such a study would be difficult to organize. Second, air pollution was not estimated for individual participants. However, we aimed to investigate the effects of long-term air pollution on lung function by comparing children residing in regions that differed substantially in long-term air quality. The air quality data obtained from the Chief Inspectorate of Environmental Protection (Figure 1) showed that the air quality in the entire Zabrze area was poorer than that in Gdynia, which makes the comparison between the two cities valid. Third, children in Zabrze were more exposed to air pollutants inside their homes (wood-burning stoves, heating furnaces in living areas). However, the differences in spirometry variables between Zabrze and Gdynia were significant after adjusting for these confounders. The use of objective measures of lung function is one of the strengths of our study.
In conclusion, this study showed that lung function measured by spirometry was impaired in children from Zabrze, which was likely due to the poor air quality. Moreover, the frequencies of seasonal rhinorrhea and coughing episodes were significantly higher in Zabrze than in Gdynia. We hope that our findings will motivate efforts to improve air quality in Silesia, because better air quality means better lung function [40].
Acknowledgments
We thank all participants.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/jcm10112375/s1: Table S1: Regression model for forced vital capacity (FVC, liters). Table S2: Regression model for forced expiratory volume in 1 s (FEV1, liters). Table S3: Regression model for FEV1/FVC. Table S4: Regression model for peak expiratory flow (PEF, liters/min). Table S5: Air pollution on spirometry days.
Author Contributions
Conceptualization, P.D., A.B., Ł.A., and D.M.; Methodology, P.D., A.B., Ł.A., D.M., and M.S.; Formal Analysis, P.D. and P.O.C.; Investigation, P.D. and M.S.; Resources, P.D., Ł.A., and D.M.; Data Curation, P.D., A.B., and M.S.; Writing—Original Draft Preparation, P.D., A.B., P.O.C., A.C., and M.S.; Writing—Review and Editing, Ł.A., D.M., P.D., A.B., P.O.C., and M.S.; Visualization, P.O.C. and M.S.; Supervision, P.D.; Project Administration, P.D.; Funding Acquisition, P.D. and A.B. All authors have read and agreed to the published version of the manuscript.
Funding
The study was conducted under the grant “Integrated support system for policies and programs Limiting Low Emissions—ZONE” and co-financed by the National Center for Research and Development under the strategic and R&D work “Social and economic development of Poland in the conditions of globalizing markets” GOSPOSTRATEG with the number Gospostrateg1/385807/4/2018/NCBR.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Bioethics Committee of the Military Institute of Medicine (49/WIM/2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The sponsors had no role in the design, execution, interpretation, or writing of the study.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization Air Pollution and Child Health: Prescribing Clean Air. World Health Organization; Geneva, Switzerland: 2019. [Google Scholar]
- 2.Committee on Environmental Health Ambient Air Pollution: Health Hazards to Children. Pediatrics. 2004;114:1699–1707. doi: 10.1542/peds.2004-2166. [DOI] [PubMed] [Google Scholar]
- 3.Hwang B.-F., Chen Y.-H., Lin Y.-T., Wu X.-T., Leo Lee Y. Relationship between exposure to fine particulates and ozone and reduced lung function in children. Environ. Res. 2015;137:382–390. doi: 10.1016/j.envres.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Urman R., McConnell R., Islam T., Avol E.L., Lurmann F.W., Vora H., Linn W.S., Rappaport E.B., Gilliland F.D., Gauderman W.J. Associations of children’s lung function with ambient air pollution: Joint effects of regional and near-roadway pollutants. Thorax. 2014;69:540–547. doi: 10.1136/thoraxjnl-2012-203159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao Y., Chan E.Y.Y., Li L.P., He Q.Q., Wong T.W. Chronic effects of ambient air pollution on lung function among Chinese children. Arch. Dis. Child. 2013;98:128–135. doi: 10.1136/archdischild-2011-301541. [DOI] [PubMed] [Google Scholar]
- 6.Rice M.B., Rifas-Shiman S.L., Litonjua A.A., Oken E., Gillman M.W., Kloog I., Luttmann-Gibson H., Zanobetti A., Coull B.A., Schwartz J., et al. Lifetime Exposure to Ambient Pollution and Lung Function in Children. Am. J. Respir. Crit. Care Med. 2016;193:881–888. doi: 10.1164/rccm.201506-1058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Badyda A.J., Dąbrowiecki P., Czechowski P.O., Majewski G. Risk of bronchi obstruction among non-smokers—Review of environmental factors affecting bronchoconstriction. Respir. Physiol. Neurobiol. 2015;209:39–46. doi: 10.1016/j.resp.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 8.Dąbrowiecki P., Mucha D., Gayer A., Adamkiewicz Ł., Badyda A.J. Assessment of Air Pollution Effects on the Respiratory System Based on Pulmonary Function Tests Performed During Spirometry Days. Adv. Exp. Med. Biol. 2015;873:43–52. doi: 10.1007/5584_2015_152. [DOI] [PubMed] [Google Scholar]
- 9.Bergstra A.D., Brunekreef B., Burdorf A. The effect of industry-related air pollution on lung function and respiratory symptoms in school children. Environ. Health. 2018;17:30. doi: 10.1186/s12940-018-0373-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asgari M.M., Dubois A., Asgari M., Gent J., Beckett W.S. Association of Ambient Air Quality with Children’s Lung Function in Urban and Rural Iran. Arch. Environ. Health Int. J. 1998;53:222–230. doi: 10.1080/00039899809605699. [DOI] [PubMed] [Google Scholar]
- 11.Priftis K.N., Anthracopoulos M.B., Paliatsos A.G., Tzavelas G., Nikolaou-Papanagiotou A., Douridas P., Nicolaidou P., Mantzouranis E. Different effects of urban and rural environments in the respiratory status of Greek schoolchildren. Respir. Med. 2007;101:98–106. doi: 10.1016/j.rmed.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Sonnappa S., Lum S., Kirkby J., Bonner R., Wade A., Subramanya V., Lakshman P.T., Rajan B., Nooyi S.C., Stocks J. Disparities in Pulmonary Function in Healthy Children across the Indian Urban–Rural Continuum. Am. J. Respir. Crit. Care Med. 2015;191:79–86. doi: 10.1164/rccm.201406-1049OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jakubiak-Lasocka J., Lasocki J., Badyda A.J. The influence of particulate matter on respiratory morbidity and mortality in children and infants. Adv. Exp. Med. Biol. 2015;849:39–48. doi: 10.1007/5584_2014_93. [DOI] [PubMed] [Google Scholar]
- 14.Badyda A.J., Feleszko W., Ratajczak A., Czechowski P.O., Czarnecki A., Dubrawski M., Dąbrowska A. Influence of Particulate Matter on the Occurrence of Upper Respiratory Tract Symptoms in Children Aged 3–12 Years. Am. J. Respir. Crit. Care Med. 2020;201:A6346. doi: 10.1164/ajrccm-conference.2020.201.1_MeetingAbstracts.A6346. [DOI] [Google Scholar]
- 15.Kurt O.K., Zhang J., Pinkerton K.E. Pulmonary health effects of air pollution. Curr. Opin. Pulm. Med. 2016;22:138–143. doi: 10.1097/MCP.0000000000000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Badyda A.J., Dąbrowiecki P., Czechowski P.O., Majewski G., Doboszyńska A. Traffic-related air pollution and respiratory tract efficiency. Adv. Exp. Med. Biol. 2015;834:31–38. doi: 10.1007/5584_2014_13. [DOI] [PubMed] [Google Scholar]
- 17.Provost E.B., Int Panis L., Saenen N.D., Kicinski M., Louwies T., Vrijens K., De Boever P., Nawrot T.S. Recent versus chronic fine particulate air pollution exposure as determinant of the retinal microvasculature in school children. Environ. Res. 2017;159:103–110. doi: 10.1016/j.envres.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 18.Pieters N., Koppen G., Van Poppel M., De Prins S., Cox B., Dons E., Nelen V., Panis L.I., Plusquin M., Schoeters G., et al. Blood Pressure and Same-Day Exposure to Air Pollution at School: Associations with Nano-Sized to Coarse PM in Children. Environ. Health Perspect. 2015;123:737–742. doi: 10.1289/ehp.1408121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Five Things We Learned from the World’s Biggest Air Pollution Database—Unearthed. [(accessed on 2 November 2020)]; Available online: https://unearthed.greenpeace.org/2018/05/02/air-pollution-cities-worst-global-data-world-health-organisation/
- 20.Global Ambient Air Quality Database (Update 2018) World Health Organization; Geneva, Switzerland: 2019. [Google Scholar]
- 21.Parascandola M. Ambient air pollution and lung cancer in Poland: Research findings and gaps. J. Health Inequalities. 2018;4:3–8. doi: 10.5114/jhi.2018.77639. [DOI] [Google Scholar]
- 22.Kupczyk M., Hofman A., Kołtowski Ł., Kuna P., Łukaszyk M., Buczyłko K., Bodzenta-Łukaszyk A., Nastałek P., Soliński M., Dąbrowiecki P. Home self-monitoring in patients with asthma using a mobile spirometry system. J. Asthma. 2021;58:505–511. doi: 10.1080/02770903.2019.1709864. [DOI] [PubMed] [Google Scholar]
- 23.Graham B.L., Steenbruggen I., Miller M.R., Barjaktarevic I.Z., Cooper B.G., Hall G.L., Hallstrand T.S., Kaminsky D.A., McCarthy K., McCormack M.C., et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019;200:e70–e88. doi: 10.1164/rccm.201908-1590ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xing Y.F., Xu Y.H., Shi M.H., Lian Y.X. The impact of PM2.5 on the human respiratory system. J. Thorac. Dis. 2016;8:E69–E74. doi: 10.3978/j.issn.2072-1439.2016.01.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wigenstam E., Elfsmark L., Bucht A., Jonasson S. Inhaled sulfur dioxide causes pulmonary and systemic inflammation leading to fibrotic respiratory disease in a rat model of chemical-induced lung injury. Toxicology. 2016;368:28–36. doi: 10.1016/j.tox.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 26.Petit P.C., Fine D.H., Vásquez G.B., Gamero L., Slaughter M.S., Dasse K.A. The Pathophysiology of Nitrogen Dioxide During Inhaled Nitric Oxide Therapy. ASAIO J. 2017;63:7–13. doi: 10.1097/MAT.0000000000000425. [DOI] [PubMed] [Google Scholar]
- 27.Gauderman W.J., Avol E., Gilliland F., Vora H., Thomas D., Berhane K., McConnell R., Kuenzli N., Lurmann F., Rappaport E., et al. The Effect of Air Pollution on Lung Development from 10 to 18 Years of Age. N. Engl. J. Med. 2004;351:1057–1067. doi: 10.1056/NEJMoa040610. [DOI] [PubMed] [Google Scholar]
- 28.Rojas-Martinez R., Perez-Padilla R., Olaiz-Fernandez G., Mendoza-Alvarado L., Moreno-Macias H., Fortoul T., McDonnell W., Loomis D., Romieu I. Lung Function Growth in Children with Long-Term Exposure to Air Pollutants in Mexico City. Am. J. Respir. Crit. Care Med. 2007;176:377–384. doi: 10.1164/rccm.200510-1678OC. [DOI] [PubMed] [Google Scholar]
- 29.Lee Y.L., Wang W.-H., Lu C.-W., Lin Y.-H., Hwang B.-F. Effects of ambient air pollution on pulmonary function among schoolchildren. Int. J. Hyg. Environ. Health. 2011;214:369–375. doi: 10.1016/j.ijheh.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 30.Gehring U., Gruzieva O., Agius R.M., Beelen R., Custovic A., Cyrys J., Eeftens M., Flexeder C., Fuertes E., Heinrich J., et al. Air Pollution Exposure and Lung Function in Children: The ESCAPE Project. Environ. Health Perspect. 2013;121:1357–1364. doi: 10.1289/ehp.1306770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He Q.-C., Lioy P.J., Wilson W.E., Chapman R.S. Effects of Air Pollution on Children’s Pulmonary Function in Urban and Suburban Areas of Wuhan, People’s Republic of China. Arch. Environ. Health Int. J. 1993;48:382–391. doi: 10.1080/00039896.1993.10545959. [DOI] [PubMed] [Google Scholar]
- 32.Rusconi F., Catelan D., Accetta G., Peluso M., Pistelli R., Barbone F., Di Felice E., Munnia A., Murgia P., Paladini L., et al. Asthma Symptoms, Lung Function, and Markers of Oxidative Stress and Inflammation in Children Exposed to Oil Refinery Pollution. J. Asthma. 2011;48:84–90. doi: 10.3109/02770903.2010.538106. [DOI] [PubMed] [Google Scholar]
- 33.Wiwatanadate P., Trakultivakorn M. Air pollution-related peak expiratory flow rates among asthmatic children in Chiang Mai, Thailand. Inhal. Toxicol. 2010;22:301–308. doi: 10.3109/08958370903300327. [DOI] [PubMed] [Google Scholar]
- 34.Oftedal B., Brunekreef B., Nystad W., Madsen C., Walker S.-E., Nafstad P. Residential Outdoor Air Pollution and Lung Function in Schoolchildren. Epidemiology. 2008;19:129–137. doi: 10.1097/EDE.0b013e31815c0827. [DOI] [PubMed] [Google Scholar]
- 35.Hasibuan I.E., Supriatmo M.N., Faisal A., Panggabean G., Daulay R.M., Siregar Z., Lubis H.M. Peak expiratory flow rate of primary school children in high and low air pollution level areas. Paediatr. Indones. 2016;43:10. doi: 10.14238/pi43.1.2003.10-13. [DOI] [Google Scholar]
- 36.Croft D.P., Zhang W., Lin S., Thurston S.W., Hopke P.K., Masiol M., Squizzato S., van Wijngaarden E., Utell M.J., Rich D.Q. The Association between Respiratory Infection and Air Pollution in the Setting of Air Quality Policy and Economic Change. Ann. Am. Thorac. Soc. 2018;16:321–330. doi: 10.1513/AnnalsATS.201810-691OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hwang B.-F., Jaakkola J.J., Lee Y.-L., Lin Y.-C., Leon Guo Y. Relation between air pollution and allergic rhinitis in Taiwanese schoolchildren. Respir. Res. 2006;7:23. doi: 10.1186/1465-9921-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calderón-Garcidueñas L., Mora-Tiscareño A., Fordham L.A., Chung C.J., Valencia-Salazar G., Flores-Gómez S., Solt A.C., Campo A.G., Jardón-Torres R., Henríquez-Roldán C., et al. Lung Radiology and Pulmonary Function of Children Chronically Exposed to Air Pollution. Environ. Health Perspect. 2006;114:1432–1437. doi: 10.1289/ehp.8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Araviiskaia E., Berardesca E., Bieber T., Gontijo G., Sanchez Viera M., Marrot L., Chuberre B., Dreno B. The impact of airborne pollution on skin. J. Eur. Acad. Dermatol. Venereol. 2019;33:1496–1505. doi: 10.1111/jdv.15583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gauderman W.J., Urman R., Avol E., Berhane K., McConnell R., Rappaport E., Chang R., Lurmann F., Gilliland F. Association of Improved Air Quality with Lung Development in Children. N. Engl. J. Med. 2015;372:905–913. doi: 10.1056/NEJMoa1414123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.