Abstract
The main objective of this study was to prepare and characterize oleogel as potential carrier for quercetin skin delivery. The formulations were prepared by adding olive oil (5–30%) to Pluronic F127 hydrogel and were evaluated for particle size, zeta potential, viscosity in vitro quercetin release and stability, and were compared with that of Pluronic F127 hydrogel. The selected formulation was characterized for its interaction possibility, ex vivo skin permeation and skin histological changes and safety. The particle sizes ranged from 345.3 ± 5.3 nm to 401.5 ± 2.8 nm, and possessed negative charges. The viscosities of the formulations were found in the range of 6367–4823 cps with inverse proportionality to olive oil percentage while the higher percentages showed higher quercetin release. Percentages of 25% and 30% olive oil showed instability pattern under the conditions of accelerated stability studies. Differential scanning calorimetry verified the existence of quercetin in micellar aggregation and the network in the case of hydrogel and oleogel respectively. Ex vivo skin permeation showed an improved skin permeation of quercetin when 20% olive oil containing oleogel was used. Skin histology after 10 days of application showed stratum corneum disruption and good safety profile. Based on these findings, the proposed oleogel containing 20% olive oil denotes a potential carrier for topical delivery of quercetin.
Keywords: oleogel, skin delivery, quercetin, olive oil
1. Introduction
The skin is the largest organ of human body and acts as biological barrier which protects the body any external threatening hazards. The external hazardous stimuli may be in the form of ultraviolet and visible radiation, microbial invaders, ionizing radiation and oxidants (reactive oxygen species; ROS) [1,2]. However, self-defending action specially against ROS was reported by the antioxidant effect of endogenous superoxide dismutase, metallothionein and melanin [3]. Nevertheless, if the oxidative stress of ROS overweighs skin self-protective effect, skin harms may take place such as lipid peroxidation, DNA breakage, tumor promotion [4] and, hence, skin aging. Therefore, assistance of the skin self-protective systems by exogenous antioxidants could preclude ROS-induced cuteneous damage. Polyphenolic compounds, including flavonoids, are well known as powerful antioxidants and are generally used for topical use as a protectants against natural skin damage (as photo-ageing), skin cancer prevention and skin cosmetic-care [5,6,7].
Flavonoids, a class of phenolic compounds, are considered powerful antioxidants. Their action depends upon several mechanisms including chain-breaking inhibition of the peroxidation process, scavenging free radicals and iron chelating effect leading to antilipoperoxidative action [8]. However, their actions against lipid peroxidation of the biomembranes rely on their molecular structure and capability to interact and infiltrate the lipid bilayer [9]. Among those, quercetin (QT; 3,3’,4’,5,7-pentahydroxyflavone) is considered as one of the most potent natural antioxidants [10]. It also has anti-inflammatory, anticarcinogenic, antibacterial and antiviral effects [11]. However, despite the documented QT beneficial therapeutic actions, its limited aqueous solubility (~2 µg/mL) hinders its skin absorption and effectiveness [12]. Several approaches have been implemented seeking for enhancing QT skin permeability, including combined microneedles and lipid microparticles [13], sodium hyaluronate-chitosan multilayered liposome [14], lipid-based nanosystems [15] and essential oil-based microemulsions [16].
Oleogels (organogels) are semi-solid systems prepared by the encapsulation of organic liquids, such as vegetable oils, in a three-dimensional polymer network [17]. They possess unique characteristics such as stability which is attributed to physical interactions such as hydrogen bonding, Van der Waals forces or chemical interactions as covalent bonds [18], biocompatibility, non-irritating nature and easiness of fabrication. Therefore, thanks to their core nature, oleogels represent a potential system possessing outstanding characteristics for skin delivery. They have been mostly formulated and studied for the cutaneous administration of active pharmaceutical ingredients (APIs) such as sumatriptan [19], melatonin [20], silymarin [21] and mefenamic acid [22].
Therefore, it seems quite justified to develop oleogels from Pluronic F127 and different concentrations of olive oil, one of the natural oils that enhance skin permeation [23], to be used as a platform for skin delivery of QT at concentrations of 5% to 30% (w/w) in relation to olive oil. Formulations were characterized for particle size, zeta potential, viscosity in vitro QT release and stability, and compared with these findings of Pluronic F127 hydrogel. The findings were utilized to select the most appropriate composition in the development of cutaneous QT delivery.
2. Materials and Methods
2.1. Materials
Quercetin (QT) and pluronic F127 were purchased from Sigma Aldrich (India). Olive oil (extra virgin) was purchased from Loba Chemie (India). All other chemicals were in analytical grades.
2.2. Preparation of QT-Loaded Pluronic F127/Olive Oil Oleogels
All the batches were fabricated in two steps. Firstly, the aqueous phase was prepared dispersing Pluronic F127 (slowly to avoid clumping of the particles; 20% w/v) in phosphate buffer under magnetic stirring. After obtaining a homogeneous hydrogel the solution was kept at 4 C overnight to obtain transparent hydrogel. Secondly, QT was solubilized in olive oil under magnetic stirring. QT oily dispersion (in different ratios) was added into aqueous solution (in 1:4 ratio respectively) with aid of homogenization (yellow Line, GmbH, Germany) at 5000 rpm for 10 min. The compositions of the batches are defined in Table 1 with 0.2% of QT in the final preparation.
Table 1.
Compositions, mean particle size, zeta potential and drug content % (means ± SD, n = 3) of different formulations.
| Code | Olive Oil % | Pluronic F127 % | Particle Size (nm) | Zeta Potential (mV) | Drug Content % |
|---|---|---|---|---|---|
| F1 | - | 20 | 345.3 ± 5.3 | −15.1 ± 2.3 | 96.5 ± 2.8 |
| F2 | 5 | 20 | 375.9 ± 5.7 | −19.7 ± 3.7 | 95.1 ± 4.6 |
| F3 | 10 | 20 | 389.4 ± 3.8 | −19.2 ± 2.5 | 95.7 ± 7.3 |
| F4 | 15 | 20 | 372.8 ± 7.9 | −16.1 ± 3.3 | 96.0 ± 9.2 |
| F5 | 20 | 20 | 369.8 ± 8.5 | −17.3 ± 6.1 | 97.1 ± 2.8 |
| F6 | 25 | 20 | 401.5 ± 2.8 | −15.5 ± 1.2 | 96.1 ± 4.9 |
| F7 | 30 | 20 | 392.8 ± 9.7 | −17.2 ± 3.1 | 97.6 ± 8.5 |
2.3. Particle Size and Zeta Potential
The prepared QT-loaded pluronic F127–olive oil oleogels were diluted 200 times with double distilled water, vortexed (Dragonlab cortex, Beijing, China) and kept in bath sonicator (specification) for 5 min. The dispersions were analyzed for particle size, polydispersity index (PDI) and surface using Zetasizer analyzer (Malvern Zetasizer Nano ZS90, Worcestershire, UK) at room temperature. All measurements were performed in triplicate.
2.4. QT Content
An accurately weighed 0.5 g of each batch was dissolved in 100 mL of absolute ethyl alcohol, sonicated and filtered to get rid of the residues. Drug free formulation was used as zero drug content formulation and subjected to the same procedure. QT level was measured by UV-visible spectrophotometer (Genesys 10S UV-VIS, Thermo Scientific, Shanghai, China) at 372 nm [24].
2.5. Organoleptic Characteristics
The prepared formulations were investigated for color, odor, softness, appearance and ease of application [25].
2.6. pH Determination
The prepared formulations were investigated for pH using a pH-meter (Hanna Instruments, Woonsocket, RI, USA) by direct dipping of the electrode into the formulation. All the measurements were made in triplicate.
2.7. Viscosity Measurement
The prepared formulations were investigated for viscosity by Brookfield rheometer (DV3T Rheometer, Middleboro, MA, USA) using spindle no. 7 with a speed of 20 rpm at room temperature. All the measurements were made in triplicate.
2.8. In Vitro QT Release
The in vitro QT release from different batches was performed using dialysis bag method. Accurately weighed 100 mg of oleogels was put inside cellulose acetate dialysis bags (M. wt. cut off: 12,000–14,000 Da, Livingstone, Australia). The bags were dipped into 200 mL of receptor solution consisting of PBS (pH 7.4) and absolute ethyl alcohol (70:30, v/v respectively) to increase the solubility of QT and maintain the sink conditions. The receptor solution was kept stirred at 50 rpm and temperature was maintained at 37 °C. At predetermined time intervals (30, 60, 120, 240, 360, 480, 600, 720 and 1440 min), 1 mL was withdrawn from the receptor solution to determine QT concentration spectrophotometerically with replenishment of receptor solution with the same volume of fresh media. All samples were measured in triplicate.
2.9. Accelerated Stability Test
Accelerated stability studies were performed using freeze–thaw cycles. Briefly, the prepared formulations were incubated at 70 °C for 15 min then transferred to −20 °C for 15 min. This cycle was done for five times. Afterwards, the batches were checked for phase separation or any other instability features.
2.10. Differential Scanning Calorimetry (DSC)
The thermal analyses of QT, Pluronic F127, F1 and selected formulation (F4) were conducted by a differential scanning calorimeter (DSC3, Mettler Toledo, Greifensee, Switzerland). Six milligrams of each sample were separately placed in an aluminum pan. Heating scan rate was adjusted 20 °C/min and temperature was recorded between 25 °C and 350 °C. Data were assessed with STARe 15.00 software.
2.11. Fourier Transform-Infrared Spectroscopy
Fourier transform infrared spectroscopy (ATR-FTIR; Thermo Scientific) was used to examine system compatibility based on wavelength function group. QT, Pluronic F127, olive oil, F1 and F4 were analyzed between 4400 and 400 cm−1 in transmission mode.
2.12. Ex Vivo Skin Permeation
The animals used during the experiments were obtained from the Animal House Colony of College of Pharmacy, Jouf University. The study was performed according to ethical procedures and policies of Jouf University (Approval code; LCBE6/5/42), which verified the laboratory animal care obeyed the guidelines of the Declaration of Helsinki and the Guiding Principles for the Care and Use of Laboratory Animals. The procedures also followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 8023, revised 1978).
The skin permeation of QT was studied using Franz diffusion cells (Logan DHC-6T Dry Heat Transdermal System) equipped with temperature control, autosampler, and reservoir replacement automatic system. The temperature was maintained at 37 ± 1 °C. After sacrificing the animals, the hairs on the dorsal side of animals were removed. The skin was harvested, freed of adhering fat layers and mounted on Franz diffusion cells. The receptor compartment was filled with twelve milliliters of a (PBS)/absolute ethyl alcohol (pH = 7.4, 70:30) with 0.11% (w/v) formaldehyde as a preservative [26]. Freshly excised full thickness rat skin was cut into pieces with dimensions relevant to cell diffusion area (1.13 cm2) at which stratum corneum (SC) faced the formulation. Ten mg of various formulations were applied onto the skin in the donor compartment. At 30, 60, 120, 180, 240, 300 and 360 min time points, aliquots of 0.5 mL were withdrawn to be assayed spectrophotometerically. The same volume of fresh receptor medium was added to receptor compartment to maintain constant receptor volume.
2.13. Permeation Kinetics
After plotting of the mean cumulative QT permeated against time points, different kinetic models were applied to check the best fitting model depending on the correlation coefficient (r2). In that regard, zero-order model, first-order model, Higuchi’s model and Korsmeyer–Peppas (KP) model were applied.
2.14. Histological Changes and Safety
In this section we investigated the histological changes and safety of the formulations through 10 days of daily application (at days 3, 7 and 10). At predetermined days, the application areas were visually checked for any noticeable changes such as erythema. The average erythemal scores were noted (ranging from zero to four) based upon the degree of redness. Afterwards, the animals were sacrificed, areas of application were excised, fixed with 10% formalin solution and stained with H&E for the histopathological investigation.
2.15. Statistical Analysis
In this study, the results are presented as means ± standard deviations (SD). The statistical analysis was performed using one-way ANOVA and means were compared using Tukey’s multiple comparison testing with GraphPad Prism v.5. Software. p < 0.05 was considered a significant difference.
3. Results and Discussion
3.1. Preparation and Formulation of QT Loaded Olive Oil/Pluronic Oleogels
Among the various drug delivery systems, oleogels were selected as promising system to deliver QT to the skin. Oleogels are potential candidates for dermocosmetics and personal care products [27,28,29]. Table 1 shows the constitutions of seven different QT loaded hydrogel and oleogels formulated for the current study, which were designed on the basis of olive oil percentage (5–30%) aiming to study the effect of the these percentages on different physicochemical characteristics and skin permeability.
3.2. Particle Size and Zeta Potential
The particle size and zeta potential are key elements for estimation of the stability of the colloidal delivery system during storage [30]. Additionally, both parameters also were reported to affect cutaneous drug delivery across skin barrier [31,32]. Table 1 shows the particle size and zeta potential of all investigated formulations. It was clear that the particle size they in nanosized range (in range from 345.3 ± 5.3 nm to 401.5 ± 2.8 nm) while the zeta potential was negative and in range from −17.2 ± 3.1 mV to −15.1 ± 2.3 mV. Olive oil addition (by any percentage) did not significantly (p > 0.05) influence both parameters.
3.3. QT Content and Organoleptic Characteristics
The drug content of the prepared oleogels was found to be high and uniform in range from 95.1 ± 4.6% to 97.6 ± 8.5, as shown in Table 1. It is clear that addition of olive oil at any level did not significantly (p < 0.05) influence the drug content. This behavior was attributed to the fact that Pluronic formed homogenous distribution of QT throughout the matrix and could incorporate most of the amount of QT added [30]. On the other hand, olive oil/Pluronic could not be confirmed at this step and would be checked by further experiments. Changes in organoleptic properties based on the concentration of olive oil are depicted in Table 2. Under the experimental conditions, all the prepared formulations were grittiness free with a smooth feel and did not show phase separation of exudates. All formulations were non-greasy except F6 (30% olive oil content) which showed less greasiness. The consistencies of the formulations were decreased with the increase in olive oil concentration. The color of olive oil free formulation (F1) was brownish while olive oil-containing formulations were light yellow.
Table 2.
Organoleptic characteristics, pH and viscosity (means ± SD, n = 3) of different formulations.
| Code | Organoleptic Characteristics | pH | Viscosity (cp) | ||||
|---|---|---|---|---|---|---|---|
| Phase Separation | Greasiness | Grittiness | Consistency | Exudate | |||
| F1 | None | None | None | Viscous, spreadable gel | Nil | 6.6 ± 0.8 | 6367 ± 28 |
| F2 | None | None | None | Viscous, spreadable gel | Nil | 6.5 ± 0.2 | 6241 ± 34 |
| F3 | None | None | None | Less viscous, spreadable gel | Nil | 6.3 ± 0.7 | 5836 ± 27 |
| F4 | None | None | None | Less viscous, spreadable gel | Nil | 6.2 ± 0.4 | 5633 ± 49 |
| F5 | None | None | None | Thin, fluid | Nil | 6.0 ± 0.4 | 5289 ± 23 |
| F6 | None | None | None | Thin, fluid | Nil | 6.0 ± 1.1 | 5003 ± 36 |
| F7 | None | Less observable | None | More thin and fluid | Nil | 5.8 ± 0.6 | 4823 ± 29 |
3.4. pH and Viscosity Measurements
The pH values of the Pluronic F127 hydrogel and oleogels were slightly acidic and found to be in the range of 6.6 ± 0.8 and 5.8 ± 0.6 (Table 2). It is clear that the value of pH decreased with the increase in olive oil concentration. Acidity rise may be ascribed to the content of fatty acids. It was reported that olive oil contains a high percentage of monounsaturated fatty acids (MUFA) which are accounting about 85% of its composition. The main fatty acids are oleic acid (C18:1), which might range between 70% and 85%, and other fatty acids such as linoleic or palmitoleic acid [33]. Generally, this pH range is safe and expected to induce no dermal irritation reactions [34]. Viscosity profiles (Table 2) indicated that the viscosity of the hydrogel was higher than oleogels and higher concentration of olive oil produced lower viscosity. The viscosities of the hydrogel and oleogels were found in the range of 6367–4823 cps. In hydrogel, adding Pluronic F127 in critical micelle concentration into water led to formation of micelles [35] and the viscosity raised. It was reported that aqueous solution of Pluronic F127 at 20–40% concentration resulted in a face-centered cubic structure of micelle [36] and hence high viscosity [37]. As adding olive oil to Pluronic decreased the percentage of solids in relation to liquid, it resulted in consistency lowering [19]. Additionally, increasing the percentage of olive oil from 5% to 30% could decrease the water percentage in the formulation resulting in disturbance in the entanglement of tubular micelles. This result is in good accordance with the previous study [25].
3.5. In Vitro Release Studies
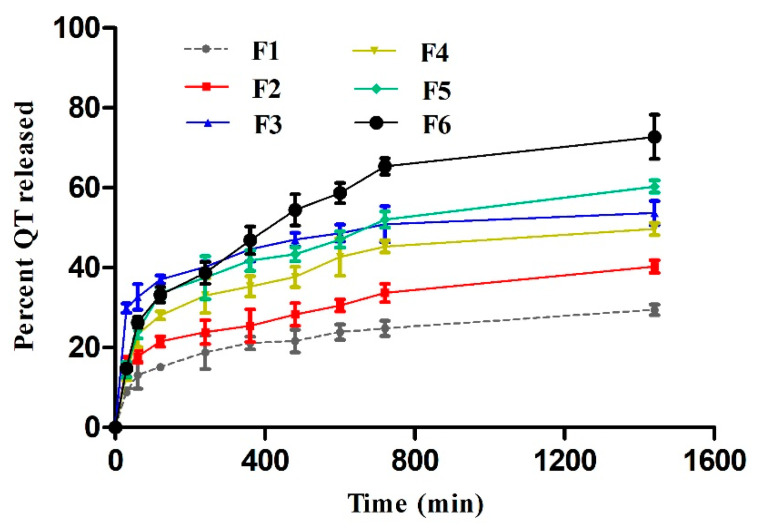
In vitro QT release from different formulations was assessed over a period of 24 h. As depicted in Figure 1, the cumulative percentages of QT released were plotted against predetermined time intervals. The results showed that the highest percentage of QT release (72.7%) after 24 h was observed from F6; the formula which contained the highest percentage of olive oil while the lowest release percentage (29.4%) was observed in olive oil free Pluronic F127 hydrogel. By another way, the release of QT was olive oil concentration dependent; the higher the olive oil concentration, the higher QT release percentage. It is suggested that the viscosity played a crucial in controlling drug release [19]. As mentioned above, olive oil decreased the water percentage in the formulation resulting in disturbance in the entanglement of tubular micelles then lowering the formulation viscosity. On the other hand, 20% Pluronic F127 is a thermogelling system and can form gels above its critical micelle concentration (CMC). In addition, keeping Pluronic F127 solution for 24 h at 37 °C triggered gelation and viscosity elevation owing to dehydration of polypropylene oxide block in the polymer micelles’ extra aggregation [38]. This pattern encouraged us to measure the viscosity after performing the release experiment. As expected, F1 hydrogel viscosity was significantly (p < 0.05) increased to 8564 ± 69 cp while the other formulations were not significantly changed. This in turn suggested the micellisation disturbance in the presence of olive oil, which needed further assessment. Anyhow, shifting into higher viscosities extended the release of the active drug from the gelling matrix.
Figure 1.
In vitro release profiles of QT from different formulations (mean values ± SD, n = 3).
3.6. Accelerated Stability Test
Accelerated stability testing of the prepared formulations was performed by freeze–thaw cycles to expect the long-term stability of the prepared oleogels. The formulations were checked for any signs of instability during the five cycles. Two oleogels (F5 and F6) were unable to preserve their structural integrity and phase separation was observed at the end of the period while the other formulations maintained the stability features. F5 and F6 were of 25% and 30% olive oil contents, respectively. Although it has been reported that the gelation of the oil helps prevent the migration of oils in the pharmaceutical products and thus improves the shelf-life stability of the products [39], higher concentrations of olive oil provoked product instability. Based on the results described above, F4 was selected for the subsequent studies as it contained the highest percentage of olive oil with good release and stability behavior.
3.7. Thermal Analysis
The thermal behavior of pure QT, Pluronic F127, F1 and F4 was appraised by evaluating DSC thermograms. However, olive oil (liquid state) could not be registered using the defined temperatures and analytical circumstances [40]. Figure 2 shows the thermograms of samples under investigation. Pure QT showed main sharp endothermic at 316 °C (Figure 2) confirming its crystallinity. Pluronic F127 showed the peak at 92 °C. For F1 and F4, broad peaks at 116 °C were observed (peak onset at 81.7 °C) which might be due to overlapped typical Pluronic F127 peak with another peak representing the loss of moisture from hydrogel and oleogel. Furthermore, the intensity of the peak in the case of F1 was more pronounced in F1 than that in F4, which was suggested to be due to higher water content in hydrogel (F1) when compared to oleogel (F4) [41]. Interestingly, Hydrogel (F1) showed faint endothermic peak at 284 °C while oleogel (F4) theromgram was deprived from such peak. This behavior of F1 was suggested to be due to breakdown of the reverse micellar structures [42]. This could partly explain the micellisation interference effect of olive oil and consequent lower consistency of oleogels when compared to hydrogel. On the other hand, disappearance of QT main peak could be due to incorporation of micelles in the case of F1 and presence of amorphous oil soluble form in the case of F4.
Figure 2.
DSC thermograms of of QT, Pluronic F127, F4 and F1.
3.8. FT-IR
An interaction between the components was studied using FT-IR spectroscopy in transmittance mode. Figure 3 represents fingerprint region spectra of QT, olive oil, Pluronic F127, F1 and F4. The FT-IR spectrum of raw QT displayed main peaks at 3379 cm−1 (revealing free hydroxyl bond vibration), 1654 cm−1 (revealing band stretching vibration of carbonyl group), 1618 cm−1 (revealing C–C stretching vibration of phenyl ring), 1512 cm−1 (revealing aromatic group), at 1315 cm−1, 1160 cm−1 (revealing C–O–C vibration), and at 996 cm−1 (revealing aromatic C–H group) [43]. Olive oil spectrum showed strong band absorption peaks at 2920 cm−1 and 2854 cm−1 (revealing C–H stretching vibration of methylene and methyl groups respectively), 1741 cm−1 (revealing carbonyl double bond stretching vibration) [44]. Pluronic F127 spectrum exhibited at 2824 cm−1 (revealing C-H stretching), 1281 cm−1 and 1239 cm−1 (revealing the stretching of C–O–C bonds) and 1105 cm−1, (revealing C-O stretching) [45]. In the spectra of F1 and F4, they presented similarly except for the appearance of new peaks in F4 spectrum at 2920 cm−1 and 1753 cm−1 correspond to the typical stretching vibrations of olive oil (dashed lines). On the other hand, most of the QT characteristic peaks disappeared in both F1 and F4 with slight shifting in few absorption peaks. This might be attributed to the alteration in the chemical environment around the components of the oleogels. Additionally, broader peaks (when compared to raw QT) at 3332 cm−1 and 3348 cm−1 were observed in spectra of F1 and F4, respectively, which supposed the occurrence of intramolecular/intermolecular hydrogen bonding among the oleogel constituents [46]. It was also observed that no additional peaks in F1 and F4 spectra which might be ascribed to lower concentration of QT when compared to oleogel forming matrix and hence QT peaks were lessened owing to the strong peaks of the other constituents [41].
Figure 3.
FT-IR spectra of QT, olive oil, Pluronic F127, F1 and F4.
3.9. Ex Vivo Skin Permeation
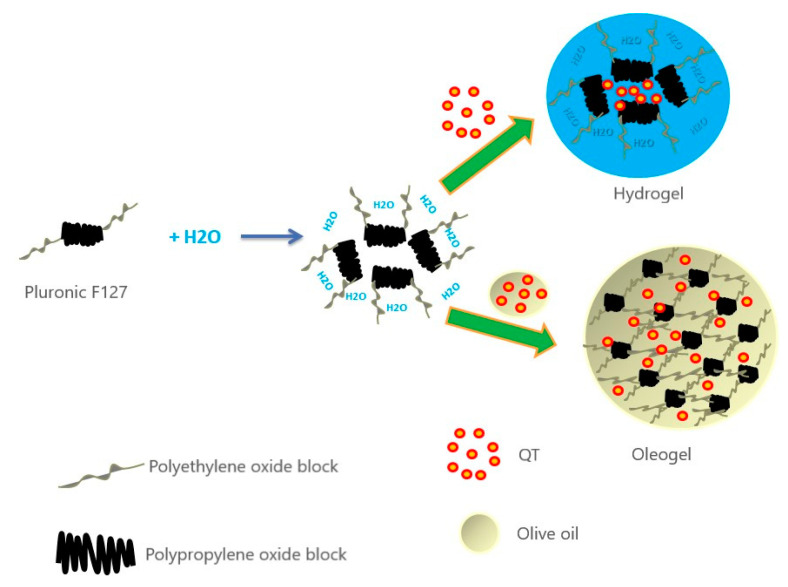
In vitro permeation profiles of F1 and F4 were achieved using Franz diffusion cells to appraise the influence of both formulations on the diffusion of QT through the full-thickness skin under a non-occlusive application. Figure 4 shows the percentage of the amount of QT that permeated through the skin versus predetermined time points. The cumulative percentages of QT which permeated through the skin into receptor compartment from F1 and F4 after 6 h were 21.2 ± 1.4% and 43.6 ± 6.2%, respectively. It is obvious that QT permeation from F4 was about two times higher than F1. Formulation/skin interaction was particularly related to its structure and components. F1 (hydrogel) is structurally aggregative micelles encapsulating the hydrophobic drug in the hydrophobic block core of Pluronic F127 (polypropylene oxide) [47] while F4 (oleogel) is self-sustained thermo-reversible network (represented by Pluronic F127) which immobilizes an organic compound (represented by olive oil) [48] (Figure 5). In the case of F1, QT escaping was confined by aqueous external surroundings and high consistency, while escaping from F4 was higher due to easier release and low consistency of the matrix when compared to F1. Regarding the components, Pluronic F127 is surface active agent and can interact with the skin, causing disturbance in the lipid barrier in the horny layer and increase skin permeability. Adding olive oil (oleic acid rich oil) to Pluronic F127 could trigger the skin permeability through disrupting the cellular arrangement of the stratum corneum irreversibly and thereby allowed better penetration through the different skin layers [49]. Additionally, the miscibility/mixing of olive oil with stratum corneum lipids could also promote skin penetration. Furthermore, the packing pattern of fatty acids altered the fluidity of skin lipid structure and assisted QT skin absorption [50].
Figure 4.
Representative graphs of the cumulative amounts of QT permeated through full thickness skin samples versus time for F1 and F4 (mean values ± SD, n = 3).
Figure 5.
Schematic representation of the mechanism of formation of pluronic olive oil oleogels.
Table 3 shows the kinetic parameters obtained by fitting the release pattern to different models. From the results we can say that the model that F1 and F4 followed the Korsmeyer–Peppas model as they displayed higher r2 values of 0.986 and 0.996 respectively, which are close to 1.0. F1 showed a value of n = 0.413 (less than 0.5), indicating that release kinetics of QT from the hydrogel containing the highest amount of particles was controlled by Fickian diffusion. On the other hand, F4 showed a value of n = 0.562 (intermediate value between 0.50 and 1.00) indicating an anomalous behavior (non-Fickian kinetics corresponding to the phenomena of diffusion and relaxation of the polymer chains) [51].
Table 3.
Kinetic parameters for the ex vivo skin permeation of QT from formulations F1 and F4.
| Code | Zero Order Kinetics | First Order Kinetics | Higuchi Model | KP Model | ||||
|---|---|---|---|---|---|---|---|---|
| r2 | K0 | r2 | K1 | r2 | KH | r2 | n | |
| F1 | 0.630 | 0.07 | 0.697 | 0.001 | 0.973 | 1.19 | 0.986 | 0.413 |
| F2 | 0.855 | 0.14 | 0.934 | 0.002 | 0.992 | 2.29 | 0.996 | 0.562 |
3.10. Histopathology
Skin histopathology was implemented to study the effects of F1 and F4 on the structure of rat skin. Figure 6A shows the histopathology of control untreated skin which exhibited a clear delineation between the epidermis and dermis, with a compact stratum corneum and well-arranged corneocytes. However, application of F1 (Figure 6B–D) and F4 (Figure 6E–G) for 10 consecutive days did not induce significant changes at the level of dermis. The difference was clearly induced at the level of epidermis. F4 showed more predominant disruption of the stratum corneum integrity and swelling when compared to F1. This increase in the thickness of the stratum corneum was attributed occlusive effect of olive oil which could increase skin hydration by precluding trans-epidermal water loss, leading to swelling effect [52]. This in turn could weaken the core barrier of the stratum corneum and improve the skin delivery, which was ascertained by ex vivo skin permeation studies.
Figure 6.
Microscopic pictures of H&E-stained skin sections from control rats (A) and rats exposed to F1 for 3 days (B), 7 days (C) and 10 days (D) and F4 for 3 days (E), 7 days (F) and 10 days (G) showing no structural damage in dermal elements (magnification 100×, bar 100).
4. Conclusions
In the present work, oleogels containing different amounts of olive oil were formulated and characterized. The prepared formulation possessed particle size in nano-range and negative surface charges. The viscosities of the formulations were found in an inverse proportionality to olive oil percentage while the higher percentages showed higher quercetin release. Percentages of 25% and 30% olive oil showed instability pattern under the conditions of accelerated stability studies. DSC results indicated that QT was embedded into in micellar aggregation and the polymeric network in the case of hydrogel and oleogel respectively. Oleogel containing 20% olive oil showed an improved skin permeation presenting more than 2-fold when compared to hydrogel. It also showed good safety profile on the skin without showing significant dermatotoxicity. Overall results suggest that 20% olive oil in 20% Pluronic F127 oleogel is a good candidate for improving topical QT skin delivery. The potential of the fabricated system could be further investigated by carrying out clinical trials and scale-up studies.
Acknowledgments
The authors would like to extend their sincere appreciation to the central laboratory at Jouf University for their support for this study.
Author Contributions
Conceptualization, M.E. and A.M.; methodology, T.S.A. and K.S.; software, S.A.F.; validation, M.E., M.M.A.F. and A.S.; formal analysis, M.M.A.F.; investigation, M.M.A.-S. and T.S.A.; resources, M.A.A.; data curation, M.E.; writing—original draft preparation, M.G.; writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this work through the project number “375213500”.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Jouf University (protocol code LCBE6/5/42).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors have no conflict of interest to declare.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.English J.S.C., Dawe R.S., Ferguson J. Environmental effects and skin disease. Br. Med. Bull. 2003;68:129–142. doi: 10.1093/bmb/ldg026. [DOI] [PubMed] [Google Scholar]
- 2.Bickers D.R., Athar M. Oxidative stress in the pathogenesis of skin disease. J. Investig. Dermatol. 2006;126:2565–2575. doi: 10.1038/sj.jid.5700340. [DOI] [PubMed] [Google Scholar]
- 3.Čáp M., Váchová L., Palková Z. Reactive oxygen species in the signaling and adaptation of multicellular microbial communities. Oxid. Med. Cell Longev. 2012;2012:976753. doi: 10.1155/2012/976753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valko M., Rhodes C.J., Moncol J., Izakovic M.M., Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 5.Svobodová A., Psotová J., Walterová D. Natural phenolics in the prevention of UV-induced skin damage. A review. Biomed. Pap. 2003;147:137–145. doi: 10.5507/bp.2003.019. [DOI] [PubMed] [Google Scholar]
- 6.Pinnell S.R. Cutaneous photodamage, oxidative stress, and topical antioxidant protection. J. Am. Acad. Dermatol. 2003;48:1–22. doi: 10.1067/mjd.2003.16. [DOI] [PubMed] [Google Scholar]
- 7.Nayak B.S., Ramdath D.D., Marshall J.R., Isitor G.N., Eversley M., Xue S., Shi J. Wound-healing activity of the skin of the common grape (Vitis Vinifera) variant, cabernet sauvignon. Phyther. Res. 2010;24:1151–1157. doi: 10.1002/ptr.2999. [DOI] [PubMed] [Google Scholar]
- 8.Morel I., Lescoat G., Cogrel P., Sergent O., Pasdeloup N., Brissot P., Cillard P., Cillard J. Antioxidant and iron-chelating activities of the flavonoids catechin, quercetin and diosmetin on iron-loaded rat hepatocyte cultures. Biochem. Pharmacol. 1993;45:13–19. doi: 10.1016/0006-2952(93)90371-3. [DOI] [PubMed] [Google Scholar]
- 9.Santos A.C., Uyemura S.A., Lopes J.L.C., Bazon J.N., Mingatto F.E., Curti C. Effect of naturally occurring flavonoids on lipid peroxidation and membrane permeability transition in mitochondria. Free Radic. Biol. Med. 1998;24:1455–1461. doi: 10.1016/S0891-5849(98)00003-3. [DOI] [PubMed] [Google Scholar]
- 10.Bonina F., Lanza M., Montenegro L., Puglisi C., Tomaino A., Trombetta D., Francesco C., Saija A. Flavonoids as potential protective agents against photo-oxidative skin damage. Int. J. Pharm. 1996;145:87–94. doi: 10.1016/S0378-5173(96)04728-X. [DOI] [Google Scholar]
- 11.Boots A.W., Haenen G.R.M.M., Bast A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008;585:325–337. doi: 10.1016/j.ejphar.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Bose S., Du Y., Takhistov P., Michniak-Kohn B. Formulation optimization and topical delivery of quercetin from solid lipid based nanosystems. Int. J. Pharm. 2013;441:56–66. doi: 10.1016/j.ijpharm.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 13.Paleco R., Vučen S.R., Crean A.M., Moore A., Scalia S. Enhancement of the in vitro penetration of quercetin through pig skin by combined microneedles and lipid microparticles. Int. J. Pharm. 2014;472:206–213. doi: 10.1016/j.ijpharm.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 14.Jeon S., Yoo C.Y., Park S.N. Improved stability and skin permeability of sodium hyaluronate-chitosan multilayered liposomes by Layer-by-Layer electrostatic deposition for quercetin delivery. Colloids Surfaces B Biointerfaces. 2015;129:7–14. doi: 10.1016/j.colsurfb.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 15.Caddeo C., Díez-Sales O., Pons R., Fernàndez-Busquets X., Fadda A.M., Manconi M. Topical anti-inflammatory potential of quercetin in lipid-based nanosystems: In vivo and in vitro evaluation. Pharm. Res. 2014;31:959–968. doi: 10.1007/s11095-013-1215-0. [DOI] [PubMed] [Google Scholar]
- 16.Lv X., Liu T., Ma H., Tian Y., Li L., Li Z., Gao M., Zhang J., Tang Z. Preparation of Essential Oil-Based Microemulsions for Improving the Solubility, pH Stability, Photostability, and Skin Permeation of Quercetin. AAPS PharmSciTech. 2017;18:3097–3104. doi: 10.1208/s12249-017-0798-x. [DOI] [PubMed] [Google Scholar]
- 17.Hughes N.E., Marangoni A.G., Wright A.J., Rogers M.A., Rush J.W.E. Potential food applications of edible oil organogels. Trends Food Sci. Technol. 2009;20:470–480. doi: 10.1016/j.tifs.2009.06.002. [DOI] [Google Scholar]
- 18.Ghosh S., Das Mahapatra R., Dey J. Thermoreversible as well as thermoirreversible organogel formation by L-cysteine-based amphiphiles with poly (ethylene glycol) tail. Langmuir. 2014;30:1677–1685. doi: 10.1021/la404258v. [DOI] [PubMed] [Google Scholar]
- 19.Agrawal V., Gupta V., Ramteke S., Trivedi P. Preparation and evaluation of tubular micelles of pluronic lecithin organogel for transdermal delivery of sumatriptan. Aaps PharmSciTech. 2010;11:1718–1725. doi: 10.1208/s12249-010-9540-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flo A., Calpena A.C., Halbaut L., Araya E.I., Fernández F., Clares B. Melatonin delivery: Transdermal and transbuccal evaluation in different vehicles. Pharm. Res. 2016;33:1615–1627. doi: 10.1007/s11095-016-1901-9. [DOI] [PubMed] [Google Scholar]
- 21.Mady F.M., Essa H., El-Ammawi T., Abdelkader H., Hussein A.K. Formulation and clinical evaluation of silymarin pluronic-lecithin organogels for treatment of atopic dermatitis. Drug Des. Dev. Ther. 2016;10:1101. doi: 10.2147/DDDT.S103423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jhawat V., Gupta S., Saini V. Formulation and evaluation of novel controlled release of topical pluronic lecithin organogel of mefenamic acid. Drug Deliv. 2016;23:3573–3581. doi: 10.1080/10717544.2016.1212439. [DOI] [PubMed] [Google Scholar]
- 23.Viljoen J.M., Cowley A., Du Preez J., Gerber M., Du Plessis J. Penetration enhancing effects of selected natural oils utilized in topical dosage forms. Drug Dev. Ind. Pharm. 2015;41:2045–2054. doi: 10.3109/03639045.2015.1047847. [DOI] [PubMed] [Google Scholar]
- 24.Baksi R., Singh D.P., Borse S.P., Rana R., Sharma V., Nivsarkar M. In vitro and in vivo anticancer efficacy potential of Quercetin loaded polymeric nanoparticles. Biomed. Pharmacother. 2018;106:1513–1526. doi: 10.1016/j.biopha.2018.07.106. [DOI] [PubMed] [Google Scholar]
- 25.Balata G., El Nahas H.M., Radwan S. Propolis organogel as a novel topical delivery system for treating wounds. Drug Deliv. 2014;21:55–61. doi: 10.3109/10717544.2013.847032. [DOI] [PubMed] [Google Scholar]
- 26.Kanikkannan N., Singh J., Ramarao P. In vitro transdermal iontophoretic transport of timolol maleate: Effect of age and species. J. Control. Release. 2001;71:99–105. doi: 10.1016/S0168-3659(01)00208-5. [DOI] [PubMed] [Google Scholar]
- 27.Plamen K., Le C.A.K., Halima R., Rum S., Carla V., Haftek M., Pirot F. Organogels for cosmetic and dermo-cosmetic applications. Househ. Pers. Care Today. 2015;10:16–20. [Google Scholar]
- 28.Kirilov P., Rum S., Gilbert E., Roussel L., Salmon D., Abdayem R., Serre C., Villa C., Haftek M., Falson F., et al. Aqueous dispersions of organogel nanoparticles–potential systems for cosmetic and dermo-cosmetic applications. Int. J. Cosmet. Sci. 2014;36:336–346. doi: 10.1111/ics.12131. [DOI] [PubMed] [Google Scholar]
- 29.Singh V.K., Pramanik K., Ray S.S., Pal K. Development and characterization of sorbitan monostearate and sesame oil-based organogels for topical delivery of antimicrobials. Aaps PharmSciTech. 2015;16:293–305. doi: 10.1208/s12249-014-0223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thakur K., Mahajan A., Sharma G., Singh B., Raza K., Chhibber S., Katare O.P. Implementation of Quality by Design (QbD) approach in development of silver sulphadiazine loaded egg oil organogel: An improved dermatokinetic profile and therapeutic efficacy in burn wounds. Int. J. Pharm. 2020;576:118977. doi: 10.1016/j.ijpharm.2019.118977. [DOI] [PubMed] [Google Scholar]
- 31.Sharma G., Kamboj S., Thakur K., Negi P., Raza K., Katare O.P. Delivery of thermoresponsive-tailored mixed micellar nanogel of lidocaine and prilocaine with improved dermatokinetic profile and therapeutic efficacy in topical anaesthesia. AAPS PharmSciTech. 2017;18:790–802. doi: 10.1208/s12249-016-0561-8. [DOI] [PubMed] [Google Scholar]
- 32.Elmowafy M., Shalaby K., Ali H.M., Alruwaili N.K., Salama A., Ibrahim M.F., Akl M.A., Ahmed T.A. Impact of nanostructured lipid carriers on dapsone delivery to the skin: In vitro and in vivo studies. Int. J. Pharm. 2019;572:118781. doi: 10.1016/j.ijpharm.2019.118781. [DOI] [PubMed] [Google Scholar]
- 33.Jimenez-Lopez C., Carpena M., Lourenço-Lopes C., Gallardo-Gomez M., Lorenzo J., Barba F., Prieto M., Simal-Gandara J. Bioactive compounds and quality of extra virgin olive oil. Foods. 2020;9:1014. doi: 10.3390/foods9081014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Behera B., Patil V., Sagiri S.S., Pal K., Ray S.S. Span-60-based organogels as probable matrices for transdermal/topical delivery systems. J. Appl. Polym. Sci. 2012;125:852–863. doi: 10.1002/app.35674. [DOI] [Google Scholar]
- 35.Sharma P.K., Matthew J.E., Bhatia S.R. Structure and assembly of PEO-PPO-PEO co-polymers in mammalian cell-culture media. J. Biomater. Sci. Polym. Ed. 2005;16:1139–1151. doi: 10.1163/1568562054798545. [DOI] [PubMed] [Google Scholar]
- 36.Liu T., Chu B. Formation of homogeneous gel-like phases by mixed triblock copolymer micelles in aqueous solution: FCC to BCC phase transition. J. Appl. Crystallogr. 2000;33:727–730. doi: 10.1107/S0021889899013369. [DOI] [Google Scholar]
- 37.Dumortier G., Grossiord J.L., Agnely F., Chaumeil J.C. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm. Res. 2006;23:2709–2728. doi: 10.1007/s11095-006-9104-4. [DOI] [PubMed] [Google Scholar]
- 38.Juhasz J., Lenaerts V., Raymond P., Ong H. Diffusion of rat atrial natriuretic factor in thermoreversible poloxamer gels. Biomaterials. 1989;10:265–268. doi: 10.1016/0142-9612(89)90103-8. [DOI] [PubMed] [Google Scholar]
- 39.Wright A.J., Marangoni A.G. Formation, structure, and rheological properties of ricinelaidic acid-vegetable oil organogels. J. Am. Oil Chem. Soc. 2006;83:497–503. doi: 10.1007/s11746-006-1232-9. [DOI] [Google Scholar]
- 40.Fang J.-Y., Fang C.-L., Liu C.-H., Su Y.-H. Lipid nanoparticles as vehicles for topical psoralen delivery: Solid lipid nanoparticles (SLN) versus nanostructured lipid carriers (NLC) Eur. J. Pharm. Biopharm. 2008;70:633–640. doi: 10.1016/j.ejpb.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 41.Baran N., Singh V.K., Pal K., Anis A., Pradhan D.K., Pramanik K. Development and Characterization of Soy Lecithin and Palm Oil-based Organogels. Polym. Plast. Technol. Eng. 2014;53:865–879. doi: 10.1080/03602559.2013.869600. [DOI] [Google Scholar]
- 42.Li Y., Xu R., Couderc S., Bloor M., Wyn-Jones E., Holzwarth J.F. Binding of sodium dodecyl sulfate (SDS) to the ABA block copolymer Pluronic F127 (EO97PO69EO97): F127 aggregation induced by SDS. Langmuir. 2001;17:183–188. doi: 10.1021/la001075j. [DOI] [Google Scholar]
- 43.Zhang L., Yang X., Li S., Gao W. Preparation, physicochemical characterization and in vitro digestibility on solid complex of maize starches with quercetin. LWT Food Sci. Technol. 2011;44:787–792. doi: 10.1016/j.lwt.2010.09.001. [DOI] [Google Scholar]
- 44.Gurdeniz G., Tokatli F., Ozen B. Differentiation of mixtures of monovarietal olive oils by mid-infrared spectroscopy and chemometrics. Eur. J. Lipid Sci. Technol. 2007;109:1194–1202. doi: 10.1002/ejlt.200700087. [DOI] [Google Scholar]
- 45.Elmowafy M., Alruwaili N.K., Shalaby K., Alharbi K.S., Altowayan W.M., Ahmed N., Zafar A., Elkomy M. Long-acting paliperidone parenteral formulations based on polycaprolactone nanoparticles; the influence of stabilizer and chitosan on in vitro release, protein adsorption, and cytotoxicity. Pharmaceutics. 2020;12:160. doi: 10.3390/pharmaceutics12020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Satapathy D., Biswas D., Behera B., Sagiri S.S., Pal K., Pramanik K. Sunflower-oil-based lecithin organogels as matrices for controlled drug delivery. J. Appl. Polym. Sci. 2013;129:585–594. doi: 10.1002/app.38498. [DOI] [Google Scholar]
- 47.Zarrintaj P., Ramsey J.D., Samadi A., Atoufi Z., Yazdi M.K., Ganjali M.R., Amirabad L.M., Zangene E., Farokhi M., Formela K., et al. Poloxamer: A versatile tri-block copolymer for biomedical applications. Acta Biomater. 2020;110:37–67. doi: 10.1016/j.actbio.2020.04.028. [DOI] [PubMed] [Google Scholar]
- 48.Almeida H., Amaral M.H., Lobão P., Lobo J.M.S. Pluronic® F-127 and Pluronic Lecithin Organogel (PLO): Main features and their applications in topical and transdermal administration of drugs. J. Pharm. Pharm. Sci. 2012;15:592–605. doi: 10.18433/J3HW2B. [DOI] [PubMed] [Google Scholar]
- 49.Dhawan B., Aggarwal G., Harikumar S.L. Enhanced transdermal permeability of piroxicam through novel nanoemulgel formulation. Int. J. Pharm. Investig. 2014;4:65. doi: 10.4103/2230-973X.133053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fang J.-Y., Hong C.-T., Chiu W.-T., Wang Y.-Y. Effect of liposomes and niosomes on skin permeation of enoxacin. Int. J. Pharm. 2001;219:61–72. doi: 10.1016/S0378-5173(01)00627-5. [DOI] [PubMed] [Google Scholar]
- 51.Ritger P.L., Peppas N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control. Release. 1987;5:37–42. doi: 10.1016/0168-3659(87)90035-6. [DOI] [PubMed] [Google Scholar]
- 52.Muller R.H., Shegokar R., Keck C.M. 20 Years of Lipid Nanoparticles (SLN & NLC): Present State of Development & Industrial Applications. Curr. Drug Discov. Technol. 2011;8:207–227. doi: 10.2174/157016311796799062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.