Abstract
The peroxisome proliferator-activated receptor co-activator-1α (PGC1α) belongs to a family of transcriptional regulators, which act as co-activators for a number of transcription factors, including PPARs, NRFs, oestrogen receptors, etc. PGC1α has been implicated in the control of mitochondrial biogenesis, the regulation of the synthesis of ROS and inflammatory cytokines, as well as genes controlling metabolic processes. The levels of PGC1α have been shown to be altered in neurodegenerative disorders. In the brains of Alzheimer’s disease (AD) patients and animal models of amyloidosis, PGC1α expression was reduced compared with healthy individuals. Recently, it was shown that overexpression of PGC1α resulted in reduced amyloid-β (Aβ) generation, particularly by regulating the expression of BACE1, the rate-limiting enzyme involved in the production of Aβ. These results provide evidence pointing toward PGC1α activation as a new therapeutic avenue for AD, which has been supported by the promising observations of treatments with drugs that enhance the expression of PGC1α and gene therapy studies in animal models of AD. This review summarizes the different ways and mechanisms whereby PGC1α can be neuroprotective in AD and the pre-clinical treatments that have been explored so far.
Keywords: Alzheimer’s disease, amyloid-β, PGC1α
1. Introduction
Peroxisome proliferator-activated receptor (PPAR) gamma coactivator 1 (PGC1) is a group of transcriptional regulators for a variety of transcription factors and nuclear receptors, which consists of three subtypes, PGC1α, PGC1β, and the PGC-related coactivator (PRC) [1]. Since the early 2000s, PGC1α has attracted interest because of its important role in metabolic processes (including gluconeogenesis, glucose transport, and fatty acid oxidation), mitochondrial biogenesis, peroxisomal remodelling, and detoxification of reactive oxygen species (ROS) [2]. These effects are mediated through the regulation of a number of transcription factors, including nuclear respiratory factors (NFRs) NRF-1 and NRF-2 (interacting with Tfam, which drives transcription and replication of mtDNA), PPARs (PPARα, PPARδ/β, and PPARγ), thyroid hormone, glucocorticoid, oestrogen, and ERRs (oestrogen-related receptors) α and γ [3] (ERRα, ERRβ, and ERRγ), initiator element binding factor (YY1), myocyte-specific enhancer factors (MEF-2A, MEF-2C, MEF-2D), forkhead box O1 (FOXO1), and others [4].
PGC1α was first identified in the adipose tissue, where it mediates the shift of white adipose tissue into a brown-fat-like phenotype. Therefore, PGC1α is highly expressed in tissues with elevated energy requirements, including adipose tissue, the liver, skeletal muscle, cardiac myocytes, the kidneys, and the brain [5,6]. Alterations in the levels of PGC1α have been linked with pathologies such as metabolic syndrome and its principal complications including obesity, type 2 diabetes mellitus, cardiovascular disease, and hepatic steatosis [7].
Many signalling pathways have been proposed to regulate PGC-1α expression and activity, including calcium signalling and second messengers, cyclin-dependent kinases, and post-translational modifications, such as phosphorylation, methylation, and deacetylation, and others [3]. In particular, PGC1α levels can be modulated by fasting, physical exercise, inflammation, and drugs that affect the pathways mentioned above. Exercise induces upregulation of PGC1α in skeletal muscle, where it stimulates the expression of FNDC5 and induces the transcription of BDNF [8], by increasing the phosphorylation of PGC1α by AMP-K. Fasting induces the expression of sirtuin-1, which has been shown to mediate the deacetylation of PGC1α [9,10,11]. In this regard, PGC1α has been reported to be involved in the exercise and fasting regulation of autophagy and the unfolded protein response (UPR) [12,13].
Previous studies suggested that PGC1α in the brain is enriched in inhibitory interneurons and required for the expression of the calcium buffer parvalbumin (PV) in the cortex [14]. Conditional knockout of PGC1α in the central nervous system (CNS) has revealed limited alterations in metabolic processes and its involvement in the regulation of a different category of genes linked with brain activity, including synaptotagmin 2, complexin 1, and interneuron genes [15,16,17,18]. Therefore, it appears that the functions of PGC1α in the brain are different from peripheral tissues. PGC1α overexpression was also found to protect neural cells in culture from oxidative-stressor-mediated death [19], and increase the formation and maintenance of dendritic spines in hippocampal neurons, while the opposite effect was observed in PGC1α knockout neurons [20]. In addition, adult conditional PGC1α knockout mice resulted in the loss of dopaminergic neurons, which was accompanied by a reduction in dopamine in the striatum [21]. PGC1α is also expressed in glial cells, such as astrocytes, regulating neuroinflammation and oxidative stress [22].
In neurodegeneration and brain injury, PGC1α can promote neuronal survival by affecting the activity of NFR-2 [23]. PGC1α deficiency affects mitochondrial structure and promotes mitochondrial ROS levels, leading to cellular senescence and ageing-related disorders [24]. PGC1α expression has been reported to be altered in neurodegenerative disorders such as amyotrophic lateral sclerosis, Huntington’s disease, Parkinson’s disease, and multiple sclerosis [25], leading to mitochondrial defects and increased ROS levels [26,27,28].
In this review, we focus on the role of PGC1α in Alzheimer’s disease (AD), particularly the promising treatments based on its activation.
2. PGC1α in Alzheimer’s Disease
AD is a neurodegenerative disorder characterized by memory and neuronal loss and the deposition of amyloid-β (Aβ) plaques and neurofibrillary tangles of hyperphosphorylated tau in the brains of the patients. Aβ is generated by the sequential cleavage of the amyloid-precursor protein (APP) by two enzymes, β-APP cleaving enzyme (BACE1) and the γ-secretase complex, whose main catalytic domain relays on presenilins (PS) [29]. Animal models used for research include generally transgenic mice overexpressing the human APP form with familiar mutations or human tau mutations [30].
Several groups have reported changes in PGC1α expression in the brain of AD patients and animal models of amyloidosis. PGC1α protein levels were reduced in brains of the Tg2576 (overexpressing APP with the familiar Swedish mutation) and APP/PS1 (which also include presenilin mutations) mouse models [31,32], as well as in nuclear extracts from human AD patients [33]. In agreement, Qin et al. reported that the mRNA levels of PGC1α decreased in the AD brain and correlated with the levels of AD dementia and Aβ pathology [34].
The role of PGC1α in the pathology of AD has been associated with reductions in Aβ levels [31,33]. Conversely, crossing Tg2576 mice with mice deficient in PGC1α or silencing PGC1α using siRNA transfection in neuronal cells led to increased Aβ [33,35]. In line with this, studies of double transgenic PGC1α and Tg19959 (containing the Indiana and Swedish mutations) mice revealed reductions in the expression of Aβ40 by ELISA;, however Congo red staining for aggregated Aβ was increased [36].
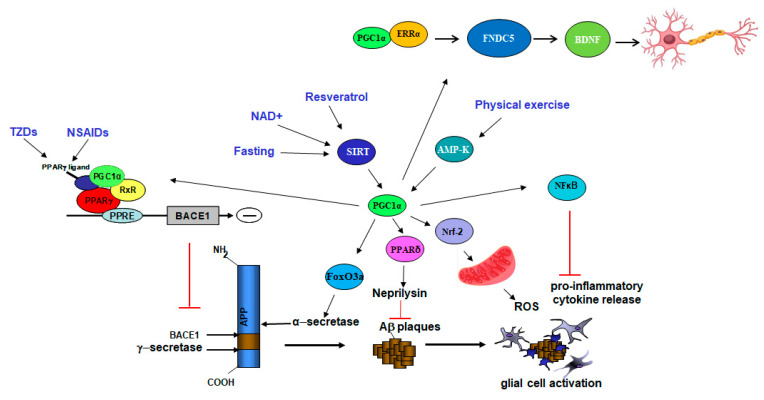
The most likely mechanism whereby PGC1α decreases the generation of Aβ seems to be by reducing the expression of the rate-limiting enzyme for Aβ production BACE1 [33,37,38] (Figure 1). In vitro, PGC1α overexpression was able to reduce BACE1 transcription and BACE1 promoter activity and the opposite effects were observed in cells transfected with PGC1α siRNA. In addition, these effects were mediated by peroxisome proliferator-activated receptor gamma (PPARγ) [33,37], since they were not detected in PPARγ-deficient cells. We previously reported that PPARγ is a repressor of BACE1 [39] and we and others found that BACE1 promoter contains PPRE domains [37,39]. However, other studies suggest that PGC1α activation may affect BACE1 proteasomal degradation through CF(Fbx2)-E3 ligase gene expression [31].
Figure 1.
Model showing the mechanistic effects of PGC1α as therapy in AD. Activation of PGC1α via interventions (in blue) such as exercise, fasting, or treatments (genetic and pharmacological) can lead to neuroprotection in AD by targeting different transcriptional pathways. Binding to PPARγ results in changes in the processing of the amyloid-precursor protein (APP) by reducing BACE1 transcription and Aβ generation. PGC1α can affect Aβ degradation by increasing neprilysin activity. In addition, the expression of neurotrophic molecules, such as sAPPα (by increasing α-secretase expression) and BDNF, are enhanced by PGC1α. Lastly, the levels of pro-inflammatory cytokines and reactive oxygen species (ROS) are also modulated by PGC1α. Adapted from Katsouri et al., 2016 (reference [38]).
Another mechanism by which PGC1α can be beneficial in AD is by affecting the non-amyloidogenic pathway. Overexpression of PGC1α via viral vectors using primary cultures of Tg2576 mice resulted in an increase in α-secretase activity [34] via suppression of FoxO3a. Increases in the levels of non-amyloidogenic soluble APPα were also detected in N2a mouse neuroblastoma cells transfected with PGC1α cDNA, although no changes in ADAM-10 expression were observed [33]. Interestingly, ADAM-10 transcription was found to be regulated by PPARα, although experiments in mouse hippocampal neurons showed that activation of PPARα induced the recruitment of PPARα to the ADAM-10 promoter without the presence of PGC1α [40].
Further studies also considered PGC1α in Aβ degradation. Transfection of PGC1α led to an increase in neprilysin activity, but not in its expression. In addition, incubation with the PGC1α activator resveratrol increased neprilysin activity [33]. Interestingly, PPARδ agonists were found to elevate neprilysin transcription in animal models of AD [41], and neprilysin promoter contains two PPRE domains [42]. PGC-1α has also been found to mediate neuroprotective effects by protecting against Aβ neurotoxicity in N2a cells [43] and in astrocytes [44], as well as by reducing neuroinflammatory cytokines [45] and the release of ROS [19].
All these results point to the potential neuroprotective effects of the activation of PGC1α in AD. In the following sections, the pre-clinical studies performed using treatments that directly or indirectly activate PGC1α as well as gene therapy studies are analysed.
3. Therapeutic Effects of Activation of PGC1α in Alzheimer’s Disease
Over the years, studies have demonstrated that PGC1α can be modulated by both pharmacological and non-pharmacological approaches. For instance, PGC1α expression can be induced by exercise, fasting, and cold exposure [46,47,48]. Pharmacological activation of PGC1α can be achieved using compounds and drugs, including resveratrol and PPARγ agonists [49,50]. In addition, gene therapy approaches showed promising results in AD models [38] but small benefits in clinical trials (Table 1 and Table 2).
Table 1.
Treatments targeting PGC1α in Alzheimer’s Disease in vitro and in vivo.
| Treatment Affecting PGC1α (Dose) | Method | Outcome on AD | Ref. |
|---|---|---|---|
| Resveratrol (20–40 µM) Resveratrol (100 µM) |
In vitro Hek293 and N2a cell lines expressing APP695 N2a cell lines expressing APP695 |
↓ Aβ, promoting clearance ↔ APP processing ↑ Neprilysin activity |
[33,41] |
| Gene therapy (lentivirus carrying hPGC1α) |
In vivo APP23 mice (8 months old) |
Improved memory Rescued neuronal loss ↓ Aβ and BACE1 expression ↑ BDNF and NGF levels |
[38] |
| Gene therapy (AAV carrying PGC1α) | 2xTg-AD mice (6 months old) | ↓ Aβ and ROS | [32] |
| Resveratrol (diet with 0.35% resveratrol) | APP/PS1 mice (4 months old) | ↓ Aβ deposition | [51] |
| Nicotinamide riboside (250 mg) | Tg2576 mice (8 months old) | ↑ PGC1α expression Improved memory ↓ BACE1 expression and Aβ |
[35] |
| Pioglitazone (40 mg/kg/day) and Ibuprofen (62.5 mg/kg/day) | APPV717I transgenic mice (10 months old) | ↓ BACE1 expression and Aβ ↓ Glial activation |
[50] |
| Pioglitazone (20 mg/kg/day) and Ibuprofen (62.5 mg/kg/day) | Tg2576 mice (11 months old) | ↓ SDS-soluble Aβ42 and Aβ40 | [52] |
| Pioglitazone (18 mg/kg) | 3xTg-AD mice (10 months old) | Improved memory Enhanced long-term plasticity ↓ Aβ and tau deposition |
[53] |
| Rosiglitazone (5 mg/g/day) | J20 mice (9 months old) | Improved memory ↓ Aβ deposition ↓ Neuroinflammation |
[54] |
| Rosiglitazone (diet of 30 mg/kg) | Tg2576 mice (4 months old) | Enhanced learning and memory ↓ Aβ levels ↑ IDE mRNA and activity |
[55] |
↑ = increased, ↓ = decreased, and ↔ = no changes.
Table 2.
Clinical trials with treatments targeting PGC1α in Alzheimer’s disease.
| Treatment Affecting PGC1α (Dose) | Subject | Benefits on AD | Ref. |
|---|---|---|---|
| Resveratrol (500 mg orally once daily) |
Clinical trials Mild to moderate AD (n = 119) |
mall functional benefits | [62] |
| Rosiglitazone (4 mg orally once daily) | MCI or mild AD (n = 30) | Small functional benefits | [63] |
| Rosiglitazone (2, 4 or 8 mg daily) | Mild to moderate AD (n = 511) | Small cognitive benefits in the ApoEe4-treated group | [64] |
| Pioglitazone (15 mg daily) | Mild to moderate AD (n = 29) | No benefits | [65] |
| Ibuprofen (400 mg twice daily) | Mild to moderate AD (n = 132) | No benefits | [66] |
| Indomethacin (100 mg daily) | Mild to moderate AD (n = 51) | No benefits | [67] |
| Naproxen (220 mg once daily) | Mild to moderate AD (n = 40) | Small functional and cognitive benefits | [68] |
3.1. Non-Pharmacological Approaches
Gene therapy studies from our laboratory demonstrated that PGC1α gene delivery using lentiviral vectors in the APP23 model of amyloidosis (overexpressing APP with the Swedish mutation) at pre-symptomatic stages of AD resulted in decreased Aβ plaques, neuronal loss, and improved memory [38]. PGC1α overexpression in the cortex and hippocampus of APP23 mice led to decreased expression of BACE1, without changes in the mechanisms of amyloid degradation or in mitochondrial markers. In addition, PGC1α-injected mice showed reduced inflammatory markers and neuronal loss in pyramidal neurons of the CA3 area of the hippocampus and improved spatial and recognition memory compared with control APP23 mice, associated with an increased expression of neurotrophic factors. The effects on factors such as BDNF can be mediated through the PGC1α/FNDC5/BDNF pathway [8].
Recently, PGC1α was shown to display beneficial effects by regulating the expression of vitamin D receptors. Overexpression of PGC1α in APP/PS1 mice by hippocampal injection of AAV-PGC-1α resulted in an increase in the expression of VDR and a decrease in the levels of Aβ plaques [32].
Thus, there is now substantial evidence indicating that modulation of PGC1α levels in the brain may be an effective approach, although the type of viral vectors used, as well as the brain area targeted, are critical in order to obtain the expected beneficial effects. Too much overexpression of PGC1α can lead to damaging effects in particular cell types, such as dopaminergic neurons, which are more prone to degeneration [56].
3.2. Pharmacological Approaches
3.2.1. Resveratrol
Resveratrol is a polyphenol produced in several plants, especially grape skin and seeds. Accumulating evidence has highlighted the neuroprotective effects of resveratrol in neurodegenerative diseases, such as AD [57,58]. Special attention has been focused on resveratrol due to its multiple biological properties, including its antioxidant, anti-inflammatory, and neuroprotective effects [59,60].
Among the mechanisms underlying the neuroprotective effects exerted by resveratrol on AD, studies have suggested the activation of AMP-activated protein kinase (AMPK) and the indirect activation of silent information regulator 1 (SIRT1), as a critical pathway on AD [51,61]. SIRT1 is a nicotinamide adenosine dinucleotide (NAD)-dependent deacetylase that regulates the activity of several proteins by removing acetyl groups from them, including PGC1α [49]. In vitro, resveratrol was reported to have a potent anti-amyloidogenic activity, reducing the levels of Aβ in N2a and HEK293Tcells expressing human Swedish mutation APP695 by promoting Aβ clearance but not affecting APP processing [41]. Furthermore, as described in the previous section, we showed that incubation of N2asw cells with resveratrol increased neprilysin activity, yet no changes were observed in the expression or in the mRNA levels of the enzyme, indicating that PGC-1α effects on neprilysin activity may be linked to transcriptional-independent mechanisms [33]. In vivo, oral treatment with resveratrol significantly reduced Aβ levels and deposition in the cortex of APP/PS1 mice through activation of AMPK, confirming not only the anti-amyloidogenic potential of resveratrol, but also its ability to cross the blood-brain-barrier [51]. However, a 52 week randomised phase 2 clinical trial of resveratrol in individuals with mild to moderate AD detected a low concentration of resveratrol in cerebrospinal fluid (CSF), although a high daily dose of oral resveratrol was administrated, suggesting a poor bioavailability of oral treatment with resveratrol in humans [62]. However, it was still effective in stabilising the decline in CSF and plasma Aβ40 levels and attenuating the decline in a functional measurement test [62]. Although several studies have indicated the protective involvement of resveratrol in the pathophysiology of AD, more studies are required to determine the bioavailability of SIRT1 activators, such as resveratrol.
3.2.2. Nicotinamide Riboside
Nicotinamide riboside, the precursor of NAD+, has been reported to increase PGC1α levels through NAD-dependent deacetylase SIRT1. NAD levels have been associated with reductions in Aβ toxicity in AD models [69,70]. Pharmacological stimulation of PGC1α synthesis with 250 mg/kg/day of nicotinamide riboside, the precursor of NAD+, for 3 months resulted in reduced Aβ levels and attenuated cognitive deterioration in Tg2576 mice. These changes were associated with reduced BACE1 expression [35].
3.2.3. Sildenafil
Sildenafil (Viagra), a drug used to treat erectile dysfunction and pulmonary arterial hypertension (particularly at low doses), likely activates PGC1α by affecting sirtuin-1 activation and PGC1α deacetylation. In transgenic mice, sildenafil appeared to reduce neuroinflammatory markers, increase neurogenesis, and improve behaviour, without evident changes in amyloid deposition [71].
3.2.4. PPARγ Agonists
As indicated above, the main transcription factor regulated by PGC1α is PPARγ, and the effects of PGC1α on BACE1 expression are controlled by PPARγ [33]. PPARγ activators comprise different groups of drugs, including thiazolidinediones (TZDs) and certain nonsteroidal anti-inflammatory drugs (NSAIDs), such as ibuprofen, naproxen, and indomethacine [72,73].
TZD drugs are synthetic agonists of PPAR-γ and are the most potent activators than any endogenous ligand of PPARγ, including rosiglitazone, troglitazone, and pioglitazone. The anti-inflammatory actions of pioglitazone occur, in part, through suppression of NF-kβ and the sequestering of co-activators necessary for inflammatory gene activation. Short-term treatment with pioglitazone suppressed neuroinflammation and decreased mRNA and protein level of BACE1 in APPV717I-transgenic mice (overexpressing APP with the V171I mutation) [50]. Furthermore, long-term treatments with pioglitazone in animal models of AD resulted in reduced amyloid deposition and neuroinflammation and ameliorated learning and memory in the 3xtg-AD model [52,53]. Similarly, chronic treatment of Tg2576 and J20 mice with rosiglitazone, a high-affinity PPARγ agonist, rescued memory impairment concomitant with a reduction in cortical Aβ levels and Aβ plaque deposition [54,55]. Although TZDs have demonstrated beneficial effects in animals models of AD, clinical trials have only reported mild improvements in memory, due to the low permeability of these compounds (see [72] and Table 2).
NSAIDs were first postulated to protect from AD due to the extensive benefits in counteracting neuroinflammation through cyclooxygenase (COX) mediated inhibition [74]. Lately, additional attention was paid to certain NSAIDs, such as ibuprofen or indomethacin, due to their protective effects on AD pathology by lowering Aβ peptide levels, independent of COX activity [75,76]. NSAIDs treatments have been widely tested in animal models of AD and its effects on Aβ levels and deposition are likely dependent on the length of the treatment [77]. For example, acute-fed-treatment with ibuprofen (62.5 mg/kg/day) resulted in decreased microglial activation and slight reduction in BACE1 levels and Aβ deposition in the brain of APP transgenic mice [50]. Conversely, chronic treatment with ibuprofen or indomethacin led to a more robust reduction in amyloid deposition and activated microglia, and a decrease in inflammatory mediators in mouse models of AD [29,72]. Long-term treatment with high doses of ibuprofen (56 mg/kg and 62.5 mg/kg), sufficient to activate PPARγ, delayed neuroinflammation and Aβ deposition in the Tg2576 mouse model for AD and in vitro [52,78]. However, both drugs, ibuprofen and indomethacin, failed to demonstrate efficiency in slowing the progression of AD in patients with mild to moderate AD, in 12 month randomised clinical trials [66,67]. Naproxen, another well-established NSAID, is a non-selective COX inhibitor known to reduce inflammation through inhibiting prostaglandin synthesis and activating PPAR-γ [79]. In vivo, early treatment reduced inflammatory response, without affecting APP processing and Aβ metabolism in APP transgenic mice model of AD [80]. Evidence suggests that NSAIDs use is more effective at preventing AD before disease onset. For instance, naproxen underwent a clinical trial to assess its ability to slow cognitive decline in patients with mild to moderate AD; however, it was discontinued due to lack of efficiency [68].
4. Conclusions
In conclusion, PGC1α activation, either via drugs that increase its levels (such as resveratrol or nicotinamide riboside) or the activation of transcription factors regulated by PGC1α (such as PPARγ agonists), results in reductions in Alzheimer pathology and improvements in behaviour. However, although gene therapy approaches appear promising, this approach should be taken with caution, because the procedures for gene delivery are highly invasive and high overexpression of PGC1α may result in deleterious effects.
Future studies on PGC1α-based therapies should investigate the effect of other pathological hallmarks present in AD brains such as tau pathology. In addition, it would be worth exploring the effect of PGC1α activation in ageing and in animal models at late stages of the disease. The potential therapeutic value of this molecule at these stages is based on the effect in the expression of growth factors, such as BDNF, which can affect neurogenesis and protect against neuronal loss, as well as the potential anti-inflammatory effects of PGC1α.
Acknowledgments
We thank the Dunhill Foundation Medical Trust for funding B.M.’s salary.
Author Contributions
B.C.M. wrote the section of therapeutic effects and organised the references and the tables, and M.S. wrote the rest. All authors have read and agreed to the published version of the manuscript.
Funding
Dunhill Foundation Medical Trust (grant reference R591/0717).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Handschin C., Spiegelman B.M. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr. Rev. 2006;27:728–735. doi: 10.1210/er.2006-0037. [DOI] [PubMed] [Google Scholar]
- 2.Puigserver P., Spiegelman B.M. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): Transcriptional coactivator and metabolic regulator. Endocr. Rev. 2003;24:78–90. doi: 10.1210/er.2002-0012. [DOI] [PubMed] [Google Scholar]
- 3.Ventura-Clapier R., Garnier A., Veksler V. Transcriptional control of mitochondrial biogenesis: The central role of PGC-1alpha. Cardiovasc. Res. 2008;79:208–217. doi: 10.1093/cvr/cvn098. [DOI] [PubMed] [Google Scholar]
- 4.McMeekin L.J., Fox S.N., Boas S.M., Cowell R.M. Dysregulation of PGC-1alpha-Dependent Transcriptional Programs in Neurological and Developmental Disorders: Therapeutic Challenges and Opportunities. Cells. 2021;10:352. doi: 10.3390/cells10020352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esterbauer H., Oberkofler H., Krempler F., Patsch W. Human peroxisome proliferator activated receptor gamma coactivator 1 (PPARGC1) gene: cDNA sequence, genomic organization, chromosomal localization, and tissue expression. Genomics. 1999;62:98–102. doi: 10.1006/geno.1999.5977. [DOI] [PubMed] [Google Scholar]
- 6.Tritos N.A., Mastaitis J.W., Kokkotou E.G., Puigserver P., Spiegelman B.M., Maratos-Flier E. Characterization of the peroxisome proliferator activated receptor coactivator 1 alpha (PGC 1alpha) expression in the murine brain. Brain Res. 2003;961:255–260. doi: 10.1016/S0006-8993(02)03961-6. [DOI] [PubMed] [Google Scholar]
- 7.Rius-Pérez S., Torres-Cuevas I., Millán I., Ortega Á.L., Pérez S. PGC-1 α, Inflammation, and Oxidative Stress: An Integrative View in Metabolism. Oxid. Med. Cell. Longev. 2020;2020:1452696. doi: 10.1155/2020/1452696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wrann C.D., White J.P., Salogiannnis J., Laznik-Bogoslavski D., Wu J., Ma D., Lin J.D., Greenberg M.E., Spiegelman B.M. Exercise induces hippocampal BDNF through a PGC-1alpha/FNDC5 pathway. Cell Metab. 2013;18:649–659. doi: 10.1016/j.cmet.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nemoto S., Fergusson M.M., Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1α. J. Biol. Chem. 2005;280:16456–16460. doi: 10.1074/jbc.M501485200. [DOI] [PubMed] [Google Scholar]
- 10.Herzig S., Long F., Jhala U.S., Hedrick S., Quinn R., Bauer A., Rudolph D., Schutz G., Yoon C., Puigserver P., et al. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature. 2001;413:179–183. doi: 10.1038/35093131. [DOI] [PubMed] [Google Scholar]
- 11.Handschin C., Lin J., Rhee J., Peyer A.K., Chin S., Wu P.H., Meyer U.A., Spiegelman B.M. Nutritional regulation of hepatic heme biosynthesis and porphyria through PGC-1alpha. Cell. 2005;122:505–515. doi: 10.1016/j.cell.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 12.Halling J.F., Ringholm S., Nielsen M.M., Overby P., Pilegaard H. PGC-1alpha promotes exercise-induced autophagy in mouse skeletal muscle. Physiol. Rep. 2016;4:e12698. doi: 10.14814/phy2.12698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu J., Ruas J.L., Estall J.L., Rasbach K.A., Choi J.H., Ye L., Bostrom P., Tyra H.M., Crawford R.W., Campbell K.P., et al. The unfolded protein response mediates adaptation to exercise in skeletal muscle through a PGC-1alpha/ATF6alpha complex. Cell Metab. 2011;13:160–169. doi: 10.1016/j.cmet.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cowell R.M., Blake K.R., Russell J.W. Localization of the transcriptional coactivator PGC-1alpha to GABAergic neurons during maturation of the rat brain. J. Comp. Neurol. 2007;502:1–18. doi: 10.1002/cne.21211. [DOI] [PubMed] [Google Scholar]
- 15.Lucas E.K., Dougherty S.E., McMeekin L.J., Trinh A.T., Reid C.S., Cowell R.M. Developmental alterations in motor coordination and medium spiny neuron markers in mice lacking pgc-1alpha. PLoS ONE. 2012;7:e42878. doi: 10.1371/journal.pone.0042878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lucas E.K., Reid C.S., McMeekin L.J., Dougherty S.E., Floyd C.L., Cowell R.M. Cerebellar transcriptional alterations with Purkinje cell dysfunction and loss in mice lacking PGC-1alpha. Front. Cell. Neurosci. 2014;8:441. doi: 10.3389/fncel.2014.00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui L., Jeong H., Borovecki F., Parkhurst C.N., Tanese N., Krainc D. Transcriptional repression of PGC-1alpha by mutant huntingtin leads to mitochondrial dysfunction and neurodegeneration. Cell. 2006;127:59–69. doi: 10.1016/j.cell.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 18.McMeekin L.J., Li Y., Fox S.N., Rowe G.C., Crossman D.K., Day J.J., Li Y., Detloff P.J., Cowell R.M. Cell-Specific Deletion of PGC-1alpha from Medium Spiny Neurons Causes Transcriptional Alterations and Age-Related Motor Impairment. J. Neurosci. 2018;38:3273–3286. doi: 10.1523/JNEUROSCI.0848-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.St-Pierre J., Drori S., Uldry M., Silvaggi J.M., Rhee J., Jager S., Handschin C., Zheng K., Lin J., Yang W., et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 20.Cheng A., Wan R., Yang J.L., Kamimura N., Son T.G., Ouyang X., Luo Y., Okun E., Mattson M.P. Involvement of PGC-1alpha in the formation and maintenance of neuronal dendritic spines. Nat. Commun. 2012;3:1250. doi: 10.1038/ncomms2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang H., Kang S.U., Zhang S., Karuppagounder S., Xu J., Lee Y.K., Kang B.G., Lee Y., Zhang J., Pletnikova O., et al. Adult Conditional Knockout of PGC-1alpha Leads to Loss of Dopamine Neurons. eNeuro. 2016;3 doi: 10.1523/ENEURO.0183-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nijland P.G., Witte M.E., van het Hof B., van der Pol S., Bauer J., Lassmann H., van der Valk P., de Vries H.E., van Horssen J. Astroglial PGC-1alpha increases mitochondrial antioxidant capacity and suppresses inflammation: Implications for multiple sclerosis. Acta Neuropathol. Commun. 2014;2:170. doi: 10.1186/s40478-014-0170-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiong W., MacColl Garfinkel A.E., Li Y., Benowitz L.I., Cepko C.L. NRF2 promotes neuronal survival in neurodegeneration and acute nerve damage. J. Clin. Investig. 2015;125:1433–1445. doi: 10.1172/JCI79735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim S.B., Heo J.I., Kim H., Kim K.S. Acetylation of PGC1alpha by Histone Deacetylase 1 Downregulation Is Implicated in Radiation-Induced Senescence of Brain Endothelial Cells. J. Gerontol. A Biol. Sci. Med. Sci. 2019;74:787–793. doi: 10.1093/gerona/gly167. [DOI] [PubMed] [Google Scholar]
- 25.Witte M.E., Nijland P.G., Drexhage J.A., Gerritsen W., Geerts D., van Het Hof B., Reijerkerk A., de Vries H.E., van der Valk P., van Horssen J. Reduced expression of PGC-1alpha partly underlies mitochondrial changes and correlates with neuronal loss in multiple sclerosis cortex. Acta Neuropathol. 2013;125:231–243. doi: 10.1007/s00401-012-1052-y. [DOI] [PubMed] [Google Scholar]
- 26.Pacelli C., De Rasmo D., Signorile A., Grattagliano I., di Tullio G., D’Orazio A., Nico B., Comi G.P., Ronchi D., Ferranini E., et al. Mitochondrial defect and PGC-1alpha dysfunction in parkin-associated familial Parkinson’s disease. Biochim. Biophys. Acta. 2011;1812:1041–1053. doi: 10.1016/j.bbadis.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 27.Che H.V., Metzger S., Portal E., Deyle C., Riess O., Nguyen H.P. Localization of sequence variations in PGC-1alpha influence their modifying effect in Huntington disease. Mol. Neurodegener. 2011;6:1. doi: 10.1186/1750-1326-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eschbach J., Schwalenstocker B., Soyal S.M., Bayer H., Wiesner D., Akimoto C., Nilsson A.C., Birve A., Meyer T., Dupuis L., et al. PGC-1alpha is a male-specific disease modifier of human and experimental amyotrophic lateral sclerosis. Hum. Mol. Genet. 2013;22:3477–3484. doi: 10.1093/hmg/ddt202. [DOI] [PubMed] [Google Scholar]
- 29.Sastre M., Walter J., Gentleman S.M. Interactions between APP secretases and inflammatory mediators. J. Neuroinflamm. 2008;5:25. doi: 10.1186/1742-2094-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gotz J., Bodea L.G., Goedert M. Rodent models for Alzheimer disease. Nat. Rev. Neurosci. 2018;19:583–598. doi: 10.1038/s41583-018-0054-8. [DOI] [PubMed] [Google Scholar]
- 31.Gong B., Chen F., Pan Y., Arrieta-Cruz I., Yoshida Y., Haroutunian V., Pasinetti G.M. SCFFbx2-E3-ligase-mediated degradation of BACE1 attenuates Alzheimer’s disease amyloidosis and improves synaptic function. Aging Cell. 2010;9:1018–1031. doi: 10.1111/j.1474-9726.2010.00632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J., Guo M.N., Liu Z.Z., Ma S.F., Liu W.J., Qian J.J., Zhang W.N. PGC-1alpha reduces Amyloid-beta deposition in Alzheimer’s disease: Effect of increased VDR expression. Neurosci. Lett. 2021;744:135598. doi: 10.1016/j.neulet.2020.135598. [DOI] [PubMed] [Google Scholar]
- 33.Katsouri L., Parr C., Bogdanovic N., Willem M., Sastre M. PPARgamma co-activator-1alpha (PGC-1alpha) reduces amyloid-beta generation through a PPARgamma-dependent mechanism. J. Alzheimers Dis. 2011;25:151–162. doi: 10.3233/JAD-2011-101356. [DOI] [PubMed] [Google Scholar]
- 34.Qin W., Haroutunian V., Katsel P., Cardozo C.P., Ho L., Buxbaum J.D., Pasinetti G.M. PGC-1alpha expression decreases in the Alzheimer disease brain as a function of dementia. Arch Neurol. 2009;66:352–361. doi: 10.1001/archneurol.2008.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gong B., Pan Y., Vempati P., Zhao W., Knable L., Ho L., Wang J., Sastre M., Ono K., Sauve A.A., et al. Nicotinamide riboside restores cognition through an upregulation of proliferator-activated receptor-gamma coactivator 1alpha regulated beta-secretase 1 degradation and mitochondrial gene expression in Alzheimer’s mouse models. Neurobiol. Aging. 2013;34:1581–1588. doi: 10.1016/j.neurobiolaging.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dumont M., Stack C., Elipenahli C., Jainuddin S., Launay N., Gerges M., Starkova N., Starkov A.A., Calingasan N.Y., Tampellini D., et al. PGC-1alpha overexpression exacerbates beta-amyloid and tau deposition in a transgenic mouse model of Alzheimer’s disease. FASEB J. 2014;28:1745–1755. doi: 10.1096/fj.13-236331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang R., Li J.J., Diao S., Kwak Y.D., Liu L., Zhi L., Bueler H., Bhat N.R., Williams R.W., Park E.A., et al. Metabolic stress modulates Alzheimer’s beta-secretase gene transcription via SIRT1-PPARgamma-PGC-1 in neurons. Cell Metab. 2013;17:685–694. doi: 10.1016/j.cmet.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Katsouri L., Lim Y.M., Blondrath K., Eleftheriadou I., Lombardero L., Birch A.M., Mirzaei N., Irvine E.E., Mazarakis N.D., Sastre M. PPARgamma-coactivator-1alpha gene transfer reduces neuronal loss and amyloid-beta generation by reducing beta-secretase in an Alzheimer’s disease model. Proc. Natl. Acad. Sci. USA. 2016;113:12292–12297. doi: 10.1073/pnas.1606171113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sastre M., Dewachter I., Rossner S., Bogdanovic N., Rosen E., Borghgraef P., Evert B.O., Dumitrescu-Ozimek L., Thal D.R., Landreth G., et al. Nonsteroidal anti-inflammatory drugs repress beta-secretase gene promoter activity by the activation of PPARgamma. Proc. Natl. Acad. Sci. USA. 2006;103:443–448. doi: 10.1073/pnas.0503839103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Corbett G.T., Gonzalez F.J., Pahan K. Activation of peroxisome proliferator-activated receptor alpha stimulates ADAM10-mediated proteolysis of APP. Proc. Natl. Acad. Sci. USA. 2015;112:8445–8450. doi: 10.1073/pnas.1504890112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marambaud P., Zhao H., Davies P. Resveratrol promotes clearance of Alzheimer’s disease amyloid-beta peptides. J. Biol. Chem. 2005;280:37377–37382. doi: 10.1074/jbc.M508246200. [DOI] [PubMed] [Google Scholar]
- 42.Kalinin S., Richardson J.C., Feinstein D.L. A PPARdelta agonist reduces amyloid burden and brain inflammation in a transgenic mouse model of Alzheimer’s disease. Curr. Alzheimer Res. 2009;6:431–437. doi: 10.2174/156720509789207949. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y., Chen C., Jiang Y., Wang S., Wu X., Wang K. PPARgamma coactivator-1alpha (PGC-1alpha) protects neuroblastoma cells against amyloid-beta (Abeta) induced cell death and neuroinflammation via NF-kappaB pathway. BMC Neurosci. 2017;18:69. doi: 10.1186/s12868-017-0387-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aguirre-Rueda D., Guerra-Ojeda S., Aldasoro M., Iradi A., Obrador E., Ortega A., Mauricio M.D., Vila J.M., Valles S.L. Astrocytes protect neurons from Abeta1-42 peptide-induced neurotoxicity increasing TFAM and PGC-1 and decreasing PPAR-gamma and SIRT-1. Int. J. Med. Sci. 2015;12:48–56. doi: 10.7150/ijms.10035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Handschin C., Spiegelman B.M. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature. 2008;454:463–469. doi: 10.1038/nature07206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jung S., Kim K. Exercise-induced PGC-1alpha transcriptional factors in skeletal muscle. Integr. Med. Res. 2014;3:155–160. doi: 10.1016/j.imr.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin J., Handschin C., Spiegelman B.M. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1:361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 48.Puigserver P., Wu Z., Park C.W., Graves R., Wright M., Spiegelman B.M. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/S0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 49.Lagouge M., Argmann C., Gerhart-Hines Z., Meziane H., Lerin C., Daussin F., Messadeq N., Milne J., Lambert P., Elliott P., et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 50.Heneka M.T., Sastre M., Dumitrescu-Ozimek L., Hanke A., Dewachter I., Kuiperi C., O’Banion K., Klockgether T., Van Leuven F., Landreth G.E. Acute treatment with the PPARgamma agonist pioglitazone and ibuprofen reduces glial inflammation and Abeta1-42 levels in APPV717I transgenic mice. Brain. 2005;128:1442–1453. doi: 10.1093/brain/awh452. [DOI] [PubMed] [Google Scholar]
- 51.Vingtdeux V., Giliberto L., Zhao H., Chandakkar P., Wu Q., Simon J.E., Janle E.M., Lobo J., Ferruzzi M.G., Davies P., et al. AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-beta peptide metabolism. J. Biol. Chem. 2010;285:9100–9113. doi: 10.1074/jbc.M109.060061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yan Q., Zhang J., Liu H., Babu-Khan S., Vassar R., Biere A.L., Citron M., Landreth G. Anti-inflammatory drug therapy alters beta-amyloid processing and deposition in an animal model of Alzheimer’s disease. J. Neurosci. 2003;23:7504–7509. doi: 10.1523/JNEUROSCI.23-20-07504.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Searcy J.L., Phelps J.T., Pancani T., Kadish I., Popovic J., Anderson K.L., Beckett T.L., Murphy M.P., Chen K.C., Blalock E.M., et al. Long-term pioglitazone treatment improves learning and attenuates pathological markers in a mouse model of Alzheimer’s disease. J. Alzheimers Dis. 2012;30:943–961. doi: 10.3233/JAD-2012-111661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Escribano L., Simon A.M., Gimeno E., Cuadrado-Tejedor M., Lopez de Maturana R., Garcia-Osta A., Ricobaraza A., Perez-Mediavilla A., Del Rio J., Frechilla D. Rosiglitazone rescues memory impairment in Alzheimer’s transgenic mice: Mechanisms involving a reduced amyloid and tau pathology. Neuropsychopharmacology. 2010;35:1593–1604. doi: 10.1038/npp.2010.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pedersen W.A., McMillan P.J., Kulstad J.J., Leverenz J.B., Craft S., Haynatzki G.R. Rosiglitazone attenuates learning and memory deficits in Tg2576 Alzheimer mice. Exp. Neurol. 2006;199:265–273. doi: 10.1016/j.expneurol.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 56.Ciron C., Lengacher S., Dusonchet J., Aebischer P., Schneider B.L. Sustained expression of PGC-1alpha in the rat nigrostriatal system selectively impairs dopaminergic function. Hum. Mol. Genet. 2012;21:1861–1876. doi: 10.1093/hmg/ddr618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Caruana M., Cauchi R., Vassallo N. Putative Role of Red Wine Polyphenols against Brain Pathology in Alzheimer’s and Parkinson’s Disease. Front. Nutr. 2016;3:31. doi: 10.3389/fnut.2016.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Karuppagounder S.S., Pinto J.T., Xu H., Chen H.L., Beal M.F., Gibson G.E. Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimer’s disease. Neurochem. Int. 2009;54:111–118. doi: 10.1016/j.neuint.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gambini J., Ingles M., Olaso G., Lopez-Grueso R., Bonet-Costa V., Gimeno-Mallench L., Mas-Bargues C., Abdelaziz K.M., Gomez-Cabrera M.C., Vina J., et al. Properties of Resveratrol: In Vitro and In Vivo Studies about Metabolism, Bioavailability, and Biological Effects in Animal Models and Humans. Oxid. Med. Cell. Longev. 2015;2015:837042. doi: 10.1155/2015/837042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gülçin İ. Antioxidant properties of resveratrol: A structure–activity insight. Innov. Food Sci. Emerg. Technol. 2010;11:210–218. doi: 10.1016/j.ifset.2009.07.002. [DOI] [Google Scholar]
- 61.Beher D., Wu J., Cumine S., Kim K.W., Lu S.C., Atangan L., Wang M. Resveratrol is not a direct activator of SIRT1 enzyme activity. Chem. Biol. Drug Des. 2009;74:619–624. doi: 10.1111/j.1747-0285.2009.00901.x. [DOI] [PubMed] [Google Scholar]
- 62.Turner R.S., Thomas R.G., Craft S., van Dyck C.H., Mintzer J., Reynolds B.A., Brewer J.B., Rissman R.A., Raman R., Aisen P.S., et al. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology. 2015;85:1383–1391. doi: 10.1212/WNL.0000000000002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Watson G.S., Cholerton B.A., Reger M.A., Baker L.D., Plymate S.R., Asthana S., Fishel M.A., Kulstad J.J., Green P.S., Cook D.G., et al. Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: A preliminary study. Am. J. Geriatr. Psychiatry. 2005;13:950–958. doi: 10.1176/appi.ajgp.13.11.950. [DOI] [PubMed] [Google Scholar]
- 64.Risner M.E., Saunders A.M., Altman J.F., Ormandy G.C., Craft S., Foley I.M., Zvartau-Hind M.E., Hosford D.A., Roses A.D., Rosiglitazone in Alzheimer’s Disease Study G. Efficacy of rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer’s disease. Pharm. J. 2006;6:246–254. doi: 10.1038/sj.tpj.6500369. [DOI] [PubMed] [Google Scholar]
- 65.Geldmacher D.S., Fritsch T., McClendon M.J., Landreth G. A randomized pilot clinical trial of the safety of pioglitazone in treatment of patients with Alzheimer disease. Arch. Neurol. 2011;68:45–50. doi: 10.1001/archneurol.2010.229. [DOI] [PubMed] [Google Scholar]
- 66.Pasqualetti P., Bonomini C., Dal Forno G., Paulon L., Sinforiani E., Marra C., Zanetti O., Rossini P.M. A randomized controlled study on effects of ibuprofen on cognitive progression of Alzheimer’s disease. Aging Clin. Exp. Res. 2009;21:102–110. doi: 10.1007/BF03325217. [DOI] [PubMed] [Google Scholar]
- 67.de Jong D., Jansen R., Hoefnagels W., Jellesma-Eggenkamp M., Verbeek M., Borm G., Kremer B. No effect of one-year treatment with indomethacin on Alzheimer’s disease progression: A randomized controlled trial. PLoS ONE. 2008;3:e1475. doi: 10.1371/journal.pone.0001475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aisen P.S., Schafer K.A., Grundman M., Pfeiffer E., Sano M., Davis K.L., Farlow M.R., Jin S., Thomas R.G., Thal L.J., et al. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: A randomized controlled trial. JAMA. 2003;289:2819–2826. doi: 10.1001/jama.289.21.2819. [DOI] [PubMed] [Google Scholar]
- 69.Kim D., Nguyen M.D., Dobbin M.M., Fischer A., Sananbenesi F., Rodgers J.T., Delalle I., Baur J.A., Sui G., Armour S.M., et al. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J. 2007;26:3169–3179. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qin W., Yang T., Ho L., Zhao Z., Wang J., Chen L., Zhao W., Thiyagarajan M., MacGrogan D., Rodgers J.T., et al. Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J. Biol. Chem. 2006;281:21745–21754. doi: 10.1074/jbc.M602909200. [DOI] [PubMed] [Google Scholar]
- 71.Sanders O. Sildenafil for the Treatment of Alzheimer’s Disease: A Systematic Review. J. Alzheimers Dis. Rep. 2020;4:91–106. doi: 10.3233/ADR-200166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lleo A., Galea E., Sastre M. Molecular targets of non-steroidal anti-inflammatory drugs in neurodegenerative diseases. Cell. Mol. Life Sci. 2007;64:1403–1418. doi: 10.1007/s00018-007-6516-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sastre M., Gentleman S.M. NSAIDs: How they Work and their Prospects as Therapeutics in Alzheimer’s Disease. Front. Aging Neurosci. 2010;2:20. doi: 10.3389/fnagi.2010.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sastre M., Klockgether T., Heneka M.T. Contribution of inflammatory processes to Alzheimer’s disease: Molecular mechanisms. Int. J. Dev. Neurosci. 2006;24:167–176. doi: 10.1016/j.ijdevneu.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 75.Eriksen J.L., Sagi S.A., Smith T.E., Weggen S., Das P., McLendon D.C., Ozols V.V., Jessing K.W., Zavitz K.H., Koo E.H., et al. NSAIDs and enantiomers of flurbiprofen target gamma-secretase and lower Abeta 42 in vivo. J. Clin. Investig. 2003;112:440–449. doi: 10.1172/JCI18162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weggen S., Eriksen J.L., Das P., Sagi S.A., Wang R., Pietrzik C.U., Findlay K.A., Smith T.E., Murphy M.P., Bulter T., et al. A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity. Nature. 2001;414:212–216. doi: 10.1038/35102591. [DOI] [PubMed] [Google Scholar]
- 77.Kukar T., Golde T.E. Possible mechanisms of action of NSAIDs and related compounds that modulate gamma-secretase cleavage. Curr. Top. Med. Chem. 2008;8:47–53. doi: 10.2174/156802608783334042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lim G.P., Yang F., Chu T., Chen P., Beech W., Teter B., Tran T., Ubeda O., Ashe K.H., Frautschy S.A., et al. Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer’s disease. J. Neurosci. 2000;20:5709–5714. doi: 10.1523/JNEUROSCI.20-15-05709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jaradat M.S., Wongsud B., Phornchirasilp S., Rangwala S.M., Shams G., Sutton M., Romstedt K.J., Noonan D.J., Feller D.R. Activation of peroxisome proliferator-activated receptor isoforms and inhibition of prostaglandin H(2) synthases by ibuprofen, naproxen, and indomethacin. Biochem. Pharmacol. 2001;62:1587–1595. doi: 10.1016/S0006-2952(01)00822-X. [DOI] [PubMed] [Google Scholar]
- 80.Varvel N.H., Bhaskar K., Kounnas M.Z., Wagner S.L., Yang Y., Lamb B.T., Herrup K. NSAIDs prevent, but do not reverse, neuronal cell cycle reentry in a mouse model of Alzheimer disease. J. Clin. Investig. 2009;119:3692–3702. doi: 10.1172/JCI39716. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.