Abstract
This review aimed to identify, evaluate, and synthesize the scientific literature on mobile health (mHealth) interventions to promote physical activity (PA) or reduce sedentary behavior (SB) in cancer survivors. We searched six databases from 2000 to 13 April 2020 for controlled and non-controlled trials published in any language. We conducted best evidence syntheses on controlled trials to assess the strength of the evidence. All 31 interventions included in this review measured PA outcomes, with 10 of them also evaluating SB outcomes. Most study participants were adults/older adults with various cancer types. The majority (n = 25) of studies implemented multicomponent interventions, with activity trackers being the most commonly used mHealth technology. There is strong evidence for mHealth interventions, including personal contact components, in increasing moderate-to-vigorous intensity PA among cancer survivors. However, there is inconclusive evidence to support mHealth interventions in increasing total activity and step counts. There is inconclusive evidence on SB potentially due to the limited number of studies. mHealth interventions that include personal contact components are likely more effective in increasing PA than mHealth interventions without such components. Future research should address social factors in mHealth interventions for PA and SB in cancer survivors.
Keywords: fitness tracker, exercise, mobile health, mobile application, health behavior
1. Introduction
In 2020, it was estimated that there were about 19.3 million new cancer cases and almost 10.0 million cancer deaths worldwide [1]. Cancer affects all regions of the world and is a major cause of morbidity and mortality. This is concerning considering that it is predicted that an estimated 28.4 million new cancer cases are expected to occur worldwide in 2040. This is a 47% increase in annual cases from the 19.3 million cases reported in 2020 [1].
Although there is a trend of increasing cancer survival [2], cancer survivors must cope with cancer complications and treatments that impact health and quality of life [3]. For example, 25% and 10% of cancer survivors reported poor physical and mental health, respectively. This stands in contrast to 10% and 6% in adults without cancer [4]. Cancer survivors are also at increased risk of recurrent cancer and other diseases, such as cardiovascular disease, diabetes, and osteoporosis [5]. Increasing physical activity (PA) and reducing sedentary behavior (SB) can improve the health and quality of life of cancer survivors.
The value of PA, defined as any bodily movement produced by skeletal muscles that need energy expenditure [6], on cancer survivors have been reported by numerous studies [7]. Engaging in PA after a cancer diagnosis has been found to be associated with decreased risk of cancer-specific and all-cause mortality among individuals with breast, colon, and prostate cancer [8]. Engaging in approximately 150 min per week of moderate PA associated with improved survival rate in breast cancer [9] and colon cancer [10]. In addition, there is strong evidence that engaging in PA during and after cancer treatments is associated with improved physiologic and psychosocial outcomes, including aerobic fitness, body composition, fatigue, mood, and quality of life [11]. This is particularly true for breast and colon cancers.
In addition to studies on PA, SB has received recent research attention. SB, as distinct from insufficient PA, is defined as any waking behavior in a sitting or reclining posture that requires energy expenditure ≤1.5 times the basal metabolic rate [12]. This includes, among others, using electronic devices while sitting, reclining or lying down; sitting at work; reading while lying down, and sitting during transportation. Although limited studies are examining SB and its association with health and wellbeing in cancer survivors, there is evidence that high levels of SB are linked to increased all-cause mortality, sleep disturbance, and depression symptoms in breast cancer survivors and mortality in colorectal cancer survivors [13,14,15].
The American Cancer Society Guidelines on Nutrition and Physical Activity for Cancer Survivors recommend that cancer survivors should engage in at least 150 min of moderate-intensity PA or 75 min of vigorous-intensity PA per week [16]. Unfortunately, many cancer survivors do not meet these guidelines. For example, only 33% of cancer survivors in the United States and 26% in South Korea met the PA recommendation [16,17]. In addition, the World Health Organization recommends to limit SB and/or replace sedentary time with any PA while aiming to do more moderate-to-vigorous PA (MVPA), defined as PA that is performed at >3 total metabolic equivalents (METs), for minimizing detrimental effects of high levels of SB [17]. Many cancer survivors spent a large part of their day being sedentary. Cancer survivors, independent of cancer type, have been found to spend longer than eight hours per day being sedentary [18].
The proliferation of mobile devices has contributed to the popularity of mobile health (mHealth) as a novel mode of delivering health and healthcare [19]. mHealth is defined as using mobile phones, patient monitoring devices, personal digital assistants, and other wireless technologies to support medical and public health practice [20]. The increased ubiquity of mHealth approaches is also apparent in the PA and SB literature, where the number of scientific publications has risen sharply in recent years [21]. Despite this, there are still gaps in behavioral mHealth research. For example, the effects of specific mHealth intervention modalities and components and how mHealth intervention effects may vary by population group are still debatable topics [22]. mHealth interventions have been reported to have small to moderate effects on PA and SB in healthy populations [23].
The evidence on mHealth interventions to address PA and SB among cancer survivors is elusive. To our knowledge, two previous reviews have focused on digital interventions among cancer survivors, and both reported some positive effects only on PA [24,25]. Further, one review focused exclusively on activity tracker interventions among cancer survivors and PA and also showed similar promising effects [26]. None of these reviews have comprehensively analyzed the effects of mHealth interventions on PA and SB in cancer survivors. This is an important gap as extrapolating findings from other populations and intervention modes is not warranted. In terms of populations, this is so because interventions that target cancer survivors need to be specifically designed to account for the distinct challenges to health behavior change they face. These challenges include cancer-related fatigue and lack of support from healthcare providers [27].
The lack of a comprehensive overview of mHealth interventions on PA and SB in cancer survivors warrants the current work. This systematic review aims to identify, evaluate, and synthesize the scientific literature on mHealth interventions to promote PA or reduce SB in cancer survivors. In the Methods section, we outline our inclusion criteria, search strategy, screening procedures as well as analysis approach. The results introduce the descriptive information of the included studies, as well as the data synthesis. Results are discussed in the Discussion section.
2. Materials and Methods
This systematic review is registered with the prospective international register of systematic reviews PROSPERO network (registration no. CRD42020167694) and followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement for reporting systematic reviews [28].
2.1. Study Inclusion Criteria
2.1.1. Study Designs
Eligible studies employed experimental or quasi-experimental designs, including randomized controlled trials (RCT), controlled trials, pre-and-post trials, and crossover trials.
2.1.2. Participants
Studies were eligible for inclusion if participants were cancer survivors of any age (persons who have been diagnosed with cancer, from the time of diagnosis through the remainder of their lives) [29]. Thus, persons of any age with any type of cancer, either living with cancer, undergoing, or those having completed cancer treatment, were included.
2.1.3. Interventions
This review included studies that implemented an intervention that featured an mHealth component to address PA and/or SB in cancer survivors. mHealth components could have been delivered via mobile devices, such as mobile phones, smartphones, tablets, personal digital assistants, mobile apps, short messaging services, or wearable activity trackers. In this review, we did not consider pedometers as an mHealth component due to their non-interactive nature to communicate electronically with mobile devices or the Internet [30].
2.1.4. Comparator(s)
Studies that compared mHealth interventions with any type of control were included in this review. This may be an electronic health (eHealth) intervention (e.g., a web-based app, email, website), a non-eHealth intervention (e.g., face-to-face, pamphlets, brochures), or a no-intervention control. Studies without a comparison group (e.g., a non-controlled study) were also included.
2.1.5. Outcomes
This review included studies that reported changes in terms of PA or SB following mHealth interventions. For PA, this included changes in energy expenditure, step counts, PA level, daily time PA in minutes/hours, PA frequency, and PA intensity. For SB, this included sitting time/day, sedentary breaks, bouts of prolonged sitting, and screen time. Studies that measured PA and SB outcomes objectively (e.g., via accelerometers) or via self-report (e.g., questionnaires) were included.
2.2. Search Strategy
The electronic search strategy aimed to locate published scientific studies in any language from 2000 until 13 April 2020. The year 2000 was chosen because a previous bibliometric analysis of studies on e- and mHealth for PA, SB, and diet showed that almost no studies were published before that year [21]. The following six databases were systematically searched: Web of Science, PubMed, Scopus, CINAHL, Cochrane Central Register of Controlled Trials (CENTRAL), and SPORTDiscus. The search strategy presented in Supplementary Material S1 consists of three main categories, namely (1) “cancer” population, (2) “mHealth” intervention method, and (3) “physical activity” or “sedentary behavior” outcome variable and their synonym keywords in each category. Reference lists of relevant articles were also searched to identify additional articles. Unpublished studies, preprints and gray literature were not included in this review.
2.3. Study Selection
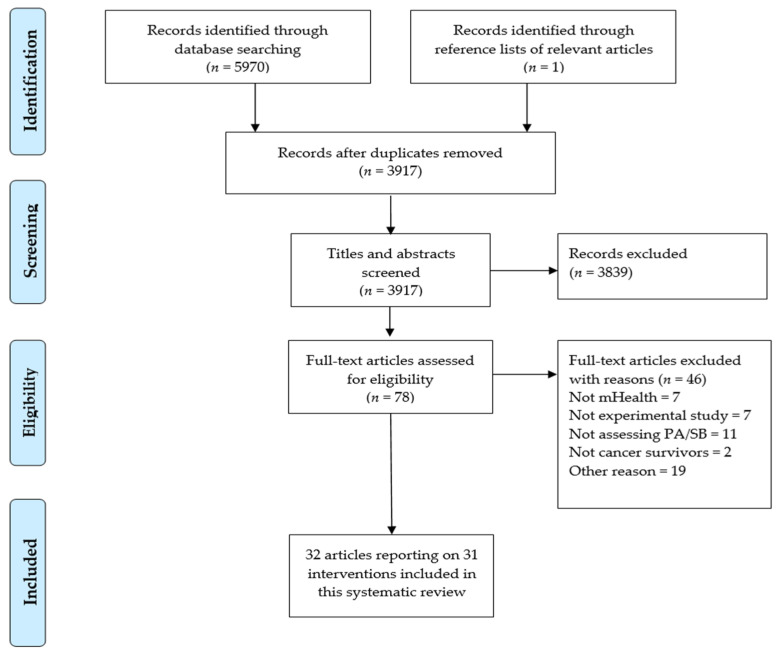
Following the search, references were imported into Zotero 5.0.89 (Corporation for Digital Scholarship, Vienna, Virginia 22182, USA), and duplicates were removed. Two independent reviewers (N.M., M.A.) screened titles and abstracts of potentially relevant articles against the inclusion criteria for the review. Disagreements were resolved by a third reviewer (A.M.M.) with vast experience in the research area and systematic review methods. Full texts of potentially relevant papers were retrieved and screened similarly. As only articles published in English were included in full-text screening, no interpretation was conducted for different language articles. The number of articles at each screening stage is shown in Figure 1.
Figure 1.
PRISMA diagram illustrating the flow of records.
2.4. Data Extraction
Data were extracted from included studies by one reviewer (N.M.) and checked for completeness and accuracy by a second reviewer (P.A.) using a standardized data extraction form adapted from a checklist presented in the Cochrane Handbook for Systematic Reviews of Interventions [31]. The extracted information of included studies included: author, year of publication, country of study, study aim, study design, sample characteristics, intervention characteristics, comparator information, outcomes measured, and results. Any disagreements that arose were resolved through consultation with a third reviewer (A.M.M.).
2.5. Risk of Bias Appraisal
Included studies were critically appraised by two independent reviewers (S.K., N.M.) using the revised Cochrane risk-of-bias tool for randomized trials (RoB 2) [32] and the risk of bias in nonrandomized studies of interventions (ROBINS-I) [33]. RoB 2 includes the following domains: randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of reported results. ROBINS-I contains seven domains, including bias due to confounding, bias in the selection of participants into the study, bias in classification of interventions, bias due to deviations from intended interventions, bias due to missing data, bias in the measurement of outcomes, and bias in the selection of the reported result. Any disagreements that arose between the reviewers were resolved through discussion with a third reviewer (A.M.M.).
2.6. Analysis
Study and intervention characteristics are described narratively. We decided against conducting a meta-analysis due to the significant heterogeneity of eligible studies in terms of intervention length, design, comparators, and outcomes. We categorized studies based on study design: controlled and non-controlled trials. We further grouped them according to intervention components: interventions that only used an mHealth component; interventions that used mHealth in addition to non-mHealth components that involved no personal contact (e.g., printed material or a website); interventions that used mHealth in addition to non-mHealth components that involved personal contact (e.g., group meetings, phone consultations).
To establish the effectiveness of interventions based on the above categorization, we conducted best evidence synthesis [34] following guidelines by Fanchini et al. [35] and Kuijer et al. [36], which were adapted from van Tulder et al. [37]. We only considered controlled trials when evaluating the evidence due to their inherently lower risk of bias. We considered the risk of bias of these controlled studies when judging the evidence. For this, the proportion of RCT with low risk of bias and some concerns raised as well as quasi-experimental trials with low risk of bias, were the basis for judging the strength of the evidence. We decided that low-risk of bias quasi-experimental trials are likely comparable to RCT with some risk [33]. We defined the following adapted evidence categories and accompanying criteria from previous guidelines [35,36,37].
-
(1)
Strong evidence: at least 66% (2/3) of controlled trials with low/some risk of bias show effect in the same direction;
-
(2)
Moderate evidence: 50% to 65% of controlled trials with low/some risk of bias show effect in the same direction;
-
(3)
Limited evidence: less than three low/some concerns risk of bias controlled trials available;
-
(4)
Inconclusive evidence: other findings not applicable to strong, moderate, or limited. For example, inconsistent findings in multiple studies (two of five or 40% low-risk studies show effect in the same direction).
3. Results
3.1. Study Selection
A total of 5971 articles were identified, and after the removal of duplicates, 3917 articles were screened for eligibility, of which 3839 were excluded. Following the first screening of titles and abstracts, 78 articles were eligible for full-text screening. After the full-text screening, 32 articles reporting on 31 interventions were included in this review. The study by Lynch et al. [38] was reported in another article [39]. Thus both will be reported as one study in this review. The PRISMA flowchart (see Figure 1) summarizes the study selection.
3.2. Study Characteristics
Of the 31 included studies, 14 were conducted in the United States [10,40,41,42,43,44,45,46,47,48,49,50,51,52], five in Australia [38,53,54,55,56], four in Republic of Korea [57,58,59,60], two each in Canada [61,62] and France [63,64], and one each in The Netherlands [65], Germany [66], Spain [67], and United Kingdom [68]. All studies were conducted between 2015 and 2020. Sixteen of the included studies were RCT [38,40,42,44,46,47,48,49,51,52,53,56,61,64,65,69], whereas 12 studies employed a pre-post [41,43,45,50,54,55,59,60,62,63,67,68], and three studies applied a quasi-experimental design [57,58,66]. The study duration ranged from two weeks [55] to six months [41,45,46,48,51,54,63]. Comparator groups included active controls in 10 studies [42,46,49,51,53,56,57,64,66,69] and inactive controls in eight studies [38,40,44,47,52,58,61,65]. In one study where more than one comparator group was used, one control group was active and the other inactive [48].
3.3. Participant Characteristics
A total of 1977 participants were recruited into the included studies at baseline, with a sample size ranging from 10 [55] to 356 [57]. Most study participants were adults/older adults with two studies, including only children/adolescents [40,45] and another study, including adolescents/young adults [41]. About a third of the studies were conducted among breast cancer survivors [38,49,51,52,53,54,57,58,61,63,67], two studies mainly included prostate cancer survivors [47,62], and another two studies included colorectal/colon cancer survivors [46,59,69]. Eight studies included survivors of various cancer types [40,41,43,44,55,64,65,68]. Several studies focused on two cancer types, such as colorectal cancer with gynecologic cancer survivors [56], breast cancer with colorectal cancer survivors [42] and breast cancer with endometrial cancer survivors [50]. The remaining studies were conducted among survivors of brain tumors [45], endometrial cancer [48], pediatric cancer [66], and hepatocellular carcinoma [60].
3.4. Intervention Characteristics
The majority (25/31) of the included studies implemented multicomponent interventions where the mHealth component was combined with or without personal contact [38,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,61,62,63,64,66,69]. Six studies exclusively relied on mHealth intervention components [58,59,60,65,67,68]. The most commonly used mHealth technology was activity trackers with 10 studies using only activity trackers [41,42,45,49,52,53,55,56,62,66], six studies using activity trackers and affiliated app [38,51,59,60,61,63], and four studies combined activity trackers with text messages [43,44,47,69]. Eight studies featured a smartphone app in their interventions [46,50,55,57,58,65,67,68], whereas three studies used text messages [48,54,64]. One study combined multiple mHealth components, namely activity trackers, app, and text messages [40]. Details of the included studies are presented in Table 1.
Table 1.
Characteristics of included studies.
| First Author, Year, Country | Study Design | Sample Size; Age Group; Cancer Type(s) |
Intervention Group | Control Group | PA/SB Outcome | Other Outcome |
|---|---|---|---|---|---|---|
| Cadmus-Bertram, 2019, United States [42] | 12 weeks RCT (Pilot) |
Baseline: n = 50 Analyzed: n = 47; Adults/older adults; Breast, colorectal |
mHealth: Activity tracker non-mHealth component: Printed material, in-person session, social support, email-based coaching and electronic health record linked to an activity tracker |
Printed material and email | PA | Weight |
| Cheong, 2018, Republic of Korea [59] | 12 weeks pre-post | Baseline/analyzed: n = 75; Adults/older adults; Colorectal |
mHealth: app providing daily exercise program and activity tracker to record activity | Not applicable | PA | Muscle strength, cardiorespiratory fitness, QoL |
| Chung, 2019, Republic of Korea [58] | 12 weeks Quasi-experimental | Baseline: n = 54 Analyzed: n = 37; Adults/older adults; Breast |
mHealth: WalkOn R app to promote healthy activities in a mobile community | No intervention | PA | Not applicable |
| Delrieu, 2020, France [63] | 6 months Pre-Post | Baseline: n = 49 Analyzed: n = 44; Adults/ older adults; Breast |
mHealth: Activity tracker and affiliated app non-mHealth component: In-person or phone sessions on performance feedback or recommendations of PA and SB |
Not applicable | PA, SB | Cardiorespiratory fitness, strength, anthropometry, QoL, fatigue |
| Gell, 2017, United States [43] | 4 weeks pre-post (Pilot) |
Baseline/Analyzed: n = 24; Adults/ older adults; Various |
mHealth: Activity tracker and text messages non-mHealth component: Health coaching phone calls |
Not applicable | PA | Self-regulation, fatigue, depression, acceptability |
| Gell, 2020, United States [44] | 8 weeks RCT (Pilot) |
Baseline: n = 66 Analyzed: n = 59; Adults/older adults; Various |
mHealth: Activity tracker and text messages non-mHealth component: Phone calls from health coach |
No intervention | PA, SB | Adherence wearing activity tracker, intervention satisfaction |
| Götte, 2018, Germany [66] | 10 weeks Quasi-experimental | Baseline: n = 40 Analyzed: n = 39; Children/adolescents; pediatric |
mHealth: Activity tracker non-mHealth component: Supervised exercise intervention during and after acute cancer treatment |
Exercise intervention after acute cancer treatment | PA | QoL, motor performance, acceptability |
| Haggerty, 2017, United States [48] | 6 months RCT (Pilot) |
Baseline: n = 41 Analyzed: n = 32; Adults/older adults; Endometrial |
mHealth: Text messages to support weight loss non-mHealth component: Conventional weighing scale |
Telemedicine (active CG) and no intervention (inactive CG) | PA | Weight loss, anthropometry, QoL |
| Hartman, 2018, United States [52] | 12 weeks RCT | Baseline/Analyzed: n = 87; Adults/ older adults; Breast |
mHealth: Activity tracker non-mHealth component: In- person session to set PA goals |
No intervention | PA | BMI, neurocognitive functioning |
| Kenfield, 2019, United States [47] | 12 weeks RCT (Pilot) |
Baseline: n = 76 Analyzed: n = 65; Adults/older adults; Prostate |
mHealth: Activity tracker and text messages non-mHealth component: Website providing behavioral and social support information |
No intervention | PA | Acceptability |
| Kim, 2020, Republic of Korea [60] | 12 weeks pre-post | Baseline/Analyzed: n = 31; Adults/older adults; Hepatocellular carcinoma |
mHealth: Care app and activity tracker prescribing exercise program | Not applicable | PA | Cardiorespiratory fitness, strength, anthropometry, QoL |
| Le, 2017, United States [41] | 6 months Pre-post (pilot) | Baseline: n = 19 Analyzed: n = 15; Adolescents/young adults; Various |
mHealth: Activity tracker to record PA non-mHealth component: Website and instruction to adhere to exercise recommendations |
Not applicable | PA | Cardiorespiratory fitness, barriers to exercise |
| Lozano-Lozano, 2019, Spain [67] | 8 weeks pre-post | Baseline: n = 80 Analyzed: n = 76; Adults/older adults; Breast |
mHealth: BENECA mHealth app to monitor and provide feedback on healthy eating and PA | Not applicable | PA | QoL, self-efficacy, anthropometry |
| Lynch, 2019, Australia [38,39] | 12 weeks RCT | Baseline: n = 83 Analyzed: n = 80; Adults/older adults; Breast |
mHealth: Activity tracker and affiliated app non-mHealth component: In-person session and telephone-health coaching sessions |
No intervention | PA, SB | Not applicable |
| Maxwell–Smith, 2019, Australia [56] | 12 weeks RCT | Baseline: n = 68 Analyzed: n = 67; Adults/older adults; Colorectal, gynecologic (at risk of cardiovascular disease) |
mHealth: Activity tracker non-mHealth component: Dashboard to collect PA engagement data, printed material, group sessions and phone call at Week 8 |
Printed material | PA, SB | BMI, blood pressure |
| Mayer, 2018, United States [46] | 6 months RCT | Baseline: n = 284 Analyzed: n = 227; Adults/older adults; Colon |
mHealth: SurvivorCHESS app to help increase daily activity levels non-mHealth component: Printed material, self-learning audio program for cancer survival and pedometer |
Printed material, self-learning program for cancer survival and pedometer | PA | QoL, distress |
| McCarroll, 2015, United States [50] | 4 weeks pre-post | Baseline: n = 50 Analyzed: n = 35; Adults/older adults; Breast, endometrial |
mHealth: LoseIt! app to support weight-loss non-mHealth component: Weighing scale to track weight |
Not applicable | PA | QoL, self-efficacy, anthropometry |
| McNeil, 2019, Canada [61] | 12 weeks RCT (Pilot) |
Baseline: n = 45 Analyzed: n = 41; Adults/older adults; Breast |
mHealth: Activity tracker and app to prescribe exercise non-mHealth component: Diary and phone call or e-mail |
No intervention | PA, SB | Anthropometry, cardiorespiratory fitness |
| Mendoza, 2017, United States [40] | 10 weeks RCT (Pilot) |
Baseline/analyzed: n = 59; Children/adolescents; Various |
mHealth: Activity tracker with app and text messages non-mHealth component: Facebook support group and phone call to set a daily step goal |
No intervention | PA, SB | QoL, acceptability |
| Ormel, 2018, Netherlands [65] | 12 weeks RCT | Baseline/analyzed: n = 32; Adults/older adults; Various |
mHealth: RunKeeper app for self-monitoring PA | No intervention | PA, SB | Usability of app |
| Ovans, 2018, United States [45] | 12 weeks Pre-post (pilot) | Baseline/analyzed: n = 15; Children/ adolescents; Brain tumor |
mHealth: Activity tracker non-mHealth component: Phone or in-person coaching to encourage PA |
Not applicable | PA | Cardiorespiratory fitness, QoL, fatigue |
| Pope, 2018, United States [49] | 10 weeks RCT | Baseline: n = 30 Analyzed: n = 20; Adults/older adults; Breast |
mHealth: Activity tracker non-mHealth component: Facebook group providing PA tips |
Facebook group | PA, SB | Anthropometry, cardiorespiratory fitness |
| Puszkiewic, 2016, United Kingdom [68] | 6 weeks pre-Post | Baseline/Analyzed: n = 11; Adults/older adults; Various |
mHealth: GAINFitness app that provides a PA program | Not applicable | PA | Anthropometry, QoL, sleep quality, app engagement |
| Short, 2018, Australia [55] | 2 weeks pre-post (pilot) | Baseline/analyzed: n = 10; Adults/older adults; Various |
mHealth: app to support PA. non-mHealth component: In- person consultation, handout, and telephone or email. |
Not applicable | PA | Acceptability |
| Singh, 2020, Australia [53] | 12 weeks RCT | Baseline: n = 52 Analyzed: n = 50; Adults/older adults; Breast (after completed supervised exercise intervention) |
mHealth: Activity tracker non-mHealth component: Counselling session and printed material |
Counselling session and printed material | PA | Acceptability |
| Spark, 2015, Australia [54] | 6 months pre-post | Baseline: n = 25 Analyzed: n = 23; Adults/older adults; Breast |
mHealth: Text messages to promote weight loss, PA and dietary behavior change non-mHealth component: Phone call to tailor self-regulation strategies |
Not applicable | PA | Weight |
| Trinh, 2018, Canada [62] | 12 weeks pre-post (pilot) | Baseline/Analyzed: n = 46, Adults/older adults; Prostate |
mHealth: Activity tracker non-mHealth component: Web-based SB intervention |
Not applicable | PA, SB | QoL, feasibility |
| Uhm, 2017, Republic of Korea [57] | 12 weeks Quasi-experimental | Baseline: n = 356 Analyzed: n = 339; Adults/older adults; Breast |
mHealth: Smart After Care exercise app non-mHealth component: Pedometer |
Printed material | PA | BMI, blood pressure, heart rate, strength, cardiorespiratory fitness, QoL, user satisfaction |
| Valle, 2017, United States [51] | 6 months RCT (Pilot) |
Baseline: n = 35 Analyzed: n = 33; Adults/older adults; Breast |
mHealth: Activity tracker interfaced with app non-mHealth component: In-person session, wireless weighing scale, and email-delivered behavioral lessons |
Self-regulation intervention group and wireless weighing scale | PA | BMI |
| Van Blarigan, 2019, United States [69] | 12 weeks RCT (Pilot) |
Baseline: n = 41 Analyzed: n = 39; Adults/older adults; Colorectal |
mHealth: Activity tracker and daily text messages non-mHealth component: Printed material on PA after cancer |
Printed material on PA after cancer | PA | Feasibility and acceptability |
| Villaron, 2018, France [64] | 8 weeks RCT (Pilot) |
Baseline/Analyzed: n = 43; Adults/older adults; Various |
mHealth: Weekly PA encouraging text messages non-mHealth component: PA recommendations, pedometer and printed material |
Pedometer | PA | QoL, fatigue |
BMI: body mass index; CG: control group; PA: physical activity; QoL: quality of life; RCT: randomized control trial; SB: sedentary behavior.
3.5. Intervention Effects on PA Outcomes
All 31 included studies measured PA outcomes. MVPA was the most frequently reported PA outcome (n = 18) [38,40,41,42,43,44,46,47,49,52,53,54,55,56,61,62,67,69], followed by total activity (n = 13) [42,48,49,50,51,52,53,57,59,60,61,63,65], and step counts (n = 11) [42,43,45,47,49,53,58,62,64,67,69].
3.5.1. Intervention Effects on PA Outcomes in Controlled Trials
Out of eight controlled trials, seven reported significant effects of mHealth interventions with personal contact on MVPA [38,42,44,52,53,56,61]. From these trials, two interventions featuring activity trackers and apps with personal contact reported a significant increase in MVPA daily minutes [38,61] when compared to inactive control groups. Similarly, interventions that combined activity trackers with personal contact showed significant MVPA increases compared to both active [42,53,56] and inactive [52] controls. In an intervention that used activity trackers and text messages in conjunction with personal contact, MVPA improved significantly compared to the inactive control group [44].
Some controlled trials also reported PA outcomes as total PA (n = 6) [42,48,52,53,61,65], total energy expenditure [49,51], or METs [57]. From these studies, only two mHealth interventions showed a significant increase in total activity [52,53]. These interventions employed activity trackers and also had a personal contact component. However, there was no effect on total PA when activity trackers were the only intervention component [49].
Step counts were reported as an outcome in seven controlled trials [42,47,49,53,58,64,69]. Only two of these controlled trials reported significant increases in step counts of interventions. One intervention only featured mHealth components [58], and the other intervention had other non-mHealth components without personal contact [42]. Further details are reported in Table 2, where interventions are categorized by intervention components: mHealth only interventions, multicomponent interventions featuring mHealth and other non-personal contact components, multicomponent interventions featuring mHealth and personal contact components.
Table 2.
Physical activity (PA) and sedentary behavior (SB) intervention effects were reported in controlled trials.
| mHealth Only Interventions | |||||||
| First Author, Year | mHealth Intervention | Control | Effects | Risk of Bias | |||
| PA | SB | Results Summary | |||||
| Chung, 2019 [58] | App to motivate and provide information on PA, healthy diet and distress | Inactive |
Significant effect Step counts (steps/w) O |
Not applicable | Significant between-group increase in step count favoring the intervention group | No information ** (NI,?,+,+,?,?,+) |
|
| Ormel, 2018 [65] | App for PA self-monitoring | Inactive |
No significant effect Total PA (min/w) S, Physical activity scale for the elderly (sum score) S |
No significant effect Sitting time (min/w) S |
No significant between-group change in either outcome | Some concerns * (+,+,+,?,+) |
|
| Multicomponent interventions with mHealth component | |||||||
| First Author, Year | Intervention | Control | Effects | Risk of Bias | |||
| mHealth | Non-mHealth Component | PA | SB | Results Summary | |||
| Multicomponent intervention without personal contact (e.g., No phone and in-person contact) | |||||||
| Haggerty, 2017 [48] | Daily text messages providing feedback, support, and strategies to adhere to behavior change | Conventional weighing scale | Active and inactive |
Significant effect Walking activity, vigorous PA (METs/w) S No significant effect Total PA (METs/w) S |
Not applicable | Significant between-group increase in walking favoring intervention group with a significant increase of vigorous PA in favor of the inactive control group | High * (+,+,?,?,+) |
| Kenfield, 2019 [47] | Activity tracker and text messages to motivate behavioral changes following recommendations | Website | Inactive |
No significant effect MVPA (min/d) O, Step counts (steps/d) O |
Not applicable | No significant between-group changes in either outcome | Low * (+,+,+,+,+) |
| Mayer, 2018 [46] | Apps providing information and support to increase daily activity levels | Printed material, self-learning audio program and pedometer | Active |
No significant effect MVPA (min/w) S |
Not applicable | No significant between-group changes in outcome | Some concerns * (?,+,+,+,+) |
| Pope, 2018 [49] | Activity tracker monitoring PA and health metrics | Facebook group | Active |
No significant effect Light PA, MVPA (min/d) O, Energy expenditure (kcal/d) O, Step counts (Steps/d) O |
No significant effect SB (min/d) O |
No significant between-group changes in either outcome | Low * (+,+,+,+,+) |
| Uhm, 2017 [57] | Apps providing information and monitor the prescribed exercises |
Pedometer and printed material | Active |
No significant effect Total metabolic equivalent (METs/w) S |
Not applicable | No significant between-group changes in outcome | Serious ** (/,?,+,+,+,?,+) |
| Van Blarigan, 2019 [69] | Activity tracker to assess PA and daily text messages providing PA information | Printed material | Active |
No significant effect MVPA, moderate PA, vigorous PA (min/d) O, Step counts (steps/d) O |
Not applicable | No significant between-group changes in either outcome | Low * (+,+,+,+,+) |
| Villaron, 2018 [64] | Text messages on recommendations to increase PA | Printed material | Active |
No significant effect Step counts (steps/w) O |
Not applicable | No significant between-group changes in outcome | High * (?,+,+,+,?) |
| Multicomponent intervention with personal contact | |||||||
| Cadmus-Bertram, 2019 [42] | Activity tracker for self-monitoring PA and developing self-regulatory skills |
Printed material, in-person session, social support and email | Active |
Significant effect MVPA, moderate PA (min/w)O, MVPA in bouts (min/d) O, Step counts (steps/d) O No significant effect Total PA, vigorous PA, light PA (min/w) O |
Not applicable | Significant between-group increase in MVPA, moderate PA and step counts favoring the intervention group | Low * (+,+,+,+,+) |
| Gell, 2020 [44] | Activity tracker for self-monitoring PA level and text messages to support PA engagement | Phone call | Inactive |
Significant effect MVPA (min/w) O No significant effect Light PA (min/d) O, Adjusted light intensity time (% of day) O |
No significant effect Sedentary time (min/d O) Adjusted sedentary time (% of day O) |
Significant between-group increase in MVPA favoring the intervention group | Some concerns * (+,+,+,+,?) |
| Götte, 2018 [66] | Activity trackers assessing and providing feedback on PA during and after cancer treatment | In-person session | Active |
No significant effect Daily step goal, active time goal (Goals achievement percentage,?) O |
Not applicable | No significant between-group changes in either outcome | High ** (?,?,?,+,+,?,+) |
| Hartman, 2018 [52] | Activity tracker promoting behavior change | Phone calls and emails | Inactive |
Significant effect MVPA, total activity (min/d) O, ?Number of participants meeting 150 min/w (n,?) |
Not applicable | Significant between-group increase in MVPA, total activity and number of participant meeting 150 min/w favoring the intervention group | Low * (+,+,+,+,+) |
| Lynch, 2019 [38,39] | Activity tracker with the app providing inactivity alerts and assess PA | In-person session and phone call coaching | Inactive |
Significant effect MVPA (min/w) O |
Significant effect Sitting time (min/d) O |
Significant between-group increase MVPA and decrease sitting time favoring the intervention group | Low * (+,+,+,+,+) |
| Maxwell–Smith, 2019 [56] | Activity tracker to record the daily activity and as an encouragement to increase PA target | Printed material, group sessions and phone call | Active |
Significant effect MVPA, moderate PA (min/w) O No significant effect Proportion of MV10: MVPA accrued in bouts of at least 10 min (min/d) O |
No significant effect SB (hours/w) O |
Significant between-group increase in MVPA and moderate PA favoring the intervention group | Low * (+,+,+,+,+) |
| Mcneil, 2019 [61] | Activity tracker with app prescribing exercise intensity either lower or higher-intensity IG | Diary and phone call or email | Inactive |
Significant effect MVPA (min/d) O No significant effect Total PA, Light–intensity PA (min/d) O |
Significant effect Sedentary time (min/d) O |
Significant between-group increase in MVPA and decrease in sedentary time favoring the low-intensity intervention group | Low * (+,+,+,+,+) |
| Mendoza, 2017 [40] | Activity tracker with an app to show goal progression and text messages for PA encouragement | Facebook group and phone call | Inactive |
No significant effect MVPA (min/d) O |
No significant effect SB (min/d) O |
No significant between-group changes in outcome | Low * (+,+,+,+,+) |
| Singh, 2020 [53] | Activity tracker for self-monitoring and manage PA maintenance following supervised exercise intervention | In-person session and printed material | Active |
Significant effect Walking, moderate PA, MVPA, total activity (min/w) S, Vigorous PA and MVPA (min/w) O No significant effect Vigorous PA (min/w) S, Moderate PA (min/w) O, Step counts (steps/d) O |
Not applicable | Significant between-group increase in MVPA S, total activity S, moderate PA S, walking S, MVPA O and vigorous PA O favoring the intervention group | Some concerns * (+,+,+,?,+) |
| Valle, 2017 [51] | Activity tracker interfaced with an app to assess PA and providing feedback on PA | In-person session, wireless weighing scale and email | Active |
No significant effect Energy expenditure (kcal/w) S |
Not applicable | No significant between-group changes in outcome | Low * (+,+,+,+,+) |
S: subjective; O: objective; kcal/w: kilocalories per week; METs/w: total metabolic equivalent per week; min/d: minutes per day; min/w: minutes per week; steps/d: step counts per day; steps/w: step counts per week. * Assessed using RoB2.0 (Randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, selection of the reported result); +: low-risk of bias; /: some concerns; -: high-risk of bias. ** Assessed using ROBINS-I (bias due to confounding, bias in the selection of participants, bias in classification of interventions, bias due to deviations from intended interventions, bias due to missing data, bias in the measurement of outcomes, bias in the selection of reported results). +: low-risk of bias; /: moderate risk of bias; ?: serious risk of bias; -: critical risk of bias: NI: No information.
3.5.2. Effects on PA Outcomes in Non-Controlled Trials
In total, six non-controlled trials reported on MVPA, of which two reported a significant effect favoring the mHealth intervention [62,67]. A mHealth only intervention featuring an app to monitor and provide feedback on PA increased MVPA [67]. Another intervention that used activity trackers in conjunction with other non-personal contact intervention components showed significant MVPA improvements [62].
Total PA [50,63] and the total metabolic equivalent [59,60] as outcomes were reported in four non-controlled trials. Out of these, only one intervention that relied solely on mHealth components leads to increased total energy expenditure [60]. This finding is in contrast to a similar study, which employed a similar intervention approach and found no effects on energy expenditure [59]. Step counts were also reported in four noncontrolled trials [43,45,62,67]. Only one of these reported significant effects on weekly step counts following an intervention [62]. Further details are reported in Table 3.
Table 3.
Physical activity (PA) and sedentary behavior (SB) interventions effects were reported in non-controlled trials.
| mHealth Only Intervention | ||||||
| First Author, Year | mHealth Intervention | Effects | Risk of Bias | |||
| PA | SB | Results Summary | ||||
| Cheong, 2018 [59] | Activity tracker with an app providing exercise program |
No significant effect Total metabolic equivalent (METs/w) S |
Not applicable | No significant change pre to post-intervention in an outcome | High * (?,?,?,?,?,?,+) |
|
| Kim, 2020 [60] | Activity tracker with app prescribing exercise program |
Significant effect Total metabolic equivalent (METs/w) S |
Not applicable | Significant increase of METs pre to post-intervention | No information * (NI,?,+,+,?,?,+) |
|
| Lozano-Lozano, 2019 [67] | App to monitor and provide feedback on health behaviors |
Significant effect MVPA weekday (min/d) O No significant effect MVPA weekend, MVPA global (min/d) O Steps weekday, steps weekend, steps global (steps/d) O |
Not applicable | Significant increase of MVPA weekday pre to post-intervention | No information * (NI,?,+,+,+,?,+) |
|
| Puszkiewicz, 2016 [68] | App to provide PA program |
Significant effect Strenuous PA, mild PA (min/w)S No significant effect Moderate PA (min/w) S |
Not applicable | Significant increase in strenuous PA and a significant decrease in mild PA pre to post-intervention | High * (?,?,+,+,+,?,+) |
|
| mHealth and multicomponent intervention | ||||||
| First Author, Year | mHealth intervention | Non-mHealth Intervention | Effects | Risk of Bias | ||
| PA | SB | Results Summary | ||||
| mHealth without personal contact intervention | ||||||
| Le, 2017 [41] | Motivational activity tracker to record PA | Website and exercise recommendations |
No significant effect MVPA (min/d, min/wk) O Proportion of average total daily time doing MVPA and MVPA for at least 10 min day/w S |
No significant effect Proportion of average screen time/day watching TV and days playing computer, video games, surfing the Internet S |
No significant change pre to post-intervention in either outcome | No information * (NI,?,?,?,?,?,+) |
| McCarroll, 2015 [50] | App giving motivational feedback | Weighing scale |
No significant effect PA times (min/w) S, Caloric expenditure (kcals/w) S |
Not applicable | No significant change pre to post-intervention in either outcome | No information * (NI,?,+,+,+,?,+) |
| Trinh, 2018 [62] | Activity tracker to assess PA and provide alert to decrease SB | Web-based intervention |
Significant effect MVPA (min/w) S, Step counts (steps/w) O No significant effect Light PA (min/w) O, Number of bouts spent in MVPA ≥ 10 min |
Significant effect Sedentary time (min/w) S No significant effect Total time spent in SB bouts of ≥30 min (min/w) O, Number of breaks in time spent in SB bouts of ≥30 min |
Significant increase of MVPA and step counts with significant reduction of sedentary time pre to post-intervention | No information * (NI,?,+,+,?,?,+) |
| mHealth with personal contact intervention | ||||||
| Delrieu, 2020 [63] | Activity tracker and app to monitor activity level | In-person or phone sessions |
Significant effect Domestic PA (min/w) S No significant effect Total PA, recreational PA, moderate PA, vigorous PA, walking PA (MET-min/w) S, Participants proportions in low, moderate, and vigorous PA, n (%) |
Significant effect Sitting time (min/w) S |
Significant decrease in sitting time with significantly favoring a decrease in domestic PA pre to post-intervention | High * (-,?,+,+,+,?,+) |
| Gell, 2017 [43] | Activity tracker for self-monitoring activity and text messages to support PA | Phone call |
No significant effect MVPA (min/w) O, Step counts (steps/d) O |
Not applicable | No significant change pre to post-intervention in either outcome | No information * (NI,?,+,+,+,?,+) |
| Ovans, 2018 [45] | Activity tracker to show feedback and progress toward a goal | In-person or phone coaching |
No significant effect Step counts (steps/d) O, Leisure score index S |
Not applicable | No significant change pre to post-intervention in either outcome | High * (?,?,+,+,?,?,+) |
| Short, 2018 [55] | Recommended app according to individual characteristics | In-person session, printed material and a phone call or email |
No significant effect MVPA, walking, moderate PA, vigorous PA (min/w) S |
Not applicable | No significant change pre- to post-intervention in either outcome | No information * (NI,?,+,+,+,?,+) |
| Spark, 2015 [54] | Text messages for promoting behavioral change. | Phone call |
No significant effect MVPA (min/d) O |
Not applicable | No significant change pre to post-intervention in an outcome | High * (?,?,+,+,+,?,+) |
S: subjective; O: objective; min/d: minutes per day; min/w: minutes per week; steps/d: step counts per day; kcals/w: kilocalories per week. * Assessed using ROBINS-I (Bias due to confounding, Bias in election of participants, Bias in classification of interventions, bias due to deviations from intended interventions, bias due to missing data, bias in measurement of outcomes, Bias in selection of reported results). +: low-risk of bias; /: moderate risk of bias; ?: serious risk of bias; -: critical risk of bias: NI: No information.
3.6. Intervention Effects on SB Outcomes
Ten out of 31 of included studies reported on the effects of mHealth interventions on SB outcomes [38,40,41,44,49,56,61,62,63,65]. Overall, only four studies reported a significant decrease in SB following an mHealth intervention. Two controlled trials that used activity trackers with apps and personal contact reported decreased daily sedentary time when compared to inactive comparators [38,61]. Reduction in weekly sedentary time was also reported in a non-controlled trial in which an intervention featuring an activity tracker and web-based components was implemented [62]. Finally, one non-controlled study reported a significant reduction in weekly sitting time following an mHealth intervention [63].
3.7. Risk of Bias Assessment
Ten out of 16 RCT were considered to have a low risk of bias. Four RCT were judged to have some concerns arising from the randomization process [46], outcome measurement [53,65], and selection of the reported result [44]. A high risk of bias was present in two RCT, which had several concerns due to lack of information in the randomization process [48,64] and selective in reporting the results [64]. The study by Haggerty et al. [48] had a high risk of bias due to a lack of information on subjective measurement of outcome. Of the non-RCT included, there were three quasi-experimental [57,58,66] and 12 pre-post studies [41,43,45,50,54,55,59,60,62,63,67,68]. All the non-RCT had either high-risk of bias [45,54,59,63,66,68] or no information [41,43,50,55,58,60,62,67] except one in which risk was slightly lower [57]. Details of the risk of bias assessment are presented in Supplementary Material S2.
3.8. Best Evidence Synthesis
There is strong evidence that mHealth interventions in conjunction with personal contact components can lead to increases in MVPA among cancer survivors based on seven out of eight (87.5%) controlled trials with either low-risk or bias [38,42,52,56,61] or some concerns raised [44,53] reporting positive effects. There is inconclusive evidence on mHealth interventions with personal contact to impact total PA/activity among cancer survivors based on two out of six (33.3%) controlled trials with low risk of bias [52] and some concerns raised [53] reporting positive effects. There is limited evidence on the effectiveness of mHealth interventions with a personal contact for step counts among cancer survivors, as only one out of two controlled trials with low-risk reported an increase in step counts [42]. There is inconclusive evidence that mHealth interventions with personal contact are effective in reducing sedentary time among cancer survivors, as only two out of six (33.3%) low-risk-controlled trials showed significant effects. There is limited evidence on the effects of mHealth only interventions due to a lack of studies. In addition, there is inconclusive evidence on the effects of mHealth interventions without personal contacts components for PA due to inconsistent findings in studies. There were no studies, which examined the effects of mHealth interventions without personal contacts components for SB, and this hindered the evidence synthesis.
4. Discussion
The aim of this review was to identify, evaluate, and synthesize the scientific literature on mHealth interventions to promote PA or reduce SB in cancer survivors. In brief, 31 studies of mHealth interventions were included and systematically analyzed. All the studies evaluated the effects of mHealth interventions on PA outcomes, and only 10 of these also reported on SB outcomes. To evaluate the effects of mHealth interventions, we conducted the best evidence synthesis for which we only considered controlled trials due to their inherently lower risk of bias. The synthesis revealed that only for interventions that employed mHealth components in conjunction with personal contact components, there was strong evidence of effects on MVPA.
From our best evidence synthesis, it suggests that the implementation of mHealth interventions with personal contact may be effective in increasing MVPA among cancer survivors. This finding is consistent with a previous meta-analysis that reported digital interventions to increase MVPA by approximately 40 min per week among cancer survivors [25]. It is also in line with reviews on eHealth interventions in cancer survivors [24,70]. However, these earlier reviews did not examine the effects of how interventions were implemented (mHealth only, mHealth plus non-personal contact components, mHealth plus personal contact components), and as such, direct comparisons are difficult to make. This mHealth interventions that also employed personal contact components increased MVPA is intriguing because it suggests that in-person sessions, phone calls, or group consultations are to be considered when designing interventions. It is probable that such an approach will yield greater effects on health behaviors as research has shown a link between social support from others and PA in cancer survivors.
Cancer survivors whose social interactions were limited tend to engage in less PA [71]. Cancer survivors have specific support needs [27] that may not be fulfilled by purely digital interventions. Incorporating personal contact elements into mHealth interventions can be done in various ways. Personal contact in this review was defined as any person-to-person contact involving nondigital/traditional modes of communication (i.e., phone calls and face-to-face meetings). Lynch et al. [38] incorporated an in-person goal-setting session and phone call behavioral counseling to facilitate adaptation to and maintenance of PA. It is likely that these interactions provided social support because they allowed participants to receive feedback and encouragement [72]. Social support through direct interaction has repeatedly been shown to positively influence PA in various populations [73]. Other interventions included follow-up discussions [61], over the phone as well as in-person counseling [53], and goal-setting sessions [42], which all fall within the domain of social support.
Researchers that assessed an mHealth intervention and its effects on MVPA in controlled studies were often 12 weeks long. Most reported significant effects of mHealth interventions that incorporated personal contact after this time and for this outcome [38,42,52,53,56,61]. However, one 8-weeks controlled study with a similar intervention mode also showed increased MVPA [44]. Although it is not possible to provide an intervention duration that is optimal for increasing PA, 12 weeks appears to be a reasonable length that may also be feasible to implement. However, it is important to consider that the intervention duration may be less important than engaging participants effectively [74]. Interventions of short duration may be highly effective if they greatly engage participants.
There was inconclusive or limited evidence regarding the other intervention modes (mHealth only and mHealth without personal contact) and other outcomes. As such, it is not clear whether cancer survivors will benefit from mHealth interventions with or without other components in terms of total activity, step counts, and SB. It was surprising that a very limited number of studies evaluated interventions that solely relied on mHealth components. However, findings from only mHealth interventions in three non-controlled trials showed promising improvement in PA outcomes [60,67,68], warranting more research.
Despite a plethora of research on mHealth interventions targeting PA, there is still limited research on interventions targeting SB. There was inconclusive evidence in relation to mHealth interventions with personal contacts on SB. Controlled studies that incorporated this mode of intervention mainly only target PA behavioral changes and showed no effects on SB [44,49]. In contrast, findings from mHealth interventions with [63] and without [62] personal contacts targeting both PA and SB behavioral changes in non-controlled trials showed a promising reduction in sedentary time. However, SB is distinct from physical inactivity [75], whereas sitting time is unassociated with inadequate amounts of MVPA [76]. Further, it is reported that sitting time takes the most proportion of the waking day and displaces light–intensity PA [77]. Thus, a feasible approach to reduce SB among cancer survivors could be conducted in the future by replacing sitting time with light-intensity PA before gradually shifting to MVPA.
Findings from this review may not be generalizable to all cancer survivors because studies were only conducted in high-income countries. This is disappointing and indicates that the inherent potential of mHealth interventions in many lower-income countries has not yet been utilized. Using the available mobile network infrastructure in many countries to support cancer survivors in resource-constrained contexts should be considered. Although there may still be barriers in terms of the accessibility of more advanced personal mobile devices, such as fitness trackers [78], lower-end technology can also be utilized. This digital behavioral intervention can yield positive effects in middle- to low-income countries, as has been reported previously [79].
This review has several strengths. An extensive search strategy in six large databases using broad search terms was conducted. The procedures of this review were in line with the PRISMA guidelines, which strengthens the methodology of the review. We also included all experimental studies, which assessed PA and/or SB outcomes following the breadth of mHealth interventions in cancer survivors. This leads to a more comprehensive overview of mHealth as an intervention to promote PA and reduce SB for cancer survivors. In this review, the findings were grouped into intervention modes (mHealth only, mHealth plus non-personal contact components, mHealth plus personal contact components) and their effect on the outcomes. Limitations of this review are the variety of study designs, including several with a small sample size due to its pilot nature. This systematic review shows the heterogeneity in the methodology and study designs of the selected studies likely become its weakness. These issues make a comparison between selected studies difficult and why a meta-analysis could not be conducted.
In the future, larger studies with higher quality study designs are needed to generate findings that encompass the whole spectrum of movement behaviors and mHealth interventions. The effects of mHealth interventions targeting SB are still unclear. Thus more studies focusing on mHealth interventions reducing SB should be conducted. As only studies from high-income countries were included in this review, it is uncertain if the findings also apply to middle- to low-income countries. Social support when paired with mHealth intervention components has potential for promoting PA among cancer survivors, while effects on SB are still elusive. It is recommended to investigate the cost-effectiveness of mHealth interventions implementation with virtual or non-virtual social aspects in-depth. Finally, large-scale implementations, which consider a thorough cost-effectiveness analysis of mHealth interventions targeting both PA and SB in all ranges of income countries, require attention in future mHealth research involving cancer survivors.
5. Conclusions
We systematically reviewed the scientific literature on mHealth interventions that aimed to promote PA and/or reduce SB in cancer survivors. mHealth interventions with personal contact appeared to have a positive effect on MVPA among cancer survivors. The evidence for this observation was strong. Further, mixed findings for other PA outcomes like total activity and step counts were observed, while the evidence on SB outcomes was inconclusive due to the lack of studies. More research is needed to establish the optimal mHealth intervention mode for various PA outcomes in cancer survivors. Interventions that aim to reduce SB among cancer survivors are also highly encouraged as cancer survivors may be more likely to engage in changing lower-threshold health behavior. Finally, researchers may focus on the cost-effectiveness of interventions because mHealth interventions may need to incorporate personal contact components, which is more costly.
Acknowledgments
A.M.M. would like to acknowledge his little toddler, Lia Yihan, who grows and develops at lightning speed; you are my sunshine when skies are blue.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijerph18115798/s1, Supplementary S1: Search strategy, Supplementary S2: Risk of Bias Summary.
Author Contributions
Conceptualization, S.K., N.M. and A.M.M.; methodology, P.A., M.A.-K. and A.M.M.; validation, M.A.-K. and A.M.M.; writing—original draft preparation, N.M.; writing—review and editing, S.K.; supervision, S.K.; funding acquisition, S.K. and A.M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the University of Malaya Impact-Oriented Interdisciplinary Research Grant (IIRG025C-2019). A.M.M. was supported by the Singapore Ministry of Health’s National Medical Research Council, Fellowship Program by the Singapore Population Health Improvement Center: NMRC/CG/C026/2017_NUHS.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used during the current study are available from the corresponding authors on reasonable request.
Conflicts of Interest
The funders had no role in designing the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021 doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Allemani C., Matsuda T., Di Carlo V., Harewood R., Matz M., Nikšić M., Bonaventure A., Valkov M., Johnson C.J., Estève J. Global Surveillance of Trends in Cancer Survival 2000–14 (CONCORD-3): Analysis of Individual Records for 37 513 025 Patients Diagnosed with One of 18 Cancers from 322 Population-Based Registries in 71 Countries. Lancet. 2018;391:1023–1075. doi: 10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kleckner I.R., Dunne R.F., Asare M., Cole C., Fleming F., Fung C., Lin P.-J., Mustian K.M. Exercise for Toxicity Management in Cancer-A Narrative Review. Oncol. Hematol. Rev. 2018;14:28–37. doi: 10.17925/OHR.2018.14.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weaver K.E., Forsythe L.P., Reeve B.B., Alfano C.M., Rodriguez J.L., Sabatino S.A., Hawkins N.A., Rowland J.H. Mental and Physical Health–Related Quality of Life among US Cancer Survivors: Population Estimates from the 2010 National Health Interview Survey. Cancer Epidemiol. Biomark. Prev. 2012;21:2108–2117. doi: 10.1158/1055-9965.EPI-12-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demark-Wahnefried W., Pinto B.M., Gritz E.R. Promoting Health and Physical Function among Cancer Survivors: Potential for Prevention and Questions That Remain. J. Clin. Oncol. 2006;24:5125–5131. doi: 10.1200/JCO.2006.06.6175. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. [(accessed on 18 May 2021)]; Available online: https://www.who.int/news-room/fact-sheets/detail/physical-activity.
- 7.Friedenreich C.M., Stone C.R., Cheung W.Y., Hayes S.C. Physical Activity and Mortality in Cancer Survivors: A Systematic Review and Meta-Analysis. JNCI Cancer Spectrum. 2020;4 doi: 10.1093/jncics/pkz080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel A.V., Friedenreich C.M., Moore S.C., Hayes S.C., Silver J.K., Campbell K.L., Winters-Stone K., Gerber L.H., George S.M., Fulton J.E. American College of Sports Medicine Roundtable Report on Physical Activity, Sedentary Behavior, and Cancer Prevention and Control. Med. Sci. Sports Exerc. 2019;51:2391–2402. doi: 10.1249/MSS.0000000000002117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jung A.Y., Behrens S., Schmidt M., Thoene K., Obi N., Hüsing A., Benner A., Steindorf K., Chang-Claude J. Pre-to Postdiagnosis Leisure-Time Physical Activity and Prognosis in Postmenopausal Breast Cancer Survivors. Breast Cancer Res. 2019;21:117. doi: 10.1186/s13058-019-1206-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Blarigan E.L., Fuchs C.S., Niedzwiecki D., Zhang S., Saltz L.B., Mayer R.J., Mowat R.B., Whittom R., Hantel A., Benson A., et al. Association of Survival With Adherence to the American Cancer Society Nutrition and Physical Activity Guidelines for Cancer Survivors After Colon Cancer Diagnosis: The CALGB 89803/Alliance Trial. JAMA Oncol. 2018;4:783–790. doi: 10.1001/jamaoncol.2018.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown J.C., Winters-Stone K., Lee A., Schmitz K.H. Cancer, Physical Activity, and Exercise. Compr. Physiol. 2012;2:2775–2809. doi: 10.1002/cphy.c120005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tremblay M.S., Aubert S., Barnes J.D., Saunders T.J., Carson V., Latimer-Cheung A.E., Chastin S.F.M., Altenburg T.M., Chinapaw M.J.M. Sedentary Behavior Research Network (SBRN)—Terminology Consensus Project Process and Outcome. Int. J. Behav. Nutr. Phys. Act. 2017;14:75. doi: 10.1186/s12966-017-0525-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ehlers D.K., Fanning J., Sunderlage A., Severson J., Kramer A.F., McAuley E. Influence of Sitting Behaviors on Sleep Disturbance and Memory Impairment in Breast Cancer Survivors. Cancer Med. 2020;9:3417–3424. doi: 10.1002/cam4.3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sabiston C.M., Lacombe J., Faulkner G., Jones J., Trinh L. Profiling Sedentary Behavior in Breast Cancer Survivors: Links with Depression Symptoms during the Early Survivorship Period. Psychooncology. 2018;27:569–575. doi: 10.1002/pon.4520. [DOI] [PubMed] [Google Scholar]
- 15.Swain C.T., Nguyen N.H., Eagles T., Vallance J.K., Boyle T., Lahart I.M., Lynch B.M. Postdiagnosis Sedentary Behavior and Health Outcomes in Cancer Survivors: A Systematic Review and Meta-analysis. Cancer. 2020;126:861–869. doi: 10.1002/cncr.32578. [DOI] [PubMed] [Google Scholar]
- 16.Rock C.L., Doyle C., Demark-Wahnefried W., Meyerhardt J., Courneya K.S., Schwartz A.L., Bandera E.V., Hamilton K.K., Grant B., McCullough M. Nutrition and Physical Activity Guidelines for Cancer Survivors. CA Cancer J. Clin. 2012;62:242–274. doi: 10.3322/caac.21142. [DOI] [PubMed] [Google Scholar]
- 17.Bull F.C., Al-Ansari S.S., Biddle S., Borodulin K., Buman M.P., Cardon G., Carty C., Chaput J.-P., Chastin S., Chou R., et al. World Health Organization 2020 Guidelines on Physical Activity and Sedentary Behaviour. Br. J. Sports Med. 2020;54:1451. doi: 10.1136/bjsports-2020-102955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim R.B., Phillips A., Herrick K., Helou M., Rafie C., Anscher M.S., Mikkelsen R.B., Ning Y. Physical Activity and Sedentary Behavior of Cancer Survivors and Non-Cancer Individuals: Results from a National Survey. PLoS ONE. 2013;8:e57598. doi: 10.1371/journal.pone.0057598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marcolino M.S., Oliveira J.A.Q., D’Agostino M., Ribeiro A.L., Alkmim M.B.M., Novillo-Ortiz D. The Impact of mHealth Interventions: Systematic Review of Systematic Reviews. JMIR Mhealth Uhealth. 2018;6:e23. doi: 10.2196/mhealth.8873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. [(accessed on 12 April 2020)]; Available online: https://www.who.int/goe/publications/goe_mhealth_web.pdf.
- 21.Müller A.M., Maher C.A., Vandelanotte C., Hingle M., Middelweerd A., Lopez M.L., DeSmet A., Short C.E., Nathan N., Hutchesson M.J., et al. Physical Activity, Sedentary Behavior, and Diet-Related eHealth and mHealth Research: Bibliometric Analysis. J. Med. Internet Res. 2018;20:e8954. doi: 10.2196/jmir.8954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vandelanotte C., Müller A.M., Short C.E., Hingle M., Nathan N., Williams S.L., Lopez M.L., Parekh S., Maher C.A. Past, Present, and Future of eHealth and mHealth Research to Improve Physical Activity and Dietary Behaviors. J. Nutr. Educ. Behav. 2016;48:219–228.e1. doi: 10.1016/j.jneb.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Direito A., Carraça E., Rawstorn J., Whittaker R., Maddison R. MHealth Technologies to Influence Physical Activity and Sedentary Behaviors: Behavior Change Techniques, Systematic Review and Meta-Analysis of Randomized Controlled Trials. Ann. Behav. Med. 2017;51:226–239. doi: 10.1007/s12160-016-9846-0. [DOI] [PubMed] [Google Scholar]
- 24.Haberlin C., O’Dwyer T., Mockler D., Moran J., O’Donnell D.M., Broderick J., O’Dwyer T., O’Donnell D.M. The Use of EHealth to Promote Physical Activity in Cancer Survivors: A Systematic Review. Support. Care Cancer. 2018;26:3323–3336. doi: 10.1007/s00520-018-4305-z. [DOI] [PubMed] [Google Scholar]
- 25.Roberts A.L., Fisher A., Smith L., Heinrich M., Potts H.W. Digital Health Behaviour Change Interventions Targeting Physical Activity and Diet in Cancer Survivors: A Systematic Review and Meta-Analysis. J. Cancer Surviv. 2017;11:704–719. doi: 10.1007/s11764-017-0632-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coughlin S.S., Caplan L.S., Stone R. Use of Consumer Wearable Devices to Promote Physical Activity among Breast, Prostate, and Colorectal Cancer Survivors: A Review of Health Intervention Studies. J. Cancer Surviv. 2020;14:386–392. doi: 10.1007/s11764-020-00855-1. [DOI] [PubMed] [Google Scholar]
- 27.Corbett T., Cheetham T., Müller A.M., Slodkowska-Barabasz J., Wilde L., Krusche A., Richardson A., Foster C., Watson E., Little P., et al. Exploring Cancer Survivors’ Views of Health Behaviour Change: “Where Do You Start, Where Do You Stop with Everything?”. Psychooncology. 2018;27:1816–1824. doi: 10.1002/pon.4732. [DOI] [PubMed] [Google Scholar]
- 28.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Denlinger C.S., Carlson R.W., Are M., Baker K.S., Davis E., Edge S.B., Friedman D.L., Goldman M., Jones L., King A. Survivorship: Introduction and Definition. J. Natl. Compr. Cancer Netw. 2014;12:34–45. doi: 10.6004/jnccn.2014.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ganesan A.N., Louise J., Horsfall M., Bilsborough S.A., Hendriks J., McGavigan A.D., Selvanayagam J.B., Chew D.P. International Mobile-Health Intervention on Physical Activity, Sitting, and Weight: The Stepathlon Cardiovascular Health Study. J. Am. Coll. Cardiol. 2016;67:2453–2463. doi: 10.1016/j.jacc.2016.03.472. [DOI] [PubMed] [Google Scholar]
- 31.Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A., editors. [(accessed on 23 April 2021)];Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (Updated September 2020) 2020 Cochrane. Available online: www.training.cochrane.org/handbook.
- 32.Higgins J.P., Sterne J.A., Savovic J., Page M.J., Hróbjartsson A., Boutron I., Reeves B., Eldridge S. A Revised Tool for Assessing Risk of Bias in Randomized Trials. Cochrane Database Syst. Rev. 2016;10:29–31. [Google Scholar]
- 33.Sterne J.A., Hernán M.A., Reeves B.C., Savović J., Berkman N.D., Viswanathan M., Henry D., Altman D.G., Ansari M.T., Boutron I. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ. 2016;355 doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Slavin R.E. Best Evidence Synthesis: An Intelligent Alternative to Meta-Analysis. J. Clin. Epidemiol. 1995;48:9–18. doi: 10.1016/0895-4356(94)00097-A. [DOI] [PubMed] [Google Scholar]
- 35.Fanchini M., Steendahl I.B., Impellizzeri F.M., Pruna R., Dupont G., Coutts A.J., Meyer T., McCall A. Exercise-Based Strategies to Prevent Muscle Injury in Elite Footballers: A Systematic Review and Best Evidence Synthesis. Sports Med. 2020;50:1653–1666. doi: 10.1007/s40279-020-01282-z. [DOI] [PubMed] [Google Scholar]
- 36.Kuijer P.P.F.M., Gouttebarge V., Brouwer S., Reneman M.F., Frings-Dresen M.H.W. Are Performance-Based Measures Predictive of Work Participation in Patients with Musculoskeletal Disorders? A Systematic Review. Int. Arch. Occup. Environ. Health. 2012;85:109–123. doi: 10.1007/s00420-011-0659-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Tulder M., Furlan A., Bombardier C., Bouter L. Updated Method Guidelines for Systematic Reviews in the Cochrane Collaboration Back Review Group. Spine. 2003;28:1290–1299. doi: 10.1097/01.BRS.0000065484.95996.AF. [DOI] [PubMed] [Google Scholar]
- 38.Lynch B.M., Nguyen N.H., Moore M.M., Reeves M.M., Rosenberg D.E., Boyle T., Vallance J.K., Milton S., Friedenreich C.M., English D.R. A Randomized Controlled Trial of a Wearable Technology-Based Intervention for Increasing Moderate to Vigorous Physical Activity and Reducing Sedentary Behavior in Breast Cancer Survivors: The ACTIVATE Trial. Cancer. 2019;125:2846–2855. doi: 10.1002/cncr.32143. [DOI] [PubMed] [Google Scholar]
- 39.Lynch B.M., Nguyen N.H., Moore M.M., Reeves M.M., Rosenberg D.E., Boyle T., Milton S., Friedenreich C.M., Vallance J.K., English D.R. Maintenance of Physical Activity and Sedentary Behavior Change, and Physical Activity and Sedentary Behavior Change after an Abridged Intervention: Secondary Outcomes from the ACTIVATE Trial. Cancer. 2019;125:2856–2860. doi: 10.1002/cncr.32142. [DOI] [PubMed] [Google Scholar]
- 40.Mendoza J.A., Baker K.S., Moreno M.A., Whitlock K., Abbey-Lambertz M., Waite A., Colburn T., Chow E.J. A Fitbit and Facebook MHealth Intervention for Promoting Physical Activity among Adolescent and Young Adult Childhood Cancer Survivors: A Pilot Study. Pediatr. Blood Cancer. 2017;64 doi: 10.1002/pbc.26660. [DOI] [PubMed] [Google Scholar]
- 41.Le A., Mitchell H., Zheng D.J., Rotatori J., Fahey J.T., Ness K.K., Kadan-Lottick N.S., Mitchell H.-R., Kadan-Lottick N.S. A Home-Based Physical Activity Intervention Using Activity Trackers in Survivors of Childhood Cancer: A Pilot Study. Pediatr. Blood Cancer. 2017;64:387–394. doi: 10.1002/pbc.26235. [DOI] [PubMed] [Google Scholar]
- 42.Cadmus-Bertram L., Tevaarwerk A.J., Sesto M.E., Gangnon R., Van Remortel B., Date P. Building a Physical Activity Intervention into Clinical Care for Breast and Colorectal Cancer Survivors in Wisconsin: A Randomized Controlled Pilot Trial. J. Cancer Surviv. 2019;13:593–602. doi: 10.1007/s11764-019-00778-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gell N., Grover K., Humble M., Sexton M., Dittus K., Gell N.M., Grover K.W. Efficacy, Feasibility, and Acceptability of a Novel Technology-Based Intervention to Support Physical Activity in Cancer Survivors. Support. Care Cancer. 2017;25:1291–1300. doi: 10.1007/s00520-016-3523-5. [DOI] [PubMed] [Google Scholar]
- 44.Gell N.M., Grover K.W., Savard L., Dittus K. Outcomes of a Text Message, Fitbit, and Coaching Intervention on Physical Activity Maintenance among Cancer Survivors: A Randomized Control Pilot Trial. J. Cancer Surviv. 2020;14:80–88. doi: 10.1007/s11764-019-00831-4. [DOI] [PubMed] [Google Scholar]
- 45.Ovans J.A., Hooke M.C., Bendel A.E., Tanner L.R. Physical Therapist Coaching to Improve Physical Activity in Children With Brain Tumors: A Pilot Study. Pediatr. Phys. Ther. 2018;30:310–317. doi: 10.1097/PEP.0000000000000531. [DOI] [PubMed] [Google Scholar]
- 46.Mayer D.K., Landucci G., Awoyinka L., Atwood A.K., Carmack C.L., Demark-Wahnefried W., McTavish F., Gustafson D.H. SurvivorCHESS to Increase Physical Activity in Colon Cancer Survivors: Can We Get Them Moving? J. Cancer Surviv. 2018;12:82–94. doi: 10.1007/s11764-017-0647-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kenfield S., Van Blarigan E., Ameli N., Lavaki E., Cedars B., Paciorek A., Monroy C., Tantum L., Newton R., Signorell C., et al. Feasibility, Acceptability, and Behavioral Outcomes from a Technology-Enhanced Behavioral Change Intervention (Prostate 8): A Pilot Randomized Controlled Trial in Men with Prostate Cancer. Eur. Urol. 2019 doi: 10.1016/j.eururo.2018.12.040. [DOI] [PubMed] [Google Scholar]
- 48.Haggerty A., Hagemann A., Barnett M., Thornquist M., Neuhouser M., Horowitz N., Colditz G., Sarwer D., Ko E., Allison K. A Randomized, Controlled, Multicenter Study of Technology-Based Weight Loss Interventions among Endometrial Cancer Survivors. Obesity. 2017;25(Suppl. 2):S102–S108. doi: 10.1002/oby.22021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pope Z.C., Zeng N., Zhang R., Lee H.Y., Gao Z. Effectiveness of Combined Smartwatch and Social Media Intervention on Breast Cancer Survivor Health Outcomes: A 10-Week Pilot Randomized Trial. J. Clin. Med. 2018;7:140. doi: 10.3390/jcm7060140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCarroll M.L., Armbruster S., Pohle-Krauza R.J., Lyzen A.M., Min S., Nash D.W., Roulette G.D., Andrews S.J., von Gruenigen V.E. Feasibility of a Lifestyle Intervention for Overweight/Obese Endometrial and Breast Cancer Survivors Using an Interactive Mobile Application. Gynecol. Oncol. 2015;137:508–515. doi: 10.1016/j.ygyno.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 51.Valle C.G., Deal A.M., Tate D.F. Preventing Weight Gain in African American Breast Cancer Survivors Using Smart Scales and Activity Trackers: A Randomized Controlled Pilot Study. J. Cancer Surviv. 2017;11:133–148. doi: 10.1007/s11764-016-0571-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hartman S.J., Nelson S.H., Myers E., Natarajan L., Sears D.D., Palmer B.W., Weiner L.S., Parker B.A., Patterson R.E. Randomized Controlled Trial of Increasing Physical Activity on Objectively Measured and Self-Reported Cognitive Functioning among Breast Cancer Survivors: The Memory & Motion Study. Cancer. 2018;124:192–202. doi: 10.1002/cncr.30987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singh B., Spence R.R., Sandler C.X., Tanner J., Hayes S.C. Feasibility and Effect of a Physical Activity Counselling Session with or without Provision of an Activity Tracker on Maintenance of Physical Activity in Women with Breast Cancer—A Randomised Controlled Trial. J. Sci. Med. Sport. 2020;23:283–290. doi: 10.1016/j.jsams.2019.09.019. [DOI] [PubMed] [Google Scholar]
- 54.Spark L.C., Fjeldsoe B.S., Eakin E.G., Reeves M.M. Efficacy of a Text Message-Delivered Extended Contact Intervention on Maintenance of Weight Loss, Physical Activity, and Dietary Behavior Change. JMIR Mhealth Uhealth. 2015;3:e88. doi: 10.2196/mhealth.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Short C.E., Finlay A., Sanders I., Maher C. Development and Pilot Evaluation of a Clinic-Based MHealth App Referral Service to Support Adult Cancer Survivors Increase Their Participation in Physical Activity Using Publicly Available Mobile Apps. BMC Health Serv. Res. 2018;18 doi: 10.1186/s12913-017-2818-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maxwell-Smith C., Hince D., Cohen P.A., Bulsara M.K., Boyle T., Platell C., Tan P., Levitt M., Salama P., Tan J., et al. A Randomized Controlled Trial of WATAAP to Promote Physical Activity in Colorectal and Endometrial Cancer Survivors. Psychooncology. 2019;28:1420–1429. doi: 10.1002/pon.5090. [DOI] [PubMed] [Google Scholar]
- 57.Uhm K., Yoo J., Chung S., Lee J., Lee I., Kim J., Lee S., Nam S., Park Y., Lee J., et al. Effects of Exercise Intervention in Breast Cancer Patients: Is Mobile Health (MHealth) with Pedometer More Effective than Conventional Program Using Brochure? Breast Cancer Res. Treat. 2017;161:443–452. doi: 10.1007/s10549-016-4065-8. [DOI] [PubMed] [Google Scholar]
- 58.Chung I.Y., Jung M., Park Y.R., Cho D., Chung H., Min Y.H., Park H.J., Lee M., Lee S.B., Chung S., et al. Exercise Promotion and Distress Reduction Using a Mobile App-Based Community in Breast Cancer Survivors. Front. Oncol. 2019;9:1505. doi: 10.3389/fonc.2019.01505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cheong I.Y., An S.Y., Cha W.C., Rha M.Y., Kim S.T., Chang D.K., Hwang J.H. Efficacy of Mobile Health Care Application and Wearable Device in Improvement of Physical Performance in Colorectal Cancer Patients Undergoing Chemotherapy. Clin. Colorectal Cancer. 2018;17:e353–e362. doi: 10.1016/j.clcc.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 60.Kim Y., Seo J., An S.-Y., Sinn D.H., Hwang J.H. Efficacy and Safety of an MHealth App and Wearable Device in Physical Performance for Patients With Hepatocellular Carcinoma: Development and Usability Study. JMIR Mhealth Uhealth. 2020;8:e14435. doi: 10.2196/14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McNeil J., Brenner D.R., Stone C.R., O’Reilly R., Ruan Y., Vallance J.K., Courneya K.S., Thorpe K.E., Klein D.J., Friedenreich C.M. Activity Tracker to Prescribe Various Exercise Intensities in Breast Cancer Survivors. Med. Sci. Sports Exerc. 2019;51:930–940. doi: 10.1249/MSS.0000000000001890. [DOI] [PubMed] [Google Scholar]
- 62.Trinh L., Arbour-Nicitopoulos K.P., Sabiston C.M., Berry S.R., Loblaw A., Alibhai S.M.H., Jones J.M., Faulkner G.E. RiseTx: Testing the Feasibility of a Web Application for Reducing Sedentary Behavior among Prostate Cancer Survivors Receiving Androgen Deprivation Therapy. Int. J. Behav. Nutr. Phys. Act. 2018;15 doi: 10.1186/s12966-018-0686-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Delrieu L., Pialoux V., Perol O., Morelle M., Martin A., Friedenreich C., Febvey-Combes O., Perol D., Belladame E., Clemencon M., et al. Feasibility and Health Benefits of an Individualized Physical Activity Intervention in Women With Metastatic Breast Cancer: Intervention Study. JMIR Mhealth Uhealth. 2020;8:e12306. doi: 10.2196/12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Villaron C., Cury F., Eisinger F., Cappiello M.-A., Marqueste T. Telehealth Applied to Physical Activity during Cancer Treatment: A Feasibility, Acceptability, and Randomized Pilot Study. Support. Care Cancer. 2018;26:3413–3421. doi: 10.1007/s00520-018-4191-4. [DOI] [PubMed] [Google Scholar]
- 65.Ormel H.L., van der Schoot G.G.F., Westerink N.-D.L., Gietema J.A., Walenkamp A.M.E., Sluiter W.J. Self-Monitoring Physical Activity with a Smartphone Application in Cancer Patients: A Randomized Feasibility Study (SMART-Trial) Support. Care Cancer. 2018;26:3915–3923. doi: 10.1007/s00520-018-4263-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Götte M., Kesting S.V., Gerss J., Rosenbaum D., Boos J. Feasibility and Effects of a Home-Based Intervention Using Activity Trackers on Achievement of Individual Goals, Quality of Life and Motor Performance in Patients with Paediatric Cancer. BMJ Open Sport Exerc. Med. 2018;4:e000322. doi: 10.1136/bmjsem-2017-000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lozano-Lozano M., Cantarero-Villanueva I., Martin-Martin L., Galiano-Castillo N., Sanchez M.-J., Fernandez-Lao C., Postigo-Martin P., Arroyo-Morales M. A Mobile System to Improve Quality of Life Via Energy Balance in Breast Cancer Survivors (BENECA MHealth): Prospective Test-Retest Quasiexperimental Feasibility Study. JMIR Mhealth Uhealth. 2019;7:e14136. doi: 10.2196/14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Puszkiewicz P., Roberts A.L., Smith L., Wardle J., Fisher A. Assessment of Cancer Survivors’ Experiences of Using a Publicly Available Physical Activity Mobile Application. JMIR Cancer. 2016;2:e7. doi: 10.2196/cancer.5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Van Blarigan E.L., Chan H., Van Loon K., Kenfield S.A., Chan J.M., Mitchell E., Zhang L., Paciorek A., Joseph G., Laffan A., et al. Self-Monitoring and Reminder Text Messages to Increase Physical Activity in Colorectal Cancer Survivors (Smart Pace): A Pilot Randomized Controlled Trial. BMC Cancer. 2019;19:218. doi: 10.1186/s12885-019-5427-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dorri S., Asadi F., Olfatbakhsh A., Kazemi A. A Systematic Review of Electronic Health (eHealth) Interventions to Improve Physical Activity in Patients with Breast Cancer. Breast Cancer. 2020;27:25–46. doi: 10.1007/s12282-019-00982-3. [DOI] [PubMed] [Google Scholar]
- 71.Kim B.H., Wallington S.F., Makambi K.H., Adams-Campbell L.L. Social Networks and Physical Activity Behaviors among Cancer Survivors: Data from the 2005 Health Information National Trends Survey. J. Health Commun. 2015;20:656–662. doi: 10.1080/10810730.2015.1018576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smith L., Croker H., Fisher A., Williams K., Wardle J., Beeken R.J. Cancer Survivors’ Attitudes towards and Knowledge of Physical Activity, Sources of Information, and Barriers and Facilitators of Engagement: A Qualitative Study. Eur. J. Cancer Care. 2017;26:e12641. doi: 10.1111/ecc.12641. [DOI] [PubMed] [Google Scholar]
- 73.McNeill L.H., Kreuter M.W., Subramanian S.V. Social Environment and Physical Activity: A Review of Concepts and Evidence. Soc. Sci. Med. 2006;63:1011–1022. doi: 10.1016/j.socscimed.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 74.Yardley L., Spring B.J., Riper H., Morrison L.G., Crane D.H., Curtis K., Merchant G.C., Naughton F., Blandford A. Understanding and Promoting Effective Engagement With Digital Behavior Change Interventions. Am. J. Prev. Med. 2016;51:833–842. doi: 10.1016/j.amepre.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 75.Panahi S., Tremblay A. Sedentariness and Health: Is Sedentary Behavior More than Just Physical Inactivity? Front. Public Health. 2018;6:258. doi: 10.3389/fpubh.2018.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Keadle S.K., Conroy D.E., Buman M.P., Dunstan D.W., Matthews C.E. Targeting Reductions in Sitting Time to Increase Physical Activity and Improve Health. Med. Sci. Sports Exerc. 2017;49:1572–1582. doi: 10.1249/MSS.0000000000001257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Craft L.L., Zderic T.W., Gapstur S.M., Vaniterson E.H., Thomas D.M., Siddique J., Hamilton M.T. Evidence That Women Meeting Physical Activity Guidelines Do Not Sit Less: An Observational Inclinometry Study. Int. J. Behav. Nutr. Phys. Act. 2012;9:122. doi: 10.1186/1479-5868-9-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cho Y.-M., Lee S., Islam S.M.S., Kim S.-Y. Theories Applied to M-Health Interventions for Behavior Change in Low-and Middle-Income Countries: A Systematic Review. Telemed. J. E Health. 2018;24:727–741. doi: 10.1089/tmj.2017.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Müller A.M., Alley S., Schoeppe S., Vandelanotte C. The Effectiveness of E-& MHealth Interventions to Promote Physical Activity and Healthy Diets in Developing Countries: A Systematic Review. Int. J. Behav. Nutr. Phys. Act. 2016;13:1–14. doi: 10.1186/s12966-016-0434-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used during the current study are available from the corresponding authors on reasonable request.