Abstract
This study examined the relationship between plasma amino acid (AA) concentrations, including branched-chain AAs, and brown adipose tissue density (BAT-d). One hundred and seventy-three subjects (69 men, 104 women) aged 22–68 years were recruited during the winter season. AAs were comprehensively quantified using liquid chromatography-time-of-flight-mass spectrometry. The total hemoglobin concentration in the supraclavicular region ([total-Hb]sup), an indicator of BAT-d, was assessed using near-infrared time-resolved spectroscopy. Anthropometric parameters, including age, percentage of body fat, and visceral fat, were evaluated. Factors associated with higher (≥74 µM) or lower (<74 µM) [total-Hb]sup were investigated by multiple logistic regression models that included AA concentrations alone (model 1) or AA concentrations and anthropometric parameters (model 2) as independent variables. When adjusted for the false discovery rate, [total-Hb]sup was positively correlated with glycine and asparagine levels in men and with the serine level in both men and women and was negatively correlated with the branched-chain AA concentration in men. Models 1 and 2 correlated with higher or lower BAT-d for men (r = 0.73, p = 0.015) and women (r = 0.58, p = 0.079) and for men (r = 0.82, p = 0.0070) and women (r = 0.70, p = 0.020), respectively. A combination of anthropometric parameters and plasma AA concentrations could be a reliable biomarker for higher and lower BAT-d.
Keywords: amino acid concentrations, brown adipose tissue, plasma, biomarker, anthropometric parameters
1. Introduction
Brown adipose tissue (BAT) dissipates heat and secretes anti-inflammatory cytokines, known as BATkines, and it is a potential strategy for promoting cardiometabolic health [1,2]. BAT is reported to be related to less adiposity, including the percentage of whole body fat (%BF) and visceral fat area (VFA) [3,4,5], in healthy individuals and to increased glucose sensitivity in their obese counterparts [6] and patients with type 2 diabetes [7]. Therefore, increasing the activity or volume of BAT may help to combat obesity and certain chronic diseases, such as type 2 diabetes mellitus. The activity of BAT can be evaluated by 18F-fluorodeoxyglucose (FDG)-positron emission tomography (PET)/computed tomography (CT) (18FDG-PET/CT) in cold-stimulated environments [3,8,9]. Other non-invasive technologies used to evaluate the characteristics of BAT in humans include magnetic resonance imaging [10], skin thermal measurements [11], infrared thermography [12], near-infrared time-resolved spectroscopy (NIRTRS) [13,14], and contrast-enhanced ultrasonography [15]. However, each method has limitations, including high cost, ionizing radiation exposure, being time-intensive to perform, and/or requirement for acute cold exposure to stimulate BAT [3,8,9].
Clinically relevant biomarkers that can be used to evaluate the activity of BAT in individual subjects have long been sought. BAT mainly utilizes fatty acids as a primary energy source and glucose as a secondary source for non-shivering thermogenesis [8,16]. A recent study demonstrated that BAT contributes to the utilization of certain serum amino acids (AAs), such as valine and leucine, which are known as branched-chain AAs (BCAAs), especially in subjects with high BAT following 2 h of cold exposure [17]. However, that study included a small sample size comprised only of men and did not investigate thermoneutral BCAA profiles related to the activity of BAT in blood.
In this study, NIRTRS was used to monitor the vascular density of BAT (BAT-d), given that microvascular beds, evaluated based on the total hemoglobin (Hb) concentration in the supraclavicular region ([total-Hb]sup), are more abundant in BAT than in white adipose tissue [13].The reliability of [total-Hb]sup compared with BAT activity/volume parameters determined using cold-induced FDG-PET/CT measurements has been confirmed in previous studies [13,14,18].
In this research, we were attempting to find a clinically practical and simple biomarker for BAT-d without acute cold exposure, which is needed for 18FDG-PET/CT measurements. We hypothesized that the plasma AA profile would correlate with [total-Hb]sup and would be a practical biomarker in the clinical setting. Therefore, this study examined the relationship between the plasma AA profile under thermoneutral conditions (normal room temperature) and NIRTRS-determined BAT-d in a large sample of men and women.
2. Materials and Methods
2.1. Participants and Diagnosis
The study participants were recruited by placing advertisements on posters, the Internet, or via direct contact. A total of 241 volunteers were recruited, 68 of whom were excluded because they were pregnant (n = 15) or refused to provide a blood sample (n = 53), leaving 173 subjects (69 men, 104 women, aged 22–68 years) for enrollment in the study. When each subject arrived in the laboratory, where the room temperature was regulated at 23–27 °C, the following parameters were measured: body height, body weight, %BF, VFA, subcutaneous adipose tissue thickness (SATsup) in the supraclavicular region, BAT-d, and plasma AA concentrations.
The study design and protocols were approved by the institutional review board of Tokyo Medical University (approval no. 2017–199 and 2019–0028) and conducted in accordance with the ethical principles of the Declaration of Helsinki. Written informed consent was obtained from all study participants. The study was conducted during the winter season (December to April) between 2018 and 2020.
2.2. Measurement of Anthropometric Parameters
Body mass index (BMI) was calculated as body weight in kilograms divided by the square of height in meters (kg/m2). The %BF was estimated using the multifrequency bioelectric impedance method (Inbody 720 Body Composition Analyzer; InBody Japan, Tokyo, Japan). VFA was estimated using bioelectrical impedance analysis (EW-FA90; Panasonic, Osaka, Japan). The SATsup was measured by B-mode ultrasonography (Vscan Dual Probe; GE Vingmed Ultrasound AS, Horten, Norway) using the attached distance measuring system and calculated as the mean value of two measurements.
BAT-d, evaluated by the [total-Hb]sup using NIRTRS (TRS-20; Hamamatsu Photonics K.K., Hamamatsu, Japan), was measured for 1 min at room temperature (23–27 °C). The probes were placed on the skin in the supraclavicular region, which potentially contains BAT. Participants were required to remain seated in an upright position during the measurements, as previously described [13,19]. NIR wavelengths of 760, 800, and 830 nm were used to evaluate the concentrations of oxygenated Hb, deoxygenated Hb, and total Hb. With the 3-cm probe used in this study, light can reach a mean depth of 2 cm [20], where a certain amount of BAT may be located [21].
The tissue was illuminated using a 200-µm core diameter optical fiber by pulsed light generated from ps light pulses, with 100 ps full width at half-maximum, a 5-MHz repetition rate, and an average power of 80 µW for each wavelength. The emitted photons migrated into the tissue and were reflected by an optical bundle fiber (3-mm diameter), through which they were transferred to a photomultiplier tube for single-photon detection and processing. After the digitized temporal profile, data were fitted according to photon diffusion theory, the absorption coefficient and reduced scattering coefficient values at 760, 800, and 830 nm were obtained using the least-squares fitting method. The absolute total Hb concentration was calculated as the sum of the oxygenated and deoxygenated Hb concentrations [22]. The NIRTRS system collected data every 10 s. The coefficient of variation for repeated measurements of total Hb concentration was 4.9% [13].
In this study, the [total-Hb]sup was adjusted according to the underlying adipose tissue thickness [23]. Our previous study indicated that a cut-off [total-Hb]sup value of 74.0 µM was best for distinguishing higher BAT-d from lower BAT-d, as evaluated by 18FDG-PET/CT, with an accuracy of 82.8%, a sensitivity of 75.0%, a specificity of 100%, a positive predictive value of 100%, and a negative predictive value of 64.3% [13].
2.3. Sample Collection
Venous blood was collected from the 173 subjects and incubated for 30 min at room temperature (24 °C). Then, the plasma was collected by centrifugation for 15 min at 1000× g and stored at −80 °C until needed for metabolomic analyses.
2.4. Amino Acid Profiling
Sample processing and AA analyses were performed as described in our previous study [24] with some modifications. We measured the processed samples using only the positive mode to profile the AAs. Each plasma sample (10 μL) was mixed with methanol (90 μL) containing 1.5 μM of a standard compound. Following centrifugation at 20,380× g for 10 min at 4 °C, 90 μL of the supernatant were transferred to a fresh tube and vacuum-dried. The sample was reconstituted with 90% methanol (10 μL) and water (190 μL), vortexed, and centrifuged at 20,380× g for 10 min at 4 °C. For liquid chromatography-time-of-flight mass spectrometry (LC-TOF-MS) analysis, 1 μL of each supernatant was injected into the system.
The LC-TOF-MS instrument [19] and its parameters [24] have been described elsewhere. Briefly, a 1290 Infinity LC system and G6230B time-of-flight MS (TOF-MS; Agilent Technologies, Santa Clara, CA, USA) were used. Chromatographic separation was performed using an ACQUITY BEH C18 column (2.1 mm i.d. × 50 mm, 1.7 μm; Waters, Milford, MA, USA) at 40 °C.
Peaks derived from the AAs and internal standards were analyzed using MassHunter Qualitative Analysis software (version B.08.00; Agilent Technologies). The peaks were automatically integrated by the software but were also curated manually by the analysts. The peak areas of the AAs were divided by one of the internal standards (d6-N1,N8-diacetylspermidine) to yield relative areas. The absolute concentrations of each AA were calculated by comparing the relative area of each sample with that of the standard mixture.
2.5. Data Analysis
The relationship between BAT-d and the quantified AA values was evaluated. The BCAAs were evaluated together and for leucine, isoleucine, and valine individually. To eliminate fluctuation in the AA concentrations in each subject, the absolute concentration was divided by the total AA concentration, yielding the relative concentration. Relative concentrations were used for all analyses. These anthropometric parameters were categorized lower and higher groups using cut-off values for obesity [25]; BMI was divided by 25, %BF was divided by 25 in men and 30 in women, and VFA was divided by 100.
Spearman correlation coefficients were calculated for BAT-d and for the concentration of each AA or BCAA. In view of the multiple independent tests, p-values were adjusted using the false discovery rate method (Benjamini and Hochberg) to yield Q-values. Multiple logistic regression analyses (MLRs) were developed using the forced entry method to discriminate higher (≥74 µM) and lower (<74 µM) [total-Hb]sup using [total-Hb]sup as the dependent variable and the concentration of each plasma AA as the independent variable and using [total-Hb]sup as the dependent variable and age, %BF, VFA, and each plasma AA as independent variables. Receiver-operating characteristic (ROC) curves were analyzed by plotting true-positive rates (sensitivity) against false-positive rates (1 − specificity) to compare the discriminatory accuracy of [total-Hb]sup for survival and event-free survival.
All statistical analyses were performed using IBM SPSS Statistics version 27 (IBM Japan, Tokyo, Japan).
3. Results
The subject characteristics are summarized in Table 1. The height, weight, body mass index, and VFA were significantly higher, and %BF was significantly lower in men than in women. No significant sex-related differences were found in age or [total-Hb]sup.
Table 1.
Demographic and anthropometric characteristics of the study participants.
| Men (n = 69) | Women (n = 104) | All (n = 173) | p-Value * | |
|---|---|---|---|---|
| Age (years) | 38.12 ± 10.11 | 37.86 ± 7.30 | 37.96 ± 8.51 | 0.999 |
| Height (cm) ** | 172.53 ± 5.69 | 159.18 ± 5.46 | 164.50 ± 8.58 | <0.001 |
| Body weight (kg) ** | 69.54 ± 10.42 | 54.96 ± 8.62 | 60.78 ± 11.77 | <0.001 |
| BMI ** | 23.38 ± 3.48 | 21.67 ± 3.00 | 22.35 ± 3.30 | 0.001 |
| %BF (%) ** | 20.92 ± 6.44 | 28.74 ± 6.04 | 25.60 ± 7.28 | <0.001 |
| VFA (cm2) ** | 66.51 ± 39.00 | 34.37 ± 23.62 | 47.27 ± 34.45 | <0.001 |
| [total-Hb]sup (µM) | 69.48 ± 24.76 | 64.06 ± 18.95 | 66.22 ± 21.55 | 0.216 |
Values are shown as the mean ± standard deviation. * p < 0.05, ** p < 0.01 in men versus women. %BF, percentage of body fat; BMI, body mass index; [total-Hb]sup, total hemoglobin concentration in the supraclavicular region (an indicator of brown adipose tissue density); VFA, visceral fat area.
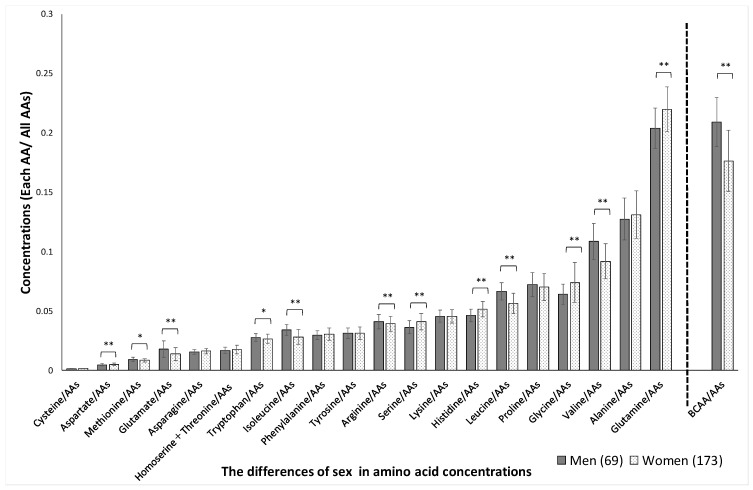
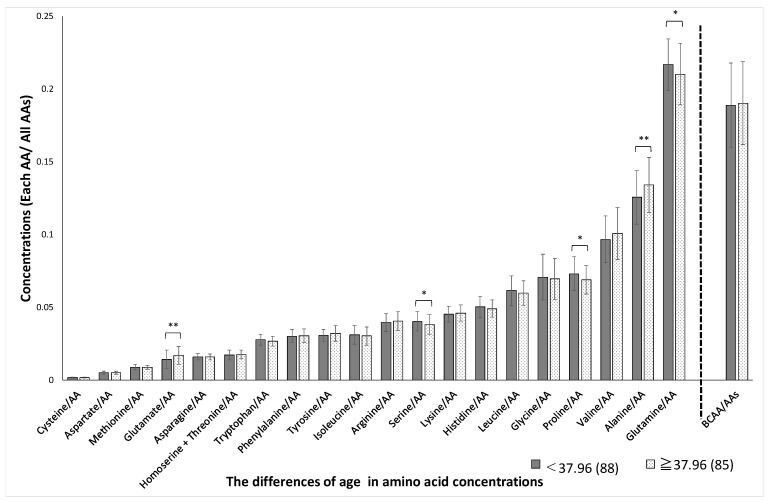
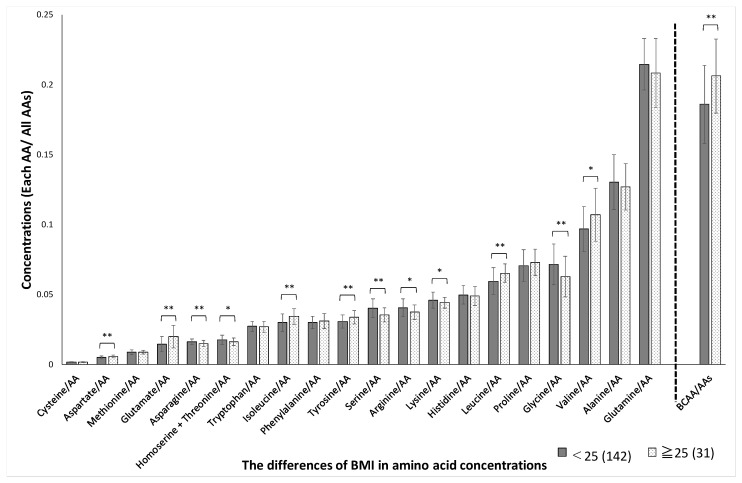
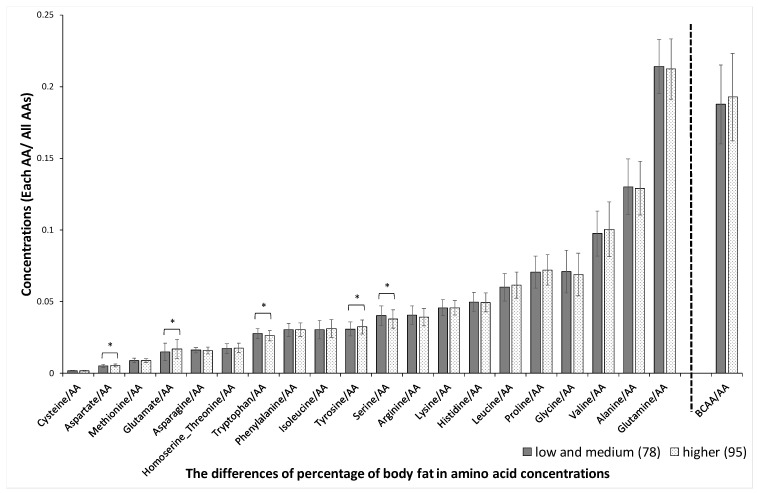
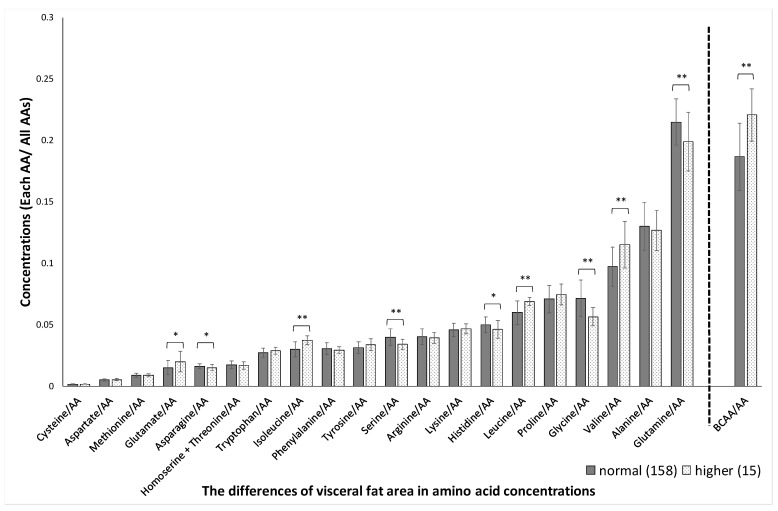
Significant sex-related differences were found in the concentrations of aspartate, methionine, glutamate, tryptophan, isoleucine, arginine, serine, histidine, leucine, glycine, valine, glutamine, and BCAAs, as shown in Figure 1. The variation (SD) in plasma amino acid concentrations between men and women was not significantly different (p = 0.53). Significant age-related differences were found in the concentrations of glutamate, serine, proline, alanine, and glutamine, as shown in Figure 2. Significant BMI-related differences were found in the concentrations of aspartate, glutamate, asparagine, homoserine + threonine, isoleucine, tyrosine, serine, arginine, lysine, leucine, glycine, valine, and BCAA, as shown in Figure 3. Significant %BF-related differences were found in the concentrations of aspartate, glutamate, tryptophan, tyrosine, and serine, as shown in Figure 4. Significant VFA related differences were found in the concentration of glutamate, asparagine, isoleucine, serine, histidine, leucine. glycine, valine, glutamine, and BCAA, as shown in Figure 5.
Figure 1.
Sex-related differences in amino acid concentrations. The absolute concentration was divided by the total AA concentration, yielding the relative concentration. AAs, amino acids; BCAAs, branched-chain amino acids. * p < 0.05, ** p < 0.01 in men versus women.
Figure 2.
Age-related differences in amino acid concentrations. The absolute concentration was divided by the total AA concentration, yielding the relative concentration. Participants were divided into two groups based on age, with a median of 37.96 years. AAs, amino acids; BCAAs, branched-chain amino acids. * p < 0.05, ** p < 0.01 in age < 37.96 versus age ≥ 37.96.
Figure 3.
BMI-related differences in amino acid concentrations. The absolute concentration was divided by the total AA concentration, yielding the relative concentration. Participants were divided into two groups based on BMI 25. AAs, amino acids; BCAAs, branched-chain amino acids BCAAs, branched-chain amino acids. * p < 0.05, ** p < 0.01 in BMI < 25 versus BMI ≥ 25.
Figure 4.
Body fat percentage-related differences in amino acid concentrations. The absolute concentration was divided by the total AA concentration, yielding the relative concentration. Participants were divided into two groups based on body fat percentage, 25% in men and 30% in women. AAs, amino acids; BCAAs, branched-chain amino acids. * p < 0.05, ** p < 0.01 in body fat percentage < 25% versus body fat percentage ≥ 25% in men and in body fat percentage < 30% versus body fat percentage ≥ 30% in women.
Figure 5.
Visceral fat area-related differences in amino acid concentrations. The absolute concentration was divided by the total AA concentration, yielding the relative concentration. Participants were divided into two groups based on the visceral fat area 100. AAs, amino acids; BCAAs, branched-chain amino acids. * p < 0.05, ** p < 0.01 in visceral fat area < 100 cm2 versus ≥ 100 cm2.
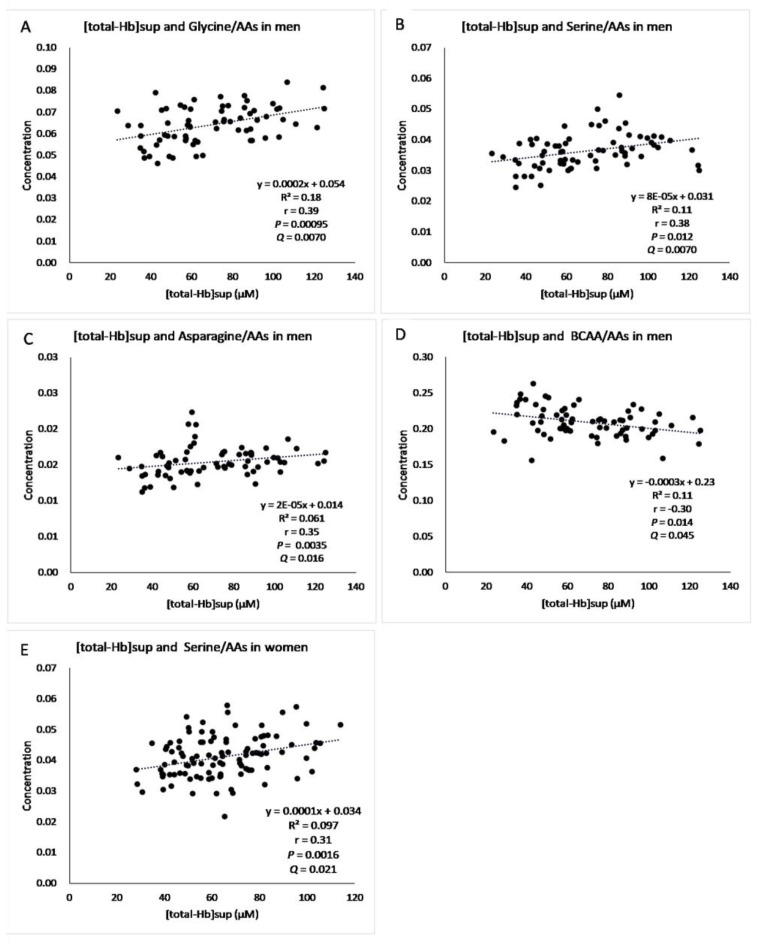
The correlations between the AA concentrations and [total-Hb]sup are shown in Table 2. In men, there were significant positive correlations of glycine (r = 0.39, p = 0.00095, Q = 0.005), serine (r = 0.38, p = 0.0012, Q = 0.0050), and asparagine (r = 0.35, p = 0.0035, Q = 0.012) with [total-Hb]sup and a negative correlation of the BCAA concentration with [total-Hb]sup (r = −0.30, p = 0.014, Q = 0.045). In women, the serine concentration (r = 0.31, p = 0.016, Q = 0.019) correlated positively with [total-Hb]sup.
Table 2.
Correlation between amino acid concentrations and total hemoglobin concentration in the supraclavicular region.
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| Valine, Leucine, and Isoleucine (BCAAs) | Valine, Leucine, and Isoleucine (BCAAs) | |||||||
| r | p-Value | Q-Value | Q-Value | r | p-Value | Q-Value | Q-Value | |
| Glycine/AAa | 0.39 ** | 0.00095 | 0.00700 | 0.00500 | 0.14 | 0.160 | 0.33 | 0.36 |
| Alanine/AAs | −0.086 | 0.48 | 0.63 | 0.55 | −0.0043 | 0.966 | 0.97 | 0.97 |
| Valine/AAs | −0.29 * | 0.016 | 0.067 | −0.18 | 0.065 | 0.20 | ||
| Leucine/AAs | −0.025 * | 0.037 | 0.13 | −0.23 * | 0.018 | 0.080 | ||
| Isoleucine/AAs | −0.19 | 0.12 | 0.32 | −0.23 * | 0.019 | 0.080 | ||
| Cysteine/AAs | −0.11 | 0.35 | 0.52 | 0.47 | −0.06 | 0.564 | 0.74 | 0.77 |
| Methionine/AAs | 0.047 | 0.70 | 0.78 | 0.67 | 0.14 | 0.172 | 0.33 | 0.36 |
| Serine/AAs | 0.38 ** | 0.0012 | 0.0070 | 0.0050 | 0.31 ** | 0.0016 | 0.021 | 0.019 |
| Homoserine + threonine/AAs | 0.23 | 0.057 | 0.17 | 0.15 | 0.071 | 0.476 | 0.67 | 0.70 |
| Aspartate/AAs | 0.038 | 0.76 | 0.80 | 0.68 | −0.090 | 0.365 | 0.59 | 0.63 |
| Glutamate/AAs | −0.15 | 0.22 | 0.44 | 0.41 | −0.19 * | 0.049 | 0.17 | 0.19 |
| Asparagine/AAs | 0.35 ** | 0.0035 | 0.016 | 0.012 | 0.14 | 0.143 | 0.33 | 0.36 |
| Glutamine/AAs | 0.170 | 0.16 | 0.38 | 0.37 | 0.048 | 0.630 | 0.77 | 0.78 |
| Arginine/AAs | 0.075 | 0.54 | 0.63 | 0.55 | 0.12 | 0.241 | 0.42 | 0.46 |
| Lysine/AAs | −0.14 | 0.24 | 0.44 | 0.41 | 0.016 | 0.869 | 0.91 | 0.92 |
| Histidine/AAs | 0.094 | 0.44 | 0.62 | 0.55 | 0.044 | 0.657 | 0.77 | 0.78 |
| Phenylalanine/AAs | 0.140 | 0.25 | 0.44 | 0.41 | 0.025 | 0.801 | 0.89 | 0.90 |
| Tyrosine/AAs | −0.130 | 0.29 | 0.46 | 0.42 | −0.079 | 0.427 | 0.64 | 0.68 |
| Tryptophan/AAs | −0.080 | 0.52 | 0.63 | 0.55 | 0.24 * | 0.015 | 0.08 | 0.10 |
| Proline/AAs | −0.031 | 0.80 | 0.80 | 0.68 | −0.14 | 0.167 | 0.33 | 0.36 |
| BCAAs/AAs | −0.30 * | 0.014 | 0.045 | −0.22 * | 0.028 | 0.13 | ||
p-values were adjusted using the false discovery rate method (Benjamini and Hochberg) to yield Q-values. The Q-value was calculated from the AA and total hemoglobin concentrations. The BCAAs were evaluated together and for leucine, isoleucine, and valine individually (according to Figure 1, Figure 2, Figure 3, Figure 4 and Figure 5). BCAAs, branched-chain amino acids. * p < 0.05, ** p < 0.01 in p-Values.
The correlation between [total-Hb]sup and age was not significant (r = −0.11, p = 0.161). The correlations between the AA concentrations and age were significant for alanine/AA (r = 0.21, p = 0.0061), serine/AA (r = −0.19, p = 0.0012), glutamate/AA (r = 0.27, p = 0.00039), asparagine/AA (r = −0.15, p = 0.048), glutamine/AA (r = −0.20, p = 0.010), tyrosine/AA (r = 0.17, p = 0.023), and proline/AA (r = −0.16, p = 0.042).
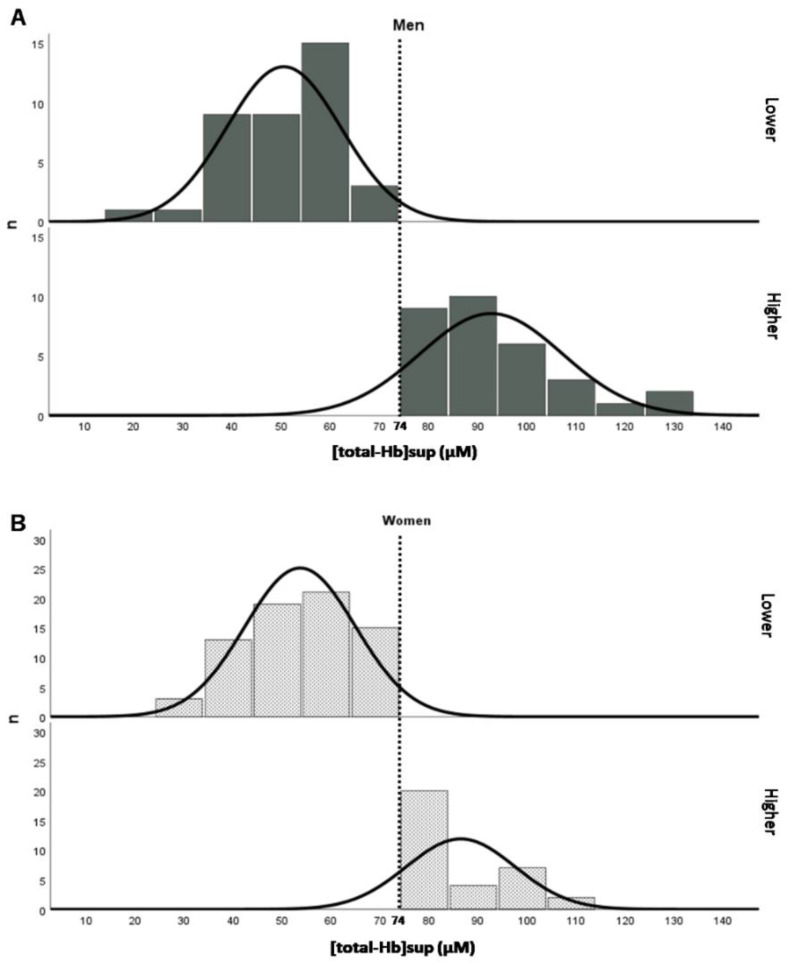
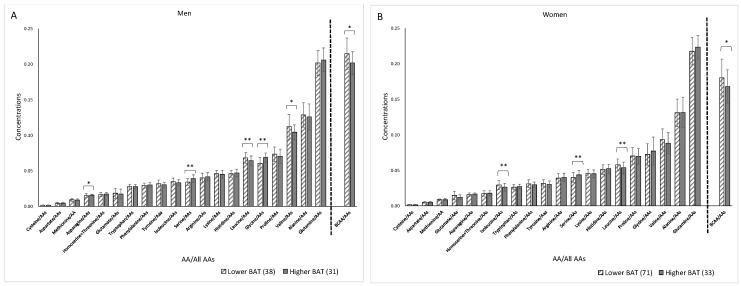
Significant (Q < 0.05) relationships were found between [total-Hb]sup and glycine, asparagine, and BCAA concentrations in men and between [total-Hb]sup and serine concentrations in both men and women (Figure 6). Figure 7 shows a histogram of [total-Hb]sup according to whether BAT-d was higher or lower in men and women. Figure 8 shows the differences in the concentrations of AAs between higher and lower BAT-d separately for men and women. Significant differences were observed in the concentrations of asparagine, serine, leucine, glycine, valine, and BCAA in men and in the concentrations of isoleucine, serine, leucine, and BCAA in women according to whether the BAT-d was higher or lower. MLR analysis with plasma AA concentrations as the independent variable correlated significantly with higher or lower BAT-d in men (r = 0.73, p = 0.015) but not in women (r = 0.58, p = 0.079) (Table S1). MLR that included both plasma AA concentrations and anthropometric parameters, including age, %BF, and VFA as independent variables revealed significant correlations with higher or lower BAT-d in men (r = 0.82, p = 0.0070) and in women (r = 0.70, p = 0.020) (Table S2); when using both plasma AA and anthropometric parameters, the correlation coefficient was higher than that when using only anthropometric parameters for both men (r = 0.54, p = 0.0020) and women (r = 0.43, p = 0.0051) (Table S3).
Figure 6.
Significant relationships between [total−Hb]sup and glycine/amino acids (AAs) in men (A), serine/AAs in men (B), asparagine/AAs in men (C), branched-chain amino acid (BCAA)/AAs in men (D), serine/AAs in women (E) when adjusted by the false discovery rate (Q < 0.05). [total-Hb]sup, total hemoglobin concentration in the supraclavicular region.
Figure 7.
Histograms showing the [total-Hb]sup, a parameter of brown adipose tissue density (BAT-d), in men (A) and women (B) according to whether BAT was higher (>74 µM) or lower (<74 µM). [total-Hb]sup, total hemoglobin concentration in the supraclavicular region.
Figure 8.
Differences in concentrations in amino acids (AAs) between higher BAT-d (>74 µM of [total-Hb]sup) and lower BAT-d (<74 µM of [total-Hb]sup) separately for men (A) and women (B). The numbers of participants in the higher and lower BAT-d groups were 31 and 38 in men and 33 and 71 in women, respectively. BCAAs, branched-chain amino acids; [total-Hb]sup, total hemoglobin concentration in the supraclavicular region. * p < 0.05, ** p < 0.01 in higher BAT-d (>74 µM of [total-Hb]sup) versus lower BAT-d (<74 µM of [total-Hb]sup).
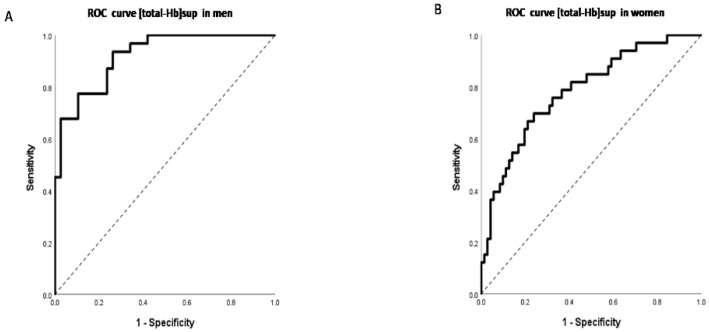
The area under the ROC curve determined by [total-Hb]sup to be closest to (0, 1) for the predicted formula determined by the AAs and anthropometric parameters provided an area under the ROC curve (AUC) of 0.92 (95% confidence interval [CI] 0.86–0.98, p < 0.001) in men and 0.78 (95% CI 0.69–0.88, p < 0.001) in women if the cut-off value of total Hb was set to 74.0 μM. The sensitivity was 94% with a specificity of 74% in men and 70% with a specificity of 76% in women (Figure 9A,B).
Figure 9.
Receiver-operating characteristic (ROC) curves for total hemoglobin in the supraclavicular region ([total-Hb]sup) in men (A) and women (B). The area under the ROC curve determined by [total-Hb]sup closest to (0, 1) for the predicted formula determined by the amino acid (AA) and anthropometric parameters was selected.
4. Discussion
In this study, when the data were corrected by the false discovery rate to yield a Q-value, we found significant positive correlations of glycine, serine, and asparagine concentrations with [total-Hb]sup in men, a significant negative correlation of the BCAA concentration with [total-Hb]sup in men, and a significant positive correlation of the serine concentration with [total-Hb]sup in women. The precision for prediction of higher or lower BAT-d was greater in both sexes when the independent variable included both anthropometric parameters and plasma AA concentrations (men, r = 0.82; women, r = 0.70) than when plasma AA concentrations were used alone (men, r = 0.73; women, r = 0.58). This finding indicates that a combination of anthropometric parameters and plasma AA concentrations could serve as a reliable biomarker for discriminating higher from lower BAT-d.
AAs are essential for protein synthesis in the body, and AA supplementation has been reported to have beneficial effects on muscle growth and function and on energy metabolism [26,27]. By contrast, elevated plasma BCAA levels have been found to have an adverse effect on glucose metabolism and to be associated with type 2 diabetes in humans and rodents [28,29]. The amount of BCAAs increases in the presence of insulin-resistant polymorphisms [30]. Mitochondrial BCAA enzymes in white adipose tissue are decreased under conditions of obesity and diabetes, suggesting that adipose tissue regulates BCAA levels in the blood [31,32]. Furthermore, a recent study demonstrated a significant negative correlation of BAT activity with a cold-induced reduction in the concentration of serum BCAAs, including valine and leucine, when examined in men and in those of valine, leucine, and isoleucine in obese mice [17]. In this study, we were attempting to identify a clinically practical and simple biomarker for BAT-d and therefore did not include 2 h of cold exposure. However, our results are similar to those obtained in a previous study that reported significant (p < 0.05) negative correlations of [total-Hb]sup with BCAA concentrations in both men and women that remained significant for men (Q = 0.045) but not for women (Q = 0.133) after correcting for the false discovery rate.
We found that only plasma serine remained significantly correlated with [total-Hb]sup (Q < 0.05) in both men and women. Insulin resistance has been associated with a decrease in arginine, aspartate, and serine concentrations and an increase in proline and tyrosine concentrations, independent of age. Expression of phosphoserine aminotransferase 1, which is involved in the synthesis of serine, is reduced in mice with diabetes induced by a high-fat diet [33]. Serine is a nonessential AA, which can be synthesized in the human body from other metabolites, such as glycine, the plasma concentration of which was only significantly (Q < 0.05) correlated with [total-Hb]sup in men in this study. By contrast, under insulin-resistant conditions, metabolic utilization of serine for gluconeogenesis and production of glutathione from glycine may be higher, resulting in a decrease in the plasma serine concentration [34]. It is interesting that BCAA was inversely correlated with BAT-d, whereas glycine was positively correlated with BAT-d. A previous report indicates that the concentration of glycine is decreased in obese individuals and that BCAAs are a source of glycine synthesis [35]. Therefore, the results of the present study suggest that BAT contributes to catabolism of BCAAs as a metabolic sink, and the catabolized BCAAs may be used for synthesis of glycine, thereby involving insulin resistance [36]. Overall, it is reasonable to conclude that attenuated plasma serine and glycine concentrations are associated with lower BAT-d in both men and women.
We examined the relationship between plasma AA profiles under thermoneutral conditions and NIRTRS-determined BAT-d in our large sample of men and women. MLR, with plasma AAs as the independent variable, revealed a significant correlation of AA concentrations with higher or lower BAT-d in men (r = 0.73, p = 0.015) but not in women (r = 0.58, p = 0.079). A previous study found that anthropometric parameters were independently correlated with [total-Hb]sup [19], with a correlation coefficient of r = 0.54 (p = 0.0020) in men and r = 0.43 (p = 0.0051) in women, which is in line with our present data. Therefore, we examined whether the precision for predicting BAT-d could be increased by using a combination of anthropometric parameters and plasma AA concentrations as the independent variable and found better correlations with higher or lower BAT-d for both men (r = 0.82, p = 0.0070) and women (r = 0.70, p = 0.020).
Despite the relevance of biomarkers for evaluation of BAT, each methodology has several limitations [3]. Our results indicate that a combination of anthropometric parameters and plasma AA concentrations provides a clinically practical and simple biomarker for BAT-d with low cost and no ionizing radiation exposure that is not time-consuming to perform and does not require acute cold exposure to stimulate BAT. Previous studies have identified other plasma biomarkers, including miRNA122 [37], LysoPC-acyl C16:0 [38], 12,13-diHOME [39], and androgens in men [19], to be potential candidates for identifying the characteristics of BAT when combined with anthropometric parameters as in the present study.
In another study, cold-induced FDG-PET/CT measurements showed a significant correlation between [total-Hb]sup under thermoneutral conditions and parameters of BAT activity/volume [13]. In our study, we also confirmed a significant correlation between cold-induced thermogenesis, which is an indicator of BAT activity [18], and [total-Hb]sup. Furthermore, a longitudinal study found that [total-Hb]sup and 18FDG-PET/CT parameters increased in parallel during chronic intake of thermogenic capsinoids, which are known to increase the mass and activity of BAT, and decreased after cessation of intake [40,41]. Therefore, we believe that [total-Hb]sup can be used to evaluate BAT-d under thermoneutral conditions.
The main limitation of this study is that when collecting blood samples, we did not control for the menstrual cycle. Although menstrual cycle was expected to show larger variations in plasma AA concentrations in women, we did not find a significant difference in variations (SD) in plasma AA concentrations between men and women. Although there has been a report of an age-related decrease in [total-Hb]sup that may influence plasma AA concentrations, we did not take this into account in this study. Furthermore, we found a significant biomarker only in lean healthy individuals in this study. Future research should include obese individuals and patients with cardiometabolic disease. Finally, given that the correlation coefficient for [total-Hb]sup and cold-induced FDG-PET/CT measurements was moderate (r = 0.74) [13], a detailed validation study should be conducted using FDG-PET/CT methodology in the future. The requirement for venous blood collection and the use of mass spectrometry represent additional challenges for using plasma AA profiles to predict BAT-d in clinical settings.
5. Conclusions
When the data obtained in this study were corrected by the false discovery rate, we found a significant positive correlation of glycine, serine, and asparagine concentrations and a negative correlation of the BCAA concentration with [total-Hb]sup in men. Moreover, there was a significant positive correlation of the serine concentration with [total-Hb]sup in women and in all study participants. A combination of anthropometric parameters and plasma AA concentrations may serve as a reliable biomarker for discriminating higher from lower BAT-d in both men (r = 0.82) and women (r = 0.70). The results of our study indicate that BAT-d can be estimated using the biomarker determined in this study at low cost and with no ionizing radiation exposure. Additional benefits of this biomarker are that it is not time-consuming to perform and does not require acute cold exposure to stimulate BAT.
Acknowledgments
We acknowledge a Grant-in-Aid Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (19H04061, 19K20123, 20H05743). We also would like to thank the study participants.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/jcm10112339/s1, Table S1. Results of multiple logistic regression analysis with plasma AA concentrations as the independent variable showed significant correlations with higher or lower BAT-d in men and women, Table S2. Results of multiple logistic regression analysis with plasma AA concentrations and anthropometric parameters, including age, %BF, and VFA, as independent variables showed significant correlations with higher or lower BAT-d in men and women, Table S3. Results of multiple logistic regression analysis with anthropometric parameters alone, including age, %BF, and VFA, as independent variables revealed significant correlations with higher or lower BAT-d in men and women.
Author Contributions
Conceptualization, T.H. and Y.K.; Methodology, T.H. and Y.K.; Software, Y.A., A.T. and M.A.; Validation, Y.A., A.T. and M.A.; Formal Analysis, M.K., S.H.-F., M.S., Y.A. and A.T.; Investigation, M.K., S.H.-F., Y.K., M.A., R.T., T.E. and R.K.; Resources, R.T., T.E. and R.K.; Data Curation, M.K. and S.H.-F.; Writing—Original Draft Preparation, M.K., S.H.-F., M.S. and T.H.; Writing—Review and Editing, M.K., S.H.-F. and T.H.; Visualization, M.K., S.H.-F. and T.H.; Supervision, T.H. and M.S.; Project Administration, T.H.; Funding Acquisition, T.H., M.S. and M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (19H04061, 19K20123, and 20H05743).
Institutional Review Board Statement
The study design and protocols were approved by the institutional review board of Tokyo Medical University (approval no. 2017–199 and 2019–0028) and conducted in accordance with the ethical principles of the Declaration of Helsinki. The study was conducted during the winter season (December to April) between 2018 and 2020.
Informed Consent Statement
Written informed consent to publish this paper has been obtained from the study participants.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available for privacy reasons.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cannon B., Nedergaard J. Brown adipose tissue: Function and physiological significance. Physiol. Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 2.Becher T., Palanisamy S., Kramer D.J., Eljalby M., Marx S.J., Wibmer A.G., Butler S.D., Jiang C.S., Vaughan R., Schoder H., et al. Brown adipose tissue is associated with cardiometabolic health. Nat. Med. 2021;27:58–65. doi: 10.1038/s41591-020-1126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Marken Lichtenbelt W.D., Vanhommerig J.W., Smulders N.M., Drossaerts J.M., Kemerink G.J., Bouvy N.D., Schrauwen P., Teule G.J. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 4.Virtanen K.A., Lidell M.E., Orava J., Heglind M., Westergren R., Niemi T., Taittonen M., Laine J., Savisto N.J., Enerbäck S., et al. Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 5.Matsushita M., Yoneshiro T., Aita S., Kameya T., Sugie H., Saito M. Impact of brown adipose tissue on body fatness and glucose metabolism in healthy humans. Int. J. Obes. 2014;38:812–817. doi: 10.1038/ijo.2013.206. [DOI] [PubMed] [Google Scholar]
- 6.Hanssen M.J., van der Lans A.A., Brans B., Hoeks J., Jardon K.M., Schaart G., Mottaghy F.M., Schrauwen P., van Marken Lichtenbelt W.D. Short-term cold acclimation recruits brown adipose tissue in obese humans. Diabetes. 2016;65:1179–1189. doi: 10.2337/db15-1372. [DOI] [PubMed] [Google Scholar]
- 7.Hanssen M.J., Hoeks J., Brans B., van der Lans A.A., Schaart G., van den Driessche J.J., Jörgensen J.A., Boekschoten M.V., Hesselink M.K., Havekes B., et al. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat. Med. 2015;21:863–865. doi: 10.1038/nm.3891. [DOI] [PubMed] [Google Scholar]
- 8.Cypess A.M., Lehman S., Williams G., Tal I., Rodman D., Goldfine A.B., Kuo F.C., Palmer E.L., Tseng Y.H., Doria A., et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saito M., Okamatsu-Ogura Y., Matsushita M., Watanabe K., Yoneshiro T., Nio-Kobayashi J., Iwanaga T., Miyagawa M., Kameya T., Nakada K., et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: Effects of cold exposure and adiposity. Diabetes. 2009;58:1526–1531. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holstila M., Pesola M., Saari T., Koskensalo K., Raiko J., Borra R.J., Nuutila P., Parkkola R., Virtanen K.A. MR signal-fat-fraction analysis and T2* weighted imaging measure BAT reliably on humans without cold exposure. Metabolism. 2017;70:23–30. doi: 10.1016/j.metabol.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Sun L., Verma S., Michael N., Chan S.P., Yan J., Sadananthan S.A., Camps S.G., Goh H.J., Govindharajulu P., Totman J., et al. Brown adipose tissue: Multimodality evaluation by PET, MRI, infrared thermography, and whole-body calorimetry (TACTICAL-II) Obesity. 2019;27:1434–1442. doi: 10.1002/oby.22560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El Hadi H., Frascati A., Granzotto M., Silvestrin V., Ferlini E., Vettor R., Rossato M. Infrared thermography for indirect assessment of activation of brown adipose tissue in lean and obese male subjects. Physiol. Meas. 2016;37:N118–N128. doi: 10.1088/0967-3334/37/12/N118. [DOI] [PubMed] [Google Scholar]
- 13.Nirengi S., Yoneshiro T., Sugie H., Saito M., Hamaoka T. Human brown adipose tissue assessed by simple, noninvasive near-infrared time-resolved spectroscopy. Obesity. 2015;23:973–980. doi: 10.1002/oby.21012. [DOI] [PubMed] [Google Scholar]
- 14.Hamaoka T., Nirengi S., Fuse S., Amagasa S., Kime R., Kuroiwa M., Endo T., Sakane N., Matsushita M., Saito M., et al. Near-infrared time-resolved spectroscopy for assessing brown adipose tissue density in humans: A review. Front. Endocrinol. 2020;11:261. doi: 10.3389/fendo.2020.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baron D.M., Clerte M., Brouckaert P., Raher M.J., Flynn A.W., Zhang H., Carter E.A., Picard M.H., Bloch K.D., Buys E.S., et al. In vivo noninvasive characterization of brown adipose tissue blood flow by contrast ultrasound in mice. Circ. Cardiovasc. Imaging. 2012;5:652–659. doi: 10.1161/CIRCIMAGING.112.975607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chondronikola M., Volpi E., Borsheim E., Porter C., Annamalai P., Enerback S., Lidell M.E., Saraf M.K., Labbe S.M., Hurren N.M., et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes. 2014;63:4089–4099. doi: 10.2337/db14-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoneshiro T., Wang Q., Tajima K., Matsushita M., Maki H., Igarashi K., Dai Z., White P.J., McGarrah R.W., Ilkayeva O.R., et al. BCAA catabolism in brown fat controls energy homeostasis through SLC25A44. Nature. 2019;572:614–619. doi: 10.1038/s41586-019-1503-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nirengi S., Fuse S., Amagasa S., Homma T., Kime R., Kuroiwa M., Endo T., Sakane N., Matsushita M., Saito M., et al. Applicability of supraclavicular oxygenated and total hemoglobin evaluated by near-infrared time-resolved spectroscopy as indicators of brown adipose tissue density in humans. Int. J. Mol. Sci. 2019;20:2214. doi: 10.3390/ijms20092214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuse S., Sugimoto M., Kurosawa Y., Kuroiwa M., Aita Y., Tomita A., Yamaguchi E., Tanaka R., Endo T., Kime R., et al. Relationships between plasma lipidomic profiles and brown adipose tissue density in humans. Int. J. Obes. 2020;44:1387–1396. doi: 10.1038/s41366-020-0558-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gunadi S., Leung T.S., Elwell C.E., Tachtsidis I. Spatial sensitivity and penetration depth of three cerebral oxygenation monitors. Biomed. Opt. Express. 2014;5:2896–2912. doi: 10.1364/BOE.5.002896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flynn A., Li Q., Panagia M., Abdelbaky A., MacNabb M., Samir A., Cypess A.M., Weyman A.E., Tawakol A., Scherrer-Crosbie M. Contrast-enhanced ultrasound: A novel noninvasive, nonionizing method for the detection of brown adipose tissue in humans. J. Am. Soc. Echocardiogr. 2015;28:1247–1254. doi: 10.1016/j.echo.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamaoka T., McCully K.K., Quaresima V., Yamamoto K., Chance B. Near-infrared spectroscopy/imaging for monitoring muscle oxygenation and oxidative metabolism in healthy and diseased humans. J. Biomed. Opt. 2007;12:062105. doi: 10.1117/1.2805437. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto K., Niwayama M., Kohata D., Kudo N., Hamaoka T., Kime R., Katsumura T. Optical Tomography and Spectroscopy of Tissue IV. Volume 4250. International Society for Optics and Photonics; Bellingham, WA, USA: 2001. Functional imaging of muscle oxygenation using a 200-channel cw NIRS system; pp. 142–152. [Google Scholar]
- 24.Shimizu H., Usui Y., Asakage M., Nezu N., Wakita R., Tsubota K., Sugimoto M., Goto H. Serum metabolomic profiling of patients with non-infectious uveitis. J. Clin. Med. 2020;9:3955. doi: 10.3390/jcm9123955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ministry of Health. Labour and Welfare. E-Health Net Body Fat Scale. [(accessed on 15 May 2021)]; Available online: http://www.e-healthnet.mhlw.go.jp/
- 26.Bagheri R., Forbes S.C., Candow D.G., Wong A. Effects of branched-chain amino acid supplementation and resistance training in postmenopausal women. Exp. Gerontol. 2021;144:111185. doi: 10.1016/j.exger.2020.111185. [DOI] [PubMed] [Google Scholar]
- 27.Manaf F.A., Peiffer J.J., Maker G.L., Fairchild T.J. Branched-chain amino acid supplementation improves cycling performance in untrained cyclists. J. Sci. Med. Sport. 2021;24:412–417. doi: 10.1016/j.jsams.2020.10.014. [DOI] [PubMed] [Google Scholar]
- 28.Huffman K.M., Shah S.H., Stevens R.D., Bain J.R., Muehlbauer M., Slentz C.A., Tanner C.J., Kuchibhatla M., Houmard J.A., Newgard C.B., et al. Relationships between circulating metabolic intermediates and insulin action in overweight to obese, inactive men and women. Diabetes Care. 2009;32:1678–1683. doi: 10.2337/dc08-2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang T.J., Larson M.G., Vasan R.S., Cheng S., Rhee E.P., McCabe E., Lewis G.D., Fox C.S., Jacques P.F., Fernandez C., et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 2011;17:448–453. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guizar-Heredia R., Tovar A.R., Granados-Portillo O., Pichardo-Ontiveros E., Flores-López A., González-Salazar L.E., Arteaga-Sanchez L., Medina-Vera I., Orozco-Ruiz X., Torres N., et al. Serum amino acid concentrations are modified by age, insulin resistance, and BCAT2 rs11548193 and BCKDH rs45500792 polymorphisms in subjects with obesity. Clin. Nutr. 2021 doi: 10.1016/j.clnu.2021.01.037. [DOI] [PubMed] [Google Scholar]
- 31.Lackey D.E., Lynch C.J., Olson K.C., Mostaedi R., Ali M., Smith W.H., Karpe F., Humphreys S., Bedinger D.H., Dunn T.N., et al. Regulation of adipose branched-chain amino acid catabolism enzyme expression and cross-adipose amino acid flux in human obesity. Am. J. Physiol. Endocrinol. Metab. 2013;304:E1175–E1187. doi: 10.1152/ajpendo.00630.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herman M.A., She P., Peroni O.D., Lynch C.J., Kahn B.B. Adipose tissue branched chain amino acid (BCAA) metabolism modulates circulating BCAA levels. J. Biol. Chem. 2010;285:11348–11356. doi: 10.1074/jbc.M109.075184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu J., Xiao F., Guo Y., Deng J., Liu B., Zhang Q., Li K., Wang C., Chen S., Guo F. Hepatic phosphoserine aminotransferase 1 regulates insulin sensitivity in mice via tribbles homolog 3. Diabetes. 2015;64:1591–1602. doi: 10.2337/db14-1368. [DOI] [PubMed] [Google Scholar]
- 34.Mullner E., Rohnisch H.E., von Bromssen C., Moazzami A.A. Metabolomics analysis reveals altered metabolites in lean compared with obese adolescents and additional metabolic shifts associated with hyperinsulinaemia and insulin resistance in obese adolescents: A cross-sectional study. Metabolomics. 2021;17:11. doi: 10.1007/s11306-020-01759-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White P.J., Newgard C.B. Branched-chain amino acids in disease. Science. 2019;363:582–583. doi: 10.1126/science.aav0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adeva-Andany M., Souto-Adeva G., Ameneiros-Rodriguez E., Fernandez-Fernandez C., Donapetry-Garcia C., Dominguez-Montero A. Insulin resistance and glycine metabolism in humans. Amino Acids. 2018;50:11–27. doi: 10.1007/s00726-017-2508-0. [DOI] [PubMed] [Google Scholar]
- 37.Okamatsu-Ogura Y., Matsushita M., Bariuan J.V., Nagaya K., Tsubota A., Saito M. Association of circulating exosomal miR-122 levels with BAT activity in healthy humans. Sci. Rep. 2019;9:13243. doi: 10.1038/s41598-019-49754-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boon M.R., Bakker L.E.H., Prehn C., Adamski J., Vosselman M.J., Jazet I.M., Arias-Bouda L.M.P., van Lichtenbelt W.D.M., van Dijk K.W., Rensen P.C.N., et al. LysoPC-acyl C16:0 is associated with brown adipose tissue activity in men. Metabolomics. 2017;13:48. doi: 10.1007/s11306-017-1185-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lynes M.D., Leiria L.O., Lundh M., Bartelt A., Shamsi F., Huang T.L., Takahashi H., Hirshman M.F., Schlein C., Lee A., et al. The cold-induced lipokine 12,13-diHOME promotes fatty acid transport into brown adipose tissue. Nat. Med. 2017;23:631–637. doi: 10.1038/nm.4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nirengi S., Homma T., Inoue N., Sato H., Yoneshiro T., Matsushita M., Kameya T., Sugie H., Tsuzaki K., Saito M., et al. Assessment of human brown adipose tissue density during daily ingestion of thermogenic capsinoids using near-infrared time-resolved spectroscopy. J. Biomed. Opt. 2016;21:091305. doi: 10.1117/1.JBO.21.9.091305. [DOI] [PubMed] [Google Scholar]
- 41.Fuse S., Endo T., Tanaka R., Kuroiwa M., Ando A., Kume A., Yamamoto A., Kuribayashi K., Somekawa S., Takeshita M., et al. Effects of capsinoid intake on brown adipose tissue vascular density and resting energy expenditure in healthy, middle-aged adults: A randomized, double-blind, placebo-controlled study. Nutrients. 2020;12:2676. doi: 10.3390/nu12092676. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available for privacy reasons.