Abstract
Food preservatives such as NaNO2, which are widely used in human food products, undoubtedly affect, to some extent, human organs and health. For this reason, there is a need to reduce the hazards of these chemical preservatives, by replacing them with safe natural bio-preservatives, or adding them to synthetic ones, which provides synergistic and additive effects. The Citrus genus provides a rich source of such bio-preservatives, in addition to the availability of the genus and the low price of citrus fruit crops. In this study, we identify the most abundant flavonoids in citrus fruits (hesperidin) from the polar extract of mandarin peels (agro-waste) by using spectroscopic techniques, as well as limonene from the non-polar portion using GC techniques. Then, we explore the synergistic and additive effects of hesperidin from total mandarin extract with widely used NaNO2 to create a chemical preservative in food products. The results are promising and show a significant synergistic and additive activity. The combination of mandarin peel extract with NaNO2 had synergistic antibacterial activity against B. cereus, Staph. aureus, E. coli, and P. aeruginosa, while hesperidin showed a synergistic effect against B. cereus and P. aeruginosa and an additive effect against Staph. aureus and E. coli. These results refer to the ability of reducing the concentration of NaNO2 and replacing it with a safe natural bio-preservative such as hesperidin from total mandarin extract. Moreover, this led to gaining benefits from their biological and nutritive values.
Keywords: mandarin peel, food preservatives, natural antimicrobials, sodium nitrites
1. Introduction
Food preservatives, which are widely used in food products, affect human health. Their effect varies according to age and health status. The most used preservatives are chemicals such as sodium nitrite, NaNO2, which is used in meats and fish as an antimicrobial and preservative. Unfortunately, despite its powerful preservative efficiency, NaNO2 has various worrisome hazardous effects on human health and safety [1]. In the stomach, NaNO2 produces nitrosamines or free radicals. Such products can increase lipid peroxidation, which can pose many health hazards to various body organs. Moreover, reactive nitrogen species have many toxic effects including hepatotoxicity, nephrotoxicity, and dysregulation of inflammatory responses [1].
To minimize or prevent the side effects of these synthetic preservatives, and to enhance the synergistic effect of these synthetic preservatives and natural extracts against foodborne microorganisms, it is essential to use natural food preservatives (bio-preservatives) or multiple combination systems between synthetic food preservatives and natural extracts [2,3]. Natural preservatives are believed to be healthier and have additional benefits due to their bioactivity and nutritional value. That is why a gradual shift from chemical additives to natural alternatives is required [4].
In recent years, the use of natural antimicrobials in the food industry has gained close attention by consumers and producers [5]. The plant kingdom is rich in plants that possess antimicrobial activity. This activity is related to the presence of polyphenols as they contain hydroxyl groups; these groups can interact with the cell membrane of bacteria to disrupt membrane structures and cause the leakage of cellular components [6,7]. In addition to the antimicrobial activity of polyphenols, essential oils have been reported to have antimicrobial activity, and their activity relationship was mentioned [8]. Natural products from plants have diverse biological activities [9,10]. There were 140 medicinal plants screened for their antimicrobial activity including garlic (Allium sativum), clove (Syzygium aromaticum), Feijoa sellowiana, and tee tree (Melaleuca alternifolia), and these were described as broad-spectrum antimicrobial agents. Additionally, bearberry (Arctostaphylos uva-ursi) and cranberry juice (Vaccinium macrocarpon) were reported to treat urinary tract infections [9,10,11].
The Citrus genus, one of the major fruit crops worldwide and a natural source for bio-preservatives, annually produces approximately 133 million tons of the world’s fruit crop, with grapefruit (8.4 million tons), lemons and limes (17.3 million tons), mandarins and tangerines (32.8 million tons), and oranges (73.2 million tons) [12].
The food and industrial processing of citrus peels generate a huge amount of peels as a by-product. Citrus peels are rich in bioactive components such as the hesperidin flavonoid and the limonene monoterpene [12].
Since limonene is commonly recognized as a safe (GRAS) material, it can be used as a food preservative as well as a common food additive to impart a citrus flavor. Limonene has also been tested as an antimicrobial agent, as well as an antioxidant [13].
Flavonoids are important in the defense against pathogenic microorganisms, and they can also be used as natural food preservatives due to their antimicrobial properties. Hesperidin, for example, has anti-infective and anti-replicative properties against many microorganisms [14,15].
Food researchers and the food industry are motivated to produce innovative, natural, and more efficient products as a result of today’s customer preference for natural preservatives over synthetic additives. Along with the current ones, there are undoubtedly many more natural additive ingredients yet to be identified. More research is required to discover new natural preservatives and investigate their safety [8,16].
Mandarin is a cheap fruit crop which is available and highly consumable worldwide. It produces tons of peels (agro-waste) rich in bioactive flavonoids such as hesperidin; therefore, in this work, we selected mandarin to explore the antimicrobial activity of the total extract and the major flavonoid hesperidin with NaNO2. The results exhibit significant synergistic and additive effects of both the total extract and hesperidin as antimicrobials.
2. Results and Discussion
2.1. Identification of Mandarin Oil by GC/MS
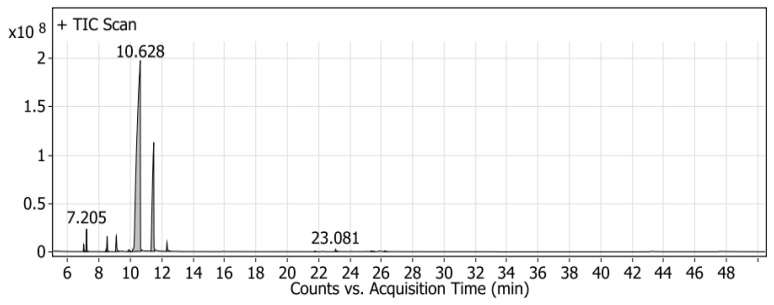
GC/MS was used to investigate the volatile compounds of the mandarin peel oil produced in Egypt (Figure 1). Thirteen compounds were detected and identified in mandarin peels by comparing the fragmentation pattern with Wiley and NIST Mass Spectral Library data (Table 1). These identified compounds contained two major compounds, namely, limonene (75.21%) and γ-terpinene (18.64%), in addition to β-myrcene (1.53%), α-pinene (1.37%), and 2-.β-pinene (1.13%). The rest of the minor compounds, present at 2.12%, are listed in Table 1.
Figure 1.
GC/MS chromatogram of mandarin volatile oils.
Table 1.
Identified volatile compounds by GC/MS spectrometry.
| Peak | RT | Name | Formula | Area | Area Sum % |
|---|---|---|---|---|---|
| 1 | 7.016 | l-Phellandrene | C10H16 | 18,336,447 | 0.47 |
| 2 | 7.205 | α-(-)-pinene | C10H16 | 53,171,439.3 | 1.37 |
| 3 | 8.453 | 3-Carene | C10H16 | 5,287,840.96 | 0.14 |
| 4 | 8.519 | 2-.β-pinene | C10H16 | 44,154,069.3 | 1.13 |
| 5 | 9.096 | β-myrcene | C10H16 | 59,501,702.7 | 1.53 |
| 6 | 9.9 | 2-Carene | C10H16 | 11,948,052.3 | 0.31 |
| 7 | 10.628 | D-Limonene | C10H16 | 2,928,075,732 | 75.21 |
| 8 | 11.488 | ɤ-Terpinene | C10H16 | 725,626,383 | 18.64 |
| 9 | 12.33 | α-Terpinolene | C10H16 | 30,695,286.9 | 0.79 |
| 10 | 21.767 | α-ylangene | C15H24 | 2,130,866.43 | 0.05 |
| 11 | 23.081 | Caryophyllene | C15H24 | 8,035,838.48 | 0.21 |
| 12 | 25.369 | 1H-Cycloprop[e]azulene, 1a,2,3,5,6,7,7a,7b-octahydro-1,1,4,7-tetramethyl-, [1aR-(1a.alpha.,7.alpha.,7a.beta.,7b.alpha.)]- | C15H24 | 3,360,910.92 | 0.09 |
| 13 | 26.211 | β-copaene | C15H24 | 3,051,328.78 | 0.08 |
According to the literature, the percent of limonene in mandarin peels extracted by different methods ranges from 68.51% to 77.59%, and the percent of ɤ-Terpinene ranges from 13.13% to 20.70% [17], which supports our method of extraction and results.
Occasionally, limonene was reported for its food preservative activity. The antibacterial activity of limonene is attributed to its ability to incorporate itself into the bacterial cell membrane lipid system and, hence, alter its properties [18].
2.2. Identification of Mandarin Peel Chemical Constituents by UPLC/MS/MS
Formulated mass spectral fragmentations of the metabolites were performed to provide a comprehensive fragmentation pattern with the retention time and MS/MS information. The freeze-dried mandarin ethanol extract MS/MS spectra imported from raw MS data to MS-DIAL 4.36 (http://prime.psc.riken.jp/) (Accessed Date: 18 February 2021) and from Global Natural Products Social Molecular Networking (GNPS, https://gnps.ucsd.edu/) (Accessed Date: 22 February 2021) were used for the identification of mandarin peels by comparing their fragmentation patterns and retention indices (RI) with available databases.
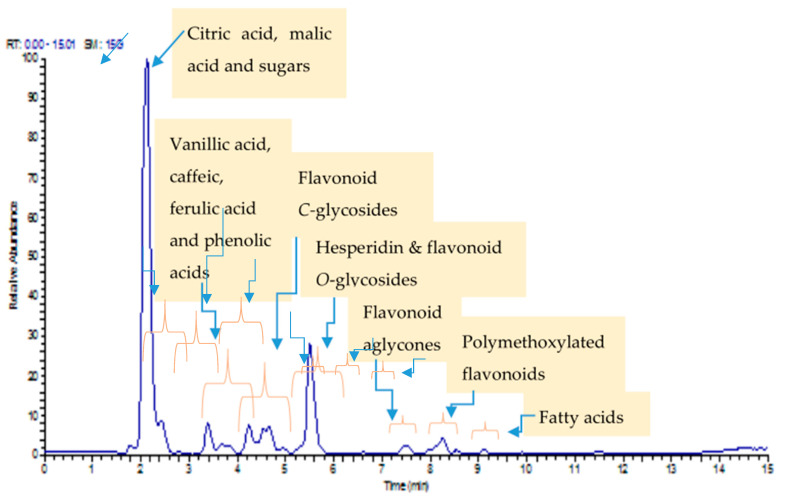
UPLC/MS/MS analysis of mandarin peels led to the identification of 33 compounds (Table 2). These compounds are mainly sugars, flavonoid glycosides, and organic acids, as well as a small amount of methoxylated flavonoids and lipids (Figure 2).
Table 2.
UPLC/MS/MS of mandarin peels.
| No | RT [Min] | Metabolite Identification | Chemical Formula | [M − H]− | Ref. | |
|---|---|---|---|---|---|---|
| Measured | Fragmentation | |||||
| 1 | 2.03 | Trehalose | C12H22O11 | 341.1092 | 179.0552 | [19] |
| 2 | 2.08 | Citric acid | C7H11O6 | 191.0552 | 173.0445 | [19] |
| 3 | 2.15 | Hexose | C6H12O6 | 179.0550 | 161.0443 | [19] |
| 4 | 2.16 | Malic acid | C4H6O5 | 133.0128 | 115.0022 | [20] |
| 6 | 3.21 | Tryptophan | C11H12N2O2 | 203.0818 | 186.0546, 159.0915, 142.0650, 116.0491 | [21] |
| 7 | 3.39 | Vanillic acid hexoside | C14H18O9 | 329.0879 | 167.0337 | [22] |
| 8 | 3.41 | Caffeic acid | C9H8O4 | 179.0551 | 134.9866 | [23] |
| 9 | 4.25 | Feruloyl quinic acid | C17H20O9 | 367.1033 | 193.0497, 191.0185 | [24] |
| 10 | 4.29 | Sinapic acid hexouronide | C17H20O11 | 399.0932 | 223.0462, 193.0497 | [25] |
| 11 | 4.46 | Apigenin-di-C-hexoside (vicenin 2) | C27H30O15 | 593.1360 | 503.1203, 473.1094, 383.0774, 353.0667 | [26] |
| 12 | 4.48 | Sinapic acid hexoside | C17H22O10 | 385.1854 | 223.1331 | [27] |
| 13 | 4.73 | Meranzin hydrate | C15H18O5 | 277.1080 | 259.0951, 233.1181, 215.1074, 189.9480, 87.0070 | [28] |
| 14 | 4.75 | Methoxyluteolin di-C-hexoside | C30H38O16 | 623.1752 | 533.1318, 503.1197, 413.6878, 383.0773 | [26] |
| 15 | 4.87 | Syringic acid | C9 H10 O5 | 197.0446 | 169.0130, 125.0227 | [22] |
| 16 | 4.89 | Orientin | C21H20O11 | 447.0935 | 357.0616, 327.0511 | [19] |
| 17 | 5.28 | Vitexin | C21H20O10 | 431.0985 | 341.0668, 311.0562 | [29] |
| 18 | 5.47 | Naringenin-7-O-rutinoside | C27H32O14 | 579.1774 | 271.0612 | [19] |
| 19 | 5.48 | Feruloyl-O-malic acid ester | C14H13O8 | 309.0616 | 193.0498, 134.0360, 115.0017 | [30] |
| 20 | 5.55 | Hesperidin | C28H34O15 | 609.1814 | 475.2522, 430.9161, 367.2440, 301.0651 | [31] |
| 21 | 5.52 | Isosakuranetin-7-O-neohesperidoside | C28H34O14 | 593.1551 | 285.0405 | [26] |
| 22 | 5.59 | Diosmetin-C-hexoside | C22H22O11 | 461.1085 | 371.0770, 341.0664, 298.0481 | [32] |
| 23 | 5.68 | Naringenin-7-O-Hexoside | C21H22O10 | 433.1142 | 271.0614 | [26] |
| 24 | 5.79 | Rhiofolin | C27H29O14 | 577.0258 | 269.0456 | [33] |
| 25 | 5.88 | Isorhamnetin-3-O-hexoside | C22H22O12 | 477.1044 | 315.0505, 314.0432, 300.0292 | [34] |
| 26 | 7.41 | Diosmetin | C16H12O6 | 299.0550 | 284.0326 | [19] |
| 27 | 7.51 | hesperitin | C16H14O6 | 301.0618 | 286.0382 | [19] |
| 28 | 8.04 | Dihydroxy trimethoxy flavone | C18H15O7 | 343.0823 | 328.0589, 313.0355, 298.0126, 285.0407 | [26] |
| 29 | 8.10 | Dihydroxy dimethoxy flavone | C17H13O7 | 329.0328 | 314.0423, 300.509, 299.0182 | [26] |
| 30 | 8.23 | Dihydroxy tetramethoxy flavone | C19H18O8 | 373.0932 | 358.0694, 343.0458, 328.0222, 300.0268 | [26] |
| 31 | 8.25 | Dihydroxy trimethoxy flavone | C18H15O7 | 343.0819 | 328.0589, 313.0355, 298.0126, 285.0407 | [26] |
| 32 | 9.12 | Hydroxy-hexadecanoic acid | C16H31O3 | 271.1914 | 253.1805, 209.1901 | [26] |
| 33 | 10.25 | Linolenic acid | C18H30O2 | 277.2170 | 233.1541, 205.1590, 59.0121 | [31] |
Figure 2.
UPLC/MS/MS of mandarin ethanol extract (base peak) chromatogram.
These compounds were dereplicated using GNPS and MS-dial and compared to literature data.
Identification of Sugars
The sugar contents seem to be high in mandarin peels, as shown in Figure 2. We identified two main sugars based on their molecular formula and fragmentation as well as their dereplication with GNPS. These compounds were identified as trehalose (C12H22O11) and hexose (C6H12O6).
It was surprisingly reported that identified sugar trehalose in the presence of fatty acids reduces the adhesion of microbial pathogens to the surfaces [35].
Identification of Organic and Fatty Acids
Two types of organic acids were detected in the mandarin peel ethanolic extract. Two organic hydroxy acids were detected and annotated according to their molecular formula, fragmentation pattern, and retention times, namely, citric acid with molecular ion [M − H]− at m/z 191 (C7H12O6) and malic acid with [M − H]− at m/z 133 (C4H6O5). It should be noted that citric acid is dominant in citrus peels [36].
Two fatty acids were also annotated and identified as hydroxy-hexadecanoic acid (C16H32O3) and linolenic acid (C18H30O2).
Organic acids, including malic and citric acids, have been widely used as food preservatives because they exhibit a broad spectrum of action against Gram-positive bacteria, Gram-negative bacteria, fungi, and yeasts [37,38].
Identification of Phenolic Acids and Phenolic Acid Conjugates
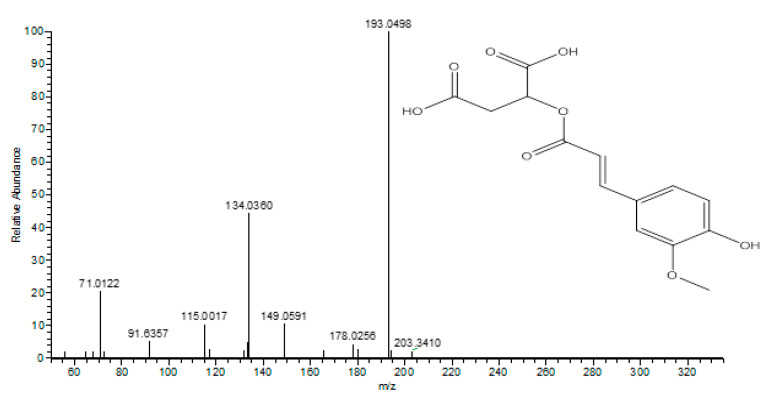
Two phenolic acids were annotated as caffeic acid (compound 8) and syringic acid (compound 16). These compounds were accompanied by the loss of [M − H − 44]−, characteristic of the loss of a carboxyl group. Compound 7 with [M-H]- at m/z 329 and the daughter ion at 167 [M − H − 162]− was assigned as vanillic acid hexoside. Compound 7 with [M − H]− at m/z 367 and the daughter ion at 193 [M − H − 191]− was assigned as feruloyl quinic acid. Compound 10 with [M − H]− at m/z 399 and the daughter ion at 223 [M − H − 176]− was assigned as sinapic acid hexuronide, which was identified for the first time from mandarin peels, and compound 12 with [M − H]− at m/z 385 and the daughter ion at m/z 223 [M − H − 162]− was assigned as sinapic acid hexoside. Sinapic acid derivatives were reported before from citrus species [39]. Compound 19, as shown in Figure 3, with [M − H]− at m/z 309 and the daughter ion at m/z 193 [M − H − 116]− was attributed to ferulic acid, and that at m/z 134 and 133 was attributed to the presence of a malic acid moiety. Therefore, compound 19 was identified as feruloyl-O-malic acid ester, which was tentatively identified for the first time from mandarin peels.
Figure 3.
UPLC/MS/MS of feruloyl-O-malic acid ester.
Moreover, cinnamic acid derivatives from plant material and their conjugates reported having antibacterial and antifungal properties [40].
Identification of Flavonoid-O-Glycosides
Flavonoid-O-glycosides in general, and especially hesperidin, are, in addition to sugars, the most abundant compounds in mandarin peels. In MS/MS fragmentation spectra, the nature of the sugars in O-glycosides could be distinguished from the elimination of the sugar residue from molecular ions, i.e., 162 dalton (glucose or galactose), and 146 dalton (deoxyhexose such as rhamnose) [26]. Therefore, six flavonoid-O-glycoside compounds (18, 20, 21, 23, 24, and 25) were annotated, according to GNPS [19,26], to naringenin-7-O-rutinoside (C27H32O14), hesperidin (C28H34O15), isosakuranetin-7-O-neohesperidoside (C28H34O14), naringenin-7-O-hexoside (C21H22O10), rhiofolin (C27H30O14), and isorhamnetin-3-O-hexoside (C22H22O12), respectively.
Identification of Flavonoid-C-Glycosides
The fragmentation patterns of C-glycoside flavonoids are [M − H − 120]− and [M − H − 90]– and were clearly observed in compounds 11, 14, 16, 17, and 22. These compounds were, respectively, identified as apigenin-di-C-hexoside (vicenin 2), methoxyluteolin di-C-hexoside (lucenin-2 4′-methyl ether), orientin, vitexin, and diosmetin-C-hexoside. It should be noted that both luteolin-C-glycosides have been reported in citrus peels [41].
Identification of Polymethoxyflavones and Flavonoid Aglycones
It should be noted that polymethylated flavones have been previously reported in citrus species [42]. In the MS/MS fragmentation spectrum of mandarin peel extract, polymethylated flavones were observed and have distinctive fragmentation patterns including [M − H − nCH3] − and [M − H − nCH3 − CO]−. These compounds are 28, 29, 30, and 31, identified as dihydroxy-trimethoxyflavone, dihydroxy-dimethoxyflavone, dihydroxy-tetramethoxyflavone, and dihydroxy-dimethoxyflavone isomer, respectively.
Identification of Coumarins
Only one compound, namely, meranzin hydrate, was detected and identified according to its molecular formula and fragmentation pattern.
Identification of Hesperidin by NMR Spectroscopy
An off-white powder of the isolated compound was obtained from mandarin peels, showing molecular ion [M − H]− at m/z 609 and accompanied by a daughter molecular ion at m/z 301. The isolated compound was further investigated by 1H and 13C NMR spectroscopy as follows:
1H NMR (400 MHz, DMSO-d6), δ 6.93 (m, 3H, H-2′, H-5′ and H-6′), 6.15 (brs, 1H, H-6), 6.13 (brs, 1H, H-8), 5.50 (dd, J = 12.3, 3.3 Hz, 1H, H-2), 2.78 (dd, J = 3.2 Hz & 17.15, 1H, H-3ax), 3.25 (dd, J = 17.16, 8.21 Hz, 1H, H-3eq), 4.98 (d, J = 7.1 Hz, 1H, H-1″), 4.54 (s, 1H, H-1‴), 1.10 (d, J = 6.1, 3H, CH3 of rhamnose). 13C NMR (101 MHz, DMSO) δ 197.46 (C-4), 165.59 (C-7), 163.50 (C-5), 162.95 (C-9), 148.42 (C-3′), 146.90 (C-4′), 131.34 (C-1′), 118.44 (C-2′), 114.60 (C-6′), 112.48 (C-5′), 103.80 (C-10), 101.05 (C-1‴), 99.92 (C-1″), 96.87(C-6), 96.03 (C-8), 78.83 (C-2), 76.73 (C-3″), 75.99 (C-5″), 73.45 (C-2″), 72.55 (C-4‴), 71.19 (C-2‴), 70.74 (C-3‴), 70.07 (C-4″), 68.79 (C-5‴), 66.50 (C-6″), 56.15 (O-Me), 42.49 (C-3), 18.29 (C-6‴) [28,43].
2.3. Antibacterial Activity of NaNO2, Mandarin Peel Extract, and Hesperidin
The antibacterial activities of sodium nitrite, mandarin peel extract, and hesperidin against four strains of foodborne pathogenic bacteria are presented in Table 3. Mandarin peel extract exhibited the highest activity against E. coli, P. aeruginosa, and B. cereus, with inhibition zones of 16.7, 13.0, and 10.2 mm, respectively. Additionally, the highest antibacterial activity of hesperidin was recorded against Gram-negative E. coli, P. aeruginosa, and P. aeruginosa, with 15.8 and 10.8 mm inhibition zones, while the highest zone of inhibition of 19.3 mm by sodium nitrite was observed against P. aeruginosa. Previously published data confirmed the antimicrobial activity of mandarin peel extract as limonene, the major volatile constituent [44], hesperidin flavonoid [45], and citric acid [46].
Table 3.
Antibacterial activity of mandarin peel extract, hesperidin, and sodium nitrite against some foodborne pathogenic bacteria.
| Bacteria | Inhibition Zone, mm (Mean ± S.E) | |||
|---|---|---|---|---|
| Negative Control | NaNO2 1 mg mL−1 |
Mandarin Peel Extract 10 mg mL−1 | Hesperidin 10 mg mL−1 |
|
| B. cereus | 0 | 15.2 ± 1.04 a | 10.2 ± 0.81 b | 8.8 ± 0.76 c |
| Staph. aureus | 0 | 17.2 ± 0.76 a | 9.3 ± 0.58 b | 9.8 ± 1.25 b |
| E. coli | 0 | 11.8 ± 0.76 c | 16.7 ± 2.46 a | 15.8 ± 0.86 b |
| P. aeruginosa | 0 | 19.3 ± 1.04 a | 13.0 ± 0.50 b | 10.8 ± 0.86 c |
n = 3, p ˂ 0.05, S.E: standard error; DMSO: negative control. Values are given as mean ± SE. Means followed by different superscripts within rows (a, b, and c) are significantly different.
2.3.1. Minimum Inhibitory Concentration and Synergy Interactions of Mandarin Peel Extract with NaNO2
As illustrated in Table 4, the MIC values obtained from sodium nitrite with mandarin peel extract against the tested pathogenic bacteria ranged between 0.77 and 1.13 mg mL−1. The lowest MIC value, 0.77 mg mL−1, was recorded against E. coli, while the highest MIC was observed against Staph. aureus, 1.13 mg mL−1. MIC values of sodium nitrite ranged from 0.67 to 1.67 mg mL−1. All combinations between mandarin peel extract and sodium nitrite were tested against all the described pathogenic strains. As shown in Table 4, a significant decrease in MIC values of sodium nitrite, between 75% and 87.5%, was recorded when combined with mandarin peel extract, this reduction depending upon the tested bacterial strain. Additionally, there was a significant decrease in the MICs of mandarin peel extract when combined with sodium nitrite.
Table 4.
Synergic interaction between mandarin peel extract with NaNO2 against the tested foodborne pathogenic bacteria.
| Bacteria | MICA mg mL−1 |
MICB mg mL−1 |
FICA | FICB | FIC Index | Interaction |
|---|---|---|---|---|---|---|
| B. cereus | 1.67 | 0.93 | 0.25 | 0.12 | 0.37 | S |
| Staph. aureus | 0.92 | 1.13 | 0.06 | 0.12 | 0.18 | S |
| E. coli | 4.76 | 0.77 | 0.13 | 0.12 | 0.25 | S |
| P. aeruginosa | 0.67 | 1.03 | 0.25 | 0.25 | 0.5 | S |
n = 3, MICA: MIC of NaNO2; MICB: MIC of mandarin peel extract; FICA: FIC of NaNO2; FICB: FIC of mandarin peel extract; S: synergistic effect, if ∑FIC index ≤ 0.5.
The fraction inhibitory concentration index (FICI) values obtained by the checkerboard assay were in the range of 0.18 to 0.5, indicating that all combinations studied had a synergistic effect (FICI ˂ 0.5) in all tested strains. Mandarin peel extract enhanced the antibacterial activity of sodium nitrite against B. cereus, Staph. aureus, E. coli, and P. aeruginosa with a synergistic effect (FICI values of 0.37, 0.18, 0.25, and 0.50, respectively).
2.3.2. Minimum Inhibitory Concentration and Synergy Interactions of Hesperidin
The MIC value of hesperidin alone and in combination with sodium nitrate was determined, as shown in Table 5. The MICs of hesperidin and sodium nitrite against the tested pathogenic bacteria varied between 0.67 and 4.76 mg mL−1. The lowest MIC value exhibited by hesperidin was against E. coli (1.13 mg mL−1), while the highest MIC observed was 1.53 mg mL−1 against Staph. aureus. Strong synergistic activity for the combination of hesperidin and sodium nitrite was shown against B. cereus and P. aeruginosa (Table 5). A significant reduction in MICs of hesperidin and sodium nitrite was observed against the tested bacteria. Fraction inhibitory concentration indices (FICIs) showed synergy of 0.37 with B. cereus and P. aeruginosa, and an additive interaction against Staph. aureus and E. coli with FICI values of 0.63 and 0.75, respectively. No antagonism was recorded from the hesperidin and sodium nitrite combination.
Table 5.
Synergic interaction between hesperidin and NaNO2 against the tested foodborne pathogenic bacteria.
| Bacteria | MICA mg mL−1 |
MICB mg mL−1 |
FICA | FICB | FIC Index | Interaction |
|---|---|---|---|---|---|---|
| B. cereus | 1.67 | 1.33 | 0.13 | 0.25 | 0.37 | S |
| Staph. aureus | 0.92 | 1.53 | 0.5 | 0.13 | 0.63 | A |
| E. coli | 4.76 | 1.13 | 0.25 | 0.5 | 0.75 | A |
| P. aeruginosa | 0.67 | 1.27 | 0.25 | 0.13 | 0.37 | S |
n = 3, p ˂ 0.05, MICA: MIC of NaNO2; MICB: MIC of hesperidin; FICA: FIC of NaNO2; FICB: FIC of hesperidin; S: synergistic effect if ∑FIC ≤ 0.5; A: additive if 0.5 < ∑FIC ≤ 1.
2.4. Time–Kill Assay
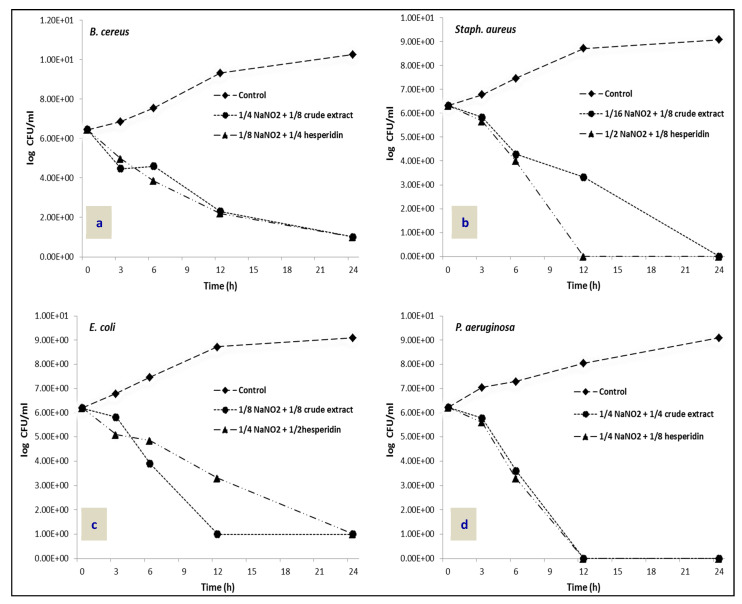
To confirm the synergistic effect of mandarin peel extract and hesperidin with NaNO2 against the tested foodborne pathogenic bacteria, a time–kill assay was conducted (Figure 4). The combination of 1/4 and 1/8 MICs of NaNO2 with 1/8 and 1/4 of mandarin peel extract and hesperidin, respectively, showed effective inhibition against B. cereus within 24 h (Figure 4a). Meanwhile, the combination of 1/16 MIC of NaNO2 and 1/8 MIC of mandarin peel extract completely inhibited Staph. aureus growth within 24 h, and the combination of 1/2 MIC of NaNO2 and 1/8 MIC of hesperidin completely inhibited the growth of Staph. aureus within 12 h (Figure 4b). The combination of 1/8 and 1/4 MICs of NaNO2 with 1/8 and 1/2 of mandarin peel extract and hesperidin, respectively, showed significant inhibition against E. coli within 24 h (Figure 4c). Additionally, the combination of 1/4 MIC of NaNO2 with 1/4 MIC of mandarin peel extract and 1/8 MIC of hesperidin required only 12 h to completely inhibit the growth of P. aeruginosa (Figure 4d).
Figure 4.
Time–kill data for synergistic combination of NaNO2 with mandarin peel extract and hesperidin against (a): B. cereus; (b): Staph. aureus; (c): E. coli; (d): P. aeruginosa.
3. Materials and Methods
3.1. Plant Materials and Extraction
The mandarin fruits were collected from the National Research Center farm, Nubaria, Egypt.
The fresh mandarin peels (1000 g) were mixed with ethanol in a blender (each 100 g:1 L of ethanol) three times, followed by filtration and evaporation at 40 °C to yield sticky material (28 g) of the total ethanolic extract.
3.2. Gas Chromatography-Mass Spectrometry Analysis (GC/MS)
To prepare a sample for GC/MS analysis, 50 g of fresh mandarin peels was mixed in a blender with 500 mL ethanol, then the filtrate was collected, and 50 mL of water was added to the filtrate. Hence, the filtrate was partitioned with hexane according to the method described in nature protocols by Kjer et al., 2010 [47]. The n-hexane fraction was evaporated under vacuum using a rotatory evaporator at 35 °C for 25 min to yield a volatile oil.
The n-hexane fraction was applied to the GC/MS system (Agilent Technologies, Santa Clara, CA 95051. USA) equipped with a gas chromatograph (7890B) and mass spectrometer detector (5977A) at Central Laboratories Network, National Research Centre, Cairo, Egypt. The GC was also equipped with an HP-5MS column (30 m × 0.25 mm internal diameter and 0.25 μm film thickness). Helium was used as a carrier gas at a flow rate of 1.0 mL/min at a split of 1:30, injection volume of 1 µL, and the following temperature program: 40 °C for 1 min; rising at 4 °C /min to 150 °C and holding for 6 min; rising at 4 °C/min to 210 °C and holding for 1 min. The injector and detector were held at 280 °C and 220 °C, respectively. Mass spectra were obtained by electron ionization (EI) at 70 eV, using a spectral range of m/z 50–900 and solvent delay of 5 min. Wiley and NIST Mass Spectral Library data were used for identification of mandarin peel volatile n-hexane fraction constituents by comparing the spectrum fragmentation pattern with those stored in the data.
3.3. Ultra-Performance Liquid Chromatography-Mass Spectrometry Analysis (UPLC/MS/MS)
UPLC/MS/MS analysis was performed according to the method described in Ammar et al., 2021 [48], in which the UPLC model, Waters (Milford, Massachusetts, MA 01757. USA) hyphenated to the Q Exactive hybrid MS/MS quadrupole - Orbitrap mass spectrometer was used. Chromatographic separation for this system was carried out using acidified water with 0.1% formic acid (solvent 1) and acetonitrile (solvent 2) with a mobile phase flow rate of 0.4 mL/min in the following gradient: 0–15 min from 50% to 50% solvent 2; 15–22 min to 98% of solvent 2, maintaining these conditions for 22 min; then, from 22 to 23 min 95% of solvent 1 to 27 min system, returning to starting conditions and re-equilibrating for 3 min with the BEH shield C18 column (150 × 2.1 mm, 1.7 μm). The Q-Exactive MS operated upon the following settings: HESI ion source voltage −3 kV or 3 kV; sheath gas (N2) flow 48 L/min; auxiliary gas flow 13 L/min; ion source capillary temperature 250 °C; auxiliary gas heater temperature 38 °C. The CID MS/MS experiments were performed using a collision energy of 15 eV.
3.4. Isolation and Identification of Hesperidin
Mandarin peel extract (25 g) was applied to polyamide 6 (Sigma-Aldrich, Munich, Germany) column chromatography (250 g), eluted by water to yield 12.5 g of sticky material, mainly sugars, and then eluted with 30% ethanol in order of decreasing polarity to yield 4.2 g of yellow amorphous powder, then 60% ethanol to yield 3.3 g, and finally 100% ethanol 2.1g after evaporation of the eluent under vacuum using a Heidolph rotatory evaporator (Schwabach, Germany).
The presence of hesperidin was checked using comparative paper chromatography (Whatman filter paper sheets No.1, United Kingdom) using 15% acetic acid as aqueous eluent and butanol/acetic acid/water (BAW) in portions (4:1:5, respectively) as organic eluent. The 30% ethanol fraction (4 g) was applied to Sephadex LH-20 column chromatography (Pharmacia Company, Uppsala, Sweden) and eluted with 50% ethanol and monitored by UV lamp (365 nm). Then, the collected fractions were checked by comparative paper chromatography using hesperidin as a standard sample (friendly, 2 mg obtained from the Department of Phytochemistry and Plant Systematics, National Research Center, Dokki, Cairo, Egypt), whereby 750 mg of hesperidin-containing sub-fractions was collected. The hesperidin sub-fraction was applied to repeated Sephadex LH-20 column chromatography using butanol saturated with water as eluent to finally yield 400 mg of hesperidin.
3.5. Nuclear Magnetic Resonance (NMR Analysis)
1H and 13C-NMR spectra were acquired using an Avance III HD 400 MHz NMR spectrometer (Bruker BioSpin GmbH, Rheinstetten, Germany). NMR spectra of the isolated compound were measured in DMSO-d6 and calibrated to the residual solvent signals resonances at δ H = 2.49 and δ C = 39.5 ppm [49].
3.6. Antibacterial Activity Assay
3.6.1. Tested Bacteria Strains
The antibacterial activity of sodium nitrite, mandarin peel extract, and hesperidin was tested on two Gram-positive bacteria (Staphylococcus aureus (ATCC 25923) and Bacillus cereus (EMCC 1080)), and two Gram-negative bacteria (Pseudomonas aeruginosa (NRRL B-272) and Escherichia coli (0157 H7 ATCC 51659)). The antibacterial assays were obtained from VACSERA (Holding Company for Biological Products and Vaccines), Egypt. The stock cultures were grown on slants of nutrient agar at 37 °C for 24 h and then kept in the refrigerator.
3.6.2. Disc Diffusion Technique
A loop full of bacteria, incubated for 24 h in a nutrient agar slant of each bacterial species, was inoculated in a test tube containing 5 mL of tryptic soy broth. Broth culture was incubated at 37 °C for 4 h until it achieved turbidity of 0.5 McFarland BaSO4 standard (108 cfu mL−1). The sensitivity tests of mandarin peel extract, hesperidin, and sodium nitrite were determined with different bacterial strains using the disc diffusion method by the Kirby–Bauer technique [50,51]. DMSO represented the negative control. After that, inoculated plates were incubated at 37 °C for 24 h. At the end of the incubation period, inhibition zones were expressed as the diameter of clear inhibition zone including the diameter of the paper disc.
3.7. Determination of Minimum Inhibitory Concentration (MIC)
Minimal inhibitory concentrations (MICs) for mandarin peel extract, hesperidin, and sodium nitrite were determined using the microbroth dilution method by Andrews et al., 2001 [52]. Two-fold serial dilutions of mandarin peel extract, hesperidin, and sodium nitrite ranging from 10 to 0.05 mg mL−1 were used. Equal volumes of tested bacteria (105 cfu mL−1) were added to each well. MIC values were taken as the lowest concentration of the antimicrobial agent that inhibited bacterial growth after 24 h of incubation at 37 °C.
3.8. Checkerboard Assay
The presence of synergism or antagonism of mandarin peel extract and hesperidin with NaNO2 was evaluated using isobolograph analyses and the checkerboard assay according to [53,54]. This method was conducted using different concentrations of samples and sodium nitrite along different axes, ensuring that each well contained different combinations of the samples and sodium nitrite. The analyses were performed using 96-well plates. Bacteria were grown to reach 2 × 108 cfu mL−1. Five microliters of each bacterial strain inoculum was added into the well containing tested samples, sodium nitrite, and Mueller Hinton Broth medium (MHB). The plates were incubated for 18 h/37 °C. MIC was determined for the combination as the lowest concentration that completely inhibited bacterial growth. Fractional inhibitory concentration (FIC) was calculated for each combination using the following formula: FICA = MICA in combination/MICA alone; FICB = MICB in combination/MICB alone; FIC index = FICA + FICB, where MICA is the MIC of NaNO2, FICA is the FIC of NaNO2, MICB is the MIC of mandarin peel extract or hesperidin, and FICB is the FIC of the mandarin peel extract or hesperidin. FIC index is the FIC added value of both NaNO2 and mandarin peel extract or hesperidin. The interaction of the antibacterial combinations was determined as previously reported by [55,56,57] by plotting an isobologram.
3.9. Time–Kill Assay
Time–kill curves were assayed using the confirmed synergistic combinations of mandarin peel extract or hesperidin with NaNO2 against tested bacteria. The overnight growth plate was inoculated in sterile MHB at 35 °C to approximate the density of 0.5 McFarland standard. The suspension was diluted 1:10 in normal saline solution to obtain a standard inoculum of 1 × 106 CFU/mL. An amount of 100 μL of the diluted bacterial suspension was added to 0.9 mL of MHB. Double dilutions for each NaNO2 and sample were prepared. Tubes containing individual NaNO2 and the combination were incubated at 35 °C for 24 h. From each tube, 100 μL of the sample was collected at 0, 3, 6, 12, and 24 h and plated to determine the count of viable cells. Additionally, growth control was included for each assay. The killing rate was determined by plotting colony viable counts (CFU/mL) against time. Synergy was defined as a ≥ 2 log10 CFU/mL reduction in viable bacteria with the combination compared with the most active single agent.
4. Conclusions
There is no escaping that food preservatives should be used to increase the shelf life of food products and to avoid the economical loss that can arise as a result of food spoilage. Additionally, there is no doubt that there are hazards of using these chemical preservatives to human health. Therefore, the solution is either finding a safe and natural substitute, which is actually tedious and costly, or decreasing the harm of these preservatives by maximizing their preservative effect at low concentrations. This work provides a solution by proving the synergistic and/or additive action of a natural by-product and it’s bioactive flavonoid (hesperidin) against selected food pathogen microbes. This study showed a significant decrease in the MIC values of sodium nitrite with mandarin peel extract, ranging between a 75% to 87.5% reduction depending on the tested strain; further, hesperidin showed both synergistic and additive activities. The application of any of them as a preservative is related to the quality measures, e.g., taste, odor, and homogeneity, that need to be achieved in food products.
Acknowledgments
We are grateful to the Deanship of Scientific Research, Najran University, for providing the funding to perform this project (NU/MID/17/052).
Author Contributions
M.A.E.R., H.A.A., and D.A.M. revised the manuscript and assumed the project profile. G.H.A., A.M.I., and M.A.M. conducted the chromatographic separation, performed the structure elucidation of the pure isolated hesperidin, and performed GC/MS identification. M.A.E.R., M.A.M., and G.H.A. performed the UPLC/MS/MS identification. D.A.M., and H.A.A. performed the antibacterial activity. All authors performed data analysis; in addition, they were responsible for drafting and writing the final version. All authors have read and agreed to the published version of the manuscript.
Funding
The Deanship of Scientific Research, Najran University, project (NU/MID/17/052).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Helal E.G.E., Mustafa R.A.A., Mohamed A., El-Gamal M.S. Adverse effects of two kinds of food additive mixtures (flavor enhancer, food preservative or food coloring agent) on physiological parameters in young male albino rats. Egypt. J. Hosp. Med. 2017;67:344–351. doi: 10.12816/0036646. [DOI] [Google Scholar]
- 2.Embaby M.A., El-Raey M.A., Zaineldain M., Almaghrabi O., Marrez D.A. Synergistic effect and efflux pump inhibitory activity of Ficus nitida phenolic extract with tetracycline against some pathogenic bacteria. Toxin Rev. 2019:1–11. doi: 10.1080/15569543.2019.1659370. [DOI] [Google Scholar]
- 3.Marrez D.A., Sultan Y.Y., Embaby M.A. Biological activity of the cyanobacterium Oscillatoria brevis extracts as a source of nutraceutical and bio-preservative agents. Int. J. Pharmacol. 2017;13:1010–1019. doi: 10.3923/ijp.2017.1010.1019. [DOI] [Google Scholar]
- 4.Carocho M., Barreiro M.F., Morales P., Ferreira I.C.F.R. Adding molecules to food, pros and cons: A review on synthetic and natural food additives. Compr. Rev. Food Sci. Food Saf. 2014;13:377–399. doi: 10.1111/1541-4337.12065. [DOI] [PubMed] [Google Scholar]
- 5.Gyawali R., Ibrahim S.A. Natural products as antimicrobial agents. Food Control. 2014;46:412–429. doi: 10.1016/j.foodcont.2014.05.047. [DOI] [Google Scholar]
- 6.Xue J., Davidson P.M., Zhong Q. Thymol nanoemulsified by whey protein-maltodextrin conjugates: The enhanced emulsifying capacity and antilisterial properties in milk by propylene glycol. J. Agric. Food Chem. 2013;61:12720–12726. doi: 10.1021/jf4043437. [DOI] [PubMed] [Google Scholar]
- 7.Tortora F., Notariale R., Maresca V., Good K.V., Sorbo S., Basile A., Piscopo M., Manna C. Phenol-rich Feijoa sellowiana (pineapple guava) extracts protect human red blood cells from mercury-induced cellular toxicity. Antioxidants. 2019;8:220. doi: 10.3390/antiox8070220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ultee A., Bennik M.H.J., Moezelaar R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002;68:1561–1568. doi: 10.1128/AEM.68.4.1561-1568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piscopo M., Tenore G.C., Notariale R., Maresca V., Maisto M., De Ruberto F., Heydari M., Sorbo S., Basile A. Antimicrobial and antioxidant activity of proteins from Feijoa sellowiana Berg. fruit before and after in vitro gastrointestinal digestion. Nat. Prod. Res. 2020;34:2607–2611. doi: 10.1080/14786419.2018.1543686. [DOI] [PubMed] [Google Scholar]
- 10.Attia G.H., Alyami H.S., Orabi M.A.A., Gaara A.H., El Raey M.A. Antimicrobial Activity of Silver and Zinc Nanoparticles Mediated by Eggplant Green Calyx. Int. J. Pharmacol. 2020;16:236–243. doi: 10.3923/ijp.2020.236.243. [DOI] [Google Scholar]
- 11.Rios J.-L., Recio M.C. Medicinal plants and antimicrobial activity. J. Ethnopharmacol. 2005;100:80–84. doi: 10.1016/j.jep.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 12.de la Rosa J.D.P., Ruiz-Palomino P., Arriola-Guevara E., García-Fajardo J., Sandoval G., Guatemala-Morales G.M. A green process for the extraction and purification of hesperidin from mexican lime peel (Citrus aurantifolia Swingle) that is extendible to the citrus genus. Processes. 2018;6:266. doi: 10.3390/pr6120266. [DOI] [Google Scholar]
- 13.Umagiliyage A.L., Becerra-Mora N., Kohli P., Fisher D.J., Choudhary R. Antimicrobial efficacy of liposomes containing d-limonene and its effect on the storage life of blueberries. Postharvest Biol. Technol. 2017;128:130–137. doi: 10.1016/j.postharvbio.2017.02.007. [DOI] [Google Scholar]
- 14.Iranshahi M., Rezaee R., Parhiz H., Roohbakhsh A., Soltani F. Protective effects of flavonoids against microbes and toxins: The cases of hesperidin and hesperetin. Life Sci. 2015;137:125–132. doi: 10.1016/j.lfs.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 15.El Dib R.A., Soliman H.S.M., Hussein M.H., Attia H.G. Two new flavonoids and biological activity of Astragalus abyssinicus (Hochst.) Steud. ex A. Rich. Aerial Parts. Drug Res. 2015;65:259–265. doi: 10.1055/s-0034-1377003. [DOI] [PubMed] [Google Scholar]
- 16.Gokoglu N. Novel natural food preservatives and applications in seafood preservation: A review. J. Sci. Food Agric. 2019;99:2068–2077. doi: 10.1002/jsfa.9416. [DOI] [PubMed] [Google Scholar]
- 17.Dugo P., Bonaccorsi I., Ragonese C., Russo M., Donato P., Santi L., Mondello L. Analytical characterization of mandarin (Citrus deliciosa Ten.) essential oil. Flavour Fragr. J. 2011;26:34–46. doi: 10.1002/ffj.2014. [DOI] [Google Scholar]
- 18.Hąc-Wydro K., Flasiński M., Romańczuk K. Essential oils as food eco-preservatives: Model system studies on the effect of temperature on limonene antibacterial activity. Food Chem. 2017;235:127–135. doi: 10.1016/j.foodchem.2017.05.051. [DOI] [PubMed] [Google Scholar]
- 19.Wang M., Carver J.J., Phelan V.V., Sanchez L.M., Garg N., Peng Y., Nguyen D.D., Watrous J., Kapono C.A., Luzzatto-Knaan T., et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016;34:828–837. doi: 10.1038/nbt.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sobeh M., Hassan S.A., El Raey M.A., Khalil W.A., Hassan M.A.E., Wink M. Polyphenolics from Albizia harveyi exhibit antioxidant activities and counteract oxidative damage and ultra-structural changes of cryopreserved bull semen. Molecules. 2017;22:1993. doi: 10.3390/molecules22111993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emam M., Abdel-Haleem D.R., Salem M.M., Abdel-Hafez L.J., Latif R.R., Farag S.M., Sobeh M., El Raey M.A. Phytochemical Profiling of Lavandula coronopifolia Poir. Aerial Parts Extract and Its Larvicidal, Antibacterial, and Antibiofilm Activity Against Pseudomonas aeruginosa. Molecules. 2021;26:1710. doi: 10.3390/molecules26061710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taamalli A., Arráez-Román D., Abaza L., Iswaldi I., Fernández-Gutiérrez A., Zarrouk M., Segura-Carretero A. LC-MS-based metabolite profiling of methanolic extracts from the medicinal and aromatic species Mentha pulegium and Origanum majorana. Phytochem. Anal. 2015;26:320–330. doi: 10.1002/pca.2566. [DOI] [PubMed] [Google Scholar]
- 23.Fang N., Yu S., Prior R.L. LC/MS/MS characterization of phenolic constituents in dried plums. J. Agric. Food Chem. 2002;50:3579–3585. doi: 10.1021/jf0201327. [DOI] [PubMed] [Google Scholar]
- 24.El Raey M.A., El-Hagrassi A.M., Osman A.F., Darwish K.M., Emam M. Acalypha wilkesiana flowers: Phenolic profiling, cytotoxic activity of their biosynthesized silver nanoparticles and molecular docking study for its constituents as Topoisomerase-I inhibitors. Biocatal. Agric. Biotechnol. 2019;20:101243. doi: 10.1016/j.bcab.2019.101243. [DOI] [Google Scholar]
- 25.González-Domínguez R., Urpi-Sarda M., Jáuregui O., Needs P.W., Kroon P.A., Andrés-Lacueva C. Quantitative dietary fingerprinting (QDF)—A novel tool for comprehensive dietary assessment based on urinary nutrimetabolomics. J. Agric. Food Chem. 2019;68:1851–1861. doi: 10.1021/acs.jafc.8b07023. [DOI] [PubMed] [Google Scholar]
- 26.Fayek N.M., Farag M.A., Monem A.R.A., Moussa M.Y., Abd-Elwahab S.M., El-Tanbouly N.D. Comparative metabolite profiling of four citrus peel cultivars via ultra-performance liquid chromatography coupled with quadrupole-time-of-flight-mass spectrometry and multivariate data analyses. J. Chromatogr. Sci. 2009;57:349–360. doi: 10.1093/chromsci/bmz006. [DOI] [PubMed] [Google Scholar]
- 27.Hegazi N.M., Sobeh M., Rezq S., El-Raey M.A., Dmirieh M., El-Shazly A.M., Mahmoud M.F., Wink M. Characterization of phenolic compounds from Eugenia supra-axillaris leaf extract using HPLC-PDA-MS/MS and its antioxidant, anti-inflammatory, antipyretic and pain killing activities in vivo. Sci. Rep. 2019;9:1–12. doi: 10.1038/s41598-019-46946-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mencherini T., Campone L., Piccinelli A.L., Garcia Mesa M., Sánchez D.M., Aquino R.P., Rastrelli L. HPLC-PDA-MS and NMR characterization of a hydroalcoholic extract of Citrus aurantium L. var. amara peel with antiedematogenic activity. J. Agric. Food Chem. 2013;61:1686–1693. doi: 10.1021/jf302815t. [DOI] [PubMed] [Google Scholar]
- 29.Ahmed R., Elkhrisy E., EL-kashak W.A., El Raey M., Nassar M., Aboutabl E.S. Structural Characterization of Polyphenolics in Livistona chinensis Using HPLC-PDA-MS. J. Adv. Pharm. Res. 2019;3:23–29. doi: 10.21608/aprh.2018.6527.1072. [DOI] [Google Scholar]
- 30.Szajwaj B., Moldoch J., Masullo M., Piacente S., Oleszek W., Stochmal A. Amides and esters of phenylpropenoic acids from the aerial parts of Trifolium pallidum. Nat. Prod. Commun. 2011;6:1293–1296. doi: 10.1177/1934578X1100600921. [DOI] [PubMed] [Google Scholar]
- 31.Li S.Z., Zeng S.L., Wu Y., Zheng G.D., Chu C., Yin Q., Chen B.Z., Li P., Lu X., Liu E.H. Cultivar differentiation of Citri Reticulatae Pericarpium by a combination of hierarchical three-step filtering metabolomics analysis, DNA barcoding and electronic nose. Anal. Chim. Acta. 2019;1056:62–69. doi: 10.1016/j.aca.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Fahmi A.A., El Raey M.A., Ibrahim A.Y., Abdelfattah M.A.O., Abdelmageed A.M., Sobeh M. A sulfated polyphenols-rich extract from Sabal yapa exhibits antitumor activities in Ehrlich ascites carcinoma. Saudi J. Biol. Sci. 2021;28:3117–3125. doi: 10.1016/j.sjbs.2021.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng G.-D., Sun C.-F., Pu J.-W., Chen J., Jiang X.-Y., Zou S.-M. Two myostatin genes exhibit divergent and conserved functions in grass carp (Ctenopharyngodon idellus) Gen. Com. Endocrinol. 2015;214:68–76. doi: 10.1016/j.ygcen.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 34.Zhang B., Schmoyer D., Kirov S., Snoddy J. GOTree Machine (GOTM): A web-based platform for interpreting sets of interesting genes using Gene Ontology hierarchies. BMC Bioinform. 2004;5:1–8. doi: 10.1186/1471-2105-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janek T., Krasowska A., Czyżnikowska Ż., Łukaszewicz M. Trehalose lipid biosurfactant reduces adhesion of microbial pathogens to polystyrene and silicone surfaces: An experimental and computational approach. Front. Microbiol. 2018;9:2441. doi: 10.3389/fmicb.2018.02441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pratiwi F., Tinata J.K., Prakasa A.W., Hartini E., Isworo S. Citric acid compounds of tangerines peel extract (Citrus reticulata) as potential materials teeth whitening. J. Phys. Conf. Ser. 2017;824:12071. doi: 10.1088/1742-6596/824/1/012071. [DOI] [Google Scholar]
- 37.Braïek O.B., Smaoui S. Chemistry, Safety, and Challenges of the Use of Organic Acids and Their Derivative Salts in Meat Preservation. J. Food Qual. 2021;2021:1–20. doi: 10.1155/2021/6653190. [DOI] [Google Scholar]
- 38.Coban H.B. Organic acids as antimicrobial food agents: Applications and microbial productions. Bioprocess Biosyst. Eng. 2020;43:569–591. doi: 10.1007/s00449-019-02256-w. [DOI] [PubMed] [Google Scholar]
- 39.Nićiforović N., Abramovič H. Sinapic acid and its derivatives: Natural sources and bioactivity. Compr. Rev. Food Sci. Food Saf. 2014;13:34–51. doi: 10.1111/1541-4337.12041. [DOI] [PubMed] [Google Scholar]
- 40.Sova M. Antioxidant and antimicrobial activities of cinnamic acid derivatives. Mini Rev. Med. Chem. 2012;12:749–767. doi: 10.2174/138955712801264792. [DOI] [PubMed] [Google Scholar]
- 41.Barreca D., Mandalari G., Calderaro A., Smeriglio A., Trombetta D., Felice M.R., Gattuso G. Citrus flavones: An update on sources, biological functions, and health promoting properties. Plants. 2020;9:288. doi: 10.3390/plants9030288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ke Z., Yang Y., Tan S., Zhou Z. Characterization of polymethoxylated flavonoids in the peels of Chinese wild mandarin (Citrus reticulata Blanco) by UPLC-Q-TOF-MS/MS. Food Anal. Methods. 2017;10:1328–1338. doi: 10.1007/s12161-016-0690-4. [DOI] [Google Scholar]
- 43.Attia G.H., Moemen Y.S., Youns M., Ibrahim A.M., Abdou R., El Raey M.A. Antiviral zinc oxide nanoparticles mediated by hesperidin and in silico comparison study between antiviral phenolics as anti-SARS-CoV-2. Colloids Surf. B Biointerfaces. 2021;203:111724. doi: 10.1016/j.colsurfb.2021.111724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Espina L., Gelaw T.K., de Lamo-Castellví S., Pagán R., García-Gonzalo D. Mechanism of bacterial inactivation by (+)-limonene and its potential use in food preservation combined processes. PLoS ONE. 2013;8:e56769. doi: 10.1371/journal.pone.0056769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abuelsaad A.S.A., Mohamed I., Allam G., Al-Solumani A.A. Antimicrobial and immunomodulating activities of hesperidin and ellagic acid against diarrheic Aeromonas hydrophila in a murine model. Life Sci. 2013;93:714–722. doi: 10.1016/j.lfs.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 46.Eliuz E. Antimicrobial activity of citric acid against Escherichia coli, Staphylococcus aureus and Candida albicans as a sanitizer agent. Eurasian J. For. Sci. 2020;8:295–301. doi: 10.31195/ejejfs.787021. [DOI] [Google Scholar]
- 47.Kjer J., Debbab A., Aly A.H., Proksch P. Methods for isolation of marine-derived endophytic fungi and their bioactive secondary products. Nat. Protoc. 2010;5:479–490. doi: 10.1038/nprot.2009.233. [DOI] [PubMed] [Google Scholar]
- 48.Ammar N.M., Hassan H.A., Mohammed M.A., Serag A., Abd El-Alim S.H., Elmotasem H., El Raey M., El Gendy A.N., Sobeh M., Abdel-Hamid A.H. Metabolomic profiling to reveal the therapeutic potency of Posidonia oceanica nanoparticles in diabetic rats. RSC Adv. 2021;11:8398–8410. doi: 10.1039/D0RA09606G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Osman S.M., El Kashak W.A., Wink M., El Raey M.A. New isorhamnetin derivatives from Salsola imbricata Forssk. leaves with distinct anti-inflammatory activity. Pharmacogn. Mag. 2016;12:S47. doi: 10.4103/0973-1296.176110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bauer R.A. Social Indicators. MIT Press; Cambridge, MA, USA: 1966. [Google Scholar]
- 51.Marrez D.A., Abdelhamid A.E., Darwesh O.M. Eco-friendly cellulose acetate green synthesized silver nano-composite as antibacterial packaging system for food safety. Food Packag. Shelf Life. 2019;20:100302. doi: 10.1016/j.fpsl.2019.100302. [DOI] [Google Scholar]
- 52.Andrews G., Issakidis C., Carter G. Shortfall in mental health service utilisation. Br. J. Psychiatry. 2001;179:417–425. doi: 10.1192/bjp.179.5.417. [DOI] [PubMed] [Google Scholar]
- 53.White M., Hu W. The sachs-wolfe effect. [(accessed on 16 September 1996)];arXiv. 1996 Available online: https://arxiv.org/abs/astro-ph/9609105.astro-ph/9609105 [Google Scholar]
- 54.Tallarida R.J. Drug synergism: Its detection and applications. J. Pharmacol. Exp. Ther. 2001;298:865–872. [PubMed] [Google Scholar]
- 55.Bansal T., Alaniz R.C., Wood T.K., Jayaraman A. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc. Natl. Acad. Sci. USA. 2010;107:228–233. doi: 10.1073/pnas.0906112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mandalari G., Tomaino A., Rich G.T., Curto R.L., Arcoraci T., Martorana M., Bisignano C., Saija A., Parker M.L., Waldron K.W., et al. Polyphenol and nutrient release from skin of almonds during simulated human digestion. Food Chem. 2010;122:1083–1088. doi: 10.1016/j.foodchem.2010.03.079. [DOI] [Google Scholar]
- 57.Petersen P., Axler S., Ribet K.A. Riemannian Geometry. Volume 171 Springer; New York, NY, USA: 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are contained within the article.