Abstract
Soft tissues have been shown to be critical for the maintenance of both teeth and implants. Currently, regenerative soft tissue techniques propose the use of collagen matrices, which can avoid the drawbacks derived from the obtainment of autogenous tissue graft. A systematic review and meta-analysis were conducted to ascertain the efficacy of collagen matrices (CM) compared to autogenous connective tissue graft (CTG) to improve soft tissue dimensions. An electronic and manual literature searches were performed to identify randomized clinical trials (RCT) or controlled clinical trials (CCT) that compared CTG and CM. Pooled data of width of keratinized tissue (KT) and mucosal thickness (MT) were collected and weighted means were calculated. Heterogeneity was determined using Higgins (I2). If I2 > 50% a random-effects model was applied. Nineteen studies were included based on the eligibility criteria. When using CTG a higher MT gain (0.32 mm, ranging from 0.49 to 0.16 mm) was obtained than when employing CM. Similar result was obtained for the width of KT gain, that was 0.46 mm higher (ranging from 0.89 to 0.02 mm) when employing CTG. However, it can be stated that, although autogenous CTG achieves higher values, CM are an effective alternative in terms of total width of KT and MT gain.
Keywords: collagen matrices, keratinized tissue, mucosal thickness, soft tissue graft
1. Introduction
Nowadays, soft tissue plays a pivotal role in maintaining and improving peri-implant and periodontal health. Adequate dimensions of soft tissue in terms of width of keratinized tissue and thickness of mucosa bring numerous benefits for maintenance, stability and prognosis of both teeth and implants. It has been reported that optimal soft tissue conditions around dental implants can contribute to an improvement in gingival and plaque index, as well as a higher stability of marginal bone in comparison to sites with minimal or lacking keratinized tissue and mucosal thickness [1,2]. Although controversial, the literature has described that a minimum keratinized tissue width of 2 mm is essential for the maintenance of the stability and health of the peri-implant soft tissues [3]. In the case of natural teeth, a poor mucogingival complex can be a predisposition toward localized inflammation resulting in the development of gingival recessions [4] or an apical shift of the gingival margin to the cemento-enamel junction, exposing the root surface. It may cause esthetic complaints, commonly associated with mechanical root wear, hypersensitivity, root caries and poor plaque control [4,5].
For all these reasons, soft tissue augmentation is generating an increasing interest. Soft tissue grafting procedures have been proposed to treat mucogingival defects and achieve both aesthetic and functional results, increasing survival rates of teeth and implants [6]. Major clinical indications could be divided into recession coverage, gain of keratinized tissue, and augmentation of soft tissue volume [7]. Many surgical techniques with different materials to produce soft tissue augmentation in thickness and in width have been described. Regardless of the technique applied, autogenous connective tissue graft (CTG) harvest from the palate is most frequently used [8,9,10,11,12]. Despite the possible benefits of the autogenous tissue graft, there are some crucial disadvantages and limitations. Namely, the morbidity and pain associated with a second operating field [12,13,14], and the limited dimensions of palate donor tissue due to different anatomical factors, covering only a few implants or teeth at one time [13].
To overcome the shortcomings of the autogenous connective tissue, the development of connective tissue substitutes of xenogeneic, allogeneic or synthetic origin, are gaining relevance [15,16]. These biomaterials can reduce the surgical time, diminish the surgical morbidity and increase patients’ acceptance [17]. However, two main criteria need to be fulfilled: good biological behavior permitting modeling and remodeling processes and a volume stability along time [12]. Suitable three-dimensional alternative structures are needed to act as scaffolds that promote cell attachment and migration, providing an appropriate environment for cell proliferation and differentiation. This allows cells to secrete their own extracellular matrix to form a tissue-like organization [18]. Consequently, collagen matrices (CM) have been described as an unlimited alternative to autogenous connective tissue grafting and have been used for soft tissue augmentation around dental implants and for root coverage therapy, showing favorable results [12,19]. Although CM have shown good volume stability allowing sufficient time for cell invasion and new tissue formation, the rapid biodegradation by the enzymatic activity jeopardizes its use as an alternative to autogenous grafting [20,21].
In this context, the aim of this systematic review and meta-analysis was to evaluate the evidence related to the efficacy of collagen matrices as an alternative to autogenous connective tissue when used as grafts for soft tissue augmentation, and to compare the clinical success of both surgical procedures.
2. Materials and Methods
2.1. Study Registration and Protocol Development
Before the execution of the study, this review proposal was registered in the International Prospective Register of Systematic Reviews (PROSPERO) with the identification number CRD42021227177. This systematic review focusing on the efficacy of CM versus CTG for soft tissue augmentation was structured according to the PRISMA-P [22], following the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions [23] and also the PRISMA [24] checklist in order to increase the quality and transparency of the study.
2.2. PICO Question and Focused Question
Population: Patients requiring soft tissue augmentation techniques to augment peri-implant or periodontal keratinized tissue width/thickness for aesthetic purpose and/or functional reasons.
Intervention: Any type of surgical procedures to augment soft tissue with the application of any type of collagen matrix at peri-implant or periodontal sites.
Comparison: Autologous connective tissue grafts.
Outcome: Soft tissue gain (width or thickness) measured in mm [25].
The focused question is: In patients requiring soft tissue augmentation techniques, how effective is the application of a collagen matrix compared to autogenous connective tissue graft in terms of keratinized mucosa height or soft tissue volume gain?
2.3. Information Sources and Screening Process
An electronic search with a time filter of 15 years was conducted by two researchers (C.V. and M.V.-R.) covering studies until February 2021 on three online data-bases: The National Library of Medicine (MEDLINE by PubMed), The Cochrane Oral Health Group Trials Register and EMBASE. Only studies published in English, between 2009 and February 2021 were included. An additional hand search was performed identifying previous systematic reviews investigating implant and root coverage procedures for soft-tissue improvements for article identification. Searches were re-run prior to the final analysis in May 2021.Details regarding the search terms are presented in Table 1.
Table 1.
Electronic databases and search strategies.
| Databases | Keywords |
|---|---|
| PUBMED | #1 “collagen matrix”[Title/Abstract] OR “extracellular membrane”[Title/Abstract] OR “extracellular matrix”[Title/Abstract] OR “xenogenic collagen matrix”[Title/Abstract] OR “acellular dermal matrix”[Title/Abstract] OR “porcine collagen matrix”[Title/Abstract] OR “porcine collagen matrices”[Title/Abstract] OR “porcine derived collagen matrix”[Title/Abstract] OR “porcine derived acellular dermal matrix”[Title/Abstract] OR “mucograft”[Title/Abstract] OR “mucoderm”[Title/Abstract]” OR “volume-stable collagen matrix”[Title/Abstract] OR “dermal substitute”[Title/Abstract] #2 “soft tissue correction”[Title/Abstract] OR “soft tissue augmentation”[Title/Abstract] OR “soft tissue transplantation”[Title/Abstract] OR “soft tissue graft”[Title/Abstract] OR “guided tissue regeneration”[Title/Abstract] #1 AND #2 |
| Cochrane Library (CENTRAL) |
#1 collagen matrix OR extracellular membrane OR extracellular matrix OR xenogenic collagen matrix OR acellular dermal matrix OR porcine collagen matrix OR porcine collagen matrices OR porcine derived collagen matrix OR porcine derived acellular dermal matrix OR mucograft OR mucoderm OR volume-stable collagen matrix OR dermal substitute #2 soft tissue correction OR soft tissue augmentation OR soft tissue transplantation OR soft tissue graft OR guided tissue regeneration #1 AND #2 |
| EMBASE | #1 “collagen matrix” OR “extracellular membrane” OR “extracellular matrix” OR “xenogenic collagen matrix” OR “acellular dermal matrix” OR “porcine collagen matrix” OR “porcine collagen matrices” OR “porcine derived collagen matrix” OR “porcine derived acellular dermal matrix” OR “mucograft” OR “mucoderm” OR “volume-stable collagen matrix” OR “dermal substitute” #2 “soft tissue correction” OR “soft tissue augmentation” OR “soft tissue transplantation” OR “soft tissue graft” OR “guided tissue regeneration” #1 AND #2 |
2.4. Eligibility Criteria
Studies were selected for inclusion if they met the following criteria: (i) human randomized clinical trials (RCT) or human controlled clinical trials (CCT), (ii) studies dealing with soft-tissue treatments to increase keratinized mucosa or mucosal thickness around teeth or implants, (iii) comparison of connective tissue grafts (control) versus xenogeneic collagen matrices (test), (iv) follow-up of at least 3 months, (v) reported outcome measures of keratinized mucosa or mucosal thickness gain following the surgical intervention. On the other hand, the exclusion criteria were: (i) in vitro and pre-clinical studies, cohort studies, case-control studies, case series, case reports, systematic reviews, (ii) full-text publications not available in English language, (iii) studies with a less than 10 patients, (iv) surgical treatment including materials for guide bone regeneration, (v) graft as a material for socket preservation.
2.5. Study Selection and Data Extraction
Two authors (C.V. and M.V-R.) independently screened the titles derived from the online search based on the inclusion criteria. Disagreements were solved by discussion. In the case where a disagreement persists, a third reviewer (R.O.) was decisive and led to an agreement. Cohen’s Kappa-coefficient was calculated as a measure of agreement between the two readers. The final selection based on inclusion/exclusion criteria was made for the full-text articles. For this purpose, all data were extracted independently by two reviewers (C.V. and M.V-R.). Information on the following parameters was acquired as follows: author(s), year of publication, study design, number of patients, age range, dropouts, mean follow-up and range, width of keratinized tissue (KT), mucosal thickness (MT), periodontal parameters, patient-reported outcomes measures (PROMs), pink esthetic score (PES) and complications.
2.6. Assessment of Risk of Bias
The assessment of the risk of bias for the included randomized clinical trials was performed using Cochrane Handbook for Systematic Reviews of Interventions [23]. With Cochrane Collaboration’s tool, each study was analyzed in relation to seven domains (sequence generation, allocation concealment, blinding of the outcome assessor, blinding of participants and personnel, incomplete outcome data, selective outcome reporting and other bias) and categorized as low, medium or high risk of bias when they met all, all but one, or all but two or more criteria, respectively.
For the included non-randomized studies of interventions, a tool called ROBINS-I was used [26]. With ROBINS-I tool, risk of bias was assessed within specified bias domains (bias due to confounding, bias in selection of participants into the study, bias in classification of interventions, bias due to deviations from intended interventions, bias due to missing data, bias in measurement of outcomes, bias in selection of the reported result) and categories for risk of bias judgements were “Low risk”, “Moderate risk”, “Serious risk” and “Critical risk” of bias.
2.7. Statistical Analysis
Descriptive statistics were used to present the primary outcome: efficacy of collagen matrix in terms of gingival thickness gain (mm) and/or changes of keratinized tissue (mm). Weighted means (CI 95%) were calculated, including total sample size, inverse variance and standard error of the treatment effect. Heterogeneity was determined using Higgins (I2). If I2 > 50% a random-effects models were applied. Statistical significance was set at 0.05. Data were analyzed with RevMan 5.4 (The Cochrane Collaboration, Oxford, UK). Funnel plot was produced by MedCalc 18.2.1 (MedCalc Software Ltd. Ostend, Belgium) to represent systematic heterogeneity.
3. Results
3.1. Study Selection
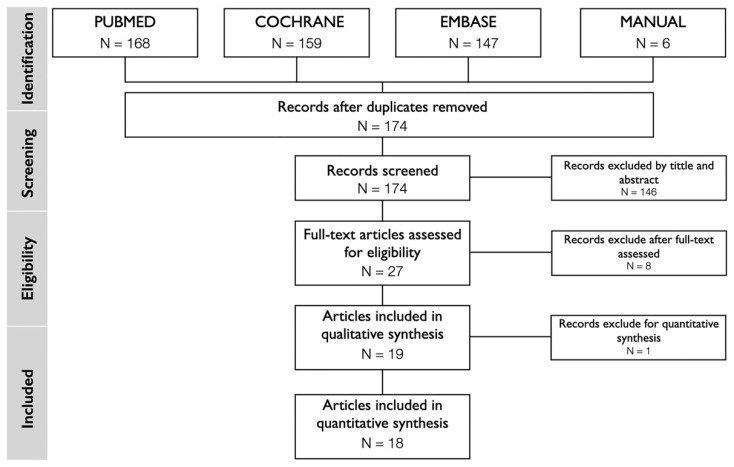
Search results based on the PRISMA guidelines are presented in Figure 1. The electronic search provided a total of 474 articles supplemented by a manual search getting 6 more articles [5,27,28,29,30,31]. After duplicates removal, a total of 174 studies were selected for screening of title and abstract. Twenty-eight articles were considered for full-text screening. Nine articles were excluded after careful reading, since they did not meet the eligibility criteria. Finally, 19 studies [12,16,19,27,28,29,31,32,33,34,35,36,37,38,39,40,41,42,43] were included in the systematic review (SR) and one of them was excluded for the quantitative analysis, so the meta-analysis is based on 18 articles [12,16,19,27,28,29,31,32,33,34,35,36,37,38,39,40,41,42]. The reasons for exclusion are reported in Table 2. The inter-reviewer agreement in the screening and inclusion process corresponded to 0.87, and 0.95 with de Cohen’s Kappa for assessment of the title and abstract, and full-text evaluation, respectively.
Figure 1.
PRISMA flow diagram for studies inclusion process.
Table 2.
Excluded studies for qualitative and quantitative synthesis with reasons.
| Stage | Reason for Exclusion | Articles |
|---|---|---|
| Qualitative synthesis |
No control group | Ozturan et al. [44]; Eeckhout et al. [45]; Ghanaati et al. [46] |
| No valid outcome for this SR | Zafiropoulos et al. [47]; McGuire y Secheyer [48]; Tonetti et al. [5]; Zuiderveld et al. [30] | |
| Use of the same data of a previous study | Puzio et al. [13] | |
| Quantitative synthesis |
Increase the follow-up of a previous study and the follow-up period not considered for meta-analysis | Thoma et al. [43] |
3.2. Study Characteristics
Varied applications for soft tissue augmentation were described in the included studies: 12 articles in relation to implant sites [12,16,19,29,31,32,34,37,38,39,42,43] and 7 concerning teeth [27,28,33,35,36,40,41]. All the included studies were RCTs except for three CCTs [31,37,42], but all of them included at least two parallel arms, the use of CTG versus CM. The different therapeutic options used for soft tissue augmentation found in the included studies are shown in Figure 2. The main characteristics for the selected trials are summarized in Table 3, and Table 4 reports on the assessment of soft tissue augmentation used in each study, as well as the primary outcome data.
Figure 2.
Different therapeutic options for soft tissue augmentation. Data are presented as a percentage of patients treated with each alternative compared to the total number of patients. Connective tissue graft (CTG), free gingival graft (FGG).
Table 3.
General overview of included studies’ design.
| Author | Study Design |
Follow-up | Patients | Inclusion Criteria | Outcome Measurements |
|---|---|---|---|---|---|
| Sanz et al., 2009 [32] |
RCT | 6 months | 20 patients | Age > 18 years Systematically healthy FMPS < 20% Presenting at least one location with minimal or no KT (1 mm). |
KMW, PD, CAL, GI, PI, pain, PAS Changes in KMW |
| Lorenzo et al., 2011 [34] |
RCT | 6 months | 24 patients | Age >18 years Systematically healthy FMPS < 20% Presenting at least one implant with minimal or no KT (1 mm). |
KMW, GI, PI, PD, CAL Changes in KMW |
| Cardaropoli et al., 2012 [33] |
RCT | 12 months | 18 patients | Age > 18 years No pregnancy or breast feeding Systematically healthy Non-smokers At least two single-rooted teeth with gingival recessions Miller class I and/or II |
GR, GT, PD, CAL, KMW Changes KMW |
| Aroca et al., 2013 [27] |
RCT split-mouth |
12 months | 22 patients | Age > 18 years Systematically healthy Healthy or treated periodontal conditions Presence of at least 3 adjacent Miller class I and II GR on both sides of the maxillary or mandibular arch with an apico-coronal extension (i.e., RD) > 2 mm FMPS < 25% |
GRD, GRW, KMW, GT, PD, CAL, PROMs |
| McGuire and Scheyer 2014 [35] |
RCT split-mouth |
6 months | 30 patients | Age > 18 years No pregnancy or breast feeding Systematically healthy Non-smokers |
PS, BOP, RD, KMW, VD, CAL, RMP, IS, Esthetics, Histological |
| Cieślik-Wegemund et al., 2016 [36] |
RCT | 6 months | 28 patients | Systematically healthy Non-smokers Absence of clinical signs of active periodontal disease An identifiable CEJ Minimum of two adjacent GR of Miller Class I or II on both sides with at least 2 mm in RD API ≤ 15% and SBI ≤ 10% |
GRD, GRW, KMW, CAL, CEJ-MGJ, PD, RA |
| Schimitt et al., 2016 [37] |
RCT | 60 months | 48 patients | Age >18 years Healthy periodontally and systemically Good plaque control. No smokers |
KMW, Appearance: color, texture |
| Thoma et al., 2016 [12] |
RCT | 3 months | 20 patients | Age > 18 years Implant placement at least 6 weeks and maximum 6 months prior enrolment Necessity of STA in a single tooth Two teeth adjacent at each side of the defect with a mean BOP of < 30% BPE < 2 |
GT, BPE, PI, KMW, BOP, PD, RD, PROMs Volumetric changes of GT |
| Cairo et al., 2017 [38] |
RCT | 6 months | 60 patients | No systemic diseases or pregnancy. Smokers ≤ 10 cigarettes/day. No probing depths ≥ 5 mm FMPS and FMBS ≤ 15% Need of STA for aesthetic and/or functional reasons in a single-tooth gap at upper and lower jaw No previous STA procedure at experimental site |
KMW, GT, BL, RD, PD, BOP, PI, PROMs |
| Zeltner et al., 2017 [39] |
RCT | 3 months | 20 patients | Age > 18 years Implant placement at least 6 weeks and maximum 6 months prior enrolment Necessity of STA in a single tooth Two teeth adjacent at each side of the defect with a mean BOP of < 30% BPE < 2 |
Volumetric changes of GT |
| Huber et al., 2018 [19] |
Follow-up of RCT | 12 months | 19 patients | Age > 18 years Necessity of STA in a single tooth Two teeth adjacent at each side of the defect with a mean BOP of < 30% BPE < 2 Final restoration inserted at implant site |
GT, BPE, PI, KMW, BOP, PD, RD, PES, PROMs Volumetric changes of GT |
| Pietruska et al., 2018 [28] |
RCT split-mouth |
12 months | 20 patients | No pregnancy or breast feeding Systematically healthy, Non-smokers At least two single-rooted teeth with GR Miller class I and/or II ≥ 1 mm deep without loss of CAL FMPS and FMBOP < 20% No active periodontal disease Detectable CEJ No caries lesions or restorations in the cervical area. |
GR, GRW, PD, CAL, KMW, GT, FMPS, FMBOP Changes KMW |
| Puzio et al.,2018 [16] |
RCT | 12 months | 22 patients | Missing single or double teeth in the anterior area of their upper or lower jaw with a proper inter arch relationship with a ridge width (bucco-lingual) greater than 5 mm at its narrowest point and a minimum height of KM of 2 mm measured buccally with a periodontal probe. | GT, gingival biotype |
| Nahas et al., 2019 [40] |
RCT split-mouth |
12 months | 15 patients | Systemically and periodontally healthy PI: ≤ 20% Multiple bilateral Class I Miller GR, involving canines and premolars (2–3 teeth) with at least one GR ≥ 3 mm Identifiable CEJ At least 1 mm KT apical to the GR |
GRD, PI, BOP, PD, CAL, KMW, PROMs Changes KMW |
| Vellis et al.,2019 [31] |
RCT split-mouth |
6 months | 30 patients | Age > 18 years Systematically healthy No pregnancy or breast feeding |
KMW, PD, pain Changes in KMW |
| Rakasevic et al., 2020 [41] |
RCT split-mouth |
12 months | 20 patients | Age > 18 years Non-smokers and light smokers (<10 cigarettes per day). Systemically and periodontally healthy FMPS < 20% and FMBOP < 20% +1 adjacent Type 1 GRs in both quadrants of the maxillary or mandibular arch with a GR depth ≥ 2 mm Identifiable CEJ Absence of the radiographic signs of periapical infection on the teeth to be treated or on the adjacent teeth. |
GRD, GRW, KMW, GT, PD, CAL, HI, RES Changes KMW |
| Schmitt et al., 2020 [42] |
CCT | 6 months | 14 patients | Age > 18 years No pregnancy or breast feeding Systematically healthy Periodontally healthy (no PD > 4 mm) Situation after early implant insertion and GBR at least 4 months and a maximum of 6 months prior to enrollment. |
Volumetric changes of GT |
| Tarasenko et al., 2020 [29] |
RCT | 6 months | 40 patients | Age >18 years Systematically healthy (ASA I-II) Non-smokers and light smokers (<10 cigarettes per day). Previous placement of one or more implants in the mandibles (3 to 6 months before the beginning of the investigation) without having yet undergone stage-two surgery FMPS and FMBS ≤ 20% |
KMW, Inflammation, PROMs, Histology Changes in KMW |
| Thoma et al., 2020 [43] |
Follow-up of RCT | 36 months | 18 patients | Age > 18 years Necessity of STA in a single tooth Two teeth adjacent at each side of the defect with a mean BOP of < 30% BPE < 2 Final restoration inserted at implant site |
GT, BPE, PI, KMW, BOP, PD, RD, PES, PROMs, MBL Volumetric changes of GT |
RCT: randomized clinical trial, FMPS: full-mouth plaque score, FMBS: full-mouth bleeding score, STA: soft tissue augmentation, KMW: keratinized mucosa width, GT: gingival thickness, BL: bono Level, RD: recession depth, PD: probing depth, BOP: bleeding on probing, PI: plaque index, PROMs: patient-reported outcomes measures, GI: gingival index, CAF: coronally advanced flap, BPE: basic periodontal examination, PES: pink esthetic score, MBL: marginal bone loss, CCT: controlled clinical trial, CAL: clinical attachment levels, PAS: participants’ aesthetic satisfaction, MBML: mid-buccal mucosal level, IML: inter-proximal mucosal levels.
Table 4.
General description of the soft tissue augmentation procedures performed in each included study.
| Author | STA/ Surgical Technique | XMC Used | Site of Treatments | Time of Grafting | Summary Results |
|---|---|---|---|---|---|
| Sanz et al., 2009 [32] |
CG: CTG (n = 10) TG: XCM (n = 10) CAF |
Mucograft® a | Maxilla and mandible | After crown placement | KW: CTG > CM |
| Lorenzo et al., 2011 [34] |
CG: CTG (n = 12) TG: XCM (n = 12) CAF |
Mucograft® | Maxilla and mandible | After crown placement | KW: CTG < CM |
| Cardaropoli et al., 2012 [33] |
CG: CTG (n = 8) TG: XCM (n = 10) CAF |
Mucograft® | Maxilla and mandible 22 GR |
NR | KW: CTG > CM * GT: CTG > CM * |
| Aroca et al., 2013 [27] |
CG: CTG (n = 22) TG: XCM (n = 22) MCAT |
Mucograft® | Maxilla and mandible | NR | KW: CTG > CM GT: CTG > CM * |
| McGuire and Scheyer 2014 [35] |
CG: FGG (n = 30) TG: CM (n = 30) |
Mucograft® | Maxilla and mandible | NR | KW: FGG > CM * |
| Cieślik-Wegemund et al., 2016 [36] |
CG: CTG (n = 14) TG: XCM (n = 14) TT |
Mucoderm® b | Maxilla and mandible CG: 47 GR; 18 in mandible, 29 in maxilla TG: 59 GR; 20 in mandible, 39 in maxilla |
NR | KW: CTG > CM |
| Schimitt et al., 2016 [37] |
CG: CTG (n = 21) TG: XCM (n = 27) CAF |
Mucograft® | Mandible (anterior region) | During 2° surgery | KW: FGG > CM |
| Thoma et al., 2016 [12] |
CG: CTG (n = 10) TG: XCM (n = 10) Sutured grafts on periosteum without periodontal dressing |
Fibro-Gide® a | Maxilla and mandible PM to PM | After implant placement | GT: CTG < CM |
| Cairo et al., 2017 [38] |
CG: CTG (n = 30) TG: XCM (n = 30) CAT |
Mucograft® | Maxilla and mandible | During 2° surgery | KW: CTG > XCM GT: CTG > XCM * |
| Zeltner et al., 2017 [39] |
CG: CTG (n = 10) TG: XCM (n = 10) Sutured grafts on periosteum without periodontal dressing |
Fibro-Gide® | Maxilla and mandible PM to PM | After implant placement | GT: CTG < CM |
| Huber et al., 2018 [19] |
CG: CTG (n = 10) TG: XCM (n = 9) Sutured grafts on periosteum without periodontal dressing |
Fibro-Gide® | Maxilla and mandible PM to PM | After implant placement | GT: CTG < CM |
| Pietruska et al., 2018 [28] |
CG: CTG (n = 20) TG: XCM (n = 20) MCAT |
Mucoderm® | Maxilla and mandible | NR | KW: CTG > CM * GT: CTG > CM * |
| Puzio et al.,2018 [16] |
CG: CTG (n = 15) TG: XCM (n = 15) CAT |
Mucograft® | Maxilla and mandible (anterior region) |
3 months after implantation | GT: CTG > CM * |
| Nahas et al., 2019 [40] |
CG: CTG (n = 15) TG: XCM (n = 15) MCAT |
Mucograft® | Maxilla and mandible 82 GR CG: 40 TG: 42 |
NR | KW: CTG > CM |
| Vellis et al.,2019 [31] |
CG: CTG (n = 30) TG: XCM (n = 30) Sutured grafts on periosteum without periodontal dressing |
Mucograft® | Maxilla and mandible (posterior region) |
After crown placement | KW: FGG > CM |
| Rakasevic et al., 2020 [41] |
CG: CTG (n = 20) TG: XCM (n = 20)MCAT |
Mucoderm® | Maxilla and mandible (114 multiple maxillary and mandibular type GR) |
NR | KW: CTG < CM GT: CTG < CM * |
| Schmitt et al., 2020 [42] |
CG: CTG (n = 17) TG: m CM (n = 17) |
Mucoderm® | NR | During 2° surgery | GT: CTG > CM |
| Tarasenko et al., 2020 [29] |
CG: FGG (n = 21) TG: CM (n = 19) CAF |
Mucograft® | Mandible | During 2° surgery | KW: FGG > CM * |
| Thoma et al., 2020 [43] |
CG: CTG (n = 10) TG: XCM (n = 8) Sutured grafts on periosteum without periodontal dressing |
Fibro-Gide® | Maxilla and mandible PM to PM | After implant placement | GT: CTG > CM |
STA: soft tissue augmentation, CG: control group, CTG: connective tissue graft, TG: test group, XCM: xenogeneic collagen matrix, CM: collagen matrix, CAT: coronally advanced tunnel, KMW: keratinized mucosa width, GT: gingival thickness, CAF: coronally advanced flap, mCM: monolayer collagen matrix, GR: gingival recession, NR: non-reported, a Geistlich Pharma AG, Wolhusen, Switzerland, b Botiss biomaterials GmbH, Zossen, Germany, * statistically significant.
3.3. Quality Assessment of the Included Studies
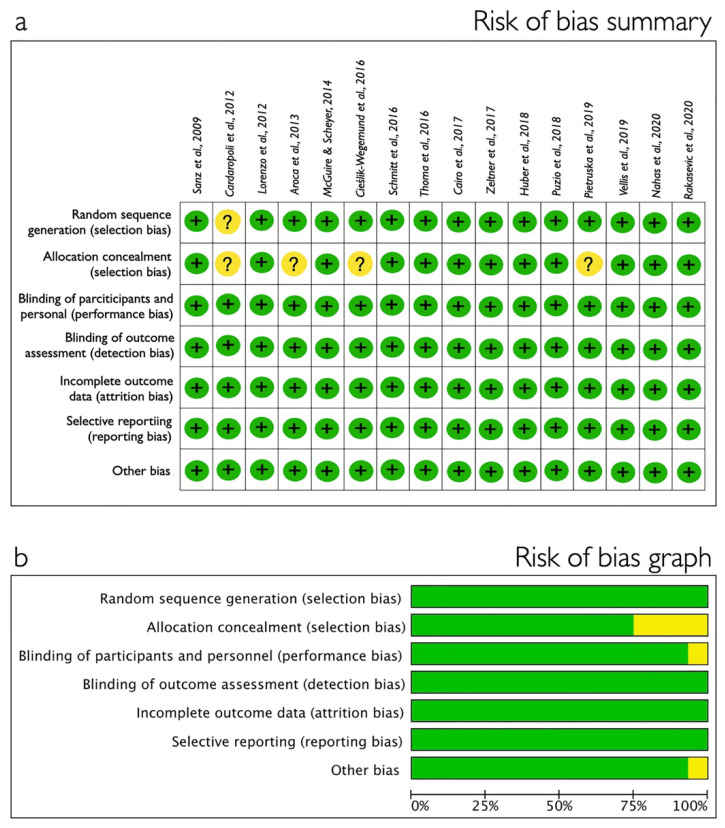
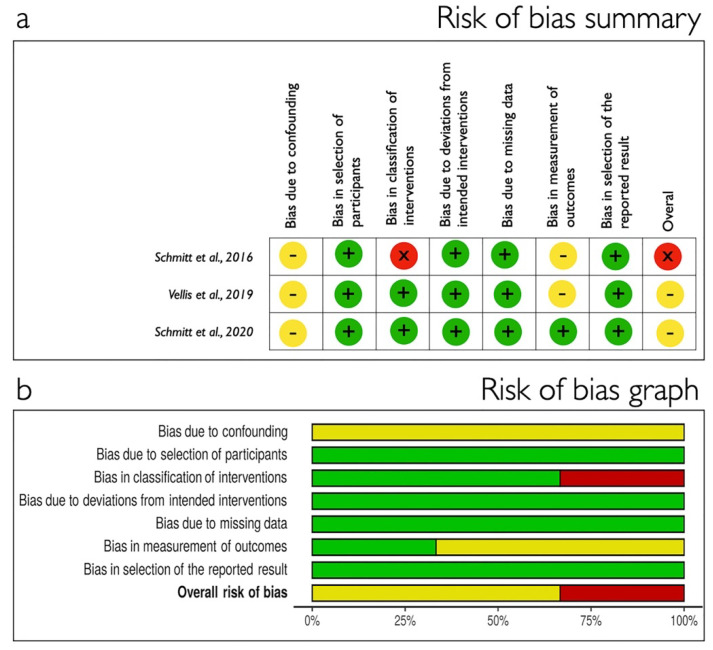
The result of the bias risk assessment for the included papers is reported in Figure 3 for RCT using Cochrane Collaboration’s tool and in Figure 4 for non-RCT in which ROBINS-I tool was used. Most of the RCTs received low risk of bias while the CCTs were classified as moderate or serious risk.
Figure 3.
Randomized clinical trial quality assessment using the Cochrane Handbook for Systematic Reviews of Interventions [23]. (a) Risk of bias summary: studies were considered as having high (red); moderate (yellow) or low (green) risk of bias. (b) Risk of bias graph: each risk of bias item presented as percentages across all included studies.
Figure 4.
Non-randomized clinical trial quality assessment using ROBINS-I tool [26]. (a) Risk of bias summary: studies were considered as having serious (red); moderate (yellow) or low (green) risk of bias. (b) Risk of bias graph each risk of bias item presented as percentages across all included studies.
3.4. Primary and Secondary Outcomes
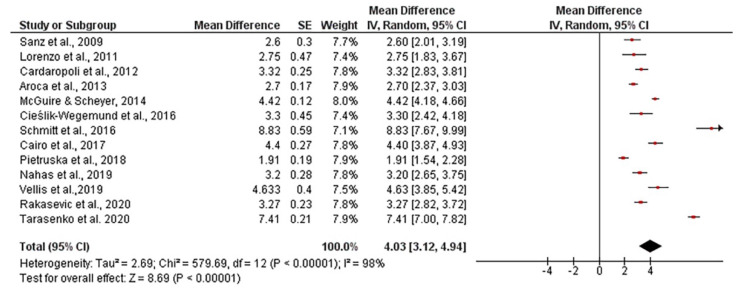
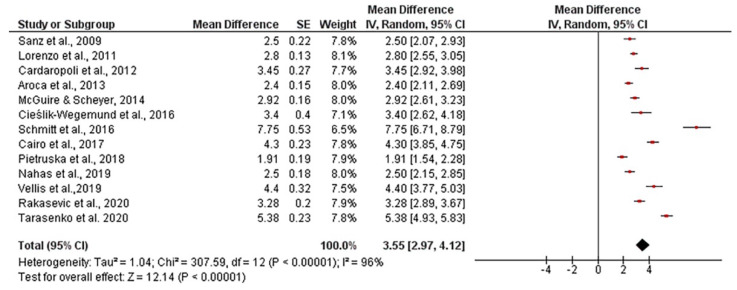
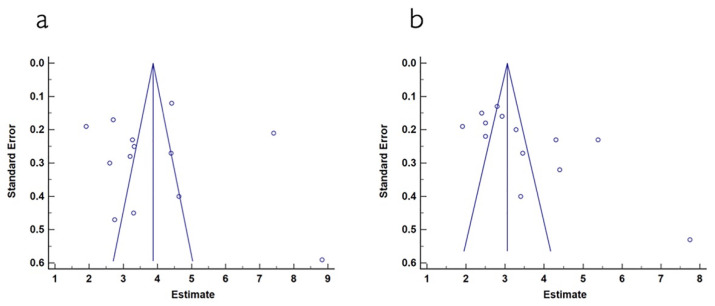
The mean width of keratinized tissue gain, when using autogenous connective tissue was 4.03 mm, ranging from 3.12 to 4.94 mm (CI 95%). Heterogeneity was I2 = 98% (CI 95%) and significance of the random-effects model was p < 0.001. When collagen matrix was employed, the mean width gain was 3.55 mm, ranging from 2.97 to 4.12 mm (CI 95%), heterogeneity was I2 = 96% (CI 95%) and significance of the random-effects model was p < 0.001. Both forest plot graphs of width of keratinized tissue are displayed in Figure 5 and Figure 6. Systematic heterogeneity is displayed at the funnel plot graphs (Figure 7). When comparing test to control groups in terms of width of keratinized tissue, the mean width gain was 0.62 mm higher (ranging from 1.09 to 0.15 mm, CI 95%) when using autogenous connective tissue in comparison to after employing collagen matrix. Heterogeneity is I2 = 83% (CI 95%) and significance of the random-effects model was p < 0.001 (Figure 8).
Figure 5.
Forest plot for keratinized mucosa width when using autogenous connective tissue. Weighted mean is presented at CI 95%. Heterogeneity was determined using Higgins (I2). A random-effects model was applied. Statistical significance was p < 0.001.
Figure 6.
Forest plot for keratinized mucosa width when collagen matrix was used. Weighted mean is presented at CI 95%. Heterogeneity was determined using Higgins (I2). A random-effects model was applied. Statistical significance was p < 0.001.
Figure 7.
(a) Funnel plot for keratinized mucosa width when using autogenous connective tissue. The estimated keratinized mucosa width measurement is on the horizontal axis and study precision (standard error) appears on the vertical axis. (b) Funnel plot for keratinized mucosa width in studies using collagen matrix. The estimated keratinized mucosa width measurement is on the horizontal axis and study precision (standard error) appears on the vertical axis.
Figure 8.
Forest plot for keratinized mucosa width gain. Test (collagen matrix) vs. control (autogenous connective tissue) groups. The weighted mean is presented at CI 95%. Heterogeneity was determined using Higgins (I2). A random-effects model was applied. Statistical significance was p = 0.04.
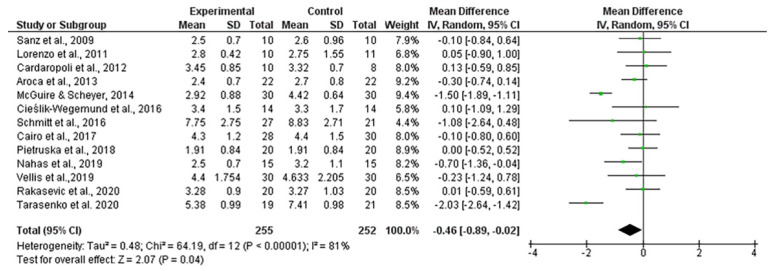
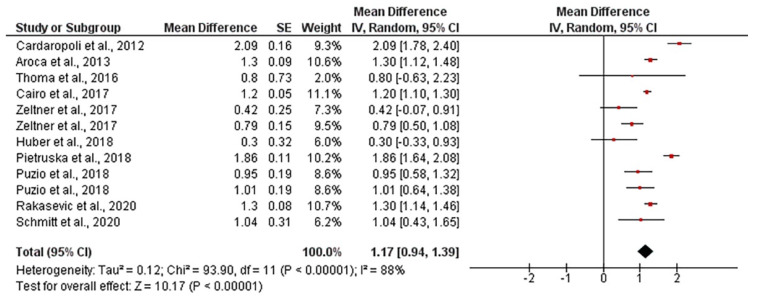
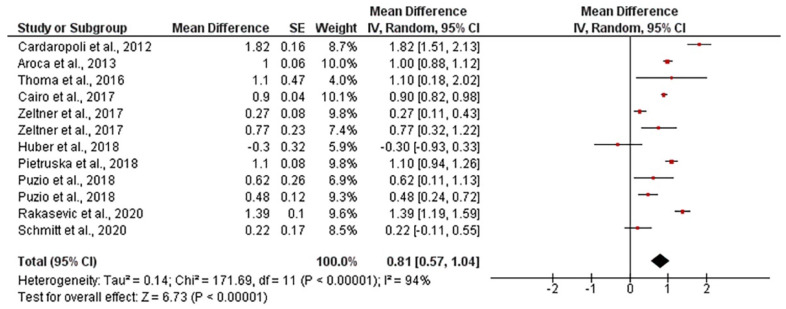
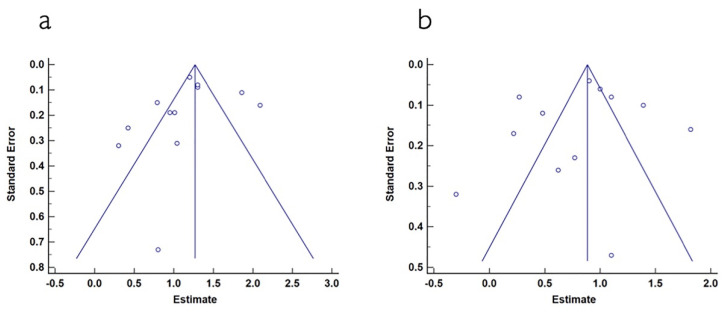
The mean gingival thickness gain when using autogenous connective tissue was 1.17 mm, ranging from 0.94 to 1.39 mm (CI 95%). Heterogeneity was I2 = 88% (CI 95%) and significance of the random-effects model was p < 0.001. After employing collagen matrixes, the mean gingival thickness gain was 0.81 mm, ranging from 0.57 to 1.04 mm (CI 95%). Heterogeneity was I2 = 94% (CI 95%) and significance of the random-effects model was p < 0.001. Both forest plot graphs of gingival are displayed in Figure 9 and Figure 10. Systematic heterogeneity is displayed at the funnel plot graphs (Figure 11). When comparing the test to the control groups, the mean gingival thickness gain was 0.32 mm (ranging from 0.49 to 0.16 mm, CI 95%) higher when using autogenous connective tissue than when employing collagen matrixes. The heterogeneity was I2 = 58% and the significance of the random-effects model was p < 0.001 (Figure 12).
Figure 9.
Forest plot for gingival thickness gain when autogenous connective tissue was used. The weighted mean is presented at CI 95%. Heterogeneity was determined using Higgins (I2). A random-effects model was applied. Statistical significance was p < 0.001.
Figure 10.
Forest plot for gingival thickness in test group (collagen matrix). The weighted mean is presented at CI 95%. Heterogeneity was determined using Higgins (I2). A random-effects model was applied. Statistical significance was set at 0.05.
Figure 11.
(a) Funnel plot for gingival thickness in control group (autogenous connective tissue). The estimated gingival thickness measurement is on the horizontal axis and the study precision (standard error) appears on the vertical axis. (b) Funnel plot for gingival thickness in the test group (collagen matrix). The estimated gingival thickness measurement is on the horizontal axis and the study precision (standard error) appears on the vertical axis.
Figure 12.
Forest plot for gingival thickness. Test (collagen matrix) vs. control (autogenous connective tissue) groups. The weighted mean is presented at CI 95%. Heterogeneity was determined using Higgins (I2). A random-effects model was applied. Statistical significance was p < 0.001.
4. Discussion
The aim of this systematic review and meta-analysis was to evidence the efficacy of collagen matrices as an alternative to autogenous connective tissue graft for soft tissue augmentation. The establishment of tight eligibility criteria resulted in a limited number of included studies: three CTs and 16 RCTs. However, it diminishes the risk of bias and strengthens the systematic review [49]. All the included studies have at least two parallel arms: the use of collagen matrix versus autogenous connective tissue graft, and the evaluation of its effectiveness in terms of mucosal thickness and/or width of keratinized mucosa gain. These clinical outcomes were selected since they are the only objective parameters which made it possible to make inter-studies comparisons [1,2,50,51]. A total of 411 patients which undergone soft tissue augmentation surgery, were analyzed. 11 studies evaluated gingival thickness [12,16,19,27,28,33,38,39,41,42,43] and 15 reported data for width of keratinized mucosa [19,27,28,29,31,32,33,34,35,36,37,38,40,41,43]. Among all the included studies, the xenogeneic collagen matrix Mucograft (Geistlich Pharma AG, Wolhusen, Switzerland) is the soft tissue substitute used the most [16,27,29,31,32,33,34,35,37,38,40]. Volume-stable collagen matrix (Fibro-Gide, Geistlich Pharma AG, Wolhusen, Switzerland) [12,19,39,43] was used in four articles. Mucoderm (Botiss biomaterials GmbH, Zossen, Germany), which is also a xenogeneic collagen matrix, was used in four other articles included [28,36,41,42].
After performing the systematic review and meta-analysis, it can be inferred that even when a high heterogeneity was attained (I2 > 50%), all the random-effects models were highly significant (p < 0.001) enough to arise differences between the groups and make it able to state that connective tissue graft is more effective than collagen matrices for soft tissues augmentation around both teeth and implants.
When connective tissue graft was used, a significant gain in gingival thickness and keratinized mucosa were obtained: 1.17 and 4.03 mm, respectively. This increase in the quality of the supportive soft tissues was significantly higher than the one obtained when collagen matrices were used, being 0.81 mm the gained gingival thickness and 3.55 mm the augmentation of the keratinized mucosa. When comparing both groups in terms of width of keratinized tissue, the mean width gain was 0.62 mm higher (ranging from 1.09 to 0.15 mm) when using autogenous connective tissue in comparison to after employing collagen matrix, gingival thickness was also higher in a range of 0.49–0.16 mm. In contrast to these results, Gargallo-Albiol et al. [2] stated that gingival thickness gain was similar (p = 0.3) when using collagen matrix or autogenous connective tissue. Other previous systematic reviews and meta-analyses also did not find significant difference between both treatments [2,3,52]. Moraschini et al. [17] and Gargallo-Albiol et al. [2] also concluded that the gain of keratinized mucosa width was similar (p = 0.14 and p = 0.62, respectively), when comparing connective tissue graft with collagen matrix. These results were probably due to the quite small number of manuscripts finally included in the mentioned systematic reviews (11 and 7 articles, respectively), that did not account for the scientific evidence. Carvalho et al. [52], in accordance with our results, disclosed that the use of connective tissue graft significantly increased keratinized mucosa width when applied to recessions ≥ 2 mm, but as the results were expressed in terms of complete root coverage, then it is not possible to ascertain the real gain using the two different surgical approaches. Therefore, although there are many reviews that analyze soft tissue augmentation in different clinical situations, this is the first study in which the quantitative differences in keratinized mucosa width and gingival thickness are calculated for collagen matrix and autogenous connective tissue, establishing a significant differential gain between each other regardless of whether the recipient is an implant or tooth.
However, the most important point is to be aware of the odds and the limitations of the studied techniques, as a balance needs to be made between the expected improvement of soft tissue dimensions and the drawbacks related to palatal harvesting with the use of autogenous connective tissue graft. It is well known that an adequate gingival thickness plays a crucial role in maintaining periodontal and peri-implant health [53]. So, this gain in soft tissue quality involves the achievement of an improvement in the aesthetic result and a better long-term prognosis of both teeth and implants. Although there is no consensus about which should be the minimal dimensions of soft tissues, it is considered that an adequate amount of keratinized tissue would be 2 mm [3,11,12]. Taking this fact into account and the results encountered by this systematic review and meta-analysis, it may be positive to design a decision tree that helped clinicians to elucidate which of the studied techniques would fit each clinical situation. The requirement of a second surgical site as a donor area increases the morbidity of this procedure, augmenting procedure time [12], post-operative discomfort and complications like bleeding, necrosis and hypo- or anaesthesia [20]. These shortcomings can affect the patient perception of the treatment and—as a matter of fact—the use of a non-autogenous graft avoids these inconveniences and permits a less invasive procedure that provides a faster and more tolerable post-operatory period. All in all, it really makes collagen matrix a valid alternative in some cases.
Although autogenous graft received the highest values in terms of gingival thickness or keratinized mucosa width, studies also assessed, as secondary outcomes, the morbidity after soft tissue augmentation surgery, showing a preference for avoiding the requirement of a second surgical site. This donor area seems to be the major triggering cause of postoperative discomfort. It was concluded by Cairo et al. [38] that the use of collagen matrix resulted in a shorter surgical time to perform the soft tissue augmentation, a reduction of analgesic consumption and a higher final patient satisfaction. In the same line, Sanz et al. [32] and Lorenzo et al. [34] established that the patients in test group (CM) perceived less pain and needed fewer anti-inflammatory drugs. In addition, 30 days after surgery, while the patients that were treated with collagen matrices did not present pain, the patients who received autografts still presented “minor pain”. Differing In contrast to the previously mentioned studies, it was stated by Thoma et al. [12] that although collagen matrix was the best rated, there were no statistical differences (p > 0.05) in patient-reported outcome measures (PROMs). Among all the included studies, only Cieślik-Wegemund et al. [36] published data against the collagen matrix in terms of pain, finding significantly greater pain in patients treated with collagen matrix. Another important factor to take into account, but infrequently evaluated across studies, is the integration of the graft in adjacent soft tissues for esthetics evaluation. When assessing this variable, good results were found in both groups [19,32,34,36,38,40,41]. These studies stated that favorable results were obtained in both groups and that when there was a blinded evaluator, both procedures were not able to be distinguished in terms of color or esthetics outcomes. This is contrast to when free gingival graft (FGG) is used. In this case, the collagen matrix shows the best results, with [31,37] reporting that one of the drawbacks of FGG is the discrepancy in tissue color between the graft and the surrounding tissue. Regarding periodontal parameters, no study included in this review found significant differences between groups, establishing a remarkable improvement in periodontal parameters such as probing depth (PD), bleeding on probing (BOP) and clinical attachment level in both study groups.
The main limitation of this systematic review and meta-analysis is the high heterogeneity, probably due to the relatively small sample sizes of the several included studies, which have an average of about 24 patients. It could also be related to the different surgical approaches, operators’ ability, outcomes measured, and data reported. It is encouraged for future researchers to perform more RCTs following the CONSORT guideline and evaluating collagen matrices with higher sample sizes and follow-up time, including patient’s assessment of the technique, postoperative period and aesthetics evaluation. Additionally, standardization the measurement tools would facilitate data extraction and could result in more conclusive outcomes.
5. Conclusions
The findings from the present systematic review and meta-analysis suggest that collagen matrix is not as effective as connective tissue graft for soft tissue augmentation, when considering both keratinized mucosa width and gingival thickness. However, collagen matrices also achieve gain values that may be considered as clinically relevant, resulting as a valid alternative for cases where the autogenous connective tissue graft may not be considered as an option, due to patient morbidity.
Author Contributions
Conceptualization, C.V., M.T. and R.O.; Formal Analysis, M.T., M.T.-O., C.V., M.V.-R., A.R.-A. and R.O.; Funding Acquisition, M.T. and R.O.; Investigation, M.T., M.T.-O., M.V.-R., C.V., A.R.-A. and R.O., Methodology, M.T.-O., C.V., M.V.-R., A.R.-A. and R.O.; Project Administration, M.T. and R.O.; Supervision, M.T., R.O. and M.T.-O.; Validation, M.T., M.T.-O. and R.O.; Visualization; M.T., M.T.-O. and R.O.; Writing—Original Draft, C.V., M.T.-O., M.V.-R., M.T., and R.O.; Writing—Review and Editing, M.T.-O., M.T., A.R.-A. and R.O. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by: (1) the Ministry of Economy and Competitiveness and European Regional Development Fund [Project MAT2017-85999-P MINECO/AEI/FEDER/UE], (2) University of Granada/Regional Government of Andalusia Research Fund from Spain and European Regional Development Fund (A-BIO-157-UGR-18/FEDER). This research is part of C.V.’s PhD research study.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors report no conflicts of interest related to this meta-analysis.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Giannobile W.V., Jung R.E., Schwarz F. Groups of the 2nd Osteology Foundation Consensus Meeting Evidence-Based Knowledge on the Aesthetics and Maintenance of Peri-Implant Soft Tissues: Osteology Foundation Consensus Report Part 1-Effects of Soft Tissue Augmentation Procedures on the Maintenance of Peri-Implant Soft Tissue Health. Clin. Oral Implant. Res. 2018;29(Suppl. 15):7–10. doi: 10.1111/clr.13110. [DOI] [PubMed] [Google Scholar]
- 2.Gargallo-Albiol J., Barootchi S., Tavelli L., Wang H.-L. Efficacy of Xenogeneic Collagen Matrix to Augment Peri-Implant Soft Tissue Thickness Compared to Autogenous Connective Tissue Graft: A Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Implant. 2019;34:1059–1069. doi: 10.11607/jomi.7497. [DOI] [PubMed] [Google Scholar]
- 3.Moraschini V., Luz D., Velloso G., dS Barboza E.P. Quality Assessment of Systematic Reviews of the Significance of Keratinized Mucosa on Implant Health. Int. J. Oral Maxillofac. Surg. 2017;46:774–781. doi: 10.1016/j.ijom.2017.02.1274. [DOI] [PubMed] [Google Scholar]
- 4.AlSarhan M.A., Al Jasser R., Tarish M.A., AlHuzaimi A.I., Alzoman H. Xenogeneic Collagen Matrix versus Connective Tissue Graft for the Treatment of Multiple Gingival Recessions: A Systematic Review and Meta-analysis. Clin. Exp. Dent. Res. 2019;5:566–579. doi: 10.1002/cre2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tonetti M.S., Cortellini P., Pellegrini G., Nieri M., Bonaccini D., Allegri M., Bouchard P., Cairo F., Conforti G., Fourmousis I., et al. Xenogenic Collagen Matrix or Autologous Connective Tissue Graft as Adjunct to Coronally Advanced Flaps for Coverage of Multiple Adjacent Gingival Recession: Randomized Trial Assessing Non-Inferiority in Root Coverage and Superiority in Oral Health-Related Quality of Life. J. Clin. Periodontol. 2018;45:78–88. doi: 10.1111/jcpe.12834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Definition and Objectives of Periodontal Plastic Surgery—Practical Periodontal Plastic Surgery—Wiley Online Library. [(accessed on 11 February 2017)]; Available online: https://onlinelibrary.wiley.co.
- 7.Thoma D.S., Naenni N., Figuero E., Hämmerle C.H.F., Schwarz F., Jung R.E., Sanz-Sánchez I. Effects of Soft Tissue Augmentation Procedures on Peri-Implant Health or Disease: A Systematic Review and Meta-Analysis. Clin. Oral Implant. Res. 2018;29(Suppl. 15):32–49. doi: 10.1111/clr.13114. [DOI] [PubMed] [Google Scholar]
- 8.Tatakis D.N., Chambrone L., Allen E.P., Langer B., McGuire M.K., Richardson C.R., Zabalegui I., Zadeh H.H. Periodontal Soft Tissue Root Coverage Procedures: A Consensus Report from the AAP Regeneration Workshop. J. Periodontol. 2015;86:S52–S55. doi: 10.1902/jop.2015.140376. [DOI] [PubMed] [Google Scholar]
- 9.Chambrone L., Tatakis D.N. Periodontal Soft Tissue Root Coverage Procedures: A Systematic Review from the AAP Regeneration Workshop. J. Periodontol. 2015;86:S8–S51. doi: 10.1902/jop.2015.130674. [DOI] [PubMed] [Google Scholar]
- 10.Prato G.P.P., Franceschi D., Cortellini P., Chambrone L. Long-Term Evaluation (20 Years) of the Outcomes of Subepithelial Connective Tissue Graft plus Coronally Advanced Flap in the Treatment of Maxillary Single Recession-Type Defects. J. Periodontol. 2018;89:1290–1299. doi: 10.1002/JPER.17-0619. [DOI] [PubMed] [Google Scholar]
- 11.Schmitt C.M., Matta R.E., Moest T., Humann J., Gammel L., Neukam F.W., Schlegel K.A. Soft Tissue Volume Alterations after Connective Tissue Grafting at Teeth: The Subepithelial Autologous Connective Tissue Graft versus a Porcine Collagen Matrix—a Pre-Clinical Volumetric Analysis. J. Clin. Periodontol. 2016;43:609–617. doi: 10.1111/jcpe.12547. [DOI] [PubMed] [Google Scholar]
- 12.Thoma D.S., Zeltner M., Hilbe M., Hämmerle C.H.F., Hüsler J., Jung R.E. Randomized Controlled Clinical Study Evaluating Effectiveness and Safety of a Volume-Stable Collagen Matrix Compared to Autogenous Connective Tissue Grafts for Soft Tissue Augmentation at Implant Sites. J. Clin. Periodontol. 2016;43:874–885. doi: 10.1111/jcpe.12588. [DOI] [PubMed] [Google Scholar]
- 13.Puzio M., Hadzik J., Błaszczyszyn A., Gedrange T., Dominiak M. Soft Tissue Augmentation around Dental Implants with Connective Tissue Graft (CTG) and Xenogenic Collagen Matrix (XCM). 1-Year Randomized Control Trail. Ann. Anat. Anat. Anz. 2020;230:151484. doi: 10.1016/j.aanat.2020.151484. [DOI] [PubMed] [Google Scholar]
- 14.Rossi R., Pilloni A., Morales R.S. Qualitative Assessment of Connective Tissue Graft with Epithelial Component. A Microsurgical Periodontal Plastic Surgical Technique for Soft Tissue Esthetics. Eur. J. Esthet. Dent. 2009;4:118–128. [PubMed] [Google Scholar]
- 15.Moharamzadeh K., Brook I.M., Van Noort R., Scutt A.M., Smith K.G., Thornhill M.H. Development, Optimization and Characterization of a Full-Thickness Tissue Engineered Human Oral Mucosal Model for Biological Assessment of Dental Biomaterials. J. Mater. Sci. Mater. Med. 2008;19:1793–1801. doi: 10.1007/s10856-007-3321-1. [DOI] [PubMed] [Google Scholar]
- 16.Puzio M., Błaszczyszyn A., Hadzik J., Dominiak M. Ultrasound Assessment of Soft Tissue Augmentation around Implants in the Aesthetic Zone Using a Connective Tissue Graft and Xenogeneic Collagen Matrix—1-Year Randomised Follow-Up. Ann. Anat. Anat. Anz. 2018;217:129–141. doi: 10.1016/j.aanat.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Moraschini V., de Almeida D.C.F., Sartoretto S., Bailly Guimarães H., Chaves Cavalcante I., Diuana Calasans-Maia M. Clinical Efficacy of Xenogeneic Collagen Matrix in the Treatment of Gingival Recession: A Systematic Review and Meta-Analysis. Acta Odontol. Scand. 2019;77:457–467. doi: 10.1080/00016357.2019.1588372. [DOI] [PubMed] [Google Scholar]
- 18.Baldino L., Cardea S., Maffulli N., Reverchon E. Regeneration Techniques for Bone-to-Tendon and Muscle-to-Tendon Interfaces Reconstruction. Br. Med. Bull. 2016;117:25–37. doi: 10.1093/bmb/ldv056. [DOI] [PubMed] [Google Scholar]
- 19.Huber S., Zeltner M., Hämmerle C.H.F., Jung R.E., Thoma D.S. Non-Interventional 1-Year Follow-up Study of Peri-Implant Soft Tissues Following Previous Soft Tissue Augmentation and Crown Insertion in Single-Tooth Gaps. J. Clin. Periodontol. 2018;45:504–512. doi: 10.1111/jcpe.12865. [DOI] [PubMed] [Google Scholar]
- 20.Rothamel D., Benner M., Fienitz T., Happe A., Kreppel M., Nickenig H.-J., Zöller J.E. Biodegradation Pattern and Tissue Integration of Native and Cross-Linked Porcine Collagen Soft Tissue Augmentation Matrices—an Experimental Study in the Rat. Head Face Med. 2014;10:10. doi: 10.1186/1746-160X-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toledano M., Toledano-Osorio M., Carrasco-Carmona Á., Vallecillo C., Lynch C.D., Osorio M.T., Osorio R. State of the Art on Biomaterials for Soft Tissue Augmentation in the Oral Cavity. Part I: Natural Polymers-Based Biomaterials. Polymers. 2020;12:1850. doi: 10.3390/polym12081850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moher D., Shamseer L., Clarke M., Ghersi D., Liberati A., Petticrew M., Shekelle P., Stewart L.A. PRISMA-P Group Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 Statement. Syst. Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins J.P.T., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D., Savovic J., Schulz K.F., Weeks L., Sterne J.A.C., et al. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moher D., Liberati A., Tetzlaff J., Altman D.G. PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 25.Schardt C., Adams M.B., Owens T., Keitz S., Fontelo P. Utilization of the PICO Framework to Improve Searching PubMed for Clinical Questions. BMC Med. Inform. Decis. Mak. 2007;7:16. doi: 10.1186/1472-6947-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sterne J.A., Hernán M.A., Reeves B.C., Savović J., Berkman N.D., Viswanathan M., Henry D., Altman D.G., Ansari M.T., Boutron I., et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aroca S., Molnár B., Windisch P., Gera I., Salvi G.E., Nikolidakis D., Sculean A. Treatment of Multiple Adjacent Miller Class I and II Gingival Recessions with a Modified Coronally Advanced Tunnel (MCAT) Technique and a Collagen Matrix or Palatal Connective Tissue Graft: A Randomized, Controlled Clinical Trial. J. Clin. Periodontol. 2013;40:713–720. doi: 10.1111/jcpe.12112. [DOI] [PubMed] [Google Scholar]
- 28.Pietruska M., Skurska A., Podlewski Ł., Milewski R., Pietruski J. Clinical Evaluation of Miller Class I and II Recessions Treatment with the Use of Modified Coronally Advanced Tunnel Technique with Either Collagen Matrix or Subepithelial Connective Tissue Graft: A Randomized Clinical Study. J. Clin. Periodontol. 2019;46:86–95. doi: 10.1111/jcpe.13031. [DOI] [PubMed] [Google Scholar]
- 29.Tarasenko S., Ashurko I., Taschieri S., Repina S., Esaya N A., Corbella S. Comparative Analysis of Methods to Increase the Amount of Keratinized Mucosa before Stage-Two Surgery: A Randomized Controlled Study. Quintessence Int. 2020;51:374–387. doi: 10.3290/j.qi.a44216. [DOI] [PubMed] [Google Scholar]
- 30.Zuiderveld E.G., Meijer H.J.A., Vissink A., Raghoebar G.M. The Influence of Different Soft-Tissue Grafting Procedures at Single Implant Placement on Esthetics: A Randomized Controlled Trial. J. Periodontol. 2018;89:903–914. doi: 10.1002/JPER.18-0061. [DOI] [PubMed] [Google Scholar]
- 31.Vellis J., Kutkut A., Al-Sabbagh M. Comparison of Xenogeneic Collagen Matrix vs. Free Gingival Grafts to Increase the Zone of Keratinized Mucosa Around Functioning Implants. Implant Dent. 2019;28:20–27. doi: 10.1097/ID.0000000000000842. [DOI] [PubMed] [Google Scholar]
- 32.Sanz M., Lorenzo R., Aranda J.J., Martin C., Orsini M. Clinical Evaluation of a New Collagen Matrix (Mucograft Prototype) to Enhance the Width of Keratinized Tissue in Patients with Fixed Prosthetic Restorations: A Randomized Prospective Clinical Trial. J. Clin. Periodontol. 2009;36:868–876. doi: 10.1111/j.1600-051X.2009.01460.x. [DOI] [PubMed] [Google Scholar]
- 33.Cardaropoli D., Tamagnone L., Roffredo A., Gaveglio L. Treatment of Gingival Recession Defects Using Coronally Advanced Flap with a Porcine Collagen Matrix Compared to Coronally Advanced Flap with Connective Tissue Graft: A Randomized Controlled Clinical Trial. J. Periodontol. 2012;83:321–328. doi: 10.1902/jop.2011.110215. [DOI] [PubMed] [Google Scholar]
- 34.Lorenzo R., García V., Orsini M., Martin C., Sanz M. Clinical Efficacy of a Xenogeneic Collagen Matrix in Augmenting Keratinized Mucosa around Implants: A Randomized Controlled Prospective Clinical Trial. Clin. Oral Implant. Res. 2012;23:316–324. doi: 10.1111/j.1600-0501.2011.02260.x. [DOI] [PubMed] [Google Scholar]
- 35.McGuire M.K., Scheyer E.T. Randomized, Controlled Clinical Trial to Evaluate a Xenogeneic Collagen Matrix as an Alternative to Free Gingival Grafting for Oral Soft Tissue Augmentation. J. Periodontol. 2014;85:1333–1341. doi: 10.1902/jop.2014.130692. [DOI] [PubMed] [Google Scholar]
- 36.Cieślik-Wegemund M., Wierucka-Młynarczyk B., Tanasiewicz M., Gilowski Ł. Tunnel Technique With Collagen Matrix Compared With Connective Tissue Graft for Treatment of Periodontal Recession: A Randomized Clinical Trial. J. Periodontol. 2016;87:1436–1443. doi: 10.1902/jop.2016.150676. [DOI] [PubMed] [Google Scholar]
- 37.Schmitt C.M., Moest T., Lutz R., Wehrhan F., Neukam F.W., Schlegel K.A. Long-Term Outcomes after Vestibuloplasty with a Porcine Collagen Matrix (Mucograft ®) versus the Free Gingival Graft: A Comparative Prospective Clinical Trial. Clin. Oral Implant. Res. 2016;27:e125–e133. doi: 10.1111/clr.12575. [DOI] [PubMed] [Google Scholar]
- 38.Cairo F., Barbato L., Tonelli P., Batalocco G., Pagavino G., Nieri M. Xenogeneic Collagen Matrix versus Connective Tissue Graft for Buccal Soft Tissue Augmentation at Implant Site. A Randomized, Controlled Clinical Trial. J. Clin. Periodontol. 2017;44:769–776. doi: 10.1111/jcpe.12750. [DOI] [PubMed] [Google Scholar]
- 39.Zeltner M., Jung R.E., Hämmerle C.H.F., Hüsler J., Thoma D.S. Randomized Controlled Clinical Study Comparing a Volume-Stable Collagen Matrix to Autogenous Connective Tissue Grafts for Soft Tissue Augmentation at Implant Sites: Linear Volumetric Soft Tissue Changes up to 3 Months. J. Clin. Periodontol. 2017;44:446–453. doi: 10.1111/jcpe.12697. [DOI] [PubMed] [Google Scholar]
- 40.Nahas R., Gondim V., Carvalho C.V., Calderero L.M., Rosa E.F., Sakiyama T., César Neto J.B., Pannuti C.M., Romito G.A. Treatment of Multiple Recessions with Collagen Matrix versus Connective Tissue: A Randomized Clinical Trial. Braz. Oral Res. 2020;33:e123. doi: 10.1590/1807-3107bor-2019.vol33.0123. [DOI] [PubMed] [Google Scholar]
- 41.Rakasevic D.L., Milinkovic I.Z., Jankovic S.M., Soldatovic I.A., Aleksic Z.M., Nikolic-Jakoba N.S. The Use of Collagen Porcine Dermal Matrix and Connective Tissue Graft with Modified Coronally Advanced Tunnel Technique in the Treatment of Multiple Adjacent Type I Gingival Recessions: A Randomized, Controlled Clinical Trial. J. Esthet. Restor. Dent. 2020;32:681–690. doi: 10.1111/jerd.12624. [DOI] [PubMed] [Google Scholar]
- 42.Schmitt C.M., Brückbauer P., Schlegel K.A., Buchbender M., Adler W., Matta R.E. Volumetric Soft Tissue Alterations in the Early Healing Phase after Peri- Implant Soft Tissue Contour Augmentation with a Porcine Collagen Matrix versus the Autologous Connective Tissue Graft: A Controlled Clinical Trial. J. Clin. Periodontol. 2021;48:146–163. doi: 10.1111/jcpe.13387. [DOI] [PubMed] [Google Scholar]
- 43.Thoma D.S., Gasser T.J.W., Jung R.E., Hämmerle C.H.F. Randomized Controlled Clinical Trial Comparing Implant Sites Augmented with a Volume-stable Collagen Matrix or an Autogenous Connective Tissue Graft: 3-year Data after Insertion of Reconstructions. J. Clin. Periodontol. 2020;47:630–639. doi: 10.1111/jcpe.13271. [DOI] [PubMed] [Google Scholar]
- 44.Ozturan S., Oztunc H., Keles Evlice B. Assessment of the Soft Tissue Volumetric Changes Following Acellular Dermal Matrix Grafts with Cone Beam Computerized Tomography. Quintessence Int. 2015;46:171–178. doi: 10.3290/j.qi.a32826. [DOI] [PubMed] [Google Scholar]
- 45.Eeckhout C., Bouckaert E., Verleyen D., De Bruyckere T., Cosyn J. A 3-Year Prospective Study on a Porcine-Derived Acellular Collagen Matrix to Re-Establish Convexity at the Buccal Aspect of Single Implants in the Molar Area: A Volumetric Analysis. J. Clin. Med. 2020;9:1568. doi: 10.3390/jcm9051568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ghanaati S., Schlee M., Webber M.J., Willershausen I., Barbeck M., Balic E., Görlach C., Stupp S.I., Sader R.A., Kirkpatrick C.J. Evaluation of the Tissue Reaction to a New Bilayered Collagen Matrix in Vivo and Its Translation to the Clinic. Biomed. Mater. 2011;6:015010. doi: 10.1088/1748-6041/6/1/015010. [DOI] [PubMed] [Google Scholar]
- 47.Zafiropoulos G.-G., Deli G., Hoffmann O., John G. Changes of the Peri-Implant Soft Tissue Thickness after Grafting with a Collagen Matrix. J. Indian Soc. Periodontol. 2016;20:441. doi: 10.4103/0972-124X.181245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McGuire M.K., Scheyer E.T. Xenogeneic Collagen Matrix With Coronally Advanced Flap Compared to Connective Tissue With Coronally Advanced Flap for the Treatment of Dehiscence-Type Recession Defects. J. Periodontol. 2010;81:1108–1117. doi: 10.1902/jop.2010.090698. [DOI] [PubMed] [Google Scholar]
- 49.Fleming P.S., Lynch C.D., Pandis N. Randomized Controlled Trials in Dentistry: Common Pitfalls and How to Avoid Them. J. Dent. 2014;42:908–914. doi: 10.1016/j.jdent.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 50.Cairo F., Barbato L., Selvaggi F., Baielli M.G., Piattelli A., Chambrone L. Surgical Procedures for Soft Tissue Augmentation at Implant Sites. A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Clin. Implant Dent. Relat. Res. 2019;21:1262–1270. doi: 10.1111/cid.12861. [DOI] [PubMed] [Google Scholar]
- 51.Bassetti R.G., Stähli A., Bassetti M.A., Sculean A. Soft Tissue Augmentation around Osseointegrated and Uncovered Dental Implants: A Systematic Review. Clin. Oral Investig. 2017;21:53–70. doi: 10.1007/s00784-016-2007-9. [DOI] [PubMed] [Google Scholar]
- 52.De Carvalho Formiga M., Nagasawa M.A., Moraschini V., Ata-Ali J., Sculean A., Shibli J.A. Clinical Efficacy of Xenogeneic and Allogeneic 3D Matrix in the Management of Gingival Recession: A Systematic Review and Meta-Analysis. Clin. Oral Investig. 2020;24:2229–2245. doi: 10.1007/s00784-020-03370-w. [DOI] [PubMed] [Google Scholar]
- 53.Jepsen S., Caton J.G., Albandar J.M., Bissada N.F., Bouchard P., Cortellini P., Demirel K., de Sanctis M., Ercoli C., Fan J., et al. Periodontal Manifestations of Systemic Diseases and Developmental and Acquired Conditions: Consensus Report of Workgroup 3 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018;89:S237–S248. doi: 10.1002/JPER.17-0733. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.