Abstract
Background: We investigated preoperative cerebral (ScO2) and abdominal (StO2) regional oxygen saturations according to cardiac diagnosis in neonates with critical CHD, their time trends, and the clinical and biochemical parameters associated with them. Methods: Thirty-seven neonates with a prenatal diagnosis of CHD were included. ScO2 and StO2 values were continuously evaluated using near-infrared spectroscopy. Measurements were obtained hourly before surgery. A linear mixed effects model was used to assess the effects of time and cardiac diagnosis on regional oxygenation and to explore the contributing factors. Results: Regional oxygenation differed according to cardiac diagnosis (p < 0.001). ScO2 was lowest in the patients with severe atrioventricular valvar regurgitation (AVVR) (48.1 ± 8.0%). StO2 tended to be lower than ScO2, and both worsened gradually during the period between birth and surgery. There was also a significant interaction between cardiac diagnosis and time. The factors related to ScO2 were hemoglobin and arterial saturation, whereas no factor was associated with StO2. Conclusions: Preoperative ScO2 and StO2 in critical CHD differed according to cardiac diagnosis. ScO2 in the patients with severe AVVR was very low, which may imply cerebral hypoxia. ScO2 gradually decreased, suggesting that the longer the time to surgery, the higher the risk of hypoxic brain injury.
Keywords: cerebral oxygen saturation, somatic oxygen saturation, congenital heart disease
1. Introduction
Neurobehavioral impairments are common in infants undergoing surgery for CHD, and they occur across a wide spectrum; cognition, motor, social interaction and behavior, language, inattention, and executive function [1,2,3,4,5,6,7,8,9,10]. Many studies have discussed the factors related to surgery or postoperative care on this issue. However, some studies suggested that brain damage may occur during the early postnatal life of infants with CHD, which may lead to impaired neurodevelopment [11,12].
Especially in patients with complex CHD, these abnormalities will be exacerbated by unstable hemodynamics before undergoing cardiac surgery. Therefore, maintaining stable hemodynamics before cardiac surgery and recognizing clinical deterioration early and taking appropriate action will yield a better outcome. Therefore, it is necessary to have an in-depth understanding of cerebral perfusion and its relationship to underlying heart diseases. Moreover, the deterioration of other-end organ perfusion, which may affect the proper timing of surgery or the outcome of the surgery, should be monitored.
Near-infrared spectroscopy (NIRS) is a tool used to continuously and non-invasively monitor the degree of oxygenation of multisite regional tissues, such as the brain, intestines, and kidneys. There have been many studies on NIRS monitoring of infants with CHD during or after surgery; however, there have been few on the preoperative cerebral and somatic oxygenation in the neonates with CHD.
The objective of this study was to investigate preoperative cerebral (ScO2) and somatic (StO2) oxygenation according to cardiac diagnosis using NIRS and the time course of these parameters. We also investigated the extent of changes in ScO2 and StO2 at the time of clinical deterioration. Moreover, we explored the clinical and biochemical parameters that correlate with ScO2 and StO2.
2. Patients and Methods
2.1. Patients
A prospective study was performed at a tertiary cardiac and neonatal intensive care unit (NICU) of Asan medical center. The study was approved by the institutional review board of Asan medical center (IRB number 2018-0817) and written informed consent was obtained.
All neonates with a prenatal diagnosis of congenital heart disease who were born at a gestational age >36 weeks between September 2018 and August 2019 were considered for inclusion. Patients with other congenital anomalies or those who were transferred to another hospital before surgery were excluded.
The study population was divided according to cardiac diagnosis:
Group 1: normal heart
Group 2: transposition of the great arteries (TGA)
Group 3: duct-dependent systemic circulation; Coarctation of aorta (CoA) with ventricular septal defect (VSD), interrupted aortic arch (IAA) with VSD, functional single ventricle (FSV) with CoA
Group 4: duct-dependent pulmonary circulation; tetralogy of fallot (TOF) with pulmonary atresia (PA) or pulmonary stenosis (PS), FSV with PA or PS
Group 5: regurgitation lesion; tricuspid dysplasia, Ebstein’s anomaly
Group 6: mixing lesion; truncus arteriosus, total anomalous pulmonary venous return (TAPVR)
2.2. Regional Oximetry
We used INVOS™ 5100c near-infrared spectrometers (Somanetics, Troy, MI, USA) with neonatal somasensors. The somasensors were placed on the two sites (forehead and abdomen) to record ScO2 and StO2 oximetries, and they were measured continuously during their stay in the NICU. The cerebral sensor was placed on the bilateral forehead and the somatic sensor was placed on the anterior abdomen (just below the umbilicus) [13]. ScO2 and StO2 were continuously monitored and recorded by the device every 6 s. For analysis, measurements were acquired hourly and at specific time points whenever an adverse event occurred.
2.3. Data Collection
We collected all available clinical and biochemical parameters that may have influenced the course of ScO2 and StO2. We reviewed the electronic medical records of the study cohort for the collection and collation of dates regarding patients’ baseline characteristics, underlying heart disease and clinical course, gestational age, birth weight, Apgar score at 5 min, respiratory support, and treatment with inotropes and sedatives.
We also measured serum lactate, hemoglobin, and hematocrit from the venous blood samples; pH, pCO2, and pO2 from the arterial, venous, or capillary blood gas measurement, as well as arterial saturation (SpO2), blood pressure, heart rate, and body temperature. Blood gas measurement was conducted upon admission to the NICU and every 6 to 12 h during the preoperative period. SpO2 and heart rate were continuously monitored. Blood pressure was measured continuously using an arterial catheter or every hour using a NIBP monitor. Body temperature was measured every 4 to 6 h. For analysis, measurement data were sampled on an hourly basis for continuous monitoring of parameters while all measurement data were used for intermittent monitoring of parameters.
2.4. Statistical Analysis
For statistical analysis, we used SPSS 21.0 (IBM Corp., Armonk, NY, USA). Continuous variables were summarized using standard descriptive statistics (mean and standard deviation), and frequencies and percentages were used for categorical variables.
We displayed the course of ScO2 and StO2 graphically per hour according to cardiac diagnosis. To analyze differences in ScO2 and StO2 according to cardiac diagnosis, we used the Wilcoxon signed rank test. The preoperative time trends in ScO2 and StO2 were examined using a linear mixed model.
We used the Pearson’s correlation test to determine the correlation between demographic and physiological parameters and regional oxygen saturation. To explore the factors contributing to regional oxygenation, a linear mixed model including a potential subset of predictors was used. The variable selection was performed using the Akaike information criterion. p-values of <0.05 were considered to be statistically significant.
3. Results
3.1. Patient Characteristics
A total of 37 neonates were included in the study. Reasons for exclusion were transfer or expiration within 72 h after birth in 2 patients, major chromosomal abnormalities in one patient and consent withdrawal after enrollment in 2 patients. Patient characteristics are presented in Table 1. The thirty-seven neonates had a gestational age of 38.8 ± 1.1 weeks, birth weight of 3.2 ± 0.5 Kg and birth height of 49.4 ± 2.6 cm. Patients were classified according to their postnatal diagnosis: normal heart (n = 3), TGA (n = 4), duct-dependent systemic circulation (n = 10), duct-dependent pulmonary circulation (n = 13), severe atrioventricular valvar regurgitation (AVVR) (n = 2), total anomalous pulmonary vein return (TAPVR) or Truncus arteriosus (n = 5).
Table 1.
Subject demographics.
| Variable | Value (n = 37) |
|---|---|
| Gestational age, weeks | 38.8 ± 1.1 |
| Birth weight, Kg | 3.2 ± 0.5 |
| Birth height (cm) | 49.4 ± 2.6 |
| Male, n (%) | 20 (54) |
| Apgar 5 min | 8.4 ± 1.0 |
| Diagnosis, n (%) | |
| Duct-dependent pulmonary circulation (TOF with PA or PS, FSV with PA or PS) | 11 (29.7) |
| TGA with IVS | 4 (8.1) |
| Mixed lesion (Truncus arteriosus, TAPVR) | 8 (21.6) |
| Duct-dependent systemic circulation (CoA with VSD, IAA with VSD, FSV with CoA) | 11 (29.7) |
| Severe valve regurgitation (TV dysplasia, Ebstein’s anomaly) | 2 (5.4) |
| Normal heart | 3 (5.4) |
| Respiratory support, n (%) | 25 (67.5) |
| Length of stay in the NICU, days | 12.9 ± 10.7 |
All values are presented as mean ± SD, unless stated otherwise. CoA, coarctation of the aorta; IVS, intact ventricular septum; FSV, functional single ventricle; TAPVR, total anomalous pulmonary venous return; PA, pulmonary atresia; PS, pulmonary stenosis; TOF, tetralogy of fallot; TGA, transposition of the great arteries.
3.2. Cerebral and Somatic Oxygenation
Table 2 and Figure 1 show the ScO2 and StO2 values according to the underlying heart disease. In Figure 1, we smoothed the raw data samples with a Gaussian-weighted moving average filter, using a window of length 10. The regional oxygenation was different according to cardiac diagnosis. ScO2 significantly decreased in the patients with severe AVVR (48.1 ± 8.0%), TGA (56.3 ± 11.3%), mixing lesions (63.2 ± 10.6%), and CHD with severe PS or PA (68.7 ± 7.5%). ScO2 values were similar between patients with aortic coarctation or interrupted aortic arch (74.8 ± 7.9%) and those with normal hearts (71.7 ± 10.3%).
Table 2.
SpO2 and cerebral and somatic tissue oxygenation measurement.
| Group 1 (N = 3, n = 479) Mean ± SD | Group 2 (N = 4, n = 466) Mean ± SD | Group 3 (N = 10, n = 1173) Mean ± SD | Group 4 (N = 13, n = 1151) Mean ± SD | Group 5 (N = 2, n = 198) Mean ± SD | Group 6 (N = 5, n = 253) Mean ± SD | p-Value | |
|---|---|---|---|---|---|---|---|
| SpO2 (%) | 92.8 ± 4.8 a,d | 84.5 ± 7.3 b | 93.5 ±5.8 d | 91.8± 7.3 a | 87.9± 6.9 c | 93.0± 4.0 d | 0.000 |
| Cerebral oximetry (ScO2) (%) | 71.7 ± 10.3 a | 56.3 ±11.3 b | 74.8± 7.9 c | 68.7± 7.5 d | 48.1± 8.0 e | 63.2 ± 10.6 f | 0.000 |
| Somatic oximetry (StO2) (%) | 61.7 ± 16.2 a | 48.6 ±15.8 b | 61.7 ± 13.8 a | 65.4± 14.2 c | 51.3± 7.5 d | 53.7± 13.9 e | 0.000 |
|
p-value (between ScO2 and StO2) |
0.000 | 0.000 | 0.000 | 0.000 | 0.715 | 0.000 |
N: number of patients, n: number of measurement samples. a, b, c, d, e and f represent the difference between groups, the same letters indicate non-significant differences among groups, while the different letters indicate the group differs significantly from others. Group 1: normal heart, Group 2: transposition of the great arteries, Group 3: duct-dependent systemic circulation, Group 4: duct-dependent pulmonary circulation, Group 5: regurgitation lesion, Group 6: mixing lesion.
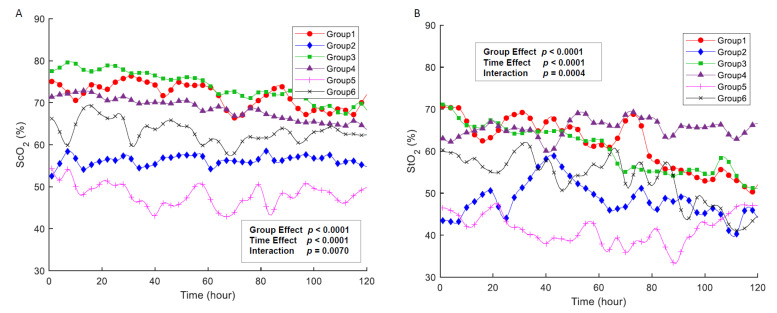
Figure 1.
Cerebral (A) and somatic (B) oxygen saturation over the 120 h after birth. ScO2 and StO2 decreased gradually with time (p = 0.029) and differed according to cardiac diagnosis (p < 0.001). There was also a significant interaction between cardiac diagnosis and time (p = 0.007). ScO2; cerebral oxygenation, StO2; Somatic oxygenation. Group 1; normal heart, Group 2; transposition of the great arteries, Group 3; duct-dependent systemic circulation, Group 4; duct-dependent pulmonary circulation, Group 5; regurgitation, Group 6; mixing lesion.
StO2 was lower on average than ScO2 in all groups except in the regurgitation lesion group. For cardiac diagnosis, we observed the lowest StO2 in the regurgitation lesion group and the highest in the duct-dependent systemic circulation group. The second lowest StO2 was observed in the TGA group, followed by the mixing lesion, duct-dependent systemic circulation, and normal heart groups (Table 2 and Figure 1).
We used a linear mixed effects model to assess the effects of time and cardiac diagnosis on ScO2 and StO2 (Figure 2, Table 3). Figure 2 shows the preoperative temporal trend of regional oxygen saturation in each group over the 120 h after birth. ScO2 and StO2 decreased gradually with time (p = 0.029), and they differed according to cardiac diagnosis (p < 0.001). There was also a significant interaction between cardiac diagnosis and time (p = 0.007); ScO2 decreased significantly with time in Groups 3 and 4.
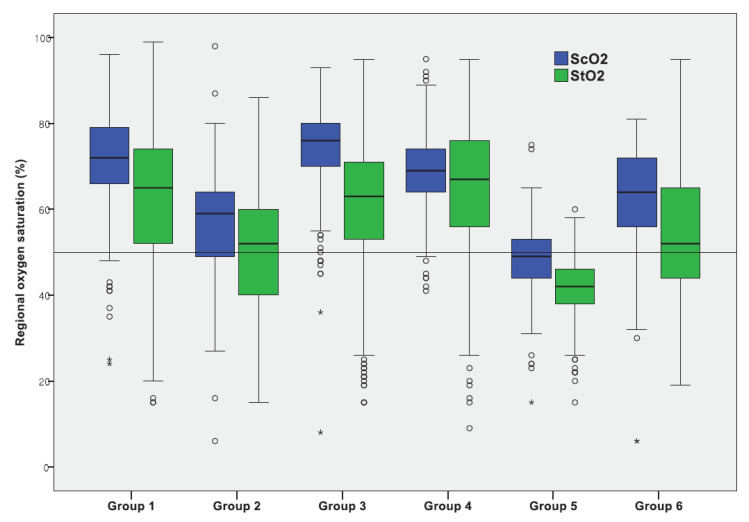
Figure 2.
Cerebral and somatic oxygen saturation according to underlying heart disease. Circles indicate outliers, asterisks are the extreme outliers. ScO2; cerebral oxygenation, StO2; Somatic oxygenation. Group 1; normal heart, Group 2; transposition of the great arteries, Group 3; duct-dependent systemic circulation, Group 4; duct-dependent pulmonary circulation, Group 5; regurgitation, Group 6; mixing lesion.
Table 3.
Parameter estimates of linear mixed model.
| ScO2 | StO2 | ||||||
|---|---|---|---|---|---|---|---|
| Effect | Estimate | SE | p-Value | Estimate | SE | p-Value | |
| Intercept | 74.917 | 1.648 | <0.0001 | 70.962 | 2.961 | <0.0001 | |
| Time point | −0.051 | 0.023 | 0.0290 | −0.150 | 0.042 | 0.0004 | |
| Group | 1 | 0.000 | 0.000 | ||||
| 2 | −19.490 | 2.343 | <0.0001 | −21.411 | 4.209 | <0.0001 | |
| 3 | 4.937 | 1.921 | 0.0106 | −1.273 | 3.451 | 0.7125 | |
| 4 | −1.985 | 1.937 | 0.3061 | −6.230 | 3.480 | 0.0743 | |
| 5 | −24.653 | 2.934 | <0.0001 | −28.278 | 5.268 | <0.0001 | |
| 6 | −9.793 | 2.678 | 0.0003 | −9.601 | 4.799 | 0.0463 | |
| Time point X Group | 1 | 0.000 | 0.000 | ||||
| 2 | 0.064 | 0.034 | 0.0606 | 0.130 | 0.060 | 0.0328 | |
| 3 | −0.045 | 0.028 | 0.1093 | 0.000 | 0.051 | 0.9939 | |
| 4 | −0.021 | 0.028 | 0.4460 | 0.161 | 0.050 | 0.0013 | |
| 5 | 0.014 | 0.046 | 0.7598 | 0.122 | 0.082 | 0.1374 | |
| 6 | 0.019 | 0.039 | 0.6202 | 0.017 | 0.070 | 0.8018 | |
| 1 | 0.000 | 0.000 | |||||
Group 1: normal heart, Group 2: transposition of the great arteries, Group 3: duct-dependent systemic circulation, Group 4: duct-dependent pulmonary circulation, Group 5: regurgitation lesion, Group 6: mixing lesion.
3.3. Association with Regional NIRS and Physiologic Parameters
Supplementary Table S1 shows the correlation between regional oxygen saturation (ScO2, StO2) and the clinical variables. Arterial saturation and hemoglobin concentration showed weak positive correlation with ScO2 and StO2, while PaCO2 showed weak negative correlation. However, blood pressure was not correlated with regional oxygenation.
We used a linear mixed model, including potential subsets of predictors, to explore the factors contributing to regional oxygenation. The factors related to ScO2 were hemoglobin (p = 0.015) or hematocrit (p = 0.019) and arterial saturation (p = 0.006), whereas there was no factor related to StO2.
The serum lactate had weak negative correlation with cerebral oxygenation (Supplementary Figure S1) (ScO2; r = −0.218, p < 0.001) and no correlation with somatic oxygenation.
3.4. Changes in Regional Oxygenation in Patients with Adverse Events
There were serious adverse events in six patients; 1 recurrent paroxysmal supraventricular tachycardia, 1 focal seizure, 2 cases of sepsis, 1 liver hematoma, and 1 hematochezia suspected of necrotizing enterocolitis (NEC). Patients with adverse events had abrupt changes in regional oxygenation during NIRS monitoring before symptoms such as seizure, fever, hematochezia, and lethargic appearance occurred. Baseline cerebral and somatic oxygenation remained stable and suddenly decreased approximately 40 min before the adverse events occurred (Supplementary Figure S2). This rapid decrease occurred in both cerebral and somatic regional oxygenation in the patients with sepsis or seizures, whereas the decrease occurred dominantly in somatic oxygenation in the patient with NEC.
Change in regional oxygenation also occurred after adequate management; in patients with TGA with intact ventricular septum (IVS) and restrictive atrial mixing, who showed very low values of ScO2 and StO2 shortly after birth, ScO2 and StO2 improved after emergency atrial septostomy.
4. Discussion
Neurobehavioral impairments are common in infants with CHD and occur across a wide spectrum [1,2,3,4,5,6,7,8,9,10]. The etiological factors of these problems are multifactorial and comprise a complex interaction between several factors before, during, and after surgery.
As preoperative factors, chronic hypoxia, acidosis, poor nutrition, and inadequate cerebral perfusion due to hemodynamic instability are the possible factors contributing to brain injury. Our study shows that cerebral oxygenation before surgery is significantly low in patients with CHD and very low in a few patients with certain CHD, which may imply cerebral hypoxia.
A few studies have reported differences in preoperative regional oxygenation in various types of CHD [14,15,16]. However, these studies only measured regional oxygenation for a very short preoperative period while focusing mainly on changes after the operative period [14,16]. Our study showed that there was a difference in regional oxygenation depending on cardiac diagnosis, even though the difference was not large in arterial oxygen saturation as measured by SpO2, and that the value of regional oxygenation was significantly lower than that in the normal heart group. The patients with severe tricuspid regurgitation (TR) (Group 5) and the patients with TGA with IVS (Group 2) had the lowest value of regional oxygenation, which is aligned with general expectation. It can be assumed that Group 5 faced the most severe low cardiac output state. During the immediate postnatal period, while pulmonary vascular resistance is still elevated, severe TR results in massive cardiomegaly and heart failure. In addition, it may be assumed that Group 2 showed a lower value due to limited effective mixing and a relatively low arterial saturation.
Normative ScO2 values have been reported as 76.8% ± 8.5% in healthy neonates. ScO2 value in many children with CHD is less than that in healthy children. In critical CHD, ScO2 was far lower than normal; mean values for ScO2 were as follows: 46.8 ± 8.9% in TGA, in hypoplastic left heart syndrome, 52.0 ± 7.2% [17], in PA, 38 ± 6%, and in TOF, 57 ± 12% [14]. Our study also showed that, overall, ScO2 in CHD patients was lower than normal. Contrary to previous reports [14,17], the value of ScO2 in our study was not significantly different to the normal values, with decreased pulmonary flow in right to left shunt lesion patients and higher than normal in left obstructive lesion patients, although our study did not include neonates with hypoplastic left heart syndrome. This can be attributed to appropriate and timely management for these patients; they were admitted to the neonatal intensive care unit (NICU) shortly after birth and prostaglandin E1 infusion was continued throughout the preoperative period to maintain ductal patency immediately after diagnosis. In addition, mechanical ventilator support and adequate inotropic helped maintain hemodynamic stability.
In our study, patients with severe AVVR, such as Ebstein’s anomaly or tricuspid valve dysplasia, showed the lowest value of ScO2 as 48.1 ± 8.0%.
Although there are no human pediatric data, Kurth et al.’s study in neonatal piglets showed that cerebral functional impairment begins at ScO2 of about 45%; there is a buffer zone between 45% and 60% whereby cerebral oxygenation is adequate for function but lower than normal. Depending on the result of that study, the value of ScO2 in the severe AVVR group is in a buffer zone. This means that the group is very susceptible to neurodevelopmental complication. In addition, ScO2 gradually decreased with time, which was similar to the results of the Lynch JM et al. Study [17,18], suggesting that the longer the time between diagnosis and surgery, the higher the risk of hypoxic brain injury. In particular, Groups 3 and 4 had a decreasing trend in ScO2 during the preoperative period. In both groups, with ductal dependent congenital heart disease, unbalanced Qp/Qs, insufficient ductal patency, or both may have resulted in such a trend.
Somatic oxygenation was lower than cerebral oxygenation in this study. In other words, this finding means that the somatic–cerebral difference is negative. The somatic–cerebral difference was 10–20% in healthy patients and decreased to 0% or negative values when in a low cardiac output state [19]. Some studies reported that a somatic–cerebral saturation difference was associated with mortality during the early postoperative period [19,20]. Our findings may imply that many patients with critical CHD are confronted with low cardiac outputs despite appropriate medical treatment. In particular, patients with severe TR during the transient circulation period are faced with severe impairment in both ScO2 and StO2.
The interest related to preoperative StO2 monitoring in the CHD is because it may be useful to predict adverse events caused by the impairment of systemic perfusion, such as acute kidney injury and NEC, which can affect the pre/post-operative course and outcome. Adverse events commonly occur in CHD patients [5,6,21], and these may originate pre, during, or post-surgery, as a result of global ischemia and hypoxia.
In our study, preoperative adverse events occurred in 6 patients (16.2%) including sepsis (n = 2), seizure (n = 1), abdominal problems (n = 2), and arrhythmia (n = 1). The value of StO2 in these patients declined abruptly in relation to an adverse event. In particular, it decreased before the onset of clinical symptoms in 2 neonates with NEC and in 1 neonate with sepsis.
However, it is difficult to define a critical threshold of adverse events. Absolute normative abdominal StO2 values do not seem to be clear. Abdominal (infraumbilical) StO2 ranged widely from 32% to 66% in healthy newborns and preterm infants, whereas renal StO2 was reported as 86.8% ± 8.1% with a range of 64% to 7% [22,23].
This difference is caused by the motility and varying luminal contents of the small bowel [24]. Therefore, the clinical utility of abdominal StO2 lies in trend monitoring rather than in critical threshold values. This enables early recognition of clinical deterioration based on the change in value from baseline. A few previous studies reported that monitoring through NIRS in pediatric intensive care units after cardiac surgery enables early prediction of serious adverse events [25,26]. Mebius et al. reported that cerebral and/or renal regional oxygenation changed 30 min or more before sudden, unexpected clinical deterioration, while other hemodynamic variables did not indicate that this deterioration was imminent.
Therefore, there remains a question of how much change in value should be considered significant. McNeill et al. reported that regional oxygenation fell by 30–35% relative to the baseline before the loss of output [15,23]. Fluctuations in regional oxygenation exceeding a reduction of 30–35% in one individual indicate the potential for decreased end-organ perfusion and consequent deterioration in clinical status. Changes in regional oxygenation using NIRS monitoring should be considered as a red flag and should be fully screened in infants, alongside physical examination, abdominal or brain ultrasound imaging study, and basic blood tests.
In this study, the factors contributing to ScO2 were hemoglobin (p = 0.015) or hematocrit (p = 0.019) and arterial saturation (p = 0.006), whereas no factor was related to StO2. This finding may indicate that certain strategies might decrease the risk of cerebral hypoxic-ischemic injury before surgery. Blood pressure was not a factor contributing to regional oxygenation; we suppose that it was kept in the cerebral autoregulation range.
Previous studies showed conflicting results regarding the association between serum lactate and cerebral near-infrared spectroscopy in infants after cardiac surgery. One study showed that an average cerebral and renal regional saturation of less than 65% as measured by NIRS predicts hyperlactatemia (>3 mmol/L) in acyanotic children after congenital heart surgery [27]. Meanwhile, other studies reported no correlation between cerebral regional saturation and serum lactate during or after cardiac surgery [28,29]. Our study showed a very weak correlation between regional oxygenation on NIRS and serum lactate. This may be because, even if global hypoperfusion occurs to the extent that lactate increases, cerebral oxygenation is maintained to some extent.
Our study is limited because it was conducted in a single center, with a small number of infants, over a short period of time, and it has location sampling bias. Additionally, poor performance may be related to the position of the NIRS probe. Increasing patient population with focused grouping remains as future work.
This study showed that preoperative ScO2 in critical CHD patients was relatively low and differed according to cardiac diagnosis. In particular, ScO2 in the patients with severe AVVR was very low, which may imply cerebral hypoxia. Abdominal StO2 tended to be lower than ScO2, and both values worsened gradually during the peri-operative periods; this finding suggests that the longer the time between birth and surgery, the higher the risk of hypoxic brain injury.
In addition, we showed that abrupt changes in regional oxygenation occurred in relation to adverse events. We believe that the continuous monitoring of regional oxygenation helps to maintain stable hemodynamics before the cardiac surgery, recognize clinical deterioration early, and take appropriate action.
Glossary
| AVVR | Atrioventricular valvar regurgitation |
| CHD | Congenital heart disease |
| CoA | Coarctation of aorta |
| FSV | Functional single ventricle |
| IAA | Interrupted aortic arch |
| NEC | Necrotizing enterocolitis |
| NICU | Neonatal intensive care unit |
| NIRS | Near-infrared spectroscopy |
| PA | Pulmonary atresia |
| PS | Pulmonary stenosis |
| Qp/Qs | Ratio of pulmonary-to-systemic flow |
| ScO2 | Cerebral oxygenation |
| StO2 | Somatic oxygenation |
| TAPVR | Total anomalous pulmonary venous return |
| TGA | Transposition of the great arteries |
| VSD | Ventricular septal defect |
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/jcm10112455/s1, Figure S1: Relationship between averaged regional NIRS and lactate, Figure S2: Changes in the cerebral and somatic oxygenation associated with adverse events, Table S1: Correlations with regional O2 saturation.
Author Contributions
M.J.K.: Data curation; Formal analysis; Investigation; Methodology; Project administration; Visualization; Writing—original draft. J.S.B.: Conceptualization; Data curation; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Visualization; Writing—original draft; Writing—review and editing; Funding acquisition. J.A.K.: Investigation. S.G.C.: Resources; Supervision; Validation; Writing—review and editing. J.J.Y.: Resources; Supervision; Validation; Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by a grant (2018IT0817) from Asan Medical Center Children’s Hospital, Seoul, Republic of Korea.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Asan medical center (IRB number 2018-0817).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data is contained within the article or Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Campbell M.J., Ziviani J.M., Stocker C.F., Khan A., Sakzewski L. Neuromotor performance in infants before and after early open-heart surgery and risk factors for delayed development at 6 months of age. Cardiol. Young. 2019;29:100–109. doi: 10.1017/S1047951118001622. [DOI] [PubMed] [Google Scholar]
- 2.Bellinger D.C., Wypij D., duPlessis A.J., Rappaport L.A., Jonas R.A., Wernovsky G., Newburger J.W. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: The Boston Circulatory Arrest Trial. J. Thorac. Cardiovasc. Surg. 2003;126:1385–1396. doi: 10.1016/S0022-5223(03)00711-6. [DOI] [PubMed] [Google Scholar]
- 3.Limperopoulos C., Majnemer A., Shevell M.I., Rohlicek C., Rosenblatt B., Tchervenkov C., Darwish H.Z. Predictors of developmental disabilities after open heart surgery in young children with congenital heart defects. J. Pediatr. 2002;141:51–58. doi: 10.1067/mpd.2002.125227. [DOI] [PubMed] [Google Scholar]
- 4.Bellinger D.C., Wypij D., Kuban K.C., Rappaport L.A., Hickey P.R., Wernovsky G., Jonas R.A., Newburger J.W. Developmental and neurological status of children at 4 years of age after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. Circulation. 1999;100:526–532. doi: 10.1161/01.CIR.100.5.526. [DOI] [PubMed] [Google Scholar]
- 5.Miller G., Eggli K.D., Contant C., Baylen B.G., Myers J.L. Postoperative neurologic complications after open heart surgery on young infants. Arch. Pediatr. Adolesc. Med. 1995;149:764–768. doi: 10.1001/archpedi.1995.02170200054008. [DOI] [PubMed] [Google Scholar]
- 6.Bellinger D.C., Jonas R.A., Rappaport L.A., Wypij D., Wernovsky G., Kuban K.C., Barnes P.D., Holmes G.L., Hickey P.R., Strand R.D., et al. Developmental and neurologic status of children after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. N. Engl. J. Med. 1995;332:549–555. doi: 10.1056/NEJM199503023320901. [DOI] [PubMed] [Google Scholar]
- 7.Gaynor J.W., Stopp C., Wypij D., Andropoulos D.B., Atallah J., Atz A.M., Beca J., Donofrio M.T., Duncan K., Ghanayem N.S., et al. Neurodevelopmental outcomes after cardiac surgery in infancy. Pediatrics. 2015;135:816–825. doi: 10.1542/peds.2014-3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uzark K., Smith C., Donohue J., Yu S., Romano J.C. Infant Motor Skills After a Cardiac Operation: The Need for Developmental Monitoring and Care. Ann. Thorac. Surg. 2017;104:681–686. doi: 10.1016/j.athoracsur.2016.12.032. [DOI] [PubMed] [Google Scholar]
- 9.Naef N., Liamlahi R., Beck I., Bernet V., Dave H., Knirsch W., Latal B. Neurodevelopmental Profiles of Children with Congenital Heart Disease at School Age. J. Pediatr. 2017;188:75–81. doi: 10.1016/j.jpeds.2017.05.073. [DOI] [PubMed] [Google Scholar]
- 10.Bellinger D.C., Wypij D., Rivkin M.J., DeMaso D.R., Robertson R.L., Jr., Dunbar-Masterson C., Rappaport L.A., Wernovsky G., Jonas R.A., Newburger J.W. Adolescents with d-transposition of the great arteries corrected with the arterial switch procedure: Neuropsychological assessment and structural brain imaging. Circulation. 2011;124:1361–1369. doi: 10.1161/CIRCULATIONAHA.111.026963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller S.P., McQuillen P.S., Vigneron D.B., Glidden D.V., Barkovich A.J., Ferriero D.M., Hamrick S.E.G., Azakie A., Karl T.R. Preoperative brain injury in newborns with transposition of the great arteries. Ann. Thorac. Surg. 2004;77:1698–1706. doi: 10.1016/j.athoracsur.2003.10.084. [DOI] [PubMed] [Google Scholar]
- 12.Licht D.J., Wang J.J., Silvestre D.W., Nicolson S.C., Montenegro L.M., Wernovsky G., Tabbutt S., Durning S.M., Shera ScD D.M., Gaynor W., et al. Preoperative cerebral blood flow is diminished in neonates with severe congenital heart defects. J. Thorac. Cardiov. Sur. 2004;128:841–849. doi: 10.1016/j.jtcvs.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 13.Kaufman J., Almodovar M.C., Zuk J., Friesen R.H. Correlation of abdominal site near-infrared spectroscopy with gastric tonometry in infants following surgery for congenital heart disease. Pediatr. Crit. Care Med. 2008;9:62–68. doi: 10.1097/01.PCC.0000298640.47574.DA. [DOI] [PubMed] [Google Scholar]
- 14.Kurth C.D., Steven J.L., Montenegro L.M., Watzman H.M., Gaynor J.W., Spray T.L., Nicolson S.C. Cerebral oxygen saturation before congenital heart surgery. Ann. Thorac. Surg. 2001;72:187–192. doi: 10.1016/S0003-4975(01)02632-7. [DOI] [PubMed] [Google Scholar]
- 15.Toet M.C., Flinterman A., van de Laar I., de Vries J.W., Bennink G.B., Uiterwaal C.S., van Bel F. Cerebral oxygen saturation and electrical brain activity before, during, and up to 36 hours after arterial switch procedure in neonates without pre-existing brain damage: Its relationship to neurodevelopmental outcome. Exp. Brain Res. 2005;165:343–350. doi: 10.1007/s00221-005-2300-3. [DOI] [PubMed] [Google Scholar]
- 16.Uebing A., Furck A.K., Hansen J.H., Nufer E., Scheewe J., Dutschke P., Jung O., Kramer H.-H. Perioperative cerebral and somatic oxygenation in neonates with hypoplastic left heart syndrome or transposition of the great arteries. J. Thorac. Cardiovasc. Surg. 2011;142:523–530. doi: 10.1016/j.jtcvs.2011.01.036. [DOI] [PubMed] [Google Scholar]
- 17.Lynch J.M., Ko T., Busch D.R., Newland J.J., Winters M.E., Mensah-Brown K., Boorady T.W., Xiao R., Nicolson S.C., Montenegro L.M., et al. Preoperative cerebral hemodynamics from birth to surgery in neonates with critical congenital heart disease. J. Thorac. Cardiovasc. Surg. 2018;156:1657–1664. doi: 10.1016/j.jtcvs.2018.04.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lynch J.M., Buckley E.M., Schwab P.J., McCarthy A.L., Winters M.E., Busch D.R., Xiao R., Goff D.A., Nicolson S.C., Montenegro L.M., et al. Time to surgery and preoperative cerebral hemodynamics predict postoperative white matter injury in neonates with hypoplastic left heart syndrome. J. Thorac. Cardiovasc. Surg. 2014;148:2181–2188. doi: 10.1016/j.jtcvs.2014.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghanayem N.S., Hoffman G.M. Near Infrared Spectroscopy as a Hemodynamic Monitor in Critical Illness. Pediatr. Crit. Care Med. 2016;17:S201–S206. doi: 10.1097/PCC.0000000000000780. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman G.M., Ghanayem N.S., Scott J.P., Tweddell J.S., Mitchell M.E., Mussatto K.A. Postoperative Cerebral and Somatic Near-Infrared Spectroscopy Saturations and Outcome in Hypoplastic Left Heart Syndrome. Ann. Thorac. Surg. 2017;103:1527–1535. doi: 10.1016/j.athoracsur.2016.09.100. [DOI] [PubMed] [Google Scholar]
- 21.Newburger J.W., Jonas R.A., Wernovsky G., Wypij D., Hickey P.R., Kuban K.C., Farrell D.M., Holmes G.L., Helmers S.L., Constantinou E., et al. A comparison of the perioperative neurologic effects of hypothermic circulatory arrest versus low-flow cardiopulmonary bypass in infant heart surgery. N. Engl. J. Med. 1993;329:1057–1064. doi: 10.1056/NEJM199310073291501. [DOI] [PubMed] [Google Scholar]
- 22.Bernal N.P., Hoffman G.M., Ghanayem N.S., Arca M.J. Cerebral and somatic near-infrared spectroscopy in normal newborns. J. Pediatr. Surg. 2010;45:1306–1310. doi: 10.1016/j.jpedsurg.2010.02.110. [DOI] [PubMed] [Google Scholar]
- 23.McNeill S., Gatenby J.C., McElroy S., Engelhardt B. Normal cerebral, renal and abdominal regional oxygen saturations using near-infrared spectroscopy in preterm infants. J. Perinatol. 2011;31:51–57. doi: 10.1038/jp.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zaleski K.L., Kussman B.D. Near-Infrared Spectroscopy in Pediatric Congenital Heart Disease. J. Cardiothorac. Vasc. Anesth. 2020;34:489–500. doi: 10.1053/j.jvca.2019.08.048. [DOI] [PubMed] [Google Scholar]
- 25.Mebius M.J., du Marchie Sarvaas G.J., Wolthuis D.W., Bartelds B., Kneyber M.C.J., Bos A.F., Kooi E.M.W. Near-infrared spectroscopy as a predictor of clinical deterioration: A case report of two infants with duct-dependent congenital heart disease. BMC Pediatr. 2017;17:79. doi: 10.1186/s12887-017-0839-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arnold D.M., Burns K.E., Adhikari N.K., Kho M.E., Meade M.O., Cook D.J., MSc (Epid) for the McMaster Critical Care Interest Group The design and interpretation of pilot trials in clinical research in critical care. Crit. Care Med. 2009;37:S69–S74. doi: 10.1097/CCM.0b013e3181920e33. [DOI] [PubMed] [Google Scholar]
- 27.Chakravarti S.B., Mittnacht A.J., Katz J.C., Nguyen K., Joashi U., Srivastava S. Multisite near-infrared spectroscopy predicts elevated blood lactate level in children after cardiac surgery. J. Cardiothorac. Vasc. Anesth. 2009;23:663–667. doi: 10.1053/j.jvca.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 28.Karaaslan P., Unlukaplan A., Vural G.B., Alkan B.T., Akcevin A. Cerebral perfusion correlations between NIRS and lactate levels during CPB in complex cardiac pathology children: 4AP3-9. Eur. J. Anaesthesiol. 2013;30:63. doi: 10.1097/00003643-201306001-00194. [DOI] [Google Scholar]
- 29.Karaaslan P., Unlukaplan A., Gokay B.V., Darçin K., H1zard1 B., Bozkaya T., Ozyuksel A., Akcevin A. Correlation between blood lactate and regional cerebral oxygen saturation in complex cardiac pathology neonates and infants: The effect on extubation time and ICU stay. Biomed. Res. 2017;28:3101–3107. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is contained within the article or Supplementary Materials.