Abstract
Aedes aegypti is the main vector of dengue globally. The variables that influence the abundance of dengue vectors are numerous and complex. This has generated a need to focus on areas at risk of disease transmission, the spatial-temporal distribution of vectors, and the factors that modulate vector abundance. To help guide and improve vector-control efforts, this study identified the ecological, social, and other environmental risk factors that affect the abundance of adult female and immature Ae. aegypti in households in urban and rural areas of northeastern Thailand. A one-year entomological study was conducted in four villages of northeastern Thailand between January and December 2019. Socio-demographic; self-reported prior dengue infections; housing conditions; durable asset ownership; water management; characteristics of water containers; knowledge, attitudes, and practices (KAP) regarding climate change and dengue; and climate data were collected. Household crowding index (HCI), premise condition index (PCI), socio-economic status (SES), and entomological indices (HI, CI, BI, and PI) were calculated. Negative binomial generalized linear models (GLMs) were fitted to identify the risk factors associated with the abundance of adult females and immature Ae. aegypti. Urban sites had higher entomological indices and numbers of adult Ae. aegypti mosquitoes than rural sites. Overall, participants’ KAP about climate change and dengue were low in both settings. The fitted GLM showed that a higher abundance of adult female Ae. aegypti was significantly (p < 0.05) associated with many factors, such as a low education level of household respondents, crowded households, poor premise conditions, surrounding house density, bathrooms located indoors, unscreened windows, high numbers of wet containers, a lack of adult control, prior dengue infections, poor climate change adaptation, dengue, and vector-related practices. Many of the above were also significantly associated with a high abundance of immature mosquito stages. The GLM model also showed that maximum and mean temperature with four-and one-to-two weeks of lag were significant predictors (p < 0.05) of the abundance of adult and immature mosquitoes, respectively, in northeastern Thailand. The low KAP regarding climate change and dengue highlights the engagement needs for vector-borne disease prevention in this region. The identified risk factors are important for the critical first step toward developing routine Aedes surveillance and reliable early warning systems for effective dengue and other mosquito-borne disease prevention and control strategies at the household and community levels in this region and similar settings elsewhere.
Keywords: Aedes aegypti; vector control; climate change; dengue; knowledge, attitudes, and practices (KAP); entomological indices
1. Introduction
Dengue is an emerging and re-emerging mosquito-borne viral disease of humans [1] that is caused by dengue viruses (DENV) from the Flavivirus genus with four serotypes: DENV 1–4. DENV is transmitted by bites of infected Aedes mosquitoes, specifically Aedes (Ae.) aegypti Linnaeus and Ae. albopictus Skuse, which are known as major and secondary dengue vectors and are also vectors of chikungunya, yellow fever, and Zika viruses [2,3]. The incidence of dengue has dramatically spread and increased globally in the past 40 years. Approximately half of the world’s population is at risk of contracting the disease, with an estimated 390 million infections occurring annually in 128 countries [4]. Dengue affects most of the world’s tropical and sub-tropical regions; Southeast Asia and the Western Pacific, in particular, have been seriously affected [5]. The disease is one of the main threats to public health and a leading cause of hospitalization in Thailand [6]. The first DENV infection was reported in 1949, the first outbreak was in 1958 [7,8], and several major outbreaks with high morbidity were documented in 1987, 1997, 1998, 2001, 2013, 2015, and 2019 across the country [9,10,11]. One of the largest dengue outbreaks in Thailand was in 1987 with 174,285 cases and 1008 deaths. According to the WHO, the country ranked sixth among the 30 most highly dengue-endemic countries in the world during 2004–2010 [12]. The Ministry of Public Health, Thailand, reported more than 72,000 dengue cases with a fatality rate of 0.13% in 2018. Recently, 71,292 dengue cases with 5151 deaths were reported in 2020 from the whole country, with all regions affected. The highest incidence rates (cases per 100,000 population) were found in the northeastern region [13]. All four DENV serotypes circulate in Thailand [9,14,15], and disease transmission is seasonal, with a peak in the rainy period from May to October [2].
The development and abundance of dengue vectors are affected by various ecological, socio-economic, and other environmental factors [14,15,16,17,18]. Ae. aegypti breeds in domestic water storage containers, which constitute major oviposition sites and an important risk factor for DENV transmission in Thailand [14]. Human-related factors that generate such artificial containers for larval development are important risk determinants of the distribution of dengue vectors. These include local socio-economic conditions, human habitats, and water storage practice-related behaviors. Socio-demographic factors also affect dengue vector production and transmission of DENV. For instance, the risk of DENV in Thailand was associated with people gaining at least a secondary education level and with households of more than four members [14,16]. It is well known that environmental factors influence diverse aspects of vector and virus biology by influencing mosquito population dynamics and virus circulation [19,20]. Furthermore, vector abundance and mosquito development vary seasonally because of local changes in temperature, humidity, and rainfall, all of which affect the availability of larval development sites and DENV transmission [21]. There have been several published studies on the climatic effect on vector abundance in Thailand [22,23,24,25,26,27,28], but there have been few detailed regional studies within Thailand.
The variables that influence the vector breeding and production of Aedes mosquitoes are numerous and complex [29]. Therefore, an in-depth understanding of fine-scale ecological, social, and other environmental risk factors that modulate vector abundance can provide vital information to fill in the gaps of our knowledge regarding the complex dynamics of dengue vectors and associated disease risk. In the absence of effective dengue treatment options and limitations of vaccines [30,31], updated information related to dengue vector abundance and associated risk factors is also essential for designing effective vector-control programs and dengue-prevention strategies. Understanding knowledge, attitudes, and practices (KAP) related to local climate change, dengue, and vector biology and control can identify perception gaps that can help guide new community engagement tools to enhance the effectiveness of vector-control efforts and disease awareness [32,33]. Notably, data on Aedes abundance and its risk factors are scanty in the northeastern region of Thailand despite it being a dengue-endemic area with a high population size and a land area proportional to the whole country. This study aimed to identify the spatiotemporal distributions and abundance of Ae. aegypti and to determine the associated predictors across urban and rural areas in northeastern Thailand.
2. Materials and Methods
2.1. Study Sites
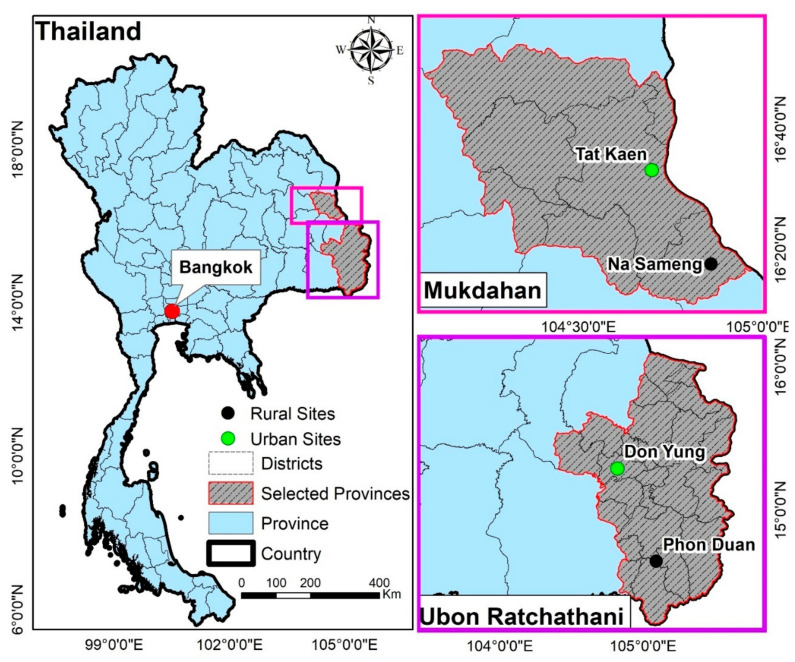
The study was conducted in four sites (two urban and two rural) in two provinces in northeastern Thailand. The selected sites were the urban Tat Khaen (16°33′18.4″ N, 104°42′01.2″ E) and rural Na Sameng (16°17′46.5″ N, 104°52′10.4″ E) in Mukdahan province and the urban Don Yung (15°17′32.9″ N, 104°49′11.8″ E) and rural Phon Duan (14°38′38.1″ N, 105°05′34.5″ E) in Ubon Ratchathani province (Figure 1). Details of the four study sites were previously described [33]. These sites were selected based on high dengue incidence during 2014–2018, feasibility, and logistics. The study area has a tropical climate with a dry season from October to April and a rainy season (dominated by the southwest monsoon) with high rainfall, high humidity, and high temperatures from May to September. DENV is mainly transmitted during the rainy season.
Figure 1.
Locations of the four data collection sites in northeastern Thailand. Data were collected from 128 households (32 households per site) in two urban and two rural study sites.
2.2. Study Design and Sample Size
The study was pursued within a larger project in Thailand and Laos (DENCLIM project; 2018–2021) that aims to evaluate the effects of climate change and variability on community vulnerability and exposure to dengue in the Southeast Asia. The total household sample size (90 households in each site) was calculated to be able to significantly detect differences in the number of dengue cases between urban and rural sites [33]. However, in this sub-study, 32 households were randomly selected for entomological, KAP, household, and climate data collection from each site, resulting in 128 households (64 urban and 64 rural). All selected households were given a unique identification number and geo-referenced using a global positioning system (GPS) device.
2.3. Entomological Survey
Monthly mosquito collections were carried out indoors and outdoors in the selected households in each study site from January to December 2019. The following data were collected: the number of adult mosquitoes (all genera and species), immature mosquitoes (larvae and pupae of all genera and species), the number of total containers, the number of wet containers, the number of mosquito-positive containers, and the characteristics of all positive containers. Adult mosquitoes were collected for 10 min indoors (in main rooms of activity, e.g., living rooms and bedrooms) and 10 min outdoors (among artificial articles, cars, motorcycles, vegetation, tree holes, roof gutters, etc.) in each household using battery-powered Prokopack aspirators [34]. Mosquito larvae and pupae were collected from larger containers (water volume of approximately >30 L) by the ‘five-sweep′ procedure using a fine-mesh hand screen and by a regular larval dipper or pipette from smaller containers (water volume approximately <30 L) [35]. Collected water samples were poured through a strain into white bowls for the better visualization, counting, and collecting of specimens [36]. The number of larvae and pupae was recorded in three categories (<10, 11–100, and >100). At least 20 larvae (if available) and all pupae were collected for further processing.
2.4. Mosquito Handling
Adult mosquitoes were stored in the aspirator collection cups in Styrofoam boxes and brought back to the laboratory. Mosquitoes from these samples were killed by freezing and then morphologically identified to Ae. aegypti, Ae. albopictus, Culex spp., or other (Armigeres spp./Anopheles spp.) using a stereomicroscope. Adult mosquitoes were sorted by sex and identified to species. Adults were stored individually in 1.5 mL micro-centrifuge tubes at −20 °C until further analysis. Immature mosquitoes were stored in separate, labeled, 100 mL Whirl-Pak plastic bags, and then they were recorded and transported to the entomology laboratory for specimen sorting and species identification. Immature Aedes were identified to species using morphological taxonomic keys [34,37].
2.5. Household and Demographic Survey
A cross-sectional survey was conducted in February 2019, and socio-demographic data were collected from household respondents of each selected household using a structured questionnaire. In addition to the semi-structured interviews, observations were made, and household information—including household characteristics (house condition, yard condition, shade condition, house type, and house materials), water management (water, hygiene, and sanitation facilities), and vector-control practices (adult and larvae control)—was recorded. The household survey also measured household wealth using a set of questions on durable asset ownership. This information was used to generate a measure of socio-economic status (SES). Housing density around sample households was calculated by counting houses within a 200-m buffer using Google Earth.
2.6. Knowledge, Attitudes and Practices Survey
Data on participants′ KAP regarding climate change and dengue were used from a broader collection of KAP surveys described in our previous study [33]. Data on climate change included knowledge about its connection to dengue, as well as local and global climate change problems, attitudes, and adaptation and mitigation practices. Data on dengue included knowledge on transmission, symptoms, and signs; most frequent bite time of mosquitoes; vector morphology and vector breeding sites; attitudes and prevention practices, such as bite prevention practices; and Aedes breeding prevention methods.
2.7. Climate Data
Weather stations were set up at the beginning of the project in three study sites located within 1–2 km from inspected houses. Meteorological data in Tat Khaen were obtained from a nearby meteorological station of the Department of Meteorology in Mukdahan (16°32′57.6″ N, 104°42′15.4″ E 16°32′ N, 104°43′ E). Meteorological data including daily maximum, minimum, and total rainfall (mm); maximum and minimum temperatures (°C); and relative humidity (%) were collected from the weather stations during the study period and aggregated at the weekly level.
2.8. Data Management and Statistical Analysis
The entomological indices calculated in this study were the house index (HI) (percentage of houses infested with larvae and/or pupae), container index (CI) (percentage of water-holding containers infested with larvae and/or pupae), Breteau index (BI) (number of positive containers per 100 houses inspected), and pupae index (PI) (number of pupae per 100 houses inspected) [36,38]. The characteristics of the most productive container types producing >60% of all pupae were also calculated. The household crowding index (HCI) was calculated using the total number of household residents (excluding newborn infants) at each monthly visit divided by the total number of rooms, excluding kitchen and bathrooms. The HCI was grouped into three categories: <1, 1–2, and >2 residents per room [39,40] (Table 1). The premise condition index (PCI) was estimated for each household and classified based on the general condition of the house, the surrounding yard area, the degree of shade, and the water management systems [41,42]. The PCI could take on a minimum value of 4 (good condition) and a maximum of 9 (bad condition) (Table 1). The SES of selected households was ranked as poor, intermediate, or wealthy. A detailed description of the method for constructing SES was provided in a previously published paper [33] (Table 1). The KAP of the 128 household respondents were assessed using the same scoring system published in our previous study [33] based on the total correct responses against the total questions (questions and summary answers shown in Additional Files 1–6: Tables S1–S6).
Table 1.
Variables used in the premise condition index (PCI), household crowding index (HCI,) and socio-economic status (SES).
| Index | Variables | Description | Classification Score |
|---|---|---|---|
| Premise condition index (PCI) | House condition | Good (well-maintained, e.g., newly painted or new house) | 1 |
| Intermediate (moderately well-maintained house) | 2 | ||
| Bad (not well-maintained house, e.g., paint peeling, broken items visible, and dilapidated old house) | 3 | ||
| Yard condition | Good (tidy yard) | 1 | |
| Intermediate (moderately tidy yard) | 2 | ||
| Bad (untidy yard) | 3 | ||
| Shade condition | Not shaded (very little or no shade) | 1 | |
| Intermediate (some shade: >25% but <50%) | 2 | ||
| Shady (plenty of shade: >50%) | 3 | ||
| Water supply and storage |
Piped water | 1 | |
| Ground water/well water supply | 2 | ||
| Rainwater and/or open water source: river/stream/lake/mountain water/river water | 3 | ||
| Household crowding index (HCI) | Co-residents | Monthly number of co-residents per household | - |
| Number of rooms | Number of rooms per household | - | |
| Socio-economic status (SES) | House roof material | Ceramic/Wood/Metal | - |
| House walls material | Plastered/cement/bricks/wood | - | |
| Ownership of durable assets |
television/VCD/refrigerator/washing machine/mobile/smartphone/computer/oven/microwave/airconditioner/car/pickup/motorcycle | - | |
| Ownership of toilet facility | Yes/no | - | |
| Toilet/bathroom floor material |
Tiles/cement/earth | - | |
| Ownership of flush toilet/squat toilet |
Yes/no | - |
Descriptive statistical analyses of mosquito collections, species composition, container characteristics, entomological indices, socio-demographics, KAP, and meteorological variables were conducted. Generalized linear models (GLMs) were fitted to investigate the association of socio-demographics, KAP, and household risk factors with the abundance of adult female and immature Ae. aegypti per household. The lag effect (0–4 weeks) of climatic factors (i.e., minimum, mean, and maximum temperatures, relative humidity, and rainfall) on both adult female and immature Ae. aegypti indices were also investigated using GLMs, assuming a negative binomial distribution with the logarithmic link function. The incidence risk ratio (IRR) was also calculated and adjusted using multivariable analysis. A negative binomial distribution was used since the response variables were over-dispersed count data (adult female Ae. aegypti per household (variance = 47.2, mean = 8.3), adult female Ae. aegypti per month (variance = 2261.9, mean = 88.8), immature Ae. aegypti per household (variance = 414.1, mean = 17.2), and immature Ae. aegypti per month (variance = 8368.8, mean = 1849)) [43]. Statistical analyses were performed using RStudio with the “MASS” package [44]. Figures were produced using the “carData,” “effects,” “ggplot2,” and “ggpbur” packages [45,46]. Maps of each study sites were created to visualize the number of adults and immature Aedes mosquitoes collected from each household over the study period.
3. Results
3.1. Entomological Collections and Indices
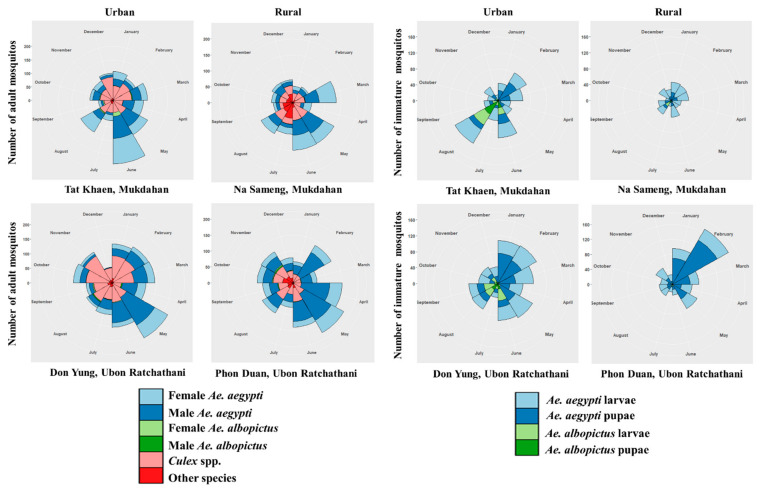
A total of 5273 adult mosquitoes were collected. The most abundant species were Ae. aegypti 2658 (50.5%), followed by Culex spp. 1979 (37.5%) and others 561 (10.6%). Ae. albopictus 75(1.4%) was the least abundant. Among the 1113 female Aedes spp. (40.7% of the total Aedes collected), 1066 (95.8%) were Ae. aegypti. An overall monthly mean of 45.9 Ae. aegypti females was collected in the urban sites, and a monthly mean of 42.9 was collected in the rural sites (Table 2).
Table 2.
Number of mosquitoes caught in 128 households in two urban and two rural study sites during monthly collections in northeastern Thailand during January–December 2019.
| Species/Stage | Total Number (%) | Monthly Range, n | Monthly Mean ± SD | |||
|---|---|---|---|---|---|---|
| Urban | Rural | Urban | Rural | Urban | Rural | |
| Adult Ae. aegypti | ||||||
| Female | 551 (18.8) | 515 (21.8) | 6–110 | 23–81 | 45.9 ± 28.2 | 42.9 ± 19.5 |
| Male | 823 (28.2) | 769 (32.7) | 11–166 | 34–153 | 64.0 ± 47.3 | 68.5 ± 36.4 |
| Adult Ae. albopictus | ||||||
| Female | 36 (1.3) | 11 (0.4) | 0–17 | 0–3 | 3.0 ± 4.6 | 0.9 ± 0.9 |
| Male | 16 (0.6) | 12 (0.5) | 0–5 | 0–4 | 1.3 ± 1.6 | 1.0 ± 1.1 |
| Culex spp. | 1302 (44.6) | 677 (28.8) | 63–149 | 40–83 | 108.5 ± 26.5 | 56.4 ± 13.6 |
| Other species | 191 (6.5) | 370 (15.8) | 6–27 | 6–54 | 15.9 ± 5.9 | 30.8 ± 19 |
| Total adult mosquitoes | 2919 (100) | 2354 (100) | 0–166 | 0–153 | 39.8 ± 45.8 | 31.6 ± 27.9 |
| Immature Ae. aegypti | ||||||
| Larvae | 647 (42.7) | 525 (48.8) | 33–75 | 23–65 | 53.9 ± 14.4 | 43.7 ± 11.3 |
| Pupae | 543 (35.8) | 495 (46.0) | 16–112 | 10–148 | 16.4 ± 17.1 | 3.0 ± 3.8 |
| Immature Ae. Albopictus | ||||||
| Larvae | 197 (12.9) | 37 (3.4) | 0–50 | 0–14 | 45.2 ± 31.7 | 41.2 ± 39.3 |
| Pupae | 131 (8.6) | 19 (1.8) | 0–57 | 0–10 | 10.9 ± 16.3 | 1.5 ± 2.7 |
| Total immature mosquitoes |
1518 (100) | 1076 (100) | 0–112 | 0–148 | 33.4 ± 32.2 | 22.4 ± 28.8 |
SD: standard deviation.
Urban sites had higher mean numbers of Ae. aegypti mosquitoes—all immature and pupae—than the rural sites. The highest numbers of adult Ae. aegypti (1374) and Ae. albopictus (52), including both females and males, were collected in the urban sites (Table 2); the numbers collected during the wet season (May–October) were 1516 and 51, respectively (Figure 2). During the 12 months of collections, a cumulative total of 856 out of 9399 inspected water-holding containers were found positive for immature mosquitoes. There were 2594 Aedes immature mosquitoes, of which 2210 (85%) were identified as Ae. aegypti and 384 (15%) as Ae. albopictus (Table 2). The highest number of immature mosquitoes (1415) was collected during the dry season (March–April), and the lowest number (1179) was collected during the wet season (Figure 2 and Figure 3). The corresponding numbers for urban and rural sites were 1518 (58.5%) and 1076 (41.5%), respectively.
Figure 2.
Monthly distribution of mosquitoes caught in a total of 128 households in two urban and two rural study sites in northeastern Thailand during January–December 2019.
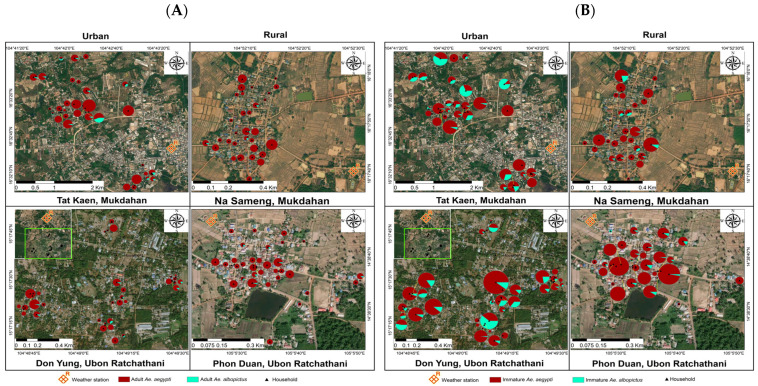
Figure 3.
Household distribution of Aedes mosquitoes caught in 128 households in two urban and two rural study sites in northeastern Thailand during January–December 2019. The green box represents the inset map of study sites: (A) adult mosquitoes; (B) immature mosquitoes.
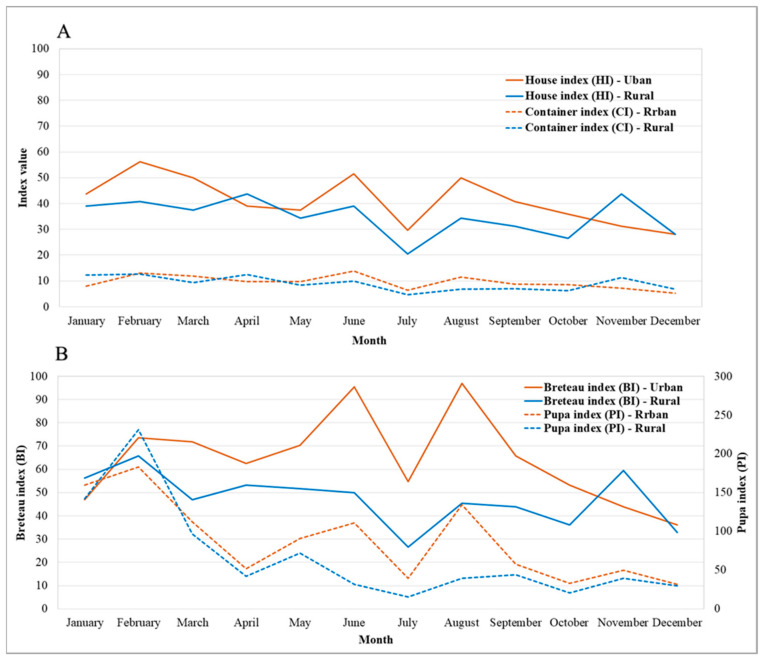
Entomological indices (HI, CI, BI, and PI) were found to be higher in urban sites than rural sites (Figure 4). The overall figures of entomological indices during January–December 2019, recorded for HI, CI, BI, and PI were urban = 41.2% and rural = 34.9%, urban = 9.4% and rural = 8.8%, urban = 64.2 and rural = 47.3, and urban = 87.8 and rural = 67.0, respectively.
Figure 4.
Entomological indices (HI, CI, BI, and PI) in urban and rural study sites in northeastern Thailand during January–December 2019.
3.2. Container Characteristics and Breeding of Dengue Vectors
Of the 856 positive containers found in both urban and rural sites during the 12 collection months of 2019, 75% of immature Aedes (pupae and larvae) were collected in round-shaped containers (jar, bucket, etc.), 14% were collected in square-shaped containers (cemented tank, flower vase/pots, etc.), and 11% were collected in other types of breeding sites (Table 3). Medium-sized (50–100 cm) containers were the highest infested containers in both urban and rural areas. Containers with any type of mosquito control were less infested with Aedes larvae (urban: 87%; rural: 81%) and pupae (urban: 90%; rural: 86%) than those without control. Containers treated with abate were the least infested containers. Uncovered and outdoor containers were the highest contributors to dengue vector breeding in both areas compared to well-covered and indoor containers.
Table 3.
Number of immature Aedes mosquitoes (%) collected in containers in 128 households in two urban and two rural study sites in northeastern Thailand during January–December 2019.
| Larvae | Pupae | Total | ||||
|---|---|---|---|---|---|---|
| Container Characteristics | Description | Urban | Rural | Urban | Rural | |
| Shape of container | Square (Cemented tank, flower vase/pots) | 27 (7) | 66 (24) | 7 (6) | 21 (26) | 121 (14) |
| Round (jar, bucket, etc.) | 292 (79) | 198 (71) | 99 (79) | 54 (66) | 643 (75) | |
| Other (tree holes, bamboo, ant traps, solid waste, etc.) | 51 (14) | 15 (5) | 19 (15) | 7 (8) | 92 (11) | |
| Size of container | Small (<50 cm) | 161 (44) | 97 (35) | 56 (45) | 31 (38) | 345 (40) |
| Medium (50–100 cm) | 200 (54) | 172 (61) | 66 (53) | 46 (56) | 484 (57) | |
| Large (>150 cm) | 9 (2) | 10 (4) | 3 (2) | 5 (6) | 27 (3) | |
| Container Cover | Good | 12 (3) | 3 (1) | 2 (2) | 2 (2) | 19 (2) |
| Poorly fitted | 34 (9) | 15 (5) | 14 (11) | 7 (9) | 70 (8) | |
| None | 324 (88) | 261 (94) | 109 (87) | 73 (89) | 767 (90) | |
| Location | Indoor | 120 (32) | 129 (46) | 37 (30) | 42 (51) | 328 (38) |
| Outdoor | 250 (68) | 150 (54) | 88 (70) | 40 (49) | 528 (62) | |
| In toilet or not | In toilet | 108 (29) | 165 (59) | 35 (28) | 42 (51) | 350 (41) |
| Not in toilet | 262 (71) | 114 (41) | 90 (72) | 40 (49) | 506 (59) | |
| Larval control types | Abate | 20 (5) | 12 (4) | 5 (4) | 4 (5) | 41 (5) |
| Larval control washed in last week | 28 (8) | 42 (15) | 8 (6) | 7 (9) | 85 (10) | |
| No larvae control | 322 (87) | 225 (81) | 112 (90) | 71 (86) | 730 (85) | |
3.3. Knowledge, Attitudes, and Practices on Climate Change
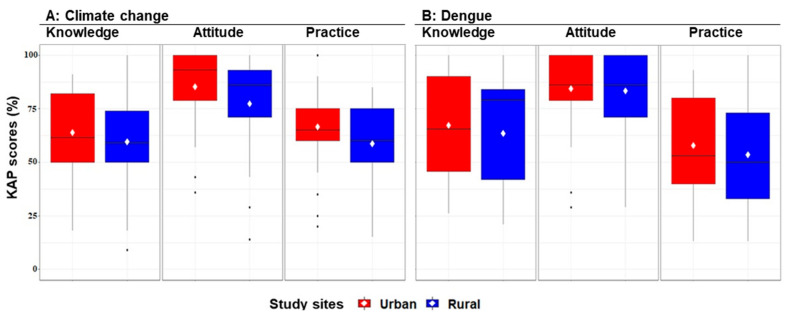
Almost all of the study respondents reported having heard about climate change (95% and 90% for urban and rural sites, respectively). More than 60% of the participants in both urban and rural sites believed that changes in climate can affect dengue fever and its vectors. However, study communities in both areas had limited knowledge and awareness about local, global, and possible future effects of climate change, as well as regarding the topics of global climate change and changing mosquito habitat suitability (Additional File 1: Table S1). More than 90% of the study respondents had a positive attitude to receiving updated information about the impacts of climate change and the mitigation of dengue risk (Additional File 2: Table S2). Only a few respondents (28% and 45% for urban and rural, respectively) took additional actions to prepare to adapt to the impact of climate change (e.g., floods, droughts, and storms) and to reduce dengue risk (Additional File 3: Table S3). Most of the respondents used some form of climate-resilient household practices regarding the spread of dengue due to climate change. The most common best practices among respondents included cleaning up drainage systems from waste (68.8% and 54.7% for urban and rural, respectively). Urban respondents had overall good level of KAP on climate change, with K = 28%, A = 67%, and p = 23%; the corresponding figures for rural respondents were K = 20%, A = 56%, and p = 13%. Detailed results are presented in Additional Files 1–3: Tables S1–S3.
3.4. Knowledge, Attitudes, and Practices on Dengue
The study respondents had limited knowledge of the transmission, symptoms, and warning signs of DENV and dengue disease (Additional File 4: Table S4). Less than 50% of the study respondents knew about DENV serotypes and the name of dengue vectors (Ae. aegypti and Ae. albopictus). Regarding vector morphology, few respondents (53% and 23% in urban and rural areas, respectively) knew that Aedes mosquitoes have white spots on their legs. However, more than 80% of the respondents knew that these mosquitoes are daytime biters. For dengue risk mitigation, the majority showed a positive attitude of requiring improved awareness and knowledge, as well as more educational programs on symptoms and treatments of dengue, including additional training on vector-control strategies (Additional File 5: Table S5). In both areas, dengue-prevention practices related to Aedes breeding sites and steps to prevent mosquito breeding during an outbreak were not satisfactory (Additional File 6: Table S6). The most common best practices among respondents included preventing mosquito–human contact followed by covering and protecting skin with clothes, using window screens and bed nets, disposing water-holding containers, covering water containers, and using insecticide sprays to reduce mosquitoes. The overall percentages of the population with a good level of KAP on dengue in urban areas were K = 45%, A = 72%, and P = 30%, and the corresponding figures for rural areas were K = 40%, A = 65%, and P = 23%. Detailed results are presented in Additional Files 4–6: Tables S4–S6.
Overall, KAP regarding climate change and dengue were low in urban and rural sites (KAP scores considered were good if ≥80 and poor if <80). Urban residents had higher mean KAP scores regarding climate change and dengue than rural residents (Figure 5), but there were no significant differences regarding KAP level between the two sites (Additional Files 1–6: Tables S1–S6).
Figure 5.
Boxplots showing the percentage and mean scores of knowledge, attitudes, and practices (KAP) in a total of 128 households in urban and rural study sites in northeastern Thailand during February–April 2019. Maximum scores are 100 for each KAP component.
3.5. Ecological and Social Determinants of the Abundance of Adult Female and Immature Ae. aegypti
Urban sites had a significantly higher abundance of adult female Ae. aegypti (IRR: 1.55; 95% confidence interval (CI): 1.21–1.98) and immature Ae. aegypti (IRR: 1.47; 95% CI: 1.01–2.17) than rural sites. Educational level was significantly associated with the abundance of both adult female and immature Ae. aegypti. The houses of respondents who had lower education levels (only primary school) were more likely to be infested with adult female (IRR: 1.39; 95% CI: 1.07–1.79) and immature (IRR: 1.49; 95% CI: 1.02–2.18) Ae. aegypti than the houses of respondents with primary education or higher. Poor households (lower SES) were found to be significantly associated with a higher abundance of immature Ae. aegypti (IRR: 2.15; 95% CI: 1.39–3.32) than wealthy households. Crowded (HCI > 3) and medium crowded (HCI > 2) households were significantly associated with a higher abundance of adult female Ae. aegypti, but results for immature mosquitoes were not as clear (Table 4). A higher PCI was also significantly associated with a higher abundance of both adult female (IRR: 1.97; 95% CI: 1.49–2.61) and immature (IRR: 1.54; 95% CI: 1–2.37) Ae. aegypti.
Table 4.
Incidence rate ratios (IRRs) for the abundance of Ae. aegypti per household in relation to socio-demographic and household risk factors using negative binomial generalized linear models. Data were collected from 128 households in northeastern Thailand during January–December 2019.
| Female Adults | Immatures | ||||
|---|---|---|---|---|---|
| Variables | n (%) | IRR (95% CI) | p-Value | IRR (95% CI) | p-Value |
| Sites types | |||||
| Urban | 64 (50) | 1.55 (1.21–1.98) | 0.000 | 1.47 (1.01–2.17) | 0.042 |
| Rural | 64 (50) | 1 | 1 | ||
| Education level | |||||
| <=Primary | 89 (69.5) | 1.39 (1.07–1.79) | 0.011 | 1.49 (1.02–2.18) | 0.032 |
| >Primary | 39 (30.5) | 1 | 1 | ||
| Socio-economic status | |||||
| Poor | 36 (28.1) | 0.94 (0.72–1.23) | 0.693 | 2.15 (1.39–3.32) | 0.001 |
| Intermediate | 52 (40.6) | 1.03 (0.81–1.3) | 0.801 | 1.63 (1.1–2.43) | 0.015 |
| Wealthy | 40 (31.3) | 1 | 1 | ||
| Household crowding index (HCI) | |||||
| 3 (Crowded) | 31 (24.2) | 1.76 (1.27–2.43) | 0.001 | 0.75 (0.46–1.23) | 0.263 |
| 2 (Medium crowded) | 65 (50.8) | 1.58 (1.22–2.05) | 0.000 | 0.50 (0.34–0.74) | 0.001 |
| 1 (Not crowded) | 32 (25.0) | 1 | 1 | ||
| Premise condition index (PCI) | |||||
| 9–10 (High) | 41 (32.0) | 1.97 (1.49–2.61) | 0.000 | 1.54 (1–2.37) | 0.043 |
| 7–8 (Medium) | 46 (36.0) | 1.20 (0.91–1.59) | 0.179 | 1.07 (0.72–1.59) | 0.730 |
| 5–6 (Low) | 41 (32.0) | 1 | 1 | ||
| House density (houses per km2) |
|||||
| 100–200 | 8 (6.3) | 1.29 (0.83–1.99) | 0.246 | 0.67 (0.34–1.34) | 0.267 |
| 201–500 | 38 (29.7) | 1.37 (1.05–1.8) | 0.021 | 0.84 (0.55–1.29) | 0.433 |
| 501–1000 | 47 (36.7) | 1.19 (0.92–1.55) | 0.167 | 1.49 (1.02–2.18) | 0.038 |
| >1000 | 35 (27.3) | 1 | 1 | ||
| House type | |||||
| Single house, one family, two floors |
64 (50) | 1.009 (0.82–1.22) | 0.931 | 1.27 (0.91–1.77) | 0.146 |
| Single house, one family, one floor |
64 (50) | 1 | 1 | ||
| Roof materials type | |||||
| Metal (e.g., corrugated iron) |
97 (75.8) | 1.14 (0.83–1.58) | 0.400 | 0.89 (0.54–1.46) | 0.659 |
| Wood | 17 (13.3) | 1.03 (0.65–1.61) | 0.889 | 0.57 (0.28–1.16) | 0.125 |
| Ceramic | 14 (10.9) | 1 | 1 | ||
| Wall type | |||||
| Wood | 12 (9.4) | 1.11 (0.79–1.57) | 0.530 | 1.68 (1.01–2.81) | 0.045 |
| Cement/bricks | 33 (25.8) | 0.95 (0.76–1.18) | 0.652 | 1.48 (1.05–2.09) | 0.022 |
| Plastered | 83 (64.8) | 1 | 1 | ||
| Location of bathroom/toilet | |||||
| Indoors | 91 (71.1) | 1.46 (1.14–1.89) | 0.003 | 1.01 (0.68–1.5) | 0.925 |
| Outdoors | 37 (28.9) | 1 | 1 | ||
| Bathroom floor type | |||||
| Cement | 54 (42.2) | 1.15 (0.91–1.47) | 0.221 | 0.73 (0.5–1.07) | 0.109 |
| Tiles | 74 (57.8) | 1 | 1 | ||
| Eaves status | |||||
| Closed | 79 (61.7) | 1.14 (0.92–1.42) | 0.201 | 0.94 (0.67–1.3) | 0.715 |
| Opened | 49 (38.3) | 1 | 1 | ||
| Windows | |||||
| Unscreened | 107 (83.6) | 1.41 (1.04–1.92) | 0.025 | 1.65 (1.05–2.6) | 0.029 |
| Screened | 21 (16.4) | 1 | 1 | ||
| Number of wet container |
|||||
| >50 | 109 (85.2) | 1.33 (1.01–1.75) | 0.037 | 1.41 (0.92–2.16) | 0.112 |
| <50 | 19 (14.8) | 1 | 1 | ||
| Use any kind of larvae control | |||||
| No | 93 (72.7) | 0.93 (0.75–1.14) | 0.503 | 1.20 (0.84–1.7) | 0.300 |
| Yes | 35 (27.3) | 1 | 1 | ||
| Use any kind of adult control | |||||
| No | 72 (56.2) | 1.24 (1.01–1.55) | 0.045 | 1.13 (0.79–1.61) | 0.496 |
| Yes | 56 (43.8) | 1 | 1 | ||
| Self-reported dengue infections | |||||
| Yes | 12 (9.4) | 1.68 (1.22–2.32) | 0.001 | 0.79 (0.46–1.35) | 0.398 |
| No | 116 (90.6) | 1 | 1 | ||
| Climate change knowledge | |||||
| Poor | 97 (75.8) | 0.87 (0.61–1.24) | 0.451 | 1.97 (1.19–3.25) | 0.008 |
| Good | 31 (24.2) | 1 | 1 | ||
| Climate change attitude | |||||
| Poor | 49 (38.3) | 0.97 (0.79–1.19) | 0.801 | 0.71 (0.52–0.99) | 0.043 |
| Good | 79 (61.7) | 1 | 1 | ||
| Climate change practice | |||||
| Poor | 105 (82.0) | 1.52 (1.07–2.16) | 0.017 | 1.84 (1.13–2.99) | 0.014 |
| Good | 23 (18.0) | 1 | 1 | ||
| Dengue knowledge | |||||
| Poor | 73 (57.0) | 0.91 (0.7–1.17) | 0.491 | 1.18 (0.8–1.75) | 0.391 |
| Good | 55 (43.0) | 1 | 1 | ||
| Dengue attitude | |||||
| Poor | 40 (31.3) | 1.24 (1.01–1.53) | 0.035 | 0.75 (0.54–1.04) | 0.092 |
| Good | 88 (68.8) | 1 | 1 | ||
| Dengue practice | |||||
| Poor | 94 (73.4) | 1.43 (1.03–1.99) | 0.029 | 1.93 (1.17–3.18) | 0.009 |
| Good | 34 (26.6) | 1 | 1 | ||
| Model fit | |||||
| Omnibus test | 131.2 | 0.000 | 957.2 | 0.000 | |
| AIC | 719.2 | 935.3 | |||
| BIC | 810.5 | 1026.6 |
CI: confidence interval; SD: standard deviation; AIC: Akaike′s information criterion; BIC: Bayesian information criterion.
Houses located in clusters with a medium housing density (201–500 houses per km2) were more significant predictors for adult females (IRR: 1.37; 95% CI: 1.05–1.8) compared to high density settings (>1000 houses per km2), whereas for immature mosquitoes, this occurred in clusters with 501–1000 houses per km2 (IRR: 1.49; 95% CI: 1.02–2.18). Houses with unscreened windows were found to be significantly associated with a higher abundance of both mosquito stages. Houses with the bathroom located indoors, with higher numbers of water-filled containers (regardless of being mosquito-positive or not) on their premises, and without any adult control interventions were more likely than not to be infested with adult female Ae. aegypti. Poor climate change adaptations and dengue preventive practices (p < 0.05) were significantly associated with higher abundances of both mosquito stages (Table 4).
3.6. Climatic Determinants of the Abundance of Adult Females and Immature Ae. aegypti
During the study period (from January to December 2019), mean monthly temperatures ranged from 23.2 to 32.6 °C and from 22.7 to 31.0 °C in urban and rural sites, respectively. The mean relative humidity levels in the urban and rural sites were 73.5% and 76.3%, respectively. The relative humidity varied between 58.0% and 88.1% in urban sites and between 60.9% and 88.7% in rural sites. The recorded mean total rainfall values in the urban and rural sites were 102.9 and 166.5 mm, respectively. The total rainfall varied between 0 and 803.3 mm in urban sites and between 0 and 1229.4 mm in rural sites (Table 5).
Table 5.
Monthly climate variables in urban and rural sites in northeastern Thailand during January–December 2019.
| Meteorological Variables | Range (n) | Mean ± SD | ||
|---|---|---|---|---|
| Urban | Rural | Urban | Rural | |
| Mean temperature (°C) | 23.2–32.6 | 22.7–31.0 | 28.0 ± 2.3 | 27.2 ± 2.2 |
| Minimum temperature (°C) | 13.2–26.7 | 9.6–23.7 | 21.6 ± 3.7 | 19.1 ± 3.9 |
| Maximum temperature (°C) | 31.0–41.8 | 33.0–41.8 | 35.5 ± 3.0 | 37.0 ± 2.6 |
| Relative humidity (%) | 58.0–88.1 | 60.0–88.7 | 73.5 ± 8.1 | 76.3 ± 8.2 |
| Total rainfall (mm) | 0–803.3 | 0–1229.4 | 102.9 ± 173.3 | 166.5 ± 254.7 |
SD: standard deviation.
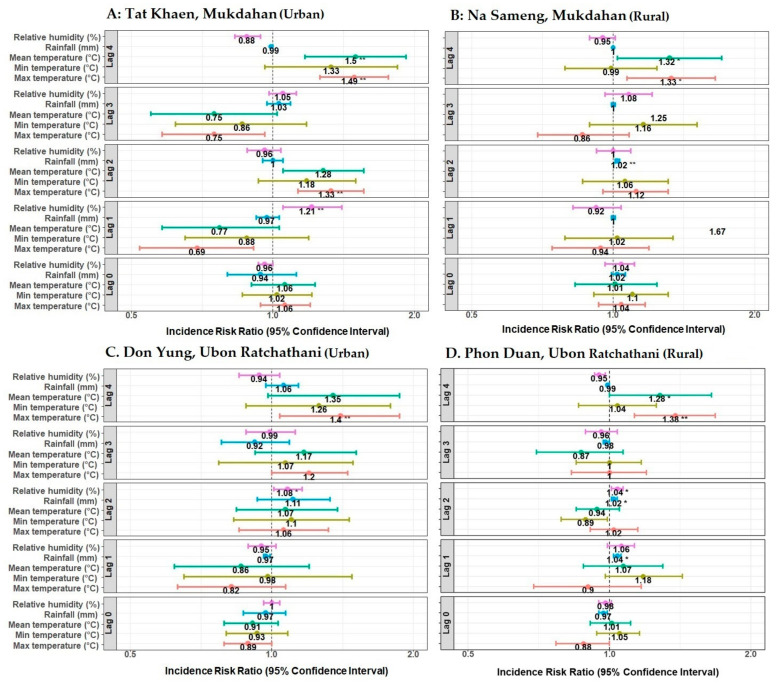
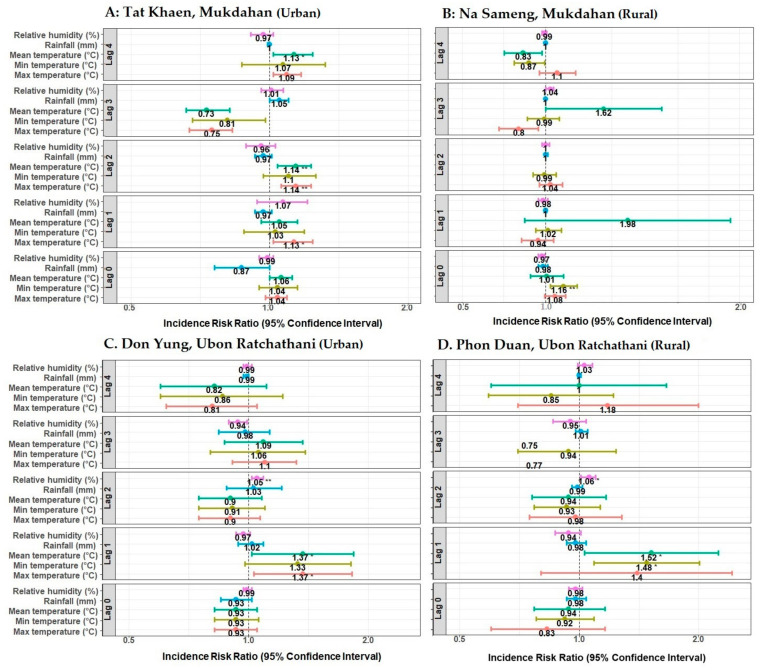
There was a considerable variation in the climate results. Though most of the relationships were non-significant, the mean and maximum temperature at four weeks of lag seemed to be generally more significant for adult mosquitoes than fewer-week lags (Figure 6). Higher temperatures were also found to be related to immature numbers at more recent times near the collection event (Figure 7). Detailed results for significant associations of climate variables at different week lags with the abundance of adult and immature mosquitoes are presented in Figure 6 and Figure 7.
Figure 6.
Effect of climate variables on the abundance of adult female Ae. aegypti mosquitoes in northeastern Thailand during January–December 2019. Incidence risk ratios were computed by negative binomial generalized linear models. Each panel shows the lag effects (0–4 weeks) of maximum, minimum, and mean temperature (°C); total rainfall (mm); and relative humidity (%). * p-value ≤ 0.05, ** p-value ≤ 0.01. Each panel (A–D) represents selected urban and rural study sites.
Figure 7.
Effect of climate variables on the abundance of immature Ae. aegypti mosquitoes in northeastern Thailand during January–December 2019. Incidence risk ratios were computed by negative binomial generalized linear models. Each panel shows the lag effects (0–4 weeks) of maximum, minimum, and mean temperature (°C); total rainfall (mm); and relative humidity (%). * p-value ≤ 0.05, ** p-value ≤ 0.01. Each panel (A–D) represents selected urban and rural study sites.
4. Discussion
The present study investigated the spatial-temporal abundance of dengue vectors and determinants of their prevalence in the selected study areas of northeastern Thailand. Ae. aegypti was a more abundant dengue mosquito species than Ae. albopictus. Ae. aegypti is the main dengue vector in Thailand and is well-adapted to human dwellings and their immediate surroundings [47]. The abundance of adult and immature Ae. aegypti was higher in urban study sites and mostly in the wet season (May–October). The higher abundance of Ae. aegypti in urban settings could be explained by differences in container characteristics and domestic water management [17,48]. Consistent with earlier studies, our study also found that there were more potential breeding containers and containers positive for Aedes vectors in urban areas during the wet monsoon season [29]. The predominant breeding sites in our urban sites were also a high number of containers (e.g., tires and discarded containers), while in rural sites, Ae. aegypti displayed behavioral plasticity in that the females lay eggs in a vast array of containers, including water storage containers and flower pots. Other risk factors might be construction sites in urban areas, where Ae. aegypti seems to be well-suited for reproduction, thus increasing the abundance of breeding sites and density at the neighborhood level [49]. Dengue transmission in Thailand is highly seasonal, with the highest incidence occurring during the rainy season [50]. This may account for the high proportion of houses with water-storage containers found positive for immature Aedes mosquitos. Different types of wet containers produced variable numbers of immature Aedes throughout the study. Round-shaped and medium-size containers were observed to produce the highest number of Aedes larvae and pupae. Water storage jars and tanks are the most commonly used containers in Thailand [17]. Participants use plastic drums, plastic buckets, and water tanks to store water from supplied piped water. Houses that used adult and larval control methods were found to be less infested with female adult and immature Ae. aegypti than houses that did not. Outdoor containers showed a higher contribution to dengue vector breeding than indoor containers. Significant numbers of outdoor containers have been reported to be positive with immature Aedes in previous studies conducted in Thailand and other countries [17,51,52,53,54]. As people become more aware of the potential oviposition sites of Aedes mosquitoes, they usually check and clean the indoor containers located inside their households. Consequently, vectors tend to shift to outdoor containers. This dichotomous treatment behavior was clearly observed in the present study. Such adaptive behavior of Aedes mosquitoes poses a severe challenge to vector-control efforts [55,56].
In the present study, most of the sampled sites had high entomological index values indicative of the risk of dengue outbreaks [57,58]. The entomological indices were observed to be higher in the urban areas relative to rural sites, indicating that urban areas are potentially more exposed to dengue risk. There may be a greater risk of dengue infection in urban areas than in rural areas because of the higher population density. Indeed, despite people living in urbanized areas people having better jobs and socio-economic conditions, a greater risk of dengue infections has been reported [59]. In contrast, other studies have found a higher risk of DENV transmission in poorer settings [60,61]. Such associations may depend on the proximity of individuals to risk factors regardless of their level of wealth. The level of education matters, as it is associated with a greater understanding of the principles of hygiene in water and food storage. Traditionally, entomological indices such as the HI, BI, CI, and PI are the chief surveillance tools of many vector-control programs in dengue-endemic countries worldwide [62]. These indices not only measure the success of vector-control strategies but also help to understand the vector ecology. However, the quantifiable association between vector indices and risk of DENV transmission has been questioned in several studies [36,62].
In this study, several suites of socio-ecological factors were associated with mosquito abundance. These findings could be readily interpreted and used to inform the design and implementation of targeted vector-control campaigns that reflect local social-ecological contexts. This study found that poor education was related to higher dengue vector infestation. Previous studies in Thailand found an association between risk of dengue and poor education level [16]. Poor climate change knowledge, practices, and attitudes about dengue were associated with higher abundances of Ae. aegypti. These findings highlight the importance of educating target populations who have poor education level and KAP on climate change and dengue. This study found a higher ratio of television and internet users among study participants who used such sources of information for climate and dengue. Social media, such as Facebook, Line, and Instagram, were especially used. To increase public awareness regarding the use of preventative measures, we advocate the use of social media and television to disseminate helpful information about adaptation and mitigation measures for climate change, as well as for monitoring and preventing dengue [33,34]. This could be a part of the government vector-control strategy that may benefit from a shift from reactive to proactive vector control. To improve the understanding of community needs and comprehension about dengue and climate, KAP should comprise a key surveillance component of any vector-control program. This allows for the development of culturally appropriate information, trust with community members, and improvements in vector-control activities through active community engagement [63].
A greater HCI was also indicative of a greater abundance of Ae. aegypti mosquitoes. Household crowding likely reflects the greater risk of exposure to infectious mosquito bites. This finding was consistent with those of other studies, both in Thailand [16] and elsewhere [64]. Our study also suggests that the HCI may be associated with higher densities of population, houses, and water storage containers; distances between houses; average tree height; and average percentage of vegetation cover for each house in urban sites compared to rural sites [48,65]. The PCI was significantly associated with both adult female and immature Ae. aegypti. Similar positive associations in Mexico and Brazil between the PCI and immature Aedes clearly showed the usefulness of the method [66,67]. Positive correlations between the PCI and house positivity for larvae, pupae, and adult A. aegypti led authors to advocate the Brazilian Dengue Control Program for the use of the PCI to schedule the vector-control teams′ visits at different frequencies based on PCI scores [42]. However, evidence about the accuracy of the PCI has been mixed [41]. Household construction may play a role in vector abundance and DENV transmission risk; a previous study found greater risk for contracting dengue among people living in two-floor houses in northeastern Thailand [47]. Interestingly, no such association was found in our study (Table 4), except for significant associations of windows screened with netting and home wall types (wood/cement/bricks) with the abundance of adult female and immature Ae. aegypti, respectively.
Global warming has had various direct and indirect effects on human health and infectious diseases. Vector-borne diseases, such as dengue, are forecasted to be most affected by the expansion of areas with vector mosquitoes and an increase in the number and feeding activity of infected mosquitoes [68]. Specifically, climate change has been suggested as one potential contributor to the relative increases in vectorial capacity for dengue vectors and dengue transmission [69]. Studies of the association between climatic variables and dengue vectors are complex, not least because the effects of climate and other environmental changes are location-specific, which can alter the geographic distribution of disease vectors and vector-borne diseases [70,71,72]. In this study, we demonstrated that a generalized linear model with lagged temperature and relative humidity as covariates were key predictors for the abundance of dengue vectors. Our study also suggests that temperature is higher in urban areas and one of the key predictors for the abundance of dengue vectors compared with rural sites. This result is valuable for vector surveillance in dengue-endemic areas with similar climates. There have been several studies on climate in Thailand and other countries that have shown that temperature is one key factor for the distribution of mosquitoes and the transmission of DENV [25,73,74,75]. Temperature modulates DENV epidemic growth rates through its effects on reproduction numbers and generation intervals [76]. The generation interval is highly sensitive to temperature, decreasing two-fold between 25 and 35 °C. Dengue epidemics may accelerate as temperatures increase, not only because of more infections per generation but also because of faster generations [76]. Temperature affects not only the survival rate of mosquitoes but also the lifecycle of the vector, including oviposition, hatching, pupation, and emergence processes [77,78,79]. As temperatures rise, the extrinsic incubation period declines, biting frequency increases, and the average life span of mosquitoes increases [80,81]. According to the Intergovernmental Panel on Climate Change (IPCC), the minimum temperature required for DENV transmission is 11.9 °C and the minimum temperature for the biological activity of Aedes mosquitoes is 6–10 °C [82]. It is predicted that at mean temperatures <18 °C, DENV transmission increases as the diurnal temperature range (DTR) increases, whereas at mean temperatures of >18 °C, larger DTR reduces DENV transmission [83]. Previous studies have shown that relative humidity is also a contributing factor for vector abundance and DENV [84,85]. Temperature defines a viable range for transmission; humidity amplifies the potential within that range [25]. Transmission-potential is regulated by temperature-humidity coupling, enabling epidemics in a limited area of weather-space [86]. However, a high relative humidity might be associated with strong rainfall events [87]. The potential impact of changing rainfall patterns on Aedes mosquitoes is more difficult to predict since Aedes larvae develop in a wide range of water-holding containers, many of which are primarily filled by humans rather than by natural precipitation [88,89]. Any change in climate resulting in the range of meteorological variables conducive for vector breeding is expected to trigger an increase in vector populations and thereby affect DENV transmission. However, the effect of climatic factors on DENV transmission and vector distribution is not consistent throughout the world [84,90,91,92]. Ecological and human factors are essential in driving vector-borne diseases [93].
This study was limited by data that were only collected in household environments and surroundings to assess Ae. aegypti habitats and that excluded other habitats in non-household environments. Immature mosquitoes were not collected from all possible breeding sites and might have been under-sampled. DENV detection in mosquitoes was not done; if available, this may have provided additional valuable information about the potential risk for dengue transmission. Despite this limitation and because no such entomology, household, KAP, and climate data were previously available in our study areas of northeastern Thailand, this study provides baseline information on the distribution of dengue vectors, overall KAP of people, and associated risk factors, all of which are essential for planning successful control operations. We expect future research endeavors to attempt to fill in the gaps in our knowledge on the complex dynamics of dengue and its vector from an entomological perspective.
5. Conclusions
The results indicate that low education level, poor socio-economic status, crowded households, poor premise conditions, surrounding house density, unscreened windows, high numbers of wet containers, lack of mosquito control, and prior DENV infections were associated with higher Ae. aegypti abundance in the study sites. We also found a strong association between mosquito abundance and household participant′s adaptive capacity to climate change and their practices related to dengue. The current study also shows that maximum and mean temperatures with a lag of four weeks are important meteorological variables that affect Ae. aegypti abundance. Understanding the KAP in communities regarding climate change and dengue is essential for improving vector-control and dengue-prevention strategies. In the absence of specific treatment and the incomplete protection provided by the currently available vaccine, these findings may contribute to the development of a reliable early warning system about the potential spread of vector-borne diseases, increasing the awareness of the general public and tourists, as well as promoting community- and individual-level preventive and control measures regarding dengue and its vector in northeastern Thailand and other dengue-endemic countries. The dataset awaits further analysis for the predictive modeling of mosquito abundance and disease risk based on socio-economic, landscape, and temporal patterns, as well as for the development of dengue early warning systems to guide vector-control operations.
Acknowledgments
The authors sincerely thank all field staff, village health volunteers, anonymous government officials, and local authorities in Thailand for their assistance and support. Particular thanks to the staff at the Office of Disease Prevention and Control 10, Ubon Ratchathani, Thailand; Tanyee Sukanda, Sirinart Aromseree, Supranee Phanthanawiboon, Dyna Doum, and Panwad Tongchai at Khon Kaen University, Thailand.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijerph18115971/s1. Additional File 1: Table S1. Summarized knowledge characteristics regarding climate change among study populations in selected urban and rural villages in northeastern Thailand (percentages in parentheses). Additional File 2: Table S2. Summarized attitude characteristics regarding climate change among study populations in selected urban and rural villages in northeastern Thailand (percentages in parentheses). Additional File 3: Table S3. Summarized practice characteristics regarding climate change among study populations in selected urban and rural villages in northeastern Thailand (percentages in parentheses). Additional File 4: Table S4. Summarized knowledge characteristics regarding dengue among study populations in selected urban and rural villages in northeastern Thailand (percentages in parentheses). Additional File 5: Table S5. Summarized attitude characteristics regarding dengue among study populations in selected urban and rural villages in northeastern Thailand (percentages in parentheses). Additional File 6: Table S6. Summarized practice characteristics regarding dengue among study populations in selected urban and rural villages in northeastern Thailand (percentages in parentheses).
Author Contributions
Conceptualization; M.S.R. and H.J.O.; data curation; M.S.R.; data analysis; M.S.R.; funding acquisition; H.J.O., U.H., M.S.R., C.P. and T.E.; investigation; M.S.R. and S.Z.; methodology; M.S.R.; project administration; M.S.R., H.J.O., C.P., T.E., O.S., S.Z. and P.P.; supervision; M.S.R., H.J.O., C.P., T.E., U.H., O.S., R.P. and J.R.; validation; M.S.R., H.J.O., C.P., T.E., S.Z., U.H., R.P. and J.R.; writing—original draft: M.S.R.; writing—review and editing: All authors. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Research Council of Norway [DENCLIM project, grant number 281077] and Khon Kaen University Faculty of Medicine Research Grant (grant number IN63312).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by Khon Kaen University Ethics Committee for Human Research (ref. no. HE611228, 2 August 2018 and HE631077, 24 March 2020) and the Regional Committees for Medical and Health Research Ethics in Norway (2018/1085/REK sør-øst C given on 27 June 2018).
Informed Consent Statement
Written informed consent has been obtained from the household head and study participants before interviews of all participating households.
Data Availability Statement
The data underlying the results presented in the study are available from the Norwegian Center for Research Data (NSD). Access to the data sets must be requested from NSD using a Data Access form at this link: https://nsd.no/nsd/english/order.html and referring to project ID NSD0000 (accessed on 22 April 2021).
Conflicts of Interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher′s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gubler D.J. The economic burden of dengue. Am. J. Trop. Med. Hyg. 2012;86:743. doi: 10.4269/ajtmh.2012.12-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dhimal M., Gautam I., Joshi H.D., O′Hara R.B., Ahrens B., Kuch U. Risk Factors for the Presence of Chikungunya and Dengue Vectors (Aedes aegypti and Aedes albopictus), Their Altitudinal Distribution and Climatic Determinants of Their Abundance in Central Nepal. PLoS Negl. Trop. Dis. 2015;9:e0003545. doi: 10.1371/journal.pntd.0003545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paul K.K., Dhar-Chowdhury P., Haque C.E., Al-Amin H.M., Goswami D.R., Kafi M.A.H., Drebot M.A., Lindsay L.R., Ahsan G.U., Brooks W.A. Risk factors for the presence of dengue vector mosquitoes, and determinants of their prevalence and larval site selection in Dhaka, Bangladesh. PLoS ONE. 2018;13:e0199457. doi: 10.1371/journal.pone.0199457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatt S., Gething P.W., Brady O.J., Messina J.P., Farlow A.W., Moyes C.L., Drake J.M., Brownstein J.S., Hoen A.G., Sankoh O., et al. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murray N.E., Quam M.B., Wilder-Smith A. Epidemiology of dengue: Past, present and future prospects. Clin. Epidemiol. 2013;5:299–309. doi: 10.2147/clep.s34440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chareonsook O., Foy H.M., Teeraratkul A., Silarug N. Changing epidemiology of dengue hemorrhagic fever in Thailand. Epidemiol. Infect. 1999;122:161–166. doi: 10.1017/S0950268898001617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waewwab P., Sungvornyothin S., Potiwat R., Okanurak K. Impact of dengue-preventive behaviors on Aedes immature production in Bang Kachao, Samut Prakan Province, Thailand: A cross-sectional study. BMC Public Health. 2020;20:905. doi: 10.1186/s12889-020-8394-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polwiang S. The time series seasonal patterns of dengue fever and associated weather variables in Bangkok (2003–2017) BMC Infect. Dis. 2020;20:208. doi: 10.1186/s12879-020-4902-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kosoltanapiwat N., Tongshoob J., Singkhaimuk P., Nitatsukprasert C., Davidson S.A., Ponlawat A.J.P. Entomological Surveillance for Zika and Dengue Virus in Aedes Mosquitoes: Implications for Vector Control in Thailand. Pathogens. 2020;9:442. doi: 10.3390/pathogens9060442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Department of Disease Control, Thailand Weekly Disease Forecast No. 231_Dengue. [(accessed on 1 January 2021)]; Available online: https://ddc.moph.go.th/uploads/files_en/28120191219084236.pdf.
- 11.Messina J.P., Brady O., Scott T.W., Zou C., Pigott D., Duda K.A., Bhatt S., Katzelnick L., Howes R.E., Battle K.E., et al. Global spread of dengue virus types: Mapping the 70 year history. Trends Microbiol. 2014;22:138–146. doi: 10.1016/j.tim.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO Global Strategy for Dengue Prevention and Control 2012–2020. [(accessed on 16 July 2020)]; Available online: https://apps.who.int/iris/bitstream/handle/10665/75303/9789241504034_eng.pdf;jsessionid=8BD7C5C13E1CC23A760ADD649728C11C?sequence=1.
- 13.MOPH Thailand Weekly Disease Forecast No. 263_Dengue. [(accessed on 27 August 2020)]; Available online: https://ddc.moph.go.th/uploads/files_en/31120200619074616.pdf.
- 14.Chareonviriyaphap T., Akratanakul P., Nettanomsak S., Huntamai S. Larval habitats and distribution patterns of Aedes aegypti (Linnaeus) and Aedes albopictus (Skuse), in Thailand. Southeast Asian J. Trop. Med. Public Health. 2003;34:529–535. [PubMed] [Google Scholar]
- 15.Kittayapong P., Strickman D. Distribution of container-inhabiting Aedes larvae (Diptera: Culicidae) at a dengue focus in Thailand. J. Med. Entomol. 1993;30:601–606. doi: 10.1093/jmedent/30.3.601. [DOI] [PubMed] [Google Scholar]
- 16.Koyadun S., Butraporn P., Kittayapong P. Ecologic and Sociodemographic Risk Determinants for Dengue Transmission in Urban Areas in Thailand. Interdiscip. Perspect. Infect. Dis. 2012 doi: 10.1155/2012/907494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vannavong N., Seidu R., Stenström T.-A., Dada N., Overgaard H. Effects of socio-demographic characteristics and household water management on Aedes aegypti production in suburban and rural villages in Laos and Thailand. Parasites Vectors. 2017;10:170. doi: 10.1186/s13071-017-2107-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ndenga B.A., Mutuku F.M., Ngugi H.N., Mbakaya J.O., Aswani P., Musunzaji P.S., Vulule J., Mukoko D., Kitron U., LaBeaud A.D. Characteristics of Aedes aegypti adult mosquitoes in rural and urban areas of western and coastal Kenya. PLoS ONE. 2017;12:e0189971. doi: 10.1371/journal.pone.0189971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuno G.J. Review of the factors modulating dengue transmission. Epidemiol. Rev. 1995;17:321–335. doi: 10.1093/oxfordjournals.epirev.a036196. [DOI] [PubMed] [Google Scholar]
- 20.Descloux E., Mangeas M., Menkes C.E., Lengaigne M., Leroy A., Tehei T., Guillaumot L., Teurlai M., Gourinat A.-C., Benzler J., et al. Climate-Based Models for Understanding and Forecasting Dengue Epidemics. PLoS Negl. Trop. Dis. 2012;6:e1470. doi: 10.1371/journal.pntd.0001470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Regis L.N., Acioli R.V., Silveira J.C., Jr., de Melo-Santos M.A.V., da Cunha M.C.S., Souza F., Batista C.A.V., Barbosa R.M.R., de Oliveira C.M.F., Ayres C.F. Characterization of the spatial and temporal dynamics of the dengue vector population established in urban areas of Fernando de Noronha, a Brazilian oceanic island. Acta Trop. 2014;137:80–87. doi: 10.1016/j.actatropica.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 22.Misslin R., Vaguet Y., Vaguet A., Daudé E. Estimating air temperature using MODIS surface temperature images for assessing Aedes aegypti thermal niche in Bangkok, Thailand. Environ. Monit. Assess. 2018;190:537. doi: 10.1007/s10661-018-6875-0. [DOI] [PubMed] [Google Scholar]
- 23.Phanitchat T., Apiwathnasorn C., Sumroiphon S., Samung Y., Naksathit A., Thawornkuno C., Sungvornyothin S. The influence of temperature on the developmental rate and survival of Aedes albopictus in Thailand. Southeast Asian J. Trop. Med. Public Health. 2017;48:799–808. [Google Scholar]
- 24.Brady O.J., Johansson M.A., Guerra C.A., Bhatt S., Golding N., Pigott D.M., Delatte H., Grech M.G., Leisnham P.T., Maciel-De-Freitas R., et al. Modelling adult Aedes aegypti and Aedes albopictus survival at different temperatures in laboratory and field settings. Parasites Vectors. 2013;6:351. doi: 10.1186/1756-3305-6-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campbell K.M., Lin C.D., Iamsirithaworn S., Scott T.W. The Complex Relationship between Weather and Dengue Virus Transmission in Thailand. Am. J. Trop. Med. Hyg. 2013;89:1066–1080. doi: 10.4269/ajtmh.13-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vargas R.E.M., Ya-Umphan P., Phumala-Morales N., Komalamisra N., Dujardin J.-P. Climate associated size and shape changes in Aedes aegypti (Diptera: Culicidae) populations from Thailand. Infect. Genet. Evol. 2010;10:580–585. doi: 10.1016/j.meegid.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Nagao Y., Thavara U., Chitnumsup P., Tawatsin A., Chansang C., Campbell-Lendrum D. Climatic and social risk factors for Aedes infestation in rural Thailand. Trop. Med. Int. Health. 2003;8:650–659. doi: 10.1046/j.1365-3156.2003.01075.x. [DOI] [PubMed] [Google Scholar]
- 28.Strickman D., Kittayapong P.J. Dengue and its vectors in Thailand: Introduction to the study and seasonal distribution of Aedes larvae. Am. J. Trop. Med. Hyg. 2002;67:247–259. doi: 10.4269/ajtmh.2002.67.247. [DOI] [PubMed] [Google Scholar]
- 29.Arunachalam N., Tana S., Espino F., Kittayapong P., Abeyewickreme W., Wai K.T., Tyagi B.K., Kroeger A., Sommerfeld J., Petzold M. Eco-bio-social determinants of dengue vector breeding: A multicountry study in urban and periurban Asia. Bull. World Health Organ. 2010;88:173–184. doi: 10.2471/BLT.09.067892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Da Silveira L.T.C., Tura B., Santos M.J. Systematic review of dengue vaccine efficacy. BMC Infect. Dis. 2019;19:750. doi: 10.1186/s12879-019-4369-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosa B.R., da Cunha A.J.L.A., de Andrade Medronho R.J.B. Efficacy, immunogenicity and safety of a recombinant tetravalent dengue vaccine (CYD-TDV) in children aged 2–17 years: Systematic review and meta-analysis. BMJ Open. 2019;9:e019368. doi: 10.1136/bmjopen-2017-019368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rahman S., Karamehic-Muratovic A., Baghbanzadeh M., Amrin M., Zafar S., Rahman N.N., Shirina S.U., Haque U. Climate change and dengue fever knowledge, attitudes and practices in Bangladesh: A social media–based cross-sectional survey. Trans. R. Soc. Trop. Med. Hyg. 2020;115:85–93. doi: 10.1093/trstmh/traa093. [DOI] [PubMed] [Google Scholar]
- 33.Rahman S., Overgaard H.J., Pientong C., Mayxay M., Ekalaksananan T., Aromseree S., Phanthanawiboon S., Zafar S., Shipin O., Paul R.E., et al. Knowledge, attitudes, and practices on climate change and dengue in Lao People′s Democratic Republic and Thailand. Environ. Res. 2021;193:110509. doi: 10.1016/j.envres.2020.110509. [DOI] [PubMed] [Google Scholar]
- 34.Rueda L.M. Pictorial keys for the identification of mosquitoes (Diptera: Culicidae) associated with Dengue Virus Transmission. Zootaxa. 2004;589:1–60. doi: 10.11646/zootaxa.589.1.1. [DOI] [Google Scholar]
- 35.Knox T.B., Yen N.T., Nam V.S., Gatton M.L., Kay B.H., Ryan P.A. Critical evaluation of quantitative sampling methods for Aedes aegypti (Diptera: Culicidae) immatures in water storage containers in Vietnam. J. Med. Entomol. 2007;44:192–204. doi: 10.1093/jmedent/44.2.192. [DOI] [PubMed] [Google Scholar]
- 36.Focks D.A. A Review of Entomological Sampling Methods and Indicators for Dengue Vectors. World Health Organization; Geneva, Switzerland: 2004. [Google Scholar]
- 37.Bangs M.J., Focks D.A. Abridged Pupa Identification Key to the Common Container-Breeding Mosquitoes in Urban Southeast Asia. J. Am. Mosq. Control Assoc. 2006;22:565–572. doi: 10.2987/8756-971X(2006)22[565:APIKTT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. [(accessed on 2 January 2021)]; Available online: http://www.ncbi.nlm.nih.gov/books/NBK143157.
- 39.Melki I.S., Beydoun H., Khogali M., Tamim H., Yunis K. Household crowding index: A correlate of socioeconomic status and inter-pregnancy spacing in an urban setting. J. Epidemiol. Community Health. 2004;58:476–480. doi: 10.1136/jech.2003.012690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Willis K., Tipple A. Economics of multihabitation: Housing conditions, household occupancy and household structure under rent control, inflation, and nonmarketability of ownership rights. World Dev. 1991;19:1705–1720. doi: 10.1016/0305-750X(91)90014-9. [DOI] [Google Scholar]
- 41.Hustedt J., Doum D., Keo V., Ly S., Sam B., Chan V., Boyer S., Liverani M., Alexander N., Bradley J., et al. Ability of the Premise Condition Index to Identify Premises with Adult and Immature Aedes Mosquitoes in Kampong Cham, Cambodia. Am. J. Trop. Med. Hyg. 2020;102:1432–1439. doi: 10.4269/ajtmh.19-0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andrighetti M.T.M., Galvani K.C., da Graça Macoris M.d.L. Evaluation of premise condition index in the context of Aedes aegypti control in Marília, São Paulo, Brazil. Dengue Bull. 2009;33:167–175. [Google Scholar]
- 43.Gardner W., Mulvey E.P., Shaw E. Regression analyses of counts and rates: Poisson, overdispersed Poisson, and negative binomial models. Psychol. Bull. 1995;118:392. doi: 10.1037/0033-2909.118.3.392. [DOI] [PubMed] [Google Scholar]
- 44.Ripley B., Venables B., Bates D.M., Hornik K., Gebhardt A., Firth D., Ripley M.B. Package ‘mass′. Cran r. 2013;538:113–120. [Google Scholar]
- 45.Wickham H. ggplot2: Elegant graPhics for Data Analysis. Springer; New York, NY, USA: 2016. [Google Scholar]
- 46.Fox J., Weisberg S. An R Companion to Applied Regression. Sage Publications; Thousand Oaks, CA, USA: 2018. [Google Scholar]
- 47.Fustec B., Phanitchat T., Hoq M.I., Aromseree S., Pientong C., Thaewnongiew K., Ekalaksananan T., Bangs M.J., Corbel V., Alexander N., et al. Complex relationships between Aedes vectors, socio-economics and dengue transmission—Lessons learned from a case-control study in northeastern Thailand. PLoS Negl. Trop. Dis. 2020;14:e0008703. doi: 10.1371/journal.pntd.0008703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Overgaard H.J., Olano V.A., Jaramillo J.F., Matiz M.I., Sarmiento D., Stenström T.A., Alexander N. A cross-sectional survey of Aedes aegypti immature abundance in urban and rural household containers in central Colombia. Parasites Vectors. 2017;10:356. doi: 10.1186/s13071-017-2295-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilke A.B.B., Chase C., Vasquez C., Carvajal A., Medina J., Petrie W.D., Beier J.C. Urbanization creates diverse aquatic habitats for immature mosquitoes in urban areas. Sci. Rep. 2019;9:15335. doi: 10.1038/s41598-019-51787-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferdousi F., Yoshimatsu S., Ma E., Sohel N., Wagatsuma Y. Identification of Essential Containers for Aedes Larval Breeding to Control Dengue in Dhaka, Bangladesh. Trop. Med. Health. 2015;43:253–264. doi: 10.2149/tmh.2015-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barrera R., Amador M., Clark G.G. Use of the pupal survey technique for measuring Aedes aegypti (Diptera: Culicidae) productivity in Puerto Rico. Am. J. Trop. Med. Hyg. 2006;74:290–302. doi: 10.4269/ajtmh.2006.74.290. [DOI] [PubMed] [Google Scholar]
- 52.Seng C.M., Setha T., Nealon J., Socheat D. Pupal sampling for Aedes aegypti (L.) surveillance and potential stratification of dengue high-risk areas in Cambodia. Trop. Med. Int. Health. 2009;14:1233–1240. doi: 10.1111/j.1365-3156.2009.02368.x. [DOI] [PubMed] [Google Scholar]
- 53.Ibarra A.M.S., Ryan S., Beltrán E., Mejía R., Silva M., Muñoz Á. Dengue Vector Dynamics (Aedes aegypti) Influenced by Climate and Social Factors in Ecuador: Implications for Targeted Control. PLoS ONE. 2013;8:e78263. doi: 10.1371/journal.pone.0078263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saifur R.G.M., Dieng H., Abu Hassan A., Salmah R.C., Satho T., Miake F., Hamdan A. Changing Domesticity of Aedes aegypti in Northern Peninsular Malaysia: Reproductive Consequences and Potential Epidemiological Implications. PLoS ONE. 2012;7:e30919. doi: 10.1371/journal.pone.0030919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chadee D.D., Huntley S., Focks D.A., Chen A.A. Aedes aegypti in Jamaica, West Indies: Container productivity profiles to inform control strategies. Trop. Med. Int. Health. 2009;14:220–227. doi: 10.1111/j.1365-3156.2008.02216.x. [DOI] [PubMed] [Google Scholar]
- 56.Bureau P.A.S. Dengue and Dengue Hemorrhagic Fever in the Americas: Guidelines for Prevention and Control. Pan American Health Organization; Washington, DC, USA: 1994. [Google Scholar]
- 57.Sanchez L., Vanlerberghe V., Alfonso L., Marquetti M.D.C., Guzman M.G., Bisset J., Van Der Stuyft P. Aedes aegypti Larval Indices and Risk for Dengue Epidemics. Emerg. Infect. Dis. 2006;12:800–806. doi: 10.3201/eid1205.050866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wijayanti S.P.M., Porphyre T., Chase-Topping M., Rainey S.M., McFarlane M., Schnettler E., Biek R., Kohl A. The Importance of Socio-Economic Versus Environmental Risk Factors for Reported Dengue Cases in Java, Indonesia. PLoS Negl. Trop. Dis. 2016;10:e0004964. doi: 10.1371/journal.pntd.0004964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Udayanga L., Gunathilaka N., Iqbal M.C.M., Lakmal K., Amarasinghe U.S., Abeyewickreme W. Comprehensive evaluation of demographic, socio-economic and other associated risk factors affecting the occurrence of dengue incidence among Colombo and Kandy Districts of Sri Lanka: A cross-sectional study. Parasites Vectors. 2018;11:478. doi: 10.1186/s13071-018-3060-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Telle O., Vaguet A., Yadav N., Lefebvre B., Daudé E., Paul R.E., Cebeillac A., Nagpal B.J. The spread of dengue in an endemic urban milieu—The case of Delhi, India. PloS ONE. 2016;11:e0146539. doi: 10.1371/journal.pone.0146539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bowman L.R., Runge-Ranzinger S., McCall P.J. Assessing the Relationship between Vector Indices and Dengue Transmission: A Systematic Review of the Evidence. PLoS Negl. Trop. Dis. 2014;8:e2848. doi: 10.1371/journal.pntd.0002848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Juarez J., Garcia-Luna S., Medeiros M., Dickinson K., Borucki M., Frank M., Badillo-Vargas I., Chaves L., Hamer G. The Eco-Bio-Social Factors That Modulate Aedes aegypti Abundance in South Texas Border Communities. Insects. 2021;12:183. doi: 10.3390/insects12020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Woolhouse M.E.J., Dye C., Etard J.-F., Smith T., Charlwood J.D., Garnett G.P., Hagan P., Hii J.L.K., Ndhlovu P.D., Quinnell R.J., et al. Heterogeneities in the transmission of infectious agents: Implications for the design of control programs. Proc. Natl. Acad. Sci. USA. 1997;94:338–342. doi: 10.1073/pnas.94.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsuda Y., Suwonkerd W., Chawprom S., Prajakwong S., Takagi M. Different Spatial Distribution of Aedes Aegypti and Aedes Albopictus along an Urban–Rural Gradient and the Relating Environmental Factors Examined in Three Villages in Northern Thailand. J. Am. Mosq. Control Assoc. 2006;22:222–228. doi: 10.2987/8756-971X(2006)22[222:DSDOAA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 65.Francisco E., Carlosmoisa H., Rafael C.J. Factors that modify the larval indices of Aedes aegypti in Colima, Mexico. Pan Am. J. Public Health. 2001;10:6–12. doi: 10.1590/s1020-49892001000700002. [DOI] [PubMed] [Google Scholar]
- 66.Nogueira L.A., Madeira N.G., Gushi L.T., Ribolla P.E.M., Miranda J.E. Application of an Alternative Aedes Species (Diptera: Culicidae) Surveillance Method in Botucatu City, São Paulo, Brazil. Am. J. Trop. Med. Hyg. 2005;73:309–311. doi: 10.4269/ajtmh.2005.73.309. [DOI] [PubMed] [Google Scholar]
- 67.Ohtomo H., Akao N. Effect of global warming on infectious diseases. Nihon Rinsho. Jpn. J. Clin. Med. 2007;65(Suppl. 3):653–658. [PubMed] [Google Scholar]
- 68.Watts N., Amann M., Ayeb-Karlsson S., Belesova K., Bouley T., Boykoff M., Byass P., Cai W., Campbell-Lendrum D., Chambers J., et al. The Lancet Countdown on health and climate change: From 25 years of inaction to a global transformation for public health. Lancet. 2018;391:581–630. doi: 10.1016/S0140-6736(17)32464-9. [DOI] [PubMed] [Google Scholar]
- 69.Kovats R.S., Campbell-Lendrum D.H., McMichel A.J., Woodward A., Cox J.S.H. Early effects of climate change: Do they include changes in vector-borne disease? Philos. Trans. R. Soc. B Biol. Sci. 2001;356:1057–1068. doi: 10.1098/rstb.2001.0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Confalonieri U., Menne B., Akhtar R., Ebi K.L., Hauengue M., Kovats R.S., Revich B., Woodward A. Human Health Climate Change 2007: Impacts, Adaptation and Vulnerability. Cambridge University Press; Cambridge, UK: 2007. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. [Google Scholar]
- 71.Woodward A., Smith K.R., Campbell-Lendrum D., Chadee D.D., Honda Y., Liu Q., Olwoch J., Revich B., Sauerborn R., Chafe Z., et al. Climate change and health: On the latest IPCC report. Lancet. 2014;383:1185–1189. doi: 10.1016/S0140-6736(14)60576-6. [DOI] [PubMed] [Google Scholar]
- 72.Fan J., Lin H., Wang C., Bai L., Yang S., Chu C., Yang W., Liu Q. Identifying the high-risk areas and associated meteorological factors of dengue transmission in Guangdong Province, China from 2005 to 2011. Epidemiol. Infect. 2014;142:634–643. doi: 10.1017/S0950268813001519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Phanitchat T., Zhao B., Haque U., Pientong C., Ekalaksananan T., Aromseree S., Thaewnongiew K., Fustec B., Bangs M.J., Alexander N. Spatial and temporal patterns of dengue incidence in northeastern Thailand 2006–2016. BMC Infect. Dis. 2019;19:743. doi: 10.1186/s12879-019-4379-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pham H.H., Doan H.T.M., Phan T.T., Minh N.N.T. Ecological factors associated with dengue fever in a central highlands Province, Vietnam. BMC Infect. Dis. 2011;11:172. doi: 10.1186/1471-2334-11-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Siraj A.S., Oidtman R.J., Huber J., Kraemer M.U.G., Brady O.J., Johansson M.A., Perkins T.A. Temperature modulates dengue virus epidemic growth rates through its effects on reproduction numbers and generation intervals. PLoS Negl. Trop. Dis. 2017;11:e0005797. doi: 10.1371/journal.pntd.0005797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alto B.W., Juliano S.A. Temperature effects on the dynamics of Aedes albopictus (Diptera: Culicidae) populations in the laboratory. J. Med. Entomol. 2001;38:548–556. doi: 10.1603/0022-2585-38.4.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Morin C.W., Comrie A.C., Ernst K. Climate and Dengue Transmission: Evidence and Implications. Environ. Health Perspect. 2013;121:1264–1272. doi: 10.1289/ehp.1306556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rohani A., Wong Y.C., Zamre I., Lee H.L., Zurainee M.N. The effect of extrinsic incubation temperature on development of dengue serotype 2 and 4 viruses in Aedes aegypti (L.) Southeast Asian J. Trop. Med. Public Health. 2009;40:942–950. [PubMed] [Google Scholar]
- 79.Halstead S.B. Dengue virus–mosquito interactions. Annu. Rev. Entomol. 2008;53:273–291. doi: 10.1146/annurev.ento.53.103106.093326. [DOI] [PubMed] [Google Scholar]
- 80.Rueda L., Patel K., Axtell R., Stinner R. Temperature-dependent development and survival rates of Culex quinquefasciatus and Aedes aegypti (Diptera: Culicidae) J. Med. Entomol. 1990;27:892–898. doi: 10.1093/jmedent/27.5.892. [DOI] [PubMed] [Google Scholar]
- 81.McCarthy J.J., Canziani O.F., Leary N.A., Dokken D.J., White K.S. Climate Change 2001: Impacts, Adaptation, and Vulnerability: Contribution of Working Group II to the Third Assessment Report of the Intergovernmental Panel on Climate Change. Volume 2 Cambridge University Press; Cambridge, UK: 2001. [Google Scholar]
- 82.Lambrechts L., Paaijmans K.P., Fansiri T., Carrington L.B., Kramer L.D., Thomas M.B., Scott T.W. Impact of daily temperature fluctuations on dengue virus transmission by Aedes aegypti. Proc. Natl. Acad. Sci. USA. 2011;108:7460–7465. doi: 10.1073/pnas.1101377108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hales S., de Wet N., Maindonald J., Woodward A. Potential effect of population and climate changes on global distribution of dengue fever: An empirical model. Lancet. 2002;360:830–834. doi: 10.1016/S0140-6736(02)09964-6. [DOI] [PubMed] [Google Scholar]
- 84.Halide H., Ridd P. A predictive model for Dengue Hemorrhagic Fever epidemics. Int. J. Environ. Health Res. 2008;18:253–265. doi: 10.1080/09603120801966043. [DOI] [PubMed] [Google Scholar]
- 85.Campbell K.M., Haldeman K., Lehnig C., Munayco C.V., Halsey E.S., Laguna-Torres V.A., Yagui M., Morrison A.C., Lin C.-D., Scott T.W. Weather Regulates Location, Timing, and Intensity of Dengue Virus Transmission between Humans and Mosquitoes. PLoS Negl. Trop. Dis. 2015;9:e0003957. doi: 10.1371/journal.pntd.0003957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mitovski T., Folkins I., Von Salzen K., Sigmond M. Temperature, relative humidity, and divergence response to high rainfall events in the tropics: Observations and models. J. Clim. 2010;23:3613–3625. doi: 10.1175/2010JCLI3436.1. [DOI] [Google Scholar]
- 87.Tun-Lin W., Lenhart A., Nam V.S., Rebollar-Téllez E., Morrison A., Barbazan P., Cote M., Midega J., Sanchez F., Manrique-Saide P. Reducing costs and operational constraints of dengue vector control by targeting productive breeding places: A multi-country non-inferiority cluster randomized trial. Trop. Med. Int. Health. 2009;14:1143–1153. doi: 10.1111/j.1365-3156.2009.02341.x. [DOI] [PubMed] [Google Scholar]
- 88.Kearney M., Porter W.P., Williams C., Ritchie S., Hoffmann A.A. Integrating biophysical models and evolutionary theory to predict climatic impacts on species′ ranges: The dengue mosquito Aedes aegypti in Australia. Funct. Ecol. 2009;23:528–538. doi: 10.1111/j.1365-2435.2008.01538.x. [DOI] [Google Scholar]
- 89.Gautam I., Aradhana K., Tuladhar R., Pandey B.D., Tamrakar A.S., Byanju R., Dhimal M., Aryal K., Kuch U. Container preference of the Asian tiger mosquito (Aedes albopictus) in Kathmandu and Lalitpur districts of Nepal. J. Nat. Hist. Mus. 2012;26:181–193. doi: 10.3126/jnhm.v26i0.14142. [DOI] [Google Scholar]
- 90.Patz J.A., Martens W.J., Focks D.A., Jetten T.H. Dengue fever epidemic potential as projected by general circulation models of global climate change. Environ. Health Perspect. 1998;106:147–153. doi: 10.1289/ehp.98106147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Banu S., Hu W., Hurst C., Tong S. Dengue transmission in the Asia-Pacific region: Impact of climate change and socio-environmental factors. Trop. Med. Int. Health. 2011;16:598–607. doi: 10.1111/j.1365-3156.2011.02734.x. [DOI] [PubMed] [Google Scholar]
- 92.Russell R.C., Currie B.J., Lindsay M.D., MacKenzie J.S., Ritchie S.A., Whelan P.I. Dengue and climate change in Australia: Predictions for the future should incorporate knowledge from the past. Med. J. Aust. 2009;190:265–268. doi: 10.5694/j.1326-5377.2009.tb02393.x. [DOI] [PubMed] [Google Scholar]
- 93.Zhou S.S., Huang F., Wang J.J., Zhang S.S., Su Y.P., Tang L.H. Geographical, meteorological and vectorial factors related to malaria re-emergence in Huang-Huai River of central China. Malar. J. 2010;9:337. doi: 10.1186/1475-2875-9-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying the results presented in the study are available from the Norwegian Center for Research Data (NSD). Access to the data sets must be requested from NSD using a Data Access form at this link: https://nsd.no/nsd/english/order.html and referring to project ID NSD0000 (accessed on 22 April 2021).