Abstract
Alginate (ALG), a polysaccharide derived from brown seaweed, has been extensively investigated as a biomaterial not only in tissue engineering but also for numerous biomedical sciences owing to its wide availability, good compatibility, weak cytotoxicity, low cost, and ease of gelation. Nevertheless, alginate lacks cell-binding sites, limiting long-term cell survival and viability in 3D culture. Collagen (Col), a major component protein found in the extracellular matrix (ECM), exhibits excellent biocompatibility and weak immunogenicity. Furthermore, collagen contains cell-binding motifs, which facilitate cell attachment, interaction, and spreading, consequently maintaining cell viability and promoting cell proliferation. Recently, there has been a growing body of investigations into collagen-based hydrogel trying to overcome the poor mechanical properties of collagen. In particular, collagen–alginate composite (CAC) hydrogel has attracted much attention due to its excellent biocompatibility, gelling under mild conditions, low cytotoxicity, controllable mechanic properties, wider availability as well as ease of incorporation of other biomaterials and bioactive agents. This review aims to provide an overview of the properties of alginate and collagen. Moreover, the application of CAC hydrogel in tissue engineering and biomedical sciences is also discussed.
Keywords: collagen–alginate composite hydrogel, tissue regeneration, wound dressing, tissue engineering, encapsulated cell therapy
1. Introduction
The vital role of biomaterials in tissue engineering and biomedical application has attracted much attention in the past decades [1]. Research into biomaterials and their application holds great promise in the future. Synthetic polymers had been extensively studied in the early twentieth century due to their better performance and ease of manufacture compared to natural materials [2,3]. Recently, naturally derived biomaterials have regained considerable interest in material science due to their extracellular matrix (ECM)-like structure that highly recapitulates in vivo environment, inherent biocompatibility, and a limited chance of immune response and immune rejection compared to human-made materials [4,5].
Alginate is a natural polysaccharide derived from brown algae with favorable properties, including broader availability, cost-effectiveness, good biocompatibility, weak cytotoxicity as well as ease of gelation in the presence of divalent cations [6]. Moreover, alginate has unique properties in comparison with other materials including, (1) ability to provide an inert aqueous environment, (2) sol-gel transition under the mild condition that enables excellent retention of bioactive molecules and cell encapsulation, (3) controllable porosity allowing adjustable molecular diffusion, (4) biodegradability under physiological environment, and (5) structural similarity to ECM [7,8,9,10]. These properties allow the wide investigation of alginate in tissue engineering and biomedical sciences, including cell encapsulation, wound dressing, tissue regeneration, and pharmaceutical application [11]. It is well known that anchorage-dependent cell death is activated in the absence of extracellular matrix [12]. Nevertheless, alginate inherently lacks cell-binding sites, thus limiting its wider-range application. Alginate modified with Arg-Gly-Asp (RGD) peptides or in combination with other biomaterials has been the focus of research.
Collagen is the primary protein found in soft connective tissue and hard connective tissue. Moreover, 33% of the protein found in the human body is collagen [13,14]. To date, 29 types of collagen are identified in the animals [15]. According to its structure, physical location in the body, and chain binding, collagen can also be divided into eight families [15]. Collagen-based hydrogel has been widely studied in tissue engineering and biomedical sciences owing to its excellent biocompatibility and weak immunogenicity [15,16,17,18,19]. Moreover, collagen, which contains RGD peptides, highly recapitulates ECM in the human body [20,21]. However, the insufficient mechanical properties limit its extensive application. It is ubiquitous to incorporate other biomaterials such as alginate, chitosan, and hyaluronic acid into collagen to improve its mechanical properties.
CAC hydrogel can be cross-linked under mild conditions. Therefore, CAC hydrogel is widely investigated for cell encapsulation and drug delivery [22,23,24,25]. What is more, the combination of collagen and alginate provides cell-binding motifs and enhances the mechanical properties [26]. There is a growing body of investigations into CAC hydrogel and its application in tissue engineering and biomedical sciences, including tissue regeneration, wound dressing, encapsulated cell therapy (ECT), and tumor biology. The composite hydrogel has been developed as a matrix scaffold to deliver a range of bioactive agents, cell populations, or in the combination of cell and bioactive factor to the defect site in tissue engineering. The composite hydrogel is also attracting much research attention in biomedical sciences. Collagen–alginate wound dressings can provide and maintain a moist environment to the wound site, promote cell migration, differentiation, thereby accelerating wound healing. CAC hydrogel is also promising for tissue regeneration due to its ease of gelling under mild conditions, which enables retention of bioactive agents and cell encapsulation. Alginate can provide encapsulation power that limits the chance of cell leakage from the ECT device. In addition, collagen can improve cell viability and proliferation. Therefore, CAC hydrogel has been exploited as a potential platform in ECT. Moreover, the composite hydrogels with tunning stiffness and higher similarity to ECM can mimic in vivo tumor cell environment. Therefore, it also explores a new field for the study on tumor biology.
This review aims to provide a comprehensive review of CAC hydrogel and its application (Figure 1). Firstly, the characteristics of alginate and collagen will be given respectively, followed by presenting the properties of CAC and the techniques. Finally, the application of CAC in tissue engineering and biomedical sciences, especially tissue regeneration, wound dressing and encapsulated cell therapy, and tumor biology, will be described.
Figure 1.
An overview of this review paper.
2. General Properties of Alginate and Collagen
2.1. Alginate
2.1.1. Alginate Sources
Alginate can be obtained from two major sources, including brown seaweed and bacteria [27]. Three species of brown seaweeds, including Laminaria Hyperborea, Ascophyllum Nodosum, and Macrocystis Pyrifera, are the primary source for commercially available alginate [28]. Alginate can also be isolated from other algae species, including Laminaria Japonica, Eclonia Maxima, Lesonia Negrescens, and Sargassum [28]. The isolation of alginate from brown seaweeds requires multiple steps and procedures. With the exception of Macrocystis Pyrifera, which is processed in wet form, brown seaweeds are first cropped and dried for further extraction. In brief, the initial removal of unwanted materials and impurities from algae is achieved by addition of dilute mineral acid [29]. The preparation of sodium alginate is done by addition of alkali solution such as NaOH, followed by centrifugation to remove sediments. The removal of homopolysaccharides is done by adding mineral acid, ethanol, or CaCl2. After these procedures, there are remaining impurities in alginates, which require further purification in order to be used in the pharmaceutic field and biomedical sciences.
Alginate can be secreted by some bacteria, including Azotobacter Vinerlandii and Pseudomonas species. The synthesis of alginate by these two bacteria is highly similar. Alginate synthesized by bacteria varies in mechanical properties and has been utilized for different applications. Alginate isolated from bacteria possesses more chemical structure and physical properties than brown algae sources. The biosynthesis of alginate by bacteria has already been illustrated. In brief, precursor substrate was firstly synthesized, followed by polymerization on the precursor. It was then transferred to the cytoplasmic membrane and periplasmic membrane for further modification, finally transported to the surroundings [30].
2.1.2. Chemical Structure and Molecular Weight
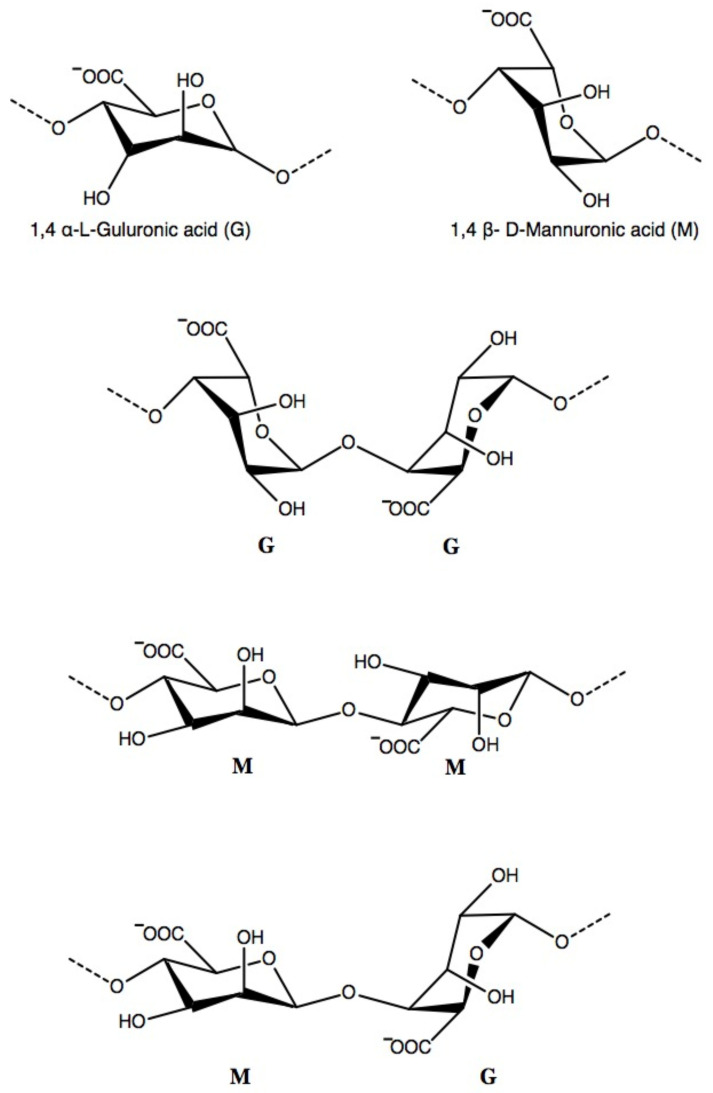
Alginate is a naturally occurring polysaccharide consisting of varying amounts of residues, including β-D-mannuronic acid (M) and 1–4 linked α-L-guluronic(G) [31]. Three different molecular structure patterns, including homogenous monomers (-GGGG-, -MMMM-) and alternating monomers (-MGMGMG-), are identified in alginates (Figure 2). M/G ratio and block length vary depending on the alginate source [32]. Up to now, over 200 alginate products are available in the market [32]. The mechanical properties of alginate are affected by the composition, length of the polymer chain, and molecular weight [33]. The molecular weight of alginate also varies with the sources. Increased proportion of G content in alginate and higher molecular weight can form strong mechanical properties.
Figure 2.
The chemical structure of 1,4 α-L-guluronic acid and β- D-mannuronic acid in alginate. The chemical arrangement of three block patterns, including homogenous monomers (-GGGG-, -MMMM-) and alternating monomers (-MGMGMG-) can be identified in alginate.
In the market, the molecular weight of alginate varies from 33,000 to 400,000 g/mol [11]. Alginate with higher molecular weight also results in greater viscosity, contributing to the difficulty in processing [34]. Meanwhile, greater shear forces are generated, which can cause undesirable damage to the encapsulated cells and proteins [35].
2.1.3. Biocompatibility and Biodegradability
Although many investigations on the biocompatibility of alginate had been undertaken in vitro and in vivo, a much-debated question about the effect of alginate composition on its biocompatibility had been discussed for many years. Until recently, it is widely accepted that alginate’s immunogenicity is associated with the presence of existing impurities, including heavy metals, endotoxins, and polyphenol [36]. Orive et al. demonstrated that poor biocompatibility, foreign body reaction, and inflammatory response of alginate primarily lie in the remaining impurities as they observed no immunological response when implanted highly purified alginate. Lee et al. further validated the importance of using purified alginate to reduce the risk of potential immunologic response by the host [37].
In human body, due to the lack of a specific enzyme named alginase to cleave alginate polymer chains, alginate is theoretically considered as nondegradable biomaterials. However, alginate cross-linked by covalent ions can be gradually dissolved by the ion-exchange reaction that continuous covalent ions release such as Ca2+ from alginate exchanges monovalent cations such as Na+ from the surroundings.
Mushollaeni et al. investigated the toxicity of alginate from two brown seaweed sources, including Sargassum and Padina by feeding Wistar mice with varying alginate concentrations. Alginate from Sargassum source ranging from 0.75% to 1% was regarded as safety concentration since the lower level of alanine aminotransferase (ALT) and aspartate transaminase (AST) was observed in the alginate-feeding group compared to the control group. However, alginate from Padina source could cause potential damage to the liver with a concentration above 0.75% [38].
2.1.4. Ionic Cross-Linking
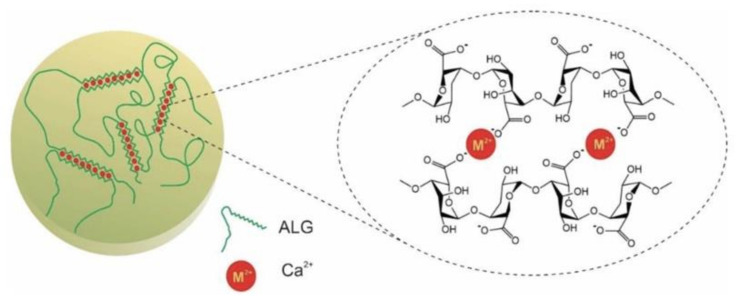
Alginate gelation can be achieved via physical and chemical methods. A 3D network of alginate is formed in the presence of divalent cation such as Ca2+, Sr2+, and Ba2+. Divalent cation can exchange Na+ from guluronate located in the separated ALG chains, forming the ‘egg-box’ structure (Figure 3) [39]. The most commonly utilized ion for alginate gelation is Ca2+ owing to its lower cytotoxicity effect, while it is reported that both Sr2+ and Ba2+-induced alginate is mechanically stronger than Ca2+-induced alginate. Gelation speed plays a critical role in controlling alginate gelation. Uniform hydrogel structure and superior mechanical integrity are achieved by decreasing the gelation speed, which is desirable for their application [40]. When directly using calcium chloride to initiate alginate gelation, it gives rise to uncontrollable and quick alginate gelling [40]. The reaction can be controlled by addition of carboxylate group and phosphate into calcium chloride solution since these ions can compete with Ca2+ to slow and delay the gelation reaction, finally forming a uniform hydrogel with greater mechanic integrity. In addition, temperature is considered another essential factor [40]. The cross-linking reaction can be slowed by low temperature as it reduces the reactivity of Ca2+. Therefore, it generates a uniform structure and greater mechanical integrity.
Figure 3.
Schematic diagram of sodium alginate cross-linking by Ca2+. In the alginate containing −COO−group, Ca2+ exchanges with Na+ from guluronate block in ALG and interacts with separated ALG chains, forming the “egg-box structure”. Reproduced from [41] with permission. Copyright (2020) Springer Nature.
Gallium has been investigated as an ionic cross-linker for alginate gelation. Like calcium ion, gallium binds with the guluronate block in the separated ALG chains, forming a 3D structure [42]. It is worth noting that alginate-based hydrogel cross-linked by gallium possesses antibacterial function against Gram-positive and Gram-negative bacteria via the interpretation of metabolic pathway mediated by iron [43,44]. Rastin et al. employed 3D bioprinting technique with Gallium-induced ALG cross-linking together. Multilayered cell-laden alginate-methylcellulose hydrogel was fabricated using 3D bioprinting followed by gallium bath [45]. Moreover, there is no significant difference in encapsulated cell viability between Gallium and Ca2+-induced gelation group over a 7-day cell culture.
2.2. Collagen
Collagen is the major component protein found in the extracellular matrix in both hard and soft connective tissues. Collagen also has a dominant role in maintaining the structural and biological integrity of ECM [46]. Until now, 29 types of collagen have been identified in which triple-helical tertiary structure is commonly present. Collagen consists of three polypeptide chains, and each chain is comprised of repetitive Glycine-X-Y sequences where X and Y are often occupied by proline and hydroxyproline [47,48]. Glycine located at every third residue enables the tight packing of the three polypeptide chains into a tropocollagen molecule. The most common types of collagen are summarized in Table 1. In addition to its favorable properties, including excellent biocompatibility, weak immunogenicity, porosity, and degradability, collagen also has a vital role in regulating cell morphology, attachment, migration as well as differentiation [49,50]. Although various collagen types have been identified, fibril-forming collagens, including type I, II, III, V, and IV, have achieved the most popularity in tissue engineering. It is worth noting that type I collagen is considered a golden material in tissue engineering, which comprises up to 90% of protein found in connective tissue [51]. The sol-gel transition of collagen happened under defined conditions, such as neutral pH and physiological temperature [52].
Table 1.
Collagen types, molecular formula, and tissue distribution. Reproduced from [46] with permission. Copyright (2010) MDPI AG.
| Type | Molecular Formula | Polymerized Form | Tissue Distribution | |
|---|---|---|---|---|
| Fibril-Forming (fibrillar) | I | [α1(I)]2α2(I) | fibril | bone, skin, tendons, ligaments, cornea (represent 90% of total collagen of the human body) |
| II | [α1(II)] | fibril | cartilage, intervertebral disc, notochord, vitreous humor in the eye | |
| III | [α1(III)]3 | fibril | skin, blood vessels | |
| V | [α1(V)]2α2(V) and α1(V)α2(V)α3(V) | fibril (assemble with type I) |
idem as type I | |
| XI | α1(XI)α2(XI)α3(XI) | fibril (assemble with type II) |
idem as type II | |
| Fibril-associated | IX | α1(IX)α2(IX)α3(IX) | lateral association with type II fibril | cartilage |
| XII | [α1(XII)]3 | lateral association with type I fibril | tendons, ligaments | |
| Network-forming | IV | [α1(IV)]2α2(IV) | sheet-like network | basal lamina |
| VII | [α1(VII)]3 | anchoring | beneath stratified squamous epithelia |
2.2.1. Collagen Source
Collagen can be obtained from various sources, including animal sources, synthetic sources, and marine sources. Type I collagen is the most used biomaterial in tissue engineering due to its widespread presence in animals and cost-effectiveness. Commercially available collagen in the market is mainly extracted from animals, including bovine, porcine, rat tail, and fish [53]. Yet, there is grave concern regarding the inflammation induced by remaining impurities in collagen, possible pathogen transmission, and batch-to-batch variability [54,55]. Indeed, it is reported that 2–4% of the population are allergic to collagen with a porcine and bovine source [56]. Recently, marine sources, including sponges and jellyfish, could be potential source candidates since there is no risk of pathogen transmission [55,57]. However, the poor thermal stability of marine-derived collagen may limit its application [58].
2.2.2. Biodegradability and Immunogenicity
The majority of collagen degradation in vivo is digested by collagenase such as matrix metalloproteinases (MMPs). There are various collagenases in human body, and it is worth noting that collagen degradation rate varies in different collagenases and cross-linking methods. The collagen degradation via collagenase includes (1) matrix metalloproteinases binding to collagen first, (2) ability to unwind three polypeptides chain into a single chain since three-helical structure blocks the enzyme active sites, and (3) MMPs acting on each strand to cleave [59]. Different types of collagen can be digested by their corresponding MMPs [60,61,62]. After the degradation by MMPs, the remaining collagen fragments can be further degraded by gelatinase and other nonspecific enzymes. It is widely accepted that collagen exhibits excellent biocompatibility since it has a limited possibility to induce immunological response. Helical structure and telopeptide are regarded as the specific regions that can cause immune rejection [54]. Since collagen has low antigenicity, collagen-based material is widely used in tissue engineering and regeneration [63].
2.2.3. Physical Cross-Linking
To date, there are various cross-linking methods for collagen including chemical cross-linking, physical cross-linking and enzyme-induced gelation. Although collagen cross-linked by chemicals including glutaraldehyde, carbodiimides, and polyepoxy compounds are more stable than other cross-linking methods, the cytotoxicity of the residuals and its byproducts after degeneration is a grave concern [64,65,66,67].
Collagen can be cross-linked by physical methods including ultraviolet (UV) light, gamma-ray radiation and dehydrothermal (DHT) [68]. Compared to chemical cross-linking, physical cross-linking is considered a begin method since it does not require cytotoxic chemicals; however, the low efficacy of physical gelation has been reported [69].
The DHT-induced collagen gelation is achieved by the formation of amide bonds between the free carboxyl group and amine group as a result of water removal under a higher temperature and vacuum condition [70]. In brief, the vacuum was set at 0.05 mbar and temperature at 40 degrees Celsius to remove water from COL molecule and prevent denaturation. After that, higher temperature (over 100 degree Celsius) was utilized to induce COL gelation further. It is worth noting that gelation and denaturation can co-occur. Collagen denaturation can occur under the higher temperature condition, finally resulting in poor-organized structure [71]. It is reported that temperature at 100 degree Celsius and 24 h treatment are the optimal parameters for DHT [72].
UV light is considered an effective and quick method compared to DHT treatment. Once collagen exposures to UV light, double bonds and aromatic rings can give rise to free radicals. The generated free radicals on the amino acids form covalent bonds between collagen molecules.
Collagen gelation via 254 nm UV light only takes 15 min, and UV-induced collagen shows higher resistance to degradation [73]. Dehydrothermal treatment is time-consuming as it requires a more extended period compared to UV light. DHT-induced collagen is more sensitive to trypsin but more resistant to degradation [74].
2.3. Collagen–Alginate Composite Hydrogel
2.3.1. Stiffness and Cell-Binding Sites
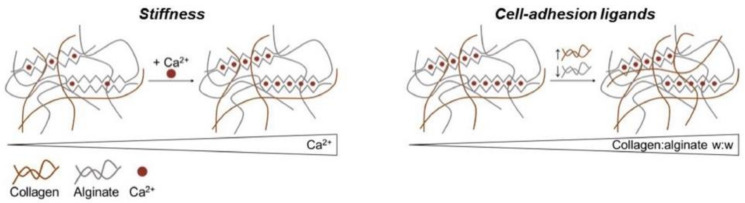
The combination of collagen with alginate overcomes the limitation of each material and integrates their favorable properties altogether. The major hurdle that restricts the widespread application of collagen is its poor mechanical properties. Incorporation with other materials can improve the mechanical properties. It is reported that collagen in the combination with alginate not only enhances the mechanical properties of the composite hydrogel but also tunes the stiffness of CAC hydrogel simply via the concentration of Ca2+ [26]. It is worth noting that stiffness is of great importance in the determination of stem cell fate [75]. Scaffold with suitable and proper mechanical properties is capable of providing the mechanical cues to stem or progenitor cell towards certain cell lineages [76]. The increase in calcium concentration improves the stiffness of the composite hydrogel and vice versa.
On account of collagen containing cell-binding ligands and the inherent lack of cell-binding motifs inside alginate, the increase in the proportion of collagens in CAC hydrogel improves cell-binding ligands, which enable more cell adhesion and attachment, thereby maintaining cell viability and promoting cell proliferation. The relationship between the stiffness of composite hydrogel and calcium concentration and the association between collagen concentration and the cell-binding site inside the composite hydrogel are illustrated in Figure 4.
Figure 4.
The relationship between the stiffness of composite hydrogel and calcium concentration and the association between collagen concentration and cell-binding sites inside the composite hydrogel. With the increase in Ca2+ concentration, the stiffness of composite hydrogel will be enhanced. Cell-adhesion ligands also increase as collagen concentration increases. Reproduced from [26] with permission, Copyright (2020) Elsevier.
2.3.2. Technique
Over the past decades, scientists have developed various techniques to manufacture CAC hydrogel on a large scale, surpassing the limitations of traditional 3D culture, which is time-consuming, technical, and laborious. In this section, 3D bioprinting on CAC hydrogel and other techniques will be discussed.
Three-Dimensional (3D) Bioprinting
Compared to traditional technique, 3D printing is an advanced technique that enables the construction of a complicated 3D structure via layer-by-layer manufacturing under the help of computer system [77]. 3D printing has achieved great success in the preparation of hydrogel scaffold in tissue engineering, especially in bone tissue and cartilage tissue engineering [78]. It is worth noting that 3D bioprinting enables the rapid fabrication of personalized implants which meet the individual need of patients [79]. The unique features of hydrogel include high water content and sensitivity to various stimuli including temperature, light as well as external signals. Therefore hydrogel is an excellent candidate in 3D bioprinting when working with cells [80].
3D printing can be classified into three categories including laser-based printing, nozzle-based printing and inkjet printer-based system [78]. Considering the good compatibility and ease of procession, alginate has received most popularity in bone tissue engineering as well as bioprinting [81,82]. Chondrocyte laden alginate hydrogel by 3D printing technique showed higher cell viability and promoted ECM deposition in vitro [83]. The main hurdle of using single materials as a bioink is poor mechanical properties [83]. Nevertheless, the poor mechanical properties of alginate usually result in instability of 3D structure, which is the main hurdle that restricts its application in hard tissue engineering [84]. Therefore, alginate in combination with other materials has received much popularity in 3D printing. Kim et al. demonstrated the prospect of a poly (ε-caprolactone) PCL/alginate scaffold with greater mechanical properties in hard tissue engineering since higher water absorption, greater retention efficacy of encapsulated cells, increased cell viability and significant calcium deposition were observed in PCL/alginate group compared to PCL group [85]. Recently, D’ Amora et al. combined 3D printing with surface modification, which enables the construction of 3D scaffold with structural and chemical gradients [86]. Moreover, recent advance also allows the incorporation of nanoparticle into the scaffold and the fabrication of injectable hydrogel [87]. Moreover, Collagen, one of the most popular bioinks, has received much research interest in tissue engineering [52].
Nozzle system is gaining most popularity in the preparation of scaffold-based hydrogel. The construction of 3D scaffold via nozzle system is based on three steps including (1) hydrogel solution was loaded into the system, (2) then it was forced out of the system into pre-designed mold, and (3) sol-gel transition. It is widely accepted that interlayer adhesion is essential for the successful fabrication of 3D structure. Besides, there are various factors which can play a dominant role in the fabrication including temperature, shear thinning, thixotropy and cross-linking methods.
A set of research groups proposed the use of dual concentric nozzle system to manufacture CAC hydrogel [88,89,90]. The dual concentric nozzle system consists of three different parts including outer syringe, inner syringe and CaCl2 bath. Collagen and alginate solution were loaded into inner syringe and outer syringe respectively to enable simultaneous injection of CAC solution into the calcium bath to form a fiber [88,89]. It is worth mentioning that fiber manufactured by dual concentric nozzle system can be molded into any irregular shapes. Besides, bioactive agents can be successfully loaded in collagen and alginate solution [89,90]. Buitrago et al. incorporated bioactive glass nanoparticle into alginate solution, which has demonstrated differentiation of stem cells towards osteogenic lineage [90].
Yao et al. adopted another electro-assisted inkjet printing to produce alginate microspheres coated with collagen [91]. Alginate microspheres were first fabricated via inkjet printing followed by collagen coating. The diameter of alginate-collagen microspheres, ranging from 245 μm to 657 μm, can be tuned by the following parameters including needle size, voltage, electrode distance and push speed.
In addition to 3D printing technique, there are other techniques to manufacture CAC hydrogel. One study applied a microconcave mold to fabricate CAC hydrogel for cell encapsulation, which overcame the traditional cell encapsulation technique [92]. In this study, islets cells were seeded into the microconcave mold followed by introduction of CAC solution. After that, CaCl2 solution was added to form islet spheroid. Microcapsules or spheroids can also be fabricated using microfluidics [93,94]. Jose et al. employed microfluidics to manufacture stem cell laden CAC microspheres. In brief, a CAC-stem cells mixture was loaded into the device. The CAC microspheres were fabricated when the solution passed through the inlet followed by shear force [94]. The techniques for the manufacture of CAC hydrogel are summarized in Table 2.
Table 2.
Summary of novel techniques for the manufacture of CAC hydrogel.
| Technique | Description | Incorporation of Bioactive Agent | Cell Type | Hydrogel Characteristics | Reference |
|---|---|---|---|---|---|
| Dual concentric nozzle system | Alginate and collagen solution were loaded into outer and inner syringe respectively. Simultaneous injection of ALG and COL solution into CaCl2 bath to form a fiber | Bioactive glass nanoparticles loaded in the shell [90] | Mesenchymal stem cells (MSCs) | CAC fiber (core diameter: 700–1000 μm and shell diameter: 200–500 μm) [88] | [88,89,90] |
| BMP loaded in the core and cobalt loaded in the shell [89] | CAC fiber (core diameter: 1000 μm and shell diameter: 150 μm [89] | ||||
| Electro-assisted inkjet printing | Alginate microsphere was fabricated by inkjet printing followed by collagen coating | Nil | Endothelial cell | CAC microsphere | [91] |
| Concave well mold | CAC containing islet cells were introduced into concave well mold followed by Ca2+ bath | Nil | Pancreatic islets cell | Islets spheroid | [92] |
| Microfluidics | CAC microspheres/spheroids were generated when pass through the inlet followed by shear force | Nil | Stem cell | CAC microsphere | [93] |
| Nil | Liver cell | CAC spheroid | [94] |
3. Application of CAC Hydrogel
3.1. Tissue Engineering
3.1.1. Bone Tissue Engineering
In bone tissue engineering, despite transplantation of autografts into defects site is the gold standard, the limited autograft sources and potential complications such as the risk of bacterial infection and donor site morbidity cannot be ignored [95]. CAC hydrogel has found prospect as their potential in treating bone defects via delivery of stem cells or progenitor cell population, bone inductive growth factors, or the combination of cells and molecules.
Many studies have demonstrated that alginate hydrogel, compared to others, can be delivered into the human body in a minimally invasive way, its powerful molding ability that enables it fitting into any irregular defect sites and its tunning porosity to release many drugs to the defects [96,97]. Nevertheless, the biological property of alginate in terms of cell adhesion and attachment is limited. Therefore, alginate combined with other biodegradable biomaterials and RGD peptides in attempt to improve long-term cell viability and survival has attracted much research attention recently.
Collagen-based hydrogel is receiving much attention in bone tissue engineering since collagen is the major component protein found in bone tissue [98]. Despite collagen owns excellent biocompatibility and ECM-like structure that facilitates cell attachment and proliferation, the limited biomechanical stability, especially in the presence of cells and the poor moldability, limit its widespread application in the field of orthopedics [99,100,101,102]. What is more, it has been reported that collagen has limited ability to induce osteogenic differentiation from stem cells in comparison to other materials [99,100,101].
The composite hydrogel integrates the merits of each material and overcomes each limitation such as enhanced mechanical properties, powerful moldability, capability to maintain cell viability and potential to induce osteogenic lineage under defined environment. The excellent biocompatibility of CAC hydrogel has been extensively examined [90,103]. A study conducted by Benayahu et al. showed that up to six-month system stability of the composite hydrogel under different storage conditions in vitro and excellent biocompatibility by the observation of the absence of adverse effect and non-cytotoxicity near the implants [103].
In an attempt to accelerate bone regeneration, the ability of CAC hydrogel to deliver cell population to the defect site has also been examined. Perez et al. developed core-shell dual-layered hydrogels using a nozzle system in which cell-containing collagen and alginate were fabricated in the core and the outer shell respectively [88]. Moreover, the nozzle system enables hydrogel to be easily molded into any irregular shape, which is desirable and corresponds with clinical needs. More importantly, the authors illustrated that compared to the non-cell-loading construct, core-shell hydrogel loaded with bone marrow mesenchymal stem cells (bMSCs) exhibited considerable bone formation at the central region of the defect site in the rat model of calvarium defect [88]. CAC can also be incorporated with other biomaterials. Bendtsen et al. incorporated hydroxyapatite, one of the major component proteins found in bone, into CAC hydrogel [104]. The development of in situ injectable hydrogels provided a candidate scaffold for bone tissue engineering [104]. Moreover, CAC hydrogel containing chitosan (ChS) and hydroxyapatite (HAp) has shown their advantages for cell infiltration and proliferation [105].
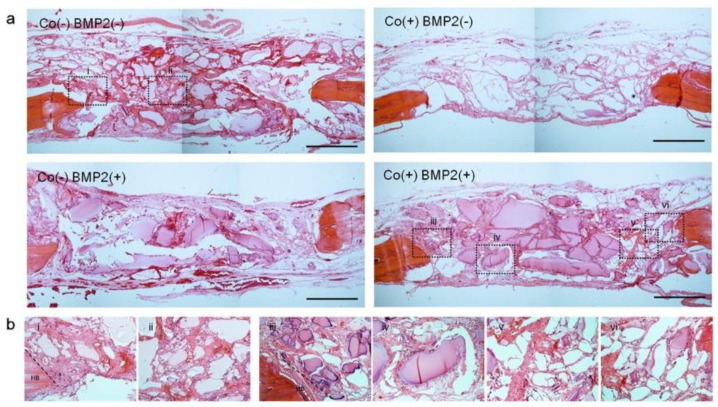
While CAC hydrogel can support bMSCs survival and proliferation, bMSCs osteogenesis happens in the presence of osteo-inductive molecules [104,106]. CAC hydrogel can be incorporated with other bioactive agents to provide mechanical cues to bMSCs. Perez et al. showed bMSCs differentiation towards osteocytes under the inductive condition in vitro [107]. CAC hydrogel is also being incorporated with novel nanoparticles to promote bone regeneration by guiding stem cell differentiation towards osteogenic lineages. The sequential delivery of cobalt ions followed by bone morphogenetic protein (BMP2) has shown promoting effect on bone regeneration in the rat model of calvarium defect (Figure 5). In particular, the author developed a core-shell CAC construct that enables the loading of Cobalt, which has a pro-angiogenic effect, and BMP-2 that stimulates bone regeneration into the outer shell and inner cone respectively. The scaffold promoted bone regeneration and angiogenesis [89]. Buitrago et al. further incorporated bioactive glass nanoparticles into the outer alginate shell using a nozzle system and showed that bioactive glass nanoparticles were capable of directing bMSCs cell differentiation into osteoblasts in vitro [90].
Figure 5.
H&E stained sections of CAC implant in vivo after 6 weeks. (a) Low magnification image of CAC hydrogel groups including Co (−) BMP (−), Co (+) BMP (−) Co (−) BMP (+), and Co (+) BMP (+), (b) high magnification image of each groups. Reproduced from [89] with permission. Copyright (2015) Elsevier.
The delivery of bone morphogenetic proteins (BMPs) has also been explored in collagen–alginate hydrogel. Quinlan et al. showed the same kinetics BMPs release from Col-HAp scaffold incorporated with ALG microparticle [108]. HAp-Col-Alg scaffold with BMPs loading was also fabricated by Sotome et al., and the results showed the composite hydrogel is a potential drug carrier scaffold for bone tissue engineering [109].
3.1.2. Cartilage Tissue Engineering
Due to its nonrenewable property, cartilage itself cannot regenerate once damaged. Therefore, cartilage regeneration poses a significant challenge in the field of orthopedics. Up to now, extensive efforts and investigations are focusing on developing alternative therapy to repair damaged cartilage. In particular, tissue engineering is considered a promising approach in cartilage regeneration.
The use of bMSCs shows great promise in cartilage regeneration as its potential differentiation into chondroblasts under certain conditions, and their favorable advantages. including ease of isolation and the limited autologous source [110]. Therefore, it is a pressing need to develop an ideal bMSC scaffold that highly recapitulates the native scenario and provides mechanical cues. Many studies have suggested that inducible molecules are essential for guiding chondrogenic differentiation of bMSCs [111]. Zhang et al. (2017) demonstrated that the CAC scaffold provides a suitable environment for bMSCs growth and proliferation. Moreover, bMSCs were capable of differentiating into chondrocytes by inducible agents secreted by co-cultured chondrocytes in vitro [111]. In addition, Ledo et al. (2020) successfully incorporated SOX9 polynucleotides, which play a vital role in chondrogenesis, into CAC hydrogel, which may provide new approach for cartilage tissue engineering [26].
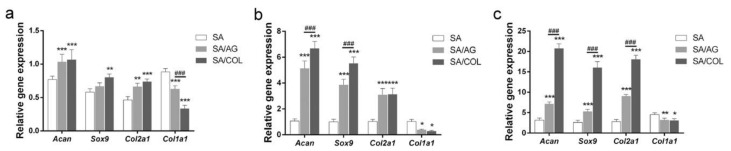
Although transplantation of chondrocytes to damaged cartilage is attractive, one major issue in cartilage regeneration is the chondrocytes’ dedifferentiation characterized by the continuous loss of chondrocyte markers [112]. A set of studies have demonstrated that alginate is capable of dedifferentiating the differentiated chondrocytes [113,114]. Nevertheless, alginate hydrogel has limited ability to maintain chondrocyte viability, restricting the application in the preservation of chondrocyte phenotype. Collagen–alginate hydrogel has the potential to preserve chondrocytes phenotypes [115]. Jin et al. (2018) demonstrated that incorporating collagen into alginate can maintain long-term chondrocytes survival and viability while preserving chondrocyte phenotype. Although collagen gel showed higher cell viability when compared to CAC hydrogel, the enhanced chondrocyte dedifferentiation was observed. Incorporating collagen into alginate, providing a suitable environment for chondrocyte growth and proliferation, inhibits chondrocyte dedifferentiation and preserves its phenotype [115]. Yang et al. (2018) employed 3D printing to fabricate cartilage tissue using CAC hydrogel (Figure 6). In comparison with ALG and ALG/agarose group, CAC group showed higher chondrocyte survival and enhanced chondrocyte gene expression including Acan, Sox9 and Col2a1 and decreased Col1a1 gene expression which is a fibroblast cell marker (Figure 7). This study may provide new approach for cartilage tissue engineering [116].
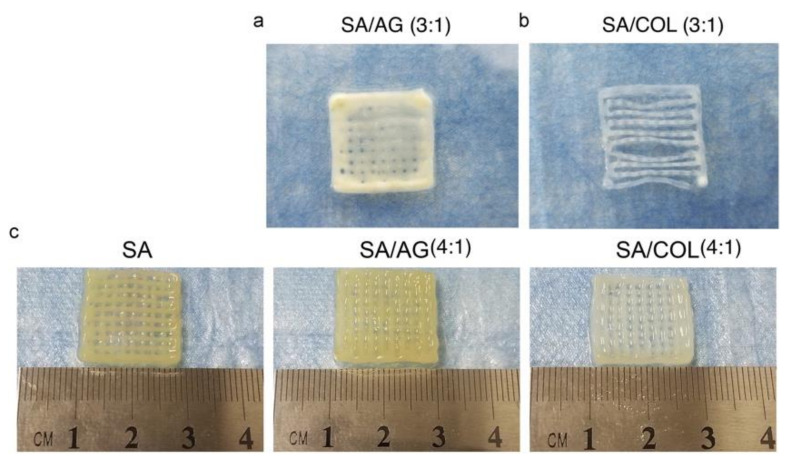
Figure 6.
Small grids by 3D printing. (a) sodium alginate/agarose (3:1), (b) sodium alginate/collagen (3:1), and (c) sodium alginate, sodium alginate/agarose (4:1) and sodium alginate/collagen (4:1). Reproduced from [116] with permission. Copyright (2018) Elsevier.
Figure 7.
Expression level of genes including Acan, Sox9, Col2a1, and Col1a1 was determined by qRT-PCR. 3D constructs including SA, SA/AG and SA/Col in vitro after 3 days (a), 7 days (b), and 14 days (c). *, **, and *** indicated p < 0.05, p < 0.01 and p < 0.001 between SA/AG and SA/COL vs. SA. ### indicated p < 0.001 between SA/AG vs. SA/COL. Reproduced from [116] with permission. Copyright (2018) Elsevier.
3.1.3. Intervertebral Disc (IVD)
Intervertebral discs lie between adjacent vertebras and function as cushions during movement, modulating spinal motion and providing flexibility [117]. An IVD consists of two distinct regions including annulus fibrous (AF) and nucleus pulposus (NP). Gelatinous nucleus pulposus (NP) is located in the inner region which is surrounded by fibrocartilaginous AF ring.
AF is a highly organized tissue composing of collagen (type I) and proteoglycans, while NP is an unaligned structure consisting of collagen (type II) and proteoglycans [118]. Low back pain is highly associated with IVD degeneration. The majority of patients with IVD degeneration suffer from low back pain, which poses a significant burden on their quality of life. Current treatment for low back pain includes analgesics, anti-inflammatory and surgical intervention. Spinal fusion is regarded as gold standard for treating low back pain caused by IVD degeneration. However, it comes with limited spinal mobility and increased chance of adjacent IVD degeneration [119].Therefore, many efforts and strategies have been placed on the development of engineered IVD to replace the degenerated IVD [120,121,122]. In particular, hydrogels including alginate, chitosan, hyaluronic acid, and collagen have been found to have potential as a scaffold in the IVD degeneration [121,123].
Collagen-based hydrogels have been widely investigated for their potential scaffold to treat IVD regeneration. Moriguchi et al. seeded AF cells into high-density collagen gel and showed that cell-loading group has the capacity of promoting IVD regeneration compared to acellular group in the rat model of IVD degeneration induced by needle puncture [124]. Hussain et al. fabricated MSCs laden high-density collagen hydrogel and showed their prospect in AF and NP regeneration in a sheep model of lumbar IVD degeneration [125].
Both alginate and collagen have been widely investigated in IVD regeneration. Tsaryk et al. engineered collagen–alginate IVD construct containing gelatin microspheres. Moreover, the composite hydrogel supported MSC cell survival and promoted its differentiation towards chondrocytes [126]. Bowles et al. seeded AF cells into composite hydrogel in which collagen and alginate were fabricated in the outer region and inner region respectively. The increased alignments of circumferential fibril were observed in the 1% collagen group in vitro [127], which provide a new approach for the engineered-IVD with more complicated structure. Gloria et al. employed 3D bioprinting to manufacture IVD construct containing PCL, collagen with low molecular weight, HA and chitosan nanoparticles [128]. The study further demonstrated that the injectability of this scaffold, similarity of compressive modulus to the natural IVD, and maintenance of cell viability in vitro [128]. Moriguchi et al. constructed engineered IVD construct consisting of outer collagen hydrogel and inner alginate hydrogel [129]. The author also seeded AF and NP cells into both inner and outer region [129]. The implantation of the designed interverbal discs into the canine model of discectomy showed a successful integration of composite hydrogel into the host tissue with no immune response [129].
3.2. Tissue Regeneration
CAC hydrogel is also being extensively explored for the potential in the field of tissue regeneration to generate a range of tissues, including vessel, neuron, liver, and vocal folds owing to its favorable properties, including gelling under mild condition, proper environment for cell growth and proliferation, and ease of incorporation of bioactive agents, and greater retention of bioactive agents.
3.2.1. Blood Vessel
In the human body, blood vessels play an important role in regulating and maintaining homeostasis, such as carrying oxygen and transporting nutrients to tissues, clearance of metabolic waste from cells, and delivering stem cells or progenitor cells to the tissues [130].
The establishment of the blood vessel network, defined by new blood vessel formation from existing blood vessels via cell proliferation, sprouting, and migration, is of great importance in the field of tissue engineering, especially in 3D constructs on a large scale since angiogenesis is crucial for improving transplanted cell survival, and normal function of newly constructed tissues [130,131]. Cells could suffer from hypoxia and receive limited nutrient supply once the distance between cells and blood vessels is over a few hundred microns (150–200 μm) [132]. The situation is even worse in 3D constructs as cells in the core receive oxygen and nutrients only via a blood perfusion reaction rather than a diffusion pathway [133]. In addition, neovascularization is also holding great promise to treat patients with myocardial infarction and stroke.
In order to achieve neovascularization, there are several strategies, including the delivery of pro-angiogenic cells to the body, transportation of angiogenic stimulating factors such as vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF), or the combination of both strategies. However, the direct delivery of cell suspension or angiogenic growth factors to the ischemic tissue comes with poor retention of pro-angiogenic cells and therapeutic factors [134]. In order to combat this issue, many efforts have been placed on achieving better retention of cells and therapeutic agents using biocompatible hydrogel in recent years.
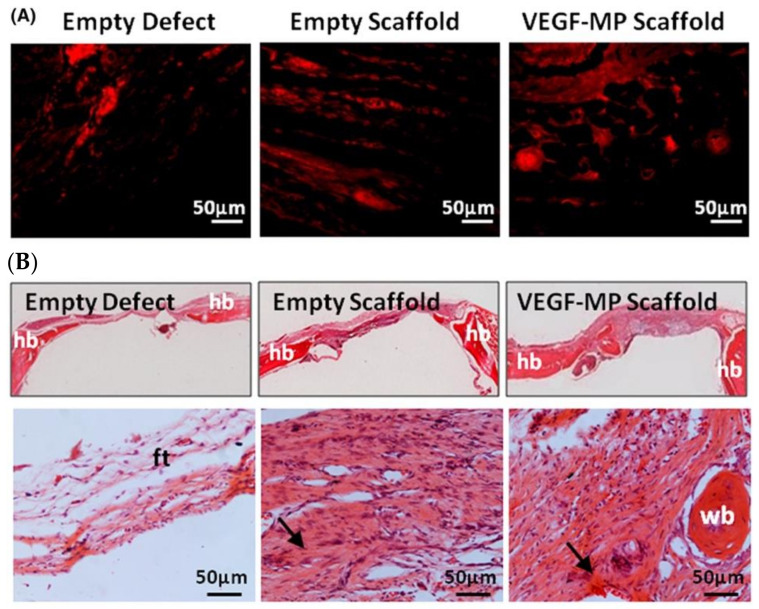
CAC hydrogel has been studied as a drug delivery platform to deliver several angiogenic growth factors to the ischemic tissues. Quinlan et al. incorporated collagen-based scaffold with spray-dried alginate microparticle to achieve long-term (at least 35 days) release of VEGF in vitro [135]. More importantly, when implanted into a rat model of calvarial bone defect, the functionalized composite scaffold was capable of promoting bone regeneration and stimulating new blood vessel formation as demonstrated by threefold increase in the number of endothelial cells labelled by CD31 in comparison to the empty scaffold group (Figure 8). Ali et al. engineered alginate collagen microspheres loaded with FGF-2 and showed at least a 7-day FGF-2 release [136]. The direct transplantation of injectable spheres into ischemic tissue enhances blood vessel formation in zebrafish [136].
Figure 8.
Immunobiological images of blood vessel formation and H&E sections. (A) Immunological images of endothelium labeled by CD31 (red). (B) H&E staining of empty defect, empty scaffold and VEGF-MP scaffold. HB, FT and WB for host bone, fibrotic tissue, and woven bone. Arrows indicated new bone formation. Reproduced from [135] with permission Copyright (2015) John Wiley & Sons, Ltd. Hoboken, NJ, USA.
3.2.2. Neuron
In comparison with other tissues, the extracellular matrix of the brain is considered complicated and soft with a heterogenous network of proteoglycan [137]. Hyaluronic acid, a mucopolysaccharide, interacted with other proteins to contribute the majority of proteoglycan [138]. Nevertheless, the cross-linking of hyaluronic acid-based hydrogel requires chemical modification or physical methods including high temperature, base and UV [139]. The cytotoxicity of the chemical cross-linker or the uncompromised physical method limits its use when working with cell [139]. Alginate is receiving research interest in neuron regeneration in light of its high structure similarity to hyaluronic acid and gelling method under mild condition [140]. Its incorporation with collagen, which contains cell binding motifs to form CAC hydrogel has also been investigated for its ability to encourage neurogenesis.
Moxon et al. demonstrated that compared to alginate hydrogel, composite hydrogel provides a suitable 3D environment that maintains encapsulated cells survival, stimulates neuronal maturation and encourages the projection from neurites as demonstrated by the significant expression of synaptophysin [25]. In addition to collagen fibrils that provide mechanical cues to iPSC-derived neuron, the study further demonstrated that increased stiffness via Ca2+ concentration significantly decreased the expression of microtubule associated protein 2 which is a marker for neurogenesis. The composite hydrogel may be a potential approach to treat patients with disorders related to damaged or degenerated nervous tissue as the work by Jose et al. demonstrated that neural stem cell encapsulated into CAC microsphere using microfluidics technology maintained at least 10-day cell viability and differentiated into neural lineage in the ex vivo model of spinal cord injury [94]. CAC hydrogel has also been combined with other biomaterials and peptides to promote neuronal differentiation. Collagen–alginate composite hydrogel containing hyaluronic acid and methacrylic anhydride has triggered the neuronal differentiation from iPSCs [141].
3.2.3. Liver
Collagen–alginate hydrogel may be a promising approach for the replacement of damaged liver tissue. Chan et al. successfully employed microfluidics technology to encapsulate hepatocytes into CAC hydrogel [93]. Moreover, hepatocytes showed greater functions [93]. In particular, when compared to the control groups including collagen and alginate group, the total albumin secretion from hepatocytes in the CAC group was significantly higher in vitro. In addition, increased cytochrome enzyme activity was observed in the CAC group.
3.2.4. Vocal Folds Restoration
The work conducted by Hahn et al. showed promise as a potential scaffold in vocal fold restoration [142]. A significant increase in synthesized reticular collagen fibrils was observed in CAC group compared to the collagen-hyaluronic acid group. In addition, CAC hydrogel maintained excellent mass and no reduction in gel size over a 42-day cell culture, further suggesting that CAC hydrogel exhibited a desirable 3D matrix for vocal folds regeneration compared to the collagen-HA scaffold.
CAC hydrogel has been found great potential as a scaffold in tissue engineering and tissue regeneration. Table 3 provides a list of existing studies of CAC hydrogel as a scaffold that can be used in different tissues.
Table 3.
Summary of CAC as a scaffold in tissue engineering and tissue regeneration.
3.3. Biomedical Sciences
3.3.1. Wound Dressing
The definition of a wound is the laceration or discontinuity of epithelium or mucosa resulting from violence, accidents or surgical procedure. Wound can be classified into two categories, including acute and chronic wound, based on the duration and nature of the healing process [143,144]. Wound healing is a complicated, intricate, and dynamic process orchestrated by an array of cells, growth factors, cytokines, and ECM, which requires an appropriate and suitable environment to assist the healing process [145]. The wound dressing is of great importance during the healing process since a proper wound dressing can accelerate the physiological reconstruction meanwhile minimizing the risk of bacterial infection and protecting wound contamination from the surroundings and retention of a moist environment for the wound [146]. The ultimate goal of wound care is to completely restore and reconstruct the damaged tissue to its prior functional level with satisfactory cosmetic results [147].
When designing an ideal wound dressing, various considerations and factors should be taken into account, including its ability to (1) keep and maintain a moist environment, (2) promote new blood vessel and physiological reconstruction of connective tissue and skins, (3) accelerate epidermal migration (4) permit gas exchange between injury site and the surroundings (5) remove easily (6) fight microbial infection (7) maintain appropriate environments such as PH and temperature and (8) offer sterilized condition with noncytotoxic and nonallergenic properties [148].
Traditional wound dressings, including gauze, and bandages, mainly provide a physiological barrier to prevent microorganism infection, meanwhile permitting exudate adsorption to maintain a dry environment for the wound site [149]. Nowadays, the market of modern wound dressing is expanding as it can provide a moist environment that stimulates cell proliferation and differentiation, therefore accelerating the healing process and minimizing the chance of bacterial infection compared with traditional wound dressing. There are growing modern wound dressing products available in the market that combine two or three natural biomaterials, including alginate, collagen, chitosan, and cellulose, since the composite hydrogel enables the integration of each individual favorable properties to meet the requirements of ideal wound dressings.
Alginate-based wound dressing has been applied as one type of modern dressings for many years since alginate provides several superior features, including biocompatibility, low cytotoxicity to mammalian cells, and moist environment retention compared to traditional ones [149]. Plenty of studies have reported that alginate exhibited a great promoting effect on the wound healing process. However, the poor hemostatic effect has also been recognized. Collagen has been studied as a biomaterial in wound dressing owing to its unique characteristics, such as excellent biocompatibility and biodegradability [150]. Collagen has been found to accelerate wound healing by increasing cell proliferation, guiding cell differentiation, promoting neovascularization, and accelerating blood coagulation [151]. Incorporating collagen with alginate has attracted much research attention recently due to its properties, including excellent biocompatibility, low immunogenicity and superb hemostatic effect. In addition to that, collagen–alginate wound dressings have exhibited unique properties, including (1) alginate could provide a moist environment which is essential and beneficial for wound healing, (2) collagen can stimulate fibroblast migration, promote re-epithelization and angiogenesis, (3) mild gelling that enables cell encapsulation and (4) ease of incorporation with other materials and antimicrobial agents (Table 4). Therefore, CAC composite hydrogel is a potential candidate in wound care. Several collagen–alginate wound dressing products, including FIBRACOL Plus, DermaCol Ag, and ColActive Plus, are commercially available.
Table 4.
Summary of CAC hydrogel in wound dressing.
| Composition | Finding | Reference |
|---|---|---|
| Collagen–alginate-AMPs | Antimicrobial activity and no cytotoxicity to fibroblasts | [152] |
| Collagen–alginate-hyaluronic acid-AMPs | Antimicrobial activity, good biocompatibility, reduced inflammation, and angiogenesis | [153] |
| Collagen–alginate wound dressing | Accelerating wound healing and great patients’ satisfaction | [154,155] |
| Aminated collagen-oxidized alginate-polymyxin B sulfate-bacitracin | Antimicrobial activity, angiogenesis, and reepithelization | [156] |
| Chitosan-collagen–alginate dressing-PU membrane | Hemocompatibility, reepithelization, and water-resistance | [157] |
| Collagen–alginate-AgNPs | Antibacterial activity but dose-dependent cytotoxicity to mammalian cells | [158] |
| Collagen–alginate-hUCMSCs | Promote wound healing, reepithelization, and angiogenesis | [159] |
Recently, a novel wound dressing comprised of two or three biomaterials such as collagen, alginate, chitosan, HA, and cellulose incorporated with functional and bioactive agents has been studied since single material cannot meet all requirements of the ideal wound dressing [160].
Carl et al. demonstrated that, compared to traditional dressing, the collagen–alginate wound dressing consisting of 90% collagen and 10% alginate presented a decreased wound healing duration and reduced chance of infection and contamination, improved patients’ compliance, and higher satisfaction in patients with chemical matricectomies [154]. In another randomized clinical trial, patients with diabetic foot ulcers were treated with traditional wound dressing (gauze moistened with 0.9% NaCl) and a modern collagen–alginate wound dressing. The results showed that a reduction of wound size in the collagen–alginate group compared to the gauze group. More patients reported having complete wound healing in the CAC group, which suggested that the collagen–alginate wound dressing has more efficacy than commercially used gauze wound dressing [155]. Moreover, greater satisfaction was shown in collagen–alginate treatment group owing to its ease of removal and limited wound care duration. Collagen–alginate dressing group showed significantly less painful index, cost-effectiveness, decreased duration of re-epithelization. The transplantation of CAC scaffold into defects promoted wound healing in vivo. Collagen–alginate wound dressings have more advantageous effects on promoting wound healing [103].
The use of stem cells in wound dressing has emerged as a promising strategy due to their potential to reconstruct tissue to a pre-injured state. A recent study by Zhang et al. demonstrated that encapsulated human umbilical cord mesenchymal stem cells (HUCMSCs) in collagen–alginate hydrogel maintained good viability and growth factors secreted by HUCMSCs inhibited immunological response, thereby promoting wound healing and tissue reconstruction [159].
CAC hydrogel can also be incorporated with other biomaterials to gain more favorable properties. A recent study by Xie et al. demonstrated that coupling chitosan, which exhibits non-cytotoxicity, antibacterial effect, hemostatic effect, and biocompatibility, into CAC wound dressing facilitated platelet aggregation and formation of a fibrin clot, accelerated cell migration including fibroblasts and endothelium to the wound site, thereby promoting wound healing in the rat model of the full-thickness wound [157]. The CCA wound dressing was further attached to polyurethanes (PU) membrane to obtain seawater resistance [157]. Patients with acute full-thickness wounds suffered from a more extended wound healing period, which increases the risk of bacterial infection, finally leading to wound ulceration, necrosis, and even life-threatening complications. It is estimated that over two million people carry wounds containing antibiotic-resistant bacteria contamination. The growing prevalence of multidrug-resistant (MDR) pathogen has led to more than twenty thousand deaths yearly, providing a strong impetus to develop modern dressing with anti-bacterial infection [161]. Up to date, the functionalization of wound dressings with antiseptic and anti-inflammatory agents are showing a more powerful effect on promoting wound healing [162]. CAC wound dressing does not possess antibacterial properties. Therefore, research into the development of novel dressing designs with antimicrobial properties by incorporating nanoparticles or antiseptic agents has received much attention. Feng et al. incorporated polymyxin B sulfate and bacitracin into CAC wound dressing. They reported that CAC-PB exhibited an excellent antibacterial effect against E. coli and S. aureus and promoted wound healing in a rat model of a full-thickness wound as the evidence of observation of granulation tissue, the new formation of the vascular network, and re-epithelization [156]. Silver has been extensively studied as its excellent antimicrobial activity against fungi, bacteria, and viruses. In a recent study by Zhang et al., AgNps, a silver nanoparticle, was introduced into a collagen–alginate wound dressing to achieve the anti-microorganism function [158]. Functionalization of CAC with AgNps showed their significant antibacterial effect against E. coli and S. aureus. Nevertheless, the dose-dependent cytotoxicity of silver nanoparticles to mammalian cells and impaired wound healing process cannot be overlooked. Therefore, scientists are searching for novel wound dressing with broad antibacterial function and weak toxicity to mammalian cells.
Antimicrobial peptides (AMPs), which belong to one part of innate immune system, have found their benefit for promoting wound healing and therapeutic potentials to treat wound infection on account of their broad-spectrum antimicrobial effect on both Gram-positive and Gram-negative bacteria and inherent favorable properties including limited cytotoxicity to human mammalian cells and regulatory effect on immune cell migration and dendritic differentiation [163]. Lin et al. conjugated AMP Tet213 with CAC hydrogel containing hyaluronic acid via the chemical bond. Moreover, slow and controlled release of AMP Tet213 from Alg/Ha/Col-AMP wound dressing showed a significant inhibitory effect on bacterial infection such as E. coli and S. aureus and promoting effect on wound healing in vivo via its regulatory role in inflammation, collagen deposition, and angiogenesis [153].
Although a vast amount of biomaterial-based wound dressings has been developed, there is limited knowledge regarding the impact of biomaterial scaffold on the wound healing process. One study conducted by Cunha et al. synthesized a set of CAC scaffolds with different storage modulus via tuning the concentration of Ca2+. The result demonstrated that varying stiffnesses caused the morphologic change on fibroblasts and regulated the secretion of inflammatory factors, therefore accelerating or inhibiting the wound dressing process [164].
3.3.2. Encapsulated Cell Therapy
CAC hydrogel has been developed as cell carriers to deliver bioactive agents secreted by encapsulated cells inside the device to treat a set of diseases including type 1 diabetes and retina degeneration.
The concept of ECT is to deliver fresh and synthesized therapeutic agents secreted by live cells either from allogeneic or xenogeneic sources that are encapsulated in a semipermeable membrane. The semipermeable membrane allows gas exchange and nutrients supply while avoiding immunological reaction from host upon transplantation via blocking the immune mediators [165]. Moreover, the function of polymers is to provide mechanical protection to the enclosed cells from shear force damage [166]. The history of cell encapsulation can be traced back to the early 1930s when Bisceglie et al. encapsulated cancer cells into the polymer scaffold and successfully transplanted the capsule into pig abdomen. The concept of “artificial cell” was firstly depicted by Tomas Chang, who subsequently proposed the idea of encapsulated cell therapy [167].
Various preclinical and clinical trials on the efficacy of ECT to treat central nervous system degeneration and retinal degeneration, including amyotrophic lateral sclerosis, Huntington’s disease, Alzheimer’s disease as well as retinitis pigmentosa have been undertaken [168]. There are plenty of biomaterials which have been investigated and developed as a scaffold for ECT device. Among these biomaterials, alginate is the most commonly used biomaterial either alone or in combination with other biomaterials. Nevertheless, the inherent lack of cell-binding sites limits its widespread application in ECT. Therefore, incorporating collagen, which contains cell-binding motifs, maintains cell viability and promotes cell proliferation via facilitating cell adhesion and attachment. CAC hydrogel is becoming the focus in ECT as composite hydrogel combines collagen and alginate merits. In this section, encapsulation of glial cell line-derived neurotrophic factor (GDNF)-secreting human embryonic kidney (HEK) cells, islet cells as an alternative therapy to treat retinal degenerative disease, and type 1 diabetes will be illustrated.
Islet Cells
The increasing number of diabetes mellitus (DM) imposes a significant burden on the healthcare system around the world. Pancreatic islets transplantation recently has been considered as a potential treatment for DM patients. Nevertheless, there remains the difficulty in the isolation of pancreatic islet cells from the donor, maintaining viability during the procedure, and the relative risk of pancreas transplantation is of grave concern. The work conducted by Lee et al. revealed the great promise of ECT as a treatment for type 1 diabetes. The author encapsulated islet cells into collagen–alginate composite hydrogel using the concave well array for manufacturing CAC hydrogel on a large scale.
The author showed the successful encapsulation of islet spheroids into CAC hydrogel. Compared to the pure alginate group, CAC showed increased islet cell viability and integrity for at least eight weeks, suggesting CAC is an excellent biomaterial scaffold for maintaining islet cell viability and function [92]. Moreover, the incorporation of collagen enhanced the inward diffusion of oxygen and nutrient to the islet cells. More importantly, encapsulated islet spheroids showed a glucose-controlling effect compared to the direct implantation of intact islets into the mice with diabetes. Direct implant of intact islets demonstrated robust and significant CD 45 fluorescent signals compared to the encapsulated islet group, strongly suggesting that CAC protected islets cells from immune attack and provided mechanical protection.
GDNF-Secreting HEK Cells
GDNF has demonstrated a neuroprotective effect on both the central nervous system (CNS) and peripheral nervous system (PNS). GDNF has been proved its neuroprotective effect in a set of animal models. Recently, GDNF has been applied into preclinical and clinic trials to treat CNS degeneration. Nevertheless, considering the relative half-life of GDNF, and the potential adverse effect of systemically administration of GDNF, ECT that enables continuous GDNF release is required. Wong et al. encapsulated GDNF-secreting HEK cells into three-dimensional collagen microspheres to achieve the continuous GDNF secretion. When enclosing GDNF-secreting HEK cells into 3D collagen microsphere, it showed dramatically increased GDNF secretion compared to monolayer cell culture since collagen recapitulates a native tissue-like environment for cells to grow and proliferate [169]. Further analysis suggested that GDNF secreted from 3D collagen microspheres possesses bioactive properties as GDNF promoted neurite extension, highly suggesting the potential of cell-collagen encapsulation as a drug delivery platform in the field of pharmaceutics.
Another study conducted by the same group introduced alginate into collagen-cell microspheres to limit cell migration from collagen scaffold to the surroundings for further application since cell migration from ECT may elicit immunological response and immune rejection [170]. In this work, the author illustrated that the collaboration of alginate with collagen is able to prevent cell migration and also allow continuous GDNF secretion from the device. Moreover, cell viability is highly associated with alginate concentration. In summary, the work developed a novel CAC ECT device as a potential drug delivery platform to treat CNS degenerative disease. Wong et al. further developed CAC ECT device from microsphere to cylinder-like structure as a potential treatment for retinal degeneration and possibly retinitis pigmentosa. The work demonstrated that long-term system stability and performance of CAC ECT design can be tuned by alginate concentration and the importance of initial cell density on the optimization of ECT device. In vivo studies demonstrated that rat vitreous can support cell survival and proliferation inside CAC ECT device and GDNF secretion from the device [22]. Wong et al. further demonstrated that the performance of CAC ECT device could be tuned by varying parameters, including initial cell density, gelation sequence, alginate molecular weight, and concentration. More importantly, continuous GDNF secretion from the CAC ECT device has a neuroprotective effect on photoreceptor both histologically and electrophysiologically in the rat model of photoreceptor degeneration, which strongly implied that CAC ECT platform is a promising treatment to treat a group of retinal degenerative diseases [23].
3.3.3. Tumor Biology
There is no doubt that cells live in the extracellular matrix that facilitates cell interaction, cell migration and cell attachment to basement, allows metabolic waste exchange and provides oxygen and nutrients to the cells. Although the vast majority of research into cancer studies are performed in two-dimensional (2D) cell culture, the limitation of 2D cancer cell studies compared to 3D culture cannot be ignored, including lack of cell interaction to ECM and cell migration, limited signal transduction, and the absence of cytoskeletal organization, which may lead to inconsistent findings between in vitro and in vivo studies.
The proliferation of tumor cells and their morphology are associated with the stiffness of 3D scaffold. A study showed that higher 3D stiffness promoted multicellular aggregate proliferation, which may provide an alternative strategy for cancer treatment and diagnosis [171]. Wang et al. further demonstrated the effect of stiffness on tumor cell migration. The study showed that higher stiffness can decrease cell volume, reducing the tumor cell migration capability [172]. Liu et al. also developed a tumor-like scaffold to mimick in vivo tumor scenario and showed tumor invasion in the 3D scaffold is similar to in vivo environment [173].
4. Conclusions and Future Perspectives
CAC hydrogel has shown its great potential in tissue engineering and biomedical sciences owing to its wide availability, excellent biocompatibility, ease of gelation, and weak cytotoxicity. The unique properties of CAC hydrogel include enhanced mechanical properties, moldability, and tunning porosity. Besides, CAC can also be incorporated with other materials, molecules, and nanoparticles to obtain more favorable features. In particular, in tissue engineering, CAC has been developed as a platform to deliver molecules or cells to the defect site. Moreover, Composite hydrogel can be further incorporated with other bioactive agents, especially in bone tissue engineering. CAC hydrogel is also being extensively explored for its potential in tissue regeneration, including angiogenesis, liver, neuron, and vocal folds. CAC hydrogel promotes wound healing by providing a moist environment and cell-binding site that enables cell migration and cell proliferation. The ease of incorporation of antibacterial agents and AMPs further expands their application in wound care. CAC hydrogel has been investigated as a drug delivery platform in the field of ECT. ECT device can be optimized by different parameters such as initial cell density, ALG concentration and molecular weight, and collagen concentration, which provides a new view for ECT application.
However, the majority of CAC studies in cartilage tissue engineering are performed in vitro. The applicability of CAC in cartilage regeneration warrants further animal investigation. 3D printing may provide an alternative approach for CAC application in cartilage tissue engineering. Although hydrogel has been investigated in IVD regeneration, the construction of IVD for clinical use is still a considerable challenge. More investigations should be placed on the generation of engineered-IVD with optimal mechanical properties, the integration of engineered-IVD to host tissue, and the maintenances of encapsulated cell viability. The performance of CAC ECT device can be tuned via initial cell number, ALG and COL concentration, and molecular weight. Therefore, the efficacy of ECT in the treatment of diseases requires more investigation. One of the merits of composite hydrogel is its tunning mechanical properties. However, there are limited studies regarding the effect of stiffness on encapsulated cells and tumor cells, which warrants more investigation.
Author Contributions
T.H. contributed to writing the manuscript. A.C.Y.L. contributed to editing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Health and Medical Research Fund, the Food and Health Bureau, The Government of the Hong Kong Special Administrative Region (06171516). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hubbell J.A. Biomaterials in tissue engineering. Bio-technology. 1995;13:565–576. doi: 10.1038/nbt0695-565. [DOI] [PubMed] [Google Scholar]
- 2.Huebsch N., Mooney D.J. Inspiration and application in the evolution of biomaterials. Nature. 2009;462:426–432. doi: 10.1038/nature08601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ratner B.D., Bryant S.J. Biomaterials: Where we have been and where we are going. Annu. Rev. Biomed. Eng. 2004;6:41–75. doi: 10.1146/annurev.bioeng.6.040803.140027. [DOI] [PubMed] [Google Scholar]
- 4.Ullah S., Chen X. Fabrication, applications and challenges of natural biomaterials in tissue engineering. Appl. Mater. Today. 2020;20:100656. doi: 10.1016/j.apmt.2020.100656. [DOI] [Google Scholar]
- 5.Porter R.M., Akers R.M., Howard R.D., Forsten-Williams K. Alginate encapsulation impacts the insulin-like growth factor-I system of monolayer-expanded equine articular chondrocytes and cell response to interleukin-1 beta. Tissue Eng. 2007;13:1333–1345. doi: 10.1089/ten.2006.0345. [DOI] [PubMed] [Google Scholar]
- 6.Wee S., Gombotz W.R. Protein release from alginate matrices. Adv. Drug Deliv. Rev. 1998;31:267–285. doi: 10.1016/s0169-409x(97)00124-5. [DOI] [PubMed] [Google Scholar]
- 7.Kim W.S., Mooney D.J., Arany P.R., Lee K., Huebsch N., Kim J. Adipose tissue engineering using injectable, oxidized alginate hydrogels. Tissue Eng. Part A. 2012;18:737–743. doi: 10.1089/ten.tea.2011.0250. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki Y., Nishimura Y., Tanihara M., Suzuki K., Nakamura T., Shimizu Y., Yamawaki Y., Kakimaru Y. Evaluation of a novel alginate gel dressing: Cytotoxicity to fibroblasts in vitro and foreign-body reaction in pig skin in vivo. J. Biomed. Mater. Res. 1998;39:317–322. doi: 10.1002/(SICI)1097-4636(199802)39:2<317::AID-JBM20>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 9.Peters M.C., Polverini P.J., Mooney D.J. Engineering vascular networks in porous polymer matrices. J. Biomed. Mater. Res. 2002;60:668–678. doi: 10.1002/jbm.10134. [DOI] [PubMed] [Google Scholar]
- 10.You J.O., Liu Y.C., Peng C.A. Efficient gene transfection using chitosan-alginate core-shell nanoparticles. Int. J. Nanomed. 2006;1:173–180. doi: 10.2147/nano.2006.1.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee K.Y., Mooney D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012;37:106–126. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee J.W., Juliano R. Mitogenic signal transduction by integrin—And growth factor receptor—Mediated pathways. Mol. Cells. 2004;17:188–202. [PubMed] [Google Scholar]
- 13.Burgeson R.E., Nimni M.E. Collagen types—Molecular-structure and tissue Distribution. Clin. Orthop. Relat. R. 1992;282:250–272. [PubMed] [Google Scholar]
- 14.Ramachandran G.N. Structure of collagen at the molecular level. In: Ramachandran G.N., editor. Treatise on Collagen. Academic Press; New York, NJ, USA: 1967. pp. 103–183. [Google Scholar]
- 15.Chattopadhyay S., Raines R.T. Collagen-based biomaterials for wound healing. Biopolymers. 2014;101:821–833. doi: 10.1002/bip.22486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hesse E., Hefferan T.E., Tarara J.E., Haasper C., Meller R., Krettek C., Lu L.C., Yaszemski M.J. Collagen type I hydrogel allows migration, proliferation, and osteogenic differentiation of rat bone marrow stromal cells. J. Biomed. Mater. Res. A. 2010;94:442–449. doi: 10.1002/jbm.a.32696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lynn A.K., Yannas I.V., Bonfield W. Antigenicity and immunogenicity of collagen. J. Biomed. Mater. Res. Part B. 2004;71B:343–354. doi: 10.1002/jbm.b.30096. [DOI] [PubMed] [Google Scholar]
- 18.Radhakrishnan S., Nagarajan S., Bechelany M., Kalkura S.N. Collagen based biomaterials for tissue engineering applications: A review. In: Frank-Kamenetskaya O., Vlasov D., Panova E., Lessovaia S., editors. Processes and Phenomena on the Boundary between Biogenic and Abiogenic Nature—Lecture Notes in Earth System Sciences. Springer; Cham, Switzerland: 2020. pp. 3–22. [Google Scholar]
- 19.Antoine E.E., Vlachos P.P., Rylander M.N. Review of collagen i hydrogels for bioengineered tissue microenvironments: Characterization of mechanics, structure, and transport. Tissue Eng. Part B Rev. 2014;20:683–696. doi: 10.1089/ten.teb.2014.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heino J. Cellular signaling by collagen-binding integrins. I Domain Integrins. 2014;819:143–155. doi: 10.1007/978-94-017-9153-3_10. [DOI] [PubMed] [Google Scholar]
- 21.Holder A.J., Badiei N., Hawkins K., Wright C., Williams P.R., Curtis D.J. Control of collagen gel mechanical properties through manipulation of gelation conditions near the sol-gel transition. Soft Matter. 2018;14:574–580. doi: 10.1039/C7SM01933E. [DOI] [PubMed] [Google Scholar]
- 22.Wong F.S.Y., Wong C.C.H., Chan B.P., Lo A.C.Y. Sustained delivery of bioactive GDNF from collagen and alginate-based cell-encapsulating gel promoted photoreceptor survival in an inherited retinal degeneration model. PLoS ONE. 2016;11:e0159342. doi: 10.1371/journal.pone.0159342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong F.S.Y., Tsang K.K., Chu A.M.W., Chan B.P., Yao K.M., Lo A.C.Y. Injectable cell-encapsulating composite alginate-collagen platform with inducible termination switch for safer ocular drug delivery. Biomaterials. 2019;201:53–67. doi: 10.1016/j.biomaterials.2019.01.032. [DOI] [PubMed] [Google Scholar]
- 24.Liu W.G., Griffith M., Li F.F. Alginate microsphere-collagen composite hydrogel for ocular drug delivery and implantation. J. Mater. Sci. Mater. Med. 2008;19:3365–3371. doi: 10.1007/s10856-008-3486-2. [DOI] [PubMed] [Google Scholar]
- 25.Moxon S.R., Corbett N.J., Fisher K., Potjewyd G., Domingos M., Hooper N.M. Blended alginate/collagen hydrogels promote neurogenesis and neuronal maturation. Mat. Sci. Eng. C. 2019;104:109904. doi: 10.1016/j.msec.2019.109904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ledo A.M., Vining K.H., Alonso M.J., Garcia-Fuentes M., Mooney D.J. Extracellular matrix mechanics regulate transfection and SOX9-directed differentiation of mesenchymal stem cells. Acta Biomater. 2020;110:153–163. doi: 10.1016/j.actbio.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strand B.L., Morch Y.A., Skjak-Braek G. Alginate as immobilization matrix for cells. Minerva Biotecnol. 2000;12:223–233. [Google Scholar]
- 28.Smidsrod O., Skjakbraek G. Alginate as immobilization matrix for cells. Trends Biotechnol. 1990;8:71–78. doi: 10.1016/0167-7799(90)90139-O. [DOI] [PubMed] [Google Scholar]
- 29.Rinaudo M. Main properties and current applications of some polysaccharides as biomaterials. Polym. Int. 2008;57:397–430. doi: 10.1002/pi.2378. [DOI] [Google Scholar]
- 30.Remminghorst U., Rehm B.H.A. Bacterial alginates: From biosynthesis to applications. Biotechnol. Lett. 2006;28:1701–1712. doi: 10.1007/s10529-006-9156-x. [DOI] [PubMed] [Google Scholar]
- 31.Martinsen A., Skjakbraek G., Smidsrod O. Alginate as immobilization material.1. Correlation between chemical and physical-properties of alginate gel beads. Biotechnol. Bioeng. 1989;33:79–89. doi: 10.1002/bit.260330111. [DOI] [PubMed] [Google Scholar]
- 32.Tonnesen H.H., Karlsen J. Alginate in drug delivery systems. Drug Dev. Ind. Pharm. 2002;28:621–630. doi: 10.1081/DDC-120003853. [DOI] [PubMed] [Google Scholar]
- 33.George M., Abraham T.E. Polyionic hydrocolloids for the intestinal delivery of protein drugs: Alginate and chitosan—A review. J. Control. Release. 2006;114:1–14. doi: 10.1016/j.jconrel.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 34.LeRoux M.A., Guilak F., Setton L.A. Compressive and shear properties of alginate gel: Effects of sodium ions and alginate concentration. J. Biomed. Mater. Res. 1999;47:46–53. doi: 10.1002/(SICI)1097-4636(199910)47:1<46::AID-JBM6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 35.Kong H.J., Smith M.K., Mooney D.J. Designing alginate hydrogels to maintain viability of immobilized cells. Biomaterials. 2003;24:4023–4029. doi: 10.1016/S0142-9612(03)00295-3. [DOI] [PubMed] [Google Scholar]
- 36.Orive G., Carcaboso A.M., Hernandez R.M., Gascon A.R., Pedraz J.L. Biocompatibility evaluation of different alginates and alginate-based microcapsules. Biomacromolecules. 2005;6:927–931. doi: 10.1021/bm049380x. [DOI] [PubMed] [Google Scholar]
- 37.Lee J., Lee K.Y. Local and sustained vascular endothelial growth factor delivery for angiogenesis using an injectable system. Pharm Res. 2009;26:1739–1744. doi: 10.1007/s11095-009-9884-4. [DOI] [PubMed] [Google Scholar]
- 38.Mushollaeni W., Supartini N., Rusdiana E. Toxicity test of alginate from Sargassum and Padina on the liver of mice. Food Public Health. 2014;4:204–208. [Google Scholar]
- 39.Grant G.T., Morris E.R., Rees D.A., Smith P.J.C., Thom D. Biological interactions between polysaccharides and divalent cations—Egg-box model. FEBS Lett. 1973;32:195–198. doi: 10.1016/0014-5793(73)80770-7. [DOI] [Google Scholar]
- 40.Kuo C.K., Ma P.X. Ionically cross-linked alginate hydrogels as scaffolds for tissue engineering: Part 1. Structure, gelation rate and mechanical properties. Biomaterials. 2001;22:511–521. doi: 10.1016/S0142-9612(00)00201-5. [DOI] [PubMed] [Google Scholar]
- 41.Abasalizadeh F., Moghaddam S.V., Alizadeh E., Akbari E., Kashani E., Fazljou S.M.B., Torbati M., Akbarzadeh A. Alginate-based hydrogels as drug delivery vehicles in cancer treatment and their applications in wound dressing and 3D bioprinting. J. Biol. Eng. 2020;14:8. doi: 10.1186/s13036-020-0227-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mourino V., Newby P., Pishbin F., Cattalini J.P., Lucangioli S., Boccaccini A.R. Physicochemical, biological and drug-release properties of gallium cross-linked alginate/nanoparticulate bioactive glass composite films. Soft Matter. 2011;7:6705–6712. doi: 10.1039/c1sm05331k. [DOI] [Google Scholar]
- 43.Hijazi S., Visaggio D., Pirolo M., Frangipani E., Bernstein L., Visca P. Antimicrobial activity of gallium compounds on ESKAPE pathogens. Front. Cell Infect. Microbiol. 2018;8:316. doi: 10.3389/fcimb.2018.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chitambar C.R. Gallium and its competing roles with iron in biological systems. BBA Mol. Cell Res. 2016;1863:2044–2053. doi: 10.1016/j.bbamcr.2016.04.027. [DOI] [PubMed] [Google Scholar]
- 45.Rastin H., Ramezanpour M., Hassan K., Mazinani A., Tung T.T., Vreugde S., Losic D. 3D bioprinting of a cell-laden antibacterial polysaccharide hydrogel composite. Carbohydr. Polym. 2021;264:117989. doi: 10.1016/j.carbpol.2021.117989. [DOI] [PubMed] [Google Scholar]
- 46.Parenteau-Bareil R., Gauvin R., Berthod F. Collagen-based biomaterials for tissue engineering applications. Materials. 2010;3:1863–1887. doi: 10.3390/ma3031863. [DOI] [Google Scholar]
- 47.Vanderrest M., Garrone R. Collagen family of proteins. FASEB J. 1991;5:2814–2823. doi: 10.1096/fasebj.5.13.1916105. [DOI] [PubMed] [Google Scholar]
- 48.Prockop D.J., Kivirikko K.I. Collagens—Molecular-Biology, diseases, and potentials for therapy. Annu. Rev. Biochem. 1995;64:403–434. doi: 10.1146/annurev.bi.64.070195.002155. [DOI] [PubMed] [Google Scholar]
- 49.Chevallay B., Herbage D. Collagen-based biomaterials as 3D scaffold for cell cultures: Applications for tissue engineering and gene therapy. Med. Biol. Eng. Comput. 2000;38:211–218. doi: 10.1007/BF02344779. [DOI] [PubMed] [Google Scholar]
- 50.Wolf K., Alexander S., Schacht V., Coussens L.M., von Andrian U.H., van Rheenen J., Deryugina E., Friedl P. Collagen-based cell migration models in vitro and in vivo. Semin. Cell Dev. Biol. 2009;20:931–941. doi: 10.1016/j.semcdb.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gelse K., Poschl E., Aigner T. Collagens—structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003;55:1531–1546. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 52.Osidak E.O., Kozhukhov V.I., Osidak M.S., Domogatsky S.P. Collagen as bioink for bioprinting: A comprehensive review. Int. J. Bioprint. 2020;6:270. doi: 10.18063/ijb.v6i3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Badylak S.F. Xenogeneic extracellular matrix as a scaffold for tissue reconstruction. Transpl. Immunol. 2004;12:367–377. doi: 10.1016/j.trim.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 54.Kumar V.A., Taylor N.L., Jalan A.A., Hwang L.K., Wang B.K., Hartgerink J.D. A Nanostructured synthetic collagen mimic for hemostasis. Biomacromolecules. 2014;15:1484–1490. doi: 10.1021/bm500091e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu X., Gan Q.L., Clough R.C., Pappu K.M., Howard J.A., Baez J.A., Wang K. Hydroxylation of recombinant human collagen type I alpha 1 in transgenic maize co-expressed with a recombinant human prolyl 4-hydroxylase. BMC Biotechnol. 2011;11:69. doi: 10.1186/1472-6750-11-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cooperman L., Michaeli D. The immunogenicity of injectable collagen. II. A retrospective review of seventy-two tested and treated patients. J. Am. Acad. Derm. 1984;10:647–651. doi: 10.1016/S0190-9622(84)80272-8. [DOI] [PubMed] [Google Scholar]
- 57.Addad S., Exposito J.Y., Faye C., Ricard-Blum S., Lethias C. Isolation, characterization and biological evaluation of jellyfish collagen for use in biomedical applications. Mar. Drugs. 2011;9:967–983. doi: 10.3390/md9060967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim B.S., Choi J.S., Kim J.D., Yoon H.I., Choi Y.C., Cho Y.W. Human collagen isolated from adipose tissue. Biotechnol. Prog. 2012;28:973–980. doi: 10.1002/btpr.1555. [DOI] [PubMed] [Google Scholar]
- 59.Lauer-Fields J.L., Fields G.B. Triple-helical peptide analysis of collagenolytic protease activity. Biol. Chem. 2002;383:1095–1105. doi: 10.1515/BC.2002.118. [DOI] [PubMed] [Google Scholar]
- 60.Fields G.B. A Model for interstitial collagen catabolism by mammalian collagenases. J. Theor. Biol. 1991;153:585–602. doi: 10.1016/S0022-5193(05)80157-2. [DOI] [PubMed] [Google Scholar]
- 61.Aimes R.T., Quigley J.P. Matrix metalloproteinase-2 Is an interstitial collagenase—Inhibitor-Free Enzyme catalyzes the cleavage of collagen fibrils and soluble native type-I collagen generating the specific 3/4-length and 1/4-length fragments. J. Biol. Chem. 1995;270:5872–5876. doi: 10.1074/jbc.270.11.5872. [DOI] [PubMed] [Google Scholar]
- 62.Ohuchi E., Imai K., Fujii Y., Sato H., Seiki M., Okada Y. Membrane type 1 matrix metalloproteinase digests interstitial collagens and other extracellular matrix macromolecules. J. Biol. Chem. 1997;272:2446–2451. doi: 10.1074/jbc.272.4.2446. [DOI] [PubMed] [Google Scholar]
- 63.Song E., Kim S.Y., Chun T., Byun H.J., Lee Y.M. Collagen scaffolds derived from a marine source and their biocompatibility. Biomaterials. 2006;27:2951–2961. doi: 10.1016/j.biomaterials.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 64.Wu X.M., Black L., Santacana-Laffitte G., Patrick C.W. Preparation and assessment of glutaraldehyde-cross-linked collagen-chitosan hydrogels for adipose tissue engineering. J. Biomed. Mater. Res. Part A. 2007;81A:59–65. doi: 10.1002/jbm.a.31003. [DOI] [PubMed] [Google Scholar]
- 65.Powell H.M., Boyce S.T. EDC cross-linking improves skin substitute strength and stability. Biomaterials. 2006;27:5821–5827. doi: 10.1016/j.biomaterials.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 66.Tu R., Quijano R.C., Lu C.L., Shen S., Wang E., Hata C., Lin D. A preliminary-study of the fixation mechanism of collagen reaction with a polyepoxy fixative. Int. J. Artif. Organs. 1993;16:537–544. doi: 10.1177/039139889301600707. [DOI] [PubMed] [Google Scholar]
- 67.Speer D.P., Chvapil M., Eskelson C.D., Ulreich J. Biological effects of residual glutaraldehyde in glutaraldehyde-tanned collagen biomaterials. J. Biomed. Mater. Res. 1980;14:753–764. doi: 10.1002/jbm.820140607. [DOI] [PubMed] [Google Scholar]
- 68.Weadock K.S., Miller E.J., Bellincampi L.D., Zawadsky J.P., Dunn M.G. Physical cross-linking of collagen fibers: Comparison of ultraviolet irradiation and dehydrothermal treatment. J. Biomed. Mater. Res. 1995;29:1373–1379. doi: 10.1002/jbm.820291108. [DOI] [PubMed] [Google Scholar]
- 69.Zork N.M., Myers K.M., Yoshida K., Cremers S., Jiang H.F., Ananth C.V., Wapner R.J., Kitajewski J., Vink J. A systematic evaluation of collagen cross-links in the human cervix. Am. J. Obs. Gynecol. 2015;212 doi: 10.1016/j.ajog.2014.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Haugh M.G., Jaasma M.J., O’Brien F.J. The effect of dehydrothermal treatment on the mechanical and structural properties of collagen-GAG scaffolds. J. Biomed. Mater. Res. Part A. 2009;89A:363–369. doi: 10.1002/jbm.a.31955. [DOI] [PubMed] [Google Scholar]
- 71.Miles C.A., Bailey A.J. Thermally labile domains in the collagen molecule. Micron. 2001;32:325–332. doi: 10.1016/S0968-4328(00)00034-2. [DOI] [PubMed] [Google Scholar]
- 72.Haugh M.G., Murphy C.M., McKiernan R.C., Altenbuchner C., O’Brien F.J. Cross-linking and mechanical properties significantly influence cell attachment, proliferation, and migration within collagen glycosaminoglycan scaffolds. Tissue Eng. Part A. 2011;17A:1201–1208. doi: 10.1089/ten.tea.2010.0590. [DOI] [PubMed] [Google Scholar]
- 73.Weadock K.S., Miller E.J., Keuffel E.L., Dunn M.G. Effect of physical cross-linking methods on collagen-fiber durability in proteolytic solutions. J. Biomed. Mater. Res. 1996;32:221–226. doi: 10.1002/(SICI)1097-4636(199610)32:2<221::AID-JBM11>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 74.Gorham S.D., Light N.D., Diamond A.M., Willins M.J., Bailey A.J., Wess T.J., Leslie N.J. Effect of chemical modifications on the susceptibility of collagen to proteolysis. II. Dehydrothermal cross-linking. Int. J. Biol. Macromol. 1992;14:129–138. doi: 10.1016/S0141-8130(05)80002-9. [DOI] [PubMed] [Google Scholar]
- 75.Lv H.W., Wang H.P., Zhang Z.J., Yang W., Liu W.B., Li Y.L., Li L.S. Biomaterial stiffness determines stem cell fate. Life Sci. 2017;178:42–48. doi: 10.1016/j.lfs.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 76.Mitrousis N., Fokina A., Shoichet M.S. Biomaterials for cell transplantation. Nat. Rev. Mater. 2018;3:441–456. doi: 10.1038/s41578-018-0057-0. [DOI] [Google Scholar]
- 77.Velasco-Hogan A., Xu J., Meyers M.A. Additive manufacturing as a method to design and optimize bioinspired structures. Adv. Mater. 2018;30:1800940. doi: 10.1002/adma.201800940. [DOI] [PubMed] [Google Scholar]
- 78.De Mori A., Fernandez M.P., Blunn G., Tozzi G., Roldo M. 3D printing and electrospinning of composite hydrogels for cartilage and bone tissue engineering. Polymers. 2018;10:285. doi: 10.3390/polym10030285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nagarajan N., Dupret-Bories A., Karabulut E., Zorlutuna P., Vrana N.E. Enabling personalized implant and controllable biosystem development through 3D printing. Biotechnol. Adv. 2018;36:521–533. doi: 10.1016/j.biotechadv.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 80.Higuchi A., Ling Q.-D., Kumar S.S., Chang Y., Kao T.-C., Munusamy M.A., Alarfaj A.A., Hsu S.-T., Umezawa A. External stimulus-responsive biomaterials designed for the culture and differentiation of ES, iPS, and adult stem cells. Prog. Polym. Sci. 2014;39:1585–1613. doi: 10.1016/j.progpolymsci.2014.05.001. [DOI] [Google Scholar]
- 81.Draget K.I., Skjåk-Bræk G., Smidsrød O. Alginate based new materials. Int. J. Biol. Macromol. 1997;21:47–55. doi: 10.1016/S0141-8130(97)00040-8. [DOI] [PubMed] [Google Scholar]
- 82.Venkatesan J., Bhatnagar I., Manivasagan P., Kang K.H., Kim S.K. Alginate composites for bone tissue engineering: A review. Int. J. Biol. Macromol. 2015;72:269–281. doi: 10.1016/j.ijbiomac.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 83.You F., Wu X., Zhu N., Lei M., Eames B.F., Chen X. 3D printing of porous cell-laden hydrogel constructs for potential applications in cartilage tissue engineering. ACS Biomater. Sci. Eng. 2016;2:1200–1210. doi: 10.1021/acsbiomaterials.6b00258. [DOI] [PubMed] [Google Scholar]
- 84.Luo Y., Wu C., Lode A., Gelinsky M. Hierarchical mesoporous bioactive glass/alginate composite scaffolds fabricated by three-dimensional plotting for bone tissue engineering. Biofabrication. 2013;5:015005. doi: 10.1088/1758-5082/5/1/015005. [DOI] [PubMed] [Google Scholar]
- 85.Kim Y.B., Kim G.H. PCL/alginate composite scaffolds for hard tissue engineering: Fabrication, characterization, and cellular activities. ACS Comb. Sci. 2015;17:87–99. doi: 10.1021/co500033h. [DOI] [PubMed] [Google Scholar]
- 86.D’Amora U., D’Este M., Eglin D., Safari F., Sprecher C.M., Gloria A., De Santis R., Alini M., Ambrosio L. Collagen density gradient on three-dimensional printed poly(ε-caprolactone) scaffolds for interface tissue engineering. J. Tissue Eng. Regen. Med. 2018;12:321–329. doi: 10.1002/term.2457. [DOI] [PubMed] [Google Scholar]
- 87.Gloria A., Russo T., Rodrigues D.F.L., D’Amora U., Colella F., Improta G., Triassi M., De Santis R., Ambrosio L. From 3D hierarchical scaffolds for tissue engineering to advanced hydrogel-based and complex devices for in situ cell or drug release. Procedia CIRP. 2016;49:72–75. doi: 10.1016/j.procir.2015.07.036. [DOI] [Google Scholar]
- 88.Perez R.A., Kim M., Kim T.H., Kim J.H., Lee J.H., Park J.H., Knowles J.C., Kim H.W. Utilizing core-shell fibrous collagen–alginate hydrogel cell delivery system for bone tissue engineering. Tissue Eng. Part A. 2014;20A:103–114. doi: 10.1089/ten.tea.2013.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Perez R.A., Kim J.H., Buitrago J.O., Wall I.B., Kim H.W. Novel therapeutic core-shell hydrogel scaffolds with sequential delivery of cobalt and bone morphogenetic protein-2 for synergistic bone regeneration. Acta Biomater. 2015;23:295–308. doi: 10.1016/j.actbio.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 90.Buitrago J.O., Perez R.A., El-Fiqi A., Singh R.K., Kim J.H., Kim H.W. Core-shell fibrous stem cell carriers incorporating osteogenic nanoparticulate cues for bone tissue engineering. Acta Biomater. 2015;28:183–192. doi: 10.1016/j.actbio.2015.09.021. [DOI] [PubMed] [Google Scholar]
- 91.Yao R., Alkhawtani A.Y.F., Chen R.Y., Luang J., Xu M.E. Rapid and efficient in vivo angiogenesis directed by electro-assisted bioprinting of alginate/collagen microspheres with human umbilical vein endothelial cell coating layer. Int. J. Bioprint. 2019;5:3–14. doi: 10.18063/ijb.v5i2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee B.R., Hwang J.W., Choi Y.Y., Wong S.F., Hwang Y.H., Lee D.Y., Lee S.H. In situ formation and collagen–alginate composite encapsulation of pancreatic islet spheroids. Biomaterials. 2012;33:837–845. doi: 10.1016/j.biomaterials.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 93.Chan H.F., Zhang Y., Leong K.W. Efficient One-step production of microencapsulated hepatocyte spheroids with enhanced functions. Small. 2016;12:2720–2730. doi: 10.1002/smll.201502932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.San Jose L.H., Stephens P., Song B., Barrow D. Microfluidic encapsulation supports stem cell viability, proliferation, and neuronal differentiation. Tissue Eng. Part C Methods. 2018;24:158–170. doi: 10.1089/ten.tec.2017.0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Henkel J., Woodruff M.A., Epari D.R., Steck R., Glatt V., Dickinson I.C., Choong P.F.M., Schuetz M.A., Hutmacher D.W. Bone regeneration based on tissue engineering conceptions—A 21st century perspective. Bone Res. 2013;1:216–248. doi: 10.4248/BR201303002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sakiyama-Elbert S.E., Hubbell J.A. Functional biomaterials: Design of novel biomaterials. Annu. Rev. Mater. Res. 2001;31:183–201. doi: 10.1146/annurev.matsci.31.1.183. [DOI] [Google Scholar]
- 97.Boontheekul T., Kong H.J., Mooney D.J. Controlling alginate gel degradation utilizing partial oxidation and bimodal molecular weight distribution. Biomaterials. 2005;26:2455–2465. doi: 10.1016/j.biomaterials.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 98.Rico-Llanos G.A., Borrego-Gonzalez S., Moncayo-Donoso M., Becerra J., Visser R. Collagen type I Biomaterials as scaffolds for bone tissue engineering. Polymers. 2021;13:599. doi: 10.3390/polym13040599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brinkman W.T., Nagapudi K., Thomas B.S., Chaikof E.L. Photo-cross-linking of type I collagen gels in the presence of smooth muscle cells: Mechanical properties, cell viability, and function. Biomacromolecules. 2003;4:890–895. doi: 10.1021/bm0257412. [DOI] [PubMed] [Google Scholar]
- 100.Sionkowska A., Kozlowska J. Properties and modification of porous 3-D collagen/hydroxyapatite composites. Int. J. Biol. Macromol. 2013;52:250–259. doi: 10.1016/j.ijbiomac.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 101.Liu X.H., Ma P.X. Polymeric scaffolds for bone tissue engineering. Ann. Biomed. Eng. 2004;32:477–486. doi: 10.1023/B:ABME.0000017544.36001.8e. [DOI] [PubMed] [Google Scholar]
- 102.Lee C.H., Singla A., Lee Y. Biomedical applications of collagen. Int. J. Pharm. 2001;221:1–22. doi: 10.1016/S0378-5173(01)00691-3. [DOI] [PubMed] [Google Scholar]
- 103.Benayahu D., Pomeraniec L., Shemesh S., Heller S., Rosenthal Y., Rath-Wolfson L., Benayahu Y. Biocompatibility of a marine collagen-based scaffold in vitro and in vivo. Mar. Drugs. 2020;18:420. doi: 10.3390/md18080420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bendtsen S.T., Wei M. Synthesis and characterization of a novel injectable alginate-collagen-hydroxyapatite hydrogel for bone tissue regeneration. J. Mater. Chem. B. 2015;3:3081–3090. doi: 10.1039/C5TB00072F. [DOI] [PubMed] [Google Scholar]
- 105.Yu C.C., Chang J.J., Lee Y.H., Lin Y.C., Wu M.H., Yang M.C., Chien C.T. Electrospun scaffolds composing of alginate, chitosan, collagen and hydroxyapatite for applying in bone tissue engineering. Mater. Lett. 2013;93:133–136. doi: 10.1016/j.matlet.2012.11.040. [DOI] [Google Scholar]
- 106.Parmentier L., Riffault M., Hoey D.A. Utilizing osteocyte derived factors to enhance cell viability and osteogenic matrix deposition within IPN hydrogels. Materials. 2020;13:1690. doi: 10.3390/ma13071690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Perez R.A., Won J.E., Knowles J.C., Kim H.W. Naturally and synthetic smart composite biomaterials for tissue regeneration. Adv. Drug Deliv. Rev. 2013;65:471–496. doi: 10.1016/j.addr.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 108.Quinlan E., Lopez-Noriega A., Thompson E., Kelly H.M., Cryan S.A., O’Brien F.J. Development of collagen-hydroxyapatite scaffolds incorporating PLGA and alginate microparticles for the controlled delivery of rhBMP-2 for bone tissue engineering. J. Control. Release. 2015;198:71–79. doi: 10.1016/j.jconrel.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 109.Sotome S., Uemura T., Kikuchi M., Chen J., Itoh S., Tanaka J., Tateishi T., Shinomiya K. Synthesis and in vivo evaluation of a novel hydroxyapatite/collagen–alginate as a bone filler and a drug delivery carrier of bone morphogenetic protein. Mat. Sci. Eng. C. 2004;24:341–347. doi: 10.1016/j.msec.2003.12.003. [DOI] [Google Scholar]
- 110.Pak J., Lee J.H., Kartolo W.A., Lee S.H. Cartilage regeneration in human with adipose tissue-derived stem cells: Current status in clinical implications. Biomed. Res. Int. 2016;2016 doi: 10.1155/2016/4702674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang L., Zheng L., Fan H.S., Zhang X.D. A scaffold-filter model for studying the chondrogenic differentiation of stem cells in vitro. Mat. Sci. Eng. C. 2017;70:962–968. doi: 10.1016/j.msec.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 112.Roach H.I., Erenpreisa J., Aigner T. Osteogenic differentiation of hypertrophic chondrocytes involves asymmetric cell divisions and apoptosis. J. Cell Biol. 1995;131:483–494. doi: 10.1083/jcb.131.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Domm C., Schunke M., Christesen K., Kurz B. Redifferentiation of dedifferentiated bovine articular chondrocytes in alginate culture under low oxygen tension. Osteoarthr. Cartil. 2002;10:13–22. doi: 10.1053/joca.2001.0477. [DOI] [PubMed] [Google Scholar]
- 114.Bonaventure J., Kadhom N., Cohensolal L., Ng K.H., Bourguignon J., Lasselin C., Freisinger P. Reexpression of cartilage-specific genes by dedifferentiated human articular chondrocytes cultured in alginate beads. Exp. Cell Res. 1994;212:97–104. doi: 10.1006/excr.1994.1123. [DOI] [PubMed] [Google Scholar]
- 115.Jin G.Z., Kim H.W. Efficacy of collagen and alginate hydrogels for the prevention of rat chondrocyte dedifferentiation. J. Tissue Eng. 2018;9 doi: 10.1177/2041731418802438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang X., Lu Z., Wu H., Li W., Zheng L., Zhao J. Collagen–alginate as bioink for three-dimensional (3D) cell printing based cartilage tissue engineering. Mater. Sci. Eng. C. 2018;83:195–201. doi: 10.1016/j.msec.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 117.Chan S.C.W., Ferguson S.J., Gantenbein-Ritter B. The effects of dynamic loading on the intervertebral disc. Eur. Spine J. 2011;20:1796–1812. doi: 10.1007/s00586-011-1827-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Eyre D.R. Biochemistry of the intervertebral disc. Int. Rev. Connect. Tissue Res. 1979;8:227–291. doi: 10.1016/b978-0-12-363708-6.50012-6. [DOI] [PubMed] [Google Scholar]
- 119.Kishen T.J., Diwan A.D. Fusion versus disk replacement for degenerative conditions of the lumbar and cervical spine: Quid est testimonium? Orthop. Clin. N. Am. 2010;41:167–181. doi: 10.1016/j.ocl.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 120.Tavakoli J., Diwan A.D., Tipper J.L. Advanced strategies for the regeneration of lumbar disc annulus fibrosus. Int. J. Mol. Sci. 2020;21:4889. doi: 10.3390/ijms21144889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Choi Y., Park M.H., Lee K. Tissue engineering strategies for intervertebral disc treatment using functional polymers. Polymers. 2019;11:872. doi: 10.3390/polym11050872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhao R.Z., Liu W.Q., Xia T.T., Yang L. Disordered mechanical stress and tissue engineering therapies in intervertebral disc degeneration. Polymers. 2019;11:1151. doi: 10.3390/polym11071151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Borzacchiello A., Gloria A., De Santis R., Ambrosio L. Spinal disc implants using hydrogels. In: Rimmer S., editor. Biomedical Hydrogels. Woodhead Publishing; Cambridge, UK: 2011. pp. 103–117. [Google Scholar]
- 124.Moriguchi Y., Borde B., Berlin C., Wipplinger C., Sloan S.R., Kirnaz S., Pennicooke B., Navarro-Ramirez R., Khair T., Grunert P., et al. In vivo annular repair using high-density collagen gel seeded with annulus fibrosus cells. Acta Biomater. 2018;79:230–238. doi: 10.1016/j.actbio.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 125.Hussain I., Sloan S.R., Wipplinger C., Navarro-Ramirez R., Zubkov M., Kim E., Kirnaz S., Bonassar L.J., Härtl R. Mesenchymal stem cell-seeded high-density collagen gel for annular repair: 6-week results from in vivo sheep models. Neurosurgery. 2019;85:E350–E359. doi: 10.1093/neuros/nyy523. [DOI] [PubMed] [Google Scholar]
- 126.Tsaryk R., Gloria A., Russo T., Anspach L., De Santis R., Ghanaati S., Unger R.E., Ambrosio L., Kirkpatrick C.J. Collagen-low molecular weight hyaluronic acid semi-interpenetrating network loaded with gelatin microspheres for cell and growth factor delivery for nucleus pulposus regeneration. Acta Biomater. 2015;20:10–21. doi: 10.1016/j.actbio.2015.03.041. [DOI] [PubMed] [Google Scholar]
- 127.Bowles R.D., Williams R.M., Zipfel W.R., Bonassar L.J. Self-assembly of aligned tissue-engineered annulus fibrosus and intervertebral disc composite via collagen gel contraction. Tissue Eng. Part A. 2010;16A:1339–1348. doi: 10.1089/ten.tea.2009.0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gloria A., Russo T., D’Amora U., Santin M., De Santis R., Ambrosio L. Customised multiphasic nucleus/annulus scaffold for intervertebral disc repair/regeneration. Connect. Tissue Res. 2020;61:152–162. doi: 10.1080/03008207.2019.1650037. [DOI] [PubMed] [Google Scholar]
- 129.Moriguchi Y., Mojica-Santiago J., Grunert P., Pennicooke B., Berlin C., Khair T., Navarro-Ramirez R., Arbona R.J.R., Nguyen J., Hartl R., et al. Total disc replacement using tissue-engineered intervertebral discs in the canine cervical spine. PLoS ONE. 2017;12:e0185716. doi: 10.1371/journal.pone.0185716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 131.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 132.Folkman J., Hochberg M. Self-regulation of growth in three dimensions. J. Exp. Med. 1973;138:745–753. doi: 10.1084/jem.138.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Carmeliet P. Angiogenesis in health and disease. Nat. Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 134.Gelmi A., Cieslar-Pobuda A., de Muinck E., Los M., Rafat M., Jager E.W.H. Direct mechanical stimulation of stem cells: A beating electromechanically active scaffold for cardiac tissue engineering. Adv. Healthc. Mater. 2016;5:1471–1480. doi: 10.1002/adhm.201600307. [DOI] [PubMed] [Google Scholar]
- 135.Quinlan E., Lopez-Noriega A., Thompson E.M., Hibbitts A., Cryan S.A., O’Brien F.J. Controlled release of vascular endothelial growth factor from spray-dried alginate microparticles in collagen-hydroxyapatite scaffolds for promoting vascularization and bone repair. J. Tissue Eng. Regen. Med. 2017;11:1097–1109. doi: 10.1002/term.2013. [DOI] [PubMed] [Google Scholar]
- 136.Ali Z., Islam A., Sherrell P., Le-Moine M., Lolas G., Syrigos K., Rafat M., Jensen L.D. Adjustable delivery of pro-angiogenic FGF-2 by alginate: Collagen microspheres. Biol. Open. 2018;7:bio027060. doi: 10.1242/bio.027060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ruoslahti E. Brain extracellular matrix. Glycobiology. 1996;6:489–492. doi: 10.1093/glycob/6.5.489. [DOI] [PubMed] [Google Scholar]
- 138.Ripellino J.A., Klinger M.M., Margolis R.U., Margolis R.K. The hyaluronic acid binding region as a specific probe for the localization of hyaluronic acid in tissue sections. Application to chick embryo and rat brain. J. Histochem. Cytochem. 1985;33:1060–1066. doi: 10.1177/33.10.4045184. [DOI] [PubMed] [Google Scholar]
- 139.Luo Y., Kirker K.R., Prestwich G.D. Cross-linked hyaluronic acid hydrogel films: New biomaterials for drug delivery. J. Control. Release. 2000;69:169–184. doi: 10.1016/S0168-3659(00)00300-X. [DOI] [PubMed] [Google Scholar]
- 140.Park H., Kang S.W., Kim B.S., Mooney D.J., Lee K.Y. Shear-reversibly cross-linked alginate hydrogels for tissue engineering. Macromol. Biosci. 2009;9:895–901. doi: 10.1002/mabi.200800376. [DOI] [PubMed] [Google Scholar]
- 141.Kuo Y.C., Hsueh C.H. Neuronal production from induced pluripotent stem cells in self-assembled collagen-hyaluronic acid-alginate microgel scaffolds with grafted GRGDSP/Ln5-P4. Mater. Sci. Eng. C. 2017;76:760–774. doi: 10.1016/j.msec.2017.03.133. [DOI] [PubMed] [Google Scholar]
- 142.Hahn M.S., Teply B.A., Stevens M.M., Zeitels S.M., Langer R. Collagen composite hydrogels for vocal fold lamina propria restoration. Biomaterials. 2006;27:1104–1109. doi: 10.1016/j.biomaterials.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 143.Robson M.C., Steed D.L., Franz M.G. Wound healing: Biologic features and approaches to maximize healing trajectories—In brief. Curr. Prob. Surg. 2001;38:65–140. doi: 10.1067/msg.2001.111167. [DOI] [PubMed] [Google Scholar]
- 144.Szycher M., Lee S.J. Modern wound dressings: A systematic approach to wound healing. J. Biomater. Appl. 1992;7:142–213. doi: 10.1177/088532829200700204. [DOI] [PubMed] [Google Scholar]
- 145.Singer A.J., Clark R.A.F. Mechanisms of disease—Cutaneous wound healing. N. Engl. J. Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 146.Jayakumar R., Prabaharan M., Kumar P.T.S., Nair S.V., Tamura H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011;29:322–337. doi: 10.1016/j.biotechadv.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 147.Pinho E., Soares G. Functionalization of cotton cellulose for improved wound healing. J. Mater. Chem. B. 2018;6:1887–1898. doi: 10.1039/C8TB00052B. [DOI] [PubMed] [Google Scholar]
- 148.Dhivya S., Padma V.V., Santhini E. Wound dressings—A review. Biomedicine. 2015;5:24–28. doi: 10.7603/s40681-015-0022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Boateng J.S., Matthews K.H., Stevens H.N.E., Eccleston G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008;97:2892–2923. doi: 10.1002/jps.21210. [DOI] [PubMed] [Google Scholar]
- 150.Liu B., Ding F.X., Hu D., Zhou Y., Long C.L., Shen L.J., Zhang Y.Y., Zhang D.Y., Wei G.H. Human umbilical cord mesenchymal stem cell conditioned medium attenuates renal fibrosis by reducing inflammation and epithelial-to-mesenchymal transition via the TLR4/NF-kappa B signaling pathway in vivo and in vitro. Stem Cell Res. Ther. 2018;9:7. doi: 10.1186/s13287-017-0760-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhu S.C., Yuan Q.J., Yin T., You J., Gu Z.P., Xiong S.B., Hu Y. Self-assembly of collagen-based biomaterials: Preparation, characterizations and biomedical applications. J. Mater. Chem. B. 2018;6:2650–2676. doi: 10.1039/C7TB02999C. [DOI] [PubMed] [Google Scholar]
- 152.Lozeau L.D., Grosha J., Smith I.M., Stewart E.J., Camesano T.A., Rolle M.W. Alginate affects bioactivity of chimeric collagen-binding LL37 antimicrobial peptides adsorbed to collagen-alginate wound dressings. ACS Biomater. Sci. Eng. 2020;6:3398–3410. doi: 10.1021/acsbiomaterials.0c00227. [DOI] [PubMed] [Google Scholar]
- 153.Lin Z.F., Wu T.T., Wang W.S., Li B.L., Wang M., Chen L.L., Xia H., Zhang T. Biofunctions of antimicrobial peptide-conjugated alginate/hyaluronic acid/collagen wound dressings promote wound healing of a mixed-bacteria-infected wound. Int. J. Biol. Macromol. 2019;140:330–342. doi: 10.1016/j.ijbiomac.2019.08.087. [DOI] [PubMed] [Google Scholar]
- 154.Van Gils C.C., Roeder B., Chesler S.M., Mason S. Improved healing with a collagen–alginate dressing in the chemical matricectomy. J. Am. Podiat. Med. Assoc. 1998;88:452–456. doi: 10.7547/87507315-88-9-452. [DOI] [PubMed] [Google Scholar]
- 155.Donaghue V.M., Chrzan J.S., Rosenblum B.I., Giurini J.M., Habershaw G.M., Veves A. A clinical evaluation of a collagen–alginate topical wound dressing in the management of diabetic foot ulcers. Diabetologia. 1996;39:1019. [PubMed] [Google Scholar]
- 156.Feng X.L., Zhang X.F., Li S.Q., Zheng Y.Q., Shi X.N., Li F., Guo S.B., Yang J.M. Preparation of aminated fish scale collagen and oxidized sodium alginate hybrid hydrogel for enhanced full-thickness wound healing. Int. J. Biol. Macromol. 2020;164:626–637. doi: 10.1016/j.ijbiomac.2020.07.058. [DOI] [PubMed] [Google Scholar]
- 157.Xie H.X., Chen X.L., Shen X.R., He Y., Chen W., Luo Q., Ge W.H., Yuan W.H., Tang X., Hou D.Y., et al. Preparation of chitosan-collagen–alginate composite dressing and its promoting effects on wound healing. Int. J. Biol. Macromol. 2018;107:93–104. doi: 10.1016/j.ijbiomac.2017.08.142. [DOI] [PubMed] [Google Scholar]
- 158.Zhang H.J., Peng M.X., Cheng T., Zhao P., Qiu L.P., Zhou J., Lu G.Z., Chen J.H. Silver nanoparticles-doped collagen–alginate antimicrobial biocomposite as potential wound dressing. J. Mater. Sci. 2018;53:14944–14952. doi: 10.1007/s10853-018-2710-9. [DOI] [Google Scholar]
- 159.Zhang Z.K., Li Z., Li Y., Wang Y.Y., Yao M.H., Zhang K., Chen Z.Y., Yue H., Shi J.J., Guan F.X., et al. Sodium alginate/collagen hydrogel loaded with human umbilical cord mesenchymal stem cells promotes wound healing and skin remodeling. Cell Tissue Res. 2021;383:809–821. doi: 10.1007/s00441-020-03321-7. [DOI] [PubMed] [Google Scholar]
- 160.Michalska-Sionkowska M., Walczak M., Sionkowska A. Antimicrobial activity of collagen material with thymol addition for potential application as wound dressing. Polym. Test. 2017;63:360–366. doi: 10.1016/j.polymertesting.2017.08.036. [DOI] [Google Scholar]
- 161.Qu J., Zhao X., Liang Y.P., Xu Y.M., Ma P.X., Guo B.L. Degradable conductive injectable hydrogels as novel antibacterial, anti-oxidant wound dressings for wound healing. Chem. Eng. J. 2019;362:548–560. doi: 10.1016/j.cej.2019.01.028. [DOI] [Google Scholar]
- 162.Zahedi P., Rezaeian I., Ranaei-Siadat S.O., Jafari S.H., Supaphol P. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym. Advan. Technol. 2010;21:77–95. doi: 10.1002/pat.1625. [DOI] [Google Scholar]
- 163.Otvos L., Jr., Ostorhazi E. Therapeutic utility of antibacterial peptides in wound healing. Expert Rev. Anti-Infect. Ther. 2015;13:871–881. doi: 10.1586/14787210.2015.1033402. [DOI] [PubMed] [Google Scholar]
- 164.Branco da Cunha C., Klumpers D.D., Li W.A., Koshy S.T., Weaver J.C., Chaudhuri O., Granja P.L., Mooney D.J. Influence of the stiffness of three-dimensional alginate/collagen-I interpenetrating networks on fibroblast biology. Biomaterials. 2014;35:8927–8936. doi: 10.1016/j.biomaterials.2014.06.047. [DOI] [PubMed] [Google Scholar]
- 165.Orive G., Hernandez R.M., Gascon A.R., Calafiore R., Chang T.M.S., De Vos P., Hortelano G., Hunkeler D., Lacik I., Shapiro A.M.J., et al. Cell encapsulation: Promise and progress. Nat. Med. 2003;9:104–107. doi: 10.1038/nm0103-104. [DOI] [PubMed] [Google Scholar]
- 166.Uludag H., De Vos P., Tresco P.A. Technology of mammalian cell encapsulation. Adv. Drug Deliv. Rev. 2000;42:29–64. doi: 10.1016/S0169-409X(00)00053-3. [DOI] [PubMed] [Google Scholar]
- 167.Chang T.M.S., Malave N. Development and First clinical use of semipermeable microcapsules (artificial cells) as a compact artificial kidney. Trans. Am. Soc. Artif. Intern. Organs. 1970;16:141–148. doi: 10.1046/j.1526-0968.2000.004002108.x. [DOI] [PubMed] [Google Scholar]
- 168.Zurn A.D., Henry H., Schluep M., Aubert V., Winkel L., Eilers B., Bachmann C., Aebischer P. Evaluation of an intrathecal immune response in amyotrophic lateral sclerosis patients implanted with encapsulated genetically engineered xenogeneic cells. Cell Transplant. 2000;9:471–484. doi: 10.1177/096368970000900404. [DOI] [PubMed] [Google Scholar]
- 169.Wong H.L., Wang M.X., Cheung P.T., Yao K.M., Chan B.P. A 3D collagen microsphere culture system for GDNF-secreting HEK293 cells with enhanced protein productivity. Biomaterials. 2007;28:5369–5380. doi: 10.1016/j.biomaterials.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 170.Lee M., Lo A.C., Cheung P.T., Wong D., Chan B.P. Drug carrier systems based on collagen–alginate composite structures for improving the performance of GDNF-secreting HEK293 cells. Biomaterials. 2009;30:1214–1221. doi: 10.1016/j.biomaterials.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 171.Khavari A., Nyden M., Weitz D.A., Ehrlicher A.J. Composite alginate gels for tunable cellular microenvironment mechanics. Sci. Rep. 2016;6:30854. doi: 10.1038/srep30854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wang M., Yang Y.W., Han L.C., Han S., Liu N., Xu F., Li F. Effect of three-dimensional ECM stiffness on cancer cell migration through regulating cell volume homeostasis. Biochem. Biophys. Res. Commun. 2020;528:459–465. doi: 10.1016/j.bbrc.2020.05.182. [DOI] [PubMed] [Google Scholar]
- 173.Liu C., Mejia D.L., Chiang B., Luker K.E., Luker G.D. Hybrid collagen alginate hydrogel as a platform for 3D tumor spheroid invasion. Acta Biomater. 2018;75:213–225. doi: 10.1016/j.actbio.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.