Abstract
A lesser known but crucially important downstream effect of Rho family GTPases is the regulation of gene expression. This major role is mediated via the cytoskeleton, the organization of which dictates the nucleocytoplasmic shuttling of a set of transcription factors. Central among these is myocardin-related transcription factor (MRTF), which upon actin polymerization translocates to the nucleus and binds to its cognate partner, serum response factor (SRF). The MRTF/SRF complex then drives a large cohort of genes involved in cytoskeleton remodeling, contractility, extracellular matrix organization and many other processes. Accordingly, MRTF, activated by a variety of mechanical and chemical stimuli, affects a plethora of functions with physiological and pathological relevance. These include cell motility, development, metabolism and thus metastasis formation, inflammatory responses and—predominantly-organ fibrosis. The aim of this review is twofold: to provide an up-to-date summary about the basic biology and regulation of this versatile transcriptional coactivator; and to highlight its principal involvement in the pathobiology of kidney disease. Acting through both direct transcriptional and epigenetic mechanisms, MRTF plays a key (yet not fully appreciated) role in the induction of a profibrotic epithelial phenotype (PEP) as well as in fibroblast-myofibroblast transition, prime pathomechanisms in chronic kidney disease and renal fibrosis.
Keywords: actin cytoskeleton, Rho GTPases, transcription factors, nucleocytoplasmic shuttling, gene expression, profibrotic epithelial phenotype, myofibroblast, kidney fibrosis
1. Introduction
Rho family GTPases are prime regulators of the cytoskeleton [1]). Precise cytoskeletal control, in turn, is a prerequisite for normal renal structure and function. For example, the delicate morphology of podocyte foot processes, the structural basis of the filtration barrier, is fundamentally dependent upon subtle and highly regulated F-actin organization [2,3,4,5]. The cytoskeleton is also the structural basis of cell motility. It is thus not surprising that alterations in the activities of Rho family proteins, either due to genetic defects or provoked by cellular injuries, are associated with, and play significant pathogenic roles in acute and chronic kidney diseases [6,7,8,9]. However, the cytoskeleton affects organ function not only through its structural roles; the cytoskeleton is a regulator of gene expression as well. It exerts this (somewhat less appreciated yet not less important) function predominantly by controlling the nucleocytoplasmic shuttling of a select set of transcription factors. This mechanism renders the cytoskeleton a cell fate-determining device, i.e., a key determinant of phenotype transitions, plasticity, cell survival/death and injury/repair, critical processes in health and disease. Based on these functions, the cytoskeleton emerges as an essential “structure-function converter”, a signaling hub that integrates an array of diverse chemical (e.g., cytokines) and mechanical (e.g., pressure, tissue stiffness) inputs and links them to gene expression [10,11,12]. Rho GTPases, responsive to both mechanical and chemical stimuli [13,14,15], represent a major afferent arm of this transcriptional regulation. Central among the Rho family/cytoskeleton-controlled transcriptional regulators (coactivators) is the myocardin-related transcription factor (MRTF) family (see Section 2, Section 3 and Section 4), comprising of two isoforms, MRTF-A and B, encoded by separate genes. In this review we use the term “MRTF” when the effect of the two isoforms is not specifically distinguished. Substantial evidence has been accumulating that MRTF, and its nucleus-resident transcription factor partner, serum response factor (SRF) play important roles in proliferation, phenotype control (e.g., epithelial-mesenchymal transition, fibroblast-myofibroblast transition), secretory profile, matrix generation, oxidative state, intermediate metabolism, cytoskeleton organization, contractility and cell migration, and thereby in the pathogenesis of multiple disease states (see Section 5). MRTF, primarily through its transcriptional and epigenetic actions, has emerged as a crucial mediator of organ fibrosis [16,17,18]. Considering the kidney, MRTF has been implicated in fibrogenesis in the context of diabetic and obstructive nephropathy as well as in the pathobiology of acute kidney injury (see Section 6). Indeed, MRTF contributes to a slew of distinct fibrogenic processes in various renal cell types, including podocytes, tubular, mesangial and endothelial cells, fibroblasts and macrophages, encompassing the glomerular, tubular, interstitial and immune compartments (Section 5 and Section 6). Ubiquitously expressed in both epithelial and mesenchymal cells, MRTF is also a key factor in the crosstalk between these tissue compartments (epithelial-mesenchymal interaction), a process of key importance during injury/repair. Finally, it is worth noting that MRTF forms interactive networks with other major fibrogenic transcriptional regulators, including the TGFβ-controlled Smad proteins [19,20], and the Hippo pathway effectors, YAP and TAZ [21,22,23], all of which are involved in the pathogenesis of (renal) fibrosis. The nuclear accumulation of YAP and TAZ are also directly regulated by the acto-myosin cytoskeleton [24], representing another major pathway whereby Rho family GTPases affect gene expression.
In this overview, we will summarize pertinent information regarding the basic biology of MRTF and its role in renal pathobiology, highlighting the underlying cellular and molecular mechanisms. We point to outstanding questions and hope to raise awareness about the central importance of this transcriptional regulator in kidney disease, and about its potential exploit as a therapeutic target.
2. MRTFs: Their Discovery and Modus Operandi
SRF was long known to activate two disparate programs: it can drive immediate early genes (like c-fos), involved in growth factor-mediated cell proliferation, and it can also induce expression of muscle genes, specifying a tissue differentiation program [25,26,27,28,29] (reviewed in [30]). However, the molecular mechanism that could act as a switch between such distinct functions remained an enigma for a while. It was then discovered that (1) RhoA-activating mediators, such as lysophosphatidic acid, can stimulate SRF-dependent transcription, and (2) the active forms of RhoA, Rac1 and Cdc42 are also potent inducers of this process [31,32]. Moreover, while SRF was known to associate with members of the ternary complex factor (TCF) family [33], and the TCF/SRF complex was shown to bind to gene promoters via neighboring cis-elements (TCE, and SRE, respectively), Rho family-induced activation of SRE was TCF-independent [31,34,35]. Importantly, actin polymerization per se was shown to drive SRF-dependent transcription [35,36]. These results forecasted the existence of another SRF interactor that might link this transcriptional pathway to Rho GTPases and the cytoskeleton. The breakthrough arrived with the near-parallel discovery of MRTF (also known as Megakarocytic Acute Leukemia (MAL); megakaryoblastic leukemia (MKL) or Basic, SAP And Coiled-Coil Domain (BSAC) Protein) and its mode of action by the Olson [37] and Treisman laboratories [38]. The identification of MRTF and its modus operandi explained both the Rho-dependence of the SRF pathway (see below), and the disparate roles of SRF as an early gene inducer vs. a differentiation driver. The former action manifests when SRF is in complex with TCF, and the latter when SRF pairs up with MRTF or myocardin, the eponymous, (cardiac) muscle-specific family member [39].
Initially myocardin was identified in a search for muscle-specific SRF activators [40]. It is expressed in smooth and cardiac muscle during development but is restricted to cardiac muscle in adult life. Myocardin binds to and activates SRF, which occupies the CC(A/T)rich6GG cis element or GArG box (the canonical SRE) on the DNA. Myocardin belongs to the SAP (SAF-A/B, Acinus, PIAS) family of chromatin-remodeling proteins, and similar to other members, it is constitutively nuclear. Importantly it is not activated by Rho GTPases. From here, the identification of MRTFs and their connection to Rho signaling followed two (converging) paths. Based on gene homology, Wang et al., from the Olson group cloned mouse MRTF-A and MRTF-B [37], and showed that these proteins are expressed in a large variety of tissues and organs, and are potent activators of SRF-dependent transcription. It was also uncovered that MRTF-A is identical with MAL, a previously cloned human protein, the gene of which undergoes chromosomal translocation (t(1;22)) and fusion with the gene encoding OTT, an RNA-binding protein [41,42]. The resulting gene product, OTT-MAL, is a deregulated, constitutive activator of SRF [43,44] and the pathogenic culprit of megakaryoblastic acute leukemia [42,44]. Finally, coming from an immunological angle, BSAC, an anti-apoptotic transcription factor was described, which exhibited strong capacity to activate CArG-containing promoters [45], and proved to be identical with MRTF.
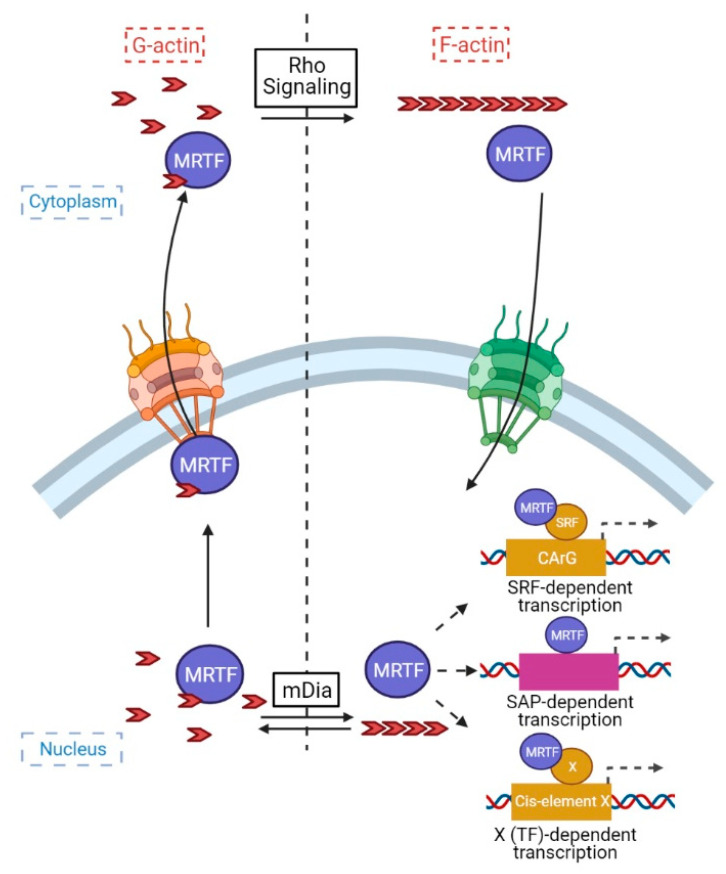
In parallel with these genetic studies, Miralles et al., in the Treisman lab [38] identified MAL (MRTF) as the RhoA/actin-regulated SRF activator, thereby finding the hitherto missing link. As opposed to myocardin, MRTF is predominantly cytosolic in resting cells and Rho activation induces its nuclear translocation (Figure 1).
Figure 1.
The regulation of MRTF subcellular localization and transcriptional activity by the actin cytoskeleton MRTF-A/B are transcriptional coactivators regulated by F/G actin ratio. Cytosolic G-actin binds MRTF masking a Nuclear Localization Sequence (NLS) (see Figure 2). Activation of small Rho GTPases promotes F-actin polymerization, thereby reducing the cytosolic G-actin pool, and causing G-actin to dissociate from MRTF to unmask the NLS, which enables importin α/β-dependent nuclear translocation. In the nucleus (1) MRTF binds SRF forming the MRTF/SRF complex which induces the transcription of various genes (SRF-dependent transcription); (2) MRTF can independently facilitate transcription of genes via its SAP domain (SAP-dependent transcription); and (3) MRTF can bind other TFs (e.g., smad3, TAZ) which induce transcription via alternative non-CArG dependent cis-elements. Additionally, the nuclear F/G actin ratio regulates MRTF function. Increased mDia activity enhances MRTF/SRF transcriptional activity, while nuclear G-actin binding to MRTF supports CMR1-dependent nuclear export. Together, the actin cytoskeleton acts as an integral regulator of MRTF-dependent gene expression (dotted arrows). (Created with BioRender.com, accessed on 15 April 2021).
Moreover, the effect of Rho is conferred by actin remodeling. MRTF is an actin monomer- (G-actin) binding protein and its association with G-actin inhibits its nuclear accumulation. This is, in part, due to G-actin-mediated masking of MRTF’s nuclear localization signal (NLS) [67] but also to the faster export of the MRTF/G-actin complex from the nucleus [68,69]. These findings unraveled a strikingly elegant and powerful mechanism. MRTF rapidly cycles across the nuclear membrane in resting cells. Upon cytosolic Rho activation, due to the ensuing F-actin assembly, G-actin “is stolen” (dissociates) from MRTF, facilitating its import; in parallel nuclear Rho activation, via the ensuing nuclear actin polymerization, inhibits MRTF efflux. Furthermore, actin binding to MRTF in the nucleus also inhibits its transcriptional activity [12,68]. Taken together, compartmentally regulated increases in actin polymerization shift MRTF to the nucleus, and unleash its transcriptional potential. A plethora of factors (e.g., guanine nucleotide exchange factors (GEFs), GTPase activating proteins (GAPs) [9,70,71,72] regulate Rho GTPases and/or induce posttranslational modification of proteins involved in actin sequestering (e.g., thymosin [73]), severing (e.g., cofilin [36,74,75,76]) and polymerization (e.g., mDIA [69,77]) or modify actin itself [78]. All of these regulate MRTF, which thus emerged as a major highway connecting cytoskeletal state to gene expression.
3. Structure
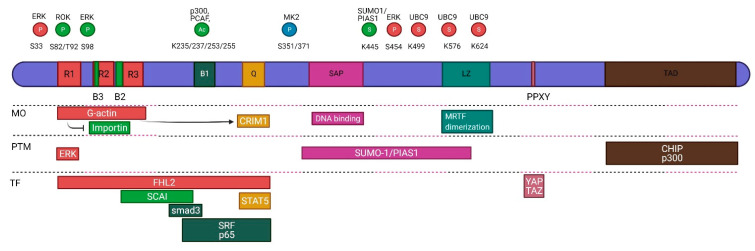
MRTF-A (MAL, MKL1) and MRTF-B (MAL16, MKL2) are encoded by different genes (chromosomes 22 and 16 in human, respectively) but share a high level of homology and a similar domain structure. They contain three N-terminal RPEL motifs (named for the corresponding amino acids), interspersed with two basic regions (B3, B2) comprising a bipartite NLS, followed by another basic region (B1), a glutamine-rich sequence (Q), the family-defining SAP domain, a coiled-coil leucine zipper (LZ) motif and a C-terminal transactivation domain (TAD) (Figure 2).
Figure 2.
Schematic of MRTF domain architecture, co-factor interaction, and posttranslational modification. MRTFs contain 3 N-term RPEL domains (R1–3) enabling actin binding, 2 interspersed basic amino acid regions (B3, B2) containing the nuclear localization sequence (NLS); Leucine Zipper (LZ) which is necessary for MRTF dimerization and MRTF/SRF-dependent transcription; B1 region, Q region, SAP domain, PPxY motif, and a Transcriptional Activation Domain (TAD), all of which are necessary for binding various regulators and co-factors to regulate MRTF-dependent transcription. Posttranslational medications (PTM) of these domains regulate MRTF nuclear accumulation and/or transcriptional activity (see Table 1). Residues indicated include those known to be modified by phosphorylation (P), acetylation (Ac) or SUMOylation (S), with the modifiers noted above. Green indicates that the residue/PTM enhances MRTF activity, red indicates that the residue/PTM suppresses MRTF activity, and blue indicates no known role. Molecules with known interaction sites within MRTF are depicted spanning the domains which they are suggested to bind. This includes: (1) regulators of MRTF activity (Figure 1); (2) regulators of PTM with known binding sites; and (3) transcription factors (TF) or co-factors, which bind to MRTF and regulate downstream gene activation (see Table 2). (Created with BioRender.com, accessed on 01 June 2021).
The RPEL motifs are the G-actin binding sites, which along with two spacer regions allow the formation of a pentameric G-actin/MRTF complex [79,80]. Occupancy of the N-terminus by 5 actins buries the bipartite NLS, thereby inhibiting importin α/β-dependent nuclear import [80,81,82]; in fact, the importin α/β complex competes with actin for the RPEL motif [81]. Association of actin with the spacer regions (between the RPELs) may also be necessary for exportin-1- (CRM-1)-mediated export [79,83]. Moreover, actin binding is cooperative, and the trimeric G-actin/MRTF complex is still mainly cytosolic. Thus, MRTF could respond sharply to changes in G-actin concentration around a threshold level [79]. Overexpression of non-polymerizable G-actin [38], or actin depolymerization by the monomer sequestering drug, latrunculin A completely abolishes nuclear MRTF accumulation, as expected. However, paradoxically, Cytochalasin D or Swinholide, two toxins that also induce F-actin disassembly, are potent inducers of MRTF nuclear translocation because they disrupt the MRTF-G-actin interaction [36,84].
The B1 basic domain is also necessary for nuclear import [38], and—together with the Q-rich stretch—for the binding of SRF [85]. The Q-domain also harbors one of the leucine-rich sequences (L2), which serves as a CRM1-dependent nuclear export signal (NES). L1 is located in the RPEL region, and disruption of either L1 or L2 induces nuclear accumulation [83]. The SAP domains function as adaptors in protein-protein interactions and have DNA binding capacities, through which they regulate chromatin organization [86,87]. Accordingly, MRTF can also directly contact DNA [85], a feature that has at least two important functional connotations: first, MRTF (partly through this mechanism) may affect transcription in an SRF-independent manner, and second, MRTF emerges as an important epigenetic factor [63,88,89,90,91] regulating the histone code (acetylation, methylation) and chromatin remodeling. These actions represent an important facet of the pathophysiologic roles of MRTF, as will be detailed in the context of various disease states. The LZ motif is involved in homo- and heterodimerization of MRTF isoforms [39,92], which may impact the rate of MRTF shuttling and thus duration of its action, constituting an understudied aspect of MRTF regulation. The TAD lends potent transcriptional activity to MRTF-interacting transcription factors, primarily to SRF but also alternative partners (e.g., SP1 and Smads, see below).
4. Regulation
MRTF activity is controlled primarily by its nuclear import and export, which are in turn dictated by the state of the actin cytoskeleton, set by a large cohort of Rho family regulators and effectors, as discussed above. Furthermore, cytoskeleton-regulating proteins may affect MRTF not only through their direct effect on cytoskeleton dynamics (actin polymerization). For example, actin nucleation-promoting factors N-WASP, WAVE2 and JMY activate MRTF because they directly compete with it for G-actin binding through their WH2 domain [93,94]. In addition to the level of available G-actin, MRTF is also regulated by two other main factors: its posttranslational modification and interaction with other regulatory proteins. These inputs impact MRTF’s localization, transcriptional activity and stability.
4.1. Posttranslational Modifications
It was observed early on that activation of either the Ras or the Rho signaling pathway elicits upward shifts in the molecular mass of MRTF, consistent with phosphorylation [38]. Indeed, MRTF contains 26 putative S/T phosphorylation sites, which can positively or negatively modify its activity [46,47] (see Table 1). Based on pharmacological and/or biochemical approaches, a variety of kinases have been implicated in MRTF phosphorylation including ERK [38,47], p38 MAPK (p38) [95] and its downstream effector MK2 [48], as well as GSK3β [49]. These can either directly or indirectly alter MRTF phosphorylation and consequently its localization or stability. Rho kinase (ROK), the most plausible link connecting Rho signaling to MRTF phosphorylation also impacts MRTF localization and activity [16,96] but it remains unclear if this involves direct phosphorylation. Some pertinent mechanisms are summarized below.
Table 1.
Posttranslational modifications/modifiers of MRTF: phosphorylation, acetylation, and SUMOylation.
| Enzyme | Site Modified | Domain Modified | Binding Site | Effect on MRTF Localization/Stability | Effect on MRTF Transcriptional Activity | Reference |
|---|---|---|---|---|---|---|
| Phosphorylation | ||||||
| ERK | S98 | between RPEL 1 and 2 | RPEL1 | Promotes MRTF nuclear import/prevents nuclear | + | [46] |
| ERK | S33 | N-term of RPEL1 | N/D | Promotes MRTF nuclear export | N/D | [46] |
| ERK | S454 | Between SAP and LZ | N/D | Promotes G-actin binding and MRTF export | N/D | [47] |
| P38 | N/D | N/D | N/D | + | ||
| MK2 | S351/371 | Between Q and SAP | N/D | N/D | No effect | [48] |
| ROK | S82/T92 | RPEL 1 | + | |||
| GSK3β | N/D | N/D | Binds MRTF via Smad3-dependent mechanism | Promotes MRTF ubiquitin-mediated degradation | − | [49] |
| Ubiquitination | ||||||
| Ubiquitinase | ||||||
| CHIP | N/D | N/D | TAD | Promotes MRTF ubiquitin-mediated degradation | − | [50] |
| Acetylation | ||||||
| Histone Acetyl Transferase (HAT) | ||||||
| P300 | K235/237/253/255 | N-term | Binds myocardin C-term | N/D | −/+ | [51] |
| Lysines are conserved between myocardin and MRTF | B1 | Binds MRTFA C-term (TAD) | N/D | + | [52] | |
| pCAF | Lysines are conserved between myocardin and MRTF | B1 | N/D | Promotes MRTF nuclear translocation | + | [53] |
| Histone Deacetyl Transferase | ||||||
| HDAC5 | N/D | N/D | Binds MRTF (domain undescribed) | Prevents MRTF-A nuclear translocation | − | [54,55] |
| HDAC6 | N/D | N/D | Binds MRTF (domain undescribed) | Regulates MRTF-A total protein (supresses) | − | [56] |
| SIRT1 | Lysines are conserved between myocardin and MRTF | B1 | Binds MRTF (domain undescribed) | N/D | + | [57] |
| SUMOylation | ||||||
| UBC9 | K499, 576, and 624 | C-term region (C-term LZ?) | N/D | No effect | − | [58] |
| SUMO-1/PIAS1 | K445 | C-term region (C-term LZ?) | 385–586 aa (C-term after SAP?) | No effect | + | [59] |
N/D, Not Determined; − or + notes if the molecule affects MRTF transcriptional activity negatively or positively.
ERK-mediated phosphorylation of S98 in the RPEL motif of MRTF-A facilitates nuclear accumulation by inhibiting actin binding [46]. This finding suggests that in addition to the cellular G-actin level, G-actin affinity of MRTF can also be modulated, which calibrates its cytoskeletal sensitivity without a change in the cytoskeleton per se. Moreover, the same kinase can target multiple sites, with differing effects: e.g., ERK-mediated phosphorylation of S454 in the LZ motif was reported to promote nuclear exit of MRTF, presumably because it facilitates G-actin binding that supports nuclear efflux [47]. Thus, ERK may induce increased nuclear import (“on” effect) followed by enhanced nuclear export (“off” effect), overall accelerating MRTF shuttling.
The activation of p38, downstream from cell-cell contact disruption [97], osmotic stress [76] or TGFβ stimulation [95] is required for nuclear accumulation and/or increased transcriptional activity of the already nuclear MRTF. We found that pharmacological inhibition or siRNA-mediated downregulation of p38 reduced the TGFβ-induced shift in the molecular mass of MRTF, indicating an effect of phosphorylation [95]. Inhibition of p38 also prevented the nuclear accumulation of MRTF in vivo in a right ventricular pressure overload-induced cardiac fibrosis model [98]. Relevantly, p38 has a paramount role in organ (e.g., renal) fibrosis [99], and the p38 inhibitor Pirfenidone—the sole antifibrotic drug in clinical practice [100,101] was also shown to reduce TGFβ-induced MRTF phosphorylation in tubular cells [95]. Nonetheless the scenario is complex as SRF is also phosphorylated and activated by p38 [102]. Finally, the p38 effector MK2 was shown to directly phosphorylate MRTF (S351, S371) but the functional consequences remain unclear [48]. Taken together, p38 has emerged as an important and therapeutically targetable modulator of MRTF, but future work is warranted to address the exact underlying mechanisms.
GSK3β was described to phosphorylate myocardin and suppress its transcriptional activity, thereby limiting atrial natriuretic factor-induced hypertrophy of cardiomyocytes [103]. Subsequently, we have shown that GSK3β is recruited to MRTF by Smad3, and induces phosphorylation, consequent ubiquitination and degradation of MRTF in tubular cells. β-catenin competes for Smad3 with GSK3β, and thus it stabilizes MRTF [49]. Since GSK3β is the key enzyme responsible for β-catenin degradation as well, and both β-catenin and MRTF are important mediators in organ (e.g., kidney) fibrosis, GSK3β could suppress fibrogenesis, in part, by promoting the degradation of these transcriptional regulators. Phosphorylation-dependent ubiquitination of myocardin is mediated by E3 ligase C terminus of Hsc70-interacting protein (CHIP) [50]. While proteasomal degradation of MRTF has been well documented [49,104], the phosphodegron and the responsible ubiquitin ligases remain to be identified.
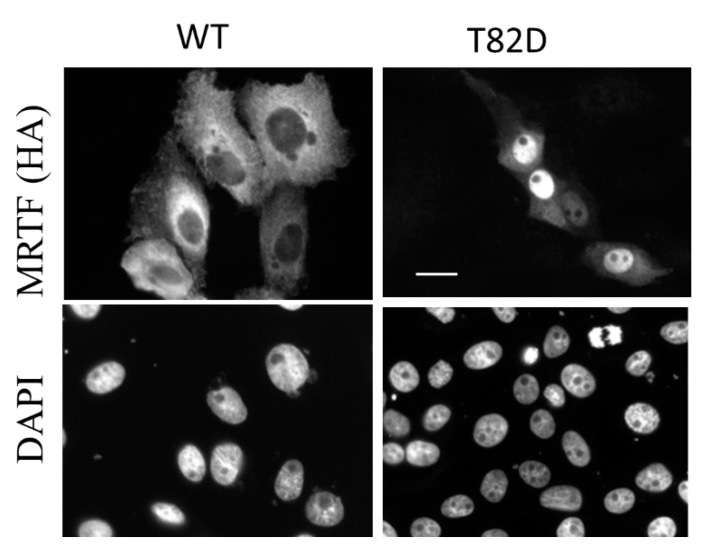
As mentioned above, active Rho provokes MRTF phosphorylation [38], and ROK inhibition abrogates S1P- [96,105], TGFβ- [106], osmotic stress- [76] or force transmission-induced [74] MRTF activity. However, to our best knowledge, direct phosphorylation of MRTF by ROK has not been shown (as yet). Nonetheless MRTF does contain ROK target motifs, and we found that phosphomimetic mutations of these (S82D and T92D) in the MRTF-B RPEL domain redistributes MRTF from the cytosol to the nucleus in tubular cells (P. Speight and A. Kapus, unpublished observation, (Figure 3). Thus, it is conceivable that ROK directly targets MRTF, which in turn might impact its actin affinity, a hypothesis worthwhile of further study.
Figure 3.
Phosphomimetic mutation of a potential ROK target site induces nuclear localization of MRTF. LLC-K1 proximal tubular cells were transfected with Hemagglutinin (HA)-tagged WT or T82D mutant MRTF-B constructs. Cells were stained for the HA epitope and nuclear marker, DAPI. Note that WT MRTF is cytosolic whereas the T82D mutant shows robust nuclear accumulation. Bar: 20 µm. (P. Speight and & A. Kapus, unpublished).
Such putative effect may be superimposed on the indirect-cytoskeletal—impact of ROK on MRTF, which seems to be brought about by two mechanisms. First, ROK promotes actin polymerization by activating LIM kinase, which phosphorylates and thereby inhibits cofilin, leading to reduced actin severing and higher F/G actin ratio [107]. Indeed, the operation of this pathway was shown to be critical for the serum induced rise in SRF activity [108] and for the force-triggered nuclear translocation of MRTF [74]. Second, ROK promotes myosin light chain phosphorylation and thereby myosin-dependent contractility [109]. Intriguingly, inhibiting myosin activity by a dominant negative myosin light chain mutant or by the drug blebbistatin dramatically reduced TGFβ- or cell contact injury-induced, MRTF-dependent activation of the α- smooth muscle actin promoter, and contact disassembly-induced MRTF translocation in kidney tubular cells [16]. These findings suggest that contractility per se might be a regulator of MRTF. While the underlying mechanisms remain to be defined, acto-myosin dependent force transmission from the extracellular matrix and the cytoskeleton to the nucleus via the linker of the nucleoskeleton to the cytoskeleton (LINC) complex [110] emerges as a significant regulator of the traffic of mechanosensitive transcription factors, including MRTF [111,112,113]. The LINC complex and the nucleoskeleton may act by modulating extra- or intranuclear actin assembly [111,112] or the permeability of the nuclear pore complexes [114,115]. Interestingly both the LINC complex and myosin were shown to regulate genome-wide transcriptional responses, with a subset of common target genes [116]. Future work should explore the role of the LINC complex and myosin in the control of MRTF-dependent transcription, a potentially important facet of mechanotransduction and the pathobiology of mechanical stress-related fibrogenic states (tissue stiffness and distension). This aspect may have particular relevance in renal pathologies since myosin mutations (e.g., in MYH9) show strong association with chronic kidney disease [117]. Considered together, phosphorylation plays a major role in MRTF regulation, and the combinatorics of 26 target residues provides enormous versatility for fine-tuning. Much remains to be learned about this type of control under physiological and pathological conditions.
In addition to phosphorylation, MRTF is controlled by acetylation (Table 1). The histone acetyl transferase (HAT) p300 was shown to acetylate myocardin [51], and it likely mediates MRTF acetylation as well, as HAT and MRTF can form a complex [52]. p300-mediated acetylation was associated with increased MRTF/SRF activity on promoters of muscle genes (e.g., MYH9, MYL9 as well as those encoding the matricellular protein Cyr61 [52]). Conversely, MRTF interacts with Histone Deacetylase 6 (HDAC6) [56], an enzyme localized predominantly in the cytosol, HDAC5 [54] (present in both cytosol and nucleus), and Sirtuin 1 (SIRT1) [57], a NAD-dependent deacetylase, localized in the nucleus. While each can deacetylate MRTF, the functional consequences were different. HDAC6 inhibition (i.e., increased acetylation) promoted MRTF activity to drive SRF-dependent α-smooth muscle actin expression; similarly HDAC5 overexpression (reduced acetylation) mitigated SRF/MRTF-mediated induction of antiapoptotic genes in neuronal cells [54]. In keeping with this, HDAC5 inhibition (increased acetylation) promoted the MRTF-dependent proinflammatory gene transcription by stimulating the activity of the MRTF/Nuclear Factor Kappa B (NFκB) (p65) complex in macrophages [55]. In contrast, SIRT1 overexpression (i.e., reduced acetylation) increased the capacity of MRTF to activate the collagen I promoter [57]. Together these results raise two important points. First, acetylation is a powerful modulator of MRTF, which may have a role in the promoter-selective regulation of its activity. The overall impact of acetylation may depend on a variety of factors including (a) the given promoter region; (b) TFs that MRTF partners with on a promoter; (c) the particular lysine residues targeted by acetylation/deacetylation; and (d) the cellular compartment in which MRTF is deacetylated. Second, MRTF forms complexes with histone-modifying enzymes at promoters, wherein its own modification by these may be a regulator of MRTF’s epigenetic and transcriptional effects.
Sumoylation of myocardin was reported to increase its activity on cardiogenic genes [59], while—interestingly—MRTF sumoylation at three lysine residues were shown to have a suppressive effect. Rho enhanced MRTF sumoylation [58], which may act as an inbuilt break in the control of MRTF activity. Again, this aspect of regulation warrants further studies.
4.2. Regulatory Protein Interactions
In addition to posttranslational modifications, MRTF is controlled by physical interactions with a number of proteins (Table 2). Four and half LIM domain protein 2 (FHL2), a multifunctional adaptor, is both a target (an MRTF/SRF-dependent gene) and a regulator of SRF/MRTF signaling. It binds to both SRF and MRTF, and while it stabilizes MRTF, it can either inhibit or stimulate the transcriptional activity of the SRF/MRTF complex, depending on the MRTF isoform and the particular promoter [60,61]. Interestingly FHL2 has been shown to be upregulated in and contribute to the pathogenesis of a variety of renal diseases, including interstitial fibrosis in diabetic, obstructive or hypertensive nephropathy [118,119,120,121,122]. Importantly, fibroblast-specific deletion of FHL2 attenuated myofibroblast accumulation (α-SMA expression) and matrix deposition (collagen, fibronectin) in the unilateral ureteral obstruction (UUO) model of renal fibrosis [122]. The likely mechanism is that FHL2 is a potent activator of the Wnt/β-catenin and TGFβ/β-catenin pathways (major drivers of renal fibrosis), and accordingly its absence abrogates the nuclear accumulation and activity of β-catenin [118,122]. FLH2 also binds to focal adhesion kinase (FAK), which in turn activates Rac; this pathway was proposed to underlie podocyte effacement in hypertensive kidney disease. Indeed, FLH2 knockout mice were partially protected against hypertension-induced albuminuria [121]. Since renal fibrogenesis is associated with MRTF overexpression [123,124], and MRTF is a driver of FHL2 gene, it is conceivable that MRTF plays a key role in increased FHL2 expression associated with these pathologies. Moreover, positive feedback loops may augment these effects, since both β-catenin and FHL2 can stabilize MRTF, contributing to its upregulation.
Table 2.
Transcription factors and transcriptional co-factors interacting with MRTF.
| Transcription Factor | Domain Bound | Effect on MRTF Localization/Stability | Effect on MRTF Transcriptional Activity | Reference |
|---|---|---|---|---|
| SRF | B1/Q | N/D | + | [37] |
| FHL2 | N-term (RPEL/B1-3/Q) | Increased myocardin protein levels | + | [60,61] |
| N-term (RPEL/B1-3/Q) | Increased MRTF-A protein levels | + | ||
| B1/Q | Decreased MRTF-B nuclear localization | − | ||
| YAP/TAZ | C-term (PPxY) | Decreased MRTF nuclear accumulation | −/+ | [22] |
| Smad3 | B1 | Promotes MRTF degradation | non-CArg = +; CArG = − | [19,20] |
| SP1 | N/D | N/D | + | [62] |
| NFκB/p65 | B1/Q | N/D | −/+ | [63,64] |
| Stat5 | Q | N/D | non-CArG/ICAM-1 = + | [65] |
| Stat3 | N/D | N/D | + | [66] |
N/D, Not Determined; − or + notes if the molecule effects MRTF transcriptional activity negatively or positively.
Suppressor of cancer cell invasion (SCAI) is a binding partner and inhibitor of MRTF, which reduces cancer cell migration primarily by suppressing the MRTF-induced β1 integrin production in cancer cells [125]. Remarkably, SCAI is downregulated during clinical and experimental renal fibrosis [126], which may be one of the reasons for enhanced MRTF signaling in these pathologies, as will be addressed further in the corresponding sections. Finally, a variety of transcription factors can bind to MRTF, including Smad3, TAZ/YAP, SP1, NFκB. These interactions impact not only (mutual) transcriptional activities but also the nuclear traffic of MRTF; the interplay between MRTF and these TFs will be further discussed in the relevant sections.
4.3. Regulation of MRTF Transcription
MRTF is also regulated at the transcriptional level, although the underlying mechanisms remain largely uncharacterized. Nonetheless, β-catenin has been recently identified as an inducer of the MRTF gene [127], suggesting that β-catenin not only stabilizes MRTF but also enhances its synthesis. While this observation was made in a tumor context, it may well be relevant in organ fibrosis as well. A very recent report shows that YAP can also activate the transcription of MRTF [128]. Since MRTF is a key transcriptional regulator of TAZ [22,95,124], MRTF and the hippo pathway effectors TAZ/YAP appear to form a positive feedback loop in each other’s transcriptional control. Future studies should address other regulators of MRTF expression. It is worth noting that several microRNAs have been implicated in MRTF expression (e.g., [129,130]). This branch of research is in a nascent state but will likely unearth key mechanisms in regulation of MRTF expression.
5. Targets, Functions, Actions
MRTF regulates a whole spectrum of processes, including development (of skeletal, cardiac, smooth muscle, neuronal and hematopoietic tissues), cell migration, phenotype shifts (e.g., EMT), mechanotransduction, wound healing/regeneration, extracellular matrix formation, cell cycle control, lipid and glucose metabolism and others. Accordingly, MRTF is involved in several pathologies, most prominently in cancer metastasis formation and organ fibrosis (for reviews of the various aspects see [11,17,18,125,131,132,133,134,135,136,137,138,139]). Overall, these widespread functions are brought about by three sets of mechanisms: MRTF can act as a
-
(1)
transcriptional co-activator for SRF or other TFs
-
(2)
regulator of epigenetic processes and chromosome organization
-
(3)
“moonlighting” protein, when it exerts its effect independent of its role in gene expression.
Each of these mechanisms plays important roles in physiological and pathological processes.
5.1. MRTF as a Transcriptional Coactivator
The most complete assembly of MRTF/SRF target genes was published by Esnault et al. [140], using MRTF chromatin-immunoprecipitation followed by deep mRNA sequencing (ChIP-seq) in fibroblasts. To identify genes likely regulated by MRTF, stringent criteria were used including (a) serum-inducibility; (b) MRTF ChIP; (c) inhibition by latrunculin A; c) stimulation by Cytochalasin D. Of the serum-inducible set, 921 genes showed at least one other criterion, and 683 genes exhibited all of them, with 398 genes containing a CArG box within 2 kB of the transcription start site (“direct”) and 285 genes within 70 Kb (“near”). Gene ontology (GO) analysis revealed that the corresponding gene products are related to cytoskeleton/focal adhesions, development, transcription, signaling, growth and metabolism, in accordance with the results of functional studies. Here we provide examples of “famous” target genes that were shown to be (a) MRTF-dependent in kidney cells and/or (b) directly related to renal pathologies. These include the contractility protein myosin [16,141], the myofibroblast hallmark α-SMA [16,20,104,142,143], actin-severing (cofilin), capping (CapZ) [20]) and bundling (filamin) [144]) proteins; focal adhesion and cell-matrix components (α and β integrins [145]), tenascin [144,146]); extracellular matrix proteins (collagen [62,147], CTGF [124,148,149,150]); caveolar proteins (caveolin 1 [144,151]), fibrogenic cytokines (TGFβ [124]), cell cycle regulators (p21 [152]), NADPH oxidases [153,154]; and transcription factors, encompassing SRF itself [20], the EMT drivers Snai1/2 and ZEB2 [155,156,157] and the Hippo effector TAZ [22,95]. Moreover, SRF/MRTF-targeted CArG motifs are frequently located in the vicinity of AP1 and TEAD sites (that are activated by YAP/TAZ), showing that these TFs often regulate the same genes, and may act synergistically with MRTF [23]. In addition to driving protein genes, MRTF was shown to induce microRNAs as well in CArG-dependent manner [158]. An intriguing example is miR-21, which is responsible for the “mechanical memory” of mesenchymal stem cells [159]. When plated on stiff surfaces these cells mobilize a fibrogenic program, and through MRTF-induced upregulation of miR-21 they “remember their stiff past” (for as long as two weeks) even when subsequently placed on soft surfaces. This mechanism is of major significance since fibrogenic phenotype shifts represent a serious impediment in stem cell therapies.
MRTF can impact gene expression not only by pairing up with the “canonical” SRF, but also with other TFs. These “alternative” interactions can also contribute to (renal) pathogenesis. In these cases MRTF does not act via the CArG box but through the cis-element targeted by its alternative partner. E.g. in kidney tubular cells TGFβ was shown to induce the interaction of MRTF with Smad3 [19,20] and this complex can drive, through a non-conventional Smad-binding element, the transcription of Snai2 (a.k.a SLUG), a key EMT-provoking gene suppressor. Snai2 in turn induces the downregulation of epithelial junction proteins (e.g., E-cadherin) [19]. Interestingly, while Smad3 is necessary for this CArG-independent MRTF action, binding of Smad3 to MRTF inhibits, the “classic” CArG-dependent effect of MRTF, e.g., the induction of the α-SMA promoter [20]. Thus, Smad3 has the capacity to act as a switch, redirecting MRTF to alternative targets (see also next section). The collagen promoter contains a non-canonical CArG box adjacent to a specificity protein-1 (SP1) sites. Interestingly, mutating these sites or the SP1 inhibitor mithramycin abrogated the MRTF-induced activation of the collagen promoter. This indicates that SP1 is necessary for the action of MRTF and the two factors work synergistically [62]. MRTF also drives the TNFα-induced expression of the RhoA/Rac GEF, GEF-H1 in tubular cells in an SP1-dependent manner (S. Venugopal, A. Kapus and K. Szaszi, manuscript in preparation). MRTF can also interact with Stat5, and this complex appears to be critical for driving fibronectin and ICAM-1 expression-through Stat5 sites—in mesangial cells stimulated by advanced glycation end product (AGEs) [65], which accumulate during diabetes and aging [65]. Further, MRTF was shown to physically interact with the NF-κB subunit p65 (RelA), resulting in a mutual inhibition of the action of these TFs. This mechanism underlies the bone morphogenic protein 4 (BMP-4)-induced anti-inflammatory response in vascular smooth muscle cells [64], and the reduction in ICAM-1 expression in endothelial cells [160]. On the other hand, MRTF was reported to promote lipopolysaccharide (LPS)-provoked, p65-dependent induction of inducible nitric oxide synthase (iNOS). Binding of MRTF to p65 modifies the histone mark at the iNOS promoter [63,91]. These studies indicate that CArG-independent actions of MRTF are also important regulators of gene expression, and they might be inductive or suppressive, depending on the particular genes or the cellular context. Furthermore, ChIP-seq analysis revealed that MRTF can act as a gene suppressor as well. This understudied area is a fertile ground for future research.
5.2. MRTF as an Epigenetic Modifier
Epigenetic control (e.g., via histone methylation, acetylation and chromatin remodeling) is a major factor in the regulation of gene expression, and MRTF impacts this process by several mechanisms (Table 3). MRTF deficiency erased key histone modifications in LPS-stimulated macrophages. As a determinant of the “trimethyl landscape”, MRTF recruits SET1, the H3K4 trimethyl transferase to NFκB sites in the promoters of proinflammatory genes [161], thereby stimulating their expression. H3K4 methylation is also enhanced via the interaction of MRTF with Ash2/Wdr5 methylation complex in response to angiotensin induction of the endothelin gene [162]. Interestingly MRTF was also reported to be important for demethylation of certain loci (H3K9) [163,164] via recruiting demethylases (e.g., KDM3A); these can also augment expression of select set of genes, e.g., those for NADPH oxidases [164]. MRTF regulates histone acetylation (e.g., at H3K9, H3K18, H3K27, H4K16) as well by recruiting various histone acetyl-transferase components, including p300, pCAF, TIP60 and MOF [52,91,123,164]. Conversely, through interaction with histone deacetylases (as detailed in Section 6) MRTF could contribute to acetylation/deacetylation cycles. Finally, MRTF controls chromatin remodeling too: e.g., it can recruit Brg1/Brm, components of the SWI/SNF ATP-dependent chromatin-remodeling complex to endothelin [165] and other promoters [162]. Since many of these modifications are relevant in various models of kidney disease, an integrated functional view will be provided in Section 6.
Table 3.
MRTF epigenetic modifiers.
| Epigenetic Modifier | Modification Type | Gene | Effect on Gene Activity | Reference |
|---|---|---|---|---|
| Methylation | ||||
| SET1 | H3K4 trimethyl transferase | Proinflammatory genes | + | [161] |
| Ash2/Wdr5 | H3K4 trimethyl transferase | Endothelin, COL1A1/COL1A2 | + | [123,162] |
| KDM3A | H3K9 demethylase | CTGF | + | [90] |
| Jmjd1a | H3K9 demethylase | SMC differentiation markers | − | [163] |
| Acetylation | ||||
| p300 | H3K18/H3K27 acetyltransferase | COL1A1/COL1A2 | + | [123] |
| TIP60 | H4K16 acetyltransferase | iNOS | + | [91] |
| MOF | H4K16 acetyltransferase | NOX1/4 | + | [164] |
− or + notes if the epigenetic modifier effects gene activity negatively or positively.
5.3. ”Moonlighting” Functions of MRTF
Our recent studies (manuscript under revision) indicate that both SRF and MRTF are necessary for serum-induced resorption of the primary cilium (PC). The PC is a microtubule-based, membrane-surrounded mechanochemical antenna [166,167] that acts as a flow sensor in the tubular epithelium [168,169], and a key regulator of cell division, differentiation and metabolism in all nucleated cells [170,171,172]. Ciliary defects are associated with a large set of severe diseases, so called ciliopathies, the prime examples of which are various forms of polycystic kidney disease (PKD) [173,174,175,176]. The PC is a dynamic organelle that develops from the basal body (BB) that arises from the membrane-anchored mother centriole as the cell leaves the cell cycle, and gets resorbed during cell cycle reentry. PC dissolution is necessary for cell proliferation because it liberates the BB, which in turn can function as a mitosis organizing center [177,178,179,180]. We found that MRTF downregulation eliminates serum-induced PC resorption and facilitates ciliogenesis. While SRF and MRTF can impact the cell cycle and may also drive genes necessary for cilium resorption, our results suggest that their role in PC regulation goes beyond such transcriptional effects. Remarkably, they localize to the PC or the BB, and MRTF physically interacts with cilium resorption proteins (e.g., Aurora A kinase), and ciliogenesis regulators (e.g., CEP290). In accordance with these findings, a recent BioID screen by the Gingras group showed that MRTF can be in complex with various BB/PC/centrosomal proteins (https://prohits-web.lunenfeld.ca (accessed on 15 April 2021). While the mechanistic details await further elucidation, these findings point to the fact that MRTF and SRF operate not only as TFs. Such moonlighting functions signify a new chapter in MRTF biology. In addition, they raise the possibility that MRTF might play a role in the pathogenesis of cystic diseases.
5.4. MRTF Knockouts
Given the wide range of regulated genes and processes, it is surprising that MRTF-A knockout mice are grossly normal. However, they are unable to nurse their offspring, because MRTF-A is indispensable for the proper development and function of mammary myoepithelial cells, and thus for milk ejection [181,182].
The likely reason for the lack of other defects is that MRTF-B (or myocardin) may fulfill most of MRTF-A’s critical functions. Nonetheless, MRTF-A KO animals show altered responses in a wide range of disease models and are useful tools to define the pathobiologic roles of MRTF (detailed for the kidney in Section 6). In contrast, MRTF-B knockouts are embryonically lethal due to serious cardiovascular defects related to the failure of differentiation of smooth muscle cells in the brachial arteries [183]. Thus, deletion of both MRTF-A and B can be achieved only in a tissue-specific manner. This has been accomplished in the context of the kidney, namely in podocytes, the highly specialized epithelial cells of the glomeruli. This cell type is a very relevant choice, as tight control of its subtle actin skeleton is indispensable for proper foot process formation, dynamics and slit diaphragm structure, which in turn are indispensable for normal glomerular filtration. A recent elegant study indicates that podocyte-specific deletion of either SRF or MRTF-A and B (but not one of them alone) causes foot process effacement, proteinuria, azotemia and reduced expression of podocyte markers [184]. These findings indicate that the SRF/MRTF system plays a fundamental physiologic role in the kidney. Currently no tubule-specific MRTF-A and/or B knockout animals exist; their generation would greatly facilitate the assessment of the physiologic and pathologic functions of MRTF in that compartment.
6. MRTF in Kidney Diseases
Considering the widespread role of MRTF in many processes, it is important to provide an overall conceptual framework to interpret its contribution to kidney disease. The major unifying notion is the role of MRTF in phenotype shifts, predominantly as it relates to organ fibrosis. To highlight these common mechanisms, first we will summarize those (mostly in vitro) studies that allowed insight into the cellular basis of this function.
6.1. The Epithelium as a Key Initiator of Fibrosis
Fibrosis is a dysregulated form of regeneration characterized by excessive ECM deposition, and the consequent disruption of tissue architecture and function [185,186]. Regarding the kidney, essentially all chronic renal diseases, from congenital disorders (like PKD or Alport syndrome) to acquired nephropathies (e.g., of diabetic, hypertensive, immune-mediated or obstructive origin) culminate in kidney fibrosis, which histologically manifests as glomerulosclerosis and/or tubulointerstitial fibrosis [187,188]. The cellular culprit of fibrosis is the myofibroblast, a contractile and ECM-producing cell type hallmarked by the expression of α-SMA. Myofibroblasts can originate from a multitude of cell types (fibroblasts, endothelial and epithelial cells, pericytes, bone-marrow derived fibrocytes), and the identification of their sources has been the focus of intensive research for over two decades (reviewed in [135,189,190,191,192]. It was recognized early on that epithelial (tubular) injury (e.g., due to high glucose levels, hypoxia, hydrostatic pressure, etc.) is one of the key initiating events of kidney fibrosis. Moreover, epithelial injury was also shown to be sufficient to trigger robust renal fibrosis. For example, proximal tubule-specific (Six2 Cre-driven) expression of diphtheria toxin (DT) receptor allows selective injury of this compartment upon administration of low dose DT. This proximal tubule-targeted insult resulted in robust tubulointerstitial fibrosis (TIF) and glomerulosclerosis [193]. Further support for the prime role of the epithelium came from genome-wide association studies (GWAS), characterizing single nucleotide polymorphism (SNPs) in chronic kidney disease (CKD). These studies revealed that approx. 20% of CKD cases can be linked to certain single gene variants, while others have a polygenic background with an overall heritability of 30–50% [194,195,196,197,198]. However, only 5% of these are localized in coding regions of the genes, pointing to the role of dysregulated expression. Recent elegant work aimed at linking a given SNP to the actual disease-causing gene built on the hypothesis that diseases are cell-type-specific, and therefore genetic variants are localized to cell type-specific regulatory regions [199]. To address this presumption, the authors used laser capture microdissection to isolate the glomerular and the tubular compartments from 151 kidneys, and then performed a compartment-specific “expression quantitative trait loci (eQTL)” analysis. Integrating the previous GWAS data, single cell RNA sequencing and epigenetic studies, they defined correlations between the observed SNPs (eVariants) and the potential corresponding target genes (eGenes) in a compartment-specific manner. This analysis identified 27 genes which showed differential expression according to the presence of the particular GWAS variants. Importantly, large portion of these occurred in (or were restricted to) the proximal tubules. They concluded that “renal proximal tubules show the greatest enrichment for GWAS–eQTL target genes (39%)”.
6.2. EMT/EMyT and PEP
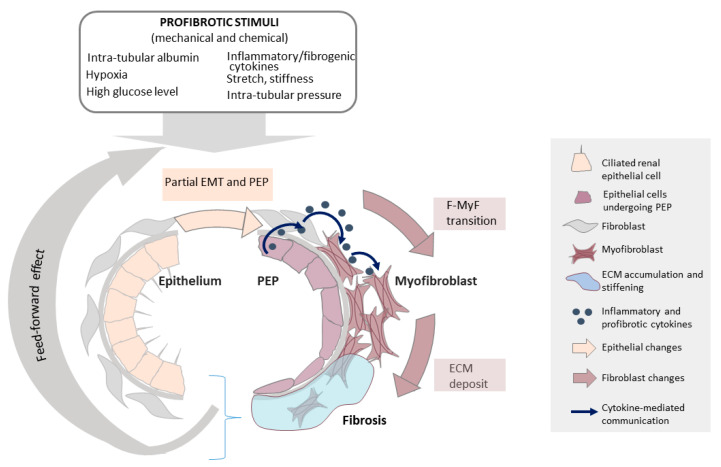
While the epithelium initiates the fibrotic process, mesenchymal cells (fibroblasts, myofibroblasts) are its executors. How is then epithelial initiation linked to mesenchymal execution? One potential explanation that dominated the field for a long time was that the epithelium is a key source of (myo)fibroblasts through epithelial-mesenchymal (EMT) or epithelial-myofibroblast (EMyT) transition (Figure 4). This idea proved to be a fertile ground to get insight into the underlying cellular pathobiology.
Figure 4.
Profibrotic epithelial phenotype (PEP) drives fibrosis. Epithelial injury can be secondary to mechanical forces (stretch and stiffness, intratubular pressure) or chemical insults (high glucose level, hypoxia), that result in the loss of cell-cell contacts, and inflammation. Together, these events drive a partial epithelial-to-mesenchymal transition, and the acquisition of the consequent profibrotic epithelial phenotype (PEP), characterized by increased production and secretion of profibrotic cytokines. PEP cells communicate with the adjacent stroma via the released cytokines (epithelial-mesenchymal crosstalk), that stimulate fibroblast-to-myofibroblast transition (F-MyF). Myofibroblasts secrete additional cytokines that trigger their proliferation, while they also deposit ECM components, leading to stiffening. On the other hand, PEP reinforces profibrotic stimuli, creating a feed-forward loop of injury, reinforcement of PEP and accelerated fibrosis.
Since the hallmark of the myofibroblast is α-SMA expression, the regulation of this process in epithelial cells emerged as a key question. The fact that renal epithelial cells can, in vitro, transform to fibroblasts and myofibroblasts have been documented by a plentitude of studies [142,200,201]. Moreover, the α-SMA gene, harboring two CArG boxes in its promoter is a typical SRF/MRTF target. Accordingly, MRTF is regulated by fibrogenic stimuli [16,97] and is indispensable for epithelial α-SMA expression [97,104]. These early studies allowed us to link MRTF to fibrosis for the first time [16,97]. The critical inducer of this transformation is TGFβ, the main fibrogenic cytokine. However, TGFβ- while necessary-is not sufficient for tubular EMT/EMyT. Remarkably the intact epithelium is resistant to such transformation, and a second hit is needed, which can be supplied by either the disruption/uncoupling/absence of the intercellular contacts (which can be induced by wounding, subconfluence or low extracellular calcium concentration) [16,20,97,202,203,204] or by mechanical stress (induced by restricted cell geometry, matrix stiffness, etc.) [22,205,206,207]. The common factor underlying this second hit is increased cellular tension or contractility, a result of small GTPase activation and cytoskeleton remodeling. All of these are potent regulators of MRTF. Interestingly, TGFβ alone is a poor inducer of MRTF nuclear translocation [20,95,104,208], while contact injury/mechanical stress triggers robust rise in nuclear MRTF levels but this is a transient phenomenon. However, TGFβ augments and prolongs the nuclear accumulation of MRTF [20]. The α-SMA promoter, which contains cis-elements for three major fibrogenic TF systems—SRF-MRTF (CArG boxes), Smad3 (SBEs) and Yap/TAZ-TEAD (TBEs) side-by-side proved to be an invaluable model to define the role of these TF pathways under various fibrogenic conditions. Investigation of the mechanism underlying the synergy between the arms (contact injury and TGFβ) of the two-hit model of α-SMA expression gave surprising results. TGFβ and injury indeed drive the α-SMA promoter synergistically but the SBEs are dispensable for this effect; synergy only requires intact CArG boxes, suggesting that in this system TGFβ acts primarily by potentiating the effect of MRTF. Moreover, these studies also allowed the definition of distinct phases of the transition. The first is an early Smad3-phase, when MRTF and Smad3 collaborate to induce a partial EMT. This results in the loss of some epithelial characteristics and enhanced mesenchymal gene expression. As mentioned above the Smad3/MRTF complex can induce the expression Snai1/2, major EMT-provoking TFs [19]. The second is a late Non-Smad3 phase, when MRTF alone turns on a myogenic program [20]. In contrast to the first phase, the second or myogenic phase is inhibited by Smad3, a finding consistent with reports showing that Smad3 levels decrease with the progression of (renal) fibrosis [209]. Later studies also revealed that combined humoral (TGFβ) and mechanical (cyclic stretch) stimulation reorders the relationship among the critical TFs. Namely, combined stimulation favors the association of Smad3 with TAZ. This has two consequences: first, it liberates MRTF from the MRTF/Smad3 and MRTF/TAZ complexes. Second it allows Smad3 and TAZ to reach their own cis-elements in gene promoters (e.g., α-SMA) [95].
While early fate-tracing studies proposed that the tubular epithelium is a major contributor to the myofibroblast population [210,211], more advanced lineage tracing methods refuted this scenario (reviewed in [135,190,212]. EMyT is a rare event in situ and only a small fraction (1–5%) of myofibroblasts is thought to be generated from the tubular epithelium in various animal models of fibrosis. Instead of a full EMyT, the epithelium undergoes a partial EMT, characterized by expression of EMT-promoting TFs (Snai1 and Twist), the loss of solute transporters, upregulation of mesenchymal markers, cell cycle arrest in G2, reduced fatty acid metabolism, and increased expression of fibrogenic mediators (TGFβ1) [156,213] and reviewed in [191] and [214]. Although partial, this mesenchymal shift is critical for fibrogenesis. Accordingly, epithelial deletion of Snai1 or Twist mitigated fibrosis in various animal models (UUO, folic acid, nephrotoxic serum), while epithelial overexpression in transgenic animals induced fibrogenesis [156,213]. These studies indicate that partial EMT is not only a feature but also a driver of renal fibrosis. How can MRTF be integrated into this refined paradigm? Two important points must be made. First, MRTF, a master regulator of EMT is a significant contributor to partial EMT as well. In fact, instead of “partial EMT” we introduced the more accurate term of “profibrotic epithelial phenotype” (PEP) [124], since the process is not restricted to the features of EMT (Figure 4). It also involves oxidative reprogramming exemplified by the increased expression of NADPH oxidases (e.g., Nox4 [153]), metabolic reprogramming, and most importantly a shift toward a secretory phenotype [124,156,215,216,217,218,219]. The injured epithelium starts producing fibrogenic cytokines (e.g., TGFβ, CTGF, PDGF, Hedgehog ligands) and MRTF silencing or pharmacological inhibition (CCG-1423) suppresses the synthesis of these mediators as well as Nox4 expression [124,153]. The produced cytokines in turn act on fibroblasts, thereby forming the link between the epithelial and the mesenchymal compartment (epithelial-mesenchymal crosstalk). MRTF also induces the expression of TAZ [22,95], another major fibrogenic TF in the kidney [124,153,203,220,221], which also contributes to cytokine production [124]. Evidence for the MRTF dependence of these processes in vivo (animal models) will be discussed in the Section 6.3. Second, the role of MRTF is likely different in the epithelial and mesenchymal compartment. While in the epithelium MRTF contributes to fibrosis via PEP (Figure 4), in most cases the process does not go “all the way”, i.e., the epithelium only rarely transforms to myofibroblast. This inbuilt break against a myogenic program, which is also manifest in the TGFβ resistance of the epithelium, is worthy of further study. In contrast, in the mesenchymal compartment MRTF is a key contributor to fibroblast-myofibroblast transition and in fact it is the main driver of this myogenic transition [17,222,223]. Indeed, MRTF has been described as major transcriptional driver of fibrosis in a wide variety of organs (including heart, lung, liver, skin, eye) and disease models [95,123,124,128,153,224,225,226,227,228,229,230,231,232,233,234,235,236,237]. Taken together, on the cellular level MRTF contributes to fibrosis both by partial EMT/PEP and by driving fibroblast-myofibroblast transition (Figure 4). In addition, as will be detailed in Section 6.3.3 it also impacts immune cell regulation.
6.3. MRTF in Animal Models of Kidney Disease
6.3.1. Diabetic Nephropathy (DN)
Diabetes is one of the leading causes of CKD, and conversely ≈30% of diabetic patients (over 460 million people worldwide) develops CKD, indicating the tremendous medical and social implications of this condition. The first direct indication that MRTF may play an important role in DN came from the studies of Fintha et al., who showed that the MRTF inhibitor protein SCAI is downregulated in the kidneys of diabetic rats concomitant with the development of fibrosis [126]. Definitive evidence was then obtained by Xu et al., who used two models of DN, high fat diet (HDF) and streptozotocin (STZ)-induced diabetes, and compared renal function and histology in WT and MRTF-A-deficient (KO) mice [123]. In both models, the absence of MRTF-A improved the serum albumin/creatinine ratio or mitigated urinary albumin excretion, reduced Type IV and Type I Collagen expression, immune cell (lymphocyte, neutrophil and macrophage) infiltration, mesangial matrix expansion and TIF. In addition, both HDF and STZ increased MRTF-A mRNA levels in WT mice. Importantly, ChIP revealed that both insults increased the binding of MRTF to the promoters of the collagen genes Col1a1 and Col1a2. To provide mechanistic insight the authors used WT or KO fibroblasts in vitro and showed that high glucose induced MRTF engagement of the collagen promoters. They then performed multiple ChIP assays using various antibodies against either particular histone marks or the enzymes involved in their generation. Overall, they found that high glucose is associated with histone acetylation (in histone 3 at lysine 18 and 27, H3K18Ac and H3K27Ac), and trimethylation (H3K4Me3). Absence or downregulation of MRTF prevented these modifications. Similar observations were made in DN mice as well. Finally, they found that high glucose triggered the recruitment of the histone acetyl transferase p300 and the histone trimethylase component WDR5 to collagen promoters, both of which were necessary for collagen promoter activation. Importantly, genetic deletion or silencing of MRTF prevented the recruitment of both enzymes. The same lab extended these studies to the induction of CTGF, a critically important fibrogenic mediator [90]. CTGF was well known to be driven by MRTF via a classic CArG box [143,238], but this recent report indicates the involvement of epigenetic mechanisms as well. CTGF induction is reduced in MRTF KO mice. In addition to the above-described changes in histone acetylation, STZ or high glucose (in vitro) decreased H3K9 dimethylation (H3K9Me2) at the CTGF promoter, and this effect was also prevented by the absence/downregulation of MRTF. These results were recapitulated in primary renal tubular epithelial cells as well, indicating the importance of MRTF in epithelial gene expression. Finally, the change in H3K9Me2 was attributed to the recruitment of the histone demethylase KDM3A, a process that required MRTF. KDM3A and MRTF formed a complex at the CTGF promoter. Taken together, MRTF expression and activity increase during DN, and MRTF significantly contributes to the ensuing pathology, at least in part, via epigenetic modulation of gene transcription. Whole body MRTF-A KO animals are substantially protected against the loss of kidney function and the development of fibrosis, which suggests that—somewhat unexpectedly—MRTF-B cannot substitute for MRTF-A in his regard. Compartment- and isoform-specific regulation remains to be elucidated. A concise summary of MRTF’s involvement and the underlying mechanisms in DN-associated and other kidney diseases is provided in Table 4.
Table 4.
MRTF in kidney diseases.
| Disease | Animal/Cell Model | Experimental Conditions | Suggested Mechanim | Reference |
|---|---|---|---|---|
| Diabetic nephropathy | REC cell model and WT rat |
SCAI overexpression, rat UUO | SCAI → blocks MRTF-A → locks fibrosis | [126] |
| Mrtf-a KO mice and fibroblasts | In vivo (STZ, high fat diet), in vitro (STZ, high glucose) |
MRTF-A is necessary to recruit histone acetyl- transferase and methyl- transferase to collagen promoters and activate type I collagen transcription | [123] | |
| MRTF-A KO mice and | In vivo (STZ, high fat diet) In vitro (STZ, high glucose) |
MRTF-A regulates histone acetylation and methylation on the CTGF promoter, partially through interacting with KDM3A | [90] | |
| Obstructive nephropathy | WT mice, REC cell model | UUO, in vitro functional studies | Epithelial MRTF-A links cytoskeletal and organization to redox state, through NOX4 | [153] |
| AMPK1α KO conditional (fibroblast) | UUO | AMPK1α → cofilin →F-actin → nuclear MRTF-A | [239] | |
| WT mice | UUO, MRTF-A inhibitor (CCG1423) | RhoA → MRTF-A → TAZ → PEP → fibrogenesis | [124,220,240,241] | |
| WT mice | UUO+ SCAI inhibition | SCAI → blocks MRTF-A → blocks fibrosis |
[126] | |
| Acute kidney injury | Macrophage-specific MRTF-A KO mice | Ischemia-reperfusion lipopolysaccharide |
MRTF-A → MYST1 → H4K16Ac at NOX → ROS | [154] |
| Polycystic kidney disease | PKD patients | Microarray comparison | MRTF-A/SRF transcription network is upregulated | [242] |
| PKD1 KO in tubules | Loss of PKD → LARG → RhoA → YAP/TAZ → c-Myc → cystogenesis | [243] | ||
| PKD1 patients PKD1 KO mice |
ROK-inhibitor (hydroxyfasudyl) treatment | Loss of PKD → ArhGAP35 → RhoA/ROK | [244] | |
| Pkd2+/−vascular smooth muscle | phenylephrin stimulation | Loss of PKD → RhoA → F-actin → αSMA |
[245,246] | |
| Pkd1 and Pkd2 KO mice | Expression and localization studies |
Increased MRTF expression and nuclear localization |
Kapus lab, unpublished data |
6.3.2. Obstructive Nephropathy (ON)
Obstructive uropathy is the most prevalent cause of CKD in children [247], and unilateral ureteric obstruction (UUO) is the most frequently used rodent model of TIF [248]. UUO is a model of mechanically induced fibrosis, inasmuch as the initiating factor is an increase in hydrostatic pressure along the nephron. The first indication of the potential involvement of MRTF came from studies showing that during UUO the expression of the NAPDH oxidase Nox4 increases in the tubular epithelium, and MRTF was shown to be necessary for the injury-induced induction of Nox4 in vitro in tubular cells [153]. In addition to MRTF, TAZ—a downstream target of MRTF—was also required for Nox4 expression, and pharmacological inhibition of TAZ eliminated Nox4 expression in the UUO-challenged epithelium. In kidney fibrosis Nox4 was proposed to play both a protective role (at low levels) and a pathogenic one (and high levels) (reviewed in [249]), implying that MRTF may be involved in these processes. Next, Wang et al. [239] proposed that MRTF might play a fibrogenic role in UUO in the fibroblast compartment. Their studies were aimed at explaining the mechanism whereby AMP-activated kinase-α1 (AMPKα1), a major sensor of cellular energy levels, contributes to renal fibrogenesis [250]. During UUO AMPKα1 levels are increased, whereas global or fibroblast-specific deletion of AMPKα1 reduces fibrosis [239,250]. The mechanism was investigated in rat kidney fibroblasts in vitro; pharmacological activation of AMPKα1 led to increased cofilin phosphorylation (i.e., less F-actin severing), thereby promoting net F-actin polymerization. Concomitantly, the AMPKα1 agonist also induced nuclear translocation of MRTF, which was proposed to be causative of myofibroblast transition. While this could be one of the MRTF-activating mechanisms, our recent studies revealed that early UUO leads to robust RhoA activation, predominantly in the tubular epithelium [124]. Accordingly, we found increased nuclear translocation of both MRTF-A and B in focal regions of the tubular epithelium, providing direct evidence for MRTF activation in UUO. Moreover, MRTF-A and MRTF-B were increased at the mRNA and protein levels as well, an effect detectable already 24 h post-injury. To assess the functional significance of MRTF signaling, mice were treated with the MRTF inhibitor CCG-1423. In control animals UUO significantly increased the mRNA (or protein) levels of fibrogenic cytokines, TGFβ, CTGF, PDGF, and Indian hedgehog, and of these, CCG-1423 significantly suppressed TGFβ and CTGF production, concomitant with mitigating ECM deposition and α-SMA expression. MRTF inhibition also suppressed the induction of TAZ in the tubular epithelium. Parallel in vitro experiments have shown that mechanical stress (cyclic stretch) increased the synthesis of all of the above-mentioned cytokines in tubular cells, and each was suppressed—to a various degree—by pharmacological inhibition or downregulation of MRTF or TAZ. To further substantiate the PEP concept in vivo, we isolated the tubular compartment using laser capture microdissection and have shown that UUO enhanced tubular TGFβ, CTGF and TAZ expression, and each of these were reduced by CCG-1423 [124]. TAZ emerges as a major effector of MRTF, as evidenced by the fact that TAZ is strongly induced in various fibrotic conditions and its inhibition mitigates renal fibrosis [220,240,241]. Finally, the MRTF inhibitor SCAI was also downregulated in UUO [126]. Taken together, these findings show that MRTF expression and activity increase in obstructive nephropathy. Several mechanisms (RhoA- and AMPKα1 mediated cytoskeleton remodeling and SCAI downregulation-induced desinhibition) contribute to this process. In the mesenchymal compartment MRTF directly facilitates myofibroblast transition, while in the tubular epithelium it promotes PEP, leading to oxidative changes, TAZ and cytokine expression. Thus, the Rho/MRTF/TAZ pathway emerges as a key mediator of fibrosis and represents a potential therapeutic target.
6.3.3. Acute Kidney Injury (AKI)
The most common causes of AKI, characterized by a sudden decrease in renal function, include ischemic insults, sepsis and poisoning, wherein hypoxia, pathogen- or damage-associated molecular patterns, inflammatory cytokines and environmental toxins or drugs damage the kidney, and particularly the tubular epithelium [251,252,253]. Its incidence is high (estimated to be 2–20% in hospitalized patients) and its mortality remains around 20% [251]. Of recent interest, AKI develops in 10% of hospitalized Covid-19 patients [254]. Beyond the acute phase, AKI was found to be associated with an 8.8-fold risk for CKD [255]. Thus, AKI represents a common and major clinical challenge. So far only one study addressed the role of MRTF in AKI. The results point to a novel cellular mechanism. Liu et al., in the Xu lab [154] used two AKI models: ischemia/reperfusion (I/R) and lipopolysaccharide (LPS) injection. Both insults caused deterioration of kidney function (blood urea nitrogen, plasma creatinine and urinary albumin/creatinine ratio), increased Kidney Injury Molecule-1 expression, high ROS production and a concomitant rise in renal Nox1 and Nox4 levels. All of these changes were ameliorated in MRTF-A knockout animals or upon MRTF inhibition by CCG-1423 in WT mice. Nox4 was upregulated in tubular cells but even more prominently in macrophages, while no change was observed in podocytes. This prompted the authors to concentrate on macrophages, showing that hypoxia/reoxygenation (H/R) induces MRTF-dependent Nox1 and Nox4 upregulation in these cells. Most importantly, myeloid cell-specific deletion of MRTF (crossing MRTF floxed mice with the Lyz2-Cre animals) was sufficient to improve renal function and reduce injury. Mechanistically, H/R caused histone modification, particularly H4K16 acetylation in the Nox promoters. The absence or inhibition of MRTF prevented these changes, as well as the recruitment of Myst1, one of the acetyl transferases that preferentially targets H4K16. Finally, a pharmacological Myst1 inhibitor ameliorated renal function and histological changes provoked by I/R. While this elegant study provided firm evidence for the role of MRTF in the pathogenesis of AKI, several questions remain open and are worthy of further study. Interestingly the major NADPH oxidase in macrophages is Nox2, whose deletion does not seem to alter the course of AKI [256]. Also, the mechanisms whereby MRTF act might be species-dependent, as the human proximal Nox4 promoter contains an SRF/MRTF-regulated GArG box [153], which is not present in the mouse promoter. The exact mechanism through which MRTF binds to promoters to recruit the epigenetic machinery also remains to be elucidated. Further, besides epigenetic regulation, MRTF might act by transactivating other TFs, such as SP1 and NFκB, both involved in Nox regulation. Indeed, NFκB was shown to bind MRTF in macrophages [257]. Accordingly, MRTF may have a broad (and largely uncharacterized) role in the regulation of inflammatory gene expression [91,258]. The role of MRTF in determining macrophage phenotype (proinflammatory vs. regenerative) also requires further study. Clearly, as Liu et al. [154] have emphasized, further studies should define “a more holistic role for MRTF-A in AKI” in particular, and in inflammation in general.
6.3.4. Polycystic Kidney Disease (PKD)—Solidifying a Hypothesis
PKD is the most common inherited renal disease (≈1:1000) [259,260] and its most prevalent form, Autosomal Dominant PKD (ADPKD) is caused by mutations in one of two primary cilium-associated membrane proteins, polycystin 1 (PC1 or PKD1, Pkd1 gene product, 85%) and polycystin 2 (PC2 or PKD2, Pkd2 gene product, 15%) [175,261]. These proteins together can form a mechanosensitive cation channel. PKD is characterized by progressive cyst formation due to enhanced and spatially distorted tubular cell proliferation [259,262]. Although a large number of mutations have been described in the two genes, ADPKD is consensually regarded as a loss-of-function disease [261]. Beside cyst formation, the major histologic feature of ADPKD is robust fibrosis [263,264]. As such, its pathobiology is a prime example of dysregulated epithelial-mesenchymal communication because the causative defect (impaired PKD1 or PKD2 function) affects predominantly tubular cells, while the fibrotic matrix is the product of mesenchymal cells. Although the underlying cell biology is complex and involves multiple pathways (e.g., cAMP, mTOR, β-catenin signaling) [259,265], there is increasing evidence that abnormal Rho signaling plays an important role in the pathogenesis of the disease. The relevant facts include the following. Gene profiling performed on cysts and minimally cystic/normal regions of five PKD1 patients indicated significant enrichment of transcripts associated with mitogen-mediated proliferation, cell cycle progression, EMT, hypoxia, aging and immune/inflammatory responses. When the identified genes were classified according to corresponding TF networks, the SRF pathway proved to be one of the strongest signatures [242]. Direct evidence of the involvement of Rho was obtained in a recent study, which investigated alterations in gene expression in a mouse model with inducible tubular deletion of Pkd1. Under these conditions YAP and TAZ signaling was strongly upregulated. Looking for a plausible mechanism, the authors investigated Rho signaling and found that Rho activity (and accordingly ROK activity and myosin phosphorylation) was increased in Pkd1-deficient cells and animals. The absence of Pkd1 resulted in the membrane translocation (activation) of the Rho-GEF LARG, suggesting that PKD1 exerts tonic inhibition on this Rho activator, a key control mechanism lost in PKD. Moreover, inhibiting ROK or LARG reduced YAP/TAZ-dependent gene expression and the cystic phenotype. The authors argued that the PKD1-inhibited LARG-RhoA-ROCK→YAP/TAZ pathway enhances the expression of the proto-oncogene c-Myc, which in turn provokes excessive tubular cell proliferation and cyst formation. A very recent study reported that the loss of PKD1 is also associated with loss of ArhGAP35, a negative regulator of Rho, from the centrosome. This is accompanied by higher RhoA activity, actin reorganization and shorter cilia. The ROK inhibitor hydroxyfasudyl reversed cyst expansion [244]. While these studies clearly suggest that Rho activation can contribute to the dysregulation of cell proliferation and thus cyst formation, we propose that it may also underlie the other major feature of the disease i.e., fibrogenesis, by promoting PEP. Moreover, MRTF may play a key role in these transitions, both because it activates the SRF-dependent genes identified above, and because it induces the expression of TAZ per se. Further rationale for this hypothesis came from earlier studies showing altered vascular responsiveness in Pkd2+/− heterozygote mice [245,246]. These animals exhibit exaggerated vasoconstriction upon phenylephrin stimulation [245], although their intracellular Ca level is lower than the WT [266]. Searching for the underlying mechanism, Du et al. [246] showed that vascular smooth muscle cells from Pkd2+/− animals expressed more αSMA and exhibited higher F/G-actin ratio under resting conditions. Intriguingly, phenylephrine induced 3-fold higher Rho activation and 5-fold higher nuclear MRTF accumulation in Pkd2+/− smooth muscle cells that in WT controls. Thus, the loss of even one Pkd2 allele is sufficient to potentiate Rho/MRTF signaling in vascular smooth muscle. Finally, our ongoing studies suggest that MRTF is both overexpressed and shows enhanced nuclear accumulation in PKD1- or 2-deficient tubular cells and in the tubular epithelium of Pkd2-/- mice (Mei D, Lichner Z, Pei Y and Kapus A). These observations give strong support to the notion that enhanced MRTF signaling may be an important pathogenic factor in PKD, which could drive both cystogenesis and fibrosis. Conversely, inhibition of MRTF may signify a potential therapy. These possibilities prompt further research.
6.3.5. Other Renal Diseases
The above sections summarize the documented involvement of MRTF in kidney diseases. However, this list likely represents only a fraction of the relevant pathologies. RhoA has been documented to play a role in a wide range of kidney pathologies, and in principle dysregulation of MRTF may play a role in each of these because dysregulated RhoA signaling can disrupt gene expression primarily via MRTF. Important examples include congenital and acquired proteinuric glomerular diseases, podocyte and mesangial cell injuries [8,9,267]. Further, any pathology associated with altered cytoskeleton organization is a potential “myrtfopathy”, impacting MRTF-dependent transcription. Examples include various forms of focal segmental glomerulosclerosis [268], or nephropathies associated with the MYH9, the myosin II heavy chain [117,268]. The emerging role of MRTF in the regulation of the primary cilium, both as a transcription factor and a cilium constituent, is fertile ground for future work. It remains to be explored whether MRTF can be linked to certain ciliopathies. Finally, new studies should be conducted to document enhanced MRTF signaling in human histological samples for various renal diseases. Such analyses will not only be important for assessing the role of MRTF in these pathologies, but may link altered MRTF signaling to different phases of these processes, thereby informing diagnosis and potentially therapy.
7. Perspectives: MRTF in Therapy
Given the substantial evidence indicating the role of MRTF in kidney diseases, a critical outstanding question is whether MRTF can be exploited as a pharmacological target in these conditions. This approach would be advantageous because it targets a hub downstream of a slew of stimuli, while at the same time it is less broad, more feasible and more appropriate for chronic conditions than inhibition of Rho itself. CCG-1423, the prototypic small molecule MRTF inhibitor was discovered as a suppressor of Rho/MRTF/SRF pathway downstream of Rho [269]. Strangely, its exact mechanism of action is yet uncertain: it has been proposed to bind directly to the RPEL motif of MRTF [270], to MICAL-2, a monooxygenase regulating actin oxidation and polymerization [78], and to pirin, a redox-sensitive nuclear protein [271]. Irrespective of the exact mechanism, chemical modification of the parent compound led to a series of MRTF inhibitors (e.g., CCG-100602, CCG-203971, CCG-222740, CCG-232601) with increased potency, better pharmacokinetics, stability, bioavailability and less toxicity [237,272,273,274]. Recently a high throughput screen identified a new and very potent class of MRTF inhibitors (5-Aryl-1,3,4-oxadiazol-2-ylthioalkanoic acids) [275]. While understanding the exact mechanisms of action of these drugs is an important goal for current research, there is firm evidence that various MRTF antagonists can mitigate tissue fibrosis in several models (lung, skin, heart, kidney, ocular, peritoneal etc.) [123,124,225,227,232,237,271,274]. They also effectively block metastasis formation in tumor models [273,276]. However, regarding kidney pathologies only the parent compound, CCG-1423 was tested, and only in a few models (DN, AKI, ON) [123,124,154]. The newer MRTF inhibitors are promising antifibrotics that deserve more attention and further scrutiny as experimentally—and potentially clinically-useful drugs. Treating fibrosis is a large unmet need, and MRTF inhibitors may, at least in part, hold the answer to this enormous problem.
Funding
Relevant work from the authors’ laboratories was supported by grants from the Canadian Institutes of Health Research (CIHR, PJT-162360, PJT-148608 and MOP-13046), the Natural Sciences and Engineering Research Council of Canada (RGPIN-2019-05222) and the Kidney Foundation of Canada to AK and CIHR grant MOP-142409 to KS.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable. No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hall A. Rho family GTPases. Biochem. Soc. Trans. 2012;40:1378–1382. doi: 10.1042/BST20120103. [DOI] [PubMed] [Google Scholar]
- 2.Perico L., Conti S., Benigni A., Remuzzi G. Podocyte-actin dynamics in health and disease. Nat. Rev. Nephrol. 2016;12:692–710. doi: 10.1038/nrneph.2016.127. [DOI] [PubMed] [Google Scholar]
- 3.Schell C., Huber T.B. The Evolving Complexity of the Podocyte Cytoskeleton. J. Am. Soc. Nephrol. 2017;28:3166–3174. doi: 10.1681/ASN.2017020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaine J., Dylewski J. Regulation of the Actin Cytoskeleton in Podocytes. Cells. 2020;9:1700. doi: 10.3390/cells9071700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sever S. Role of actin cytoskeleton in podocytes. Pediatr. Nephrol. 2020 doi: 10.1007/s00467-020-04812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moriyama T., Nagatoya K. The Rho-ROCK system as a new therapeutic target for preventing interstitial fibrosis. Drug News Perspect. 2004;17:29–34. doi: 10.1358/dnp.2004.17.1.829023. [DOI] [PubMed] [Google Scholar]
- 7.Ma S., Guan K.L. Polycystic kidney disease: A Hippo connection. Genes Dev. 2018;32:737–739. doi: 10.1101/gad.316570.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saleem M.A., Welsh G.I. Podocyte RhoGTPases: New therapeutic targets for nephrotic syndrome? F1000Research. 2019;8 doi: 10.12688/f1000research.20105.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsuda J., Asano-Matsuda K., Kitzler T.M., Takano T. Rho GTPase regulatory proteins in podocytes. Kidney Int. 2021;99:336–345. doi: 10.1016/j.kint.2020.08.035. [DOI] [PubMed] [Google Scholar]
- 10.Kustermans G., Piette J., Legrand-Poels S. Actin-targeting natural compounds as tools to study the role of actin cytoskeleton in signal transduction. Biochem. Pharmacol. 2008;76:1310–1322. doi: 10.1016/j.bcp.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 11.Olson E.N., Nordheim A. Linking actin dynamics and gene transcription to drive cellular motile functions. Nat. Rev. Mol. Cell Biol. 2010;11:353–365. doi: 10.1038/nrm2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sidorenko E., Vartiainen M.K. Nucleoskeletal regulation of transcription: Actin on MRTF. Exp. Biol. Med. 2019;244:1372–1381. doi: 10.1177/1535370219854669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoon J.L., Tan M.H., Koh C.G. The Regulation of Cellular Responses to Mechanical Cues by Rho GTPases. Cells. 2016;5:17. doi: 10.3390/cells5020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bros M., Haas K., Moll L., Grabbe S. RhoA as a Key Regulator of Innate and Adaptive Immunity. Cells. 2019;8:733. doi: 10.3390/cells8070733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burridge K., Monaghan-Benson E., Graham D.M. Mechanotransduction: From the cell surface to the nucleus via RhoA. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019;374:20180229. doi: 10.1098/rstb.2018.0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan L., Sebe A., Peterfi Z., Masszi A., Thirone A.C., Rotstein O.D., Nakano H., McCulloch C.A., Szaszi K., Mucsi I., et al. Cell contact-dependent regulation of epithelial-myofibroblast transition via the rho-rho kinase-phospho-myosin pathway. Mol. Biol. Cell. 2007;18:1083–1097. doi: 10.1091/mbc.e06-07-0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Small E.M. The actin-MRTF-SRF gene regulatory axis and myofibroblast differentiation. J. Cardiovasc. Transl. Res. 2012;5:794–804. doi: 10.1007/s12265-012-9397-0. [DOI] [PubMed] [Google Scholar]
- 18.Gasparics A., Sebe A. MRTFs- master regulators of EMT. Dev. Dyn. 2018;247:396–404. doi: 10.1002/dvdy.24544. [DOI] [PubMed] [Google Scholar]
- 19.Morita T., Mayanagi T., Sobue K. Dual roles of myocardin-related transcription factors in epithelial mesenchymal transition via slug induction and actin remodeling. J. Cell Biol. 2007;179:1027–1042. doi: 10.1083/jcb.200708174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masszi A., Speight P., Charbonney E., Lodyga M., Nakano H., Szaszi K., Kapus A. Fate-determining mechanisms in epithelial-myofibroblast transition: Major inhibitory role for Smad3. J. Cell Biol. 2010;188:383–399. doi: 10.1083/jcb.200906155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu O.M., Miyamoto S., Brown J.H. Myocardin-Related Transcription Factor A and Yes-Associated Protein Exert Dual Control in G Protein-Coupled Receptor- and RhoA-Mediated Transcriptional Regulation and Cell Proliferation. Mol. Cell. Biol. 2016;36:39–49. doi: 10.1128/MCB.00772-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Speight P., Kofler M., Szaszi K., Kapus A. Context-dependent switch in chemo/mechanotransduction via multilevel crosstalk among cytoskeleton-regulated MRTF and TAZ and TGFbeta-regulated Smad3. Nat. Commun. 2016;7:11642. doi: 10.1038/ncomms11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foster C.T., Gualdrini F., Treisman R. Mutual dependence of the MRTF-SRF and YAP-TEAD pathways in cancer-associated fibroblasts is indirect and mediated by cytoskeletal dynamics. Genes Dev. 2017;31:2361–2375. doi: 10.1101/gad.304501.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aragona M., Panciera T., Manfrin A., Giulitti S., Michielin F., Elvassore N., Dupont S., Piccolo S. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell. 2013;154:1047–1059. doi: 10.1016/j.cell.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 25.Graham R., Gilman M. Distinct protein targets for signals acting at the c-fos serum response element. Science. 1991;251:189–192. doi: 10.1126/science.1898992. [DOI] [PubMed] [Google Scholar]
- 26.Norman C., Runswick M., Pollock R., Treisman R. Isolation and properties of cDNA clones encoding SRF, a transcription factor that binds to the c-fos serum response element. Cell. 1988;55:989–1003. doi: 10.1016/0092-8674(88)90244-9. [DOI] [PubMed] [Google Scholar]
- 27.Sartorelli V., Webster K.A., Kedes L. Muscle-specific expression of the cardiac alpha-actin gene requires MyoD1, CArG-box binding factor, and Sp1. Genes Dev. 1990;4:1811–1822. doi: 10.1101/gad.4.10.1811. [DOI] [PubMed] [Google Scholar]
- 28.Sepulveda J.L., Belaguli N., Nigam V., Chen C.Y., Nemer M., Schwartz R.J. GATA-4 and Nkx-2.5 coactivate Nkx-2 DNA binding targets: Role for regulating early cardiac gene expression. Mol. Cell. Biol. 1998;18:3405–3415. doi: 10.1128/MCB.18.6.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor M., Treisman R., Garrett N., Mohun T. Muscle-specific (CArG) and serum-responsive (SRE) promoter elements are functionally interchangeable in Xenopus embryos and mouse fibroblasts. Development. 1989;106:67–78. doi: 10.1242/dev.106.1.67. [DOI] [PubMed] [Google Scholar]
- 30.Miano J.M. Serum response factor: Toggling between disparate programs of gene expression. J. Mol. Cell Cardiol. 2003;35:577–593. doi: 10.1016/S0022-2828(03)00110-X. [DOI] [PubMed] [Google Scholar]
- 31.Hill C.S., Wynne J., Treisman R. The Rho family GTPases RhoA, Rac1, and CDC42Hs regulate transcriptional activation by SRF. Cell. 1995;81:1159–1170. doi: 10.1016/S0092-8674(05)80020-0. [DOI] [PubMed] [Google Scholar]
- 32.Mack C.P., Somlyo A.V., Hautmann M., Somlyo A.P., Owens G.K. Smooth muscle differentiation marker gene expression is regulated by RhoA-mediated actin polymerization. J. Biol. Chem. 2001;276:341–347. doi: 10.1074/jbc.M005505200. [DOI] [PubMed] [Google Scholar]
- 33.Onuh J.O., Qiu H. Serum response factor-cofactor interactions and their implications in disease. FEBS J. 2020;288:3120–3134. doi: 10.1111/febs.15544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Price M.A., Hill C., Treisman R. Integration of growth factor signals at the c-fos serum response element. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1996;351:551–559. doi: 10.1098/rstb.1996.0054. [DOI] [PubMed] [Google Scholar]
- 35.Treisman R., Alberts A.S., Sahai E. Regulation of SRF activity by Rho family GTPases. Cold Spring Harb. Symp. Quant. Biol. 1998;63:643–651. doi: 10.1101/sqb.1998.63.643. [DOI] [PubMed] [Google Scholar]
- 36.Sotiropoulos A., Gineitis D., Copeland J., Treisman R. Signal-regulated activation of serum response factor is mediated by changes in actin dynamics. Cell. 1999;98:159–169. doi: 10.1016/S0092-8674(00)81011-9. [DOI] [PubMed] [Google Scholar]
- 37.Wang D.Z., Li S., Hockemeyer D., Sutherland L., Wang Z., Schratt G., Richardson J.A., Nordheim A., Olson E.N. Potentiation of serum response factor activity by a family of myocardin-related transcription factors. Proc. Natl. Acad. Sci. USA. 2002;99:14855–14860. doi: 10.1073/pnas.222561499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miralles F., Posern G., Zaromytidou A.I., Treisman R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell. 2003;113:329–342. doi: 10.1016/S0092-8674(03)00278-2. [DOI] [PubMed] [Google Scholar]
- 39.Selvaraj A., Prywes R. Megakaryoblastic leukemia-1/2, a transcriptional co-activator of serum response factor, is required for skeletal myogenic differentiation. J. Biol. Chem. 2003;278:41977–41987. doi: 10.1074/jbc.M305679200. [DOI] [PubMed] [Google Scholar]
- 40.Wang D., Chang P.S., Wang Z., Sutherland L., Richardson J.A., Small E., Krieg P.A., Olson E.N. Activation of cardiac gene expression by myocardin, a transcriptional cofactor for serum response factor. Cell. 2001;105:851–862. doi: 10.1016/S0092-8674(01)00404-4. [DOI] [PubMed] [Google Scholar]
- 41.Mercher T., Coniat M.B., Monni R., Mauchauffe M., Nguyen Khac F., Gressin L., Mugneret F., Leblanc T., Dastugue N., Berger R., et al. Involvement of a human gene related to the Drosophila spen gene in the recurrent t(1;22) translocation of acute megakaryocytic leukemia. Proc. Natl. Acad. Sci. USA. 2001;98:5776–5779. doi: 10.1073/pnas.101001498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma Z., Morris S.W., Valentine V., Li M., Herbrick J.A., Cui X., Bouman D., Li Y., Mehta P.K., Nizetic D., et al. Fusion of two novel genes, RBM15 and MKL1, in the t(1;22)(p13;q13) of acute megakaryoblastic leukemia. Nat. Genet. 2001;28:220–221. doi: 10.1038/90054. [DOI] [PubMed] [Google Scholar]
- 43.Cen B., Selvaraj A., Burgess R.C., Hitzler J.K., Ma Z., Morris S.W., Prywes R. Megakaryoblastic leukemia 1, a potent transcriptional coactivator for serum response factor (SRF), is required for serum induction of SRF target genes. Mol. Cell. Biol. 2003;23:6597–6608. doi: 10.1128/MCB.23.18.6597-6608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Descot A., Rex-Haffner M., Courtois G., Bluteau D., Menssen A., Mercher T., Bernard O.A., Treisman R., Posern G. OTT-MAL is a deregulated activator of serum response factor-dependent gene expression. Mol. Cell. Biol. 2008;28:6171–6181. doi: 10.1128/MCB.00303-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sasazuki T., Sawada T., Sakon S., Kitamura T., Kishi T., Okazaki T., Katano M., Tanaka M., Watanabe M., Yagita H., et al. Identification of a novel transcriptional activator, BSAC, by a functional cloning to inhibit tumor necrosis factor-induced cell death. J. Biol. Chem. 2002;277:28853–28860. doi: 10.1074/jbc.M203190200. [DOI] [PubMed] [Google Scholar]
- 46.Panayiotou R., Miralles F., Pawlowski R., Diring J., Flynn H.R., Skehel M., Treisman R. Phosphorylation acts positively and negatively to regulate MRTF-A subcellular localisation and activity. eLife. 2016;5 doi: 10.7554/eLife.15460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muehlich S., Wang R., Lee S.M., Lewis T.C., Dai C., Prywes R. Serum-induced phosphorylation of the serum response factor coactivator MKL1 by the extracellular signal-regulated kinase 1/2 pathway inhibits its nuclear localization. Mol. Cell. Biol. 2008;28:6302–6313. doi: 10.1128/MCB.00427-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ronkina N., Lafera J., Kotlyarov A., Gaestel M. Stress-dependent phosphorylation of myocardin-related transcription factor A (MRTF-A) by the p38(MAPK)/MK2 axis. Sci. Rep. 2016;6:31219. doi: 10.1038/srep31219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Charbonney E., Speight P., Masszi A., Nakano H., Kapus A. beta-catenin and Smad3 regulate the activity and stability of myocardin-related transcription factor during epithelial-myofibroblast transition. Mol. Biol. Cell. 2011;22:4472–4485. doi: 10.1091/mbc.e11-04-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xie P., Fan Y., Zhang H., Zhang Y., She M., Gu D., Patterson C., Li H. CHIP represses myocardin-induced smooth muscle cell differentiation via ubiquitin-mediated proteasomal degradation. Mol. Cell. Biol. 2009;29:2398–2408. doi: 10.1128/MCB.01737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cao D., Wang C., Tang R., Chen H., Zhang Z., Tatsuguchi M., Wang D.Z. Acetylation of myocardin is required for the activation of cardiac and smooth muscle genes. J. Biol. Chem. 2012;287:38495–38504. doi: 10.1074/jbc.M112.353649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He H., Wang D., Yao H., Wei Z., Lai Y., Hu J., Liu X., Wang Y., Zhou H., Wang N., et al. Transcriptional factors p300 and MRTF-A synergistically enhance the expression of migration-related genes in MCF-7 breast cancer cells. Biochem. Biophys. Res. Commun. 2015;467:813–820. doi: 10.1016/j.bbrc.2015.10.060. [DOI] [PubMed] [Google Scholar]
- 53.Yu L., Li Z., Fang M., Xu Y. Acetylation of MKL1 by PCAF regulates pro-inflammatory transcription. Biochim. Biophys. Acta Gene Regul. Mech. 2017;1860:839–847. doi: 10.1016/j.bbagrm.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 54.Li N., Yuan Q., Cao X.L., Zhang Y., Min Z.L., Xu S.Q., Yu Z.J., Cheng J., Zhang C., Hu X.M. Opposite effects of HDAC5 and p300 on MRTF-A-related neuronal apoptosis during ischemia/reperfusion injury in rats. Cell Death Dis. 2017;8:e2624. doi: 10.1038/cddis.2017.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li Z., Qin H., Li J., Yu L., Yang Y., Xu Y. HADC5 deacetylates MKL1 to dampen TNF-alpha induced pro-inflammatory gene transcription in macrophages. Oncotarget. 2017;8:94235–94246. doi: 10.18632/oncotarget.21670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang M., Urabe G., Little C., Wang B., Kent A.M., Huang Y., Kent K.C., Guo L.W. HDAC6 Regulates the MRTF-A/SRF Axis and Vascular Smooth Muscle Cell Plasticity. JACC Basic Transl. Sci. 2018;3:782–795. doi: 10.1016/j.jacbts.2018.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang Y., Li Z., Guo J., Xu Y. Deacetylation of MRTF-A by SIRT1 defies senescence induced down-regulation of collagen type I in fibroblast cells. Biochim. Biophys. Acta Mol. Basis Dis. 2020;1866:165723. doi: 10.1016/j.bbadis.2020.165723. [DOI] [PubMed] [Google Scholar]
- 58.Nakagawa K., Kuzumaki N. Transcriptional activity of megakaryoblastic leukemia 1 (MKL1) is repressed by SUMO modification. Genes Cells. 2005;10:835–850. doi: 10.1111/j.1365-2443.2005.00880.x. [DOI] [PubMed] [Google Scholar]
- 59.Wang J., Li A., Wang Z., Feng X., Olson E.N., Schwartz R.J. Myocardin sumoylation transactivates cardiogenic genes in pluripotent 10T1/2 fibroblasts. Mol. Cell. Biol. 2007;27:622–632. doi: 10.1128/MCB.01160-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hinson J.S., Medlin M.D., Taylor J.M., Mack C.P. Regulation of myocardin factor protein stability by the LIM-only protein FHL2. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H1067–H1075. doi: 10.1152/ajpheart.91421.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Philippar U., Schratt G., Dieterich C., Muller J.M., Galgoczy P., Engel F.B., Keating M.T., Gertler F., Schule R., Vingron M., et al. The SRF target gene Fhl2 antagonizes RhoA/MAL-dependent activation of SRF. Mol. Cell. 2004;16:867–880. doi: 10.1016/j.molcel.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 62.Luchsinger L.L., Patenaude C.A., Smith B.D., Layne M.D. Myocardin-related transcription factor-A complexes activate type I collagen expression in lung fibroblasts. J. Biol. Chem. 2011;286:44116–44125. doi: 10.1074/jbc.M111.276931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lin L., Zhang Q., Fan H., Zhao H., Yang Y. Myocardin-Related Transcription Factor A Mediates LPS-Induced iNOS Transactivation. Inflammation. 2020;43:1351–1361. doi: 10.1007/s10753-020-01213-0. [DOI] [PubMed] [Google Scholar]
- 64.Wang D., Prakash J., Nguyen P., Davis-Dusenbery B.N., Hill N.S., Layne M.D., Hata A., Lagna G. Bone morphogenetic protein signaling in vascular disease: Anti-inflammatory action through myocardin-related transcription factor A. J. Biol. Chem. 2012;287:28067–28077. doi: 10.1074/jbc.M112.379487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen Q., Huang J., Gong W., Chen Z., Huang J., Liu P., Huang H. MRTF-A mediated FN and ICAM-1 expression in AGEs-induced rat glomerular mesangial cells via activating STAT5. Mol. Cell Endocrinol. 2018;460:123–133. doi: 10.1016/j.mce.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 66.Xing W.J., Liao X.H., Wang N., Zhao D.W., Zheng L., Zheng D.L., Dong J., Zhang T.C. MRTF-A and STAT3 promote MDA-MB-231 cell migration via hypermethylating BRSM1. IUBMB Life. 2015;67:202–217. doi: 10.1002/iub.1362. [DOI] [PubMed] [Google Scholar]
- 67.Mouilleron S., Guettler S., Langer C.A., Treisman R., McDonald N.Q. Molecular basis for G-actin binding to RPEL motifs from the serum response factor coactivator MAL. EMBO J. 2008;27:3198–3208. doi: 10.1038/emboj.2008.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vartiainen M.K., Guettler S., Larijani B., Treisman R. Nuclear actin regulates dynamic subcellular localization and activity of the SRF cofactor MAL. Science. 2007;316:1749–1752. doi: 10.1126/science.1141084. [DOI] [PubMed] [Google Scholar]
- 69.Staus D.P., Weise-Cross L., Mangum K.D., Medlin M.D., Mangiante L., Taylor J.M., Mack C.P. Nuclear RhoA signaling regulates MRTF-dependent SMC-specific transcription. Am. J. Physiol. Heart Circ. Physiol. 2014;307:H379–H390. doi: 10.1152/ajpheart.01002.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Garcia-Mata R., Burridge K. Catching a GEF by its tail. Trends Cell. Biol. 2007;17:36–43. doi: 10.1016/j.tcb.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 71.Joo E., Olson M.F. Regulation and functions of the RhoA regulatory guanine nucleotide exchange factor GEF-H1. Small GTPases. 2020:1–14. doi: 10.1080/21541248.2020.1840889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Denk-Lobnig M., Martin A.C. Modular regulation of Rho family GTPases in development. Small GTPases. 2019;10:122–129. doi: 10.1080/21541248.2017.1294234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hinkel R., Trenkwalder T., Petersen B., Husada W., Gesenhues F., Lee S., Hannappel E., Bock-Marquette I., Theisen D., Leitner L., et al. MRTF-A controls vessel growth and maturation by increasing the expression of CCN1 and CCN2. Nat. Commun. 2014;5:3970. doi: 10.1038/ncomms4970. [DOI] [PubMed] [Google Scholar]
- 74.Zhao X.H., Laschinger C., Arora P., Szaszi K., Kapus A., McCulloch C.A. Force activates smooth muscle alpha-actin promoter activity through the Rho signaling pathway. J. Cell Sci. 2007;120:1801–1809. doi: 10.1242/jcs.001586. [DOI] [PubMed] [Google Scholar]
- 75.Thirone A.C., Speight P., Zulys M., Rotstein O.D., Szaszi K., Pedersen S.F., Kapus A. Hyperosmotic stress induces Rho/Rho kinase/LIM kinase-mediated cofilin phosphorylation in tubular cells: Key role in the osmotically triggered F-actin response. Am. J. Physiol. Cell. Physiol. 2009;296:C463–C475. doi: 10.1152/ajpcell.00467.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ly D.L., Waheed F., Lodyga M., Speight P., Masszi A., Nakano H., Hersom M., Pedersen S.F., Szaszi K., Kapus A. Hyperosmotic stress regulates the distribution and stability of myocardin-related transcription factor, a key modulator of the cytoskeleton. Am. J. Physiol. Cell. Physiol. 2013;304:C115–C127. doi: 10.1152/ajpcell.00290.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chan M.W., Chaudary F., Lee W., Copeland J.W., McCulloch C.A. Force-induced myofibroblast differentiation through collagen receptors is dependent on mammalian diaphanous (mDia) J. Biol. Chem. 2010;285:9273–9281. doi: 10.1074/jbc.M109.075218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lundquist M.R., Storaska A.J., Liu T.C., Larsen S.D., Evans T., Neubig R.R., Jaffrey S.R. Redox modification of nuclear actin by MICAL-2 regulates SRF signaling. Cell. 2014;156:563–576. doi: 10.1016/j.cell.2013.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mouilleron S., Langer C.A., Guettler S., McDonald N.Q., Treisman R. Structure of a pentavalent G-actin*MRTF-A complex reveals how G-actin controls nucleocytoplasmic shuttling of a transcriptional coactivator. Sci. Signal. 2011;4:ra40. doi: 10.1126/scisignal.2001750. [DOI] [PubMed] [Google Scholar]
- 80.Hirano H., Matsuura Y. Sensing actin dynamics: Structural basis for G-actin-sensitive nuclear import of MAL. Biochem. Biophys. Res. Commun. 2011;414:373–378. doi: 10.1016/j.bbrc.2011.09.079. [DOI] [PubMed] [Google Scholar]
- 81.Pawlowski R., Rajakyla E.K., Vartiainen M.K., Treisman R. An actin-regulated importin alpha/beta-dependent extended bipartite NLS directs nuclear import of MRTF-A. EMBO J. 2010;29:3448–3458. doi: 10.1038/emboj.2010.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nakamura S., Hayashi K., Iwasaki K., Fujioka T., Egusa H., Yatani H., Sobue K. Nuclear import mechanism for myocardin family members and their correlation with vascular smooth muscle cell phenotype. J. Biol. Chem. 2010;285:37314–37323. doi: 10.1074/jbc.M110.180786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hayashi K., Morita T. Differences in the nuclear export mechanism between myocardin and myocardin-related transcription factor A. J. Biol. Chem. 2013;288:5743–5755. doi: 10.1074/jbc.M112.408120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Posern G., Sotiropoulos A., Treisman R. Mutant actins demonstrate a role for unpolymerized actin in control of transcription by serum response factor. Mol. Biol. Cell. 2002;13:4167–4178. doi: 10.1091/mbc.02-05-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zaromytidou A.I., Miralles F., Treisman R. MAL and ternary complex factor use different mechanisms to contact a common surface on the serum response factor DNA-binding domain. Mol. Cell. Biol. 2006;26:4134–4148. doi: 10.1128/MCB.01902-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aravind L., Koonin E.V. SAP—A putative DNA-binding motif involved in chromosomal organization. Trends Biochem. Sci. 2000;25:112–114. doi: 10.1016/S0968-0004(99)01537-6. [DOI] [PubMed] [Google Scholar]
- 87.Kipp M., Gohring F., Ostendorp T., van Drunen C.M., van Driel R., Przybylski M., Fackelmayer F.O. SAF-Box, a conserved protein domain that specifically recognizes scaffold attachment region DNA. Mol. Cell. Biol. 2000;20:7480–7489. doi: 10.1128/MCB.20.20.7480-7489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fang F., Yang Y., Yuan Z., Gao Y., Zhou J., Chen Q., Xu Y. Myocardin-related transcription factor A mediates OxLDL-induced endothelial injury. Circ. Res. 2011;108:797–807. doi: 10.1161/CIRCRESAHA.111.240655. [DOI] [PubMed] [Google Scholar]
- 89.Yang Y., Cheng X., Tian W., Zhou B., Wu X., Xu H., Fang F., Fang M., Xu Y. MRTF-A steers an epigenetic complex to activate endothelin-induced pro-inflammatory transcription in vascular smooth muscle cells. Nucl. Acids Res. 2014;42:10460–10472. doi: 10.1093/nar/gku776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shao J., Xu H., Wu X., Xu Y. Epigenetic activation of CTGF transcription by high glucose in renal tubular epithelial cells is mediated by myocardin-related transcription factor A. Cell Tissue Res. 2020;379:549–559. doi: 10.1007/s00441-019-03124-5. [DOI] [PubMed] [Google Scholar]
- 91.Yang Y., Yang G., Yu L., Lin L., Liu L., Fang M., Xu Y. An Interplay Between MRTF-A and the Histone Acetyltransferase TIP60 Mediates Hypoxia-Reoxygenation Induced iNOS Transcription in Macrophages. Front. Cell Dev. Biol. 2020;8:484. doi: 10.3389/fcell.2020.00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hayashi K., Morita T. Importance of dimer formation of myocardin family members in the regulation of their nuclear export. Cell Struct. Funct. 2013;38:123–134. doi: 10.1247/csf.13001. [DOI] [PubMed] [Google Scholar]
- 93.Weissbach J., Schikora F., Weber A., Kessels M., Posern G. Myocardin-Related Transcription Factor A Activation by Competition with WH2 Domain Proteins for Actin Binding. Mol. Cell. Biol. 2016;36:1526–1539. doi: 10.1128/MCB.01097-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kluge F., Weissbach J., Weber A., Stradal T., Posern G. Regulation of MRTF-A by JMY via a nucleation-independent mechanism. Cell Commun. Signal. 2018;16:86. doi: 10.1186/s12964-018-0299-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Miranda M.Z., Bialik J.F., Speight P., Dan Q., Yeung T., Szaszi K., Pedersen S.F., Kapus A. TGF-beta1 regulates the expression and transcriptional activity of TAZ protein via a Smad3-independent, myocardin-related transcription factor-mediated mechanism. J. Biol. Chem. 2017;292:14902–14920. doi: 10.1074/jbc.M117.780502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lockman K., Hinson J.S., Medlin M.D., Morris D., Taylor J.M., Mack C.P. Sphingosine 1-phosphate stimulates smooth muscle cell differentiation and proliferation by activating separate serum response factor co-factors. J. Biol. Chem. 2004;279:42422–42430. doi: 10.1074/jbc.M405432200. [DOI] [PubMed] [Google Scholar]
- 97.Sebe A., Masszi A., Zulys M., Yeung T., Speight P., Rotstein O.D., Nakano H., Mucsi I., Szaszi K., Kapus A. Rac, PAK and p38 regulate cell contact-dependent nuclear translocation of myocardin-related transcription factor. FEBS Lett. 2008;582:291–298. doi: 10.1016/j.febslet.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kojonazarov B., Novoyatleva T., Boehm M., Happe C., Sibinska Z., Tian X., Sajjad A., Luitel H., Kriechling P., Posern G., et al. p38 MAPK Inhibition Improves Heart Function in Pressure-Loaded Right Ventricular Hypertrophy. Am. J. Respir. Cell. Mol. Biol. 2017;57:603–614. doi: 10.1165/rcmb.2016-0374OC. [DOI] [PubMed] [Google Scholar]
- 99.Ma F.Y., Sachchithananthan M., Flanc R.S., Nikolic-Paterson D.J. Mitogen activated protein kinases in renal fibrosis. Front. Biosci. 2009;1:171–187. doi: 10.2741/s17. [DOI] [PubMed] [Google Scholar]
- 100.Ruwanpura S.M., Thomas B.J., Bardin P.G. Pirfenidone: Molecular Mechanisms and Potential Clinical Applications in Lung Disease. Am. J. Respir. Cell. Mol. Biol. 2020;62:413–422. doi: 10.1165/rcmb.2019-0328TR. [DOI] [PubMed] [Google Scholar]
- 101.Haller V., Nahidino P., Forster M., Laufer S.A. An updated patent review of p38 MAP kinase inhibitors (2014–2019) Exp. Opin. Ther. Pat. 2020;30:453–466. doi: 10.1080/13543776.2020.1749263. [DOI] [PubMed] [Google Scholar]
- 102.Martin-Garrido A., Brown D.I., Lyle A.N., Dikalova A., Seidel-Rogol B., Lassegue B., San Martin A., Griendling K.K. NADPH oxidase 4 mediates TGF-beta-induced smooth muscle alpha-actin via p38MAPK and serum response factor. Free Radic. Biol. Med. 2011;50:354–362. doi: 10.1016/j.freeradbiomed.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Badorff C., Seeger F.H., Zeiher A.M., Dimmeler S. Glycogen synthase kinase 3beta inhibits myocardin-dependent transcription and hypertrophy induction through site-specific phosphorylation. Circ. Res. 2005;97:645–654. doi: 10.1161/01.RES.0000184684.88750.FE. [DOI] [PubMed] [Google Scholar]
- 104.Elberg G., Chen L., Elberg D., Chan M.D., Logan C.J., Turman M.A. MKL1 mediates TGF-beta1-induced alpha-smooth muscle actin expression in human renal epithelial cells. Am. J. Physiol. Renal Physiol. 2008;294:F1116–F1128. doi: 10.1152/ajprenal.00142.2007. [DOI] [PubMed] [Google Scholar]
- 105.Jeon E.S., Park W.S., Lee M.J., Kim Y.M., Han J., Kim J.H. A Rho kinase/myocardin-related transcription factor-A-dependent mechanism underlies the sphingosylphosphorylcholine-induced differentiation of mesenchymal stem cells into contractile smooth muscle cells. Circ. Res. 2008;103:635–642. doi: 10.1161/CIRCRESAHA.108.180885. [DOI] [PubMed] [Google Scholar]
- 106.Lee M., San Martin A., Valdivia A., Martin-Garrido A., Griendling K.K. Redox-Sensitive Regulation of Myocardin-Related Transcription Factor (MRTF-A) Phosphorylation via Palladin in Vascular Smooth Muscle Cell Differentiation Marker Gene Expression. PLoS ONE. 2016;11:e0153199. doi: 10.1371/journal.pone.0153199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Scott R.W., Olson M.F. LIM kinases: Function, regulation and association with human disease. J. Mol. Med. 2007;85:555–568. doi: 10.1007/s00109-007-0165-6. [DOI] [PubMed] [Google Scholar]
- 108.Geneste O., Copeland J.W., Treisman R. LIM kinase and Diaphanous cooperate to regulate serum response factor and actin dynamics. J. Cell. Biol. 2002;157:831–838. doi: 10.1083/jcb.200203126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Julian L., Olson M.F. Rho-associated coiled-coil containing kinases (ROCK): Structure, regulation, and functions. Small GTPases. 2014;5:e29846. doi: 10.4161/sgtp.29846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Janota C.S., Calero-Cuenca F.J., Gomes E.R. The role of the cell nucleus in mechanotransduction. Curr. Opin. Cell. Biol. 2020;63:204–211. doi: 10.1016/j.ceb.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 111.Ho C.Y., Jaalouk D.E., Vartiainen M.K., Lammerding J. Lamin A/C and emerin regulate MKL1-SRF activity by modulating actin dynamics. Nature. 2013;497:507–511. doi: 10.1038/nature12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Plessner M., Melak M., Chinchilla P., Baarlink C., Grosse R. Nuclear F-actin formation and reorganization upon cell spreading. J. Biol. Chem. 2015;290:11209–11216. doi: 10.1074/jbc.M114.627166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kassianidou E., Kalita J., Lim R.Y.H. The role of nucleocytoplasmic transport in mechanotransduction. Exp. Cell Res. 2019;377:86–93. doi: 10.1016/j.yexcr.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 114.Elosegui-Artola A., Andreu I., Beedle A.E.M., Lezamiz A., Uroz M., Kosmalska A.J., Oria R., Kechagia J.Z., Rico-Lastres P., Le Roux A.L., et al. Force Triggers YAP Nuclear Entry by Regulating Transport across Nuclear Pores. Cell. 2017;171:1397–1410 e14. doi: 10.1016/j.cell.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 115.Hoffman L.M., Smith M.A., Jensen C.C., Yoshigi M., Blankman E., Ullman K.S., Beckerle M.C. Mechanical stress triggers nuclear remodeling and the formation of transmembrane actin nuclear lines with associated nuclear pore complexes. Mol. Biol. Cell. 2020;31:1774–1787. doi: 10.1091/mbc.E19-01-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Alam S.G., Zhang Q., Prasad N., Li Y., Chamala S., Kuchibhotla R., Kc B., Aggarwal V., Shrestha S., Jones A.L., et al. The mammalian LINC complex regulates genome transcriptional responses to substrate rigidity. Sci. Rep. 2016;6:38063. doi: 10.1038/srep38063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pecci A., Ma X., Savoia A., Adelstein R.S. MYH9: Structure, functions and role of non-muscle myosin IIA in human disease. Gene. 2018;664:152–167. doi: 10.1016/j.gene.2018.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wei Y., Renard C.A., Labalette C., Wu Y., Levy L., Neuveut C., Prieur X., Flajolet M., Prigent S., Buendia M.A. Identification of the LIM protein FHL2 as a coactivator of beta-catenin. J. Biol. Chem. 2003;278:5188–5194. doi: 10.1074/jbc.M207216200. [DOI] [PubMed] [Google Scholar]
- 119.Li S.Y., Huang P.H., Tarng D.C., Lin T.P., Yang W.C., Chang Y.H., Yang A.H., Lin C.C., Yang M.H., Chen J.W., et al. Four-and-a-Half LIM Domains Protein 2 Is a Coactivator of Wnt Signaling in Diabetic Kidney Disease. J. Am. Soc. Nephrol. 2015;26:3072–3084. doi: 10.1681/ASN.2014100989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhou S.G., Ma H.J., Guo Z.Y., Zhang W., Yang X. FHL2 participates in renal interstitial fibrosis by altering the phenotype of renal tubular epithelial cells via regulating the beta-catenin pathway. Eur. Rev. Med. Pharmacol. Sci. 2018;22:2734–2741. doi: 10.26355/eurrev_201805_14970. [DOI] [PubMed] [Google Scholar]
- 121.Li S.Y., Chu P.H., Huang P.H., Hsieh T.H., Susztak K., Tarng D.C. FHL2 mediates podocyte Rac1 activation and foot process effacement in hypertensive nephropathy. Sci. Rep. 2019;9:6693. doi: 10.1038/s41598-019-42328-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Duan Y., Qiu Y., Huang X., Dai C., Yang J., He W. Deletion of FHL2 in fibroblasts attenuates fibroblasts activation and kidney fibrosis via restraining TGF-beta1-induced Wnt/beta-catenin signaling. J. Mol. Med. 2020;98:291–307. doi: 10.1007/s00109-019-01870-1. [DOI] [PubMed] [Google Scholar]
- 123.Xu H., Wu X., Qin H., Tian W., Chen J., Sun L., Fang M., Xu Y. Myocardin-Related Transcription Factor A Epigenetically Regulates Renal Fibrosis in Diabetic Nephropathy. J. Am. Soc. Nephrol. 2015;26:1648–1660. doi: 10.1681/ASN.2014070678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bialik J.F., Ding M., Speight P., Dan Q., Miranda M.Z., Di Ciano-Oliveira C., Kofler M.M., Rotstein O.D., Pedersen S.F., Szaszi K., et al. Profibrotic epithelial phenotype: A central role for MRTF and TAZ. Sci. Rep. 2019;9:4323. doi: 10.1038/s41598-019-40764-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Brandt D.T., Xu J., Steinbeisser H., Grosse R. Regulation of myocardin-related transcriptional coactivators through cofactor interactions in differentiation and cancer. Cell Cycle. 2009;8:2523–2527. doi: 10.4161/cc.8.16.9398. [DOI] [PubMed] [Google Scholar]
- 126.Fintha A., Gasparics A., Fang L., Erdei Z., Hamar P., Mozes M.M., Kokeny G., Rosivall L., Sebe A. Characterization and role of SCAI during renal fibrosis and epithelial-to-mesenchymal transition. Am. J. Pathol. 2013;182:388–400. doi: 10.1016/j.ajpath.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 127.He H., Du F., He Y., Wei Z., Meng C., Xu Y., Zhou H., Wang N., Luo X.G., Ma W., et al. The Wnt-beta-catenin signaling regulated MRTF-A transcription to activate migration-related genes in human breast cancer cells. Oncotarget. 2018;9:15239–15251. doi: 10.18632/oncotarget.23961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Francisco J., Zhang Y., Jeong J.I., Mizushima W., Ikeda S., Ivessa A., Oka S., Zhai P., Tallquist M.D., Del Re D.P. Blockade of Fibroblast YAP Attenuates Cardiac Fibrosis and Dysfunction Through MRTF-A Inhibition. JACC Basic Transl. Sci. 2020;5:931–945. doi: 10.1016/j.jacbts.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gao X., Xu D., Li S., Wei Z., Li S., Cai W., Mao N., Jin F., Li Y., Yi X., et al. Pulmonary Silicosis Alters MicroRNA Expression in Rat Lung and miR-411-3p Exerts Anti-fibrotic Effects by Inhibiting MRTF-A/SRF Signaling. Mol. Ther. Nucl. Acids. 2020;20:851–865. doi: 10.1016/j.omtn.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Holstein I., Singh A.K., Pohl F., Misiak D., Braun J., Leitner L., Huttelmaier S., Posern G. Post-transcriptional regulation of MRTF-A by miRNAs during myogenic differentiation of myoblasts. Nucleic Acids Res. 2020;48:8927–8942. doi: 10.1093/nar/gkaa596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cen B., Selvaraj A., Prywes R. Myocardin/MKL family of SRF coactivators: Key regulators of immediate early and muscle specific gene expression. J. Cell Biochem. 2004;93:74–82. doi: 10.1002/jcb.20199. [DOI] [PubMed] [Google Scholar]
- 132.Posern G., Treisman R. Actin’ together: Serum response factor, its cofactors and the link to signal transduction. Trends Cell. Biol. 2006;16:588–596. doi: 10.1016/j.tcb.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 133.Parmacek M.S. Myocardin-related transcription factors: Critical coactivators regulating cardiovascular development and adaptation. Circ. Res. 2007;100:633–644. doi: 10.1161/01.RES.0000259563.61091.e8. [DOI] [PubMed] [Google Scholar]
- 134.Scharenberg M.A., Chiquet-Ehrismann R., Asparuhova M.B. Megakaryoblastic leukemia protein-1 (MKL1): Increasing evidence for an involvement in cancer progression and metastasis. Int, J. Biochem. Cell Biol. 2010;42:1911–1914. doi: 10.1016/j.biocel.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 135.Quaggin S.E., Kapus A. Scar wars: Mapping the fate of epithelial-mesenchymal-myofibroblast transition. Kidney Int. 2011;80:41–50. doi: 10.1038/ki.2011.77. [DOI] [PubMed] [Google Scholar]
- 136.Kuwahara K., Nakao K. New molecular mechanisms for cardiovascular disease:transcriptional pathways and novel therapeutic targets in heart failure. J. Pharmacol. Sci. 2011;116:337–342. doi: 10.1254/jphs.10R28FM. [DOI] [PubMed] [Google Scholar]
- 137.Janmey P.A., Wells R.G., Assoian R.K., McCulloch C.A. From tissue mechanics to transcription factors. Differentiation. 2013;86:112–120. doi: 10.1016/j.diff.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sward K., Stenkula K.G., Rippe C., Alajbegovic A., Gomez M.F., Albinsson S. Emerging roles of the myocardin family of proteins in lipid and glucose metabolism. J. Physiol. 2016;594:4741–4752. doi: 10.1113/JP271913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gau D., Roy P. SRF’ing and SAP’ing—The role of MRTF proteins in cell migration. J. Cell Sci. 2018;131 doi: 10.1242/jcs.218222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Esnault C., Stewart A., Gualdrini F., East P., Horswell S., Matthews N., Treisman R. Rho-actin signaling to the MRTF coactivators dominates the immediate transcriptional response to serum in fibroblasts. Genes Dev. 2014;28:943–958. doi: 10.1101/gad.239327.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rozycki M., Lodyga M., Lam J., Miranda M.Z., Fatyol K., Speight P., Kapus A. The fate of the primary cilium during myofibroblast transition. Mol. Biol. Cell. 2014;25:643–657. doi: 10.1091/mbc.e13-07-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Masszi A., Di Ciano C., Sirokmany G., Arthur W.T., Rotstein O.D., Wang J., McCulloch C.A., Rosivall L., Mucsi I., Kapus A. Central role for Rho in TGF-beta1-induced alpha-smooth muscle actin expression during epithelial-mesenchymal transition. Am. J. Physiol. Renal Physiol. 2003;284:F911–F924. doi: 10.1152/ajprenal.00183.2002. [DOI] [PubMed] [Google Scholar]
- 143.Muehlich S., Rehm M., Ebenau A., Goppelt-Struebe M. Synergistic induction of CTGF by cytochalasin D and TGFbeta-1 in primary human renal epithelial cells: Role of transcriptional regulators MKL1, YAP/TAZ and Smad2/3. Cell Signal. 2017;29:31–40. doi: 10.1016/j.cellsig.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 144.Krawczyk K.M., Hansson J., Nilsson H., Krawczyk K.K., Sward K., Johansson M.E. Injury induced expression of caveolar proteins in human kidney tubules—Role of megakaryoblastic leukemia 1. BMC Nephrol. 2017;18:320. doi: 10.1186/s12882-017-0738-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pozzi A., Zent R. Integrins in kidney disease. J. Am. Soc. Nephrol. 2013;24:1034–1039. doi: 10.1681/ASN.2013010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Asparuhova M.B., Ferralli J., Chiquet M., Chiquet-Ehrismann R. The transcriptional regulator megakaryoblastic leukemia-1 mediates serum response factor-independent activation of tenascin-C transcription by mechanical stress. FASEB J. 2011;25:3477–3488. doi: 10.1096/fj.11-187310. [DOI] [PubMed] [Google Scholar]
- 147.Parreno J., Raju S., Niaki M.N., Andrejevic K., Jiang A., Delve E., Kandel R. Expression of type I collagen and tenascin C is regulated by actin polymerization through MRTF in dedifferentiated chondrocytes. FEBS Lett. 2014;588:3677–3684. doi: 10.1016/j.febslet.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 148.Iwanciw D., Rehm M., Porst M., Goppelt-Struebe M. Induction of connective tissue growth factor by angiotensin II: Integration of signaling pathways. Arterioscler. Thromb. Vasc. Biol. 2003;23:1782–1787. doi: 10.1161/01.ATV.0000092913.60428.E6. [DOI] [PubMed] [Google Scholar]
- 149.Chaqour B., Goppelt-Struebe M. Mechanical regulation of the Cyr61/CCN1 and CTGF/CCN2 proteins. FEBS J. 2006;273:3639–3649. doi: 10.1111/j.1742-4658.2006.05360.x. [DOI] [PubMed] [Google Scholar]
- 150.Mao L., Liu L., Zhang T., Wu X., Zhang T., Xu Y. MKL1 mediates TGF-beta-induced CTGF transcription to promote renal fibrosis. J. Cell. Physiol. 2020;235:4790–4803. doi: 10.1002/jcp.29356. [DOI] [PubMed] [Google Scholar]
- 151.Guan T.H., Chen G., Gao B., Janssen M.R., Uttarwar L., Ingram A.J., Krepinsky J.C. Caveolin-1 deficiency protects against mesangial matrix expansion in a mouse model of type 1 diabetic nephropathy. Diabetologia. 2013;56:2068–2077. doi: 10.1007/s00125-013-2968-z. [DOI] [PubMed] [Google Scholar]
- 152.Yang S., Liu L., Xu P., Yang Z. MKL1 inhibits cell cycle progression through p21 in podocytes. BMC Mol. Biol. 2015;16:1. doi: 10.1186/s12867-015-0029-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rozycki M., Bialik J.F., Speight P., Dan Q., Knudsen T.E., Szeto S.G., Yuen D.A., Szaszi K., Pedersen S.F., Kapus A. Myocardin-related Transcription Factor Regulates Nox4 Protein Expression: Linking cytoskeletal organization to redox state. J. Biol. Chem. 2016;291:227–243. doi: 10.1074/jbc.M115.674606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liu L., Wu X., Xu H., Yu L., Zhang X., Li L., Jin J., Zhang T., Xu Y. Myocardin-related transcription factor A (MRTF-A) contributes to acute kidney injury by regulating macrophage ROS production. Biochim. Biophys. Acta Mol. Basis Dis. 2018;1864:3109–3121. doi: 10.1016/j.bbadis.2018.05.026. [DOI] [PubMed] [Google Scholar]
- 155.Yoshino J., Monkawa T., Tsuji M., Inukai M., Itoh H., Hayashi M. Snail1 is involved in the renal epithelial-mesenchymal transition. Biochem. Biophys. Res. Commun. 2007;362:63–68. doi: 10.1016/j.bbrc.2007.07.146. [DOI] [PubMed] [Google Scholar]
- 156.Grande M.T., Sanchez-Laorden B., Lopez-Blau C., De Frutos C.A., Boutet A., Arevalo M., Rowe R.G., Weiss S.J., Lopez-Novoa J.M., Nieto M.A. Snail1-induced partial epithelial-to-mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease. Nat. Med. 2015;21:989–997. doi: 10.1038/nm.3901. [DOI] [PubMed] [Google Scholar]
- 157.Tang W.B., Zheng L., Yan R., Yang J., Ning J., Peng L., Zhou Q., Chen L. miR302a-3p May Modulate Renal Epithelial-Mesenchymal Transition in Diabetic Kidney Disease by Targeting ZEB1. Nephron. 2018;138:231–242. doi: 10.1159/000481465. [DOI] [PubMed] [Google Scholar]
- 158.Davis-Dusenbery B.N., Chan M.C., Reno K.E., Weisman A.S., Layne M.D., Lagna G., Hata A. down-regulation of Kruppel-like factor-4 (KLF4) by microRNA-143/145 is critical for modulation of vascular smooth muscle cell phenotype by transforming growth factor-beta and bone morphogenetic protein 4. J. Biol. Chem. 2011;286:28097–28110. doi: 10.1074/jbc.M111.236950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Li C.X., Talele N.P., Boo S., Koehler A., Knee-Walden E., Balestrini J.L., Speight P., Kapus A., Hinz B. MicroRNA-21 preserves the fibrotic mechanical memory of mesenchymal stem cells. Nat. Mater. 2017;16:379–389. doi: 10.1038/nmat4780. [DOI] [PubMed] [Google Scholar]
- 160.Hayashi K., Murai T., Oikawa H., Masuda T., Kimura K., Muehlich S., Prywes R., Morita T. A novel inhibitory mechanism of MRTF-A/B on the ICAM-1 gene expression in vascular endothelial cells. Sci. Rep. 2015;5:10627. doi: 10.1038/srep10627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yu L., Fang F., Dai X., Xu H., Qi X., Fang M., Xu Y. MKL1 defines the H3K4Me3 landscape for NF-kappaB dependent inflammatory response. Sci. Rep. 2017;7:191. doi: 10.1038/s41598-017-00301-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Weng X., Yu L., Liang P., Li L., Dai X., Zhou B., Wu X., Xu H., Fang M., Chen Q., et al. A crosstalk between chromatin remodeling and histone H3K4 methyltransferase complexes in endothelial cells regulates angiotensin II-induced cardiac hypertrophy. J. Mol. Cell Cardiol. 2015;82:48–58. doi: 10.1016/j.yjmcc.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 163.Lockman K., Taylor J.M., Mack C.P. The histone demethylase, Jmjd1a, interacts with the myocardin factors to regulate SMC differentiation marker gene expression. Circ. Res. 2007;101:e115–e123. doi: 10.1161/CIRCRESAHA.107.164178. [DOI] [PubMed] [Google Scholar]
- 164.Yu L., Yang G., Zhang X., Wang P., Weng X., Yang Y., Li Z., Fang M., Xu Y., Sun A., et al. Megakaryocytic Leukemia 1 Bridges Epigenetic Activation of NADPH Oxidase in Macrophages to Cardiac Ischemia-Reperfusion Injury. Circulation. 2018;138:2820–2836. doi: 10.1161/CIRCULATIONAHA.118.035377. [DOI] [PubMed] [Google Scholar]
- 165.Menendez M.T., Ong E.C., Shepherd B.T., Muthukumar V., Silasi-Mansat R., Lupu F., Griffin C.T. BRG1 (Brahma-Related Gene 1) Promotes Endothelial Mrtf Transcription to Establish Embryonic Capillary Integrity. Arterioscler. Thromb. Vasc. Biol. 2017;37:1674–1682. doi: 10.1161/ATVBAHA.117.309785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Malicki J.J., Johnson C.A. The Cilium: Cellular Antenna and Central Processing Unit. Trends Cell. Biol. 2017;27:126–140. doi: 10.1016/j.tcb.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wang L., Dynlacht B.D. The regulation of cilium assembly and disassembly in development and disease. Development. 2018;145 doi: 10.1242/dev.151407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Praetorius H.A., Spring K.R. The renal cell primary cilium functions as a flow sensor. Curr. Opin. Nephrol. Hypertens. 2003;12:517–520. doi: 10.1097/00041552-200309000-00006. [DOI] [PubMed] [Google Scholar]
- 169.Praetorius H.A. The primary cilium as sensor of fluid flow: New building blocks to the model. A review in the theme: Cell signaling: Proteins, pathways and mechanisms. Am. J. Physiol. Cell. Physiol. 2015;308:C198–C208. doi: 10.1152/ajpcell.00336.2014. [DOI] [PubMed] [Google Scholar]
- 170.Ke Y.N., Yang W.X. Primary cilium: An elaborate structure that blocks cell division? Gene. 2014;547:175–185. doi: 10.1016/j.gene.2014.06.050. [DOI] [PubMed] [Google Scholar]
- 171.Oh E.C., Vasanth S., Katsanis N. Metabolic regulation and energy homeostasis through the primary Cilium. Cell Metab. 2015;21:21–31. doi: 10.1016/j.cmet.2014.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wheway G., Nazlamova L., Hancock J.T. Signaling through the Primary Cilium. Front. Cell Dev. Biol. 2018;6:8. doi: 10.3389/fcell.2018.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Braun D.A., Hildebrandt F. Ciliopathies. Cold Spring Harb. Perspect. Biol. 2017;9:a028191. doi: 10.1101/cshperspect.a028191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Menezes L.F., Germino G.G. The pathobiology of polycystic kidney disease from a metabolic viewpoint. Nat. Rev. Nephrol. 2019;15:735–749. doi: 10.1038/s41581-019-0183-y. [DOI] [PubMed] [Google Scholar]
- 175.Douguet D., Patel A., Honore E. Structure and function of polycystins: Insights into polycystic kidney disease. Nat. Rev. Nephrol. 2019;15:412–422. doi: 10.1038/s41581-019-0143-6. [DOI] [PubMed] [Google Scholar]
- 176.McConnachie D.J., Stow J.L., Mallett A.J. Ciliopathies and the Kidney: A Review. Am. J. Kidney Dis. 2021;77:410–419. doi: 10.1053/j.ajkd.2020.08.012. [DOI] [PubMed] [Google Scholar]
- 177.Kobayashi T., Dynlacht B.D. Regulating the transition from centriole to basal body. J. Cell. Biol. 2011;193:435–444. doi: 10.1083/jcb.201101005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Vertii A., Hung H.F., Hehnly H., Doxsey S. Human basal body basics. Cilia. 2016;5:13. doi: 10.1186/s13630-016-0030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sanchez I., Dynlacht B.D. Cilium assembly and disassembly. Nat. Cell Biol. 2016;18:711–717. doi: 10.1038/ncb3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Breslow D.K., Holland A.J. Mechanism and Regulation of Centriole and Cilium Biogenesis. Annu. Rev. Biochem. 2019;88:691–724. doi: 10.1146/annurev-biochem-013118-111153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Li S., Chang S., Qi X., Richardson J.A., Olson E.N. Requirement of a myocardin-related transcription factor for development of mammary myoepithelial cells. Mol. Cell. Biol. 2006;26:5797–5808. doi: 10.1128/MCB.00211-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sun Y., Boyd K., Xu W., Ma J., Jackson C.W., Fu A., Shillingford J.M., Robinson G.W., Hennighausen L., Hitzler J.K., et al. Acute myeloid leukemia-associated Mkl1 (Mrtf-a) is a key regulator of mammary gland function. Mol. Cell. Biol. 2006;26:5809–5826. doi: 10.1128/MCB.00024-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Oh J., Richardson J.A., Olson E.N. Requirement of myocardin-related transcription factor-B for remodeling of branchial arch arteries and smooth muscle differentiation. Proc. Natl. Acad. Sci. USA. 2005;102:15122–15127. doi: 10.1073/pnas.0507346102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Guo B., Lyu Q., Slivano O.J., Dirkx R., Christie C.K., Czyzyk J., Hezel A.F., Gharavi A.G., Small E.M., Miano J.M. Serum Response Factor Is Essential for Maintenance of Podocyte Structure and Function. J. Am. Soc. Nephrol. 2018;29:416–422. doi: 10.1681/ASN.2017050473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Pakshir P., Hinz B. The big five in fibrosis: Macrophages, myofibroblasts, matrix, mechanics, and miscommunication. Matrix Biol. 2018;68–69:81–93. doi: 10.1016/j.matbio.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 186.Henderson N.C., Rieder F., Wynn T.A. Fibrosis: From mechanisms to medicines. Nature. 2020;587:555–566. doi: 10.1038/s41586-020-2938-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Humphreys B.D. Mechanisms of Renal Fibrosis. Annu. Rev. Physiol. 2018;80:309–326. doi: 10.1146/annurev-physiol-022516-034227. [DOI] [PubMed] [Google Scholar]
- 188.Gewin L.S. Renal fibrosis: Primacy of the proximal tubule. Matrix Biol. 2018;68–69:248–262. doi: 10.1016/j.matbio.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Grgic I., Duffield J.S., Humphreys B.D. The origin of interstitial myofibroblasts in chronic kidney disease. Pediatr. Nephrol. 2012;27:183–193. doi: 10.1007/s00467-011-1772-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Nakagawa N., Duffield J.S. Myofibroblasts in Fibrotic Kidneys. Curr. Pathobiol. Rep. 2013;1 doi: 10.1007/s40139-013-0025-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lovisa S., Zeisberg M., Kalluri R. Partial Epithelial-to-Mesenchymal Transition and Other New Mechanisms of Kidney Fibrosis. Trends Endocrinol. Metab. 2016;27:681–695. doi: 10.1016/j.tem.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 192.Pakshir P., Noskovicova N., Lodyga M., Son D.O., Schuster R., Goodwin A., Karvonen H., Hinz B. The myofibroblast at a glance. J. Cell Sci. 2020;133 doi: 10.1242/jcs.227900. [DOI] [PubMed] [Google Scholar]
- 193.Grgic I., Campanholle G., Bijol V., Wang C., Sabbisetti V.S., Ichimura T., Humphreys B.D., Bonventre J.V. Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int. 2012;82:172–183. doi: 10.1038/ki.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Pattaro C., Kottgen A., Teumer A., Garnaas M., Boger C.A., Fuchsberger C., Olden M., Chen M.H., Tin A., Taliun D., et al. Genome-wide association and functional follow-up reveals new loci for kidney function. PLoS Genet. 2012;8:e1002584. doi: 10.1371/journal.pgen.1002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Okada Y., Sim X., Go M.J., Wu J.Y., Gu D., Takeuchi F., Takahashi A., Maeda S., Tsunoda T., Chen P., et al. Meta-analysis identifies multiple loci associated with kidney function-related traits in east Asian populations. Nat. Genet. 2012;44:904–909. doi: 10.1038/ng.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Pattaro C., Teumer A., Gorski M., Chu A.Y., Li M., Mijatovic V., Garnaas M., Tin A., Sorice R., Li Y., et al. Genetic associations at 53 loci highlight cell types and biological pathways relevant for kidney function. Nat. Commun. 2016;7:10023. doi: 10.1038/ncomms10023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wuttke M., Kottgen A. Insights into kidney diseases from genome-wide association studies. Nat. Rev. Nephrol. 2016;12:549–562. doi: 10.1038/nrneph.2016.107. [DOI] [PubMed] [Google Scholar]
- 198.Hishida A., Nakatochi M., Akiyama M., Kamatani Y., Nishiyama T., Ito H., Oze I., Nishida Y., Hara M., Takashima N., et al. Japan Multi-Institutional Collaborative Cohort Study, G. Genome-Wide Association Study of Renal Function Traits: Results from the Japan Multi-Institutional Collaborative Cohort Study. Am. J. Nephrol. 2018;47:304–316. doi: 10.1159/000488946. [DOI] [PubMed] [Google Scholar]
- 199.Qiu C., Huang S., Park J., Park Y., Ko Y.A., Seasock M.J., Bryer J.S., Xu X.X., Song W.C., Palmer M., et al. Renal compartment-specific genetic variation analyses identify new pathways in chronic kidney disease. Nat. Med. 2018;24:1721–1731. doi: 10.1038/s41591-018-0194-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Fan J.M., Ng Y.Y., Hill P.A., Nikolic-Paterson D.J., Mu W., Atkins R.C., Lan H.Y. Transforming growth factor-beta regulates tubular epithelial-myofibroblast transdifferentiation in vitro. Kidney Int. 1999;56:1455–1467. doi: 10.1046/j.1523-1755.1999.00656.x. [DOI] [PubMed] [Google Scholar]
- 201.Yang J., Liu Y. Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. Am. J. Pathol. 2001;159:1465–1475. doi: 10.1016/S0002-9440(10)62533-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Masszi A., Fan L., Rosivall L., McCulloch C.A., Rotstein O.D., Mucsi I., Kapus A. Integrity of cell-cell contacts is a critical regulator of TGF-beta 1-induced epithelial-to-myofibroblast transition: Role for beta-catenin. Am. J. Pathol. 2004;165:1955–1967. doi: 10.1016/S0002-9440(10)63247-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Speight P., Nakano H., Kelley T.J., Hinz B., Kapus A. Differential topical susceptibility to TGFbeta in intact and injured regions of the epithelium: Key role in myofibroblast transition. Mol. Biol. Cell. 2013;24:3326–3336. doi: 10.1091/mbc.e13-04-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Dan Q., Shi Y., Rabani R., Venugopal S., Xiao J., Anwer S., Ding M., Speight P., Pan W., Alexander R.T., et al. Claudin-2 suppresses GEF-H1, RHOA, and MRTF, thereby impacting proliferation and profibrotic phenotype of tubular cells. J. Biol. Chem. 2019;294:15446–15465. doi: 10.1074/jbc.RA118.006484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Gomez E.W., Chen Q.K., Gjorevski N., Nelson C.M. Tissue geometry patterns epithelial-mesenchymal transition via intercellular mechanotransduction. J. Cell Biochem. 2010;110:44–51. doi: 10.1002/jcb.22545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.O’Connor J.W., Gomez E.W. Cell adhesion and shape regulate TGF-beta1-induced epithelial-myofibroblast transition via MRTF-A signaling. PLoS ONE. 2013;8:e83188. doi: 10.1371/journal.pone.0083188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.O’Connor J.W., Riley P.N., Nalluri S.M., Ashar P.K., Gomez E.W. Matrix Rigidity Mediates TGFbeta1-Induced Epithelial-Myofibroblast Transition by Controlling Cytoskeletal Organization and MRTF-A Localization. J. Cell. Physiol. 2015;230:1829–1839. doi: 10.1002/jcp.24895. [DOI] [PubMed] [Google Scholar]
- 208.Hinson J.S., Medlin M.D., Lockman K., Taylor J.M., Mack C.P. Smooth muscle cell-specific transcription is regulated by nuclear localization of the myocardin-related transcription factors. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H1170–H1180. doi: 10.1152/ajpheart.00864.2006. [DOI] [PubMed] [Google Scholar]
- 209.Poncelet A.C., Schnaper H.W., Tan R., Liu Y., Runyan C.E. Cell phenotype-specific down-regulation of Smad3 involves decreased gene activation as well as protein degradation. J. Biol. Chem. 2007;282:15534–15540. doi: 10.1074/jbc.M701991200. [DOI] [PubMed] [Google Scholar]
- 210.Iwano M., Plieth D., Danoff T.M., Xue C., Okada H., Neilson E.G. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J. Clin. Investig. 2002;110:341–350. doi: 10.1172/JCI0215518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kalluri R., Neilson E.G. Epithelial-mesenchymal transition and its implications for fibrosis. J. Clin. Investig. 2003;112:1776–1784. doi: 10.1172/JCI200320530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Duffield J.S., Humphreys B.D. Origin of new cells in the adult kidney: Results from genetic labeling techniques. Kidney Int. 2011;79:494–501. doi: 10.1038/ki.2010.338. [DOI] [PubMed] [Google Scholar]
- 213.Lovisa S., LeBleu V.S., Tampe B., Sugimoto H., Vadnagara K., Carstens J.L., Wu C.C., Hagos Y., Burckhardt B.C., Pentcheva-Hoang T., et al. Epithelial-to-mesenchymal transition induces cell cycle arrest and parenchymal damage in renal fibrosis. Nat. Med. 2015;21:998–1009. doi: 10.1038/nm.3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Sheng L., Zhuang S. New Insights Into the Role and Mechanism of Partial Epithelial-Mesenchymal Transition in Kidney Fibrosis. Front. Physiol. 2020;11:569322. doi: 10.3389/fphys.2020.569322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Fukuda K., Yoshitomi K., Yanagida T., Tokumoto M., Hirakata H. Quantification of TGF-beta1 mRNA along rat nephron in obstructive nephropathy. Am. J. Physiol. Renal Physiol. 2001;281:F513–F521. doi: 10.1152/ajprenal.2001.281.3.F513. [DOI] [PubMed] [Google Scholar]
- 216.Yang L., Besschetnova T.Y., Brooks C.R., Shah J.V., Bonventre J.V. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat. Med. 2010;16:535–543. doi: 10.1038/nm.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Chen Y.T., Chang F.C., Wu C.F., Chou Y.H., Hsu H.L., Chiang W.C., Shen J., Chen Y.M., Wu K.D., Tsai T.J., et al. Platelet-derived growth factor receptor signaling activates pericyte-myofibroblast transition in obstructive and post-ischemic kidney fibrosis. Kidney Int. 2011;80:1170–1181. doi: 10.1038/ki.2011.208. [DOI] [PubMed] [Google Scholar]
- 218.Fabian S.L., Penchev R.R., St-Jacques B., Rao A.N., Sipila P., West K.A., McMahon A.P., Humphreys B.D. Hedgehog-Gli pathway activation during kidney fibrosis. Am. J. Pathol. 2012;180:1441–1453. doi: 10.1016/j.ajpath.2011.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Wu C.F., Chiang W.C., Lai C.F., Chang F.C., Chen Y.T., Chou Y.H., Wu T.H., Linn G.R., Ling H., Wu K.D., et al. Transforming growth factor beta-1 stimulates profibrotic epithelial signaling to activate pericyte-myofibroblast transition in obstructive kidney fibrosis. Am. J. Pathol. 2013;182:118–131. doi: 10.1016/j.ajpath.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Szeto S.G., Narimatsu M., Lu M., He X., Sidiqi A.M., Tolosa M.F., Chan L., De Freitas K., Bialik J.F., Majumder S., et al. YAP/TAZ Are Mechanoregulators of TGF-beta-Smad Signaling and Renal Fibrogenesis. J. Am. Soc. Nephrol. 2016;27:3117–3128. doi: 10.1681/ASN.2015050499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Patel S., Tang J., Overstreet J.M., Anorga S., Lian F., Arnouk A., Goldschmeding R., Higgins P.J., Samarakoon R. Rac-GTPase promotes fibrotic TGF-beta1 signaling and chronic kidney disease via EGFR, p53, and Hippo/YAP/TAZ pathways. FASEB J. 2019;33:9797–9810. doi: 10.1096/fj.201802489RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Sandbo N., Kregel S., Taurin S., Bhorade S., Dulin N.O. Critical role of serum response factor in pulmonary myofibroblast differentiation induced by TGF-beta. Am. J. Respir. Cell. Mol. Biol. 2009;41:332–338. doi: 10.1165/rcmb.2008-0288OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ni J., Dong Z., Han W., Kondrikov D., Su Y. The role of RhoA and cytoskeleton in myofibroblast transformation in hyperoxic lung fibrosis. Free Radic. Biol. Med. 2013;61:26–39. doi: 10.1016/j.freeradbiomed.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Small E.M., Thatcher J.E., Sutherland L.B., Kinoshita H., Gerard R.D., Richardson J.A., Dimaio J.M., Sadek H., Kuwahara K., Olson E.N. Myocardin-related transcription factor-a controls myofibroblast activation and fibrosis in response to myocardial infarction. Circ. Res. 2010;107:294–304. doi: 10.1161/CIRCRESAHA.110.223172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Sakai N., Chun J., Duffield J.S., Wada T., Luster A.D., Tager A.M. LPA1-induced cytoskeleton reorganization drives fibrosis through CTGF-dependent fibroblast proliferation. FASEB J. 2013;27:1830–1846. doi: 10.1096/fj.12-219378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Velasquez L.S., Sutherland L.B., Liu Z., Grinnell F., Kamm K.E., Schneider J.W., Olson E.N., Small E.M. Activation of MRTF-A-dependent gene expression with a small molecule promotes myofibroblast differentiation and wound healing. Proc. Natl. Acad. Sci. USA. 2013;110:16850–16855. doi: 10.1073/pnas.1316764110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Haak A.J., Tsou P.S., Amin M.A., Ruth J.H., Campbell P., Fox D.A., Khanna D., Larsen S.D., Neubig R.R. Targeting the myofibroblast genetic switch: Inhibitors of myocardin-related transcription factor/serum response factor-regulated gene transcription prevent fibrosis in a murine model of skin injury. J. Pharmacol. Exp. Ther. 2014;349:480–486. doi: 10.1124/jpet.114.213520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Yu-Wai-Man C., Treisman R., Bailly M., Khaw P.T. The role of the MRTF-A/SRF pathway in ocular fibrosis. Invest. Ophthalmol. Vis. Sci. 2014;55:4560–4567. doi: 10.1167/iovs.14-14692. [DOI] [PubMed] [Google Scholar]
- 229.Tian W., Hao C., Fan Z., Weng X., Qin H., Wu X., Fang M., Chen Q., Shen A., Xu Y. Myocardin related transcription factor A programs epigenetic activation of hepatic stellate cells. J. Hepatol. 2015;62:165–174. doi: 10.1016/j.jhep.2014.07.029. [DOI] [PubMed] [Google Scholar]
- 230.Lauriol J., Keith K., Jaffre F., Couvillon A., Saci A., Goonasekera S.A., McCarthy J.R., Kessinger C.W., Wang J., Ke Q., et al. RhoA signaling in cardiomyocytes protects against stress-induced heart failure but facilitates cardiac fibrosis. Sci. Signal. 2014;7:ra100. doi: 10.1126/scisignal.2005262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Rahaman S.O., Grove L.M., Paruchuri S., Southern B.D., Abraham S., Niese K.A., Scheraga R.G., Ghosh S., Thodeti C.K., Zhang D.X., et al. TRPV4 mediates myofibroblast differentiation and pulmonary fibrosis in mice. J. Clin. Investig. 2014;124:5225–5238. doi: 10.1172/JCI75331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Sisson T.H., Ajayi I.O., Subbotina N., Dodi A.E., Rodansky E.S., Chibucos L.N., Kim K.K., Keshamouni V.G., White E.S., Zhou Y., et al. Inhibition of myocardin-related transcription factor/serum response factor signaling decreases lung fibrosis and promotes mesenchymal cell apoptosis. Am. J. Pathol. 2015;185:969–986. doi: 10.1016/j.ajpath.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Shiwen X., Stratton R., Nikitorowicz-Buniak J., Ahmed-Abdi B., Ponticos M., Denton C., Abraham D., Takahashi A., Suki B., Layne M.D., et al. A Role of Myocardin Related Transcription Factor-A (MRTF-A) in Scleroderma Related Fibrosis. PLoS ONE. 2015;10:e0126015. doi: 10.1371/journal.pone.0126015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Tian W., Fan Z., Li J., Hao C., Li M., Xu H., Wu X., Zhou B., Zhang L., Fang M., et al. Myocardin-related transcription factor A (MRTF-A) plays an essential role in hepatic stellate cell activation by epigenetically modulating TGF-beta signaling. Int, J. Biochem. Cell Biol. 2016;71:35–43. doi: 10.1016/j.biocel.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 235.Yu-Wai-Man C., Tagalakis A.D., Manunta M.D., Hart S.L., Khaw P.T. Receptor-targeted liposome-peptide-siRNA nanoparticles represent an efficient delivery system for MRTF silencing in conjunctival fibrosis. Sci. Rep. 2016;6:21881. doi: 10.1038/srep21881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Yokota S., Chosa N., Kyakumoto S., Kimura H., Ibi M., Kamo M., Satoh K., Ishisaki A. ROCK/actin/MRTF signaling promotes the fibrogenic phenotype of fibroblast-like synoviocytes derived from the temporomandibular joint. Int. J. Mol. Med. 2017;39:799–808. doi: 10.3892/ijmm.2017.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Yu-Wai-Man C., Spencer-Dene B., Lee R.M.H., Hutchings K., Lisabeth E.M., Treisman R., Bailly M., Larsen S.D., Neubig R.R., Khaw P.T. Local delivery of novel MRTF/SRF inhibitors prevents scar tissue formation in a preclinical model of fibrosis. Sci. Rep. 2017;7:518. doi: 10.1038/s41598-017-00212-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Giehl K., Keller C., Muehlich S., Goppelt-Struebe M. Actin-mediated gene expression depends on RhoA and Rac1 signaling in proximal tubular epithelial cells. PLoS ONE. 2015;10:e0121589. doi: 10.1371/journal.pone.0121589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Wang Y., Jia L., Hu Z., Entman M.L., Mitch W.E., Wang Y. AMP-activated protein kinase/myocardin-related transcription factor-A signaling regulates fibroblast activation and renal fibrosis. Kidney Int. 2018;93:81–94. doi: 10.1016/j.kint.2017.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Liang M., Yu M., Xia R., Song K., Wang J., Luo J., Chen G., Cheng J. Yap/Taz Deletion in Gli(+) Cell-Derived Myofibroblasts Attenuates Fibrosis. J. Am. Soc. Nephrol. 2017;28:3278–3290. doi: 10.1681/ASN.2015121354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Anorga S., Overstreet J.M., Falke L.L., Tang J., Goldschmeding R.G., Higgins P.J., Samarakoon R. Deregulation of Hippo-TAZ pathway during renal injury confers a fibrotic maladaptive phenotype. FASEB J. 2018;32:2644–2657. doi: 10.1096/fj.201700722R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Song X., Di Giovanni V., He N., Wang K., Ingram A., Rosenblum N.D., Pei Y. Systems biology of autosomal dominant polycystic kidney disease (ADPKD): Computational identification of gene expression pathways and integrated regulatory networks. Hum. Mol. Genet. 2009;18:2328–2343. doi: 10.1093/hmg/ddp165. [DOI] [PubMed] [Google Scholar]
- 243.Cai J., Song X., Wang W., Watnick T., Pei Y., Qian F., Pan D. A RhoA-YAP-c-Myc signaling axis promotes the development of polycystic kidney disease. Genes Dev. 2018;32:781–793. doi: 10.1101/gad.315127.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Streets A.J., Prosseda P.P., Ong A.C. Polycystin-1 regulates ARHGAP35-dependent centrosomal RhoA activation and ROCK signaling. JCI Insight. 2020;5 doi: 10.1172/jci.insight.135385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Qian Q., Hunter L.W., Du H., Ren Q., Han Y., Sieck G.C. Pkd2+/− vascular smooth muscles develop exaggerated vasocontraction in response to phenylephrine stimulation. J. Am. Soc. Nephrol. 2007;18:485–493. doi: 10.1681/ASN.2006050501. [DOI] [PubMed] [Google Scholar]
- 246.Du H., Wang X., Wu J., Qian Q. Phenylephrine induces elevated RhoA activation and smooth muscle alpha-actin expression in Pkd2+/- vascular smooth muscle cells. Hypertens. Res. 2010;33:37–42. doi: 10.1038/hr.2009.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Chadha V., Warady B.A. Epidemiology of pediatric chronic kidney disease. Adv. Chronic Kidney Dis. 2005;12:343–352. doi: 10.1053/j.ackd.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 248.Klahr S., Morrissey J. Obstructive nephropathy and renal fibrosis. Am. J. Physiol. Renal Physiol. 2002;283:F861–F875. doi: 10.1152/ajprenal.00362.2001. [DOI] [PubMed] [Google Scholar]
- 249.Holterman C.E., Read N.C., Kennedy C.R. Nox and renal disease. Clin. Sci. 2015;128:465–481. doi: 10.1042/CS20140361. [DOI] [PubMed] [Google Scholar]
- 250.Mia S., Federico G., Feger M., Pakladok T., Meissner A., Voelkl J., Groene H.J., Alesutan I., Lang F. Impact of AMP-Activated Protein Kinase alpha1 Deficiency on Tissue Injury following Unilateral Ureteral Obstruction. PLoS ONE. 2015;10:e0135235. doi: 10.1371/journal.pone.0135235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Scholz H., Boivin F.J., Schmidt-Ott K.M., Bachmann S., Eckardt K.U., Scholl U.I., Persson P.B. Kidney physiology and susceptibility to acute kidney injury: Implications for renoprotection. Nat. Rev. Nephrol. 2021 doi: 10.1038/s41581-021-00394-7. [DOI] [PubMed] [Google Scholar]
- 252.Liangos O. Drugs and AKI. Minerva Urol. Nefrol. 2012;64:51–62. [PubMed] [Google Scholar]
- 253.Morrell E.D., Kellum J.A., Pastor-Soler N.M., Hallows K.R. Septic acute kidney injury: Molecular mechanisms and the importance of stratification and targeting therapy. Crit. Care. 2014;18:501. doi: 10.1186/s13054-014-0501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Xu Z., Tang Y., Huang Q., Fu S., Li X., Lin B., Xu A., Chen J. Systematic review and subgroup analysis of the incidence of acute kidney injury (AKI) in patients with COVID-19. BMC Nephrol. 2021;22:52. doi: 10.1186/s12882-021-02244-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Coca S.G., Singanamala S., Parikh C.R. Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int. 2012;81:442–448. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.You Y.H., Okada S., Ly S., Jandeleit-Dahm K., Barit D., Namikoshi T., Sharma K. Role of Nox2 in diabetic kidney disease. Am. J. Physiol. Renal Physiol. 2013;304:F840–F848. doi: 10.1152/ajprenal.00511.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Yu L., Weng X., Liang P., Dai X., Wu X., Xu H., Fang M., Fang F., Xu Y. MRTF-A mediates LPS-induced pro-inflammatory transcription by interacting with the COMPASS complex. J. Cell Sci. 2014;127:4645–4657. doi: 10.1242/jcs.152314. [DOI] [PubMed] [Google Scholar]
- 258.Jain N., Vogel V. Spatial confinement downsizes the inflammatory response of macrophages. Nat. Mater. 2018;17:1134–1144. doi: 10.1038/s41563-018-0190-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Chapin H.C., Caplan M.J. The cell biology of polycystic kidney disease. J. Cell. Biol. 2010;191:701–710. doi: 10.1083/jcb.201006173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Suwabe T., Shukoor S., Chamberlain A.M., Killian J.M., King B.F., Edwards M., Senum S.R., Madsen C.D., Chebib F.T., Hogan M.C., et al. Epidemiology of Autosomal Dominant Polycystic Kidney Disease in Olmsted County. Clin. J. Am. Soc. Nephrol. 2020;15:69–79. doi: 10.2215/CJN.05900519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Harris P.C., Torres V.E. Polycystic kidney disease. Annu. Rev. Med. 2009;60:321–337. doi: 10.1146/annurev.med.60.101707.125712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Malekshahabi T., Khoshdel Rad N., Serra A.L., Moghadasali R. Autosomal dominant polycystic kidney disease: Disrupted pathways and potential therapeutic interventions. J. Cell. Physiol. 2019;234:12451–12470. doi: 10.1002/jcp.28094. [DOI] [PubMed] [Google Scholar]
- 263.Song C.J., Zimmerman K.A., Henke S.J., Yoder B.K. Inflammation and Fibrosis in Polycystic Kidney Disease. Results Probl. Cell Differ. 2017;60:323–344. doi: 10.1007/978-3-319-51436-9_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Xue C., Mei C.L. Polycystic Kidney Disease and Renal Fibrosis. Adv. Exp. Med. Biol. 2019;1165:81–100. doi: 10.1007/978-981-13-8871-2_5. [DOI] [PubMed] [Google Scholar]
- 265.Formica C., Peters D.J.M. Molecular pathways involved in injury-repair and ADPKD progression. Cell Signal. 2020;72:109648. doi: 10.1016/j.cellsig.2020.109648. [DOI] [PubMed] [Google Scholar]
- 266.Qian Q., Hunter L.W., Li M., Marin-Padilla M., Prakash Y.S., Somlo S., Harris P.C., Torres V.E., Sieck G.C. Pkd2 haploinsufficiency alters intracellular calcium regulation in vascular smooth muscle cells. Hum. Mol. Genet. 2003;12:1875–1880. doi: 10.1093/hmg/ddg190. [DOI] [PubMed] [Google Scholar]
- 267.Yu S.M., Nissaisorakarn P., Husain I., Jim B. Proteinuric Kidney Diseases: A Podocyte’s Slit Diaphragm and Cytoskeleton Approach. Front. Med. 2018;5:221. doi: 10.3389/fmed.2018.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Lim B.J., Yang J.W., Do W.S., Fogo A.B. Pathogenesis of Focal Segmental Glomerulosclerosis. J. Pathol. Transl. Med. 2016;50:405–410. doi: 10.4132/jptm.2016.09.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Evelyn C.R., Wade S.M., Wang Q., Wu M., Iniguez-Lluhi J.A., Merajver S.D., Neubig R.R. CCG-1423: A small-molecule inhibitor of RhoA transcriptional signaling. Mol. Cancer Ther. 2007;6:2249–2260. doi: 10.1158/1535-7163.MCT-06-0782. [DOI] [PubMed] [Google Scholar]
- 270.Hayashi K., Watanabe B., Nakagawa Y., Minami S., Morita T. RPEL proteins are the molecular targets for CCG-1423, an inhibitor of Rho signaling. PLoS ONE. 2014;9:e89016. doi: 10.1371/journal.pone.0089016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Lisabeth E.M., Kahl D., Gopallawa I., Haynes S.E., Misek S.A., Campbell P.L., Dexheimer T.S., Khanna D., Fox D.A., Jin X., et al. Identification of Pirin as a Molecular Target of the CCG-1423/CCG-203971 Series of Antifibrotic and Antimetastatic Compounds. ACS Pharmacol. Transl. Sci. 2019;2:92–100. doi: 10.1021/acsptsci.8b00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Evelyn C.R., Bell J.L., Ryu J.G., Wade S.M., Kocab A., Harzdorf N.L., Showalter H.D., Neubig R.R., Larsen S.D. Design, synthesis and prostate cancer cell-based studies of analogs of the Rho/MKL1 transcriptional pathway inhibitor, CCG-1423. Bioorg. Med. Chem. Lett. 2010;20:665–672. doi: 10.1016/j.bmcl.2009.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Bell J.L., Haak A.J., Wade S.M., Kirchhoff P.D., Neubig R.R., Larsen S.D. Optimization of novel nipecotic bis(amide) inhibitors of the Rho/MKL1/SRF transcriptional pathway as potential anti-metastasis agents. Bioorg. Med. Chem. Lett. 2013;23:3826–3832. doi: 10.1016/j.bmcl.2013.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Hutchings K.M., Lisabeth E.M., Rajeswaran W., Wilson M.W., Sorenson R.J., Campbell P.L., Ruth J.H., Amin A., Tsou P.S., Leipprandt J.R., et al. Pharmacokinetic optimitzation of CCG-203971: Novel inhibitors of the Rho/MRTF/SRF transcriptional pathway as potential antifibrotic therapeutics for systemic scleroderma. Bioorg. Med. Chem. Lett. 2017;27:1744–1749. doi: 10.1016/j.bmcl.2017.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Kahl D.J., Hutchings K.M., Lisabeth E.M., Haak A.J., Leipprandt J.R., Dexheimer T., Khanna D., Tsou P.S., Campbell P.L., Fox D.A., et al. 5-Aryl-1,3,4-oxadiazol-2-ylthioalkanoic Acids: A Highly Potent New Class of Inhibitors of Rho/Myocardin-Related Transcription Factor (MRTF)/Serum Response Factor (SRF)-Mediated Gene Transcription as Potential Antifibrotic Agents for Scleroderma. J. Med. Chem. 2019;62:4350–4369. doi: 10.1021/acs.jmedchem.8b01772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Haak A.J., Appleton K.M., Lisabeth E.M., Misek S.A., Ji Y., Wade S.M., Bell J.L., Rockwell C.E., Airik M., Krook M.A., et al. Pharmacological Inhibition of Myocardin-related Transcription Factor Pathway Blocks Lung Metastases of RhoC-Overexpressing Melanoma. Mol. Cancer Ther. 2017;16:193–204. doi: 10.1158/1535-7163.MCT-16-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable. No new data were created or analyzed in this study. Data sharing is not applicable to this article.