Abstract
Background
Many valuable and productive patented technologies have been developed to control schistosomiasis in China in the past 70 years. We conducted a research to analyse patented technologies for schistosomiasis control and prevention filed by Chinese applicants for determining the future patent layout.
Methods
The patent databases of China National Intellectual Property Administration and Baiten were comprehensively searched, and patented technologies for schistosomiasis control and prevention, published between January 1950 and December 2020 filed by Chinese applicants were sorted on 30 December 2020. The patent types, technical fields, and patent development trends were analysed using patent indexing.
Results
There are 184 valid schistosomiasis control technology patents, among them 128 invention patents. The patents related to schistosomiasis control and prevention technology have gone through the germination, growth, and maturity stages. These phases correspond with three phases in schistosomiasis control in China. The main technical aspects were fundamental research (n = 37), detection (n = 13), chemotherapy (n = 61), and armamentarium/devices (n = 73), of which the number of patents for detection for diagnosis was smaller. The top three specialised technical fields for patents subgroups, focusing on antiparasitic agents, DNA or RNA, vectors and medicines, of which schistosomicides are the major dominant subgroup.
Conclusions
We recommend that technologies to be patented for schistosomiasis control and prevention be focused on detection, preliminary studies for molecular detection methods should be significantly enhanced, and patent layout must be performed, which will, in turn, promote accuracy of early diagnosis, not only in humans but also in livestock. It is necessary to develop more anti-schistosomal drugs safely and effectively, exceptionally eco-friendly molluscicides and herbal extracts anti-schistosomes, improve treatment, develop vaccines for use in humans.
Graphic abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s40249-021-00869-6.
Keywords: Schistosomiasis, Patent analysis, Control and prevention, Technology
Background
Schistosomiasis is a zoonotic parasitic disease caused by six species of trematodes. The major three species of schistosomes, namely, Schistosoma japonicum, S. manosni, and S. haematobium cause schistosomiasis in a wide range of endemic areas and severe harm to human health [1–3]. According to World Health Organization (WHO) reports, approximately 779 million people worldwide have been infected with schistosomes, with 236.6 million cases in 2019 [4]. Schistosomiasis japonica caused by S. japonicum is predominantly prevalent in China [5–8]. A total of 30 170 advanced schistosomiasis cases was documented in China in 2019. Oncomelania hupensis, is the only intermediate host snail of S. japonica, with snail habitats 3.6 billion m2 in 2019 [9].
China has a 70-year-old history of schistosomiasis control. The national control programmes have achieved great success, and the endemic status of schistosomiasis in China is already low [10–14]. The technologies for schistosomiasis immunodiagnosis, chemotherapy, snail control, devices, and vaccines have been gradually developed, sanitation (faecal disposal) has been improved, and patents have been obtained to protect technological innovation [15–20]. However, many challenges in eliminating schistosomiasis exist [14]. Precision control of schistosomiasis still needs to be reinforced in China [9]. It's necessary to conduct a research on patented technologies for further patent payout.
In this study, we searched and analysed patent information on schistosomiasis control and prevention from the patent databases of the State Intellectual Property Office and Baiten to understand the developmental trends of schistosomiasis control and prevention in patents, analyze technical fields and patent layout. We present proposals for the structure of patented technologies, which may aid high-quality scientific and technological innovation [21] to achieve the national goal of eliminating schistosomiasis by 2030, as stated in the ‘Healthy China 2030’ Planning Outline [22].
Methods
Patent search strategy
The search was conducted in the China Patent Database from China National Intellectual Property Administration (CNIPA, weblink: https://www.cnipa.gov.cn/) and the Baiten Patent Database (weblink: https://www.baiten.cn/) independently for patents related to schistosomiasis control and prevention published between January 1950 and December 2020, using the following search terms: 'Schistosoma' (all fields) AND 'Oncomelania' (all fields) AND 'Schistosoma' (patent specification) on December 30, 2020. We only included patents filed by Chinese applicants. We also undertook an additional manual filtration by abstracts and specifications of the patents and categorised the legal status.
Study selection criteria
Only applications with patent titles were screened in the two databases with the same inclusion and exclusion criteria applied to the search strategy [23], and the duplicates were identified and deleted by using web clips in databases. We excluded foreign patents which were not filed by Chinese applicants, but passed the Patent Cooperation Treaty (PCT) into China. The patent literature was downloaded for further analysis. We deleted unrelated patents with detailed information and retained only valid patents as target patents among the total patents with three legal statuses, i.e. valid patents, invalid patents and patents under trial [24].
Data extraction and statistical methods
According to the selection criteria mentioned above, the valid patents were extracted for analysis by patent analysis methods, and the technical domains of valid patents were analysed according to the International Patent Classification (IPC) number, the most common hierarchical system of categories used in the world [23, 25, 26]. Microsoft Excel (Office professional plus 2016, Microsoft, Redmond, USA) was used as the statistical analysis software to analyse the collected data [27].
Results
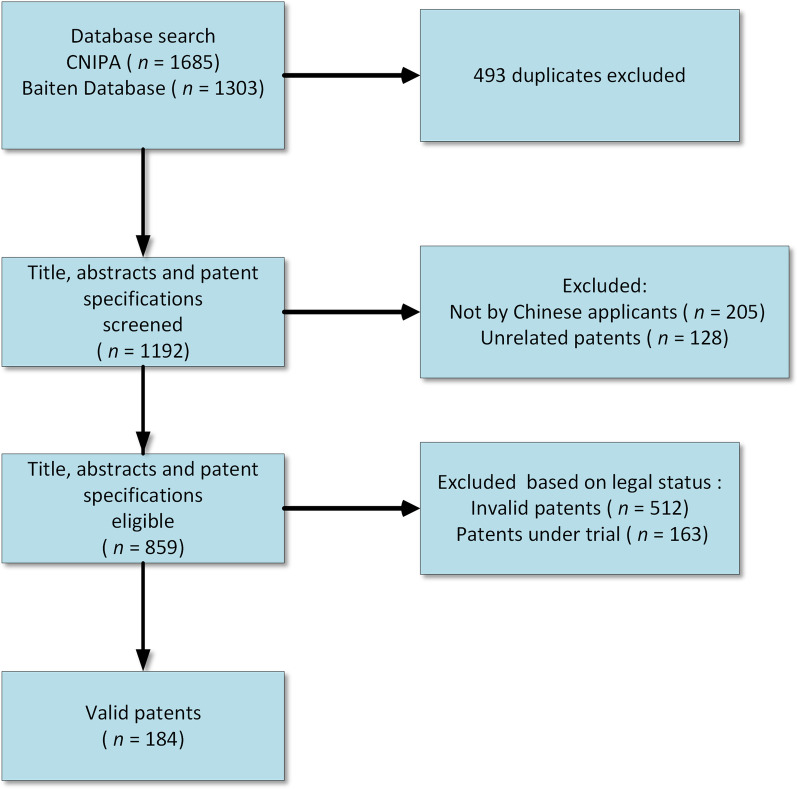
By the study selection criteria and data extraction, 382 duplicates in CNIPA database were identified and deleted. Several patents had been repeated in 'Schistosoma' (all fields) AND 'Oncomelania' (all fields) AND 'Schistosoma' (patent specification). We eliminated 111 duplicates using web clips in databases and excluded 205 patents according to the International Application Number. We deleted 128 unrelated patents and retained 184 valid patents as target patents among the 859 total patents filed by Chinese applicants (Fig. 1). A list of targeted patents is provided in Additional file 1.
Fig. 1.
Flow chart of included patents. CNIPA China Patent Database from China National Intellectual Property Administration
Overall trend analysis
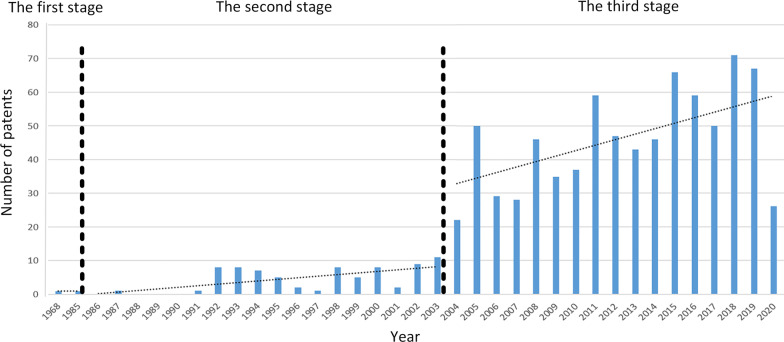
According to the data obtained, patent applications by the Chinese for schistosomiasis control and prevention technologies began in 1968 (Fig. 2). After the stages of germination, growth, and maturity, the entire research and development process, exploration, and application have been carried out for schistosomiasis-related control technologies. Specifically, there was slow progress in the development of schistosomiasis-related technologies from 1950 to 1985, with only two patents, which were still undergoing research and investigation, being introduced in this period. From 1986 to 2003, the number of patent applications increased, with some of the critical technologies related to snail control, chemotherapy, and usage of proteins and antibodies, but the overall rate of application was still low. Since 2004, there has been rapid development in schistosomiasis-related technologies. The number of patent applications increased rapidly year-on-year and peaked in 2018, with 71 patent applications on the application-oriented research and development process. A list of the number of patents applications is provided in Additional file 2.
Fig. 2.
The number of patent applications between 1968 to 2020. The first stage (from 1950 to 1985): the germination period; The second stage (from 1986 to 2003): the growth period; The third stage (2004 onwards): the maturity period
Analysis of the legal status of patents
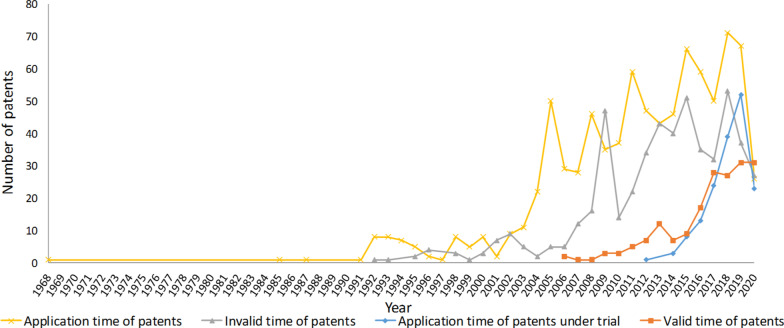
There are 184 valid patents, 512 invalid patents, and 163 patents under trial. A toal of 219 were invalid as a result of the termination of patent rights, and 214 of which were mainly due to unpaid annual fees, except 5 expired patents.
As shown in Fig. 3, there was a substantial increase in the total number of patent activities between 1968 and 2020, including the overall number of patent applications, invalid patents, valid patents, and patents under trial. However, actual trends fluctuated, followed by several years of stagnation before the 1990s, mainly because of the relatively small number of applications. Patent activities increased significantly since 2004. In 2019, the total number of patent applications were 67, 52 under trial, 31 valid patents, and 37 invalid patents, with comparatively better results than in other years.
Fig. 3.
The number of patent applications, invalid patents, patents under trial, and valid patents during 1968–2020
Analysis of valid patents
Patent types
Most of the valid patents (69.6%, 128/184) are invention patents. And they mainly consist of fundamental research and pharmaceutical technologies, with 56 utility models accounting for 30.4% of valid patents.
By analyzing the types of applicants, part of the patents is jointly applied, among which 59 patents are applied jointly by colleges and universities and 91 patents by scientific research institutions, i.e., a total of 150 patents are involved, accounting for the vast majority; enterprises apply for 38 patents, and individuals apply for only 8 patents. Generally speaking, the patents are mainly applied by scientific research institutions.
Patent indexing analysis
Among the valid patents for the control and prevention of schistosomiasis, the major ones are related to schistosomiasis japonica, which is prevalent in China, and only one patent is about schistosomiasis haematobium. The necessary technical aspects are fundamental research (37, 20.1%), detection methods (13, 7.1%), and chemotherapy (61, 33.1%), mainly focused on armamentarium and devices (73, 39.7%). Detection technologies are relatively small in number, as presented in Table 1. For more detailed information, see the Additional file 1.
Table 1.
Technology aspects of 184 valid patented technologies for schistosomiasis control and prevention
| Technology aspects | Patented technology aspects | |
|---|---|---|
| Fundamental research (n = 37, 20.1%) | Oncomelania hupensis (n = 1) | Detection: Specific gene: mitochondrial genome DNA to study genetic variation and differentiation of O. hupensis (n = 1) |
| Schistosoma japonicum (n = 36)a |
Detection methods (n = 17) Proteins: SJ16, SjSap, SjScP27, SjScP80, SjScP84, SjScP88, SjHSP90,Vamp2, Microsatellite DNA,nucleic acid,Schistosoma egg crude antigen, amino acid, nanoantibodies, transposon DNA target sequences, glutathione-S-transferase(GST), fluorescent markers |
|
|
Chemotherapy (n = 17) SJ16, SjP40, SjSAPLP4, SjSAPLP5, SjScP27, SjScP80, SjScP84, SjScP88, SjHSP60, IRF4, Frizzled 9, wingless/integrated (WNT) signaling pathways, Sj thioredoxin glutathione reductase (GR), Ad-TD-gene, O-GlcNAcylation, miRNAs, worm production | ||
|
Vaccine (n = 11) SJ16, Sj29, Sj23, SjE16, Sjp50, SjCTRL, SjSAPLP4, SjSAPLP5, SjScP27, SjScP80, SjScP84, SjScP88, SJ26 glutathione-S-transferase(GST), worm production | ||
| Detection methods (n = 13, 7.1%)b |
Molecular detection methods (n = 8) O. hupensis (n = 2)/S. japonicum (n = 6) |
LAMP technique (n = 3) |
| PCR technique (n = 4) | ||
| RPA technique (n = 1) | ||
| Serological detection methods for S. japonicum (n = 5) | Electrochemical immunosensor (n = 1) | |
| ELISA technique (n = 2) | ||
| GICA (n = 2) | ||
| Pathogenic detection methods for S. japonicum (n = 1) | Improved Kato-Katz (n = 1) | |
| Chemotherapy (n = 61, 33.1%) | Molluscicides for O. hupensis (n = 26) | Streptomyces (n = 3) |
| Niclosamide (n = 6) | ||
| Other chemical compounds or compounded with plant or nanocomposite (n = 17) | ||
| Schistosomicides for S. japonicum (n = 35) | Praziquantel (n = 4) | |
| Artemisinin/Artesunate (n = 3) | ||
|
Herbal effective ingredient extracts (n = 12) Bo Luohui/Pulsatilla/Xusuizi/betel nut/Chinese medicine combination |
||
| WNT and other chemical compounds (n = 15) | ||
| External use with gossypol (n = 1) | ||
| Armamentarium/devices (n = 73, 39.7%) | For O. hupensis (n = 34) | Prevention of spread of snails/water conservation and electricity (n = 10) |
| Molluscicidal device/crushing of O. hupensis (n = 7) | ||
| O. hupensis search/collection (n = 10) | ||
| O. hupensis tracking and raising/bioimaging/monitoring and testing (n = 7) | ||
| For S. japonicum (n = 38) | Monitoring/Observation for cercariae/miracidium (n = 5) | |
| Incubation collection and separation research for cercariae/miracidium (n = 4) | ||
| Ultrasonic larviciding (n = 1) | ||
| Device for separating, hatching, and killing S. japonicum eggs (n = 3) | ||
| Neck sleeve deinsectization device for removing schistosomes on necks of dogs (n = 1) | ||
| Oral drug delivery device for cattle, sheep, and dogs (n = 3) | ||
| Epidermal needle for the vaccine (n = 1) | ||
| Detoxification and collection of excrement (n = 4) | ||
| Kit devices for on-site test and laboratory analysis for schistosomiasis control and prevention (n = 16) | ||
| For S. haematobium (n = 1) | Device of a detection filter membrane for filtering S. haematobium eggs (n = 1) |
aThe overlap of classification for partial patents of detection methods, chemotherapy and vaccine. bOne of the detection patents is based on protein Sjp7 for both molecular detection and serological detection, with 14 detection methods in 13 patents
GST Glutathione-S-transferase, GR Glutathione reductase, LAMP Loop-mediated isothermal amplification, PCR Polymerase chain reaction, RPA Recombinase polymerase amplification, ELISA Enzyme-linked immunosorbent assay, GICA Eolloidal gold immunochromatographic assay
Fundamental research involves the extraction of specific mitochondrial genome DNA from O. hupensis (n = 1) for detection, and development of technologies for S. japonicum (n = 36), involving antigen genes, proteins, or recombinant proteins, miRNAs, transposon DNA target sequences, glutathione-S-transferase (GST), glutathione reductase (GR), nanoantibodies, wingless/integrated (WNT) signaling pathways, nucleic acid, O-GlcNAcylation, and worm production, etc., which applying detection methods (n = 17), chemotherapy (n = 17), vaccine (n = 11), respectively, with the overlap of classification for partial patents.
A total of 14 detection methods have been introduced in 13 patents, which include molecular detection methods (n = 8), and serological detection methods (n = 5), and only one pathogen detection method involving improved Kato-Katz. The molecular detection methods described in the applications include loop-mediated isothermal amplification (LAMP) kit, polymerase chain reaction (PCR) kit, and recombinase polymerase amplification (RPA) kit for the detection S. japonicum nucleic acid. The serological detection methods described primarily include the schistosomiasis electrochemical immunosensor, enzyme-linked immunosorbent assay (ELISA) kit to detect S. japonicum antibodies, colloidal gold immunochromatographic assay (GICA) to detect antigens and antibodies, and so on. There are 2 for O. hupensis (LAMP and PCR) and 12 for S. japonicum.
Chemotherapy was implemented via molluscicides (n = 26) and schistosomicides (n = 35). The introduction of Streptomyces, salicylamide esters, methyl pyridine phosphorus, chlorphenoxyacetic acid, nicotinamide, tetramethyl acetaldehyde, methyl naphthol aldicarb, chlorothalonil, macrolides nitrolime, calcium cyanamide or compounded with plant or nanocomposite as molluscicides for snail control excepted niclosamide. In addition to the highly effective praziquantel, artemisinin and artesunate as anti-schistosomal drugs, schistosomicides also include several effective herbal extracts, heterocyclic compounds, α-tocopherol, calcium cyanamide, 2-[2-(4-chloro-2-nitrophenyl)diaz-1-enyl]-4,6-difluorophenol, 1,2,5-thiadiazole-2 oxide, 1,2,5-oxadiazole-2 oxide, 4-benzyl-1-phenethyl-piperazine-2,6-dione, drug for the treatment of liver fibrosis, and so on. The external use of gossypol is also effective in this regard. In total, two cercaricides include N-(4-nitrophenyl)-5-chlorosalicylamide and supramolecular hydrogel with N,N'-diaspartic acid-perylene tetracarboxylic diimide, and tetrahydrofuran.
Most current patents are for armamentarium and devices for the control of O. hupensis (n = 34), S. japonicum (n = 38), and S. haematobium (n = 1), these technologies are related to preventing the spread of snails, molluscicidal armamentarium, the crushing of O. hupensis, search, collection, tracking, raising, bioimaging, monitoring and testing for O. hupensis, testing related equipment; monitoring, observation, incubation collection, and separation research, ultrasonic larviciding associated with cercariae and miracidium, the device for separating, hatching and killing and filtering schistosome eggs, neck sleeve deinsectization device for removing schistosomes on necks of dogs; oral drug delivery devices for cattle, sheep, and dogs; an epidermal needle for a DNA vaccine against schistosomes; detoxification and collection of excrement; and kit devices for on-site test and laboratory analysis for schistosomiasis control and prevention.
Analysis of technical field distribution
A61P33 (n = 45), C12N15 (n = 32), and A61K31(n = 26) are the top three dominant IPC subgroups for the technical field of schistosomiasis control, focusing on antiparasitic agents, DNA or RNA, vectors and medicines, of which A61P33/12, i.e., schistosomicides (n = 43) is the significant dominant subgroup by analyzing the technical field distribution of valid patents according to the IPC number.
Discussion
Analysis of patents in three phases in schistosomiasis control
The patented technologies related to schistosomiasis control and prevention have gone through three stages. These phases corresponded with three phases in schistosomiasis control in China: the first stage (transmission control strategy through snail control, from the mid-1950s to the early 1980s), the second stage (morbidity control based on chemotherapy, from the mid-1980s to 2003), and the third stage (integrated control strategy with an emphasis on infection source control, 2004 onwards) [6, 28], with the implementation of patents for interventions on infection source control, transmission control or transmission interruption, and protection of susceptible population [29]. Only two patents were applied in the first stage in China, which is the same as other outputs, such as articles. There were application patents with 77 pieces during the second stage, 30 (39.0%) related to snail control, and 13 (16.9%) related to chemotherapy. It was expected to summarise the previous stage's technologies, and thus there were significant patents that referred to snail control, the primary strategy in the first stage. During the third stage, the majority of patents (163) accompanied strategy under trial, which may become valid patents. All 184 valid patent applications were made after 2004. A total of 128 invention patents indicate more creativity with longer protective time than utility models. The number of patents has gradually increased. Through continuous research and development (R&D) and technological innovation, significant preliminary research results referring to genes, proteins, especially recombinant proteins, nucleotides, and pathways, with part of the primary research mentioned applied to multiple studies. Meanwhile, some devices used for experiments have become patents to promote basic research. The number of articles of schistosomiasis control strategy increased after 2004, with the same tendency for patents [30].
With the increasing number of imported schistosomiasis cases [31–37], there is currently a patented technology for producing a filter membrane for filtering S. haematobium eggs, and nine patents related to S. manosni and S. haematobium are under trial.
The technical field distribution from the analysis of IPC can also provide specific corroborative evidence to patent technologies. A study [24] found global patents for Schistosoma between 1985 and 2014. A similar application was also observed. The IPC classification of patents mainly concentrated around A61P33/12 (schistosomicides) and A61K39/00 (medicinal preparations containing antigens or antibodies). Patents on Schistosoma in China focused on A61P33, C12N15, and A61K31 after six years, of which A61P33/12 is the most common one. The technology subdomains are also pharmaceuticals and biotechnology.
It is necessary to carry out the patent layout for numerous invalid patented technologies for schistosomiasis control and prevention, especially the patent for which rights have been terminated without paying the annual fee (n = 214), with a high proportion of improper maintenance for a low commercial value [24]. We can explore technical points, actively pay attention to R&D trends, and seek technologies such as PCR and LAMP, etc., valuable detection and diagnosis technology points are patented.
Patents for infection source control
There are 13 patents for detecting S. japonicum infection, including pathogenic diagnosis, serological diagnosis, and molecular diagnosis. Only one valid patent referred to the traditional pathogenic method (faecal examination: detecting schistosome eggs in faeces of infected source), the Kato-Katz technique as the 'golden method' to judge whether Schistosoma is infected or not. However, the pathogen-detecting method is effort-intensive, mainly it requires faecal sample collection, a long diagnosis cycle, and low sensitivity (especially in areas where the overall endemicity has become low), the high false-negative rate of approximately 5.56–89.47% [38–40].
There are five kit patents related to serological diagnosis, and many more patents in basic research targeted this kind of diagnosis. Serological diagnosis using four methods, that is, indirect haemagglutination assay (IHA), ELISA, dye dipstick immunoassay (DDIA), dot immunogold filtration assay (DIGFA), have simple operation and high sensitivity, and have the advantages of increased compliance with the people in epidemic areas; however, they cannot distinguish current infections from past ones. Such tests proved unsatisfactory specificity and were not suitable for early diagnosis [41, 42]. Currently, there are eight test kits approved by the National Medical Products Administration, that is, DDIA (n = 1), IHA (n = 3), ELISA (n = 2), and IHA (n = 2).
Eight kit patents referred to molecular diagnostic techniques, such as LAMP, RAP, PCR, etc., which have proven significant because of their speed, high specificity, and sensitivity. However, there is no molecular detection kit approved by the National Medical Products Administration, as they are relatively expensive, require a controlled environment, and are likely to cause false positives because of contamination. Inventions described in most of these types of patents are still used for humans and one for livestock. However, domestic animals are also the primary source of infection, and relevant testing products are urgently needed.
Generally, the diagnostic options and related products are still few, especially kits for detecting schistosomiasis. A more efficient, convenient, and rapid kit is urgently required to facilitate a more sensitive and rapid diagnosis of schistosomiasis.
The intervention approaches also included chemotherapy for humans and livestock. The large-scale deployment of praziquantel to control schistosomiasis in China significantly reduced morbidity due to S. japonicum [43, 44]. To date, there are only four valid patents regarding praziquantel formulations and compounds. Three patents related to artemisinin and its derivatives (artemether and artesunate) could have anti-schistosomal properties [45]. Many patents about new chemotherapy are mainly associated with in vitro trials and effective herbal extracts for anti-schistosomsis; however, the National Medical Products Administration has approved praziquantel among 19 products as the only therapeutic drug. Giving a green passageway to speed up approval of the adaptation of older drugs artesunate and artemisinin for anti-schistosomiasis will aid in controlling schistosomiasis.
It is challenging to control livestock, an infectious source that plays a crucial role in schistosomiasis transmission, as a primary reservoir host [41]. Patented technologies for oral drug delivery devices for cattle and sheep have emerged. After control and prevention measures, infectivity in cattle has been basically controlled, but sheep have gradually shown increasing infectivity and have become the primary infection source. One reason is that sheep dung, which is scattered everywhere and is not easy to collect. The use of a patented sheep dung collection device has been promoted, and other patented technologies such as equipment for the harmless treatment of faeces, washroom pan, etc., have been used for faeces management. Finally, faecal matter with infected eggs is being prevented from contaminating water sources to cut off the transmission.
Patents for transmission control or transmission interruption
O. hupensis, the only intermediate host of S. japonicum, is not easy to control. The snail habitats in endemic regions are enormous, with approximately 3.6 billion m2 [9, 46, 47], a large majority located in the lake regions. With the restoration and protection of the ecological environment, many factors, such as temperature, rainfall, vegetation, and soil moisture, have also increased snail spreading. Thus, mass snail elimination campaigns have been developed for schistosomiasis control [41]. There are many patented technologies for snail management, and it is essential to apply scientific and technological strategies, such as a drainage system, and armamentarium for O. hupensis, and molluscicides to control snails. Patented snail control technologies that have low toxicity and are environmentally friendly [48], and effective snail surveys also have market application prospects.
One of the other primary strategies for snail control is chemotherapy. Niclosamide, the only approved molluscicide, is the most widely used in China [49], and has six patents, including patents for powder formulations, suspension concentrate formulations, and spreading oil formulations. Several new chemical compounds have been developed for snail control. Metaldehyde has excellent molluscicidal effects against O. hupensis and has low toxicity [44]. Calcium cyanamide (CaCN2) is not only a molluscicide but also can be used to kill S. japonicum eggs. It also acts as a nitrogen fertiliser and is both economical and environment-friendly. At present, further investigation is required for broader applications in the field. Several molluscicides and schistosomicides from herb extracts have been developed for patenting, which could potentially be safer. The government has been recommended to increase investment and provide supportive policy innovation services to promote effective herbal extracts.
Patents for susceptible population protection
The development of the patented schistosomiasis vaccine is an adjuvant measure of strategic significance; it protects the susceptible population and helps in the integrated control of schistosomiasis. WHO Special Programme for Research and Training in Tropical Diseases (TDR) has placed the development of schistosomiasis vaccines at the forefront of research on the control and prevention of schistosomiasis. Currently, the schistosomiasis vaccine is mainly used in animals. More clinical trials for determining safety and efficacy in humans are required [50, 51].
Limitations
This study has several limitations. First, patent retrieval is a complicated process, and it is challenging to identify targeted patents in online Chinese patent databases completely. Second, patent databases have drawbacks in terms of data collection. As databases have different retrieval standards, although the exact keywords were used, the results have significant differences. It is likely that this introduced some personal bias, as the system cannot automatically and accurately filter data.
Conclusions
Patented technologies for schistosomiasis control and prevention have been researched and developed as integrated control strategies, transitioning to precise control, which has proven useful and has played a specific role in schistosomiasis control and prevention in China. However, there are many significant challenges; therefore, it is necessary to carry out R&D for precise technology and more advanced techniques with continuous innovation. Exploring technical applications, pursuing essential research on molecular detection, and applying preliminary results for detection while patenting the technologies can improve the accuracy of early diagnosis, not only in human beings but also in livestock, and patent layout must be performed. Safe and effective drugs for S. japonicum and O. hupensis still remain to be developed, exceptionally eco-friendly molluscicides and herbal extract anti-schistosomes. In addition, strategies for sufficient treatment and vaccines for human beings should be developed for better control. And patent layout must be performed. Technologies worthy of mention are those related to S. haematobium and S. manosni control, which serve to deal with imported schistosomiasis cases brought into China as a result of travel and migration from other countries. High-quality scientific and technological innovation will positively aid in reaching the schistosomiasis elimination targets by 2030.
Supplementary Information
Additional file 1. Valid patents for schistosomiasis control and prevention filed by Chinese applicants
Additional file 2. The number of patent applications between 1968 to 2020
Acknowledgements
We are grateful to our institute for enabling this study. We would like to express our gratitude to Prof. Xiao-Nong Zhou, the Editor-in-Chief of Infectious Diseases of Poverty, for his useful comments and remarks.
Abbreviations
- WHO
World Health Organization
- PCT
Patent Cooperation Treaty
- GST
Glutathione-S-transferase
- GR
Glutathione reductase
- WNT
Wingless/integrated
- ELISA
Enzyme-linked immunosorbent assay
- LAMP
Loop-mediated isothermal amplification
- PCR
Polymerase chain reaction
- RPA
Recombinase polymerase amplification
- GICA
Colloidal gold immunochromatographic assay
- IHA
Indirect haemagglutination assays
- DDIA
Dye dipstick immunoassay
- DIGFA
Dot immunogold filtration assay
- IPC
International Patent Classification
- R&D
Research and Development
- DNA
Deoxyribose nucleic acid
- RNA
Ribonucleic acid
- TDR
Special Programme for Research and Training in Tropical Diseases
Authors' contributions
YHX,XNX,and BZ conceived and designed the study. YHX retrieved the patents and prepared the first version of the manuscript. XNX and BZ provided suggestions for revising the manuscript. YHX has revised the manuscript. All authors read and approved the final manuscript.
Funding
National Science and Technology Major Project on Important Infectious Diseases Prevention and Control (No. 2018ZX10734-404).
Availability of data and materials
The datasets analysed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors have provided consent for publication.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Wu GL. Human Parasitology. 4th edition. Beijing: People's Medical Publishing House. 2013: 277–333 (in Chinese).
- 2.Schistosomiasis. http://www.who.int/mediacentre/factsheets/fs115/en/index.html. Accessed 21 Jan 2021.
- 3.Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet. 2014;383(9936):2253–2264. doi: 10.1016/S0140-6736(13)61949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization Schistosomiasis and soil-transmitted helminthiases: numbers of people treated in 2019. Weekly Epidemiol Record. 2020;95(50):629–640. [Google Scholar]
- 5.Zhou XN, Bergquist R, Leonardo L, Yang GJ, Yang K, Sudomo M, et al. Schistosomiasis japonica control and research needs. Adv Parasitol. 2010;72:145–178. doi: 10.1016/S0065-308X(10)72006-6. [DOI] [PubMed] [Google Scholar]
- 6.Collins C, Xu J, Tang S. Schistosomiasis control and the health system in P. R. China. Infect Dis Poverty. 2012;1(1):8. doi: 10.1186/2049-9957-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006;368(9541):1106–18. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 8.Chitsulo L, Engels D, Montresor A, Savioli L. The global status of schistosomiasis and its control. Acta Trop. 2000;77(1):41–51. doi: 10.1016/S0001-706X(00)00122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang LJ, Xu ZM, Dang H, Li YL, Lv S, Xu J, et al. Endemic status of schistosomiasis in People's Republic of China in 2019. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2020;32(6):551–558. doi: 10.16250/j.32.1374.2020263. [DOI] [PubMed] [Google Scholar]
- 10.Xu J, Hu W, Yang K, Lv S, Li SZ, Zhou XN. Key points and research priorities of schistosomiasis control in China during the 14th Five-Year Plan Period. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2021;33(1):1–6. doi: 10.16250/j.32.1374.2020356. [DOI] [PubMed] [Google Scholar]
- 11.Li SZ, Xu J, Wang TP, Wen LY, Yang K, Wang W, et al. Upholding Chinese spirit on schistosomiasis control in the new era to accelerate the progress toward elimination in China. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2019;31(1):1–13. doi: 10.16250/j.32.1374.2019043. [DOI] [PubMed] [Google Scholar]
- 12.Qian MB, Chen J, Bergquist R, Li ZJ, Li SZ, Xiao N, et al. Neglected tropical diseases in the People's Republic of China: progress towards elimination. Infect Dis Poverty. 2019;8(1):86. doi: 10.1186/s40249-019-0599-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu J, Li SZ, Zhang LJ, Bergquist R, Dang H, Wang Q, et al. Surveillance-based evidence: elimination of schistosomiasis as a public health problem in the Peoples' Republic of China. Infect Dis Poverty. 2020;9(1):63. doi: 10.1186/s40249-020-00676-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu J, Lv S, Cao CL, Zhou XN. Progress and challenges of schistosomiasis elimination in China. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2018;30(6):605–609. doi: 10.16250/j.32.1374.2018249. [DOI] [PubMed] [Google Scholar]
- 15.Bergquist R, Zhou XN, Rollinson D, Reinhard-Rupp J, Klohe K. Elimination of schistosomiasis: the tools required. Infect Dis Poverty. 2017;6(1):158. doi: 10.1186/s40249-017-0370-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun LP, Wang W, Hong QB, Li SZ, Liang YS, Yang HT, et al. Approaches being used in the national schistosomiasis elimination programme in China: a review. Infect Dis Poverty. 2017;6(1):55. doi: 10.1186/s40249-017-0271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang LD, Guo JG, Wu XH, Chen HG, Wang TP, Zhu SP, et al. China's new strategy to block Schistosoma japonicum transmission: experiences and impact beyond schistosomiasis. Trop Med Int Health. 2009;14(12):1475–1483. doi: 10.1111/j.1365-3156.2009.02403.x. [DOI] [PubMed] [Google Scholar]
- 18.Seto EY, Remais JV, Carlton EJ, Wang S, Liang S, Brindley PJ, et al. Toward sustainable and comprehensive control of schistosomiasis in China: lessons from Sichuan. PLoS Negl Trop Dis. 2011;5(10):e1372. doi: 10.1371/journal.pntd.0001372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tong QB, Chen R, Zhang Y, Yang GJ, Kumagai T, Furushima-Shimogawara R, et al. A new surveillance and response tool: risk map of infected Oncomelania hupensis detected by loop-mediated isothermal amplification (LAMP) from pooled samples. Acta Trop. 2015;141(Pt B):170–177. doi: 10.1016/j.actatropica.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Wang W, Yang K. Implementation of precision control to facilitate the progress towards schistosomiasis elimination in China. Chin Trop Med. 2020;20(7):595–598. [Google Scholar]
- 21.Zhou XN. A high-quality driver to accelerate the progress towards schistosomiasis elimination by science and technology-led innovation in Jiangsu Province. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2019;31(6):573–575. doi: 10.16250/j.32.1374.2019297. [DOI] [PubMed] [Google Scholar]
- 22.The Central Committee of the Communist Party of China, The State Council of the People's Republic of China. 'Healthy China 2030 planning outline'. 2016. http://www.gov.cn/xinwen/2016-10/25/content_5124174.htm.
- 23.Eisinger D, Tsatsaronis G, Bundschus M, Wieneke U, Schroeder M. Automated patent categorization and guided patent search using IPC as inspired by MeSH and PubMed. J Biomed Semantics. 2013;4(1):S3. doi: 10.1186/2041-1480-4-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akinsolu FT, Paiva VN, Souza SS, Varga O. Patent landscape of neglected tropical diseases: an analysis of worldwide patent families. Global Health. 2017;13(1):82. doi: 10.1186/s12992-017-0306-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.International Patent Classification. http://www.wipo.int/classifications/ipc/en.
- 26.Harris CG, Arens R, Srinivasan P. Comparison of IPC and USPC classification systems in patent prior art searches. In: Proceedings of the 3rd international workshop on Patent Information Retrieval. ACM, 2010, 27–32.
- 27.Xiong YH, Zheng B, Xu XN. A study on trends in the development of techniques to prevent and control parasitic diseases based on a patent analysis. Chin J Pathogen Biol. 2018;06:621–624. [Google Scholar]
- 28.Wang W, Dai JR, Liang YS. Apropos: factors impacting on progress towards elimination of transmission of schistosomiasis japonica in China. Parasit Vectors. 2014;7:408. doi: 10.1186/1756-3305-7-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang LD, Chen HG, Guo JG, Zeng XJ, Hong XL, Xiong JJ, et al. A strategy to control transmission of Schistosoma japonicum in China. N Engl J Med. 2009;360(2):121–128. doi: 10.1056/NEJMoa0800135. [DOI] [PubMed] [Google Scholar]
- 30.Lu LT, Zhu R, Zhang LJ, Guo JG. Bibliometric study on the evolution of schistosomiasis control strategies in China. Int J Med Parasit Dis. 2014;41(3):154–158. [Google Scholar]
- 31.Lim PL. Schistosoma haematobium in China, ex-Africa: new populations at risk? J Travel Med. 2013;20(4):211–213. doi: 10.1111/jtm.12031. [DOI] [PubMed] [Google Scholar]
- 32.Hua HY, Wang W, Cao GQ, Tang F, Liang YS. Improving the management of imported schistosomiasis haematobia in China: lessons from a case with multiple misdiagnoses. Parasit Vectors. 2013;6(1):260. doi: 10.1186/1756-3305-6-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang W, Liang YS, Hong QB, Dai JR. African schistosomiasis in mainland China: risk of transmission and countermeasures to tackle the risk. Parasit Vectors. 2013;6(1):249. doi: 10.1186/1756-3305-6-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou XN, Li SZ, Xu J, Chen JX, Wen LY, Zhang RL, et al. Surveillance and control strategy of imported schistosomiasis mansoni: an expert consensus. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2019;31(6):591–595. doi: 10.16250/j.32.1374.2019248. [DOI] [PubMed] [Google Scholar]
- 35.Zhang JF, Wen LY, Xu J, Liang YS, Yan XL, Ren GH, et al. Current status and transmission risks of oversea imported schistosomiasis in China. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2019;31(1):26–32. doi: 10.16250/j.32.1374.2019021. [DOI] [PubMed] [Google Scholar]
- 36.Zhu R, Xu J. Epidemic situation of oversea imported schistosomiasis in China and thinking about its prevention and control. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2014;26(2):111–114. [PubMed] [Google Scholar]
- 37.Liang YS, Wang W, Hong QB, Dai JR. Risk assessment and control measures for import of African schistosomiasis into China. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2013;25(3):221–225. [PubMed] [Google Scholar]
- 38.Zhu HQ, Cao CL, Gao FH, Guo JG, Bao ZP, Wang XH, et al. Evaluation of effectiveness of modified Kato-Katz technique for diagnosis of schistosomiasis japonica. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2005;17(4):273–277. [Google Scholar]
- 39.Zhu H, Yu C, Xia X, Dong G, Tang J, Fang L, et al. Assessing the diagnostic accuracy of immunodiagnostic techniques in the diagnosis of schistosomiasis japonica: a meta-analysis. Parasitol Res. 2010;107(5):1067–1073. doi: 10.1007/s00436-010-1970-3. [DOI] [PubMed] [Google Scholar]
- 40.Wu GL. A historical perspective on the immunodiagnosis of schistosomiasis in China. Acta Trop. 2002;82(2):193–198. doi: 10.1016/S0001-706X(02)00010-4. [DOI] [PubMed] [Google Scholar]
- 41.Xu J, Steinman P, Maybe D, Zhou XN, Lv S, Li SZ, et al. Evolution of the National Schistosomiasis Control Programmes in the People's Republic of China. Adv Parasitol. 2016;92:1–38. doi: 10.1016/bs.apar.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 42.Zhang LJ, Li SZ, Wen LY, Lin DD, Abe EM, Zhu R, et al. The establishment and function of schistosomiasis surveillance system towards elimination in the People's Republic of China. Adv Parasitol. 2016;92:117–141. doi: 10.1016/bs.apar.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 43.Bergquist R, Utzinger J, Keiser J. Controlling schistosomiasis with praziquantel: How much longer without a viable alternative? Infect Dis Poverty. 2017;6(1):74. doi: 10.1186/s40249-017-0286-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Q, Tian LG, Xiao SH, Qi Z, Steinmann P, Mak TK, et al. Harnessing the wealth of Chinese scientific literature: schistosomiasis research and control in China. Emerg Themes Epidemiol. 2008;5:19. doi: 10.1186/1742-7622-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen XY, Wang LY, Cai JM, Zhou XN, Zheng J, Guo JG, et al. Schistosomiasis control in China: the impact of a 10-year World Bank Loan Project (1992–2001) Bull World Health Organ. 2005;83(1):43–48. [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou XN. Implementation of precision control to achieve the goal of schistosomiasis elimination in China. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2016;28(1):1–4. [PubMed] [Google Scholar]
- 47.Zhou XN, Wang LY, Chen MG, Wu XH, Jiang QW, Chen XY, et al. The public health significance and control of schistosomiasis in China–then and now. Acta Trop. 2005;96(2–3):97–105. doi: 10.1016/j.actatropica.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 48.Liang S, Abe EM, Zhou XN. Integrating ecological approaches to interrupt schistosomiasis transmission: opportunities and challenges. Infect Dis Poverty. 2018;7(1):124. doi: 10.1186/s40249-018-0506-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dai JR, Zhou XN, Liang YS, Zhang YP, Jiang YJ, Xi WP, et al. Sensitivity of Oncomelania snail to niclosamide in China. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2002;20(2):101–105. [PubMed] [Google Scholar]
- 50.Molehin AJ. Schistosomiasis vaccine development: update on human clinical trials. J Biomed Sci. 2020;27(1):28. doi: 10.1186/s12929-020-0621-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Molehin AJ, Rojo JU, Siddiqui SZ, Gray SA, Carter D, Siddiqui AA. Development of a schistosomiasis vaccine. Expert Rev Vaccines. 2016;15(5):619–627. doi: 10.1586/14760584.2016.1131127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Valid patents for schistosomiasis control and prevention filed by Chinese applicants
Additional file 2. The number of patent applications between 1968 to 2020
Data Availability Statement
The datasets analysed during the current study are available from the corresponding author upon reasonable request.