Abstract
Health care costs, health care resource utilization, and time to next treatment were compared among patients with chronic lymphocytic leukemia initiated on front-line ibrutinib single agent (N = 322) or chemoimmunotherapy (N = 839). Ibrutinib was associated with lower total health care costs driven by lower medical costs (despite higher pharmacy costs), and longer time to next treatment versus chemoimmunotherapy.
Background:
Studies assessing ibrutinib’s economic burden versus chemoimmunotherapy (CIT) focused on pharmacy costs but not medical costs. This study compared time to next treatment (TTNT), health care resource utilization (HRU), and total direct costs among patients with chronic lymphocytic leukemia (CLL) initiating front-line ibrutinib single agent (Ibr) or CIT.
Materials and Methods:
Optum Clinformatics Extended DataMart De-Identified Databases were used to identify adults with ≥ 2 claims with a CLL diagnosis initiating front-line Ibr or CIT from February 12, 2014 to June 30, 2017. Inverse probability of treatment weighting was used to control for potential differences in baseline characteristics between the Ibr and CIT cohorts. Two periods were considered: entire front-line therapy (until initiation of second-line therapy) and first 6 months of front-line therapy. Comparisons with a subgroup of CIT patients initiating bendamustine/rituximab (BR) were also conducted.
Results:
TTNT was significantly longer for Ibr (N = 322) relative to CIT (N = 839; hazard ratio, 0.54; P = .0163; Kaplan-Meier rates [24 months]: Ibr = 88.6%, CIT = 75.9%) and the subset of CIT patients treated with BR (N = 455; hazard ratio, 0.54; P = .0208; Kaplan-Meier rates [24 months]: Ibr = 89.0%, BR = 79.0%). During the entire front-line therapy, Ibr patients had significantly fewer monthly days with outpatient visits (rate ratio = 0.75; P = .0200). Ibrutinib’s higher pharmacy costs (mean monthly cost difference [MMCD] = $6,849; P < .0001) were offset by lower medical costs (MMCD = −$10,615; P < .0001), yielding net savings (MMCD = −$3,766; P < .0001) versus CIT. Ibr was associated with net savings (MMCD = −$5,569; P < .0001) versus BR. Cost savings and reductions in HRU were more pronounced during the first 6 months of front-line therapy.
Conclusion:
During front-line CLL treatment, Ibr was associated with longer TTNT, fewer monthly days with outpatient visits, and net monthly total cost reduction versus CIT and BR.
Keywords: Administrative claims data, Bruton’s tyrosine kinase inhibitor, Front-line therapy, Health care economics and outcomes research, Oral targeted therapy
Introduction
A major breakthrough in the treatment of chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL; collectively referred to as CLL) has been the advent of anti-CD20-based chemoimmunotherapy (CIT).1-3 For many patients, rituximab-based CIT, such as bendamustine plus rituximab (BR) or fludarabine cyclophosphamide plus rituximab (FCR), was, until recently, the standard of care4,5; overall, these regimens are still commonly utilized in real-world clinical practice.6 Despite this progress, BR and FCR have limited efficacy in subgroups of patients with 17p deletions (a region that contains the TP53 locus).1,7-10 In addition, long-term safety concerns, including a higher risk of secondary myelodysplastic syndrome, acute myeloid leukemia, and transformation to diffuse large B-cell lymphoma (ie, Richter’s transformation), are other limitations associated with CIT.4,11,12
More recently, the approval of new targeted therapies greatly improved the prognosis of patients with CLL.4,13 Along with venetoclax (single agent or in combination with rituximab or obinutuzumab), ibrutinib (single agent or in combination with obinutuzumab) is among the oral targeted therapies approved by the United States (US) Food and Drug Administration in the front-line setting regardless of TP53 mutation status.14-16 In addition, the National Comprehensive Cancer Network (NCCN) guidelines currently list ibrutinib as the preferred front-line regimen regardless of age, comorbidity, and 17p deletion/TP53 mutation status.17 Across 7 trials, ibrutinib demonstrated favorable clinical efficacy in patients with or without prior treatment or unfavorable genetic prognosis (ie, unmutated IGHVand/or TP53 alterations).18-24 In particular, phase III trial data demonstrated that front-line ibrutinib was associated with longer progression-free survival (PFS) than BR in patients ≥ 65 years of age,24 and that ibrutinib plus rituximab led to fewer grade 3 or 4 adverse events and to longer PFS and overall survival (OS) than FCR in patients ≤ 70 years without 17p deletion.23
Per prescribing information, ibrutinib treatment should be continued until progression.16 In contrast, CIT is administered for up to 6 28-day cycles (ie, approximately 6 months).25 Therefore, approximately 6 months after treatment initiation, the drug costs of ibrutinib will continue to accumulate, whereas those of CIT are expected to fall to zero, yielding higher total drug costs for ibrutinib. However, the superior effectiveness of ibrutinib relative to CIT,23,24 and the different routes of administration (ie, ibrutinib: oral,16 CIT: intravenous25) may translate into lower medical costs for ibrutinib compared with CIT, thereby potentially offsetting the higher drug costs. To date, studies that assessed the economic burden of patients treated with ibrutinib versus CIT focused only on pharmacy costs,26 were conducted outside of the US,27,28 or relied on data modeling rather than empirical data analyses.27,28 Notably, Chen et al concluded that oral targeted therapies will dramatically increase the overall economic burden of CLL, but relied solely on literature-derived estimates and projections without consideration for differences in drug effectiveness that may reduce medical costs.29 Thus, this study was conducted to assess and compare real-world time to next treatment (TTNT), health care resource utilization (HRU), and health care costs in patients with CLL initiated on front-line treatment with ibrutinib single-agent versus CIT regimens and versus BR specifically (as this regimen is the most commonly used type of CIT in contemporary real-world clinical practice).6,17
Materials and Methods
Data Source
The Optum Clinformatics Extended DataMart De-Identified Databases, which cover 13 million annual lives of UnitedHealth Group members in all US census regions, were used. It contains historical data on patient demographics, dates of eligibility, date of death, claims for inpatient and outpatient visits, pharmacy encounters, costs of services, and laboratory tests and results. It includes claims from both commercial and Medicare Advantage plans. Data are de-identified and comply with the patient requirements of the Health Insurance Portability and Accountability Act (HIPAA).
Patients’ disease history was identified starting from January 1, 2004. Data related to the administration of CLL treatments of interest (ie, ibrutinib and CIT) was collected from February 12, 2014 to June 30, 2017. This period was chosen to capture ibrutinib use following US approval and inclusion in the NCCN guidelines. Ibrutinib was approved for patients who received at least 1 prior therapy on February 12, 2014, for patients with 17p deletion on July 28, 2014, and as a front-line therapy on March 4, 2016. Ibrutinib was added to the NCCN treatment recommendations in 2014 for relapsed/refractory patients and for front-line therapy in patients with 17p deletion. It was recommended as a front-line therapy for all patients in 2016.
Study Design
A retrospective study design was used. The index date (between February 12, 2014 and May 31, 2017) was defined as the date of initiation of the first observed treatment with ibrutinib or CIT after the first observed CLL diagnosis. The baseline period was defined as the 12 months pre-index.
Per the prescribing information, patients treated with ibrutinib should continue treatment until progression,16 whereas CIT treatment should be given for up to 6 28-day cycles (ie, approximately 6 months).25 Thus, 2 observation periods were considered post-index: (1) From front-line treatment initiation up to the earliest of second-line treatment initiation, end of eligibility (eg, disenrollment, loss of follow-up, death), or lack of available follow-up data (hereinafter referred to as the front-line therapy period); and (2) from front-line treatment initiation up to the earliest of 6 months post-initiation, end of eligibility, or lack of available follow-up data (hereinafter referred to as the first 6-month period). The front-line therapy period was chosen to reflect a period where both cohorts should have received their index treatment for at least 6 months post-index, but those in the ibrutinib cohort should be continuing treatment, whereas those in the CIT cohort should subsequently have ceased treatment as indicated per prescribing guidelines.16 The first 6-month period reflects the period of evaluation under the new Oncology Care Model. The Oncology Care Model was issued by the Centers for Medicare and Medicaid Services in 2016 with the objective to provide higher quality and more coordinated oncology care using a payment arrangement that emphasizes financial and performance accountability for care provided to patients with cancer.30 This period was also chosen to reflect a timeframe where both treatment cohorts would likely be continuously treated.
Study Population
To be included in the study, patients were required to have ≥ 2 claims with a diagnosis of CLL (ie, International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9 CM] code: 204.1; International Classification of Diseases, Tenth Revision, Clinical Modification code [ICD-10 CM]: C91.1) or SLL (ICD-10 CM code: C83.0), have initiated front-line treatment with ibrutinib single-agent or a CIT regimen between February 12, 2014 (date of ibrutinib approval in the US for patients who received at least 1 prior therapy) and May 31, 2017; be ≥ 18 years old as of the index date, have ≥ 12 months of continuous eligibility pre-index, and have ≥ 30 days of continuous eligibility post-index (to capture all agents used as part of the front-line regimen). Patients were excluded if they had ≥ 1 claim with a diagnosis for end-stage renal disease (ie, ICD-9 CM code: 585.6; ICD-10 CM code: N18.6) at any time.
Patients were classified into 2 different cohorts based on the front-line treatment received: ibrutinib single agent (ibrutinib cohort) or CIT regimen (CIT cohort). Among the CIT cohort, the subgroup of patients treated with BR (BR cohort) was also examined separately because this regimen is the most widely used type of CIT among patients with CLL in contemporary real-world clinical practice.6
Study Measures
Study measures included TTNT, HRU, and health care costs. TTNT was used as a proxy for PFS and was defined as the time from index date to the initiation of a new second-line treatment. All-cause HRU and costs captured all types of services received and claimed for by the patient, including those related to the management of the disease, the management of adverse events, and other common services associated with the use of ibrutinib and CIT. Costs reported in the data represented payer-incurred costs.
All-cause HRU included the number of inpatient admissions, number of days of inpatient stay, number of days with outpatient services, number of days with emergency room (ER) visits, and number of days with other services. Inpatient admissions were defined as inpatient episodes occurring in an acute care hospitalization or skilled nursing facility setting. Outpatient HRU was further stratified by the number of days with antineoplastic/CIT drug administration (ie, days with claims with a procedure code for antineoplastic agents such as those used in a CIT regimen), days with services related to drug administration (ie, all other services provided during the encounter for the administration of an antineoplastic agent [eg, nurses, equipment, monitoring, professional services, facility services]), and days with other outpatient services. ER visits were defined as any facility, place of service, or professional services labeled as “emergency room.” Other services included skilled nursing facility services not classified as inpatient, home health, hospice, rehabilitation center, long-term care, drug administration not classified elsewhere, radiology services not classified elsewhere, and surgery services not classified elsewhere. In addition to all-cause HRU, cancer-related HRU was also reported. Cancer-related HRU was defined as claims with a primary or secondary diagnosis for cancer (ICD-9 CM codes: 140-239; ICD-10 CM codes: C00-D49).
All-cause total health care costs were stratified by medical and pharmacy costs. Medical costs were further stratified by inpatient, outpatient, ER costs, and other costs; outpatient costs were stratified by antineoplastic/CIT drug costs, antineoplastic/CIT drug administration costs, and other outpatient costs. In addition to all-cause health care costs, cancer-related health care costs were reported. Cancer-related medical costs were defined as claims with a primary or secondary diagnosis for cancer (ICD-9 CM codes: 140-239; ICD-10 CM codes: C00-D49). Cancer-related pharmacy costs were defined as costs of antineoplastic agents and of corticosteroids commonly used with antineoplastic agents (ie, prednisone, dexamethasone, and methylprednisolone).
Statistical Analysis
Means, medians, and standard deviations were used to report continuous variables; counts and percentages were used to report categorical variables. To account for differences in baseline characteristics between the study cohorts, inverse probability of treatment weighting (IPTW) was used. Weights were calculated based on the propensity score (PS) of being treated with ibrutinib. The PS for each patient was estimated using multivariable logistic regression adjusting for the following characteristics observed during the baseline period: age, gender, US region, month and year of index date, insurance plan type, time from first CLL diagnosis to index date, Charlson comorbidity index, baseline comorbidities (hypertension, lymphoma, deficiency anemias, diabetes, coagulation deficiency, chronic pulmonary disease), and baseline use of corticosteroids. Each patient was assigned a weight of 1/PS for those in the ibrutinib cohort and 1/(1–PS) for those in the corresponding comparator cohort; weights were then normalized by the mean weight. Consequently, the weighted sample sizes (ie, post-IPTW) were different from the original sample sizes although the same patients contributed to the analysis. In other words, before IPTW, each patient has a weight of 1 and after IPTW, each patient has a different weight. When adding the weight for each patient after IPTW in a given cohort, the sum of weights (ie, weighted sample size) may be different than the original sample size for a given cohort. The resulting differences between the weighted cohorts of interest reflect the average treatment effect.
After weighting, the baseline characteristics were compared between the 2 groups using standardized differences (Std. diff.). Characteristics with Std. diff. < 10% were considered as balanced.31,32 TTNT was described using weighted Kaplan-Meier (KM) curves and compared between cohorts using hazard ratios (HRs), 95% confidence intervals (CIs), and P-values calculated from weighted Cox proportional hazards models. Using weights obtained from IPTW, weighted generalized linear models with Poisson distribution for HRU and normal distribution for costs were used to compare outcomes between treatment groups. Non-parametric bootstrap procedures were used to evaluate statistical significance and 95% CIs. Because the duration of treatment is different between ibrutinib and CIT (ie, approximately 6 months for CIT and until progression for ibrutinib) and not all patients could be followed for the same amount of time, HRU and cost outcomes were evaluated per-patient-per-month (PPPM), an approach commonly used in non-experimental study settings. Cost outcomes were inflated to 2017 US dollars. All weighted models included 2 independent variables: an indicator for the treatment group and a continuous variable measuring baseline total health care costs.
Results
Baseline Characteristics
Of 1161 eligible patients, 322 were treated with ibrutinib single-agent and 839 were treated with a CIT regimen, including 455 treated with BR (Figure 1). Other CIT regimens observed included FCR (N = 134), and obinutuzumab-based CIT (N = 89). Given the smaller sample sizes, results were not reported separately for FCR, obinutuzumab-based CIT, and other CIT regimens observed. During the baseline period, the ibrutinib cohort comprised older patients (mean age [before IPTW] = 72.5 vs. 68.8 years; Std. diff. = 34.4%) and a lower proportion of male patients (57.8% vs. 64.2%; Std. diff. = 13.3%) compared with the CIT cohort before IPTW. Patients in the ibrutinib cohort had a longer average time from CLL diagnosis to index date (24.5 vs. 17.7 months; Std. diff. = 28.9%). After applying IPTW, the weighted sample sizes of the ibrutinib and CIT cohorts were 583 and 578, respectively. Weighted cohorts were well-balanced with respect to all demographic and clinical characteristics examined (eg, mean age = 69.9 vs. 69.6; Std. diff. = 3.2%; proportion of males = 61.2% vs. 62.4%; Std. diff. = 2.6%; and mean time from CLL diagnosis to index date = 19.4 vs. 19.1 months; Std. diff. = 1.8%) (Table 1). Conclusions similar to the ibrutinib versus CIT comparison could be drawn when comparing baseline characteristics of the ibrutinib and BR cohorts (see Supplemental Table 1 in the online version).
Figure 1. Selection of the Study Population.
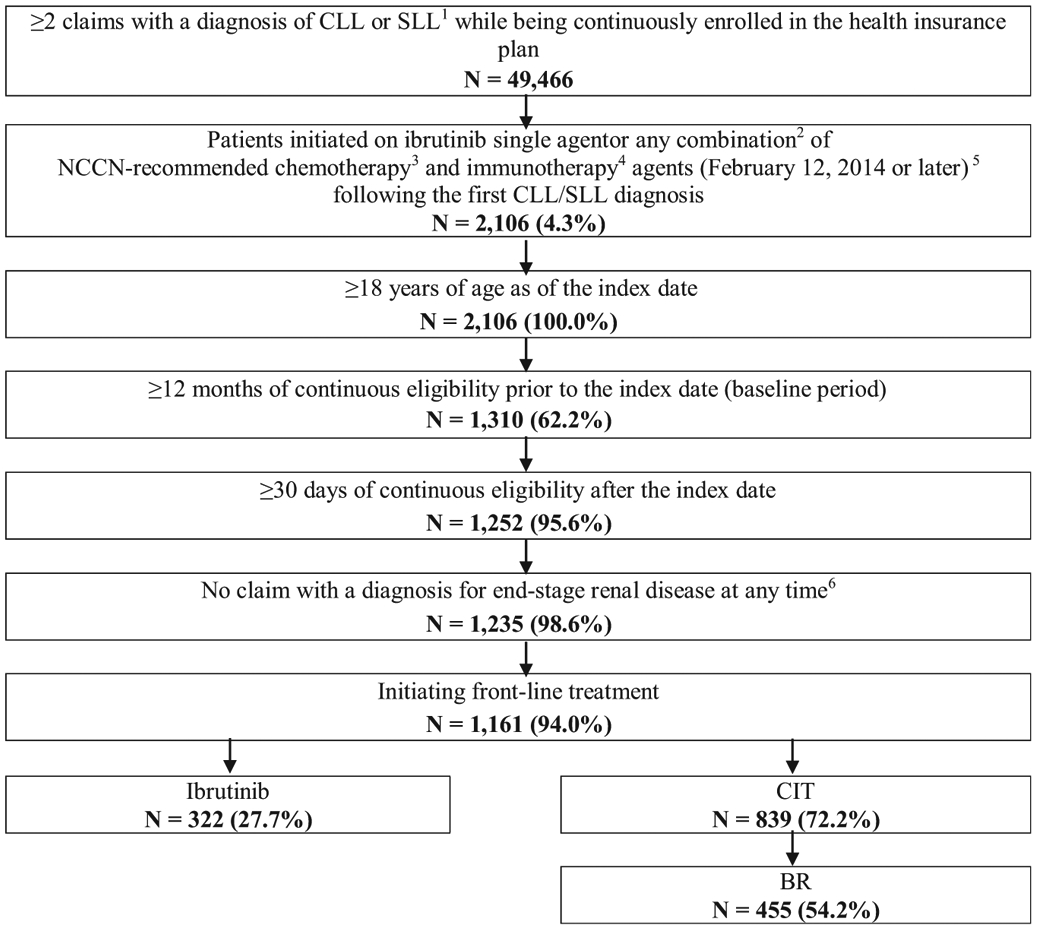
Abbreviations: BR = bendamustine/rituximab; CIT = chemoimmunotherapy; CLL = chronic lymphocytic leukemia; NCCN = National Comprehensive Cancer Network; SLL = small lymphocytic lymphoma. Notes: 1CLL diagnosis was identified using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9 CM) codes 204.1 and International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10 CM) code C91.1. SLL diagnosis was identified using ICD-10 CM code C83.0. 2For combination therapy, the prescription date of each agent had to be within 30 days apart. 3Chemotherapy agents included bendamustine, chlorambucil, cyclophosphamide, cytarabine, doxorubicin, fludarabine, oxaliplatin, pentostatin, vincristine, lenalidomide, and cladribine. 4Immunotherapy agents included alemtuzumab, obinutuzumab, ofatumumab, and rituximab. 5The date of the first claim for one of these treatments following the first CLL/SLL diagnosis is the index date. 6End-stage renal disease was identified using ICD-9 CM code 585.6 and ICD-10 CM code N18.6.
Table 1.
Baseline Demographic and Clinical Characteristics for the Ibrutinib Versus CIT Comparison
| Unweighted Populations | Weighted Populationsa | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ibrutinib | CIT | Std Diff, % | Ibrutinib | CIT | Std Diff, % | |||||
| N = 322 | N = 839 | N = 583 | N = 578 | |||||||
| n | % | n | % | n | % | n | % | |||
| Demographics | ||||||||||
| Male gender | 186 | 57.8 | 539 | 64.2 | 13.3 | 357 | 61.2 | 361 | 62.4 | 2.6 |
| Mean age,b y, ± SD [median] | 72.5 | ± 10.8 [74.0] | 68.8 | ± 10.7 [70.0] | 34.4 | 69.9 | ± 10.9 [72.0] | 69.6 | ± 10.7 [71.0] | 3.2 |
| Year of index date | ||||||||||
| 2014 | 24 | 7.5 | 192 | 22.9 | 44.0 | 100 | 17.1 | 109 | 18.9 | 4.6 |
| 2015 | 64 | 19.9 | 259 | 30.9 | 25.5 | 170 | 29.1 | 164 | 28.3 | 1.8 |
| 2016 | 147 | 45.7 | 241 | 28.7 | 35.6 | 199 | 34.2 | 190 | 32.9 | 2.8 |
| 2017 | 87 | 27.0 | 147 | 17.5 | 23.0 | 114 | 19.5 | 115 | 19.9 | 1.0 |
| Regionb | ||||||||||
| South | 109 | 33.9 | 320 | 38.1 | 8.9 | 225 | 38.6 | 214 | 37.0 | 3.4 |
| West | 94 | 29.2 | 183 | 21.8 | 17.0 | 133 | 22.8 | 138 | 23.8 | 2.4 |
| Midwest | 82 | 25.5 | 243 | 29.0 | 7.9 | 168 | 28.8 | 164 | 28.3 | 1.0 |
| Northeast | 36 | 11.2 | 87 | 10.4 | 2.6 | 53 | 9.1 | 60 | 10.3 | 4.0 |
| Unknown | 1 | 0.3 | 6 | 0.7 | 5.7 | 4 | 0.7 | 4 | 0.6 | 0.9 |
| Insurance plan typeb | ||||||||||
| Medicare | 243 | 75.5 | 535 | 63.8 | 25.6 | 388 | 66.6 | 384 | 66.4 | 0.6 |
| Commercial insurance | 79 | 24.5 | 304 | 36.2 | 25.6 | 194 | 33.4 | 195 | 33.6 | 0.6 |
| Clinical characteristics | ||||||||||
| Mean time between the first CLL diagnosis and the index date, months, ± SD [median] | 24.5 | ± 24.3 [17.8] | 17.7 | ± 22.3 [10.2] | 28.9 | 19.4 | ± 20.6 [14.4] | 19.1 | ± 23.5 [11.9] | 1.8 |
| Use of corticosteroidsc | 140 | 43.5 | 383 | 45.6 | 4.4 | 277 | 47.5 | 258 | 44.6 | 5.7 |
| Comorbiditiesd | ||||||||||
| Hypertension | 198 | 61.5 | 526 | 62.7 | 2.5 | 387 | 66.4 | 363 | 62.8 | 7.6 |
| Lymphoma | 124 | 38.5 | 484 | 57.7 | 39.1 | 299 | 51.4 | 307 | 53.0 | 3.3 |
| Deficiency anemias | 146 | 45.3 | 359 | 42.8 | 5.1 | 242 | 41.6 | 249 | 43.1 | 2.9 |
| Diabetes | 88 | 27.3 | 242 | 28.8 | 3.4 | 145 | 24.9 | 163 | 28.2 | 7.5 |
| Coagulation deficiency | 92 | 28.6 | 204 | 24.3 | 9.7 | 148 | 25.5 | 147 | 25.3 | 0.3 |
| Chronic pulmonary disease | 88 | 27.3 | 201 | 24.0 | 7.7 | 165 | 28.2 | 147 | 25.4 | 6.5 |
| CLL-related comorbiditiesd | ||||||||||
| Anemia | 147 | 45.7 | 363 | 43.3 | 4.8 | 247 | 42.3 | 252 | 43.5 | 2.4 |
| Enlarged lymph nodes | 149 | 46.3 | 470 | 56.0 | 19.6 | 306 | 52.6 | 314 | 54.4 | 3.6 |
| Abdominal pain | 68 | 21.1 | 198 | 23.6 | 6.0 | 131 | 22.5 | 135 | 23.3 | 1.9 |
| Fatigue/weakness | 115 | 35.7 | 304 | 36.2 | 1.1 | 208 | 35.7 | 210 | 36.4 | 1.4 |
| Thrombocytopenia | 79 | 24.5 | 181 | 21.6 | 7.0 | 133 | 22.8 | 130 | 22.5 | 0.8 |
| Mean Charlson comorbidity indexd ± SD [median] | 3.9 | ± 2.2 [3.0] | 3.8 | ± 2.1 [3.0] | 6.3 | 3.8 | ± 2.1 [3.0] | 3.8 | ± 2.1 [3.0] | 1.2 |
Abbreviations: CIT = Chemoimmunotherapy; CLL = chronic lymphocytic leukemia; SD = standard deviation; Std diff = standardized difference.
Baseline characteristics for the weighted populations were obtained by using inverse probability of treatment weights. The inverse probability of treatment weights were estimated based on propensity score. Variables used in the propensity score calculation included the following baseline characteristics: age, gender, region, quarter and year of index date, insurance plan type, time from first CLL diagnosis to index date, Charlson comorbidity index, comorbidities (hypertension, lymphoma, deficiency anemias, diabetes, coagulation deficiency, chronic pulmonary disease), and baseline use of corticosteroids.
Evaluated at the index date.
Evaluated between the first CLL diagnosis and index date.
Evaluated during the 12-month baseline period.
Comparison of TTNT
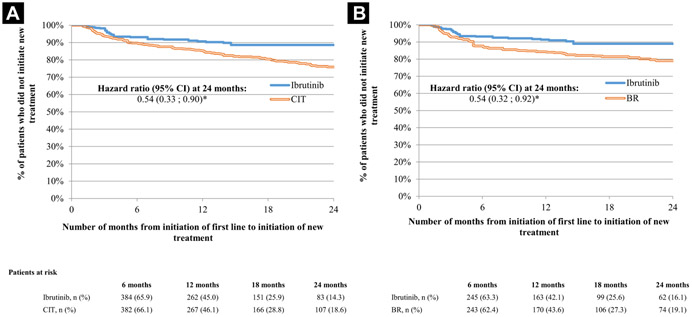
The median follow-up was 12.9 months in the ibrutinib cohort and 13.1 months in the CIT cohort. The median duration of the front-line therapy period was 10.6 months in both the ibrutinib and CIT cohorts. After 24 months of follow-up, ibrutinib-treated patients were significantly less likely to initiate a next line of therapy compared with CIT-treated patients (KM rates: 88.6% [Ibrutinib] vs. 75.9% [CIT]; HR, 0.54; 95% CI, 0.33-0.90; P = .0163) (Figure 2). Similar results were obtained when comparing the ibrutinib cohort with the BR cohort (KM rates: 89.0% [Ibrutinib] vs. 79.0% [CIT]; HR, 0.54; 95% CI, 0.32-0.92; P = .0208) (Figure 2).
Figure 2. Time to Next Treatment for Ibrutinib Versus CIT (A) and BR (B)1, 2.
Abbreviations: BR = bendamustine/rituximab; CI = confidence interval; CIT = chemoimmunotherapy. Notes: *Indicates P-value < .05. 1Weighted populations were obtained by using inverse probability of treatment weights. The inverse probability of treatment weights were estimated based on propensity score. Variables used in the propensity score calculation included the following baseline characteristics: age, gender, United States region, month and year of index date, insurance plan type, time from first chronic lymphocytic leukemia diagnosis to index date, Charlson comorbidity index, baseline comorbidities (hypertension, lymphoma, deficiency anemias, diabetes, coagulation deficiency, chronic pulmonary disease), and baseline use of corticosteroids. 2Hazard ratios were calculated using weighted Cox proportional hazards regression models adjusted for baseline total all-cause costs.
Comparison of HRU
Relative to any evaluated comparator group, patients in the ibrutinib cohort had fewer days PPPM with outpatient services, fewer days PPPM with antineoplastic drug administration, and fewer days PPPM with outpatient services related to antineoplastic drug administration during the front-line therapy period (all P < .05); the number of days PPPM with ER visits did not significantly differ during this period (Table 2). Compared with the front-line therapy period, greater reductions in PPPM HRU were observed in the ibrutinib cohort when considering the first 6-month period. In addition to fewer number of days PPPM with outpatient services, the number of days PPPM with ER visits was significantly lower in the ibrutinib cohort compared with the CIT and BR cohorts (Table 3). Most PPPM all-cause HRU claims were cancer-related. In all comparisons, similar results were found for PPPM cancer-related HRU (ie, lower HRU for ibrutinib vs. all combined CIT and vs. BR).
Table 2.
Comparison of HRU per Patient per Month Between Ibrutinib and CIT and Between Ibrutinib and BR During the Front-line Therapy Periodb, c
| RRd | 95% CIe | P Valuee | |
|---|---|---|---|
| Ibrutinib versus CIT | |||
| Monthly all-cause HRU | |||
| Number of inpatient admissions | 0.74 | 0.48-1.17 | .2400 |
| Number of days of inpatient stay | 0.92 | 0.49-1.83 | .8200 |
| Days with outpatient services | 0.75 | 0.60-0.94 | .0200a |
| Days with antineoplastic/CIT drug administration | 0.05 | 0.01-0.13 | <.0001a |
| Days with outpatient services related to the antineoplastic/CIT drug administration | 0.09 | 0.03-0.19 | <.0001a |
| Days with other outpatient services | 1.01 | 0.80-1.28 | .8240 |
| Days with ER visits | 0.89 | 0.57-1.46 | .5360 |
| Days with other services | 0.77 | 0.49-1.31 | .2520 |
| Monthly cancer-related HRUf | |||
| Number of inpatient admissions | 0.77 | 0.49-1.22 | .3080 |
| Number of days of inpatient stay | 0.93 | 0.48-1.86 | .8640 |
| Days with outpatient services | 0.64 | 0.51-0.82 | .0080a |
| Days with antineoplastic/CIT drug administration | 0.05 | 0.02-0.13 | <.0001a |
| Days with outpatient services related to the antineoplastic/CIT drug administration | 0.09 | 0.03-0.19 | <.0001a |
| Days with other outpatient services | 0.98 | 0.79-1.30 | .8400 |
| Days with ER visits | 0.78 | 0.44-1.29 | .2880 |
| Days with other services | 0.70 | 0.42-1.23 | .1720 |
| Ibrutinib versus BR | |||
| Monthly all-cause HRU | |||
| Number of inpatient admissions | 0.87 | 0.55-1.34 | .5000 |
| Number of days of inpatient stay | 1.32 | 0.70-2.37 | .3960 |
| Days with outpatient services | 0.74 | 0.57-0.91 | .0040a |
| Days with antineoplastic/CIT drug administration | 0.04 | 0.01-0.09 | <.0001a |
| Days with outpatient services related to the antineoplastic/CIT drug administration | 0.07 | 0.02-0.16 | <.0001a |
| Days with other outpatient services | 1.01 | 0.78-1.25 | .9200 |
| Days with ER visits | 1.01 | 0.56-1.74 | .8680 |
| Days with other services | 0.94 | 0.49-1.61 | .7600 |
| Monthly cancer-related HRUf | |||
| Number of inpatient admissions | 0.91 | 0.56-1.44 | .7080 |
| Number of days of inpatient stay | 1.36 | 0.72-2.45 | .3440 |
| Days with outpatient services | 0.65 | 0.51-0.79 | <.0001a |
| Days with antineoplastic/CIT drug administration | 0.04 | 0.01-0.09 | <.0001a |
| Days with outpatient services related to the antineoplastic/CIT drug administration | 0.07 | 0.02-0.16 | <.0001a |
| Days with other outpatient services | 1.04 | 0.82-1.30 | .6600 |
| Days with ER visits | 0.91 | 0.42-1.97 | .6800 |
| Days with other services | 0.88 | 0.44-1.56 | .6520 |
Abbreviations: BR = Bendamustine/rituximab; CI = confidence interval; CIT = chemoimmunotherapy; ER = emergency room; HRU = health care resource utilization; RR = rate ratio.
Indicates P-value < .05.
HRU for the weighted populations were obtained by using inverse probability of treatment weights. The inverse probability of treatment weights were estimated based on propensity score. Variables used in the propensity score calculation included the following baseline characteristics: age, gender, region, quarter and year of index date, insurance plan type, time from first chronic lymphocytic lymphoma diagnosis to index date, Charlson comorbidity index, comorbidities (hypertension, lymphoma, deficiency anemias, diabetes, coagulation deficiency, chronic pulmonary disease), and baseline use of corticosteroids.
The front-line therapy period was defined as the period from the initiation of the front-line therapy to the earliest among discontinuation of the front-line therapy (defined as a gap of more than 90 days between the last day of supply of a claim and the first day of supply of the next claim for the front-line therapy), initiation of a second-line therapy (a new antineoplastic agent not part of the front-line therapy), or end of data.
RRs were calculated using generalized linear models with Poisson distribution. Models were adjusted for baseline total all-cause costs.
CIs and P-values were obtained from non-parametric bootstrap procedures using 500 replicates.
Cancer-related HRU was defined as claims with a primary or secondary diagnosis for cancer (International Classification of Diseases, Ninth Edition Clinical Modification codes: 140-239; International Classification of Diseases, Tenth Edition Clinical Modification codes: C00-D49).
Table 3.
Comparison of HRU per Patient per Month Between Ibrutinib and CIT and Between Ibrutinib and BR During the First 6-month Periodb, c
| RRd | 95% CIe | P Valuee | |
|---|---|---|---|
| Ibrutinib versus CIT | |||
| Monthly all-cause HRU | |||
| Number of inpatient admissions | 0.62 | 0.36-1.01 | .0600 |
| Number of days of inpatient stay | 0.77 | 0.37-1.48 | .4840 |
| Days with outpatient services | 0.60 | 0.50-0.74 | <.0001a |
| Days with antineoplastic/CIT drug administration | 0.06 | 0.02-0.11 | <.0001a |
| Days with outpatient services related to the antineoplastic/CIT drug administration | 0.08 | 0.03-0.16 | <.0001a |
| Days with other outpatient services | 0.75 | 0.62-0.92 | .0200a |
| Days with ER visits | 0.55 | 0.34-0.96 | .0320a |
| Days with other services | 0.69 | 0.43-1.13 | .1000 |
| Monthly cancer-related HRUf | |||
| Number of inpatient admissions | 0.62 | 0.34-1.02 | .0600 |
| Number of days of inpatient stay | 0.77 | 0.37-1.48 | .4880 |
| Days with outpatient services | 0.51 | 0.42-0.65 | <.0001a |
| Days with antineoplastic/CIT drug administration | 0.06 | 0.02-0.11 | <.0001a |
| Days with outpatient services related to the antineoplastic/CIT drug administration | 0.08 | 0.03-0.16 | <.0001a |
| Days with other outpatient services | 0.65 | 0.54-0.83 | .0080a |
| Days with ER visits | 0.53 | 0.31-0.97 | .0240a |
| Days with other services | 0.62 | 0.35-1.03 | .0640 |
| Ibrutinib versus BR | |||
| Monthly all-cause HRU | |||
| Number of inpatient admissions | 0.71 | 0.37-1.14 | .1560 |
| Number of days of inpatient stay | 0.96 | 0.44-1.96 | .8200 |
| Days with outpatient services | 0.59 | 0.48-0.69 | <.0001a |
| Days with antineoplastic/CIT drug administration | 0.06 | 0.02-0.10 | <.0001a |
| Days with outpatient services related to the antineoplastic/CIT drug administration | 0.07 | 0.03-0.14 | <.0001a |
| Days with other outpatient services | 0.74 | 0.61-0.90 | .0040a |
| Days with ER visits | 0.53 | 0.30-0.87 | .0120a |
| Days with other services | 0.81 | 0.45-1.30 | .3440 |
| Monthly cancer-related HRUf | |||
| Number of inpatient admissions | 0.71 | 0.38-1.16 | .1640 |
| Number of days of inpatient stay | 0.97 | 0.44-1.99 | .8360 |
| Days with outpatient services | 0.50 | 0.41-0.61 | <.0001a |
| Days with antineoplastic/CIT drug administration | 0.06 | 0.02-0.10 | <.0001a |
| Days with outpatient services related to the antineoplastic/CIT drug administration | 0.07 | 0.03-0.14 | <.0001a |
| Days with other outpatient services | 0.67 | 0.55-0.81 | <.0001a |
| Days with ER visits | 0.54 | 0.30-0.94 | .0360a |
| Days with other services | 0.77 | 0.41-1.32 | .3240 |
Abbreviations: BR = Bendamustine/rituximab; CI = confidence interval; CIT = chemoimmunotherapy; ER = emergency room; HRU = health care resource utilization; RR = rate ratio.
Indicates P-value < .05.
HRU for the weighted populations were obtained by using inverse probability of treatment weights. The inverse probability of treatment weights were estimated based on propensity score. Variables used in the propensity score calculation included the following baseline characteristics: age, gender, region, quarter and year of index date, insurance plan type, time from first chronic lymphocytic lymphoma diagnosis to index date, Charlson comorbidity index, comorbidities (hypertension, lymphoma, deficiency anemias, diabetes, coagulation deficiency, chronic pulmonary disease), and baseline use of corticosteroids.
The 6-month period spanned from the initiation of front-line therapy up to 6 months after initiation. Even if treatment was stopped or changed to another regimen, the evaluation continues until the end of the 6-month period.
RRs were calculated using generalized linear models with Poisson distribution. Models were adjusted for baseline total all-cause costs.
CIs and P-values were obtained from non-parametric bootstrap procedures using 500 replicates.
Cancer-related HRU was defined as claims with a primary or secondary diagnosis for cancer (International Classification of Diseases, Ninth Edition Clinical Modification codes: 140-239; International Classification of Diseases, Tenth Edition Clinical Modification codes: C00-D49).
Comparison of Health Care Costs
During the front-line therapy period, patients in the ibrutinib cohort incurred $3,766 lower PPPM total all-cause costs versus those in the CIT cohort (P < .05). This difference was driven by $10,079 lower outpatient PPPM costs (P < .05). The difference in outpatient costs was attributable to $6,583 lower PPPM antineoplastic drug costs, $2,494 lower PPPM drug administration costs, and $1,002 lower PPPM costs for other outpatient services not related to the administration of antineoplastic drugs (all P < .05). Patients in the ibrutinib cohort incurred $6,849 higher PPPM pharmacy costs compared with patients in the CIT cohort (P < .05) (Table 4). Higher savings PPPM were observed in the ibrutinib cohort versus the CIT cohort when considering the first 6-month period (mean monthly cost difference [MMCD] = −$8,365; P < .05) (Table 5), with $15,664 savings in medical costs offsetting the $7,299 higher pharmacy costs of ibrutinib.
Table 4.
Comparison of Health Care Costs per Patient per Month Between Ibrutinib and CIT and Between Ibrutinib and BR During the Front-line Therapy Periodb ,c
| MMCDd | 95% CIe | P Valuee | |
|---|---|---|---|
| Ibrutinib versus CIT | |||
| Monthly all-cause total health care costs, US $2017 | −3,766 | −5,016 to −1,947 | <.0001a |
| Medical costs | −10,615 | −12,022 to −8,917 | <.0001a |
| Inpatient costs | −290 | −951 to 516 | .4080 |
| Outpatient costs | −10,079 | −11,208 to −8,907 | <.0001a |
| Antineoplastic/CIT drug costs | −6,583 | −7,325 to −5,906 | <.0001a |
| Costs related to the antineoplastic/CIT drug administration | −2,494 | −3,094 to −1,941 | <.0001a |
| Other outpatient costs | −1,002 | −1,749 to −276 | .0120a |
| ER costs | −150 | −333 to 96 | .1520 |
| Other services costs | −95 | −217 to 38 | .1560 |
| Pharmacy costs | 6,849 | 6,245 to 7,593 | <.0001a |
| Monthly cancer-related total health care costs,f US $2017 | −3,741 | −5,055 to −1,969 | <.0001a |
| Medical costs | −10,528 | −11,921 to −9,003 | <.0001a |
| Inpatient costs | −275 | −941 to 552 | .4400 |
| Outpatient costs | −10,008 | −11,127 to −8,967 | <.0001a |
| Antineoplastic/CIT drug costs | −6,523 | −7,257 to −5,862 | <.0001a |
| Costs related to the antineoplastic/CIT drug administration | −2,468 | −3,077 to −1,926 | <.0001a |
| Other outpatient costs | −1,016 | −1,716 to −369 | .0040a |
| ER costs | −138 | −308 to 65 | .1320 |
| Other services costs | −107 | −183 to −31 | .0160a |
| Pharmacy costs | 6,787 | 6,218 to 7,491 | <.0001a |
| Ibrutinib versus BR | |||
| Monthly all-cause total health care costs, US $2017 | −5,569 | −7,509 to −3,582 | <.0001a |
| Medical costs | −12,571 | −14,576 to −10,772 | <.0001a |
| Inpatient costs | −223 | −1,052 to 920 | .6840 |
| Outpatient costs | −12,088 | −13,766 to −10,635 | <.0001a |
| Antineoplastic/CIT drug costs | −8,683 | −9,971 to −7,642 | <.0001a |
| Costs related to the antineoplastic/CIT drug administration | −2,262 | −3,109 to −1,527 | <.0001a |
| Other outpatient costs | −1,143 | −2,068 to −190 | .0160a |
| ER costs | −216 | −623 to 52 | .1080 |
| Other services costs | −45 | −173 to 108 | .4680 |
| Pharmacy costs | 7,002 | 6,296 to 7,566 | <.0001a |
| Monthly cancer-related total health care costs,f US $2017 | −5,529 | −7,503 to −3,643 | <.0001a |
| Medical costs | −12,462 | −14,465 to −10,606 | <.0001a |
| Inpatient costs | −196 | −1,027 to 957 | .7080 |
| Outpatient costs | −11,994 | −13,626 to −10,602 | <.0001a |
| Antineoplastic/CIT drug costs | −8,600 | −9,892 to −7,544 | <.0001a |
| Costs related to the antineoplastic/CIT drug administration | −2,249 | −3,099 to −1,515 | <.0001a |
| Other outpatient costs | −1,145 | −2,034 to −233 | .0120a |
| ER costs | −199 | −595 to 43 | .1120 |
| Other services costs | −73 | −160 to 3 | .0520 |
| Pharmacy costs | 6,933 | 6,232 to 7,487 | <.0001a |
Abbreviations: BR = Bendamustine/rituximab; CI = confidence interval; CIT = chemoimmunotherapy; ER = emergency room; MMCD = mean monthly cost difference.
Indicates P-value < .05.
Health care costs for the weighted populations were obtained by using inverse probability of treatment weights. The inverse probability of treatment weights were estimated based on propensity score.Variables used in the propensity score calculation included the following baseline characteristics: age, gender, region, quarter and year of index date, insurance plan type, time from first chronic lymphocytic lymphoma diagnosis to index date, Charlson comorbidity index, comorbidities (hypertension, lymphoma, deficiency anemias, diabetes, coagulation deficiency, chronic pulmonary disease), and baseline use of corticosteroids.
The front-line therapy period was defined as the period from the initiation of the front-line therapy to the earliest among discontinuation of the front-line therapy (defined as a gap of more than 90 days between the last day of supply of a claim and the first day of supply of the next claim for the front-line therapy), initiation of a second-line therapy (a new antineoplastic agent not part of the front-line therapy), or end of data.
Cost differences were calculated using weighted generalized linear models with normal distribution. Models were adjusted for baseline total all-cause costs.
CIs and P-values were obtained from non-parametric bootstrap procedures using 500 replicates.
Cancer-related medical costs were defined as claims with a primary or secondary diagnosis for cancer (International Classification of Diseases, Ninth Edition Clinical Modification codes: 140-239; International Classification of Diseases, Tenth Edition Clinical Modification codes: C00-D49). Cancer-related pharmacy costs were defined as costs of antineoplastic agents and of corticosteroids commonly used with antineoplastic agents (ie, prednisone, dexamethasone, and methylprednisolone).
Table 5.
Comparison of Health Care Costs per Patient per Month Between Ibrutinib and CIT and Between Ibrutinib and BR During the First 6-month Period b, c
| MMCDd | 95% CIe | P Valuee | |
|---|---|---|---|
| Ibrutinib versus CIT | |||
| Monthly all-cause total health care costs, US $2017 | −8,365 | −9,975 to −6,464 | <.0001a |
| Medical costs | −15,664 | −17,399 to −13,827 | <.0001a |
| Inpatient costs | −349 | −1,206 to 730 | .4880 |
| Outpatient costs | −14,875 | −16,155 to −13,510 | <.0001a |
| Antineoplastic/CIT drug costs | −9,362 | −10,122 to −8,526 | <.0001a |
| Costs related to the antineoplastic/CIT drug administration | −3,744 | −4,560 to −2,987 | <.0001a |
| Other outpatient costs | −1,769 | −2,525 to −1,000 | <.0001a |
| ER costs | −238 | −427 to 4 | .0520 |
| Other services costs | −201 | −348 to −57 | .0160a |
| Pharmacy costs | 7,299 | 6,713 to 8,042 | <.0001a |
| Monthly cancer-related total health care costs,f US $2017 | −8,301 | −9,901 to −6,534 | <.0001a |
| Medical costs | −15,497 | −17,224 to −13,672 | <.0001a |
| Inpatient costs | −369 | −1,229 to 724 | .4840 |
| Outpatient costs | −14,741 | −16,012 to −13,458 | <.0001a |
| Antineoplastic/CIT drug costs | −9,292 | −10,013 to −8,485 | <.0001a |
| Costs related to the antineoplastic/CIT drug administration | −3,722 | −4,532 to −2,949 | <.0001a |
| Other outpatient costs | −1,726 | −2,445 to −1,027 | <.0001a |
| ER costs | −203 | −369 to 3 | .0520 |
| Other services costs | −184 | −295 to −79 | <.0001a |
| Pharmacy costs | 7,196 | 6,616 to 7,771 | <.0001a |
| Ibrutinib versus BR | |||
| Monthly all-cause total health care costs, US $2017 | −10,896 | −13,060 to −8,755 | <.0001a |
| Medical costs | −18,277 | −20,340 to −16,165 | <.0001a |
| Inpatient costs | −331 | −1,245 to 667 | .4920 |
| Outpatient costs | −17,523 | −19,402 to −15,791 | <.0001a |
| Antineoplastic/CIT drug costs | −12,055 | −13,352 to −10,980 | <.0001a |
| Costs related to the antineoplastic/CIT drug administration | −3,529 | −4,775 to −2,396 | <.0001a |
| Other outpatient costs | −1,939 | −3,015 to −905 | <.0001a |
| ER costs | −298 | −676 to −49 | .0120a |
| Other services costs | −125 | −276 to 95 | .1480 |
| Pharmacy costs | 7,381 | 6,731 to 8,009 | <.0001a |
| Monthly cancer-related total health care costs,f US $2017 | −10,805 | −12,829 to −8,767 | <.0001a |
| Medical costs | −18,088 | −20,081 to −16,047 | <.0001a |
| Inpatient costs | −322 | −1,258 to 684 | .4920 |
| Outpatient costs | −17,376 | −19,173 to −15,729 | <.0001a |
| Antineoplastic/CIT drug costs | −11,990 | −13,274 to −10,926 | <.0001a |
| Costs related to the antineoplastic/CIT drug administration | −3,517 | −4,758 to −2,390 | <.0001a |
| Other outpatient costs | −1,869 | −2,942 to −883 | <.0001a |
| ER costs | −265 | −636 to −42 | .0120a |
| Other services costs | −124 | −247 to 45 | .0960 |
| Pharmacy costs | 7,283 | 6,671 to 7,839 | <.0001a |
Abbreviations: BR = Bendamustine/rituximab; CI = confidence interval; CIT = chemoimmunotherapy; ER = emergency room; MMCD = mean monthly cost difference.
Indicates P-value < .05.
Health care costs for the weighted populations were obtained by using inverse probability of treatment weights. The inverse probability of treatment weights were estimated based on propensity score. Variables used in the propensity score calculation included the following baseline characteristics: age, gender, region, quarter and year of index date, insurance plan type, time from first chronic lymphocytic lymphoma diagnosis to index date, Charlson comorbidity index, comorbidities (hypertension, lymphoma, deficiency anemias, diabetes, coagulation deficiency, chronic pulmonary disease), and baseline use of corticosteroids.
The 6-month period spanned from the initiation of front-line therapy up to 6 months after initiation. Even if treatment was stopped or changed to another regimen, the evaluation continues until the end of the 6-month period.
Cost differences were calculated using weighted generalized linear models with normal distribution. Models were adjusted for baseline total all-cause costs.
CIs and P-values were obtained from non-parametric bootstrap procedures using 500 replicates.
Cancer-related medical costs were defined as claims with a primary or secondary diagnosis for cancer (International Classification of Diseases, Ninth Edition Clinical Modification codes: 140-239; International Classification of Diseases, Tenth Edition Clinical Modification codes: C00-D49). Cancer-related pharmacy costs were defined as costs of antineoplastic agents and of corticosteroids commonly used with antineoplastic agents (ie, prednisone, dexamethasone, and methylprednisolone).
Compared with BR patients, ibrutinib patients had significantly lower PPPM all-cause health care costs (MMCD = −$5,569) during the front-line therapy period. When evaluating other cost strata, results were largely similar to those observed for the ibrutinib versus CIT comparison (Table 4). Similar to previous comparisons, total cost savings were even more pronounced over the first 6-month period, reaching $10,896 PPPM (Table 5). In all comparisons, PPPM cancer-related costs accounted for more than 90% of PPPM all-cause costs. Total cost savings PPPM were similar when considering only cancer-related costs.
Discussion
In this claims-based study, nearly 90% of patients initiated on front-line ibrutinib did not initiate a new treatment after 24 months, suggesting only a limited proportion of patients experienced disease progression during this period. This represented a significantly higher proportion compared with CIT and BR, which is consistent with the superior efficacy observed in clinical trials for ibrutinib.18-24 Front-line treatment of CLL with ibrutinib was also associated with lower total costs when compared with CIT and BR. The higher pharmacy costs associated with ibrutinib were fully offset by lower outpatient drug administration costs in all comparisons. Cost savings related to outpatient services not related to the drug administration were also observed in the ibrutinib cohort compared with the CIT and BR cohorts. Similar conclusions could be drawn whether outcomes were evaluated during the duration of the front-line therapy period, or during the first 6 months of front-line treatment, although differences were more pronounced when considering the latter period.
Although ibrutinib is associated with longer PFS compared with previous CIT standards of care,23,24 concerns were raised regarding its high cost.26 Using a modeling approach, results from Shanafelt et al suggested that the cost of ibrutinib would likely be too prohibitive for some patients to remain on treatment, which could lead to poor medication adherence and real-world outcomes worse than anticipated based on results from randomized controlled trials (RCTs). Although PFS was not assessed in the current study, results from the present real-world study suggest ibrutinib was associated with lower total health care costs and fewer days with ER or outpatient services when compared with CIT and BR. In addition, the higher proportions of patients who remained on front-line treatment in the ibrutinib cohort 24 months post-index also argues against Shanafelt et al’s hypothesis with regards to patients’ adherence. A recent chart review study found that only 10% of patients who temporarily discontinued ibrutinib for a minimum of 60 days and restarted treatment did so owing to economic reasons.33
The results of the present study also contradict those from a recent study conducted by Chen et al, who concluded that the introduction of oral targeted therapies will dramatically increase the overall cost of CLL management over time based on a simulated model.29 Multiple reasons account for the discrepancy with findings from the current study.34 First, as acknowledged by Chen et al, the greater efficacy of new targeted agents23,24 will inevitably lead to a higher prevalence of CLL over time and higher global or lifetime costs. Such way of reporting burden-of-illness statistics is misleading as it depicts higher life expectancy as an additional societal burden. Second, the authors compared the oral targeted therapy scenario with a CIT scenario where patients who fail second-line BR/ofatumumab would transition to the model’s terminal state (ie, death). However, this does not reflect current standard of care and ignores the impact of further lines of therapy on costs. Third, a comprehensive assessment of medical costs should factor in inpatient and outpatient services, but Chen et al relied on literature-derived cost estimates for common adverse events from clinical trials, thus not capturing all types of services provided to the patient. Fourth, Chen et al’s model relied on data extracted from multiple clinical trials and observational studies. This approach may be prone to confounding given that it does not account for heterogeneity in trial design and differences in the outcomes of patients treated in a clinical trial versus those treated in clinical practice. Finally, the assumption that oral targeted therapies will reach a market share of 100% by 2019 appears to be inflated, particularly in light of the present results (in 2017: NIbrutinib = 147, NCIT = 87).
Results from the present study suggest that ibrutinib treatment leads to lower HRU and lower medical costs compared with CIT. Notably, these lower medical costs fully offset the higher pharmacy costs of ibrutinib and led to savings in total health care costs. Interestingly, although ibrutinib outpatient drug administration costs were expected to be lower than those for CIT, given the drug’s oral mode of administration, other outpatient costs not related to drug administration were also lower. Although the current study did not evaluate efficacy, these results suggest that at least part of the cost differences observed in the current study may be driven by the higher efficacy of ibrutinib relative to CIT, which was demonstrated in recent phase III trials.23,24 Further research is warranted to validate whether the longer PFS associated with ibrutinib single-agent may drive part of the cost savings observed in the current study.
Results from the current study also suggest that patients treated with front-line ibrutinib therapy were less likely to initiate second-line treatment. Almost 90% of patients initiated on front-line ibrutinib treatment did not initiate a new treatment after 2 years. This result is consistent with the high PFS rates observed across all ibrutinib trials.18-24 More specifically, this is consistent with the results of the E1912 and A041202 phase III trials, which showed that front-line ibrutinib was associated with longer PFS than BR,24 and that ibrutinib plus rituximab led to longer PFS and OS than FCR.23,24 Therefore, the present study builds on the results from these trials by providing evidence that the higher efficacy of front-line ibrutinib versus CIT may translate into the real world and lead to cost savings for payers.
Limitations
Prior to 2016, ibrutinib was approved as a front-line treatment only in patients with 17p deletions and may still be used more often in populations with a poor prognosis. As TP53 mutations are associated with an unfavorable prognosis, the conclusions of the current study are likely conservative estimates of the potential cost savings associated with ibrutinib treatment relative to CIT. Conversely, the BR cohort may include fewer patients with TP53 mutations or 17p deletions as this regimen was found poorly effective for this population in second-line.35 Given that mutation status was not available in the current study, sensitivity analyses could not be performed on subgroup of patients with different mutation profiles. Next, given the low sample of patients initiated on FCR (N = 134) or obinutuzumab-based CIT (N = 89) and the very different profile of these patients (eg, in terms of age for FCR), comparisons between ibrutinib and these 2 groups of patients were not performed. Another limitation is that despite adjusting for observed confounders using IPTW, the contribution of unobserved confounders cannot be ruled out. In the A041202 trial, median PFS was 43 months in patients treated with BR,24 with 67% of patients who completed the 6 cycles of therapy. Because the observed median duration of the front-line therapy period reached 10.6 months, this suggests that progression was not observed for many patients and that patients’ observation period has been truncated upon end of eligibility or lack of available follow-up data. Therefore, the results of the present study may not totally reflect outcomes incurred during the real front-line therapy period, which should end at the earliest of initiation of second-line therapy or death. It should also be noted that the present analyses may not be generalized to patients treated outside the US because of geographic disparities in drug costs and cost of administration of outpatient intravenous therapies in the US versus elsewhere.36 Finally, claims data may contain omissions and inaccuracies, but this is expected to equally affect all cohorts, and, thus, should not impact the overarching conclusions of this study.
Conclusions
Almost 90% of patients initiated on frontline ibrutinib did not initiate a new treatment after 2 years, which is higher than the proportion observed in CIT-treated patients. These results are consistent with the high rates of PFS observed in ibrutinib RCTs.18-22 Ibrutinib-treated patients incurred lower HRU and health care costs than CIT-treated patients during their front-line of therapy; this difference was more pronounced over the first 6 months of treatment. In addition to drug administration-related costs, outpatient costs unrelated to drug administration were also lower in ibrutinib-treated patients. Similar results were found when comparing ibrutinib with BR.
Supplementary Material
Clinical Practice Points.
For patients with CLL, rituximab-based CIT, such as BR, was, until recently, a standard of care; overall, these regimens remain commonly utilized in the real-world. However, some CITs have a poor tolerability profile or limited efficacy in specific sub-populations. Ibrutinib is the only single-agent targeted therapy approved for CLL in the front-line setting regardless of TP53 mutation. To the best of our knowledge, none of the studies that assessed the economic burden of patients treated with ibrutinib used empirical data collected in the United States and included medical costs in addition to pharmacy costs.
This study was conducted to compare TTNT, HRU, and total direct health care costs in patients with CLL initiated on frontline treatment with ibrutinib single agent versus CIT regimens and versus BR specifically.
In the present study, ibrutinib single agent was associated with longer TTNT (HR, 0.54), fewer monthly days with outpatient visits (rate ratio, 0.75), and monthly total cost savings (mean monthly cost difference = −$3,766), compared with CIT and BR during the total duration of front-line treatment. Cost savings and reductions in HRU were even more pronounced when considering only the first 6 months of front-line treatment.
These results suggest that ibrutinib single-agent is associated with lower total costs driven by lower medical costs, despite higher pharmacy costs, compared with CIT and BR. Patients may benefit from oral ibrutinib treatment, as shown by longer TTNT and lower HRU and costs compared with CIT and BR.
Acknowledgments
Medical writing assistance was provided by Samuel Rochette, an employee of Analysis Group, Inc. This work was supported by Janssen Scientific Affairs, LLC. The sponsor was involved in all steps of the present work, including the design of the study; the collection, analysis, and interpretation of data; the writing of the report; and the decision to submit the article for publication.
Footnotes
Disclosure
MS is an employee of Janssen Scientific Affairs, LLC and may hold stock in Johnson and Johnson. SW was an employee of Janssen Scientific Affairs, LLC at the time the study was conducted. BE, HR, and PL are employees of Analysis Group, Inc, a consulting company that provided paid consulting services to Janssen Scientific Affairs, LLC for the conduct of this study. AM reports the following affiliations: AbbVie (consultant/advisory board/research grant), Acerta (consultant/advisory board/research grant), BeiGene (research grant), DTRM (research grant), Johnson and Johnson (employment/ownership interest), Sunesis (research grant), and TG Therapeutics (consultant/advisory board/research grant).
Supplemental Data
Supplemental table accompanying this article can be found in the online version at https://doi.org/10.1016/jxlml.2019.08.004.
References
- 1.Hallek M, Fischer K, Fingerle-Rowson G, et al. , International Group of Investigators, German Chronic Lymphocytic Leukaemia Study Group. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet 2010; 376: 1164–74. [DOI] [PubMed] [Google Scholar]
- 2.Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med 2014; 370:1101–10. [DOI] [PubMed] [Google Scholar]
- 3.Hillmen P, Robak T, Janssens A, et al. , COMPLEMENT 1 Study Investigators. Chlorambucil plus ofatumumab versus chlorambucil alone in previously untreated patients with chronic lymphocytic leukaemia (COMPLEMENT 1): a randomised, multicentre, open-label phase 3 trial. Lancet 2015; 385:1873–83. [DOI] [PubMed] [Google Scholar]
- 4.Hallek M, Shanafelt TD, Eichhorst B. Chronic lymphocytic leukaemia. Lancet 2018; 391:1524–37. [DOI] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network. Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma version 1.2017, 2018. Available at: https://www.nccn.org/professionals/physician_gls/pdf/cll.pdf. Accessed: September 1, 2018.
- 6.Seymour EK, Ruterbusch JJ, Beebe-Dimmer JL, Schiffer CA. Real-world testing and treatment patterns in chronic lymphocytic leukemia (CLL) in the United States during the modern era: a SEER patterns of care analysis. Blood 2017; 130:3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer K, Cramer P, Busch R, et al. Bendamustine combined with rituximab in patients with relapsed and/or refractory chronic lymphocytic leukemia: a multi-center phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol 2011; 29:3559–66. [DOI] [PubMed] [Google Scholar]
- 8.Dohner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med 2000; 343:1910–6. [DOI] [PubMed] [Google Scholar]
- 9.Zenz T, Eichhorst B, Busch R, et al. TP53 mutation and survival in chronic lymphocytic leukemia. J Clin Oncol 2010; 28:4473–9. [DOI] [PubMed] [Google Scholar]
- 10.Fischer K, Bahlo J, Fink AM, et al. Long-term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: updated results of the CLL8 trial. Blood 2016; 127:208–15. [DOI] [PubMed] [Google Scholar]
- 11.Maurer C, Langerbeins P, Bahlo J, et al. Effect of first-line treatment on second primary malignancies and Richter’s transformation in patients with CLL. Leukemia 2016; 30:2019–25. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Y, Tang G, Medeiros LJ, et al. Therapy-related myeloid neoplasms following fludarabine, cyclophosphamide, and rituximab (FCR) treatment in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma. Mod Pathol 2012; 25:237–45. [DOI] [PubMed] [Google Scholar]
- 13.Boddy CS, Ma S. Frontline therapy of CLL: evolving treatment paradigm. Curr Hematol Malig Rep 2018; 13:69–77. [DOI] [PubMed] [Google Scholar]
- 14.United States Food and Drug Administration. Highlights of Prescribing Information - Venclexta (Venetoclax), 2019. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208573s000lbl.pdf. Accessed: July 1, 2019.
- 15.United States Food and Drug Administration. Medication Guide - Zydelig (Idelalisib), 2018. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/206545lbl.pdf. Accessed: July 1, 2019.
- 16.United States Food and Drug Administration. Highlights of Prescribing Information - Imbruvica (Ibrutinib), 2018. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/205552s002lbl.pdf. Accessed: July 1, 2019.
- 17.National Comprehensive Cancer Network. Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma version 4.2019, 2019. Available at: https://www.nccn.org/professionals/physician_gls/pdf/cll.pdf. Accessed: September 1, 2018.
- 18.Burger JA, Tedeschi A, Barr PM, et al. , RESONATE-2 Investigators. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med 2015; 373:2425–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Byrd JC, Brown JR, O’Brien S, et al. , RESONATE Investigators. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med 2014; 371:213–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med 2013; 369:32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chanan-Khan A, Cramer P, Demirkan F, et al. , HELIOS investigators. Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): a randomised, double-blind, phase 3 study. Lancet Oncol 2016; 17:200–11. [DOI] [PubMed] [Google Scholar]
- 22.O’Brien S, Jones JA, Coutre SE, et al. Ibrutinib for patients with relapsed or refractory chronic lymphocytic leukaemia with 17p deletion (RESONATE-17): a phase 2, open-label, multicentre study. Lancet Oncol 2016; 17:1409–18. [DOI] [PubMed] [Google Scholar]
- 23.Shanafelt TD, Wang V, Kay NE, et al. Randomized phase III study of ibrutinib (PCI-32765)-based therapy vs. standard fludarabine, cyclophosphamide, and rituximab (FCR) chemoimmunotherapy in untreated younger patients with chronic lymphocytic leukemia (CLL): a trial of the ECOG-ACRIN Cancer Research Group (E1912). Presented at the 60th American Society of Hematology (ASH) Annual Meeting, San Diego, CA, 2018. Blood 2018, 132:LBA–4A. [Google Scholar]
- 24.Woyach JA, Ruppert AS, Heerema NA, et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med 2018; 379: 2517–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.United States Food and Drug Administration. Highlights of prescribing information - Rituxan (rituximab). Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/103705s5311lbl.pdf. Accessed: July 1, 2019.
- 26.Shanafelt TD, Borah BJ, Finnes HD, et al. Impact of ibrutinib and idelalisib on the pharmaceutical cost of treating chronic lymphocytic leukemia at the individual and societal levels. J Oncol Pract 2015; 11:252–8. [DOI] [PubMed] [Google Scholar]
- 27.Mahlich J, Okamoto S, Tsubota A. Cost of illness of Japanese patients with chronic lymphocytic leukemia (CLL), and budget impact of the market introduction of ibrutinib. Pharmacoecon Open 2017; 1:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sinha R, Redekop WK. Cost-effectiveness of ibrutinib compared with obinutuzumab with chlorambucil in untreated chronic lymphocytic leukemia patients with comorbidities in the United Kingdom. Clin Lymphoma Myeloma Leuk 2018; 18: e131–42. [DOI] [PubMed] [Google Scholar]
- 29.Chen Q, Jain N, Ayer T, et al. Economic burden of chronic lymphocytic leukemia in the era of oral targeted therapies in the United States. J Clin Oncol 2017; 35: 166–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Centers for Medicare & Medicaid Services (CMS). Oncology Care Model Fact Sheet, 2016. Available at: https://www.cms.gov/newsroom/fact-sheets/oncology-care-model. Accessed: September 1, 2018.
- 31.Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Comm Stat Simul Comput 2009; 38:1228–34. [Google Scholar]
- 32.Cohen J Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988:20–6. [Google Scholar]
- 33.Mato AR, Samp JC, Gauthier G, Terasawa E, Brander DM. Drivers of treatment patterns in patients with chronic lymphocytic leukemia stopping ibrutinib or idelalisib therapies. Cancer Biol Ther 2018; 19:636–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nabhan C, Mato AR. Economic modeling of the cost of chronic lymphocytic leukemia therapy: it is about the model. J Clin Oncol 2017; 35:1863–4. [DOI] [PubMed] [Google Scholar]
- 35.Fisher RI. Advanced insights into the biology of malignant lymphomas. J Clin Oncol 2011; 29:1799–800. [DOI] [PubMed] [Google Scholar]
- 36.Hess LM, Rajan N, Winfree K, et al. Cost analyses in the US and Japan: a cross-country comparative analysis applied to the PRONOUNCE trial in non-squamous non-small cell lung cancer. Adv Ther 2015; 32:1248–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.